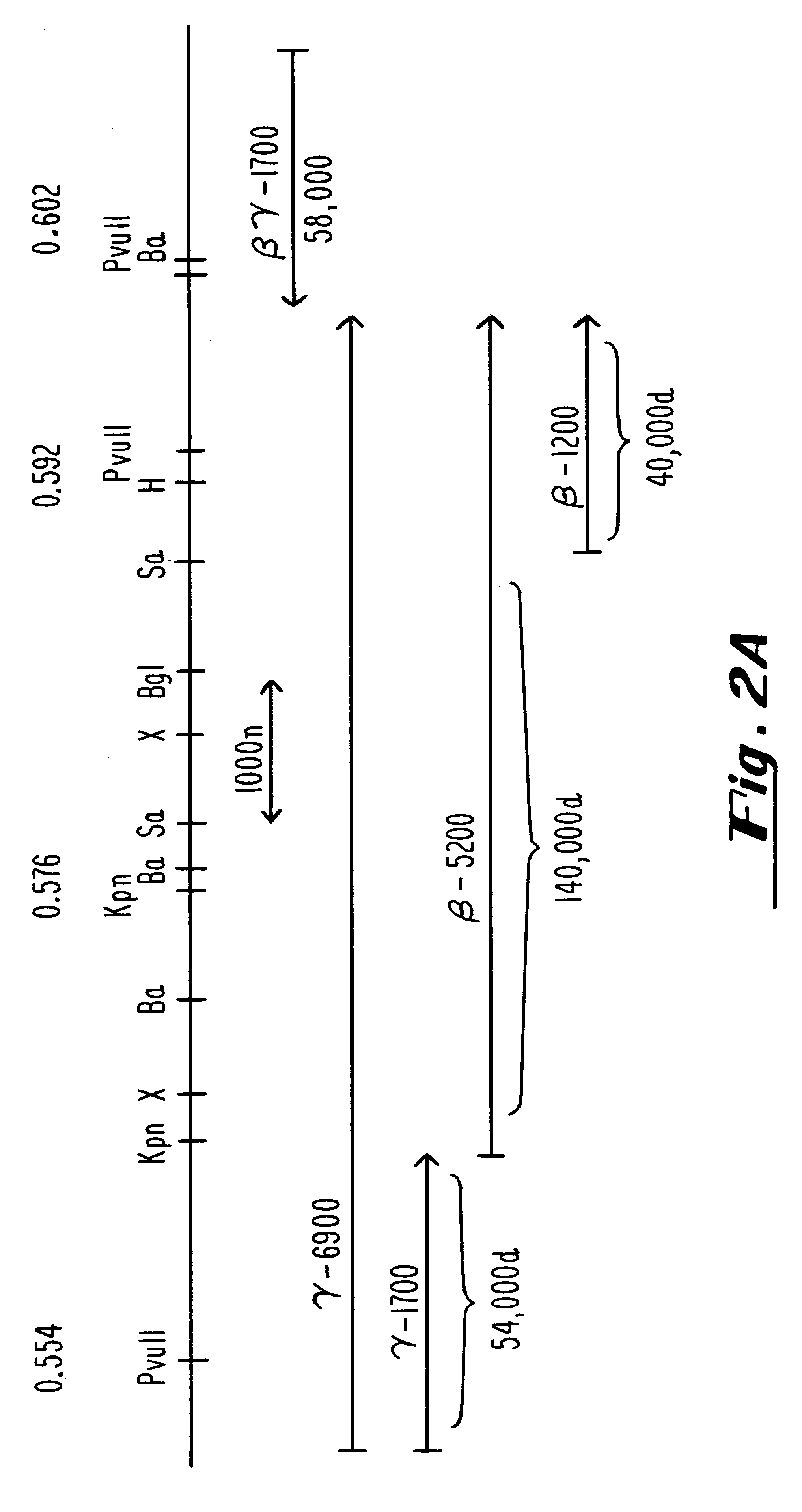
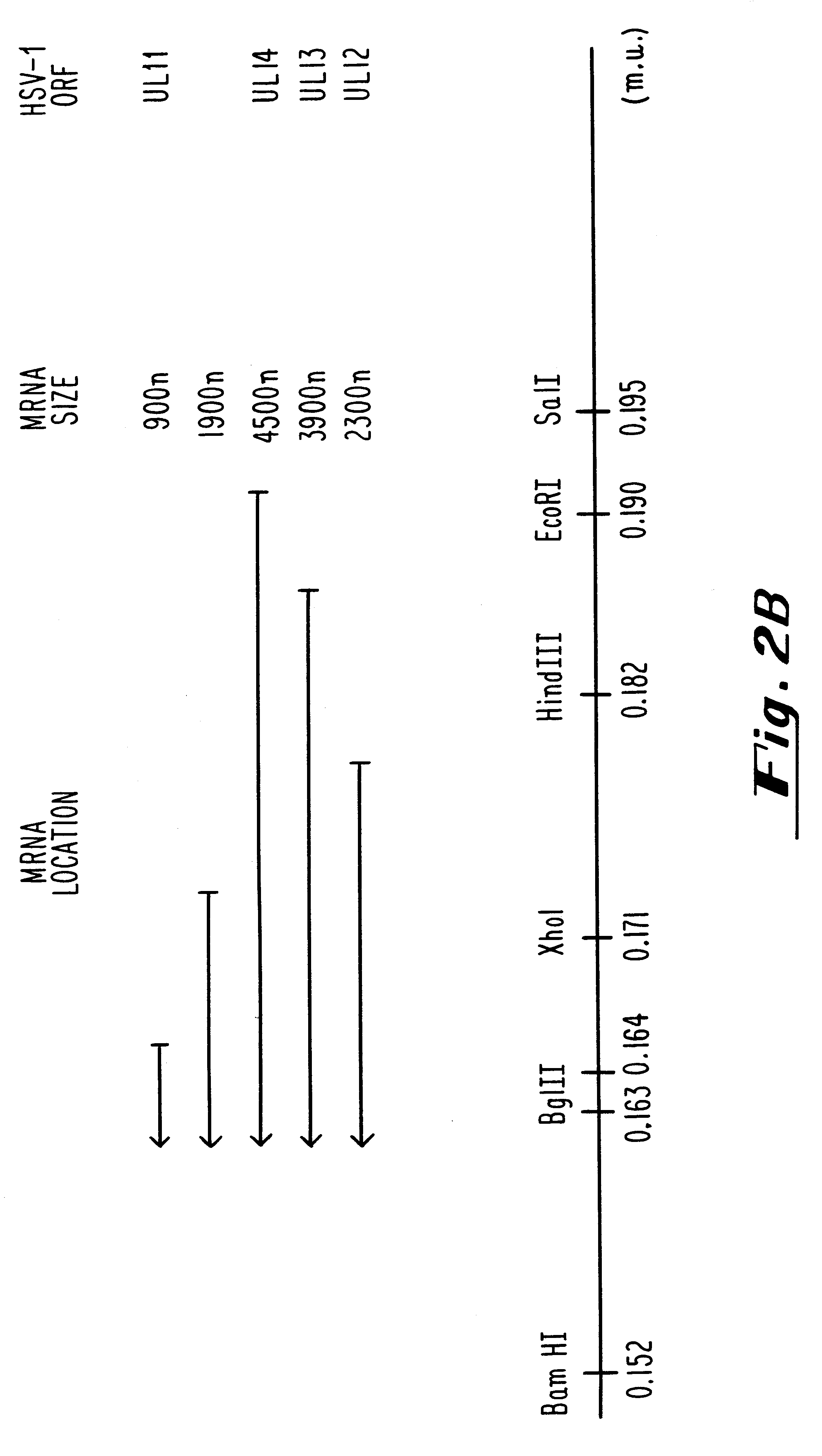
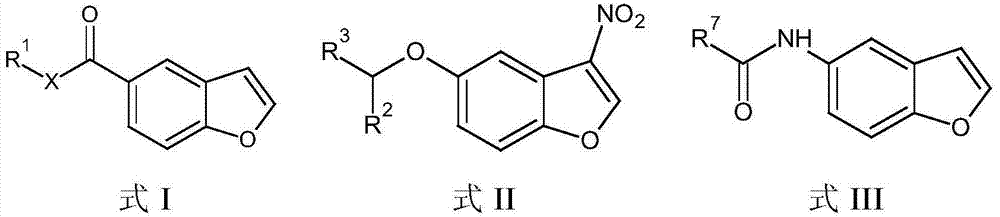
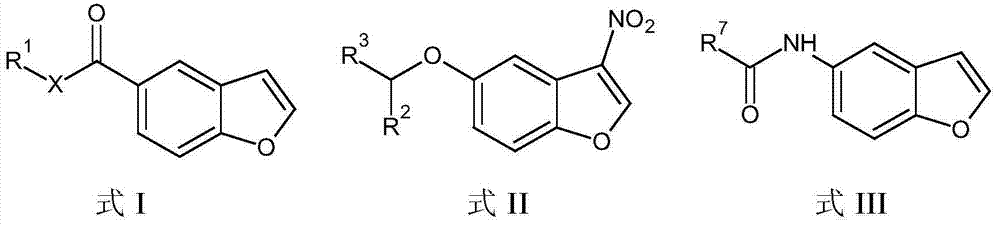
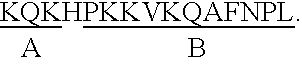Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Epstein bar virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quantitative epstein barr virus PCR rapid assay
InactiveUS6790952B2Sensitive and accurateSugar derivativesMicrobiological testing/measurementEpstein bar virusNucleotide
The present invention provides novel compositions comprising Epstein-Barr virus-specific oligonucleotides that are useful as primers to amplify particular regions of the genome during enzymatic nucleic acid amplification. The invention also provides a rapid, sensitive and specific method for the detection and quantitation of the virus which may be present in a clinical specimen, using the virus-specific primers and enzymatic nucleic acid amplification; hybridization of amplified target sequences, if present, with one or more Epstein-Barr virus-specific oligonucleotide probes which are labeled with a detectable moiety; and detection of the detectable moiety of labeled oligonucleotide probe hybridized to amplified target sequences of Epstein-Barr virus DNA.
Owner:CHILDRENS HOSPITAL RES FOUND
Methods and kits for diagnosis, prognosis or monitoring of Epstein-Barr virus (EBV)-associated cancer
InactiveUS20080206749A1Lower Level RequirementsEliminate false positive caseMicrobiological testing/measurementFermentationBacteriuriaNon invasive
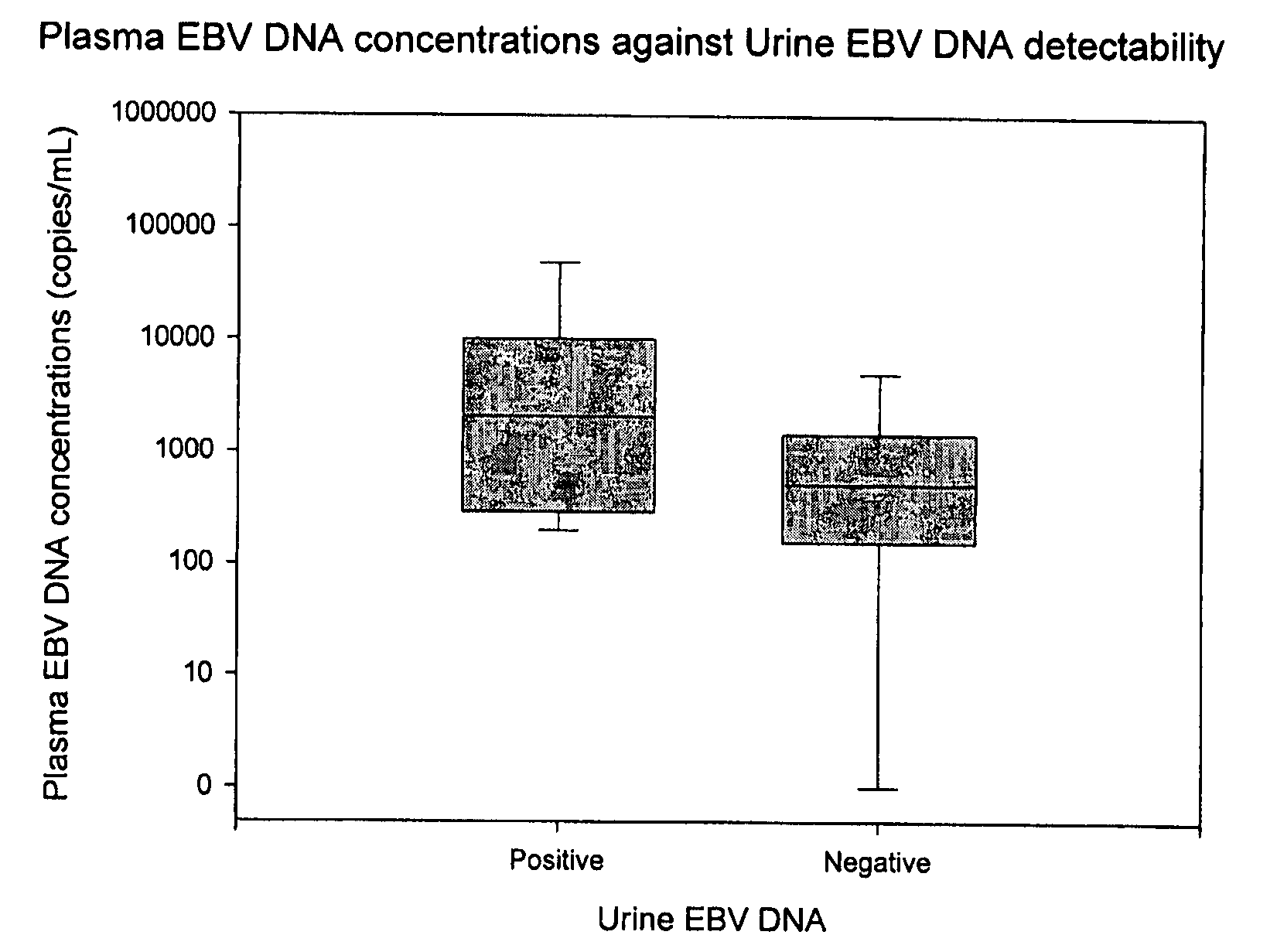
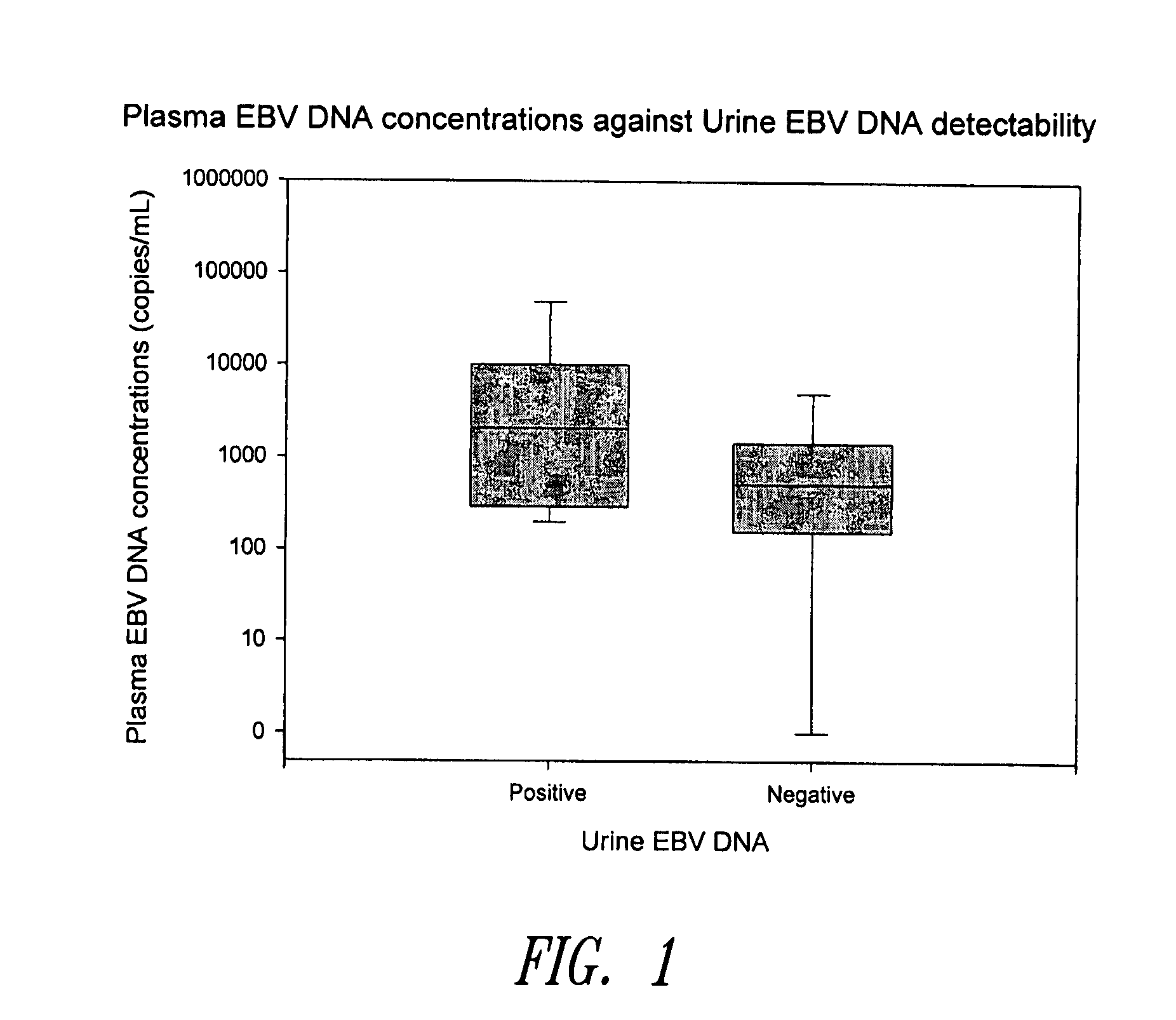
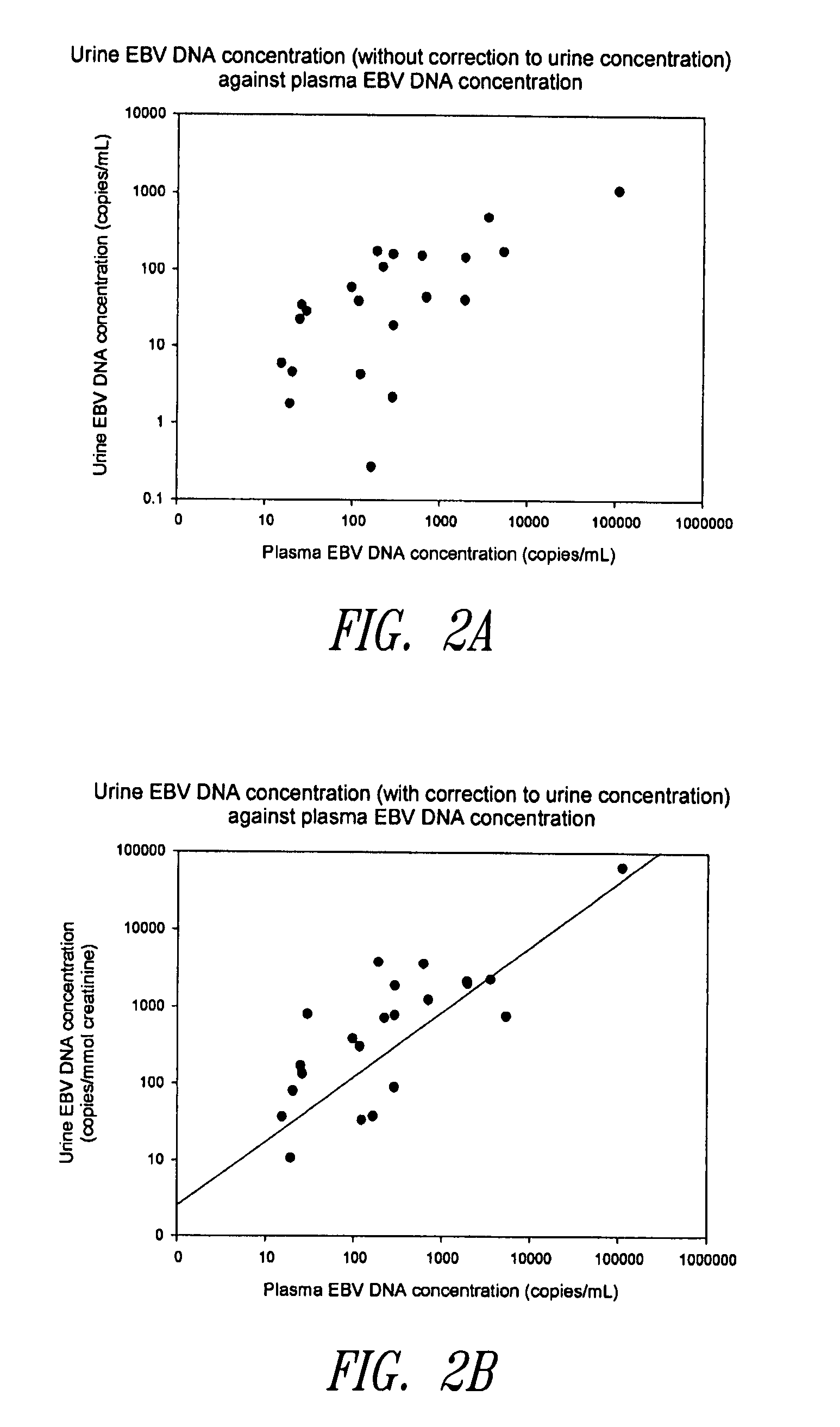
Disclosed is a non-invasive method for diagnosis, prognosis or monitoring of Epstein-Barr virus (EBV)-associated cancer by detecting and / or quantifying EBV associated nucleic acid fragments in a urine sample from an individual. Kits for diagnosis, prognosis or monitoring of cancer are also disclosed.
