Stabilized human papillomavirus formulations
A technology of human papillomavirus and preparation, which is applied in the direction of antiviral agents, viruses, and viral peptides, and can solve the problem of low expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Overexpression of recombinant HPV11L1 VLP
[0072] Construction of Synthetic L1 Genes - Overexpression and Purification of HPV11L1 VLPs are described in two U.S. Patent Applications Serial Nos. 08 / 413,571 and 08 / 413,572, filed March 30, 1995, in Examples of the present invention Mention in this section is by way of example only and by no means limiting the methods that can be used to produce recombinant HPV VLPs. In order to give an example of the preparation of the present invention, this description also discloses the use of recombinant HPV11L1 and HPV16L1. One of ordinary skill in the art should know that known recombinant DNA methods can be used to produce the recombinant HPV VLPs listed in this specification. It should also be appreciated that hosts other than yeast can be used to overexpress the HPV VLPs used in the antigenic preparations of the invention.
[0073]The 1.5 kbp open reading frame of HPV11L1 was constructed using synth...
Embodiment 2
[0105] Expression of HPV11L1 and HPV6L1 in yeast - by spheroplast method (Hinnen, et al., 1978, Proc, Natl. Acad. Sci. USA 75, 1929-1933), with plasmid D362-1 (pGAL1-10+HPV6 / 11L1 ), p329-1(pGAL1-10+wt-HPV11L1), D128(pGAL1-10+HPV6L1) and pGAL1-10 transformed Saccharomyces cerevisiae strain #1558.