Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "HPV vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human papilloma virus (HPV) vaccines are vaccines that prevent infection by certain types of human papillomavirus. Available vaccines protect against either two, four or nine types of HPV. All vaccines protect against at least HPV types 16 and 18, which cause the greatest risk of cervical cancer. It is estimated that the vaccines may prevent 70% of cervical cancer, 80% of anal cancer, 60% of vaginal cancer, 40% of vulvar cancer and possibly some mouth cancer. They additionally prevent some genital warts, with the vaccines against HPV types 4 and 9 providing greater protection.
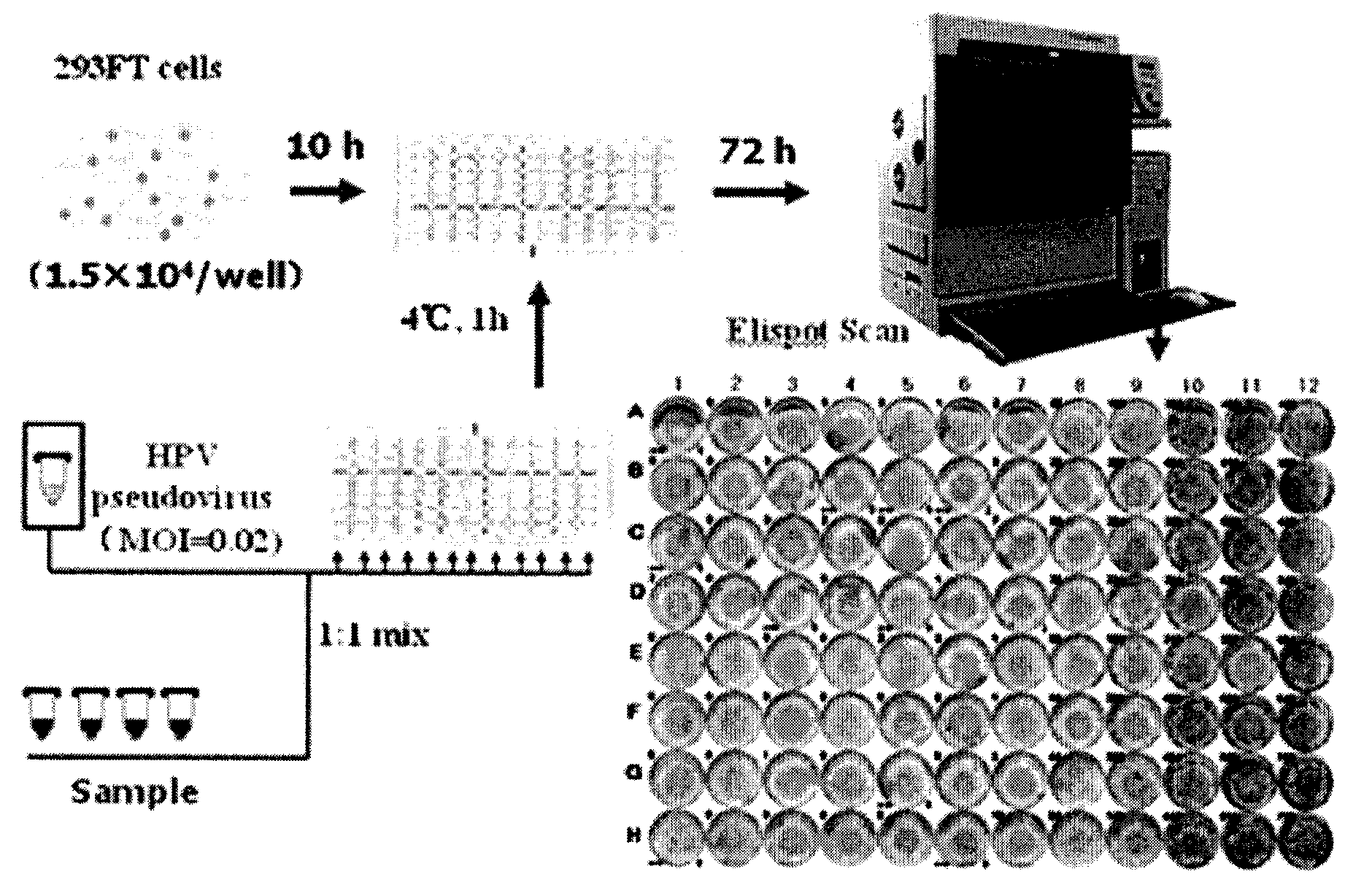
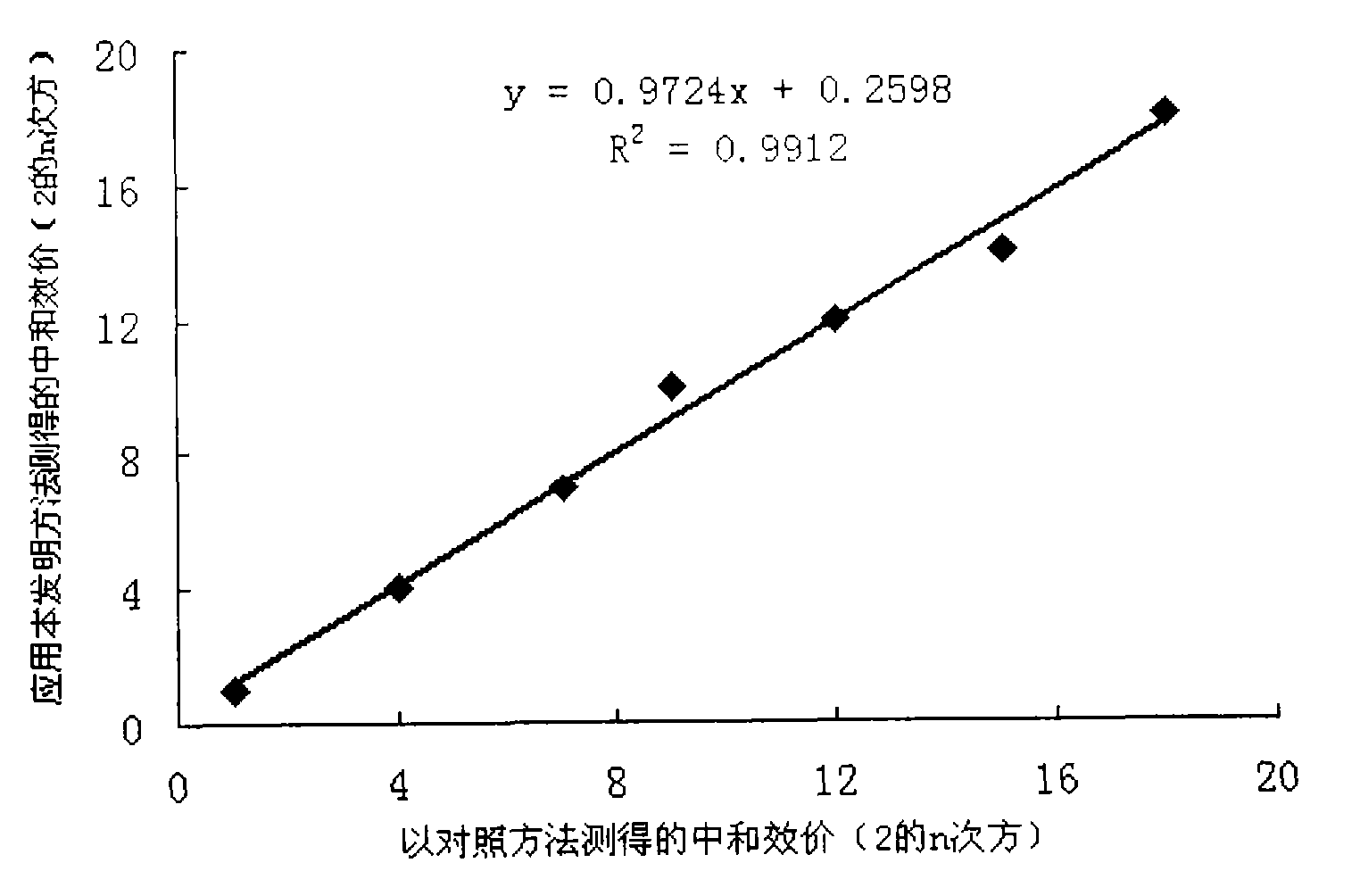
Method for detecting human papilloma virus neutralizing antibody
InactiveCN101551392AThe detection method is simpleFast detection methodBiological testingHPV vaccinesAlphapapillomavirus
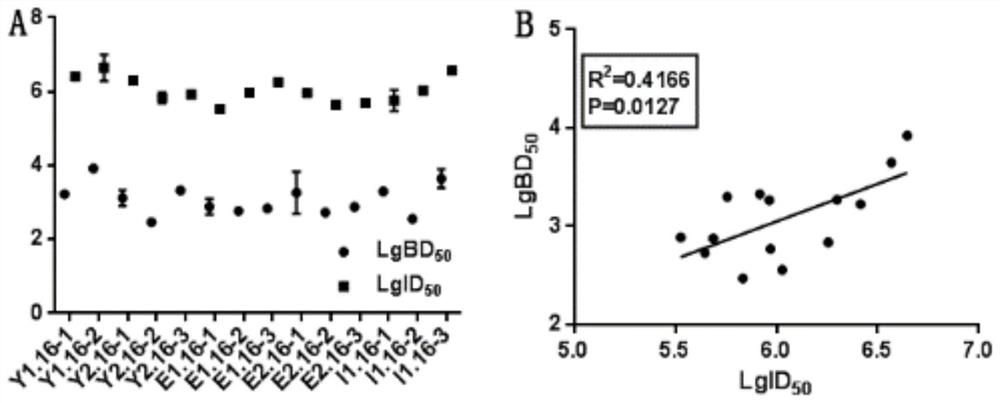
The invention relates to a high-efficiency and simple method for detecting a human papilloma virus (HPV) neutralizing antibody, which can be applicable to high-throughput detection of the human papilloma virus neutralizing antibody. The method is characterized by using a spot detection method for detecting cells infected by human papilloma virus or pseudotype virus. The invention further discloses applications of the method in screening and identifying the neutralized monoclonal antibody against HPV, evaluating the immunity protectiveness of HPV vaccine, the potency test (ED50 test) of HPV vaccine, etc.
Owner:XIAMEN UNIV +1
HPV vaccines and methods for using the same
ActiveUS8168769B2SsRNA viruses negative-senseSsRNA viruses positive-senseHPV vaccinesRecombinant vaccines
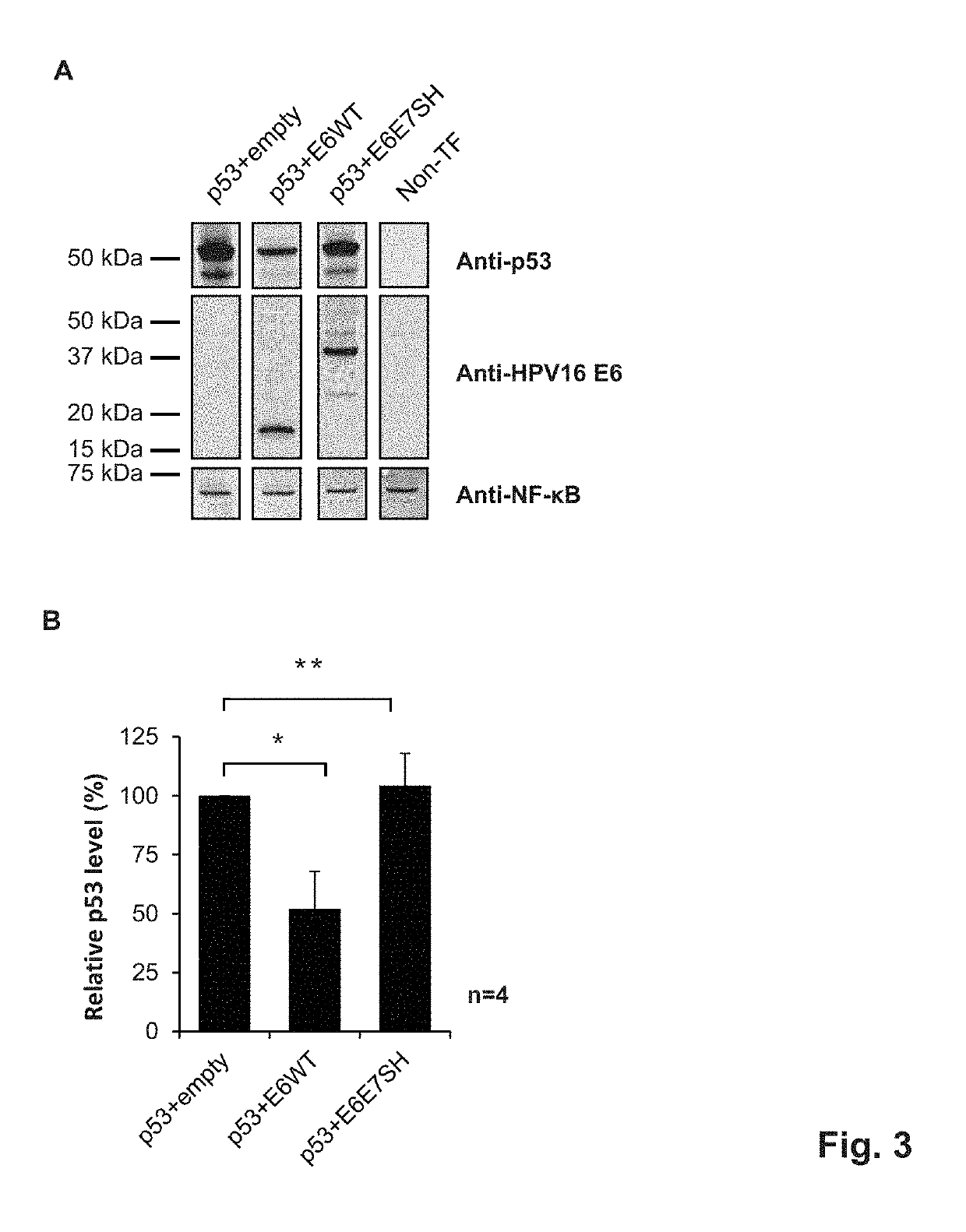
Improved anti-HIV immunogens and nucleic acid molecules that encode them are disclosed, Immunogens disclosed include those having consensus sequences for HIV Subtype A Envelope protein, those having consensus sequences for HIV Subtype B Envelope protein, those having consensus sequences for HIV Subtype C Envelope protein, those having consensus sequences for HIV Subtype D Envelope protein, those having consensus sequences for HIV Subtype B consensus Nef-Rev protein, and those having consensus sequences form HIV Gag protein subtypes A, B, C and D. Improved anti-HPV immunogens and nucleic acid molecules that encode them; improved anti-HCV immunogens and nucleic acid molecules that encode them; improved hTERT immunogens and nucleic acid molecules that encode them; and improved anti-Influenza immunogens and nucleic acid molecules that encode them are disclosed. Pharmaceutical composition, recombinant vaccines comprising and live attenuated pathogens are disclosed as well methods of inducing an immune response in an individual against HIV, HPV, HCV, hTERT and Influenza are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
HPV CD8+ T-cell epitopes
InactiveUS7153659B2Improving immunogenicitySugar derivativesPeptide/protein ingredientsEpitopeHPV vaccines
The present invention provides means to identify functional CD8+ T-cell epitopes in any protein of interest. The present invention further provides CD8+ T-cell epitopes of various proteins. In additional embodiments, the present invention provides epitopes suitable for use in prophylactic and / or therapeutic vaccines. In particularly preferred embodiments, the present invention provides modified epitopes suitable for use in prophylactic and / or therapeutic vaccines.In some preferred embodiments, the present invention provides means for the development of HPV vaccines, in particular multivalent vaccines for the prevention of infection with high-risk HPV strains. In particular, the present invention provides means to identify CD8+ T-cell epitopes in HPV strains such as HPV 16 and HPV 18. In additional embodiments, the present invention provides means for the development of therapeutic vaccines against high-risk HPV types that prevent the development of benign and / or malignant tumors in infected individuals. The present invention further provides epitopes suitable for use in prophylactic and therapeutic vaccines.