Owner:HONG KONG THE CHINESE UNIV OF
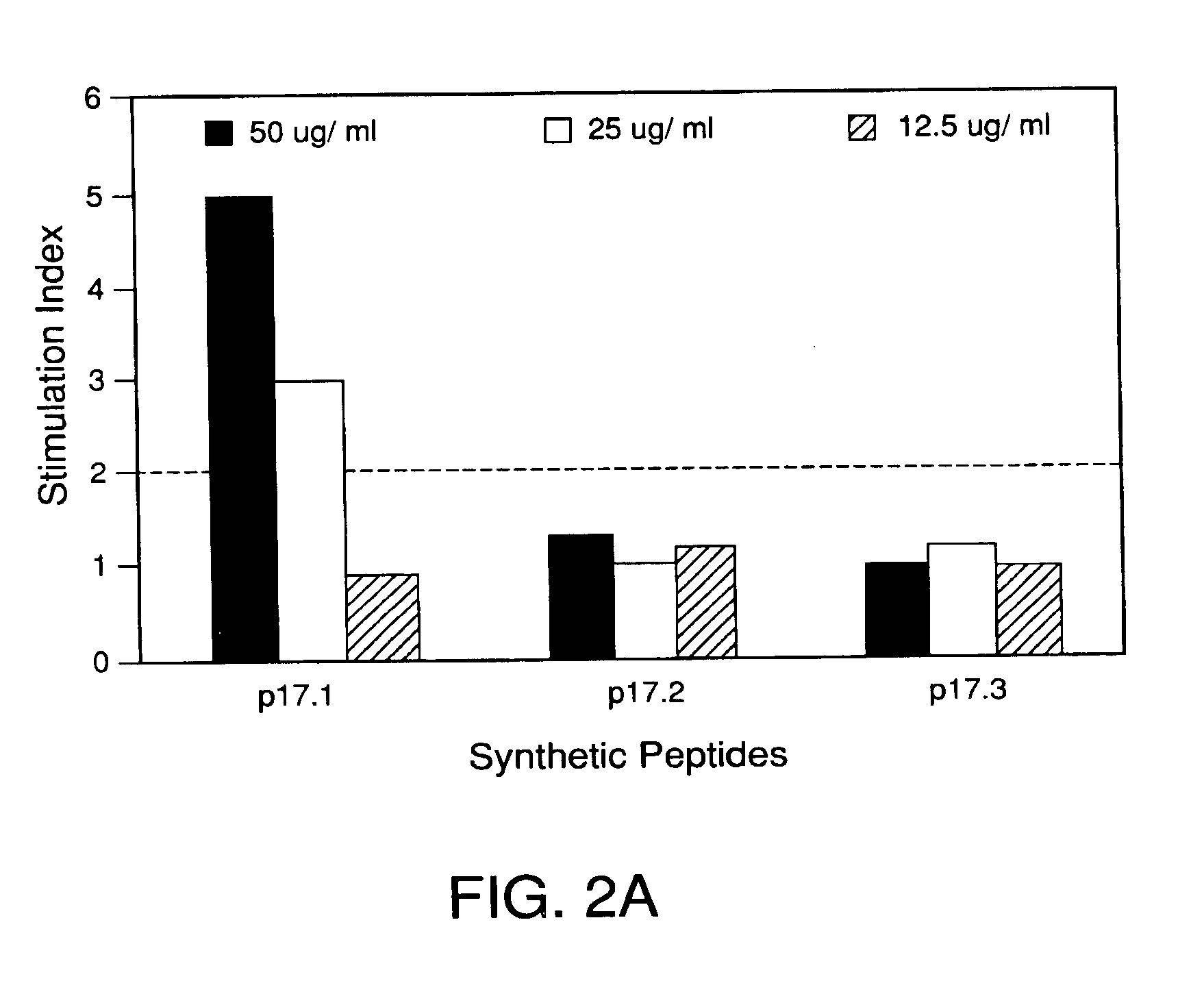
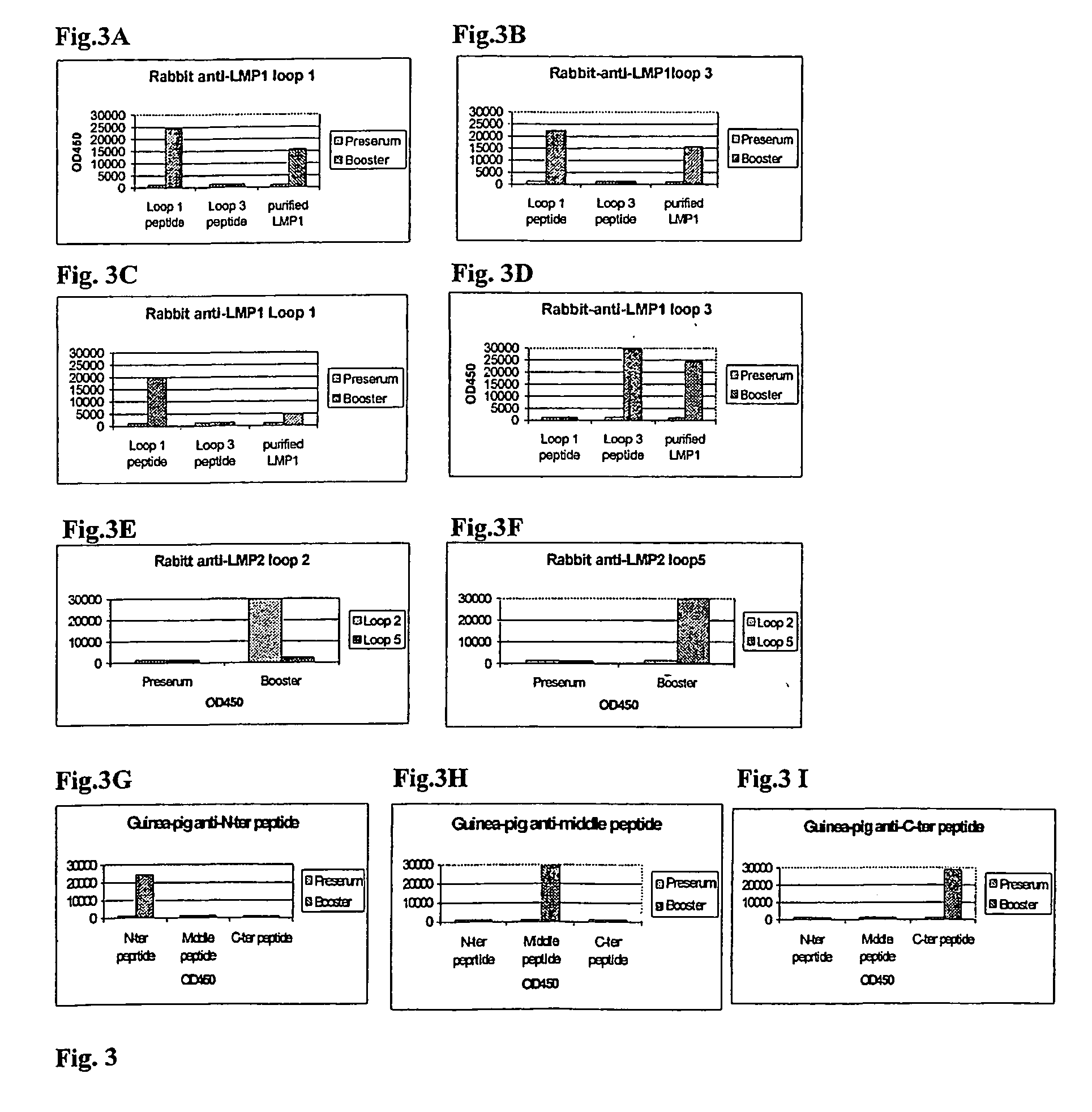
Cytotoxic t-cell epitope peptides that specifically attack epstein-barr virus-infected cells and uses thereof
InactiveUS20090305324A1Peptide/protein ingredientsGenetic material ingredientsEpitopeNatural Killer Cell Inhibitory Receptors
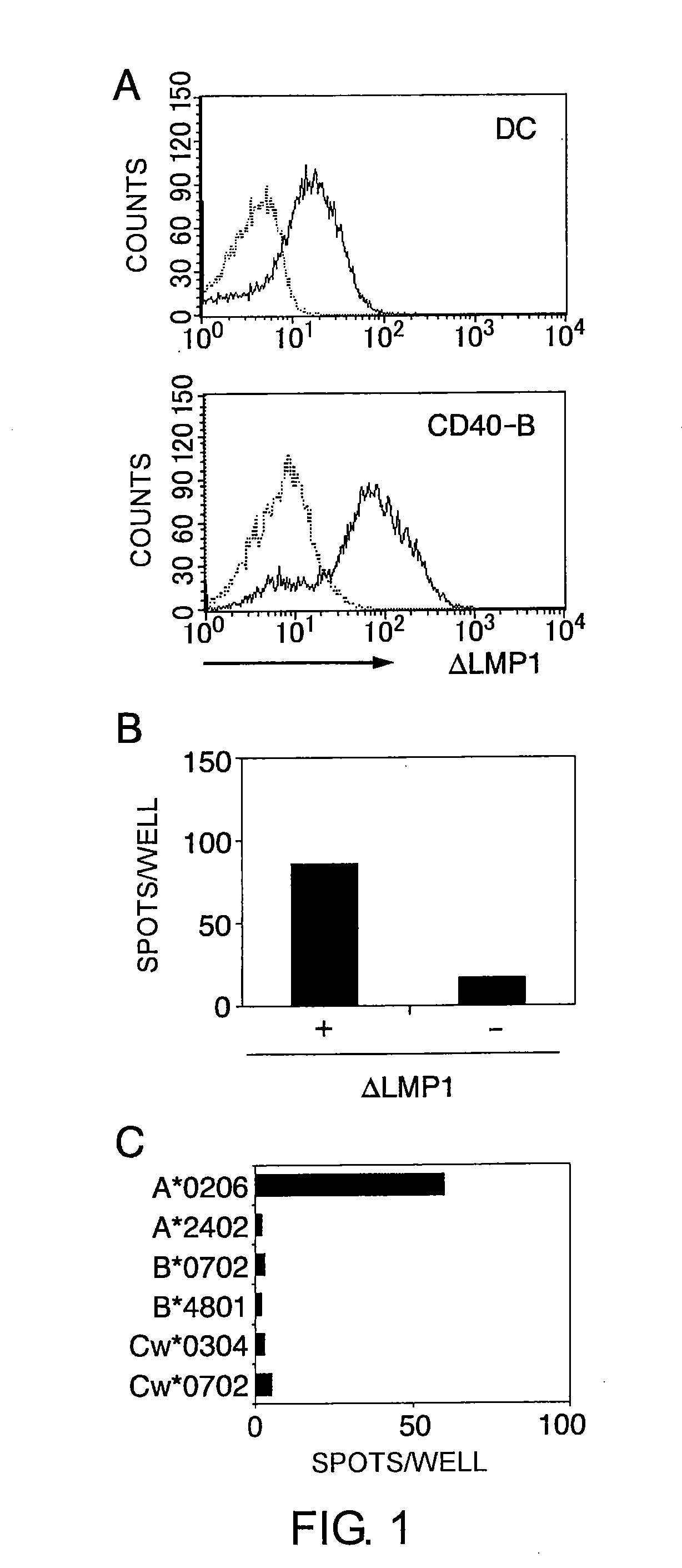
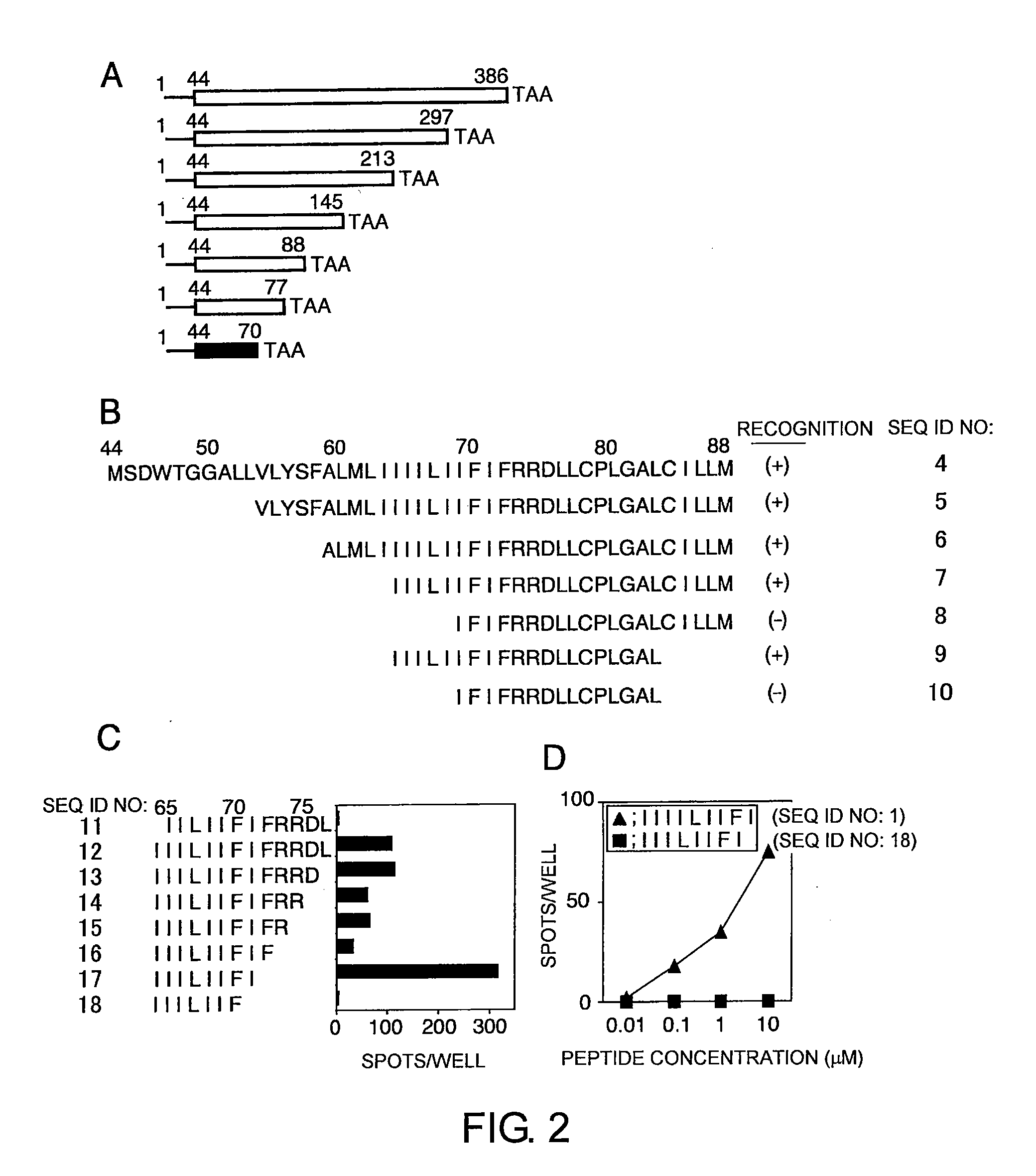
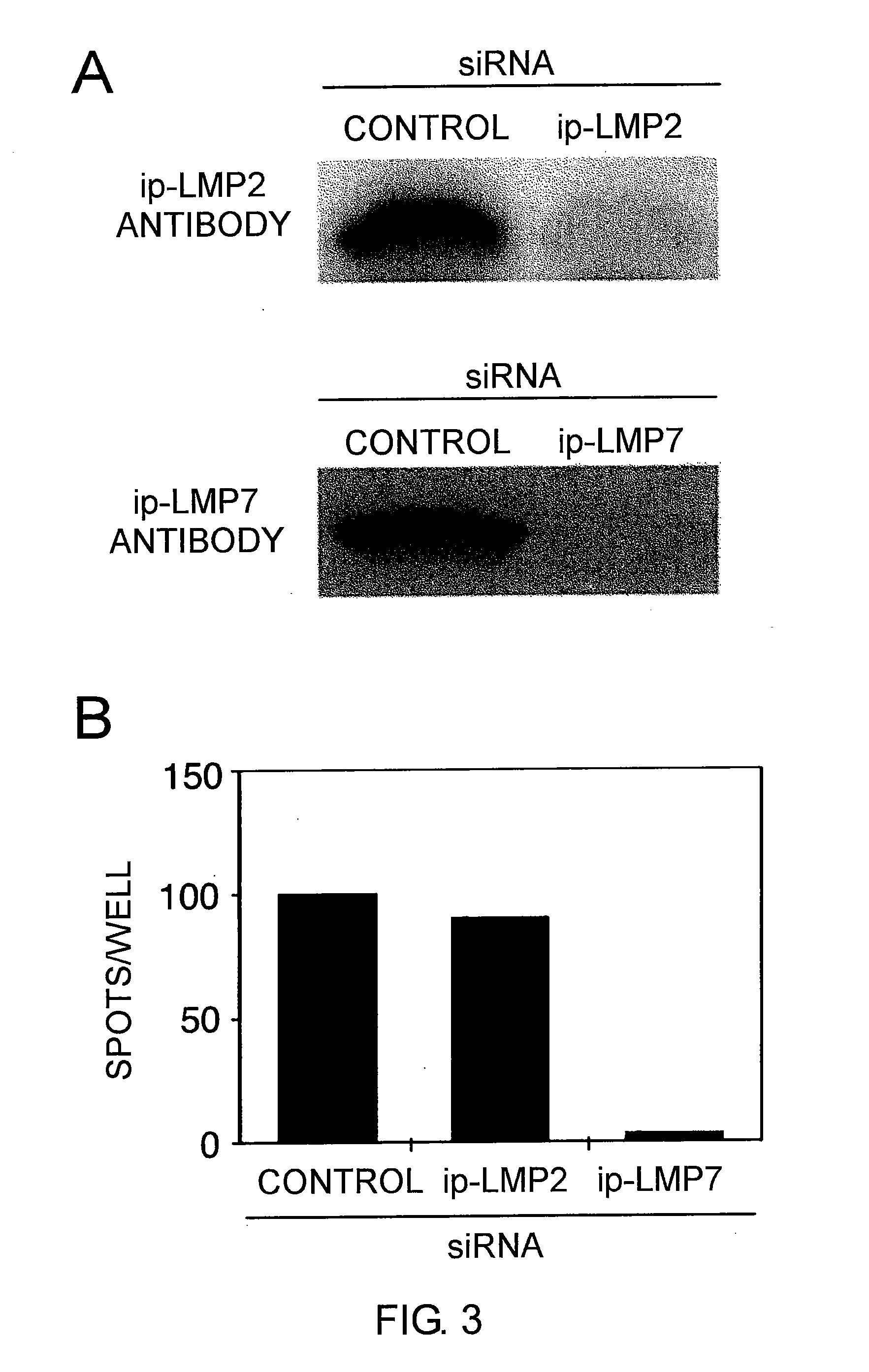
The present inventors introduced mRNAs for the Epstein-Barr virus proteins LMP1 and EBNA1 into antigen-presenting cells, and as a result, demonstrated that the cells induced Epstein-Barr virus-specific cytotoxic T cells. The present inventors also demonstrated that the cytotoxic T cells recognized epitope peptides presented via HLA-A*0206, HLA-Cw*0303, or HLA-Cw*0304, inhibited the outgrowth of Epstein-Barr virus-infected B cells, and lysed Epstein-Barr virus-infected NK lymphomas and NK cells.
