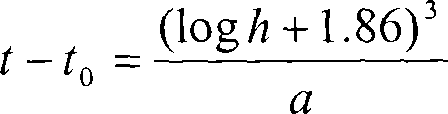Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Human Females" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
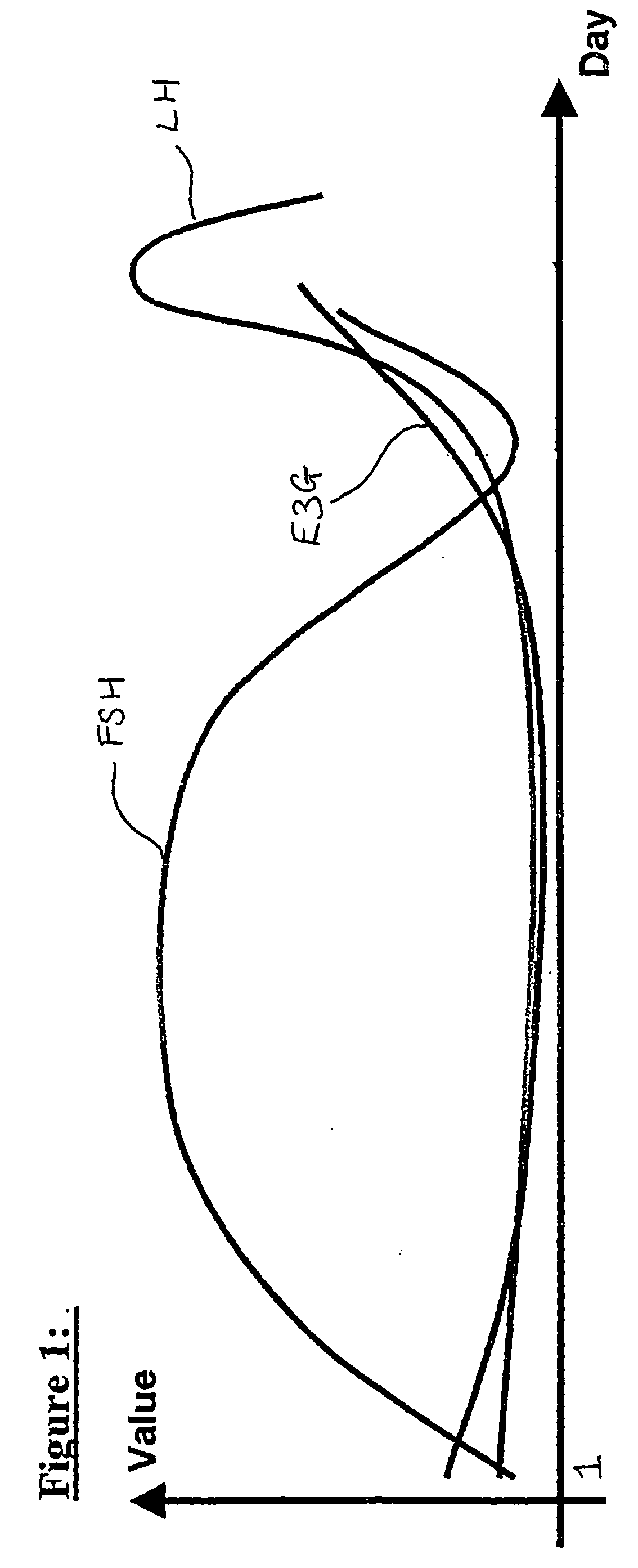
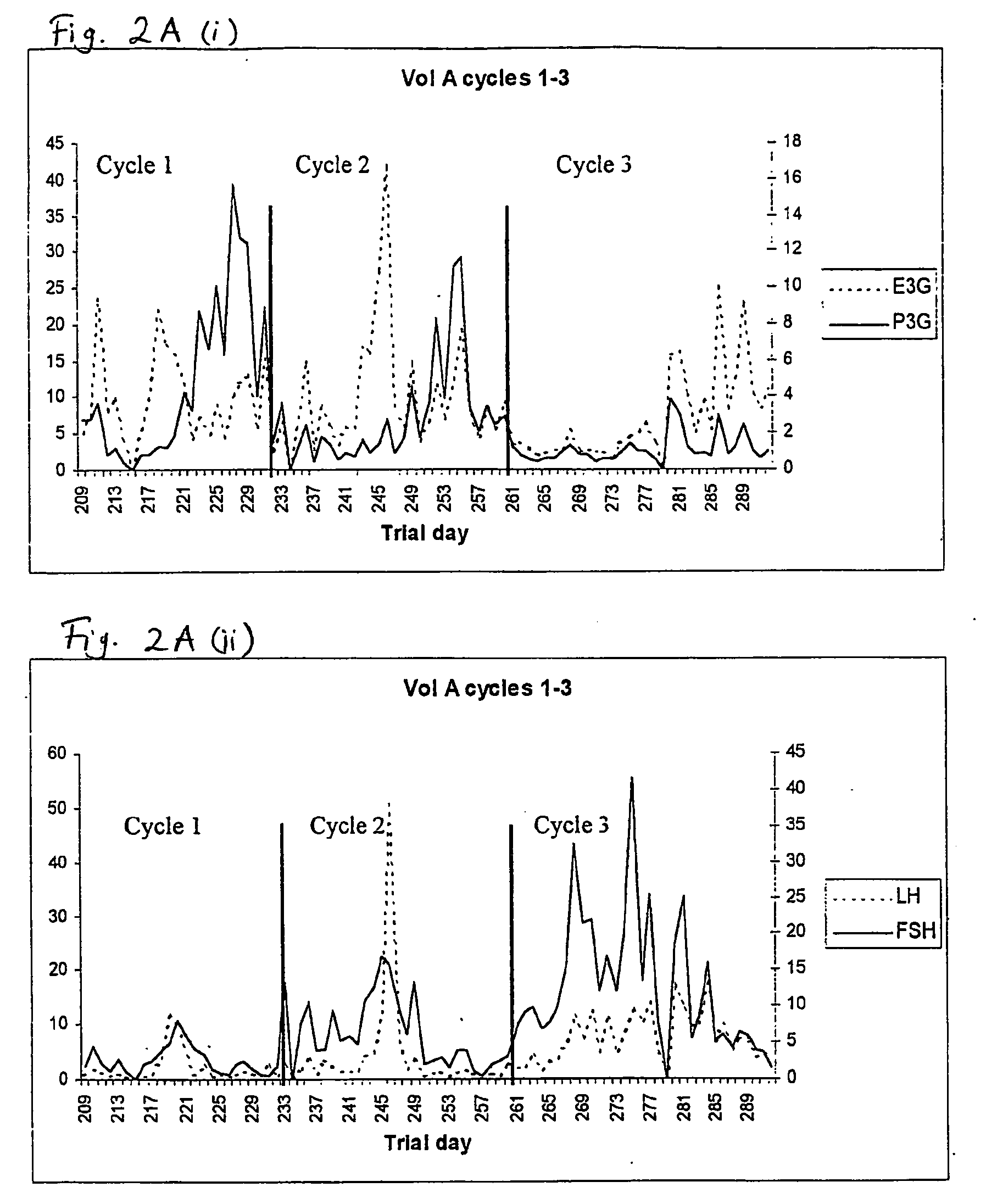
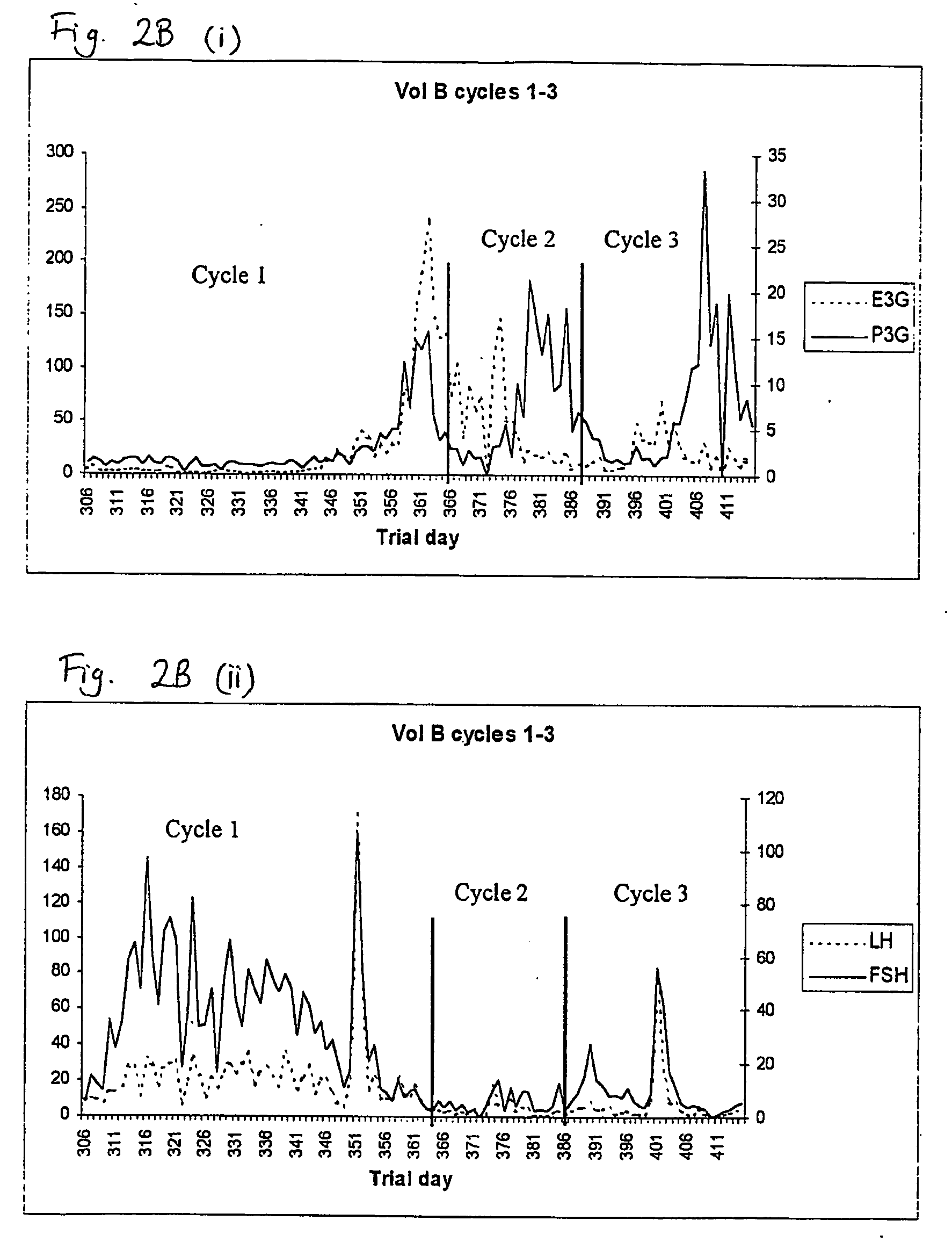
Used for all-female populations when sex is pertinent to the focus of the study. For comparison of sexes use HUMAN SEX DIFFERENCES.
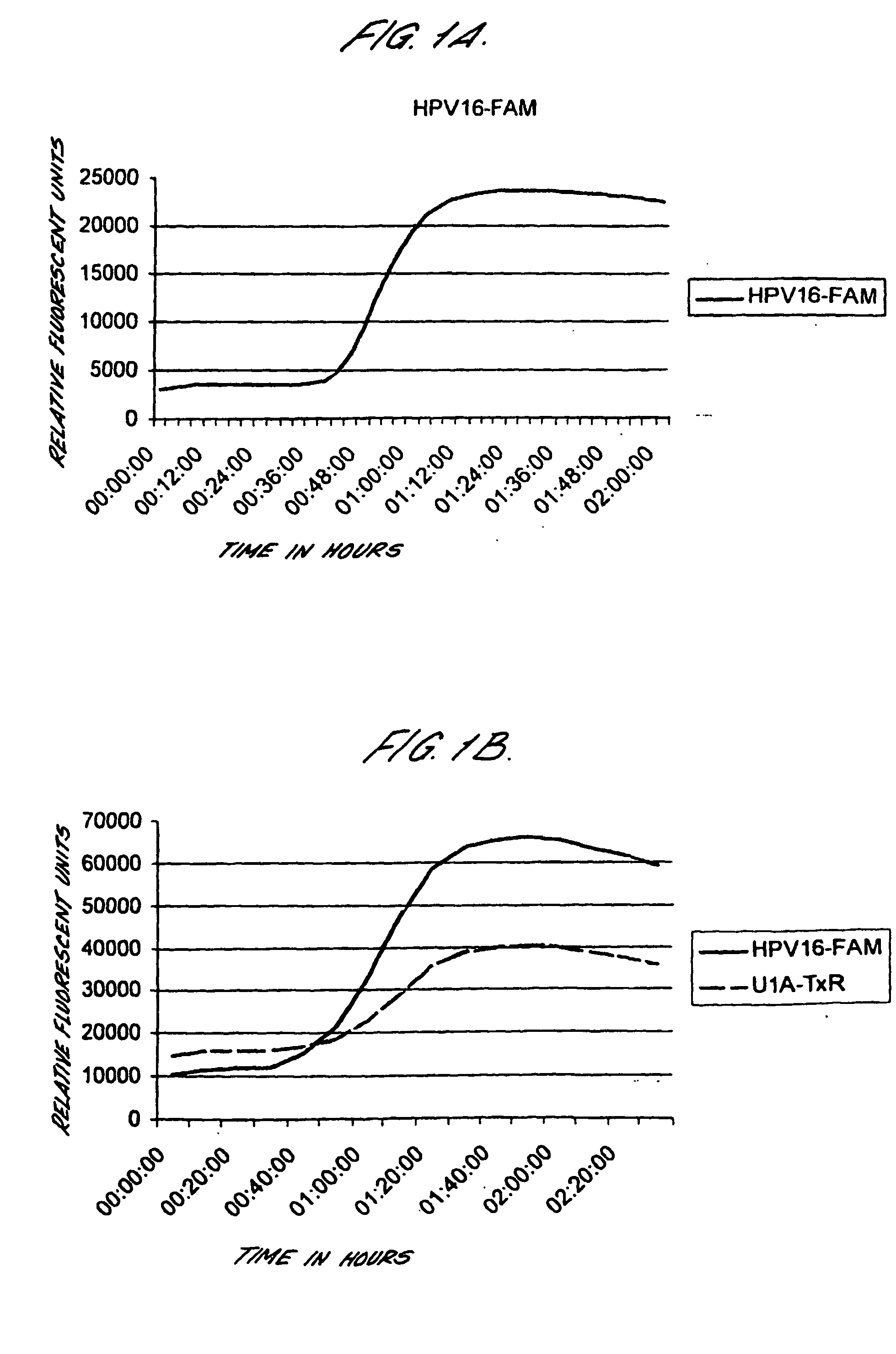
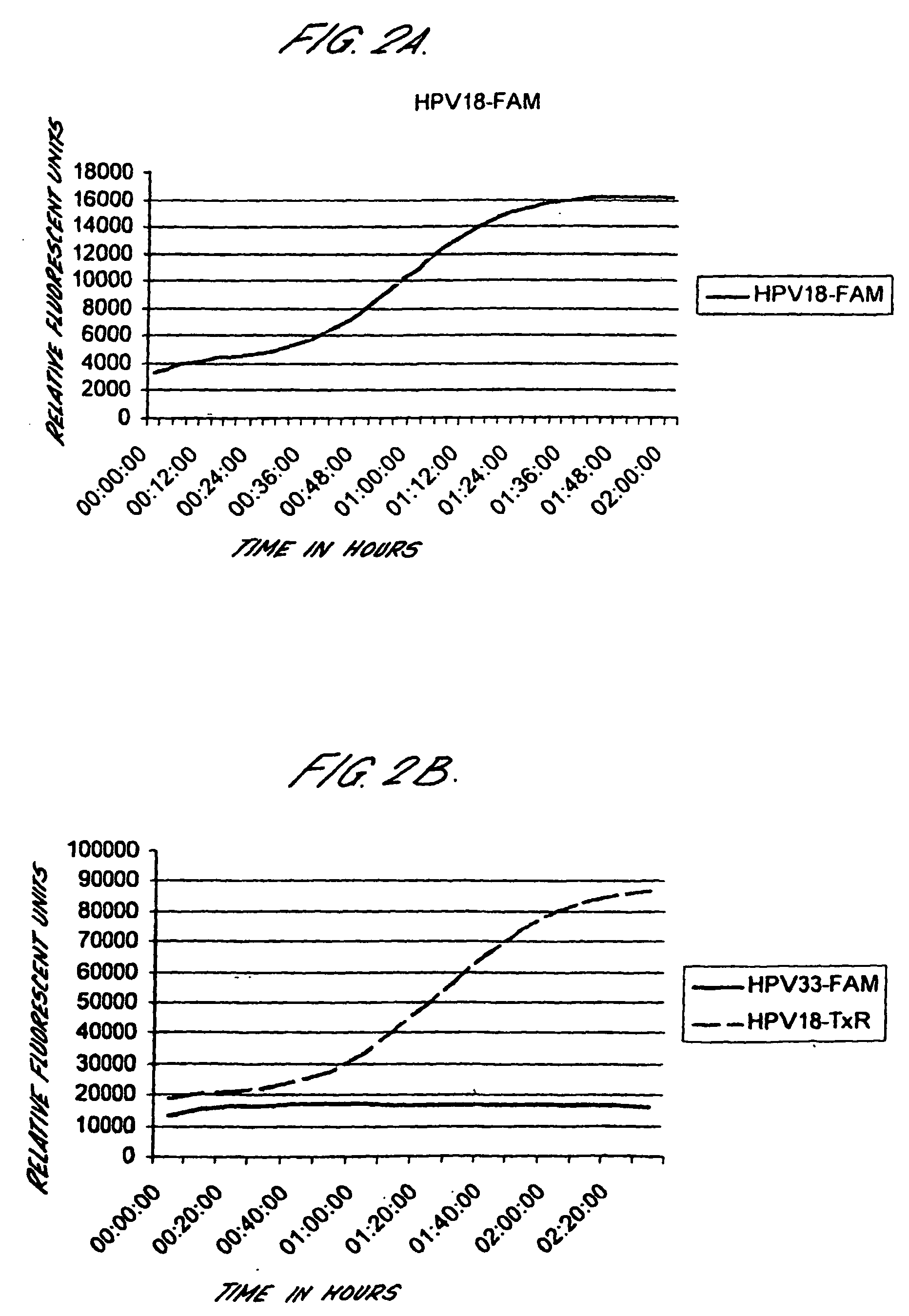
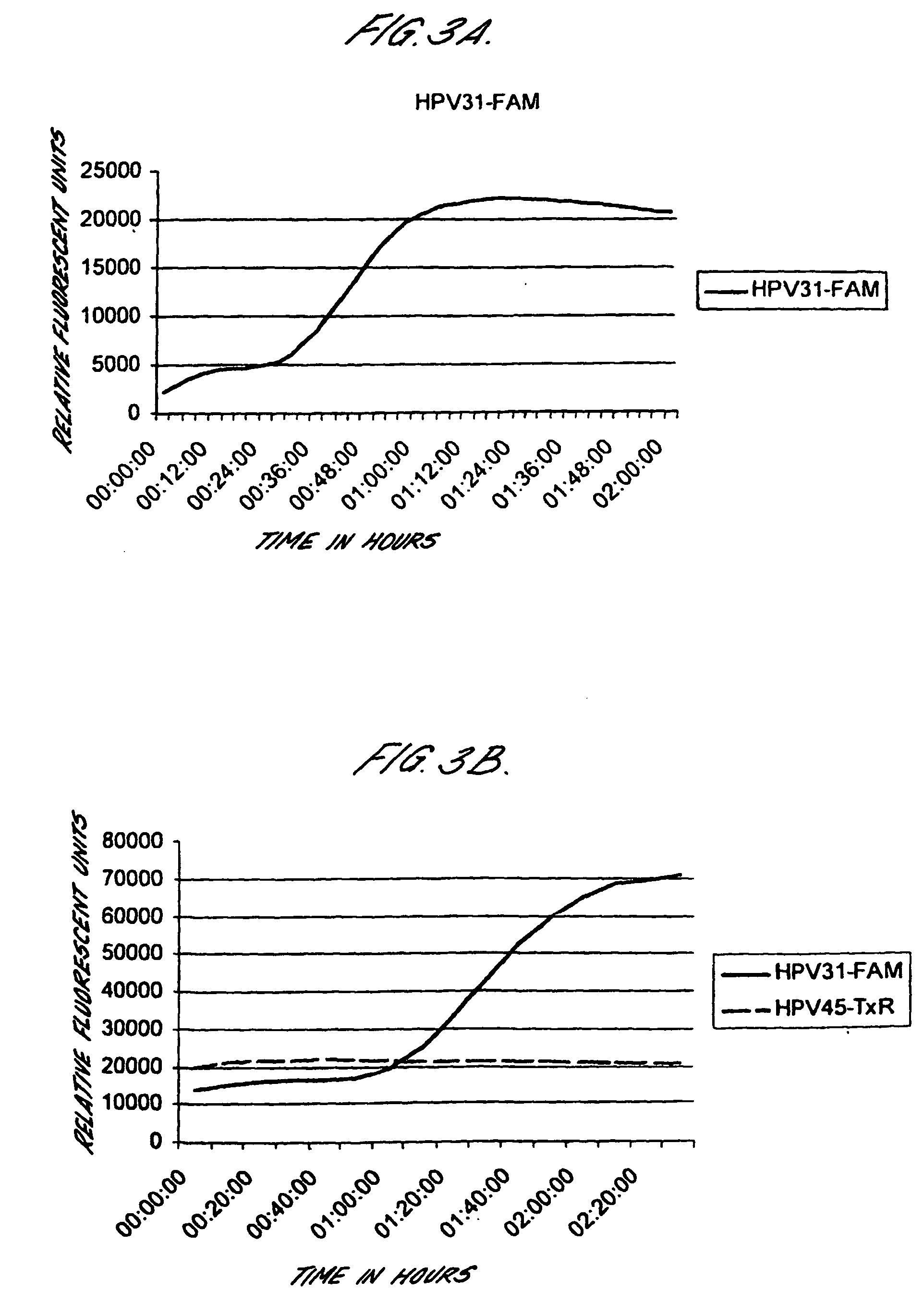
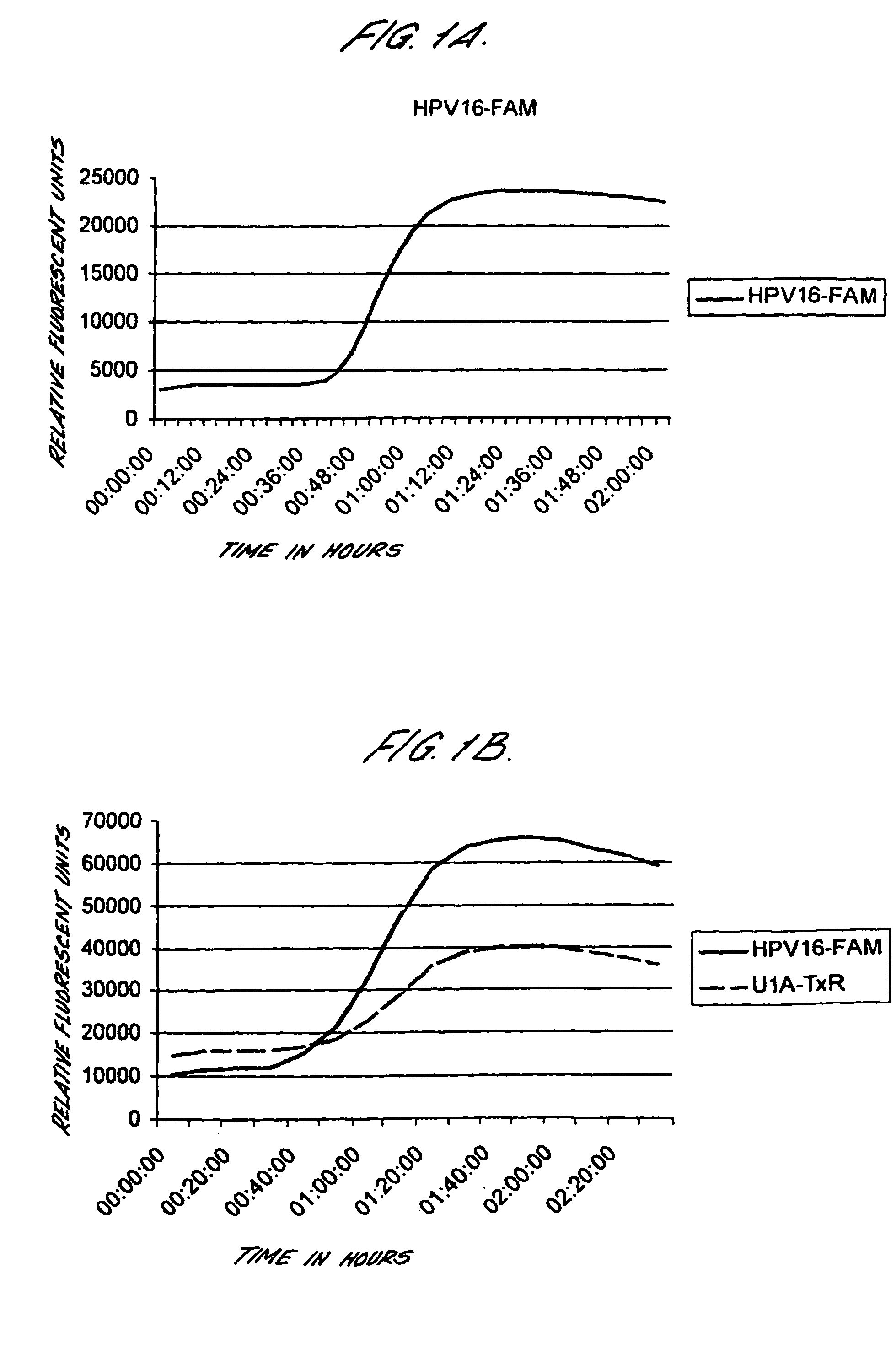
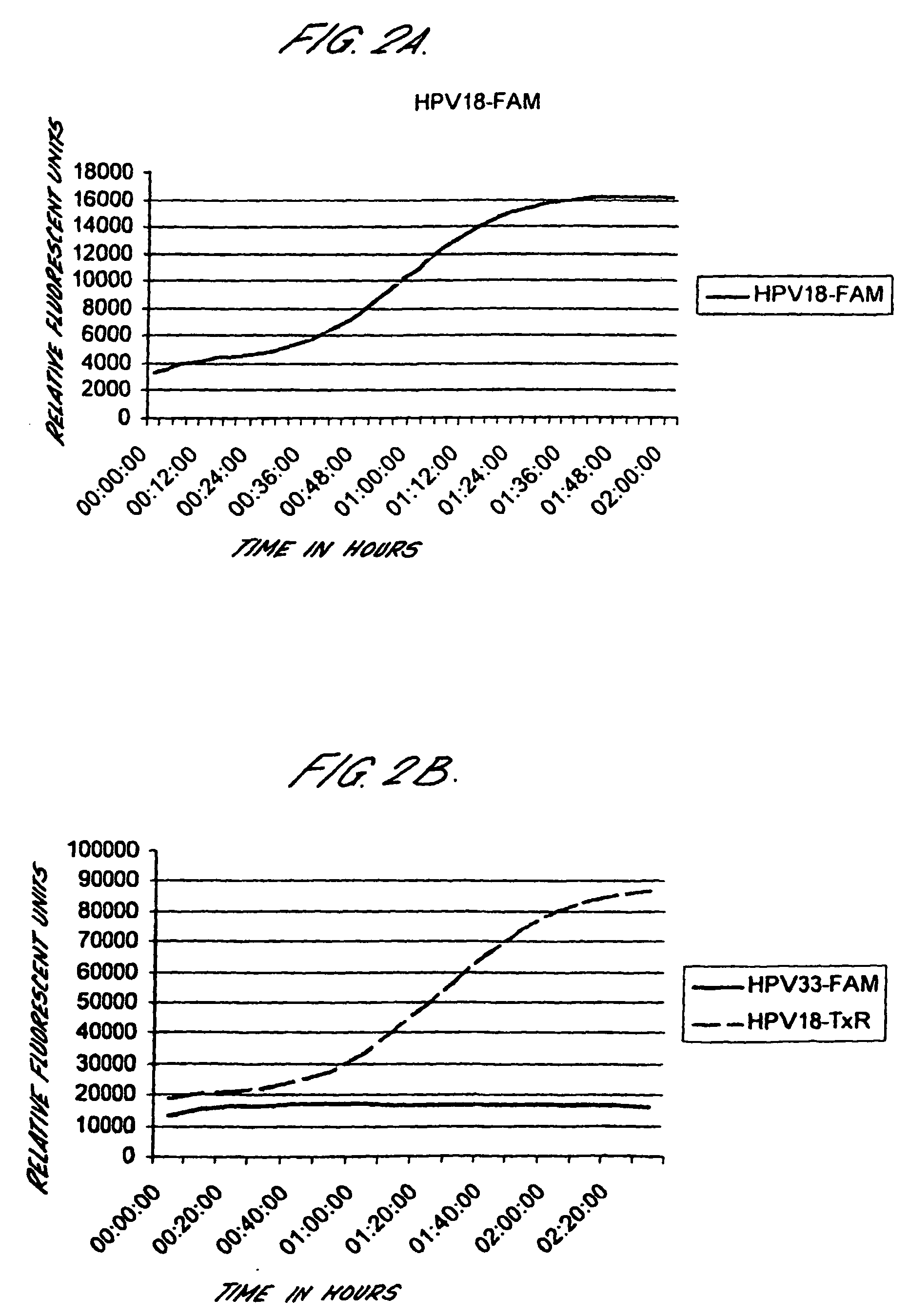
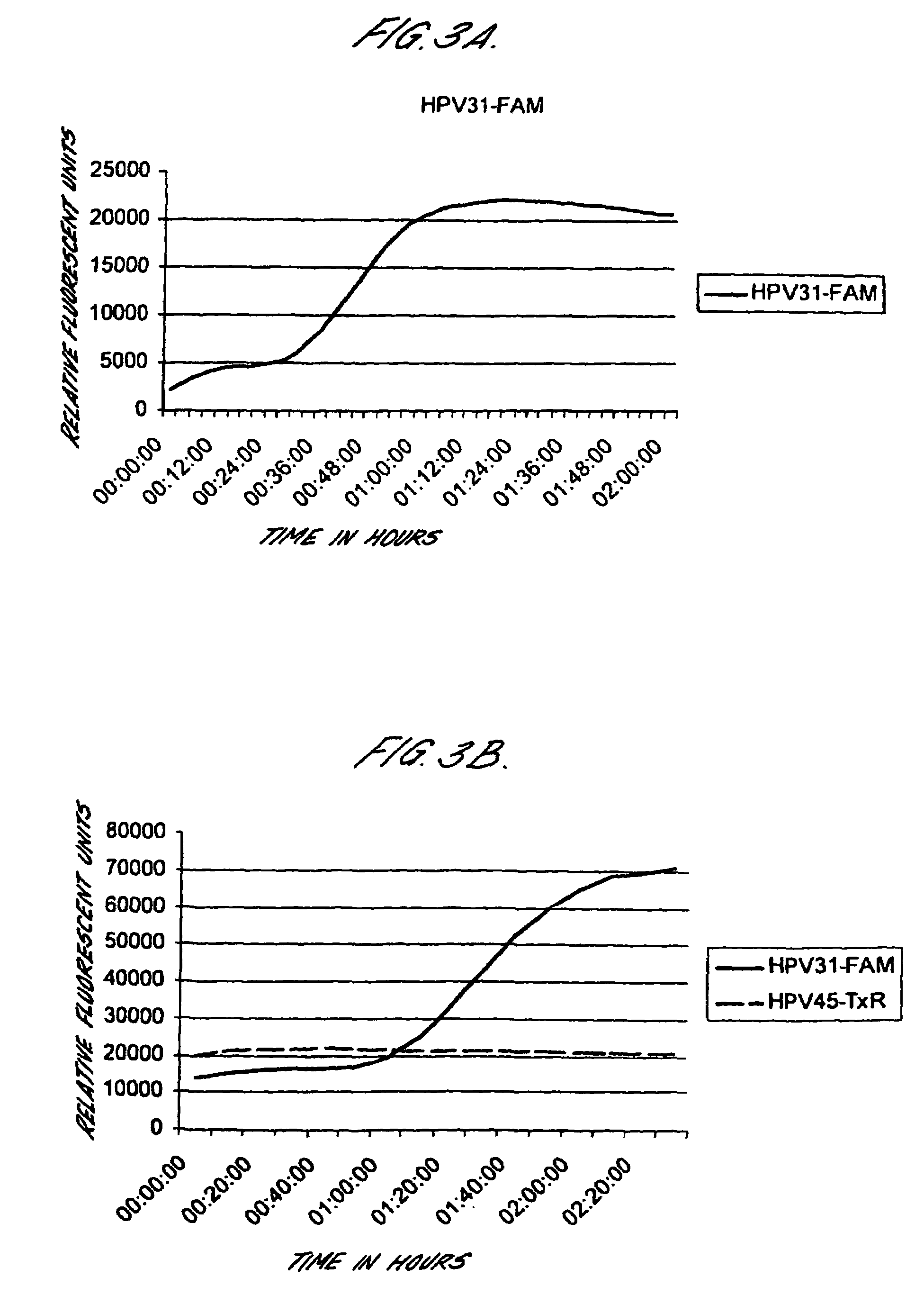
Method for detecting human papillomavirus mrna
InactiveUS20050118568A1High expressionIncrease probabilityMicrobiological testing/measurementEnzymologyHuman papillomavirusHuman Females
An in vitro method is provided for screening human female subjects to assess their risk of developing cervical carcinoma which comprises screening the subject for expression of mRNA transcripts from the E6 and optionally the L1 gene of human papillomavirus, wherein subjects positive for expression of L1 and / or E6 mRNA are scored as being at risk of developing cervical carcinoma. Kits for carrying out such methods are also provided.