Owner:GENENCOR INT INC
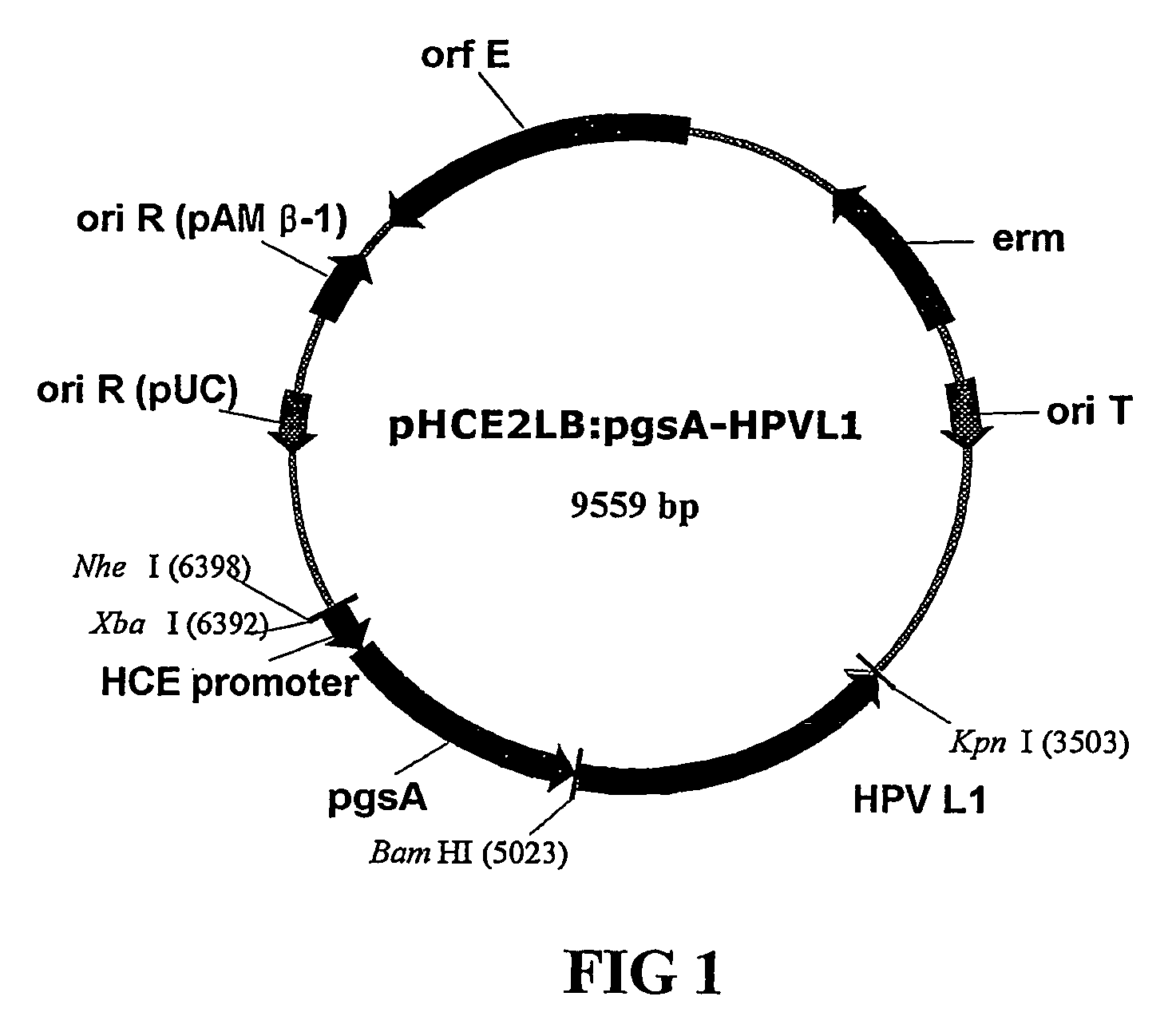
Vector for anti-hpv vaccine and transformed microorganism by the vector
ActiveUS20050249752A1Effective preventionInduce responseOrganic active ingredientsBacteriaTumor-Related ProteinSurface display
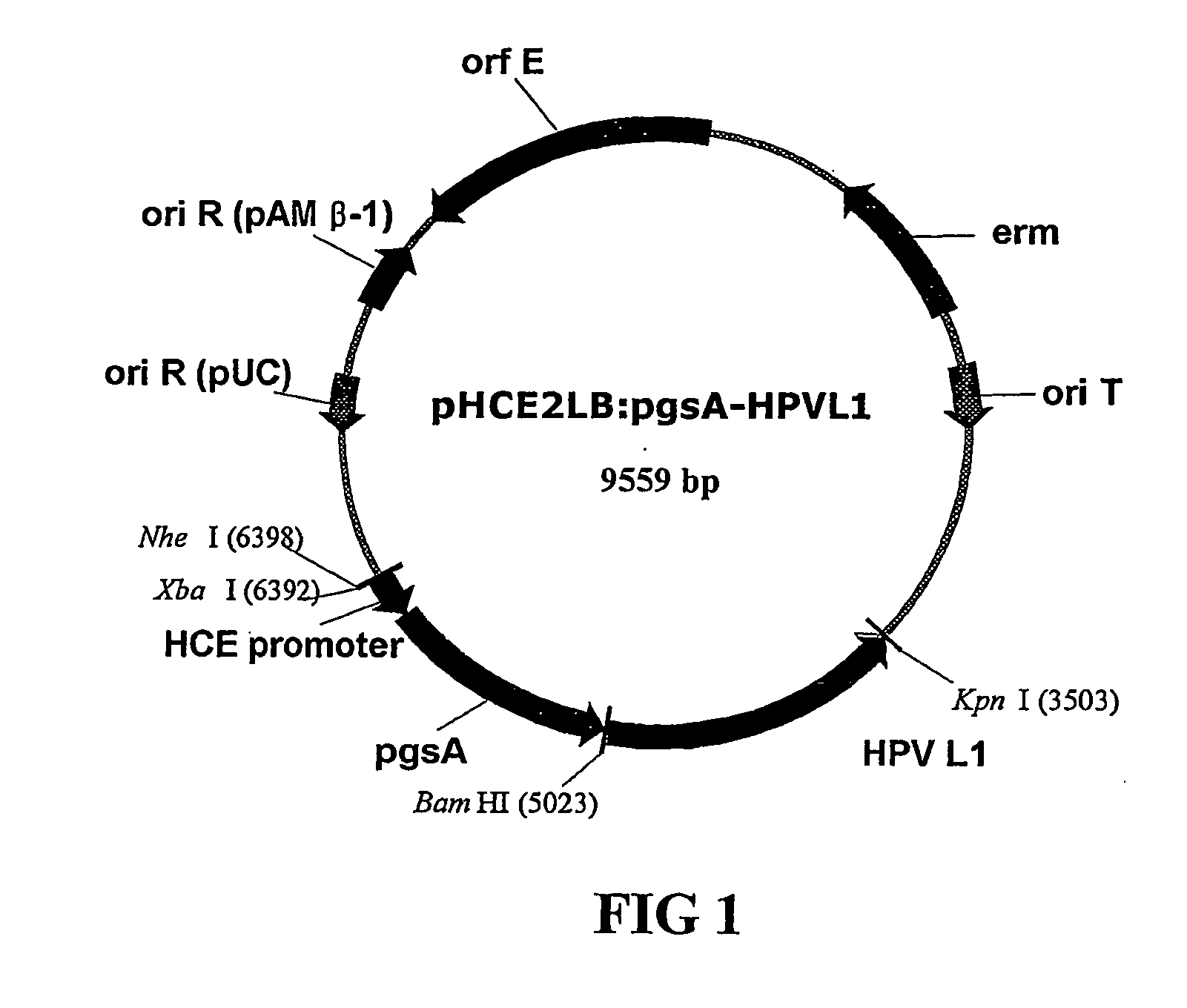
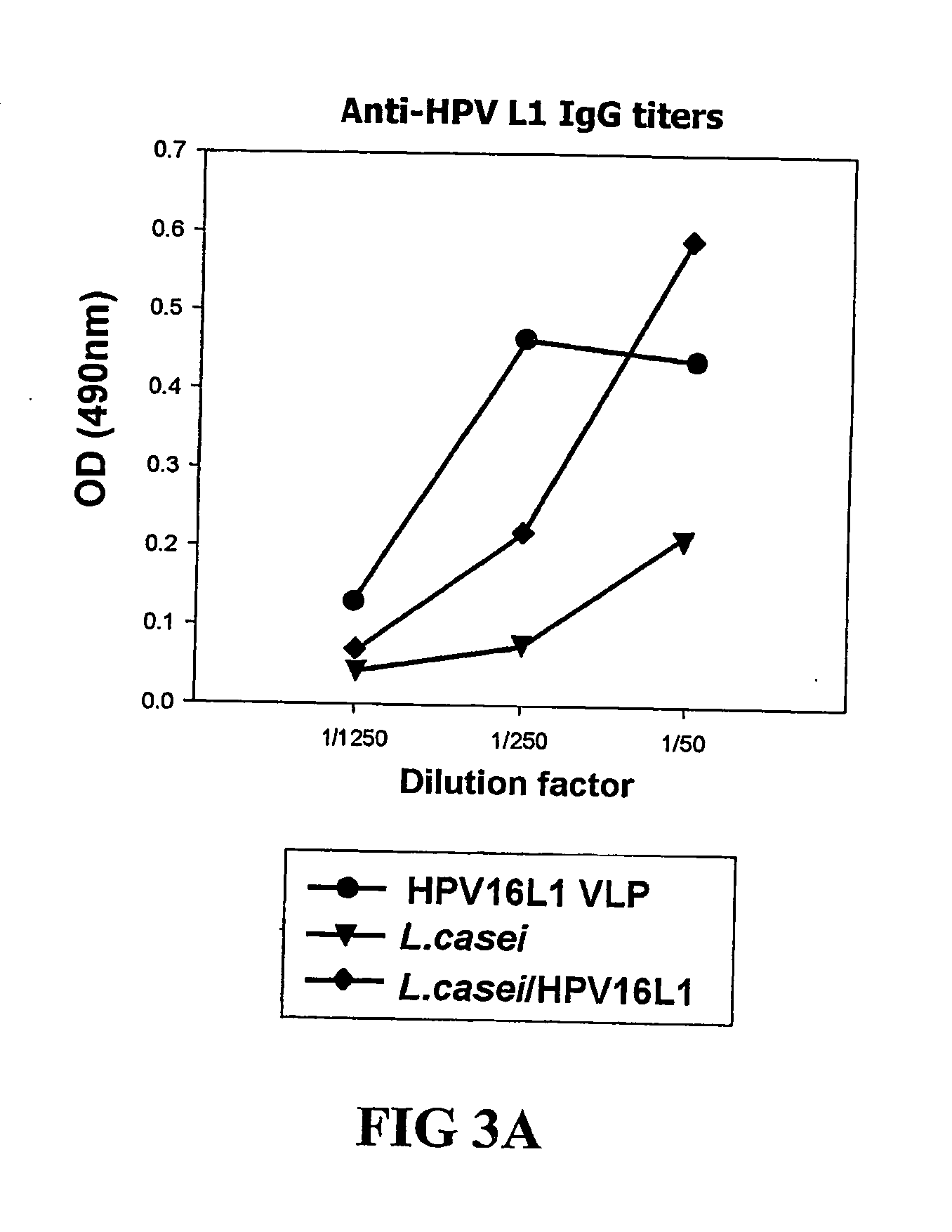
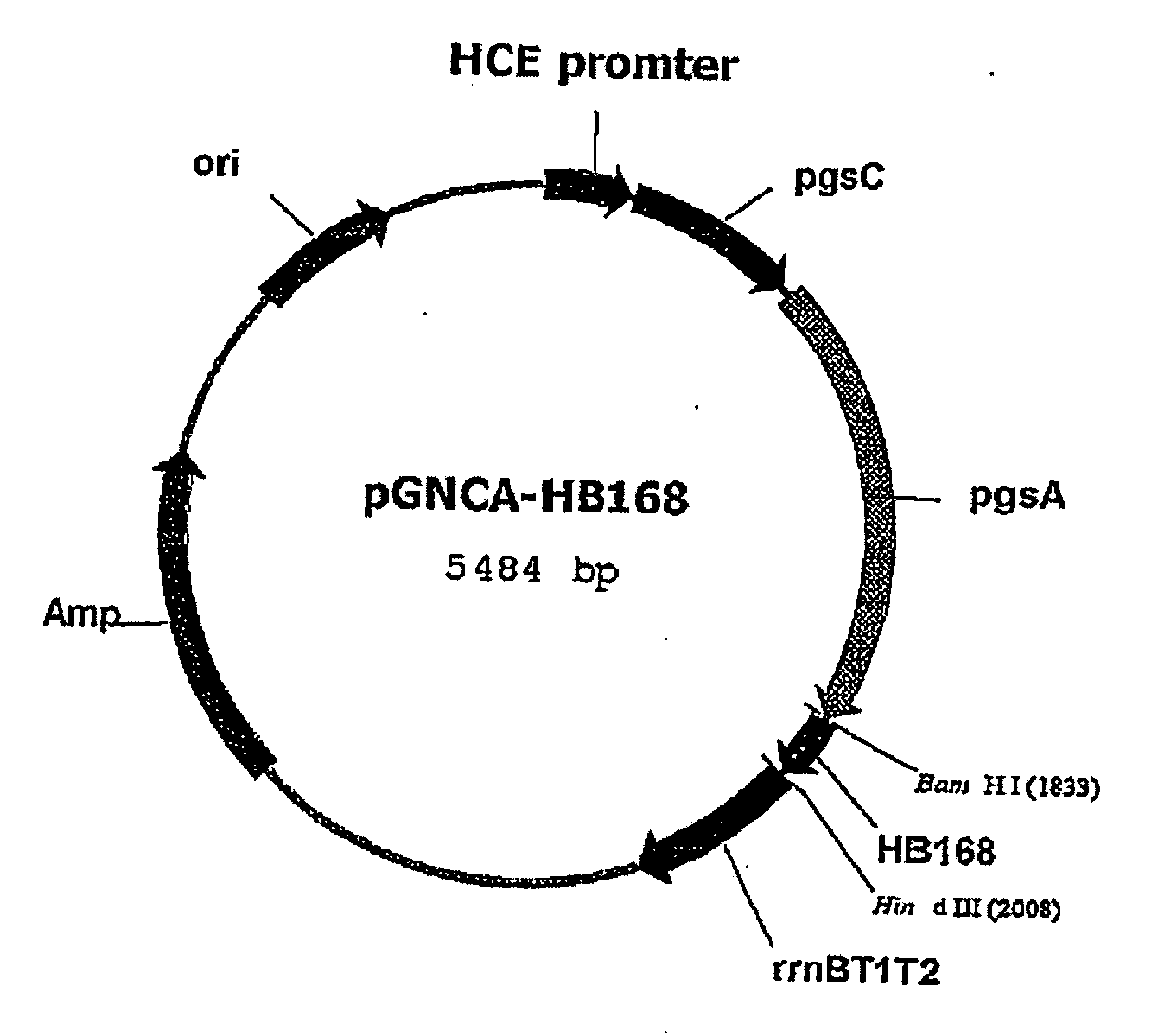
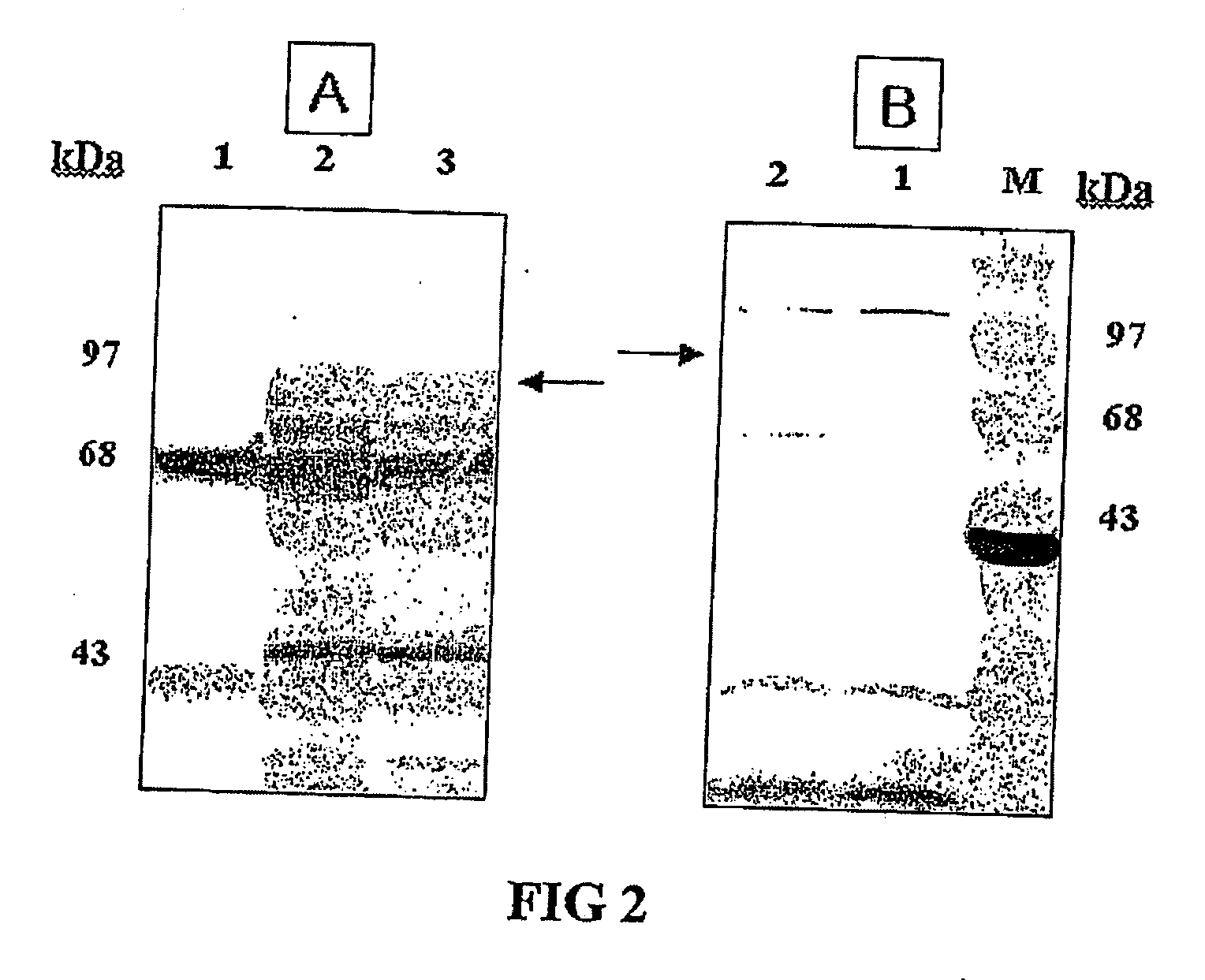
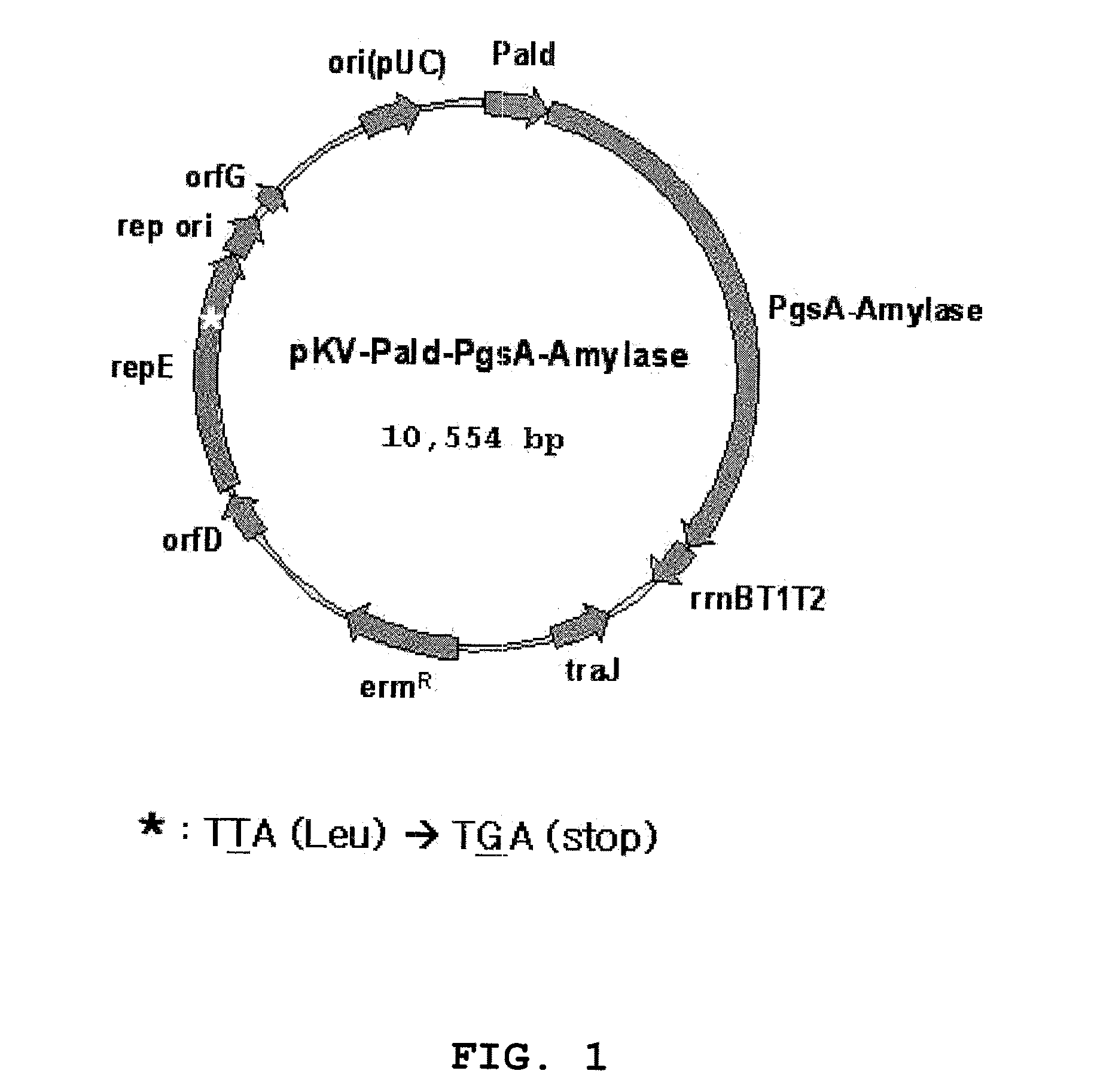
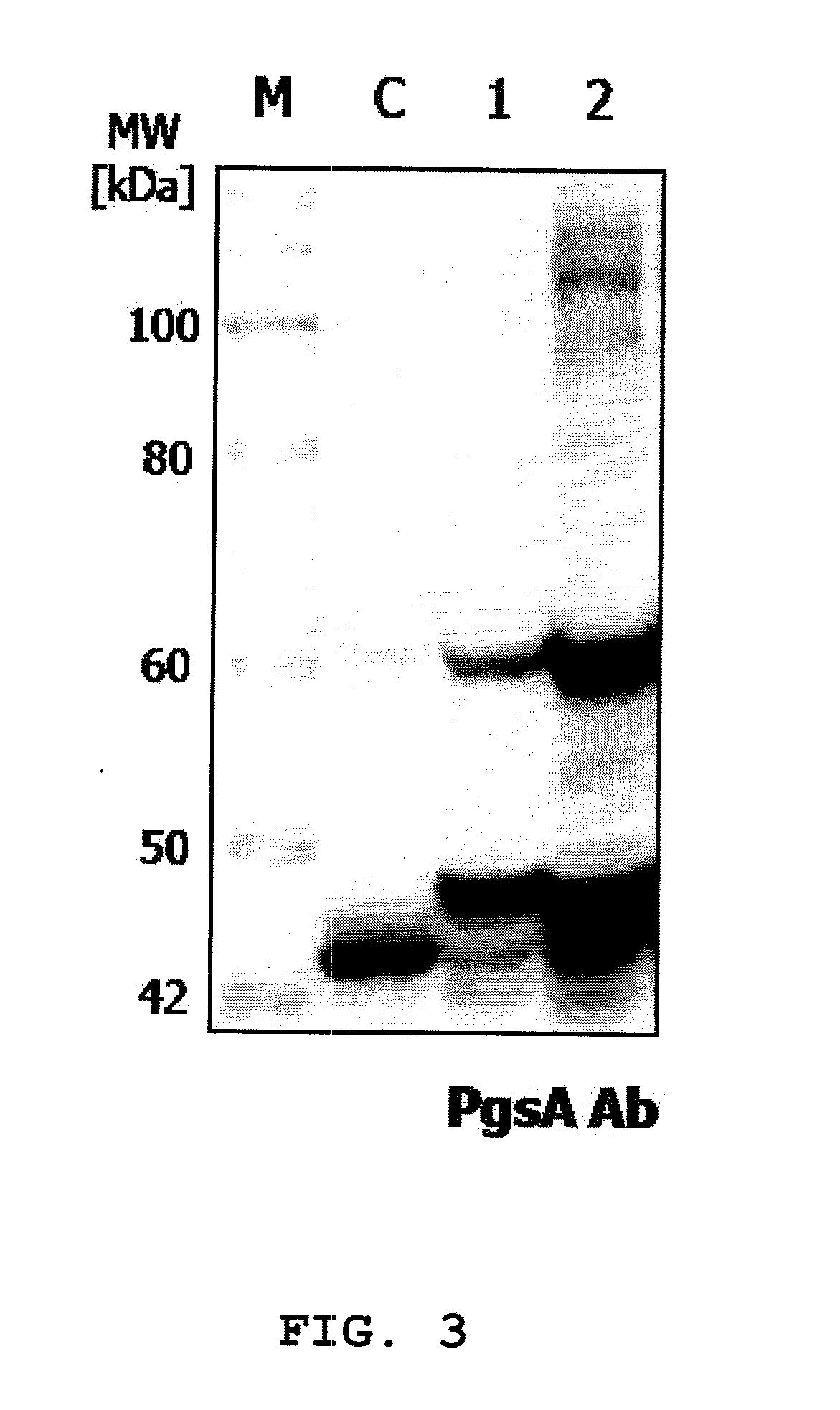
Expression vectors are described that can efficiently produce virion capsid protein, tumor-associated protein of human papillomavirus on a microbial surface. Bacterial strains harboring such surface display vectors, and the use of the bacterial strains or their extracts or purified products as complex vaccines, are also described. The surface display vectors contain one or more than two genes selected from among pgsB, pgsC and pgsA, encoding a poly-χ-glutamic acid synthetase complex (pgsBCA) of a Bacillus sp. strain, and genes that encode virion capsid proteins, tumor-associated proteins of human papillomavirus. Methodology for preparing the foregoing vectors, vaccines and transformed microorganisms are also described.
Owner:BIOLEADERS CORP
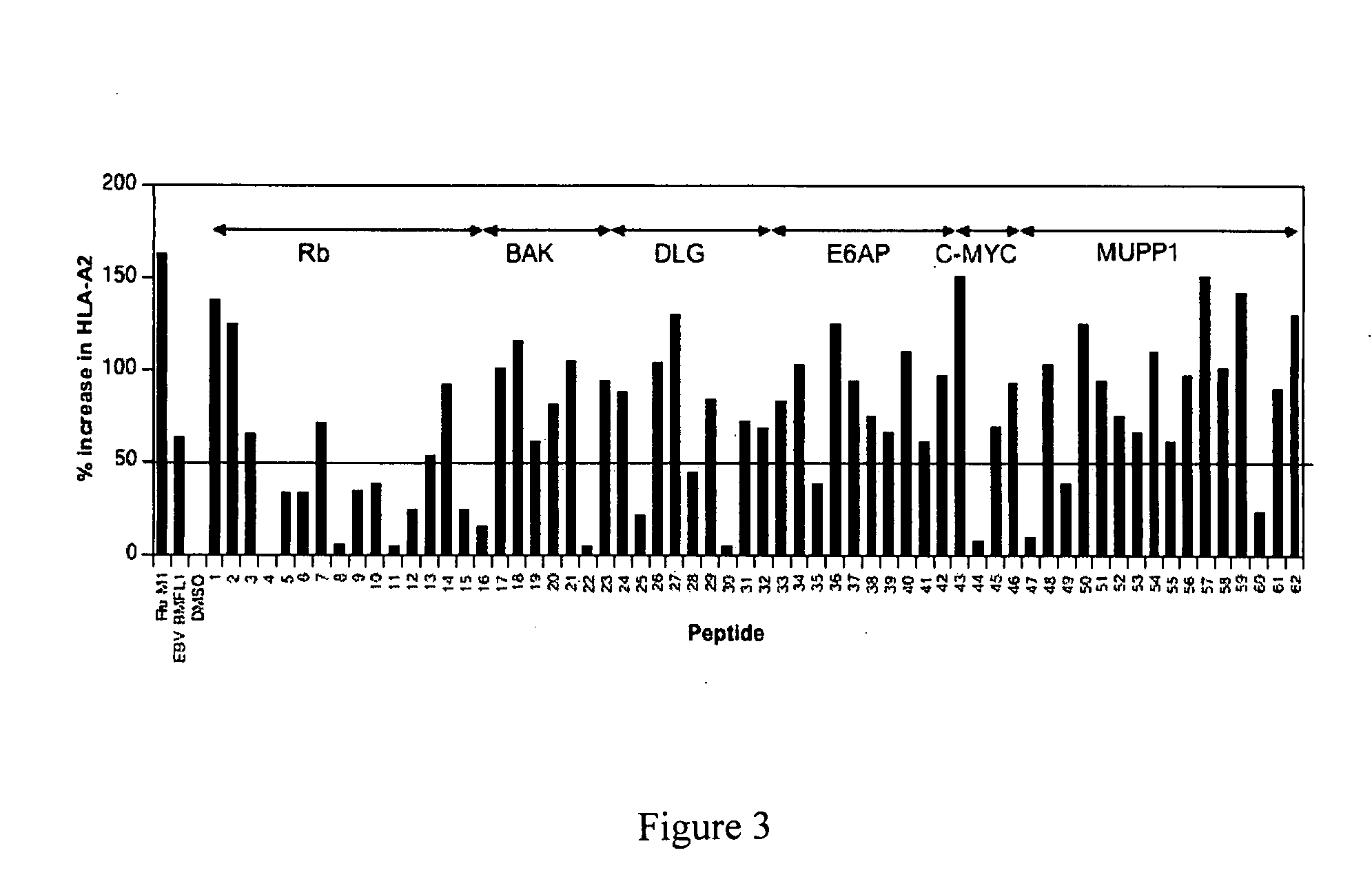
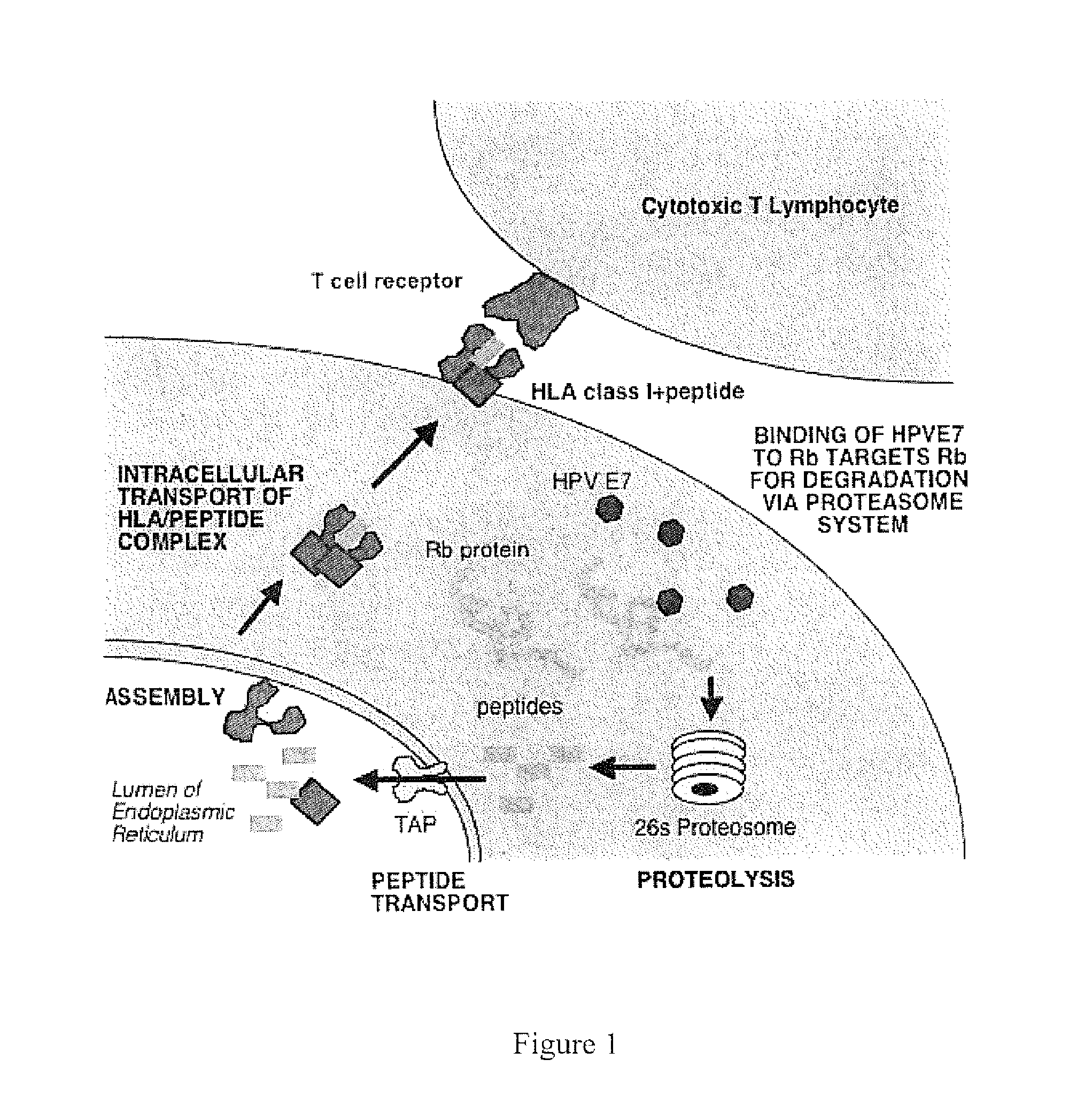
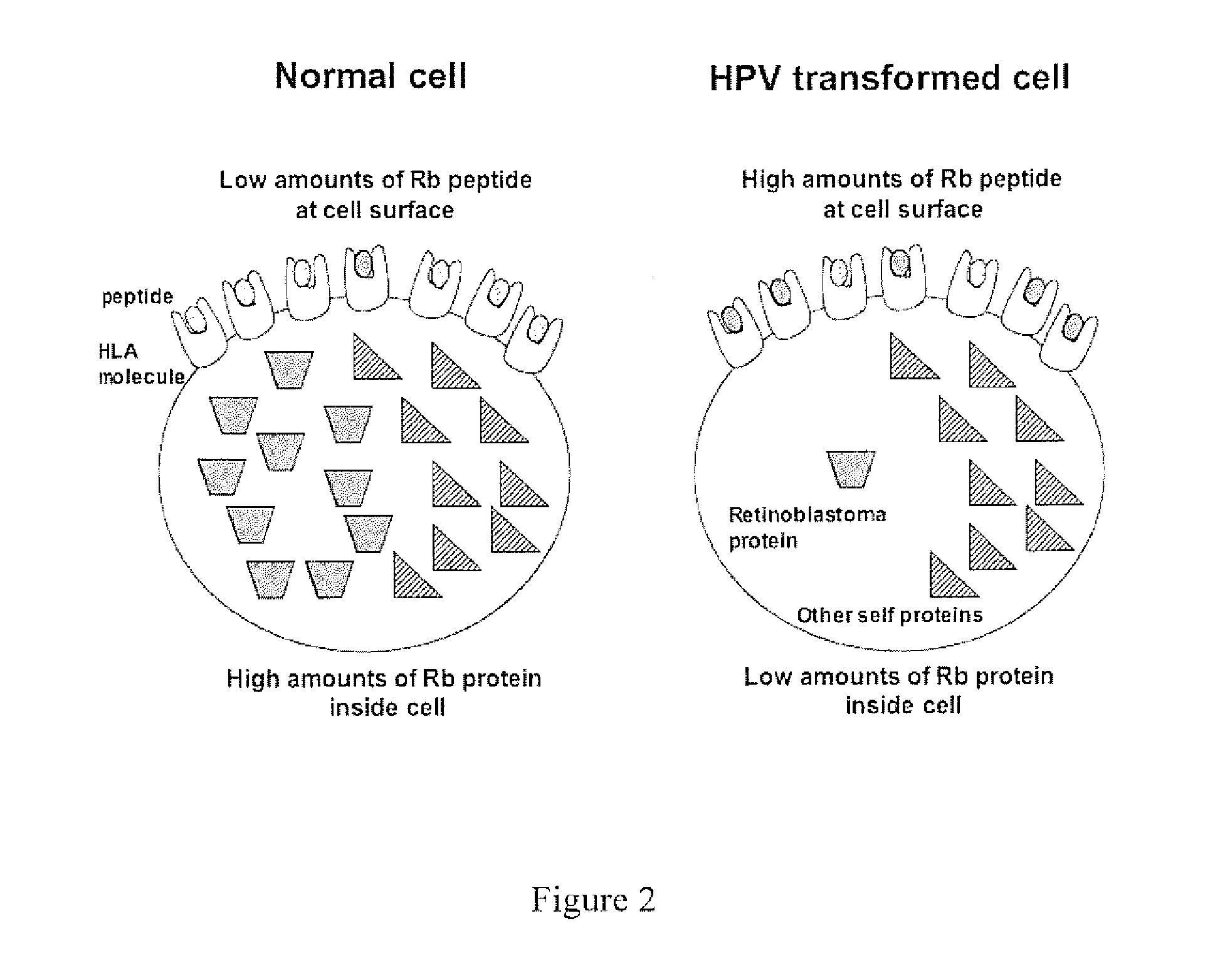
HPV vaccine comprising peptides from host cell proteins
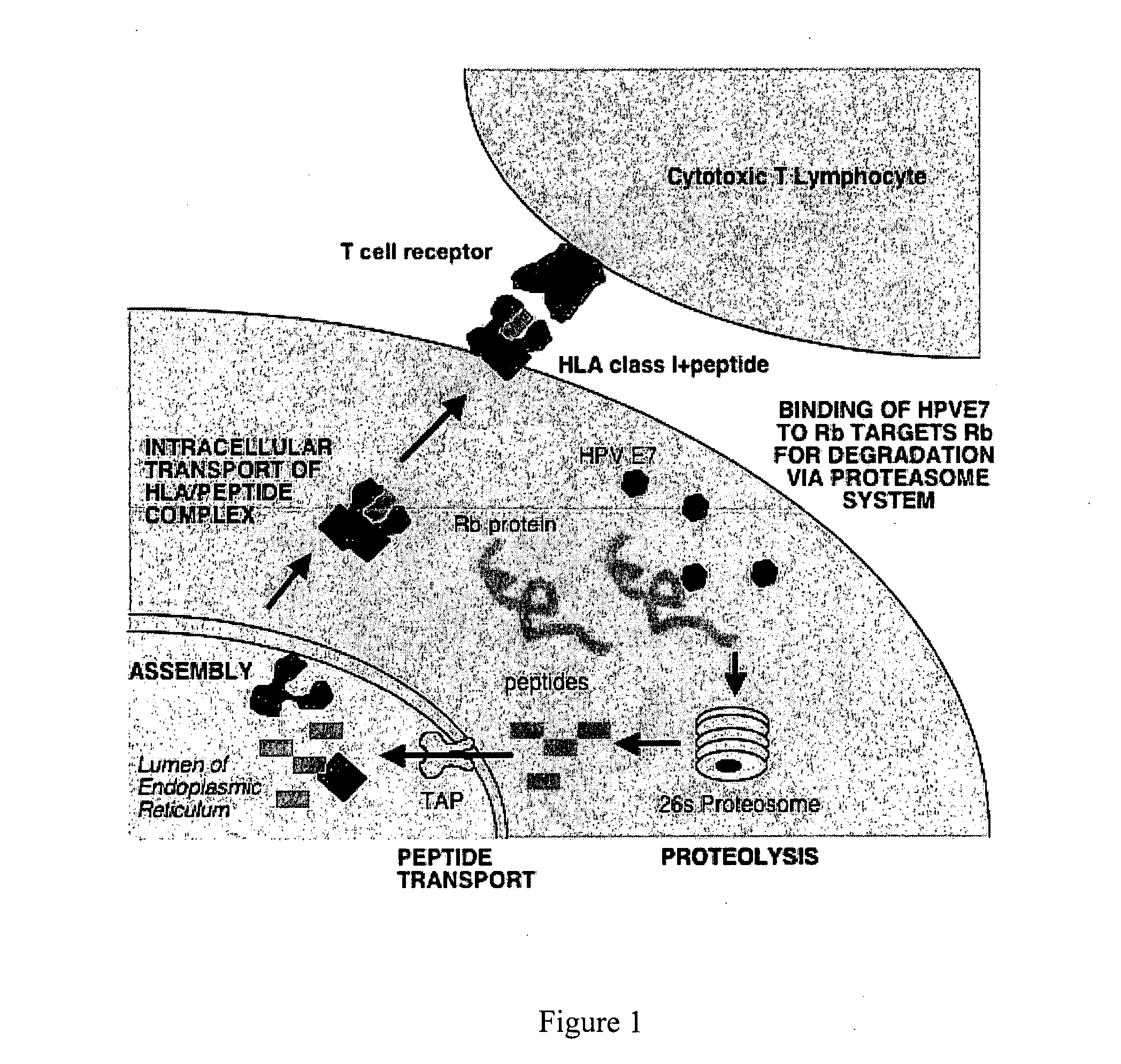