Owner:AICHI PREFECTURE +1
Monoclonal antibody production by ebv transformation of b cells
ActiveUS20100021470A1Improve efficiencyRapid isolationImmunoglobulins against bacteriaImmunoglobulins against virusesEpstein bar virusLymphocyte
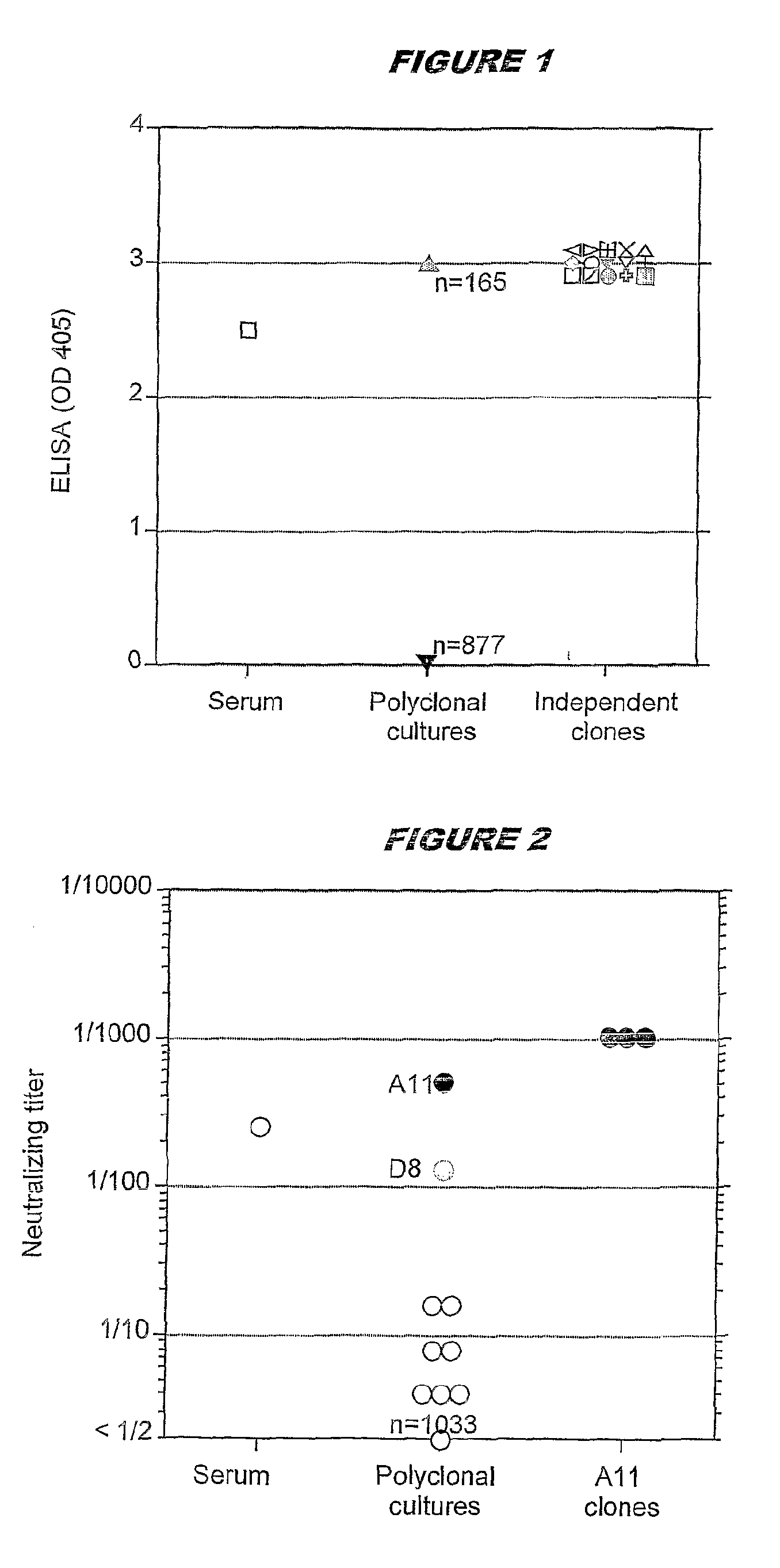
A method for producing a clone of an immortalised human B memory lymphocyte, comprising the step of transforming human B memory lymphocytes using Epstein Barr Virus (EBV) in the presence of a polyclonal B cell activator. The method is particularly useful in a method for producing a clone of an immortalised human B memory lymphocyte capable of producing a human monoclonal antibody with a desired antigen specificity, comprising the steps of: (i) selecting and isolating a human memory B lymphocyte subpopulation; (ii) transforming the subpopulation with Epstein Barr Virus (EBV) in the presence of a polyclonal B cell activator; (iii) screening the culture supernatant for antigen specificity; and (iv) isolating an immortalised human B memory lymphocyte clone capable of producing a human monoclonal antibody having the desired antigen specificity.
Owner:INSTITUTE FOR RESEARCH IN BIOMEDECINE
Protective antigen of Epstein Barr Virus
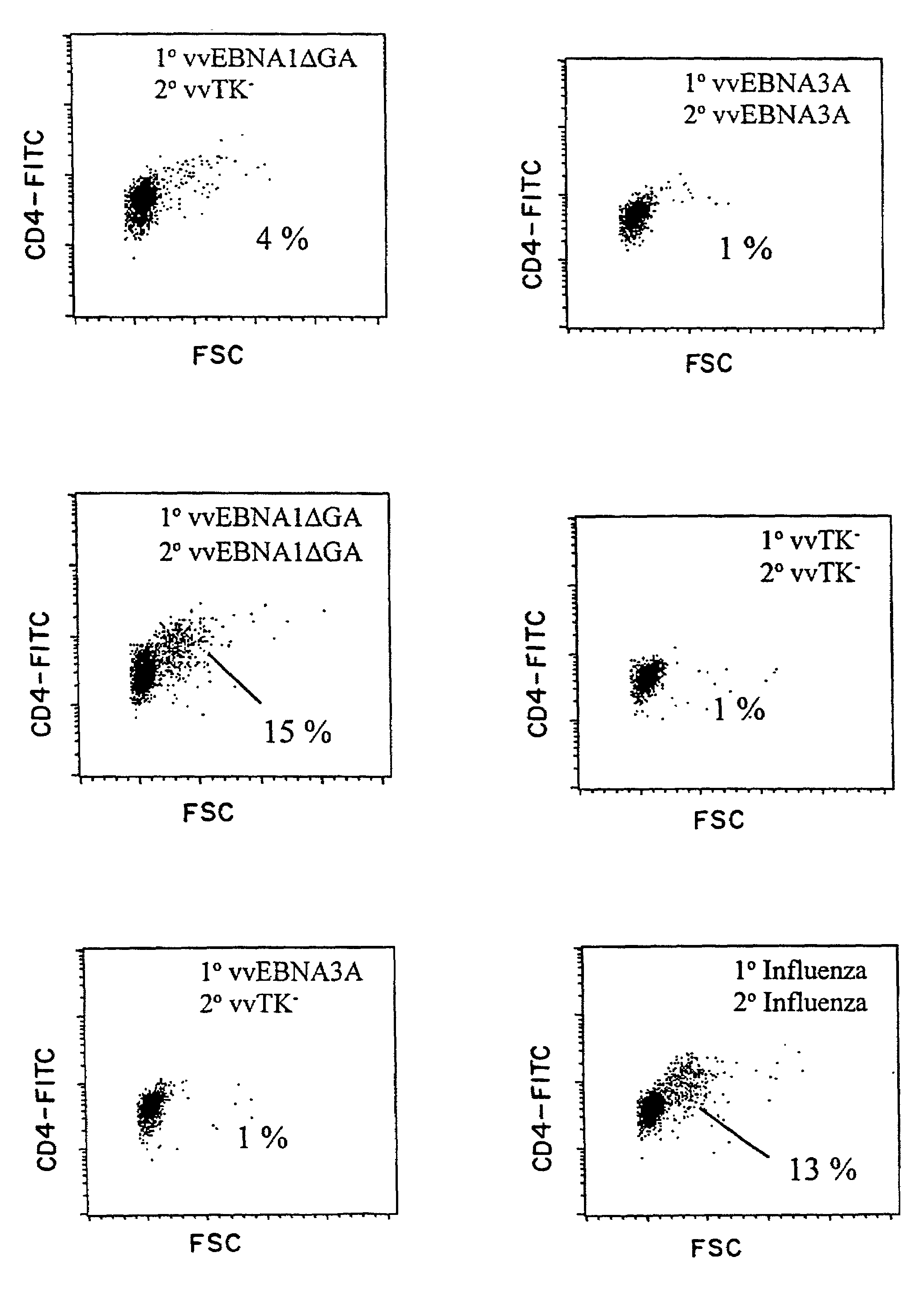
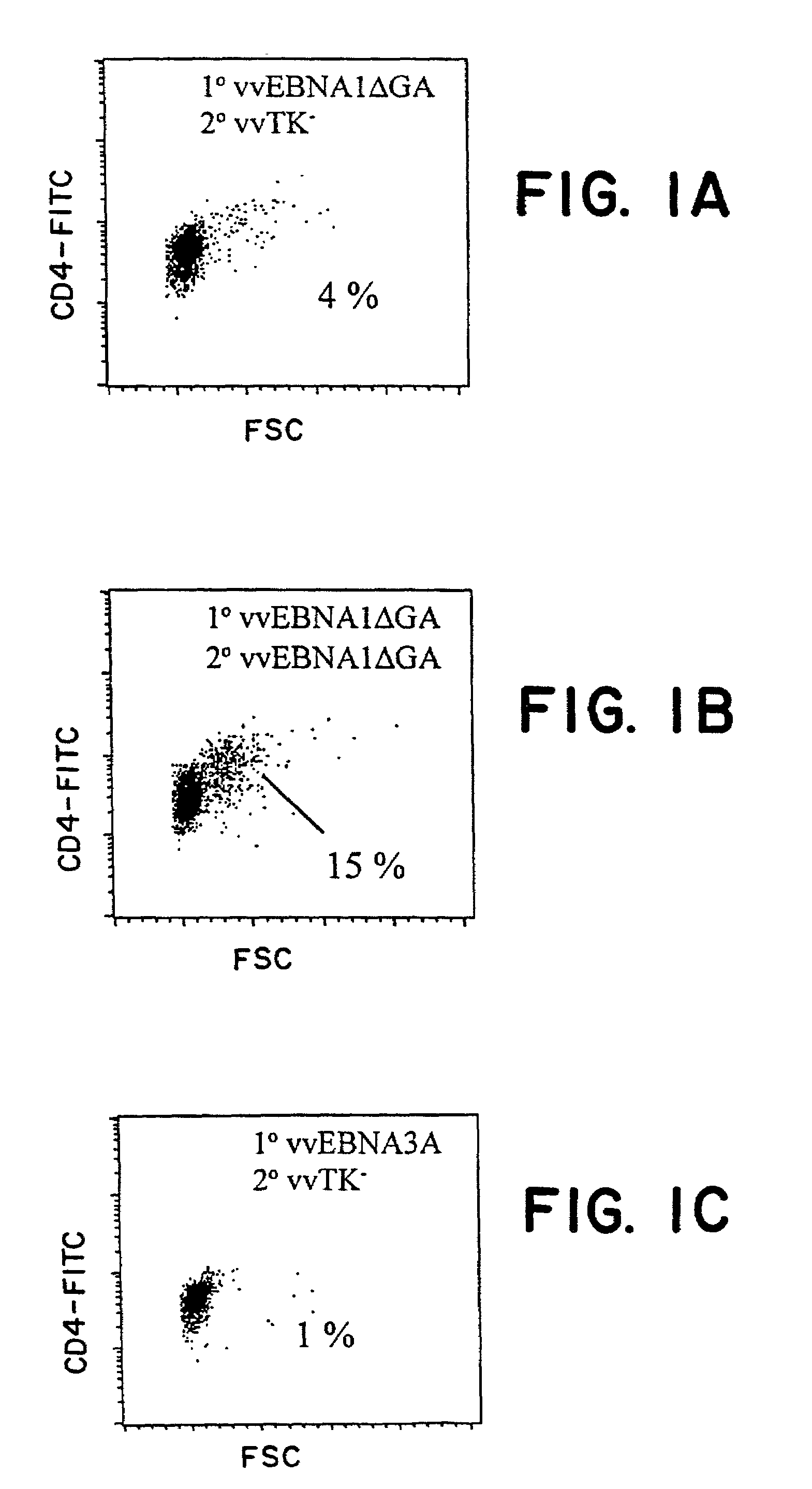
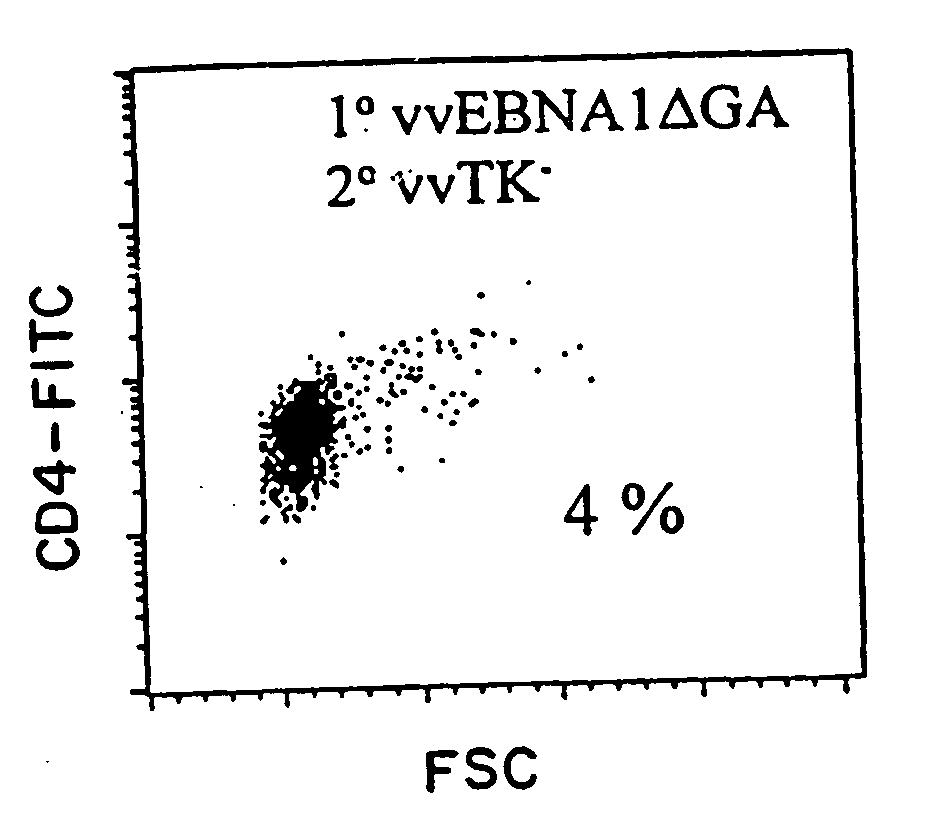
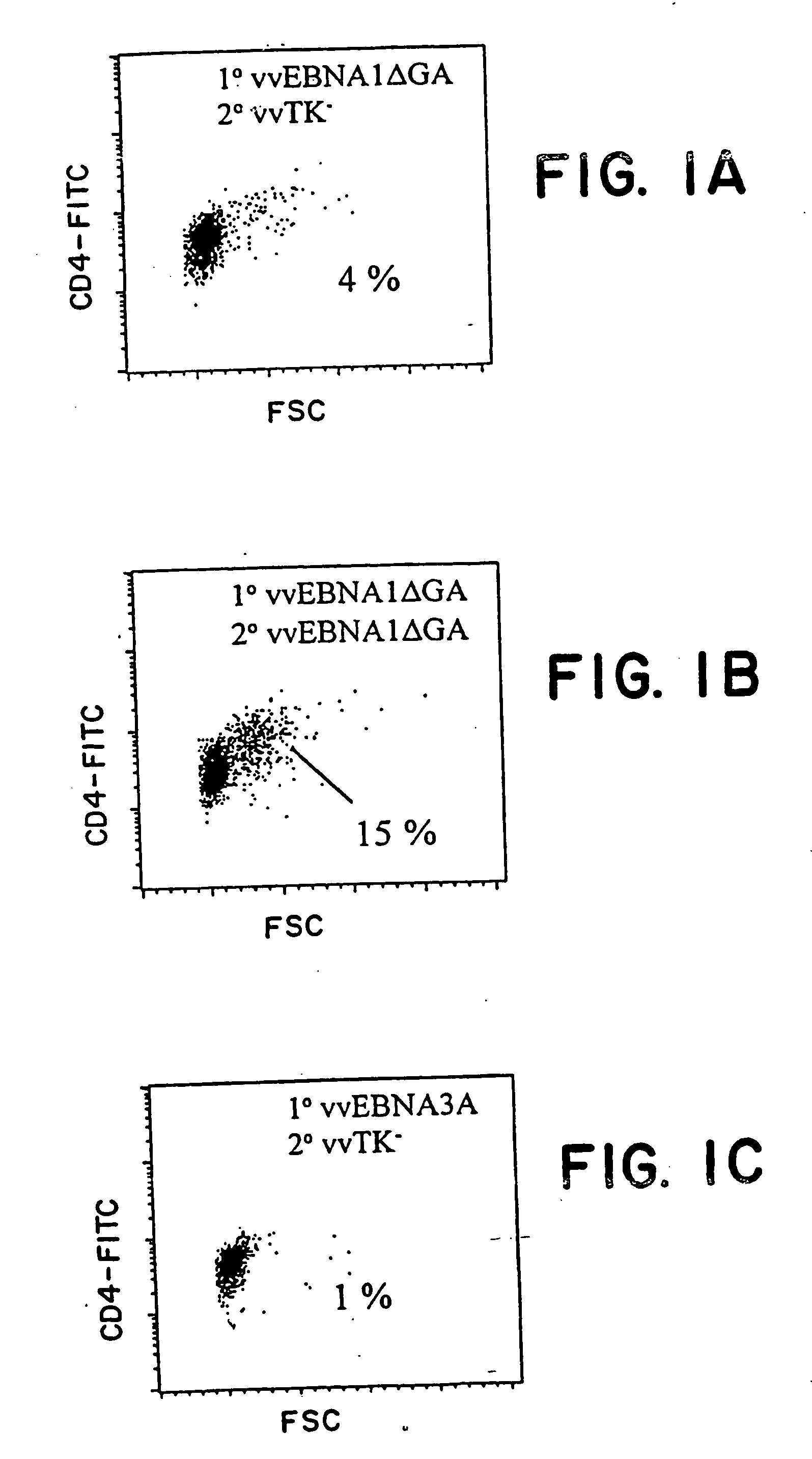
The present invention relates to the identification of a subunit vaccine to prevent or treat infection of Epstein Barr Virus. In particular, EBNA-1 was identified as a vaccine antigen. In a specific embodiment, a purified protein corresponding to EBNA-1 elicited a strong CD4+ T cell response. The responsive CD4+ T cell are primarily TH1 in function. EBNA-1 is an attractive candidate for a protective vaccine against EBV, and for immunotherapy of EBV infection and neoplasms, particularly with dendritic cells charged with EBNA-1.