Owner:NORCHIP AS
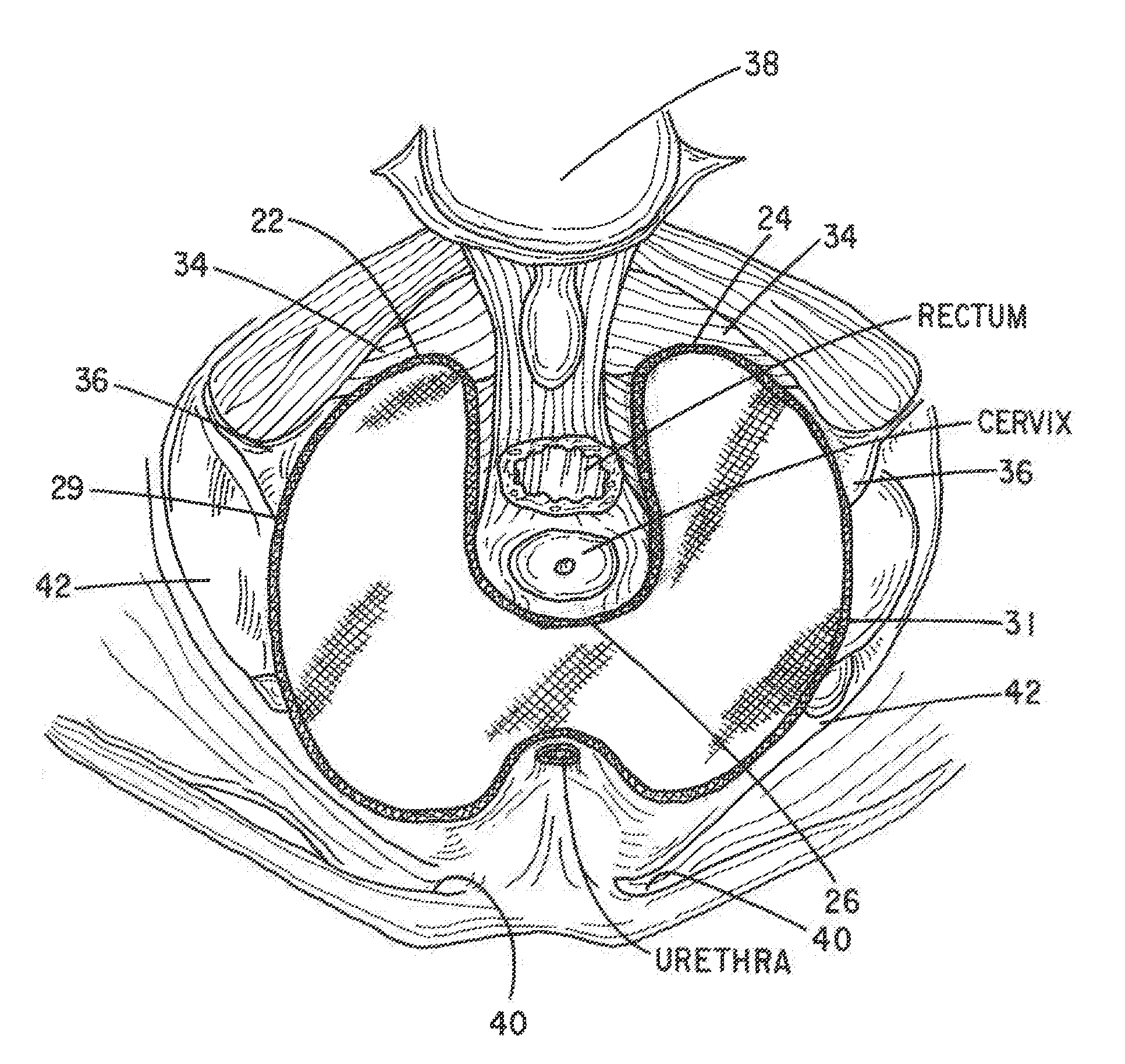
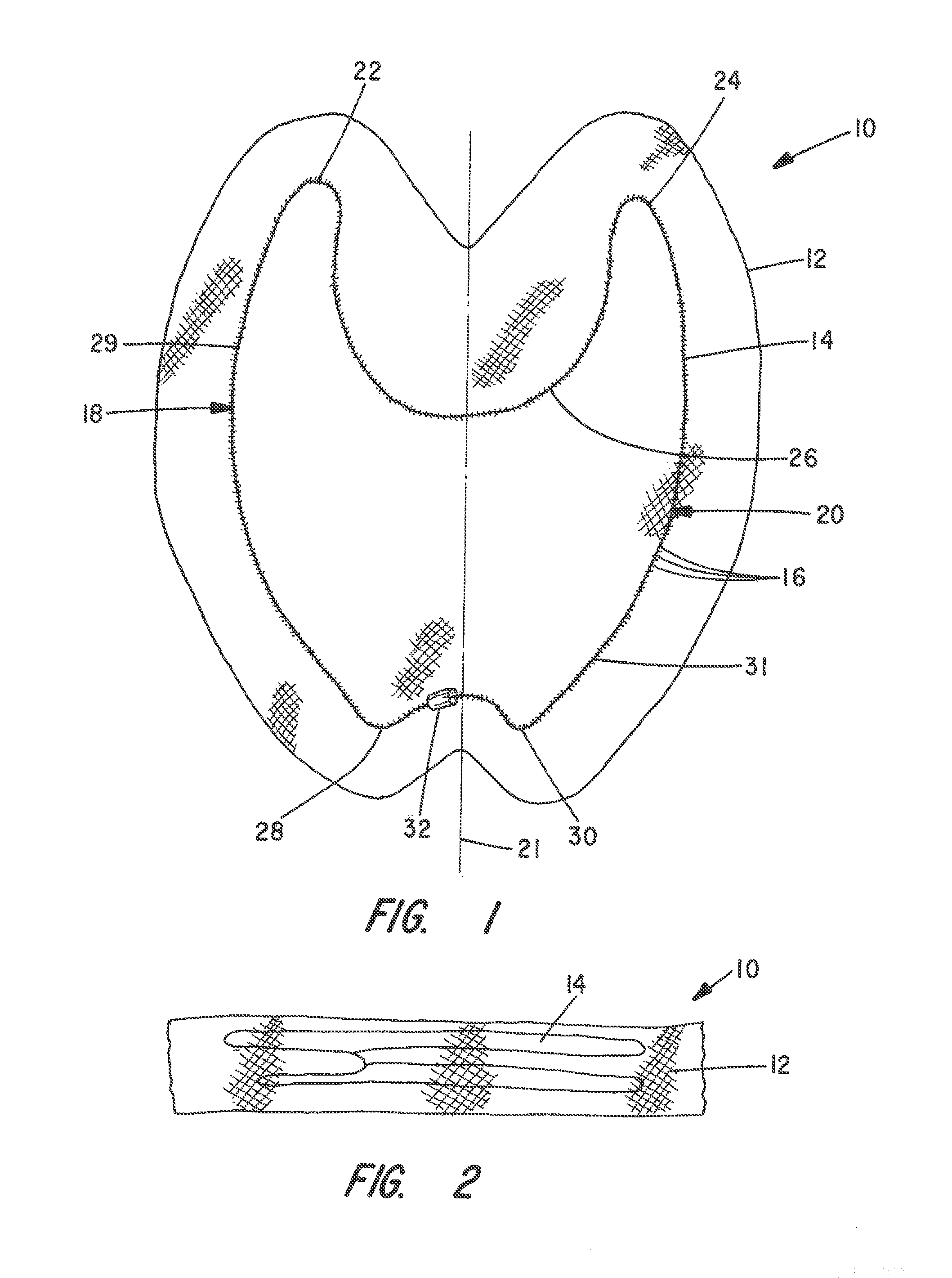
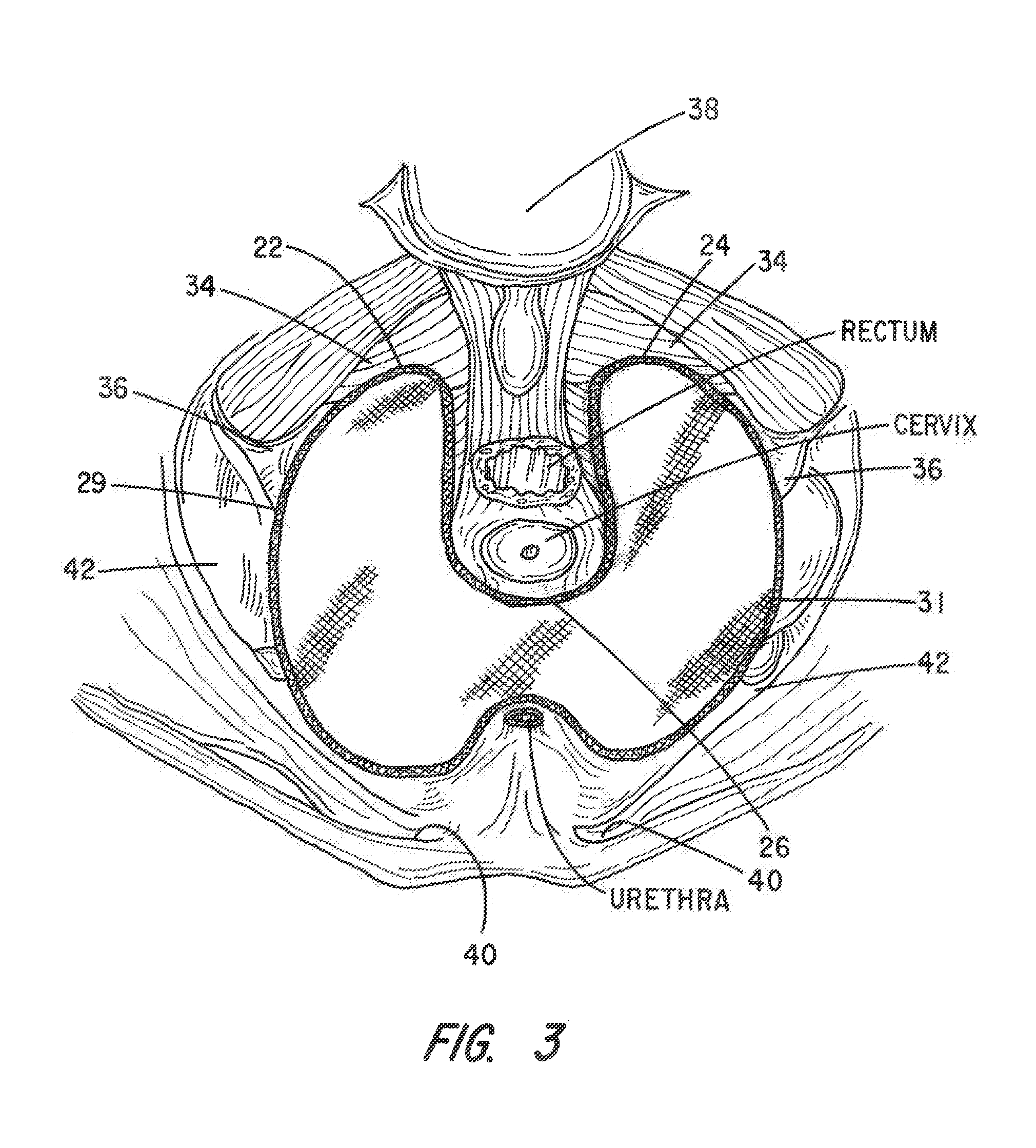
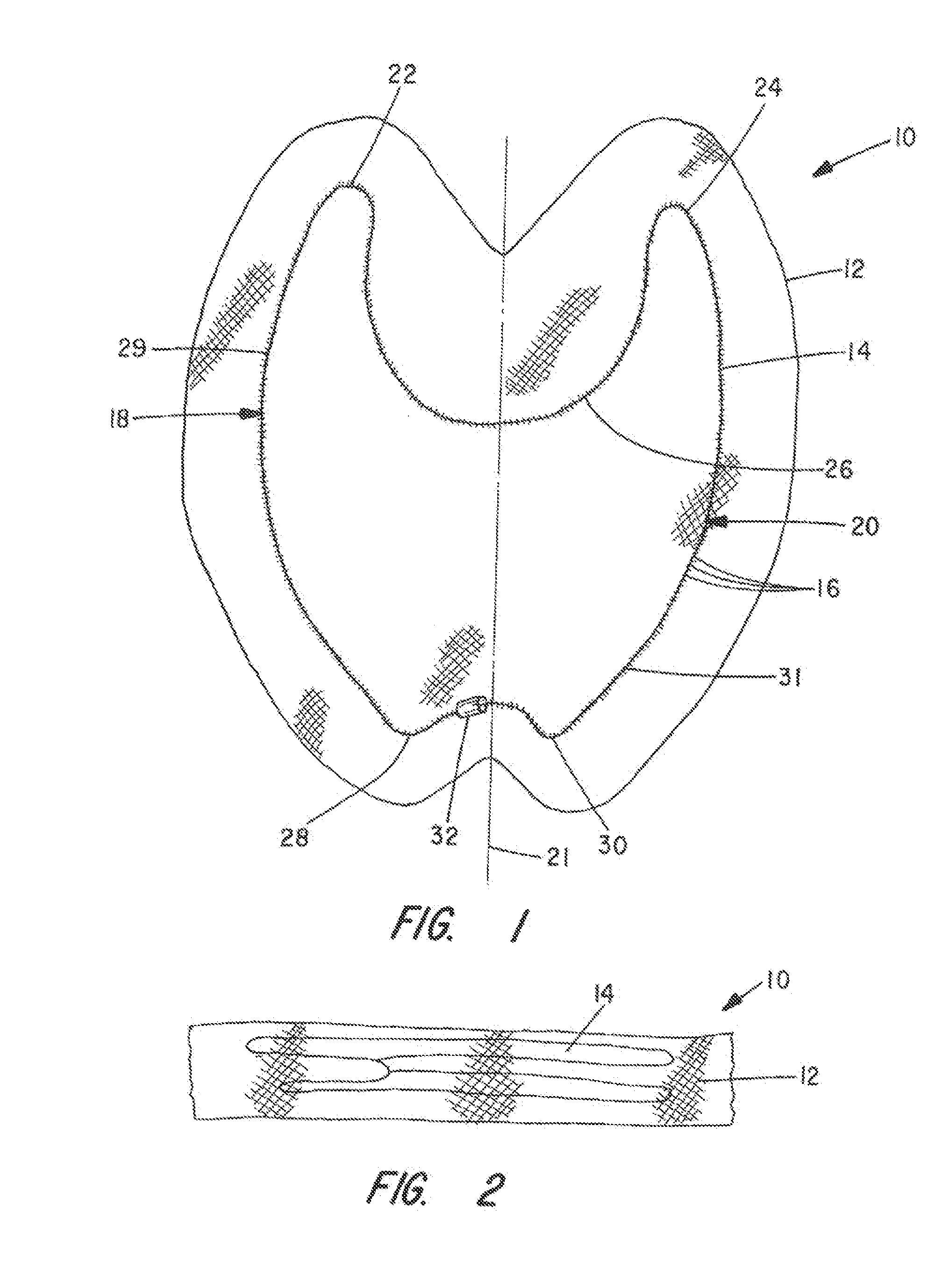
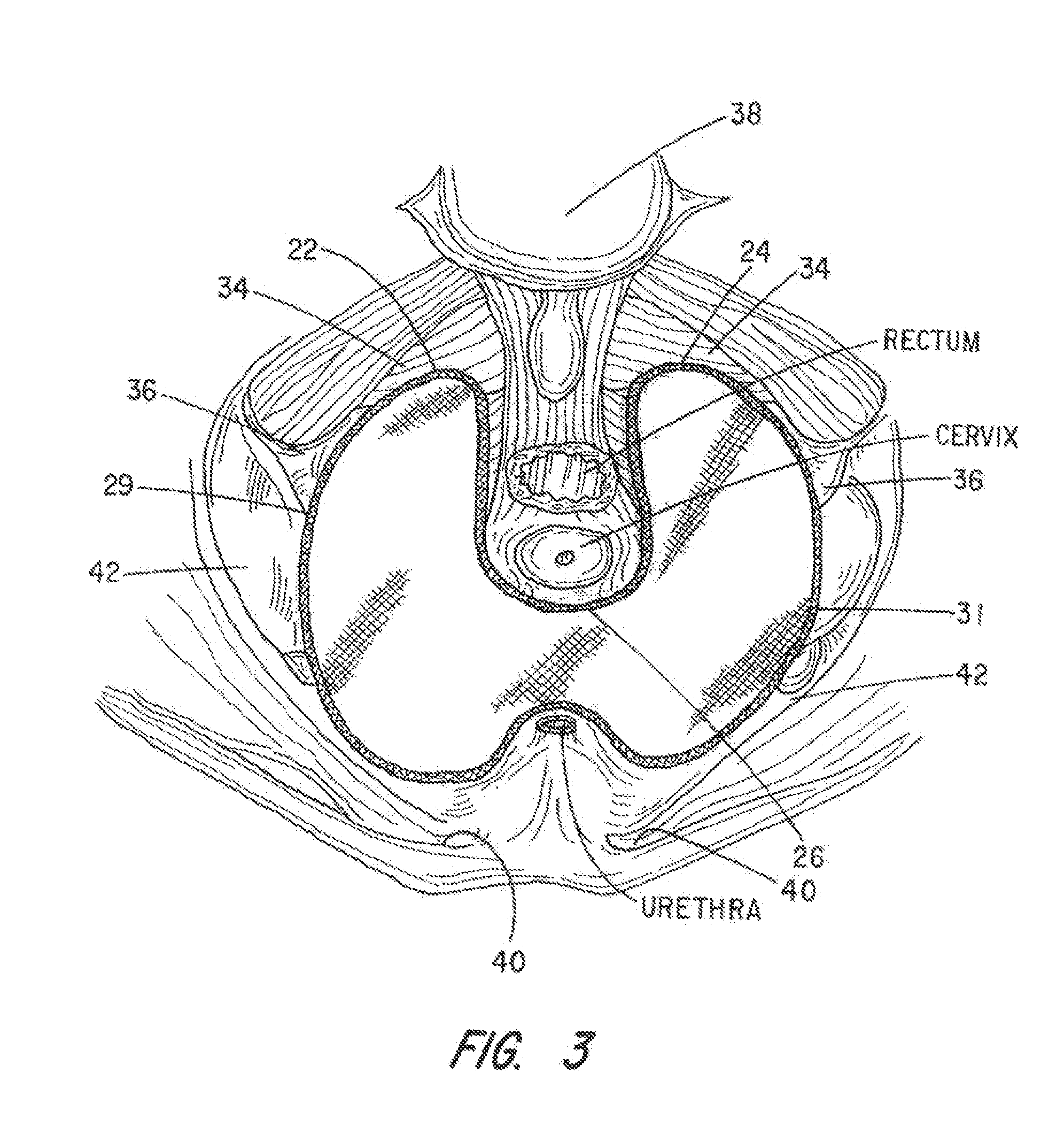
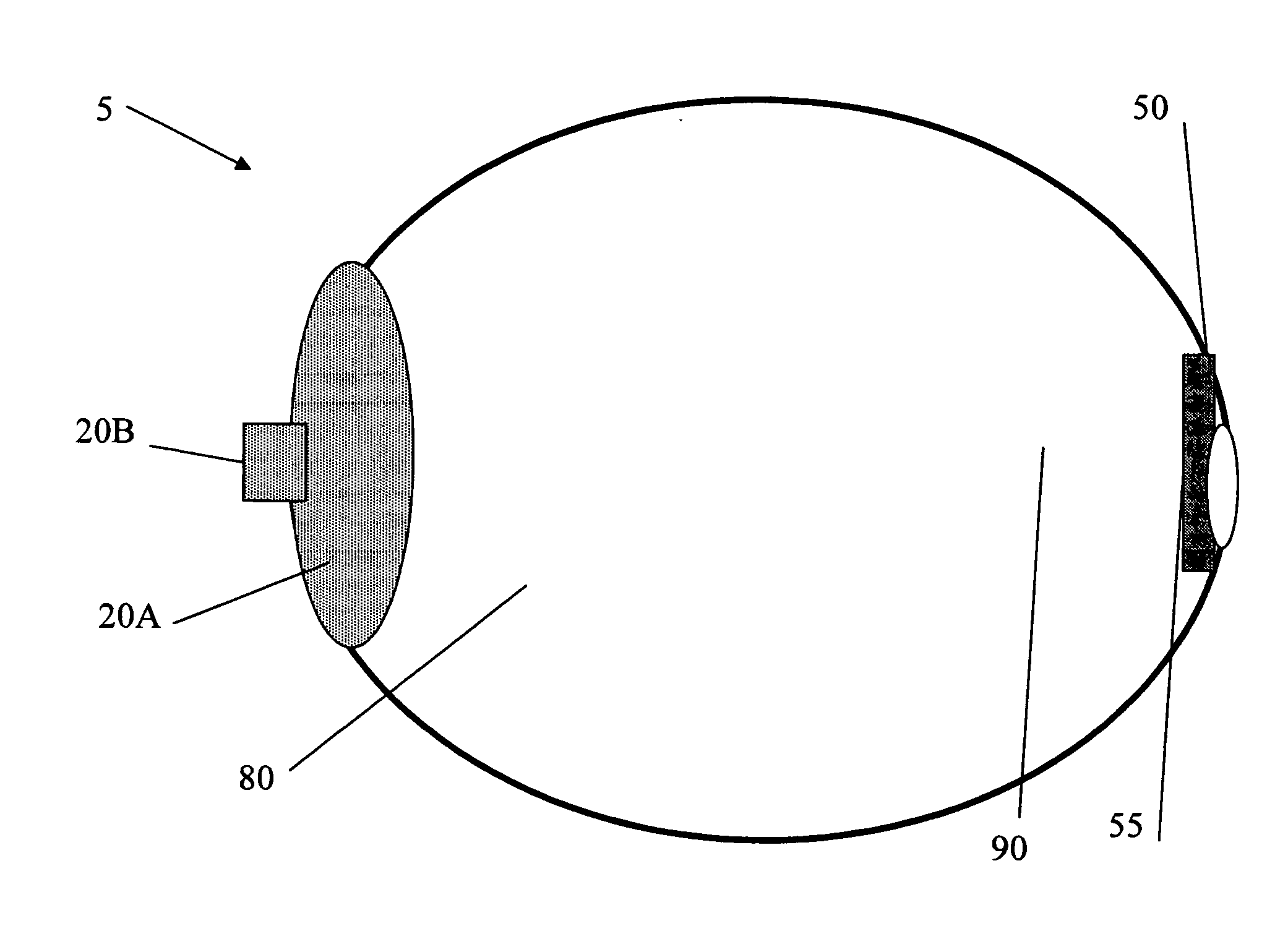
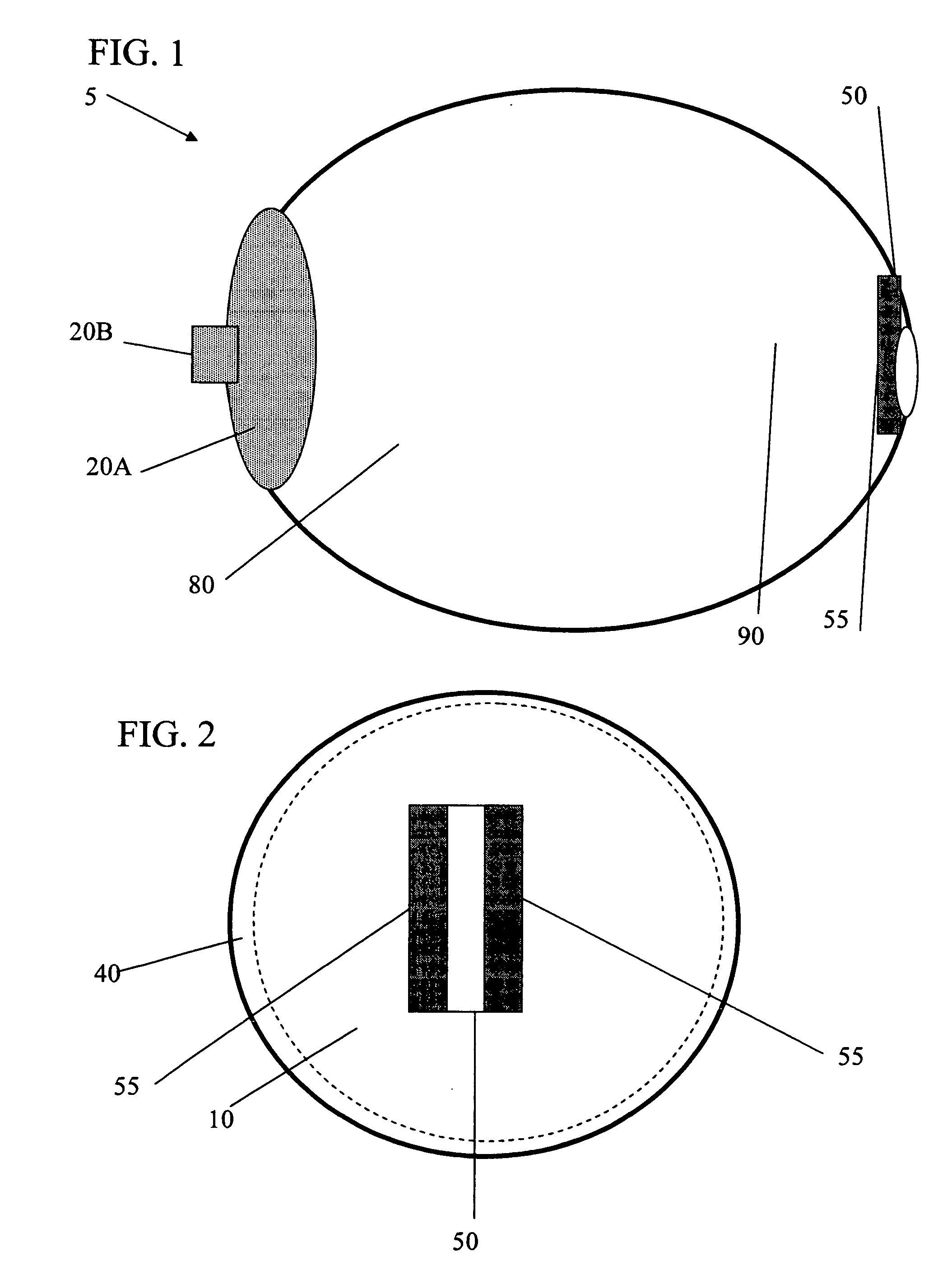
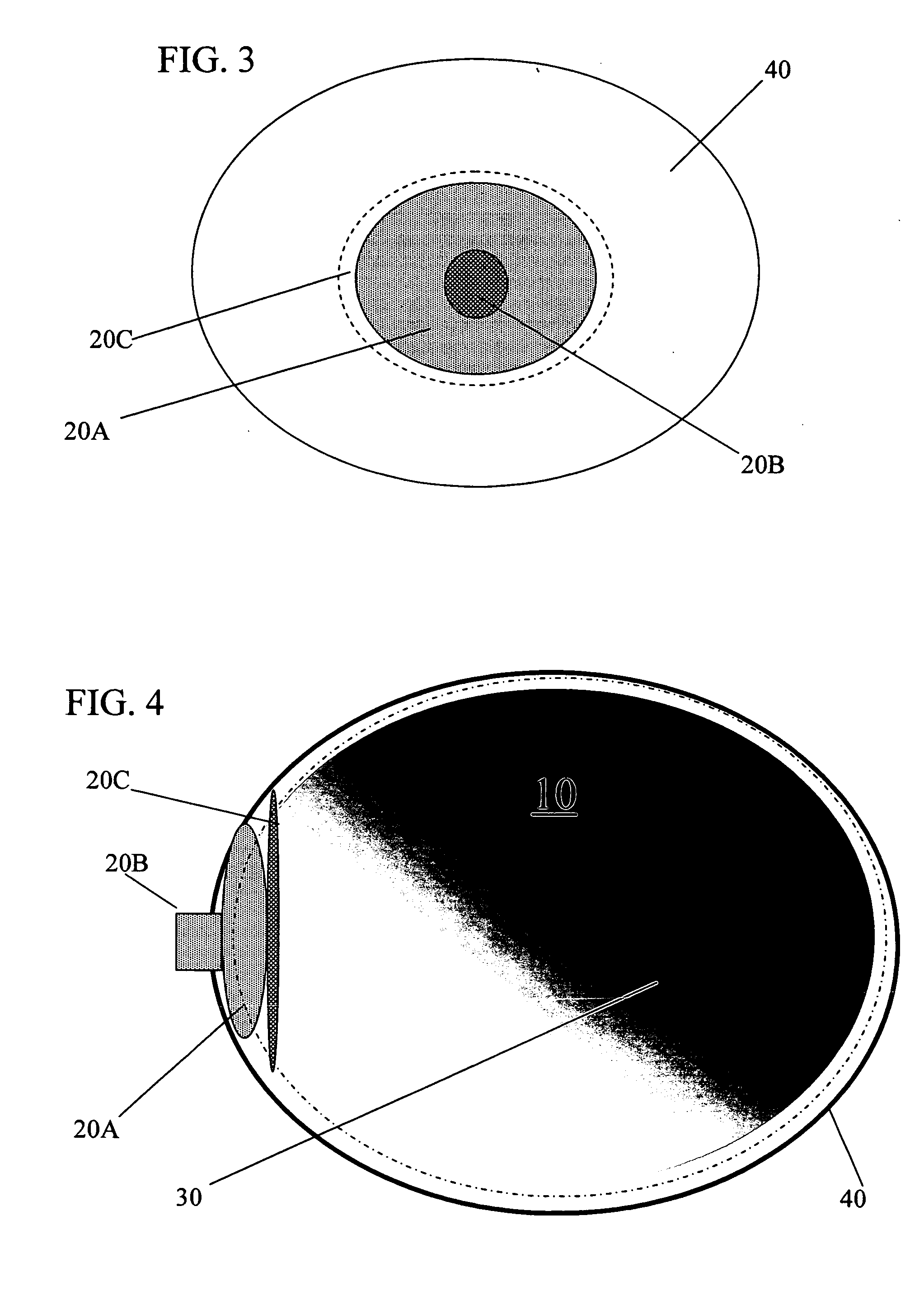
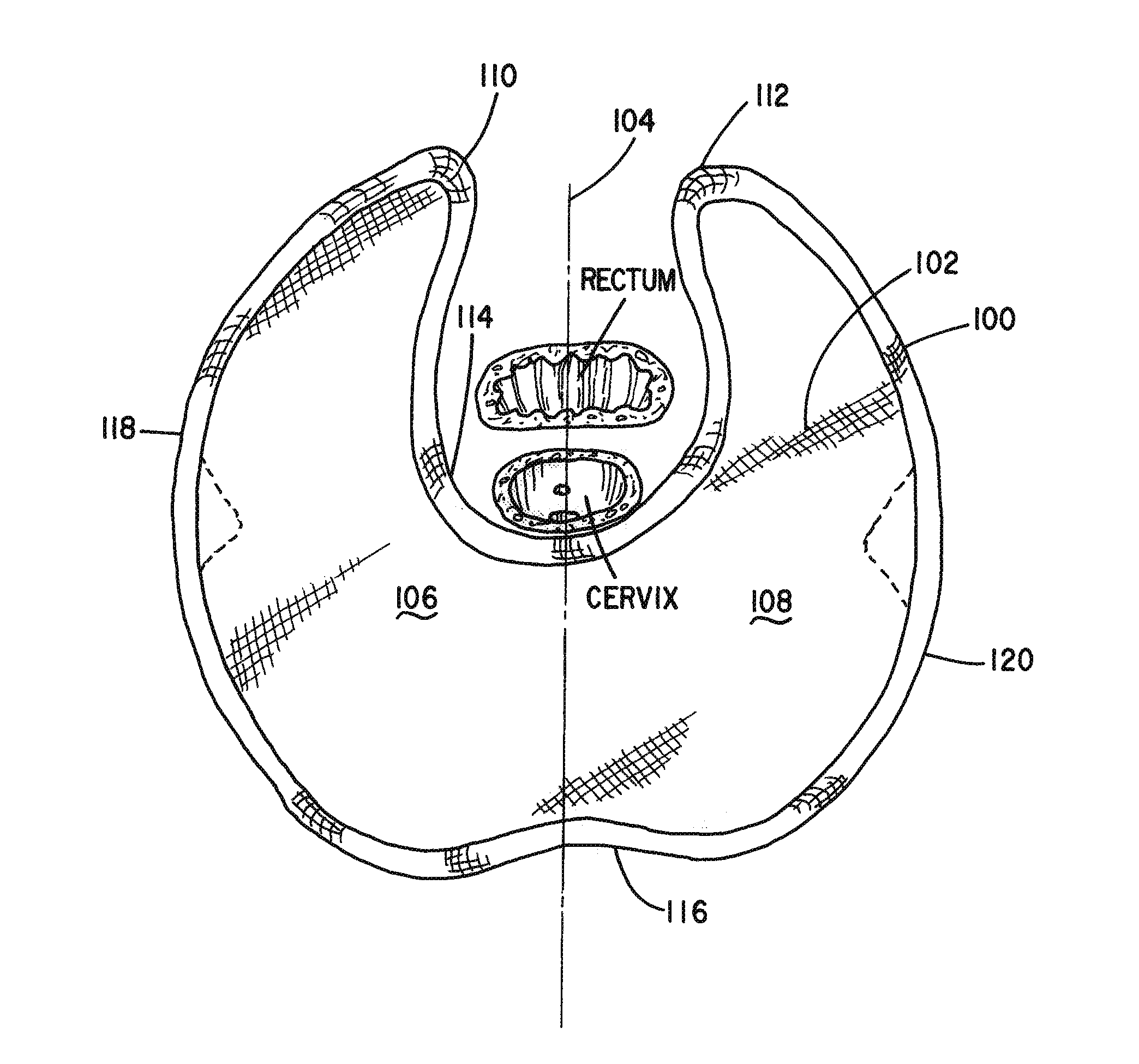
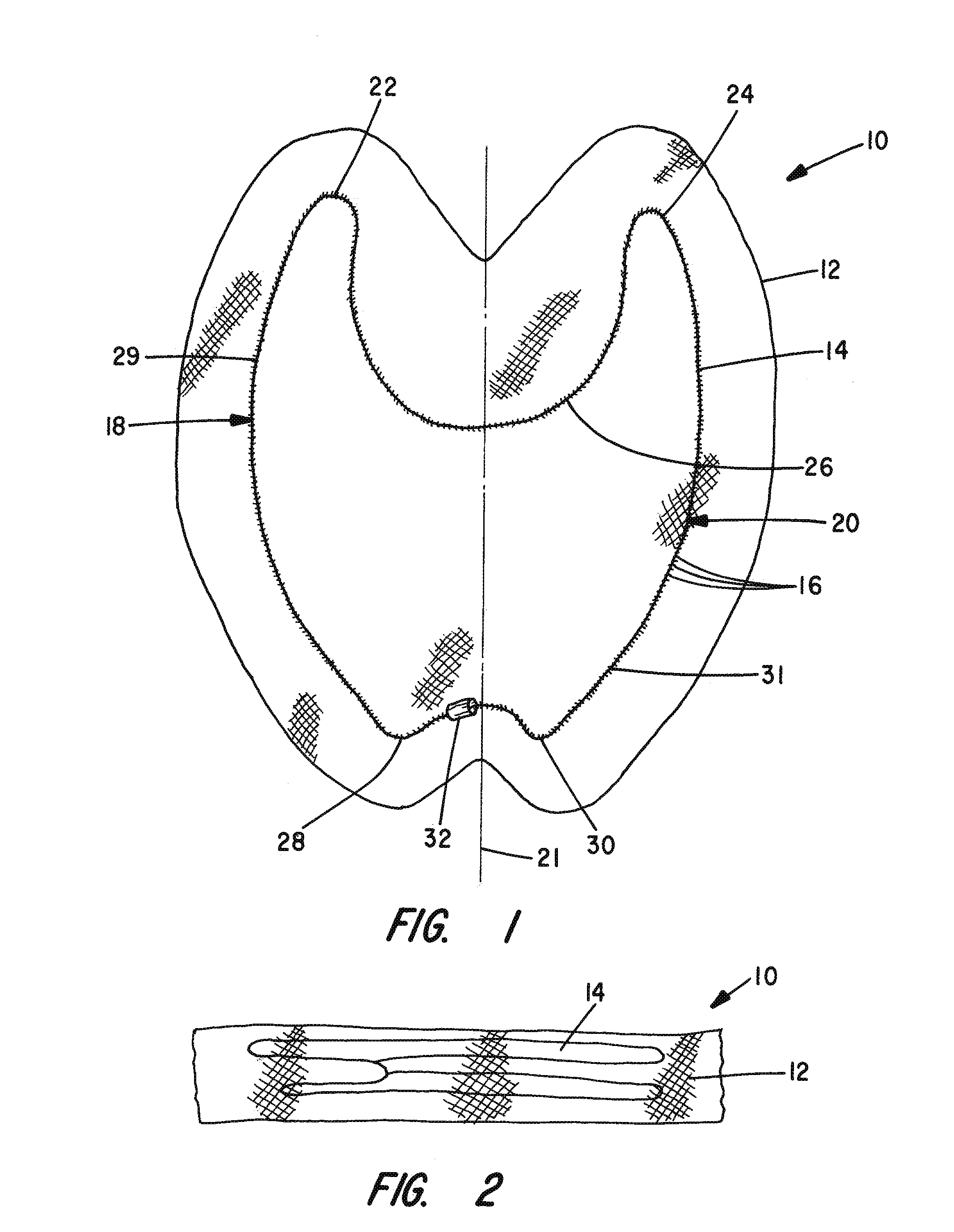
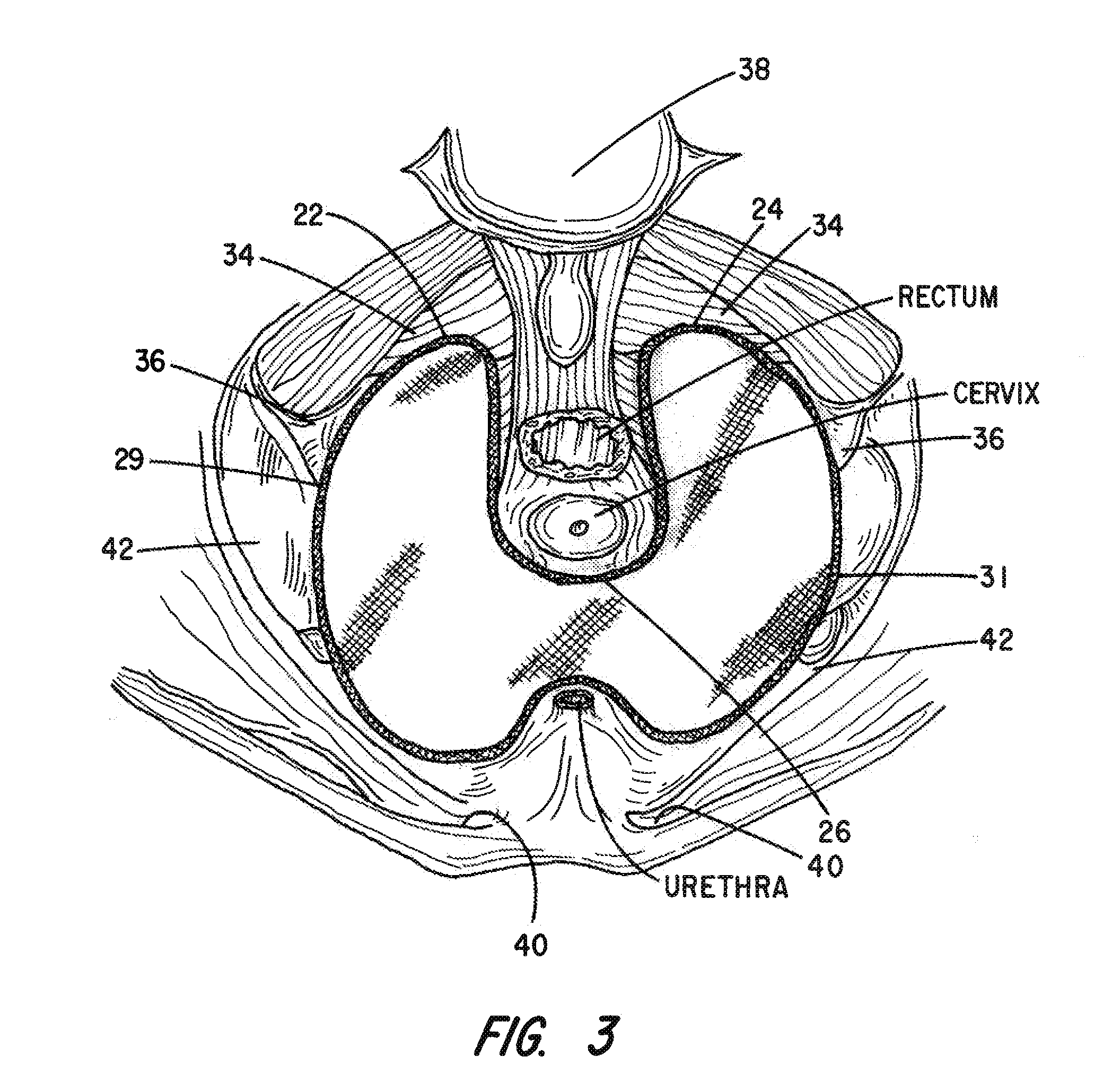
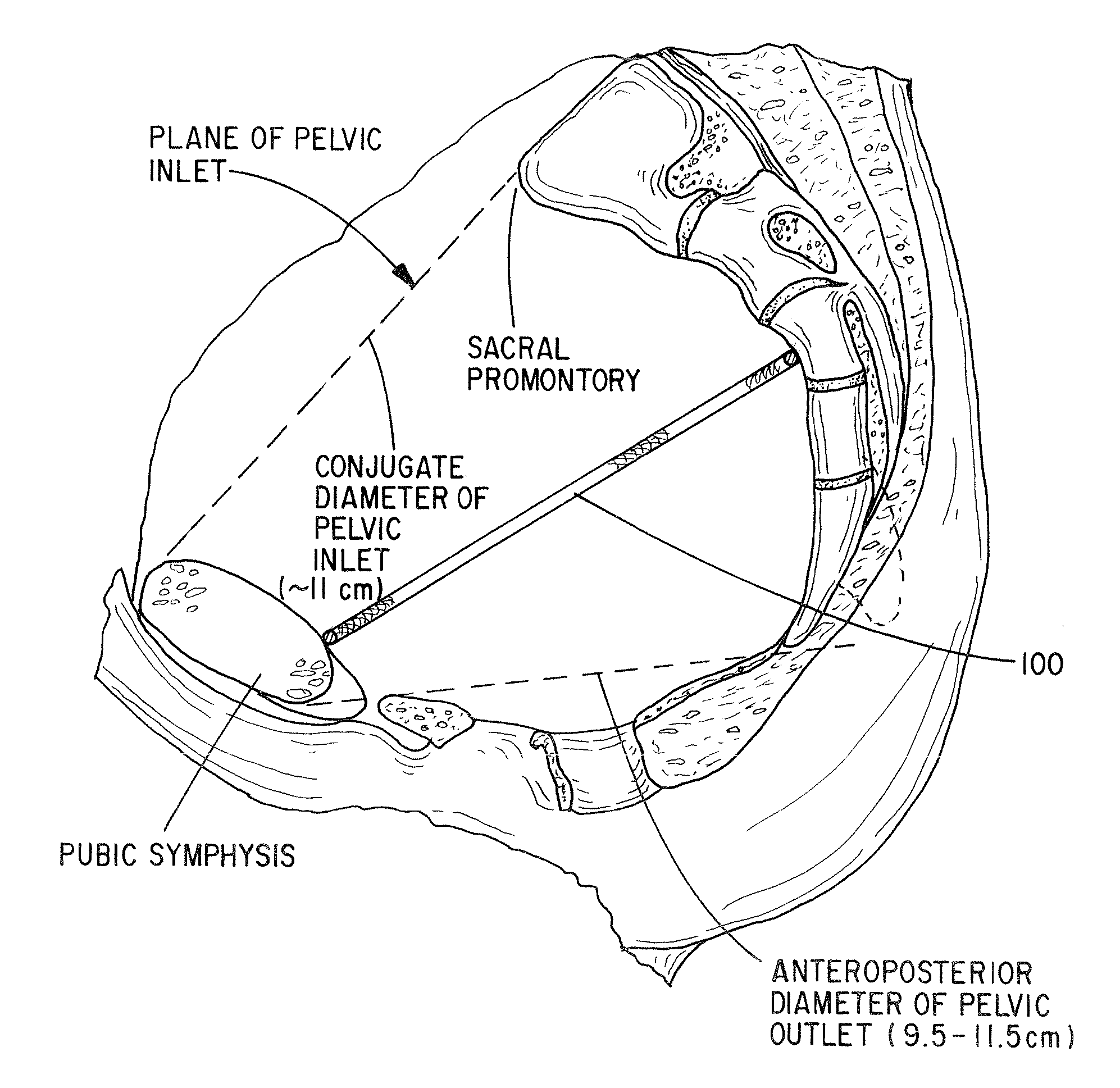
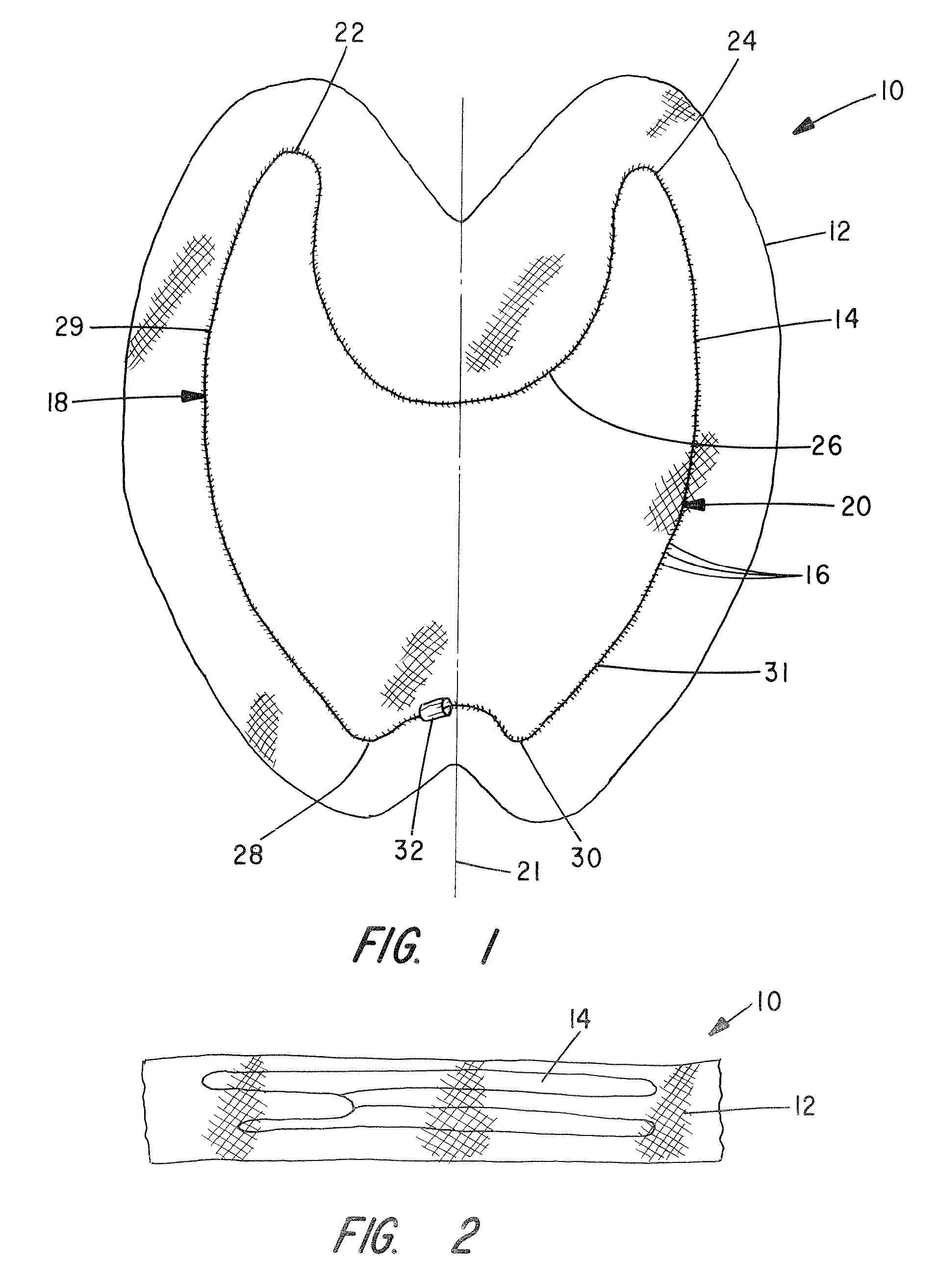
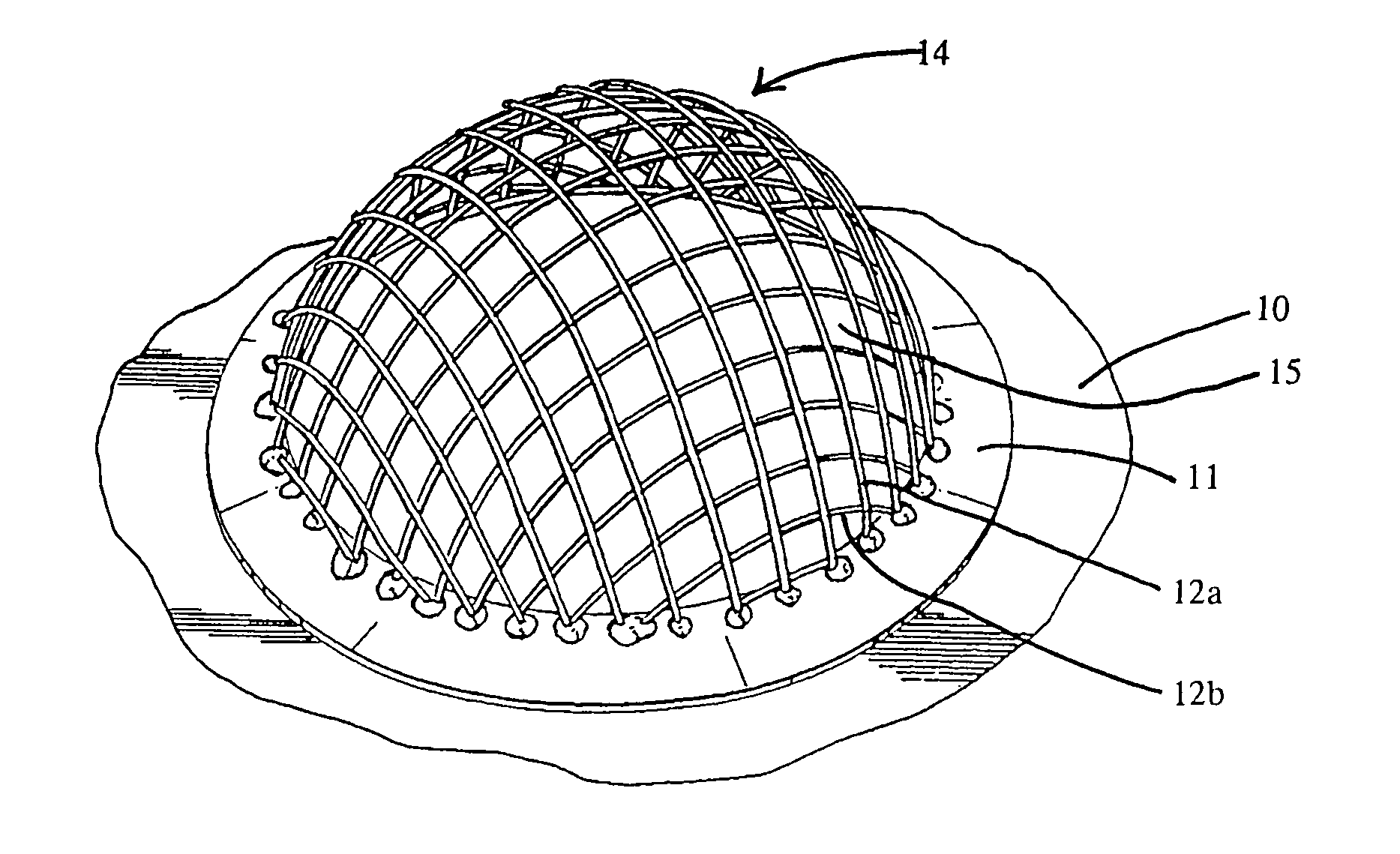
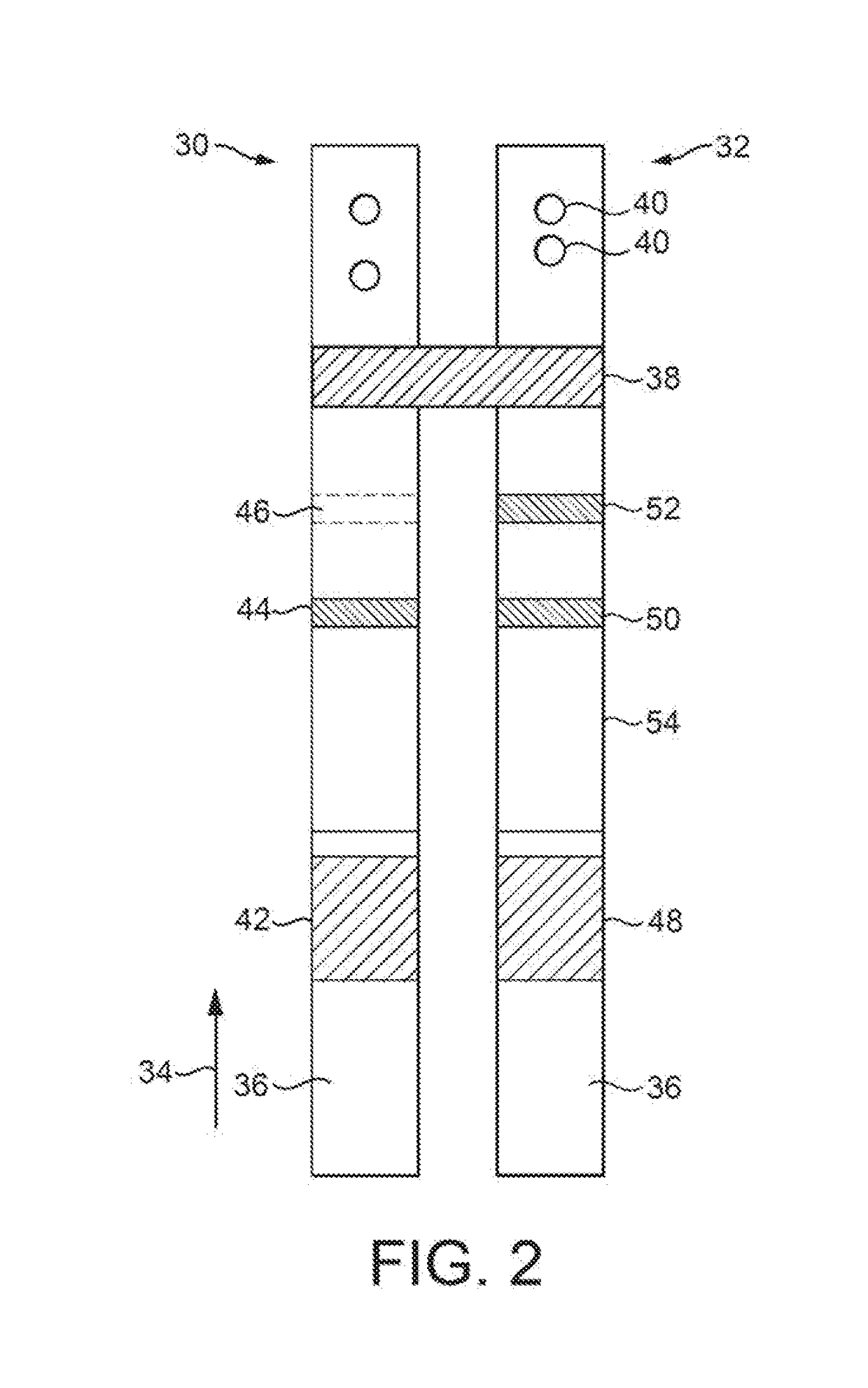
Apparatus and Method for Pelvic Floor Repair in the Human Female
A prosthesis for addressing pelvic organ prolapse in females comprises a frame fabricated from a shape memory material that supports a thin, flexible mesh sheet in a stretched condition when the frame is unconstrained. The mesh sheet is formed with two finger receiving pockets proximate its posterior periphery to be used by the surgeon in steering the prosthesis to a desired disposition within the pelvic basin. The frame is shaped so as to conform to and be supported by bone structures and muscle tissue in the pelvic basin while providing needed support to pelvic organs to maintain them in a proper position. The use of a shape memory material allows the prosthesis to be rolled or folded into a reduced size for ease of placement through a small incision in the wall of the vagina, but that springs back to its memorized shape following deployment from a delivery sheath.
Owner:MINNESOTA MEDICAL DEV
Apparatus and method for pelvic floor repair in the human female
A prosthesis for addressing pelvic organ prolapse in females comprises a frame fabricated from a shape memory material that supports a thin, flexible mesh sheet in a stretched condition when the frame is unconstrained. The mesh sheet is formed with two finger receiving pockets proximate its posterior periphery to be used by the surgeon in steering the prosthesis to a desired disposition within the pelvic basin. The frame is shaped so as to conform to and be supported by bone