The present invention relates to a human papillomavirus (HPV) vaccine that comprises peptides from host cell proteins and more particularly, a vaccine that is directed against cancers that are associated with HPV infections, such as cervical cancer, head and neck cancer and skin cancers. The peptides comprise fragments of host cell proteins that have been targeted for degradation by HPV proteins, such as E6 and E7 and are presented on the surface of HPV infected cells in relatively large amounts. These peptides can be recognised by CTL and elicit an immune response, and are therefore ideal tumour-specific markers. The invention also relates to novel peptide: peptide complexes such as peptide / HLA complexes and their use in a tumour-specific vaccine.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD
Cd4+ human papillomavirus (hpv) epitopes
InactiveUS20070037151A1Improving immunogenicityViral antigen ingredientsMicrobiological testing/measurementAbnormal tissue growthHPV vaccines
The present invention provides CD4+ T-cell epitopes in E6, E7 and E2 proteins from various strains of human papillomavirus (HPV). In some preferred embodiments, the present invention provides means for the development of HPV vaccines, in particular multivalent vaccines for the prevention of infection with high-risk HPV strains. In additional embodiments, the present invention provides means for the development of therapeutic vaccines against high-risk HPV types that prevent the development of benign and / or malignant tumors in infected individuals. The present invention further provides epitopes suitable for use in prophylactic and therapeutic vaccines.
Owner:GENENCOR INT INC
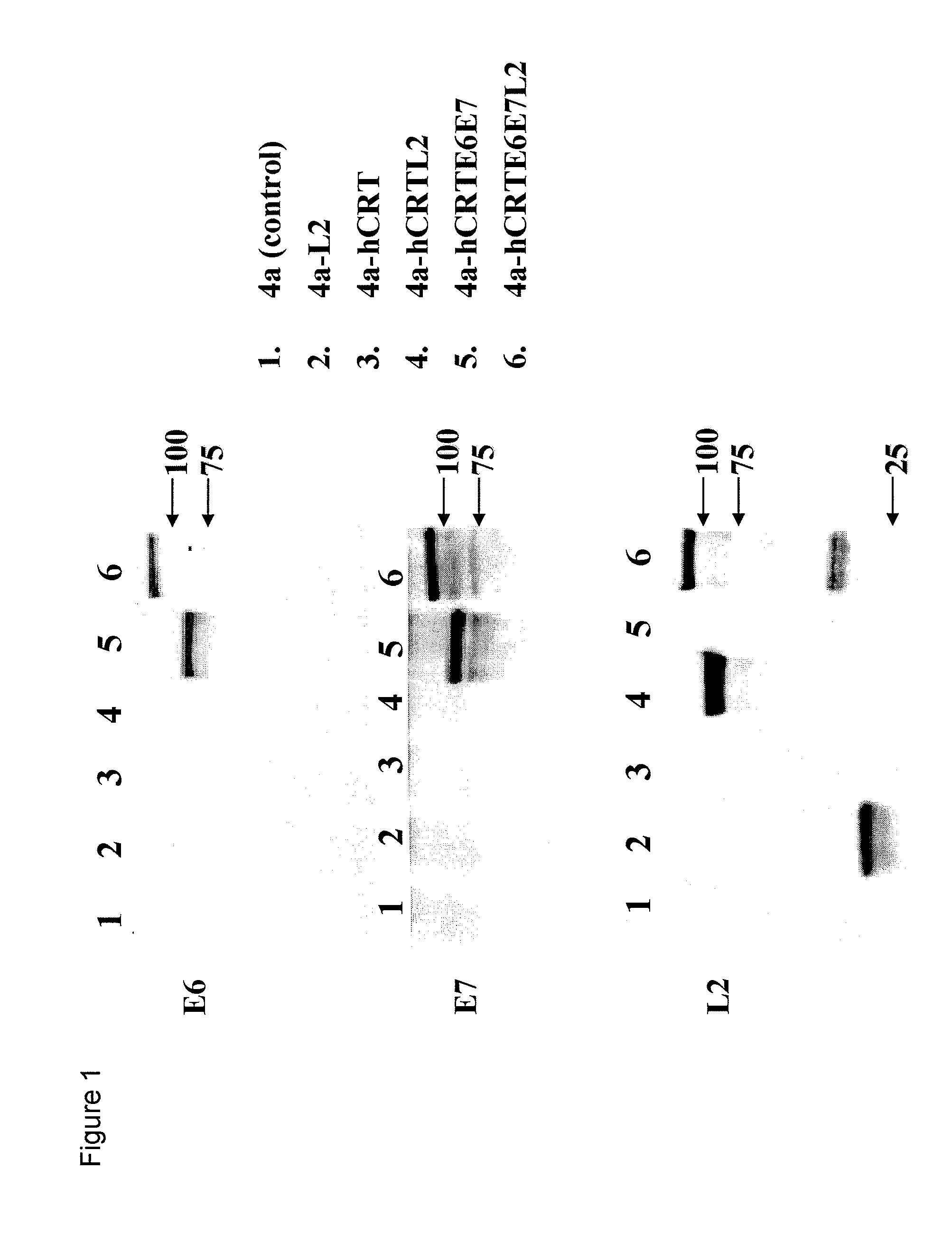
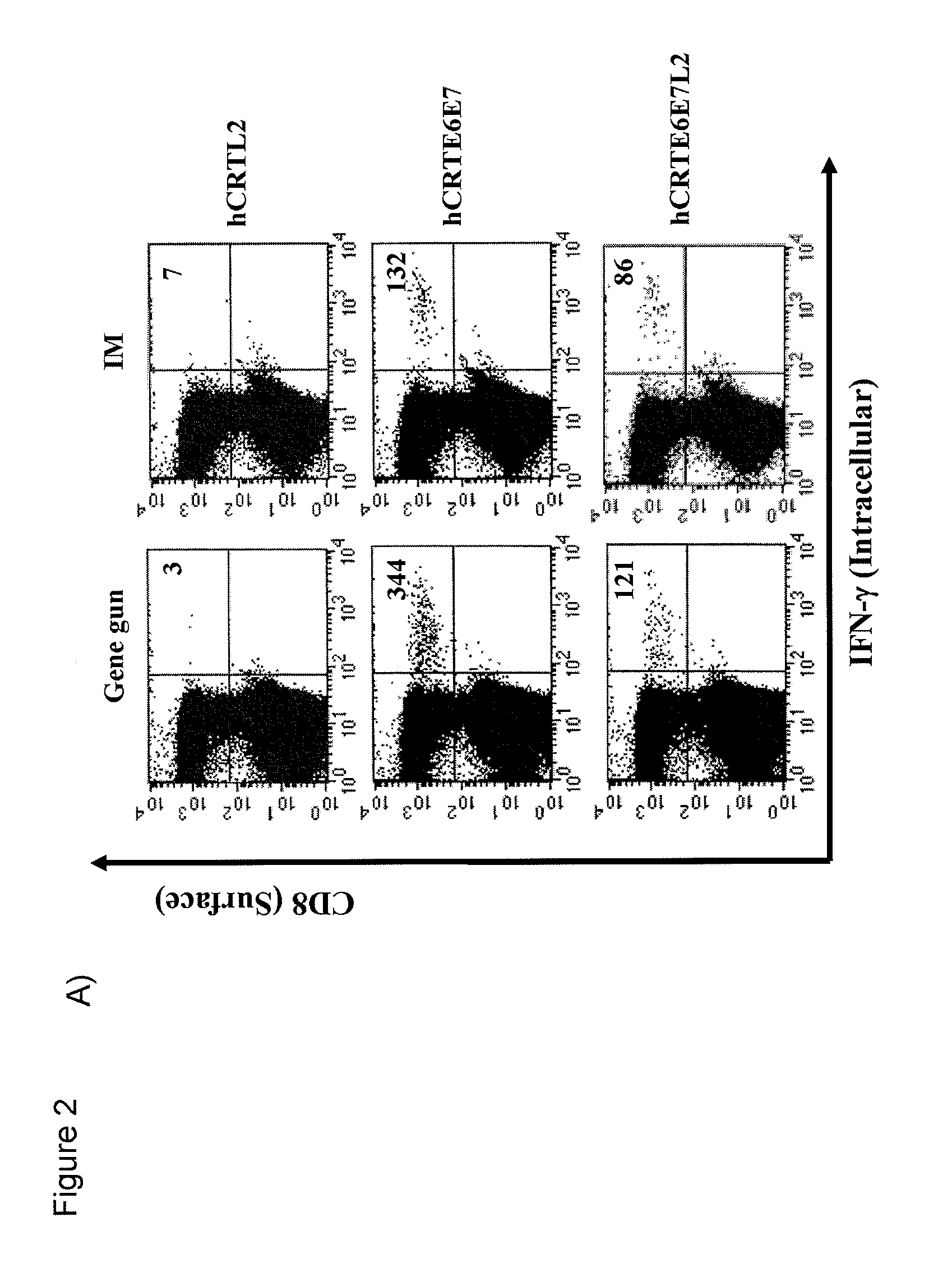
HPV DNA Vaccines and Methods of Use Thereof
