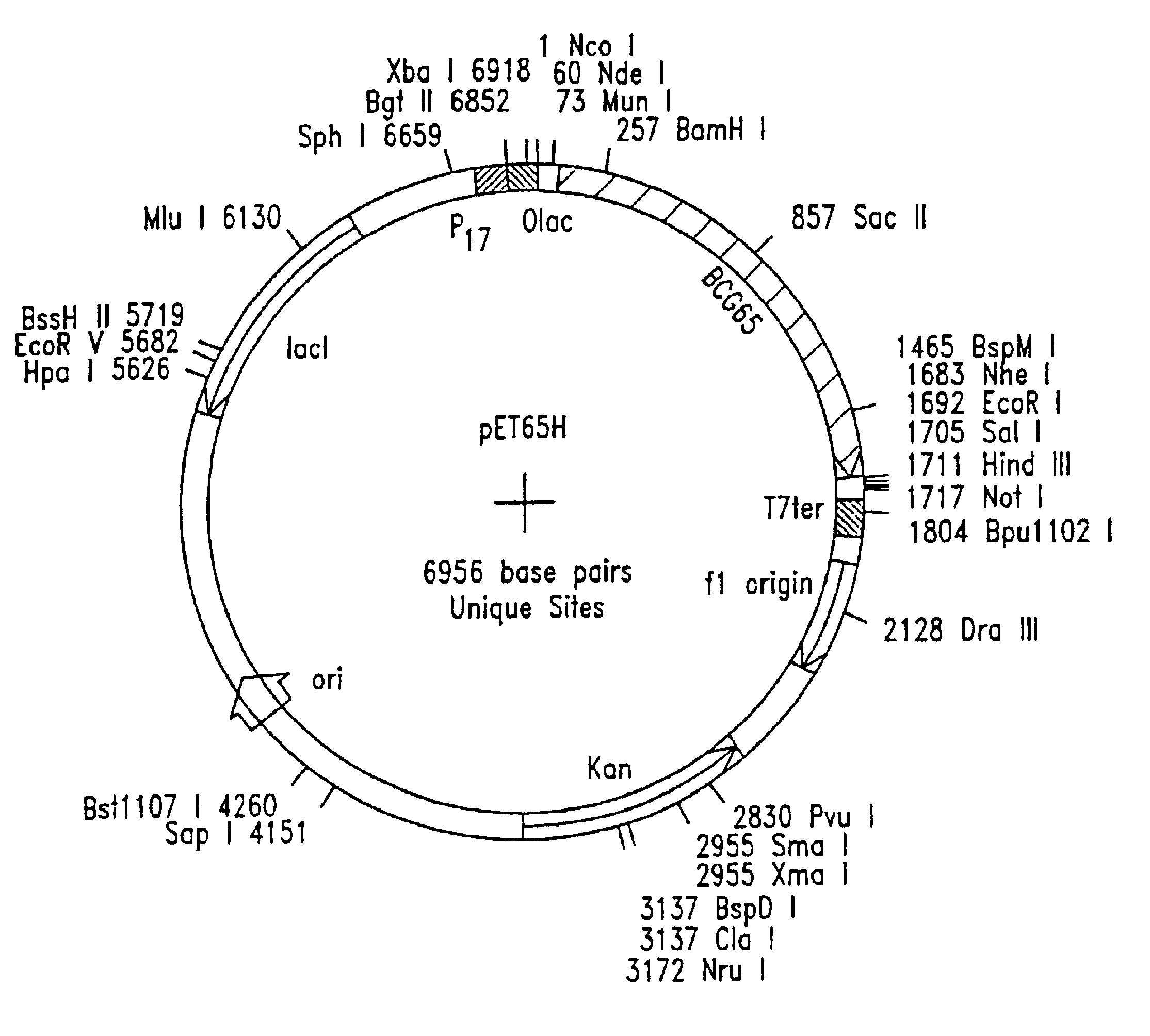
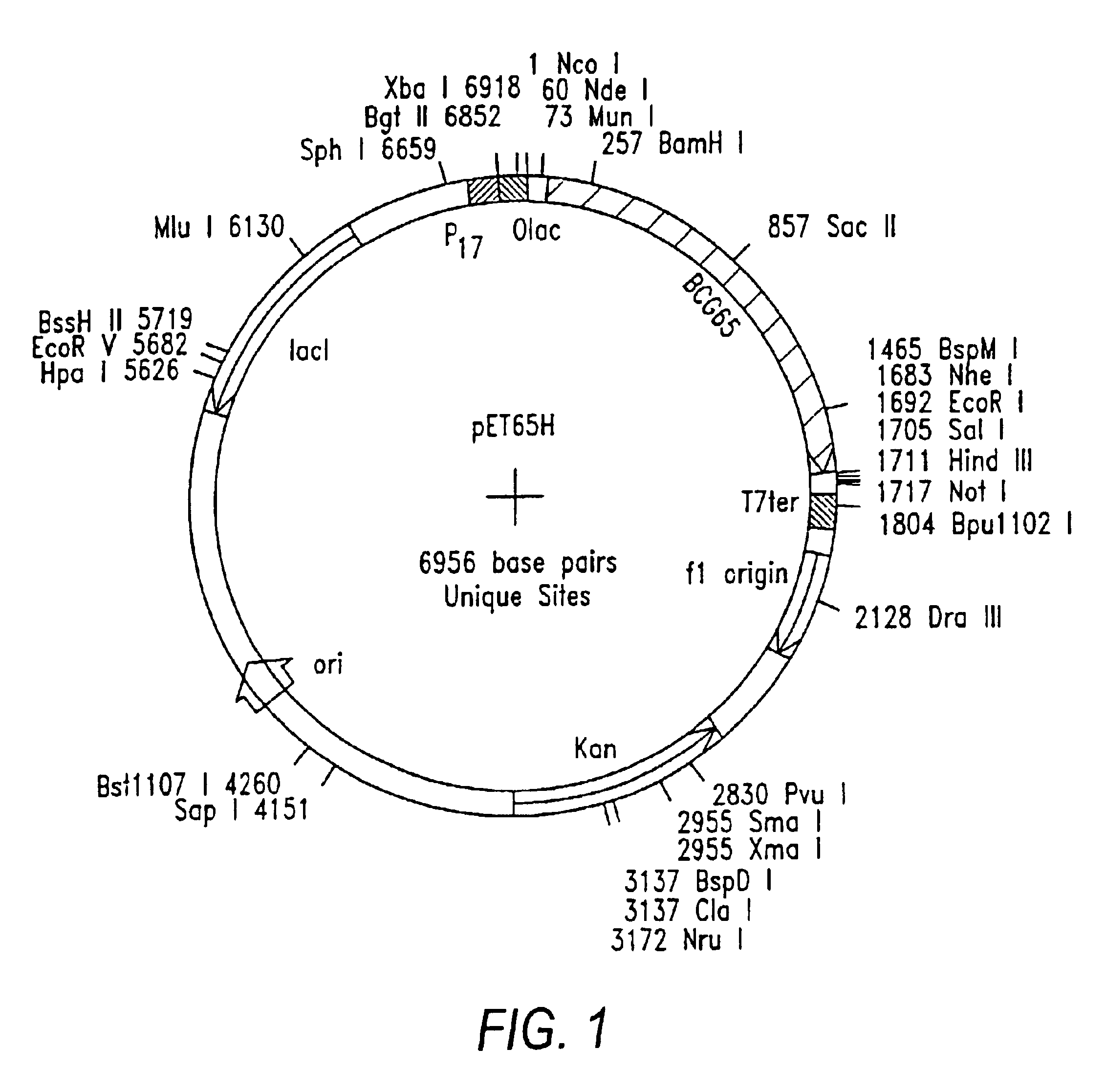
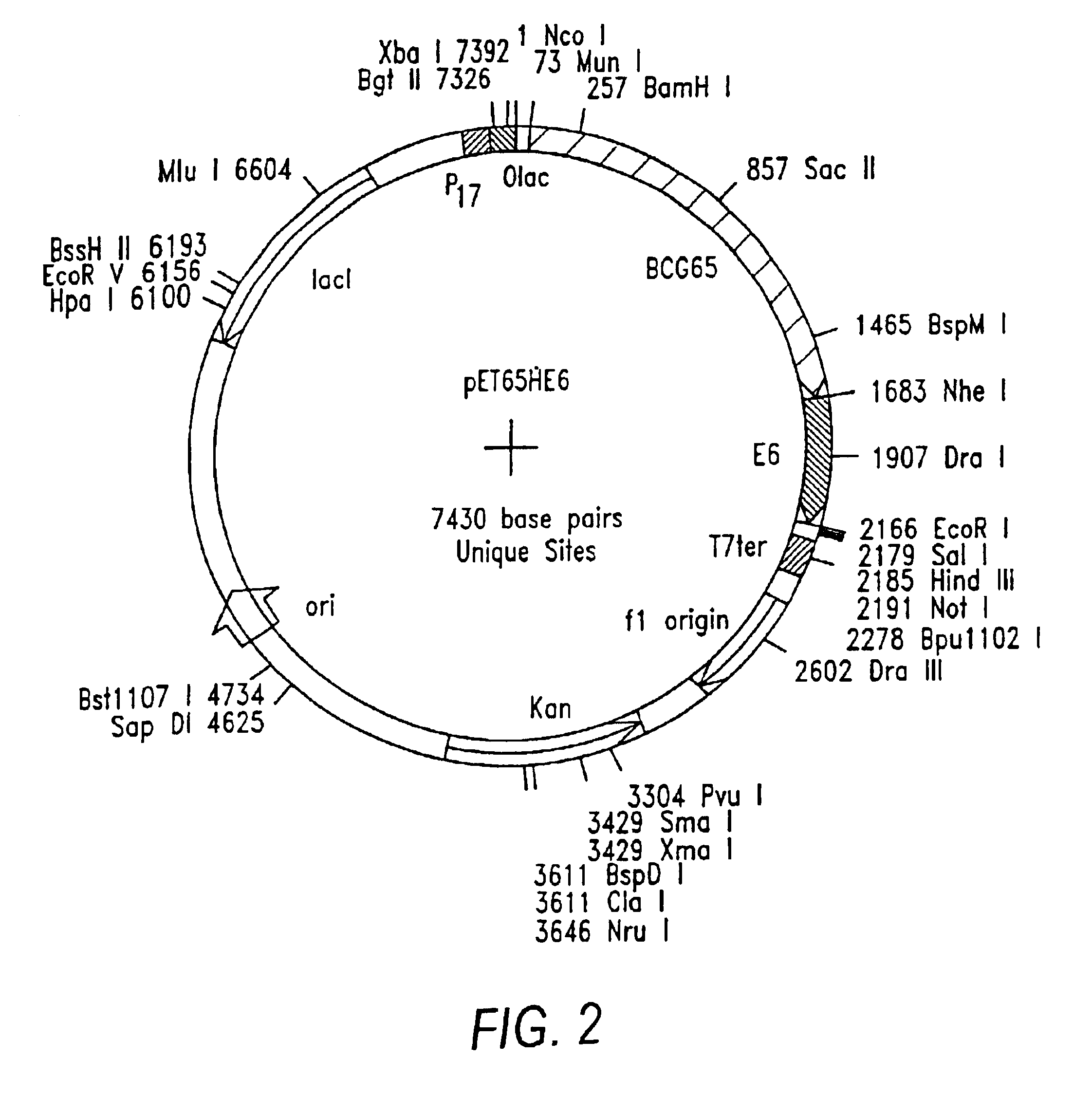
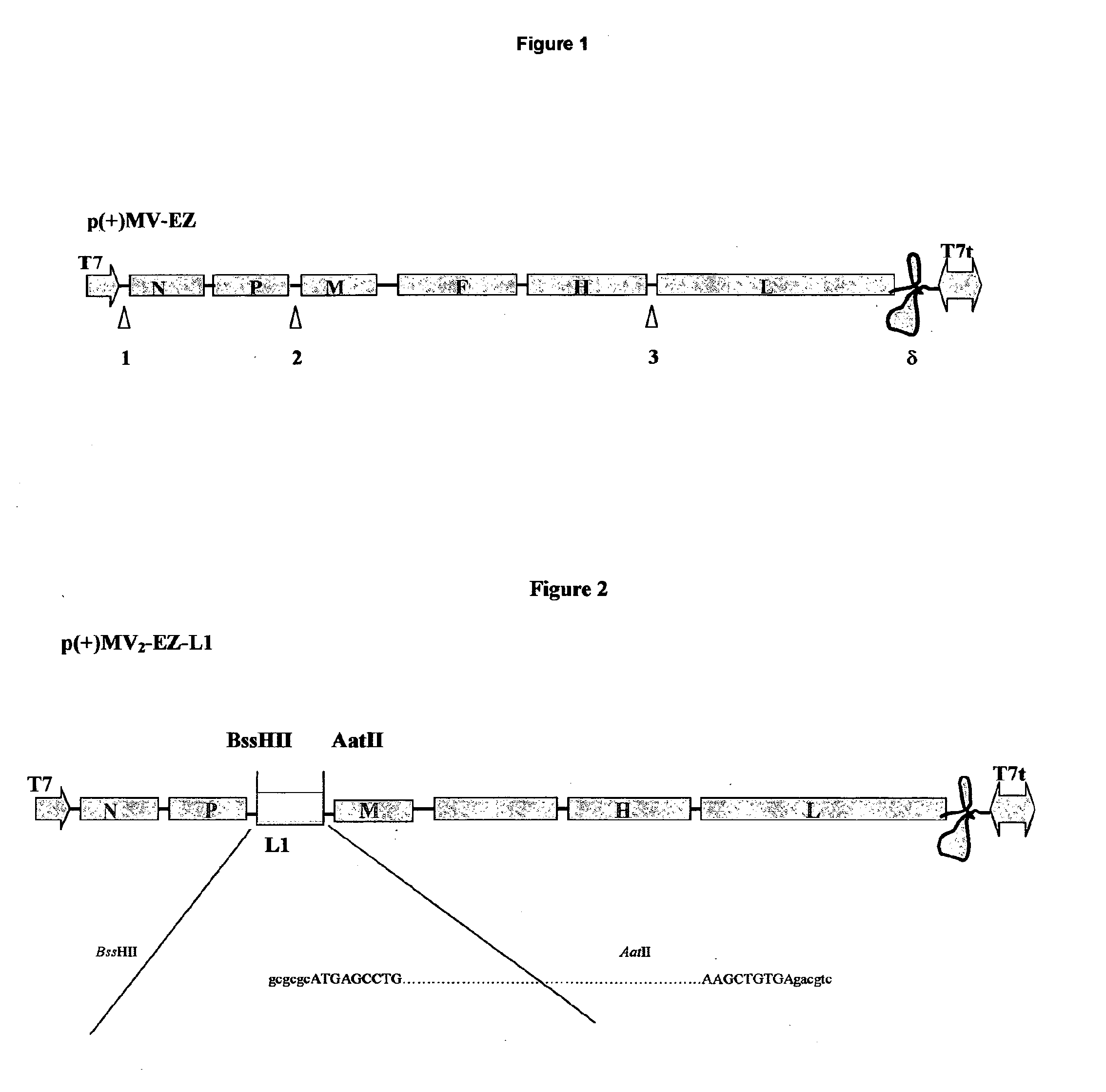
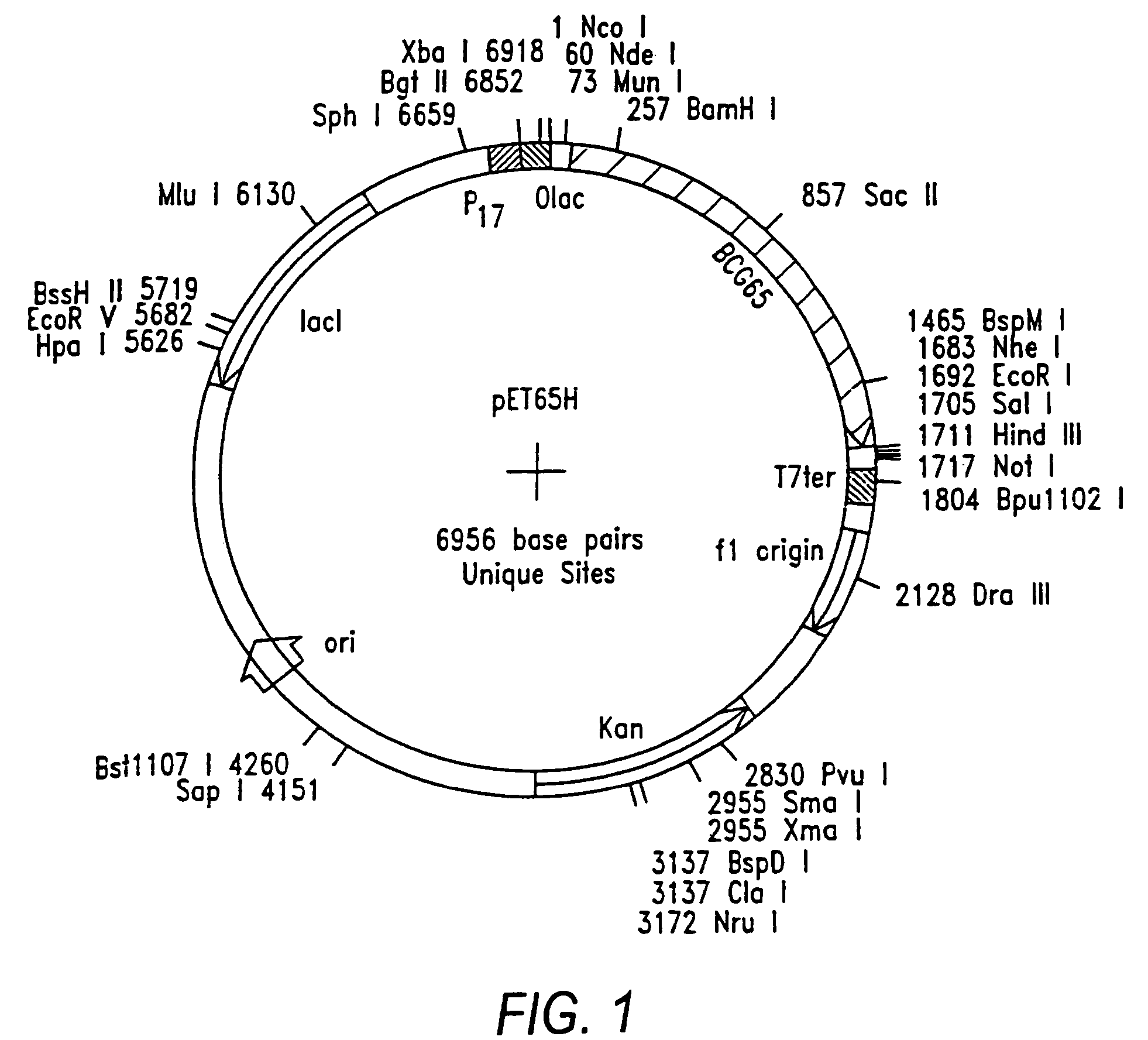
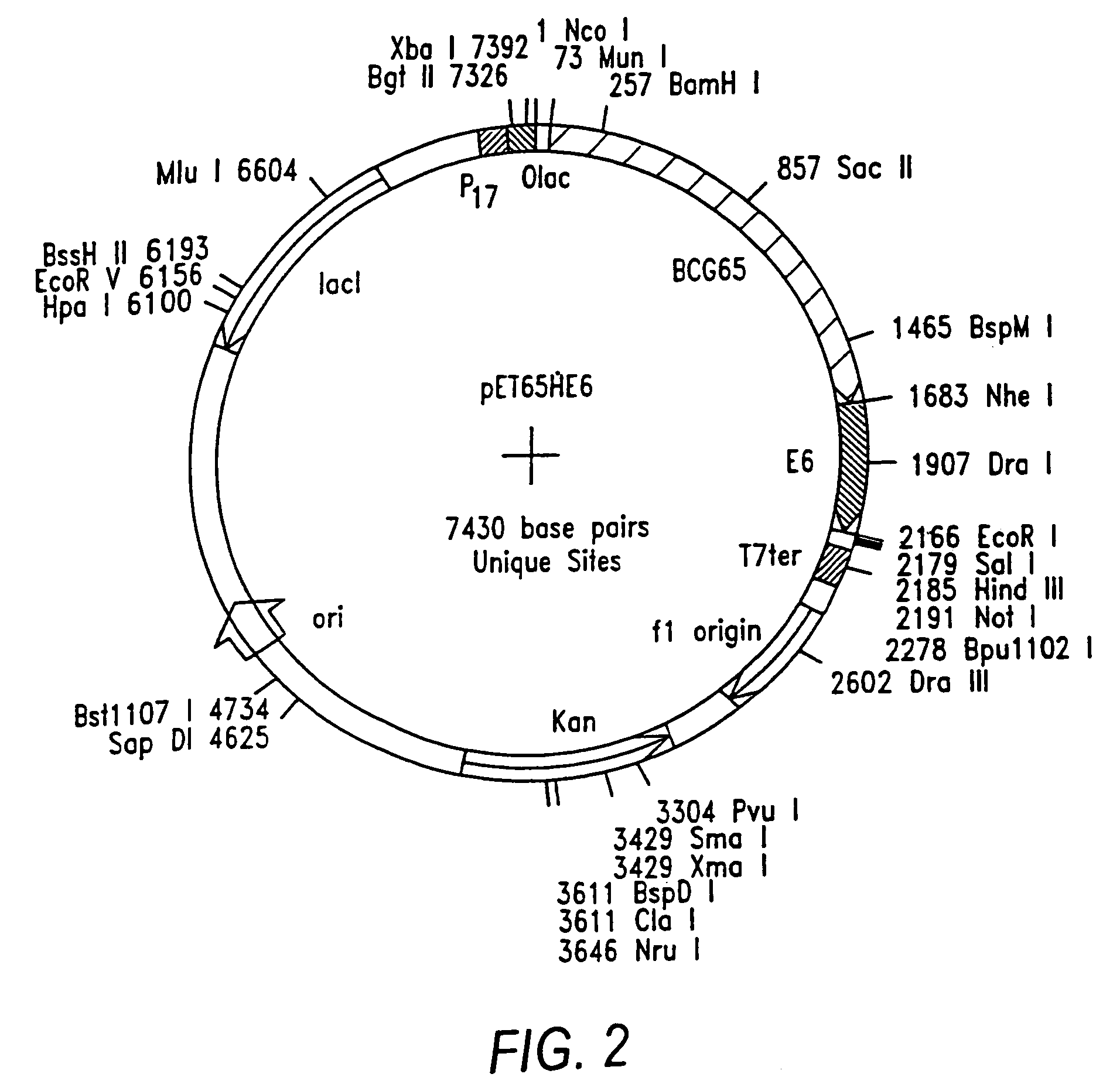
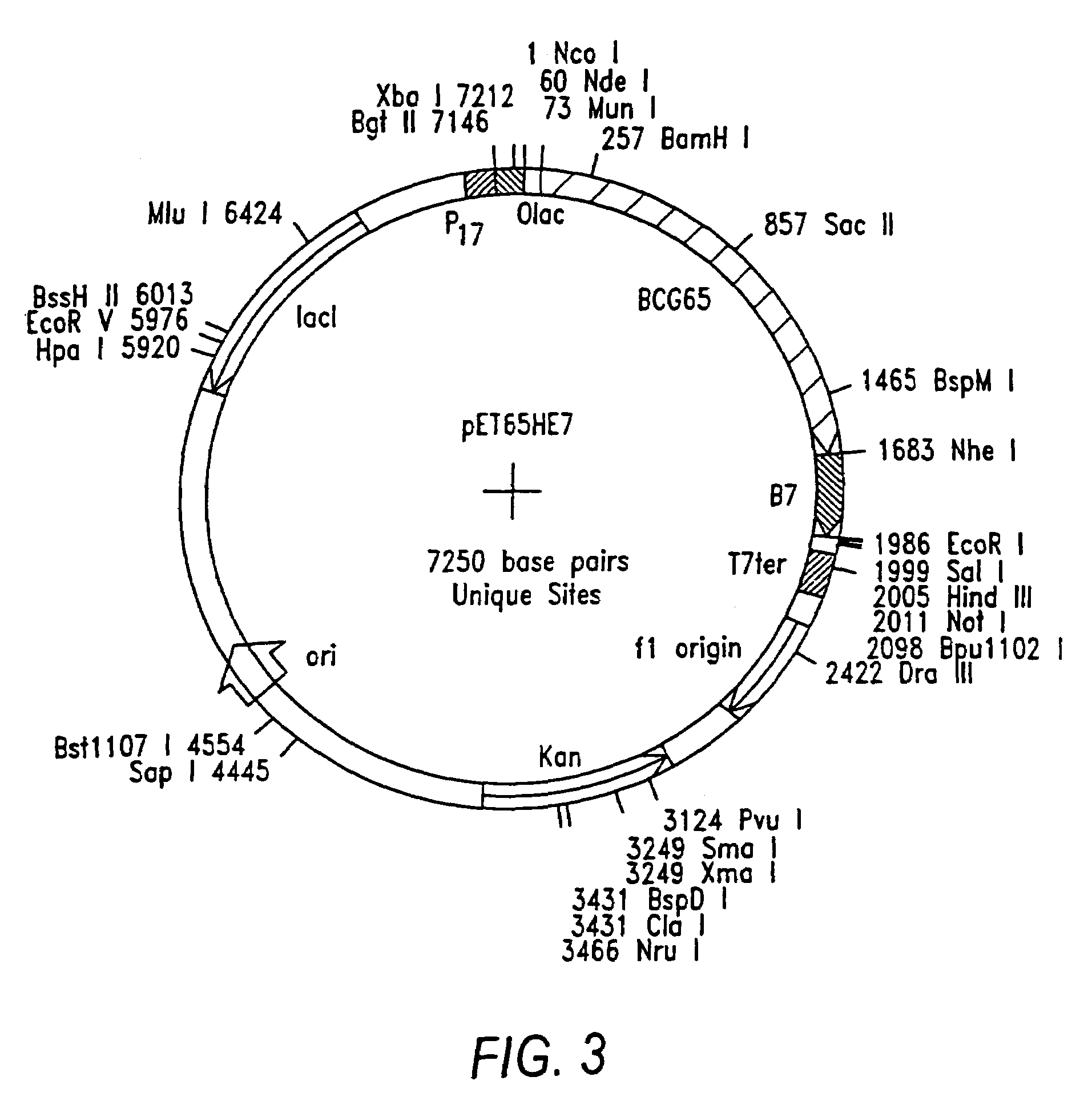
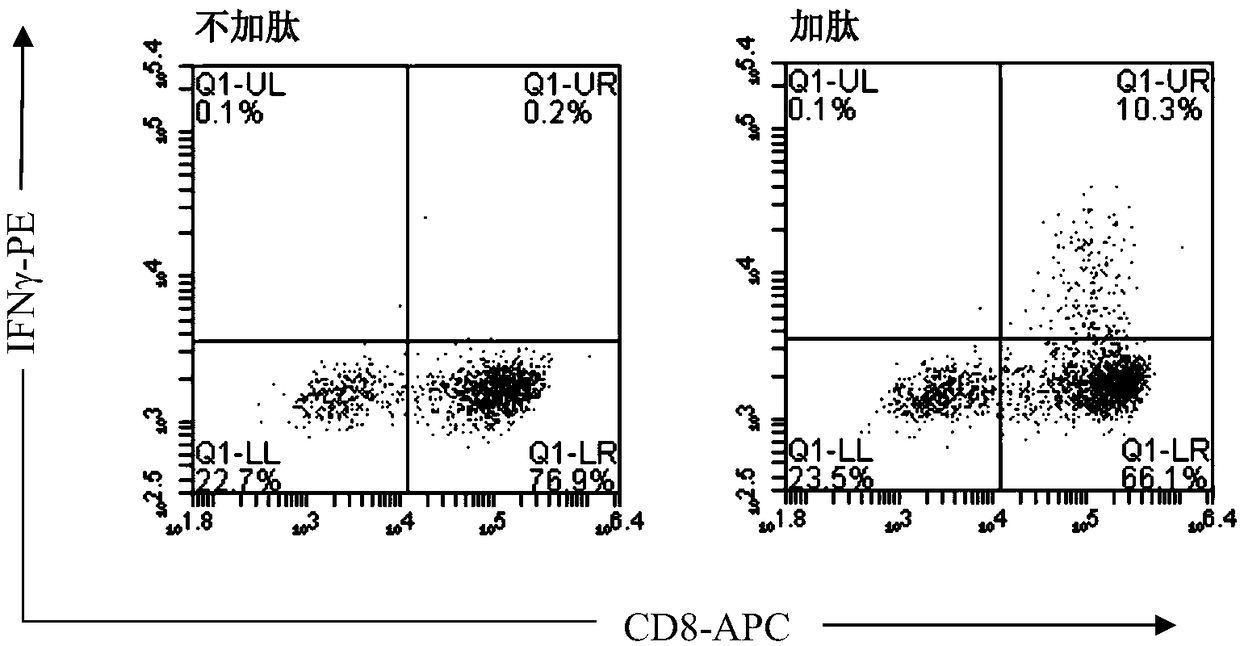
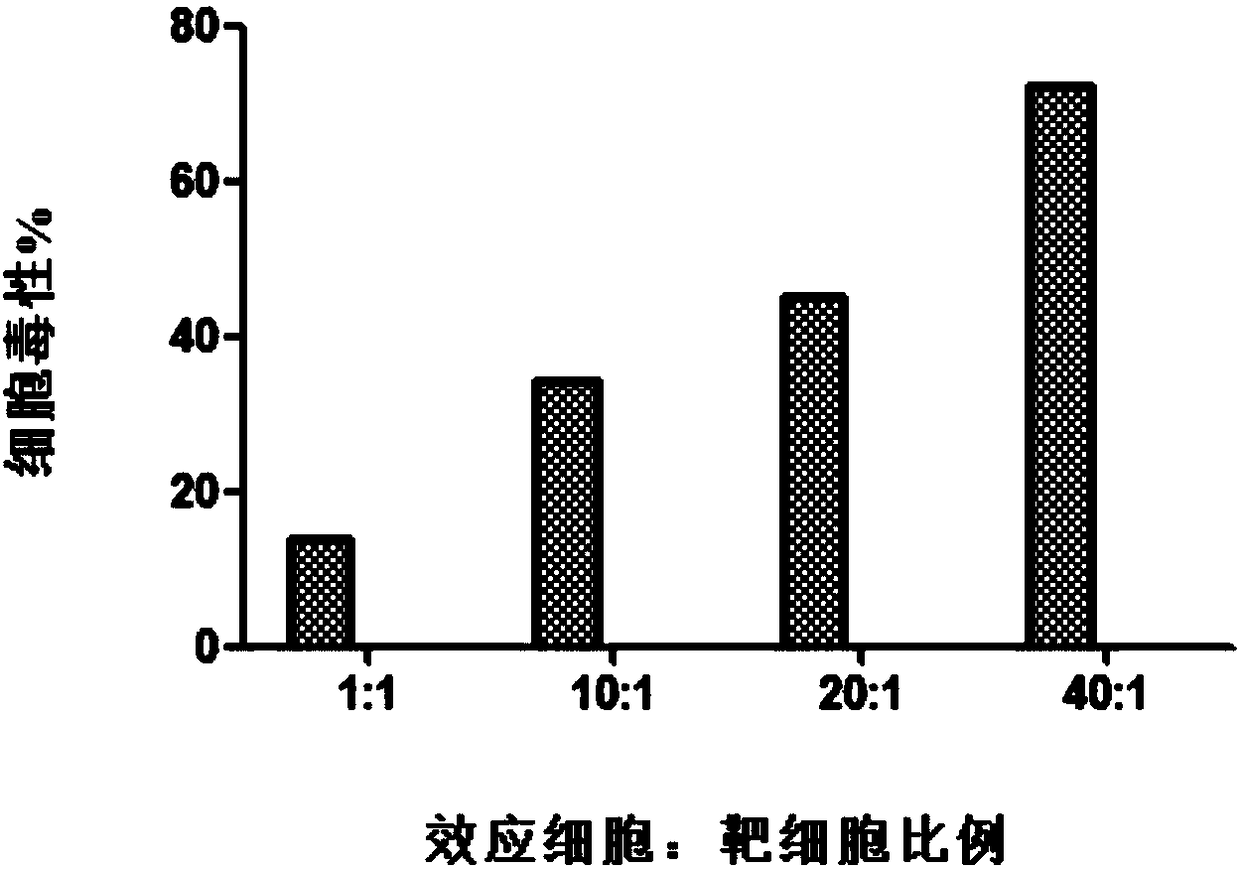
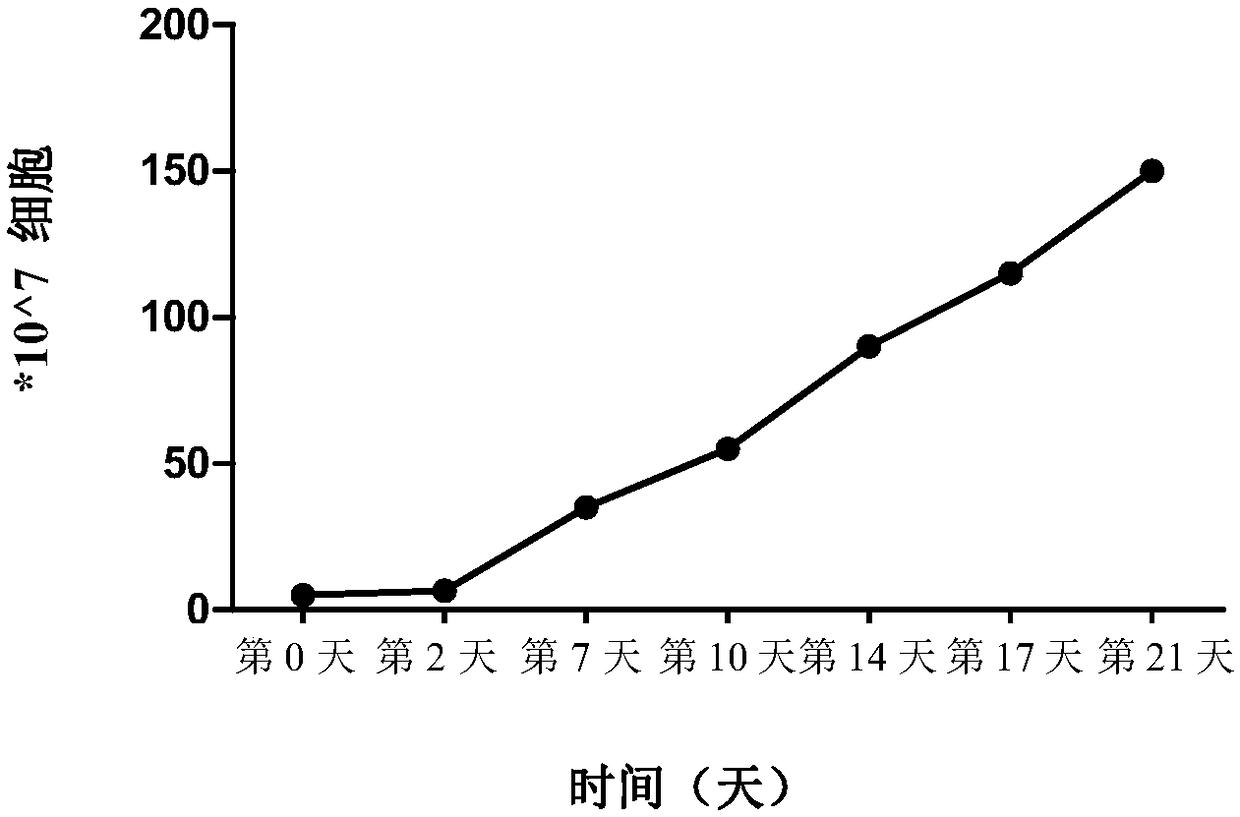
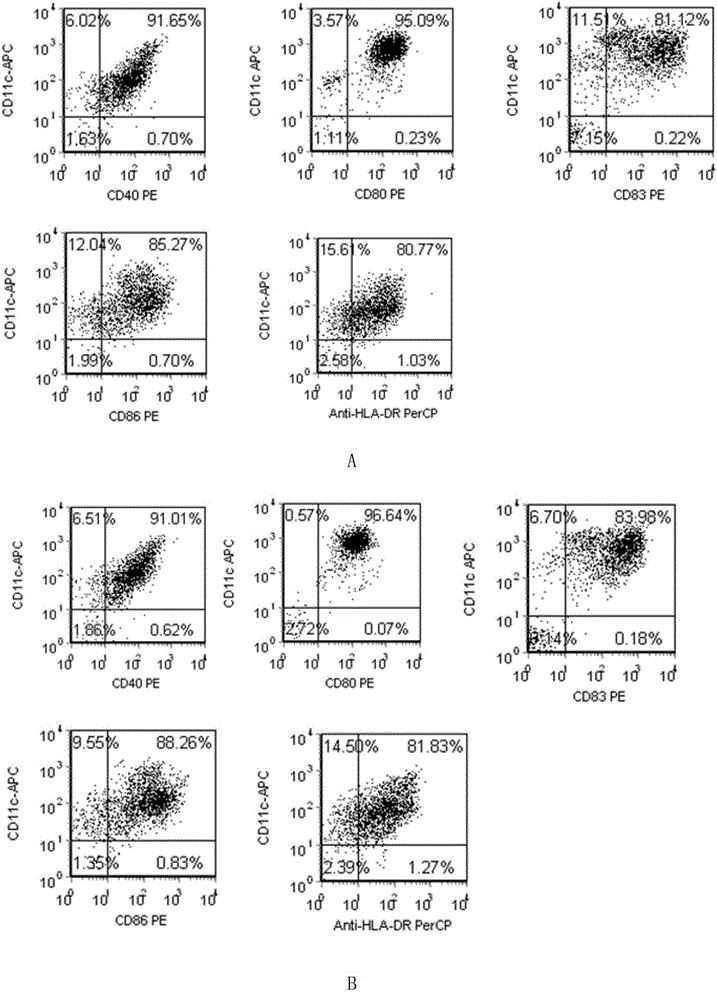
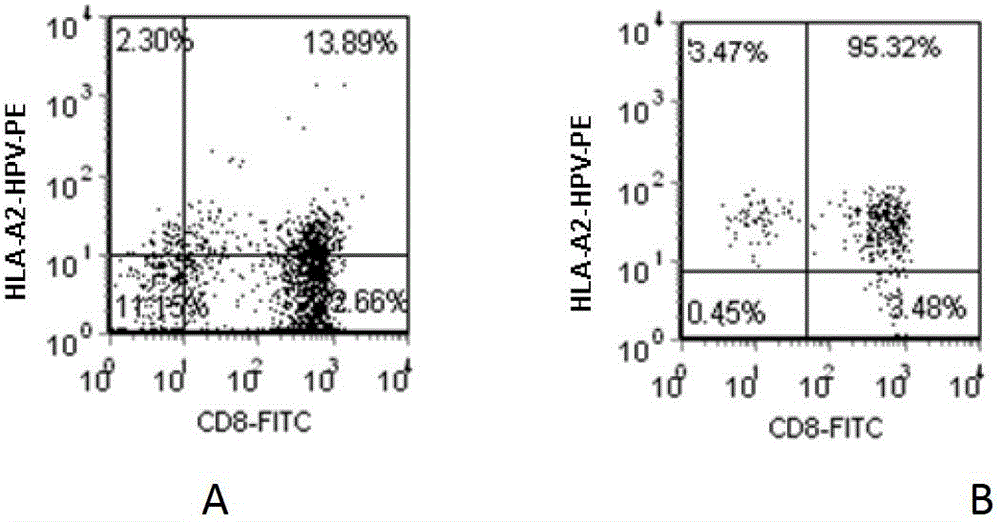
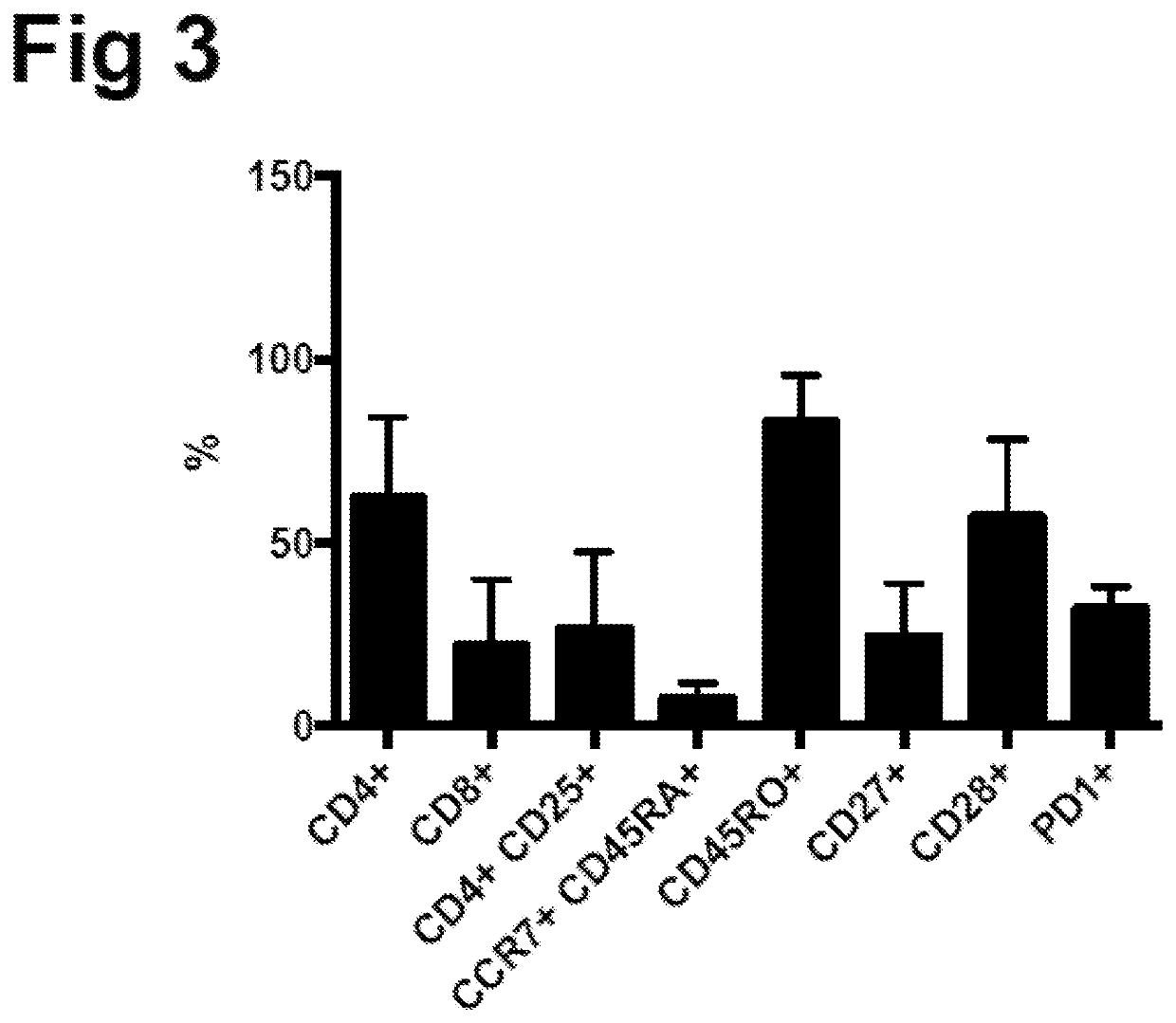
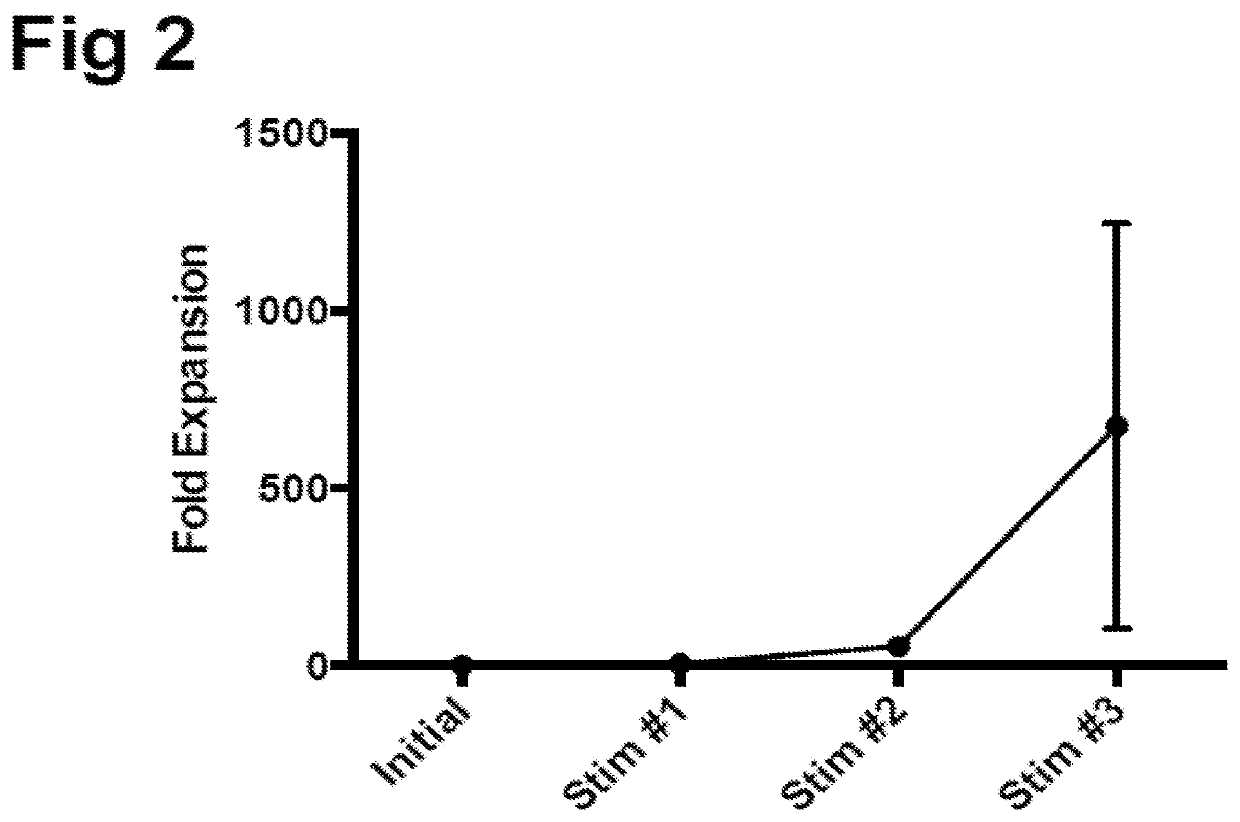
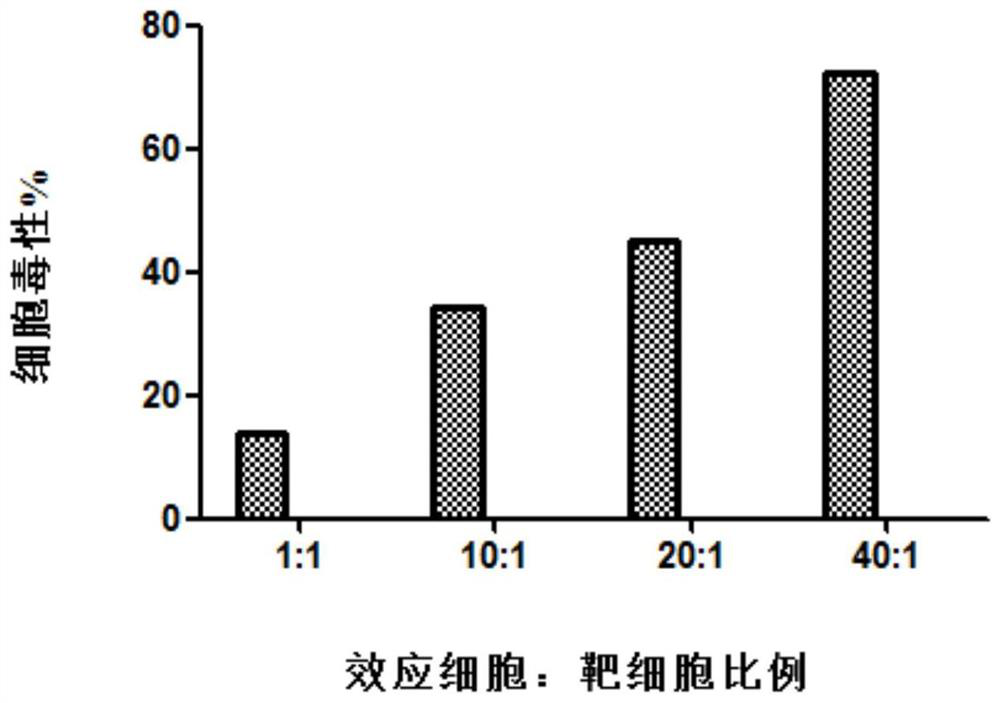
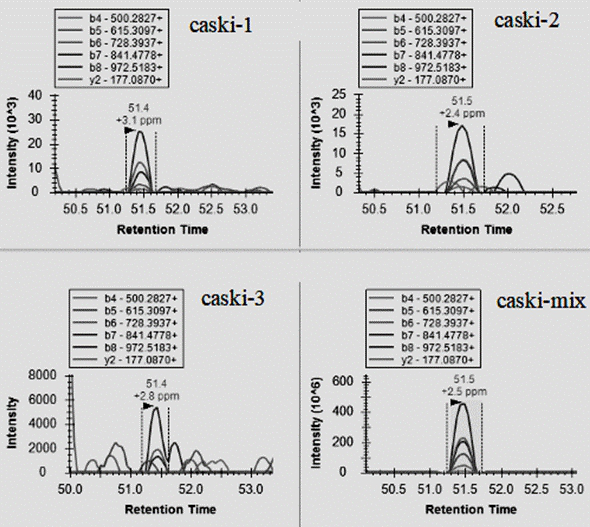
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "HPV Antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immune responses against HPV antigens elicited by compositions comprising an HPV antigen and a stress protein or an expression vector capable of expression of these proteins
InactiveUS6900035B2Enhance immune responseVirusesAntibody mimetics/scaffoldsHPV AntigenInfected cell
This document describes compositions and methods for inducing an immune response (e.g., a cellular response such as a cell-mediated cytolytic immune response) to a human papillomavirus (HPV) antigen, which can be displayed by HPV or exhibited by infected cells (e.g., cells from cervical and other tumors). The HPV protein can be joined to a stress protein by chemical conjugation or noncovalently using linking moieties, or by fusion (e.g., a recombinant fusion protein). Also described are expression vectors containing sequences encoding HPV antigens and stress proteins, which can be introduced into cells of a subject or cells ex vivo. Also described are compositions that include a stress protein linked to an HPV antigen and another pharmacologically acceptable component and stress protein—HPV antigen fusions and conjugates. These compositions can be used to induce or enhance an immune response against HPV and cells that exhibit HPV antigens, including HPV-associated tumors.
