Detection kit, detection system and detection method for 19 high-risk human papilloma viruses (HPVs)
A technology of human papillomavirus and kit, applied in the biological field, can solve the problems of high false negative rate and false positive rate, poor specificity, and low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] In this embodiment, 19 high-risk human papillomavirus DNA plasmids in the whole genome type reference product are used as templates, and the kit of the present invention is used for real-time fluorescent PCR detection:
[0049] (1) Establish PCR reaction system
[0050] Using the probes and specific primers designed above, take 5 μL of reference DNA as a reaction template, and use the following PCR reaction system for amplification. The PCR amplification reaction system is:
[0051]
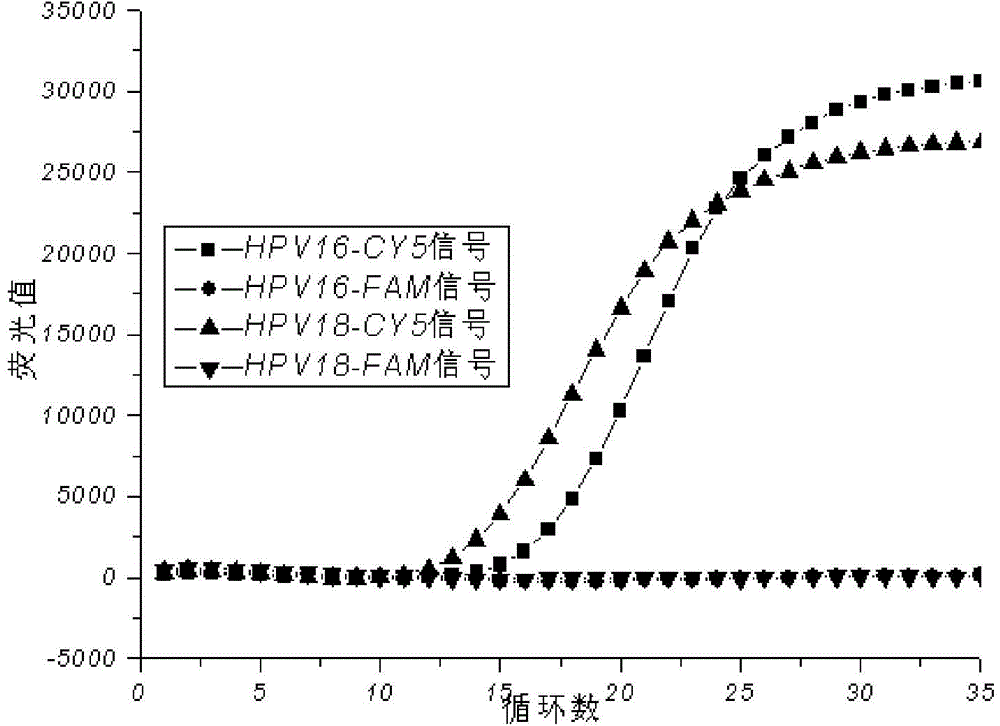
[0052] Put the template-added PCR reaction tube into the real-time fluorescent PCR detector, and proceed according to the following amplification procedures: first stage: 50°C for 2 minutes, 95°C for 5 minutes, 1 cycle; second stage: 95°C for 5s, 40°C 30s, 72°C for 30s, 10 cycles; third stage: 95°C for 5s, 60°C for 35s, 72°C for 30s, 35 cycles; fluorescence collection: collect FAM, HEX (or VIC) and CY5 signals at 60°C in the third stage .
[0053] (2) Judge the detection result accordin...
Embodiment 2
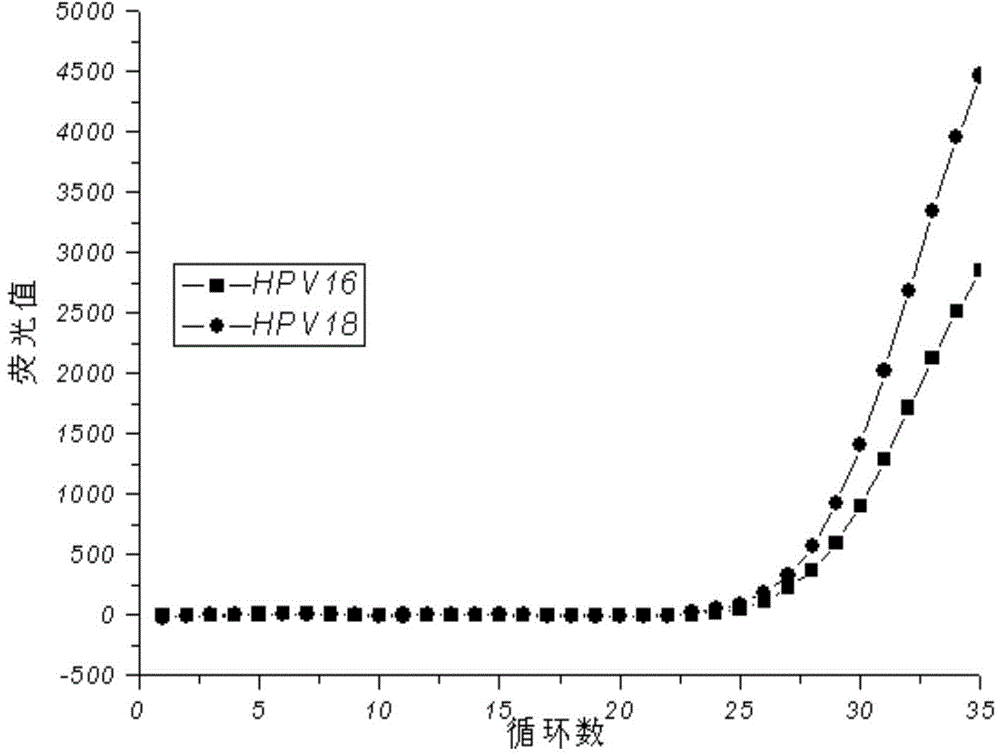
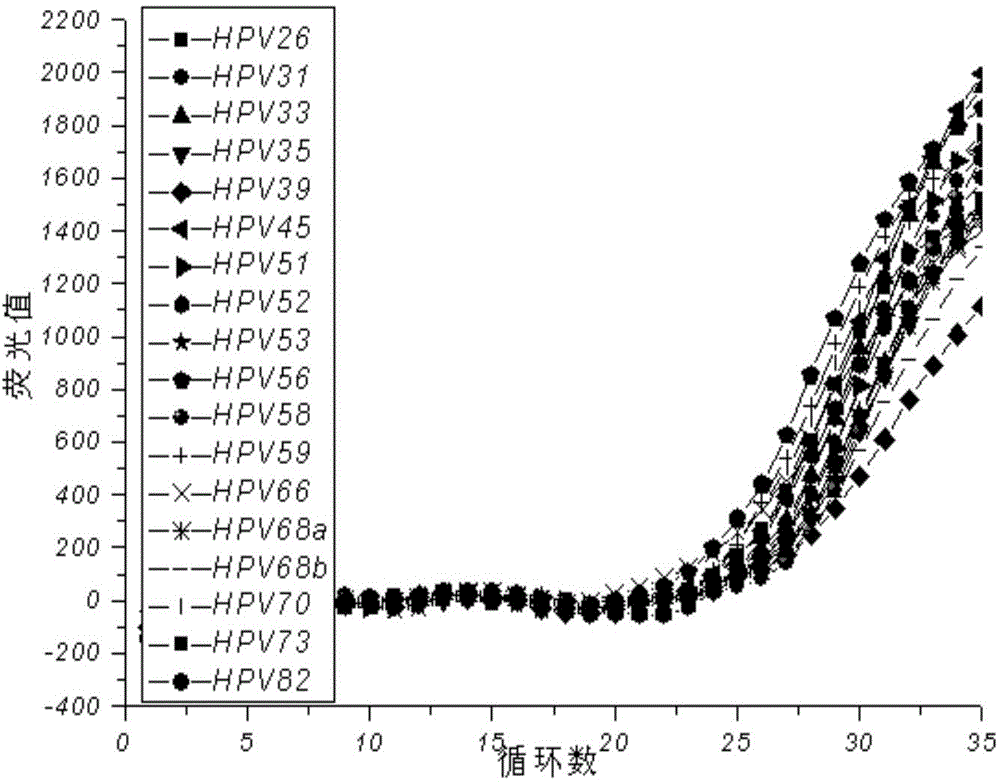
[0061] The reaction system is as in Example 1, using the kit of the present invention to detect each component in the national reference product for the full genome type of human papillomavirus, and investigating the effect of the final determined reaction system on the national reference product for the full genome type of human papillomavirus The detection situation, the test results are as follows Figure 3-7 .
