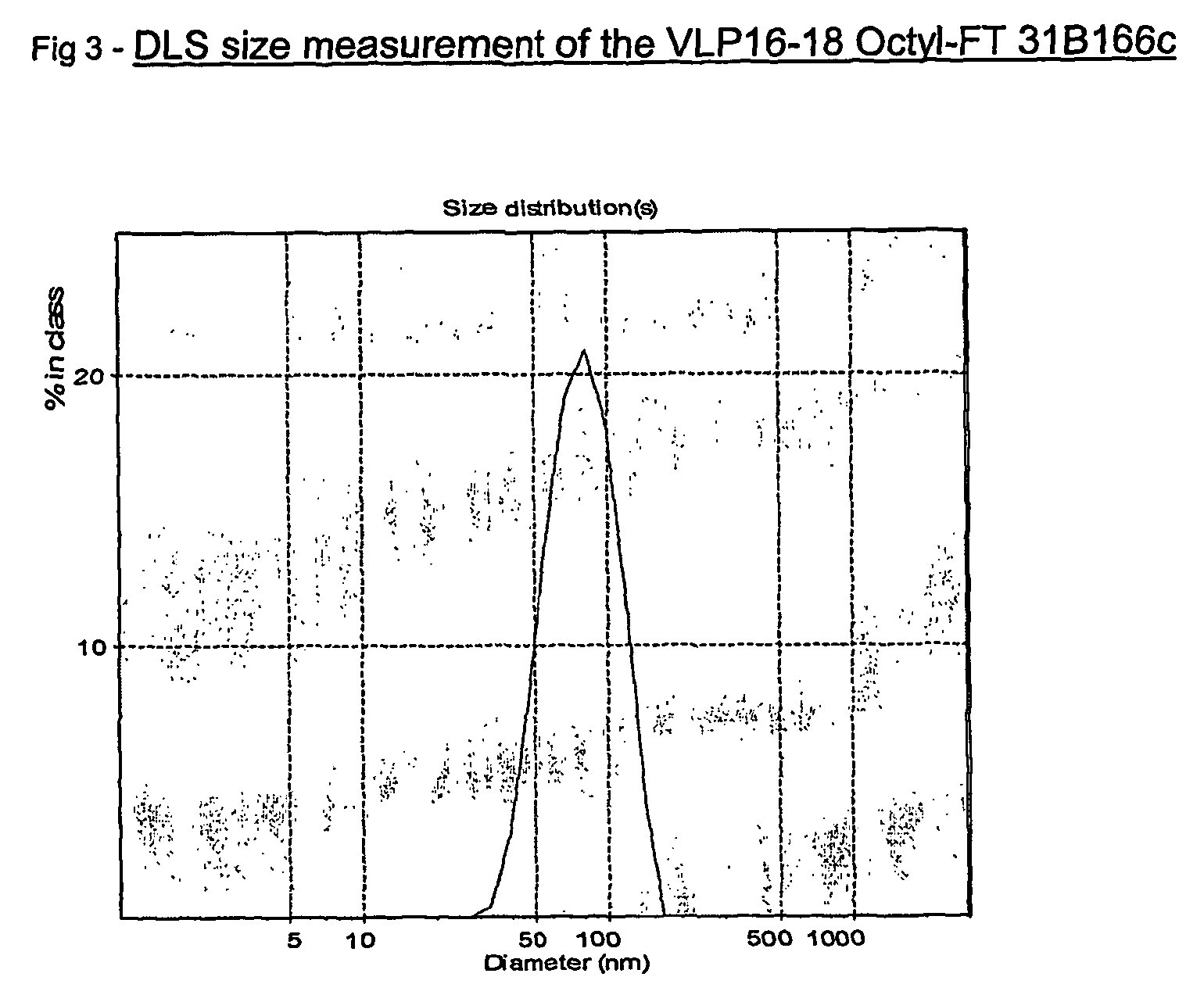
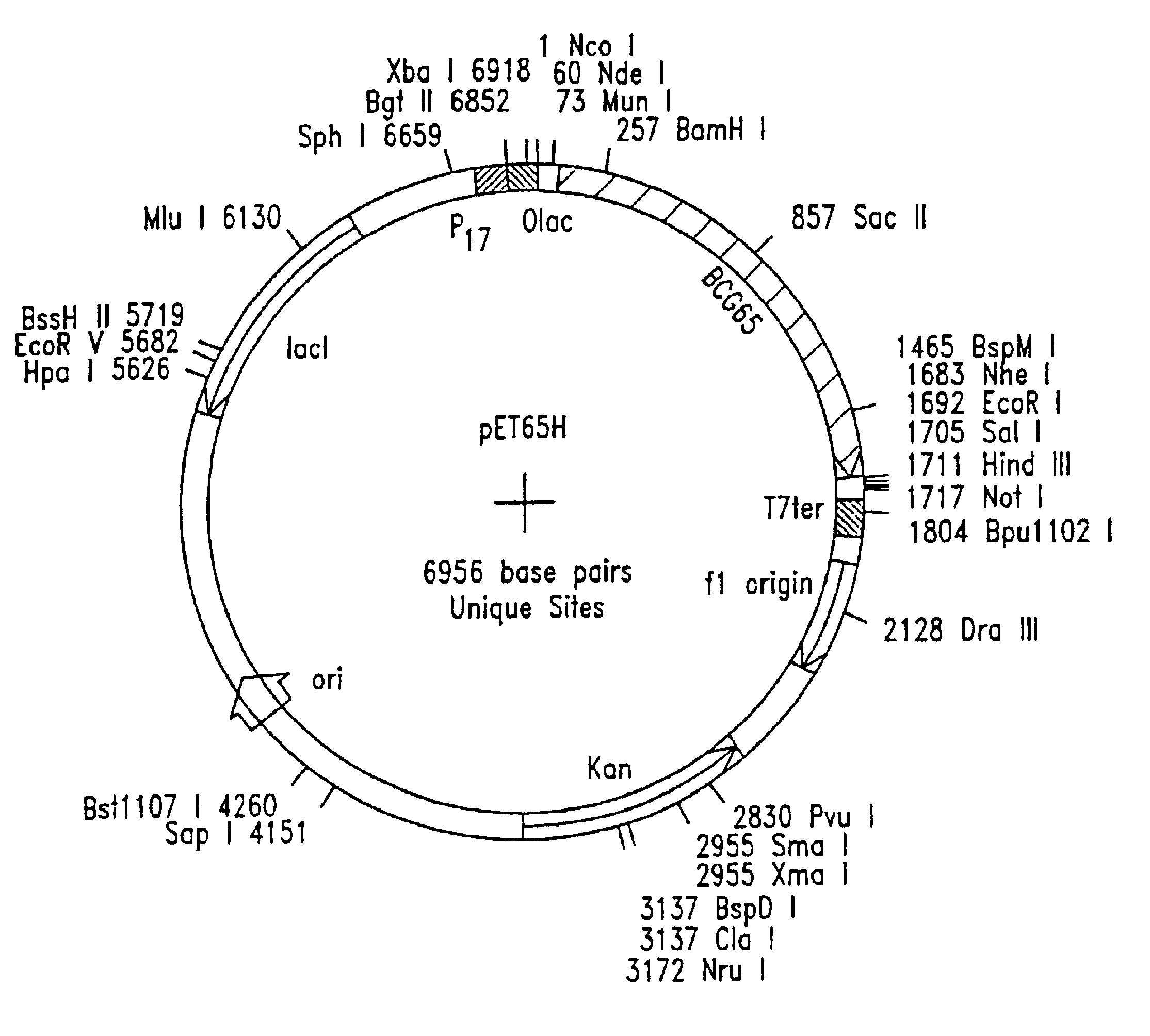
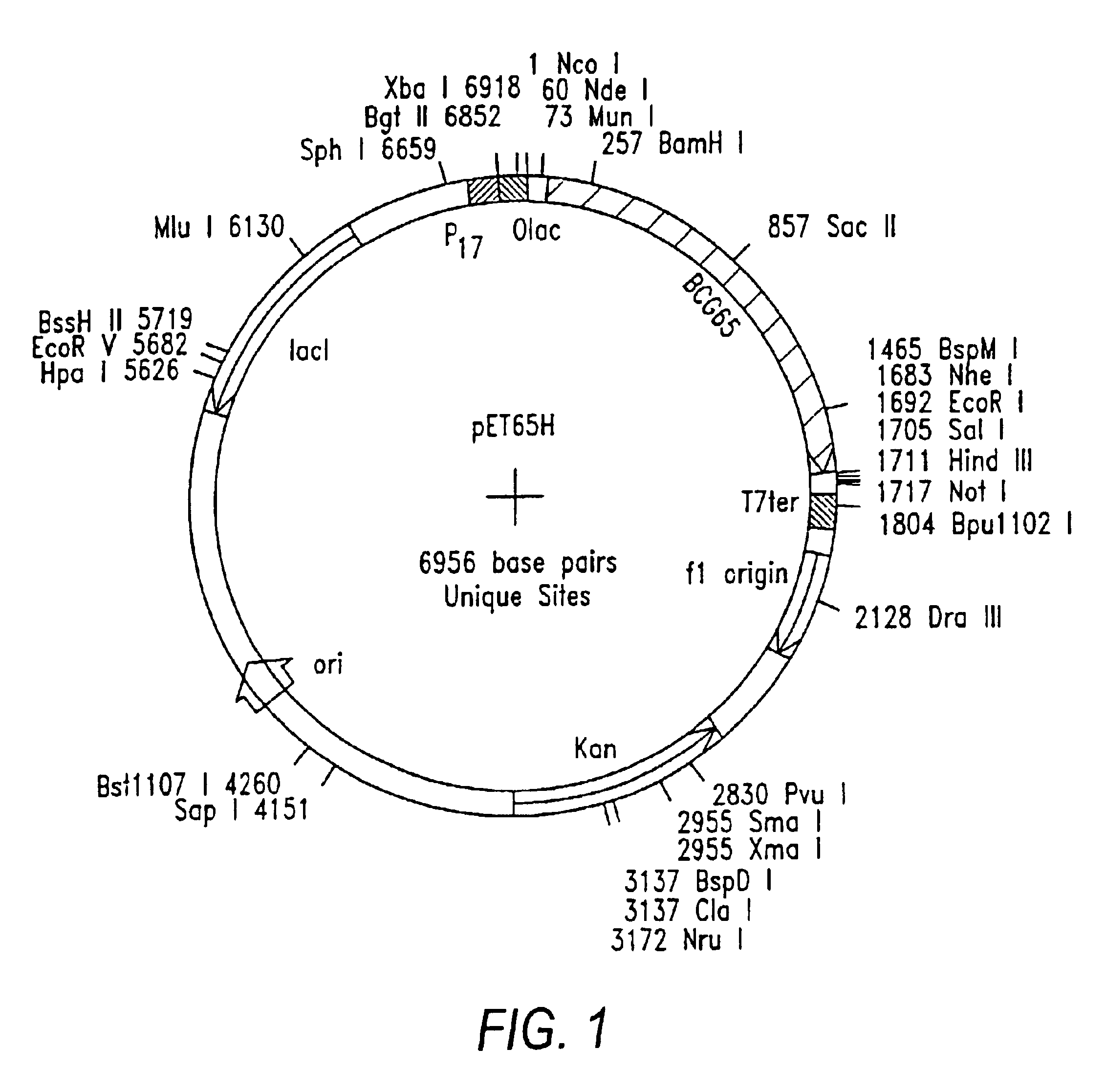
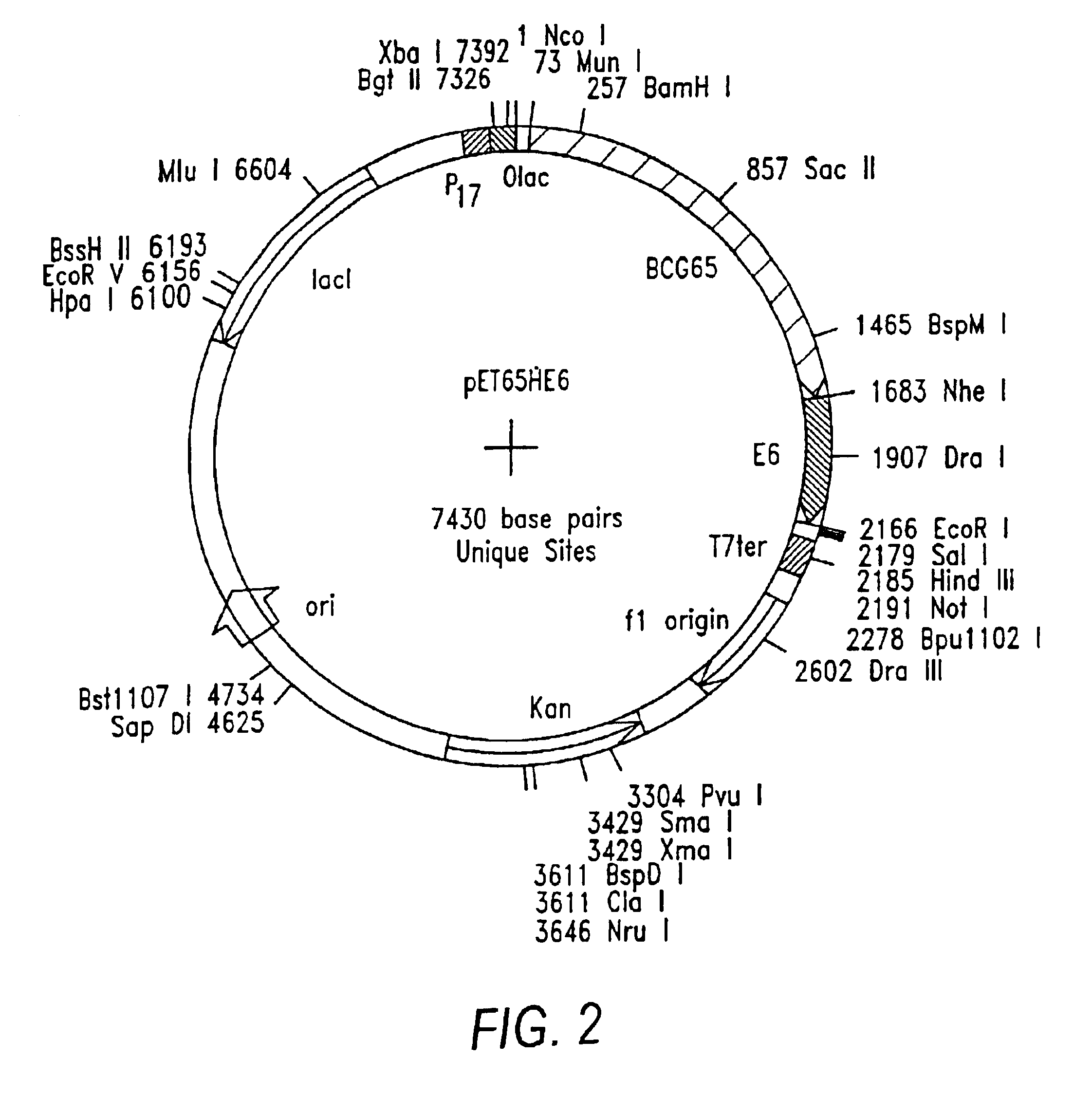
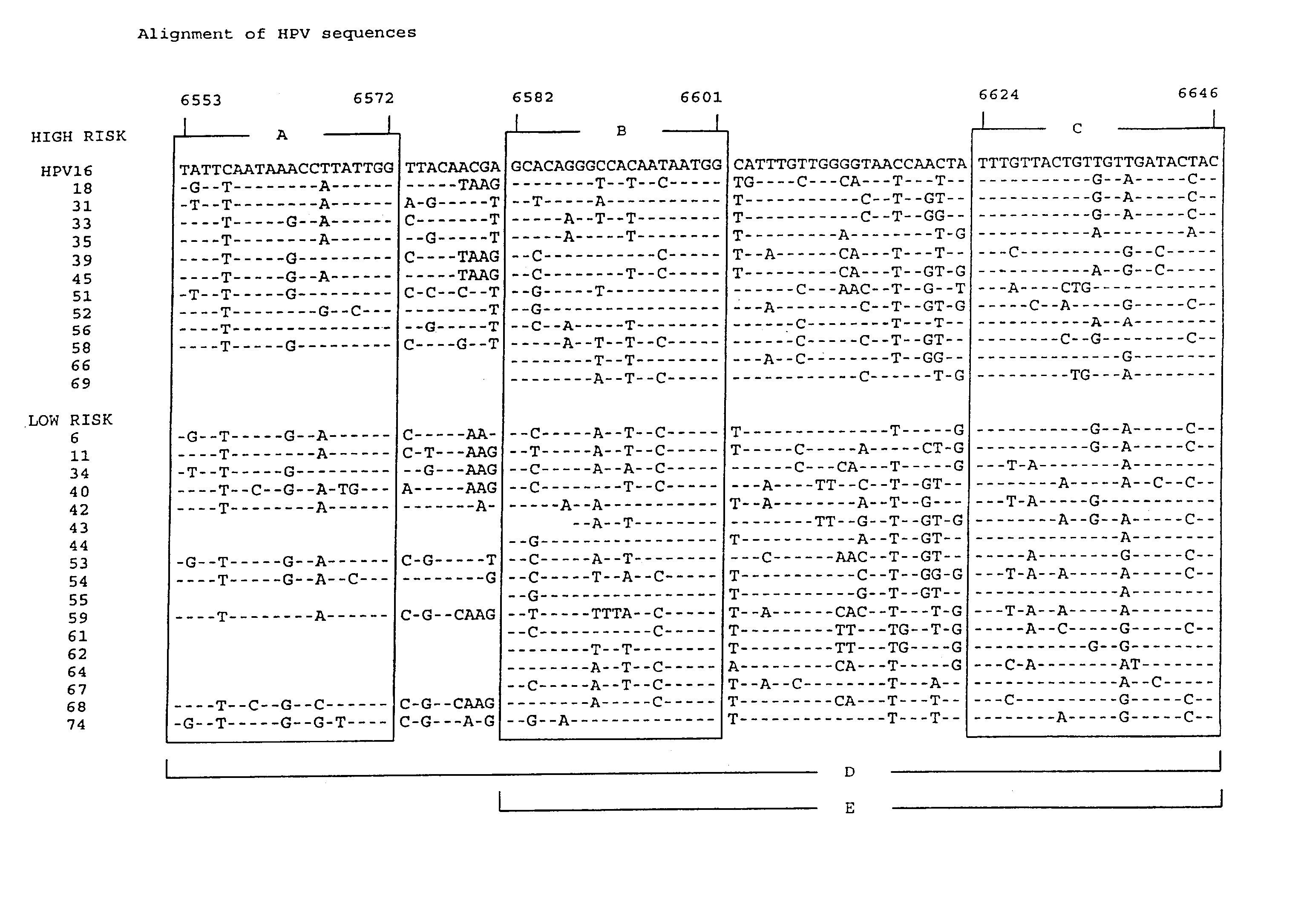
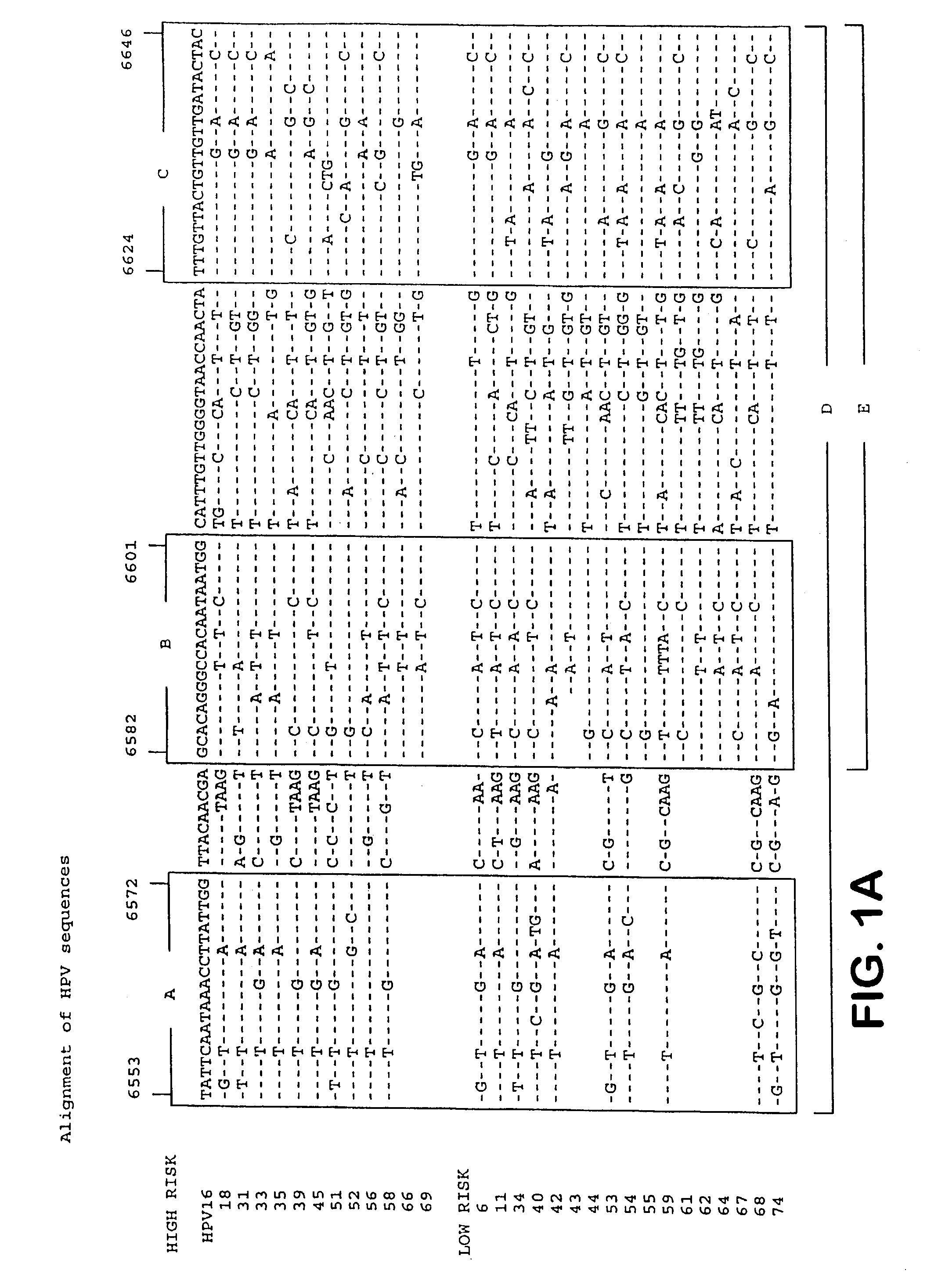
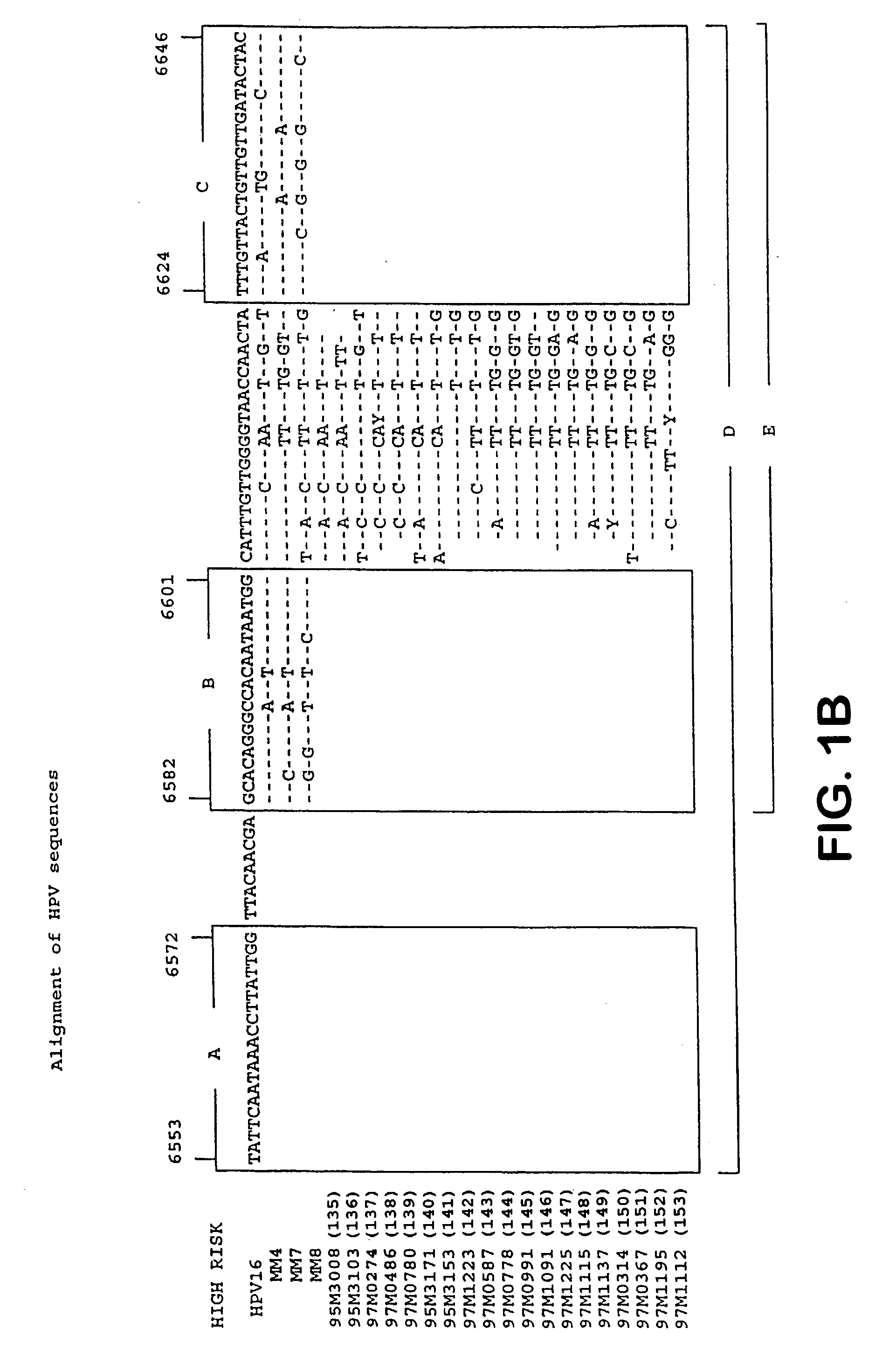
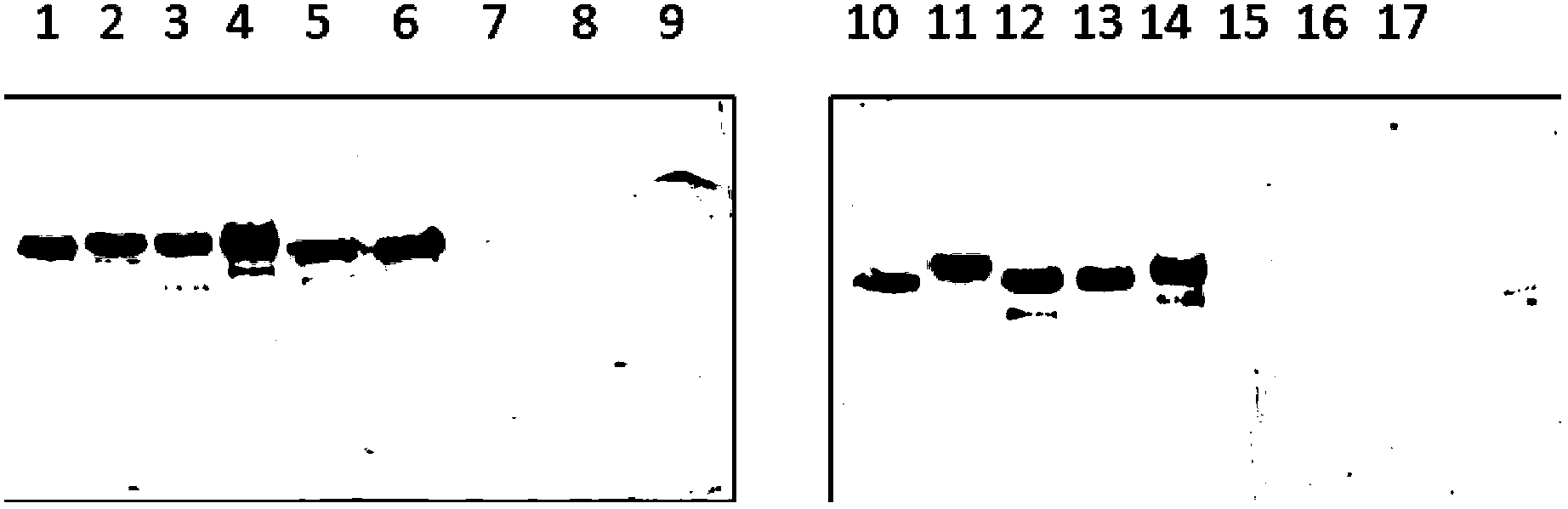
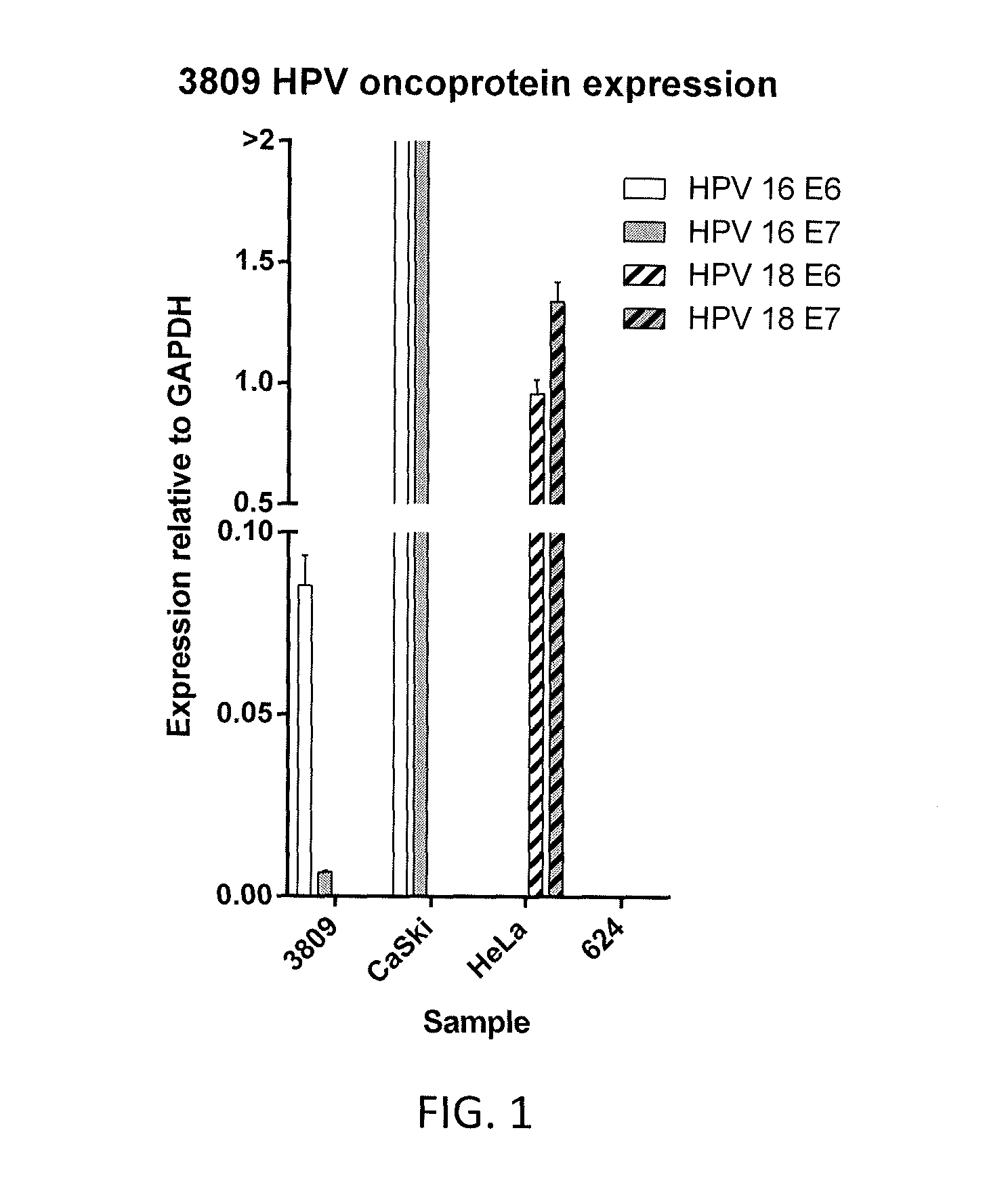
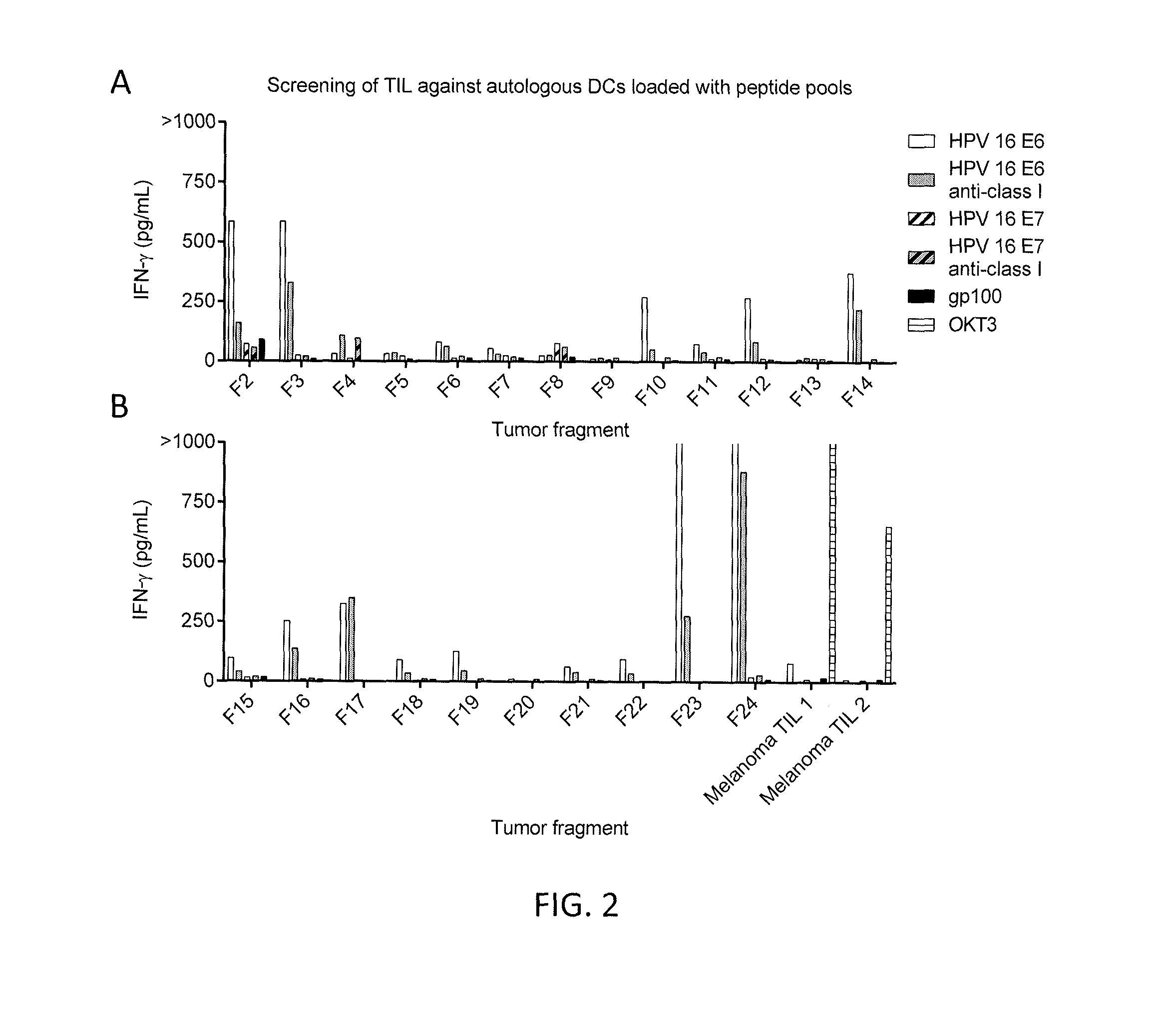
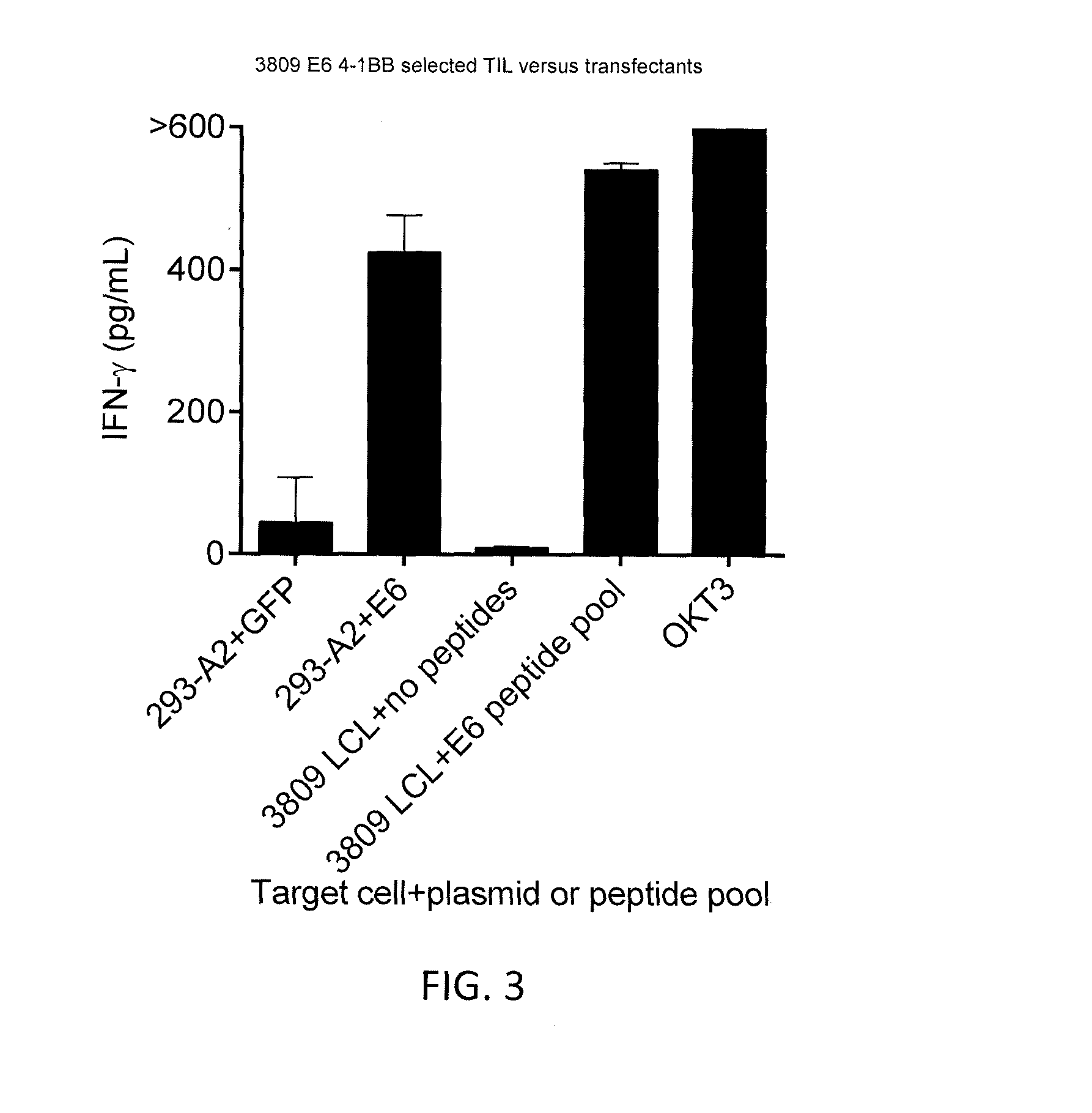
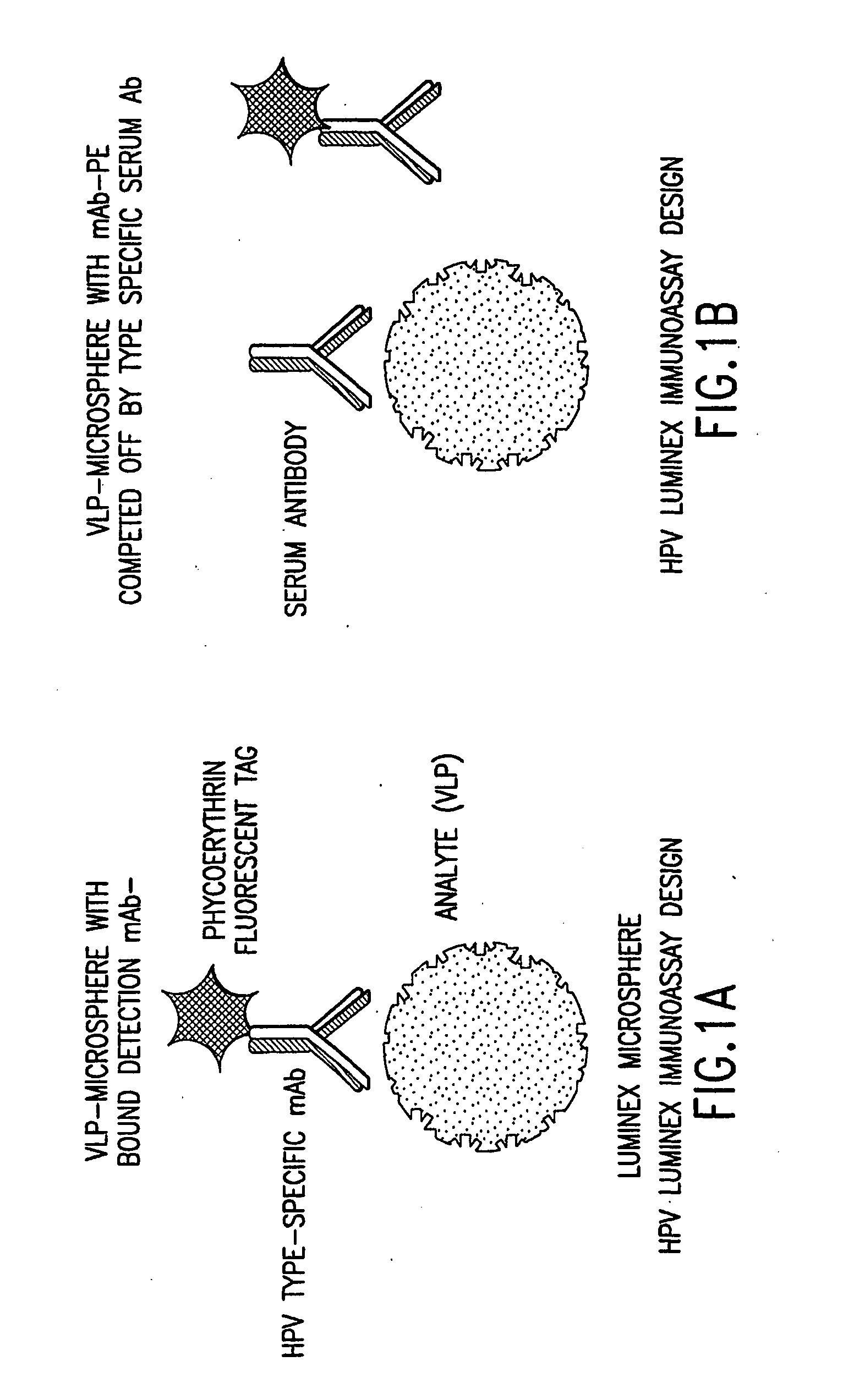
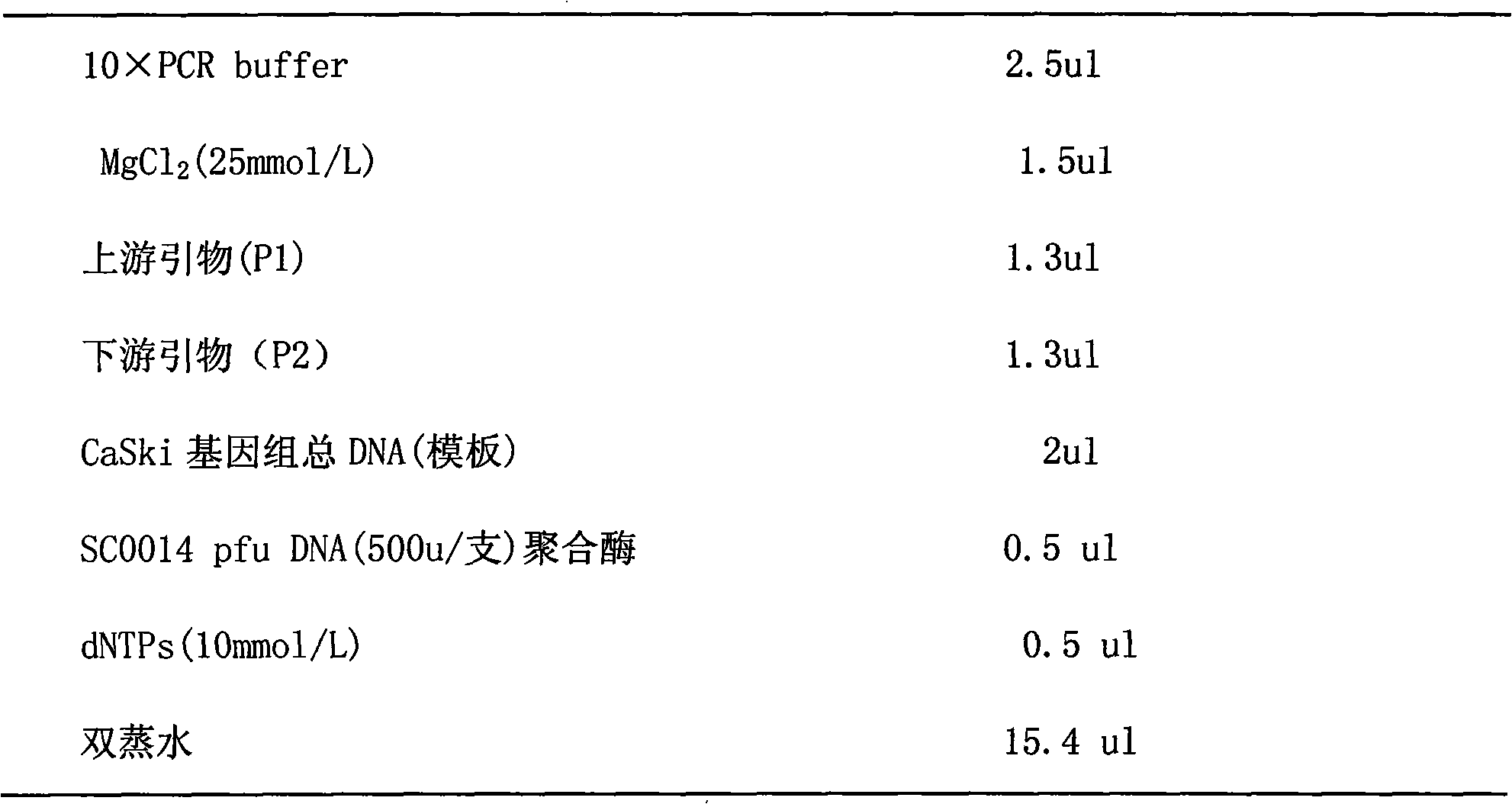
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
236 results about "PAPILLOMAVIRUS HUMAN" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human papillomavirus (HPV) is a virus from the papillomavirus family that is capable of infecting humans. Like all papillomaviruses, HPVs establish productive infections only in keratinocytes of the skin or mucous membranes.
Inducing cellular immune responses to human papillomavirus using peptide and nucleic acid compositions
InactiveUS20070014810A1Reduce the possibilityImproving immunogenicitySugar derivativesViral antigen ingredientsEpitopeT cell
This invention uses our knowledge of the mechanisms by which antigen is recognized by T cells to identify and prepare human papillomavirus (HPV) epitopes, and to develop epitope-based vaccines directed towards HPV. More specifically, this application communicates our discovery of pharmaceutical compositions and methods of use in the prevention and treatment of HPV infection.
Owner:GENIMMUNE NV +1
Mixed Virus-like particles
The present invention relates to mixed VLPs, and to a process for the production of a mixed VLP, the VLPs comprising L1 proteins from at least 2 different types of human papillomavirus.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Immune responses against HPV antigens elicited by compositions comprising an HPV antigen and a stress protein or an expression vector capable of expression of these proteins
