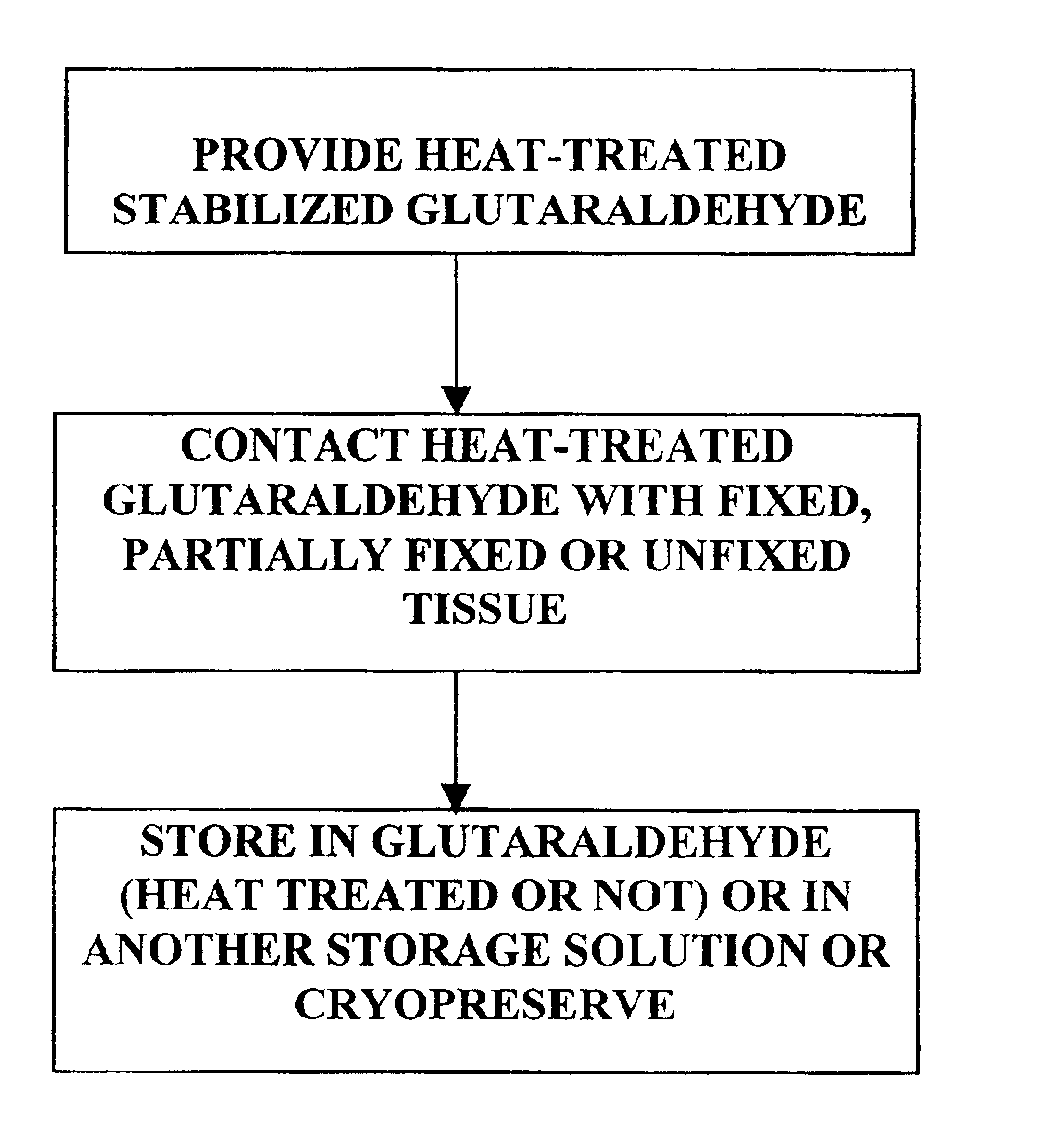
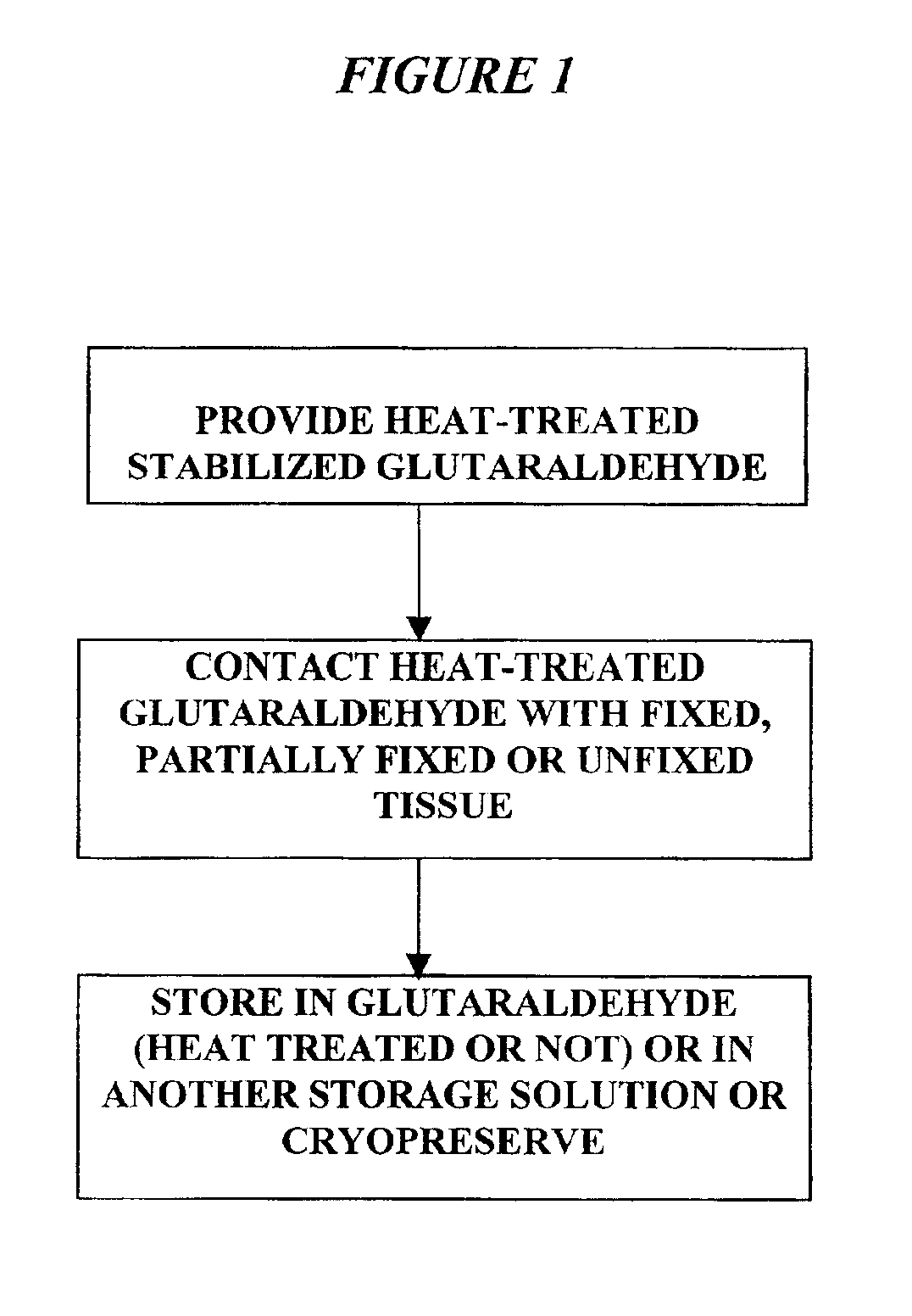
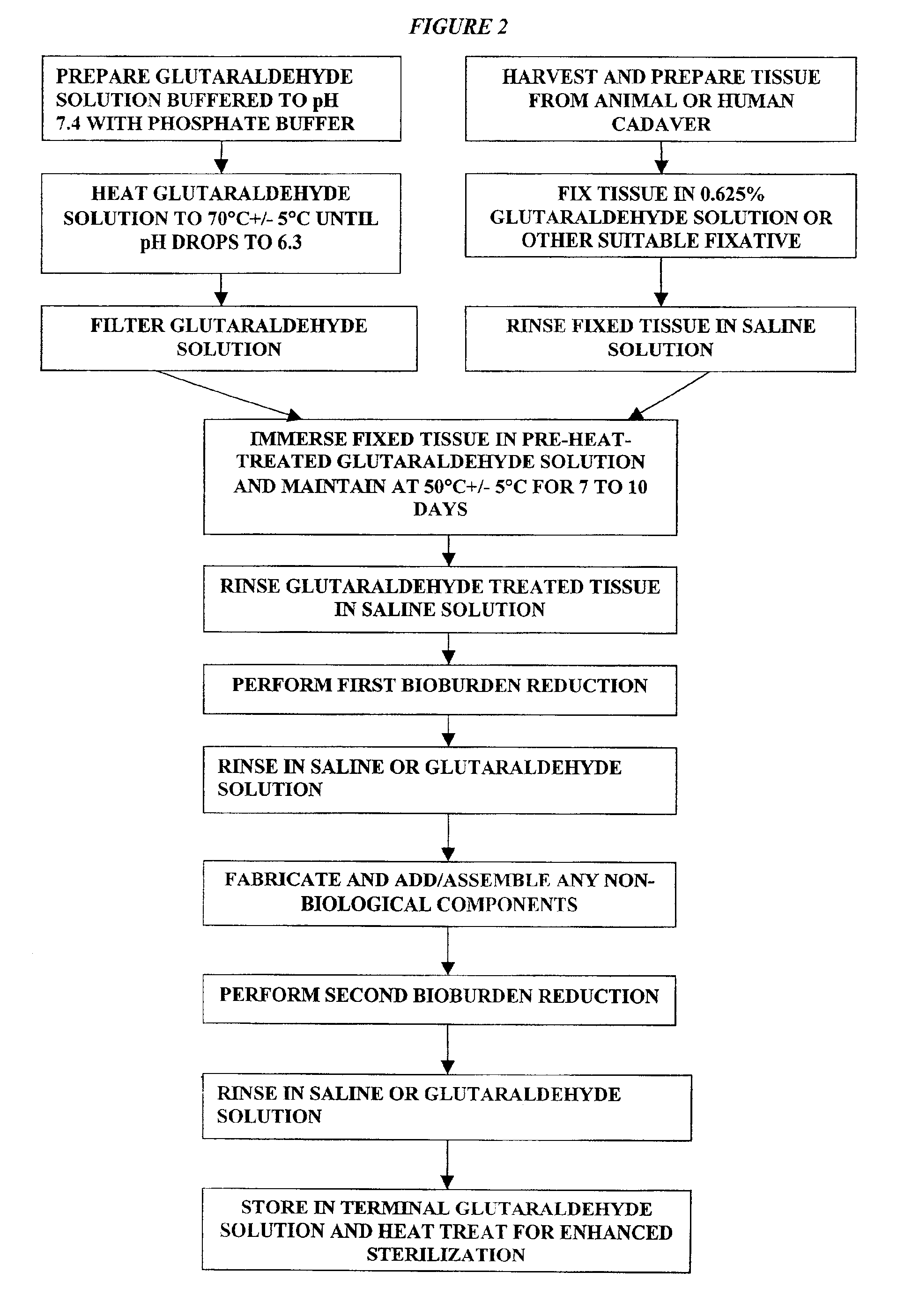
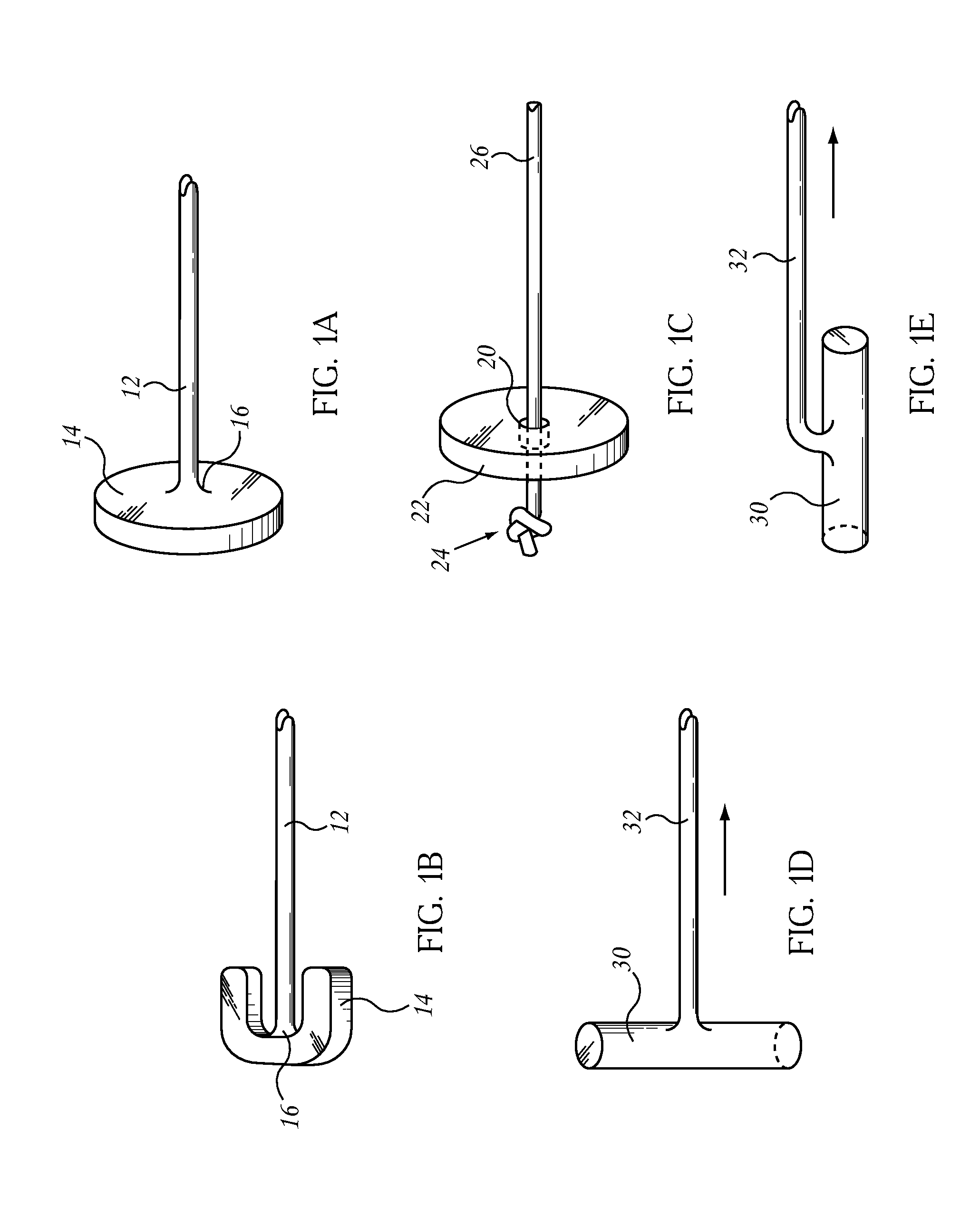
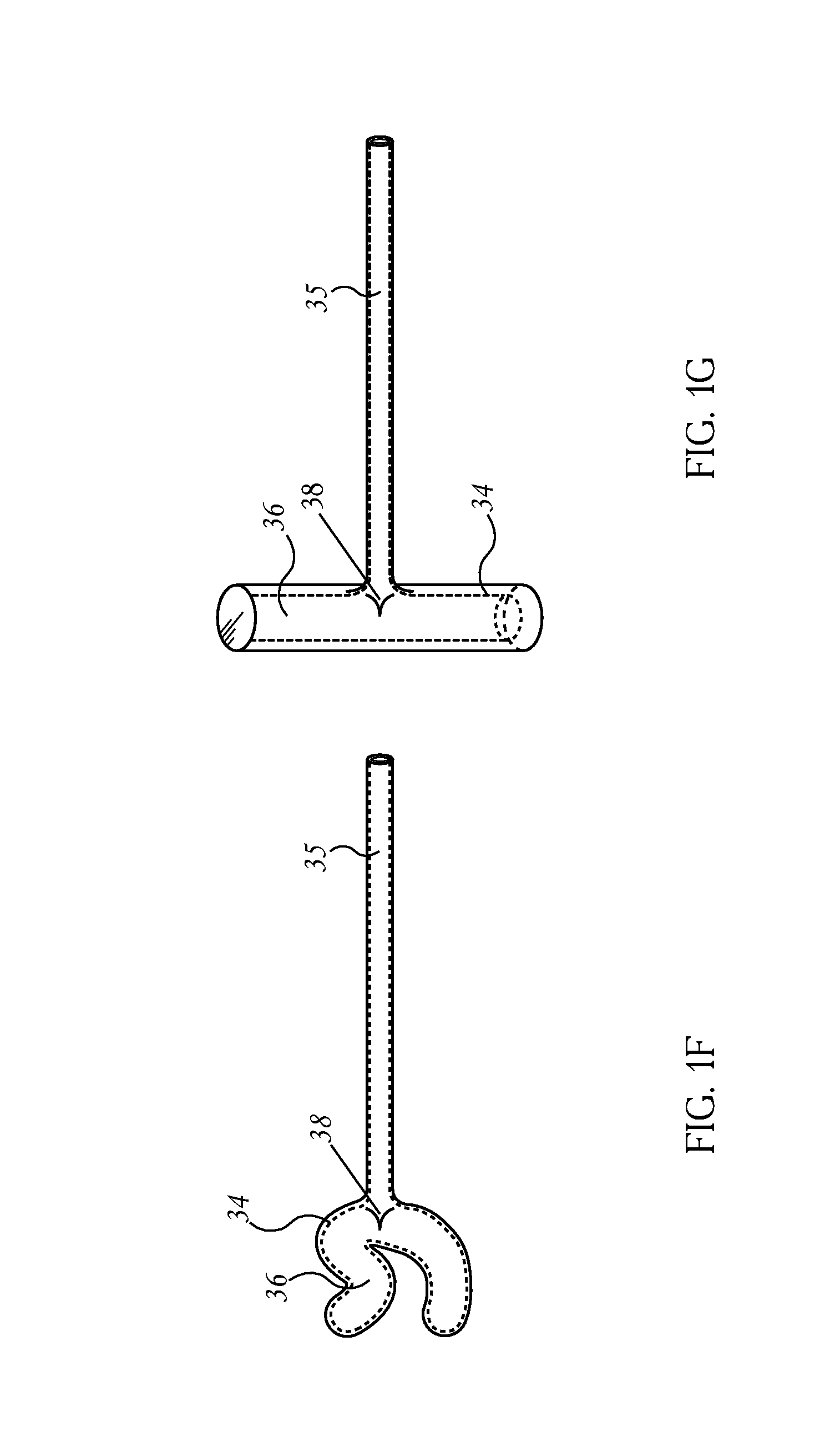
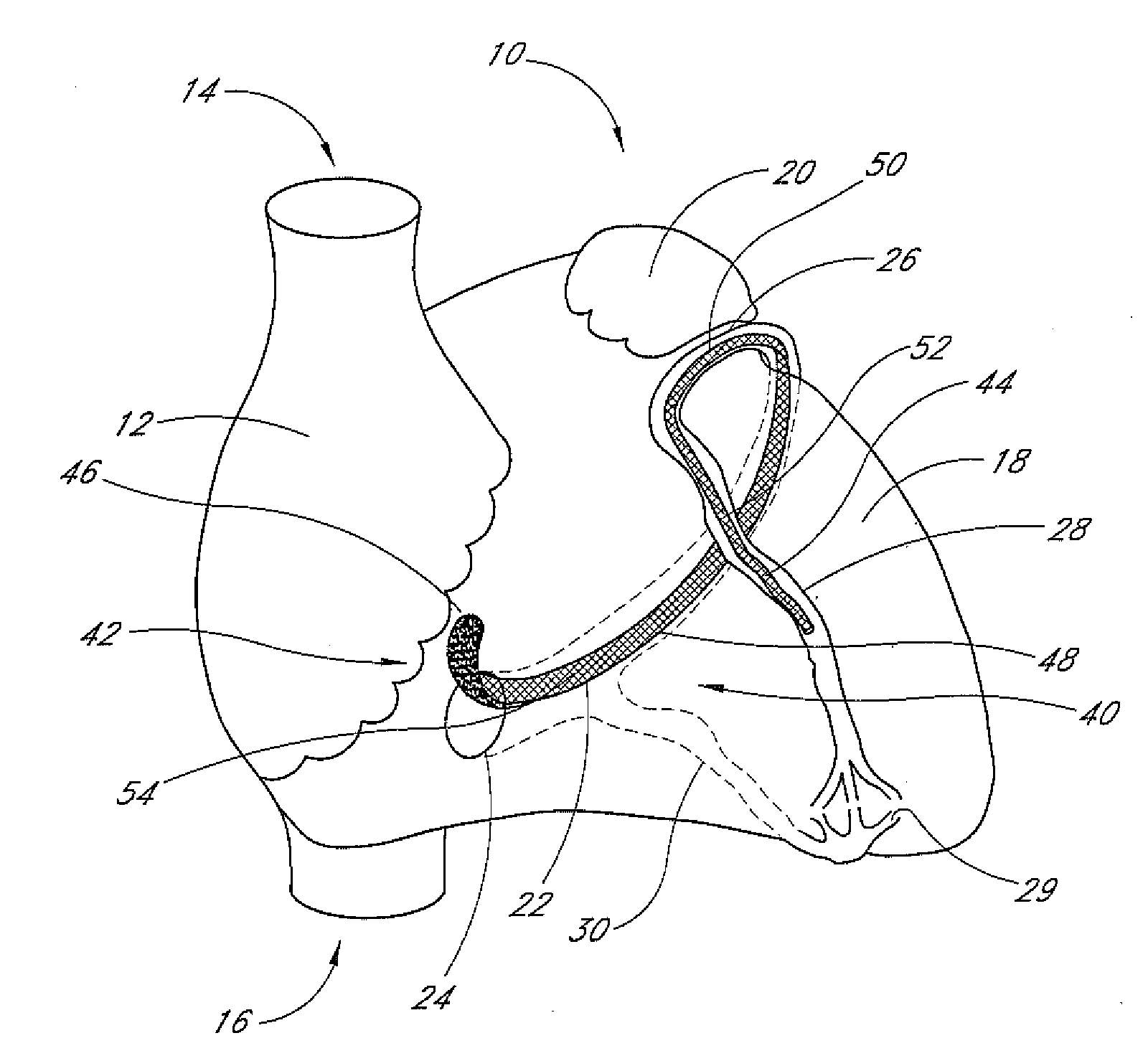
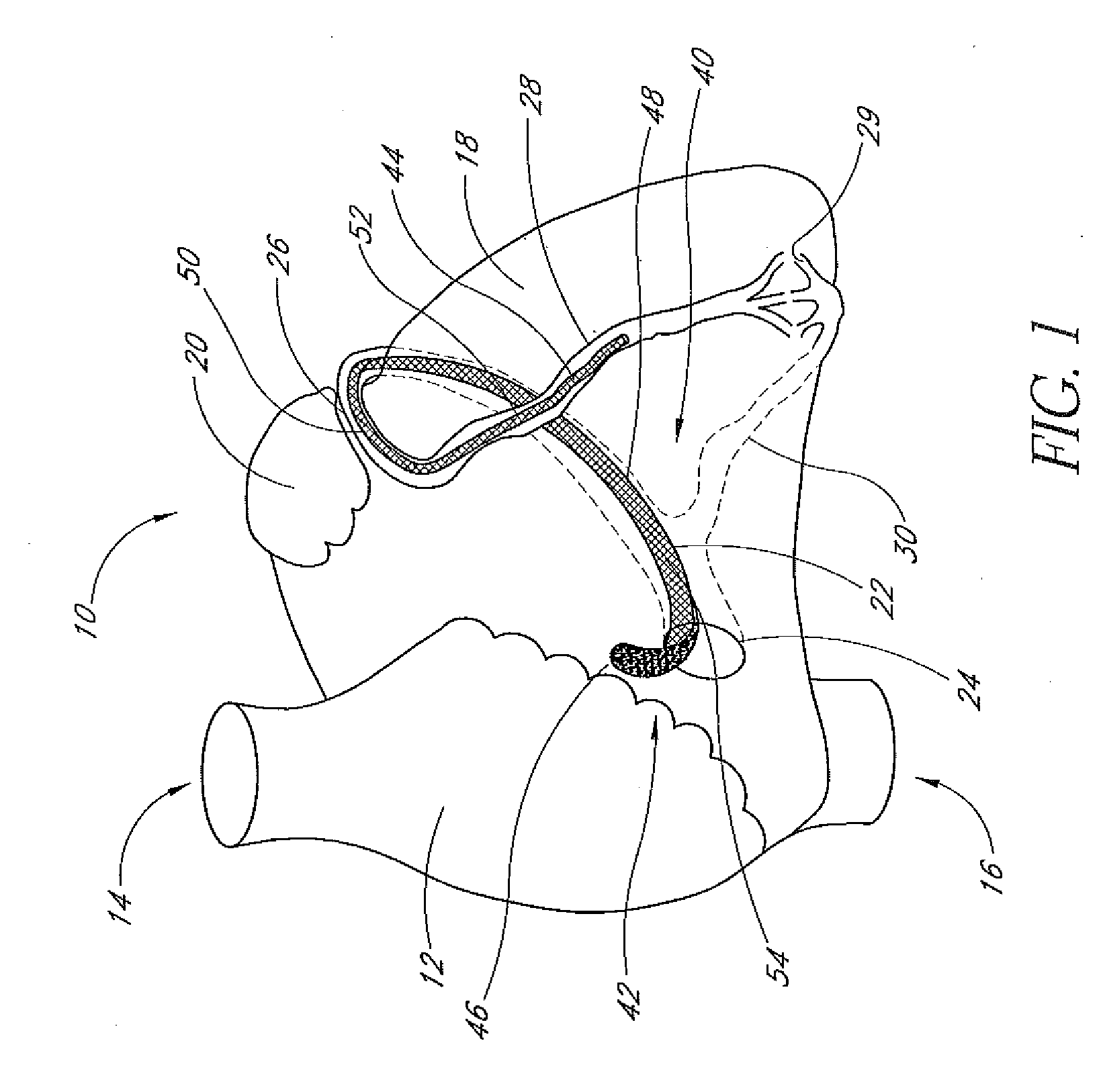
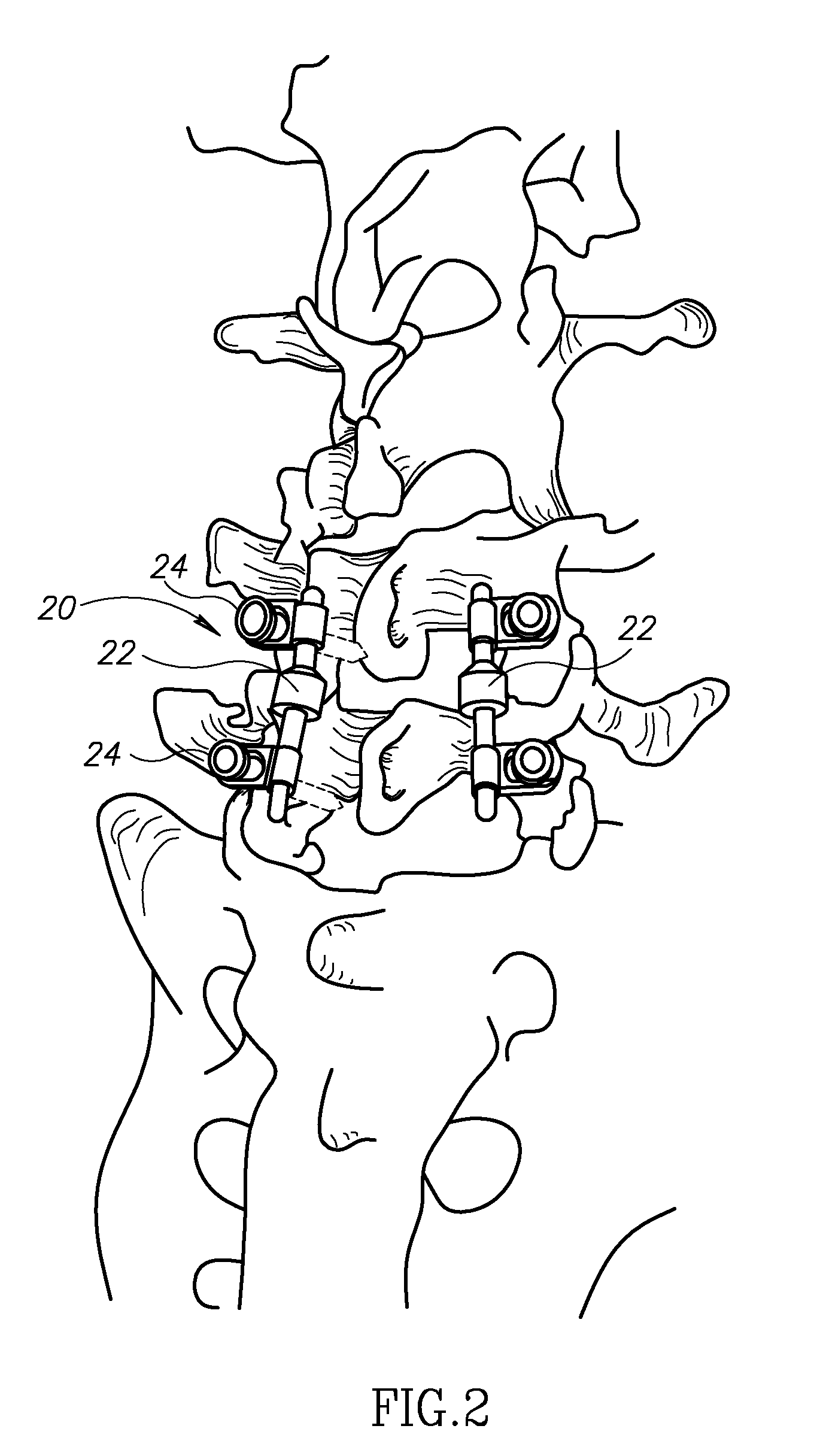Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
282 results about "Post implantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
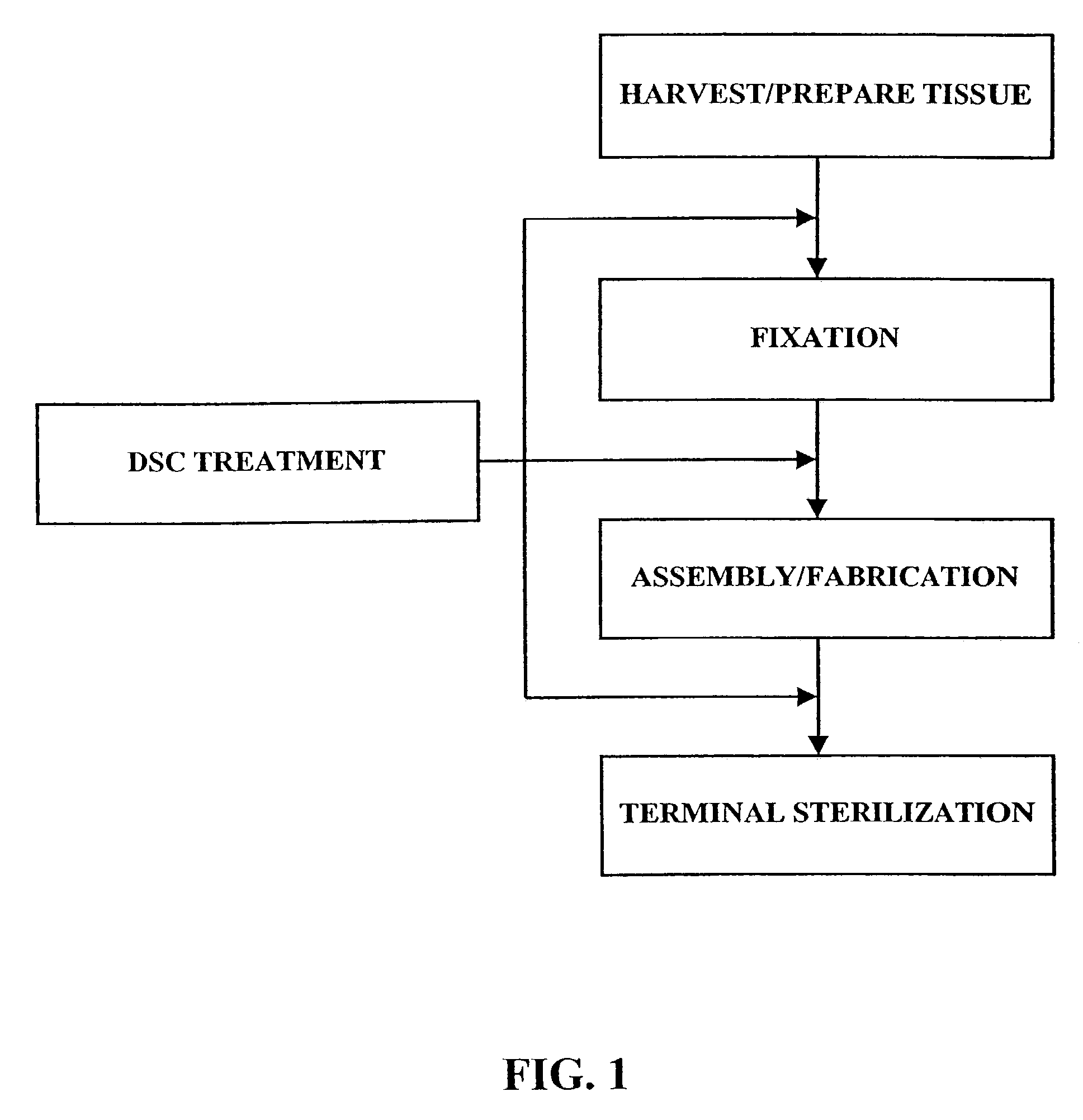
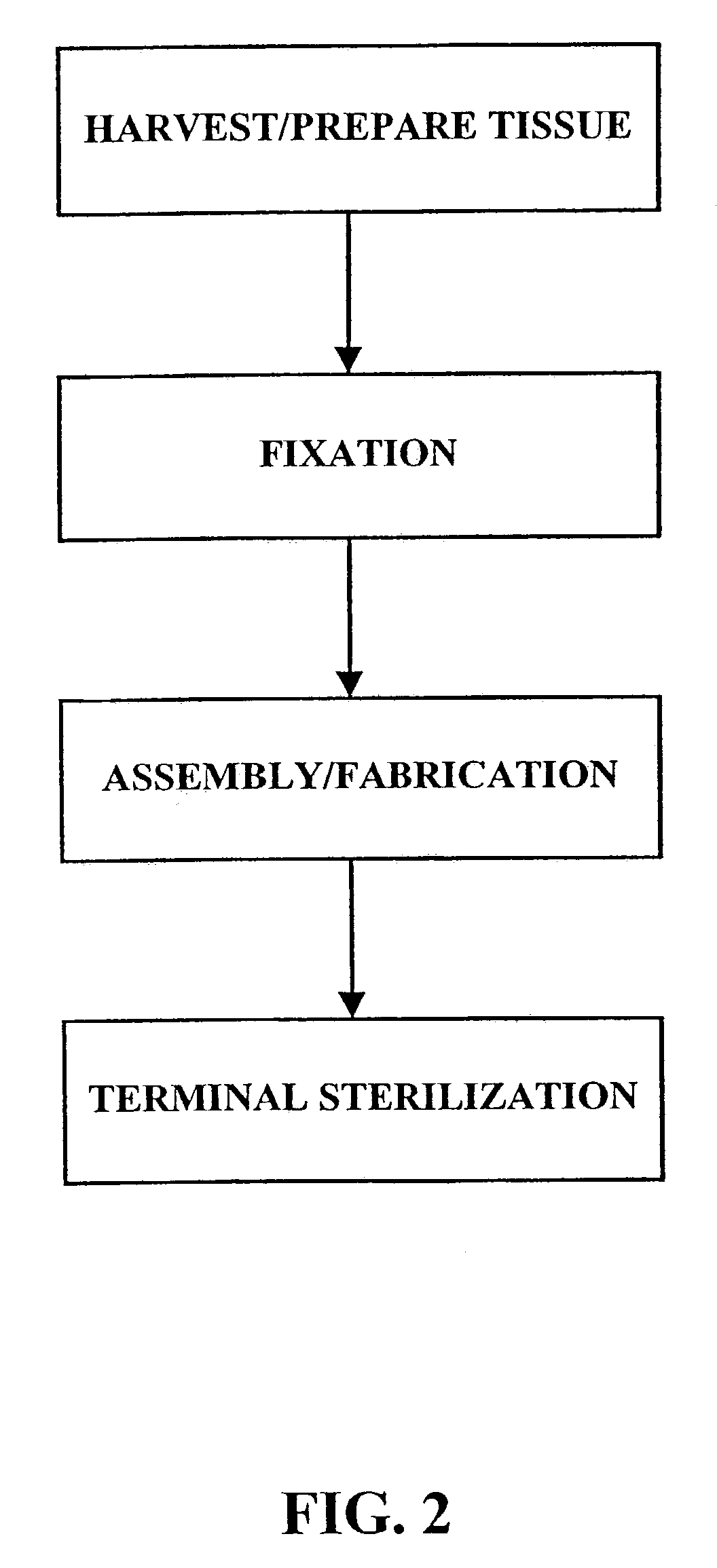
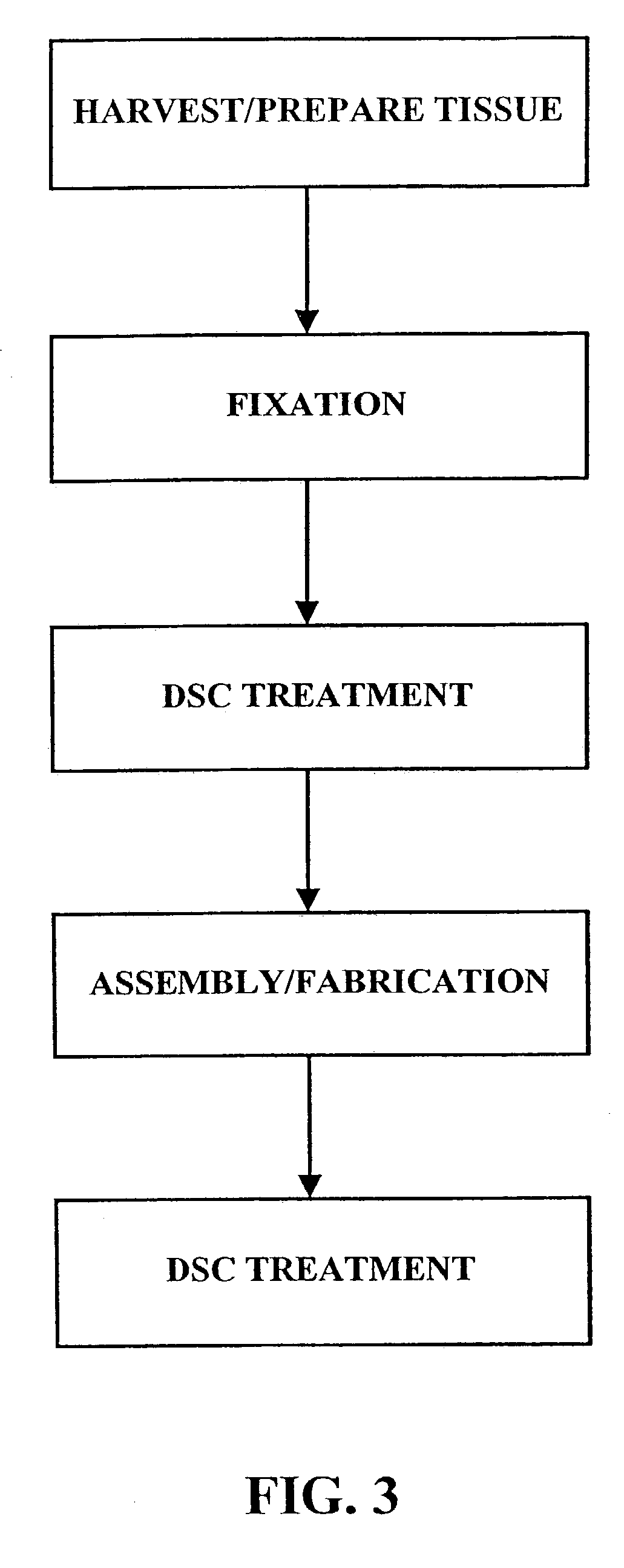
Implantation is the initial stage of pregnancy that occurs approximately on the 7-12 day post conception. The ovum cells divide a lot of times until it becomes a zygote, which is then attached to the uterine wall.
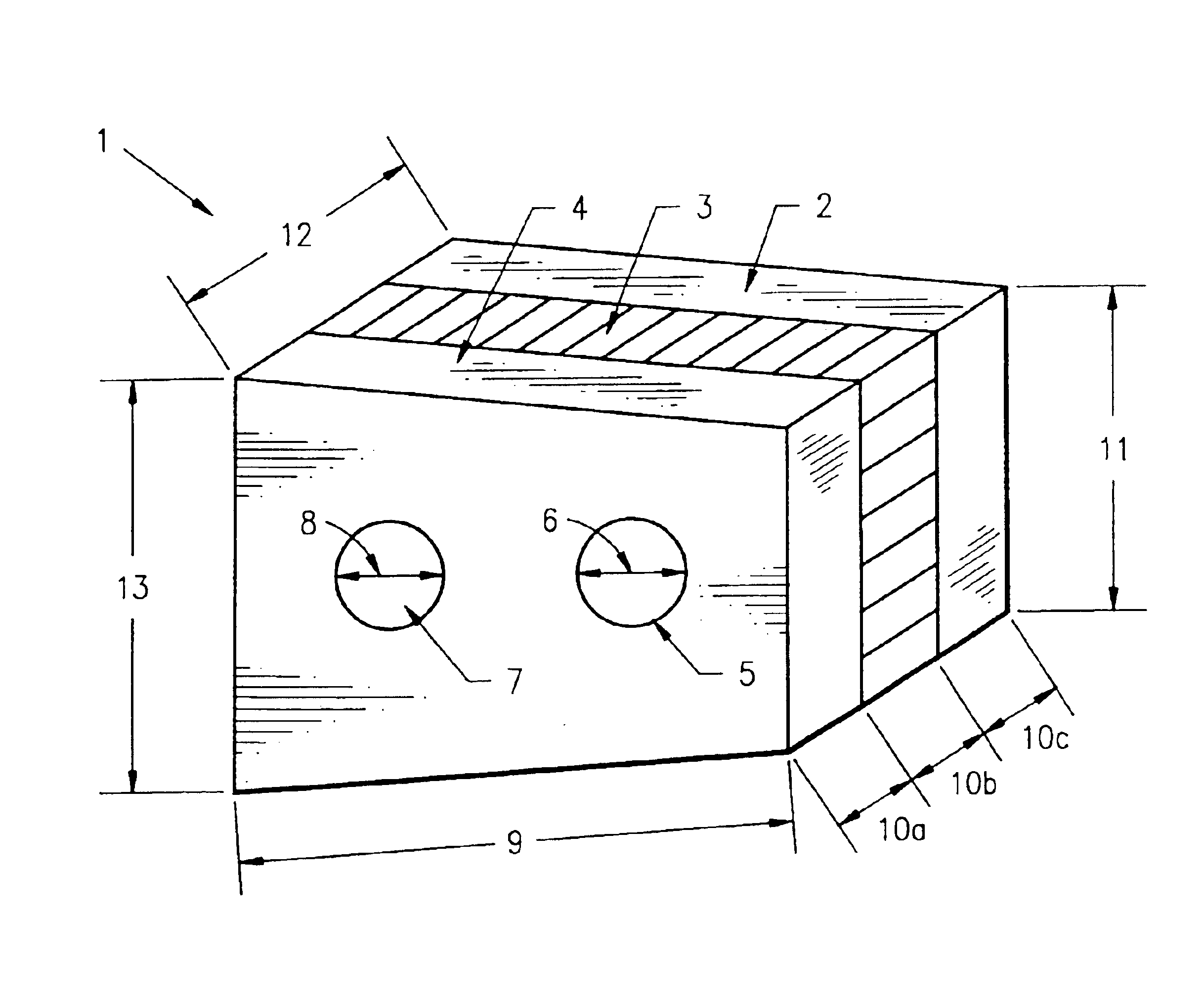
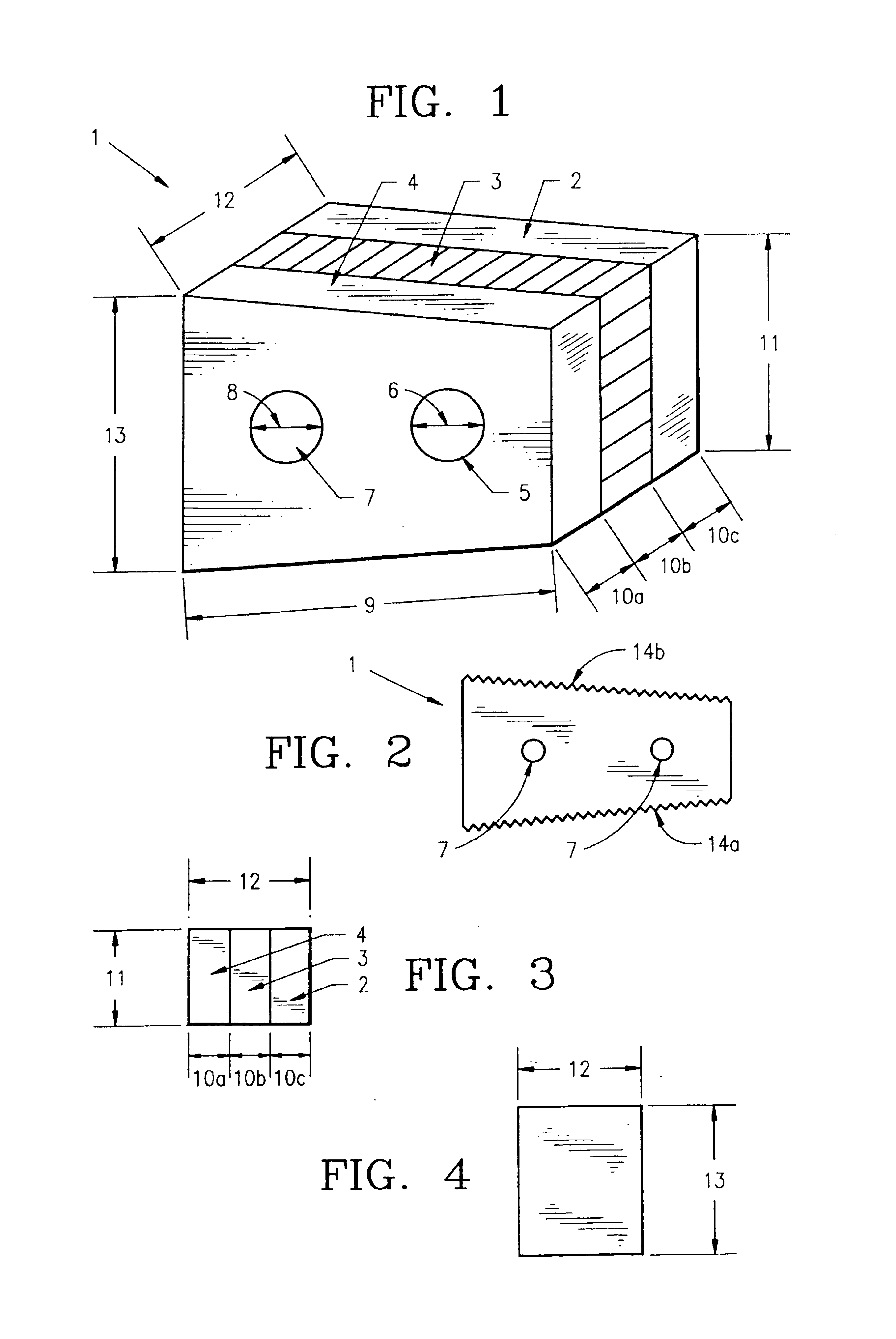
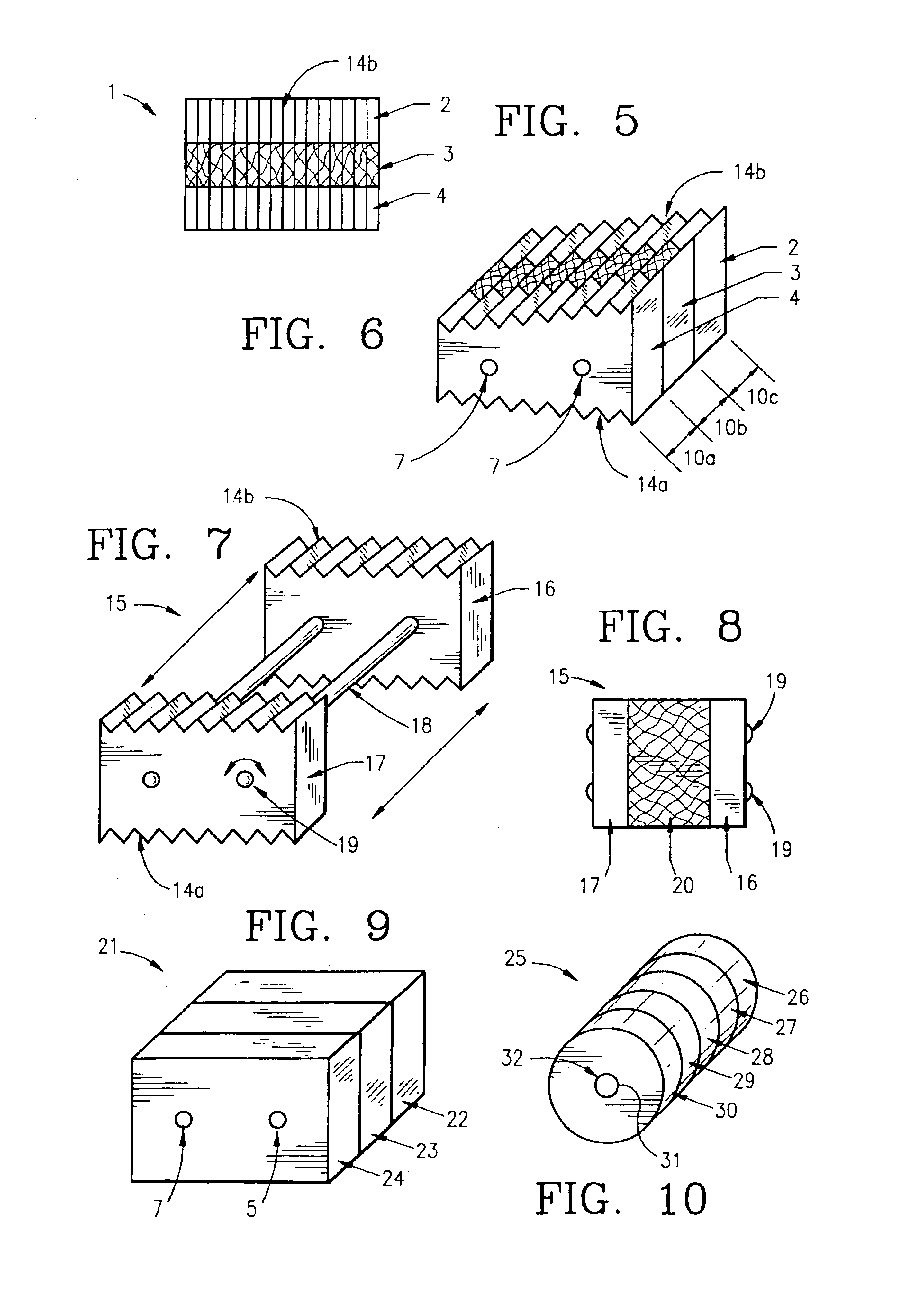
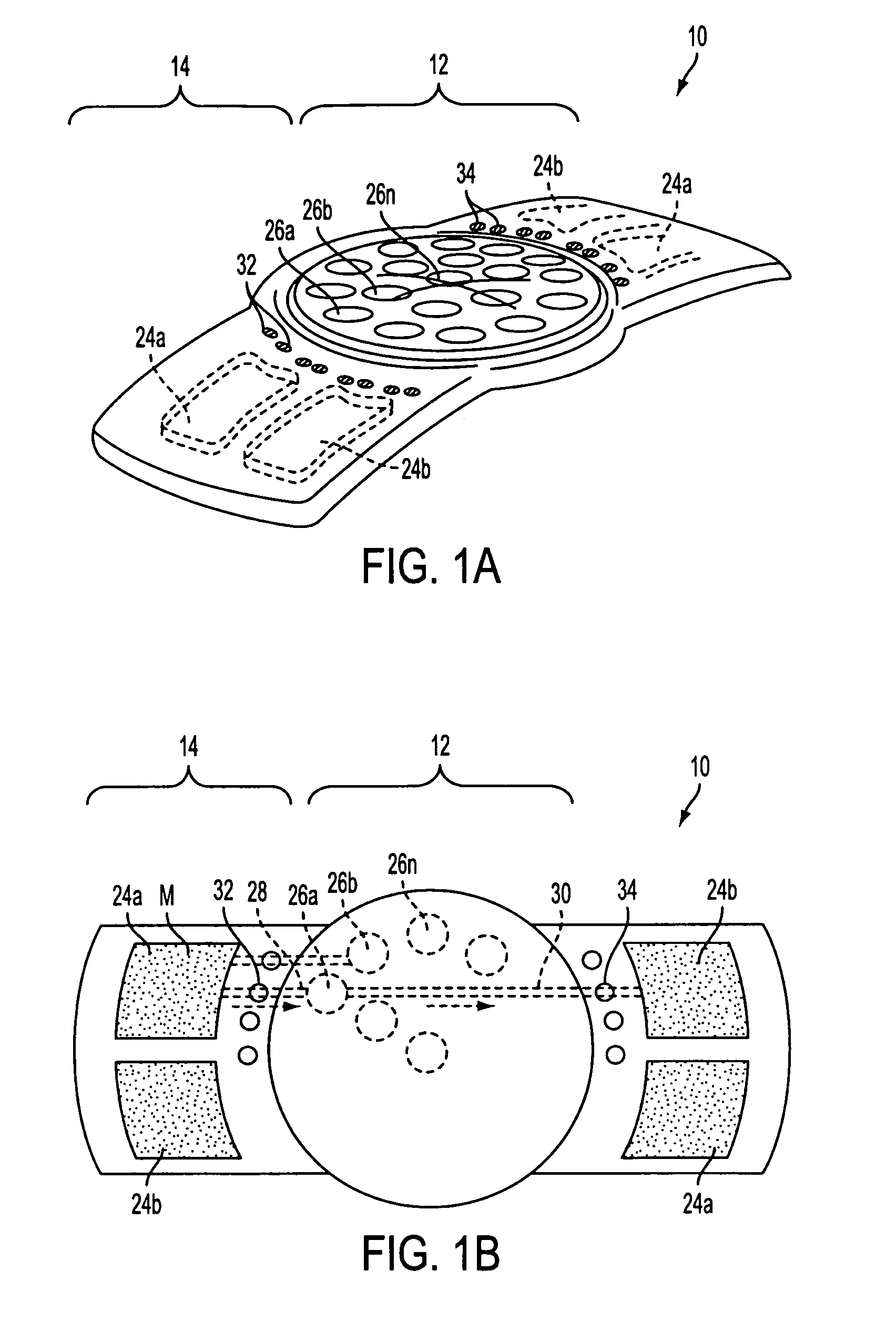
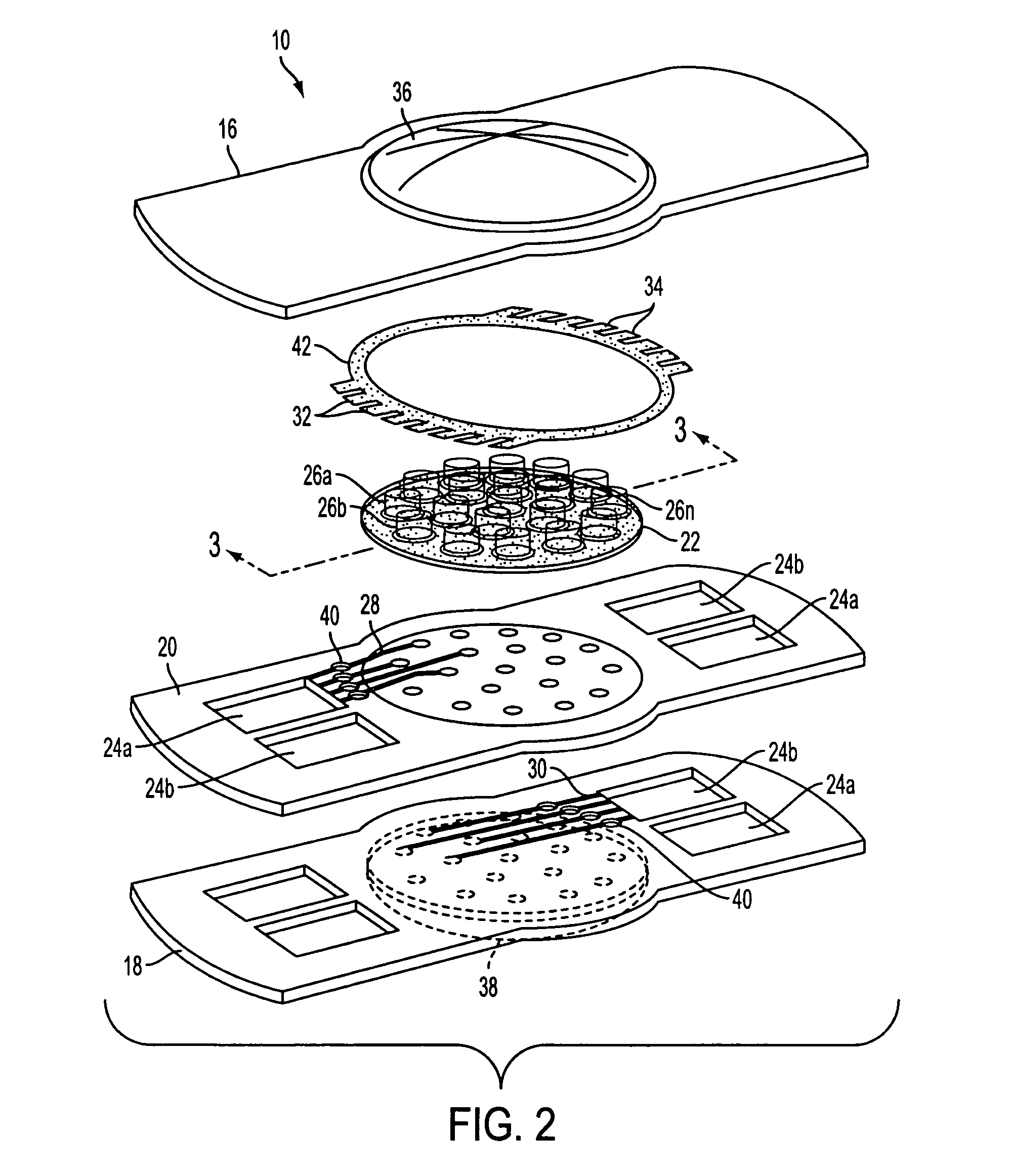