structures and muscle tissue in the pelvic basin while providing needed support to pelvic organs to maintain them in a proper position. The use of a shape memory material allows the prosthesis to be rolled or folded into a reduced size for ease of placement through a small incision in the wall of the vagina, but that springs back to its memorized shape following deployment from a delivery sheath.
Owner:MINNESOTA MEDICAL DEV
Pregnancy test device and method
InactiveUS20150094227A1Avoid false negative resultsImprove the level ofLibrary screeningBiological material analysisPregnancy testsMetabolite
Disclosed is a test device to detect pregnancy in a human female subject, the test device comprising:an assay means to measure the absolute or relative amount of hCG in a sample from the subject;an assay means to measure the absolute or relative amount of FSH in a sample from the subject;and an assay means to measure the absolute or relative amount of one or more progesterone metabolites in a sample from the subject.
Owner:SPD SWISS PRECISION DIAGNOSTICS
Compositions and methods for amelioration of human female sexual dysfunction
InactiveUS20050004226A1Modulate sexual responseIncrease secretionBiocideElcosanoid active ingredientsDiseaseFemale Sexual Arousal Disorder
The invention provides compositions and methods suitable for ameliorating female sexual dysfunction, and in particular, female sexual arousal disorder. In preferred embodiments, the invention provides a semisolid composition suitable for topical application comprising: an effective amount of a vasoactive prostaglandin, a penetration enhancer, a polymer thickener, a lipophilic component, and an acidic buffer system. In other embodiments, the invention provides a method of treating female sexual arousal disorder by applying an effective dose of a topical semisolid prostaglandin composition to the anterior wall of the vagina.
Owner:NEXMED HLDG INC
Method for detecting human papillomavirus mRNA