InactiveUS6900035B2Enhance immune responseVirusesAntibody mimetics/scaffoldsHPV AntigenInfected cell
This document describes compositions and methods for inducing an immune response (e.g., a cellular response such as a cell-mediated cytolytic immune response) to a human papillomavirus (HPV) antigen, which can be displayed by HPV or exhibited by infected cells (e.g., cells from cervical and other tumors). The HPV protein can be joined to a stress protein by chemical conjugation or noncovalently using linking moieties, or by fusion (e.g., a recombinant fusion protein). Also described are expression vectors containing sequences encoding HPV antigens and stress proteins, which can be introduced into cells of a subject or cells ex vivo. Also described are compositions that include a stress protein linked to an HPV antigen and another pharmacologically acceptable component and stress protein—HPV antigen fusions and conjugates. These compositions can be used to induce or enhance an immune response against HPV and cells that exhibit HPV antigens, including HPV-associated tumors.
Owner:NVENTA BIOPHARMACEUTICALS CORP
Selective Reaction Monitoring (SRM) Derived Protein Profiles for Cancer and other Pathologic Entities
InactiveUS20130288233A1Microbiological testing/measurementBiological material analysisProtein proteinSpecific protein
The invention relates to a method of detecting and quantifying small peptides derived from proteins from a range of different clinical samples using the Selective Reaction Monitoring (SRM) profiling technique. By targeting these unique peptides which specifically identify particular