Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
154 results about "Alphapapillomavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
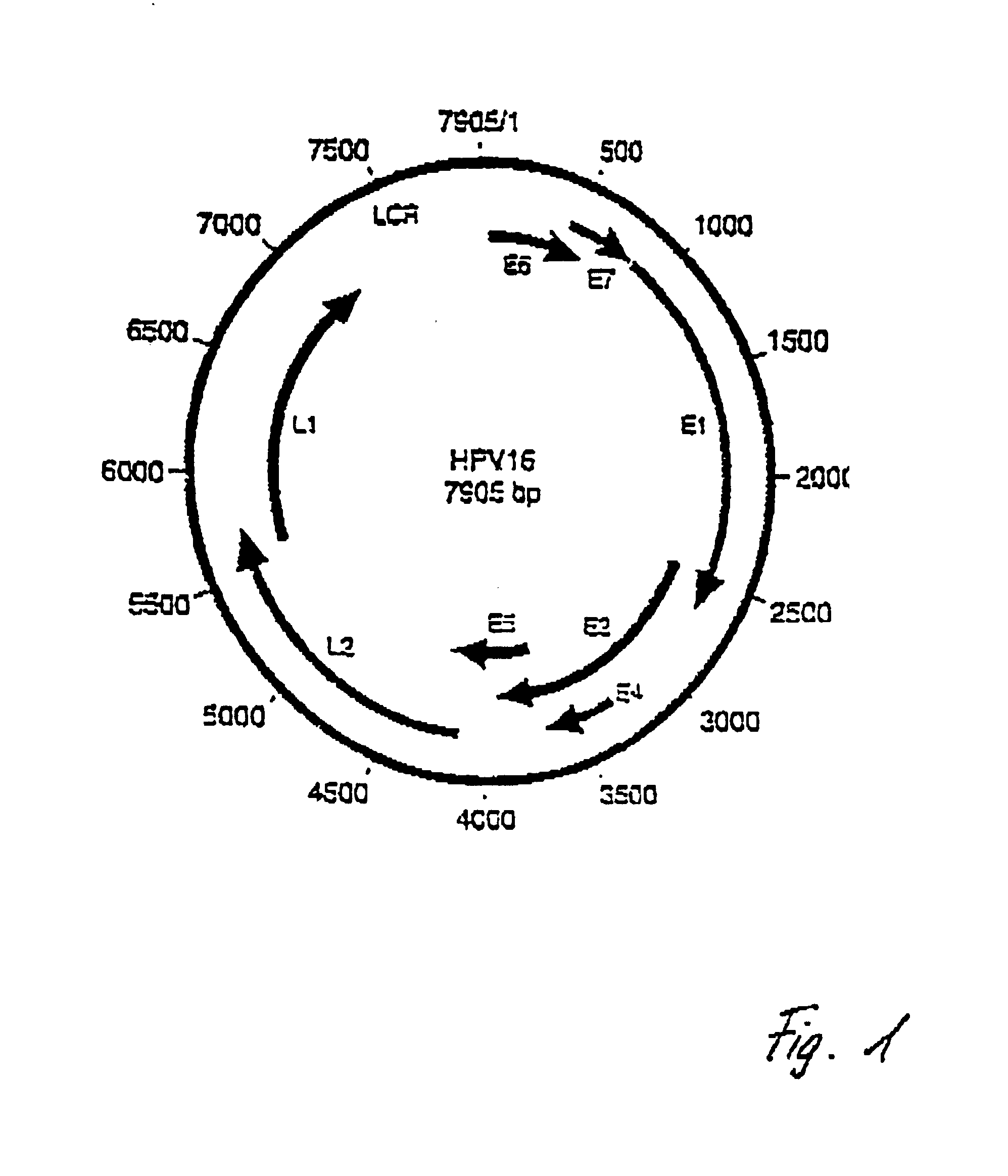
Alphapapillomavirus is a genus of viruses, in the family Papillomaviridae. Human and monkeys serve as natural hosts. There are currently 14 species in this genus including the type species Alphapapillomavirus 1. Diseases associated with this genus include: warts, papilloma, malignant tumours. Genital-type, high-risk of malignancy (cervical cancer): HPV-16, 18. Genital-type, low-risk of malignancy (genital warts): HPV-6, 11.
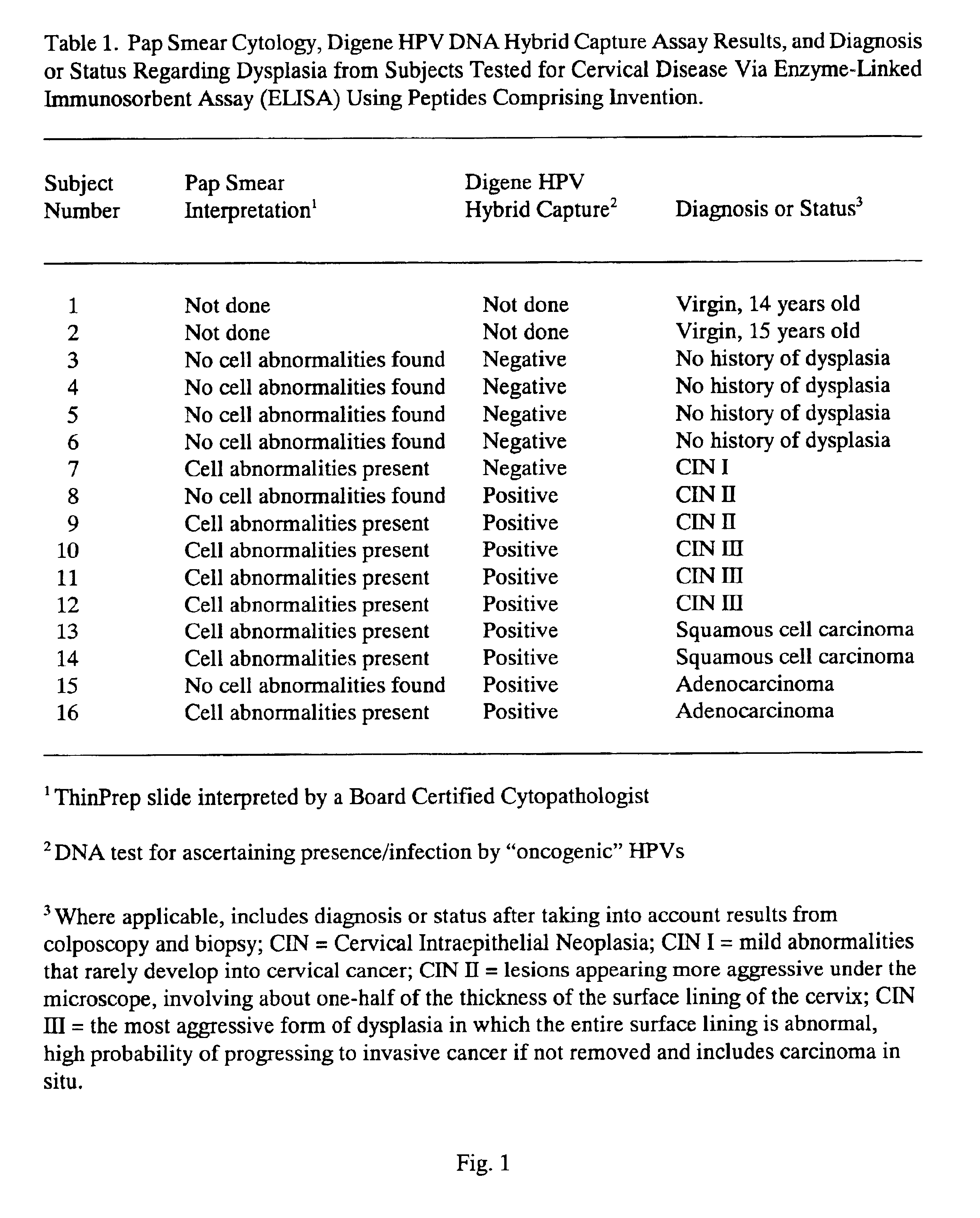
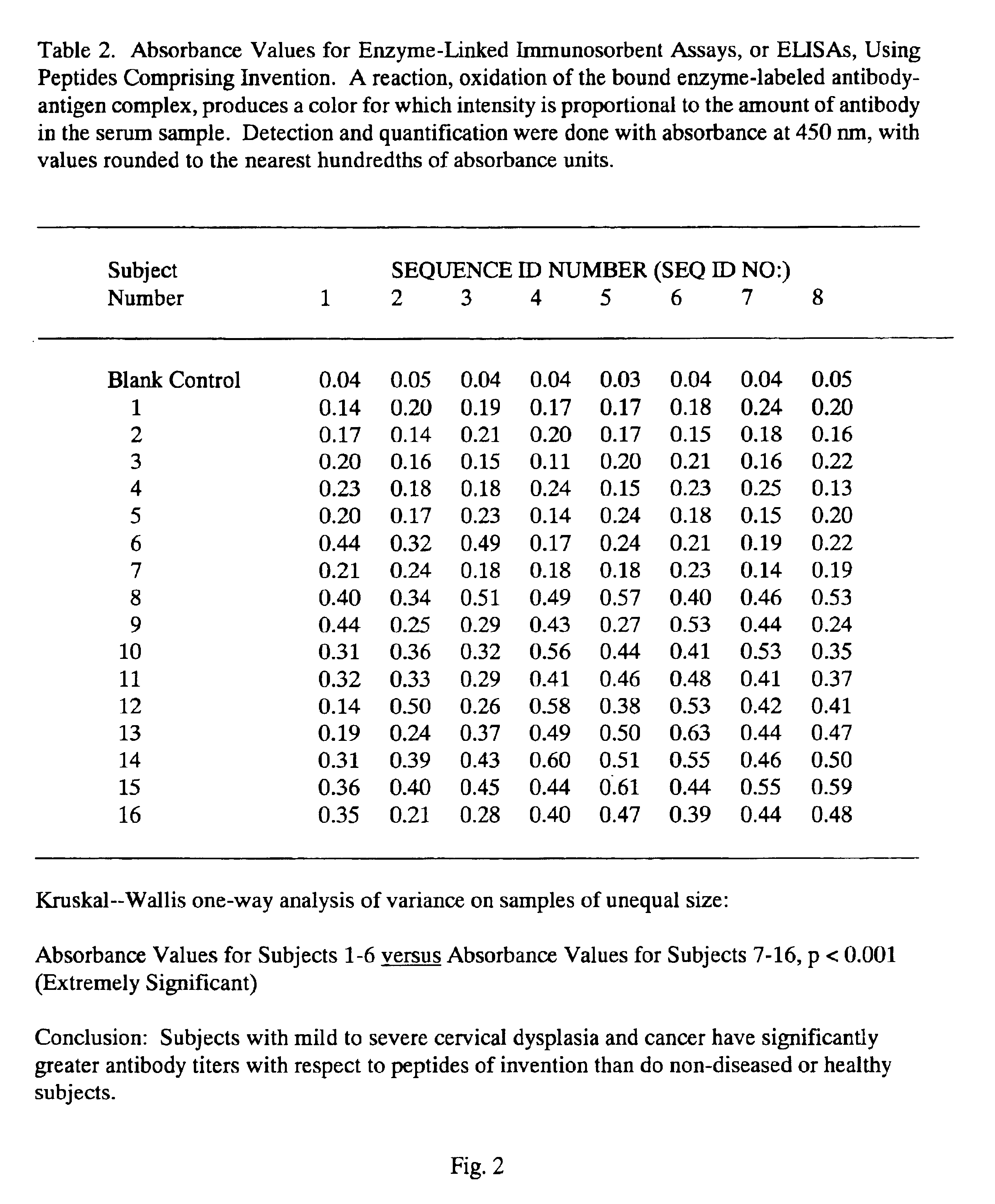
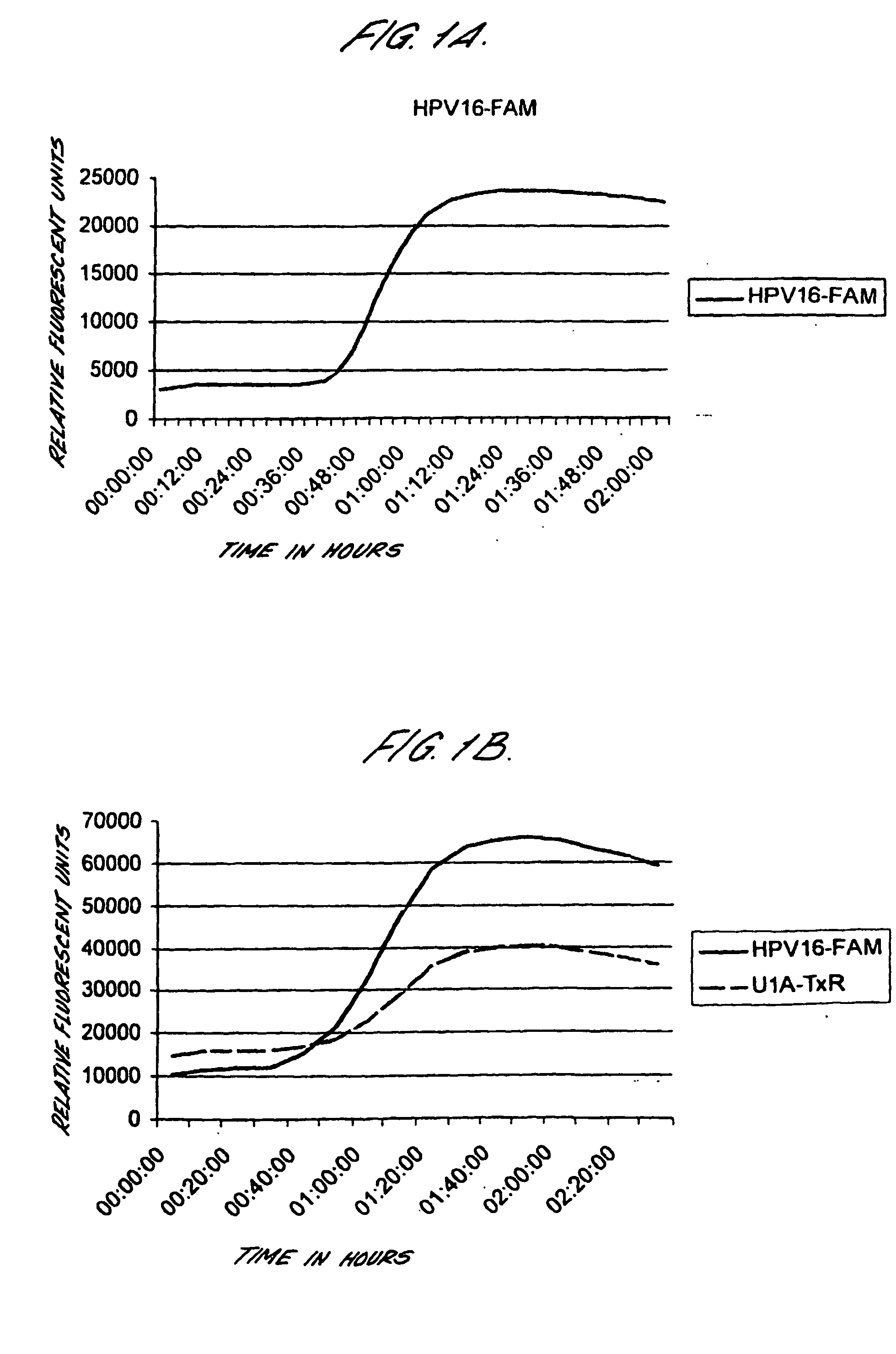
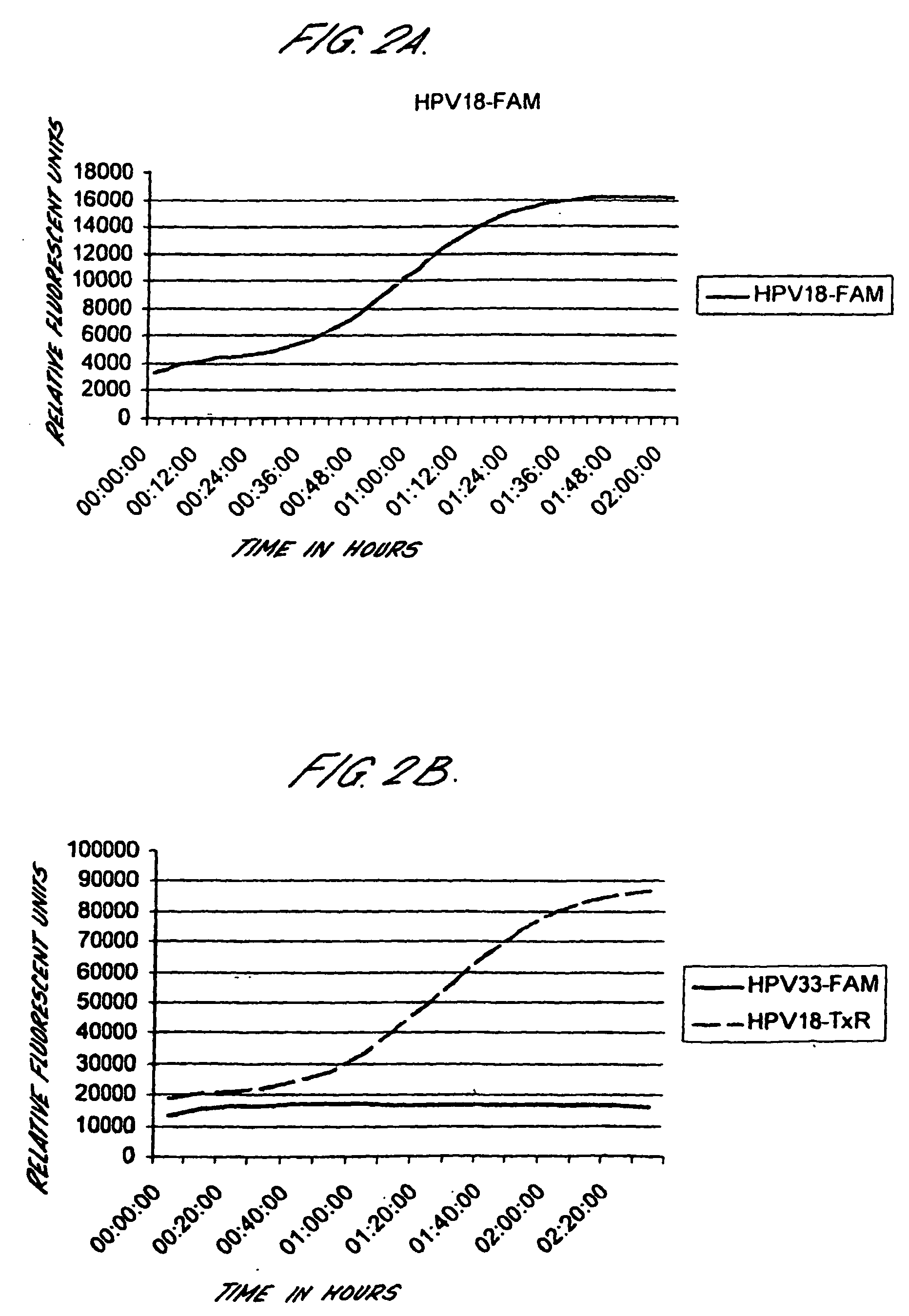
Peptides from the E2, E6, and E7 proteins of human papilloma viruses 16 and 18 for detecting and/or diagnosing cervical and other human papillomavirus associated cancers
InactiveUS6933123B2Simple and rapid and and more testMicrobiological testing/measurementVirus peptidesCysteine thiolateTryptophan
Owner:HU YAO XIONG
Virion Derived Protein Nanoparticles For Delivering Diagnostic Or Therapeutic Agents For The Treatment Of Dermatology Related Genetic Diseases
InactiveUS20130012450A1Peptide/protein ingredientsViral/bacteriophage medical ingredientsBasal membraneDelivery system
This invention relates to a transdermal delivery system for treating skin related genetic diseases. More specifically, the present invention provides particles and methods for using pseudo-viruses, including those derived from the herpes and papillomaviruses, to deliver drugs to keratinocytes and basal membrane cells for the treatment of skin genetic disorders including Pachyonychia Congenita and Xeroderma Pigmentosum.
Owner:AURA BIOSCI
Method for detecting human papillomavirus mrna
InactiveUS20050118568A1High expressionIncrease probabilityMicrobiological testing/measurementEnzymologyHuman papillomavirusHuman Females
An in vitro method is provided for screening human female subjects to assess their risk of developing cervical carcinoma which comprises screening the subject for expression of mRNA transcripts from the E6 and optionally the L1 gene of human papillomavirus, wherein subjects positive for expression of L1 and / or E6 mRNA are scored as being at risk of developing cervical carcinoma. Kits for carrying out such methods are also provided.
Owner:NORCHIP AS
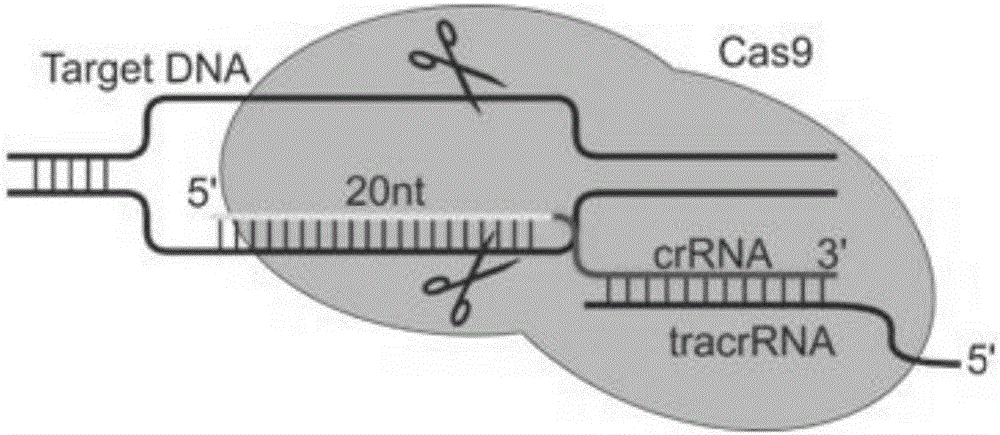
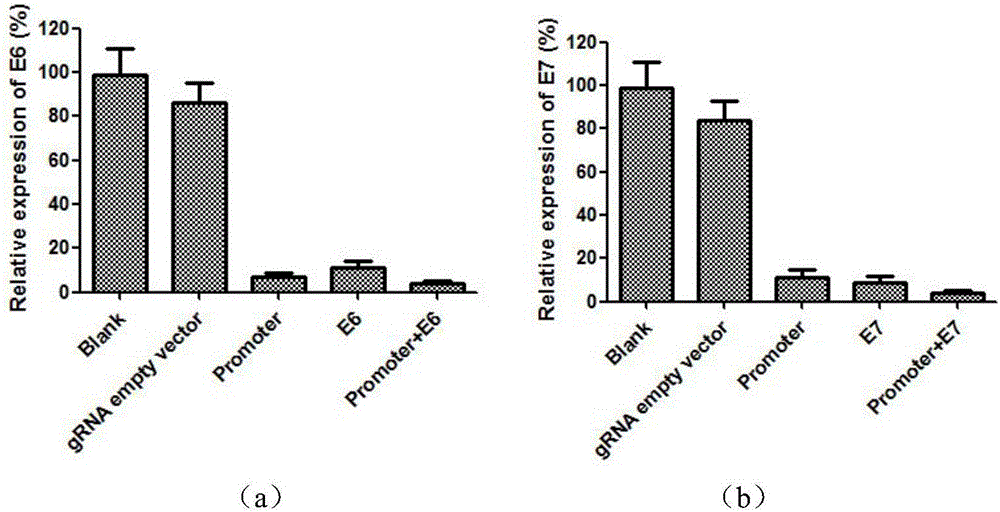
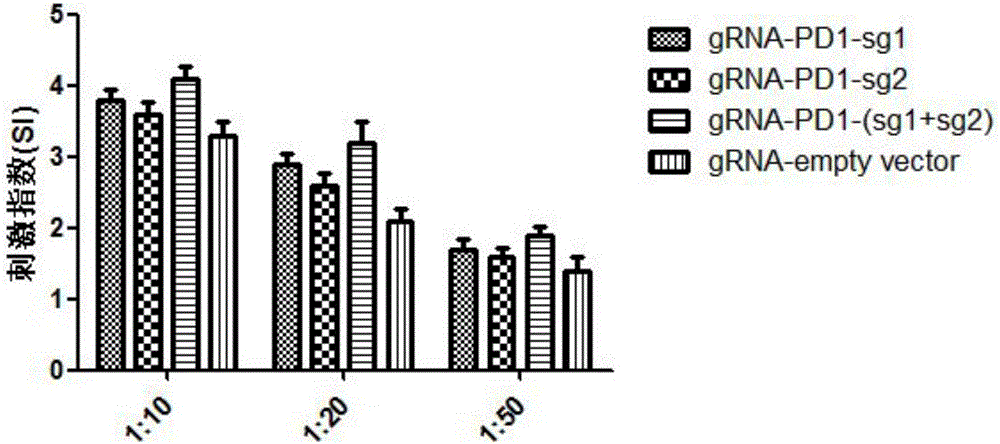
SgRNA combined with immunogene to inhibit high risk HPV expression, gene knockout vector thereof, and application of sgRNA and vector