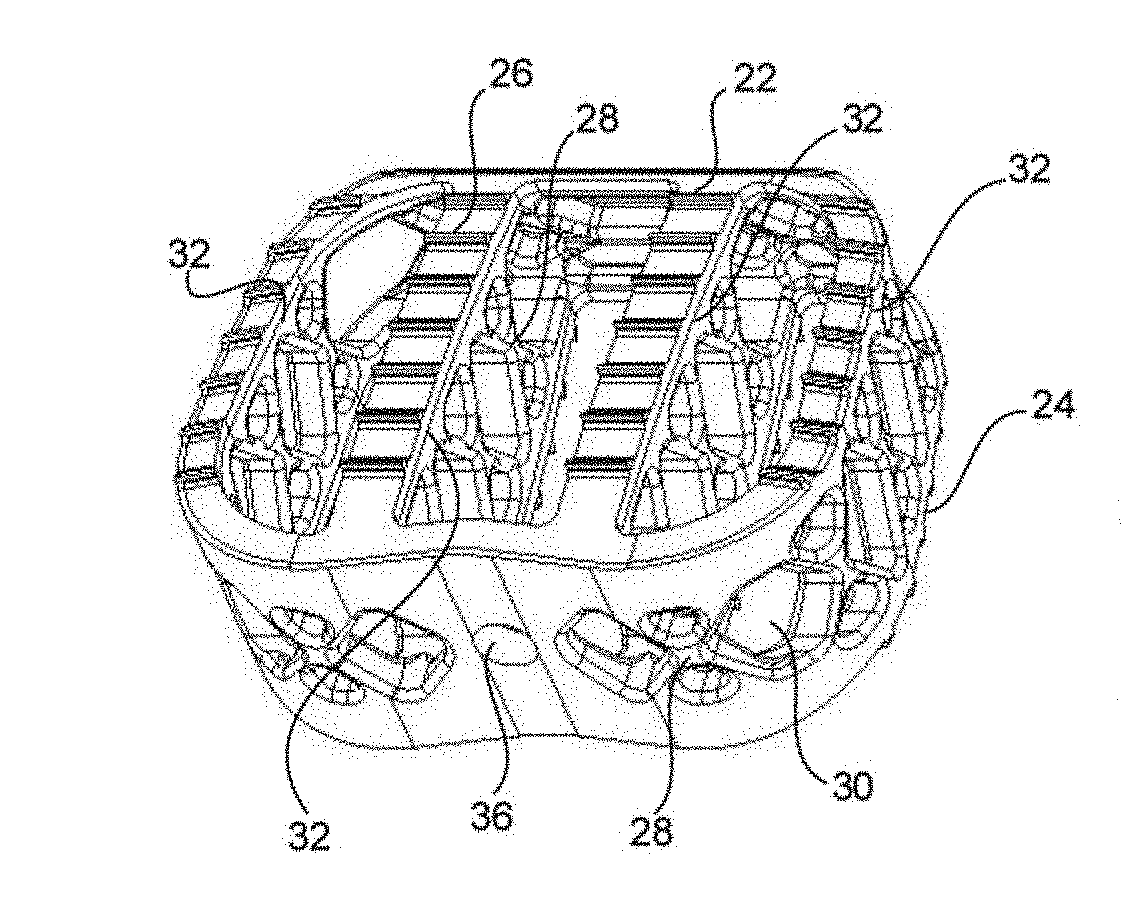
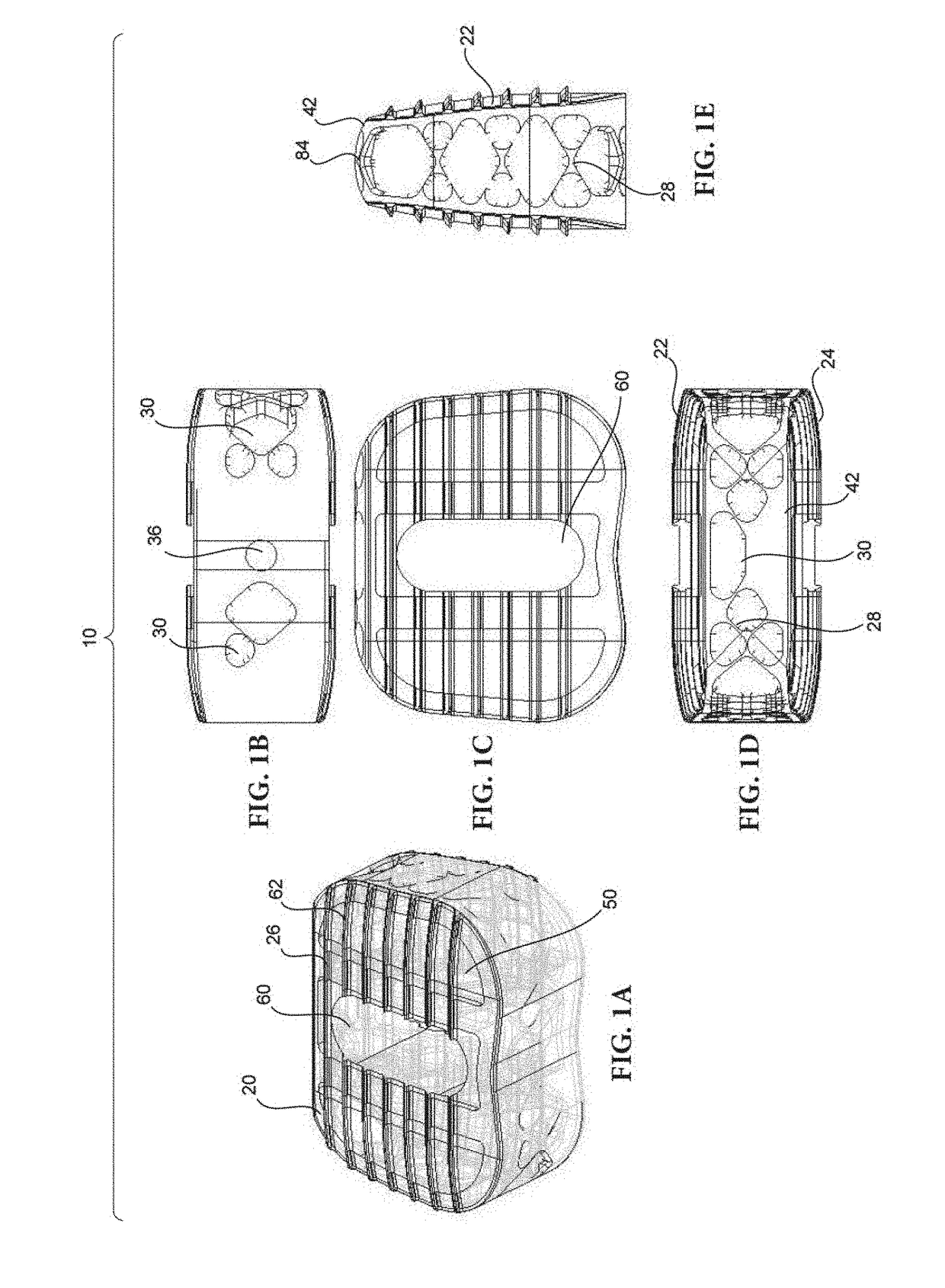
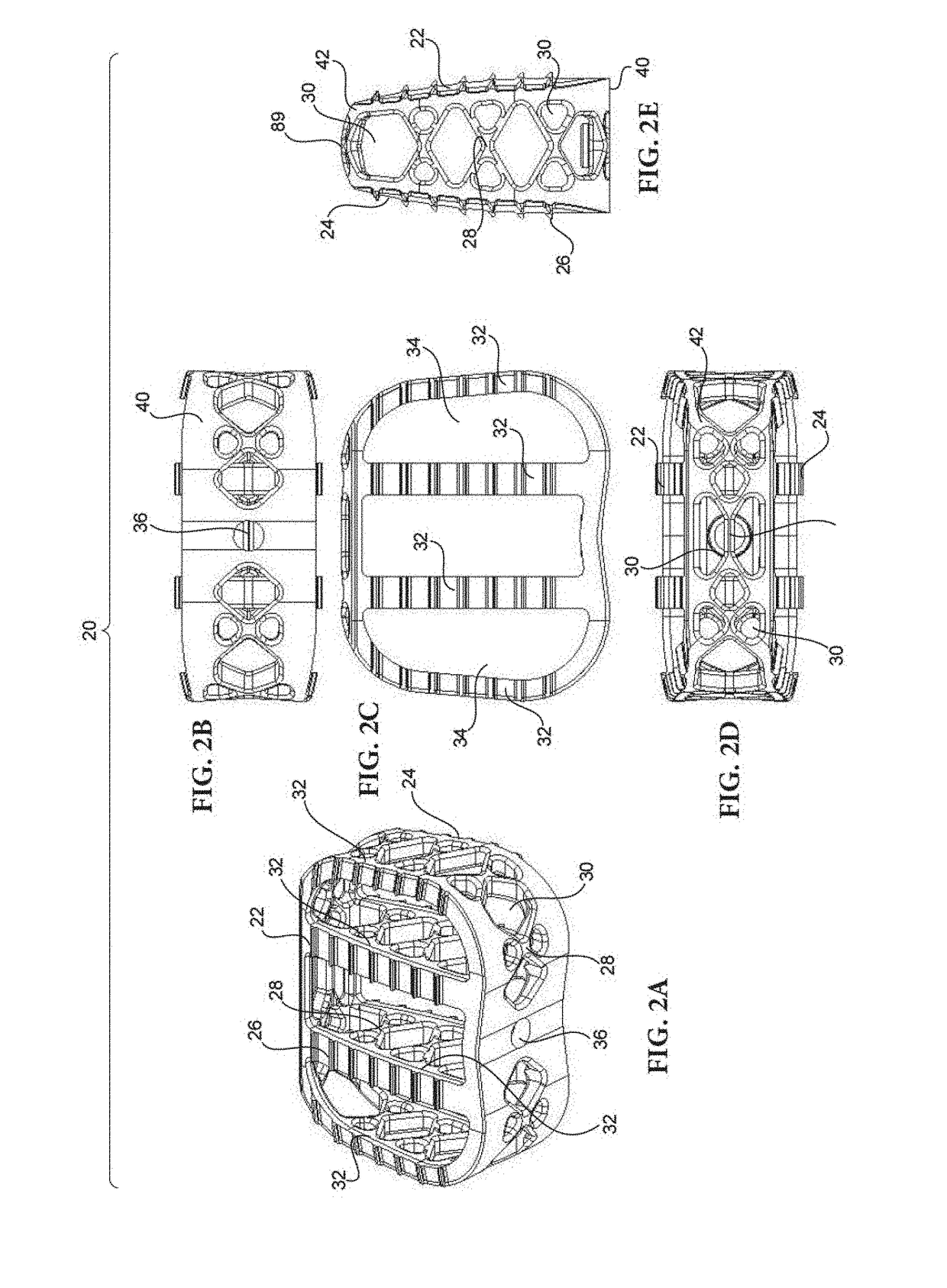
Method of forming an implantable device
ActiveUS9352071B2Greater and beneficial tissue ingrowthLow melting pointWeft knittingMedical devicesImplanted devicePliability
Owner:ETHICON INC
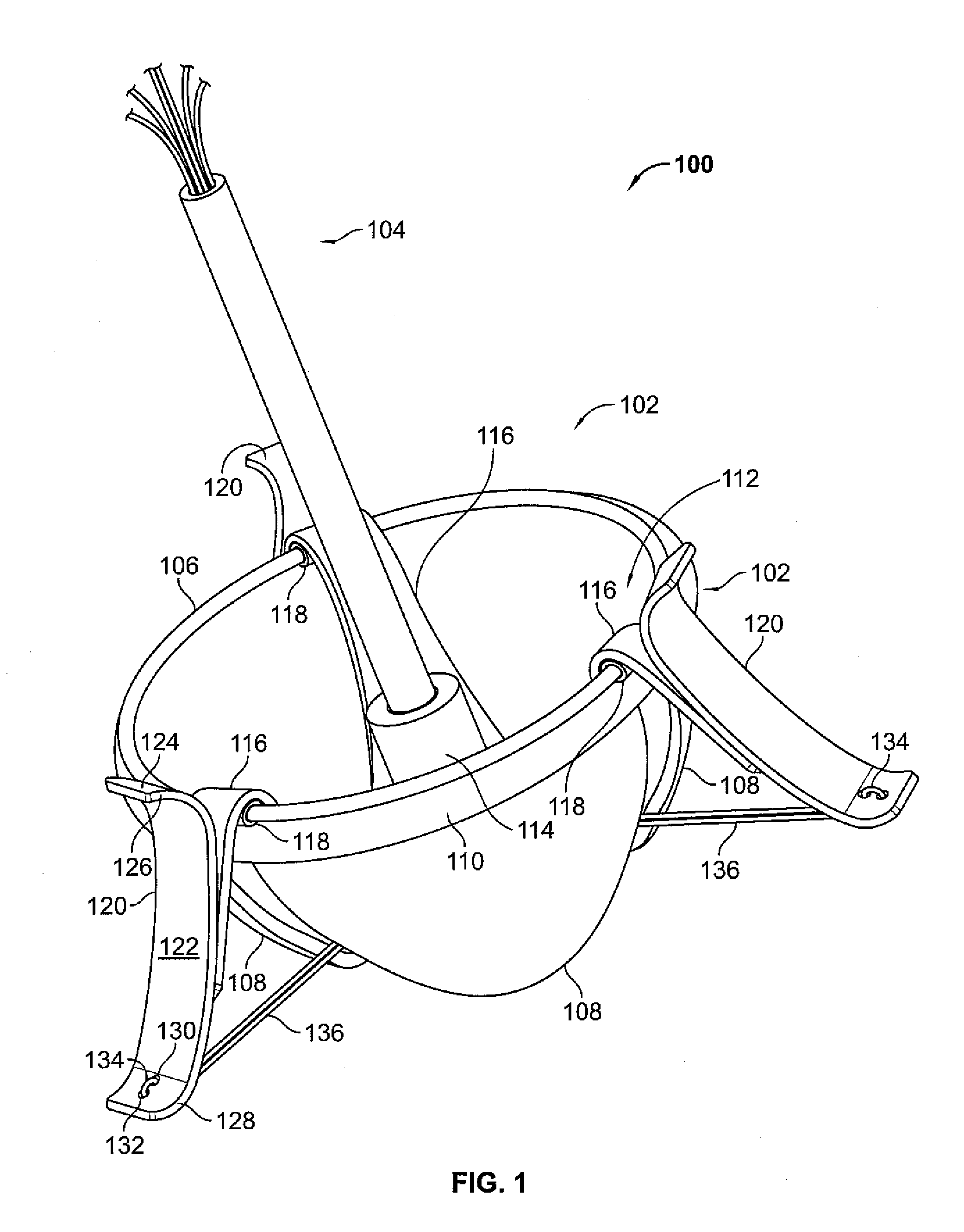
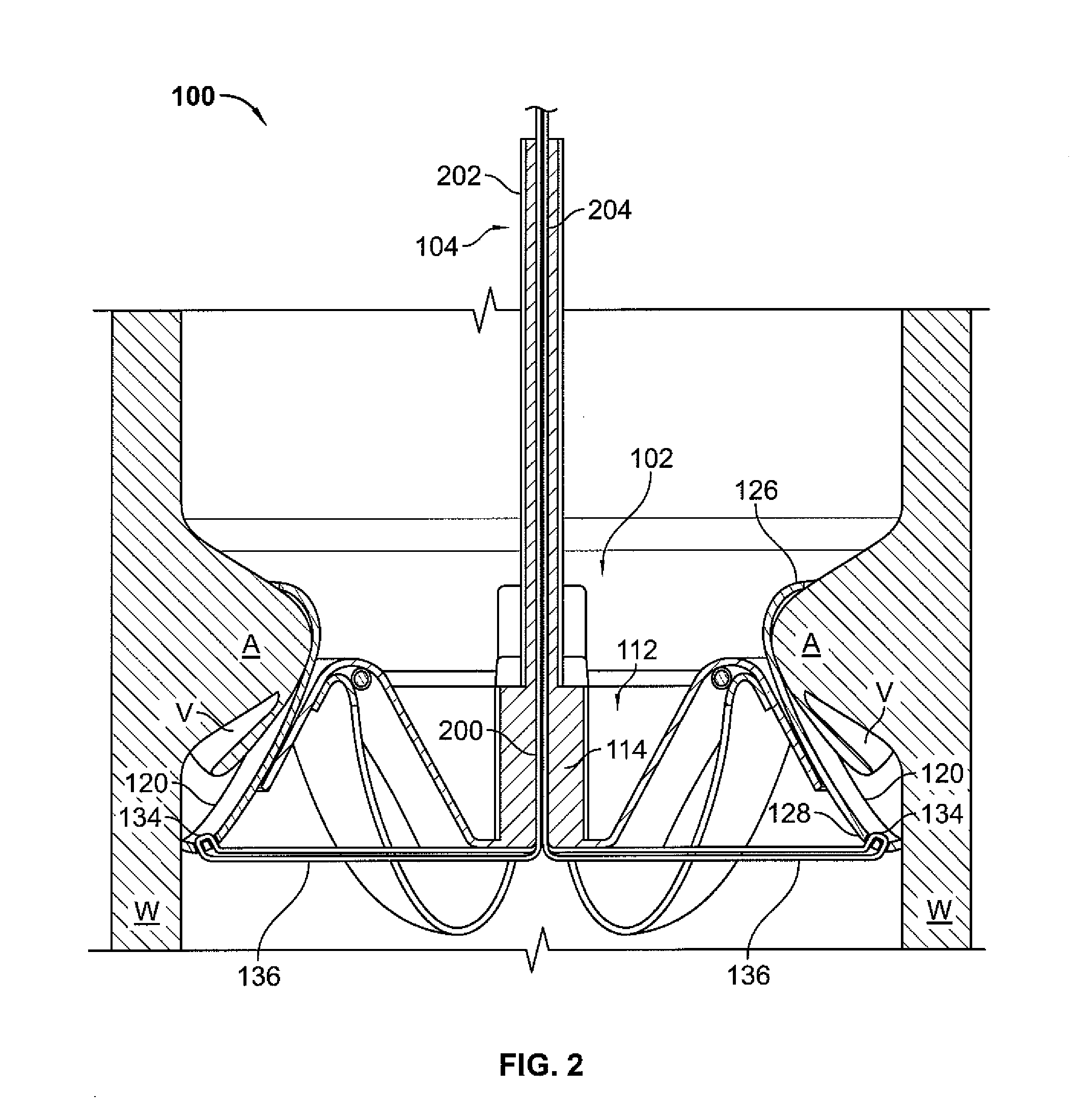
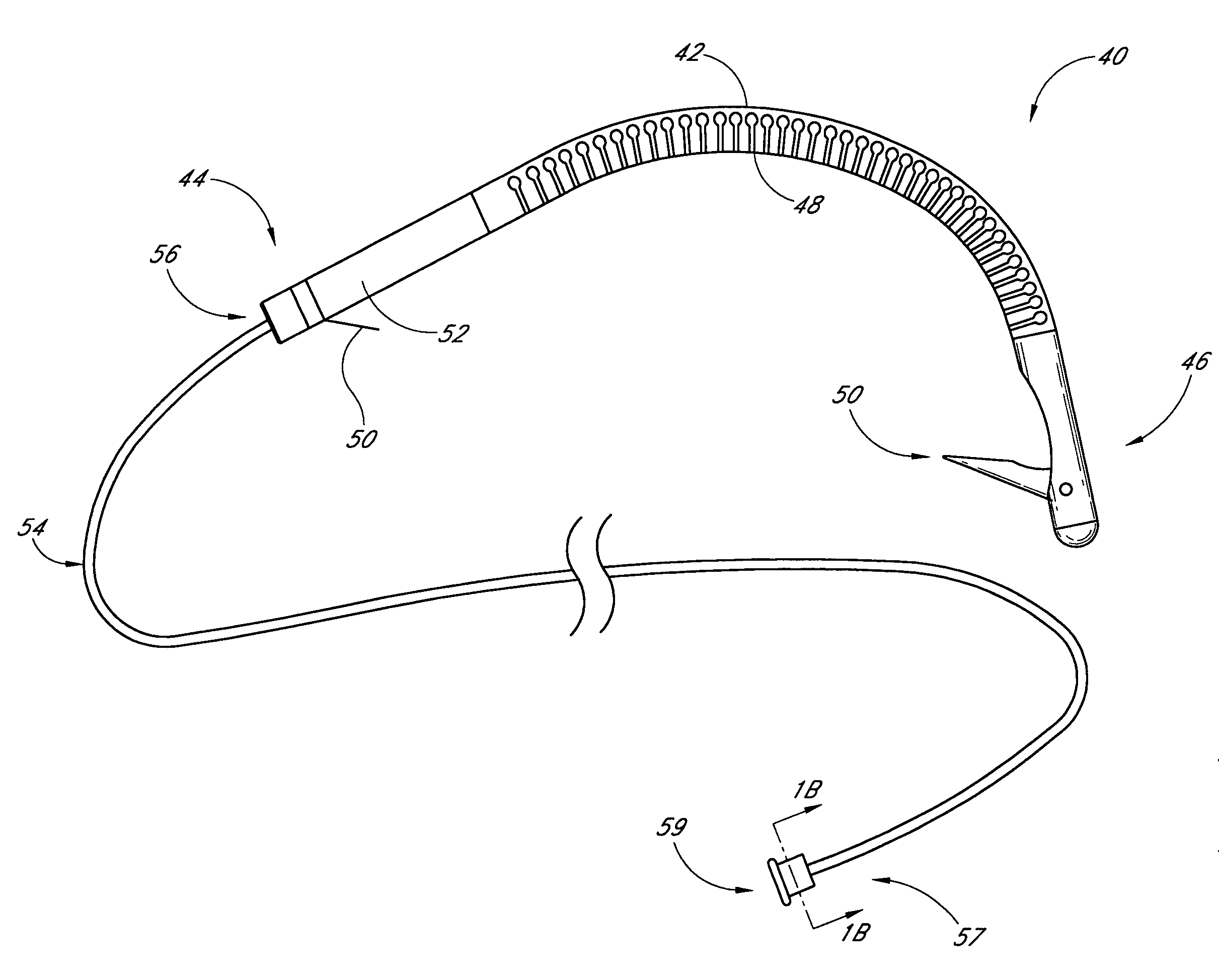
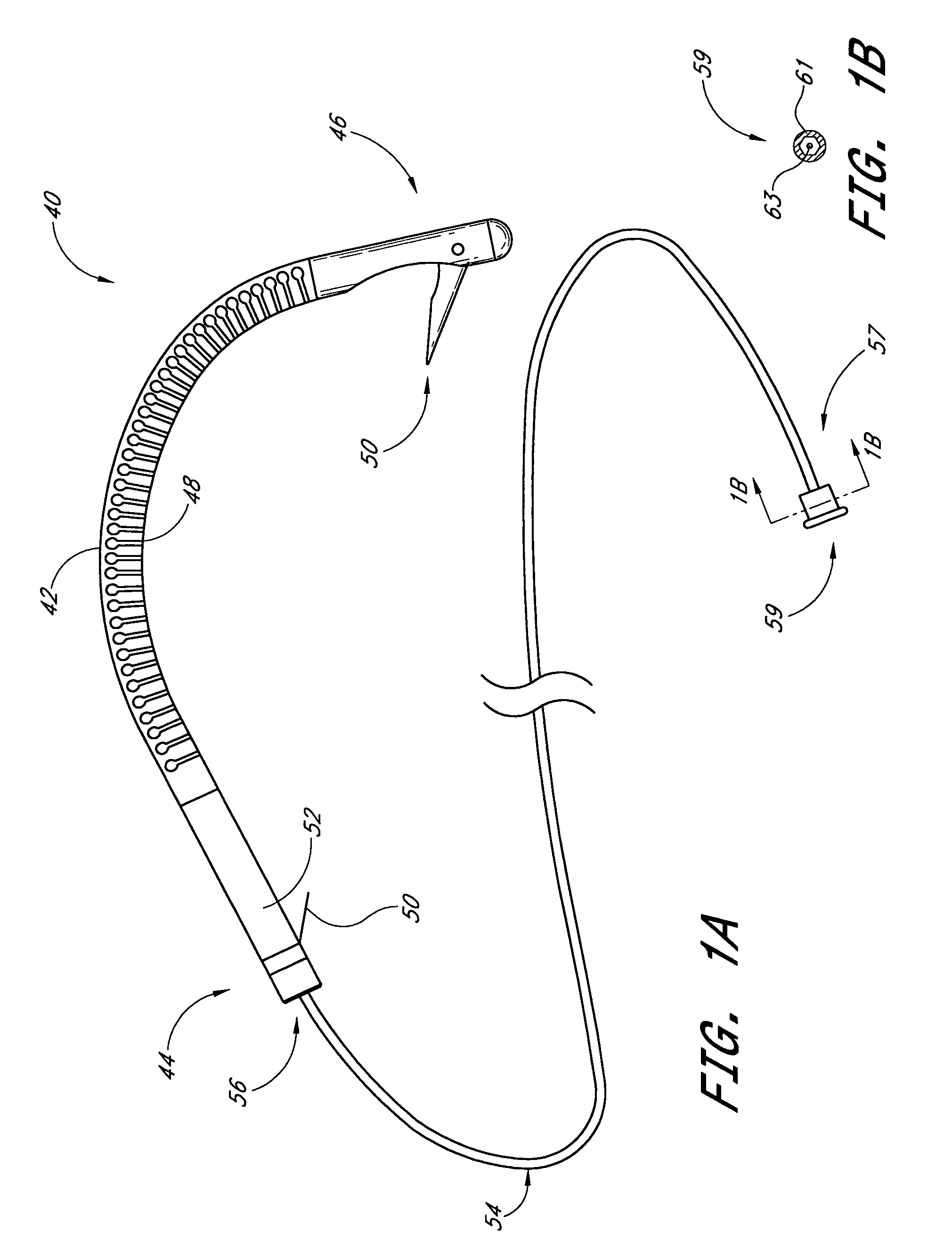
Systems and Methods For Placement of Valve Prosthesis System
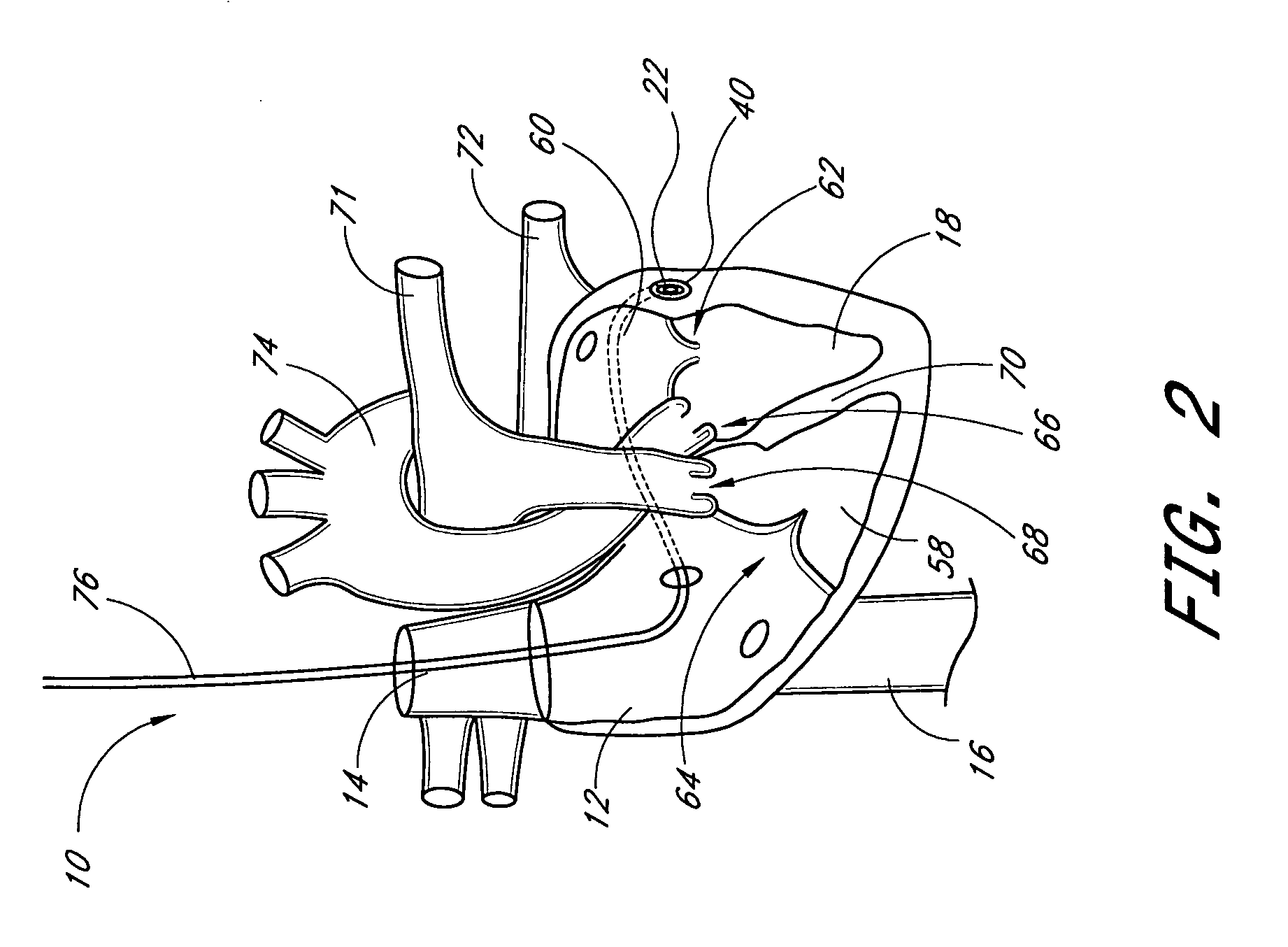
InactiveUS20080208328A1Facilitate efficient, reliable and minimally invasive delivery modalitiesReduce deliveryHeart valvesAnatomical structuresPost implantation
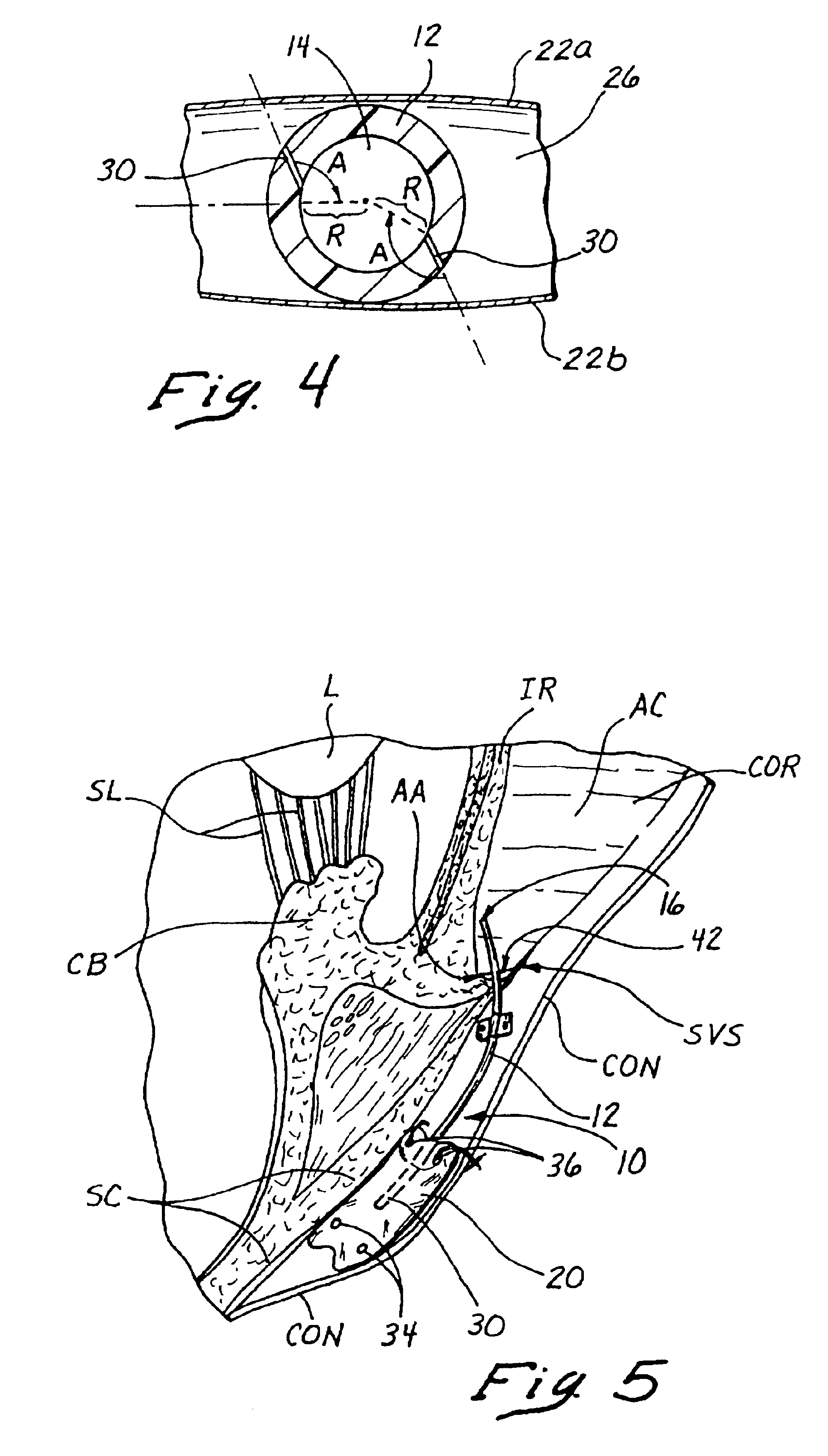
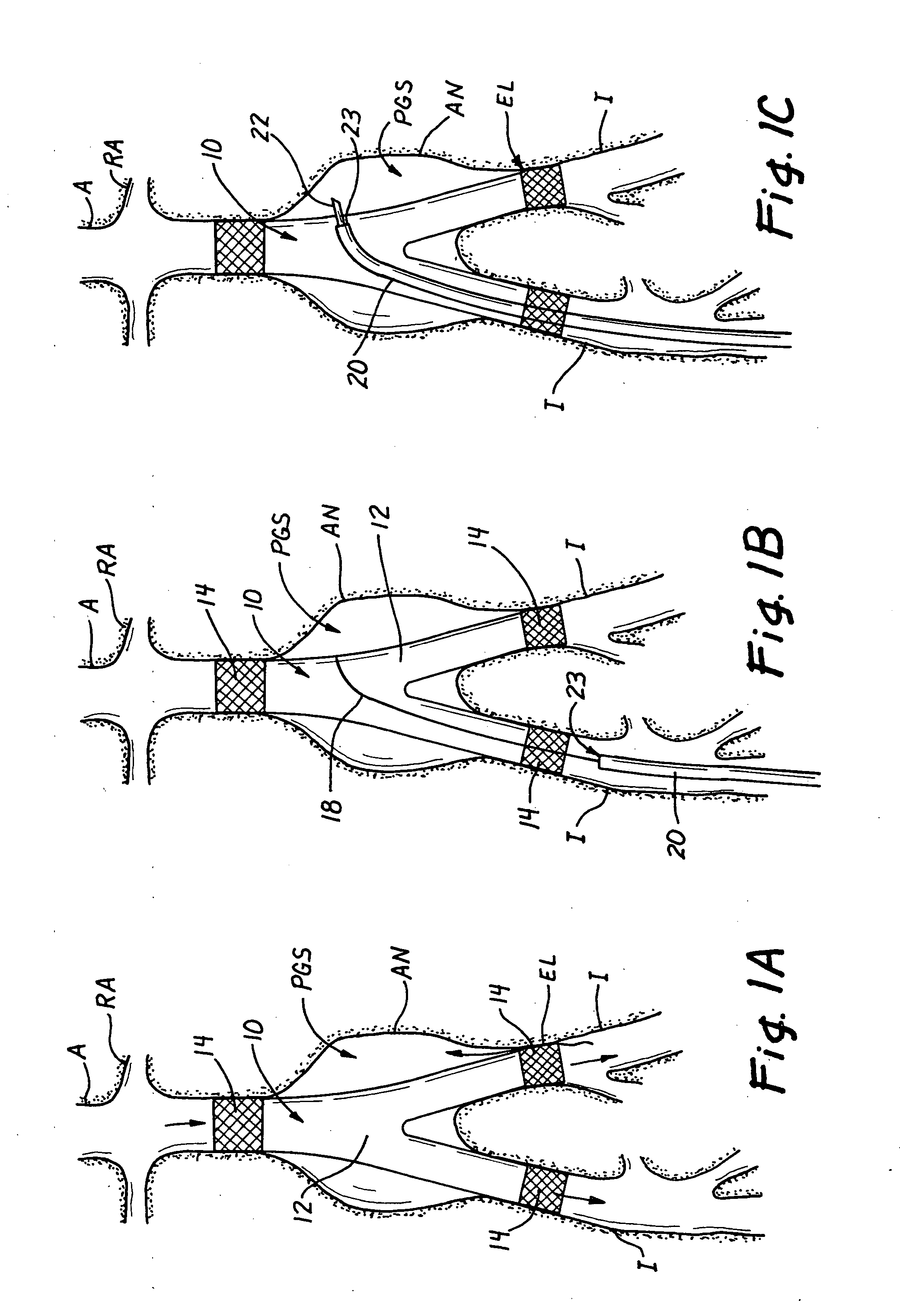
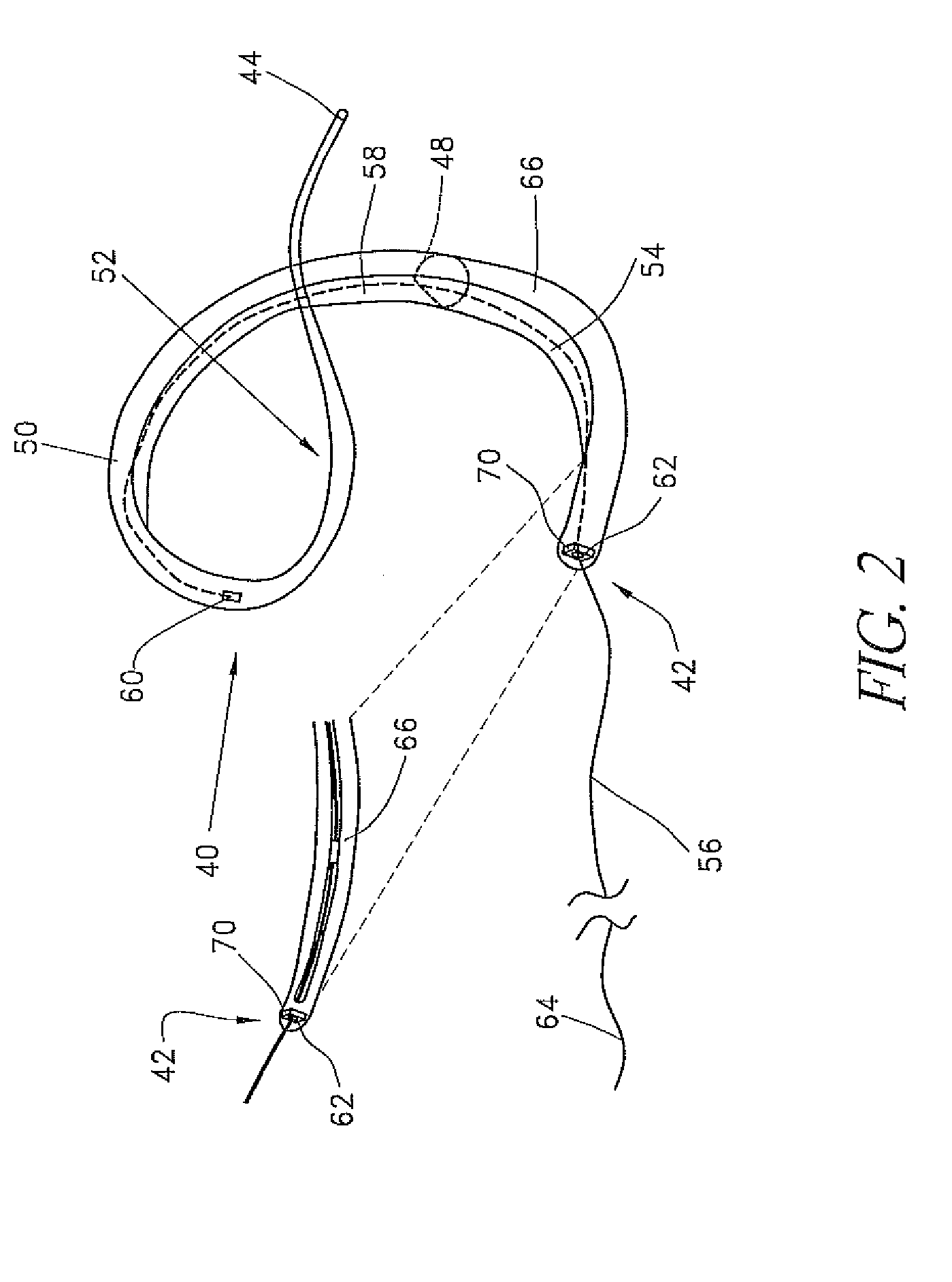
Valve prosthesis systems and methods / systems for placement of such valve prostheses are provided that facilitate efficient, reliable and minimally invasive delivery