proteins, the present invention enables multiple samples to be run in a multiplexed fashion in order to identify, diagnose, quantitate and profile a full range of benign and pathologic entities, including but not limited to, the complete range of cancers and the spectrum of inflammatory diseases, including inflammatory cell typing and bone marrow cell typing. The SRM assay is capable of performing clinical blood typing and it can also act as a diagnostic test to identify women at highest risk for cervical cancer base on Human Papillomavirus (HPV) testing.
Owner:MAP IP HLDG
Effective Sensitizing Dose of a Gelled Immunomodulating Topical Composition
InactiveUS20110268761A1Reduction in tumor massImprove the quality of lifeBiocideViral antigen ingredientsHpv human papillomavirusHuman papilloma virus
The present invention relates to compositions and methods of treating warts and other human papilloma virus (HPV) skin infections. The present invention relates to compositions and methods of treating skin cancer.
Owner:HAPTEN PHARMA
Method of increasing yield of human papilloma virus L1 albumen pronucleus expression
ActiveCN101016542ALow costHigh expressionViral antigen ingredientsAntiviralsTGE VACCINENucleic acid sequence
The invention discloses a method to increase human papilloma virus L1 protein pronucleus expression productivity and also discloses a encode HPV L1 protein codon majorizing nucleic acid sequence from this method, which is characterized by the following: supplying expression carrier and host cell and HPVL1 protein poly body of nucleic acid sequence; disclosing appliance in preparing vaccine, drug compound and immunodiagnosis or antibody. The nucleic acid sequence expressing quantity possesses distinctive improvement, which decreases the preparing cost effectively.