Owner:NVENTA BIOPHARMACEUTICALS CORP
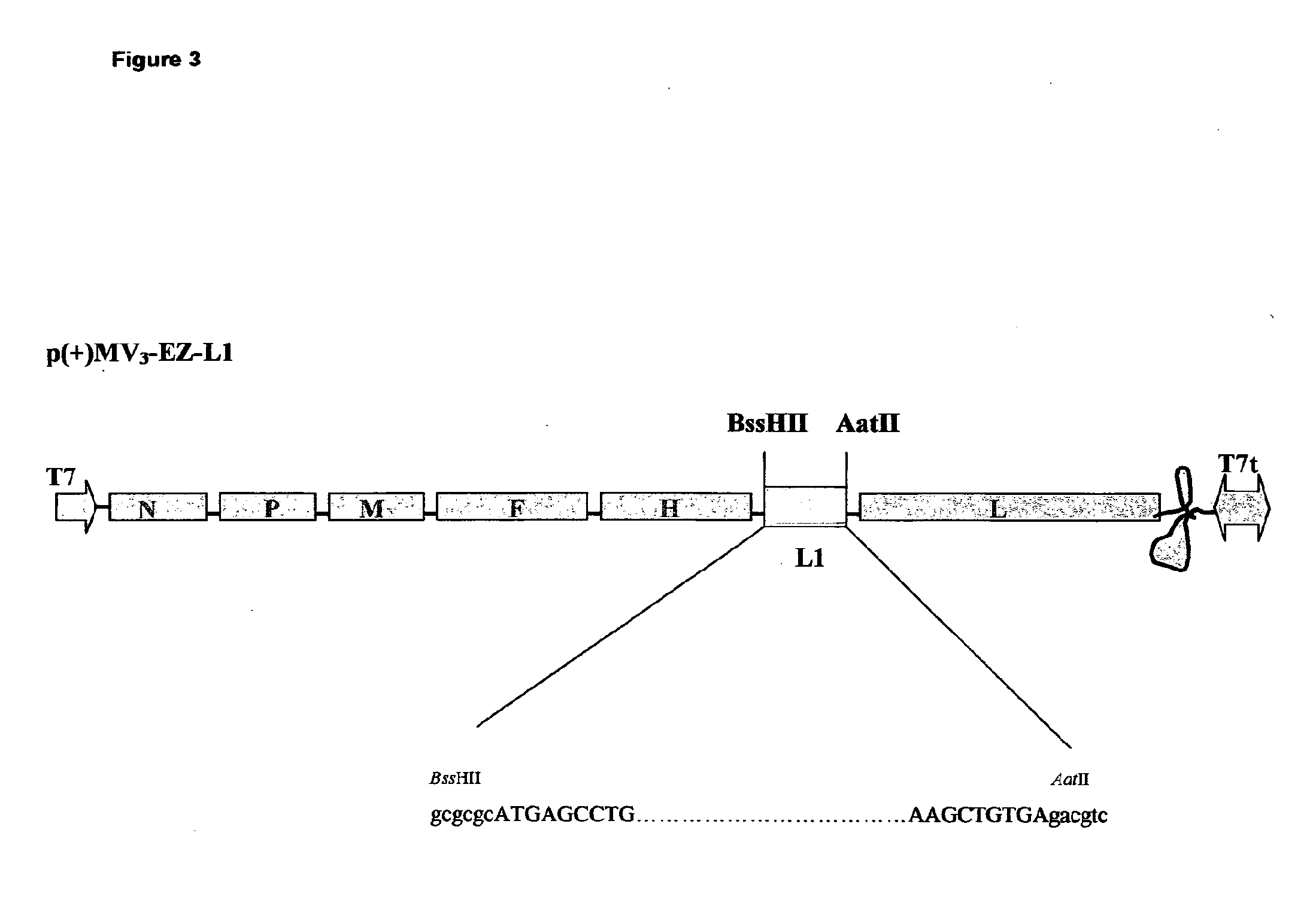
Combined measles-human papilloma vacine
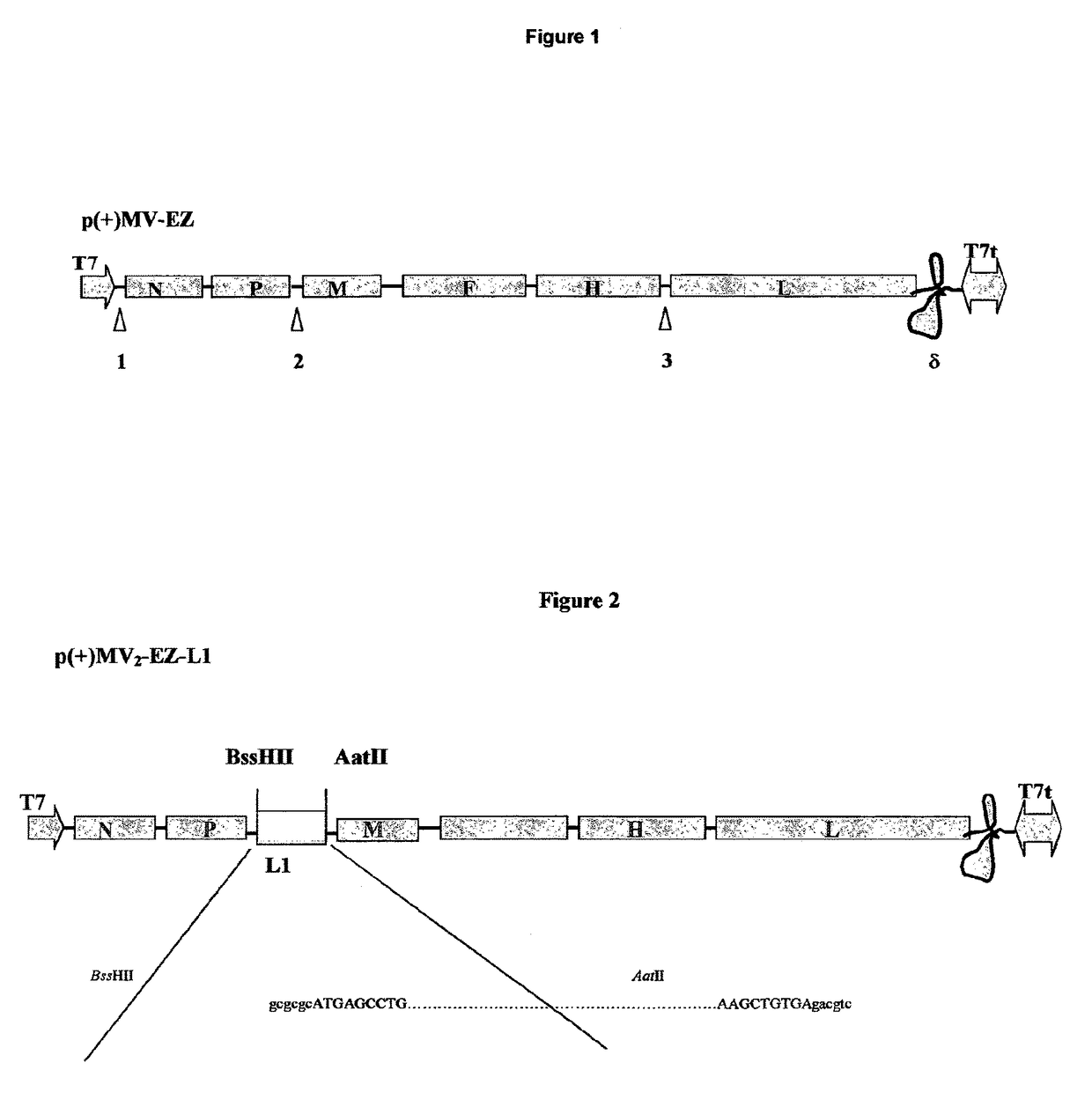
The present invention relates to combined vaccines against measles and human papilloma virus (HPV). In particular, the invention relates to recombinant measles virus vectors containing heterologous nucleic acid encoding single or several antigens derived from HPV, preferably, the major capside antigen L1, the minor capside antigen L2, the early gene E6 and the early gene E7 oncoproteins of HPV type 16, and optionally of types 18, 6 and 11. In a first embodiment, prophylactic vaccines are generated expressing HPV antigens, preferably L1 and / or L2 such that they induce a potent long-lasting immune response in mammals, preferably humans, to protect against HPV and MV infection. In another embodiment, therapeutic vaccines are generated expressing E6 and E7 proteins, and optionally L1 and L2, such that they induced strong immune responses will resolve persistent HPV infections at early or late stages, including HPV-induced cervical carcinoma. In a preferred embodiment, the combined vaccines are easy to produce on a large scale and can be distributed at low cost.
Owner:CADILA HEALTHCARE LTD
HPV antigens, vaccine compositions, and related methods
InactiveUS20080279877A1Reduces and eliminates riskLess considerationVirus peptidesAntiviralsHPV AntigenHuman papilloma virus infection
The present invention relates to the intersection of the fields of immunology and protein engineering, and particularly to antigens and vaccines useful in prevention of infection by human papilloma virus. Provided are recombinant protein antigens, compositions, and methods for the production and use of such antigens and vaccine compositions.
Owner:FRAUNHOFER USA
Methods and means for the treatment of HPV induced intraepithelial neoplasia
InactiveUS20090060870A1Superior clinical responsePromote resultsBiocidePeptide/protein ingredientsHPV AntigenTopical treatment
The current invention provides improved methods and means for the treatment of virally induced intraepithelial neoplasias of the ano-genital tract, such as HPV induced vulvar-, cervical-, vaginal-, penile- and anal intraepithelial neoplasias (VIN, CIN, VAIN, PIN and AIN). The invention provides a method of treatment of a subject suffering from an anogenital intraepithelial neoplasia comprising at least the steps of first determining whether the subject has a T-cell reactivity for viral early antigens, in particular high risk type HPV antigens; and subsequently a local treatment of the neoplasia with immune modulating compounds eliciting local inflammation if the subject scores positive for the T-cell reactivity, preferably a CD4+response against HPV early antigens. The invention also comprises methods and means to induce or further stimulate a cellular immune response against HPV antigens, prior to or during treatment with the immune modulating compound capable of eliciting a local inflammatory response.
Owner:LEIDEN UNIV (MEDICAL CENT)
Methods and means for the treatment of HPV induced intraepithelial neoplasia
InactiveUS7807369B2Enhance immune responseEnhance and prolong immune responseOrganic active ingredientsPeptide/protein ingredientsHPV AntigenVirus
The current invention provides improved methods and means for the treatment of virally induced intraepithelial neoplasias of the ano-genital tract, such as HPV induced vulvar-, cervical-, vaginal-, penile- and anal intraepithelial neoplasias (VIN, CIN, VAIN, PIN and AIN). The invention provides a method of treatment of a subject suffering from an anogenital intraepithelial neoplasia comprising at least the steps of first determining whether the subject has a T-cell reactivity for viral early antigens, in particular high risk type HPV antigens; and subsequently a local treatment of the neoplasia with immune modulating compounds eliciting local inflammation if the subject scores positive for the T-cell reactivity, preferably a CD4+response against HPV early antigens. The invention also comprises methods and means to induce or further stimulate a cellular immune response against HPV antigens, prior to or during treatment with the immune modulating compound capable of eliciting a local inflammatory response.