modalities. The placement systems and methods permit remote manipulation and positioning of the valve prosthesis such that desirable placement relative to anatomical structures, e.g., the heart annulus, may be achieved. The valve prosthesis includes a resilient ring, a plurality of leaflet membranes mounted with respect to the resilient ring, and a plurality of positioning elements movably mounted with respect to the flexible ring. The delivery system includes a first elongate element that terminates at the valve prosthesis and is manipulable by an operator to remotely rotate the positioning elements relative to the flexible ring. A second elongate element terminates at the valve prosthesis and is manipulable by an operator to remotely advance the valve prosthesis downward into an anatomical annulus. The second elongate element may be manipulated to remotely advance the valve prosthesis into the anatomical annulus to assume a position for supporting post-implantation function of the valve prosthesis in situ. The first elongate element may be further manipulated to remotely rotate the positioning element relative to the flexible ring to cause the positioning element to engage tissue associated with the anatomical annulus and to thereby maintain the post-implantation position of the valve prosthesis in situ. Methods for valve prosthesis deployment are also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
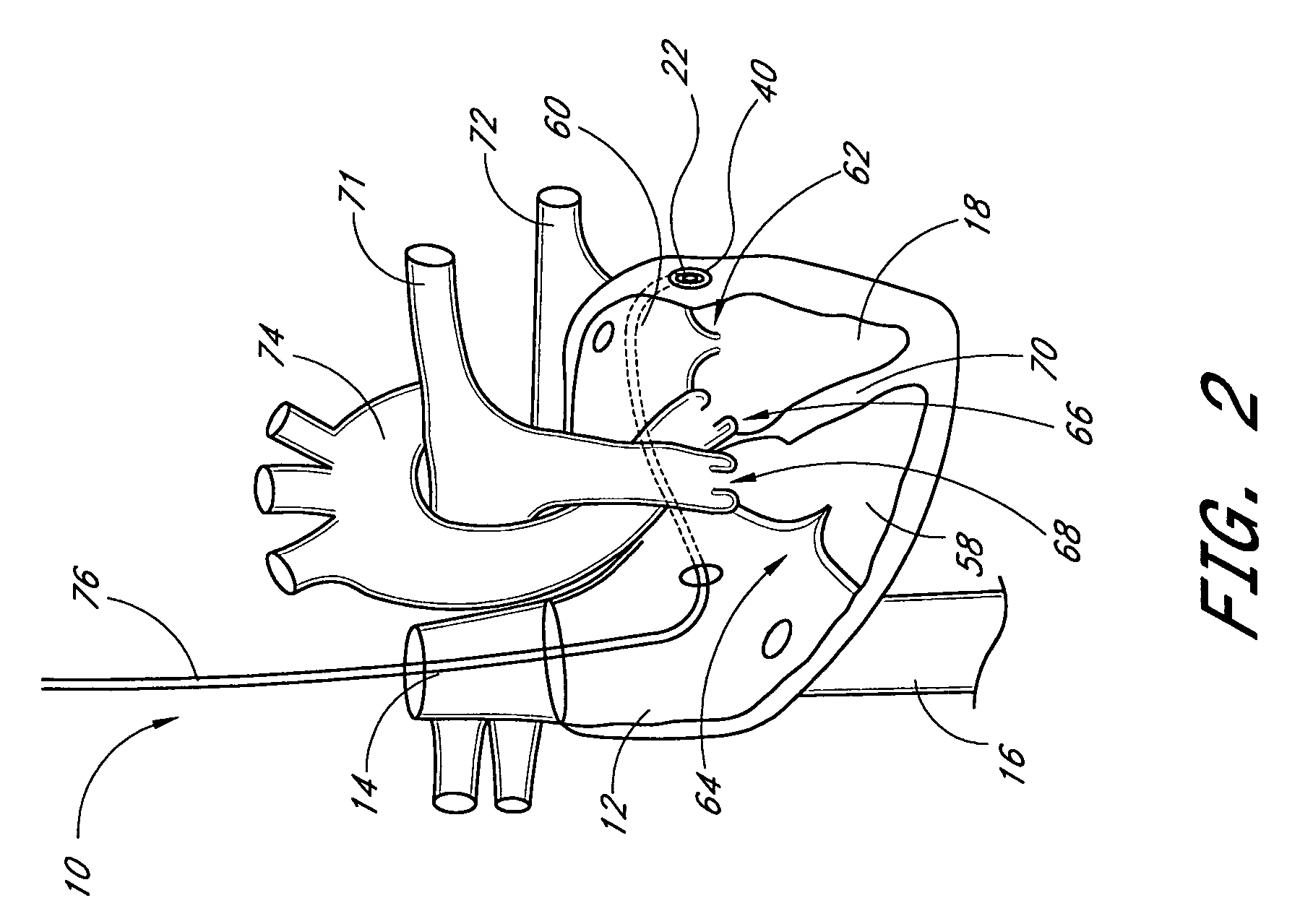
Remotely adjustable coronary sinus implant
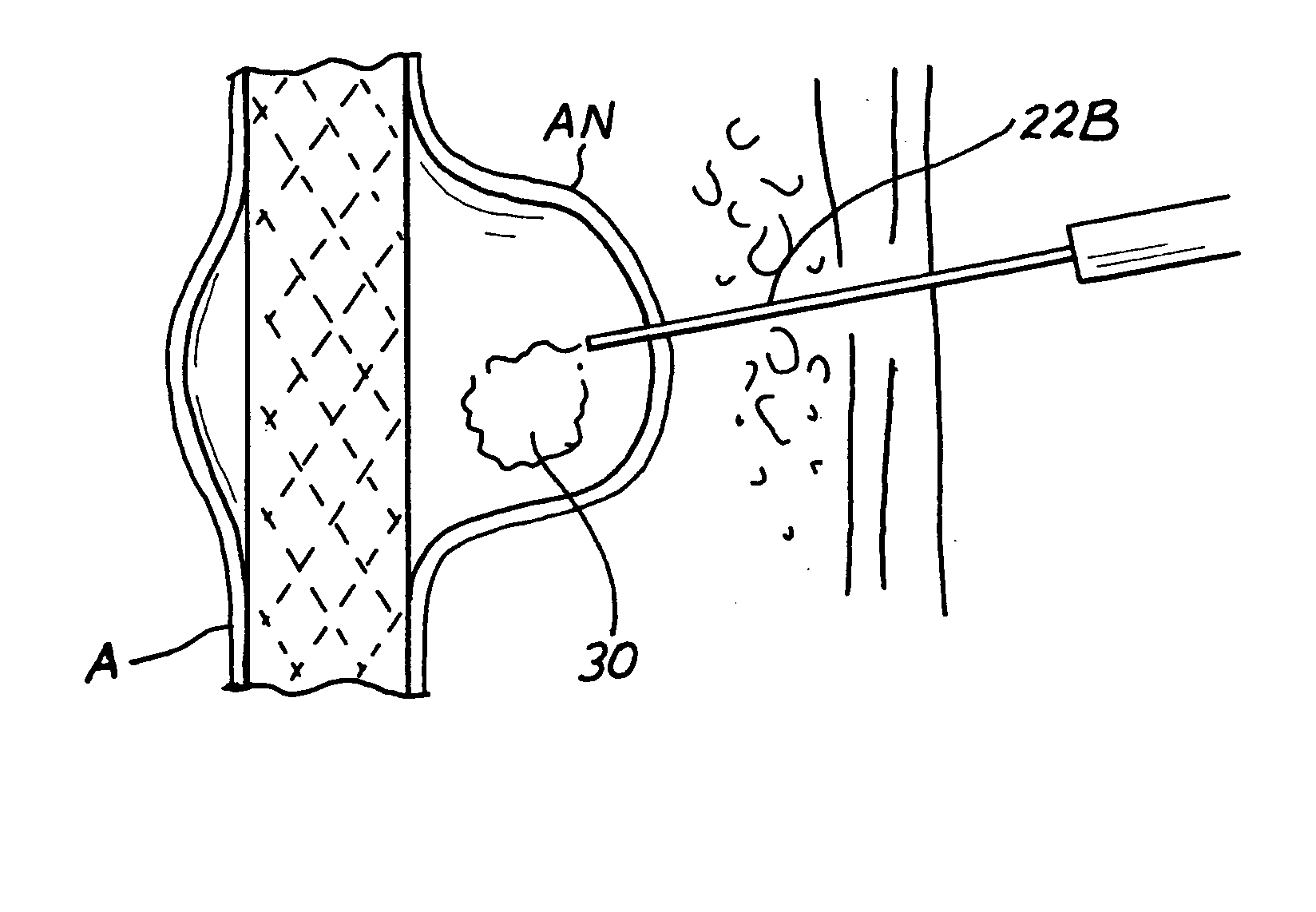
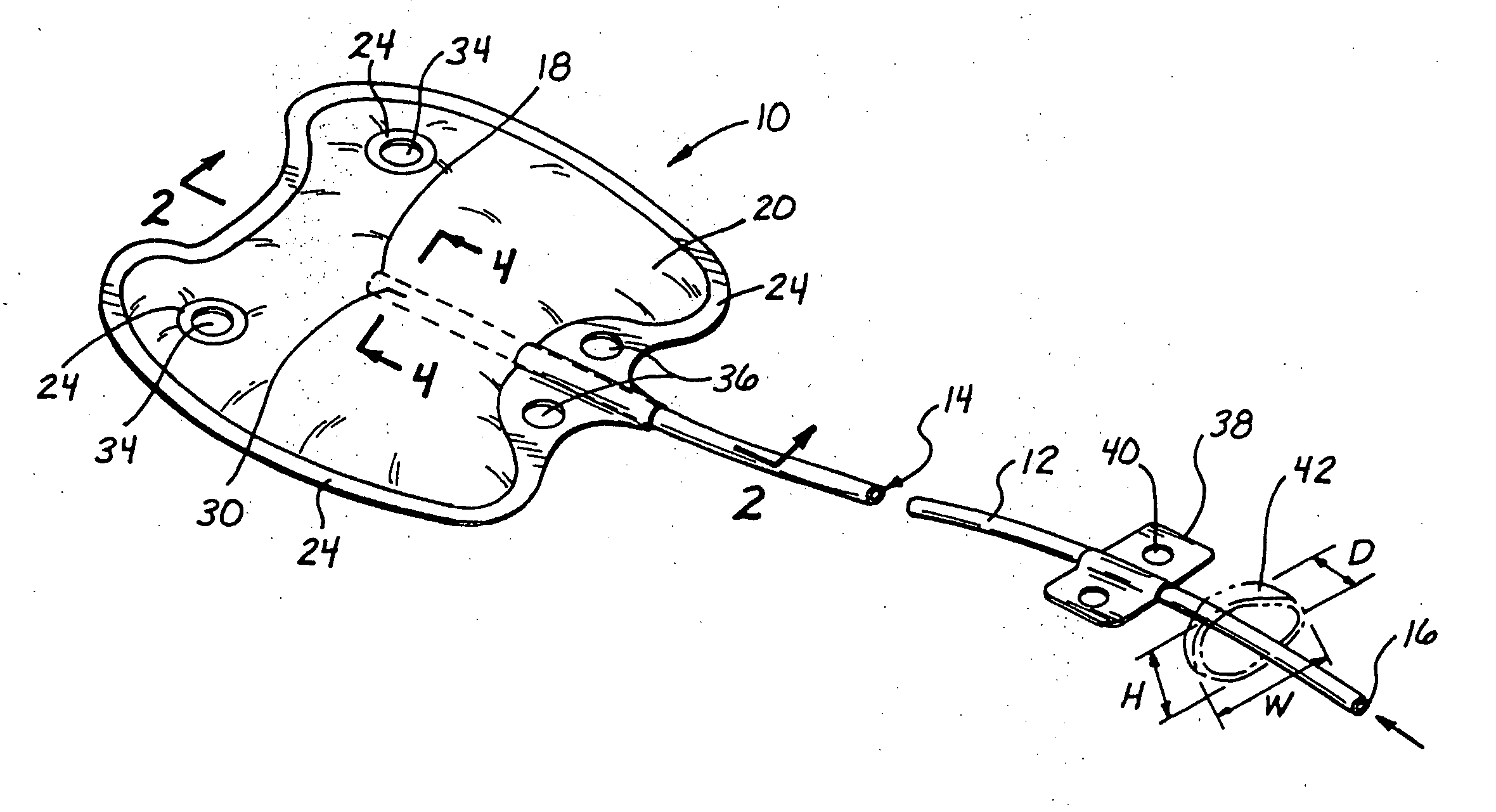
Disclosed are methods and devices for applying pressure to an adjacent tissue structure, such as the annulus of the mitral valve. An adjustable implant is provided with an elongate control line having a distal end connected to the implant and a proximal end spaced apart from the implant. The device enables post implantation adjustment, by accessing the proximal end of the control line and manipulating the control line to adjust the implant.