Owner:THE ROCKEFELLER UNIV
Protective antigen of epstein barr virus
The present invention relates to the identification of a subunit vaccine to prevent or treat infection of Epstein Barr Virus. In particular, EBNA-1 was identified as a vaccine antigen. In a specific embodiment, a purified protein corresponding to EBNA-1 elicited a strong CD4+ T cell response. The responsive CD4+ T cell are primarily TH1 in function. EBNA-1 is an attractive candidate for a protective vaccine against EBV, and for immunotherapy of EBV infection and neoplasms, particularly with dendritic cells charged with EBNA-1.
Owner:THE ROCKEFELLER UNIV
Immunoreactive peptides from epstein-barr virus
Owner:ORTHO-CLINICAL DIAGNOSTICS
Antiviral nucleosides
InactiveUS7589077B2Superior anti-HBV activityHigh anti-EBV propertyBiocideSugar derivativesHerpes simplex virus DNAHepatitis B virus
Disclosed are nucleosides which are useful in diagnosing and treating viral infections, for example, infections caused by hepatitis B virus (HBV), and herpes viruses including Epstein Barr virus.
Owner:KUMAR RAKESH
Oligonucleotide therapies for modulating the effects of herpesviruses
InactiveUS6310044B1Conveniently and desirably presentedFaster replicationPeptide/protein ingredientsGenetic material ingredientsOpen reading frameHerpesvirus infection
Compositions and methods are provided for the treatment and diagnosis of herpesvirus infections. In accordance with preferred embodiments, oligonucleotides are provided which are specifically hybridizable with RNA or DNA deriving from a gene corresponding to one of the open reading frames UL5, UL8, UL9, UL13, UL29, UL30, UL39, UL40, UL42 AND UL52 of herpes simplex virus type 1. The oligonucleotide comprises nucleotide units sufficient in identity and number to effect said specific hybridization. In other preferred embodiments, the oligonucleotides are specifically hybridizable with a translation initiation site; it is also preferred that they comprise the sequence CAT. Methods of treating animals suspected of being infected with herpesvirus comprising contacting the animal with an oligonucleotide specifically hybridizable with RNA or DNA deriving from one of the foregoing genes of the herpesvirus are disclosed. Methods for treatment of infections caused by herpes simplex virus type 1, herpes simplex virus type 2, cytomegalovirus, human herpes virus 6, Epstein Barr virus or varicella zoster virus are disclosed.
Owner:IONIS PHARMA INC
Compositions and methods for modulating the activity of epstein-barr nuclear antigen 1
InactiveCN103717757AOrganic chemistryMicrobiological testing/measurementEpstein bar virusPharmaceutical drug
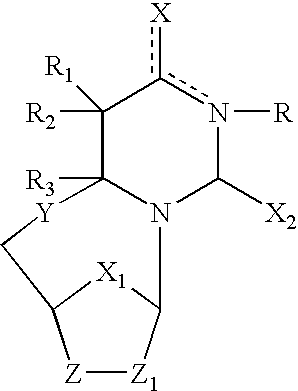
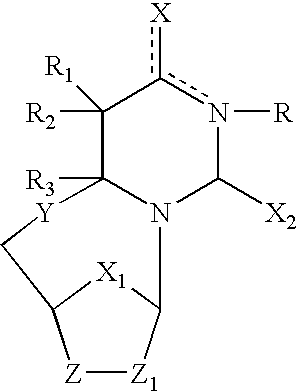
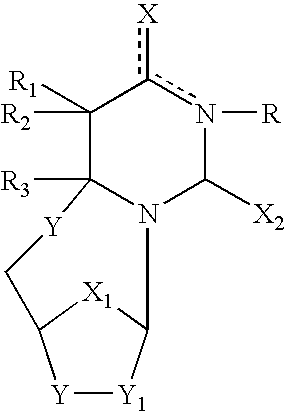
The present invention embraces compounds that modulate the activity of Epstein-Barr Nuclear Antigen 1 (BBNA1 ) protein and use thereof in methods for treating latent Epstein-Barr virus infection. R7 is a substituted or unsubstituted phenyl, pyridyl, or pyrimidinyl group. A pharmaceutical composition comprising a compound of the invention in admixture with a pharmaceutically acceptable carrier is also provide as are methods for modulating the activity of Epstein-Barr Nuclear Antigen 1 (EBNA1 ) protein and treating a latent EpsteinBarr virus infection with a composition of the present invention.
Owner:WISTAR INSTITUTE
Improved human herpesvirus immunotherapy
An isolated protein comprises respective amino acid sequences of each of a plurality of CTL epitopes from two or more different herpesvirus antigens and further comprises an intervening amino acid or amino acid sequence between at least two of said CTL epitopes comprising proteasome liberation amino acids or amino acid sequences and, optionally, Transporter Associated with Antigen Processing recognition motifs. The isolated protein is capable of rapidly expanding human cytotoxic T lymphocytes (CTL) in vitro and eliciting a CTL immune response in vivo upon administration to an animal as an exogenous protein. Typically, the isolated protein comprises no more than twenty (20) CTL epitopes derived from cytomegalovirus and / or Epstein-Barr virus antigens.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
5-(E)-bromovinyl uracil analogues and related pyrimidine nucleosides as anti-viral agents and methods of use
InactiveUS20040053891A1Extended half-lifeImprove retentionOrganic active ingredientsBiocideHalf-lifeUracil analog
The present invention relates to pyrimidine nucleoside compounds and their use to treat viral infections of Varicella Zoster Virus, Epstein Barr Virus and Kaposi's Sarcoma virus, also known as HV-8 and related complications of these viral infections. In another aspect of the present invention, the use of one or more nucleoside compound to increase the retention or half-life of 5-fluorouracil (FU) in patients is also described.
Owner:CHENG YUNG CHI +3
Monoclonal antibody production by EBV transformation of B cells
ActiveUS8071371B2Improve efficiencyRapid isolationMicrobiological testing/measurementGenetically modified cellsEpstein bar virusPolyclonal B cell activator
A method for producing a clone of an immortalized human B memory lymphocyte, comprising the step of transforming human B memory lymphocytes using Epstein Barr Virus (EBV) in the presence of a polyclonal B cell activator. The method is particularly useful in a method for producing a clone of an immortalized human B memory lymphocyte capable of producing a human monoclonal antibody with a desired antigen specificity, comprising the steps of: (i) selecting and isolating a human memory B lymphocyte subpopulation; (ii) transforming the subpopulation with Epstein Barr Virus (EBV) in the presence of a polyclonal B cell activator; (iii) screening the culture supernatant for antigen specificity; and (iv) isolating an immortalized human B memory lymphocyte clone capable of producing a human monoclonal antibody having the desired antigen specificity.
Owner:INSTITUTE FOR RESEARCH IN BIOMEDECINE
Immunoreactive peptides from Epstein-Barr virus
Epstein-Barr virus (EBV) specific polypeptides consisting of a series of one to 1000 peptide units selected from the group consisting of peptide units Φ, Γ, Δ and Ω, wherein Φ is 25 amino acids or less and has the formula (αETFTETWNRFITHTEβ) (SEQ ID NO:1), Γ is 25 amino acids or less and has the formula (αGMLEASEGLDGWIHQβ) (SEQ ID NO:2), Δ is 25 amino acids or less and has the formula (αHQQGGWSTLIEDNIβ) (SEQ ID NO:3), Ω is 25 amino acids or less and has the formula (αKQKHPKKVKQAFNPLβ) (SEQ ID NO:4), α and β are each independently from 0 to 5 naturally occurring amino acids, and the polypeptide is capable of binding antibody in a specimen from an individual with Epstein-Barr virus (EBV)-associated disease are disclosed. Also disclosed are the use of these polypeptides for the production of polypeptide-specific antibodies and the diagnosis and treatment of EBV-associated disease.
Owner:ORTHO DIAGNOSTICS +1
Oligonucleotides for the amplification and detection of epstein barr virus (EBV) nucleic acid
InactiveUS20040180331A1Microbiological testing/measurementRecombinant DNA-technologyDiseaseMalignancy
The present invention is concerned with oligonucleotides that can be used as in the amplification and detection of Epstein Barr Virus (EBV) nucleic acid, in particular RNA-specific sequences. Furthermore a method for the diagnosis of EBV associated malignant and non-malignant diseases is provided. The oligonucleotides according to the present invention are specifically suited for the detection of EBV gene expression in circulating peripheral blood cells, in human (tumor) tissue samples and thin sections thereof using "in solution" amplification or "in situ" amplification techniques and in other biological samples potentially containing EBV-infected cells.
Owner:VERVOORT M B H J MARCEL BARTOLINA HENDRIKUS JOHANNES +2
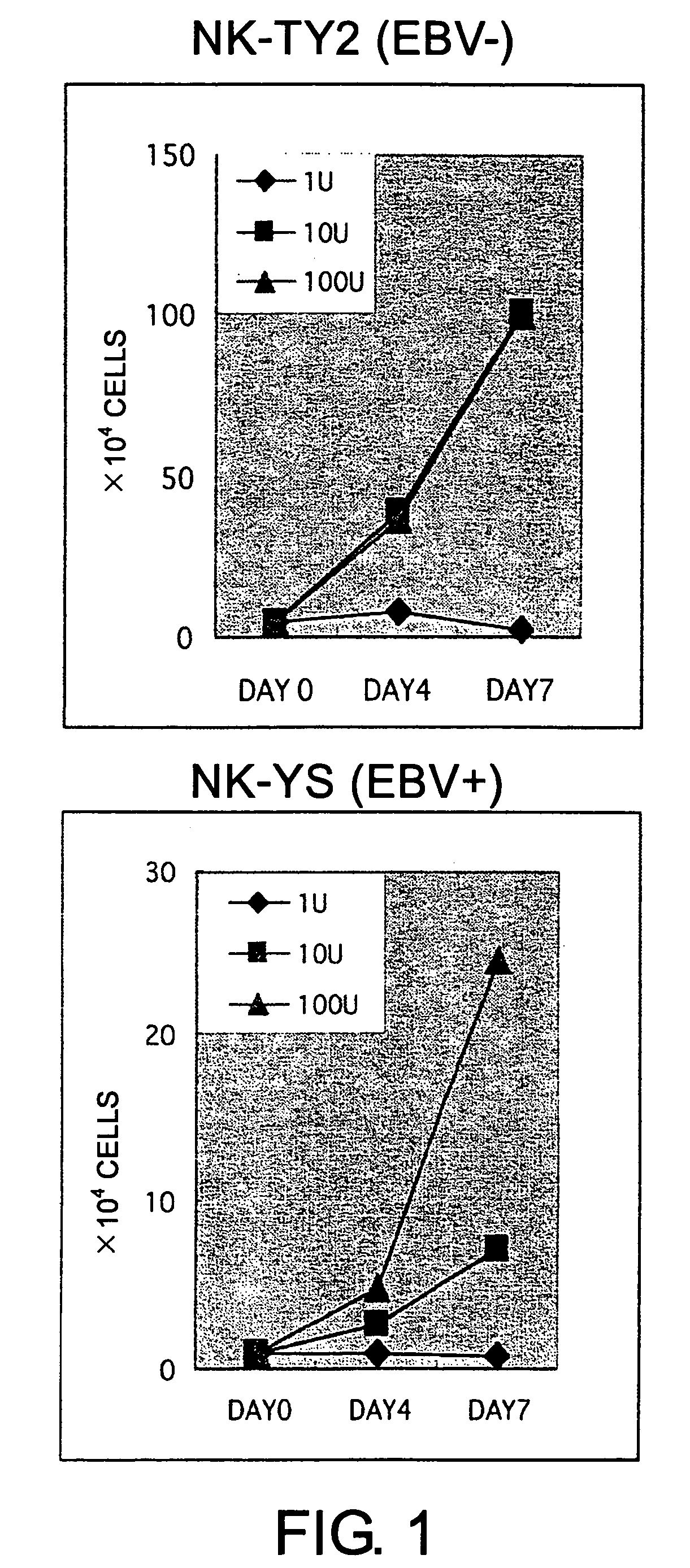
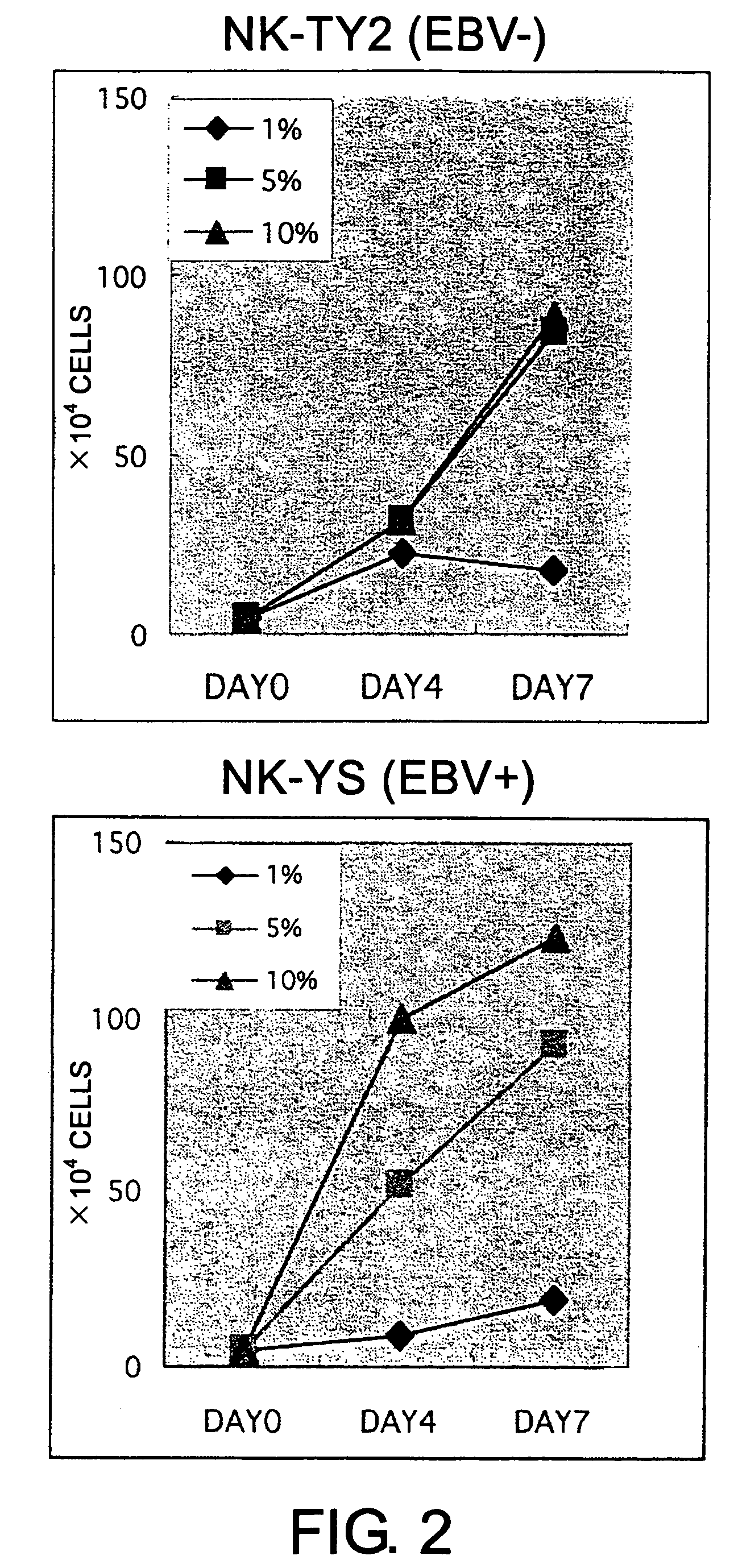
Epstein-Barr virus-negative NK cell line
ActiveUS7781210B2High sensitivity measurementEasy to measureGenetically modified cellsMicrobiological testing/measurementEpstein bar virusNatural Killer Cell Inhibitory Receptors
The present invention provides Epstein-Barr virus (EBV)-negative NK cell lines. The NK cell lines of the present invention are useful for screening factors associated with the proliferation and expression functions of NK cells and to discover factors produced by the NK cells. In addition, the cell lines are immortalized despite the fact that they are EBV-negative. Thus, unknown mechanisms of oncogenesis may be elucidated through an understanding of the mechanisms underlying the immortalization of the cell lines of the present invention.
Owner:JUNJIRO TSUCHIYAMA
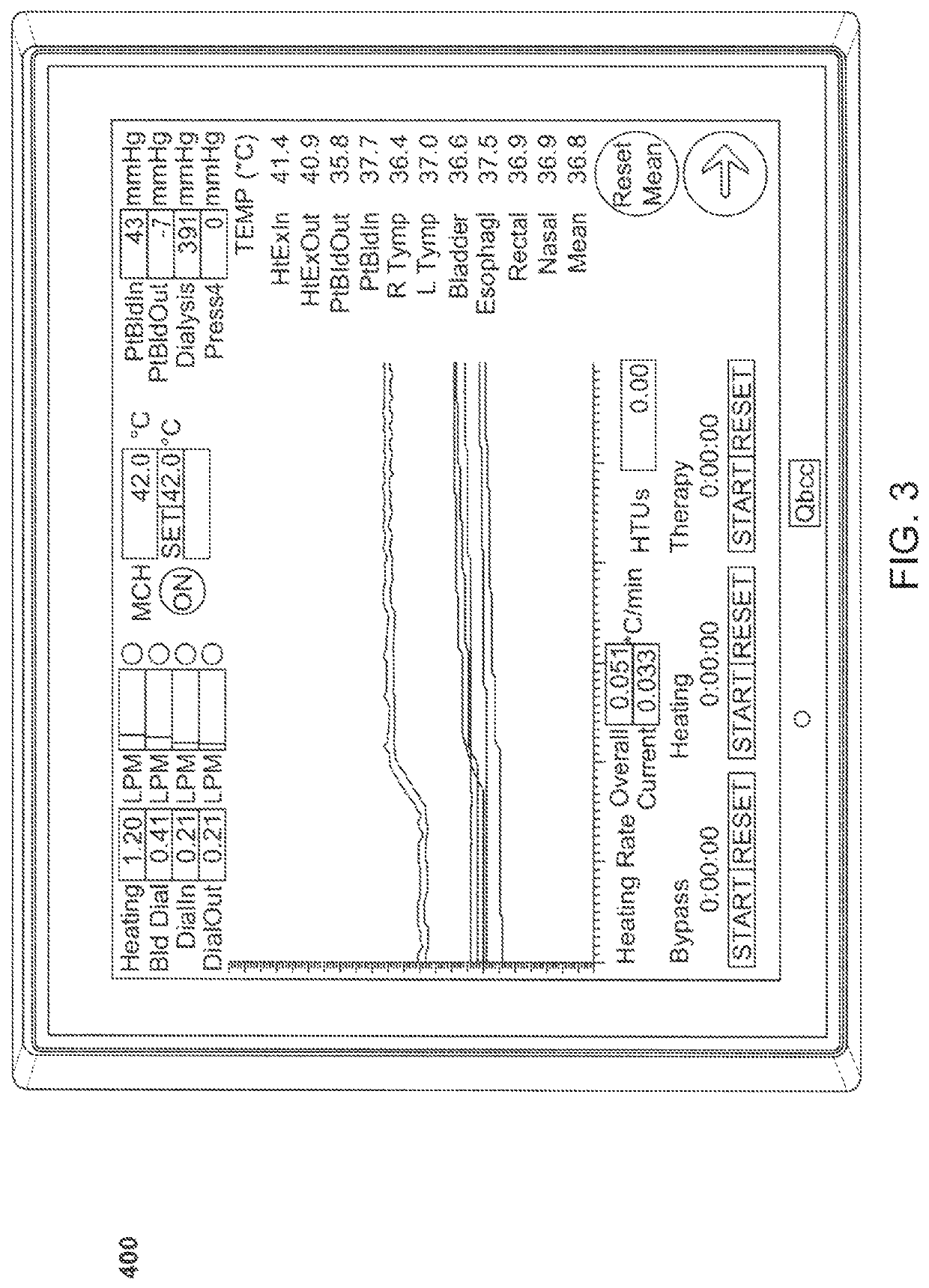
Method and system for controlled hyperthermia
Methods and for treatment of cancer and other diseases including complications from late stage viral infections by inducing hyperthermia in a patient relying on withdrawing blood from the patient and returning the withdrawn blood to the patient to establish an extracorporeal flow circuit. Blood is heated by passing through the extracorporeal circuit at a controlled rate until a target body core temperature in is achieved. Usually, the blood will be subjected to a continuously re-circulating dialysis to balance electrolytes. Additionally, the blood will be subjected to a continuously recirculating regeneration through a carbon sorbent column where toxins and contaminants are removed. The blood temperature is maintained at the target blood temperature for a treatment period, and the blood is cooled after the treatment period has been completed. The method can also be effective in treating rheumatoid arthritis, scleroderma, hepatitis, sepsis, the Epstein-Barr virus, and patients with life threatening complications from other viruses, including the COVID-19 virus. A method for removing viruses from the blood supply in an external circuit is also presented.