Owner:LEIDEN UNIV (MEDICAL CENT)
HPV (human papillomavirus) immune colloidal gold diagnostic test strip, method for preparing same and detection method implemented by HPV immune colloidal gold diagnostic test strip
InactiveCN106053806ANo training requiredStrong specificityBiological material analysisBiological testingHPV AntigenHpv detection
The invention discloses a quick and accurate HPV (human papillomavirus) immunochromatographic detection test strip, a method for preparing the same and a HPV detection method on the basis of immunochromatographic technologies. The immunochromatographic technologies are applied to detecting HPV antigen protein for the first time, and accordingly the HPV immunochromatographic detection test strip has high-specificity and high-sensitivity detection performance. Compared with Pasteur and HPV-DNA (deoxyribonucleic acid) detection reported in existing literature, the HPV immunochromatographic detection test strip, the method and the HPV detection method have the main advantages that the HPV immunochromatographic detection test strip and the HPV detection method are short in detection time (5-20 minutes); optional special instruments can be omitted; the HPV immunochromatographic detection test strip is easy and convenient to operate and low in detection cost, only one-step reaction is required, and operators do not need to be trained; special temperature requirements and freezing can be omitted, and accordingly the HPV immunochromatographic detection test strip is convenient to store and transport and can be stored at the room temperatures for 24 months.
Owner:SUZHOU DONGNI BIOTECH CO LTD
Immune responses against HPV antigens elicited by compositions comprising an HPV antigen and a stress protein or an expression vector capable of expression of these proteins
InactiveUS7262014B2Enhance immune responseAntibody mimetics/scaffoldsGenetic material ingredientsHPV AntigenInfected cell
This document describes compositions and methods for inducing an immune response (e.g., a cellular response such as a cell-mediated cytolytic immune response) to a human papillomavirus (HPV) antigen, which can be displayed by HPV or exhibited by infected cells (e.g., cells from cervical and other tumors). The HPV protein can be joined to a stress protein by chemical conjugation or noncovalently using linking moieties, or by fusion (e.g., a recombinant fusion protein). Also described are expression vectors containing sequences encoding HPV antigens and stress proteins, which can be introduced into cells of a subject or cells ex vivo. Also described are compositions that include a stress protein linked to an HPV antigen and another pharmacologically acceptable component and stress protein—HPV antigen fusions and conjugates. These compositions can be used to induce or enhance an immune response against HPV and cells that exhibit HPV antigens, including HPV-associated tumors.
Owner:NVENTA BIOPHARMACEUTICALS CORP
Vaccine composition useful for HPV and hepatitis B infections and a method for preparing the same
InactiveCN102112152AAntibody mimetics/scaffoldsViral antigen ingredientsHPV AntigenHepatitis B virus
The invention describes a vaccine compositions comprising chimeric fusions of the HPV antigens with viral or bacterial proteins conferring enhanced immunogenicity useful for Hepatitis B virus as well as human papillomavirus (HPV) infections.
Owner:BHARAT BIOTECH INTERNATIONAL
Method for preparing HPV (human papillomavirus) antigen specific CTL (cytotoxic T lymphocyte)
ActiveCN108300692AEnhance antigen presentationInduced proliferationMammal material medical ingredientsBlood/immune system cellsHPV AntigenDisease
The invention discloses a method for preparing HPV (human papillomavirus) antigen specific CTL (cytotoxic T lymphocyte) and particularly discloses a preparation method of HLA-A2402 restrictive anti-HPV antigen specific CTL. According to the method, a peripheral blood mononuclear cell is collected through single blood collection or venous blood collection, the antigen presentation function of B cells is enhanced with CpG ODN 2395, the B cells supporting HLA-A2402 restrictive HPV antigen peptide are used for stimulating the peripheral blood mononuclear cell, rhIL-2, rhIL-7, rhIL-15 and rhIL-21 are jointly used to promote growth of the T cell. The target CTL prepared with the method has the characteristics that preparation is simple, the preparation cycle is short, the cost is low, the multiplication capacity and the killing activity are high, the cell viability is high and the like, and can be used for immunotherapy of HPV infection related diseases including cervical cancer.
Owner:JILIN TUO HUA BIOTECH
Preparation method of HLA-A0201-restricted anti-HPV (human papillomavirus) antigen-specific CTL
ActiveCN102719402ASimple and fast operationEfficient activationBlood/immune system cellsHPV AntigenDendritic cell
The invention discloses a preparation method of HLA-A0201-restricted anti-HPV (human papillomavirus) antigen-specific CTL. The preparation method comprises the following steps of: collecting peripheral blood mononuclear cells through apheresis, and enriching and purifying CD8+T lymphocytes; stimulating the CD8 + T lymphocytes by mature dendritic cells of a loaded HLA-A0201-restricted HPV antigen polypeptide; and promoting the T-cell growth through combined use of rhIL-2 and rhIL-7; purifying a target CTL by a Tetramer marking method and a flow cytometry sorting technique; stimulating the growth of the target CTL by solid phase packaged anti-human CD3 monoclonal antibody and IL-2; adding autologous PBMCs (peripheral blood mononuclear cell) after gamma-ray irradiation for enhancing activation of the target CTL; and adding rhIL-15, cultivating and amplifying, and collecting and identifying. The target CTL prepared by the prepared method has high purity, high proliferative capacity, high killing activity and a high CTL-CM ratio and can be applied to immunotherapy of tumors and the like.
Owner:江苏得康生物科技有限公司
Methods for treating hpv-associated diseases
PendingUS20210038709A1Enhance immune responseViral antigen ingredientsInorganic active ingredientsHPV AntigenDisease
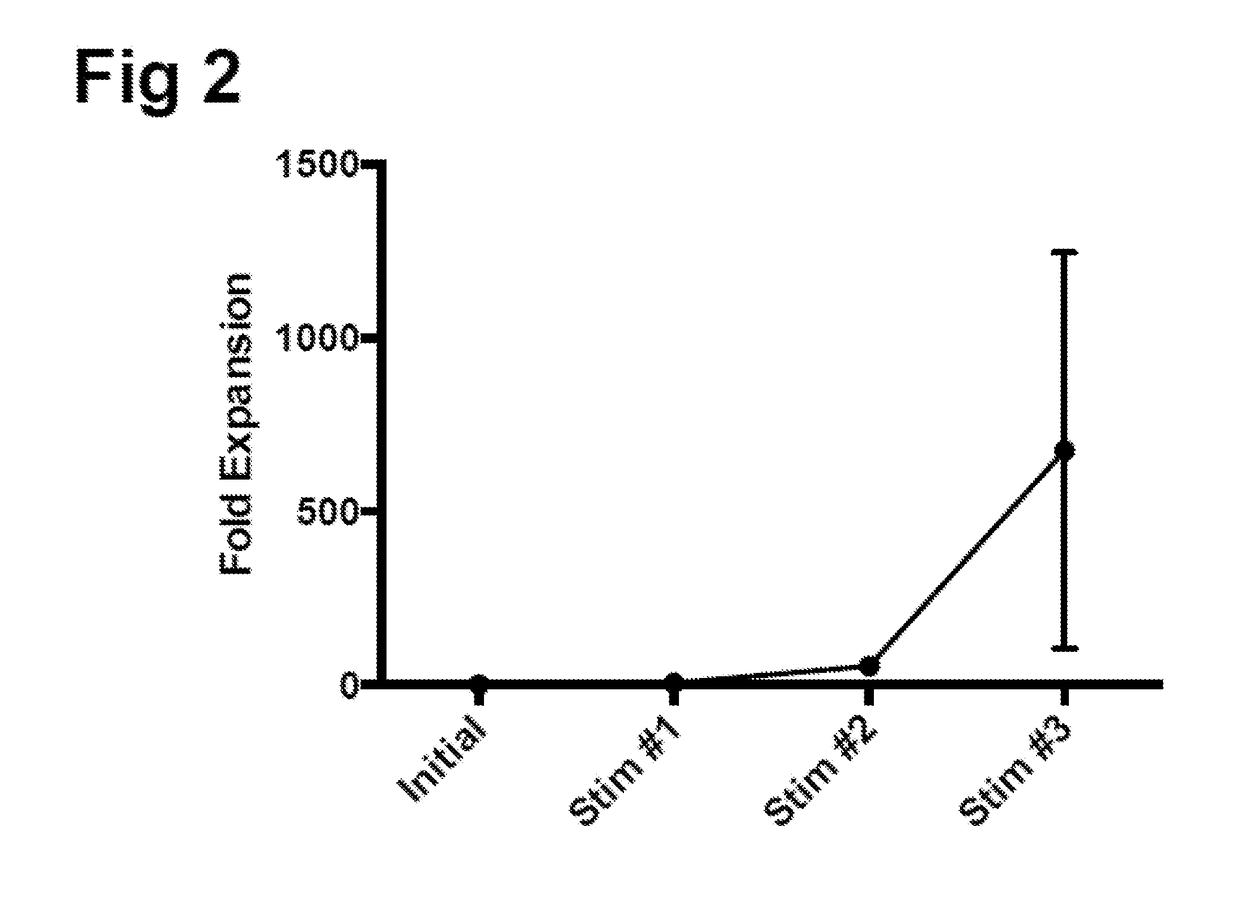
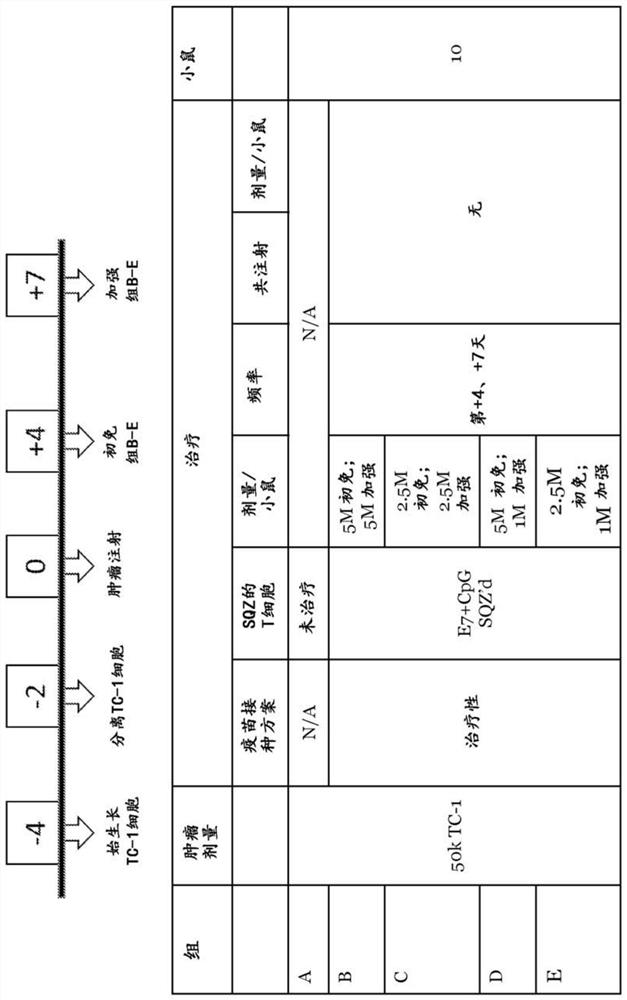
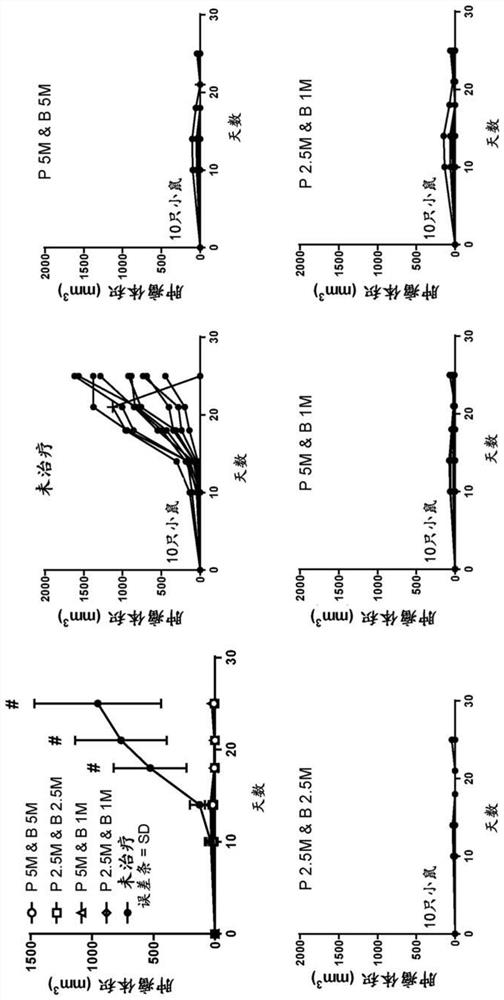
The present application provides immune cells comprising an HPV antigen and an adjuvant, methods of manufacturing such modified immune cells, and methods of using such modified immune cells for treating an HPV-associated disease, preventing an HPV-associated disease and / or for modulating an immune response in an individual with an HPV-associated disease.