ActiveCN105821040AImprove targetingGrowth inhibitionOrganic active ingredientsGenetic material ingredientsWilms' tumorCas9
The invention provides an sgRNA combined with immunogene to inhibit high risk HPV expression, a gene knockout vector thereof, and an application of the sgRNA and the vector. The sgRNA sequences of a human papilloma virus 16 type gene suitable for CRISPR-Cas9 targeting editing and a human PD-1 gene are selected, and the plasmid vector of the sgRNA and a CRISPR-Cas9 nuclease gene expression vector are transferred to HPV16+ human cervical carcinoma cells and HPV16+ transplantation tumor-bearing mice, and can obviously inhibit HPV16 expression and inhibit tumor growth. The gene expression vector prepared in the invention has the advantages of simple method steps, good sgRNA targeting property, high CRISPR-Cas9 knockout efficiency, accurate targeting splicing of high risk HPV16 type and PD-1 genes, efficient reduction of the expression of the high risk HPV16 type gene, and obvious inhibition of the tumor growth through combined application.
Owner:李旭
Human papillomavirus inhibitors
The present invention provides systems for identifying anti-viral agents. In particular, the invention encompasses reagents and strategies for identifying agents that inhibit or disrupt key protein-protein interactions that are important in the life cycle of papillomaviruses. The invention allows identification, production, and / or use of agents that reduce or inhibit the replication of HPV by inhibiting (e.g., precluding, reversing, or disrupting) the formation of the E1-E2 protein-protein complex. The invention also provides specific inhibitory agents, pharmaceutical compositions, and methods of using these inhibitors and pharmaceutical compositions for inhibiting viral replication in vitro. Methods are also described for the treatment and prevention of HPV infections and HPV-related diseases in patients.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Compositions, methods and products comprising human papillomavirus for detecting and treating a cancer
InactiveUS20060029943A1Microbiological testing/measurementBiological material analysisHuman papillomavirusAlphapapillomavirus
Owner:HERMONAT PAUL +2
Synthetic human papillomavirus genes
InactiveUS7001995B1Easy to insertEasy to removeBiocideGenetic material ingredientsPolynucleotide VaccinesHuman papillomavirus
Synthetic DNA molecules encoding papillomavirus proteins are provided. The codons of the synthetic molecules are codons preferred by the projected host cell. The synthetic molecules may be used as a polynucleotide vaccine which provides effective immunoprophylaxis against papillomavirus infection through stimulation of neutralizing antibody and cell-mediated immunity.