InactiveUS20080260765A1Enhance antigen-specific immune responseEnhance immune responseSugar derivativesViral antigen ingredientsDiseaseTreatment effect
Human papillomavirus (HPV) infection is the etiological factor for cervical cancer. Provided are HPV vaccines that generate a humoral immune response to prevent new infection, as well as cell-mediated immunotherapy to eliminate established infection or HPV-related disease. HPV vaccines include nucleic acid sequences encoding HPV16 early proteins E6 and E7. Additional nucleic acid sequences in the vaccines include sequences encoding calreticulin and / or the HPV16 late protein L2. Methods using these vaccines are provided that result in therapeutic effects.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Vector for anti-HPV vaccine and transformed microorganism by the vector
ActiveUS7425438B2Effective preventionInduce responseOrganic active ingredientsBacteriaTumor-Related ProteinSurface display
Expression vectors are described that can efficiently produce virion capsid protein, tumor-associated protein of human papillomavirus on a microbial surface. Bacterial strains harboring such surface display vectors, and the use of the bacterial strains or their extracts or purified products as complex vaccines, are also described. The surface display vectors contain one or more than two genes selected from among pgsB, pgsC and pgsA, encoding a poly-χ-glutamic acid synthetase complex (pgsBCA) of a Bacillus sp. strain, and genes that encode virion capsid proteins, tumor-associated proteins of human papillomavirus, Methodology for preparing the foregoing vectors, vaccines and transformed microorganisms are also described.
