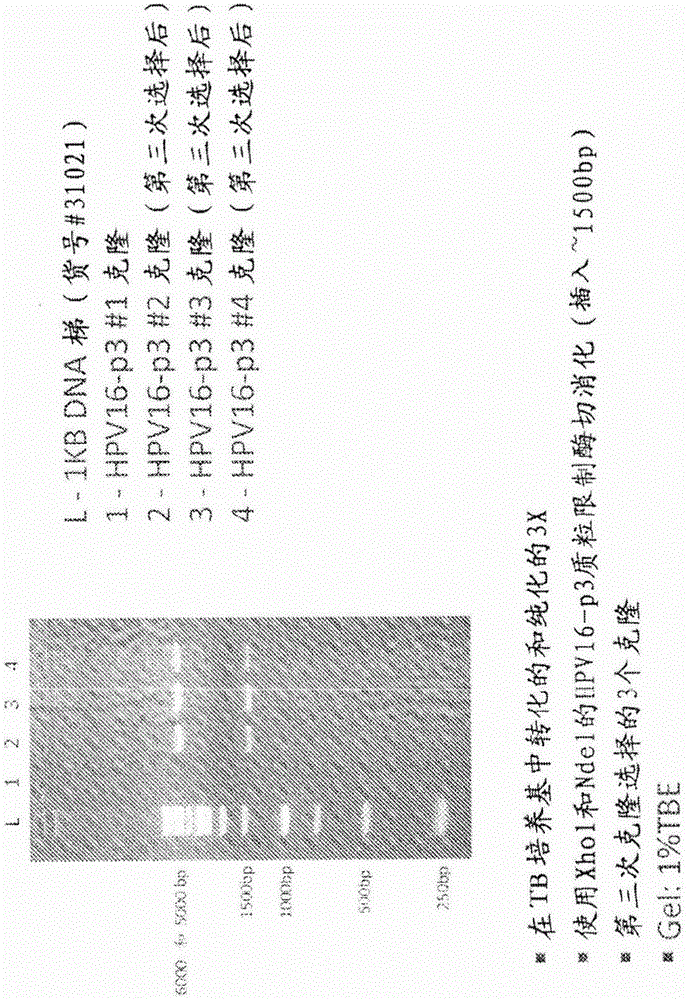
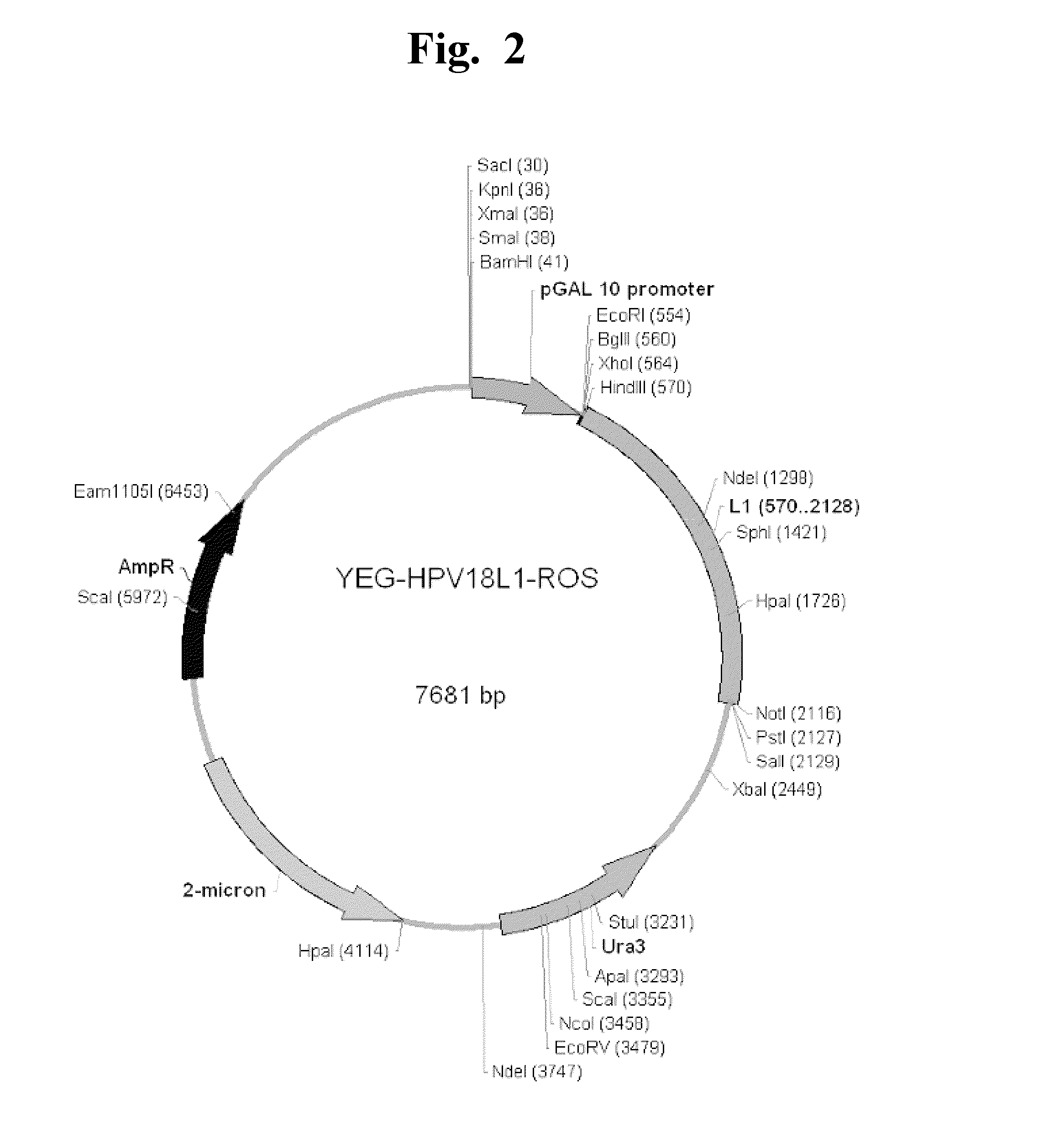
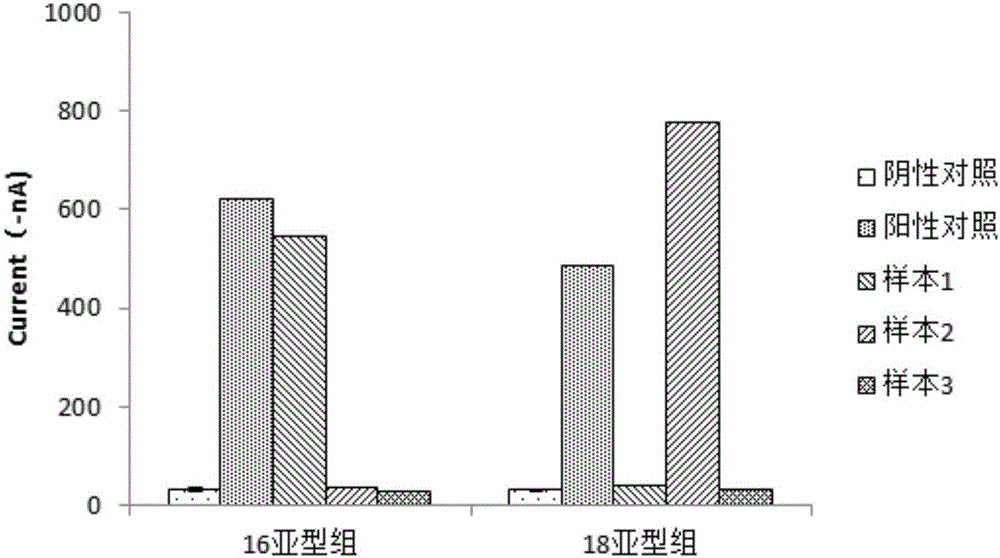
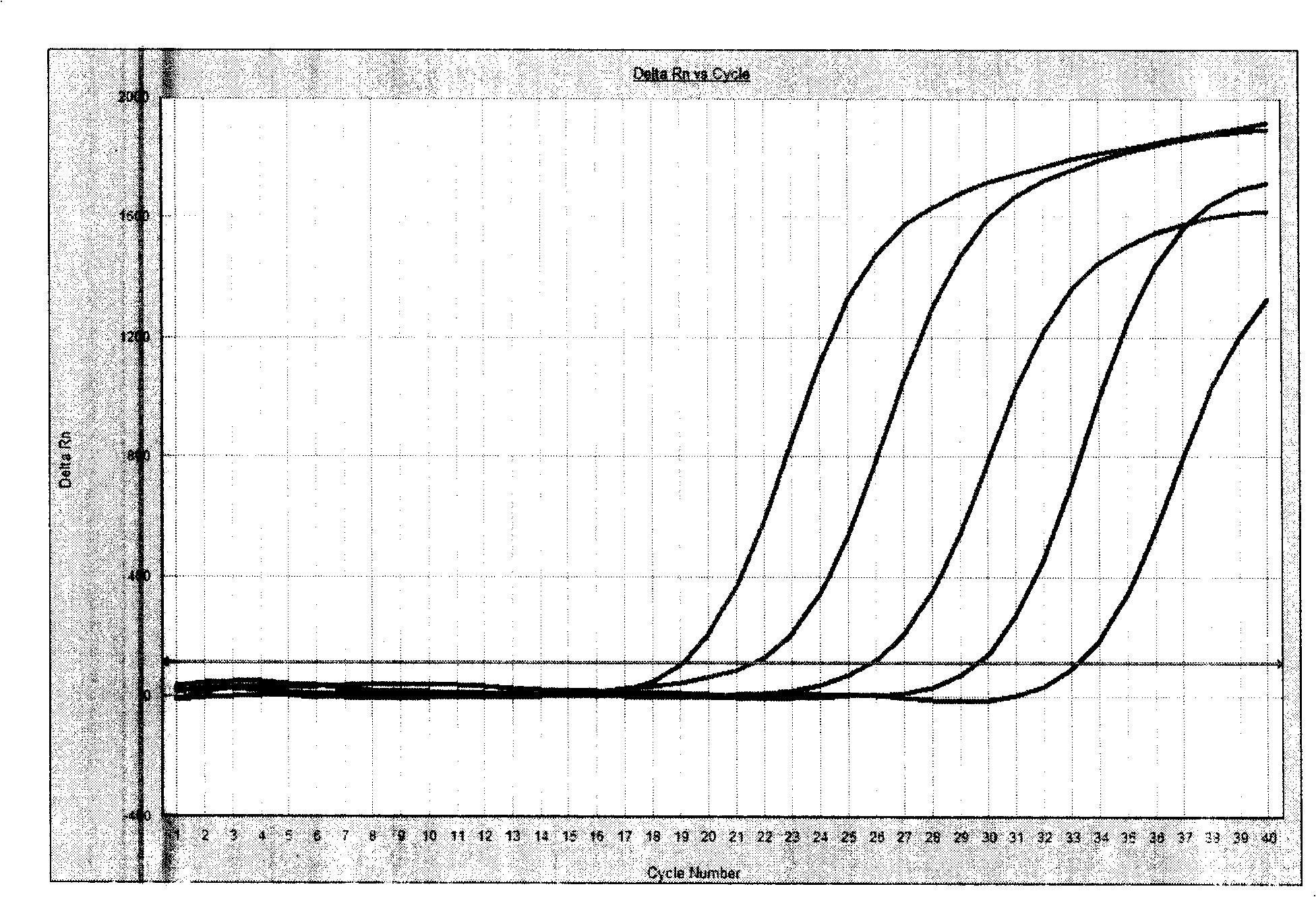