Owner:MERCK SHARP & DOHME CORP
Detection of human papilloma virus
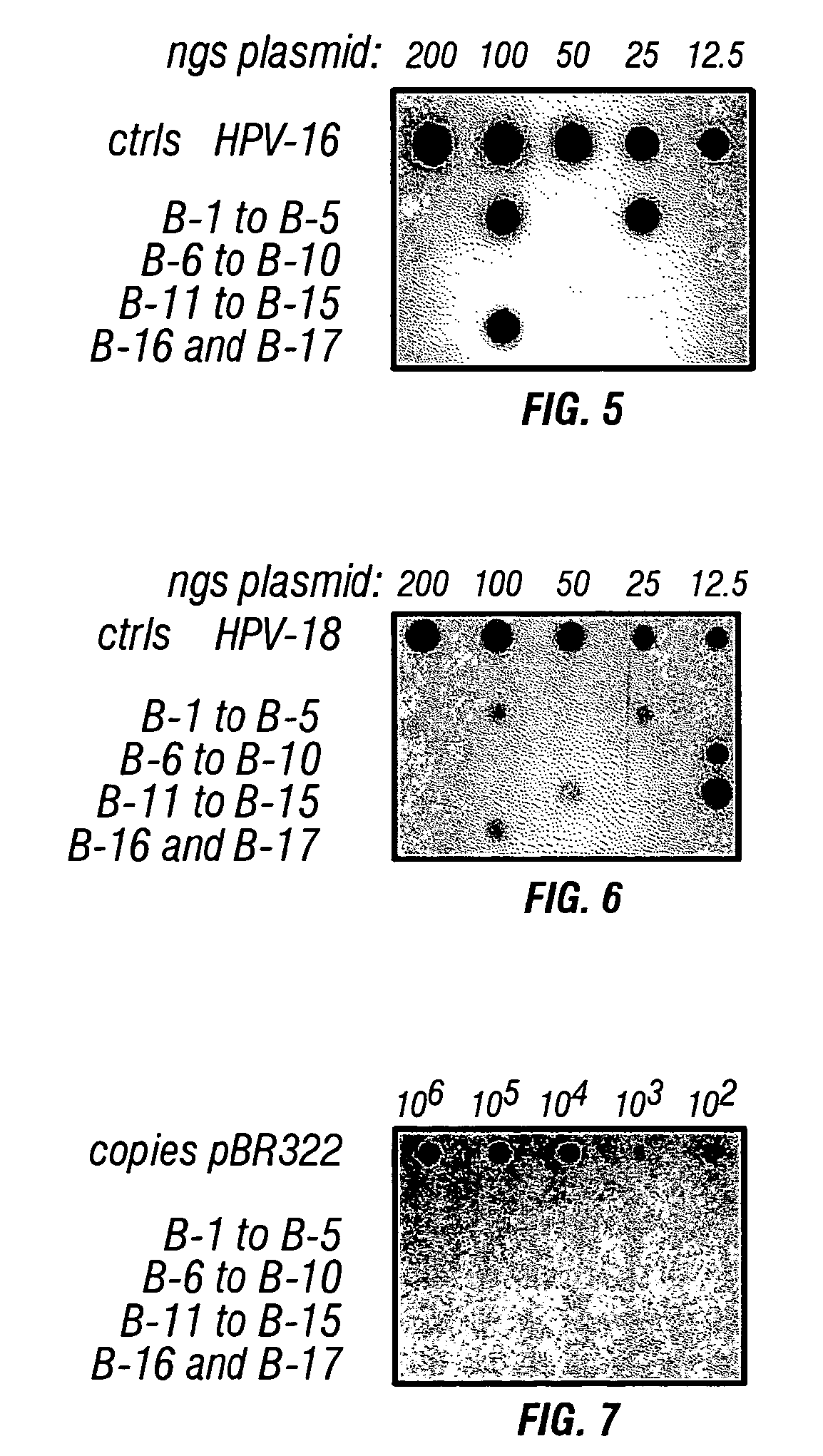
InactiveUS20090029346A1Reduce in quantityMicrobiological testing/measurementCytosineAlphapapillomavirus
An assay for detecting HPV comprising treating the viral nucleic acid with an agent that modifies cytosine to form derivative viral nucleic acid, amplifying at least a part of the derivative viral nucleic acid to form an HPV-specific nucleic acid molecule, and looking for the presence of an HPV-specific nucleic acid molecule, wherein detection of the HPV-specific nucleic acid molecule is indicative HPV.
Owner:HUMAN GENETIC SIGNATURES PTY LTD
Gelled immunomodulating topical compositions and a method of treating warts and other human papilloma virus skin infections
Topical drug compositions of this invention contain delayed type contact sensitizing haptens in a unique non-flowable, non-toxic, non-volatile, anhydrous gel composition to achieve retained site application on warts and other human papilloma virus (HPV) skin infections. The preferred gelled compositions contain, but are not limited to, the sensitizing haptens, squaric acid dibutylester and diphenylcyclopropenone in optimized blends of Polysorbate 80, Isopropyl myristate uniquely gelled with Polyoxyl 40 stearate to form a penetrant of keratinized epitheliiuim of warts for direct application wherein virucidal pharlacologic action is induced by Th-1 cell mediated immune responses with resultant releases of CD4 helper T cells, CD8 killer T cells and cytokines to attack the human papilloma viruses. The commonly used vehicles with these contact sensitizers are acetone, petrolatum, or water containing emulsion creams which do not have the capacity to penetrate the keratinized wart surfaces and are therefore minimally effective in treating warts.
Owner:HAPTEN PHARMA
Method for purifying human papilloma virus advanced protein from prokaryote
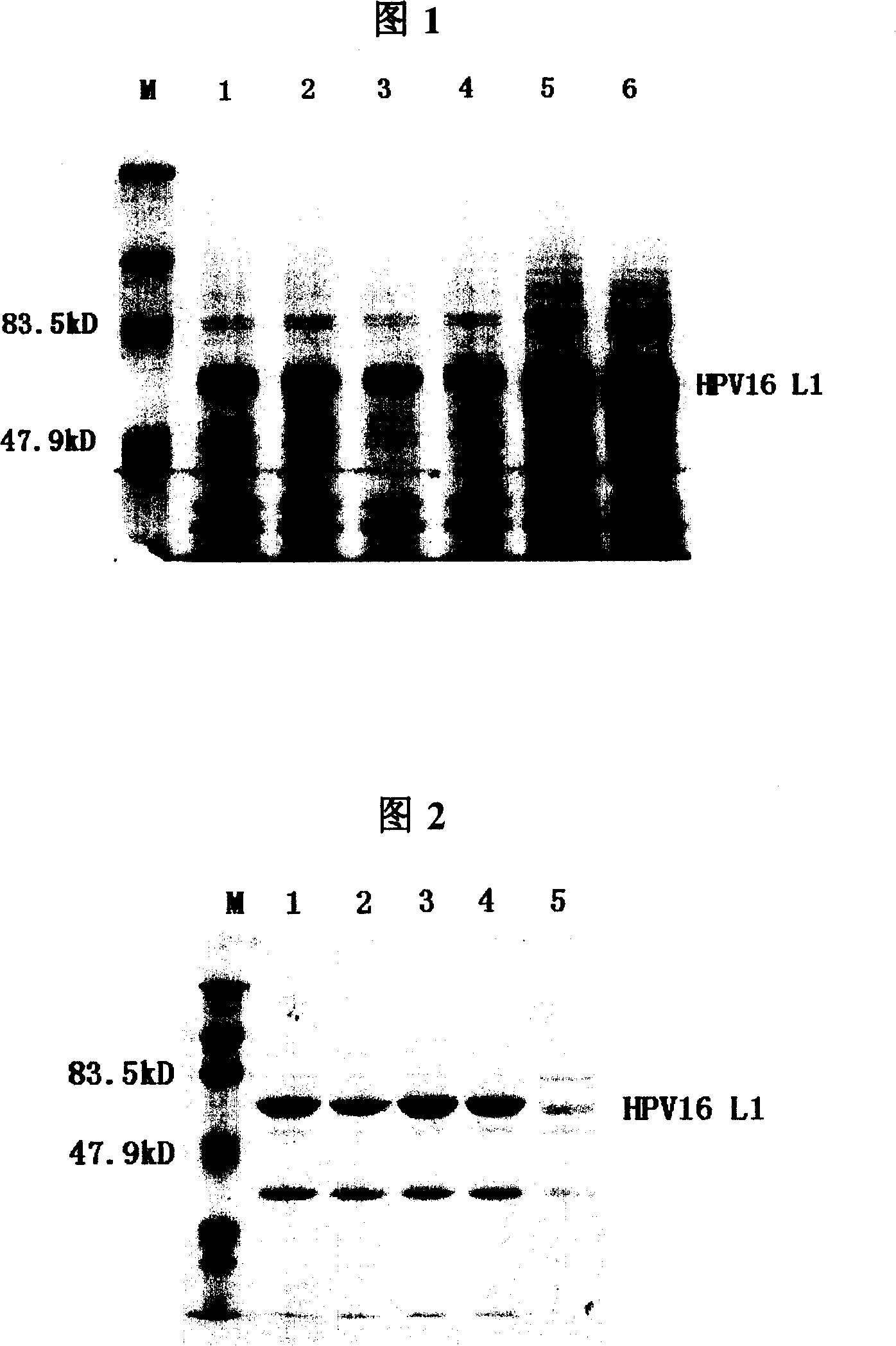
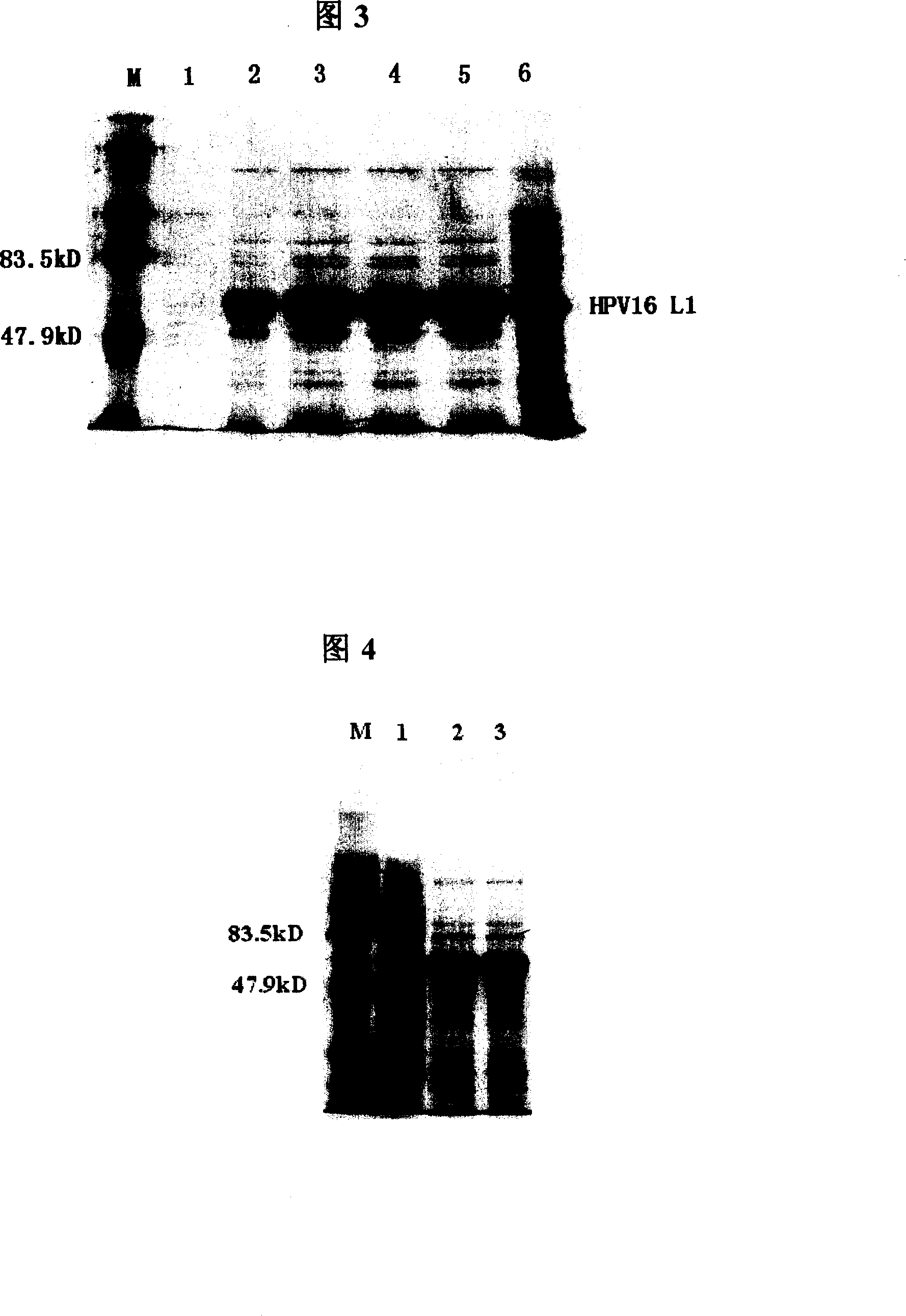
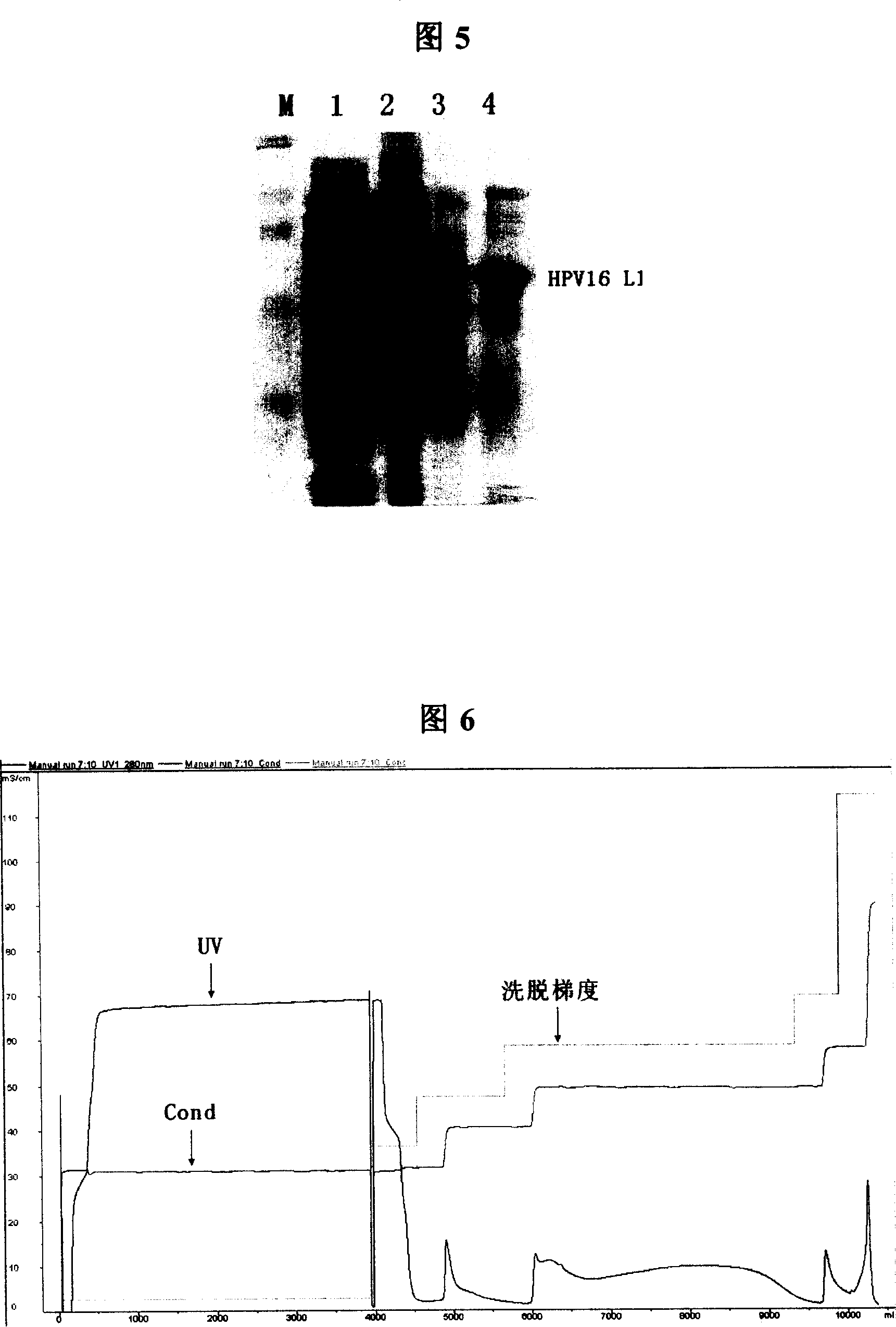
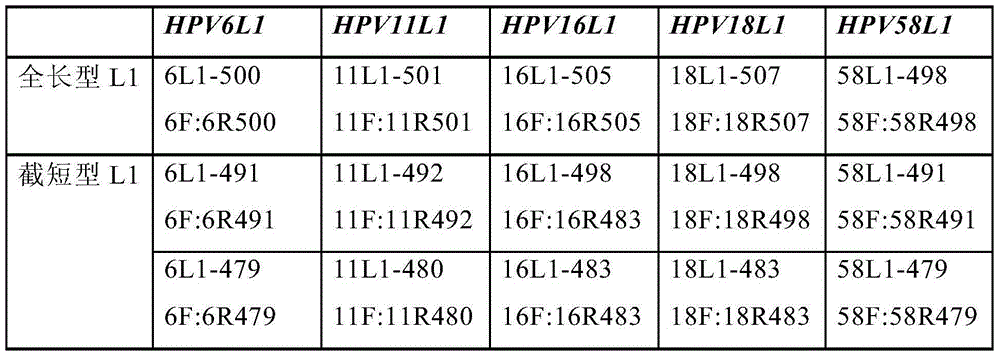
The present invention provides a purification method of human papillomavirus late protein L1 from escherichia coli. Virus-like Particles; the virus-like Particles with elctrophoresis purity more than 98 percent can be produced in a large scale through salt free precipitation, re-dissolving, ion exchange chromatography, hydrophobic interaction chromatography and renaturation of the L1 protein in the lysate supernatant of escherichia coli. With good immunogenicity, the virus particles can induce neutralizing antibodies towards homologous HPV virus, and can be used as a vaccine for preventing the infection of HPV.