Owner:VERTHERMIA ACQUISITION INC
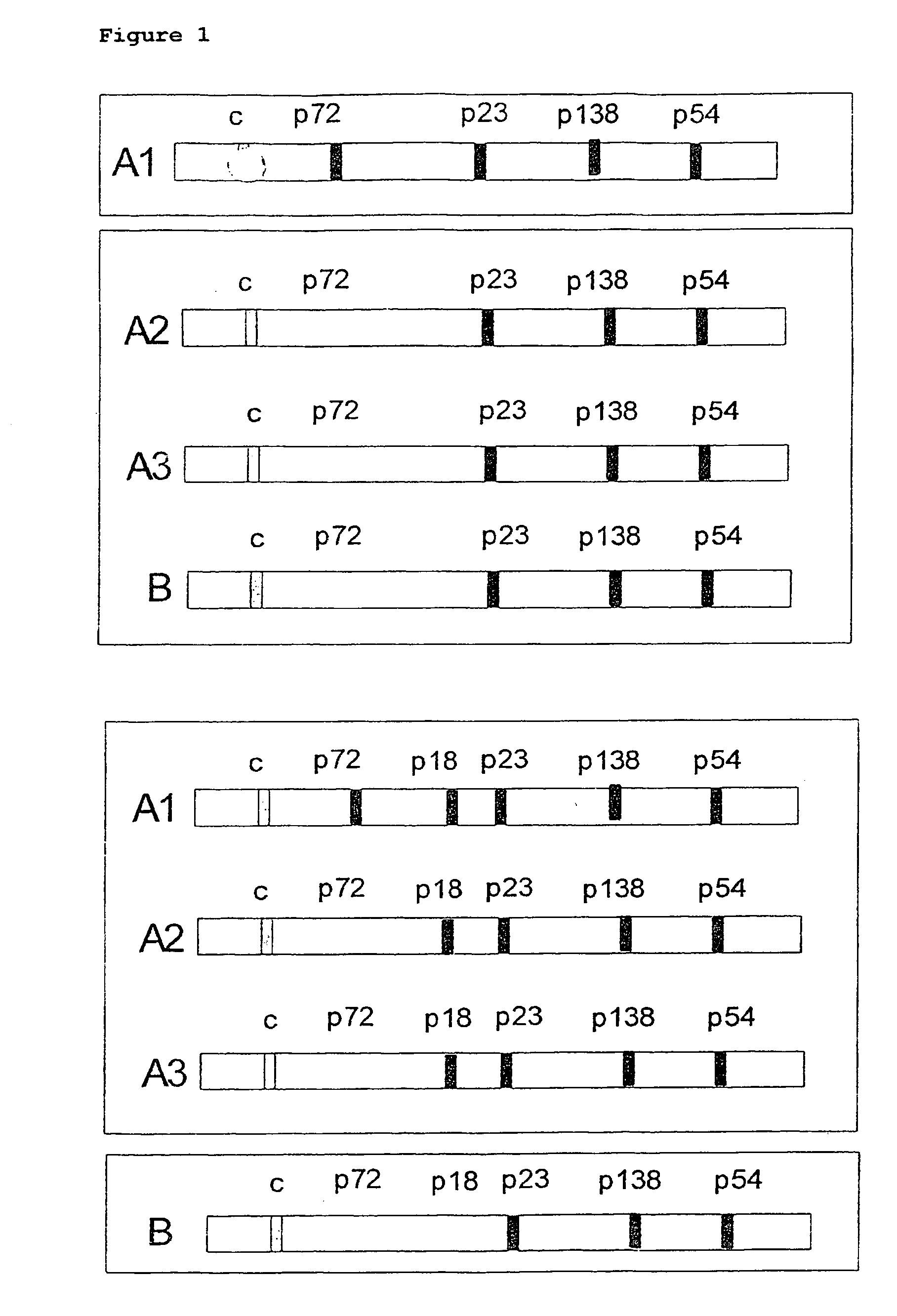
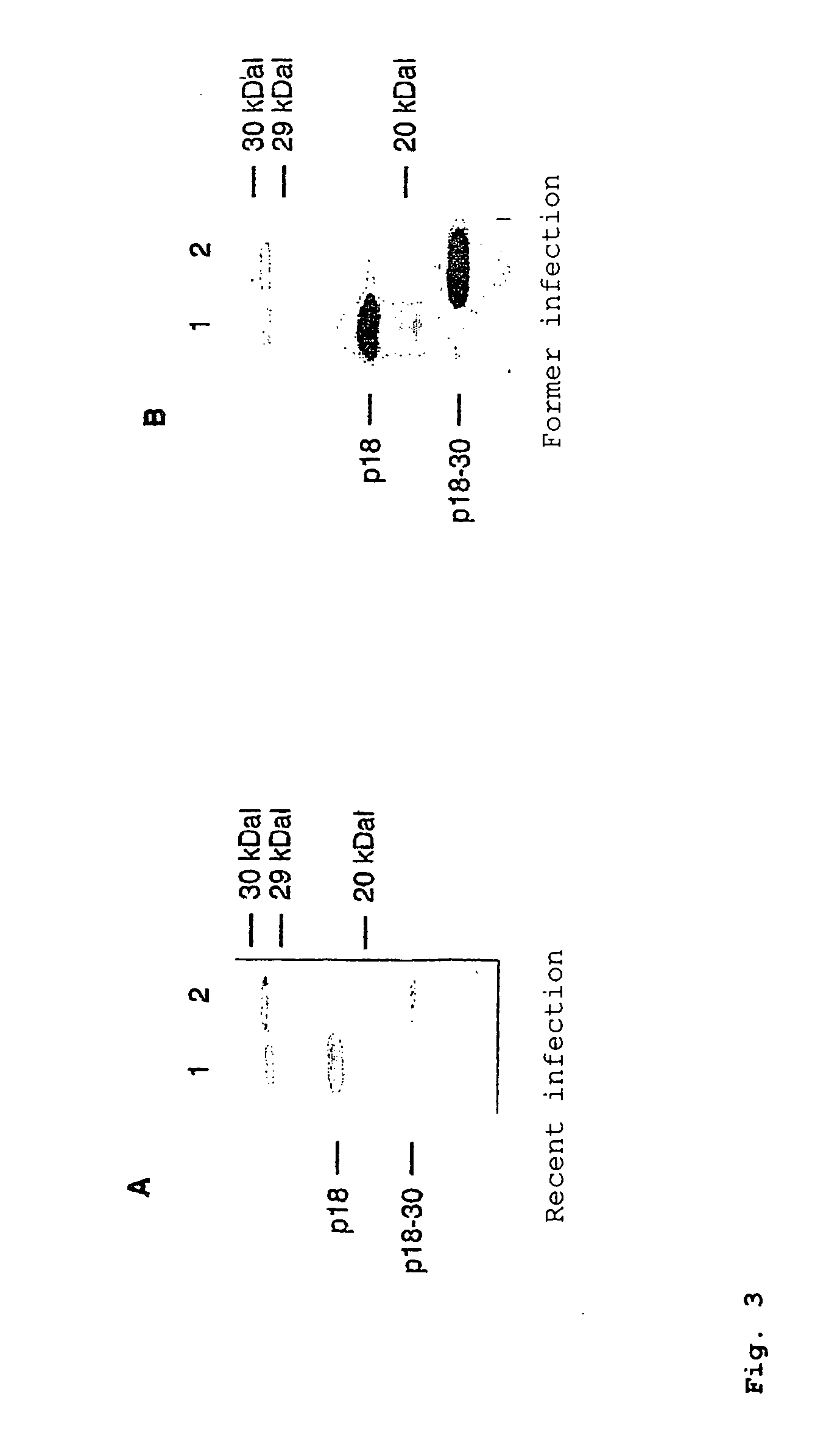
Peptides derived from capsid antigens of the Epstein-Barr virus and the use thereof
ActiveUS20060057563A1Safe differentiationSugar derivativesMicrobiological testing/measurementAntigenEpstein bar virus
In the area of virus diagnosis, in particular Epstein-Barr virus (EBV) diagnosis, methods for the detection of EBV and agents suitable for this purpose are provided, including peptides which are derived from p18-VCA and permit discrimination between acute and past EBV infection.
Owner:MIKROGEN MOLEKULARBIOLOGISCHE ENTWICKLUNGS GMBH
Compositions and methods for detecting Epstein Barr Virus (EBV)
PendingCN114174540AMicrobiological testing/measurementMicroorganism based processesEpstein bar virusAssay
Described herein are methods for the rapid detection of the presence or absence of Epstein Barr virus (EBV) in a biological or non-biological sample. The method may include performing an amplification step, a hybridization step, and a detection step. In addition, EBV-targeting primers and probes as well as kits designed to detect a target region of EBV are provided. Kits, reaction mixtures, and oligonucleotides (e.g., primers and probes) for amplifying and detecting EBV are also described. Primers and probes that detect different regions of EBV and that can be employed in a dual target assay to simultaneously detect two different and non-overlapping target regions of EBV are also described.
Owner:F HOFFMANN LA ROCHE & CO AG
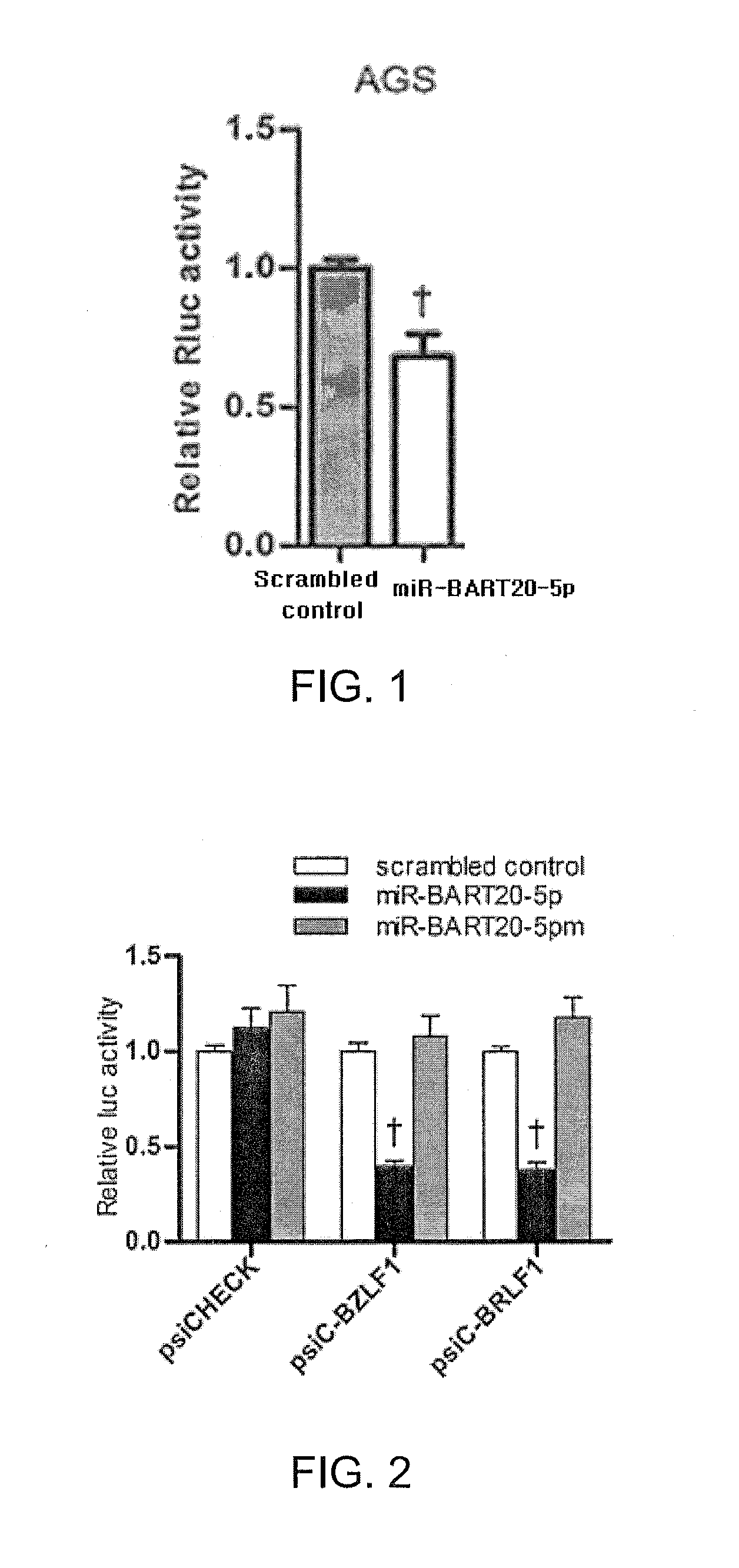
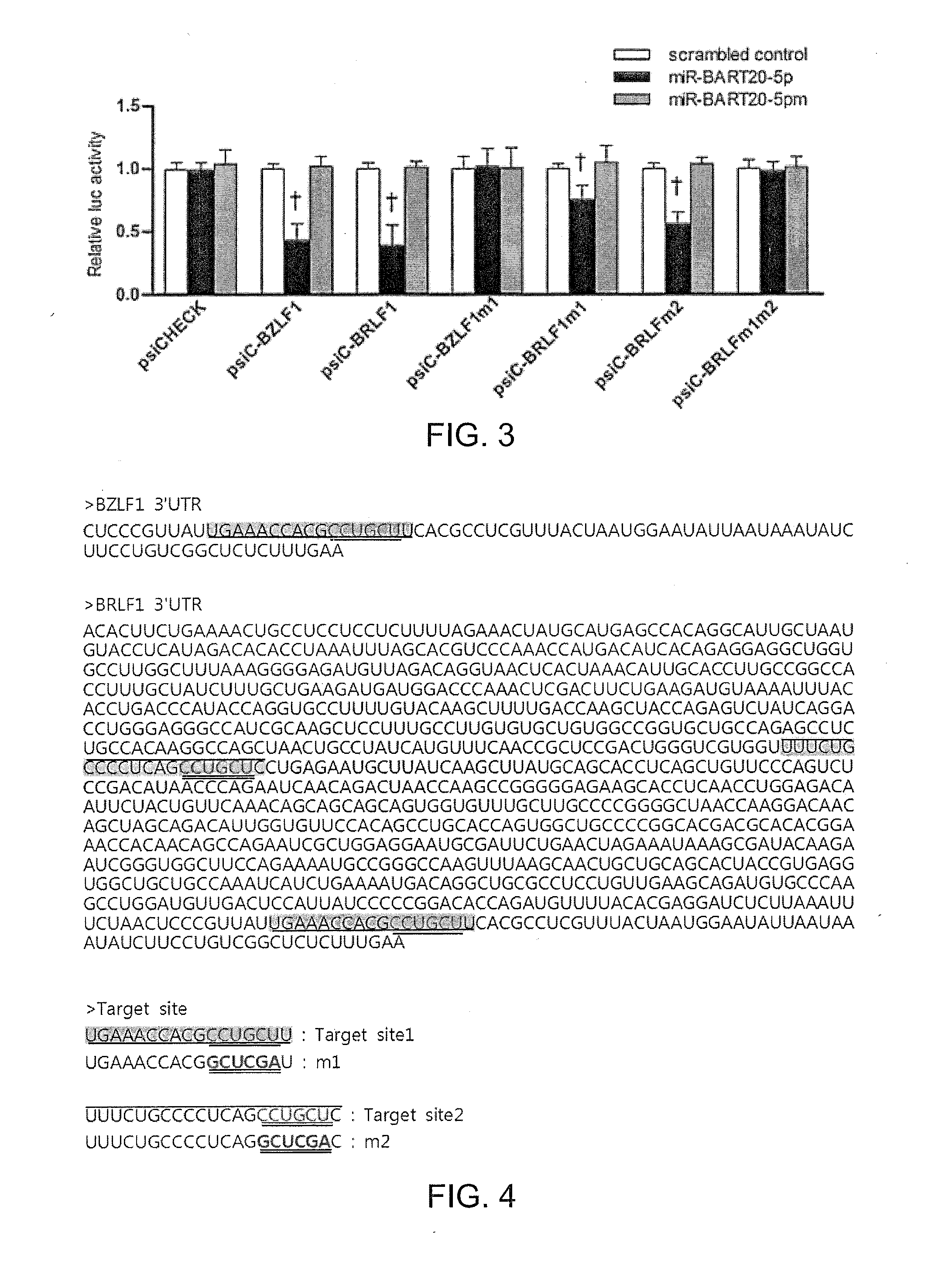
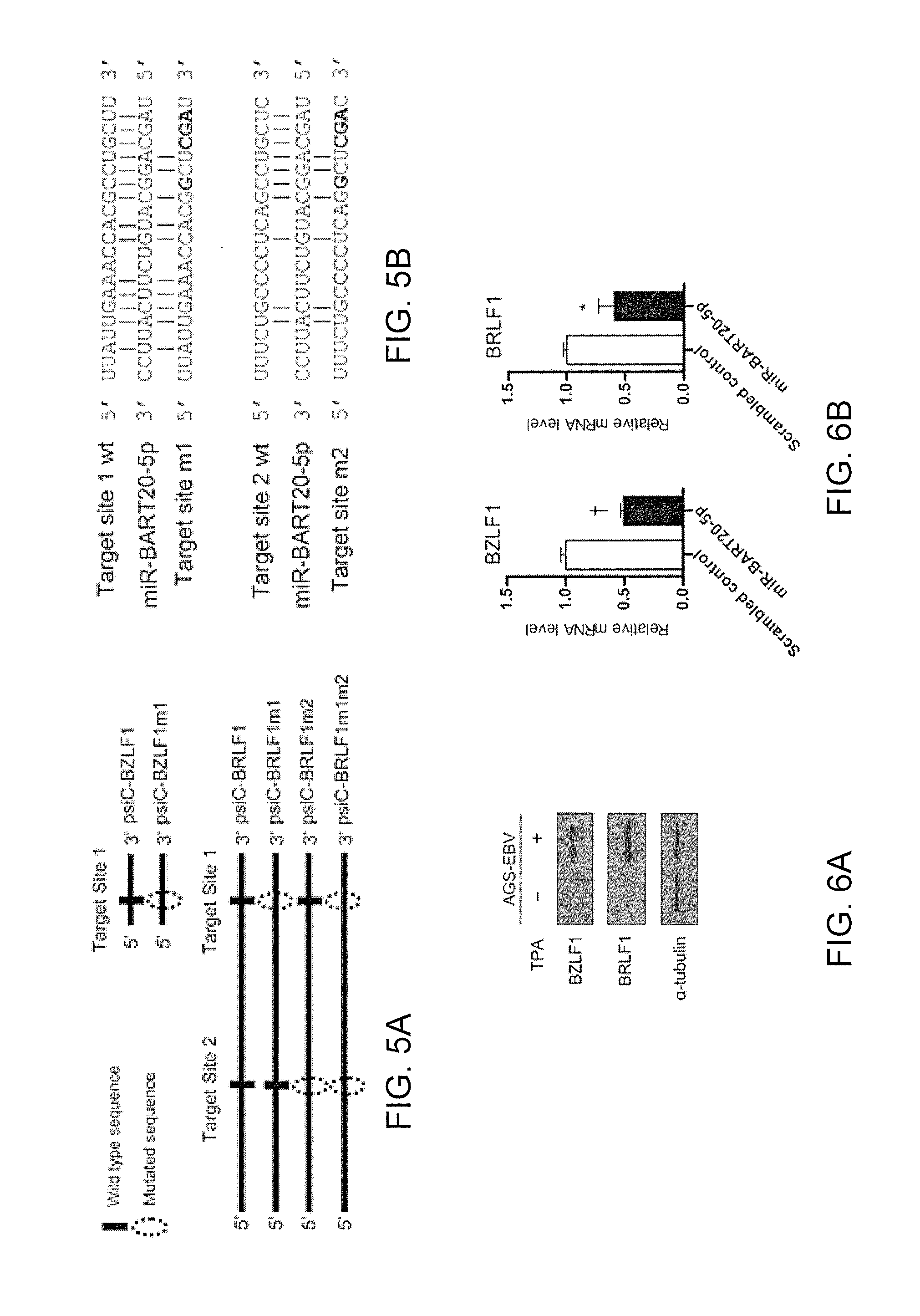
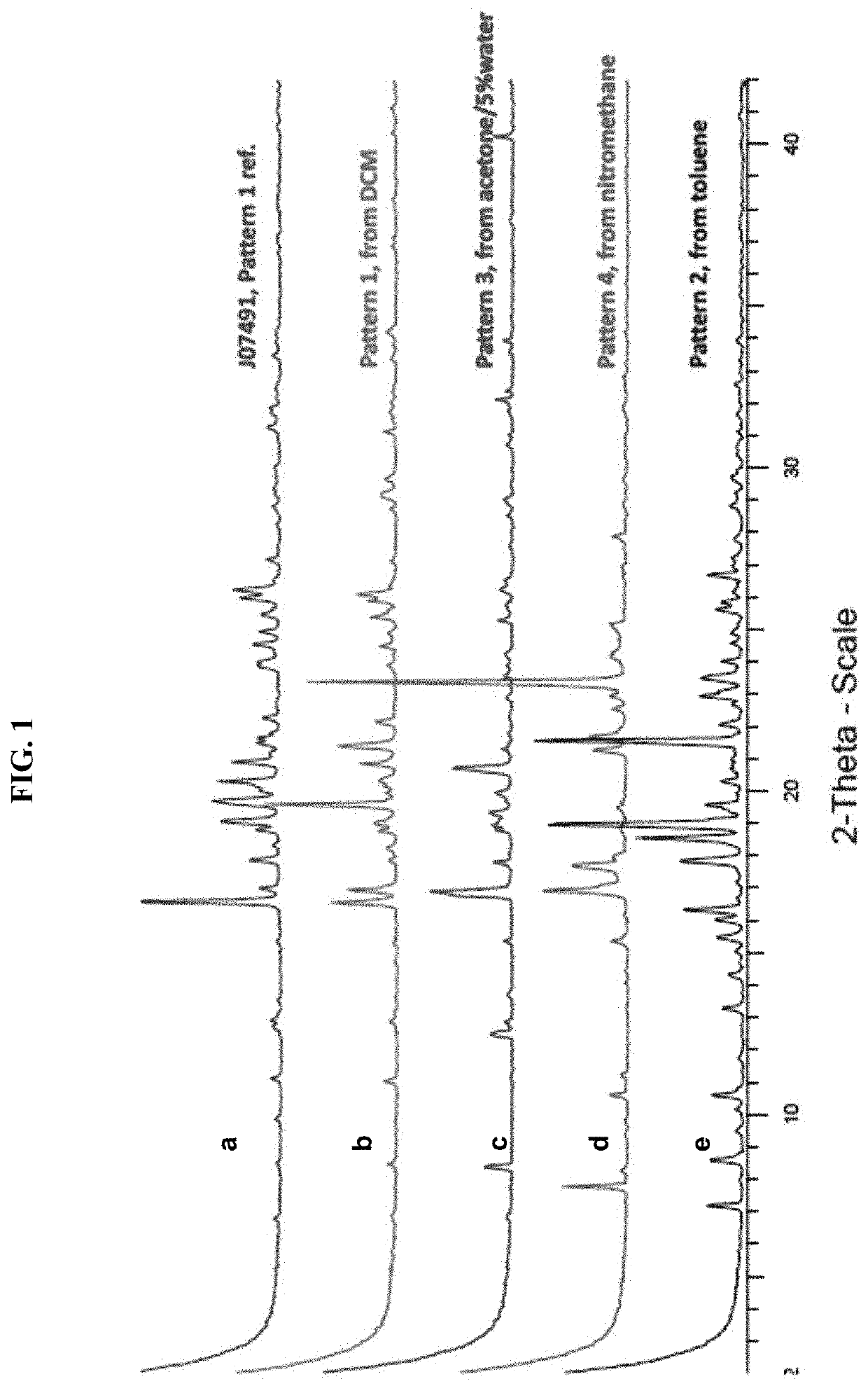
Composition for treating epstein-barr virus infection, comprising epstein-barr virus micro RNA inhibitor
InactiveUS20150329865A1Good antiviral effectEffective preventionMicrobiological testing/measurementInactivation/attenuationDiseaseMicroRNA