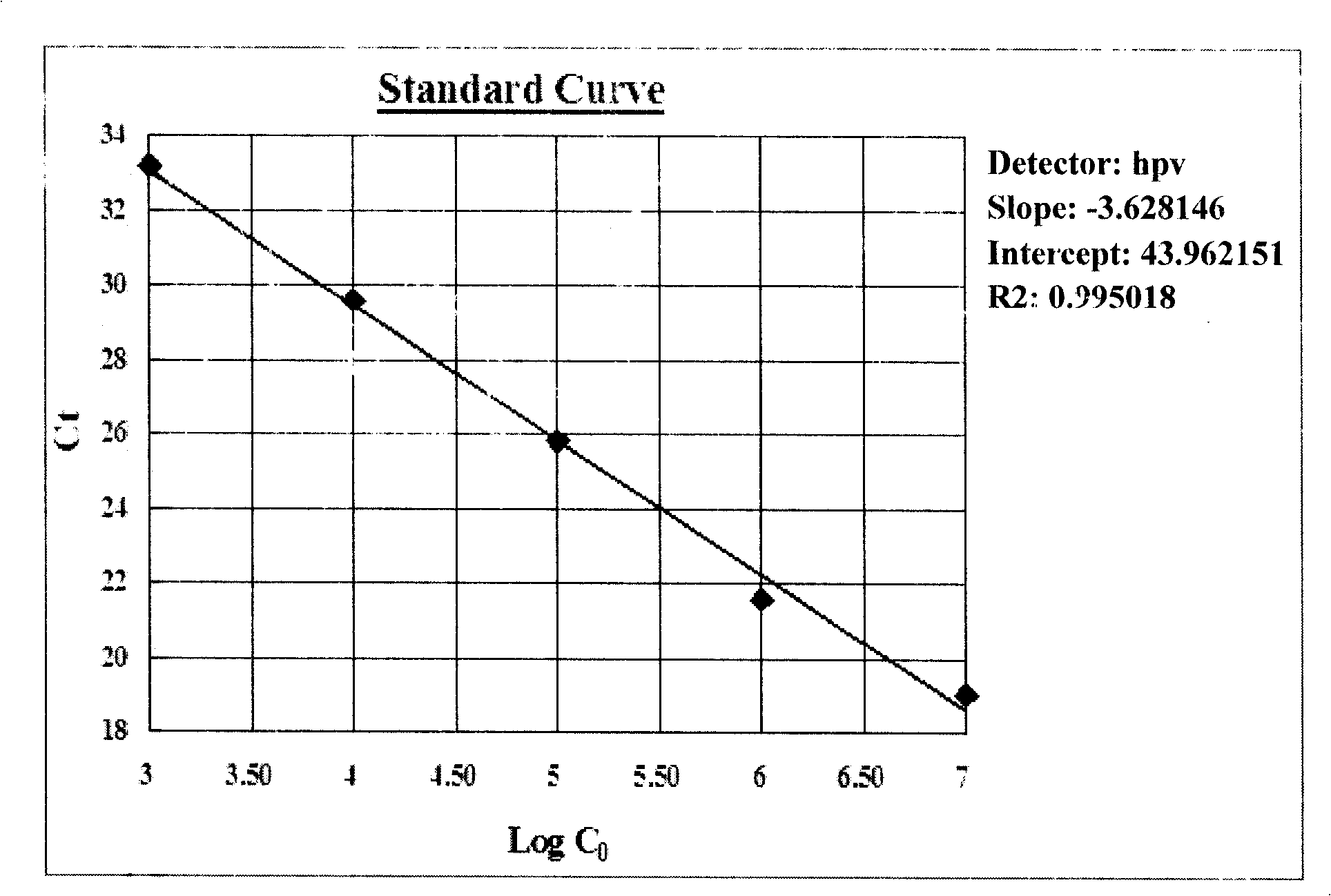
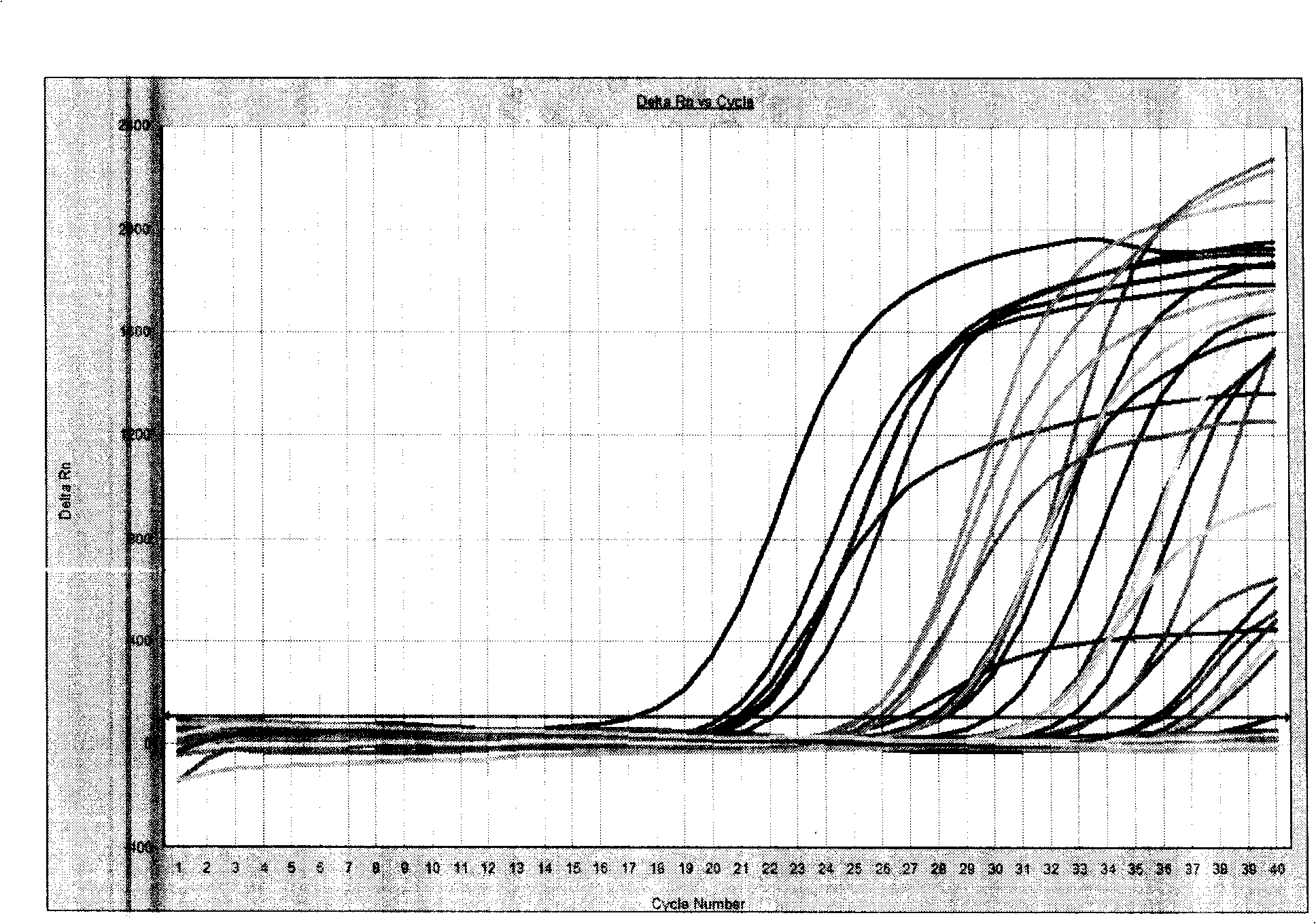
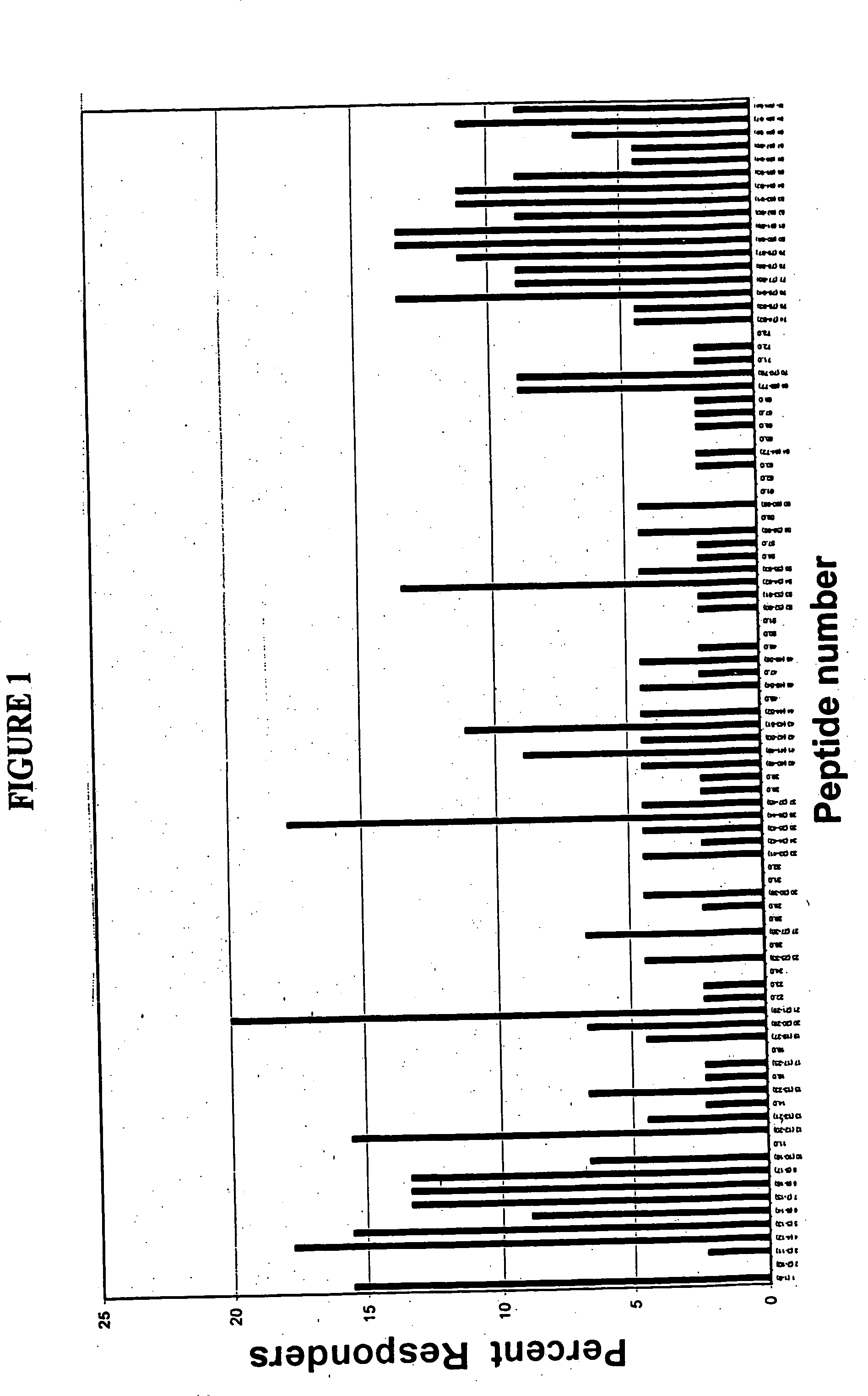
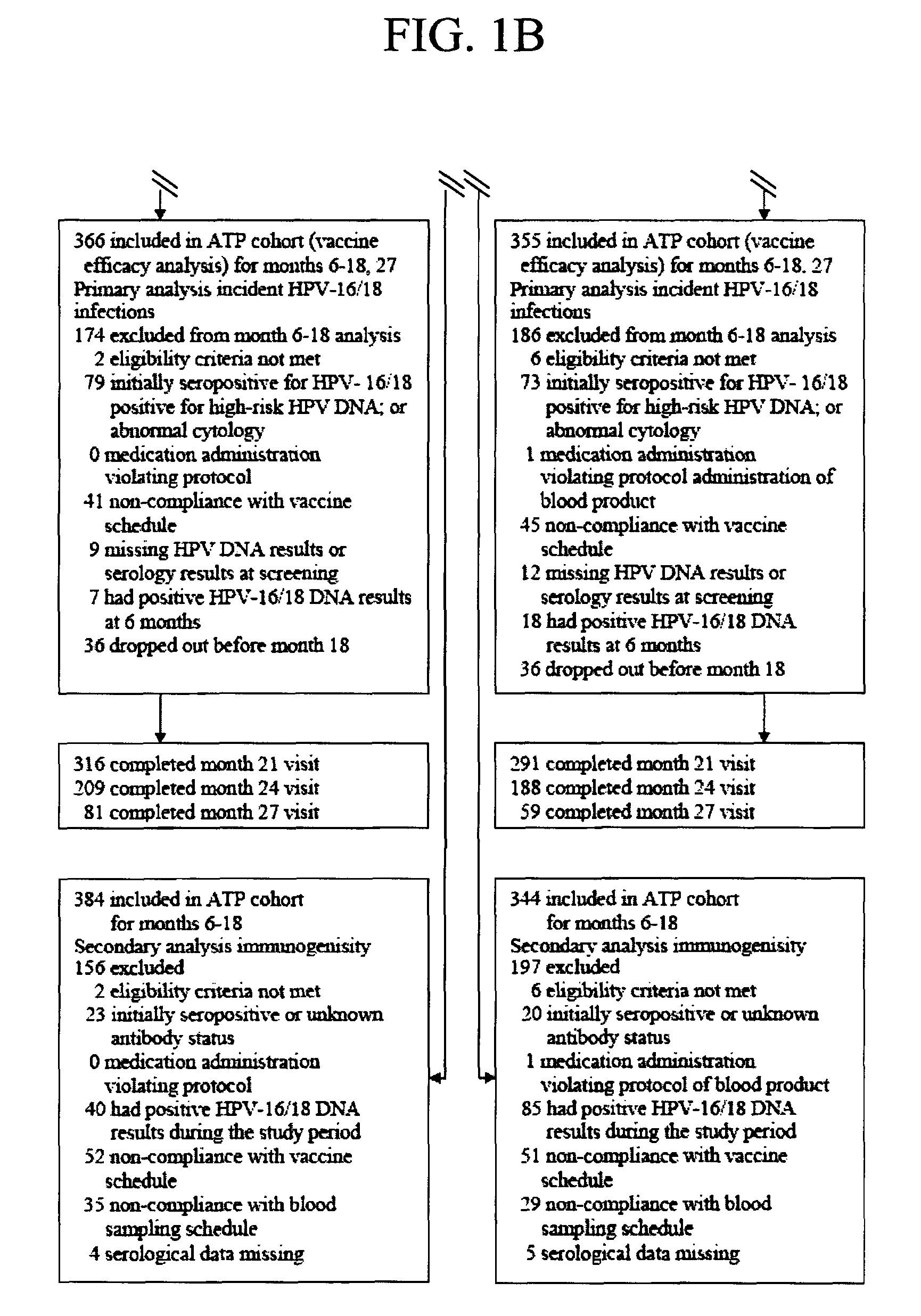