[0062] The test results show that this kit is negative for the negative reference products N1-N5 in the national reference products of the full genome type of human papillomavirus, and it is negative for the 7 low-risk samples in the national reference products of the full genome type of human papillomavirus. Types (HPV6, 11, 61, 67, 69, 71, 81) positive reference products were all tested negative, and the 13 high-risk types (HPV16, 18, 26, 31 . 100%.
Embodiment 3
[0064] Using the present invention to detect clinical samples, a total of 80 clinical samples were detected, and the human papillomavirus nucleic acid detection kit (PCR-fluorescent probe method) (type 17) of Yaneng Biotechnology (Shenzhen) Co., Ltd. was used as a contrast . The steps of using the real-time fluorescent PCR system of the present invention to detect 80 cases of clinical samples are as follows.
[0065] (1) Detection sample DNA extraction
[0066] Test samples include cotton swabs of urogenital secretions, genital scrapings, tissue biopsy specimens, etc.
[0067] Transfer 1 mL of the collected sample to a clean centrifuge tube, centrifuge at 12,000 rpm for 1 minute; carefully suck away 700 μL of supernatant, and add 350 μL of Buffer VDL12 (with absolute ethanol added) to the remaining precipitate; shake and mix for 10 seconds , centrifuge in a palm centrifuge for 5-10s, place in an incubator for digestion at 80°C for 3min; shake and mix for 10s, centrifuge in a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com