Preparation method of HPV (human papilloma virus)-specific antibody and freeze-dried preparation of HPV-specific antibody
A specific, HPV-16 technology, used in the preparation of HPV-specific antibodies and the field of freeze-dried preparations, can solve the problems of difficulty in inducing immunity of various vaccines, increase the difficulty of preparation, and increase the cost of vaccines, and achieve low immunogenicity. Sexual, short acting time, fast onset effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
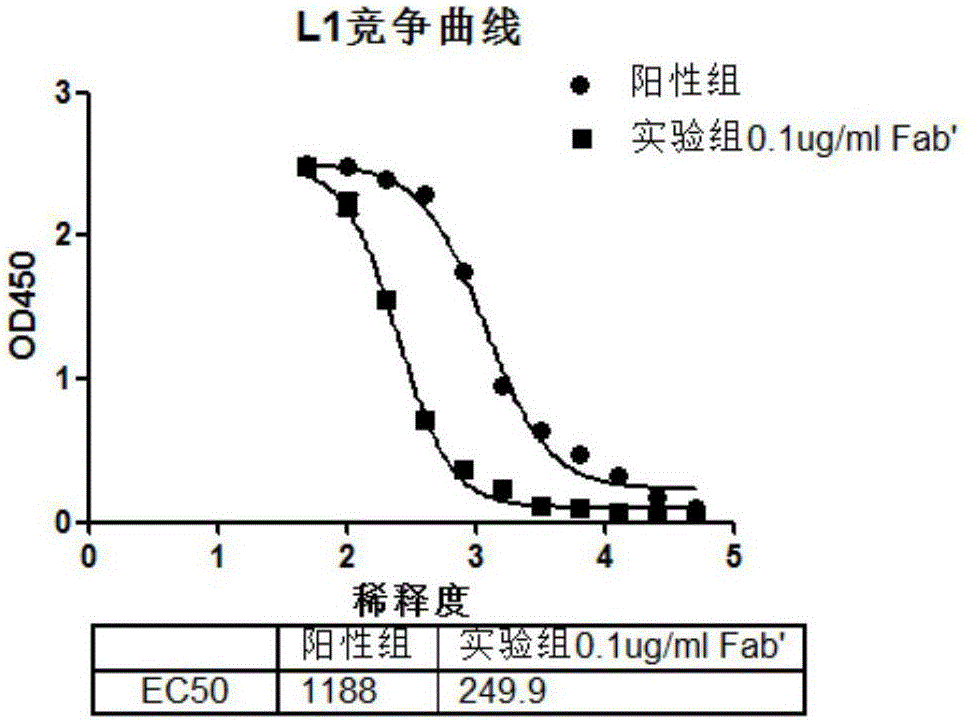
Image
Examples
Embodiment 1
[0023] One, a kind of preparation method of the specific antibody (Fab' fragment) of anti-HPV infection
[0024] 1. Gene cloning and protein separation and purification
[0025] 1.1 Query the gene sequence of related protein molecules through NCBI / Genebank, including: HPV (low-risk type, high-risk type) L1, L2 full sequence, heparan sulfate proteoglycan and furin-furin, and obtain the complete gene through gene synthesis sequence.
[0026] Wherein: HPV type-16L1 gene sequence: (SEQ ID NO: 1).
[0027] HPV type-16L2 gene sequence: (SEQ ID NO: 2).
[0028] 1.2 After the L1 and L2 of the above-mentioned heparan sulfate proteoglycans, furin- and HPV-16 subtypes are synthesized by genes, they are cloned into prokaryotic expression vectors or eukaryotic expression vectors (or have SV40 promoter and Eukaryotic enhancers, and appropriate reporter genes such as HRP, fluorescent proteins, etc.). The genetic engineering steps include: designing PCR primers according to the gene seque...
Embodiment 2
[0082] The preparation method of a freeze-dried preparation of anti-HPV infection antibody is as follows: the specific Fab' is mixed with other auxiliary materials and filled. Weigh according to the ratio in Table 1 below.
[0083] Table 1
[0084] raw material name
content
Specific Antibody Fab' Fragments
0.01-0.5mg / ml
polyethylene glycol
0.5-8%
5-80%
1-70%
0.1-10%
0.1-5%
0.1-5%
Buffered salt (PBS phosphate buffered saline)
0.01-1%
[0085]Inject the purified water of 50% batching volume into the dosing tank, add the mannose, trehalose, sucrose of formula quantity. After stirring to dissolve, add the glycine and arginine in the batching amount, and stir again to dissolve. The phosphate of the batching amount is injected into 20%-30% and dissolved in the batching tank. After the dissolution is complete, it is injected into the main b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com