Owner:BEIJING HEALTH GUARD BIOTECH
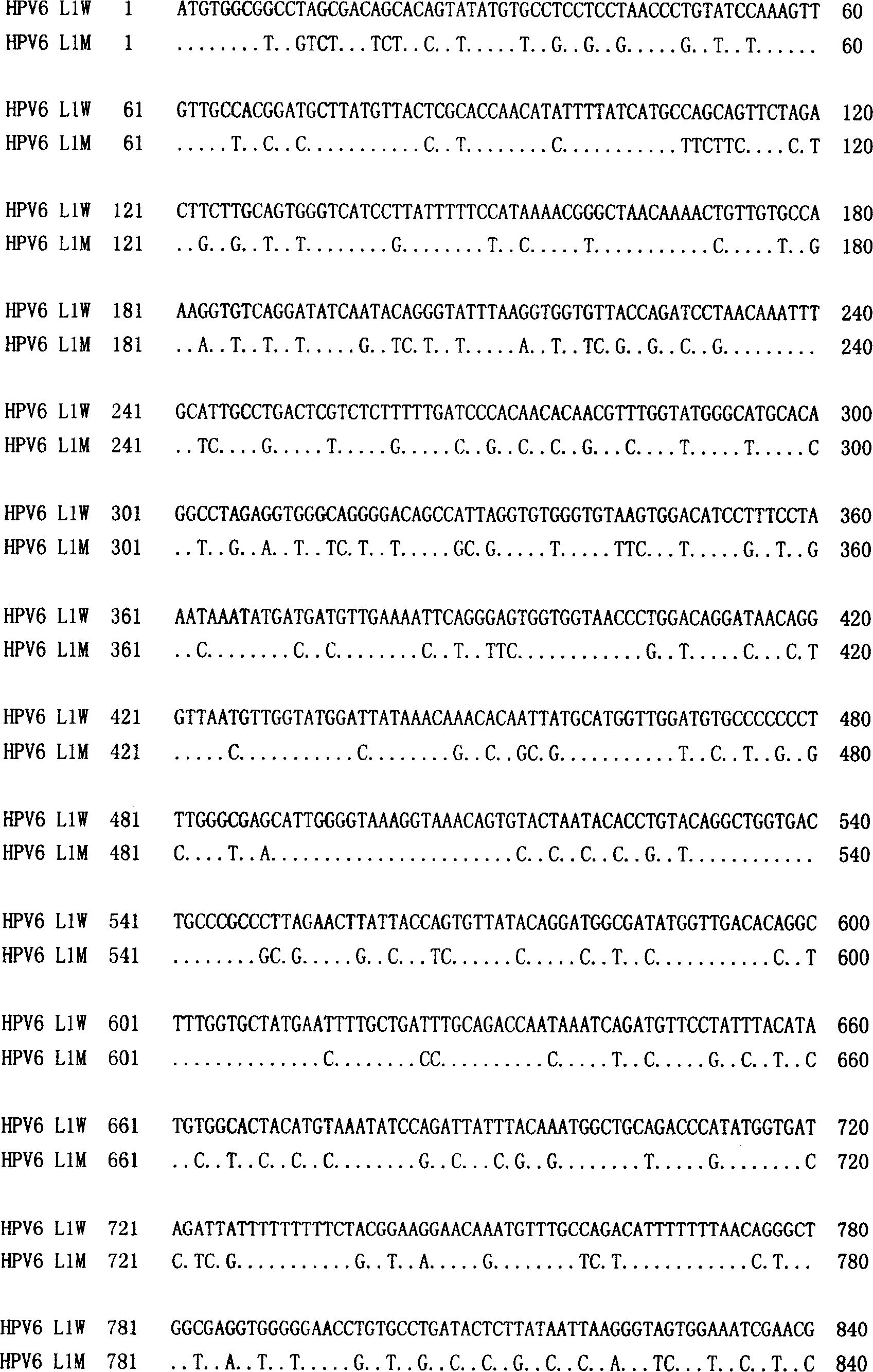
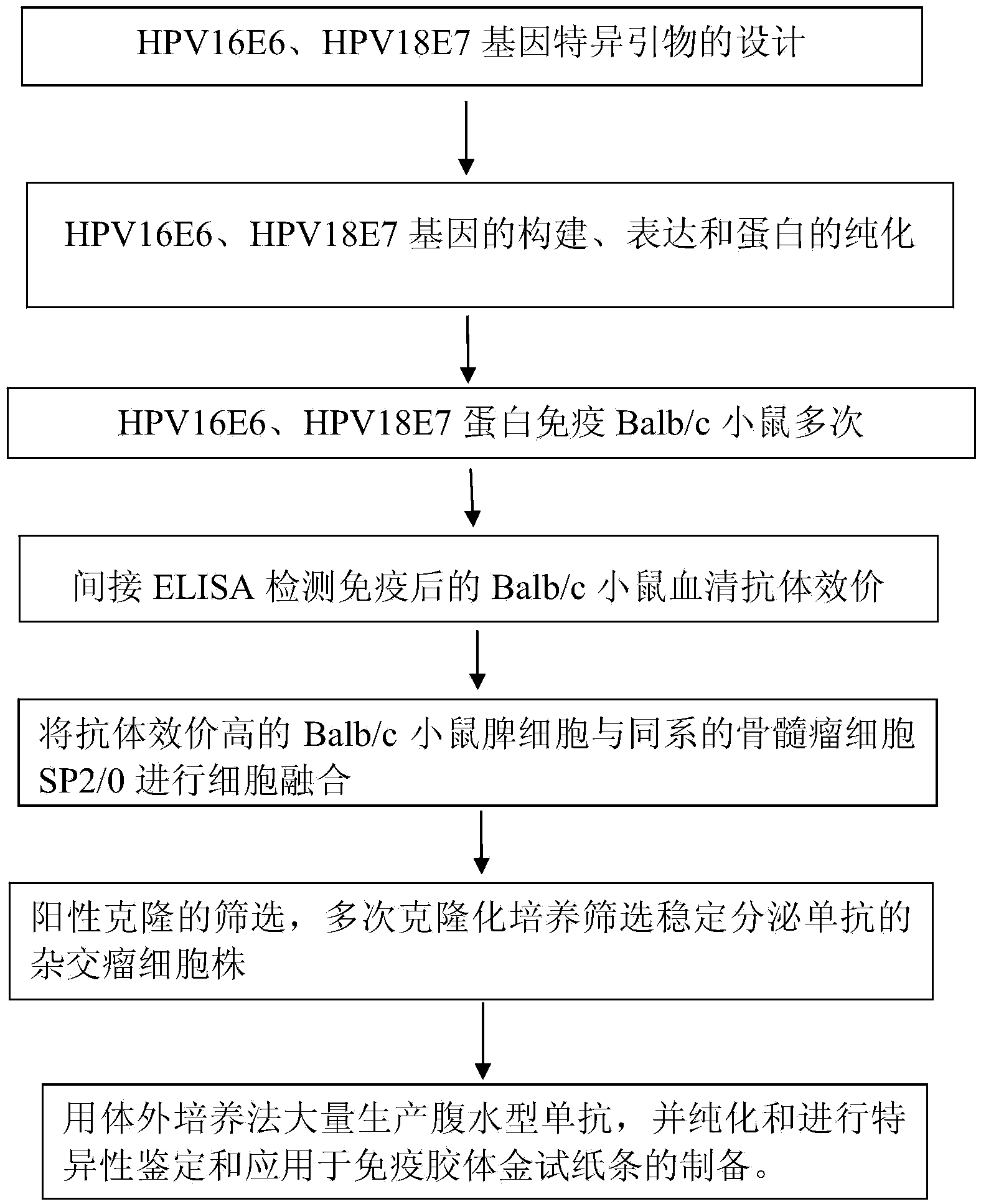
Monoclonal antibodies for resisting high-risk human papillomavirus proteins and application of monoclonal antibodies
InactiveCN103865883AStrong specificityImprove bindingImmunoglobulins against virusesMicroorganism based processesHuman papillomavirusLatex particle
The invention relates to monoclonal antibodies for resisting high-risk human papillomavirus proteins HPV16E6 and HPV18E7, a hybridoma cell strain secreting the monoclonal antibodies and the application of the monoclonal antibodies. The monoclonal antibodies can be used for specifically detecting the proteins HPV16E6 and HPV18E7. The two antibodies can be prepared into immunochromatographic test strips for rapidly detecting the proteins HPV16E6 and HPV18E7 by virtue of labeled colloidal gold or color latex particles. The monoclonal antibodies for detecting the proteins HPV16E6 and HPV18E7 have the characteristics of high speed (results can be obtained within 10 minutes), simplicity, specificity, sensitivity, low cost and easiness in popularization.
Owner:CHONGQING UNIV OF TECH
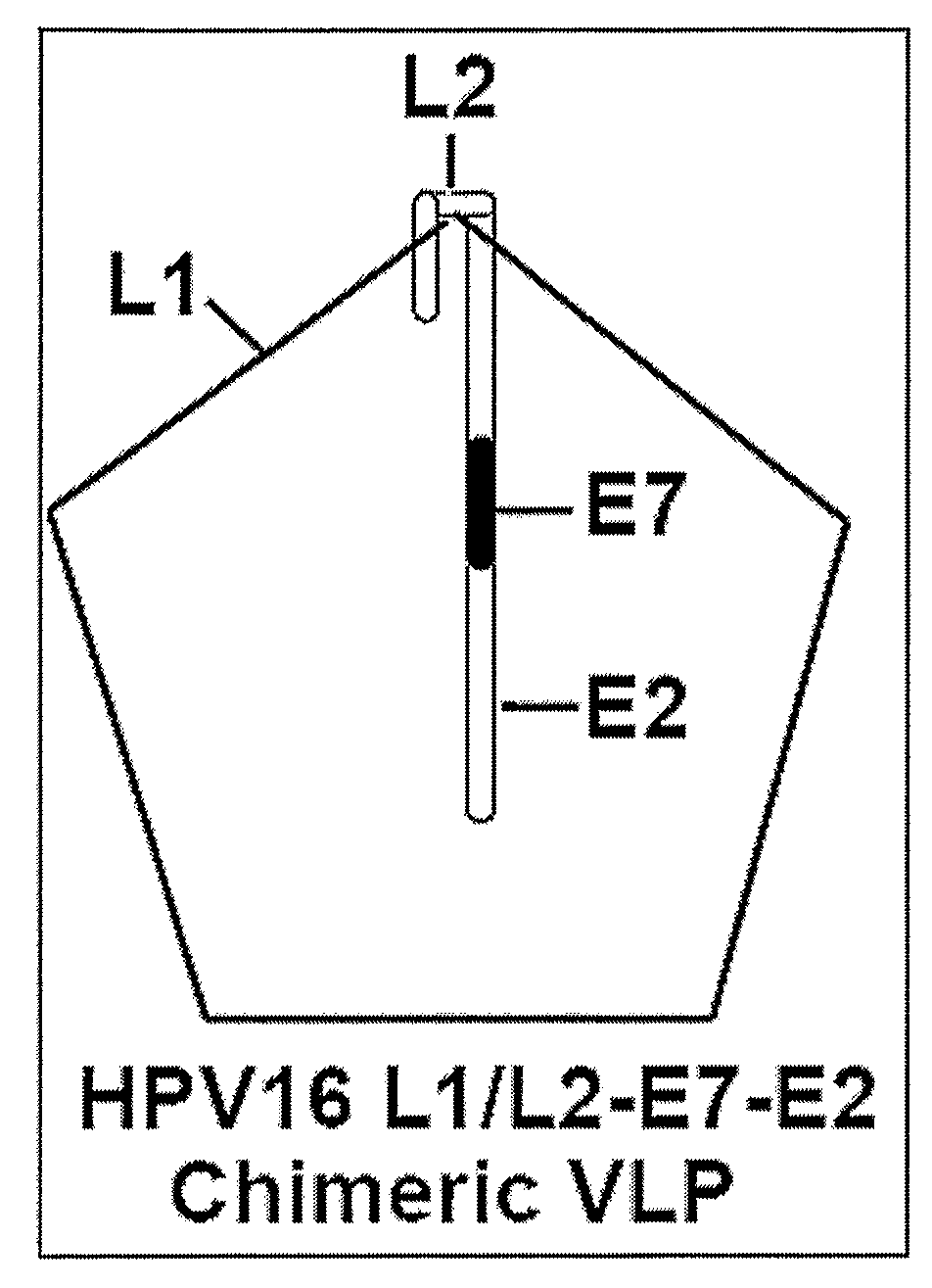
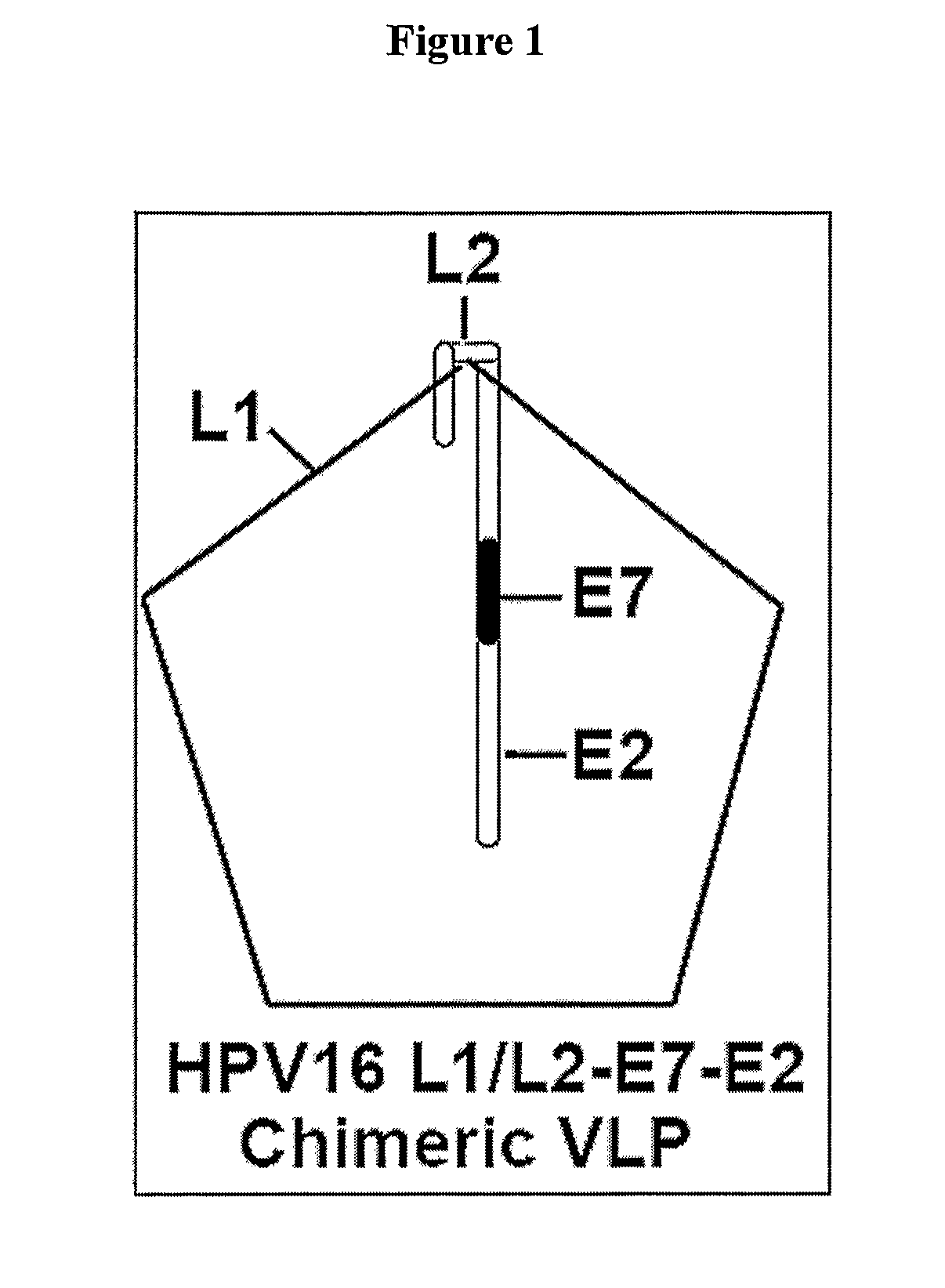
Protein delivery system using human papillomavirus virus-like particles
Human Papillomavirus virus like particles (VLPs) have been constructed so that they contain a modified L2 protein. The L2 protein has been minimized and is fused to a second protein or peptide. The fused protein is incorporated into the VLP and the VLP can deliver the protein to a cell. The modified VLPs can be used to increase the breadth of immune response in vaccine preparations or to deliver other proteins of interest.
Owner:MERCK SHARP & DOHME CORP
Detection and identification of human papillomavirus by PCR and type-specific reverse hybridization
InactiveUS20030165821A1Rapid and reliable for detectionMicrobiological testing/measurementFermentationHuman papillomavirusType specific
A method for detection and / or identification of HPV present in a biological sample comprising amplification of HPV polynucleic acids and of hybridization of said amplified polynucleic acids to a number of probes whereby a short fragment of the L1 gene of HPV is amplified after which, the amplimers are contacted with probes that specifically hybridize to the said short fragment of the L1 gene of at least one HPV type and a diagnostic kit to perform said method and primers and probes used in the said method.
Owner:INNOGENETICS NV
Inducing cellular immune responses to human papillomavirus using peptide and nucleic acid compositions
ActiveUS20070053922A1Reduce the possibilityImproving immunogenicityPeptide/protein ingredientsVirus peptidesEpitopeT cell
This invention uses our knowledge of the mechanisms by which antigen is recognized by T cells to identify and prepare human papillomavirus (HPV) epitopes, and to develop epitope-based vaccines directed towards HPV. More specifically, this application communicates our discovery of pharmaceutical compositions and methods of use in the prevention and treatment of HPV infection.
Owner:EPIMMUNE
Broad spectrum monoclonal antibodies or antigen binding fragments thereof of anti-HPV L1 protein, and applications thereof
ActiveCN103483447AAdvantage detectionPredominant diagnosisFungiBacteriaAntigen Binding FragmentAntigen binding
The invention relates to monoclonal antibodies and antigen binding fragments thereof, which can have a broad spectrum combination with human papilloma virus (HPV) L1 protein, coding of the sequences of the monoclonal antibodies and the antigen binding fragments thereof, generation of cell strains of the monoclonal antibodies and the antigen binding fragments thereof, and methods and applications of the monoclonal antibodies and the antigen binding fragments thereof on diagnosis, prevention or treatment.