Owner:BIOLEADERS CORP
HPV CD8+ T-cell epitopes
InactiveUS20050181458A1Improving immunogenicityPeptide/protein ingredientsVirus peptidesEpitopeHPV vaccines
The present invention provides means to identify functional CD8+ T-cell epitopes in any protein of interest. The present invention further provides CD8+ T-cell epitopes of various proteins. In additional embodiments, the present invention provides epitopes suitable for use in prophylactic and / or therapeutic vaccines. In particularly preferred embodiments, the present invention provides modified epitopes suitable for use in prophylactic and / or therapeutic vaccines. In some preferred embodiments, the present invention provides means for the development of HPV vaccines, in particular multivalent vaccines for the prevention of infection with high-risk HPV strains. In particular, the present invention provides means to identify CD8+ T-cell epitopes in HPV strains such as HPV 16 and HPV 18. In additional embodiments, the present invention provides means for the development of therapeutic vaccines against high-risk HPV types that prevent the development of benign and / or malignant tumors in infected individuals. The present invention further provides epitopes suitable for use in prophylactic and therapeutic vaccines.
Owner:GENENCOR INT INC
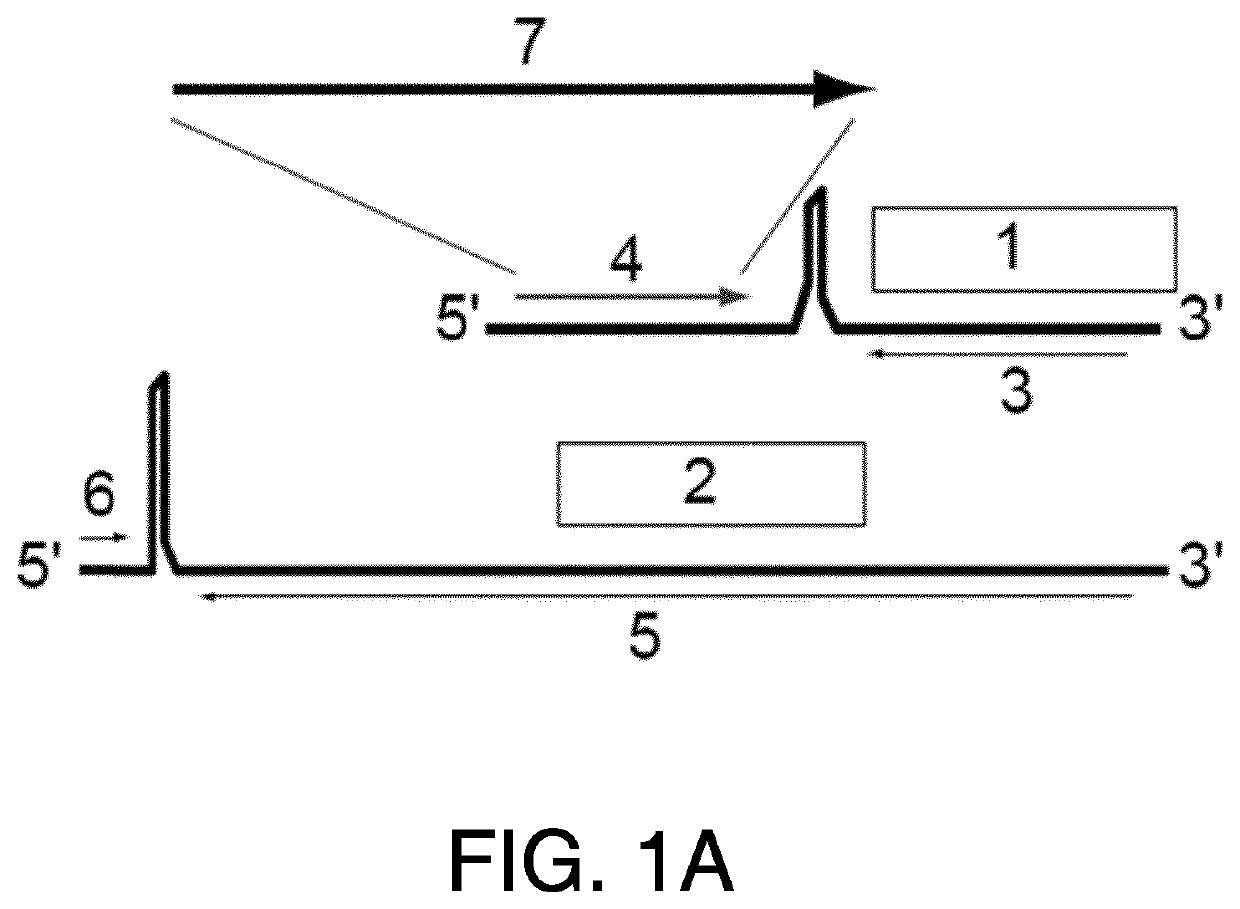
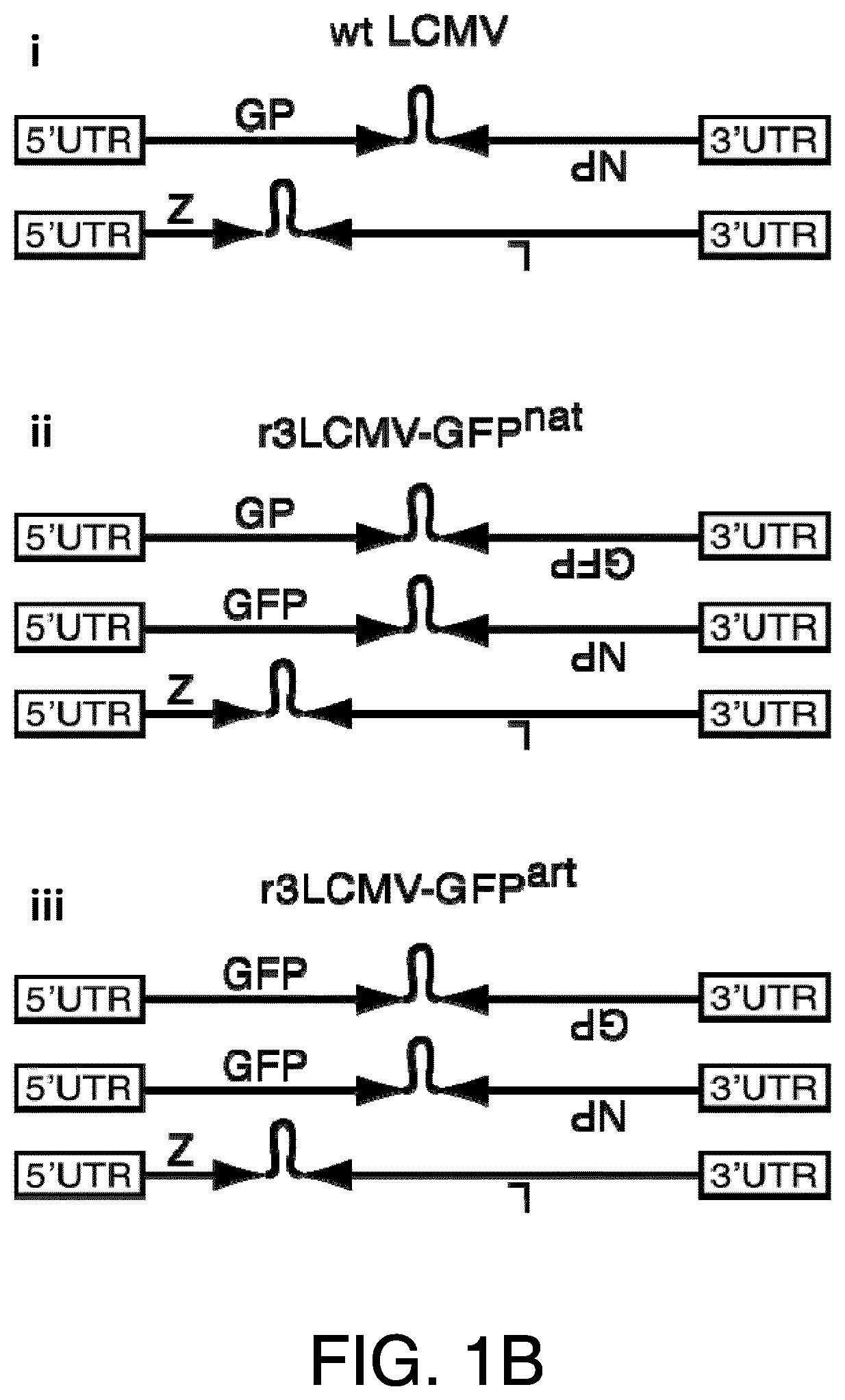
HPV vaccines
ActiveUS10669315B2Reduce severityReduce frequencySsRNA viruses negative-senseViral antigen ingredientsDiseaseHuman papilloma virus infection
Provided herein are genetically modified arenaviruses suitable as vaccines against neoplastic diseases or cancer. The invention also relates to pharmaceutical compositions and methods for the prevention or treatment of certain infections causing neoplastic diseases or cancer, such as infections with oncogenic viruses. Specifically, provided herein are pharmaceutical compositions, vaccines, and methods of preventing or treating diseases and conditions caused by and associated with infections with Human Papillomavirus (HPV), such as cervical cancer, anogenital cancer, head and neck cancer and skin cancers. Also provided herein are immunotherapies for the treatment of a neoplastic disease, such as a neoplastic disease caused by infection with oncogenic viruses.
Owner:HOOKIPA BIOTECH GMBH
Dry Powder Formulations, Vaccines and Methods
Respirable dry powder formulations comprise myo-inositol and leucine. Dry powder human papillomavirus (HPV) vaccine formulations comprise a least one HPV capsid protein and a carrier comprising myo-inositol and leucine. Methods of administering an HPV vaccine to an individual comprise inhalation administration to the individual of a dry powder HPV vaccine formulation comprising a least one HPV capsid protein and a carrier comprising myo-inositol and leucine.
Owner:UNIV OF COLORADO THE REGENTS OF
Preparation method of HPV (human papilloma virus)-specific antibody and freeze-dried preparation of HPV-specific antibody
InactiveCN105859877AHigh potencyLow immunogenicityPowder deliveryEgg immunoglobulinsSucroseSaccharum
The invention relates to the technical field of biological pharmacy, particularly a preparation method of HPV (human papilloma virus)-specific antibody and a freeze-dried preparation of the HPV-specific antibody. The preparation method comprises the following steps: preparing an antigen, immunizing a chicken with the antigen to obtain an antibody, and carrying out enzymolysis purification to obtain the HPV-specific antibody. The freeze-dried preparation of the anti-HPV-infection antibody is prepared from the following raw materials in parts by weight: 0.5-8 parts of polyethyleneglycol, 5-80 parts of mannitose, 1-70 parts of trehalose, 0.1-10 parts of sucrose, 0.1-5 parts of glycine, 0.1-5 parts of arginine and 0.01-1 part of buffer salt. The freeze-dried preparation also comprises the 0.01-0.5 mg / ml HPV-specific antibody. Compared with the preventive HPV vaccine, the HPV-specific antibody has the advantages of short action time, quick effect taking and the like, and is free from the restriction of the ages.
Owner:LANJIATANG BIOLOGICAL MEDICINE FUJIAN CO LTD
HPV vaccines
ActiveUS20210024584A1SsRNA viruses negative-senseViral antigen ingredientsDiseaseHuman papilloma virus infection
Provided herein are genetically modified arenaviruses suitable as vaccines against neoplastic diseases or cancer. The invention also relates to pharmaceutical compositions and methods for the prevention or treatment of certain infections causing neoplastic diseases or cancer, such as infections with oncogenic viruses.Specifically, provided herein are pharmaceutical compositions, vaccines, and methods of preventing or treating diseases and conditions caused by and associated with infections with Human Papillomavirus (HPV), such as cervical cancer, anogenital cancer, head and neck cancer and skin cancers. Also provided herein are immunotherapies for the treatment of a neoplastic disease, such as a neoplastic disease caused by infection with oncogenic viruses.