Provided is a composition comprising an Epstein-Barr virus microRNA inhibitor for treating Epstein-Barr virus infection, and a method using Epstein-Barr virus microRNA for screening a therapeutic agent for treating Epstein-Barr virus infection. The provided composition enables one to induce the lytic cycle of EBV such that EBV-infected cells are destroyed by a host immune system. Therefore, the composition can be effectively used for the prevention or treatment of diseases, including various cancers, caused by EBV infection. Moreover, the provided method enables one to screen a therapeutic agent having excellent antiviral effect for treating Epstein-Barr virus infection by inducing Epstein-Barr virus lytic cycle.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOP FOUND
EBNA1 Inhibitor Crystalline Forms, and Methods of Preparing and Using Same
ActiveUS20190352285A1Avoid infectionOrganic active ingredientsNervous disorderMedicineEBV Infections
The invention relates, in certain aspects, to developable forms of certain compounds that are useful to treat and / or prevent EBV infection and related conditions in a subject. The invention further provides EBNA1 inhibitors, and / or pharmaceutical compositions comprising the same, that are useful for the treatment of diseases caused by latent Epstein-Barr Virus (EBV) infection and / or lytic EBV infection.
Owner:WISTAR INSTITUTE
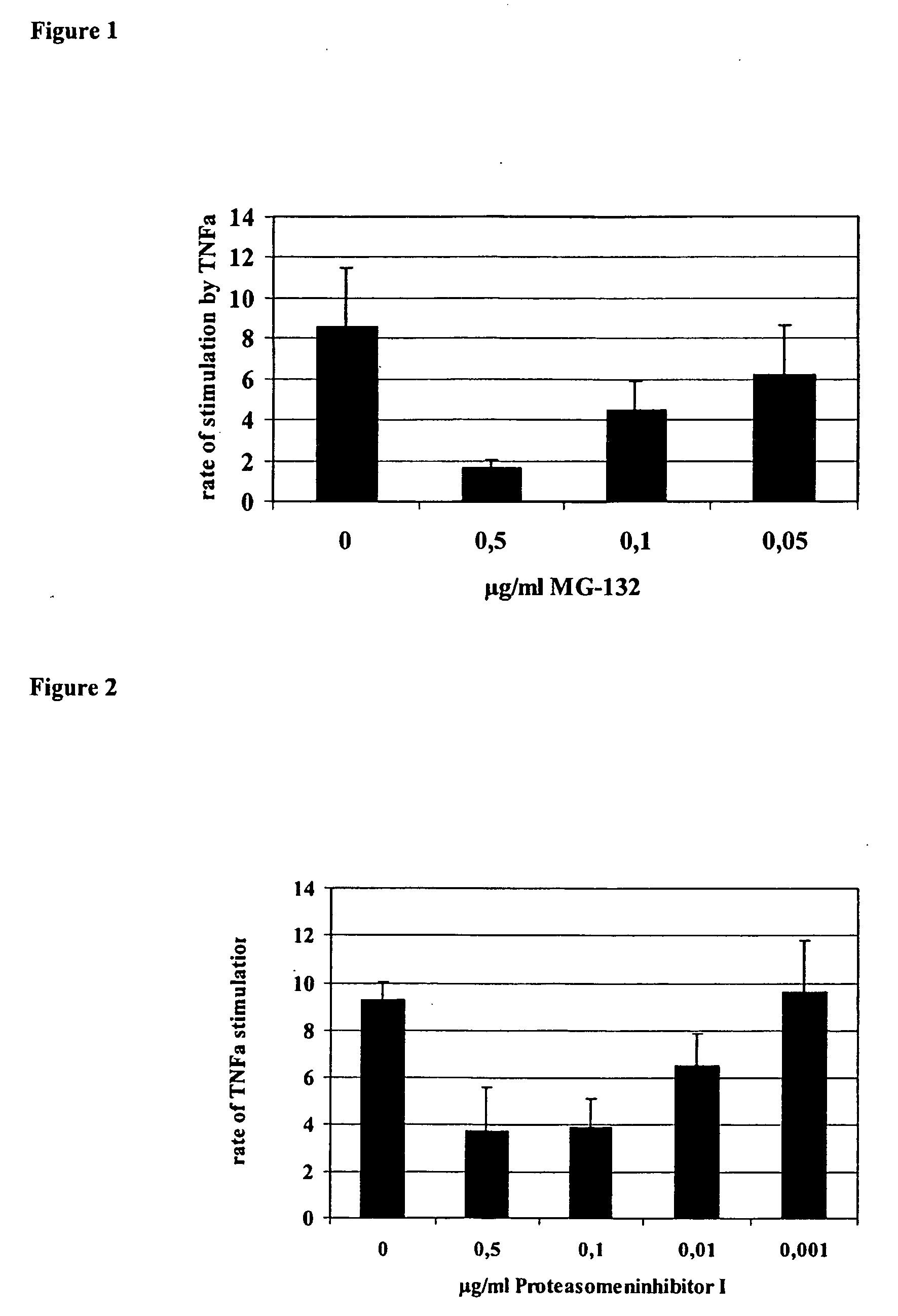
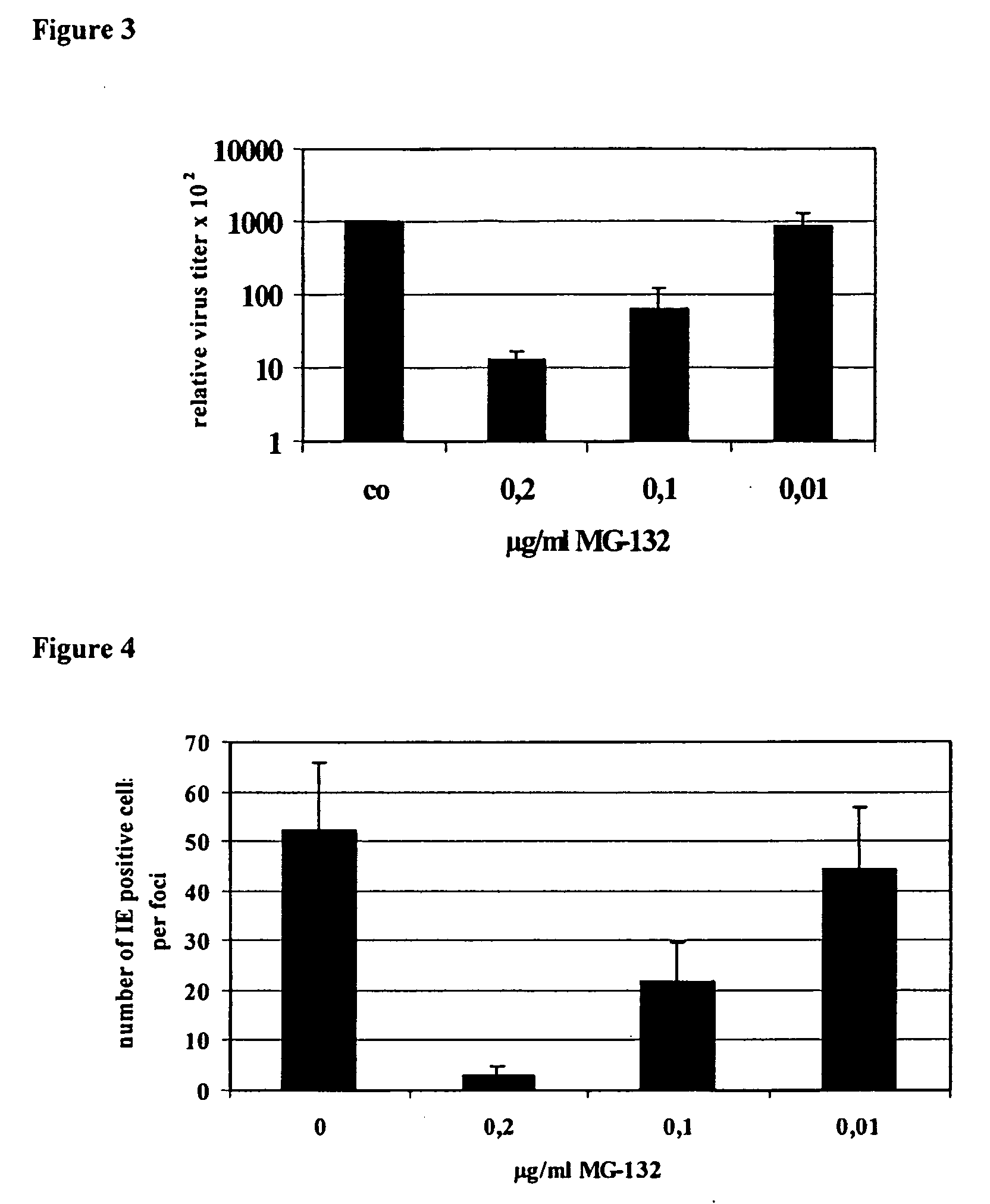
Proteaseome inhibitors for the treatment of herpesviridae infected individuals
InactiveUS20070042967A1Reduce duplicationReduce expressionBiocideDipeptide ingredientsEpstein bar virusHuman cytomegalovirus
The present invention relates to the use of a substance or composition comprising one or more proteasome inhibitors for the manufacture of a medicament for the treatment of an individual infected with a virus selected from the group comprising varicella zoster virus, human cytomegalovirus, human herpesvirus 6 and 7 and Epstein-Barr virus and Karposi's sarcoma herpesvirus. The invention further relates to methods of treatment of individuals infected with a virus selected from the group comprising varicella zoster virus, human cytomegalovirus, human herpesvirus 6 and 7 and Epstein-Barr virus and Karposi's sarcoma herpesvirus.