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
93 results about "Hpv types" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
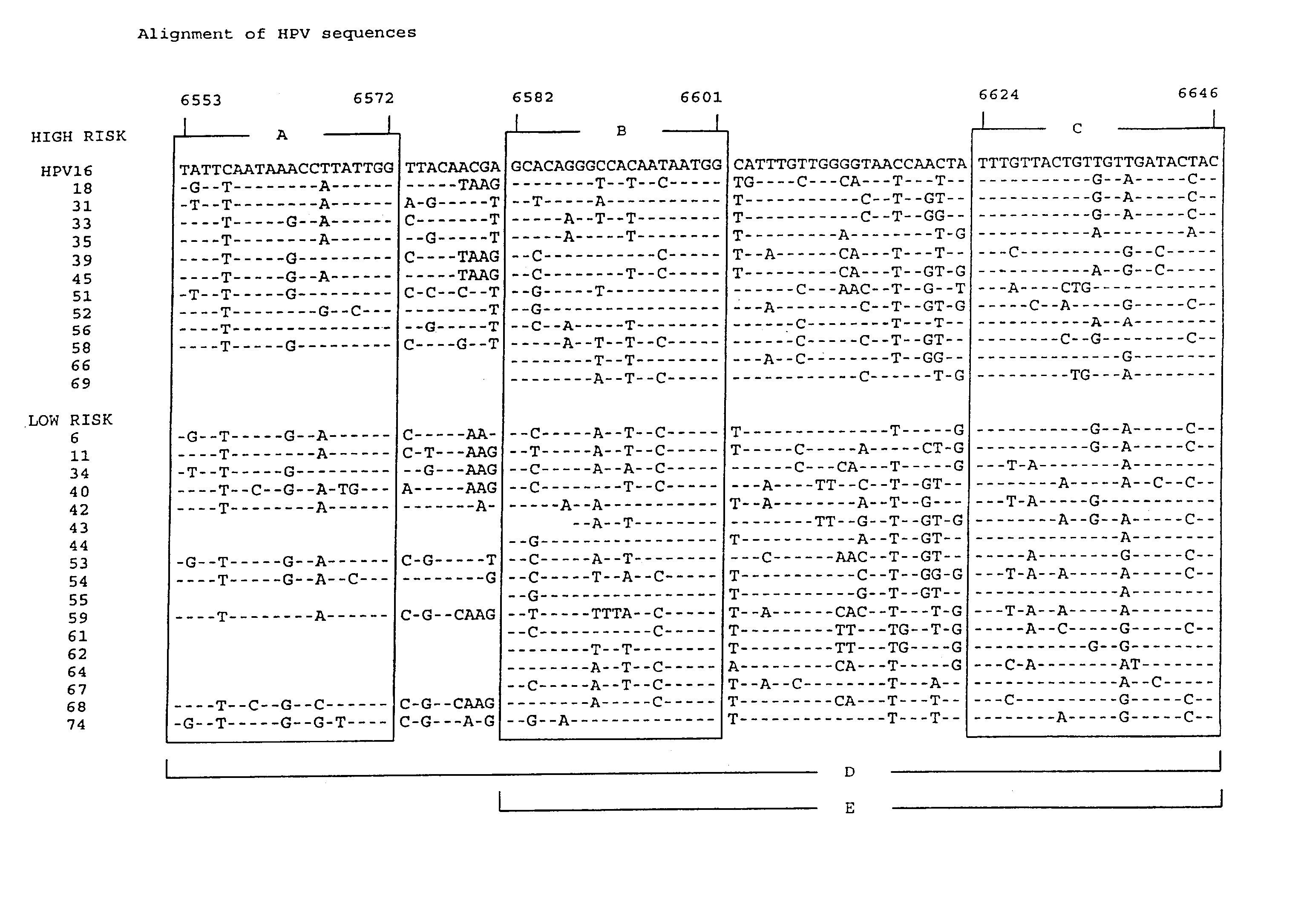
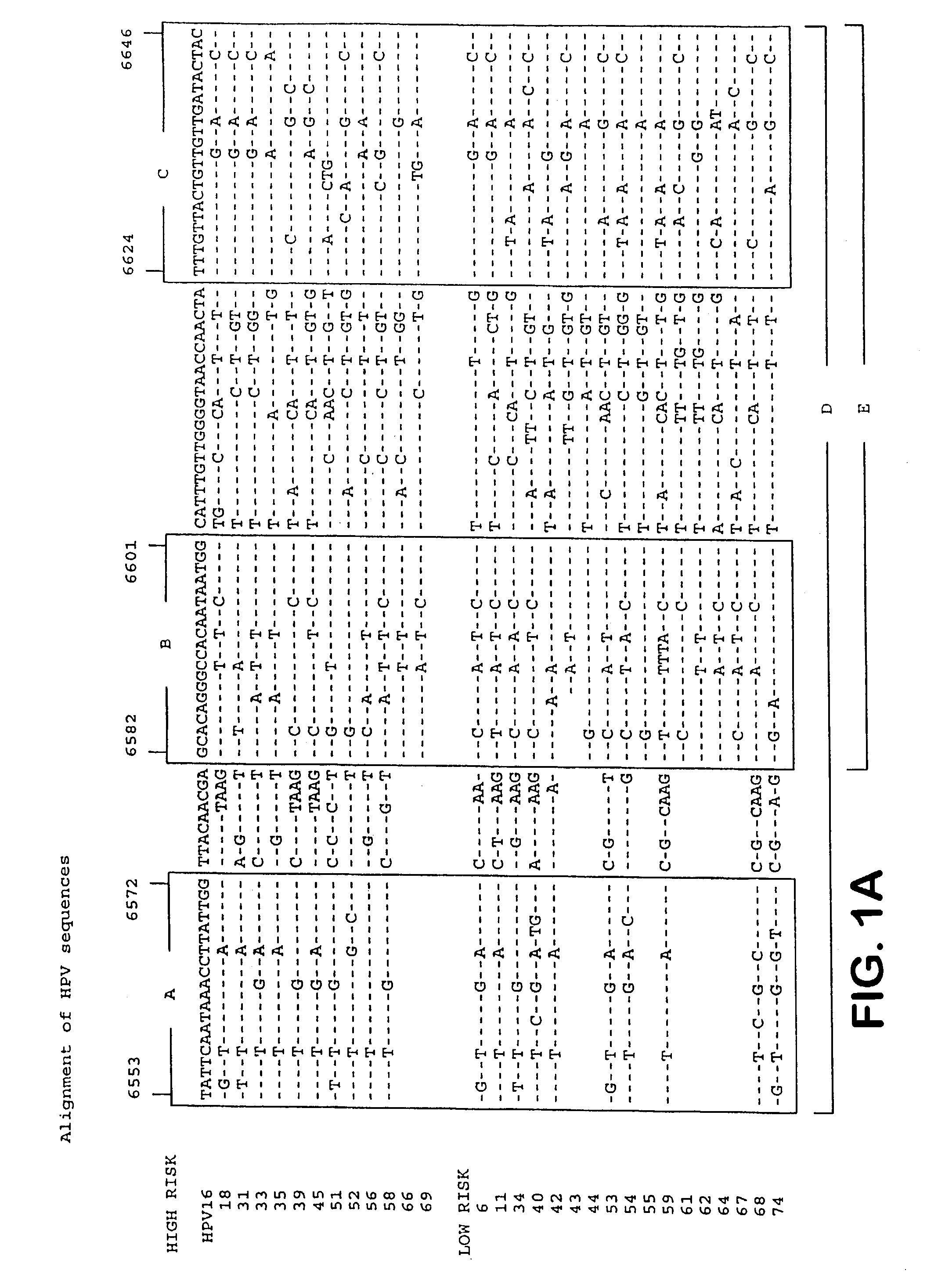
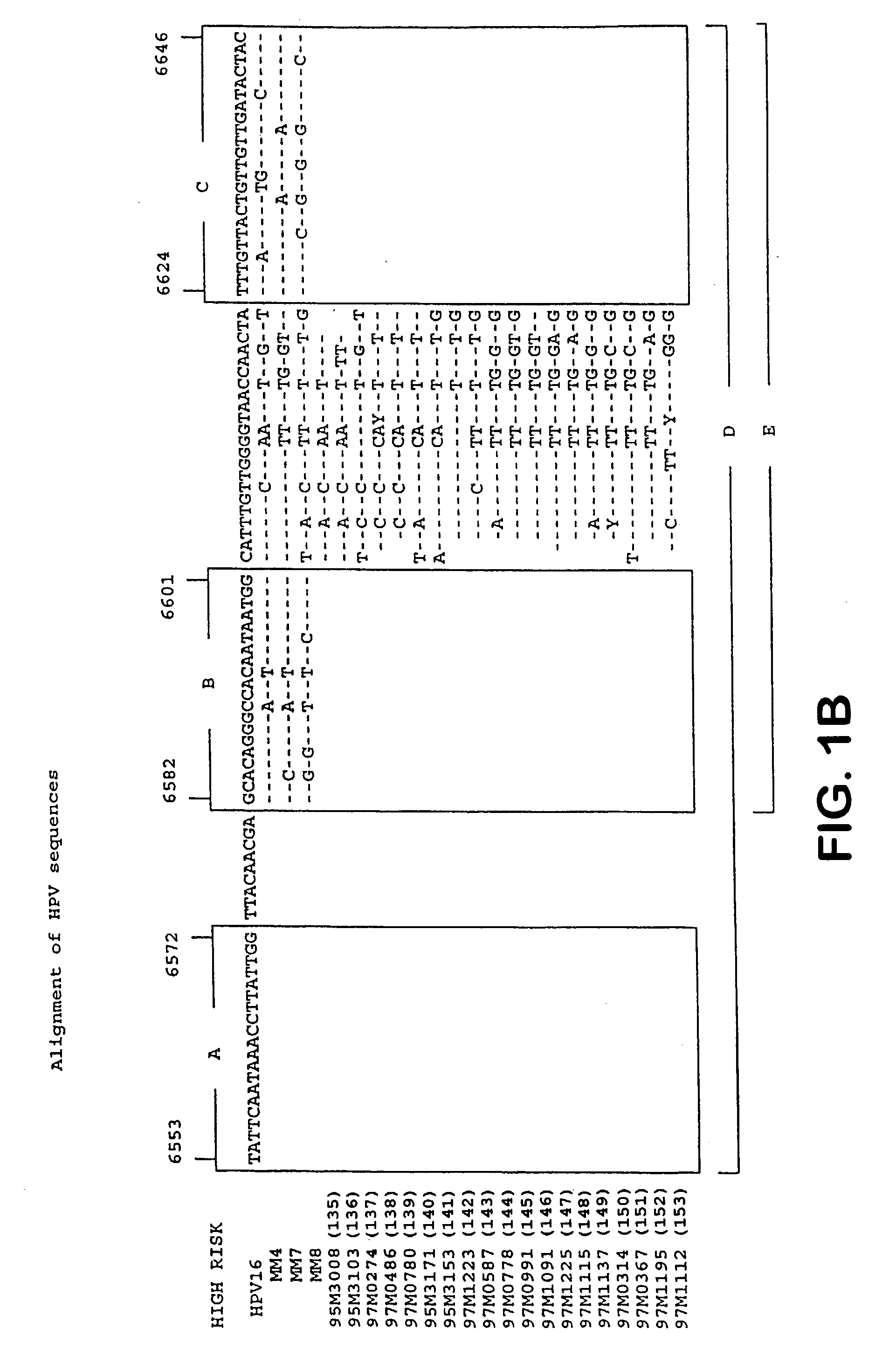
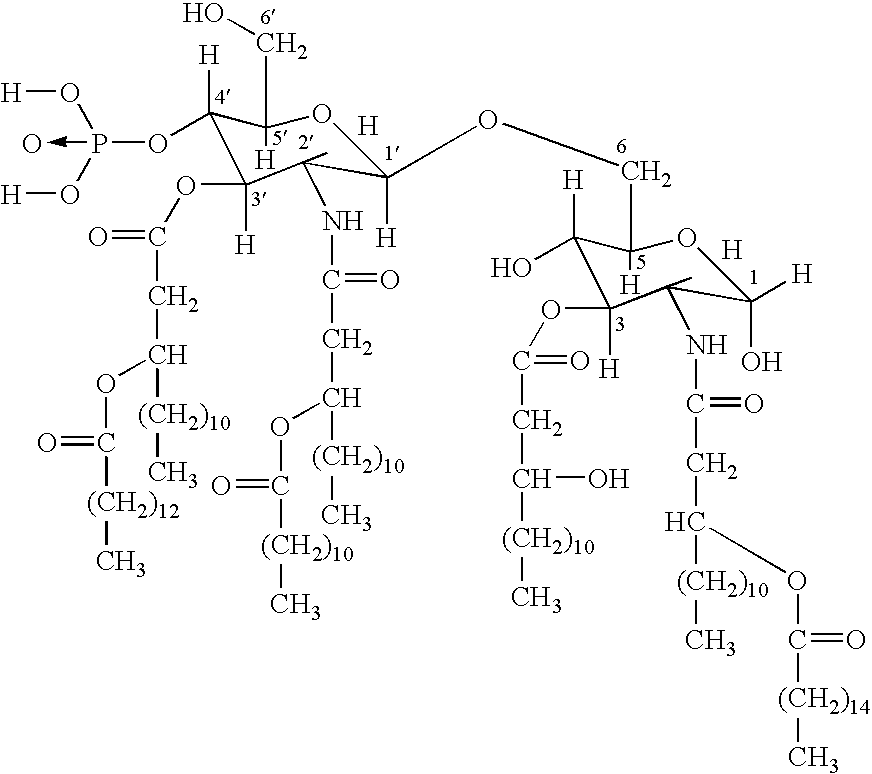
HPV is short for human papilloma (pap-uh-LO-muh) virus. HPVs are a large group of related viruses. Each virus in the group is given a number, which is called an HPV type.