Owner:HOOKIPA BIOTECH GMBH
HPV vaccine comprising peptides from host cell proteins
The present invention relates to an immunogenic composition for a human papillomavirus (HPV) vaccine that comprises BAX peptides from BAX host cell proteins and more particularly, a vaccine including those peptides that is directed against cancers that are associated with HPV infections, such as cervical cancer, head and neck cancer and skin cancers. The BAX peptides comprise fragments of BAX host cell proteins that have been targeted for degradation by HPV proteins, such as E6 and E7 and are presented on the surface of HPV infected cells in relatively large amounts. These peptides can be recognised by CTL and elicit an immune response, and are therefore ideal tumour-specific markers. The invention also relates to novel peptide: peptide complexes such as BAX peptide / HLA complexes and their use in a tumour-specific vaccine.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD
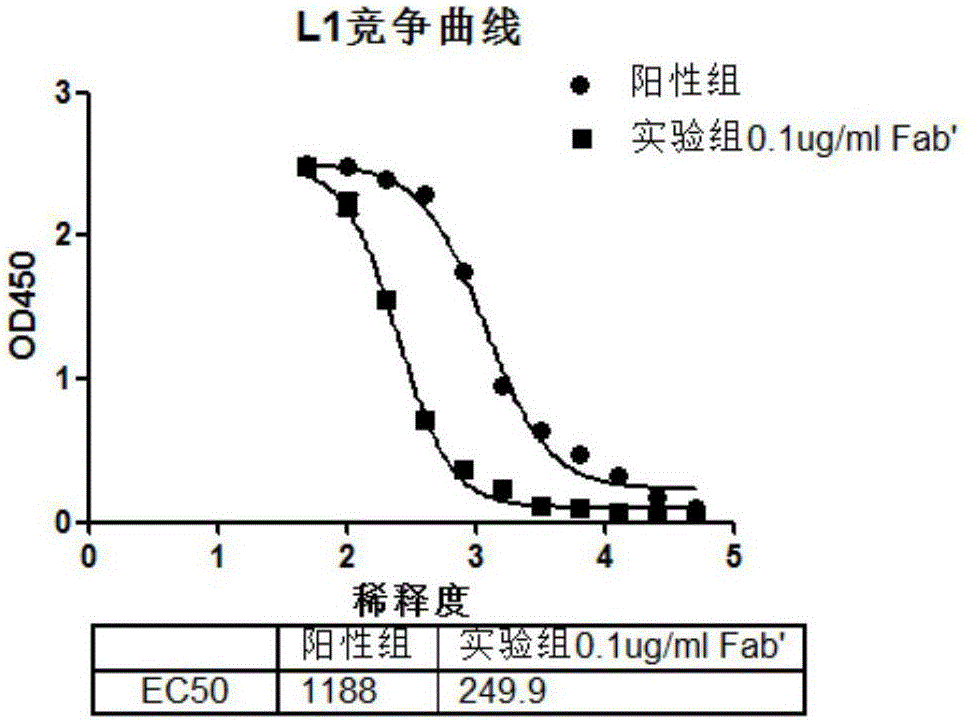
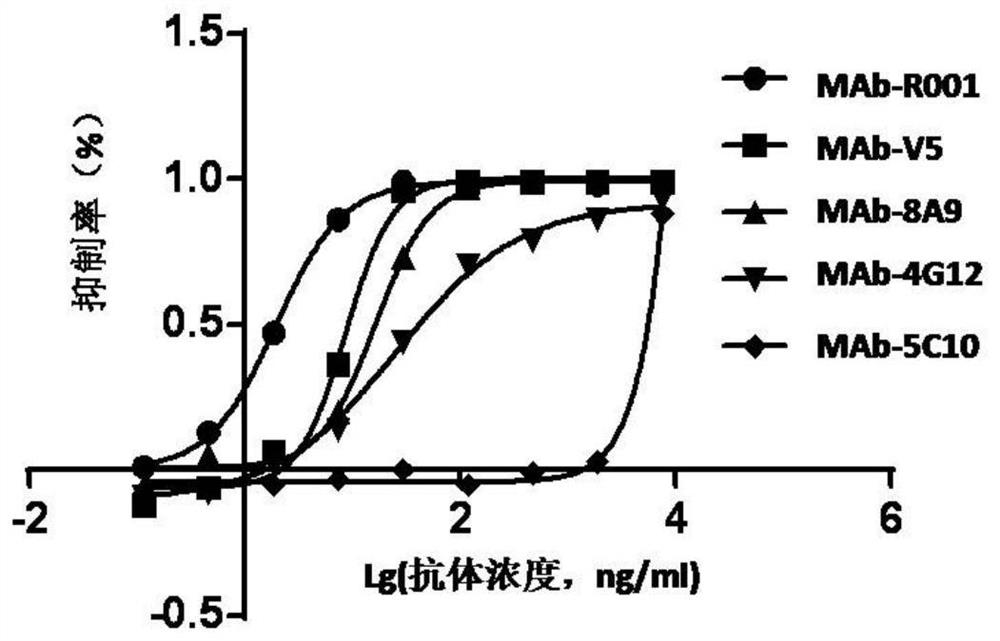
Anti-HPV16 L1 protein monoclonal antibody and detection method applying same
ActiveCN112125972AStrong specificityAdvantages of specific detectionImmunoglobulins against virusesFermentationAntigen Binding FragmentAntigen binding
The invention provides an anti-human papilloma virus (HPV) L1 protein monoclonal antibody, an antigen binding fragment and a detection method applying the antibody. The antibody has strong binding force with an HPV16 L1 antigen, has no cross reaction with HPV6, 11, 18, 31, 33, 35, 39, 45, 52, 56, 58 and 59 types, can specifically neutralize HPV16 pseudotype viruses, and has neutralizing activity.The monoclonal antibody obtained by screening of the invention shows good reaction consistency and neutralization activity on virus-like particle antigens derived from different expression systems; the recognized epitope is a dominant epitope in both immune guinea pig serum and immune human serum; and the monoclonal antibody is suitable for being used as a detection antibody in ELISA quantification to perform immunogenicity evaluation on HPV vaccines.
Owner:SINO CELL TECH INC
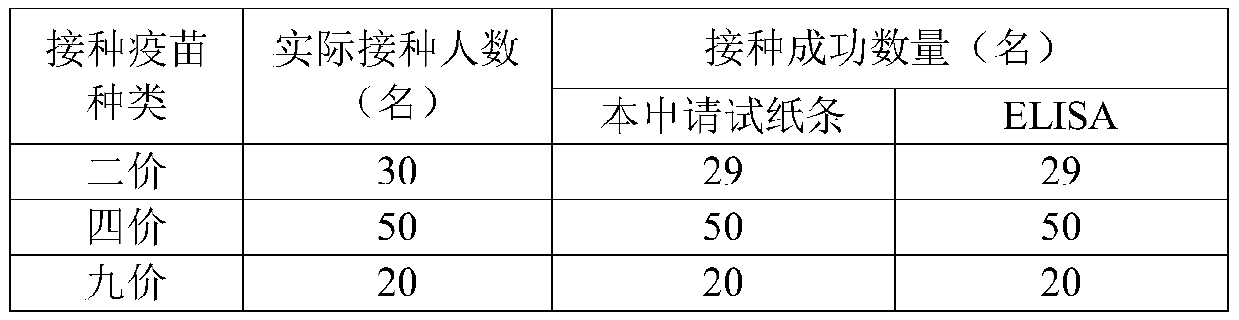
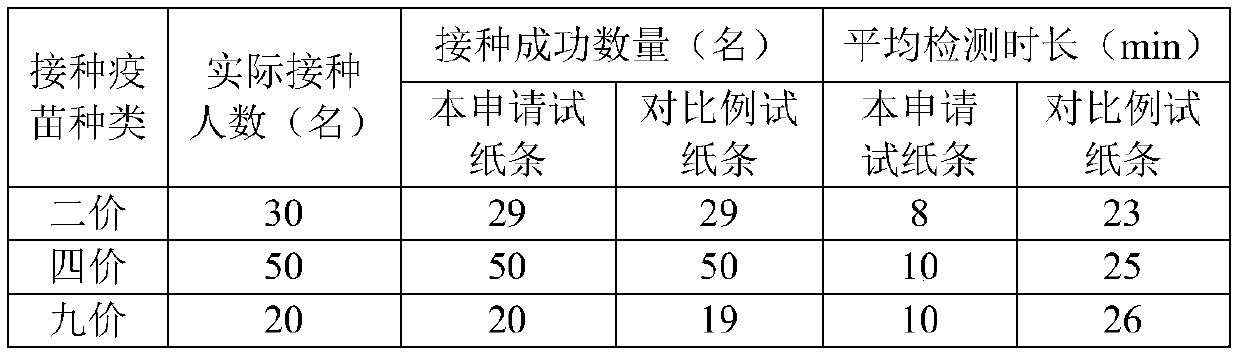
Rapid detection test strip for HPV antibody, and preparation method and use method thereof
ActiveCN110297087AEfficient detectionEasy to measure in batchesMaterial analysisHPV vaccinesElectrospinning
The invention relates to the technical field of HPV antibody detection, in particular to a rapid detection test strip for an HPV antibody, and a preparation method and a use method thereof. The test strip comprises a sample pad, a bonding pad, an analysis membrane, a water absorption pad and a handle, which are arranged in sequence along a chromatographic direction, citric acid-sodium citrate is attached to the surface of the sample pad, anti-human IgG antibody-nanogold particles are arranged on the bonding pad, a detection band and a quality control band are arranged on the analysis membrane,the main component of the detection band is HPV L1, and the main component of the quality control band is a secondary antibody bound with an anti-human IgG antibody. According to the test strip, thecorresponding sample pad, the bonding pad, the analysis membrane and the water absorption pad are prepared in an electrostatic spinning manner, so that it is convenient to measure the inoculation effect of HPV vaccine in batches, the detection efficiency of the inoculation effect of the HPV vaccine is improved, and the test strip has the advantages of simple preparation and convenience for batch production, and is suitable for hospitals, disease control, grass root, health unit detection and household use.
Owner:瓦至医学科技(上海)有限公司
Vector for Anti-hpv vaccine and transformed microorganism by the vector
ActiveUS20090117151A1Effective preventionInduce responseOrganic active ingredientsVirusesTumor-Related ProteinHuman papillomavirus
Owner:BIOLEADERS CORP
Vector for treatment vaccine for stable and constitutive high-expression cervical cancer and recombinant lactobacillus transformed by same
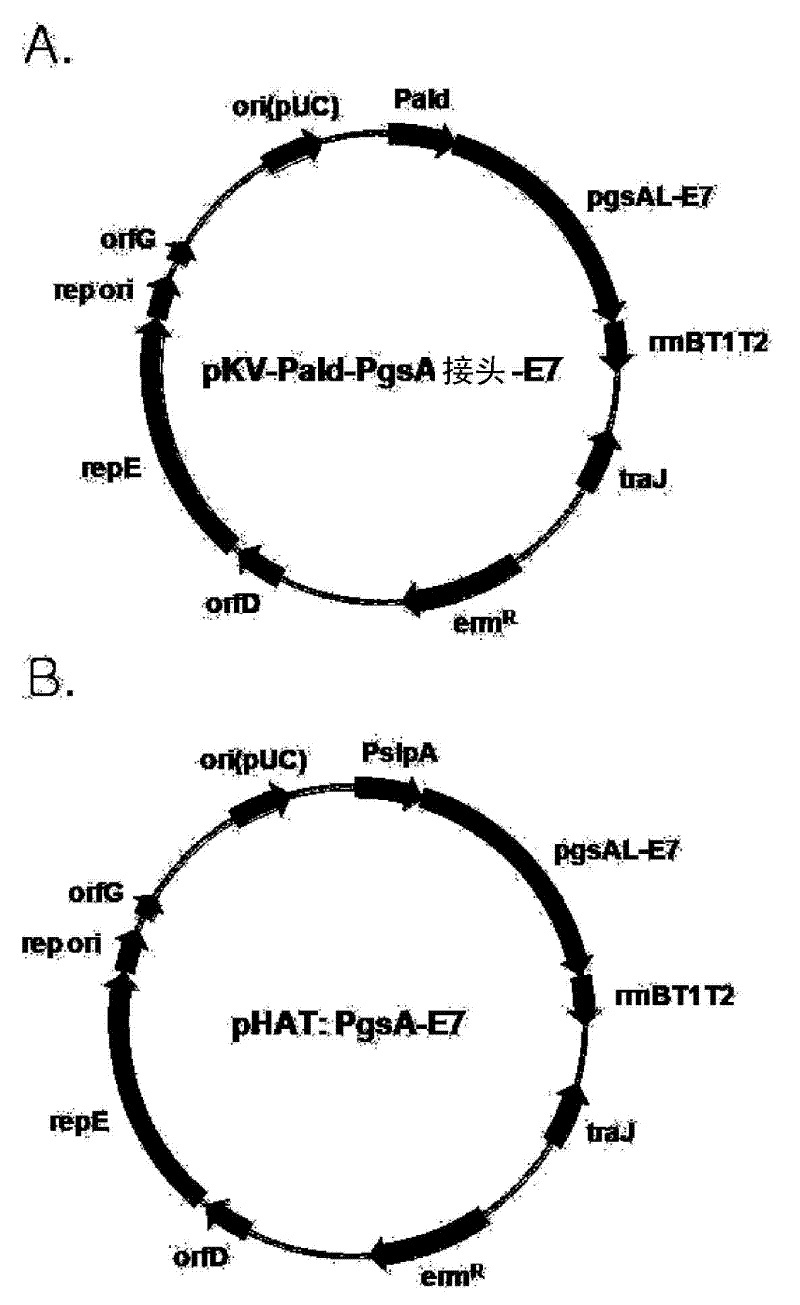
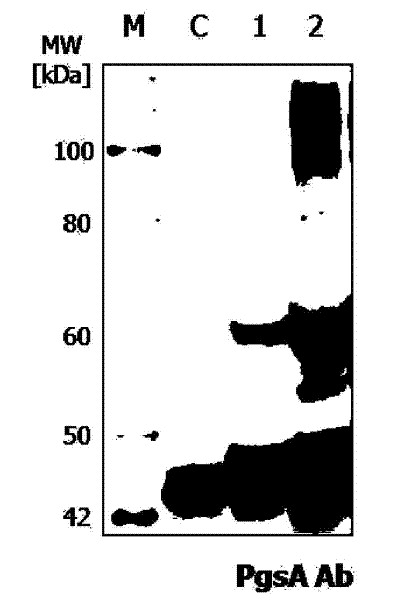
The present invention relates to a surface expression vector for HPV vaccine preparation including a gene that encodes an antigen protein related to the occurrence of human papilloma virus tumors, connected with: a composite gene with repE mutation gene having the amino sequence of sequence No. 1, a promoter, a poly-gamma-glutamate synthetase complex, and a poly-gamma-glutamate synthetase complex gene. The recombinant lactobacillus of the present invention that has been transformed into a surface expression vector of the antigen protein of the human papilloma virus, and the composition using the same, may be utilized as a treatment vaccine for cervical cancer and directly applied to the cervical or vaginal area, so that it has very economical effects.
Owner:BIOLEADERS CORP
Vaccines against HPV and HPV-related diseases
ActiveUS10286058B2Small sizeReduce the total massAntibody mimetics/scaffoldsFusion with post-translational modification motifDiseaseDendritic cell
Owner:BAYLOR RES INST
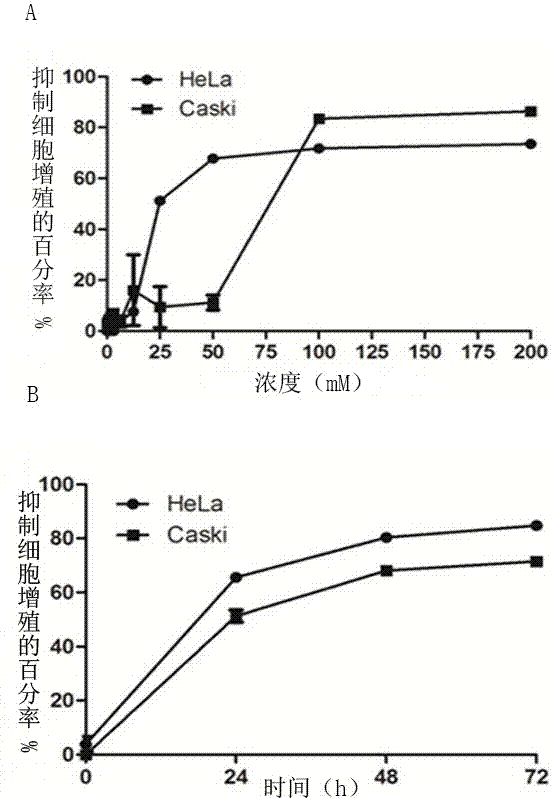
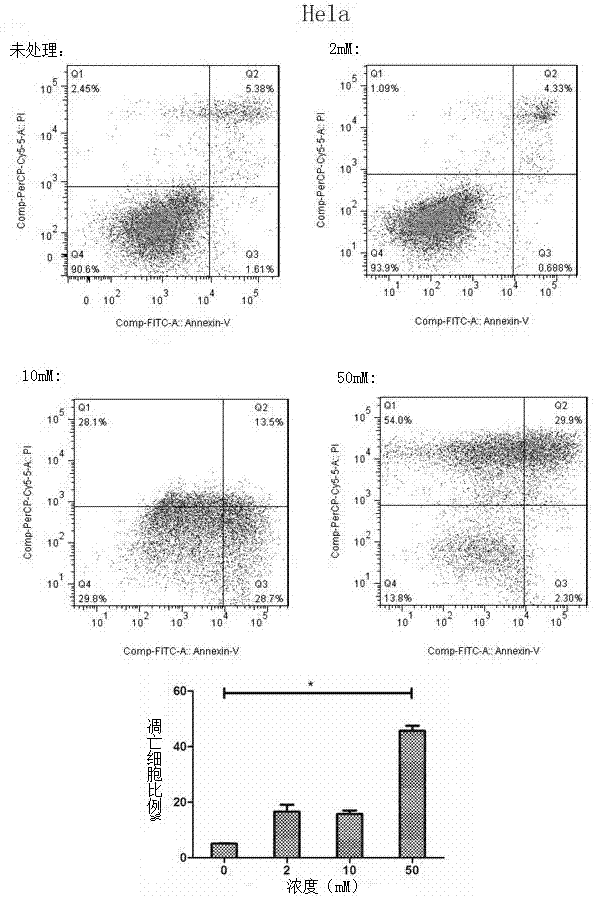
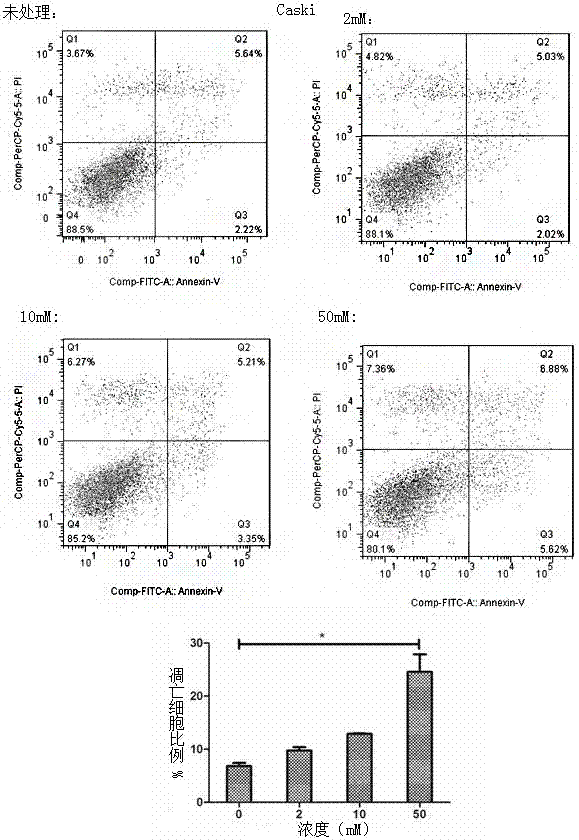
Application of metformin in preparation of drug for cervical cancer
InactiveCN107281172ALittle side effectsLow priceOrganic active ingredientsAntineoplastic agentsSide effectCervical cancer prevention
The invention discloses application of metformin in preparation of a drug for cervical cancer. Metformin hydrochloride or a composition of the metformin hydrochloride serves as the drug, and by inhibiting PI3K and up-regulating p53 and p21 protein expression, the drug inhibits the proliferation of cervical cancer cells, promotes the apoptosis of the cervical cancer cells, and induces cervical cancer cell cycle arrest to realize prevention and treatment of the cervical cancer. The side effect of the metformin is smaller than that of first-line drugs for the cervical cancer such as platinum drugs, the price of the metformin is lower than that of HPV (human papillomavirus) vaccine, and the metformin has a great anti-cervical cancer potential and a good development prospect.