Owner:SQZ BIOTECH CO
New HPV epitope
InactiveCN108164587AEfficient detectionImprove the test positive rateViral antigen ingredientsVirus peptidesHPV AntigenEpitope
The present invention relates to new HPV epitope, wherein the sequence of the new HPV epitope is an amino acid sequence selected from SEQ ID NO:1-5 or an amino acid sequence having at least 80% identity with a sequence selected from SEQ ID NO:1-5. The invention further relates to applications of the epitope in detection of the presence of antibodies against HPV, particularly high-risk HPV, in samples, and in detection of the infection of HPV, particularly high-risk HPV, in subjects.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Immunity enhancing therapeutic vaccine for HPV and related diseases
ActiveUS20160324952A1Easy to degradeEasy to presentViral antigen ingredientsAntibody mimetics/scaffoldsDiseaseHPV Antigen
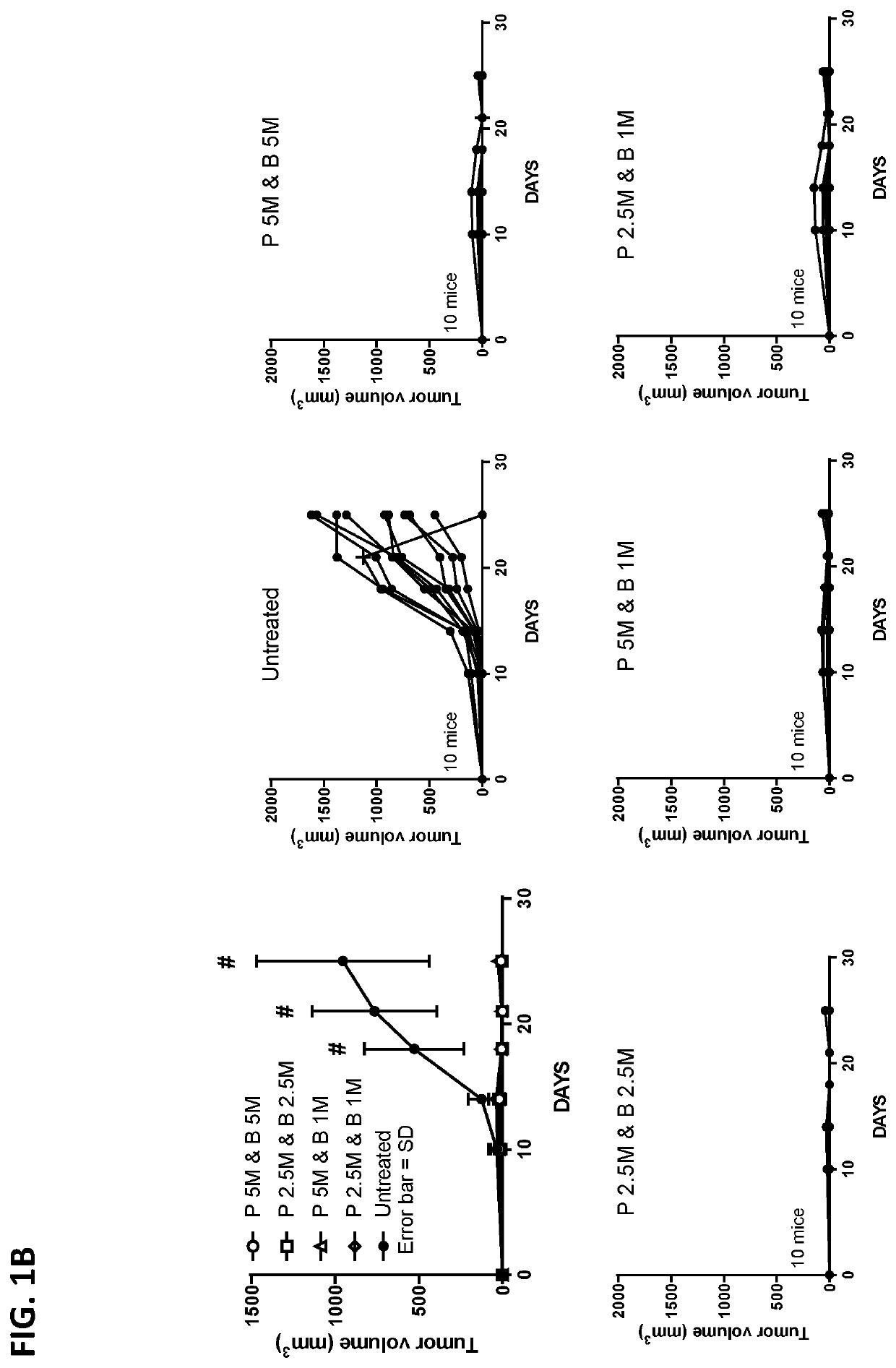
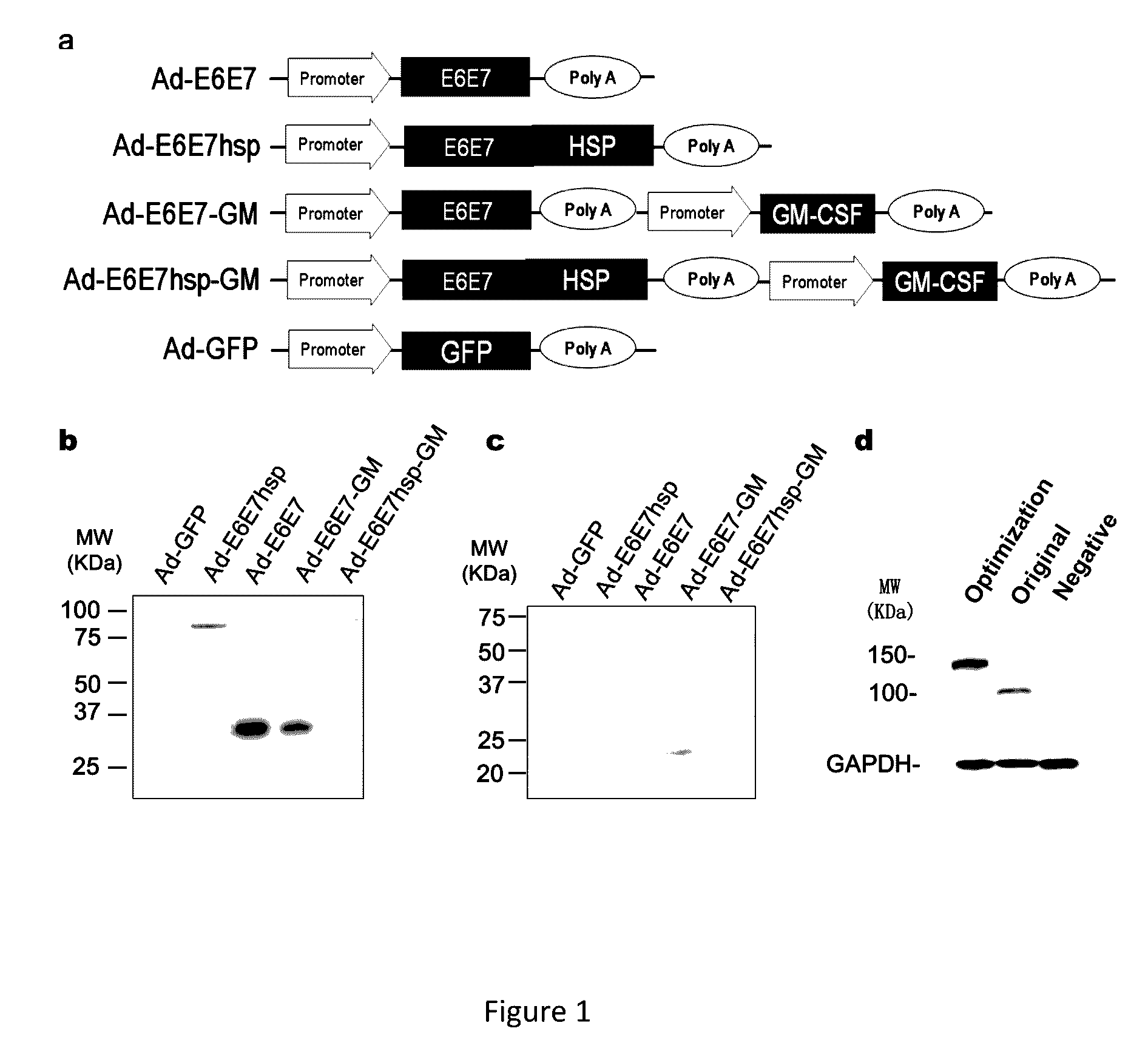
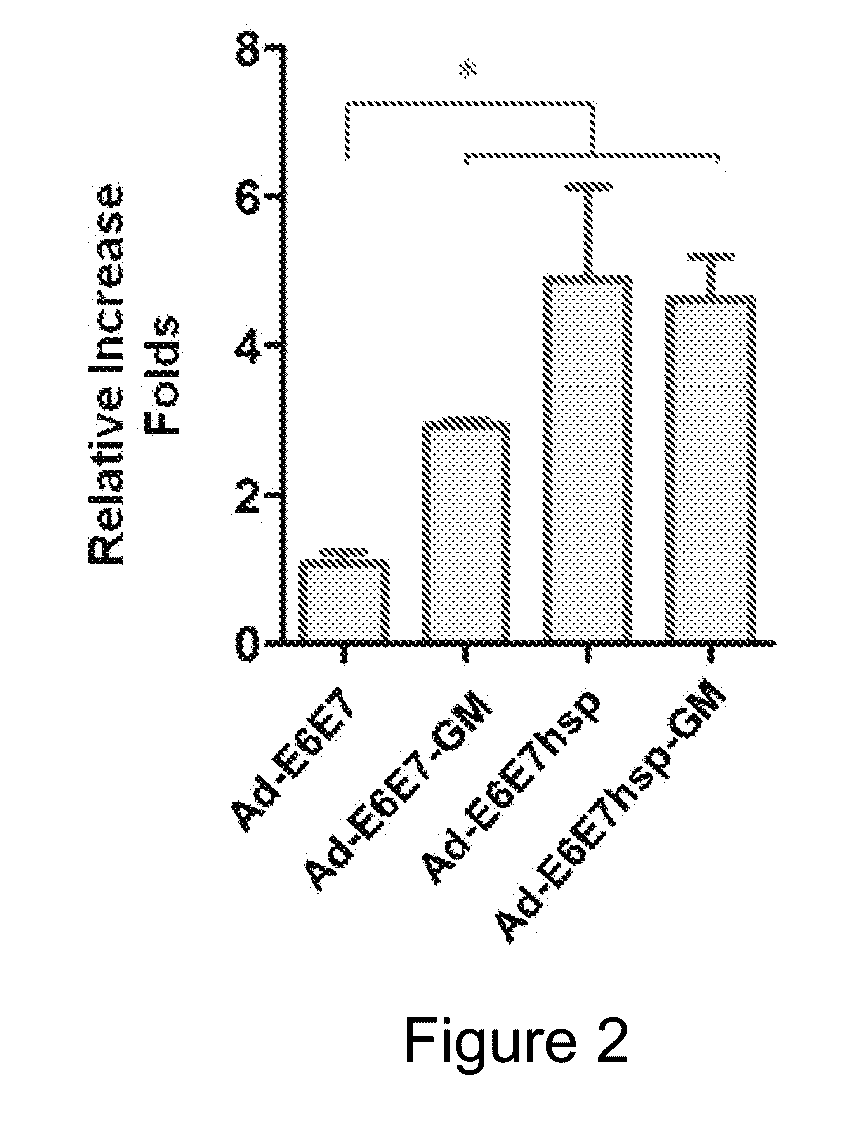
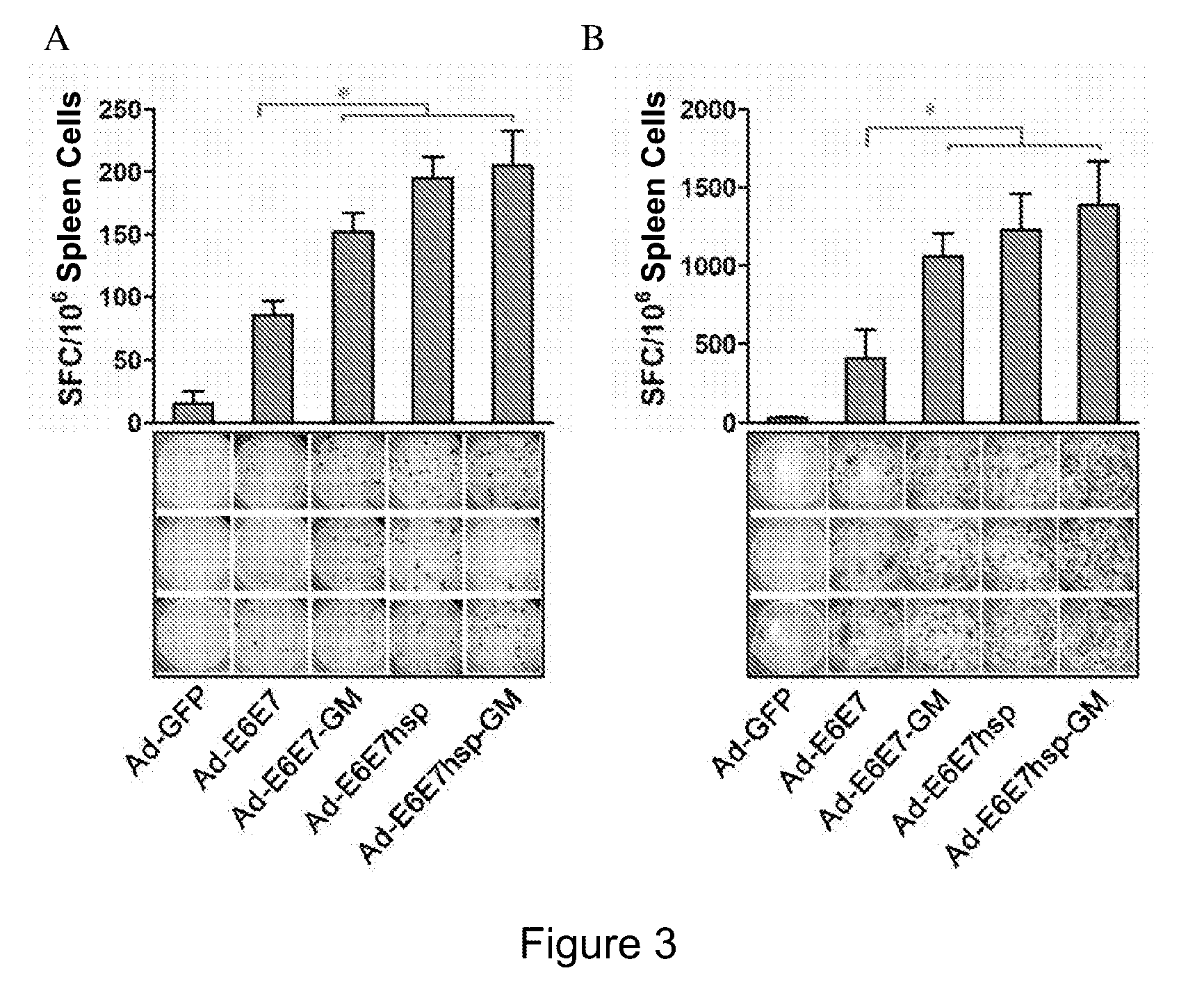
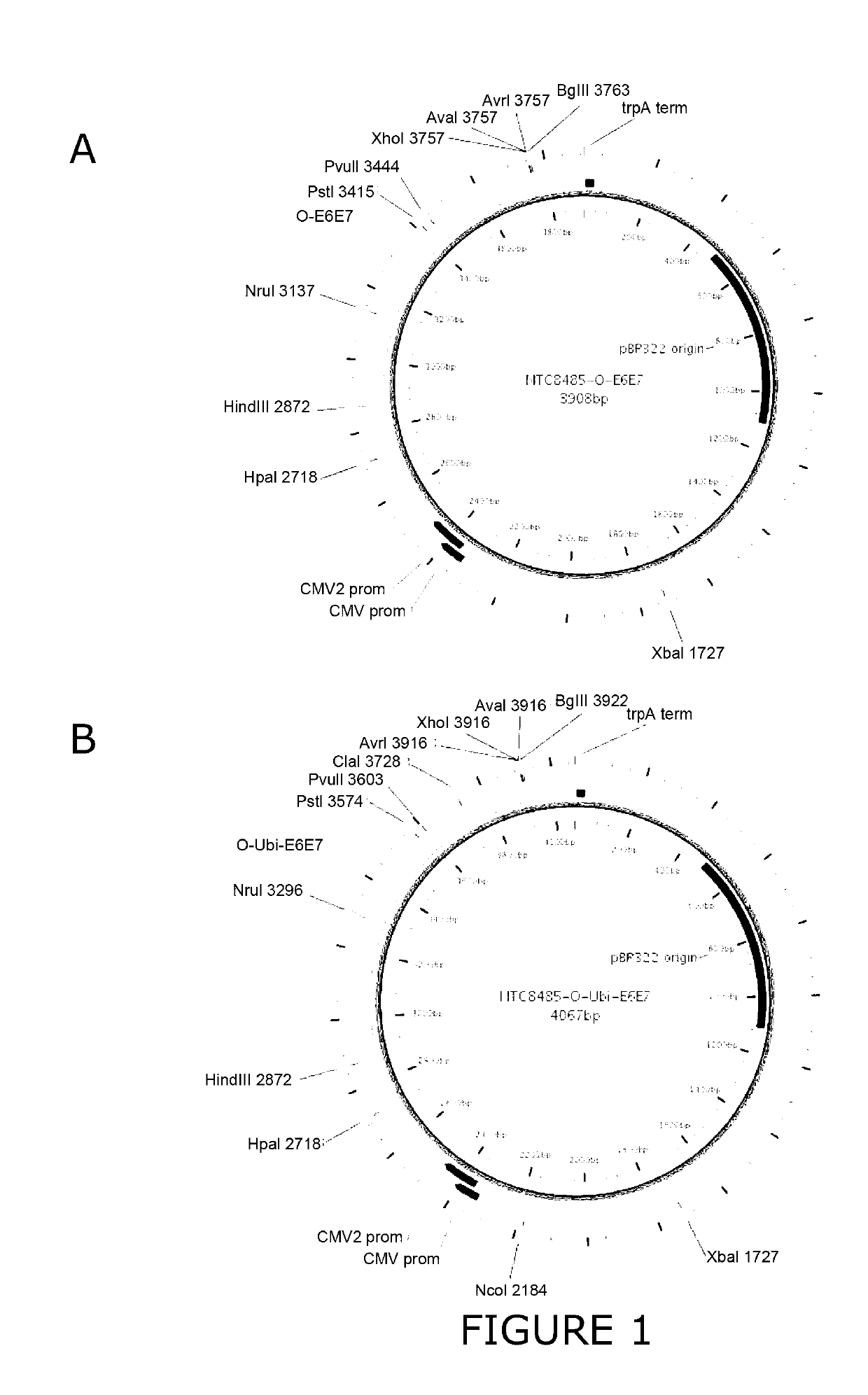
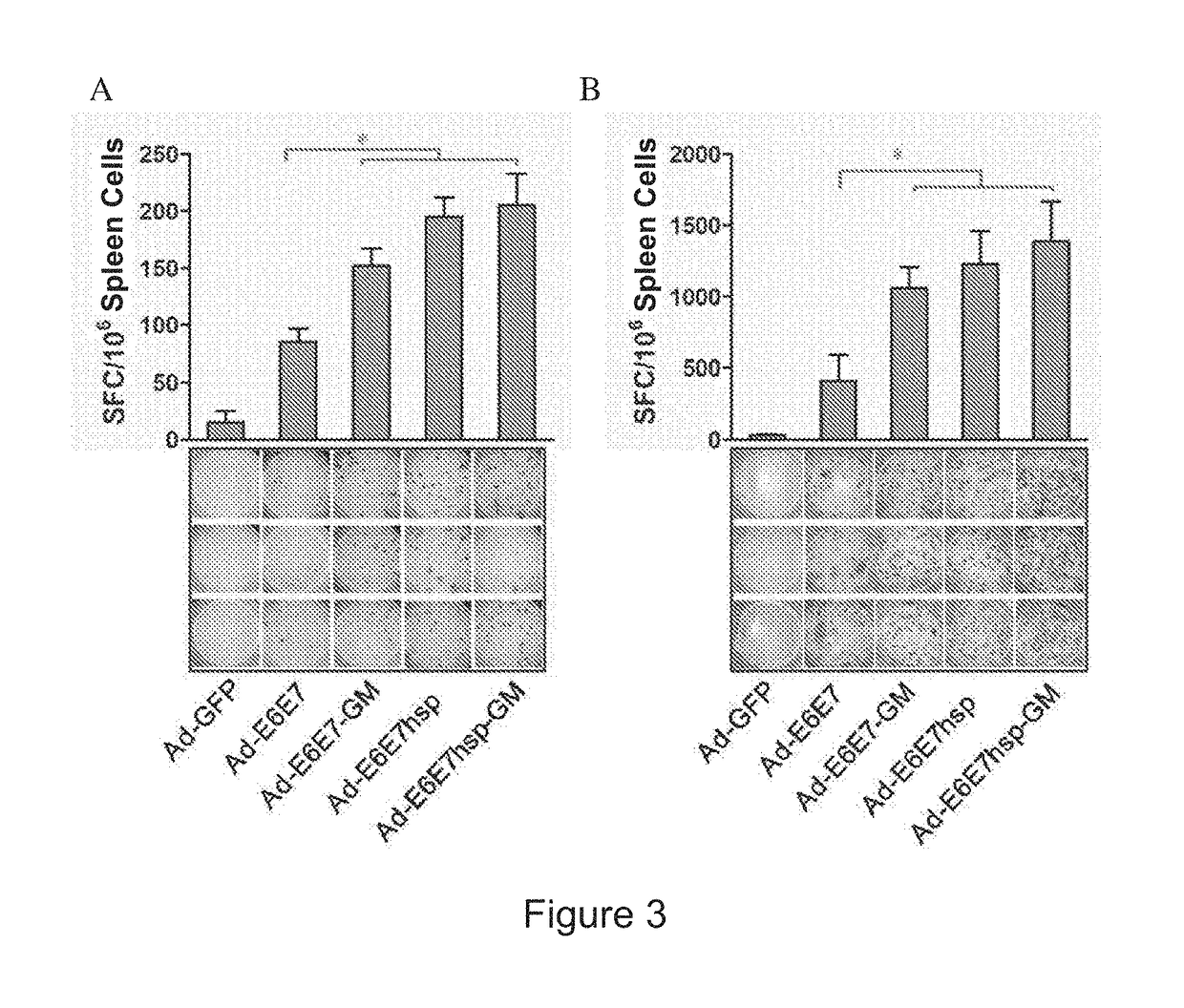
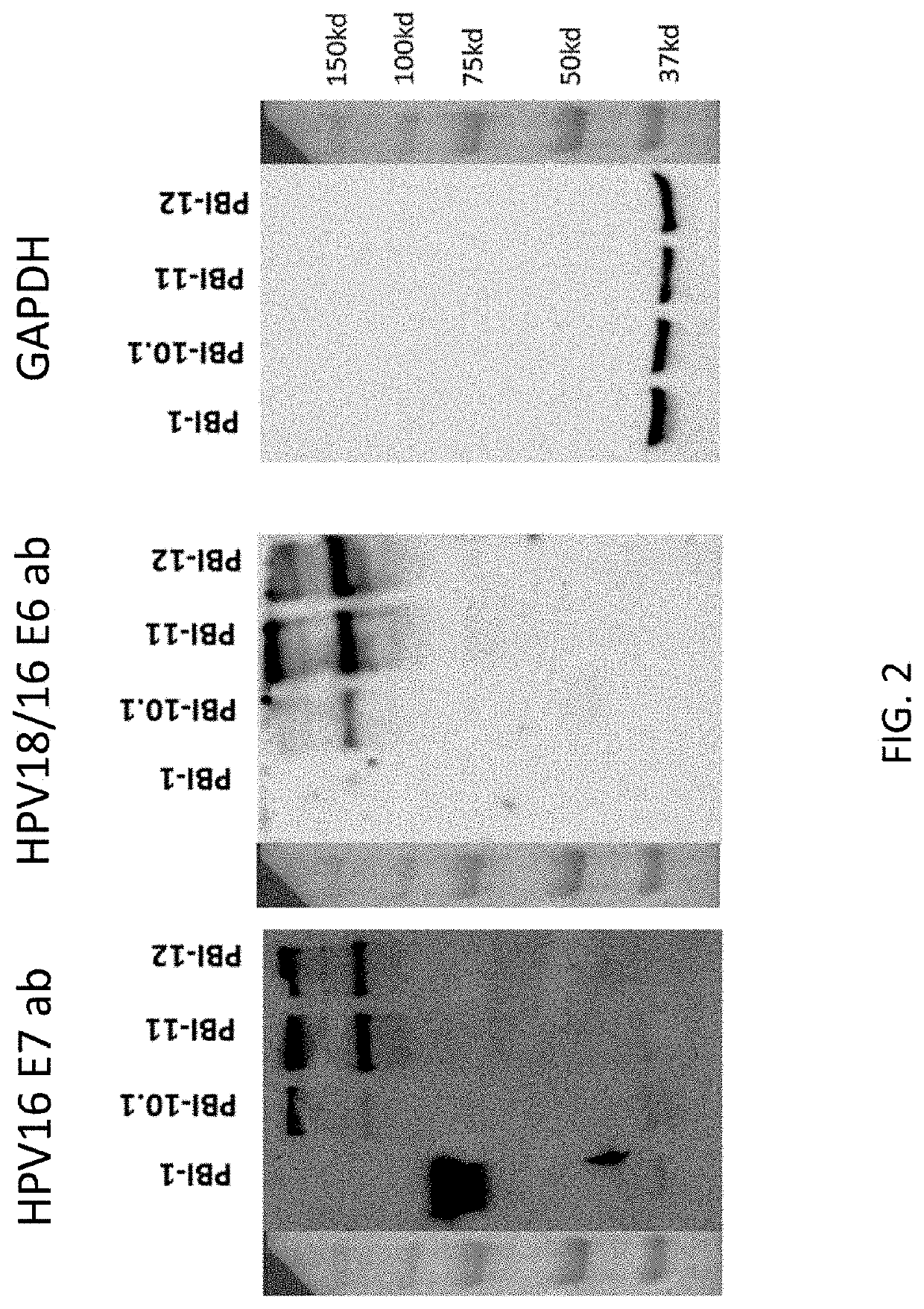
An immunity enhancing therapeutic vaccine includes a gene vector based on recombinant adenovirus and three elements: 1) an HPV antigen including the E6 and E7 multivalent fusion proteins of HPV types 16 and 18, 2) an immunologic adjuvant protein fused with said antigen, which protein may be a heat shock protein (HSP) of Mycobacterium tuberculosis, and 3) an immunostimulant, which may be granulocyte-macrophage colony stimulating factor (GM-CSF). The vaccine is used for the treatment of human papilloma virus infections and related diseases.
Owner:MYGT BIOPHARM LLC
Generating HPV antigen-specific t cells from a naïve t cell population
Safe, rapid, and efficient methods for producing antigen-specific T cells recognizing human papilloma virus (HPV antigens); HPV-specific T cells, and methods for treating HPV infections and HPV-related malignancies by adoptive transfer of HPV-specific T cells.
Owner:CHILDRENS NAT MEDICAL CENT
Combined measles-human papilloma vaccine
The present invention relates to combined vaccines against measles and human papilloma virus (HPV). In particular, the invention relates to recombinant measles virus vectors containing heterologous nucleic acid encoding single or several antigens derived from HPV, preferably, the major capside antigen L1, the minor capside antigen L2, the early gene E6 and the early gene E7 oncoproteins of HPV type 16, and optionally of types 18, 6 and 11. In a first embodiment, prophylactic vaccines