Owner:XIAMEN UNIV +1
Method for detecting and typing 26 human papillomaviruses
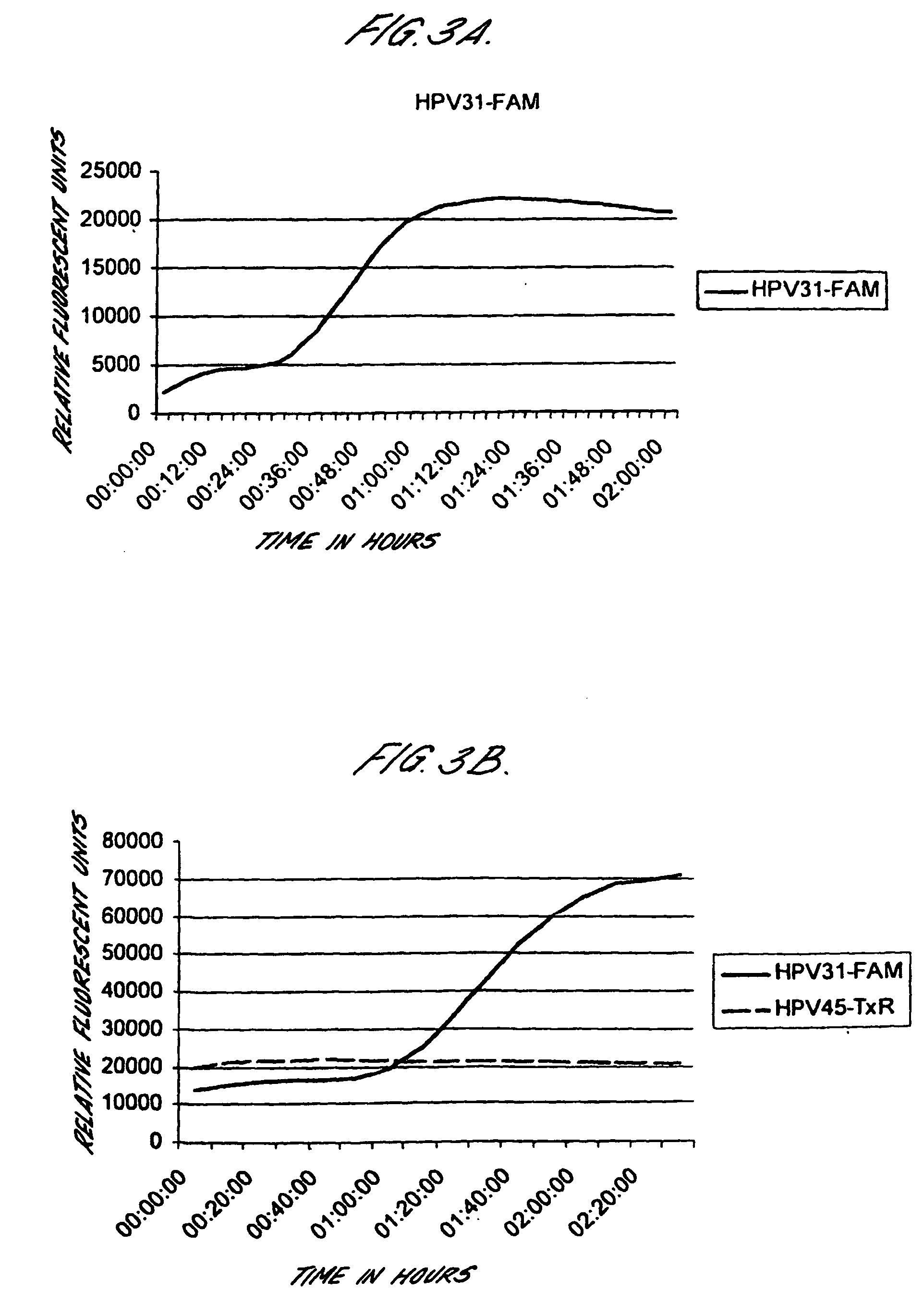
InactiveCN1814796AImprove detection efficiencyShorten detection timeMicrobiological testing/measurementFluorescence/phosphorescenceHuman papillomavirusType specific
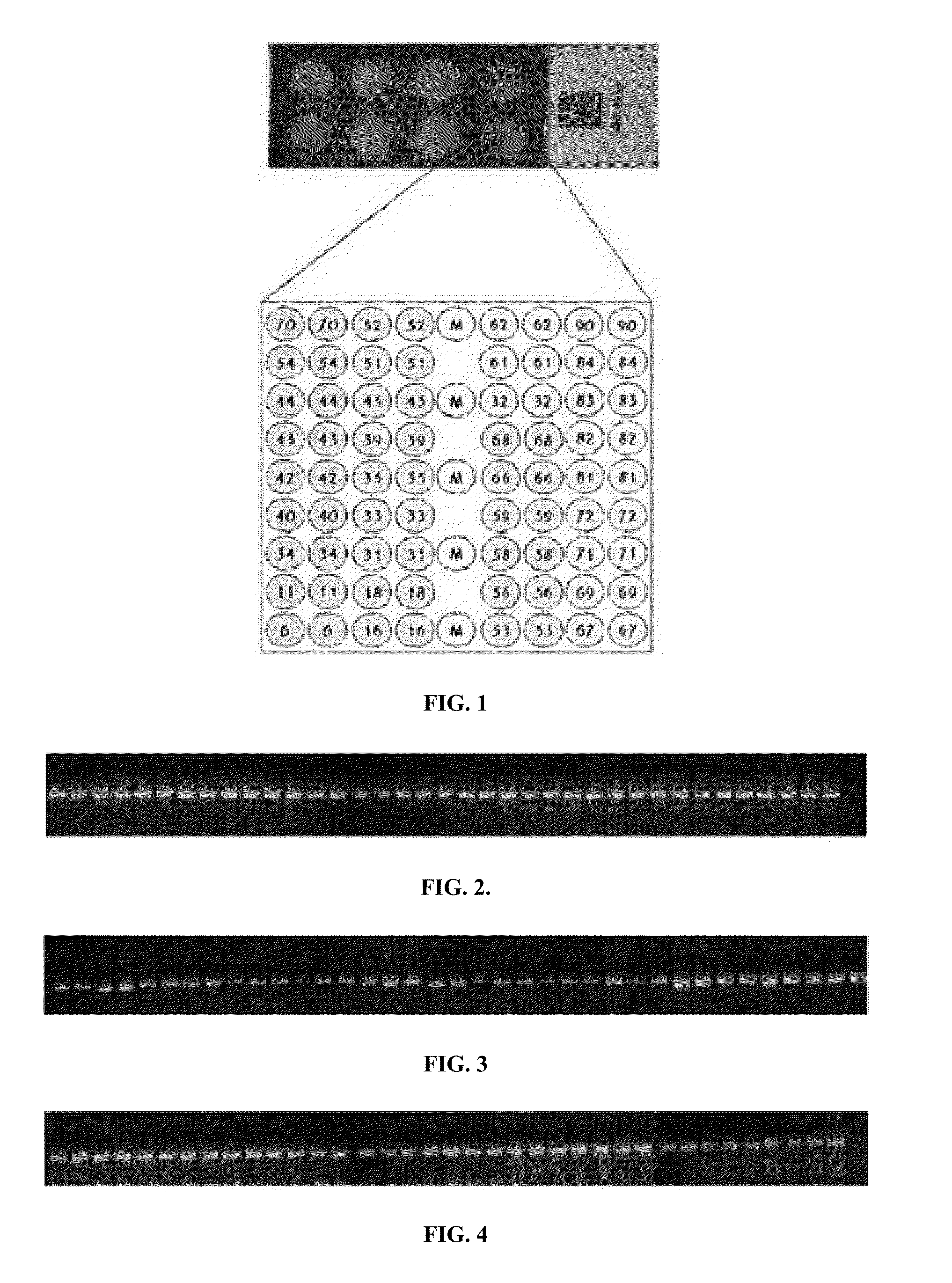
This invention relates to a suspension chip technology for the gene test to human papillomavirus used in testing and typing 26 kinds of papillomaviruses, in which, specific probes of which are crosslinked on 26 kinds of fluorescent microspheres to be reacted with the tested specimens then reacted with the report molecules labeled by the fluorescein to test the type specific nucleic acid of the virus by a fluorescent test device, which can test 26 kinds of ordinary HPV once and increases the test efficiency greatly and overcomes the shortcoming of missing testing the potential infections in the serology and immunity test method to realize early diagnosis to HPA diseases.
Owner:ZHEJIANG UNIV
Truncated L1 proteins of human papilloma virus (HPV), virus-like particles as well as preparation method and application of virus-like particles
InactiveCN104418942AHigh expressionViral antigen ingredientsVirus peptidesVirus-like particleAlphapapillomavirus
The invention relates to truncated L1 proteins of a human papilloma virus (HPV), virus-like particles as well as a preparation method and application of virus-like particles. The invention particularly relates to HPV6-type, HPV11-type, HPV16-type, HPV18-type or HPV58-type truncated L1 proteins of the HPV, the virus-like particles as well as the preparation method and application of the virus-like particles.