(MATa, leu2-04, prb1::HIS3, mnn9:: URA3, adel, cir°). The #1558 yeast strain transformed with plasmid D362-1 was designated strain 1782. For RNA studies, yeast clonal isolates were grown in YEH complex medium (Carty et al., 1987, J.Ind.Micro.2, 117-121) containing 0.1M sorbitol and 2% glucose or galactose Incubate at 30°C for 26 hours. After harvesting the cells, yeast RNA was extracted by the hot acid phenol method (Current Protocols In Molicular Biology, vol. 2, Current Protocols, 1993). For protein analysis, the same isolates were cultured in YEH complex medium containing 0.1M sorbitol, 2% glucose and 2% galactose at 30°C for 70 hours. After the cells were harvested, the cell pel...
Embodiment 3
[0116] Dependence of aggregation on protein concentration
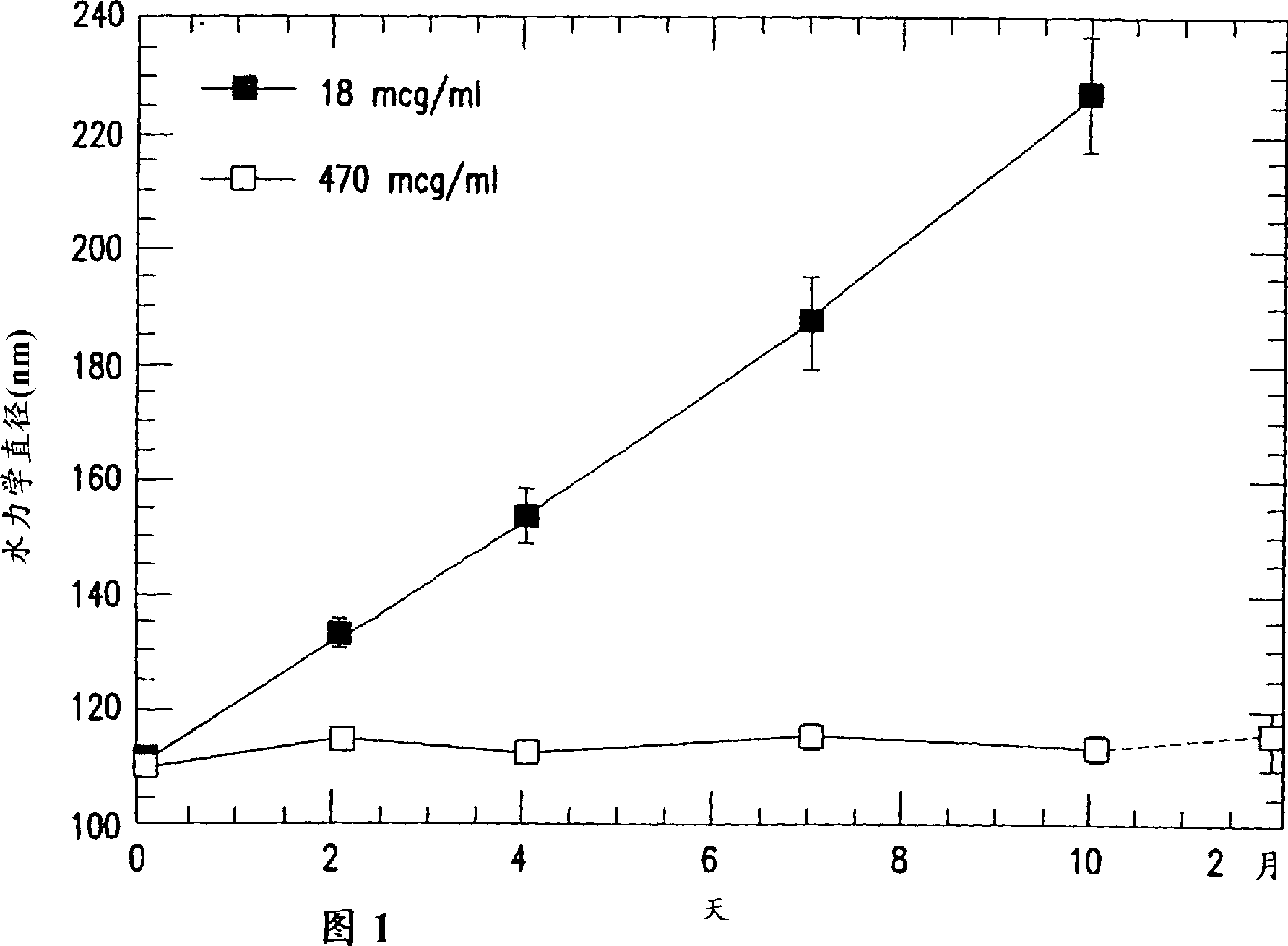
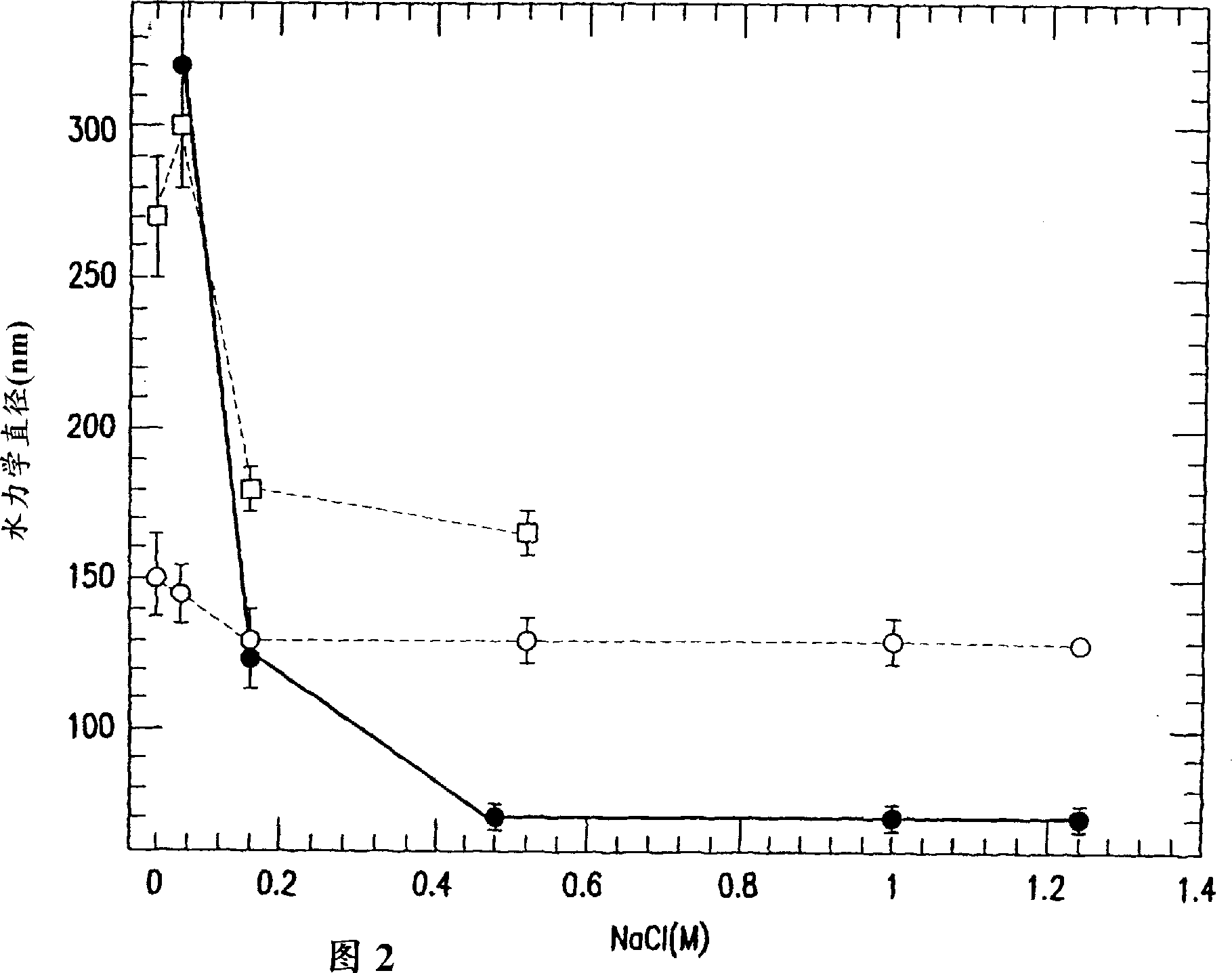
[0117] A batch of HPV11 VLPs was prepared as described above and stored at a concentration of 0.47 mg / mL in 50 mM MOPS containing 1.25 M NaCl. Thaw this stock and dilute a portion of it to 18mcg / mL with the same buffer. Within 2 months at 4°C, the hydraulic diameter (Dh) in the solution with high protein concentration remained unchanged in the range of 107-115 nm (measured by dynamic light scattering), while the solution with low protein concentration remained unchanged even at the salt concentration. Under the condition of 1.25M NaCl, agglomeration still occurred after a period of time (Fig. 1). Figure 1 shows that at low protein concentrations even high salt concentrations were unable to prevent aggregation of HPV during storage at 4°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com