An in vitro method is provided for screening human female subjects to assess their risk of developing cervical carcinoma which comprises screening the subject for expression of mRNA transcripts from the E6 and optionally the L1 gene of human papillomavirus, wherein subjects positive for expression of L1 and / or E6 mRNA are scored as being at risk of developing cervical carcinoma. Kits for carrying out such methods are also provided.
Owner:NORCHIP AS
Simulated breast comfort aide system
An improved comfort aide system for providing breastfed babies with the comforting sensory experience of non-feeding suckling at a breast resembling that of a mother's breast in: smell, form, feel and function. Comprising a main body portion of latex or silicone material, filled with liquid or other filler substance, shaped like a human female breast. A cover having the tactility of human skin encloses the main body. The cover may be saturated, sprayed or sprinkled with mom's breast milk. Cover dries and retains the smell of mom and her milk. Cover has a rear opening flap for permitting removal of the main body. Cover has a hole placed opposite to opening flap for nipple placement. The nipple, comprising areola, teat, and nipple base is attached through cover hole and is suckled by baby in the same manner baby would suckle to mom's natural lactating breast.
Owner:GARRETT VANESSA LYNN BLEVINS
Apparatus and Method for Pelvic Floor Repair in the Human Female
A prosthesis for addressing pelvic organ prolapse in females comprises a frame comprising first and second segments or halves fabricated from a shape memory material that together support a thin, flexible sheet in a stretched condition when the frame is unconstrained. The frame is shaped so as to conform to and be supported by bone structures and muscle tissue in the pelvic basin while providing needed support to pelvic organs to maintain them in a proper position. The use of a shape memory material allows the prosthesis to be rolled or folded into a reduced size for ease of placement through a small incision in the wall of the vagina, but that springs back to its memorized shape following deployment from a. delivery sheath. By providing a two-piece segmented frame, removal of the frame structure post implantation of the prosthesis is facilitated.