Owner:CHANGCHUN BCHT BIOTECH +1
Polypeptides useful as immunotherapeutic agents and methods of polypeptide preparation
InactiveUS6123948AGood effectStimulate immune responseAntibody mimetics/scaffoldsVirus peptidesAdjuvantHuman papillomavirus
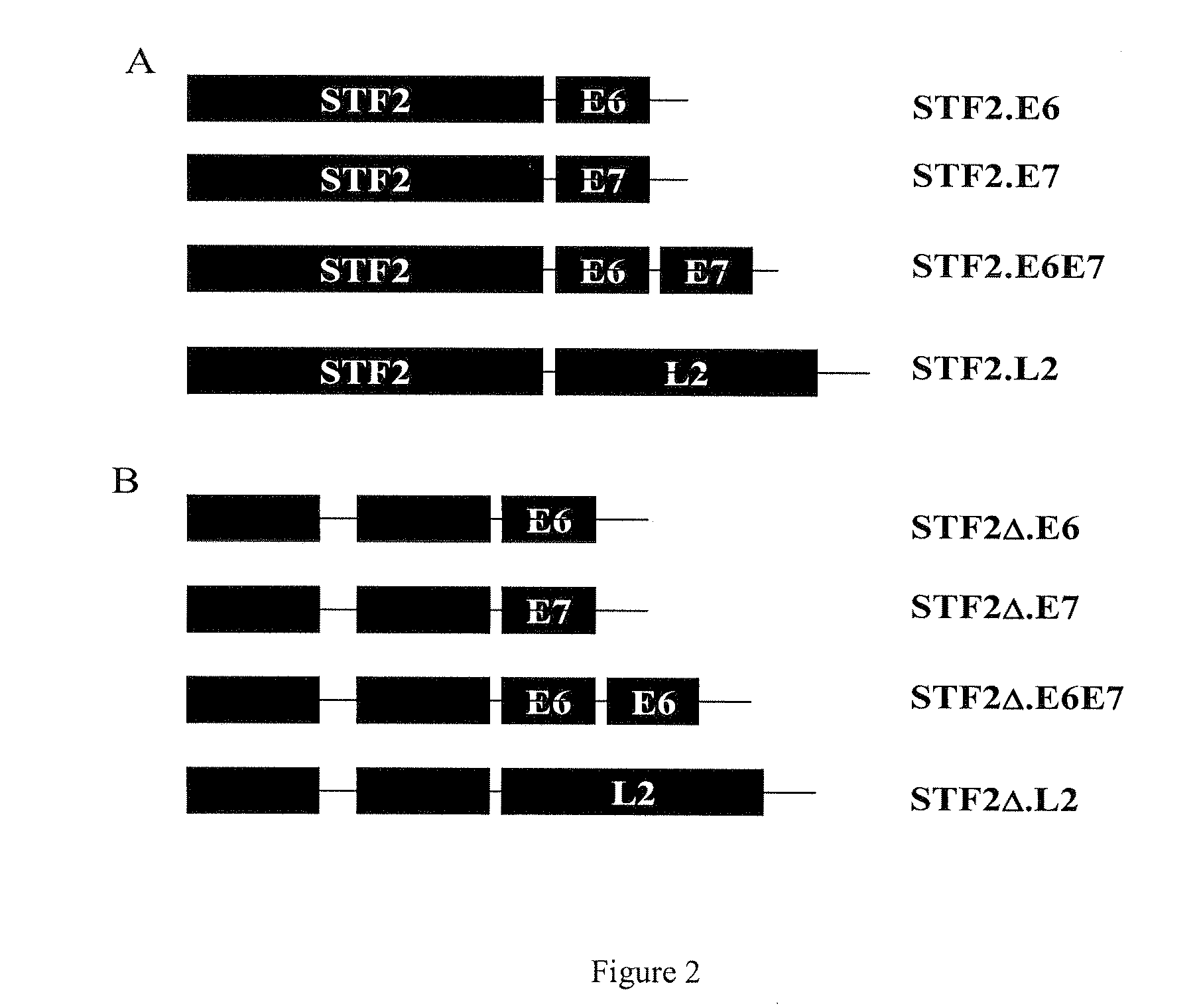
Fusion polypeptides and aggregates of polypeptides comprising papillomavirus-derived antigens, and compositions thereof and their use e.g. with adjuvants for immunogenic and vaccine purposes in eliciting e.g. HPV-specific immune responses. The polypeptides can be purified to result in aggregates which when in solution or dispersion can pass through a sterilisation filter, and in amorphous aggregates. An example of such a polypeptide is a fusion protein of human papillomavirus proteins L2 and E7.
Owner:CANTAB PHARMA RES
Detection of human papillomaviruses
InactiveUS6902899B2Reliable detectionMicrobiological testing/measurementRecombinant DNA-technologyNucleotideAlphapapillomavirus
Owner:UNIV TUBINGEN
Human papillomavirus shell protein L1 short peptide and application thereof
InactiveCN101186636ASerum immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAlphapapillomavirusCoat protein
The invention relates to a polypeptide, in particular to a human papilloma virus coat protein L1 short peptide and relative application. The invention is characterized in that the sequence of the human papilloma virus coat protein L1 short peptide is N-EVNLKEKFSADLDQFPLGRKFLLQAGLKAK-C. The invention can simultaneously induce human papilloma virus coat protein L1 short peptide with immunity reaction on high risk type and low risk type, which can induce and form the antibody for various human papilloma virus (HPV) and coat protein (HPV L1), to check various HPV or HPV L1, while the antibody can be used in biological pharmaceutical engineering, to purify and prepare various HPV or HPV L1.
Owner:CHINA THREE GORGES UNIV
Methods for detecting papillomavirus DNA in blood plasma and serum
InactiveUS7183053B2Assess prognosisAntibody mimetics/scaffoldsMicrobiological testing/measurementDiseaseAlphapapillomavirus
This invention relates to the detection of extracellular papillomavirus DNA in blood plasma or serum from a human or animal. In particular, the invention relates to the detection, identification, evaluation, or monitoring of neoplastic, premalignant or malignant disease associated with a papillomavirus. The invention thereby provides methods for the identification of individuals at risk for, or having, cervical dysplasia, cervical intraepithelial neoplasia, or cervical cancer.
Owner:PENN STATE RES FOUND
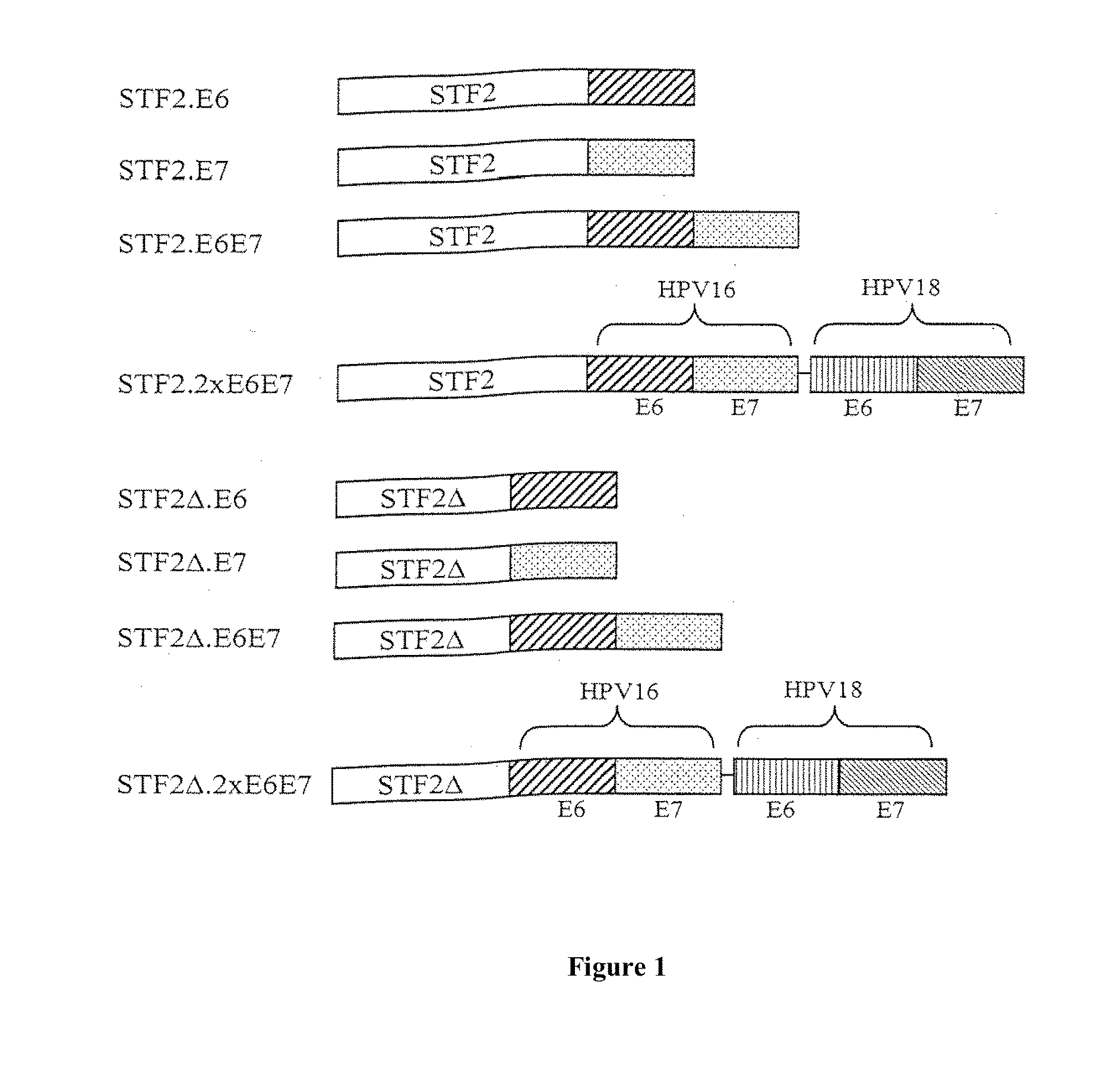
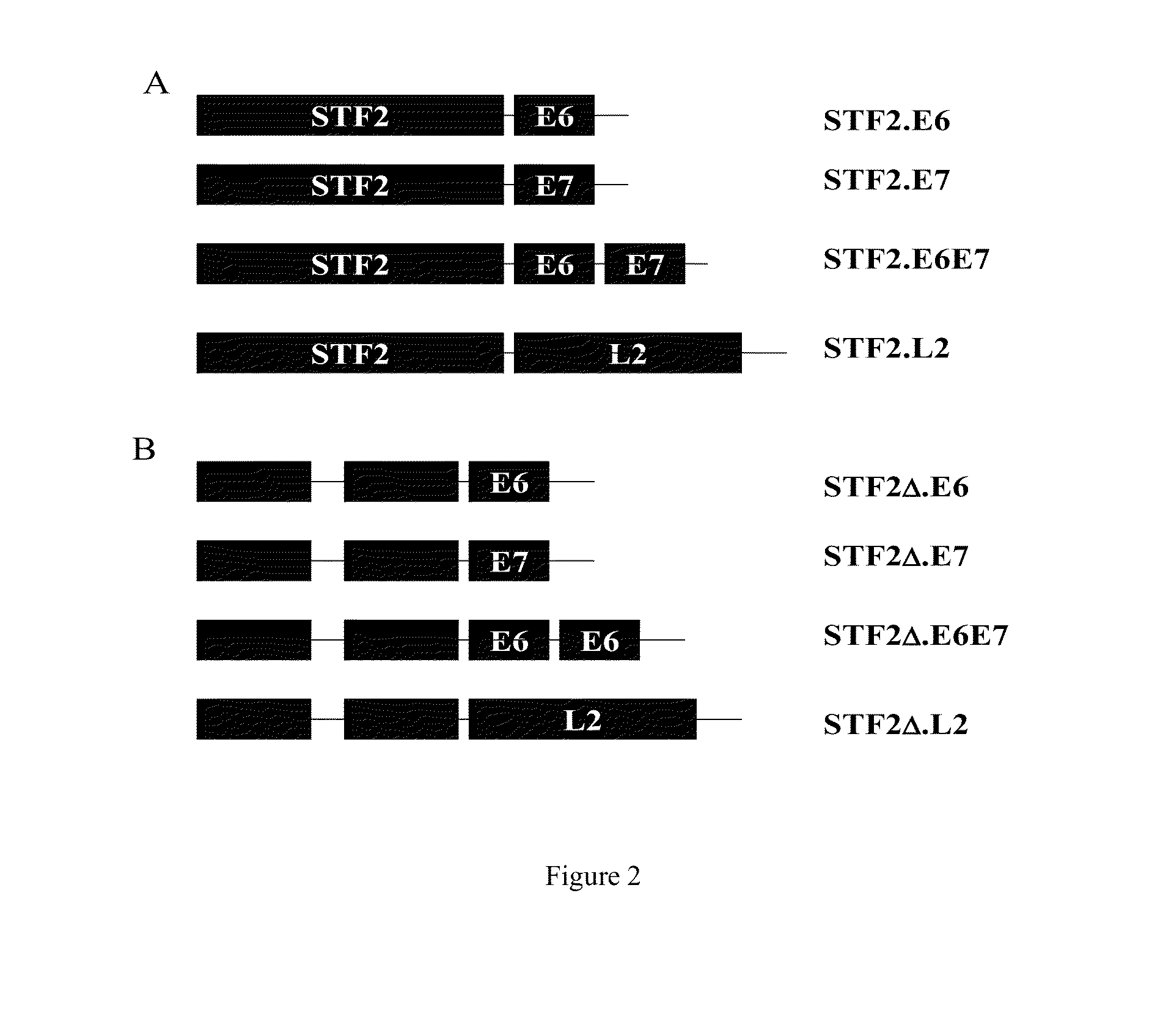
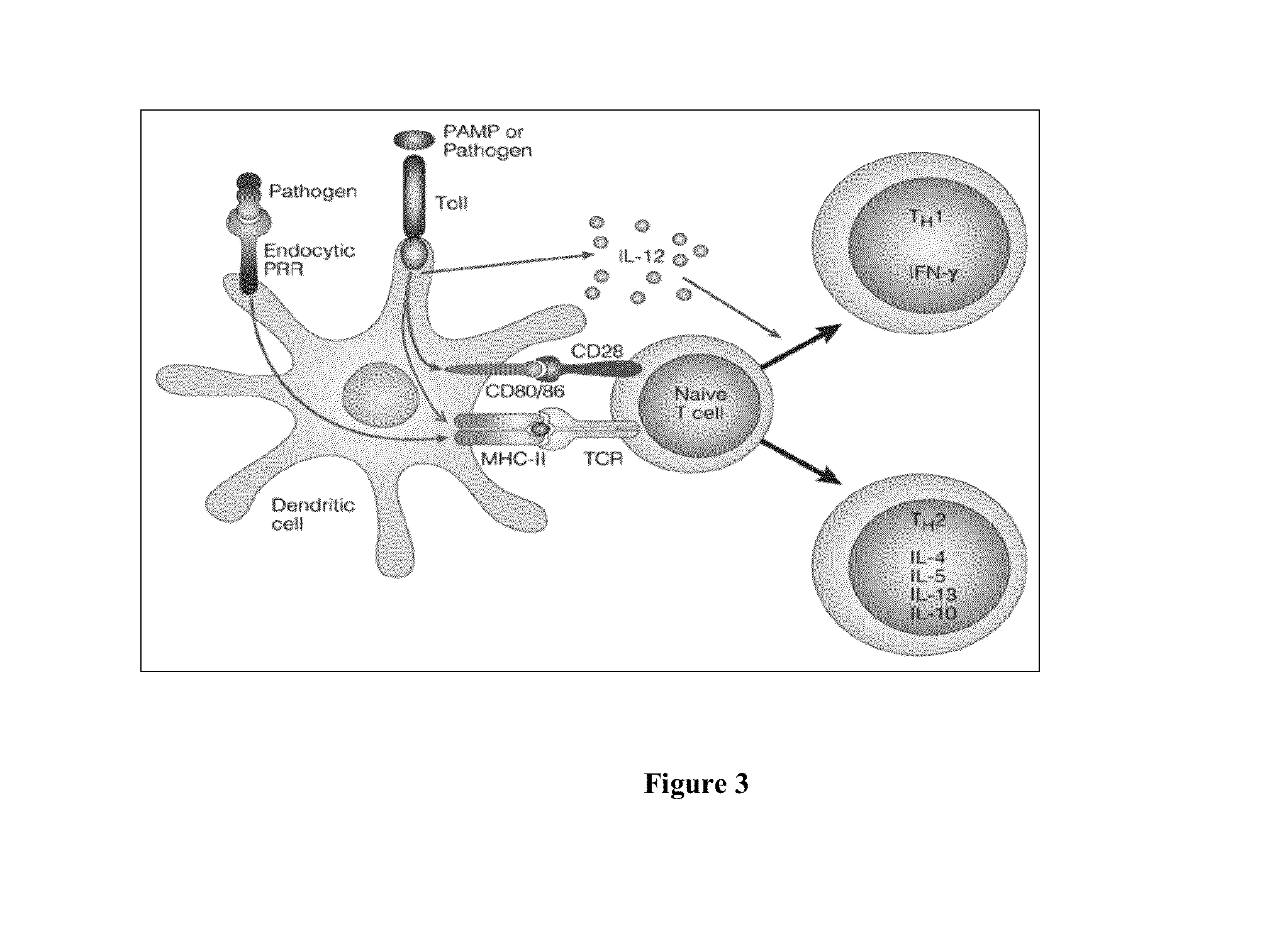
Compositions of flagellin and papillomavirus antigens
InactiveUS20130331548A1Stimulate immune responseCost effectiveAntibody mimetics/scaffoldsVirus peptidesAntigenAdjuvant
Compositions that include at least one protein that activates a Toll-like Receptor and includes at least a portion of at least one Toll-like Receptor agonist and at least a portion of at least one papillomavirus suppressor binding protein, papillomavirus transforming protein and papillomavirus capsid protein can be employed in methods that stimulate an immune response in a subject, in particular, a protective immune response in a subject. Compositions can further include an adjuvant and a carrier protein.