Owner:CHARITE UNIVS MEDIZIN BERLIN
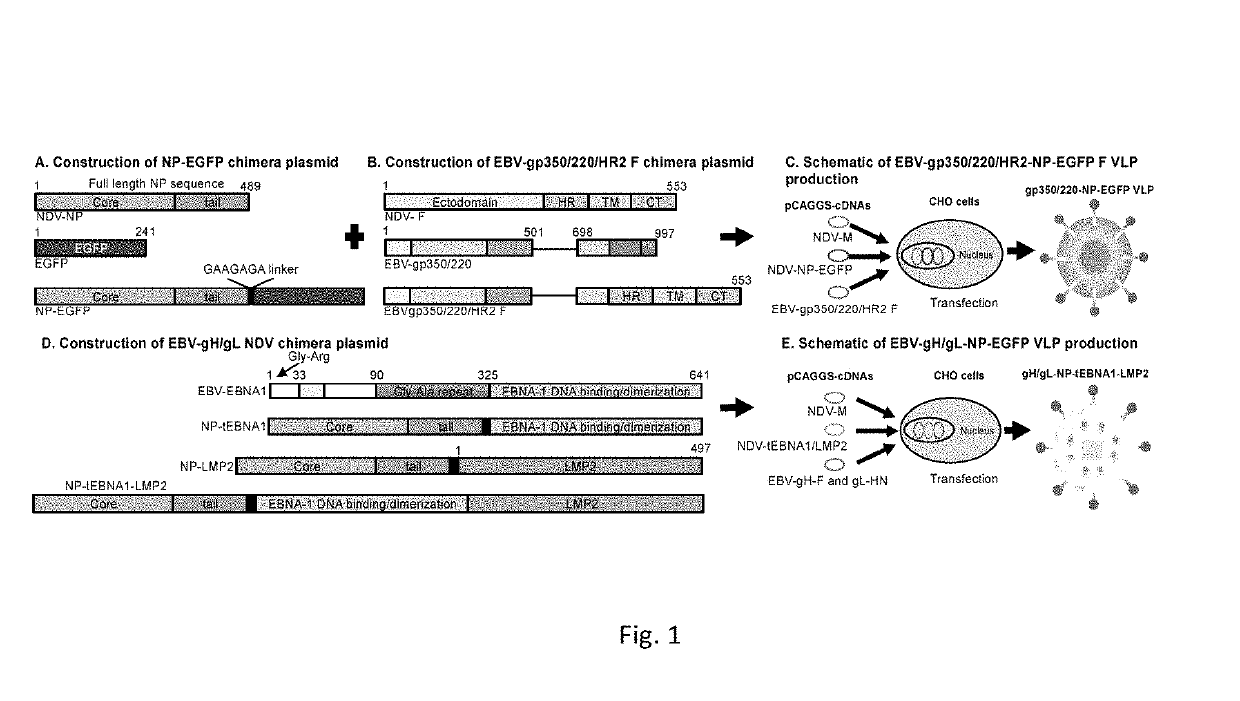
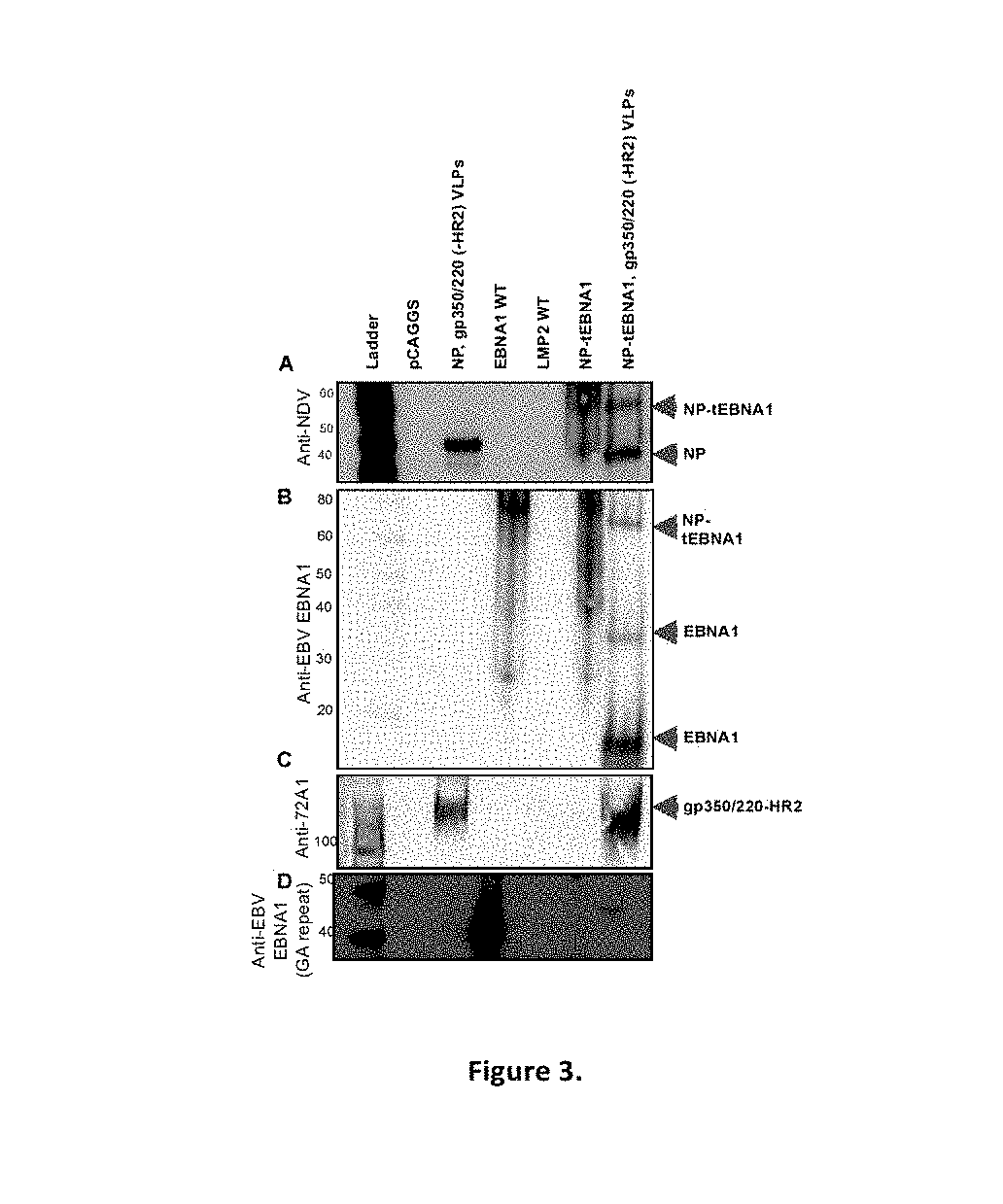
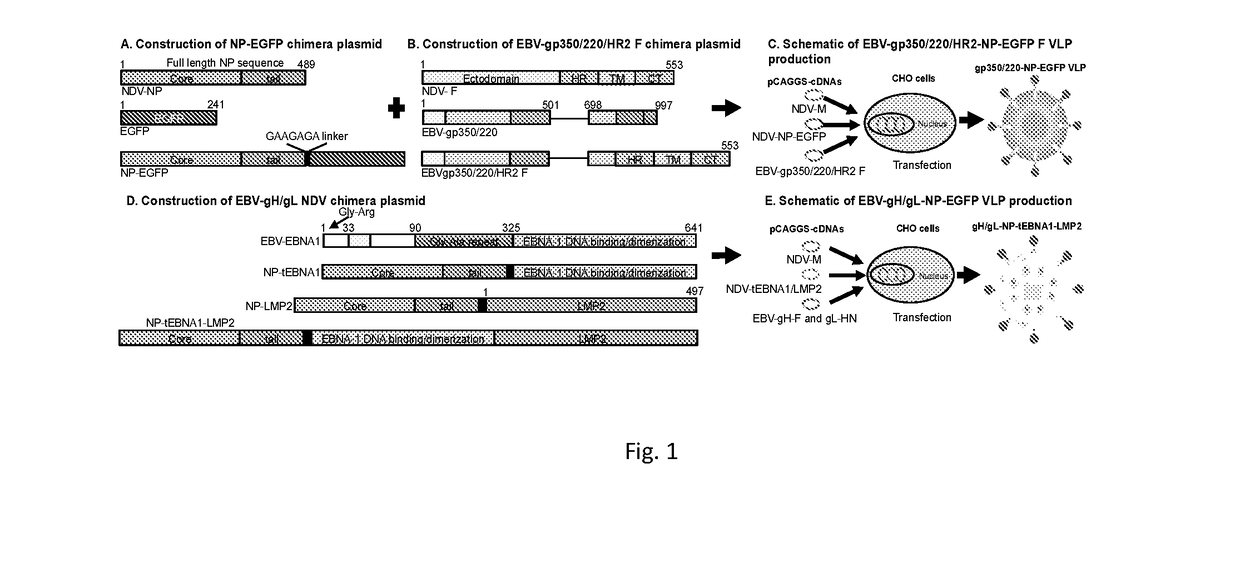
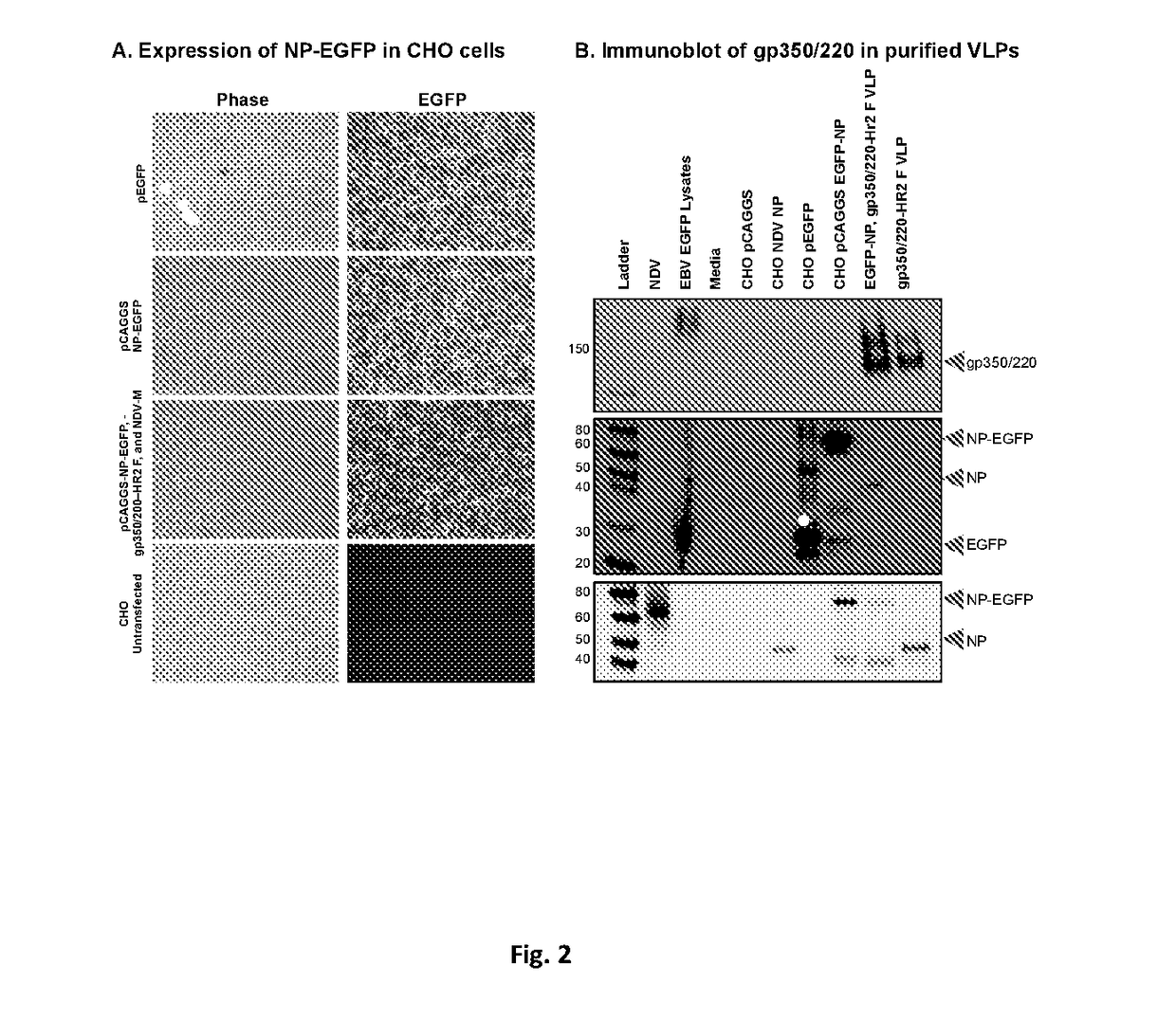
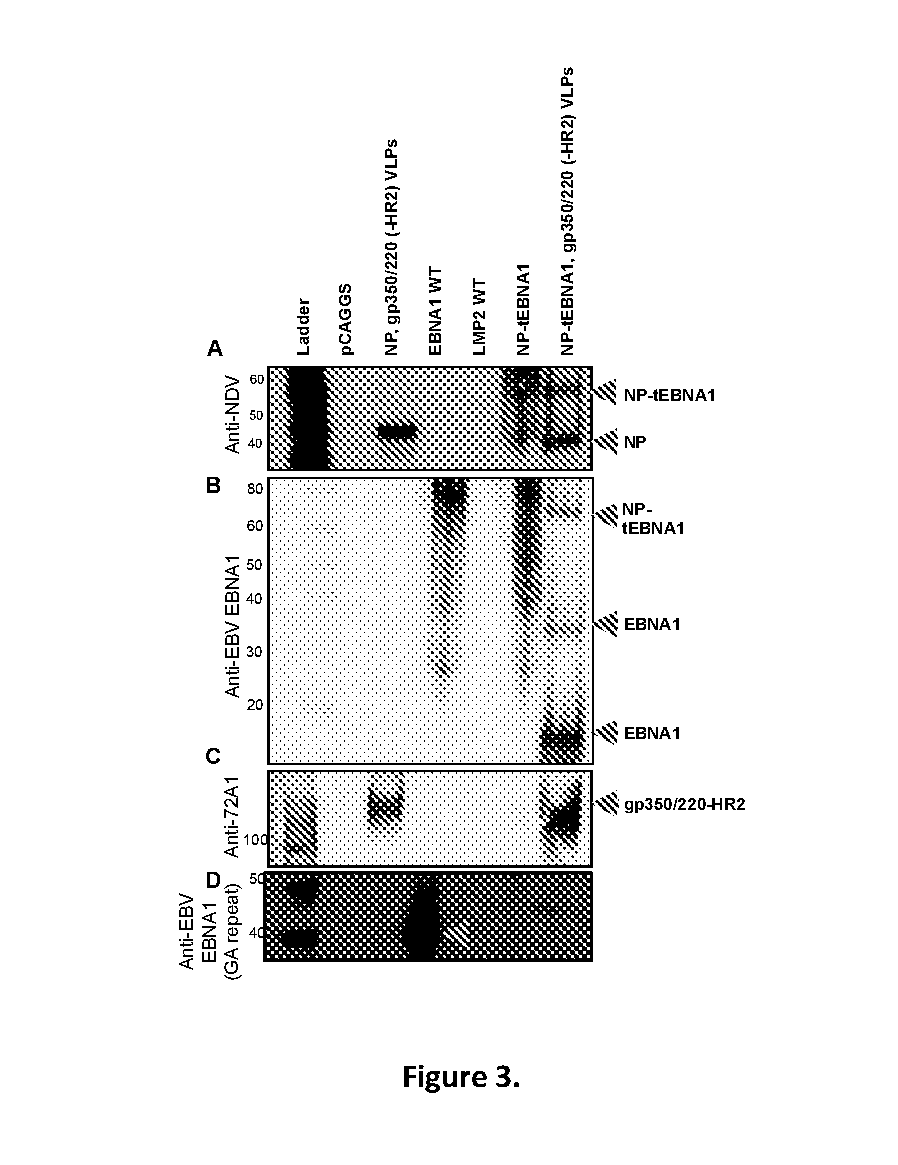
Virus-like particle compositions and vaccines against Epstein-Barr virus infection and disease
ActiveUS10314906B2SsRNA viruses negative-senseViral antigen ingredientsDiseaseNewcastle disease virus NDV
The present inaveation relates to prophylactic and / or therapeutic vaccines thatpoatairj Newcastle disease Virus (NDV) virus-like particles (VLPs) comprising one or more Epstein-Barr Virus (EBV) antigens, in one embodiment, the invention provides a recombinant virus-like particle (V'UP) comprising, i is operable combination, a) Newcastle disease virusΛ iNDVj matrix (M) protein, and b) one or more Epstein-Barr Virus (BBV) antigens. The im'eniion's prophylactic and / or therapeutic vacclrses are useful for preventing asc / or treatmg, infection with EBY aixi / or disease associated Epstein-Barr Virus, such as cancer.
Owner:UNIV OF MASSACHUSETTS
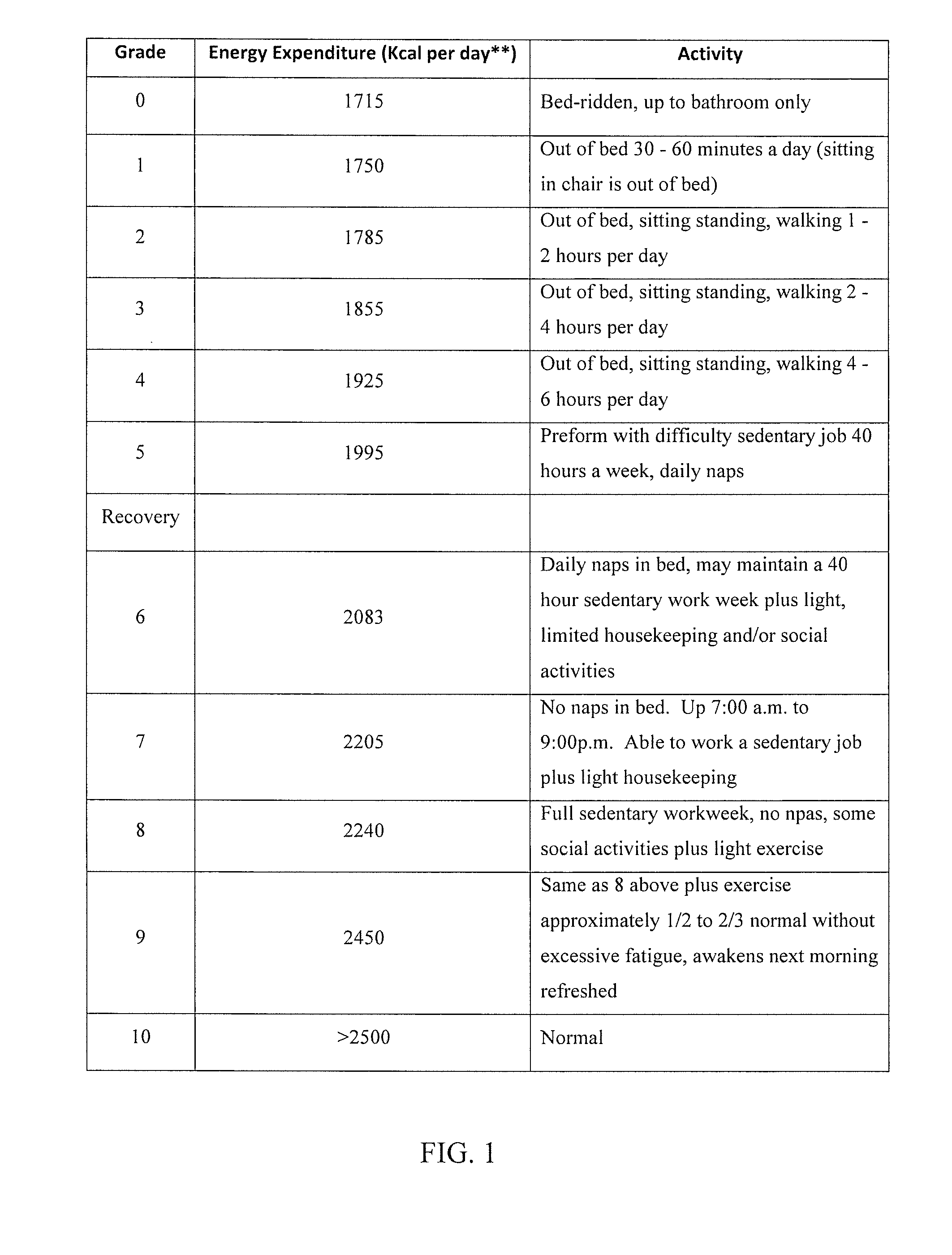
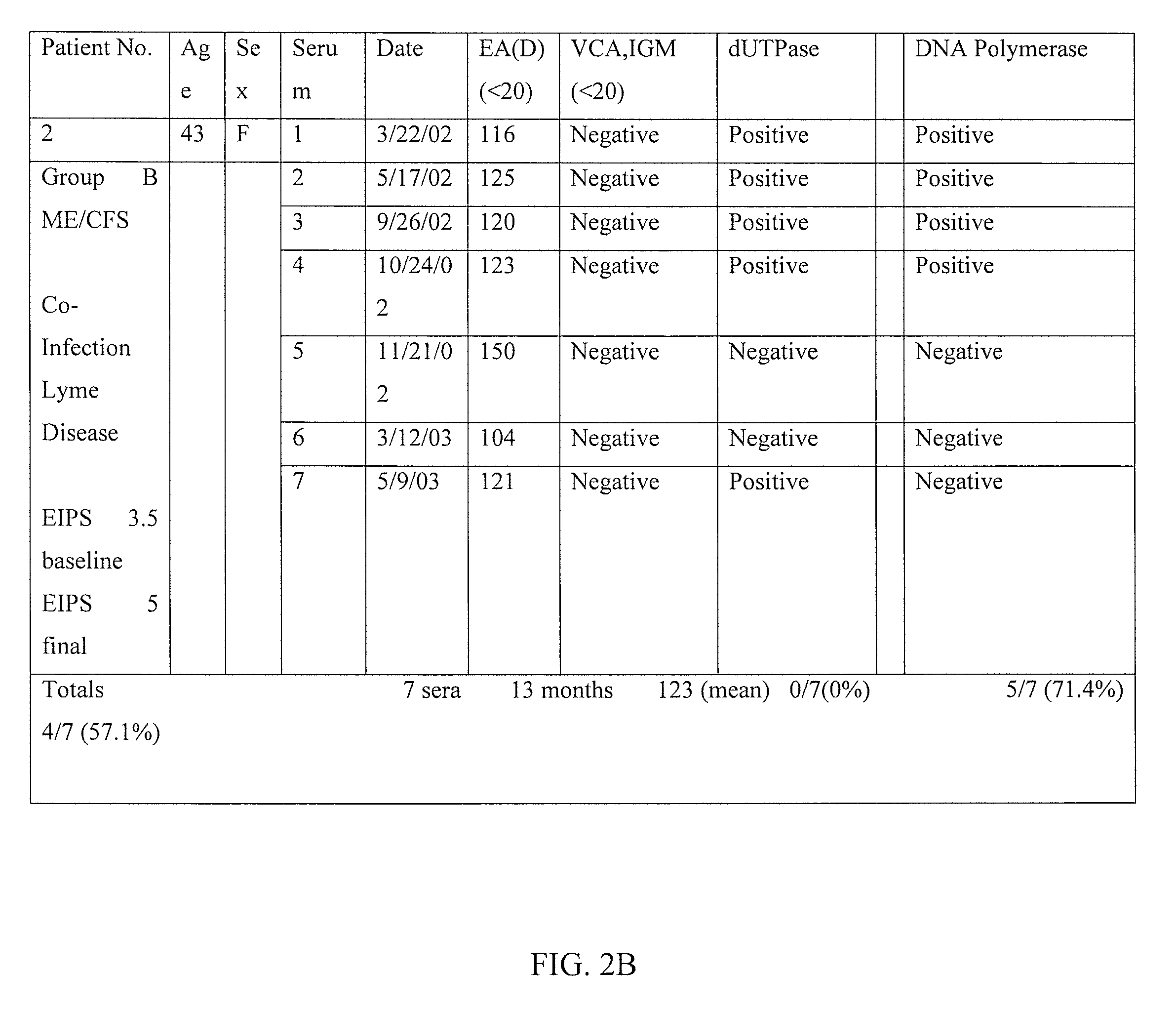
Method of diagnosing and treating epstein barr virus-based myalgic encephalomyelitis chronic fatigue syndrome patients using a serum antibody assay
InactiveUS20160258954A1Peptide/protein ingredientsBiological material analysisEpstein bar virusCFS - Chronic fatigue syndrome
Owner:CFS
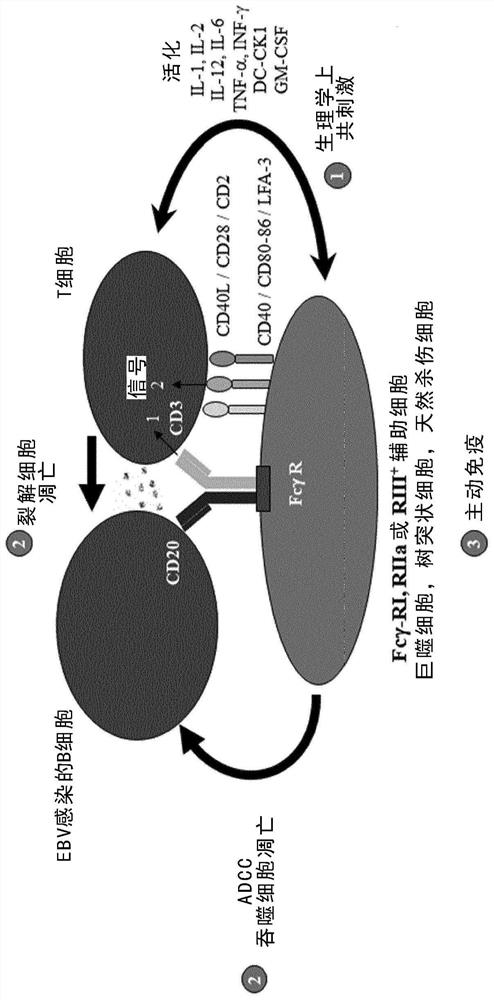
Pharmaceutical preparation for use in treating epstein-barr virus positive patients with reactivation phenomenon-associated diseases
The present invention discloses an isolated trifunctional bispecific antibody for use in a method of treating a patient suffering from a disease and / or a disorder associated with reactivation of Epstein-Barr virus (EBV) in at least B cells and potentially also other susceptible cells such as susceptible epithelial cells, comprising: providing an autologous cell preparation of enriched B cells of said patient; incubating said enriched B cells with the trifunctional bispecific antibody for a time-period sufficient to establish a physical interaction between said trifunctional bispecific antibodyand said enriched B cells to obtain an incubation mixture; transferring said incubation mixture obtained after incubation into the same patient, wherein said trifunctional bispecific antibody comprises: (a) a first binding arm which binds to a B cell via a B cell surface antigen; (b) a second binding arm which binds to a T cell via a T cell surface antigen; (c) an Fc-portion which binds to an Fcreceptor-positive cell. The present invention also discloses a pharmaceutical composition comprising the said isolated trifunctional bispecific antibody for use in the treatment of a patient sufferingfrom a disease and / or a disorder associated with reactivation of EBV in B cells, and an ex-vivo method for preparing the said pharmaceutical composition.