are generated expressing HPV antigens, preferably L1 and / or L2 such that they induce a potent long-lasting immune response in mammals, preferably humans, to protect against HPV and MV infection. In another embodiment, therapeutic vaccines are generated expressing E6 and E7 proteins, and optionally L1 and L2, such that they induced strong immune responses will resolve persistent HPV infections at early or late stages, including HPV-induced cervical carcinoma. In a preferred embodiment, the combined vaccines are easy to produce on a large scale and can be distributed at low cost.
Owner:CADILA HEALTHCARE LTD
Immunomodulating composition for treatment
ActiveUS10596248B2Treat infectionAvoid unnecessary expensesViral antigen ingredientsFusion with degradation motifHPV AntigenImmunomodulations
Owner:JINGANG MEDICINE AUSTRALIA PTY LTD
Generating HPV antigen-specific cells from a naive T cell population
ActiveUS10934525B2Rapid and robust expansion of HPV-recognizingCell receptors/surface-antigens/surface-determinantsViral antigen ingredientsHPV AntigenVirus
Safe, rapid and efficient methods for producing antigen-specific T cells recognizing human papilloma virus or HPV antigens.
Owner:CHILDRENS NAT MEDICAL CENT
Generating HPV antigen-specific cells from a naive t cell population
PendingUS20210155900A1Rapid and robust expansion of HPV-recognizingCell receptors/surface-antigens/surface-determinantsAntiviralsHPV AntigenVirus
Safe, rapid and efficient methods for producing antigen-specific T cells recognizing human papilloma virus or HPV antigens.
Owner:CHILDRENS NAT MEDICAL CENT
Method for enhancing immunogenicity of epitope peptide of HPV antigen, virus-like particle, and method for preparing HPV vaccine
ActiveUS20170166612A1Improving immunogenicityHigh recovery rateAntibody mimetics/scaffoldsViral antigen ingredientsHPV AntigenEpitope
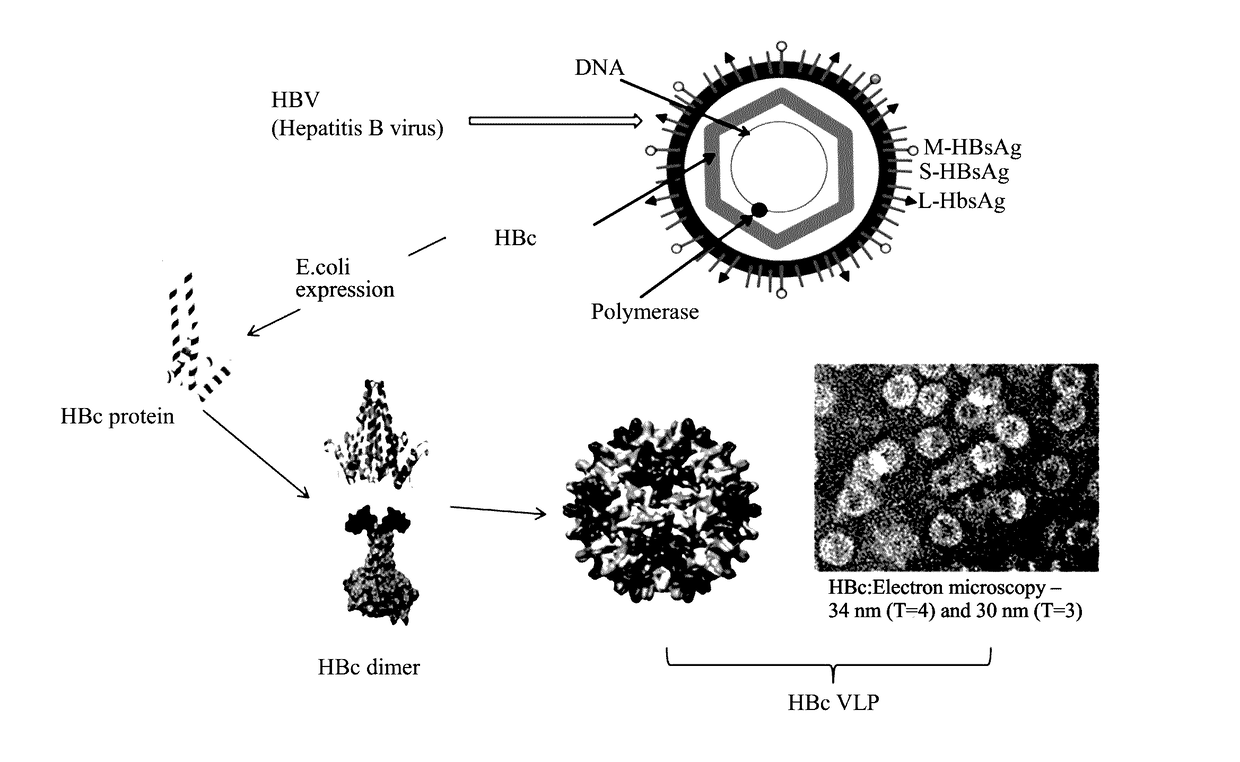
A method for enhancing immunogenicity of an epitope peptide of an HPV antigen, the method including: assembling a gene of an HPV antigen into a gene of HBc, exogenously expressing a resulting assembled gene to acquire a fusion protein, allowing the fusion protein to automatically assemble to form a virus-like particle including the HPV antigen on a surface of the virus-like particle, to obtain HBc-HPV virus-like particle. A virus-like particle capable of expressing the antigen peptide of HPV acquired by the method. The virus-like particle includes a HBc-L2 fusion protein. A genome for encoding the HBc-L2 fusion protein is represented by SEQ ID NO. 3. A method for preparing an HPV vaccine includes using the virus-like particle.