Owner:EDWARDS LIFESCIENCES AG
Graft Fixation Device
InactiveUS20080228186A1Avoid damageSuture equipmentsPharmaceutical delivery mechanismBone implantPost implantation
In one aspect a device is disclosed for use as a bone implant comprising, a body having a pre-implantation shape and a post-implantation shape different from the pre-implantation shape. The body is configured to change from the pre-implantation shape to the post-implantation shape in response to the body being activated. The body is configured to be inserted in a bone recess while the body is in the pre-implantation shape. In another aspect a method is disclosed comprising inserting a cable member into a recess in a bone, inserting a retention device into the recess, the retention device containing a shape memory material, and activating the shape memory material.
Owner:UNIV OF COLORADO THE REGENTS OF
Treatment of bioprosthetic tissues to mitigate post implantation calcification
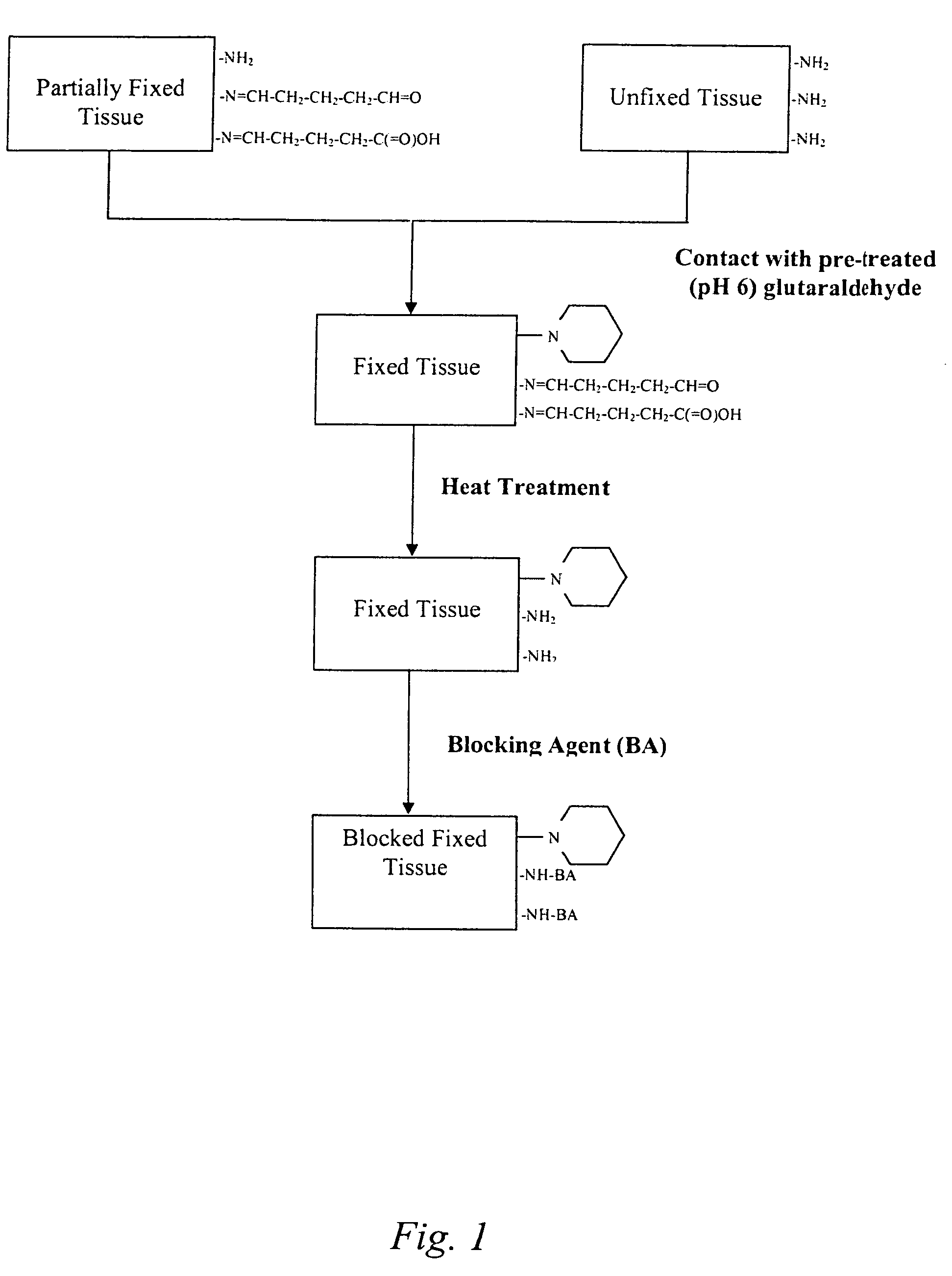
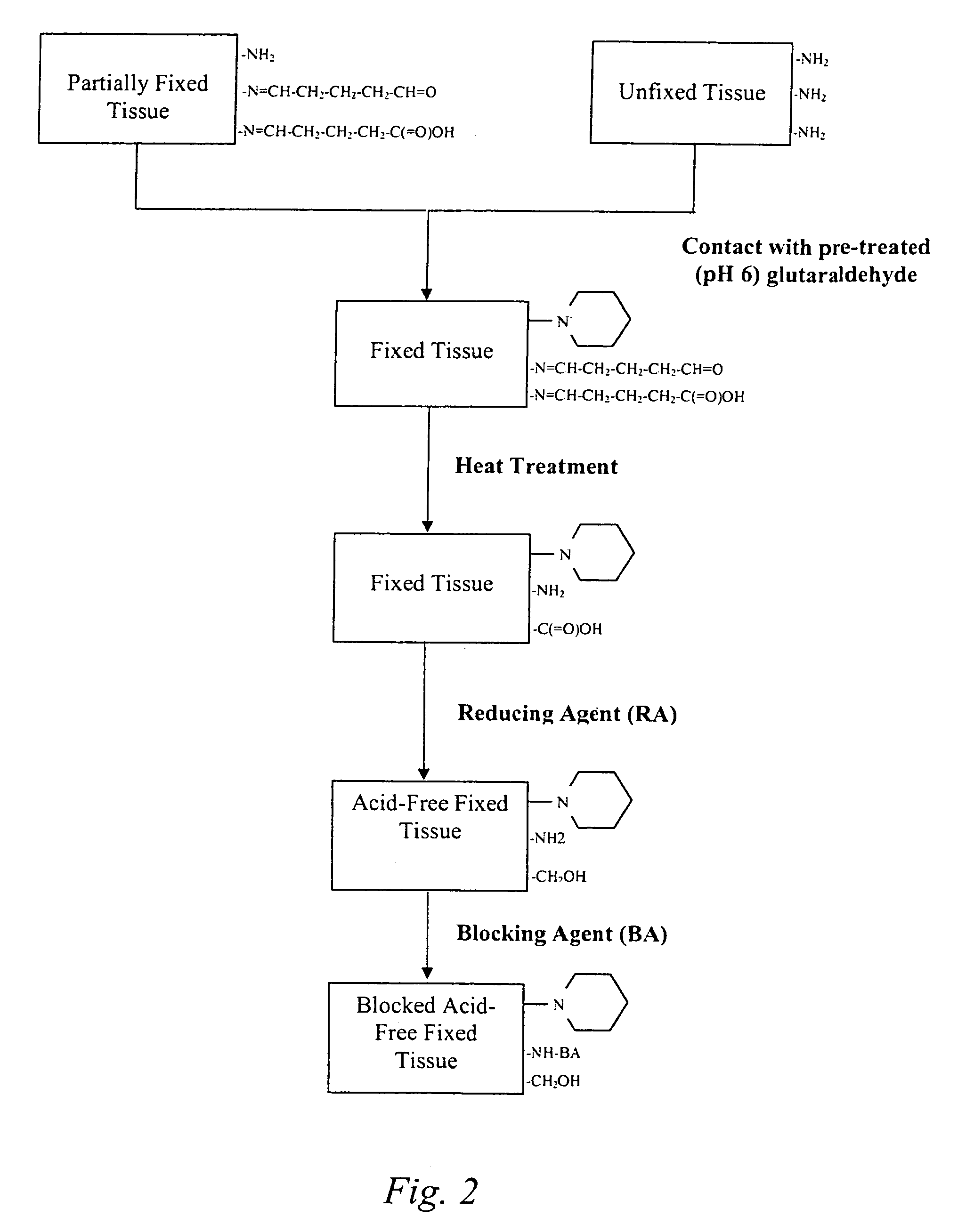
The present invention provides methods for treating tissue to inhibit post-implant calcification of a biological tissue. In one method of this invention, a tissue is immersed in or otherwise contacted with a pretreated glutaraldehyde solution, i.e., a heat-treated or pH-adjusted glutaraldehyde solution. The tissue may be partially fixed with glutaraldehyde prior to, after, or concurrently with the step of contacting the tissue with the pretreated gluteraldehyde. Contact with the pretreated gluteraldehyde produces free amine groups on the tissue, which are subsequently blocked by contacting the crosslinked tissue with a blocking agent. In another embodiment, a tissue is contacted with either a non-pretreated glutaraldehyde or a pH-adjusted glutaraldehyde solution for a period of time sufficient to crosslink the tissue. The crosslinked tissue is then treated with a reducing agent that reduces aldehyde and carboxylic acid groups on the fixed tissue.
Owner:EDWARDS LIFESCIENCES CORP
Implant that can be implanted in osseous tissue and method for producing said implant corresponding implant
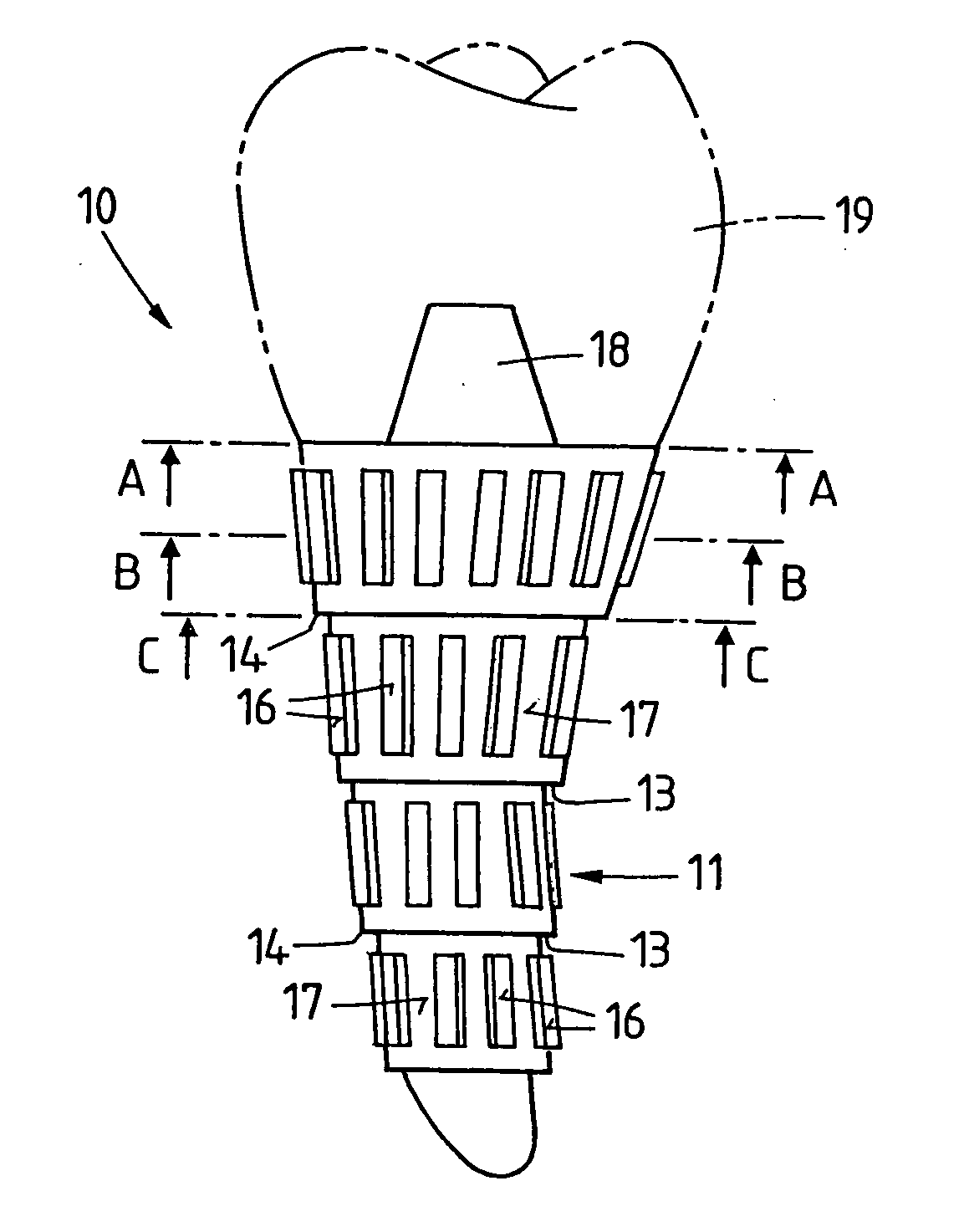
ActiveUS20060105295A1Strong long-term anchoringProcess stabilityDental implantsInternal osteosythesisBone implantCavity wall
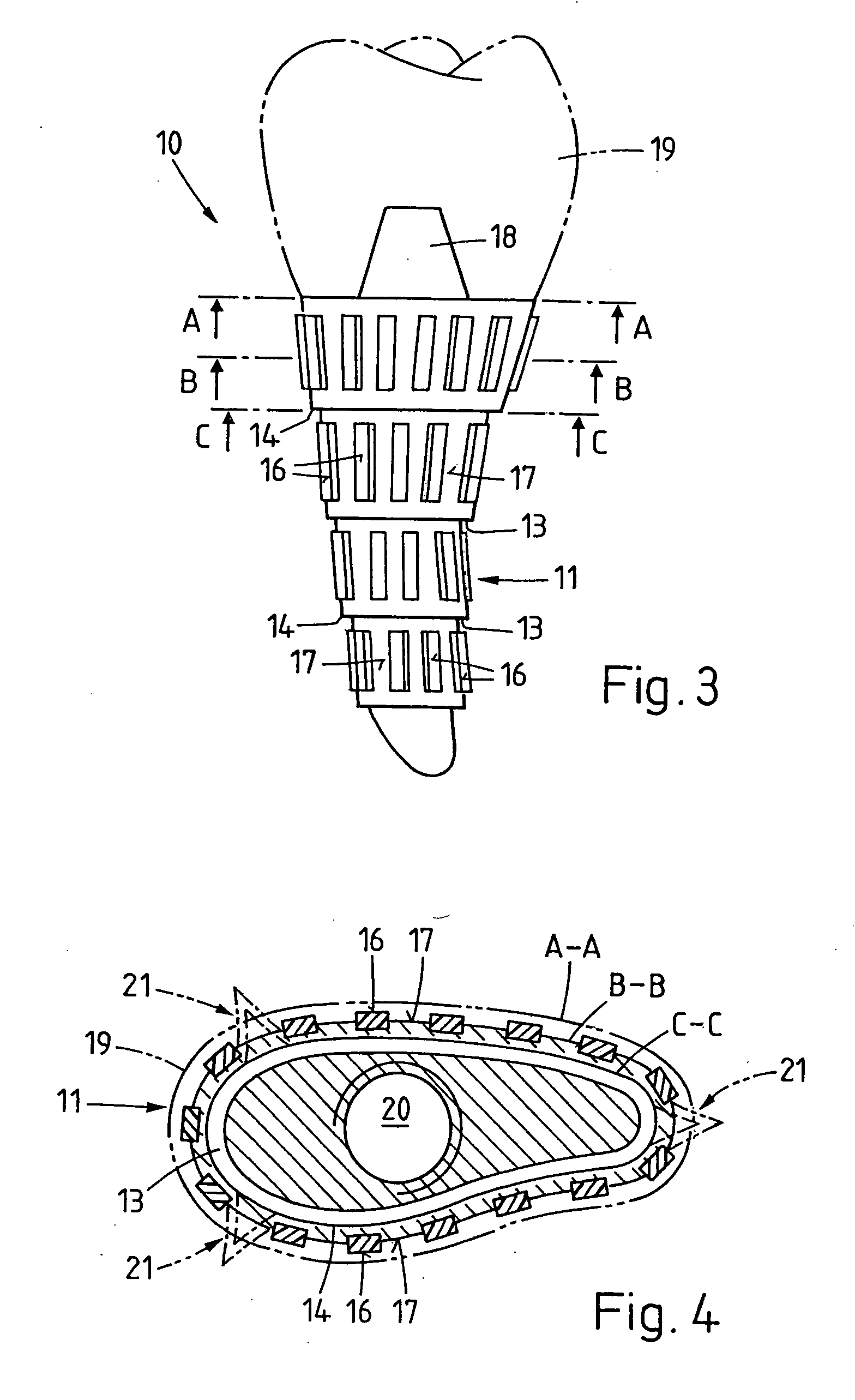
A bone implant (10) is implanted in a cavity parallel to an implant axis (I) and without substantial rotation. The implant includes, on an implant portion to be implanted, cutting edges (14), which do not extend in a common plane with the implant axis and are facing toward the distal end of the implant. The implant also includes surface ranges (16) of a material that is liquefiable by mechanical oscillations. The cutting edges (14) are dimensioned such that they are lodged in the cavity wall after implantation. For implantation, the implant is impinged with mechanical oscillations, resulting in the thermoplastic material being at least partially liquefied and pressed into unevennesses and pores of the cavity wall to form a form-fit and / or material-fit connection between implant (10) and cavity wall, when re-solidified. The cutting edges (14) anchor the implant in the cavity wall.
Owner:WOODWELDING
Lens system and method for power adjustment
A lens is provided that having optical parameters that may be adjusted in-situ, and is particularly useful as an IOL for use in cataract patients that require an adjustment in the optical power of the lens post-implantation. In one embodiment, the lens body carries an array of interior fluid-filled cells in which fluid is controllably moved upon application of energy from an external source to move a fluid media into or out of the cells to thereby alter the lens surface shape.
Owner:ALCON INC
Composite bone graft, method of making and using same
InactiveUS6902578B1Avoid significant donor site morbidityHigh mechanical strengthBone implantJoint implantsDiseaseOssicular Prosthesis Implantation
The invention is directed to a composite bone graft for implantation in a patient, and methods of making and using the composite bone graft, along with methods for treating patients by implanting the composite bone graft at a site in a patient. The composite bone graft includes two or more connected, discrete, bone portions, and includes one or more biocompatible connectors which hold together the discrete bone portions to form the composite bone graft. The composite bone graft may include one or more textured bone surfaces. The textured surface preferably includes a plurality of closely spaced protrusions, preferably closely spaced continuous protrusions. The composite bone graft is useful for repairing bone defects caused by congenital anomaly, disease, or trauma, in a patient, for example, for restoring vertical support of the anterior and / or posterior column. Implantation of the composite bone graft results in improved graft stability and osteoinductivity, without a decrease in mechanical strength. The composite bone graft does not shift, extrude or rotate, after implantation. The present composite bone graft can be appropriately sized for any application and can be used to replace traditional non-bone prosthetic implants.
Owner:LIFENET HEALTH
Remotely adjustable coronary sinus implant
Disclosed are methods and devices for applying pressure to an adjacent tissue structure, such as the annulus of the mitral valve. An adjustable implant is provided with an elongate control line having a distal end connected to the implant and a proximal end spaced apart from the implant. The device enables post implantation adjustment, by accessing the proximal end of the control line and manipulating the control line to adjust the implant.
Owner:EDWARDS LIFESCIENCES AG
Shape Memory Polymer Medical Devices
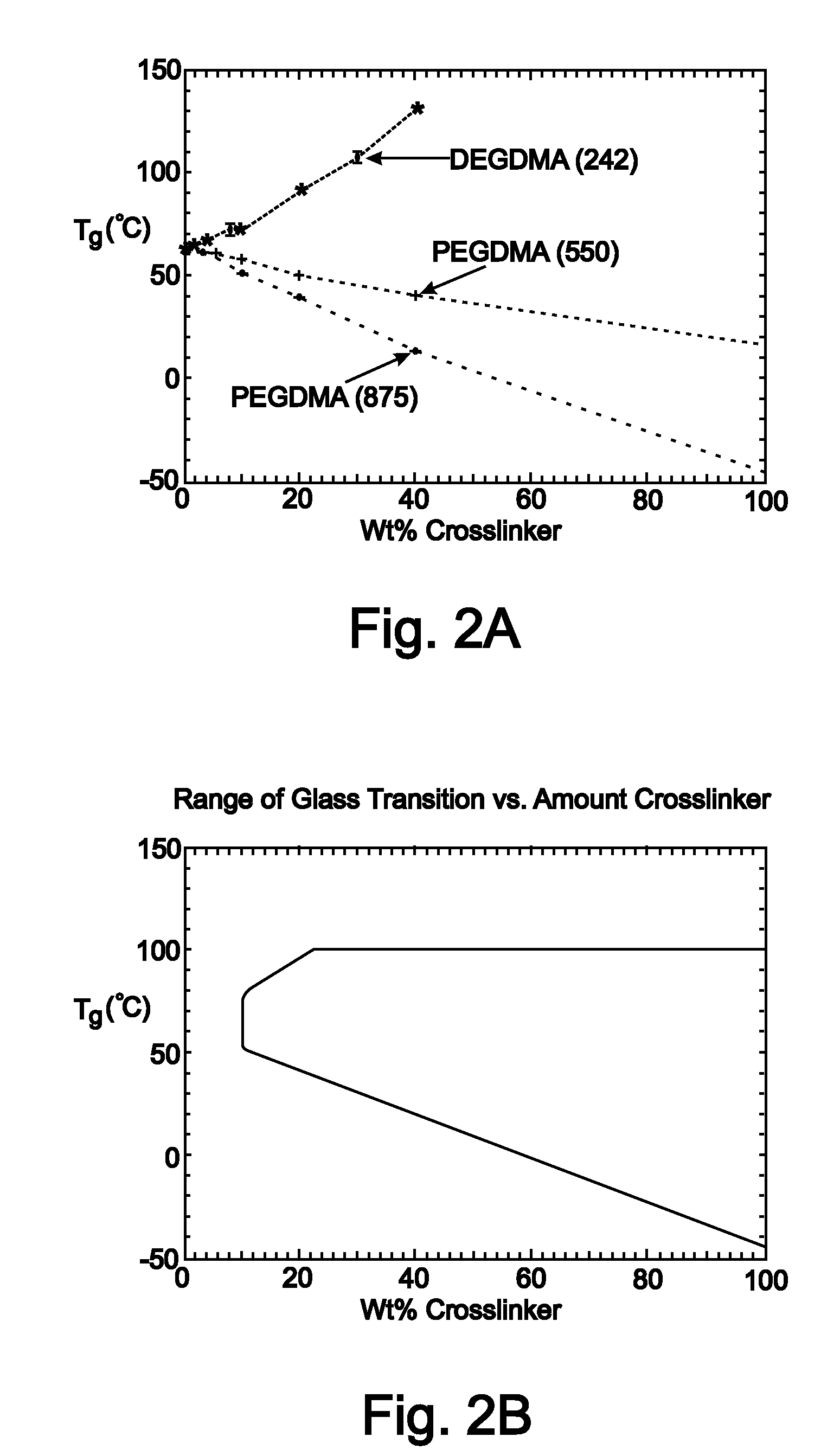
Medical devices for in vivo medical applications are disclosed. The medical devices are constructed of shape memory polymer (SMP) materials capable of assuming a memory shape at physiological temperatures. These medical devices may be used in surgical procedures and in both vascular and non-vascular applications. These SMP medical devices have a post-implantation memory shape that is substantially identical to or slightly larger than the insertion site to adapt to vessel growth or size changes. SMP medical devices may be formed as stents or occlusion devices (i.e., plugs) having a number of different structural features. The SMP medical devices may be formed from a first monomer and a second cross-linking monomer, wherein the weight percentages of the first and second monomers are selected by performing an iterative function to reach a predetermined glass transition temperature (Tg) and a predetermined rubbery modulus to optimize post-implantation memory shape properties of the devices.