Owner:XIAMEN UNIV +1
Human papilloma virus genes, vector, strain, and expression method thereof
ActiveCN104513826AImprove translationPromote research and developmentFungiViral antigen ingredientsPichia pastorisHuman Papillomavirus Major Capsid Protein L1
The present invention relates to genes of the Pichia pastoris expression codon-optimized main capsid protein L1 of human papilloma virus types 52,31 and 45, a vector containing the genes, a strain, a preparation method, and an expression method thereof.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
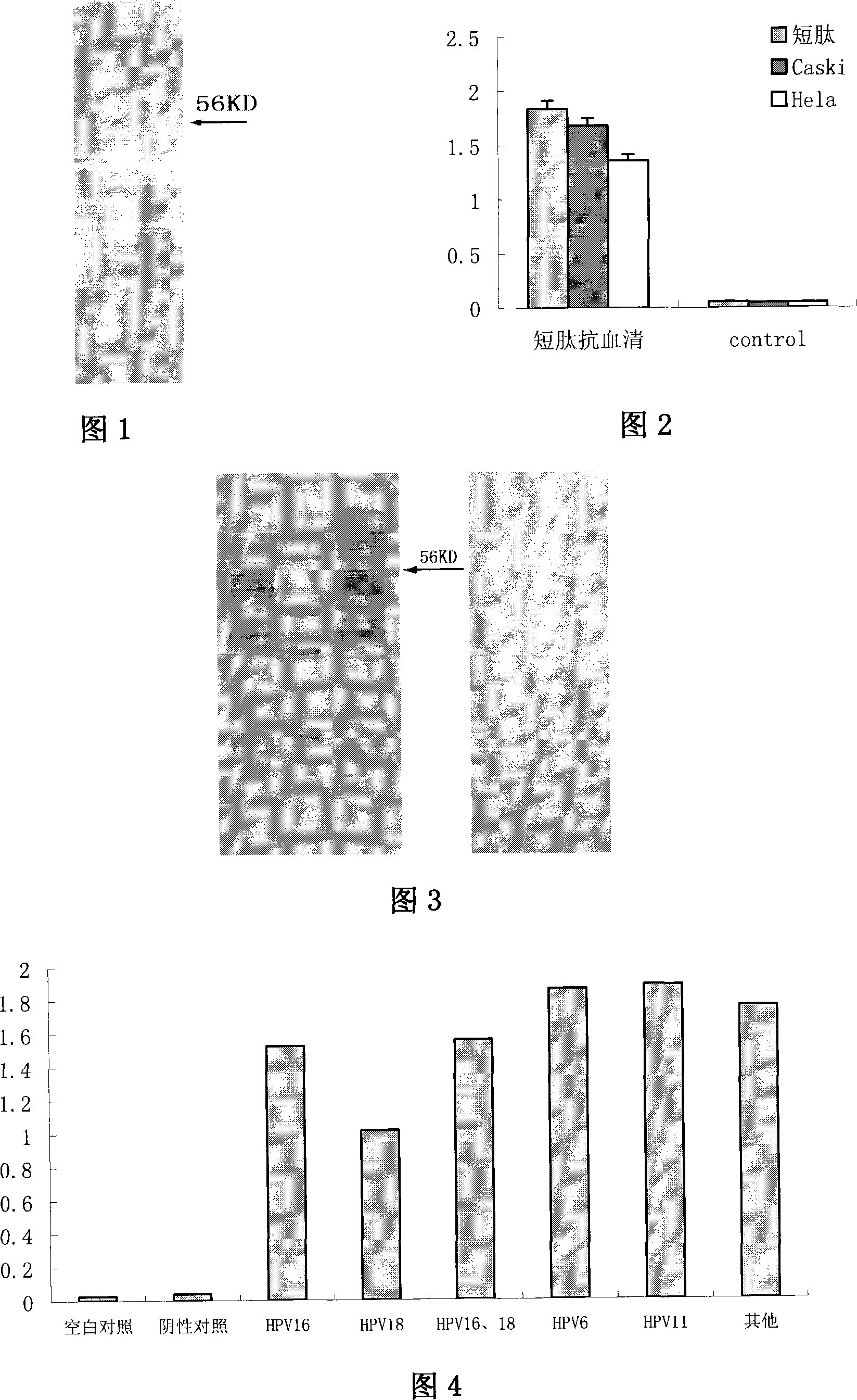
Human papillomavirus shell protein L1 short peptide and application thereof
InactiveCN101186636ASerum immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAlphapapillomavirusCoat protein
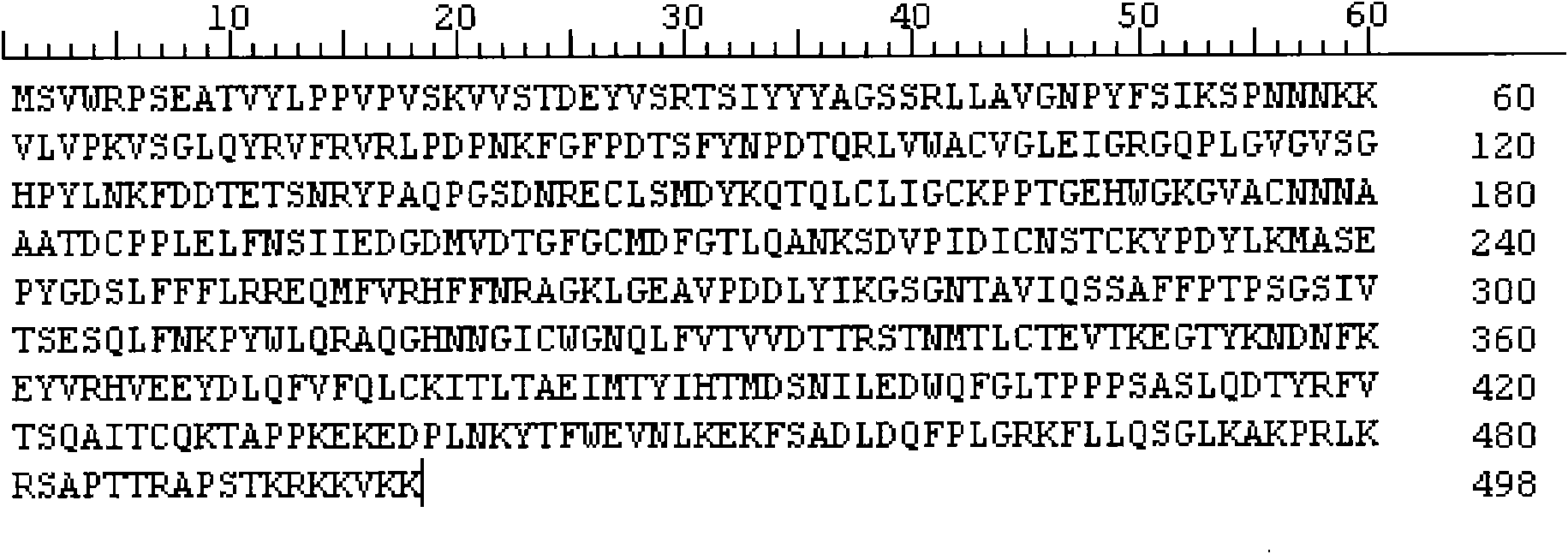
The invention relates to a polypeptide, in particular to a human papilloma virus coat protein L1 short peptide and relative application. The invention is characterized in that the sequence of the human papilloma virus coat protein L1 short peptide is N-EVNLKEKFSADLDQFPLGRKFLLQAGLKAK-C. The invention can simultaneously induce human papilloma virus coat protein L1 short peptide with immunity reaction on high risk type and low risk type, which can induce and form the antibody for various human papilloma virus (HPV) and coat protein (HPV L1), to check various HPV or HPV L1, while the antibody can be used in biological pharmaceutical engineering, to purify and prepare various HPV or HPV L1.
Owner:CHINA THREE GORGES UNIV
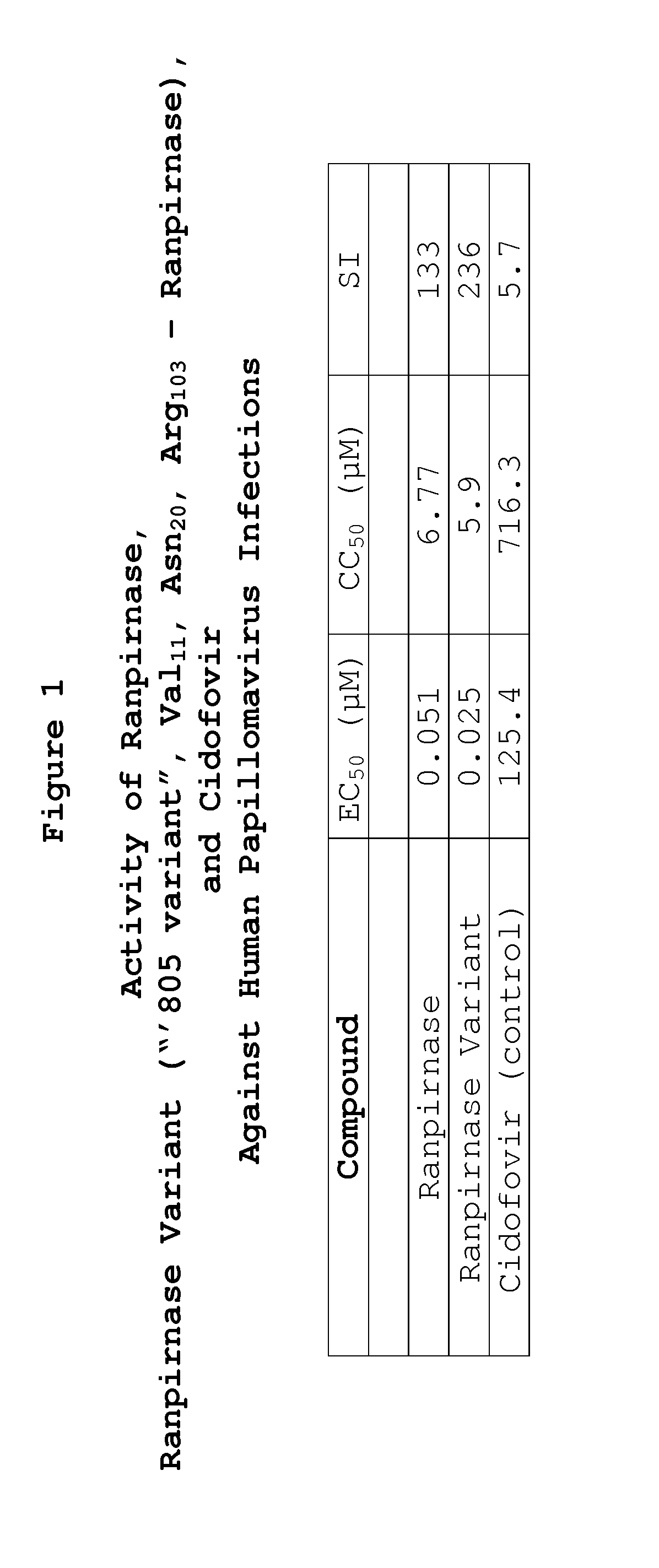
Methods of treating human papillomavirus
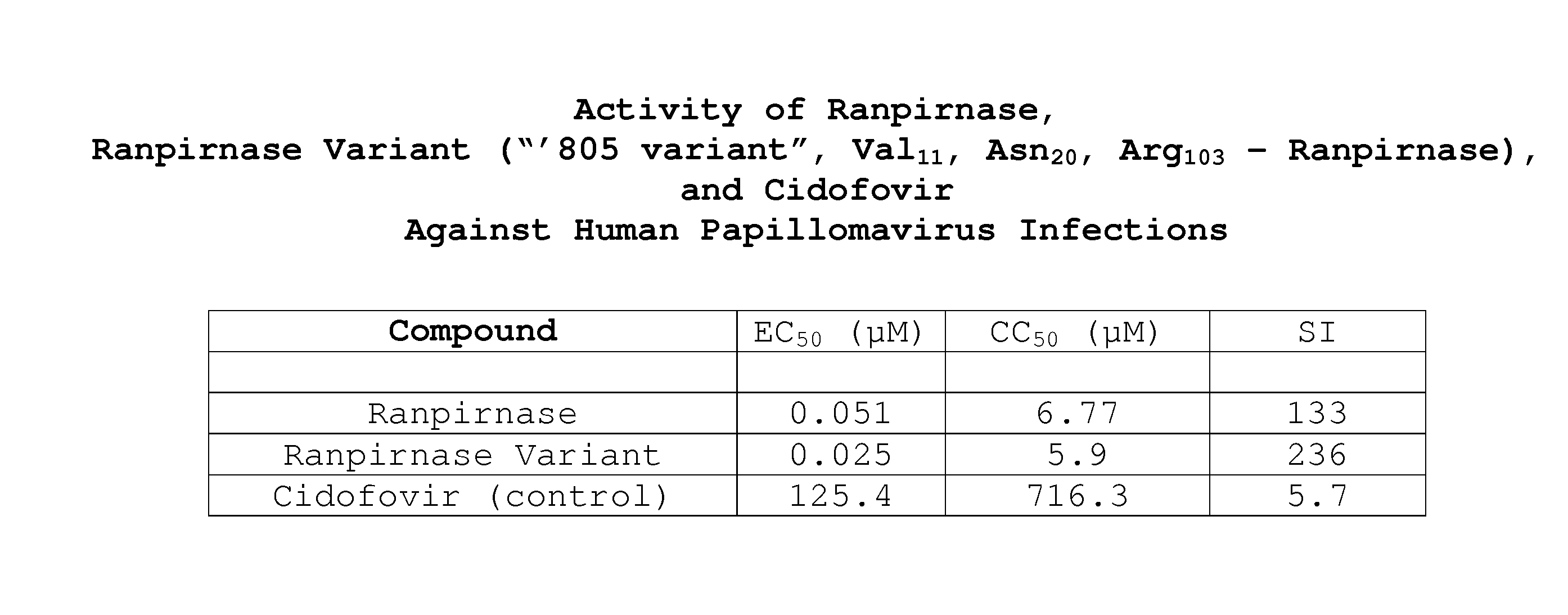
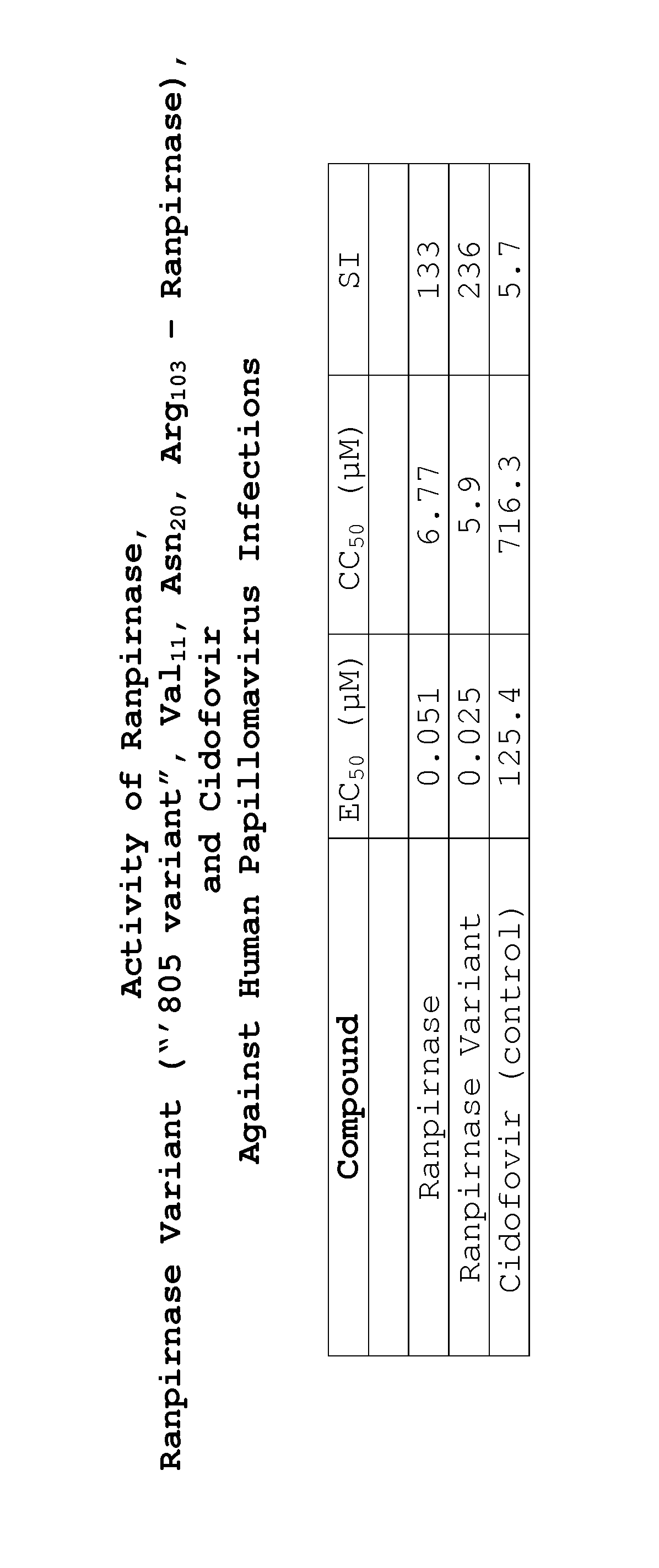
Two RNases (ranpirnase and the second embodiment disclosed in U.S. Pat. No. 5,728,805) are tested against human papillomavirus infections. QRT-PCR assays indicate that the RNases have anti-viral activity against type 11 HPV.