Owner:VAXINNATE
Primer, probe, DNA chip containing the same and method for detecting human papillomavirus and detection kit thereof
InactiveUS20100285485A1Strong specificityShorten the timeSugar derivativesMicrobiological testing/measurementHpv testingTrue positive rate
The present invention discloses a primer, a probe, a DNA chip, and a test kit for diagnosis and genotyping of Human Papilloma Virus (HPV) as well as a method for testing HPV genotype. According to the present invention, it is possible to screen HPV genotypes with high sensitivity and specificity, and to diagnose infection by multiple HPV types which was not possible in other conventional HPV testing methods
Owner:JUNG WOON WON +1
Method for preparing vaccine for anti-HPV 18 infection by pichia yeast expression system
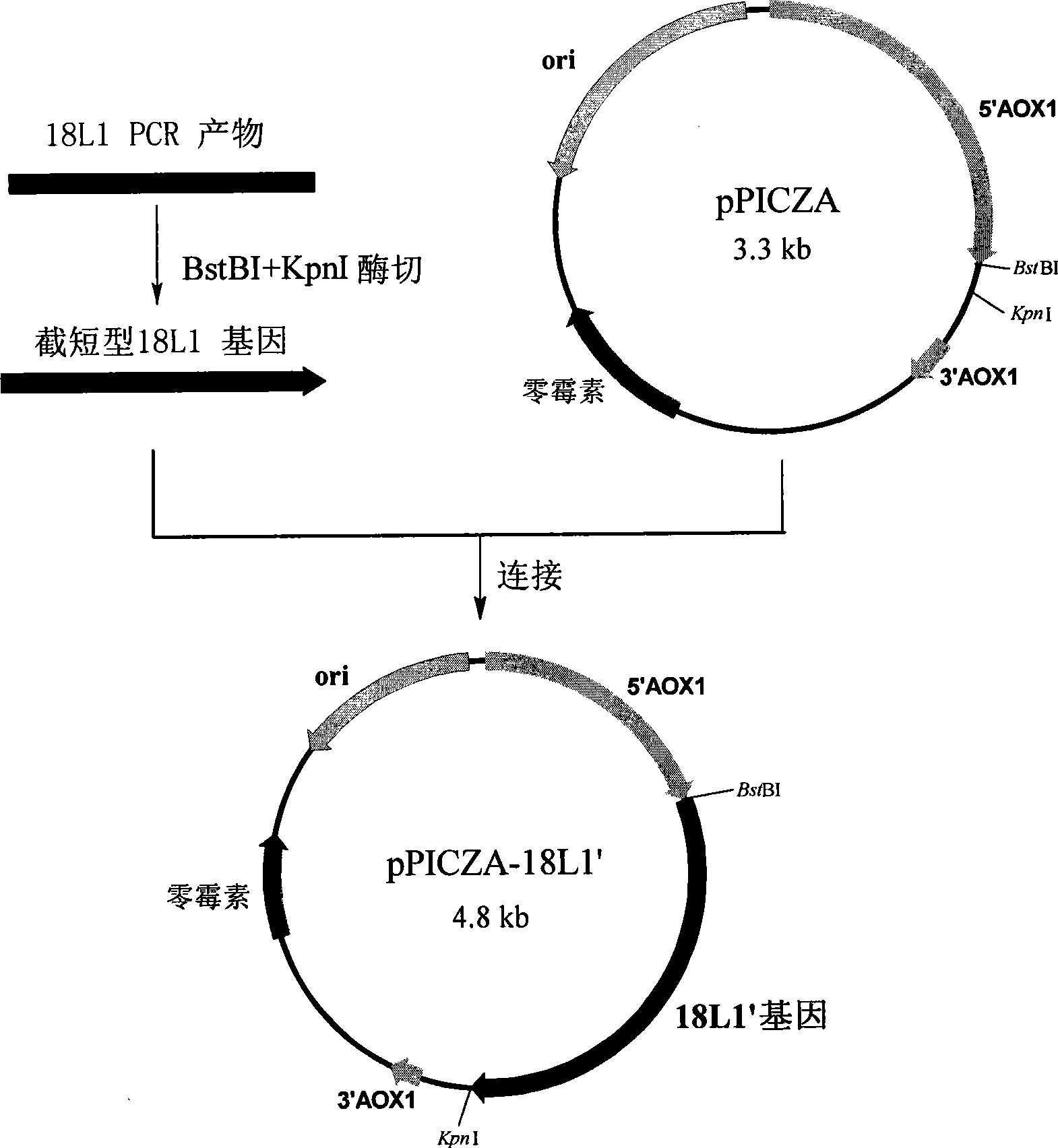
ActiveCN101487010AGood neutralizing activityAvoid enteringViral antigen ingredientsAntiviralsYeastHuman papillomavirus
The invention discloses a method for preparing a recombinant human papillomavirus 18-typed L1 protein with a pichia pastoris expression system and comprises the steps of loading an HPV 18 L1 gene in accordance with optimal design into an expression carrier, transforming pichia pastoris and cultivating a transformant, thus obtaining the recombinant human papillomavirus 18-typed L1 protein, which is self-assembled into virus-like particles inside a pichia pastoris body; wherein, nucleotide sequences of the HPV 18 L1 gene in accordance with optimal design are shown in SEQ ID NO.1, SEQ ID NO.2, SEQ ID NO.3, SEQ ID NO.4, SEQ ID NO.5 or SEQ ID NO.6. The HPV 18 L1protein that is prepared by the method of the invention has high expressed quantity. The invention further discloses a method for preparing a papillomavirus 18-typed infection vaccine with better activity by utilizing the virus-like particles of the recombinant HPV 18 L1 protein.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Compositions of toll-like receptor agonists and papillomavirus antigens and methods of use thereof
InactiveUS20100303847A1Avoid serious illnessAvoid deathAntibody mimetics/scaffoldsViral antigen ingredientsAntigenAdjuvant
Compositions that include at least one protein that activates a Toll-like Receptor and includes at least a portion of at least one Toll-like Receptor agonist and at least a portion of at least one papillomavirus suppressor binding protein, papillomavirus transforming protein and papillomavirus capsid protein can be employed in methods that stimulate an immune response in a subject, in particular, a protective immune response in a subject. Compositions can further include an adjuvant and a carrier protein.
Owner:VAXINNATE
Human papilloma virus (HPV) capsid protein L1 polypeptide and preparation and application thereof
The invention relates to a human papilloma virus capsid protein L1 (HPVL1) peptide and preparation and application thereof. The HPVL1 peptide is a peptide segment of which the amino acid of the sequence is N end-Cys Thr Leu Thr Ala Asp Val Met listed Thr Tyr Ile His-C end, or is a peptide segment comprising the amino acid of the sequence and with a length less than 50 amino acids. In the invention, the peptide which is a very conservative peptide segment located on the surface of protein is sieved out by performing epitope forecasting and multi-sequencing comparison on the L1 proteins of 18 low-risk types and high-risk type HPV subtypes (6, 11, 16, 18, 52, 58 and the like); the antibody generated from induction can react with multiple types of HPVL1, and can be used for the detection of the multiple types of HPVL1, in particular to the application of precancerous lesions detection of human cervical carcinoma; and the antibody aiming at the peptide segment also can be used for purifying and preparing the multiple types of HPVL1. In addition, the invention also provides the applications of the peptide segment used as multiple types of HPV antiserum and detected matters of monoclonal antibody.
Owner:SICHUAN UNIV
Truncated l1 protein of human papillomavirus type 11
ActiveUS20100291141A1Reduced expression levelLow costVirus peptidesAntiviralsVirus-like particleAlphapapillomavirus
The invention relates to a truncated L1 protein of the Human Papillomavirus Type 11, a virus-like particle consisting of the protein, a vaccine comprising said virus-like particle, and the use of the vaccine in the prevention of condyloma acuminatum or HPV infections.
Owner:XIAMEN INNOVAX BIOTECH +1
Antisense oligonucleotide inhibition of papillomavirus
InactiveUS6174870B1Rapid clearanceAltered sugar moieties or inter-sugar linkagesSugar derivativesMicrobiological testing/measurementMessenger RNAAlphapapillomavirus
Oligonucleotides and oligonucleotide analogs are provided which are capable of antisense interaction with messenger RNA of papillomavirus. Such oligonucleotides or oligonucleotide analogs can be used for diagnostics and therapeutics as well as for research purposes. In accordance with preferred embodiments of this invention, oligonucleotide or oligonucleotide analog is provided which is hybridizable with a messenger RNA from a papillomavirus. The oligonucleotide or oligonucleotide analog is able to inhibit the function of the RNA, and accordingly is useful for therapy for infections by such papillomavirus.In accordance with a preferred embodiment, portions of the papillomavirus are targeted for antisense attack. Thus oligonucleotides are preferably provided which hybridize with the E2, E1, E7, E6 or E6-7 messenger RNAs.
Owner:IONIS PHARMA INC
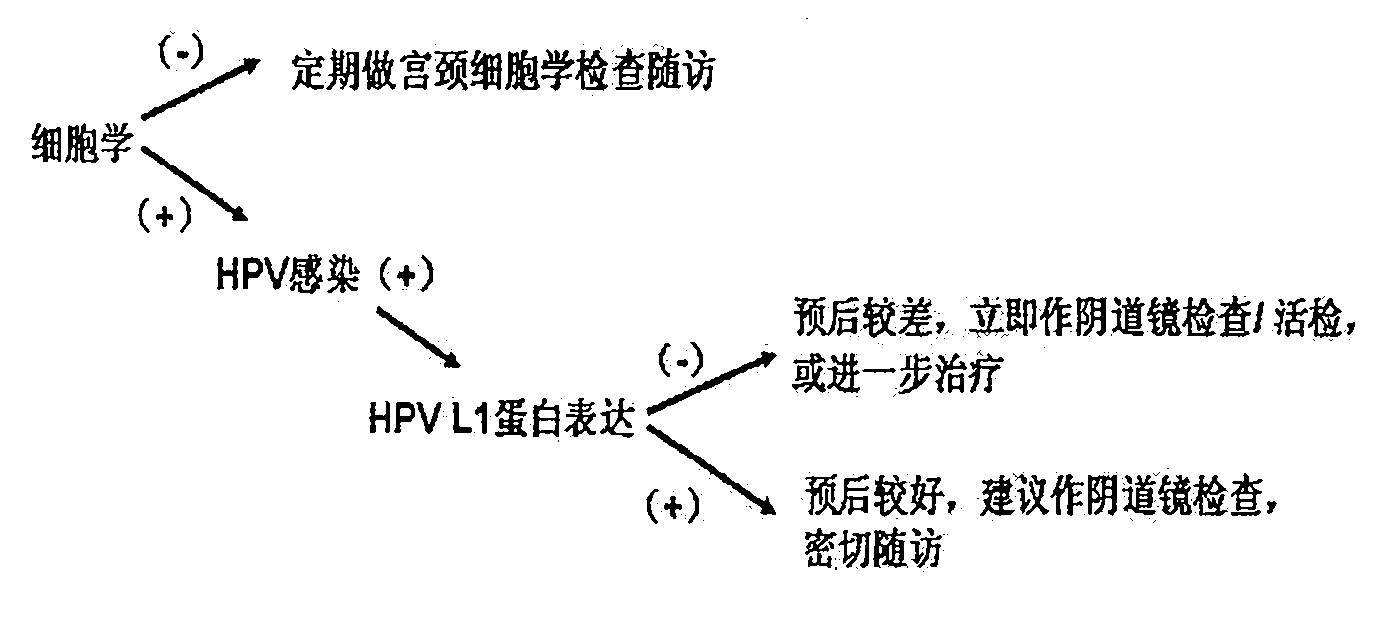
Novel approach to molecular diagnosis of human papillomavirus-related diseases
ActiveUS20070243552A1Accurate sensitive toolReduce in quantitySugar derivativesMicrobiological testing/measurementProtein markersHuman papillomavirus
The present invention relates to an accurate, sensitive, and efficient sequential or concurrently sequential method for molecular diagnosis of human papillomavirus (HPV)-based disease, where the method improves the accuracy and reliability of diagnostic and prognostic assessments of HPV-based disease. The method of the invention comprises a primary screen of a sample for HPV nucleic acids, followed by a secondary screen for molecular markers, such as proliferation and cell cycle control group protein markers. The sequential or concurrently sequential method significantly reduces the number of false positive results.