Owner:FOSHAN MATERNAL & CHILD HEALTH CARE HOSPITAL
Therapeutic HPV vaccine based on chimpanzee adenovirus vector as well as preparation method and application of therapeutic HPV vaccine
InactiveCN112138150AEasy to makeImprove securityViral antigen ingredientsVirus peptidesVaccine PotencyAdjuvant
The invention discloses a therapeutic HPV vaccine based on a chimpanzee adenovirus vector as well as a preparation method and application of the therapeutic HPV vaccine. The therapeutic HPV vaccine isan immunogenic HPV recombinant adenovirus vaccine obtained by packaging and processing HPV virus vaccine vector plasmids, and the HPV virus vaccine vector is a replication-defective chimpanzee adenovirus vector. The HPV therapeutic vaccine is constructed by using the replication-defective chimpanzee adenovirus vector, the influence of the pre-stored immunity of a human adenovirus vector in a human body on the vaccine efficacy is overcome, and a vaccine product which is simple to prepare, low in cost, good in safety and free of adjuvants is expected to be obtained. The therapeutic HPV vaccinehas good immunogenicity and anti-tumor efficacy, can induce high-level antigen-specific T cell reaction and anti-tumor efficacy in a mouse body through single muscle immunization, and is expected to show good humoral and cellular immunotherapy effect can be embodied in human clinical tests.
Owner:IMMUNE PATH BIOTECHNOLOGY SUZHOU CO LTD
HPV vaccines
ActiveUS10744196B2Viral antigen ingredientsAntibody mimetics/scaffoldsAntigenHuman papilloma virus infection
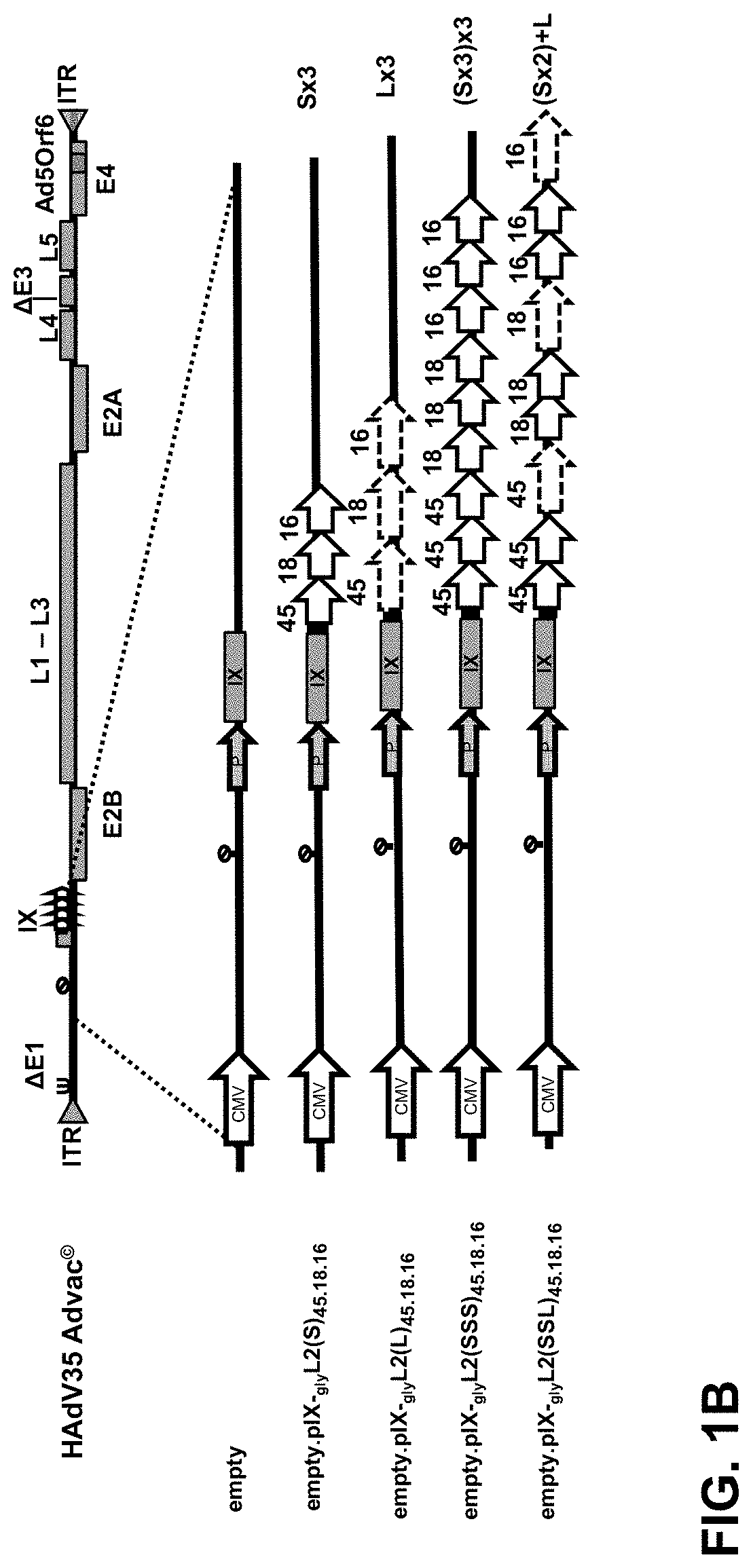
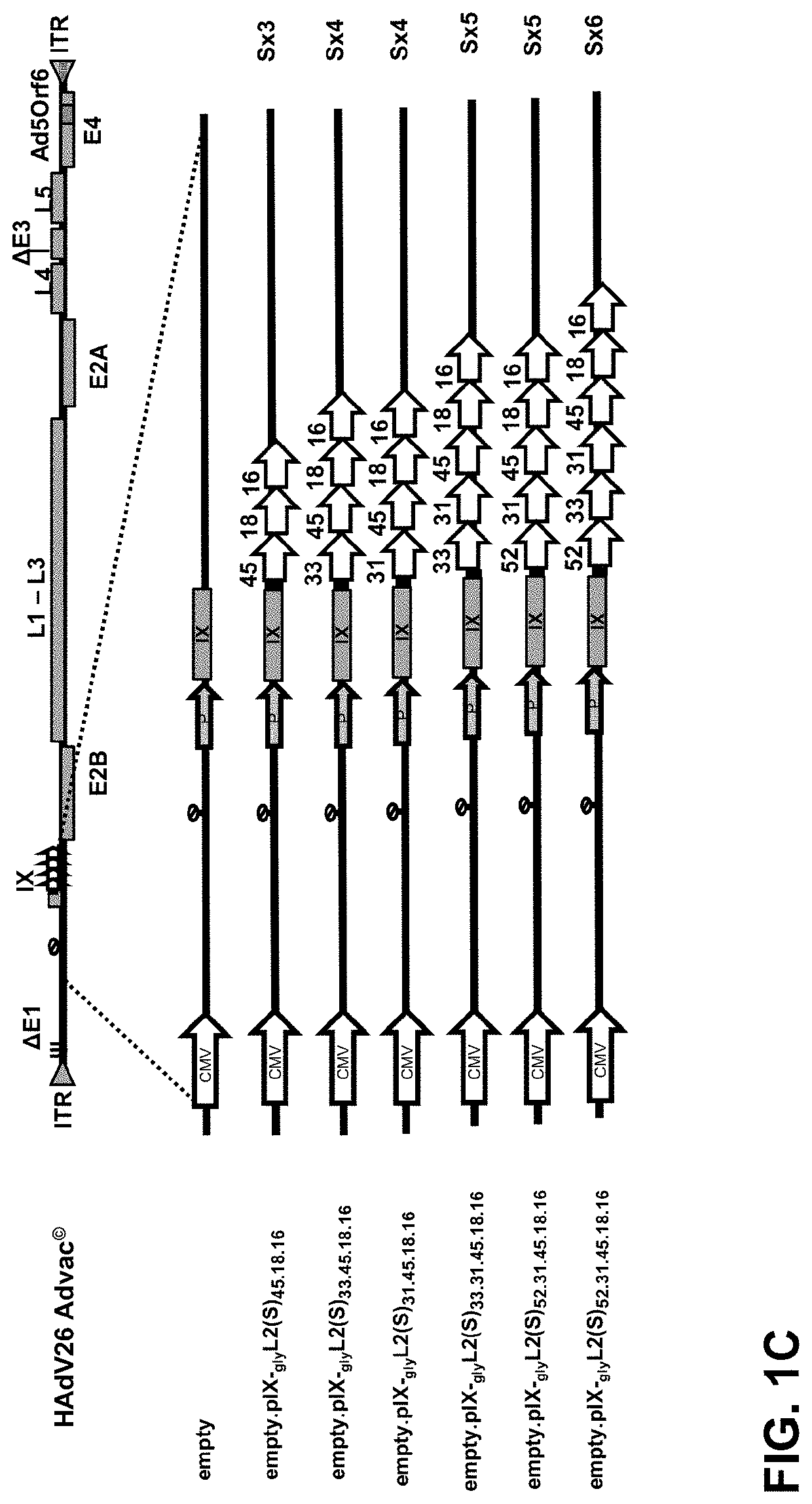
The present invention relates to novel vaccines against Human papillomavirus (HPV) infections, based on recombinant capsid-display adenovirus vectors. Described are capsid modified replication deficient adenovirus particles encoding and displaying multiple HPV L2 antigenic fragments, via a minor capsid protein IX, and their use for eliciting an immune response in order to provide protection against infections from multiple HPV types.
Owner:JANSSEN VACCINES & PREVENTION BV
Therapeutic HPV vaccine combinations
ActiveUS20190142933A1Minimize the numberStimulate immune responseViral antigen ingredientsAntiviralsHPV vaccinesVirology
Owner:JANSSEN VACCINES & PREVENTION BV +1
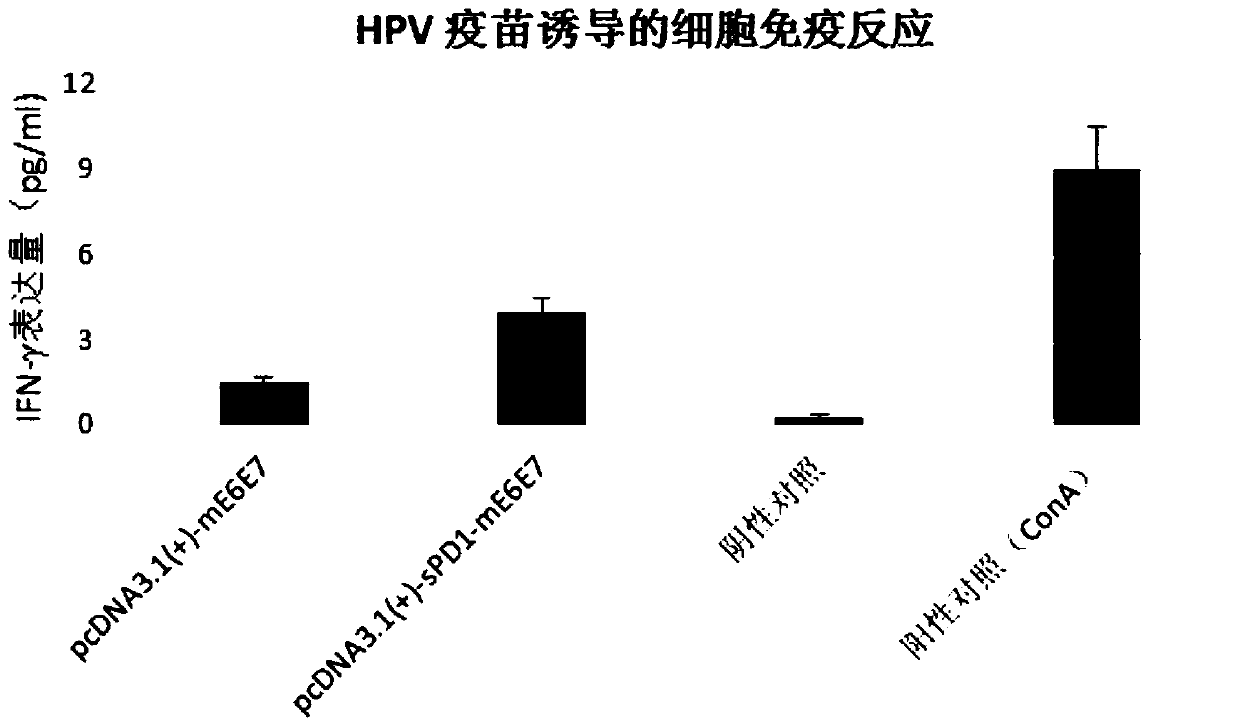
Application of sPD1 protein and/or sPD1 gene as immunologic adjuvant
ActiveCN109568574AImproving immunogenicityImprove securityAntibody mimetics/scaffoldsViral antigen ingredientsHPV vaccinesNucleotide
The invention belongs to the field of biological medicine, and particularly relates to application of an sPD1 protein and / or an sPD1 gene as an immunologic adjuvant to preparation of a recombinant HPVvaccine. In application, the sPD1 protein contains an amino acid sequence shown as SEQ ID NO:3, or an amino acid sequence with the same function, wherein the amino acid sequence with the same function is obtained after deletion, insertion or replacement of the amino acid sequence shown as SEQ ID NO:3; and the sPD1 gene contains an amino acid sequence shown as SEQ ID NO:1, or an amino acid sequence with the same function, wherein the amino acid sequence with the same function is obtained after deletion, insertion or replacement of the amino acid sequence shown as SEQ ID NO:1. sPD1 serves as the immunologic adjuvant to enhance the ability to stimulate an organism to generate an antibody by the HPV vaccine and induce the organism to generate humoral immune response, stimulate lymphocytes tosecrete IFN-gamma, and stimulate the organism to generate cellular immune response.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Minimal motifs of linear B-cell epitopes in L1 protein from human papillomavirus type 58 and their applications
InactiveUS8715681B2Peptide/protein ingredientsViral antigen ingredientsLinear epitopePeptide vaccine
Minimal Motifs of linear B-cell epitopes in L1 structure protein from human papillomavirus type 58 (HPV 58) and their applications are disclosed. Eighteen linear epitope motifs and their extended 8-mer peptides in the L1 protein from HPV 58 are described, which can be used as antigens separately or in combination to specifically detect the serum from subjects with HPV 58 infection, or to develop preventive or therapeutic multi-epitope peptide vaccines against HPV 58 by inducing humoral immunity. Of the eighteen B-cell epitope motifs, ten of them are 100% conservative and one is highly conservative among many homologous proteins of high-risk HPVs. They can be used as candidate “universal” epitopes to develop preventive or therapeutic HPV vaccines. The amino acid sequences of the epitope motifs and the 8-mer peptide formula of the invention are shown below in SEQ ID No. 1-2, 2B, 3, 3B, 4-18, 18B, 19, 19B, and 20-32.