Owner:ORGENESIS INC
Anti-human papillomavirus 16 e7 t cell receptors
ActiveUS20170145070A1Minimize ToxicityImprove abilitiesPeptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeHuman papillomavirus
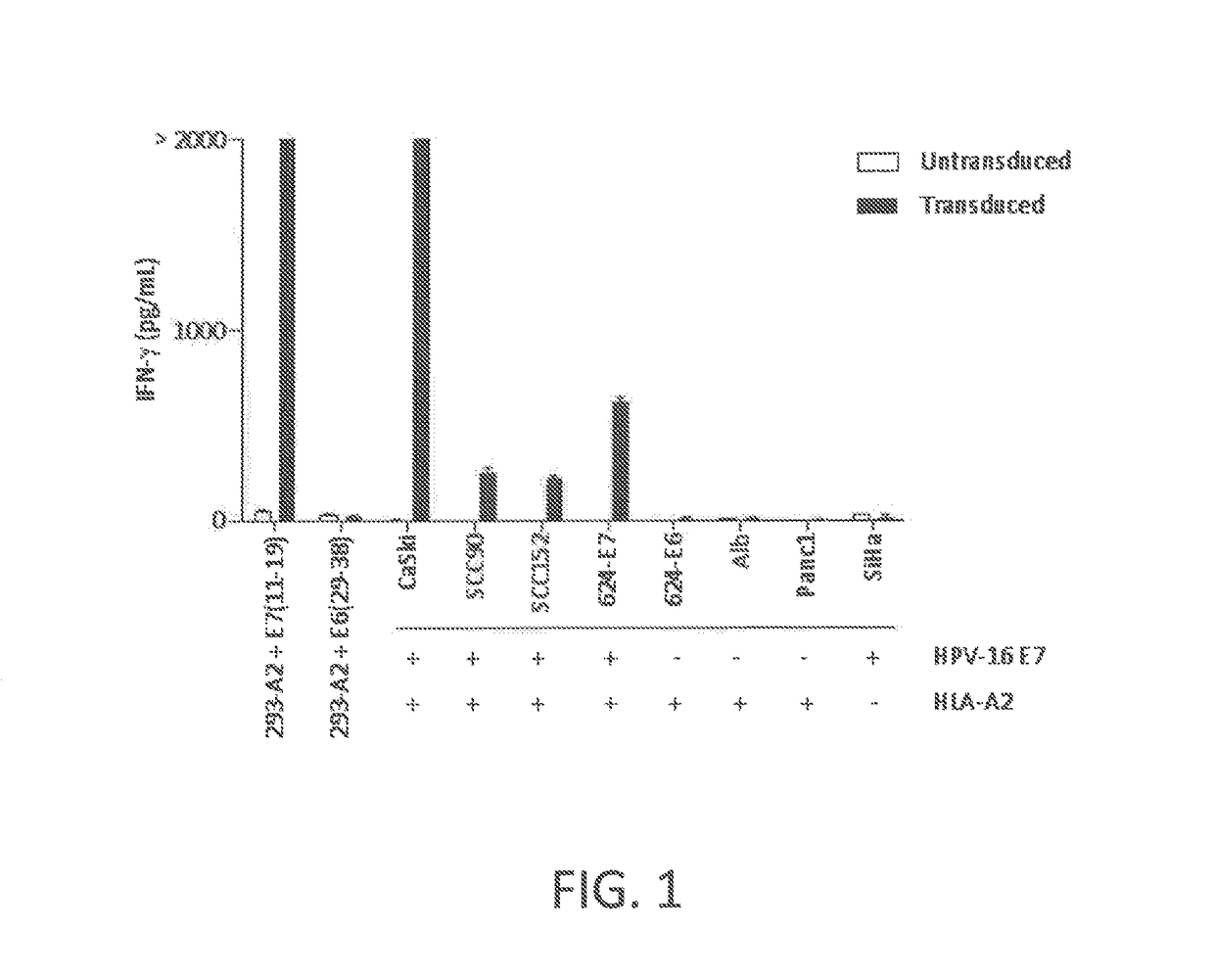
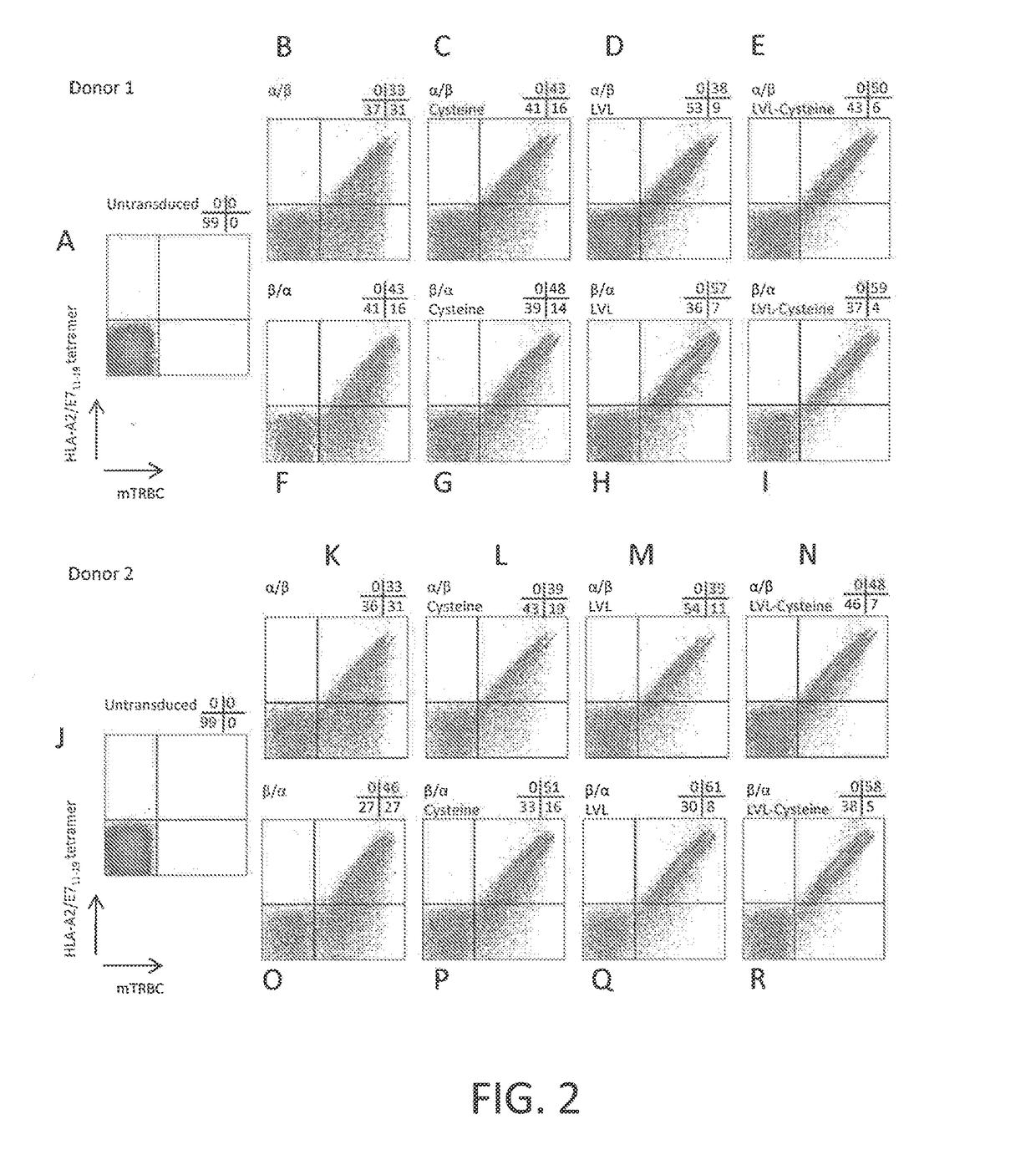
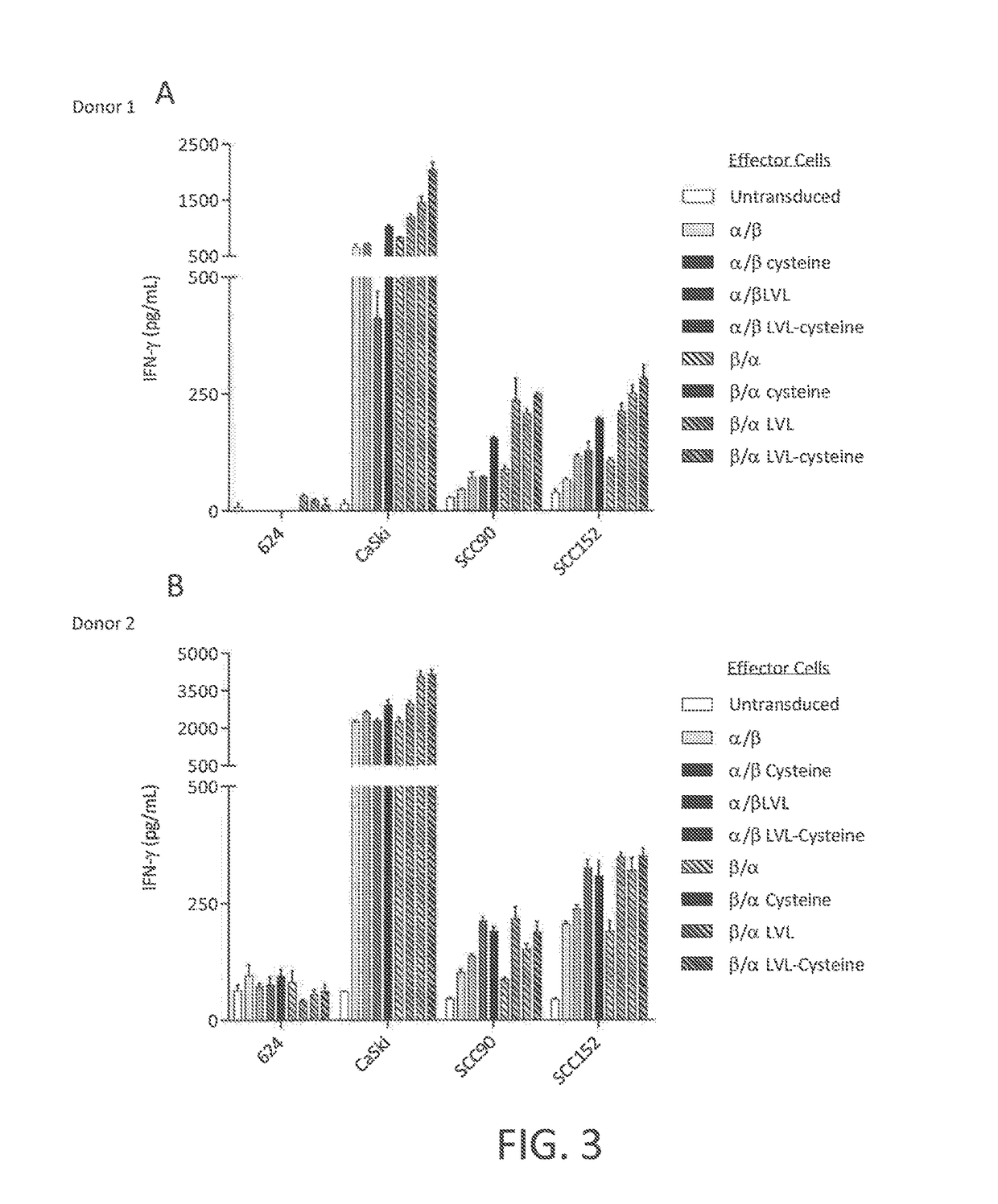
Disclosed is a synthetic T cell receptor (TCR) having antigenic specificity for an HLA-A2-restricted epitope of human papillomavirus (HPV) 16 E7, E711-19. Related polypeptides and proteins, as well as related nucleic acids, recombinant expression vectors, host cells, and populations of cells are also provided. Antibodies, or an antigen binding portion thereof, and pharmaceutical compositions relating to the TCRs of the invention are also provided. Also disclosed are methods of detecting the presence of a condition in a mammal and methods of treating or preventing a condition in a mammal, wherein the condition is cancer, HPV 16 infection, or HPV-positive premalignancy.
Owner:UNITED STATES OF AMERICA
Novel bis-Benzylidine Piperidone Proteasome Inhibitor with Anticancer Activity
ActiveUS20160106725A1Reduced potencyReducing potencyBiocideOrganic chemistryHuman papillomavirus19S regulatory particle
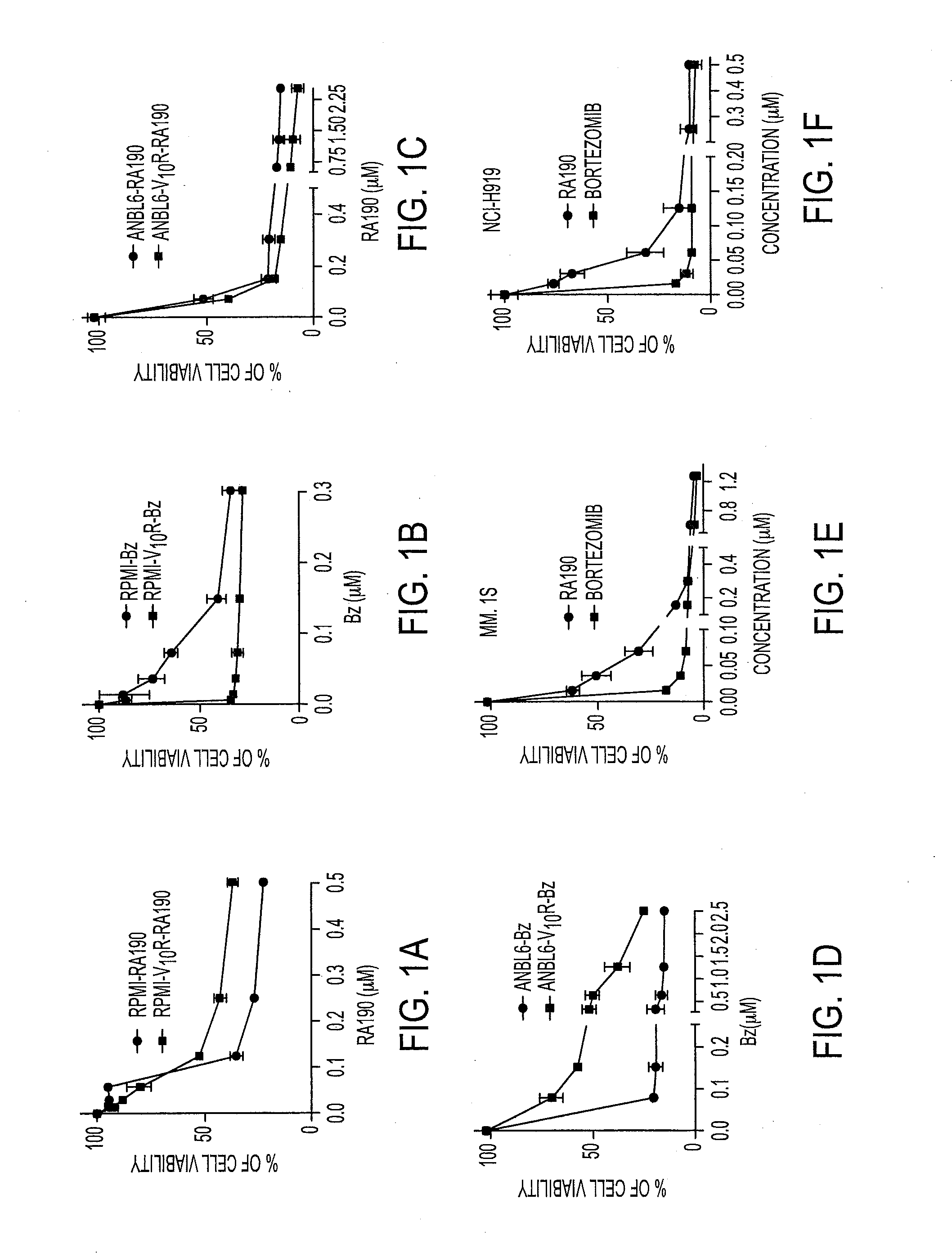
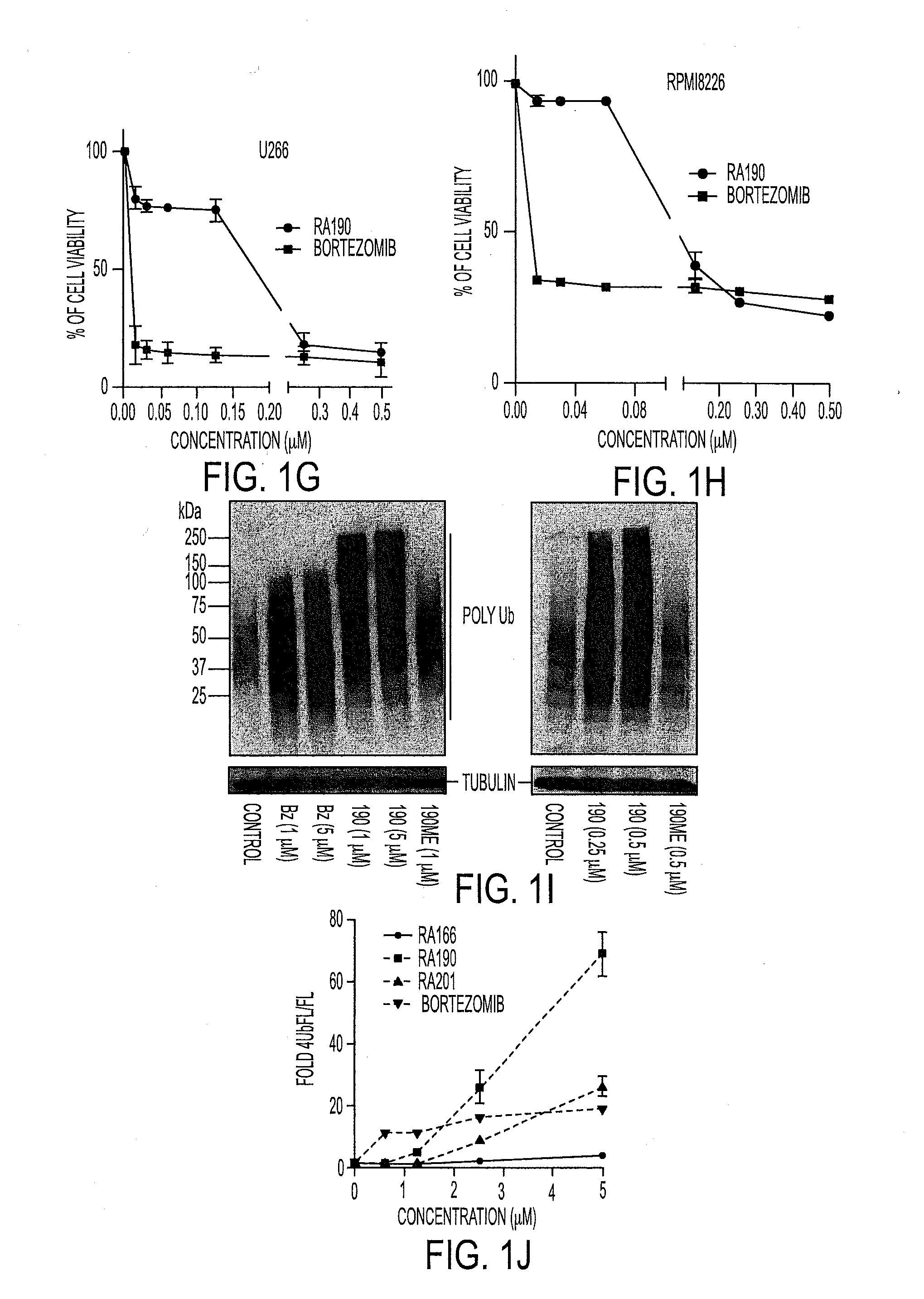
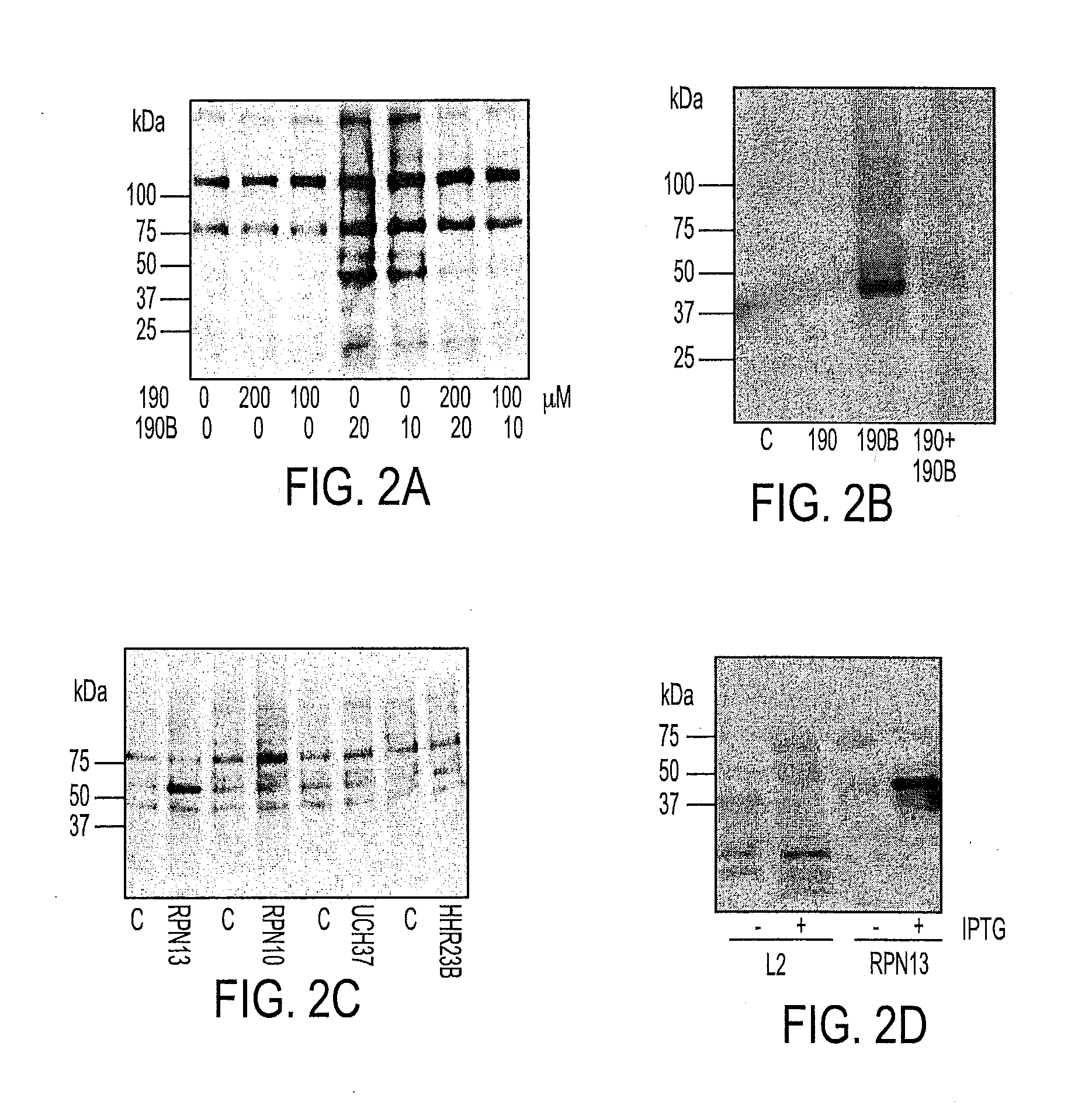
We describe a bis-benzylidine piperidone, RA190, which covalently binds to the ubiquitin receptor RPN13 (ADRM1) in the 19S regulatory particle and inhibits proteasome function, triggering rapid accumulation of polyubiquitinated proteins. Multiple myeloma lines, even those resistant to bortezomib, were sensitive to RA190 via ER stress-related apoptosis. RA190 stabilized targets of human papillomavirus (HPV) E6 oncoprotein, and preferentially killed HPV-transformed cells. After p.o. or i.p. dosing of mice, RA190 distributed to plasma and major organs excepting brain, and potently inhibited proteasome function in skin and muscle. RA190 administration i.p. profoundly reduced growth of multiple myeloma and ovarian cancer xenografts, and oral RA190 treatment retarded HPV+ syngeneic mouse tumor growth, without impacting spontaneous HPV-specific CD8+ T cell responses, suggesting its therapeutic potential. The bis-benzylidine piperidone RA190 is a new orally-available proteasome inhibitor. Multiple myeloma, cervical and ovarian cancers are particularly sensitive to RA190.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods of treating human papillomavirus
Owner:ORGENESIS INC
Approach to molecular diagnosis of human papillomavirus-related diseases
ActiveUS7361460B2Accurate sensitive toolReduce in quantitySugar derivativesMicrobiological testing/measurementProtein markersSequential method
The present invention relates to an accurate, sensitive, and efficient sequential or concurrently sequential method for molecular diagnosis of human papillomavirus (HPV)-based disease, where the method improves the accuracy and reliability of diagnostic and prognostic assessments of HPV-based disease. The method of the invention comprises a primary screen of a sample for HPV nucleic acids, followed by a secondary screen for molecular markers, such as proliferation and cell cycle control group protein markers. The sequential or concurrently sequential method significantly reduces the number of false positive results.