Owner:MINNESOTA MEDICAL DEV
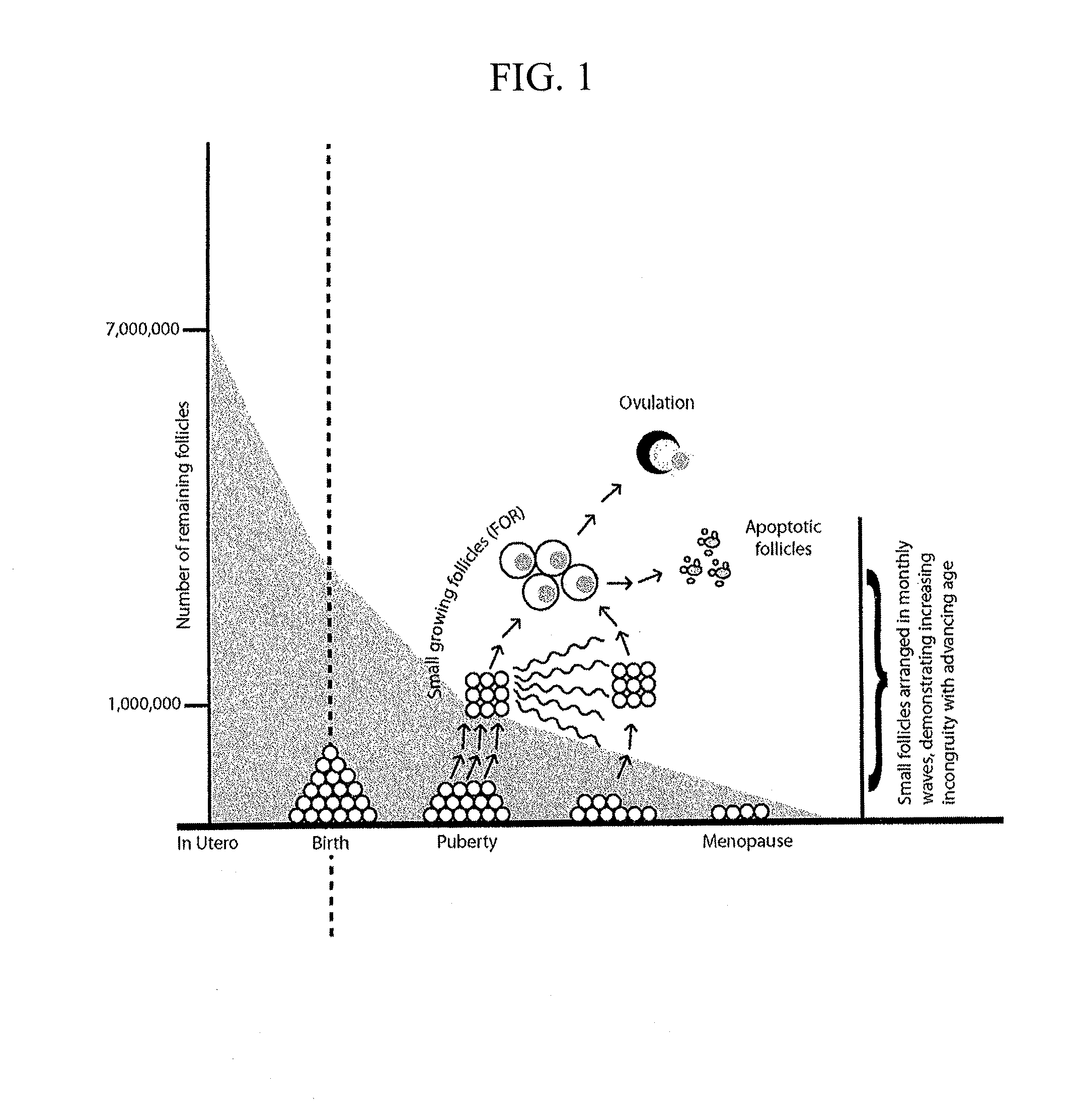
Oocytes derived from ovarian culture initially containing no oocytes
Ovarian germ-line-competent embryonic stem cells (GLC-ESC) are cultured, either in the presence or absence of a compound having estrogenic activity. The GLC-ESC are either collected prior to specific commitment or are permitted to remain in the culture medium for a time sufficient to develop into oocytes, and the oocytes may be fertilized by adding sperm to the culture medium. The fertilized oocytes may be permitted to develop into embryos, which may be transferred into the uterus of an adult human female or frozen for later use. The invention provides a method for obtaining by in vitro fertilization an embryo that is genetically related to a human female who is not producing oocytes.
Owner:OVASCI
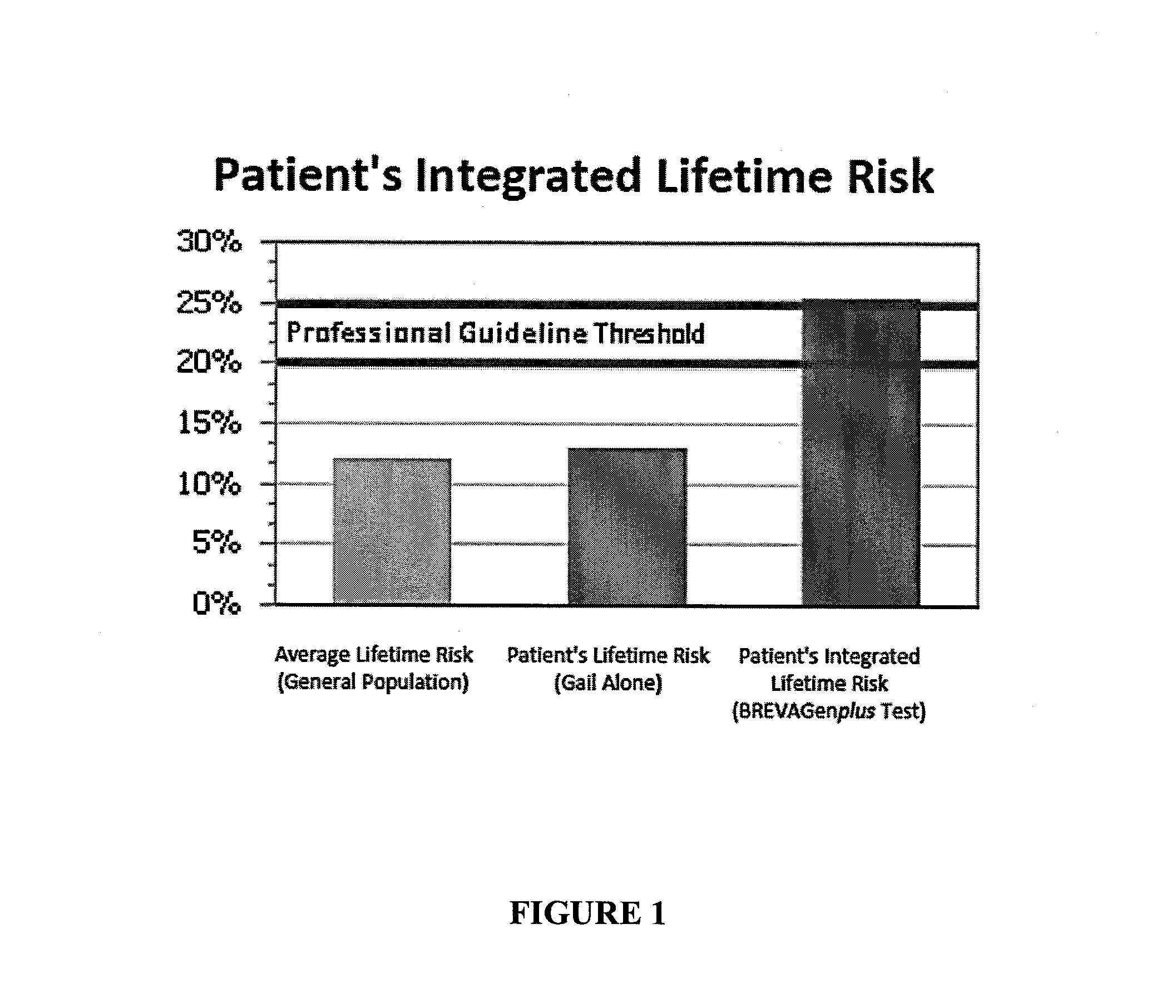
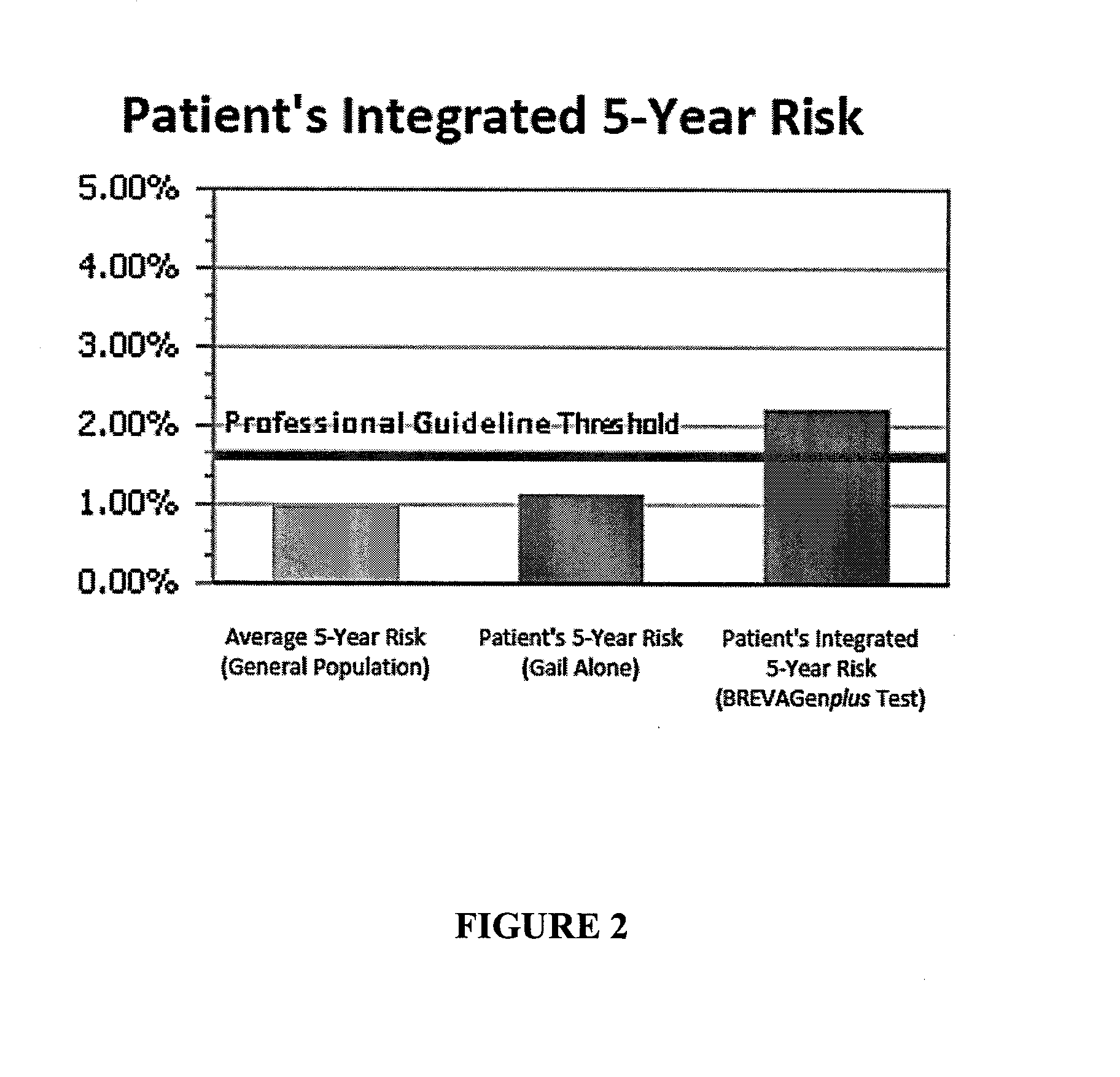
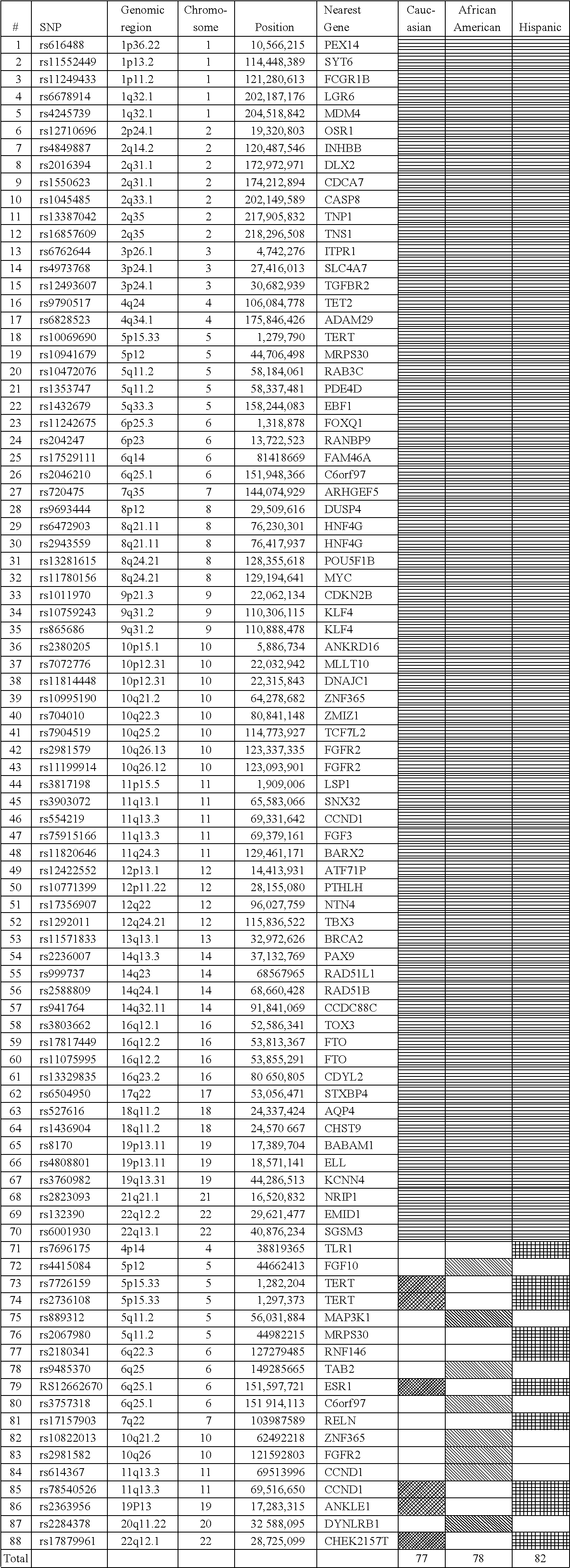
Methods for breast cancer risk assessment
InactiveUS20110294681A1Microbiological testing/measurementLibrary screeningGenetic riskHuman Females
The present invention relates to methods and systems for assessing the overall risk of a human female subject for developing a breast cancer phenotype. In particular, the present invention relates to combining clinical risk assessment and genetic risk assessment to improve risk analysis.
Owner:GENETIC TECHNOLOGIES LIMTIED
Method of controlled ovarian hyperstimulation and pharmaceutical kit for use in such method
InactiveUS7815912B2Easy to solveIncrease dosePeptide/protein ingredientsDepsipeptidesGanirelixCo administration
One aspect of the present invention is concerned with a method of controlled ovarian hyperstimulation in a mammalian female, said method comprising the co-administration to said female of —a substance having follicle stimulating hormone activity (FSH substance) in an amount effective to stimulate multiple follicular development; —gonadotropin releasing hormone (GnRH) antagonist in an amount equivalent to a daily subcutaneous dose of at least 0.5 mg ganirelix to prevent a premature LH-surge; and —a LH substance in an amount effective to prevent or suppress symptoms of luteinising hormone (LH) deficiency resulting from the administration of the GnRH antagonist; followed by administering a meiosis and luteinisation inducing substance (ML substance) in an amount effective to stimulate resumption of meiosis and luteinisation, and wherein the LH substance is not obtained from the urine of human females. Another aspect of the to invention relates to a pharmaceutical kit for use in a method of controlled hyperstimulation, which kit comprises: —at least one parenteral or oral dosage unit containing one or more FSH substances in an amount equivalent to a subcutaneous dose of 50-1500 I.U. FSH; —at least one parenteral dosage unit containing one or more GnRH antagonists in an amount equivalent to a subcutaneous dose of 0.5-25 mg ganirelix; —at least one parenteral dosage unit containing one or more LH substances in an amount equivalent to a subcutaneous dose of 50-3000 I.U. recombinant LH; wherein the LH substance is not obtained from the urine of human females.
Owner:ZONE IND DE IOURIETTAZ
Diagnostic markers of human female infertility
Methods and reagents for the diagnosis of female infertility, prognostic indicators for female infertility, compounds for the treatment of female infertility, compounds and methods for contraception. Methods and compounds are based on the levels of ebaf in endometrial tissue. Methods for diagnosing endometrial receptivity and bleeding function by screening a biological sample such as an endometrial tissue sample, or bodily fluid for the presence of ebaf. A contraceptive compound containing an effective amount of ebaf and a pharmaceutically acceptable carrier. A diagnostic kit for timing contraception containing reagents for screening a sample for the presence of ebaf. A method of treating endometrial irregularities by down-regulating the expression of ebaf.
Owner:UNIV OF SOUTH FLORIDA
Device for sizing a human female breast
InactiveUS20080125675A1Guaranteed accuracySee clearlyMammary implantsPerson identificationHuman FemalesGynecology
A device for intraoperative sizing of a human female breast during resizing and / or shaping of the breast composes: a (a) substantially hemispherical, rigid, cup-shaped foraminous body, i.e., a foraminous cup, defining a desired size and shape of the breast; and (b) a rim member to which the foraminous cup is affixed.
Owner:ACCURATE SURGICAL & SCI INSTR CORP
Diagnostic markers of human female infertility
InactiveUS20080200379A1Peptide/protein ingredientsMicrobiological testing/measurementHuman FemalesEndometrial receptivity
The subject invention pertains to methods and reagents for the diagnosis of female infertility, prognostic indicators for female infertility, compounds for the treatment of female infertility, compounds and methods for contraception. Methods and compounds are based on the levels of ebaf in endometrial tissue. Methods for diagnosing endometrial receptivity and bleeding function by screening a biological sample such as an endometrial tissue sample, or bodily fluid for the presence of ebaf. A contraceptive compound containing an effective amount of ebaf and a pharmaceutically acceptable carrier. A diagnostic kit for timing contraception containing reagents for screening a sample for the presence of ebaf. A method of treating endometrial irregularities by down-regulating the expression of ebaf.