Owner:TRION RES +1
Virus-like particle compositions and vaccines against epstein-barr virus infection and disease
ActiveUS20180078634A1Reduce the amount requiredSsRNA viruses negative-senseViral antigen ingredientsDiseaseNewcastle disease virus NDV
The present invention relates to prophylactic and / or therapeutic vaccines that contain Newcastle disease virus (NDV) virus-like particles (VLPs) comprising one or more Epstein-Barr Virus (EBV) antigens. In one embodiment, the invention provides a recombinant virus-like particle (VLP) comprising, in operable combination, a) Newcastle disease virus (NDV) matrix (M) protein, and b) one or more Epstein-Barr Virus (EBV) antigens. The invention's prophylactic and / or therapeutic vaccines are useful for preventing and / or treating infection with EBV and / or disease associated Epstein-Barr Virus, such as cancer.
Owner:UNIV OF MASSACHUSETTS
Modified epstein-barr virus DNA polymerase and methods for isothermal DNA amplification
Modified Epstein Barr Virus DNA polymerase for use in nucleic acid amplification, including isothermal nucleic acid amplification, in vitro are provided. Methods using and kits comprising Epstein Barr Virus DNA polymerase and its variants of this invention for nucleic acid amplification in vitro, including isothermal DNA amplification, are also provided.
Owner:UNIV OF CONNECTICUT
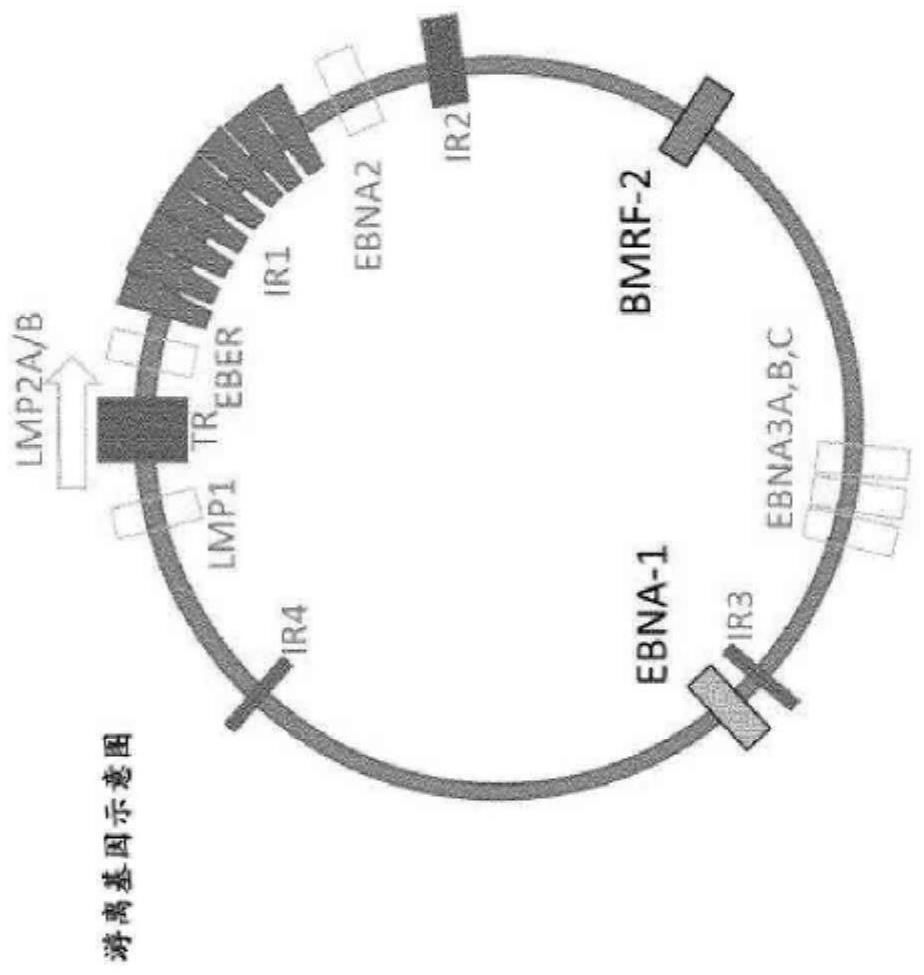
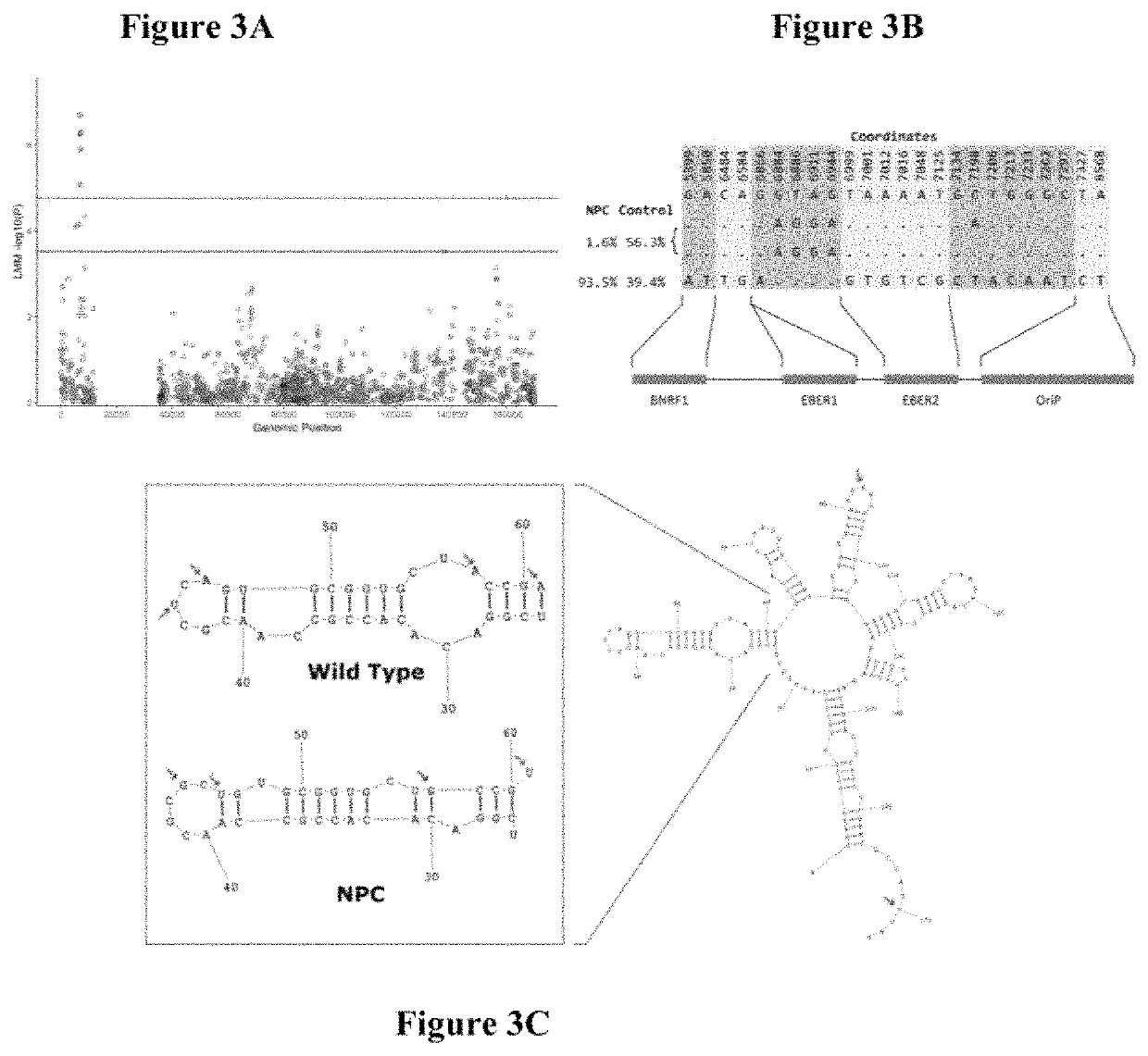
Epstein-barr virus variants
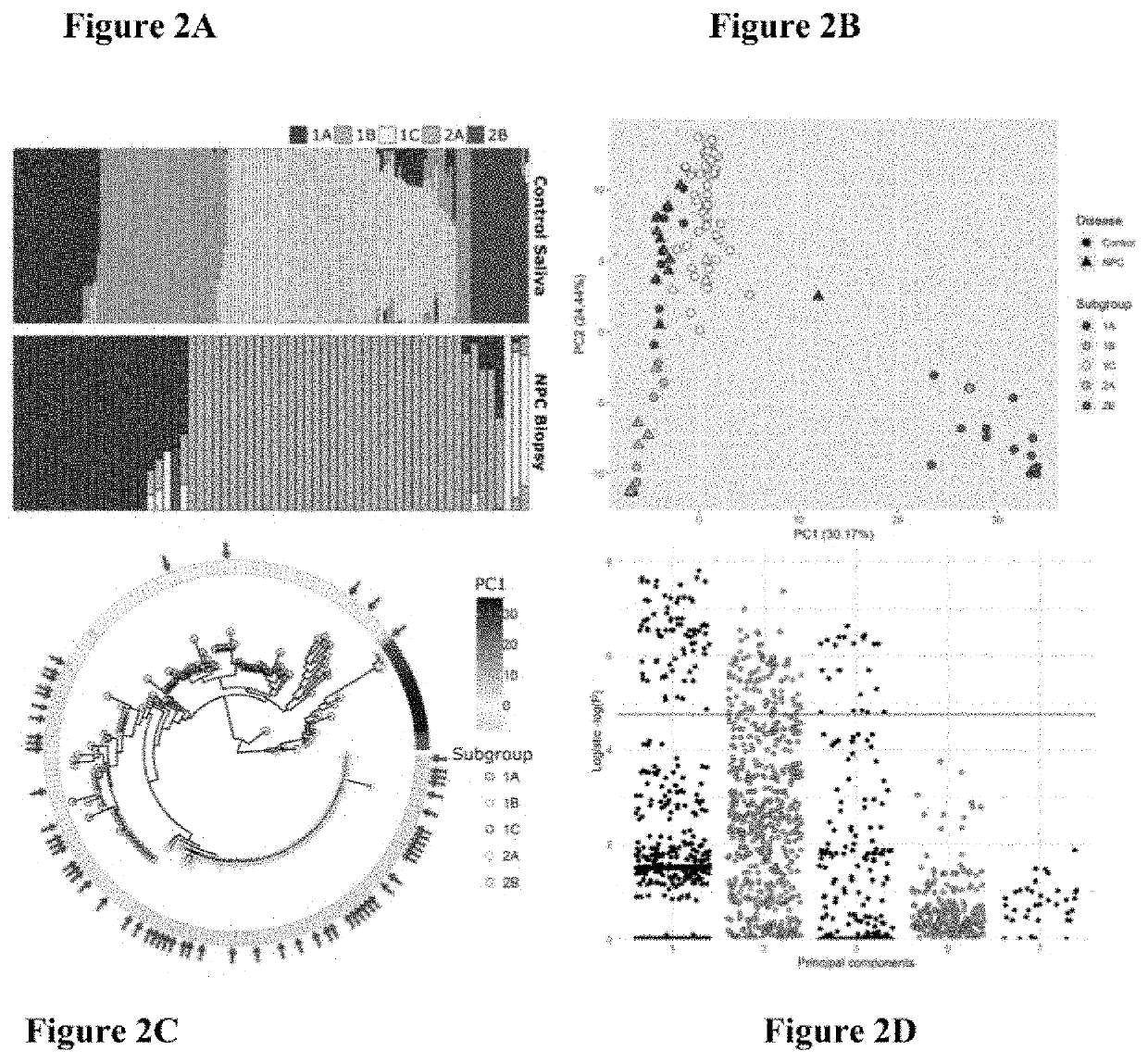
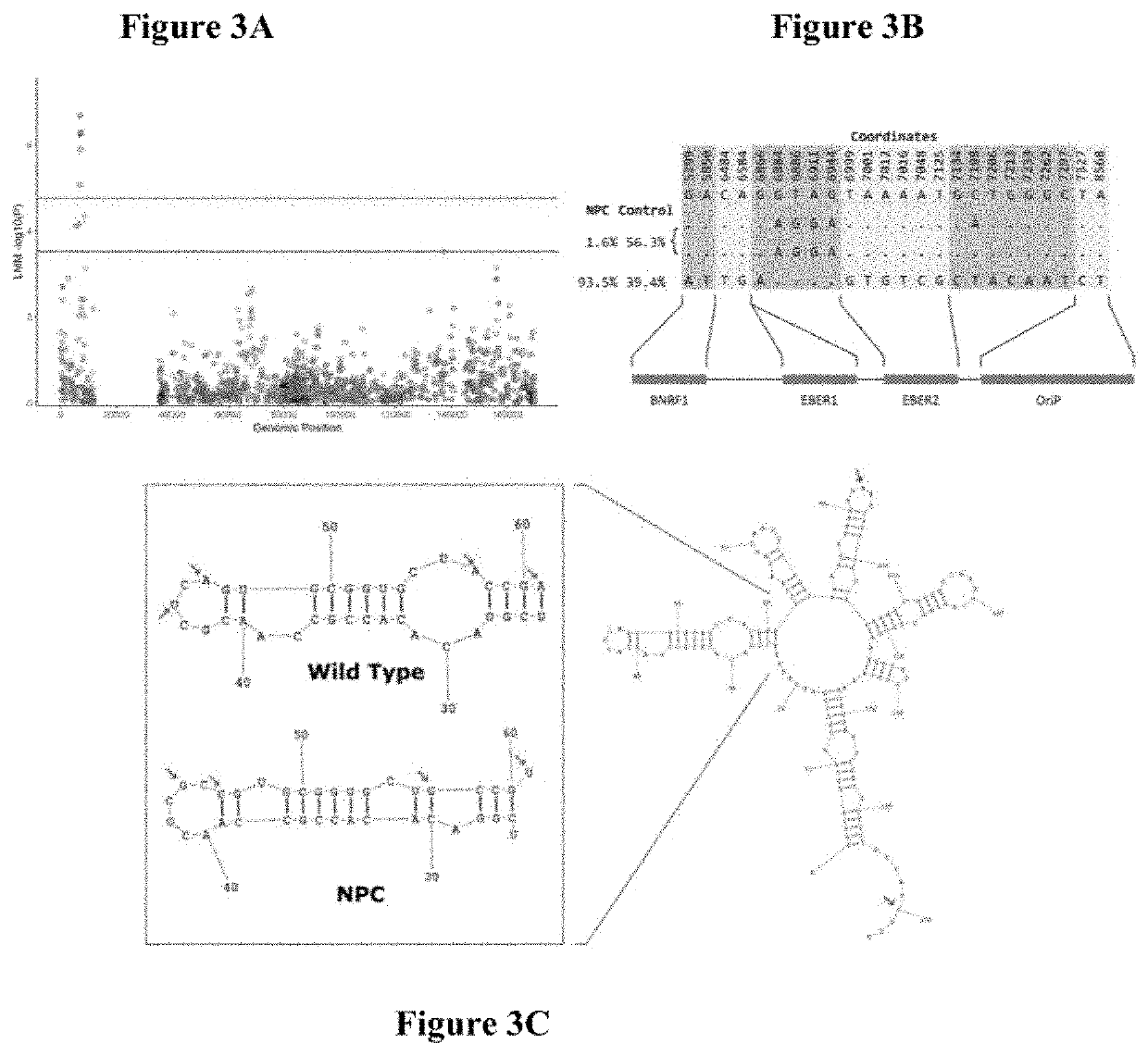
The present invention provides novel Epstein Barr virus (EBV) variants including those associated with increased risk for developing nasopharyngeal carcinoma (NPC).
Owner:THE UNIVERSITY OF HONG KONG
Epstein-barr virus variants
ActiveUS20200140961A1Microbiological testing/measurementDisease diagnosisEpstein bar virusBioinformatics
The present invention provides novel Epstein Barr virus (EBV) variants including those associated with increased risk for developing nasopharyngeal carcinoma (NPC).
Owner:THE UNIVERSITY OF HONG KONG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com