Owner:DIGENE CORP
Anti-human papillomavirus 16 e6 t cell receptors
ActiveUS20160152681A1Highly avid recognitionMinimize destructionPeptide/protein ingredientsAntibody mimetics/scaffoldsEpitopeHuman papillomavirus
Disclosed is a T cell receptor (TCR) having antigenic specificity for an HLA-A2-restricted epitope of human papillomavirus (HPV) 16 E6, E629-38. Related polypeptides and proteins, as well as related nucleic acids, recombinant expression vectors, host cells, and populations of cells are also provided. Antibodies, or an antigen binding portion thereof, and pharmaceutical compositions relating to the TCRs of the invention are also provided. Also disclosed are methods of detecting the presence of a condition in a mammal and methods of treating or preventing a condition in a mammal, wherein the condition is cancer, HPV 16 infection, or HPV-positive premalignancy.
Owner:UNITED STATES OF AMERICA
Human papillomavirus multiplexed assay
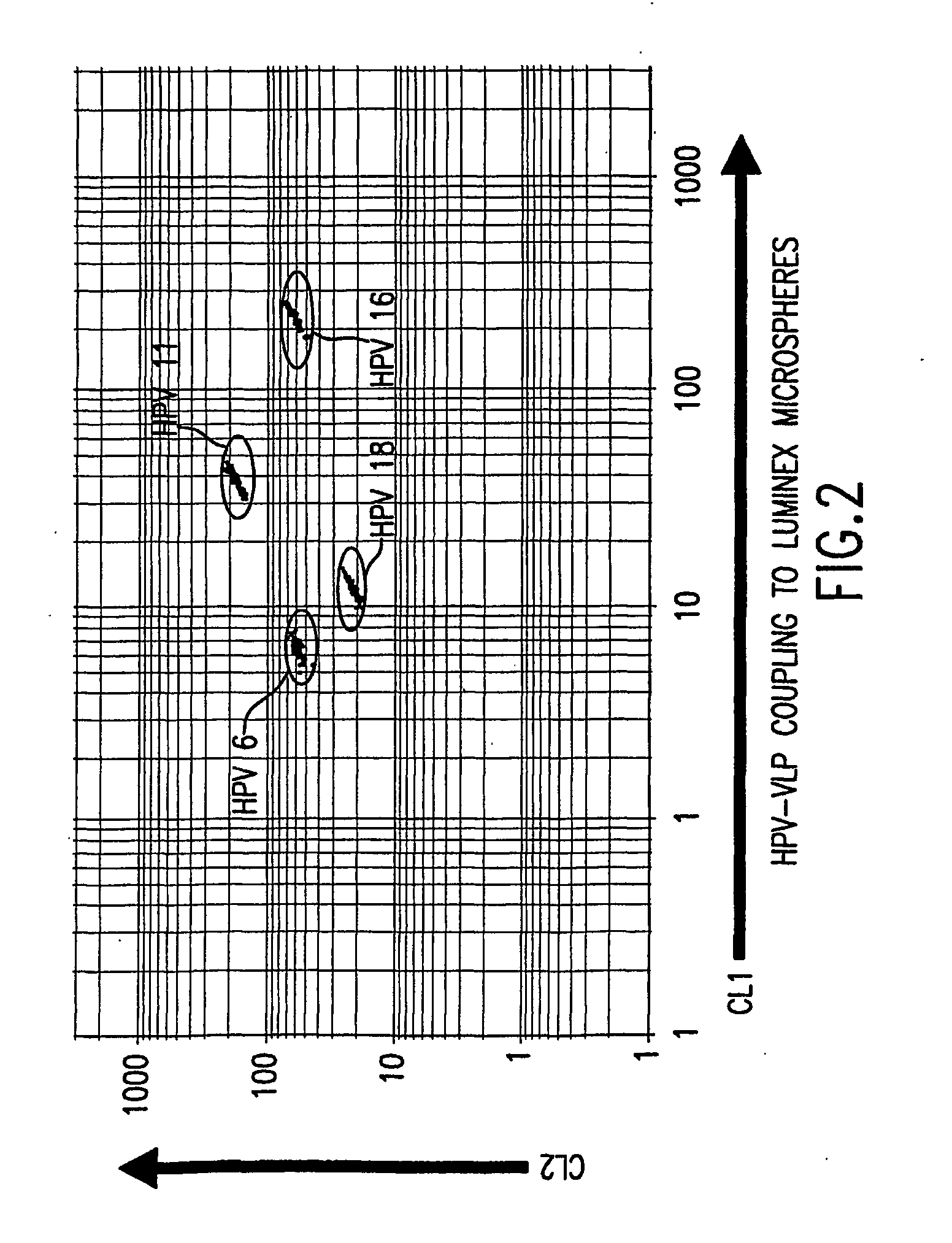
InactiveUS20050147961A1Inducing high titres of neutralizing antibodiesAnimal cellsHybrid immunoglobulinsMicrosphereFluorescence
The present invention relates to an immunoassay for simultaneously measuring the presence of antibodies to a plurlity of HPV types that utilizes particle-based flow cytometric analysis. The presence and / or titre of neutralizing antibodies in a test sample are determined in a competitive format, where known, type-specific, fluorescently labeled neutralizing monoclonal antibodies compete with antibodies within a test sample for binding to conformationally sensitive, neutralizing epitopes on specific HPV-VLPs. The invention also provides a microsphere complex comprising a microsphere coupled to an HPV VLP.
Owner:MERCK SHARP & DOHME CORP
Human papillomavirus HPV DNA fragment, specific primer and application thereof
InactiveCN101851630ASimple methodMature technologyMicrobiological testing/measurementMicroorganism based processesDiseaseHuman papillomavirus DNA
The invention provides a human papillomavirus HPV DNA fragment, a mix primer for human papillomavirus HPV DNA detection and / or parting and application thereof. The mixed primer can specifically amplify to obtain one or a plurality of DNA sequences from HPV6, HPV 11, HPV 16, HPV 18, HPV 26, HPV 31, HPV 33, HPV 35, HPV 39, HPV 40, HPV 42, HPV 43, HPV 44, HPV45, HPV 51, HPV 52, HPV 53, HPV 54, HPV 55, HPV 56, HPV 58, HPV 59, HPV 61, HPV 66, HPV 68, HPV 70, HPV 72, HPV 73, HPV 81, HPV 82 and HPV 83, can be used for the human papillomavirus HPV DNA detection and / or parting and prepared into a PCR kit. The invention can detect common types of HPV at a time, greatly improve the HPV detection efficiency, overcome the defect of missed detection of latent infection by a serology and IHC detection method, realize early diagnosis of HPV diseases, and is suitable for clinical HPV gene detection and parting and guides prevention and cure of cervical carcinoma.
Owner:白向阳 +1
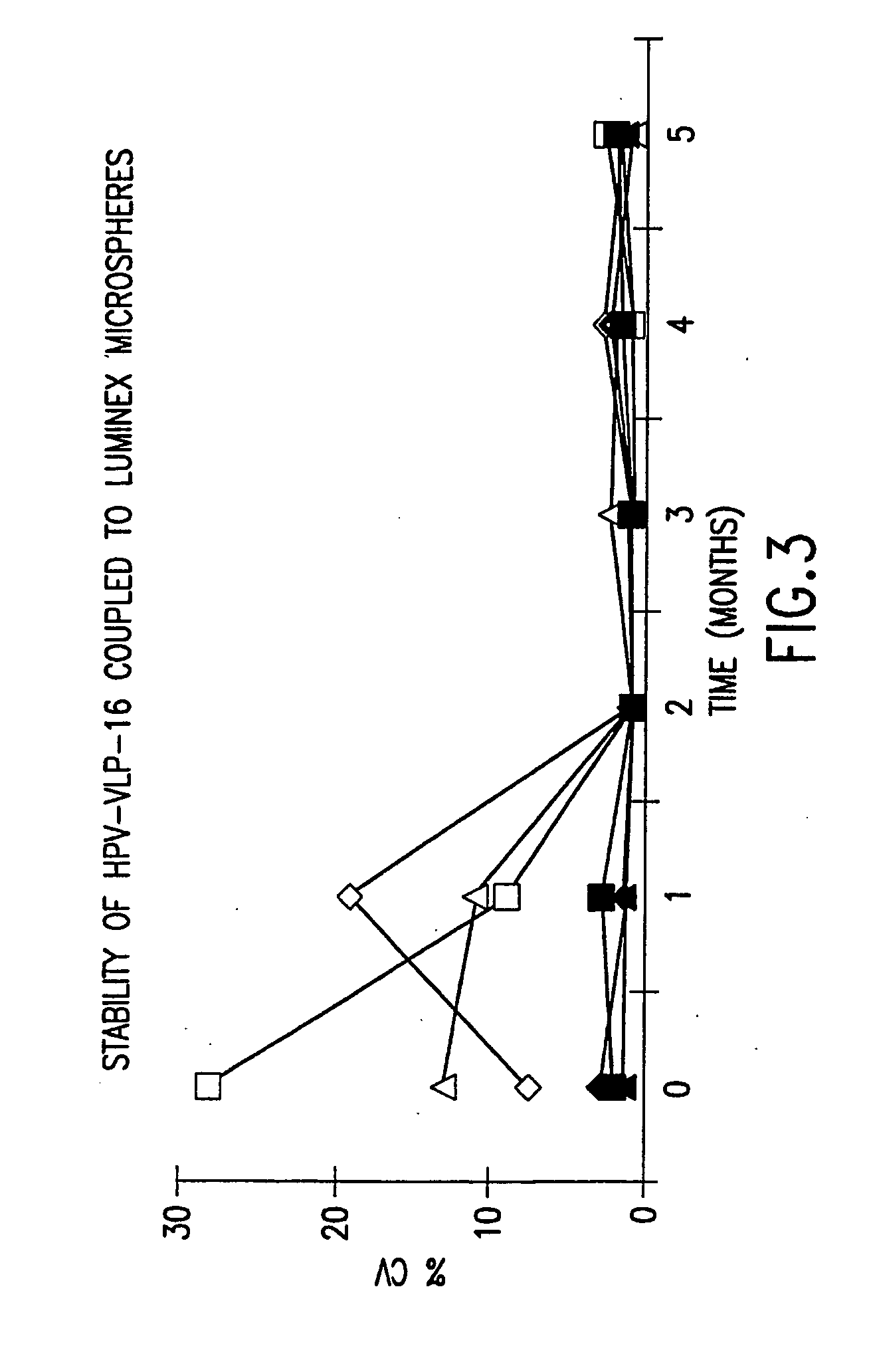
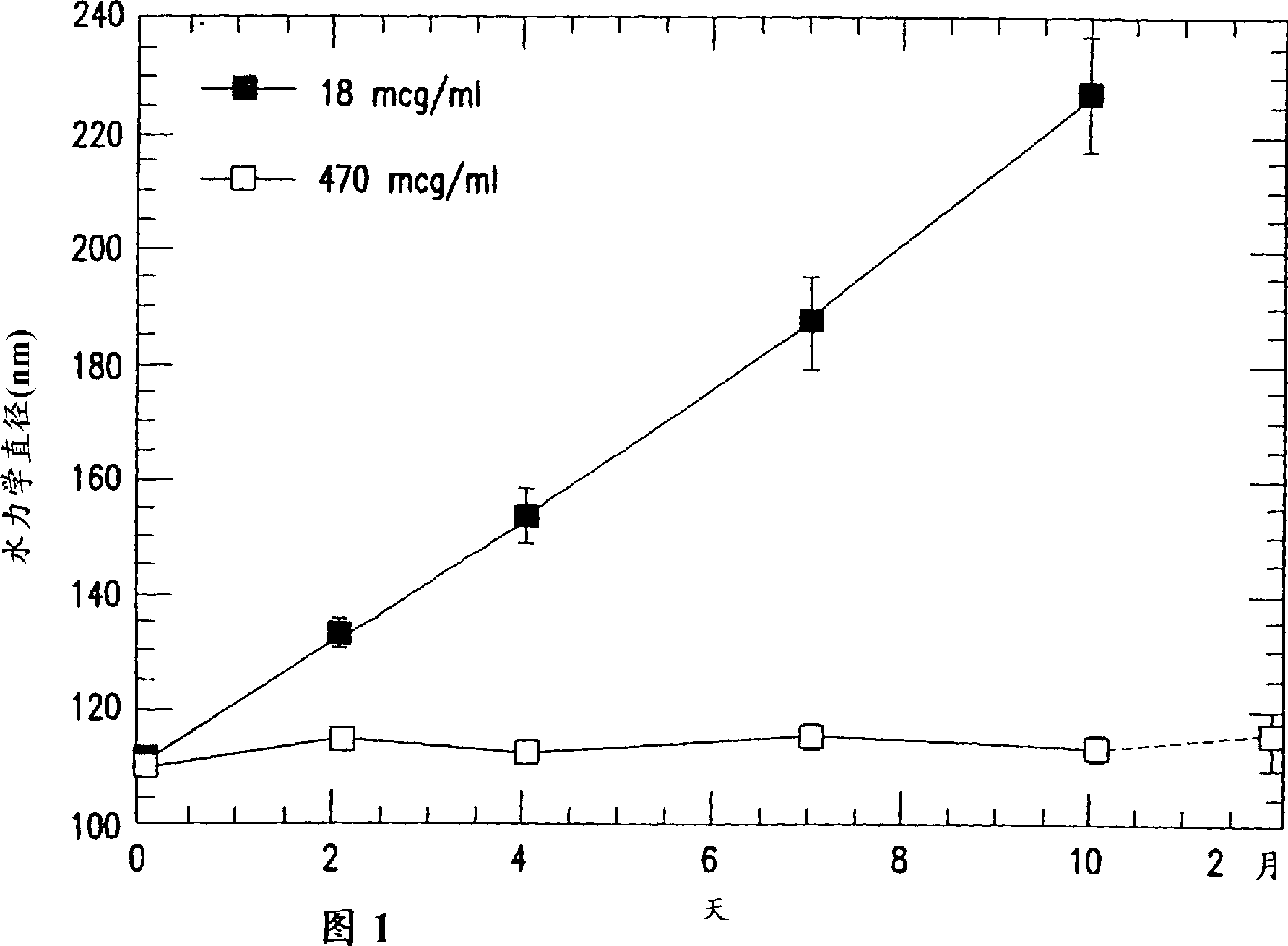
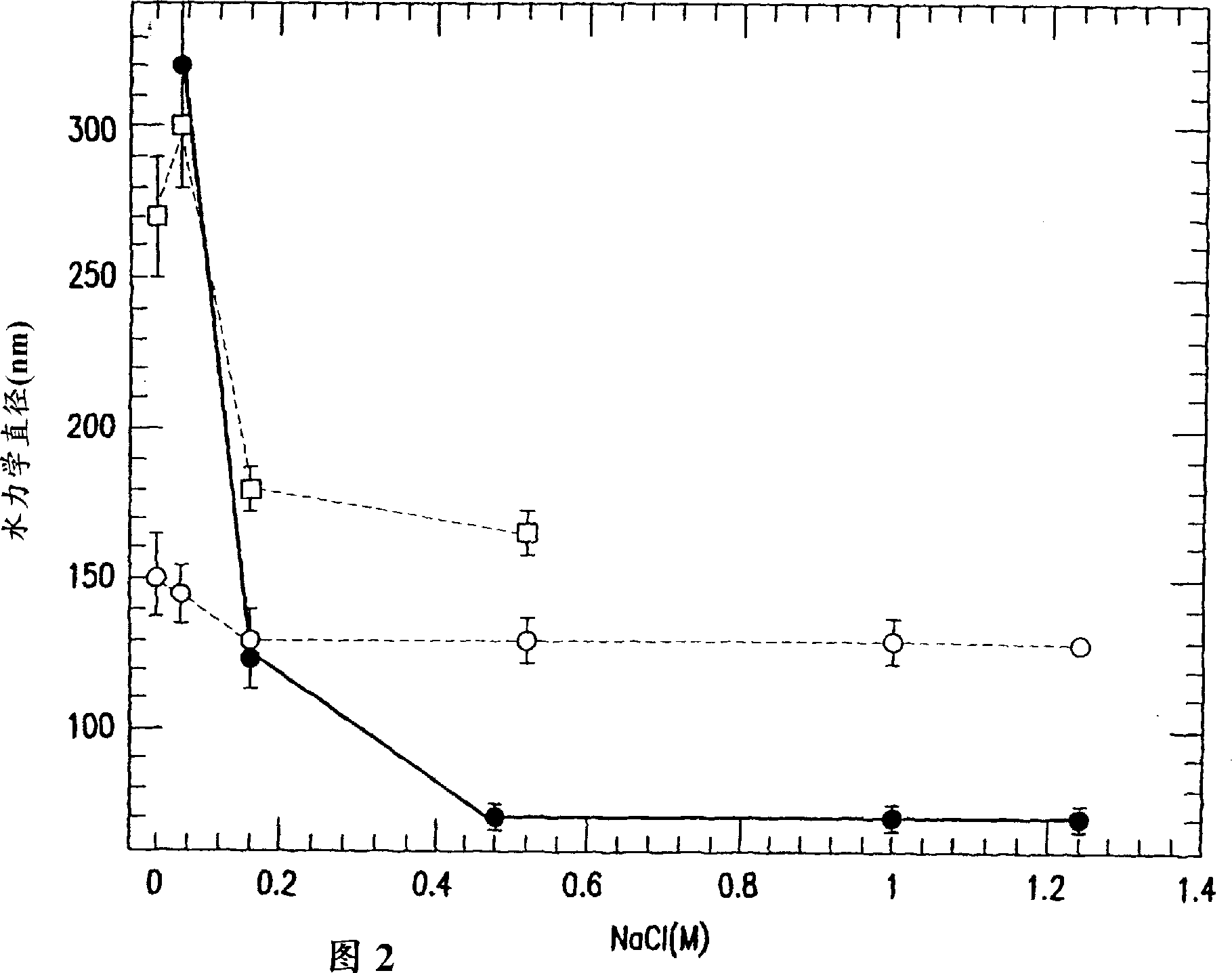
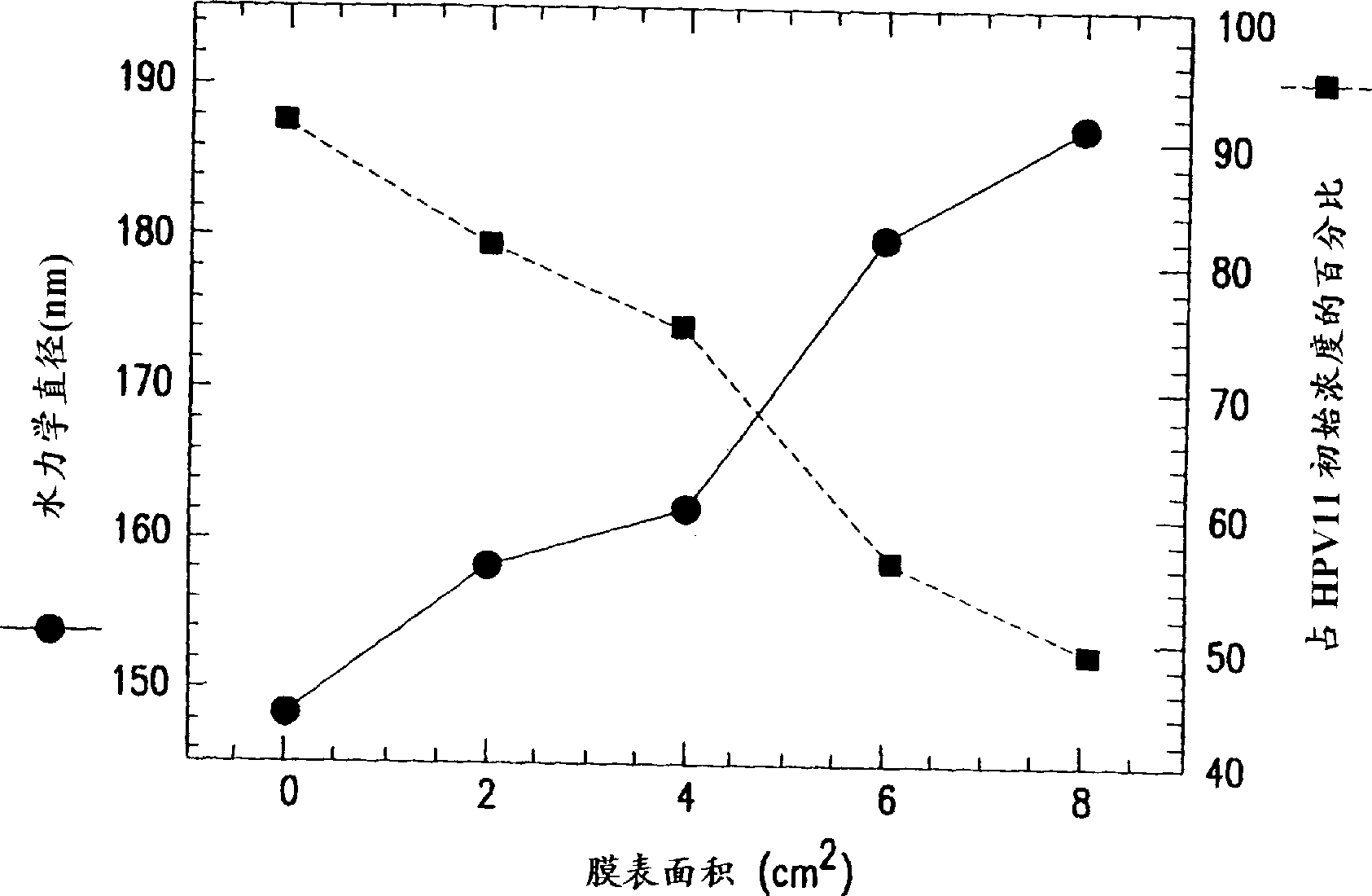
Stabilized human papillomavirus formulations
The invention discloses human papillomavirus (HPV) antigen formulations which prevent protein aggregation and show prolonged stability as aqueous solutions. These formulations comprise a salt (such as sodium chloride) and a non ionic surfactant (Polysorbate 80 such as Tween 803) in physiologically acceptable concentrations.
Owner:MERCK & CO INC
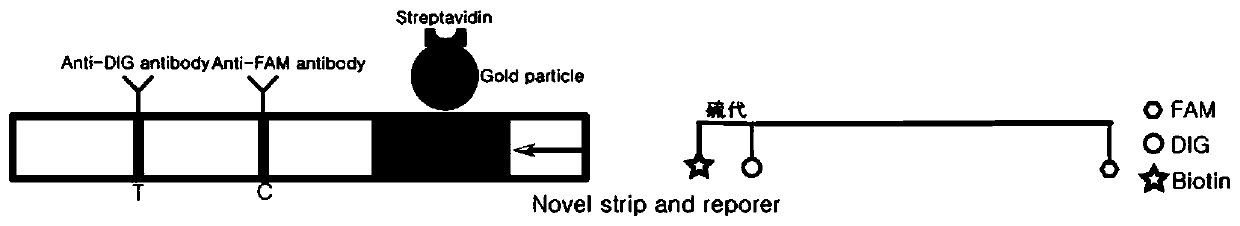
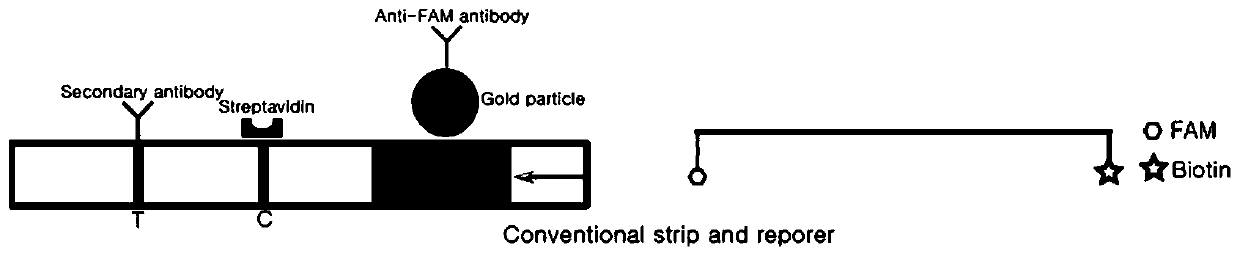
Detection method based on CRISPR/Cas and nucleic acid test paper and human papilloma virus detection kit
PendingCN111560482AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesNucleic acid testSingle strand
The invention relates to the technical field of gene detection, in particular to a nucleic acid detection method based on CRISPR / Cas and nucleic acid test paper and a human papilloma virus rapid detection kit. The human papilloma virus detection kit comprises crRNA, Cas12, RPA upstream and downstream primers, a buffer system and a single-stranded DNA reporter molecule, the sequence of the reportermolecule is 5 '-NNN......-3', the length of the reporter molecule is 3-30 basic groups, FAM, DIG and Biotin labeling is carried out, and meanwhile modification for preventing DNA enzyme or cas12 degradation after activation is carried out on nucleic acid molecules. The CRISPR / Cas and nucleic acid test paper-based nucleic acid detection method and the human papilloma virus rapid detection kit havethe advantages that innovative reporter molecule design and modification are adopted, and corresponding test paper strips are combined, so that non-specific color development is completely eradicated, and the kit has remarkable significance in clinical practical application.