Owner:QIAGEN GAITHERSBURG
Method for detecting human papilloma virus neutralizing antibody
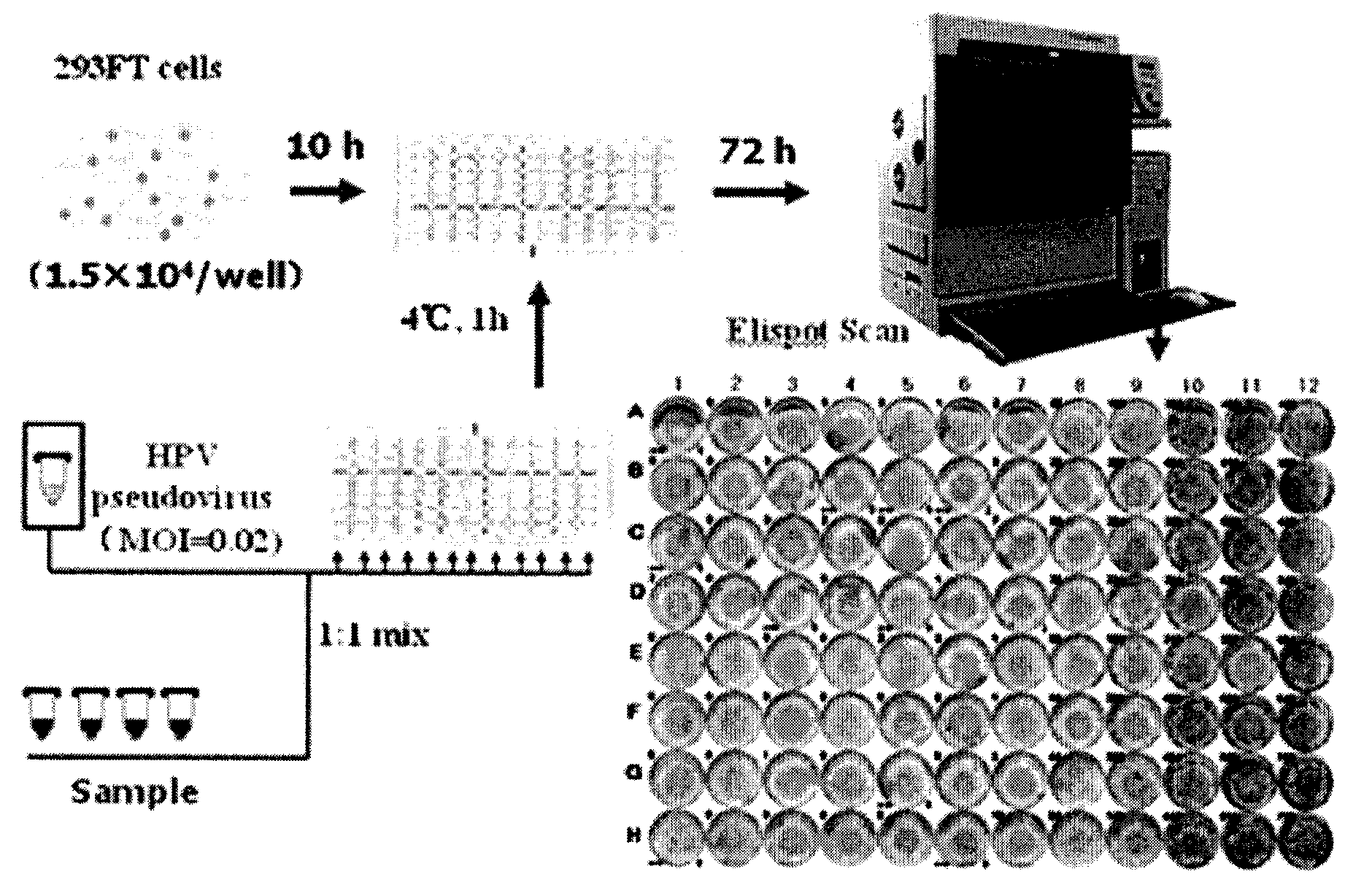
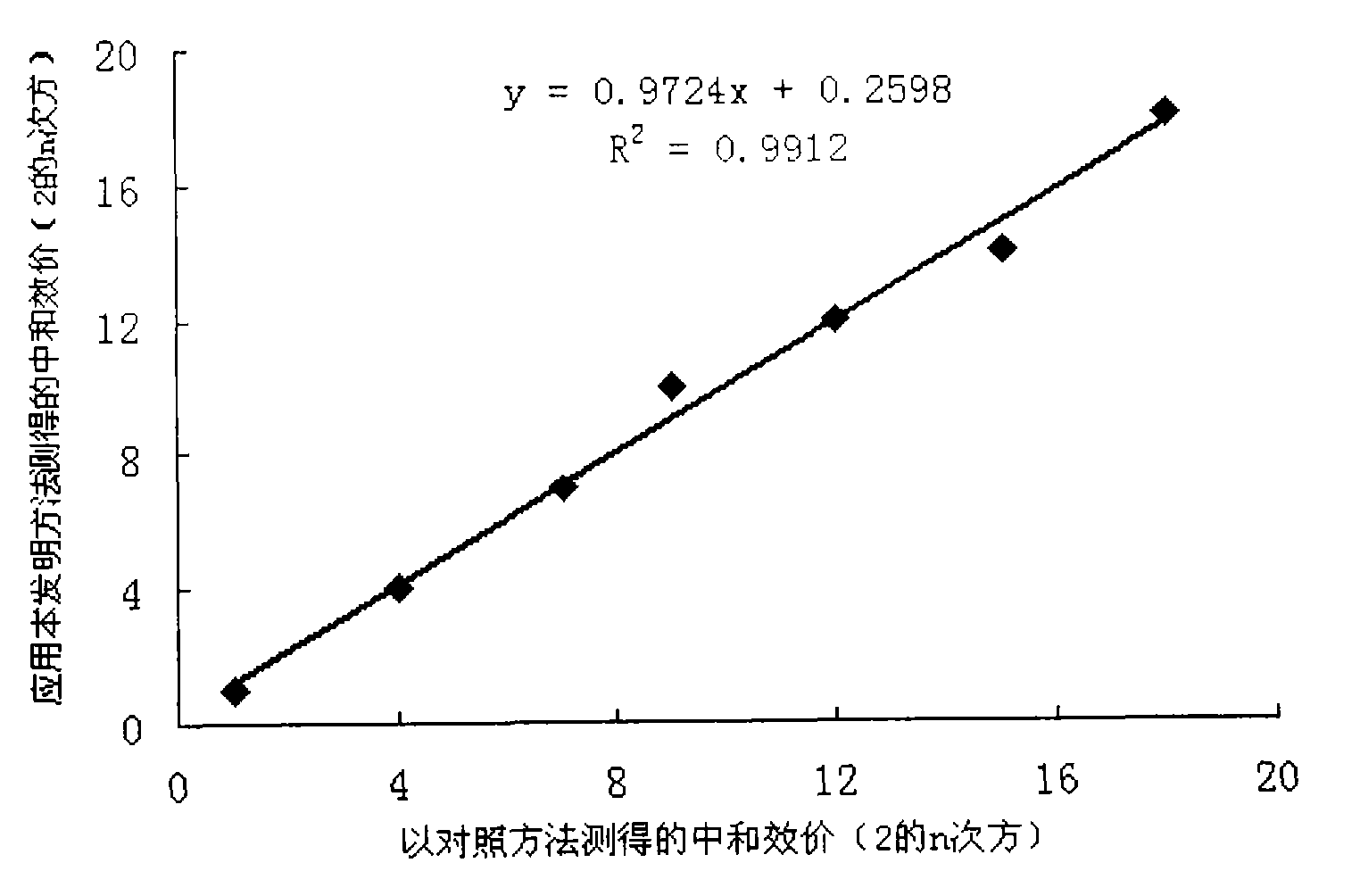
InactiveCN101551392AThe detection method is simpleFast detection methodBiological testingHPV vaccinesAlphapapillomavirus
The invention relates to a high-efficiency and simple method for detecting a human papilloma virus (HPV) neutralizing antibody, which can be applicable to high-throughput detection of the human papilloma virus neutralizing antibody. The method is characterized by using a spot detection method for detecting cells infected by human papilloma virus or pseudotype virus. The invention further discloses applications of the method in screening and identifying the neutralized monoclonal antibody against HPV, evaluating the immunity protectiveness of HPV vaccine, the potency test (ED50 test) of HPV vaccine, etc.
Owner:XIAMEN UNIV +1
Immunological methodology for discerning human papillomavirus contemplating peptides from the E7 early coding region of HPV 16
InactiveUS20050042600A1Positive immunological resultImprove the level ofMicrobiological testing/measurementVirus peptidesEpithelial cell abnormalityHuman papillomavirus
Owner:HU YAO
Compounds and methods of early diagnosis of cervical cancer and genital condyloma with HPV, CHSP60 tumor suppressor h-ras, k-ras and PTEN derived peptides modified
InactiveUS20060154238A1Easy to synthesizeStrong specificityAntibody mimetics/scaffoldsViral antigen ingredientsAbnormal tissue growthHuman papillomavirus
An isolated sequence or peptide isolated from an E2, E4, E6, E7 early or late coding region of human papillomavirus (HPV) that is soluble in aqueous medium, and characterized by a linkage to another protein sequence or peptide isolated from the E2, E4, E6, E7 early or late coding region of HPV by a spacer sequence, wherein the isolated protein sequence or peptide consists of more than 50% hydrophilic amino acids, and is recognized by a specific antibody of HPV. Also disclosed are isolated protein sequences or peptides from Harvey Ras (H-Ras), Kirsten Ras (K-Ras), and phosphatase and tensin homologue (PTEN) tumor suppressor proteins and Chlamydia trachomatis heat shock protein 60 (CHSP60 groEL1) and methods for detecting or diagnosing cancer or cellular abnormalities.
Owner:HU YAO XIONG
Gene chip, reagent and kit thereof for high-throughput typing detection of human papilloma virus
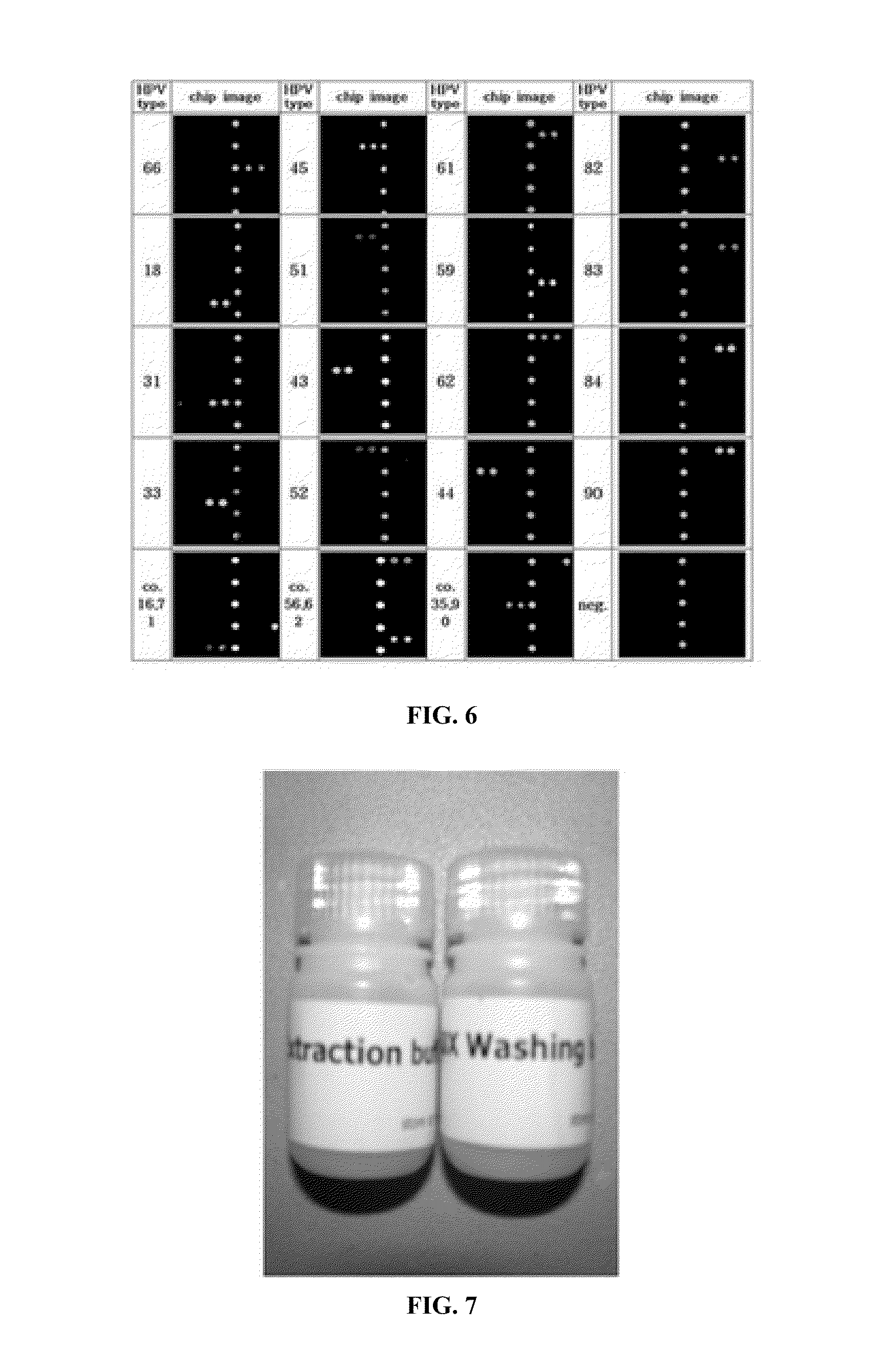
ActiveCN103409553ANucleotide librariesMicrobiological testing/measurementNucleotideAlphapapillomavirus
The invention discloses a gene chip, a reagent and a kit for high-throughput typing detection of a human papilloma virus. The gene chip comprises a carrier membrane substrate, and a probe fixed on the substrate, wherein the probe is nucleotide which can hybridize with various types of DNA special segments of human papilloma virus; 5' end of the probe is labeled by amino, and a nucleotide sequence of the probe is any one of probe nucleotide sequences in an attached sequence list. The invention provides the gene chip for detecting various types of the human papilloma virus, and simultaneously provides a detection reagent for rapid and parallel detection of various types of HPVs (human papilloma virus) as well as for high-throughput analysis of a great sample size.