Owner:UNIV OF COLORADO THE REGENTS OF
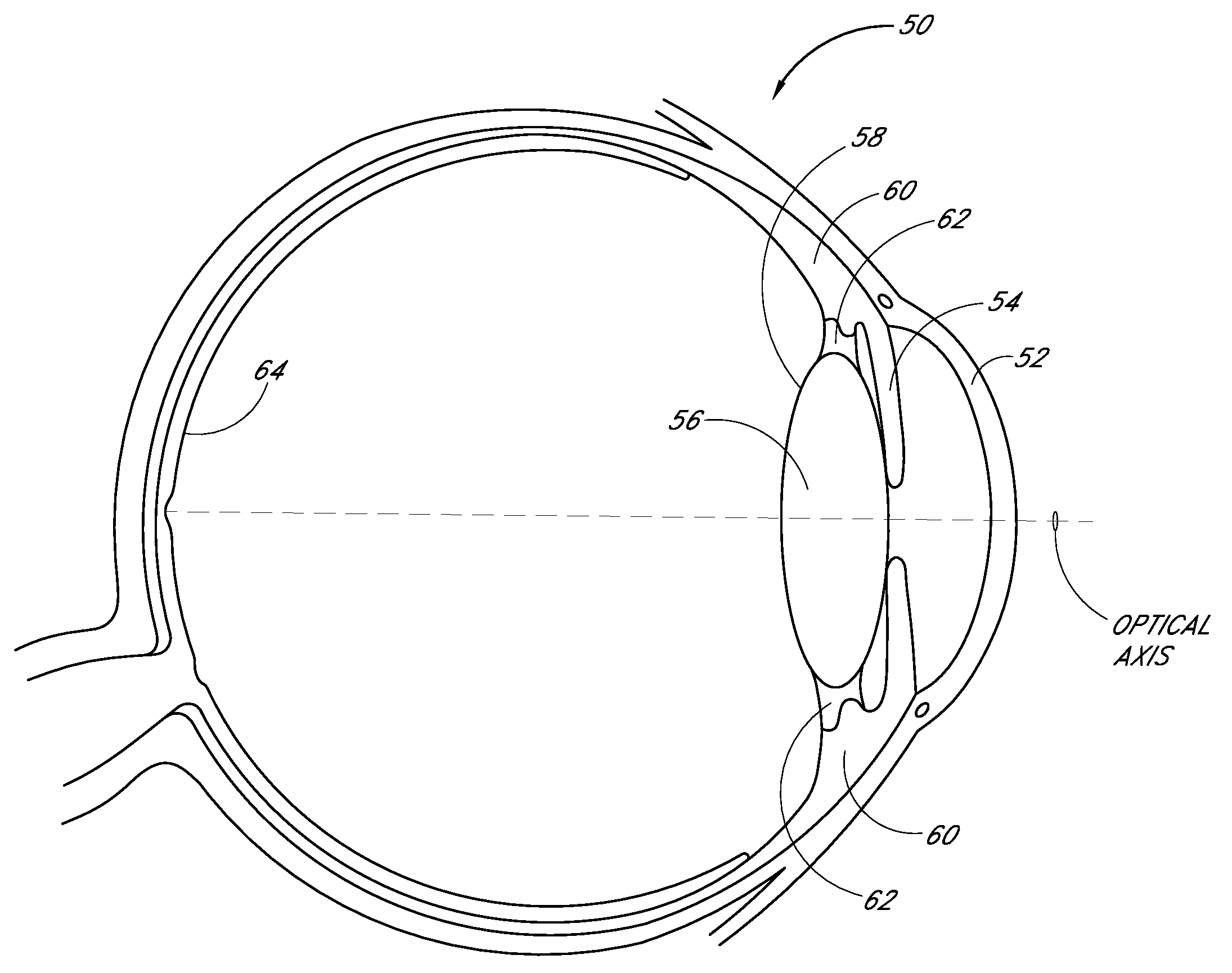
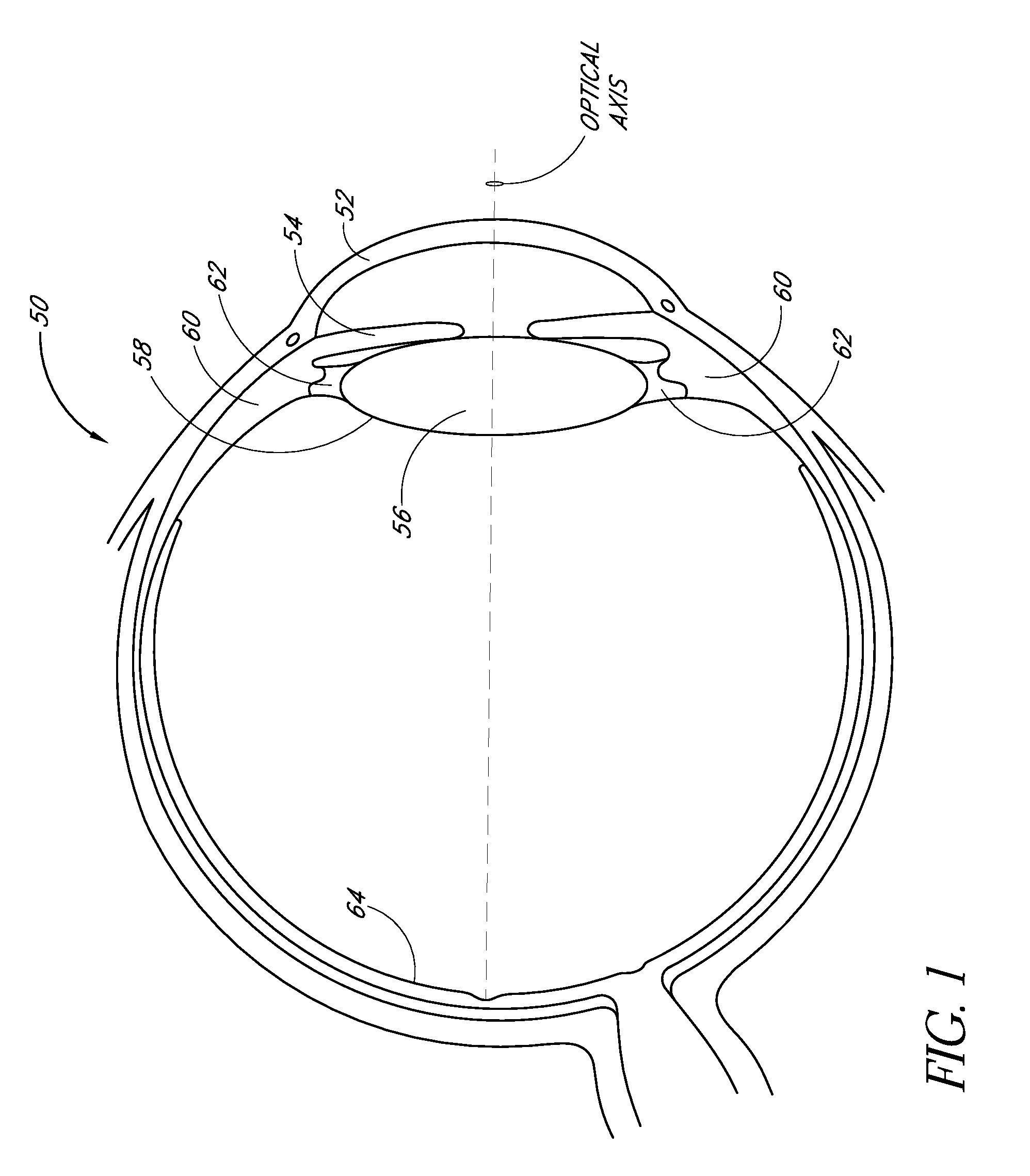
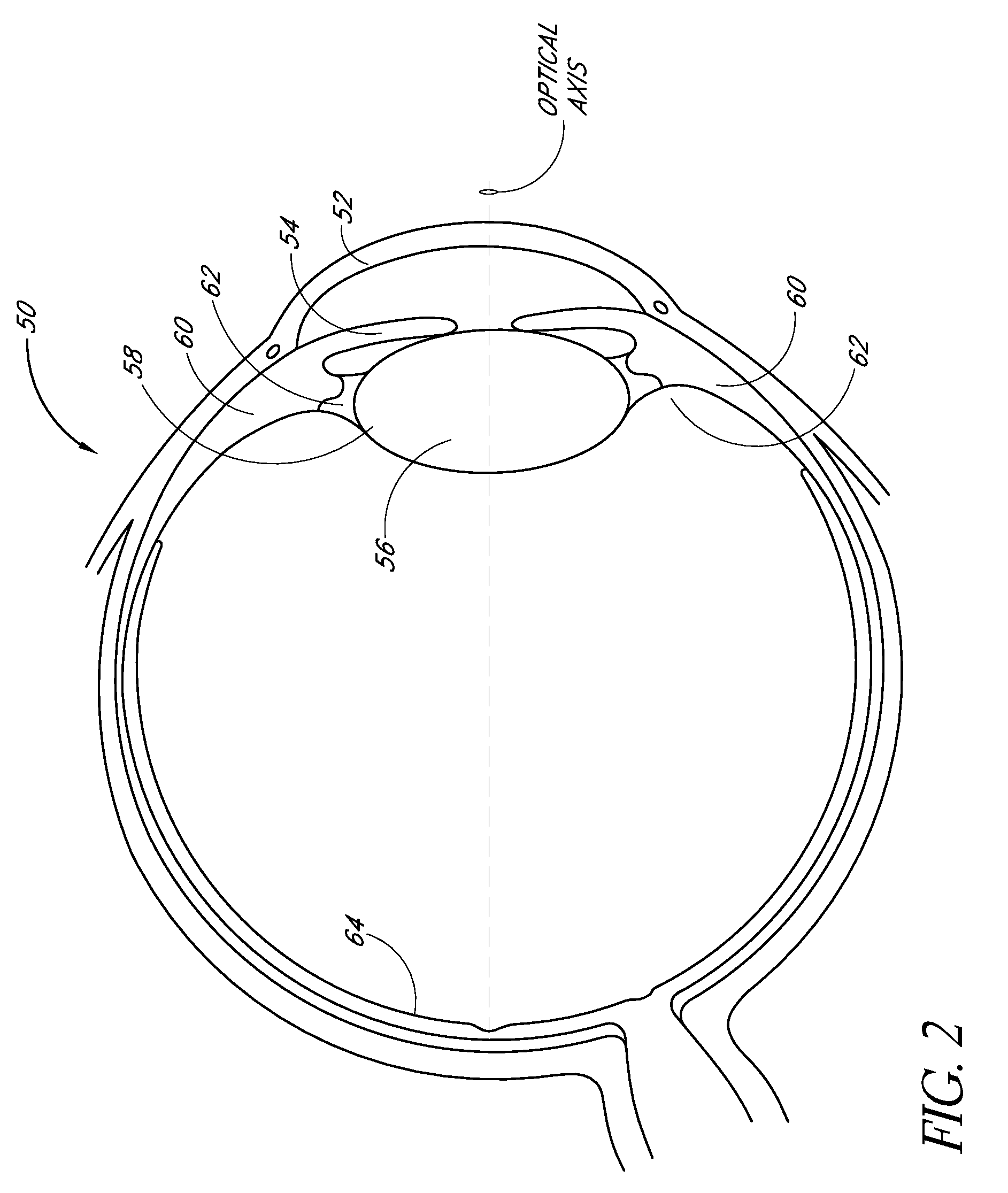
Intraocular Lenses and Methods of Accounting for Capsule Size Variability and Post-Implant Changes in the Eye
Accommodating intraocular lenses and methods of use which account for changes to a capsular bag post-implantation as well as a mismatch is size between the accommodating intraocular lens and capsule.
Owner:ALCON INC
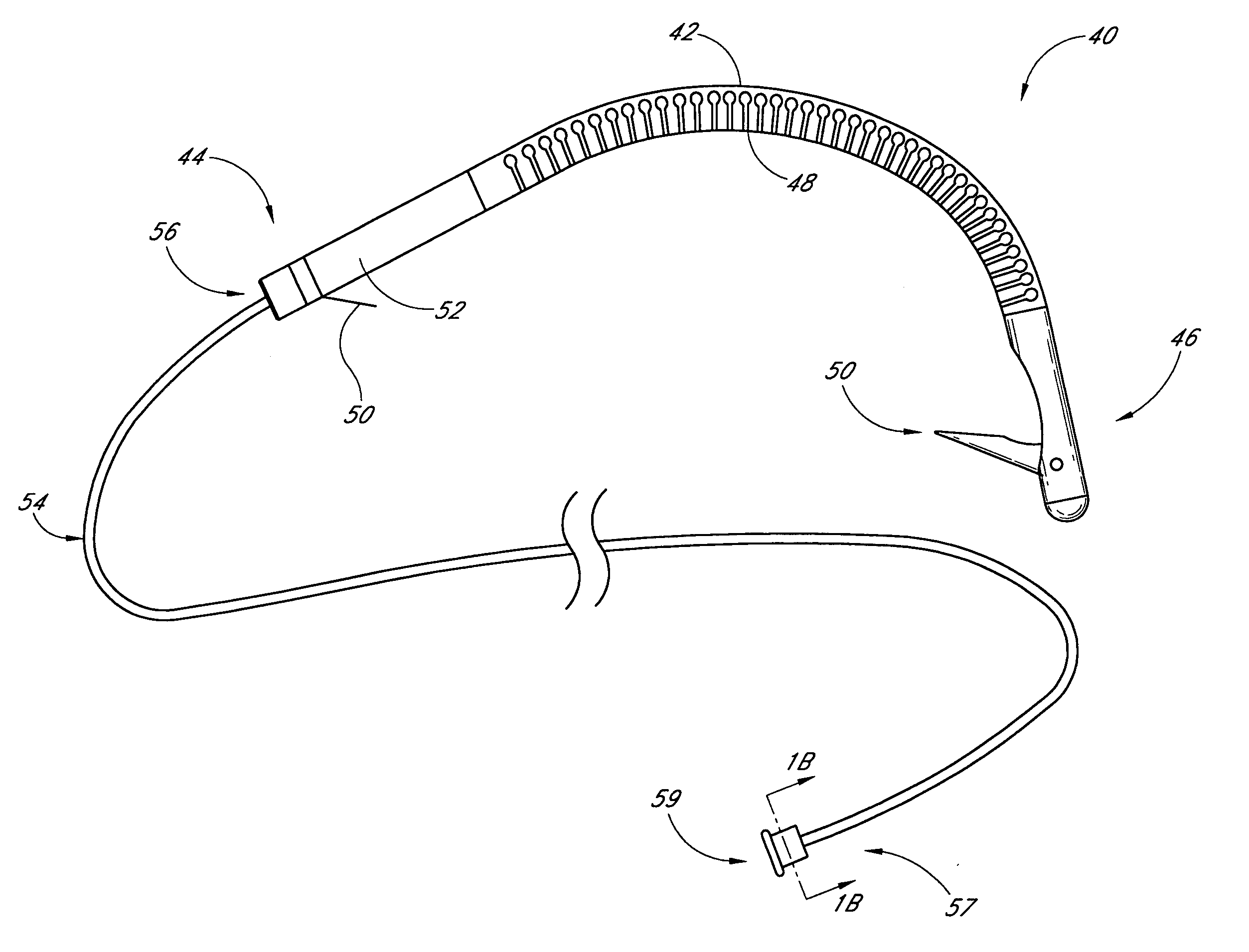
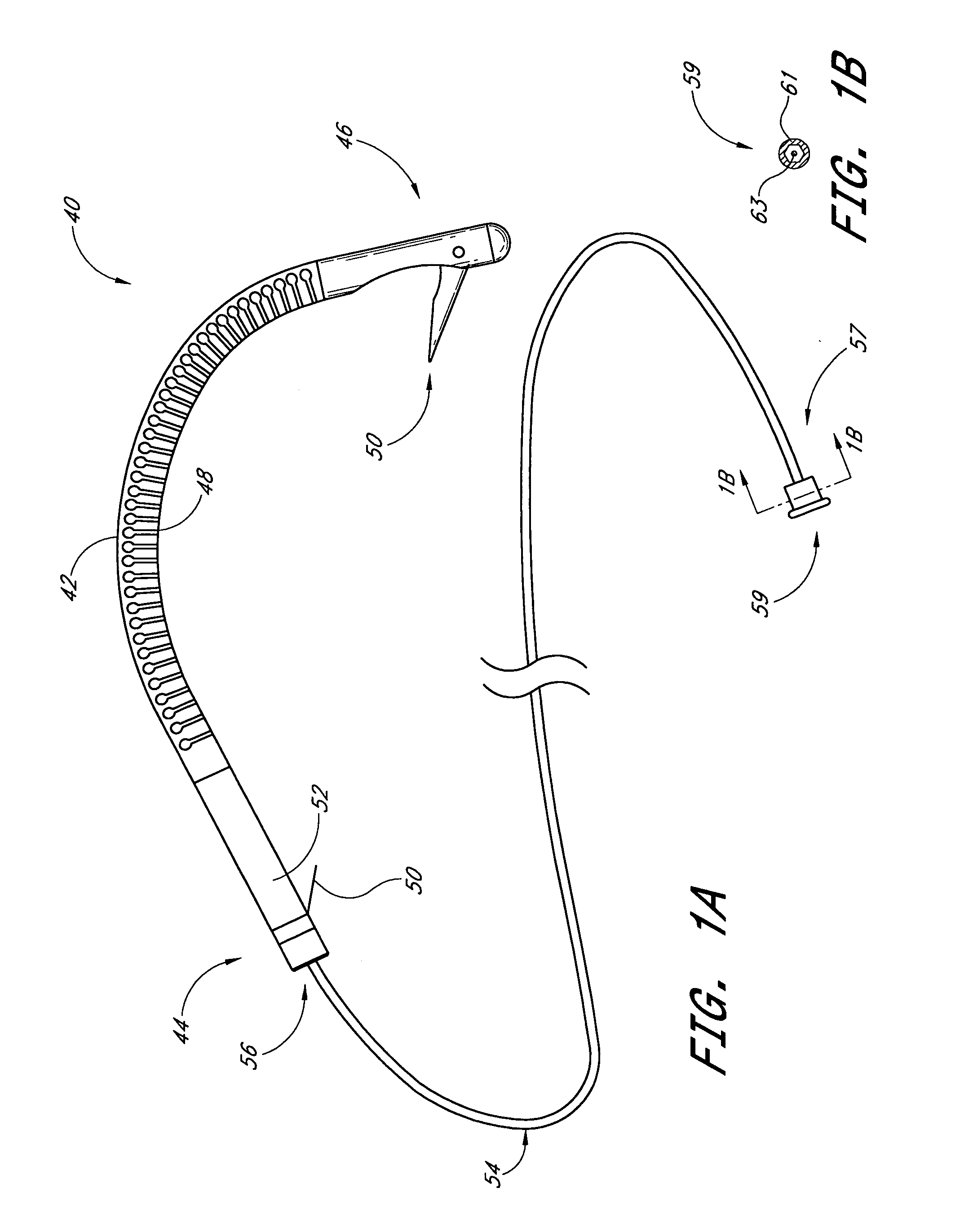
Implantable devices useful for reinforcing a surgically created stoma
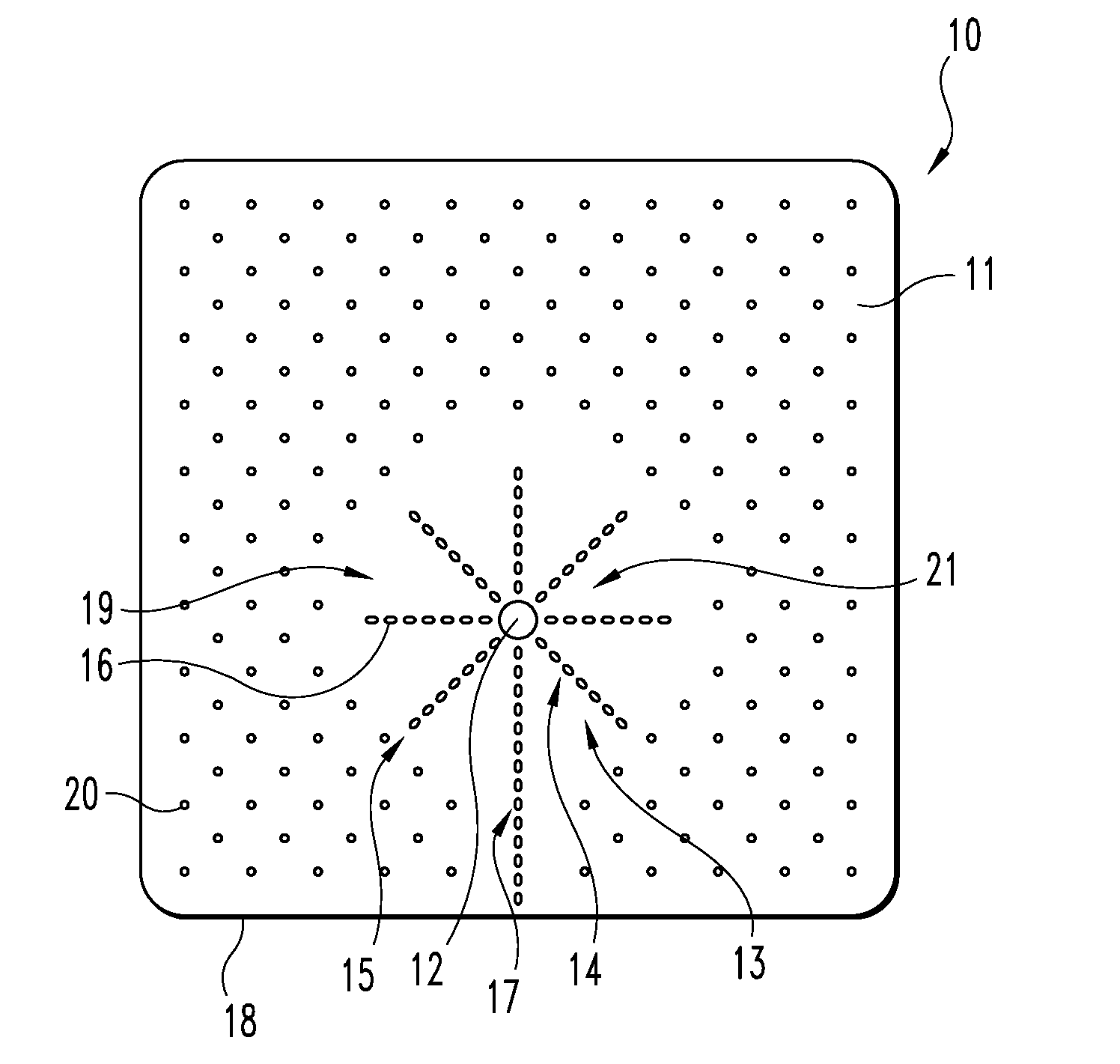
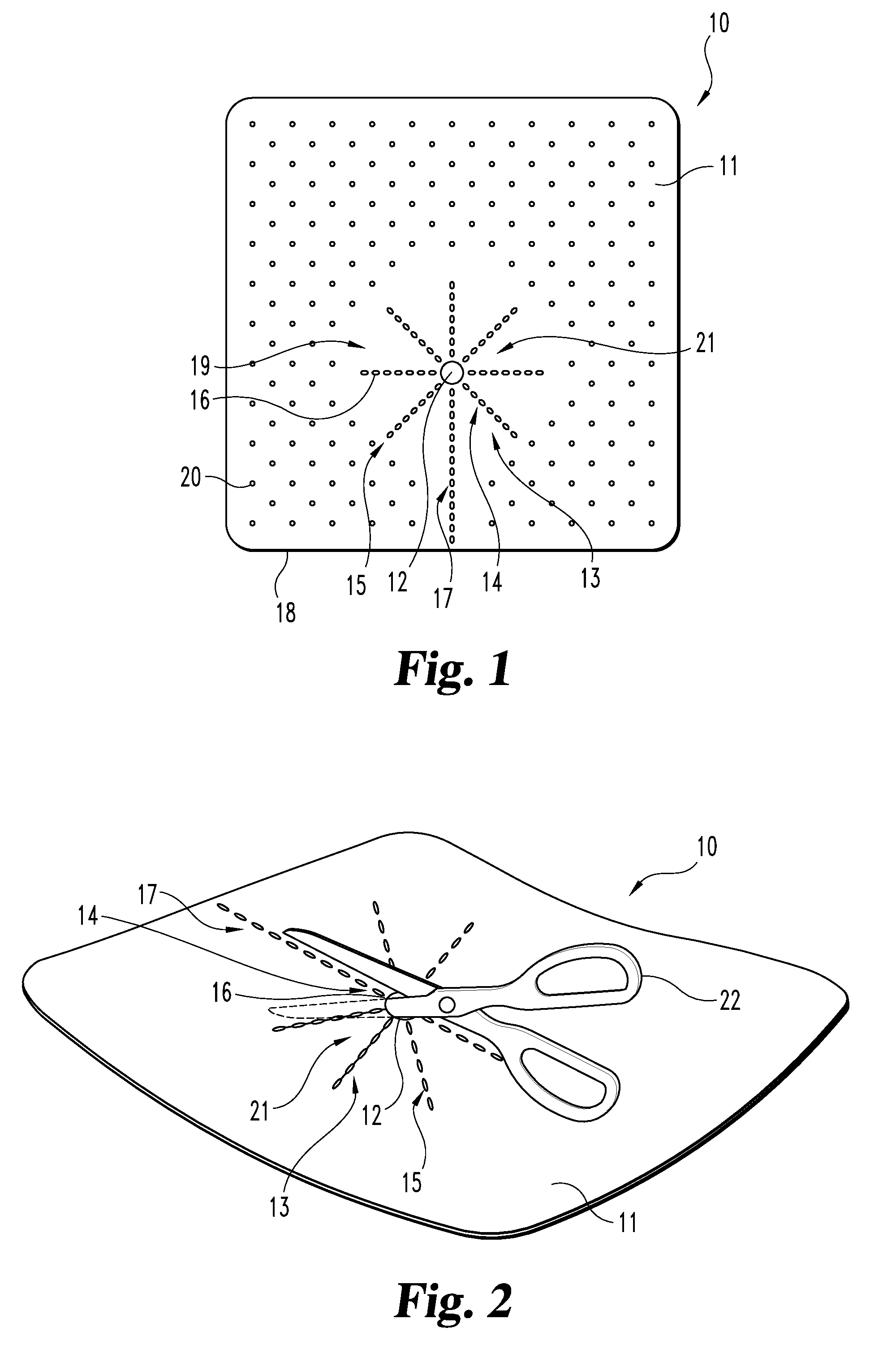
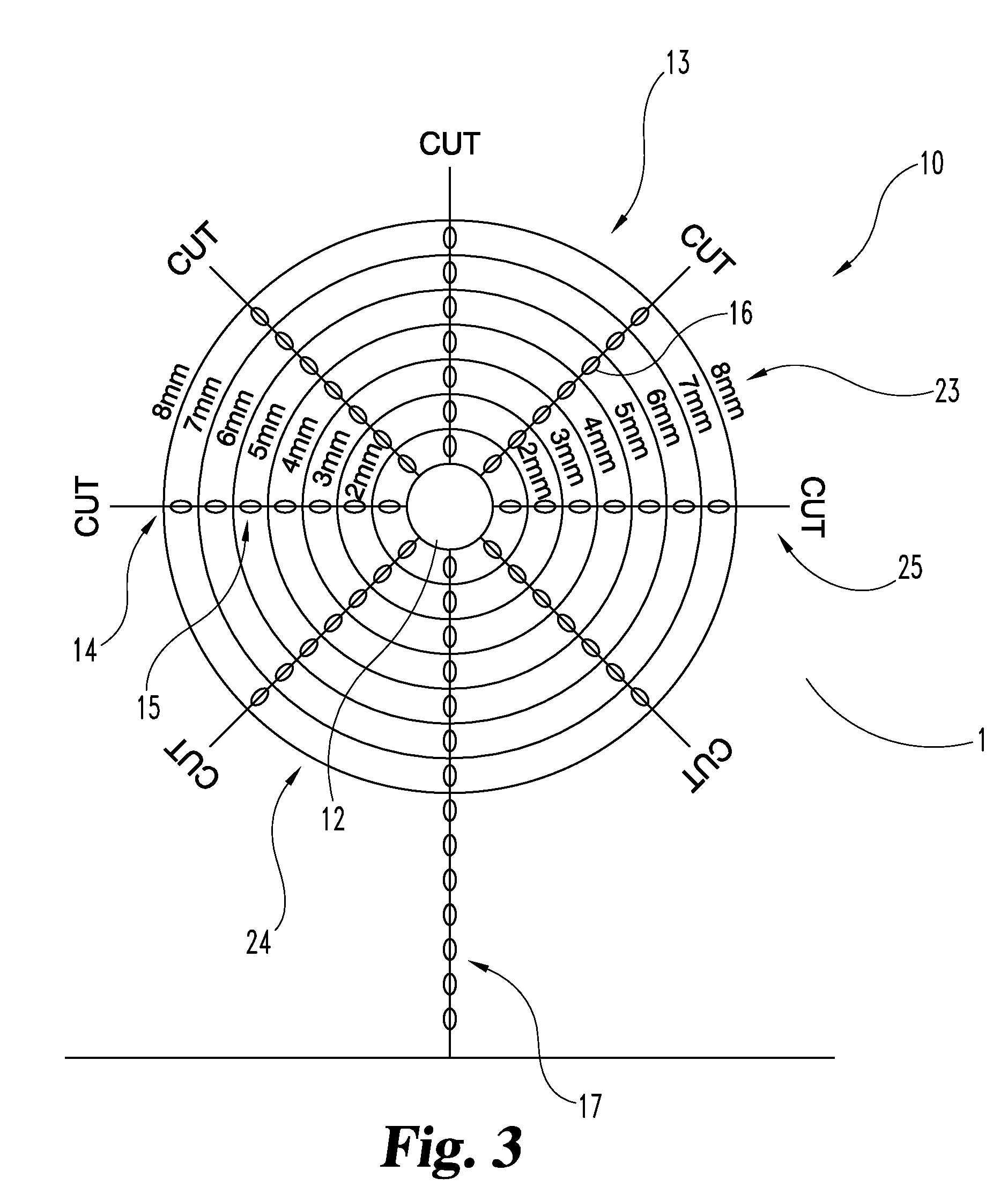
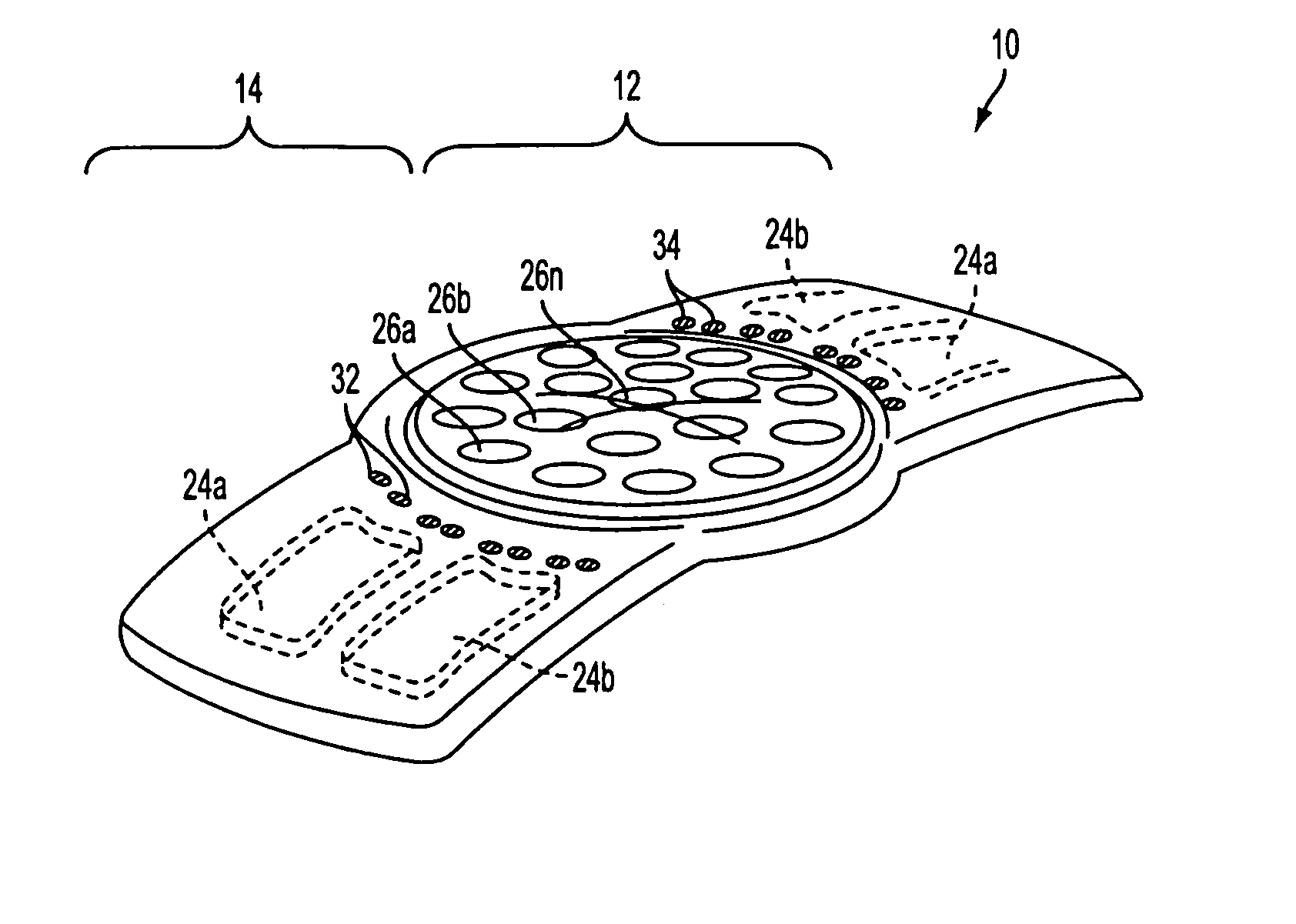
An implantable tissue reinforcement device useful for treating or preventing the formation of parastomal hernias forming about a surgically created stoma. The tissue reinforcement device can comprise a synthetic or mammalian-derived sheet-like graft member, such as a resorbable material that promotes tissue ingrowth, wherein a sizing pattern is included that comprises linear indicia radiating outward from a stomal aperture. The linear indicia facilitate creation of a resized stomal opening in the graft member sufficiently large to allow passage of the resected bowel portion. A series of cuts made along the indicia create a plurality of flaps about the resized opening that are configured to abut the bowel portion and enhance reinforcement the stomal region after implantation. In one embodiment, the linear indicia comprises a series of weakened (e.g., perforated) lines that facilitate the cutting of the material by a scalpel, scissors, etc. The clinician resizes the stomal opening to a known diameter using reference indicia, such as diameter indicia and circular guide indicia. The sizing pattern can be imprinted on or etched into the material of the graft member, or it can be at least partially located on a separate template that is either laid over the graft member, preattached as a cuttable overlay, or used as a reference guide.
Owner:COOK BIOTECH
Lens system and method for power adjustment using externally actuated micropumps
An intraocular lens is provided that having optical parameters that may be adjusted in-situ, and is particularly useful in cataract patients that require an adjustment in the optical power of the lens post-implantation. The lens body carries an array of interior fluid-filled cells in which fluid is controllably moved by micropumps upon application of energy from an external source to move a fluid media into the cells to thereby alter the lens surface shape.
Owner:ALCON INC
Sutureless implantable device and method for treatment of glaucoma
InactiveUS6881197B1Easy to installControl pressureEye surgeryWound drainsDiffusion barrierMaximum pressure
Sutureless, implantable fluid shunting devices and associated methods for controlling the pressure of fluids within anatomical spaces or cavities of the body. The device generally comprises a tube having a diffusion barrier (e.g., diffusion chamber) formed on a proximal end thereof. Fluid which flows through the tube will collect within the diffusion chamber and will diffuse outwardly therethrough. However, the presence of the diffusion chamber will prevent microbes, cells or other matter from interfering with or backflowing through the tube. Additionally, the tube may be provided with a pressure-openable aperture through which fluid from the tube may flow into the diffusion chamber. Such pressure-openable aperture will remain closed, until the pressure of fluid within the tube exceeds a predetermined maximum pressure PMAX. In this manner, the pressure-openable aperture will limit the amount of fluid drained from the anatomical space or cavity of the body, thereby avoiding hypotony within such anatomical space or cavity. The diffusion barrier of the device is preferably configured to fit between, and to be engaged by, adjacent recti muscles of the eye. Such engagement of the diffusion barrier with the adjacent recti muscles serves to prevent unwanted migration or post-implantation movement of the device, without the need for suturing of the device to the tissue of the eye.
Owner:REVISION OPTICS
Methods, materials and apparatus for deterring or preventing endoleaks following endovascular graft implantation