Owner:CANSINO BIOLOGICS INC
Vector for treatment vaccine for stable and constitutive high-expression cervical cancer and recombinant lactobacillus transformed by the same
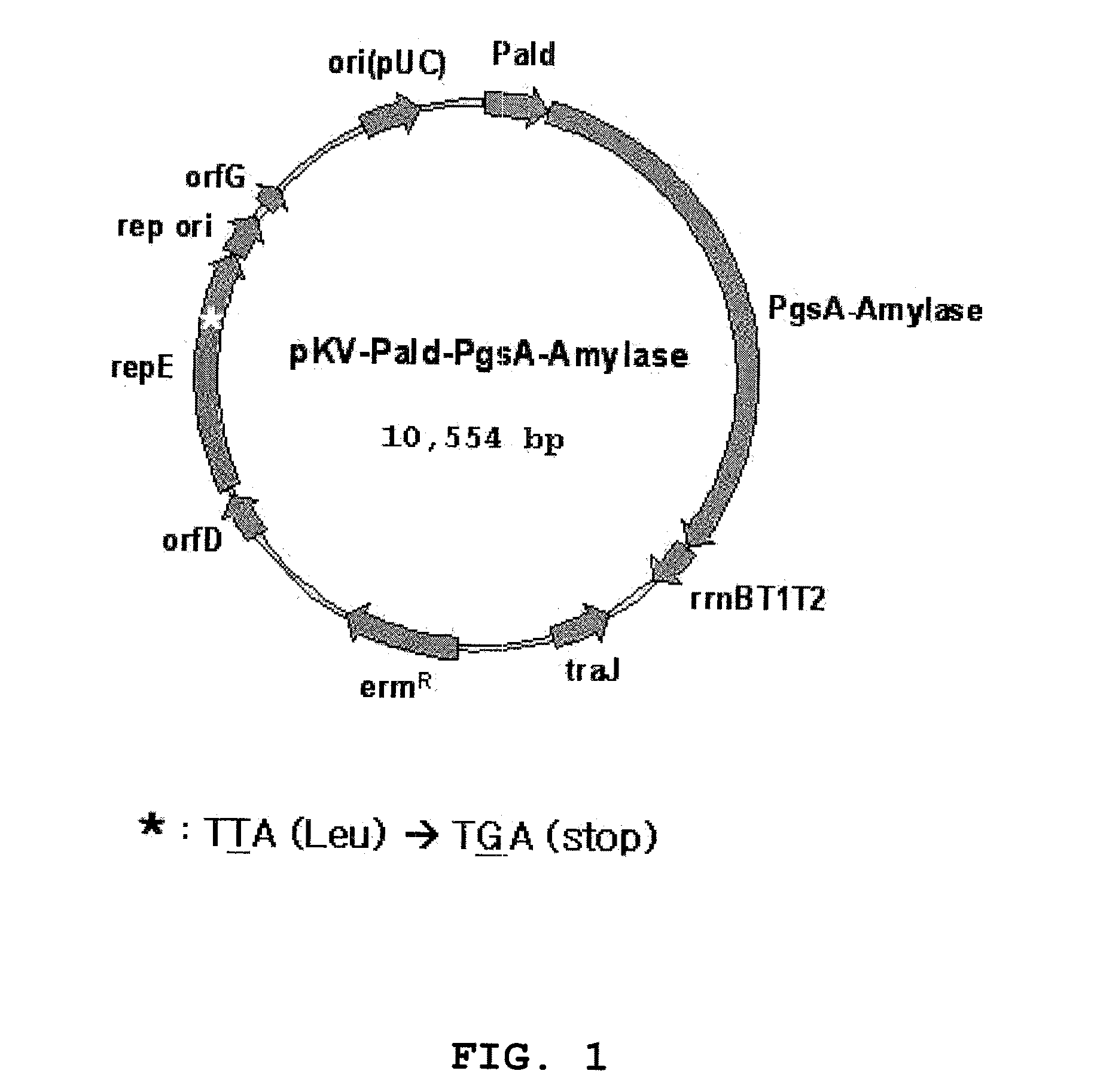
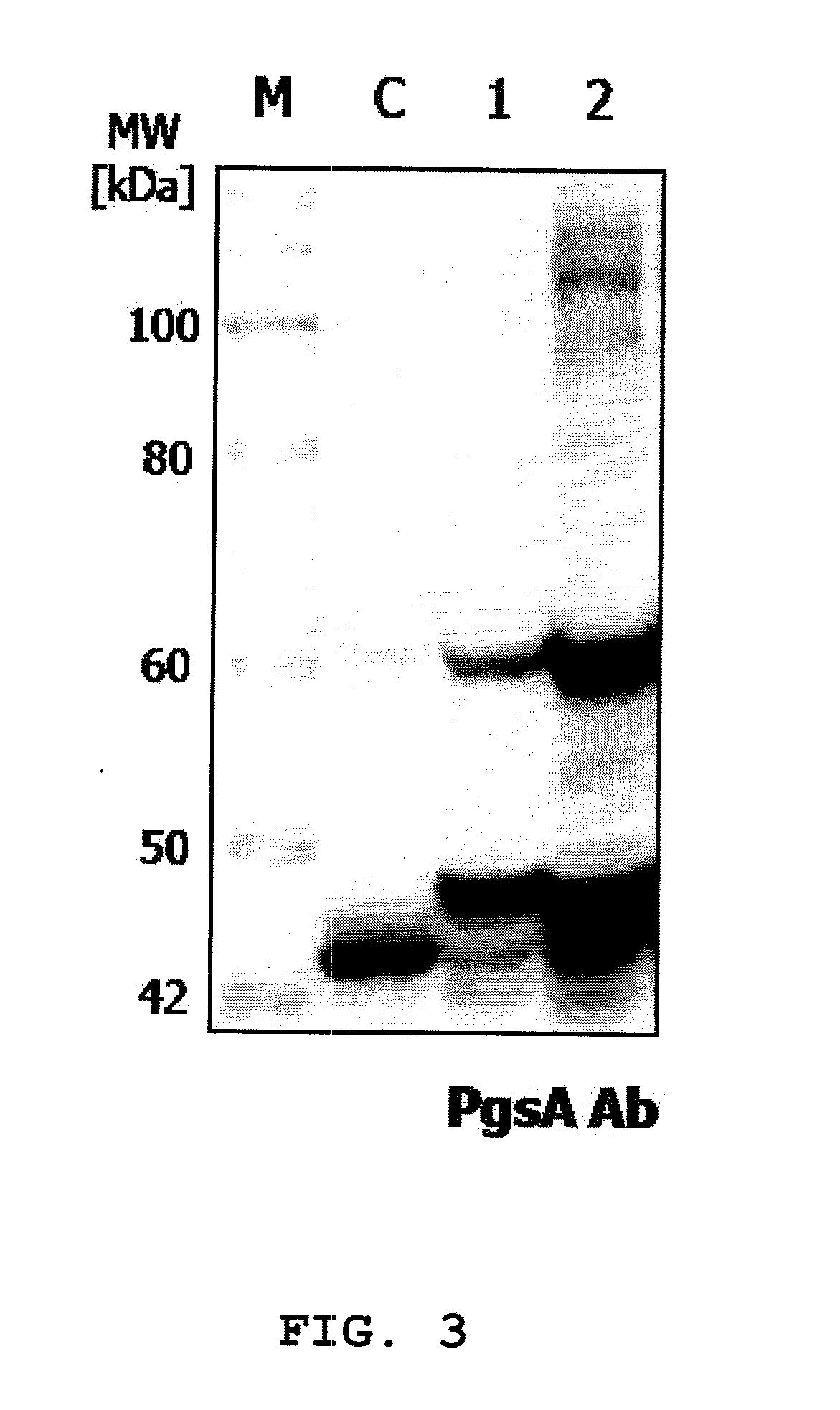
A surface expression vector for preparing HPV vaccines, in which the surface expression vector contains a gene encoding a repE mutant protein having an amino acid sequence of SEQ ID NO: 1, a promoter, a poly-gamma-glutamate synthetase complex gene, and a gene which is linked with the poly-gamma-glutamate synthetase complex gene and encodes a tumor induction-associated antigen protein of human papillomavirus.An expression vector constitutively expressing a high level of the human papillomavirus (HPV) antigen protein is provided. Also, a recombinant lactic acid bacteria, transformed with the expression vector and expressing the HPV antigen protein on the surface thereof, and a composition comprising the recombinant lactic acid bacteria are provided. The recombinant lactic acid bacteria and the composition are very effective as a vaccine for the treatment of cervical cancer, because they can be applied orally or directly to the vagina.
Owner:BIOLEADERS CORP
Generating HPV antigen-specific cells from a naive t cell population
ActiveUS20180312807A1Rapid and robust expansion of HPV-recognizingCell receptors/surface-antigens/surface-determinantsMammal material medical ingredientsHPV AntigenNaive T cell
Safe, rapid and efficient methods for producing antigen-specific T cells recognizing human papilloma virus or HPV antigens.
Owner:CHILDRENS NAT MEDICAL CENT
Methods for treating hpv-associated diseases
The present application provides immune cells comprising an HPV antigen and an adjuvant, methods of manufacturing such modified immune cells, and methods of using such modified immune cells for treating an HPV-associated disease, preventing an HPV-associated disease and / or for modulating an immune response in an individual with an HPV-associated disease.
Owner:SQZ BIOTECH CO
Preparation method of HLA-A0201-restricted anti-HPV (human papillomavirus) antigen-specific CTL
ActiveCN102719402BImprove proliferative abilityHigh purityBlood/immune system cellsHPV AntigenDendritic cell
The invention discloses a preparation method of HLA-A0201-restricted anti-HPV (human papillomavirus) antigen-specific CTL. The preparation method comprises the following steps of: collecting peripheral blood mononuclear cells through apheresis, and enriching and purifying CD8+T lymphocytes; stimulating the CD8 + T lymphocytes by mature dendritic cells of a loaded HLA-A0201-restricted HPV antigen polypeptide; and promoting the T-cell growth through combined use of rhIL-2 and rhIL-7; purifying a target CTL by a Tetramer marking method and a flow cytometry sorting technique; stimulating the growth of the target CTL by solid phase packaged anti-human CD3 monoclonal antibody and IL-2; adding autologous PBMCs (peripheral blood mononuclear cell) after gamma-ray irradiation for enhancing activation of the target CTL; and adding rhIL-15, cultivating and amplifying, and collecting and identifying. The target CTL prepared by the prepared method has high purity, high proliferative capacity, high killing activity and a high CTL-CM ratio and can be applied to immunotherapy of tumors and the like.
Owner:江苏得康生物科技有限公司
Method for enhancing immunogenicity of epitope peptide of HPV antigen, virus-like particle, and method for preparing HPV vaccine
ActiveUS10125175B2Improving immunogenicityLow immunogenicityAntibody mimetics/scaffoldsViral antigen ingredientsHPV AntigenEpitope
A method for enhancing immunogenicity of an epitope peptide of an HPV antigen, the method including: assembling a gene of an HPV antigen into a gene of HBc, exogenously expressing a resulting assembled gene to acquire a fusion protein, allowing the fusion protein to automatically assemble to form a virus-like particle including the HPV antigen on a surface of the virus-like particle, to obtain HBc-HPV virus-like particle. A virus-like particle capable of expressing the antigen peptide of HPV acquired by the method. The virus-like particle includes a HBc-L2 fusion protein. A genome for encoding the HBc-L2 fusion protein is represented by SEQ ID NO. 3. A method for preparing an HPV vaccine includes using the virus-like particle.
Owner:CANSINO BIOLOGICS INC
A method for preparing HPV antigen-specific cytotoxic T lymphocytes
ActiveCN108300692BEnhance antigen presentationInduced proliferationMammal material medical ingredientsBlood/immune system cellsHPV AntigenDisease
The invention discloses a method for preparing HPV antigen-specific cytotoxic T lymphocytes. Specifically, the present invention discloses a preparation method of HLA-A2402-restricted anti-HPV antigen-specific CTL. In this method, peripheral blood mononuclear cells were collected by apheresis or venous blood sampling, the antigen presentation function of B cells was enhanced with CpG ODN 2395, and peripheral blood mononuclear cells were stimulated with B cells loaded with HLA-A2402-restricted HPV antigen peptides, and rhIL ‑2, rhIL‑7, rhIL‑15, rhIL‑21 combined to promote T cell growth. The target CTL prepared by the method has the characteristics of simple preparation, short preparation cycle, low cost, high proliferation ability, high killing activity, high cell viability and the like, and can be used for immunotherapy of diseases related to HPV infection including cervical cancer.
Owner:JILIN TUO HUA BIOTECH
HPV (human papillomavirus) antigen epitope as well as identification method and application thereof
The invention provides an HPV (human papillomavirus) antigen epitope as well as an identification method and application thereof. The HPV antigen epitope comprises 1) 75-83 amino acids of HPV E7 protein; 2) 75-82 amino acids of the HPV E7 protein; 3) 77-86 amino acids of the HPV E7 protein; 4) 77-90 amino acids of the HPV E7 protein; 5) 79-88 amino acids of the HPV E7 protein; and 6) 4-11 amino acids of the HPV E7 protein. An HPV antigen epitope is obtained by utilizing an identification method of the screened and optimized HPV antigen epitope, the obtained HPV antigen epitope is applied, various dominant mutants are further obtained, drugs and vaccines prepared from the HPV antigen epitope or the mutants can effectively treat various cancers, and the HPV antigen epitope or the mutants are higher in safety and smaller in side effect.
Owner:SHENZHEN GINO BIOTECHNOLOGY CO LTD +1
Immunomodulating composition for treatment
ActiveUS20190134181A1Treat infectionAvoid unnecessary expensesViral antigen ingredientsFusion with degradation motifHPV AntigenNucleic acid molecule
Disclosed are therapeutic compositions and methods for inducing an immune response to human papillomavirus (HPV). More particularly, disclosed is a method for inducing an immune response in a subject by introducing and expressing a nucleic acid molecule encoding an immunogenic HPV antigen.
Owner:JINGANG MEDICINE AUSTRALIA PTY LTD
Immunity enhancing therapeutic vaccine for HPV and related diseases
ActiveUS10071150B2Easy to degradeEasy to presentAntibody mimetics/scaffoldsViral antigen ingredientsDiseaseHPV Antigen
An immunity enhancing therapeutic vaccine includes a gene vector based on recombinant adenovirus and three elements: 1) an HPV antigen including the E6 and E7 multivalent fusion proteins of HPV types 16 and 18, 2) an immunologic adjuvant protein fused with said antigen, which protein may be a heat shock protein (HSP) of Mycobacterium tuberculosis, and 3) an immunostimulant, which may be granulocyte-macrophage colony stimulating factor (GM-CSF). The vaccine is used for the treatment of human papilloma virus infections and related diseases.
Owner:MYGT BIOPHARM LLC
DNA vaccine for human papillomavirus and method for using the same
The present disclosure provides a DNA vaccine for a subject having a human papillomavirus (HPV)-associated disease. The DNA vaccine may include a DNA construct including a fusion gene. The fusion gene may be a subsegment of the DNA construct that includes an optimized HPV subsequence encoding at least one HPV antigen. The optimized HPV subsequence may include one or more of: an HPV-16 E6 expressing gene set forth in SEQ ID NO: 1, an HPV-16 E7 expressing gene set forth in SEQ ID NO: 2, an HPV-18 E6 expressing gene set forth in SEQ ID NO: 3, and an HPV-18 E7 expressing gene set forth in SEQ ID NO: 4.
Owner:PAPIVAX BIOTECH INC
Use of mutant hiv-1 protease or siv protease as an adjuvant
InactiveUS20110059127A1Enhance cell-mediated immune responseEfficient use ofAntiviralsWhole-cell/virus/DNA/RNA ingredientsHPV AntigenImmunodeficiency virus
The present invention relates to a mutant HIV-1 (Human immunodeficiency virus-1) protease capable of effectively enhancing cell-mediated immune responses to DNA vaccination, and use of a nucleic acid encoding the same as a vaccine adjuvant. The mutant HIV-1 protease according to the present invention has inactivated or attenuated proteolytic activity, while retaining chaperone-like activity. When the mutant HIV-1 protease is used together with a DNA vaccine against the HIV-1 envelope protein or the HPV antigen (E6 or E7), cell-mediated immune responses can be effectively enhanced for the prevention or treatment of AIDS or cervical cancer.
Owner:POSTECH ACAD IND FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com