Owner:亚能生物技术(深圳)有限公司
Methods and Compositions for Producing an Enhanced Immune Response to a Human Papillomavirus Immunogen
InactiveUS20090068214A1Viral antigen ingredientsMicrobiological testing/measurementCo administrationHuman papillomavirus
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Vaccine composition comprising virus-like particles of human papillomavirus
The present invention relates to a vaccine composition comprising VLPs containing L1 proteins or functional L1 protein derivatives from HPV 16, HPV 18, HPV 31 and HPV 45 genotypes.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
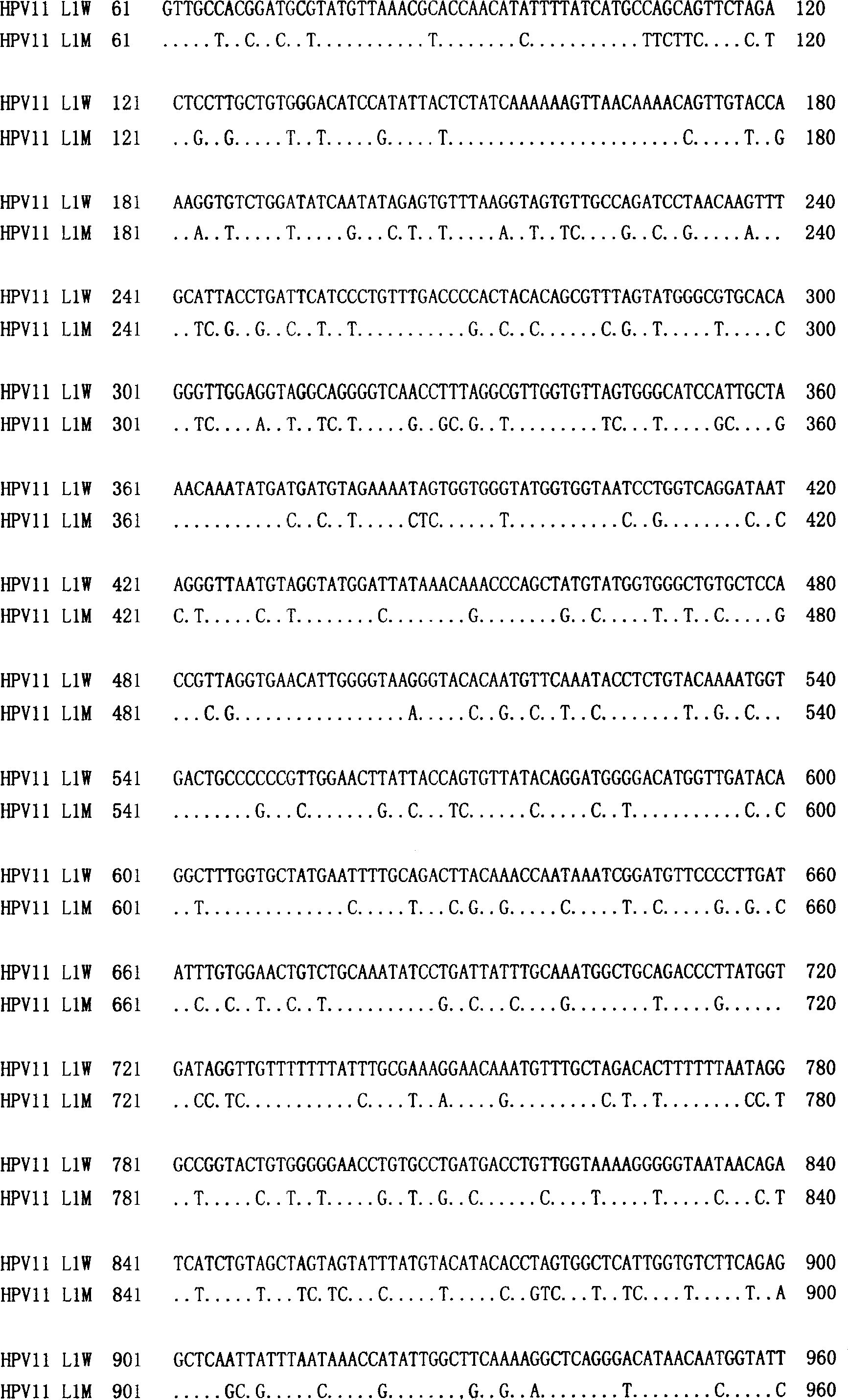
Truncated l1 protein of human papillomavirus type 11
ActiveUS20100291141A1Reduced expression levelLow costVirus peptidesAntiviralsVirus-like particleAlphapapillomavirus
The invention relates to a truncated L1 protein of the Human Papillomavirus Type 11, a virus-like particle consisting of the protein, a vaccine comprising said virus-like particle, and the use of the vaccine in the prevention of condyloma acuminatum or HPV infections.
Owner:XIAMEN INNOVAX BIOTECH +1
Recombinant listeria vaccine strains and methods of using the same in cancer immunotherapy
ActiveUS20160367650A1Reduce severityReduce needOrganic active ingredientsViral antigen ingredientsListeria booriaeVaccine strain
The present invention provides methods of treating, protecting against, and inducing an immune response against a human papillomavirus-associated oropharyngeal tumor or cancer, comprising the step of administering to a subject a recombinant Listeria strain expressing a human papillomavirus antigen.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Detection kit, detection system and detection method for 19 high-risk human papilloma viruses (HPVs)
ActiveCN104818342AHigh sensitivityImprove featuresMicrobiological testing/measurementMicroorganism based processesRisk typeBioinformatics
The invention discloses a detection kit, detection system and detection method for 19 high-risk HPVs. The 19 high-risk HPVs comprise HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73 and 82. According to the invention, a plurality of general primers are employed for amplification of target fragments, and specific fluorescence probes are used for real-time detection of the amplified fragments; since the general primers are employed, the primer amount of the system and complexity of the system are reduced, cost is saved, and all the high risk types are detected through combined action of 10 primers; and 20 specific fluorescence probes are used to distinguish different types. With the detection kit, detection system and detection method provided by the invention, the 19 high-risk HPVs can be detected in a reaction tube; high sensitivity and good specificity are obtained; operation is simple and fast; and for patients, screening cost is saved.
Owner:AMOY DIAGNOSTICS CO LTD
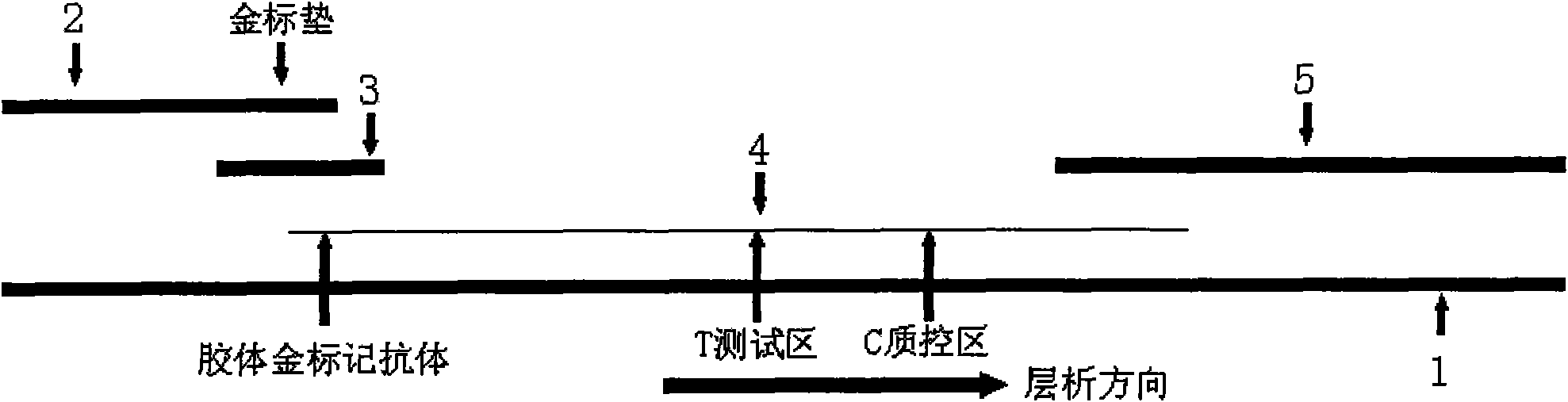
Immune colloidal gold test card for testing high-risk type human papillomavirus and test method thereof
The invention provides an immune colloidal gold test card for testing high-risk type human papillomavirus. A test strip thereof consists of a back lining, a sample pad, a combining pad, an NC membrane and a water-absorbing pad; the upper middle part of the back lining is provided with the NC membrane; the two ends of the NC membrane are respectively connected with the combining pad and water-absorbing pad in a neighboring way; the sample pad is arranged above the combining pad; a gold-labelled antibody G-Ab1 is sprayed on the combining pad; the coated gold-labelled antibody G-Ab1 is the monoclonal antibody of the high-risk type human papillomaviru virus HPV16; and the NC membrane is respectively sprayed with the polyclonal antibody Ab2 of the high-risk type human papillomaviru virus HPV16 and the antibody of the gold-labelled antibody, namely the IgG antibody of a rabbit antimouse. The invention applies the technology of immune colloidal gold into the quick testing of the high-risk type human papillomaviru virus; moreover, the test strip for quick testing the high-risk type human papillomaviru virus does not need a test device, thus being convenient for the application to the clinic quick diagnosis and suitable to hospitals of various grades to screen cervical carcinoma.
Owner:CHONGQING UNIV OF TECH
HPV58 L1 gene, vector, strain and expression method
The invention relates to a gene of the main capsid protein of 58 type human papillomavirus, which is optimized through a pichiapastoris expression codon, a vector containing the gene, a strain and an expression method thereof.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com