Methods and apparatus for treating or preventing endoleaks after an endovascular graft (e.g., a stent, tubular graft, stent-graft, coated stent, covered stent, intravascular flow modifier or other endovascular implant that affects, limits or prevents blood flow into a vascular defect such as an aneurysm, arterio-venous fistula, arterio-venous malformation, vessel wall perforation, etc.) has been implanted in the vasculature of a human or veterinary patient. An expansile polymeric material, such as a swellable polymer (e.g., a hydrogel), a flexible or elastomeric polymer foam (e.g. silicone, polyurethane, etc.) or a carrier member (e.g, a coil, filament, wire, etc) that carries a quantity of such expansile polymer is delivered into a perigraft space (i.e., space between the endovascular graft and the surrounding blood vessel wall) such that the polymeric material expands in situ to substantially fill the perigraft space or a portion thereof. The expansile polymeric material is delivered into he perigraft space through a catheter and / or cannula that is placed prior to, during or after the implantation of the endovascular graft. The invention includes an injector apparatus that is useable to deliver the expansile polymeric material through the wall of a previously implanted graft. After delivery into the perigraft space, the expanded polymeric material expands so as to fill all or an intended portion of the perigraft space in a manner that substantially prevents additional blood from leaking or flowing into such perigraft space. One type of blood-absorbing, porous, expansile polymeric material useable in this invention is a super-expansile hydrogel.
Owner:MICROVENTION INC
Sutureless implantable device and method for treatment of glaucoma
InactiveUS20050182350A1Easy to installMinimal discomfort to the patientEye surgeryWound drainsDiffusion barrierMaximum pressure
Sutureless, implantable fluid shunting devices and associated methods for controlling the pressure of fluids within anatomical spaces or cavities of the body. The device generally comprises a tube having a diffusion barrier (e.g., diffusion chamber) formed on a proximal end thereof. Fluid which flows through the tube will collect within the diffusion chamber and will diffuse outwardly therethrough. However, the presence of the diffusion chamber will prevent microbes, cells or other matter from interfering with or backflowing through the tube. Additionally, the tube may be provided with a pressure-openable aperture through which fluid from the tube may flow into the diffusion chamber. Such pressure-openable aperture will remain closed, until the pressure of fluid within the tube exceeds a predetermined maximum pressure PMAX. In this manner, the pressure-openable aperture will limit the amount of fluid drained from the anatomical space or cavity of the body, thereby avoiding hypotony within such anatomical space or cavity. The diffusion barrier of the device is preferably configured to fit between, and to be engaged by, adjacent recti muscles of the eye. Such engagement of the diffusion barrier with the adjacent recti muscles serves to prevent unwanted migration or post-implantation movement of the device, without the need for suturing of the device to the tissue of the eye.
Owner:REVISION OPTICS
Systems and methods for enhanced implantation of electrode leads between tissue layers
ActiveUS20180008311A1Reduce riskRecovery functionSpinal electrodesSurgical needlesAnatomical structuresMedicine
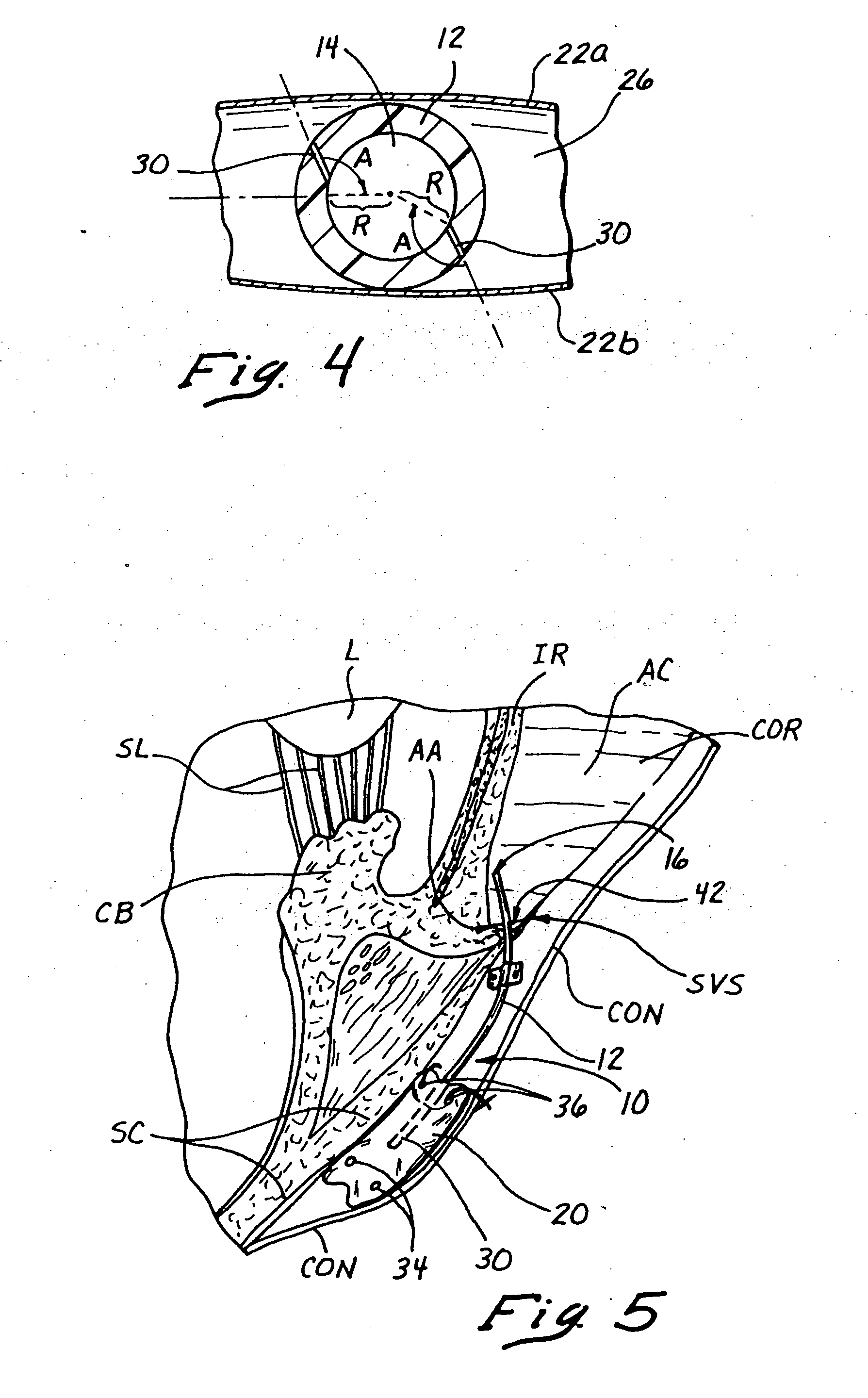
Systems and methods for enhanced implantation of an electrode lead for neuromuscular electrical stimulation of tissue associated with control of the lumbar spine for treatment of back pain, in a midline-to-lateral manner are provided. The implanted lead may be secured within the patient and used to restore muscle function of local segmental muscles associated with the lumbar spine stabilization system without disruption of the electrode lead post-implantation due to anatomical structures.
Owner:MAINSTAY MEDICAL
Implantable Prosthetic Valve
InactiveUS20090276039A1Reduce thrombosisImprove survivalJoint implantsVenous valvesProsthetic valveThrombus
An implantable prosthetic valve for regulating fluid flow through a body vessel is provided. The prosthetic valve comprises an anchoring member, at least one leaflet, and a restraining member capable of temporarily preventing substantial movement of the leaflet between and open and closed position so as to allow fluid flow in the antegrade and retrograde directions. In various embodiments, the prosthetic valve reduces the risk of thrombosis. In various embodiments, the prosthetic valve reduces the appearance of potentially thrombogenic abnormal flow patterns at the site of implantation immediately following the implantation, allows cell deposition making the valve more biocompatible, less thrombogenic before flow changes resulting from valving action set in and allows tissue growth so that a partially or completely biological functioning valve may form on the scaffold provided by the implant.
Owner:CLINASYS
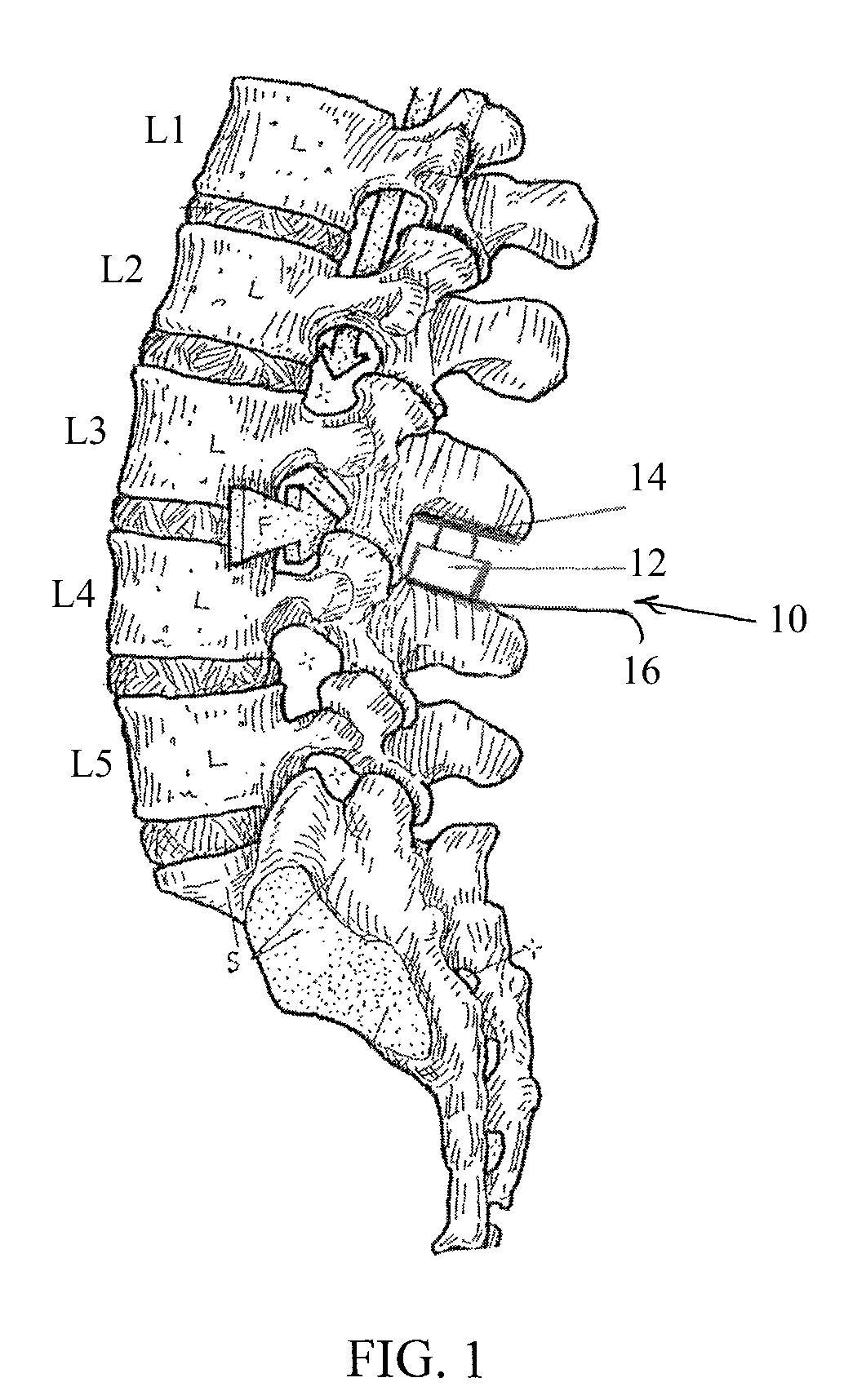
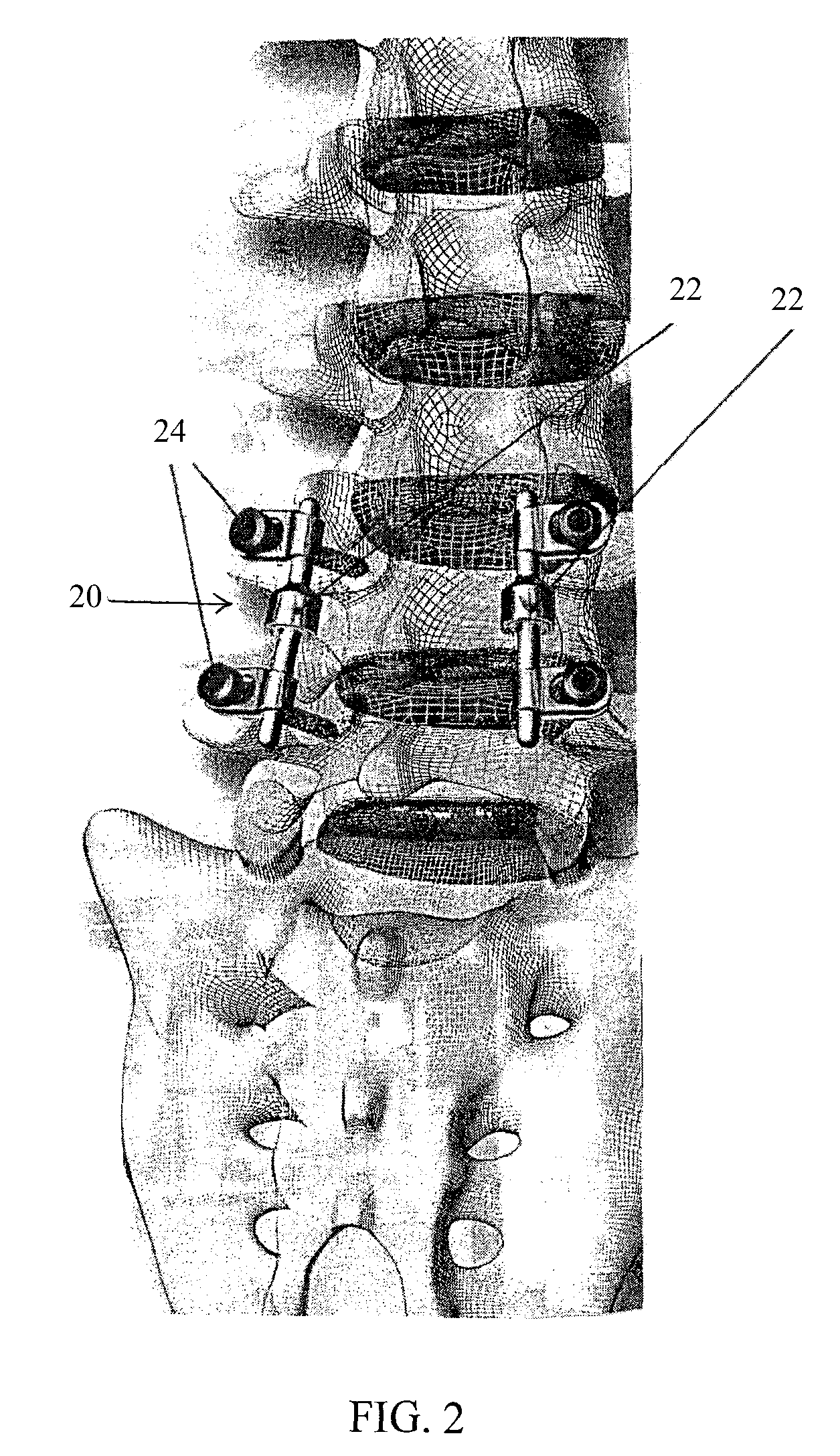
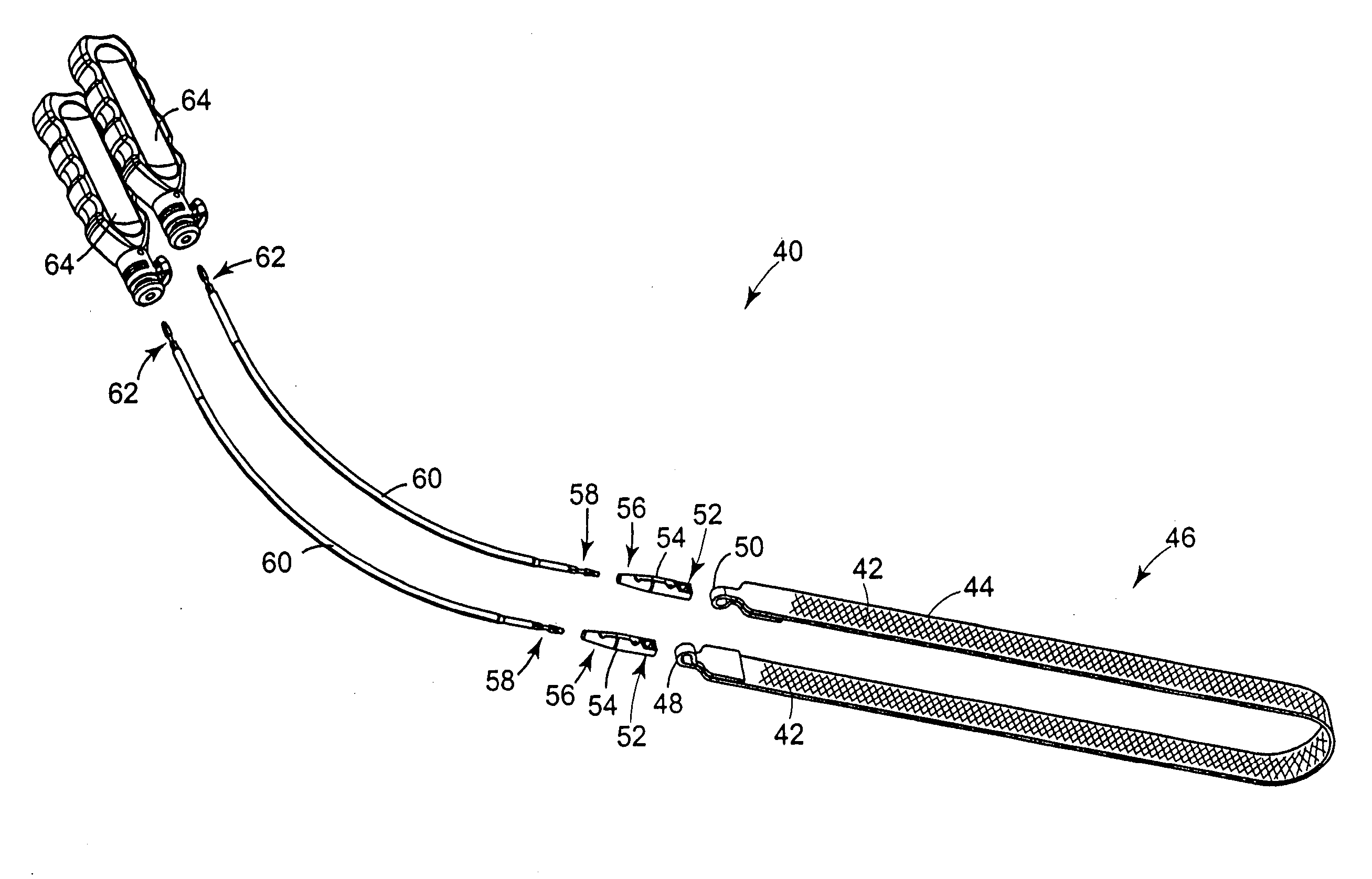
Spinal implant having a post-operative adjustable dimension
ActiveUS8241331B2Suture equipmentsInternal osteosythesisSpinal columnPhysical medicine and rehabilitation
A spinal implant including first spinal attachment member for attaching to a first spinal portion, second spinal attachment member for attaching to a second spinal portion, and a post-implantation variable dimension device disposed between the first and second spinal attachment members, which is operable after completing surgery in which said spinal implant was installed into a patient, to cause relative movement between the first and second spinal attachment members.