Owner:UNIV OF SOUTH FLORIDA
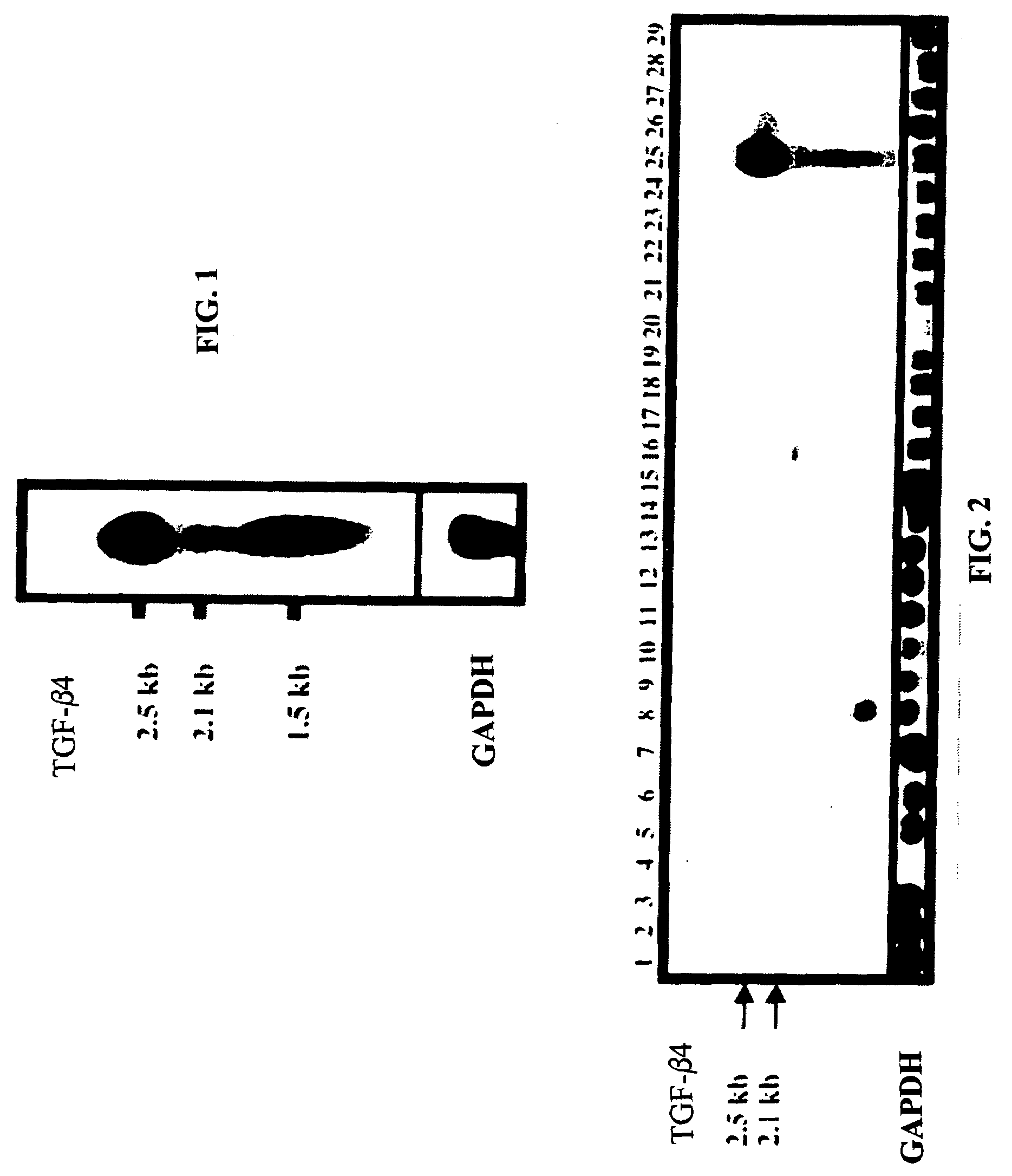
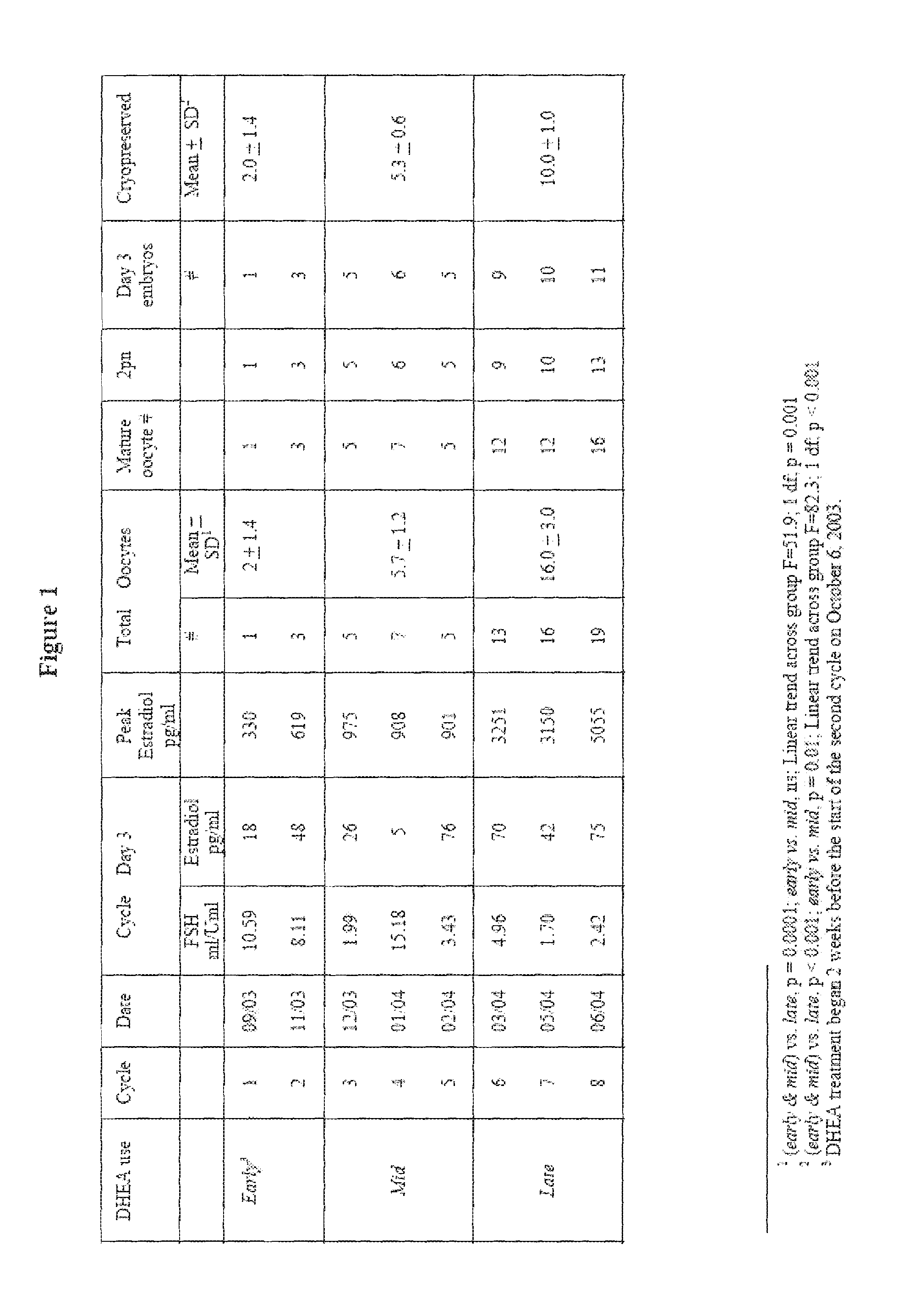
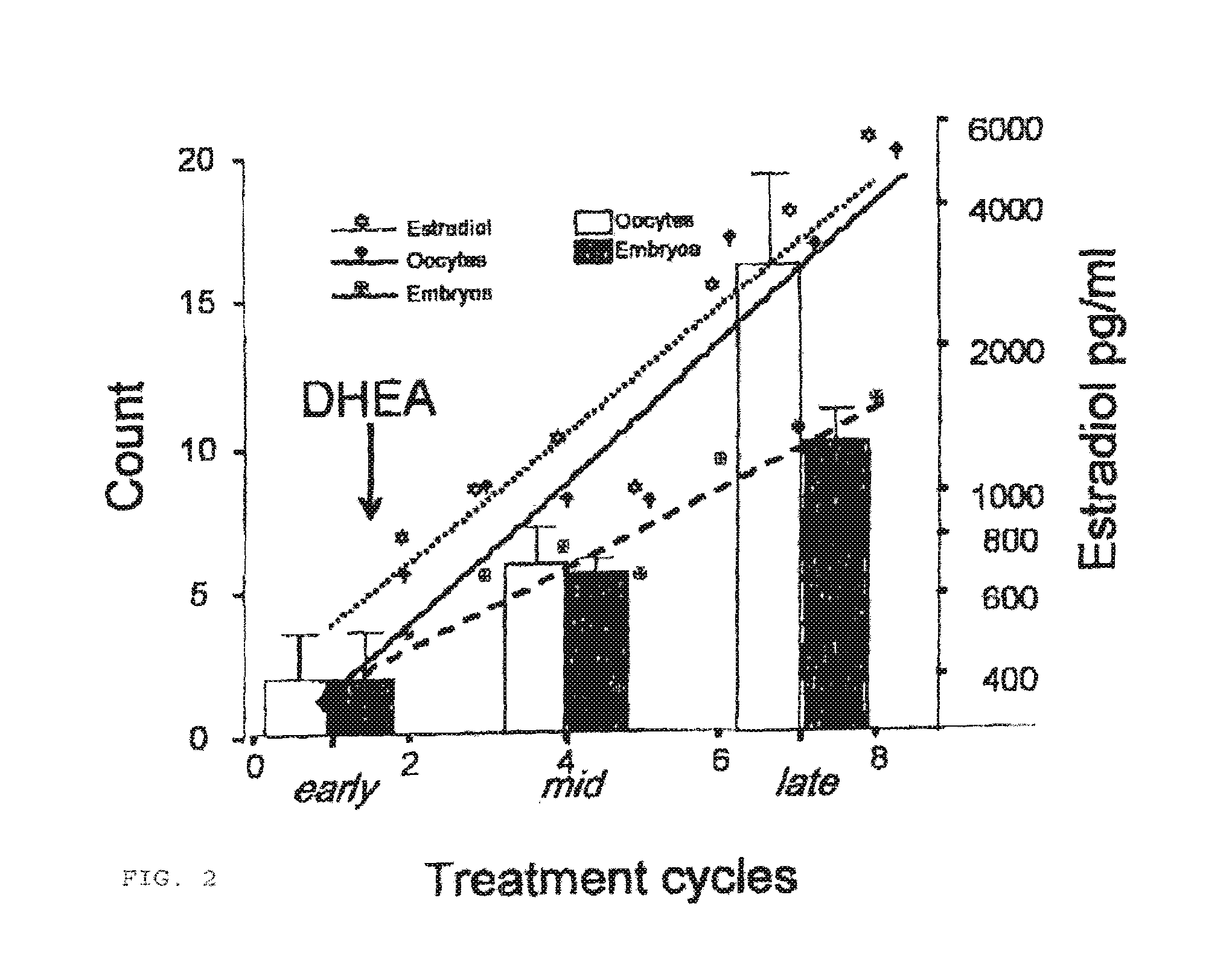
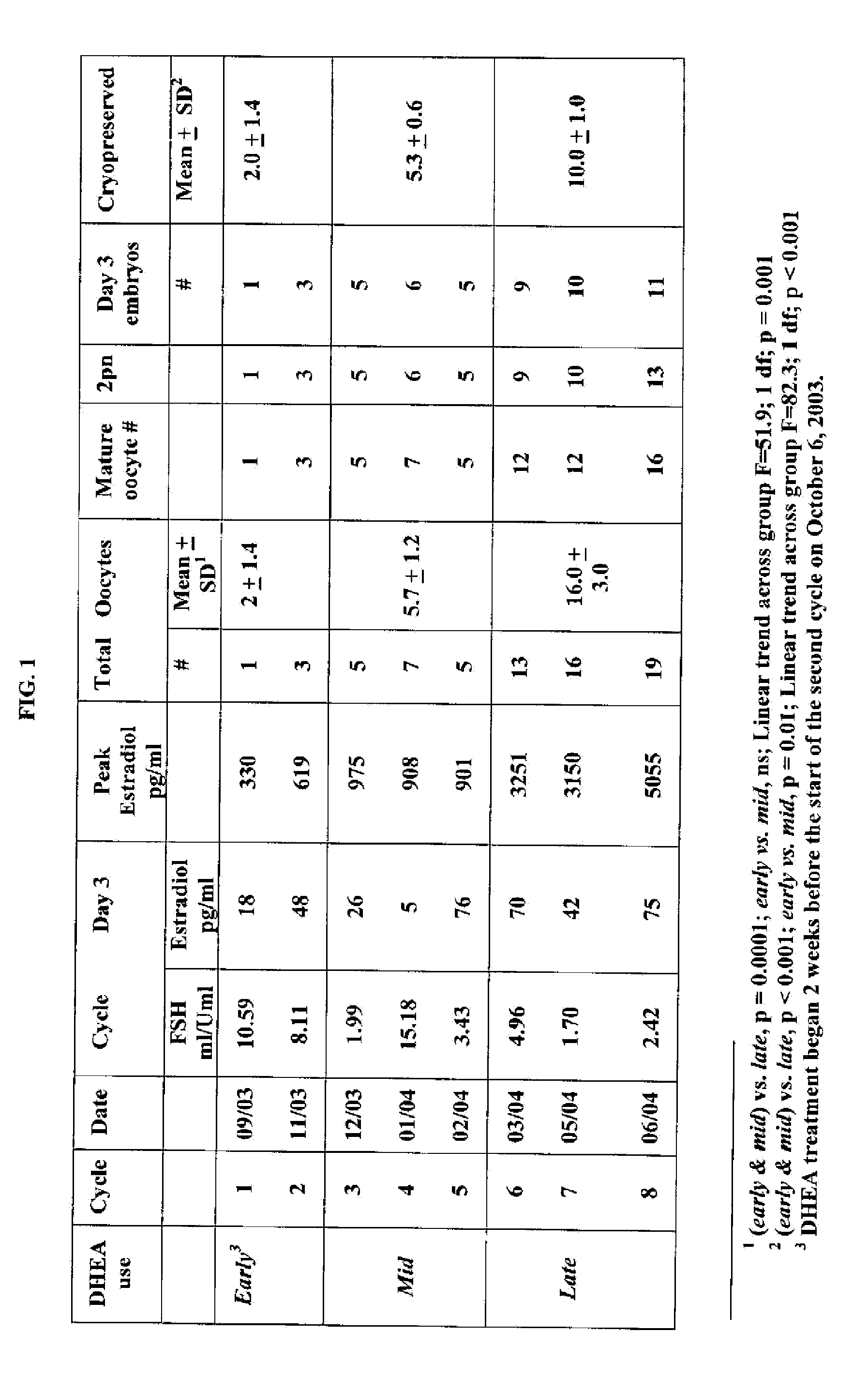
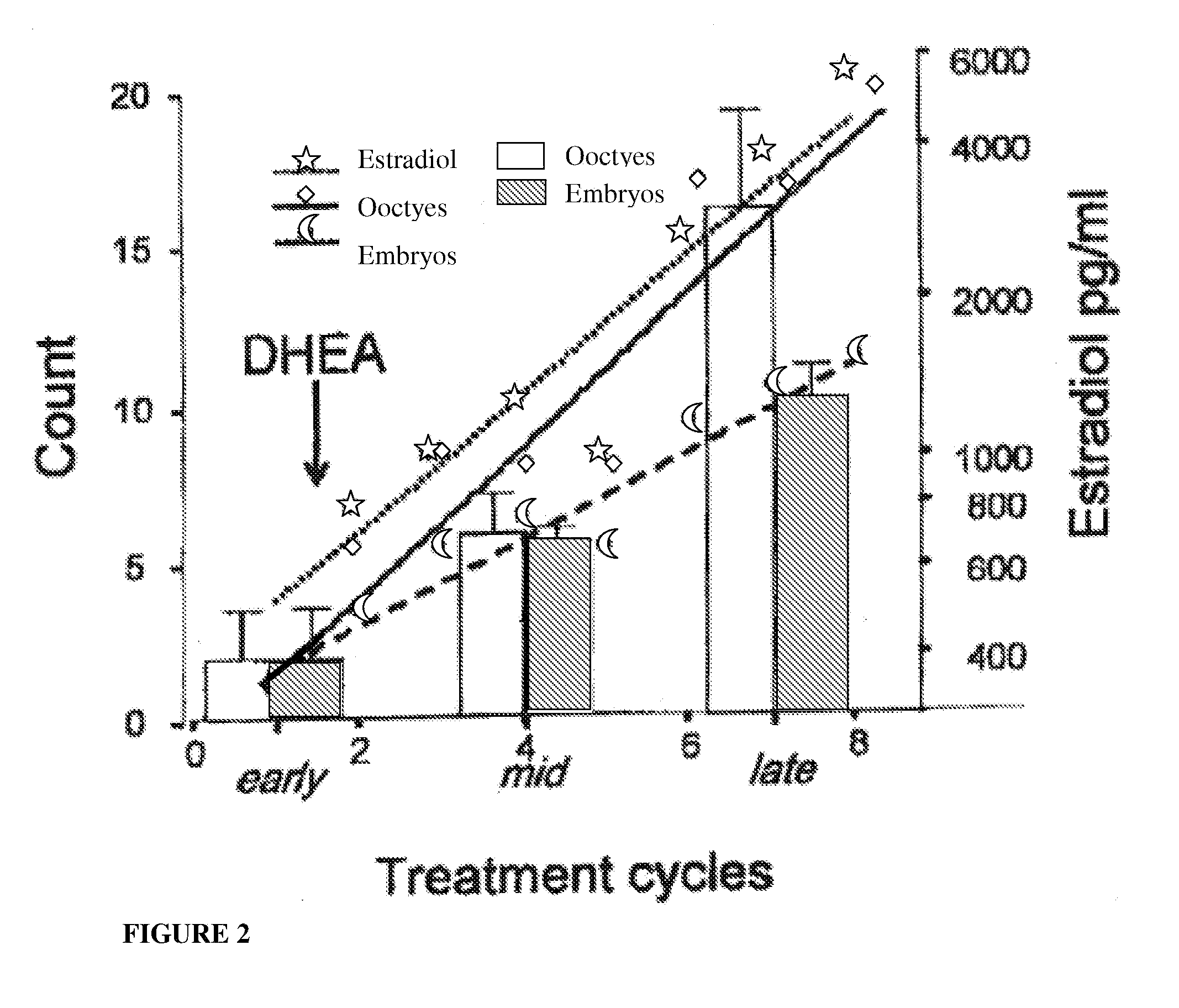
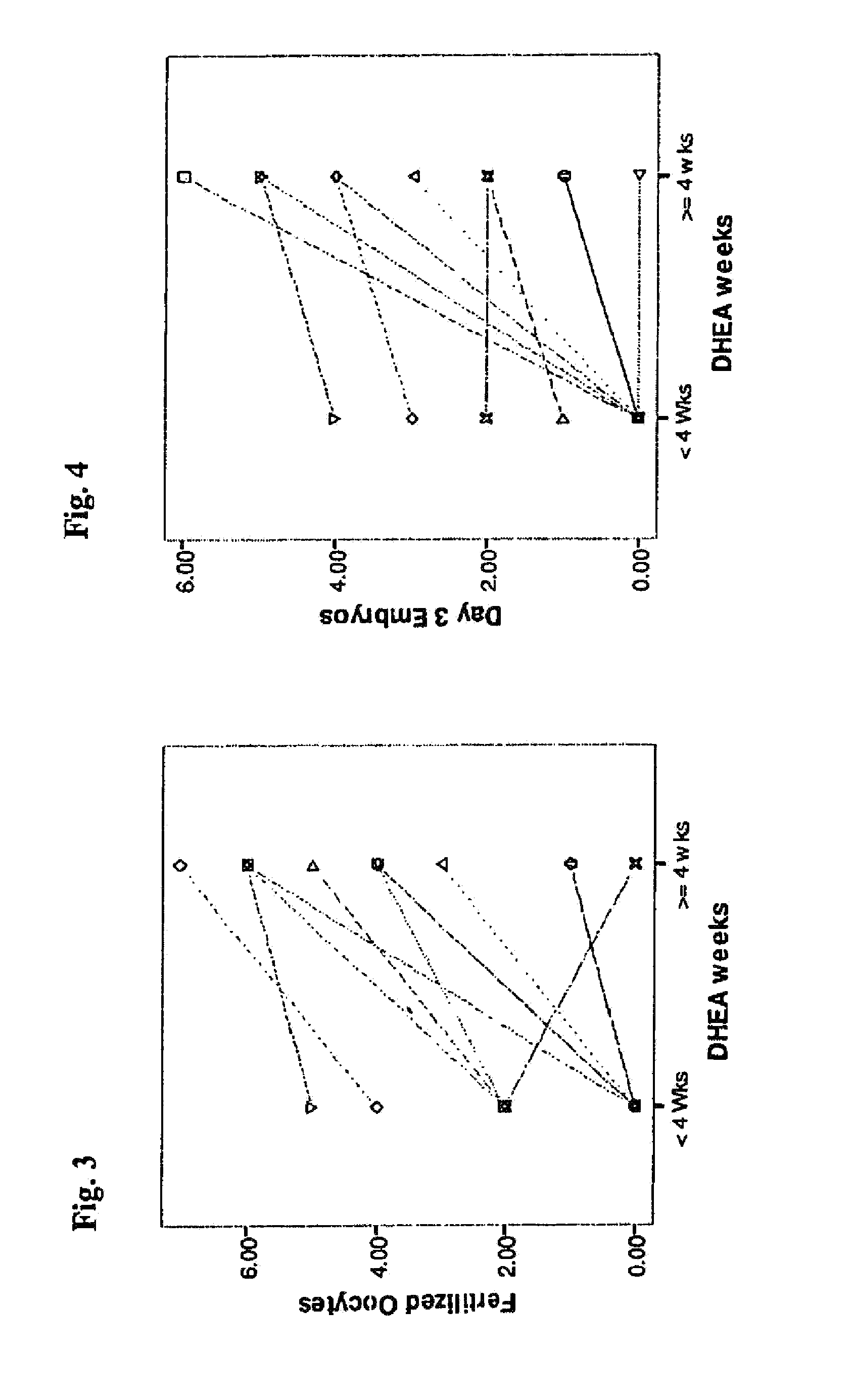
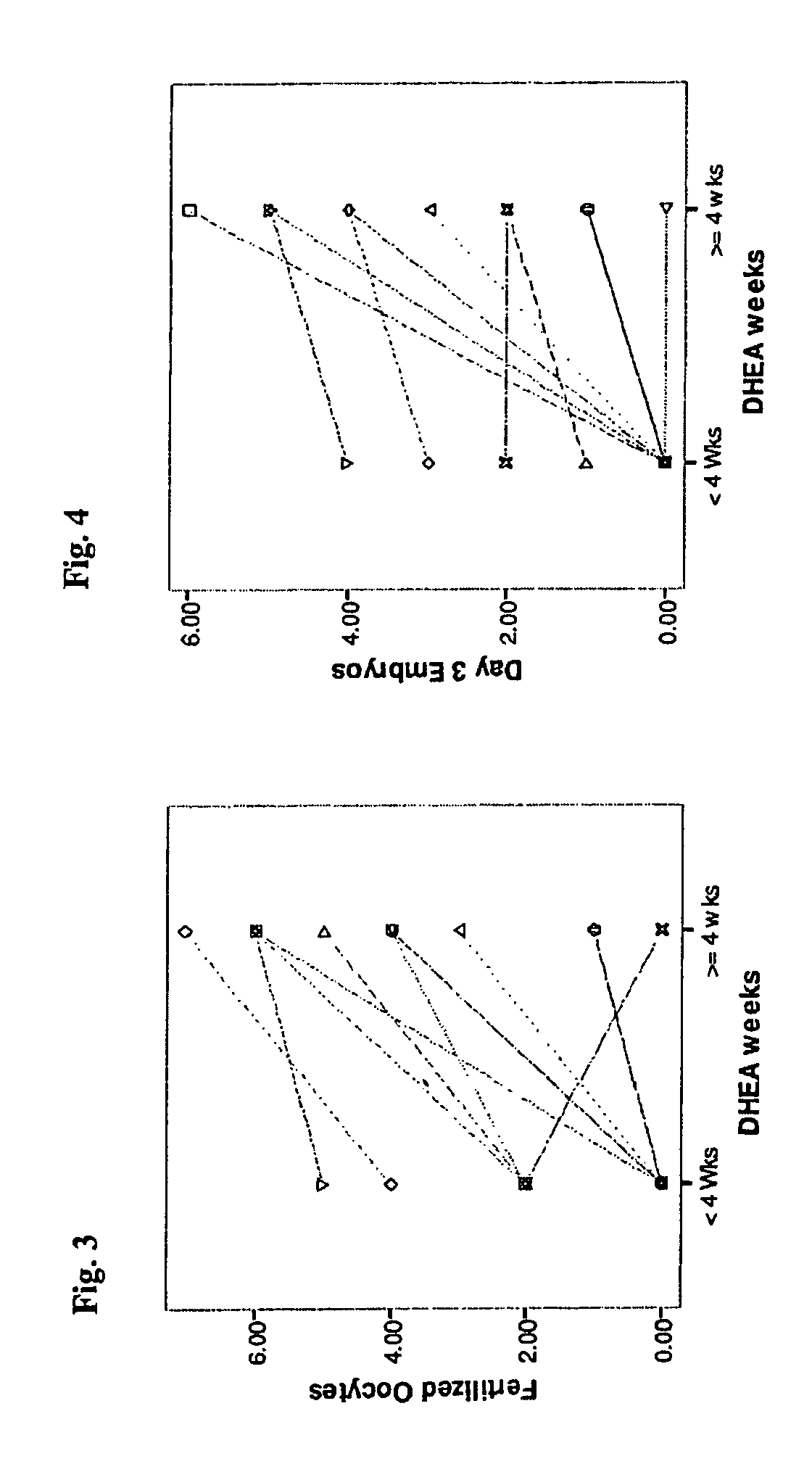
Androgen Treatment in Females
InactiveUS20070155710A1Increasing inhale fetus sex ratioReduce the possibilityOrganic active ingredientsPeptide/protein ingredientsHuman FemalesEmbryo
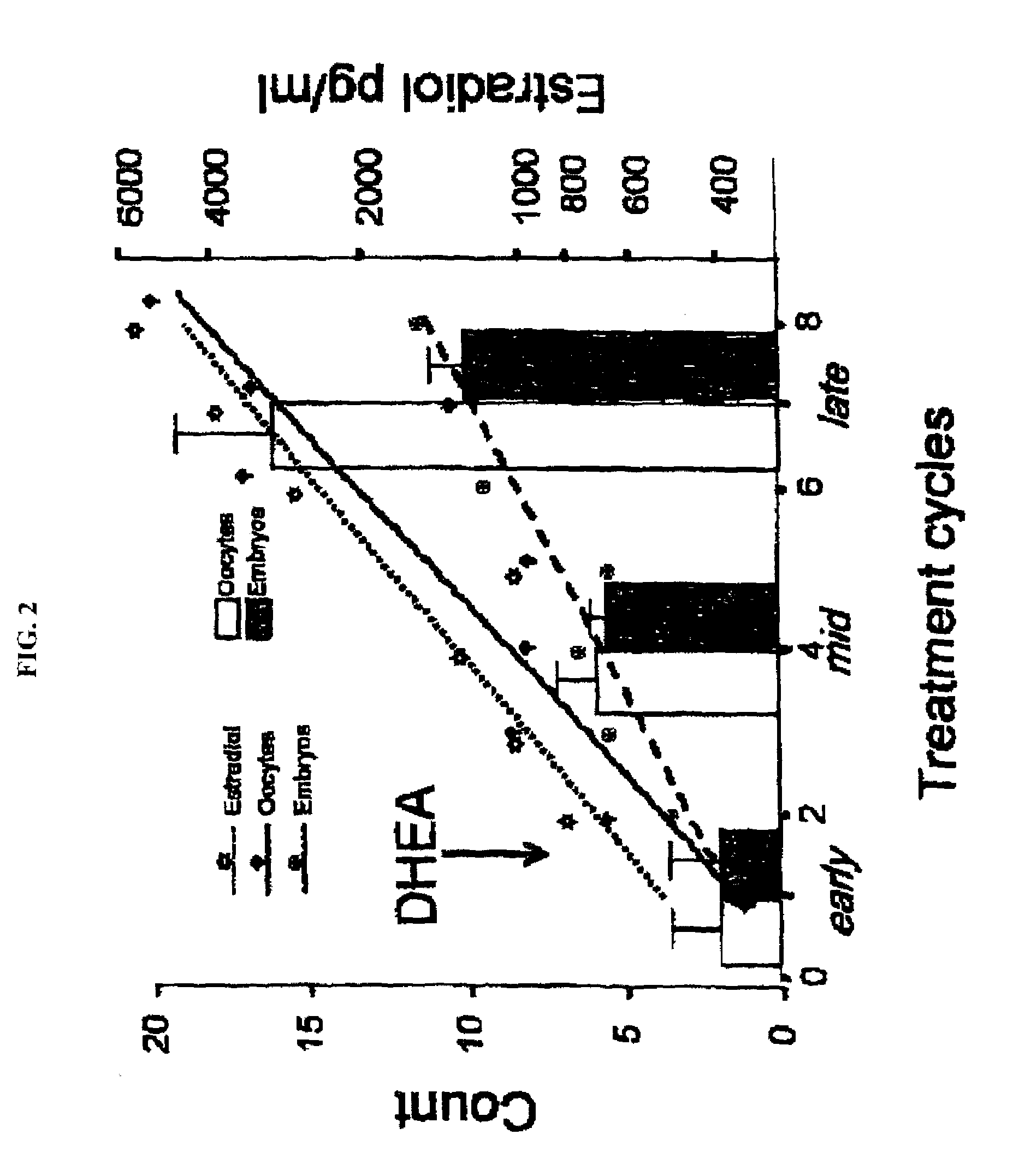
A method of improving cumulative embryo score may comprise administering an androgen to a human female, for example, DHEA, for at least about four consecutive months followed by harvesting and fertilizing oocytes and forming embryos. Between about 50 mg and about 100 mg of DHEA may be administered to a human female per day. Moreover, a method of increasing the quantity of fertilized oocytes in one cycle of in vitro fertilization may comprise administering an androgen to a human female for at least about four consecutive months, harvesting and fertilizing the oocytes. Furthermore, a method of increasing the quantity of day 3 embryos from one cycle of in vitro fertilization may comprise administering an androgen for at least about four consecutive months, harvesting and fertilizing the oocytes and forming day 3 embryos. A method of normalizing ovarian DHEA also may include administering an androgen for at least about four consecutive months. A method of increasing the rate and number of euploid oocytes may include administering an androgen for at least about four consecutive weeks. In addition, a method of increasing male fetus sex ratio may comprise raising baseline androgen levels in a female prior to or at time of embryo implantation.
Owner:AMERICAN INFERTILITY OF NEW YORK
Androgen Treatment in Females
ActiveUS20110207708A1Organic active ingredientsMicrobiological testing/measurementGynecologyHuman Females
The present invention is directed to a method of using dehydroepiandrosterone to treat a human female with diminished ovarian reserve. The method includes administering about 25 milligrams three times a day of dehydroepiandrosterone per day to the female for at least four weeks to reduce human embryo aneuploidy. The present invention further is directed to a method of treating a human female with diminished ovarian reserve to improve the female's diminished ovarian reserve.
Owner:AMERICAN INFERTILITY OF NEW YORK
Apparatus and method for pelvic floor repair in the human female
A prosthesis for addressing pelvic organ prolapse in females comprises a frame fabricated from a shape memory material that supports a thin, flexible sheet in a stretched condition when the frame is unconstrained. The frame is shaped so as to conform to and be supported by bone structures and muscle tissue in the pelvic basin while providing needed support to pelvic organs to maintain them in a proper position. The use of a shape memory material allows the prosthesis to be rolled or folded into a reduced size for ease of placement through a small incision in the wall of the vagina, but that springs back to its memorized shape following deployment from a delivery sheath.
Owner:MINNESOTA MEDICAL DEV
Method of improving cumulative embryo score and quantity of fertilized oocytes, increasing euploidy rate and of normalizing ovarian function using an androgen such as dehydroepiandrosterone
A method of improving cumulative embryo score may comprise administering an androgen to a human female, for example, DHEA, for at least about four consecutive months followed by harvesting and fertilizing oocytes and forming embryos. Between about 50 mg and about 100 mg of DHEA may be administered to a human female per day. Moreover, a method of increasing the quantity of fertilized oocytes in one cycle of in vitro fertilization may comprise administering an androgen to a human female for at least about four consecutive months, harvesting and fertilizing the oocytes. Furthermore, a method of increasing the quantity of day 3 embryos from one cycle of in vitro fertilization may comprise administering an androgen for at least about four consecutive months, harvesting and fertilizing the oocytes and forming day 3 embryos. A method of normalizing ovarian DHEA also may include administering an androgen for at least about four consecutive months. A method of increasing the euploidy rate in embryos may include administering an androgen for at least about four consecutive weeks.
Owner:AMERICAN INFERTILITY OF NEW YORK
Determining the estimated date of embryo implantation and related dates
InactiveUS20050196812A1Biological testingSpecial data processing applicationsHuman FemalesChorionic gonadotrophin
A method of determining the estimated date of implantation of an embryo may include testing a sample of a body fluid from a pregnant human female subject so as to obtain data indicative of the concentration of human chorionic gonadotrophin (hCG) in the sample, and calculating an estimated date of implantation from the hCG concentration data.
Owner:INVERNESS MEDICAL SWITZERLAND GMBH
Androgen treatment in females
ActiveUS20100113407A1Improve human folliculogenesisBiocideOrganic active ingredientsHuman FemalesPhysiology
The present invention is directed to a method of using dehydroepiandrosterone (DHEA) to treat a human female with diminished ovarian reserve. The method includes measuring a baseline follicle stimulating hormone (FSH) level of the human female, and when the baseline FSH level is below about 40.0 mIU / ml, administering about 75 milligrams of DHEA per day to the female for at least four months to treat ovarian follicles in at least one ovary of the female to improve human folliculogenesis during the at least four months. The present invention further is directed to a method of restoring the ovarian environment of an older human female to that of a younger human female. The method includes administering about 75 milligrams of DHEA per day to the female for at least four months.
Owner:AMERICAN INFERTILITY OF NEW YORK
Androgen treatment in females
The present invention is directed to a method of improving ovarian reserve in a human female with diminished ovarian reserve as measured by the female's anti-Müllerian hormone level. The method may include evaluating a first anti-Müllerian hormone level of the female, administering dehydroepiandrosterone to the female for at least about one month, and evaluating a second anti-Müllerian hormone level of the female, wherein the second anti-Müllerian hormone level is greater than the first anti-Müllerian hormone level.
Owner:AMERICAN INFERTILITY OF NEW YORK
Methods for Assessing Risk of Developing Breast Cancer
ActiveUS20160222469A1Nucleotide librariesMicrobiological testing/measurementGenetic riskHuman Females