Owner:HENGSUN PHARMA TECH
Recombinant expression vector system, recombinant engineering bacterium and preparation method and application of recombinant expression vector system or recombinant engineering bacterium
The invention provides a recombinant expression vector system, a recombinant engineering bacterium and a preparation method and application of the recombinant expression vector system or the recombinant engineering bacterium. The recombinant expression vector system at least comprises two recombinant expression vectors; each recombinant expression vector includes one or more nucleic acid expression cassettes; each nucleic acid expression cassette includes a target gene, and a promoter operably linked to the target gene; each target gene is independently selected from one of HPV6 L1, HPV11 L1,HPV16 L1, HPV18 L1, HPV31 L1, HPV33 L1, HPV35 L1, HPV39 L1, HPV45 L1, HPV51 L1, HPV52 L1, HPV53 L1, HPV56 L1, HPV58 L1, HPV59 L1, HPV66 and HPV68 L1, and all the target genes are different from each other. The recombinant engineering bacterium can be obtained by converting the recombinant expression vector system into an engineering bacterium. The recombinant engineering bacterium can express various HPV L1 proteins at the same time, and is used for preparing HPV vaccines.
Owner:重庆博唯佰泰生物制药有限公司 +1
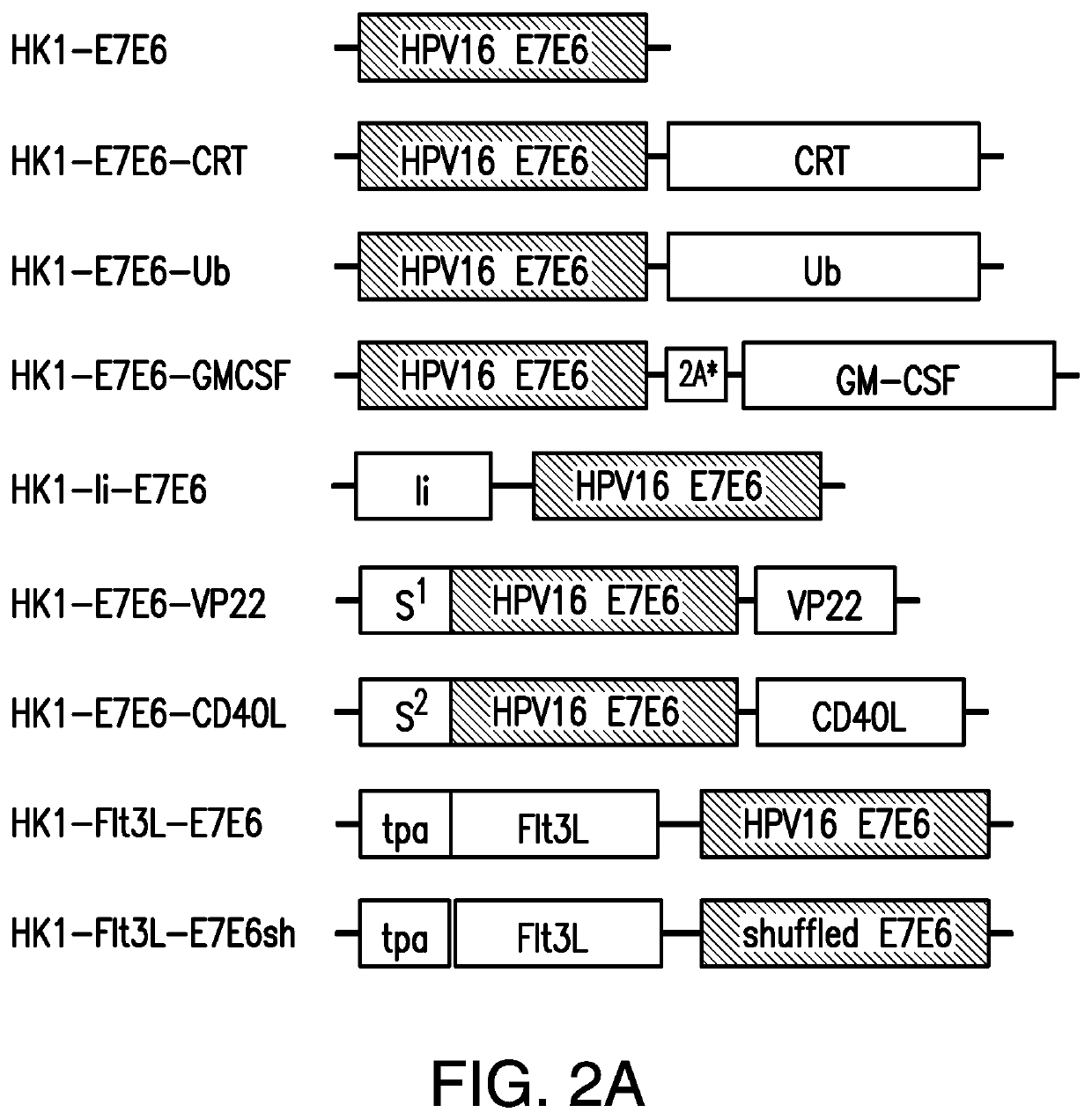
Novel vaccines against HPV and HPV-related diseases
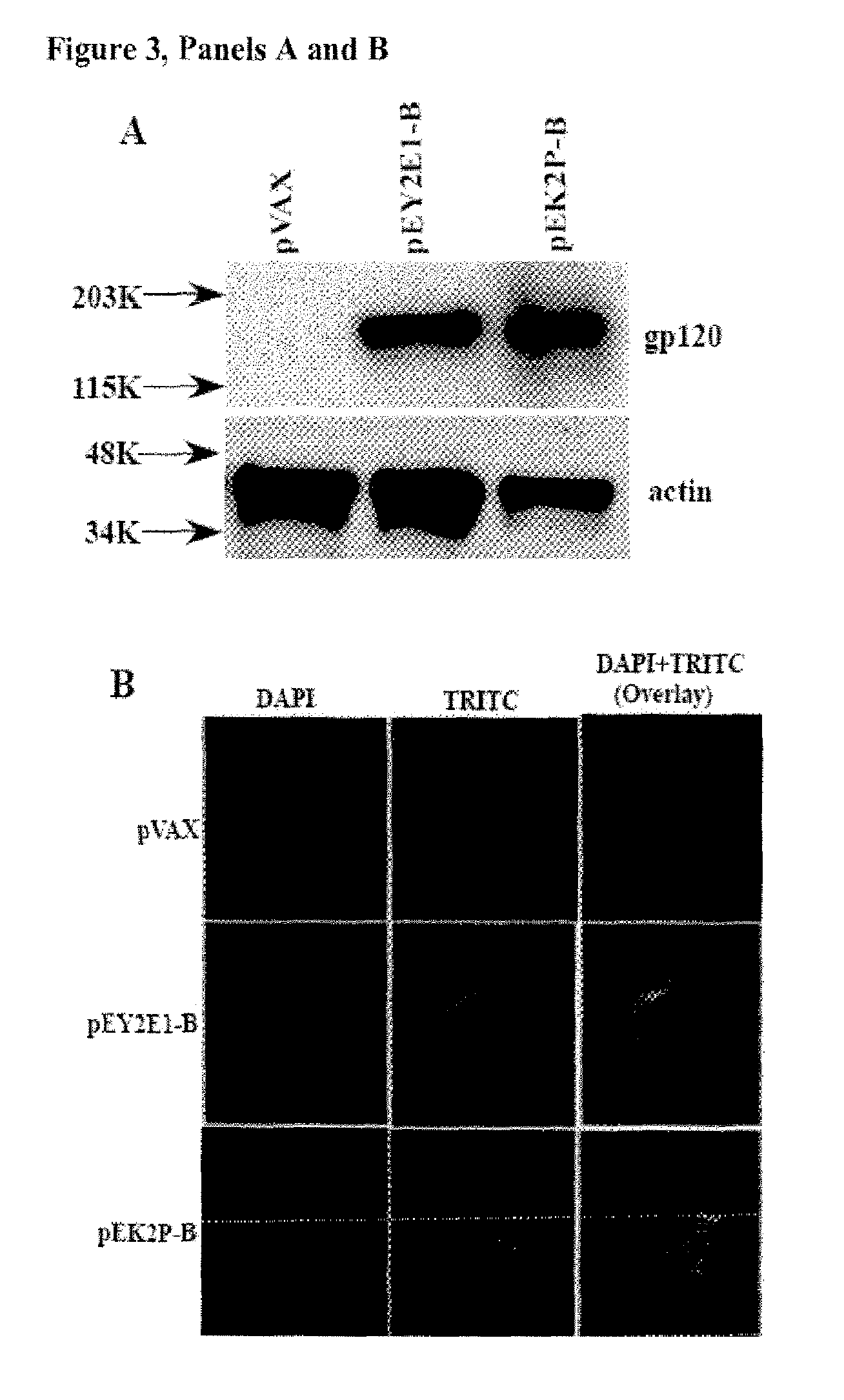
ActiveUS20160331825A1Small sizeReduce the total massViral antigen ingredientsAntibody mimetics/scaffoldsHuman papillomavirusDendritic cell
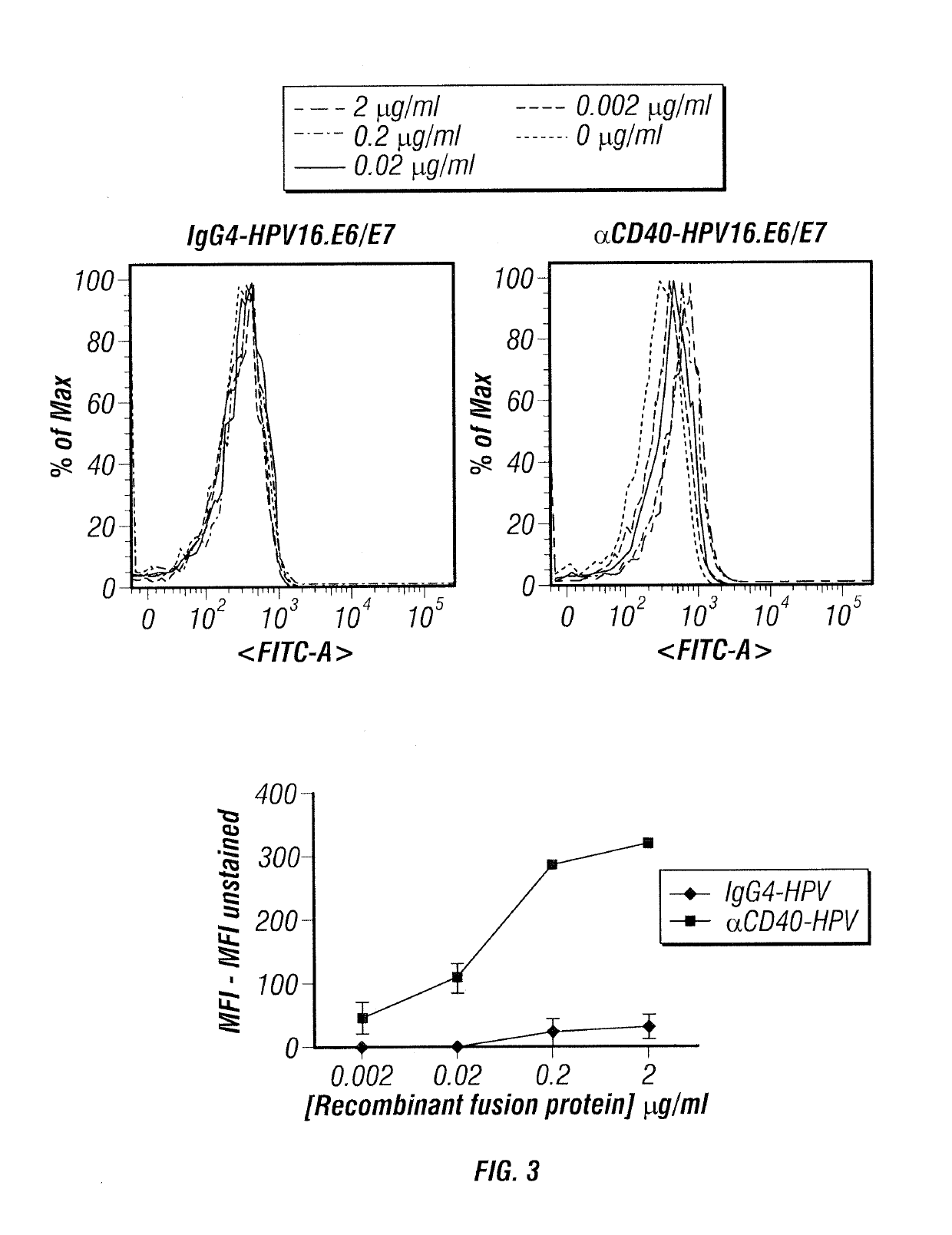
Embodiments relate to novel vaccines against human papillomavirus (HPV) and HPV-related diseases, including multiple types of cancers. The HPV vaccines are composed of anti-human dendritic cell (DC) surface receptor antibodies, including CD40, and E6 / 7 proteins of HPV 16 and 18. The technology described is not limited to making vaccines against HPV16- and HPV18-related diseases and can be applied to making vaccines carrying E6 / 7 from any type of HPV. The HPV vaccines described can target DCs, major and professional antigen presenting cells (APCs), and can induce and activate potent HPV E6 / 7-specific and strong CD4+ and CD8+ T cell responses. The HPV vaccines can be used for the prevention of HPV infection and HPV-related diseases as well as for the treatment of HPV-related diseases, including cancers.
Owner:BAYLOR RES INST
Detection method for residual amount of MOPS (3-(N-Morpholino)Propane-Sulfonic acid)
ActiveCN105548420AEnsure safetyHigh detection sensitivityComponent separationHuman papillomavirusBiologic Products
The invention provides a detection method for a residual amount of MOPS (3-(N-Morpholino)Propane-Sulfonic acid), which is used for detecting with a high performance liquid chromatography. The MOPS is quantified by adopting a linear normalization method. The high performance liquid chromatography adopts an Acclaim Trinity P1 chromatographic column and an electric spraying detector; a mobile phase is a mixed solution of acetonitrile and an ammonium acetate solution, and the volume ratio of the acetonitrile to the ammonium acetate solution is (1:1) to (2:1); and the flow speed of the mobile phase is 0.4mL / min-0.6mL / min. The detection method for the residual amount of the MOPS, provided by the invention, can be used for detecting trace MOPS components in an HPV (Human Papillomavirus) vaccine, which cannot be detected by a chemical titration method and a C18 column liquid chromatography; and the detection method has good detection precision and high detection sensitivity and accuracy, and can be widely applied to the detection of the residual amount of the MOPS of biological products.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Method for enhancing immunogenicity of epitope peptide of HPV antigen, virus-like particle, and method for preparing HPV vaccine
ActiveUS20170166612A1Improving immunogenicityHigh recovery rateAntibody mimetics/scaffoldsViral antigen ingredientsHPV AntigenEpitope
A method for enhancing immunogenicity of an epitope peptide of an HPV antigen, the method including: assembling a gene of an HPV antigen into a gene of HBc, exogenously expressing a resulting assembled gene to acquire a fusion protein, allowing the fusion protein to automatically assemble to form a virus-like particle including the HPV antigen on a surface of the virus-like particle, to obtain HBc-HPV virus-like particle. A virus-like particle capable of expressing the antigen peptide of HPV acquired by the method. The virus-like particle includes a HBc-L2 fusion protein. A genome for encoding the HBc-L2 fusion protein is represented by SEQ ID NO. 3. A method for preparing an HPV vaccine includes using the virus-like particle.
Owner:CANSINO BIOLOGICS INC
Vector for treatment vaccine for stable and constitutive high-expression cervical cancer and recombinant lactobacillus transformed by the same
A surface expression vector for preparing HPV vaccines, in which the surface expression vector contains a gene encoding a repE mutant protein having an amino acid sequence of SEQ ID NO: 1, a promoter, a poly-gamma-glutamate synthetase complex gene, and a gene which is linked with the poly-gamma-glutamate synthetase complex gene and encodes a tumor induction-associated antigen protein of human papillomavirus.An expression vector constitutively expressing a high level of the human papillomavirus (HPV) antigen protein is provided. Also, a recombinant lactic acid bacteria, transformed with the expression vector and expressing the HPV antigen protein on the surface thereof, and a composition comprising the recombinant lactic acid bacteria are provided. The recombinant lactic acid bacteria and the composition are very effective as a vaccine for the treatment of cervical cancer, because they can be applied orally or directly to the vagina.
Owner:BIOLEADERS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com