Owner:GENETEL PHARMA SHENZHEN
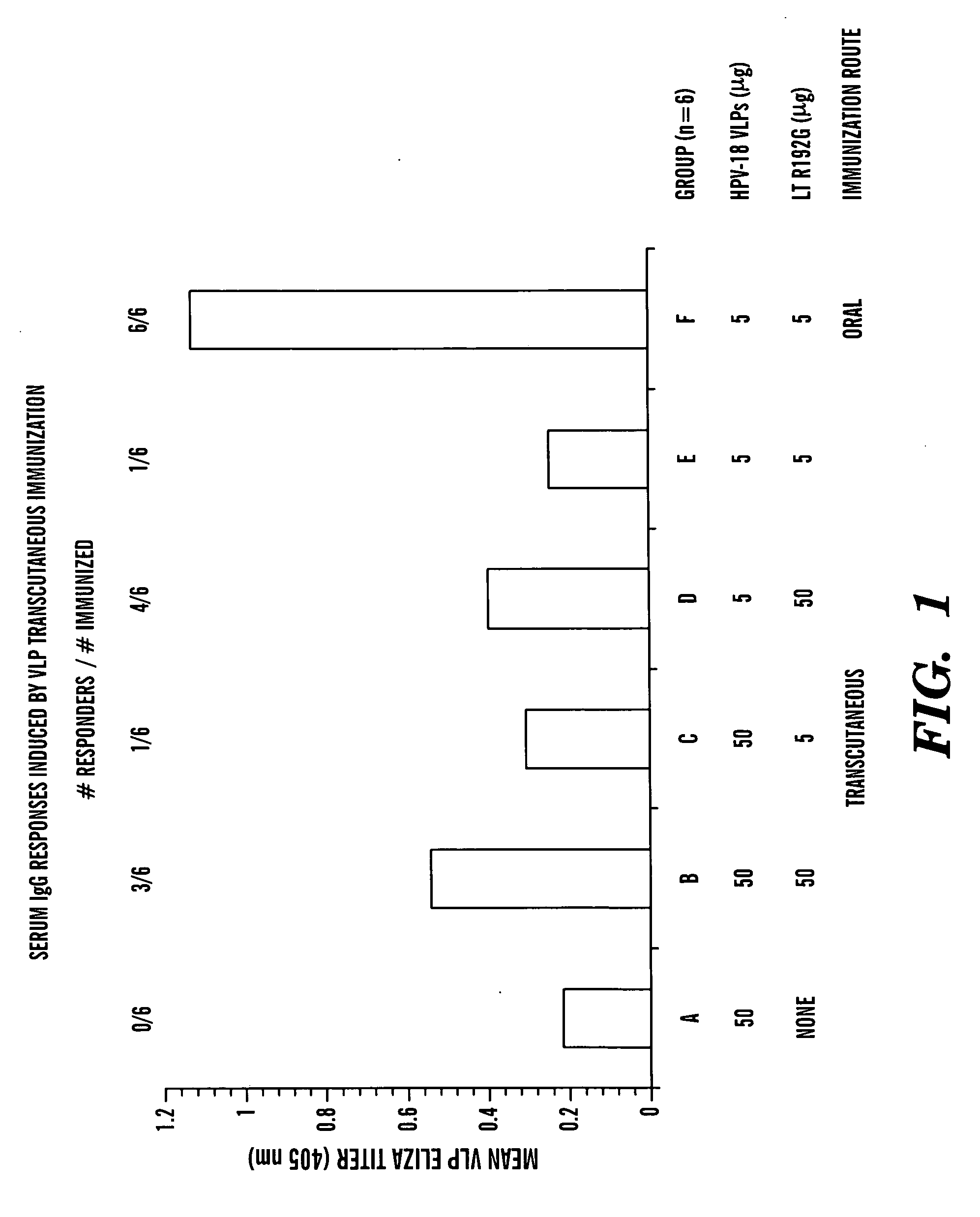
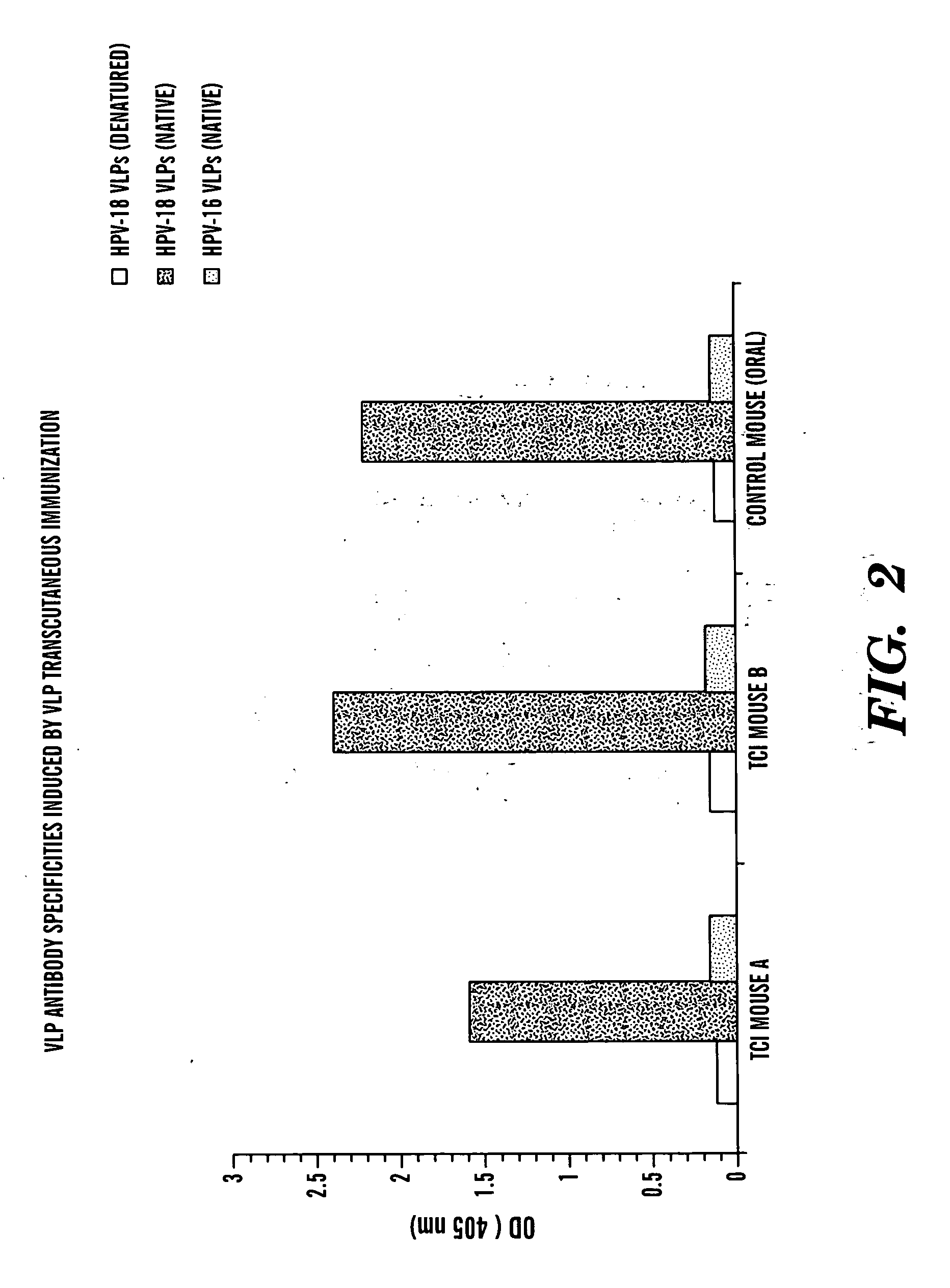
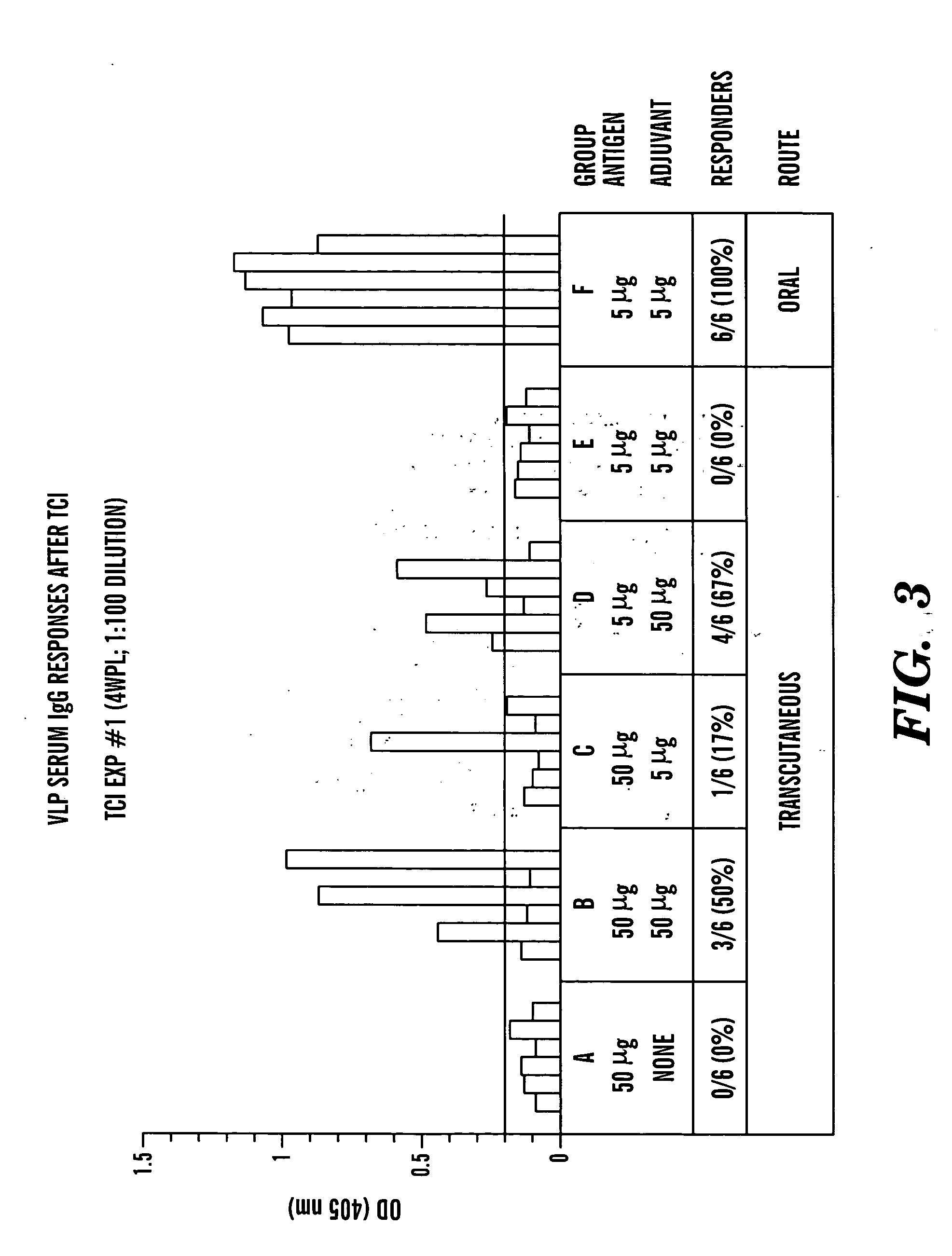
Transcutaneous immunization against papillomavirus with papillomavirus virus-like particles
InactiveUS20050013832A1Reduce morbidityEfficient methodViral antigen ingredientsMicrobiological testing/measurementMammalVirus-like particle
Owner:UNIVERSITY OF ROCHESTER
Compound vaccine composition for preventing and controlling human viral infection, compound vaccine vaginal mist and uses thereof
ActiveCN101181636ABroad immune spectrumFor personal usePeptide/protein ingredientsViral antigen ingredientsHuman papilloma virus infectionHuman papillomavirus
The invention relates to a compound vaccine composition for preventing and treating human papillomavirus infection, a compound vaccine vaginal spray and uses thereof, and belongs to the field of vaccine preparations with targeted treatment indications. The product of the present invention can prevent cervical cancer and treat HPV infection; it is suitable for married women of any age; it can prevent and treat chronic cervicitis and cervical epithelial dysplasia caused by sexually transmitted HPV infection, including CIN I, II, III Degree, inhibit HPV reproduction, prevent cervical cancer.
Owner:北京金迪克生物技术有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com