The present disclosure relates to methods and systems for assessing the risk of a human female subject for developing breast cancer. In particular, the present disclosure relates to combining clinical risk assessment and genetic risk assessment to improve risk analysis.
Owner:GENETIC TECHNOLOGIES LIMTIED
Detection of impaired fertility
InactiveUS20050130311A1Analysis using chemical indicatorsAnalysis by subjecting material to chemical reactionHuman FemalesAnalyte
Disclosed is a method of detecting a retarded cycle in a human female subject experiencing same, the method comprising the steps of obtaining a sample of body fluid from the subject on each of a plurality of, but not less than three, days; testing each of the plurality of samples to determine the concentration therein of at least one analyte of significance in the ovulatory cycle; comparing a result determined from said testing with a predetermined threshold value; and, if said determined result is different from the threshold value; declaring the cycle, during which the samples were taken, to be a retarded cycle.
Owner:INVERNESS MEDICAL SWITZERLAND GMBH
Device for sizing a human female breast
InactiveUS7955274B2Reduce weight and expenseEasy to cutMammary implantsPerson identificationHuman FemalesGynecology
A device for intraoperative sizing of a human female breast during resizing and / or shaping of the breast composes: a (a) substantially hemispherical, rigid, cup-shaped foraminous body, i.e., a foraminous cup, defining a desired size and shape of the breast; and (b) a rim member to which the foraminous cup is affixed.
Owner:ACCURATE SURGICAL & SCI INSTR CORP
Pregnancy test device & method
ActiveUS20180088136A1Avoid false negative resultsImprove the level ofBiological material analysisBiological testingObstetricsPregnancy test
Disclosed is a test device to detect pregnancy In a human female subject, the test device comprising: an assay means to measure the absolute or relative amount of hCG m a sample from the subject; an assay means to measure the absolute or relative amount of FSH in a sample from the subject; and m assay means to measure the absolute or relative amount of one or mere progesterone metabolites I>> a sample from the subject.
Owner:SPD SWISS PRECISION DIAGNOSTICS
Determining the estimated date of embryo implantation and related dates
InactiveCN101147069ABiological material analysisBiological testingHuman FemalesChorionic gonadotrophin
A method of determining the estimated date of implantation of an embryo may include testing a sample of a body fluid from a pregnant human female subject so as to obtain data indicative of the concentration of human chorionic gonadotrophin (hCG) in the sample, and calculating an estimated date of implantation from the hCG concentration data.
Owner:INVERNESS SWITZERLAND GMBH
Detection of infertility risk and premature ovarian aging
ActiveUS20140206756A1Microbiological testing/measurementGenetic material ingredientsRegimenHuman Females
Method of early detection of risk of infertility and ovarian aging in and treatment of a human female who has not experienced infertility and is not otherwise indicated to have premature ovarian aging. A number of CGG repeats on each allele of the isolated FMR1 gene is measured by using an assay, and a testing regimen is performed only when the determined number of CGG repeats on one of the FMR1 gene alleles is less than 26. The testing regimen includes periodically measuring serum level of a hormone related to fertility, such as Anti-Müllerian Hormone, Follicle Stimulating Hormone and / or estradiol over a period of about three to eight years and, after each measurement, determining if the measured serum level is less than a set confidence interval for a human female of the same age of the female. If so, the human female is treated for premature ovarian aging.
Owner:THE FOUND FOR REPRODUCTIVE MEDICINE INC
Infant Development Toy
InactiveUS20140194034A1Optimal sucking motionPromote proper breathingDollsDomestic articlesControlled breathingHuman Females
A development toy that is made of soft, light BPA-free silicone and / or clear plastic that is one solid piece with no moving parts or attachments. The toy is intended to sooth an infant and / or toddler when frustrated from teething pains in their gum and oral area. The toy will be made in various different animal, bird, fish and machine shapes with appendages in the shape of a human female “mother's” nipple to provide the familiar sensation of sucking on mother's breast. The toy will be made in non-toxic soft materials, clear plastic and / or silicone resembling candy in bright soft pastels in a cartoon formed body with easily gripping areas. All parts of the toy provide a safe form of soothing and controlled breathing areas.
Owner:CASTANEDA ROBERT
Androgen treatment in females
ActiveUS8067400B2Maximize inductionIncreasing euploid number and rateBiocideOrganic active ingredientsHuman FemalesMiscarriage
A method of improving cumulative embryo score may comprise administering an androgen to a human female for at least about four consecutive months followed by harvesting and fertilizing oocytes and forming embryos. A method of increasing the quantity of fertilized oocytes in one cycle of in vitro fertilization may comprise administering an androgen to a human female for at least about four consecutive months, harvesting and fertilizing the oocytes. A method of normalizing ovarian DHEA may include administering an androgen for at least about four consecutive months. A method of decreasing the time to pregnancy and increasing the rate of pregnancy by administering an androgen for at least about two months. A method of decreasing miscarriage rates may comprise administering an androgen for at least about two months to a female. Moreover, a method of decreasing aneuploidy rates in human embryos may comprise administering an androgen to a female for at least about two months.
Owner:AMERICAN INFERTILITY OF NEW YORK
Method of improving ovulation induction using an androgen such as dehydroepiandrosterone
InactiveUS20060089308A1Maximize ovulation inductionBiocideOrganic active ingredientsLeuprorelinHuman Females
A method of preconditioning ovulation induction in a human female comprises of administering an androgen, for example, DHEA, for at least about four consecutive months. DHEA may be administered along with high dose gonadotrophins in ovulation induction treatments. Moreover, DHEA may be administered with follicle stimulating hormone, human menopausal gonadotrophin, norethindrone acetate, leuprolide acetate, and human chorionic gonadotrophin in ovulation induction treatments.
Owner:AMERICAN INFERTILITY OF NEW YORK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com