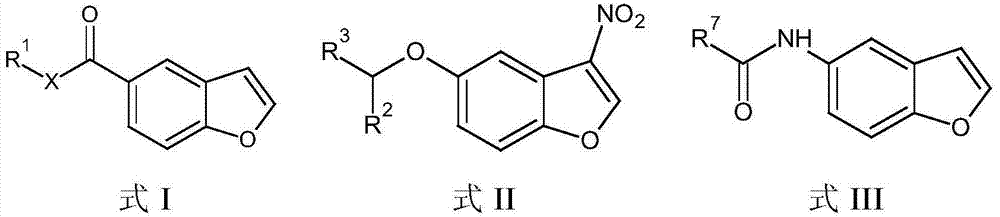
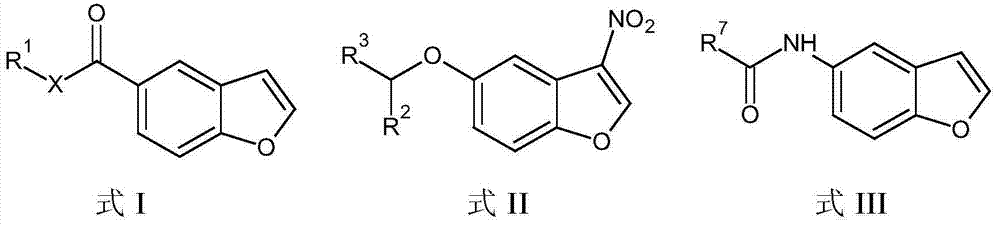
Compositions and methods for modulating the activity of epstein-barr nuclear antigen 1
A composition and compound technology, applied in the direction of biochemical equipment and methods, drug combination, antineoplastic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
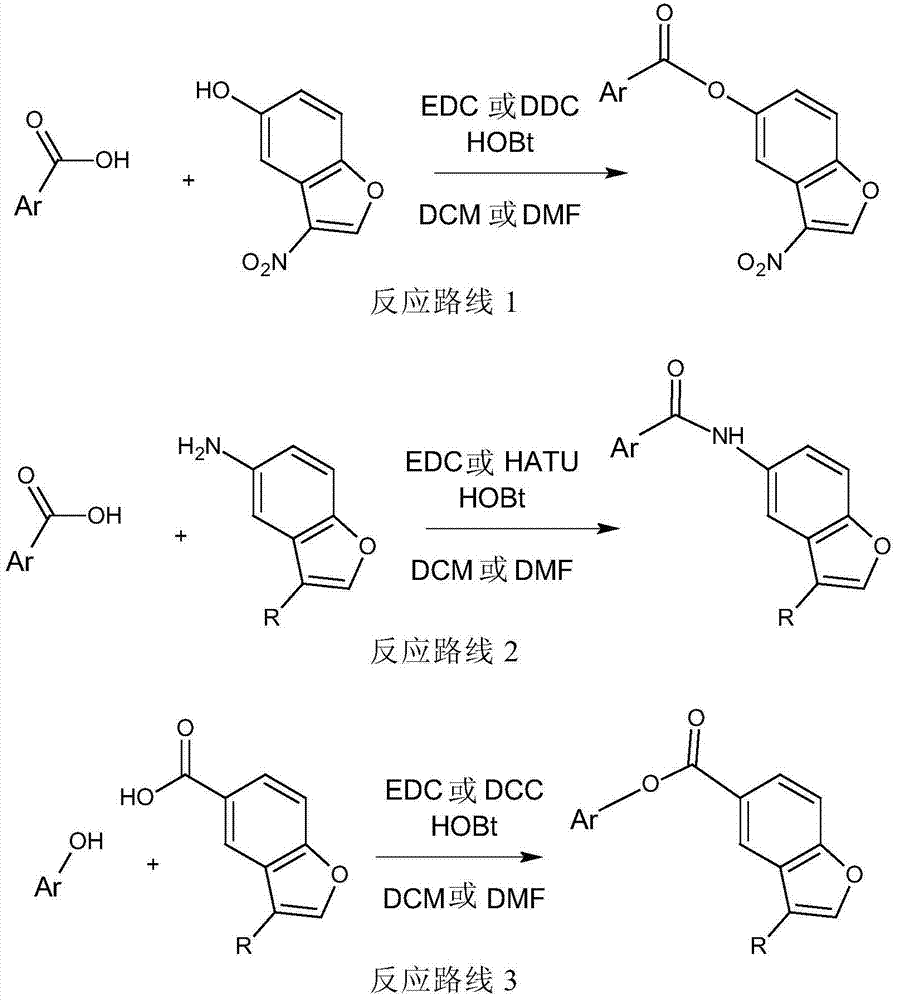
[0054] Example: Parallel Synthesis of Compounds
[0055]
[0056]
[0057] The substituents of the compounds synthesized according to Schemes 1-7 are listed in Table 1.
[0058] Table 1
[0059]
[0060] In addition to 3-nitro-benzofuran, other core structures are also envisioned, including but not limited to the following structures.
[0061]
[0062]
Embodiment 2
[0063] Example 2: Materials and methods
[0064] Expression and purification of recombinant EBNA1DBD. Amino acids 454 to 607 of EBNA1 encoding the DNA binding domain (GENBANK accession number YP_401677; SEQ ID NO: 1) were expressed in E. coli as a hexahistidine fusion protein. Expression was in Rosetta2 cells induced with 0.3 mM IPTG for 3 hours at 25°C. Soluble protein was recovered and purified by Ni-NTA agarose following standard methods (Frangioni & Neel (1993) Anal. Biochem. 210:179-87). The bound protein was extensively washed with 20 mM HEPES, pH 7.9, 1 M NaCl, 5 mM 2-mercaptoethanol, 40 mM imidazole and 10% glycerol to dissociate non-specific DNA bound to EBNA1, and then washed in a buffer containing 250 mM imidazole take off. Peak fractions of the eluted protein were pooled and dialyzed against 20 mM HEPES, pH 7.9, 500 mM NaCl, 5 mM 2-mercaptoethanol, 10% glycerol and 0.2 mM PMSF.
[0065]FP assay. The FP assay uses a probe with a non-palindromic site with two hi...
Embodiment 3
[0072] Example 3: Activity of Compounds
[0073] Compounds are screened for activity in the cell-based assays disclosed herein. Compound IC 50 The values are listed in Table 2.
[0074] Table 2
[0075]
[0076]
[0077]
[0078]
[0079]
[0080] IC 50 Compounds at 125000 nM were inactive.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com