Owner:SPINE21 LTD
Navigational markers in implants
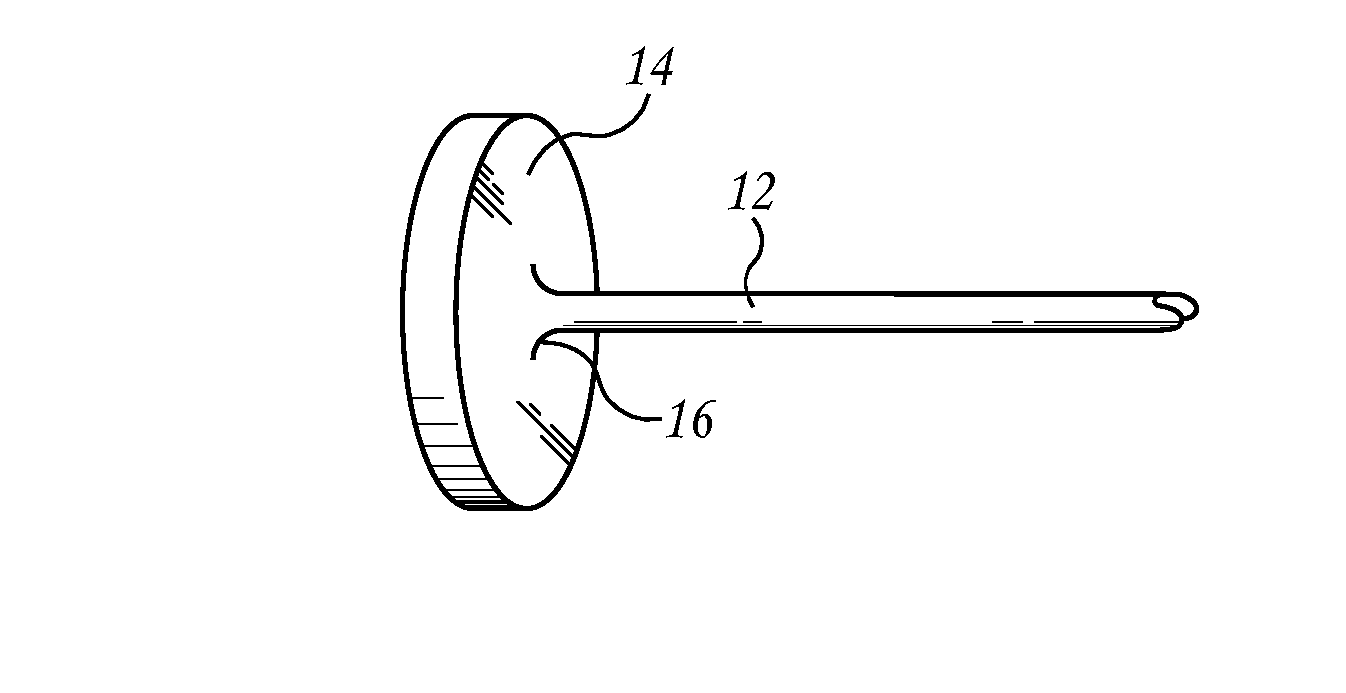
A prosthetic implant for the body having an associated gyroscope, accelerometer and / or magnetometer as the sensor to detect changes in position of the implant. The device incorporates a miniature gyroscope, accelerometer, and / or magnetometer as a permanent part of the prosthesis for navigational information. Alternatively, the magnetometer can be external to the implant when the implant has magnetic elements associated with it. The sensors can be microelectromechanical system (MEMS) sensors placed within a drilled hole and sealed with a screw or a cap (20). The implant can be responsive to an external interrogation and powering systems. The device provides information so as to help to properly place the prosthetic implant during surgery and to assess proper functioning. The gyroscope, accelerometer, and / or magnetometer are used after implantation to provide diagnostic information in situ based upon the changed positioning or motion of the device in the prosthesis. The device can be a total joint replacement, dental implant or other type of prosthetic implant.
Owner:JOHNSON LANNY L
Treatment of bioprosthetic tissues to mitigate post implantation calcification
InactiveUS6878168B2Lower potentialReduce the temperatureSuture equipmentsPharmaceutical delivery mechanismTreatment resultsMedicine
Bioprosthetic tissues are treated by immersing or otherwise contacting fixed, unfixed or partially fixed tissue with a glutaraldehyde solution that has previously been heat-treated or pH adjusted prior to its contact with the tissue. The prior heat treating or pH adjustment of the glutaraldehyde solution causes its free aldehyde concentration to decrease by about 25% or more, preferably by as much as 50%, and allows a “stabilized” glutaraldehyde solution to be obtained at the desired concentration and pH for an optimal fixation of the tissue at high or low temperature. This treatment results in a decrease in the tissue's propensity to calcify after being implanted within the body of a human or animal patient. The heat-treated or pH adjusted glutaraldehyde solution may, in some cases, also be used as a terminal sterilization solution such that the calcification-decreasing treatment with the previously treated glutaraldehyde and a terminal sterilization may be carried out simultaneously and / or in a single container.
Owner:EDWARDS LIFESCIENCES CORP
Variable cohesive gel form-stable breast implant
ActiveUS20070135916A1Maintain formReduce and eliminate effectMammary implantsBreast implantSuperior pole
A variable cohesive gel form stabilizing implant is disclosed for augmentation or reconstruction of the breast. The prosthesis of this invention comprises an implantable shell or envelope (not limited to a single shell or envelope), filled with a biocompatible gel, or gels, having alterations in gel cohesiveness to maintain stable form, shape, and dimension after surgical implantation. The gel cohesiveness may increase, with increased volume or dimension of the prosthesis. The variable cohesiveness of the gel filler material may be altered by any means (i.e. chemical, fabrication, etc.). The variable cohesive gel form stabilizing implant has shape retention characteristics to maintain its form, thereby reducing or eliminating the undesirable effects of shell wrinkling, knuckling, scalloping or deformation, which can occur at the upper or lower pole of the prostheses, along the perimeter of the shell or at the base, post-implantation. Finally, the variable cohesive gel form stabilizing implant provides new control and possibilities for achieving and preserving the most natural breast shape.
Owner:ALLERGAN INC
Spinal implant having a post-operative adjustable dimension
A spinal implant (40) including first spinal attachment member (44) for attaching to a first spinal portion (41), second spinal attachment member (46) for attaching to a second spinal portion (42), and a post-implantation variable dimension device disposed between the first and second spinal attachment members, which is operable after completing surgery in which said spinal implant was installed into a patient, to cause relative movement between the first and second spinal attachment members.
Owner:SPINE21 LTD
Method for treatment of biological tissues to mitigate post-implantation calcification and thrombosis
A method of treating a biological tissue including contacting the biological tissue with an aqueous sterilizing solution, and maintaining the aqueous sterilizing solution at a temperature of about 50° C. for a time period of about 1 to 2 days. The method of treating a biological tissue may be utilized as a terminal sterilization step in a method for fixation of biological tissues, and bioprosthetic devices may be prepared by such fixation method. The fixation method may include the steps of A) fixing the tissue, B) treating the tissue with a mixture of i) a denaturant, ii) a surfactant and iii) a crosslinking agent, C) fabricating or forming the bioprosthesis (e.g., forming the tissue and attaching any non-biological components thereto) and D) subjecting the bioprosthesis to the terminal sterilization method. The aqueous sterilizing solution may be glutaraldehyde of about 0.625 weight percent buffered to a pH of about 7.4.
Owner:EDWARDS LIFESCIENCES CORP
Intraocular lens with post-implantation adjustment capabilities
Disclosed are accommodating intraocular lenses for implantation in an eye having an optical axis. In certain embodiments, an intraocular lens includes an anterior optic, a posterior optic, and a support structure configured to move the optics relative to each other along an optical axis between an accommodated state and an unaccommodated state. In certain embodiments, at least a portion of the support structure can be modified in situ to alter reaction forces between the support structure and at least one structure of the eye. In certain embodiments, a refractive property of one of the anterior or posterior optics can be modified in situ while leaving the refractive properties of the remaining one of the anterior or posterior optics substantially unaffected. Additional embodiments and methods are also disclosed.
Owner:VISIOGEN
Methods and Devices For to Treatment of Obesity
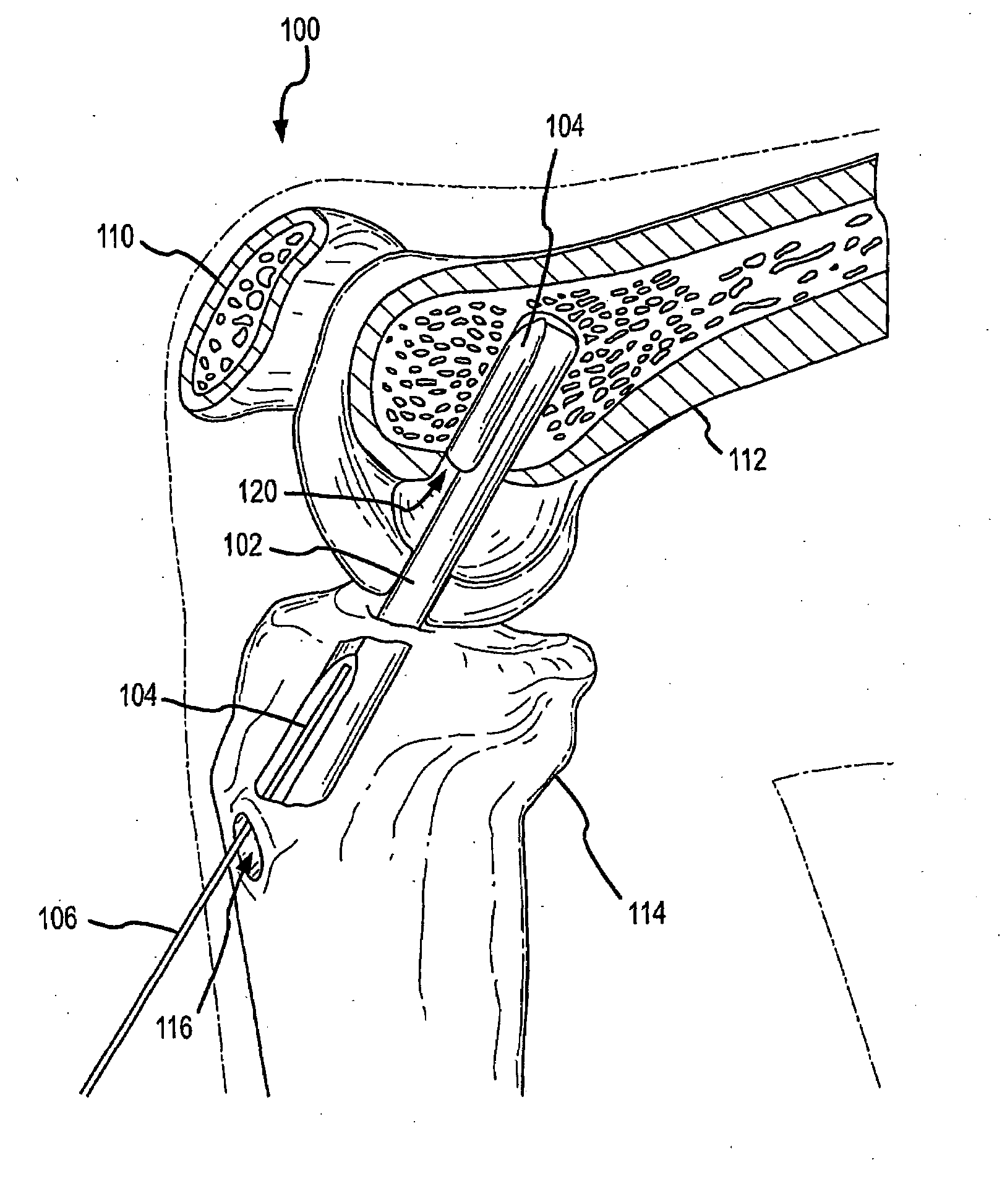
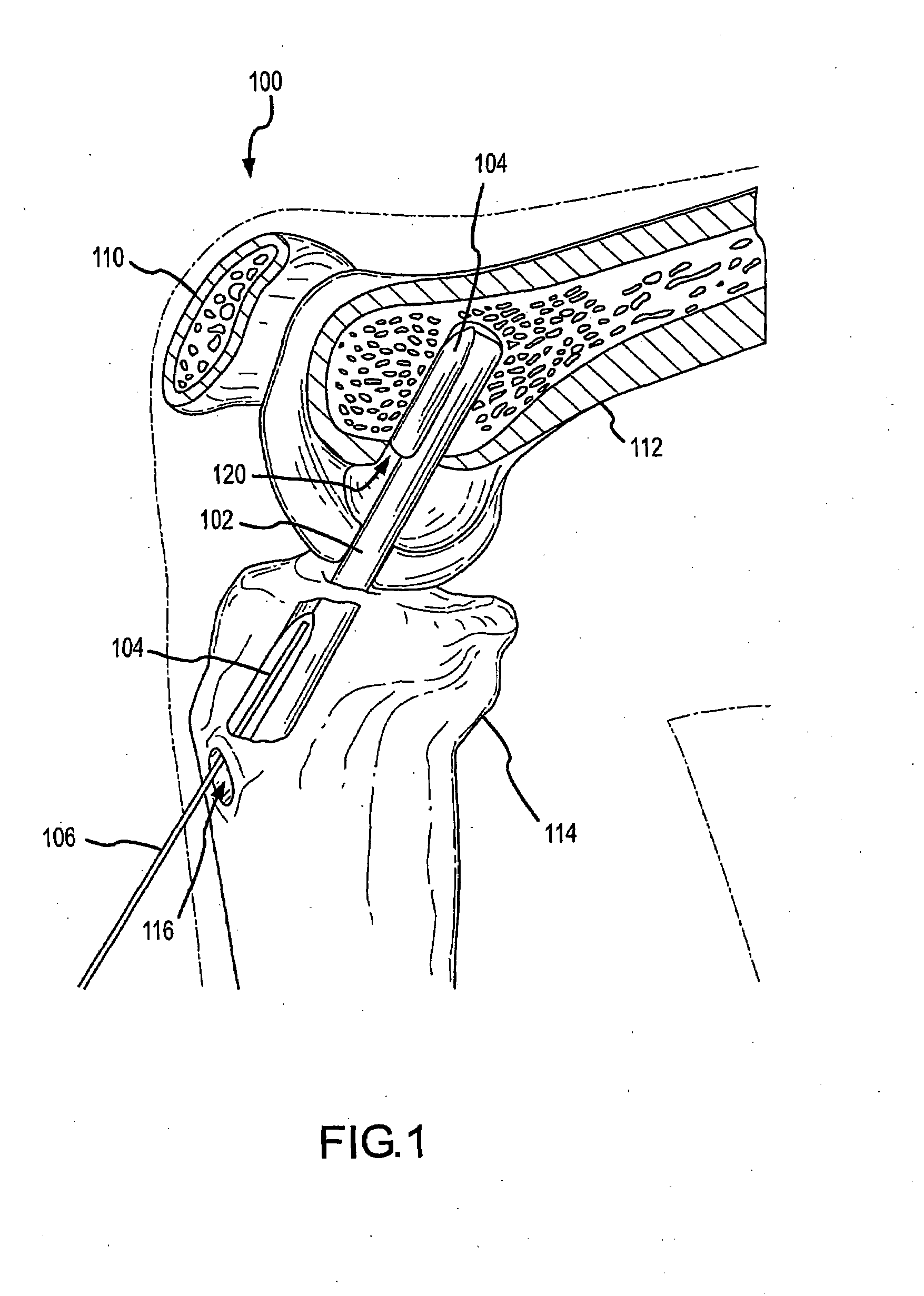
InactiveUS20080051824A1Increase heightLonger-term implantationSuture equipmentsElectrotherapyStomach partCatheter
Methods and devices to externally create a restriction on the stomach are described. In some embodiments, the devices are contoured to fit the stomach and can be further anchored to the stomach. In further embodiments, the degree of deployment of the extragastric restriction device is controllable after implantation. In other embodiments, specialized wires, catheters, ports, and trocars specific for placement of extragastric restriction devices are presented. In still further embodiments, systems are described in which adjustability of the devices is provided along with sensing and actuating ability.
Owner:GERTNER MICHAEL
Percutaneous mitral annulplasty with cardiac rhythm management
InactiveUS20110009957A1Increase frictionTransvascular endocardial electrodesHeart valvesCoronary sinusHemodynamics
A minimally invasive method of performing mitral annuloplasty is disclosed. An implantable device is positioned within the coronary sinus and tightened around the mitral annulus. Mitral valve regurgitation is monitored before, during, and / or after the tightening step. An on-going drug therapy may be determined, taking into account post-implantation hemodynamic function.
Owner:EDWARDS LIFESCIENCES AG
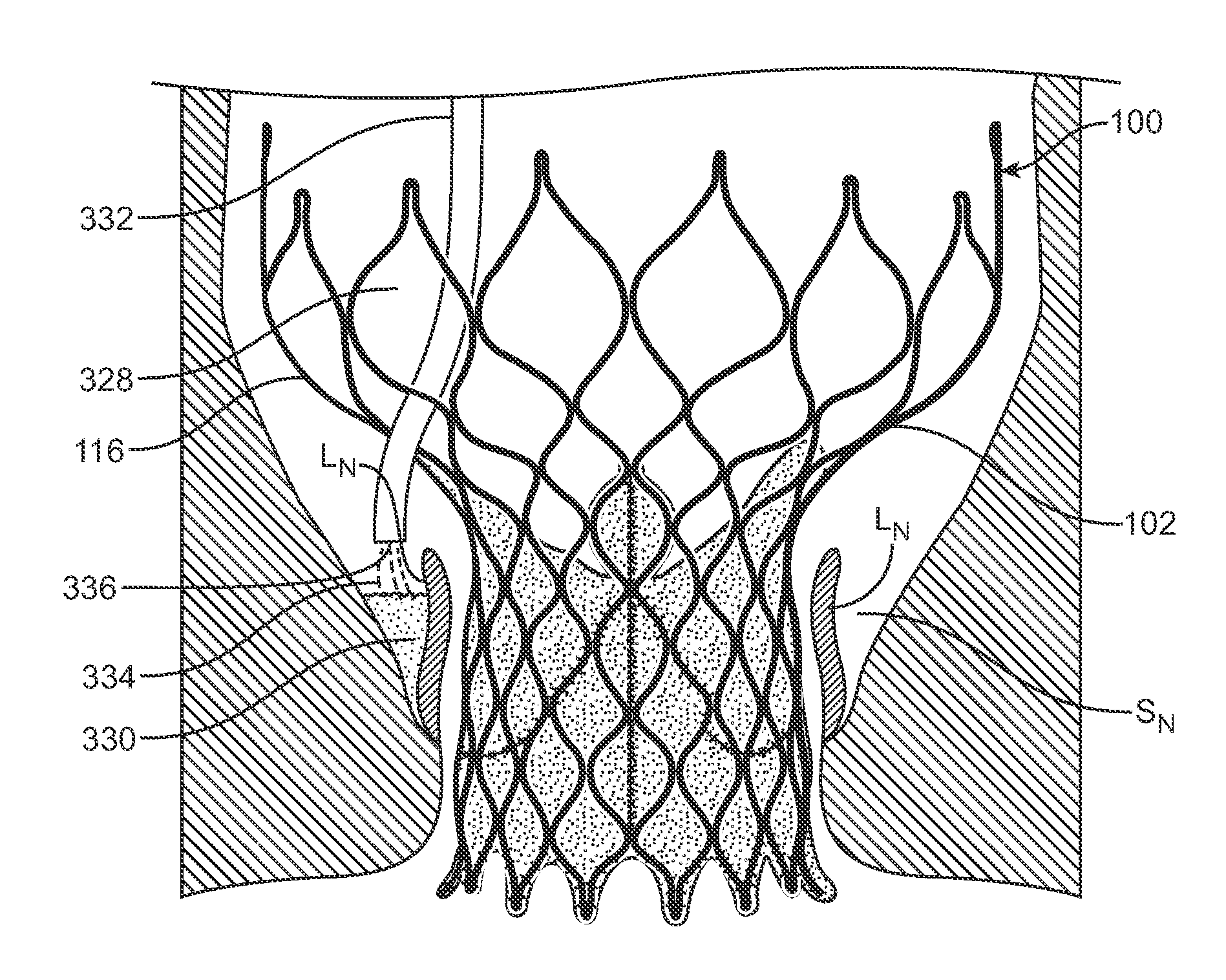
Methods and Devices for Repairing and/or Preventing Paravalvular Leakage Post-Implantation of a Valve Prosthesis
ActiveUS20140114402A1Prevent leakageBalloon catheterHeart valvesParavalvular leakagePost implantation
Devices and methods for delivering a sealing element post-implantation of valve prosthesis that functions to occlude or fill gaps present between the valve prosthesis and the native valve tissue, thereby reducing, minimizing, or eliminating leaks there through. In a first method, an injectable material is placed within a native valve sinus to form a sealing element that presses native valve leaflets against an outer surface of a heart valve prosthesis. In a second method, an injectable sealing material or a preformed annular sealing component is positioned between the outer surface or perimeter of a heart valve prosthesis and native heart valve tissue. In a third method, a preformed sealing element or component is placed within a heart valve prosthesis to press the prosthesis against native valve tissue.
Owner:MEDTRONIC INC
Partially resorbable implants and methods
ActiveUS20170182222A1Reduce stress peaksReduce and eliminate subsidenceSpinal implantsTissue regenerationSide effectPost implantation
Implants including non-resorbable frameworks and resorbable components, as well as methods of use thereof are disclosed. The embodiments include different combinations of a non-resorbable framework (in some case structural and in other cases non-structural), and a resorbable component embedded within and / or around the framework (again, in some cases structural and in other cases non-structural). The disclosed implants provide an efficient means of providing structural support for the vertebral bodies post-implantation, as well as encouraging resorption of the implant and fusion of the associated vertebral bodies without negative side effects and / or failure, such as subsidence of the implant or cracking / fracturing of a portion of the implant when implanted.
Owner:STRYKER EURO OPERATIONS HLDG LLC
Sling delivery system and method of use
InactiveUS20060015001A1Reduce the amount requiredWithout increasing profileSuture equipmentsSurgical furnitureDiseaseSide effect
An apparatus and method of use are disclosed to treat urological disorders. The biocompatible device includes a handle, needle, dilator and sling assembly configured to be minimally invasive and provide sufficient support to the target site. In addition, the configuration of the sling assembly also allows the sling to be adjusted during and / or after implantation. The device and treatment procedure are highly effective and produce little to no side effects or complications. Further, operative risks, pain, infections and post operative stays are reduced, thereby improving patient quality of life.
Owner:STASKIN DAVID MD DR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com