Application of sPD1 protein and/or sPD1 gene as immunologic adjuvant
An immune adjuvant and protein technology, applied in the field of biomedicine, can solve the problems of limited selection of preventive and therapeutic vaccine X
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] On the other hand, the present invention also provides a method for preparing a recombinant protein vaccine, comprising the step of transfecting host cells with the above-mentioned recombinant plasmid and expressing it. Specifically, after the recombinant plasmid was transfected into 293T cells, the expression was identified, and the specific operation method was as follows: according to the routine method of molecular cloning in the laboratory, the endotoxin-free pcDNA3.1(+)-sPD1-mE6E7 plasmid was transfected to a growth density of 80%. After culturing 293T cells with 10% FBS DEME medium at 5% CO2 and 37°C for 48 hours, separate and collect the cell culture supernatant and cells, use E6 monoclonal antibody, use Western Blot to analyze and identify the cells and cell culture The expression of sPD1-mE6E7 fusion protein in the supernatant, SDS-PAGE electrophoresis and Western Blot were performed according to the molecular cloning experiment guide, the concentration of the ...
Embodiment 1
[0051] The recombinant expression vectors pcDNA3.1(+)-mE6E7 and pcDNA3.1(+)-sPD1-mE6E7 were transfected into 293T cells and their expression was identified.
[0052] First, transfect the endotoxin-free pcDNA3.1(+)-mE6E7 or pcDNA3.1(+)-sPD1-mE6E7 recombinant plasmid into 293T cells with a growth density of 80% according to the conventional method of molecular cloning in the laboratory. %CO 2 1. After culturing in 10% FBS DEME medium for 48 hours at 37° C., the cell culture supernatant and cells were separated and collected. Then, utilize E6 monoclonal antibody (commercially purchased, the brand is Abcam), adopt Western Blot to analyze and identify the expression of mE6E7 protein and sPD1-mE6E7 fusion protein in the cell and cell culture supernatant, SDS-PAGE electrophoresis and Western Blot method reference molecule The cloning experiment guide is carried out, the concentration of the stacking gel is 5%, the concentration of the separation gel is 12%, the concentration gel is ...
Embodiment 2
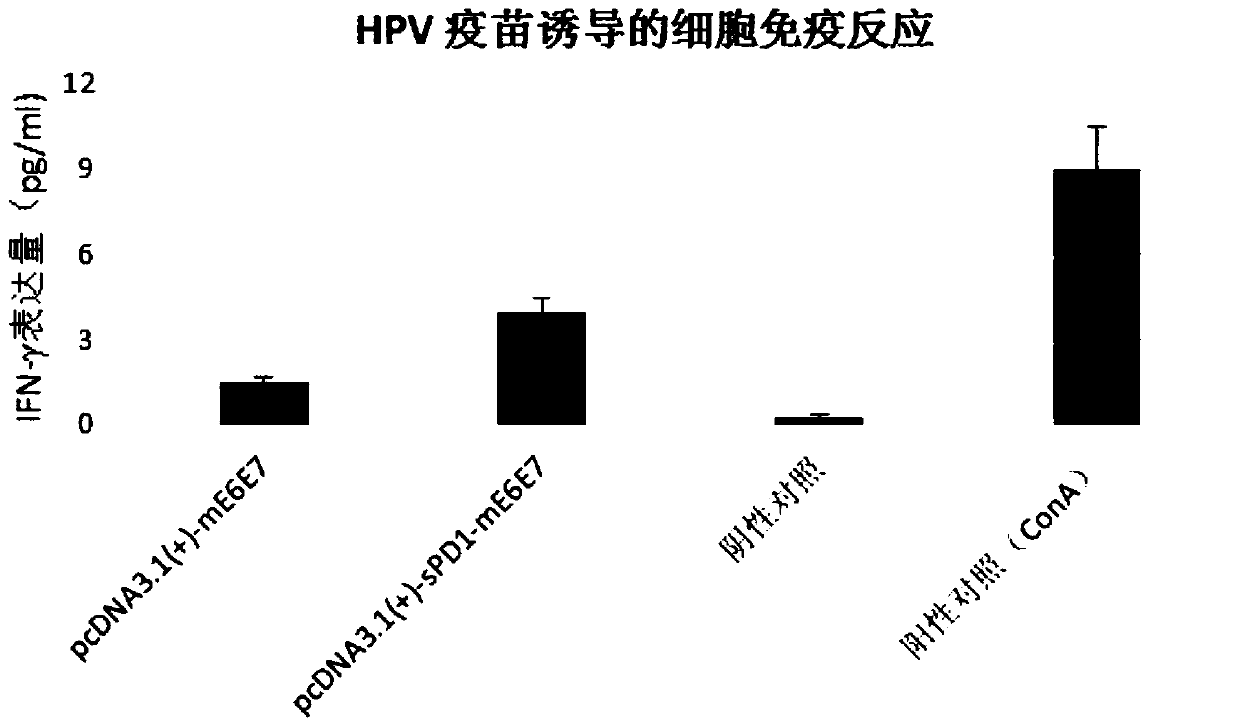
[0055] To verify the effect of PD1 on the immune adjuvant of HPV E6E7 RNA vaccine.
[0056] Construct the pcDNA3.1(+)-sPD1-mE6E7 plasmid containing the sPD1 gene coding sequence and the pcDNA3.1(+)-mE6E7 plasmid not containing the sPD1 coding sequence according to the method described in the instructions, and transcribe the two plasmids into RNA in vitro The corresponding RNA vaccines were obtained, and the specific vaccination scheme for mice is shown in Table 1:
[0057] Table 1
[0058]
[0059] The above-mentioned HPVmE6E7RNA vaccine group and sPD1+mE6E7RNA vaccine group were immunized with four injections. First, the vaccine was injected intramuscularly, and then the NEPA GENE animal electroporation device was used for electroporation stimulation. At a voltage of 80V, the pulse was 6 times, and the time interval between each pulse was 25ms. , the pulse frequency is 2Hz, and the electroporation is stimulated by the animal electroporation device to improve the immune ef...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com