Detection and identification of human papillomavirus by PCR and type-specific reverse hybridization
InactiveUS20030165821A1Rapid and reliable for detectionMicrobiological testing/measurementFermentationHuman papillomavirusType specific
A method for detection and / or identification of HPV present in a biological sample comprising amplification of HPV polynucleic acids and of hybridization of said amplified polynucleic acids to a number of probes whereby a short fragment of the L1 gene of HPV is amplified after which, the amplimers are contacted with probes that specifically hybridize to the said short fragment of the L1 gene of at least one HPV type and a diagnostic kit to perform said method and primers and probes used in the said method.
Owner:INNOGENETICS NV
Method and kit for quantitative and qualitative determination of human papillomavirus
ActiveUS20070037137A1Minimize the numberRoutinely usedSugar derivativesMicrobiological testing/measurementHuman papillomavirusBiology
The present invention relates to a method and kit for quantitative and qualitative determination of human papillomavirus, HPV, in a sample. More precisely, for quantitative and qualitative determination of oncogenic HPV to predict the risk of HPV infection resulting in cervical carcinoma. The method and kit enable simultaneous measurement of several oncogenic HPV types.
Owner:CEPHEID INC
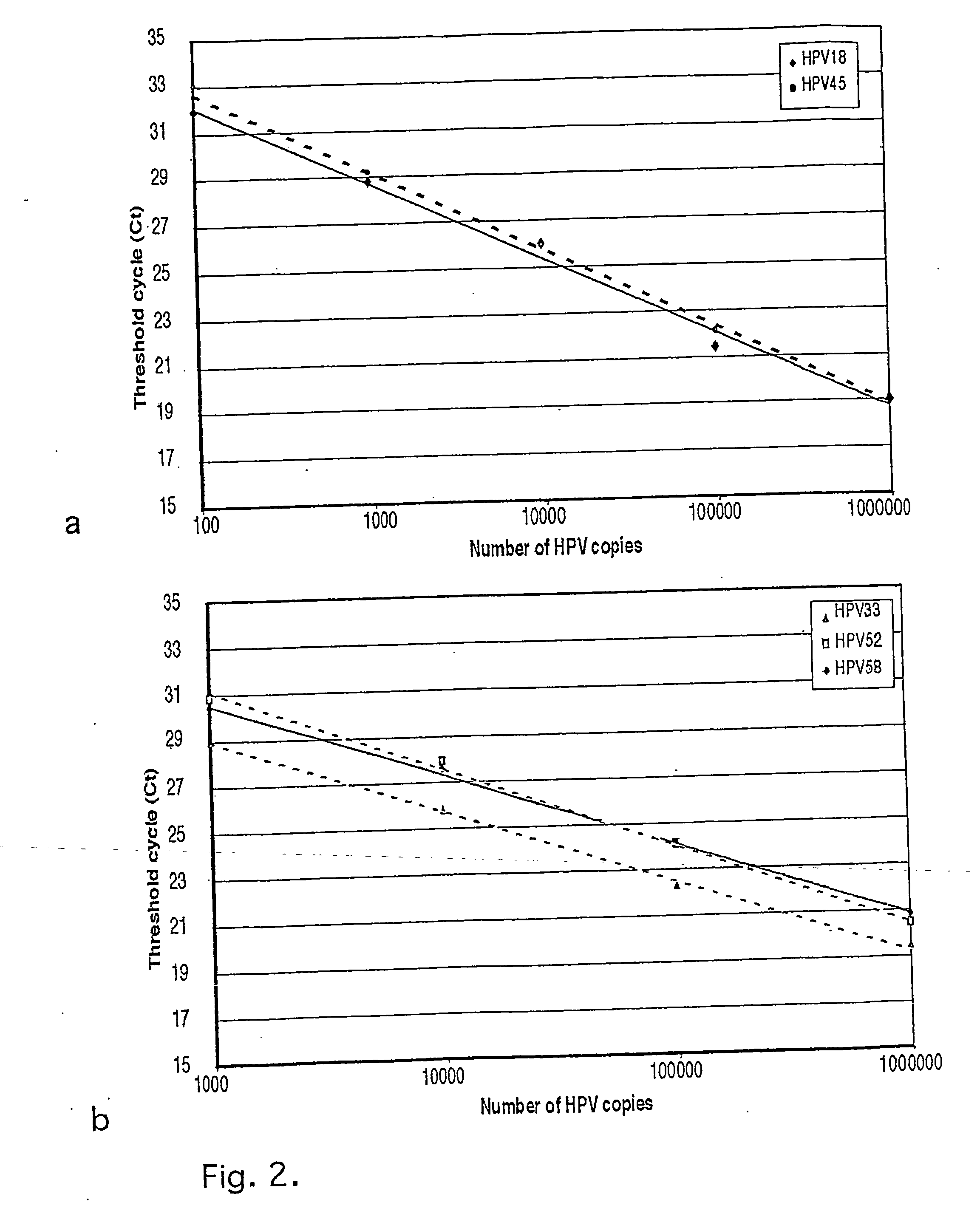
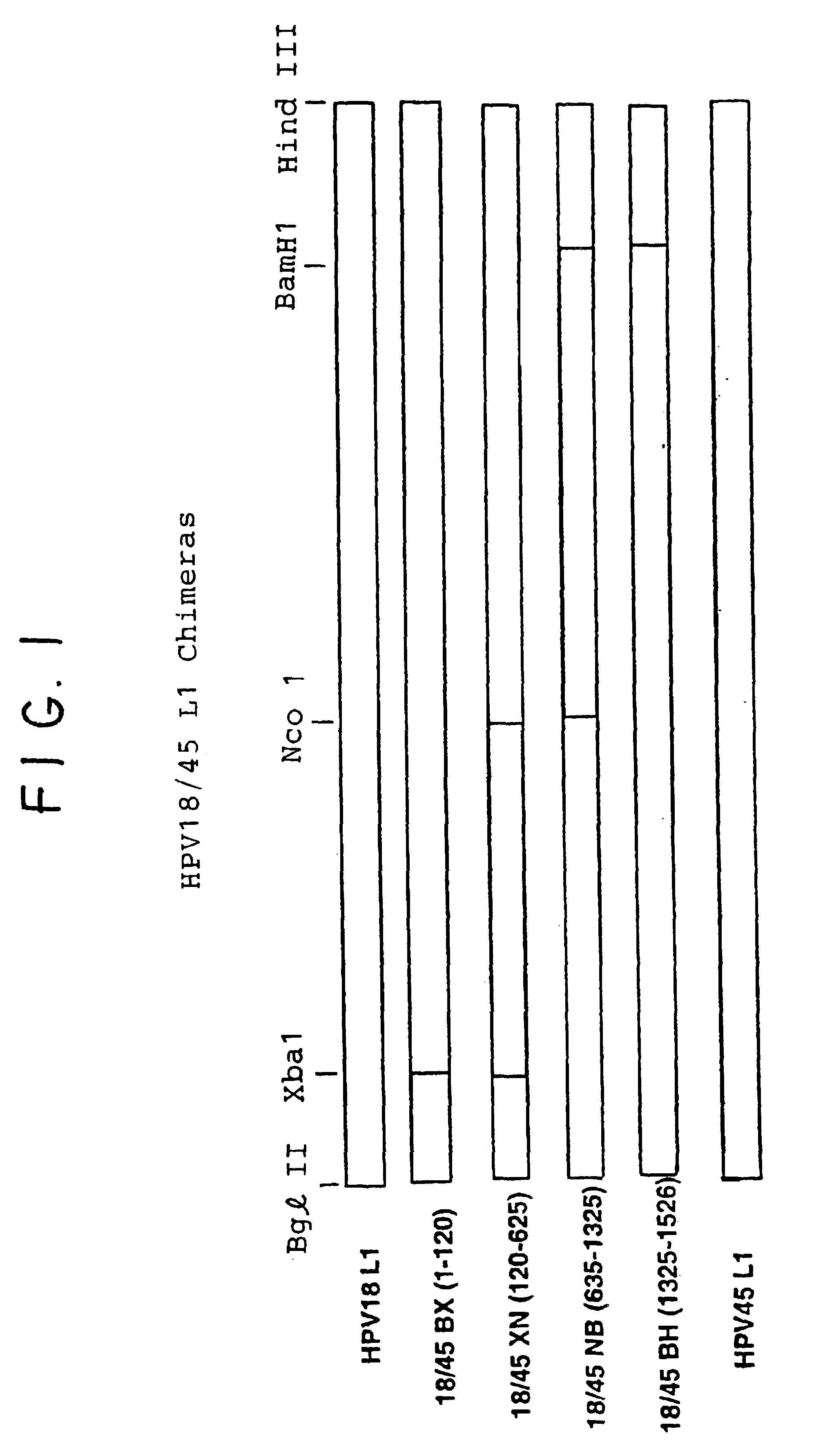
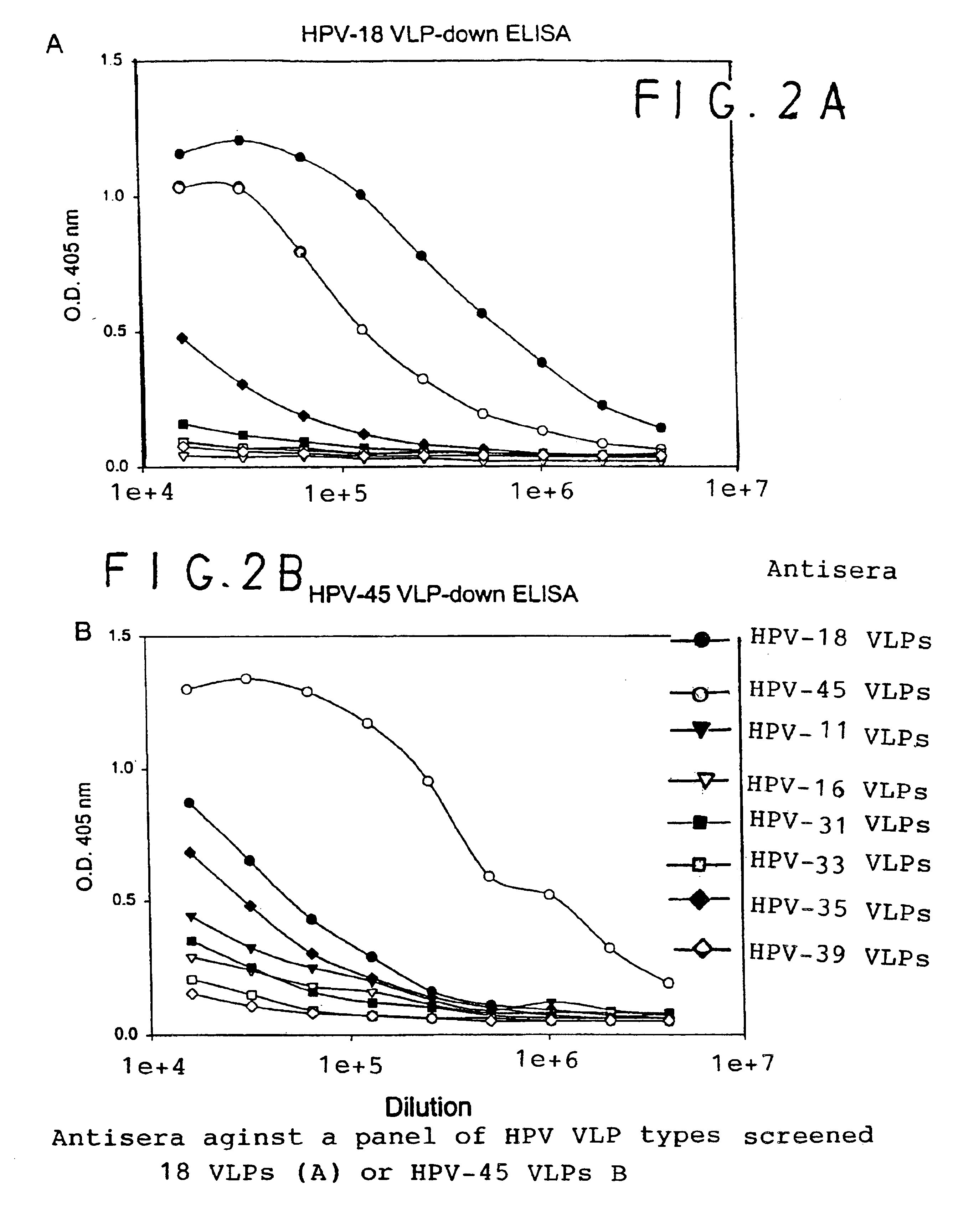
Chimeric human papillomavirus (HPV) L1 molecules and uses therefor
InactiveUS6908613B2Need can be quite largeLevel of protectionPeptide/protein ingredientsAntibody mimetics/scaffoldsHuman papillomavirusVirus-like particle
Owner:MEDIMMUNE LLC
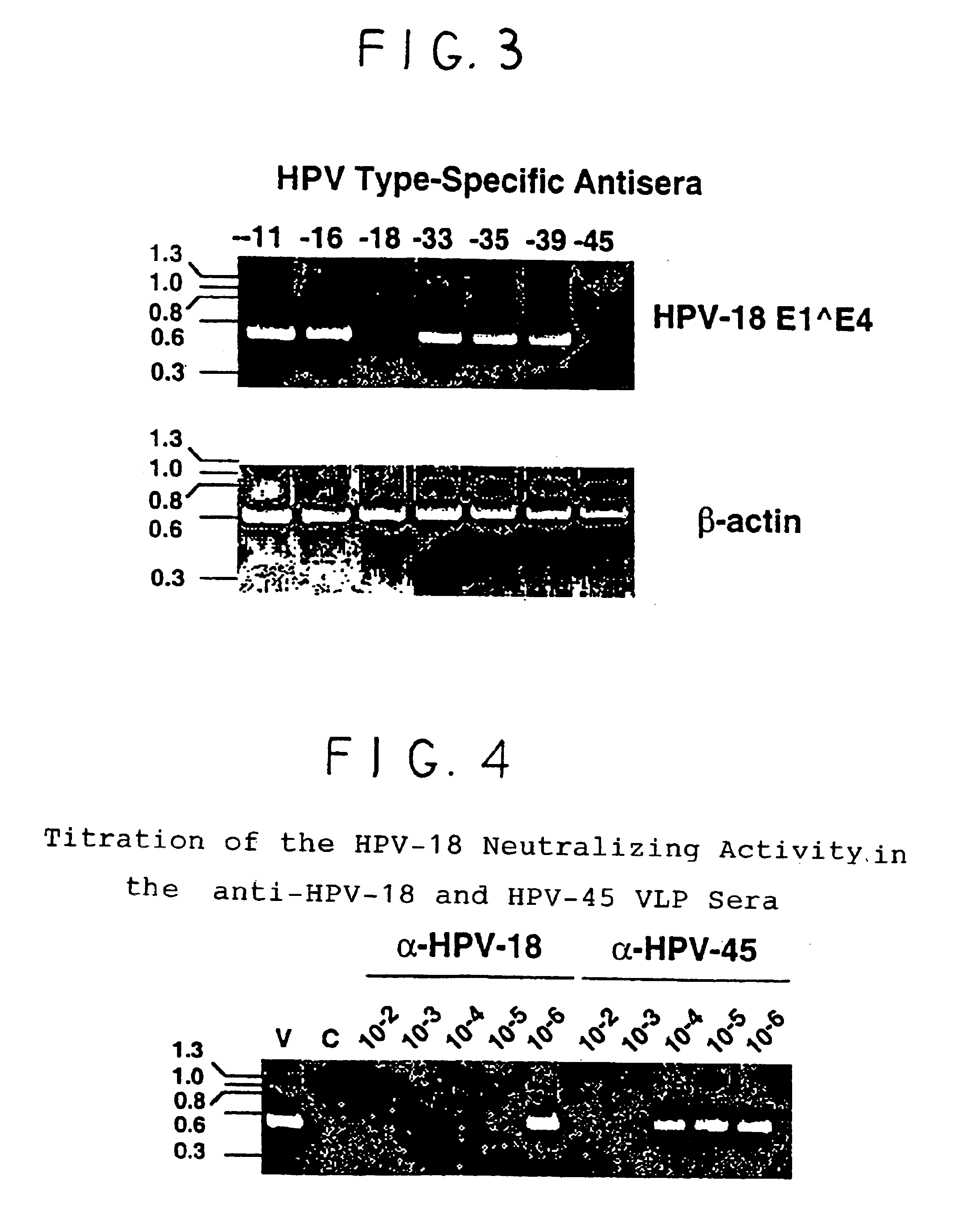
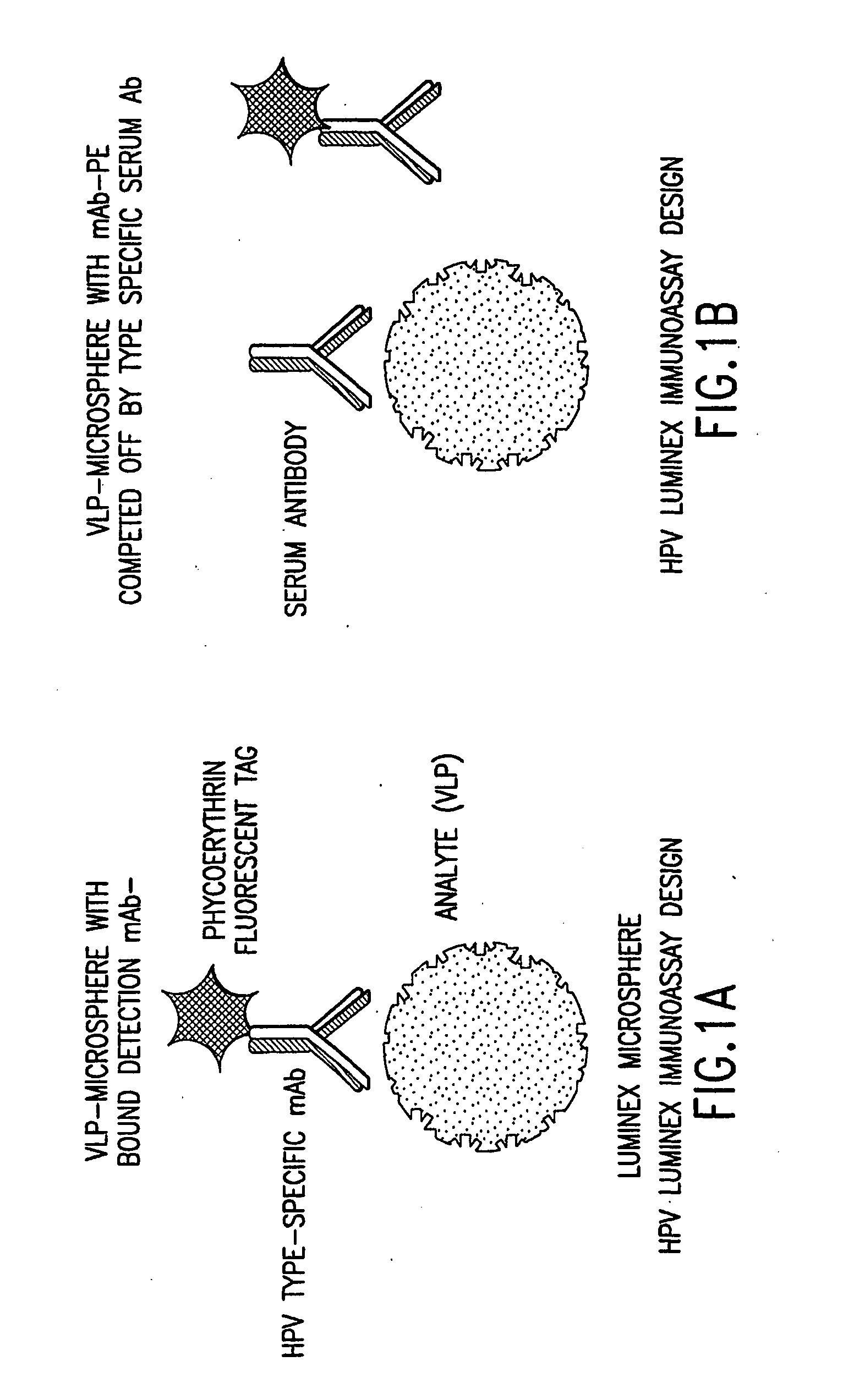
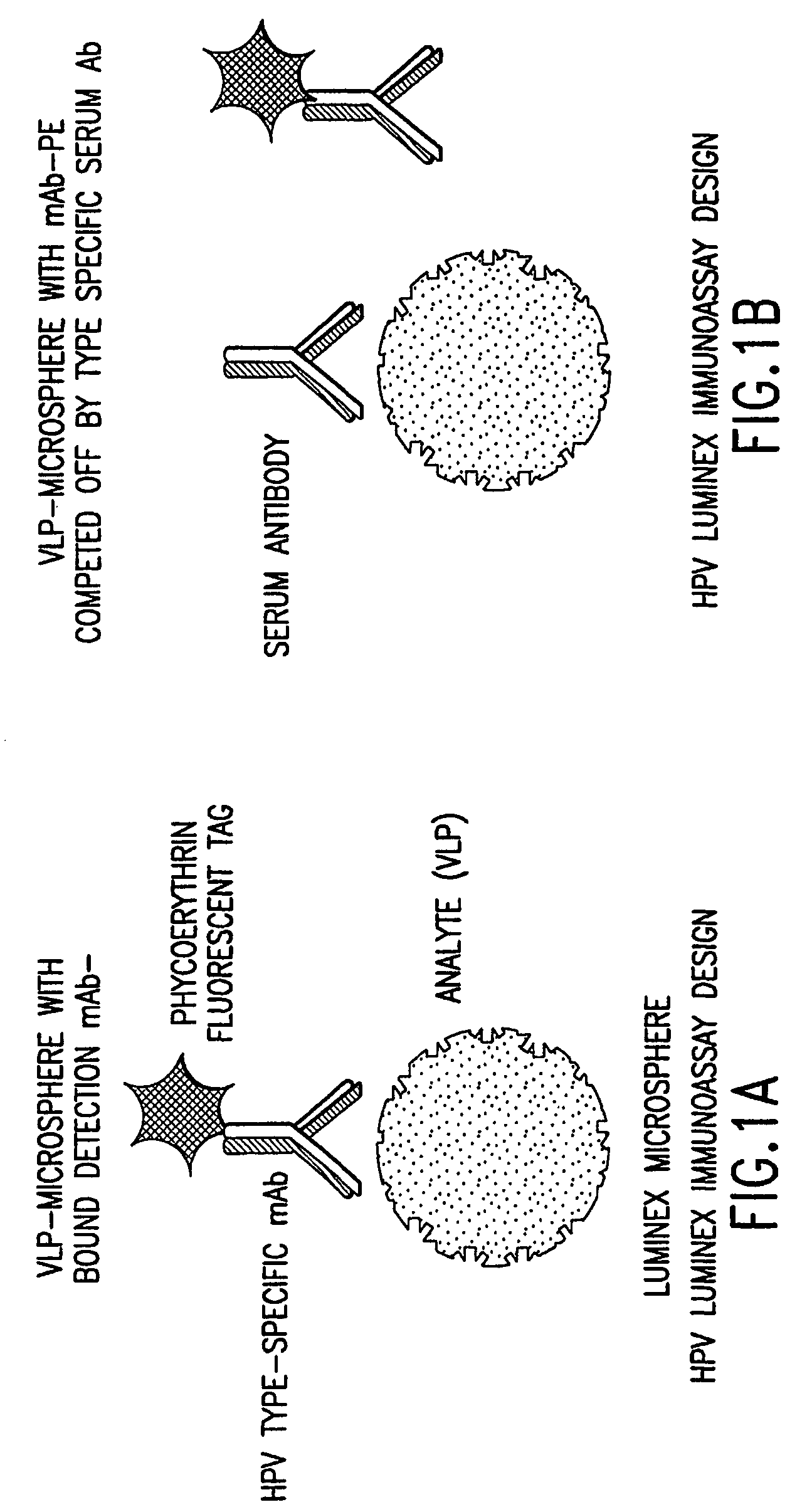
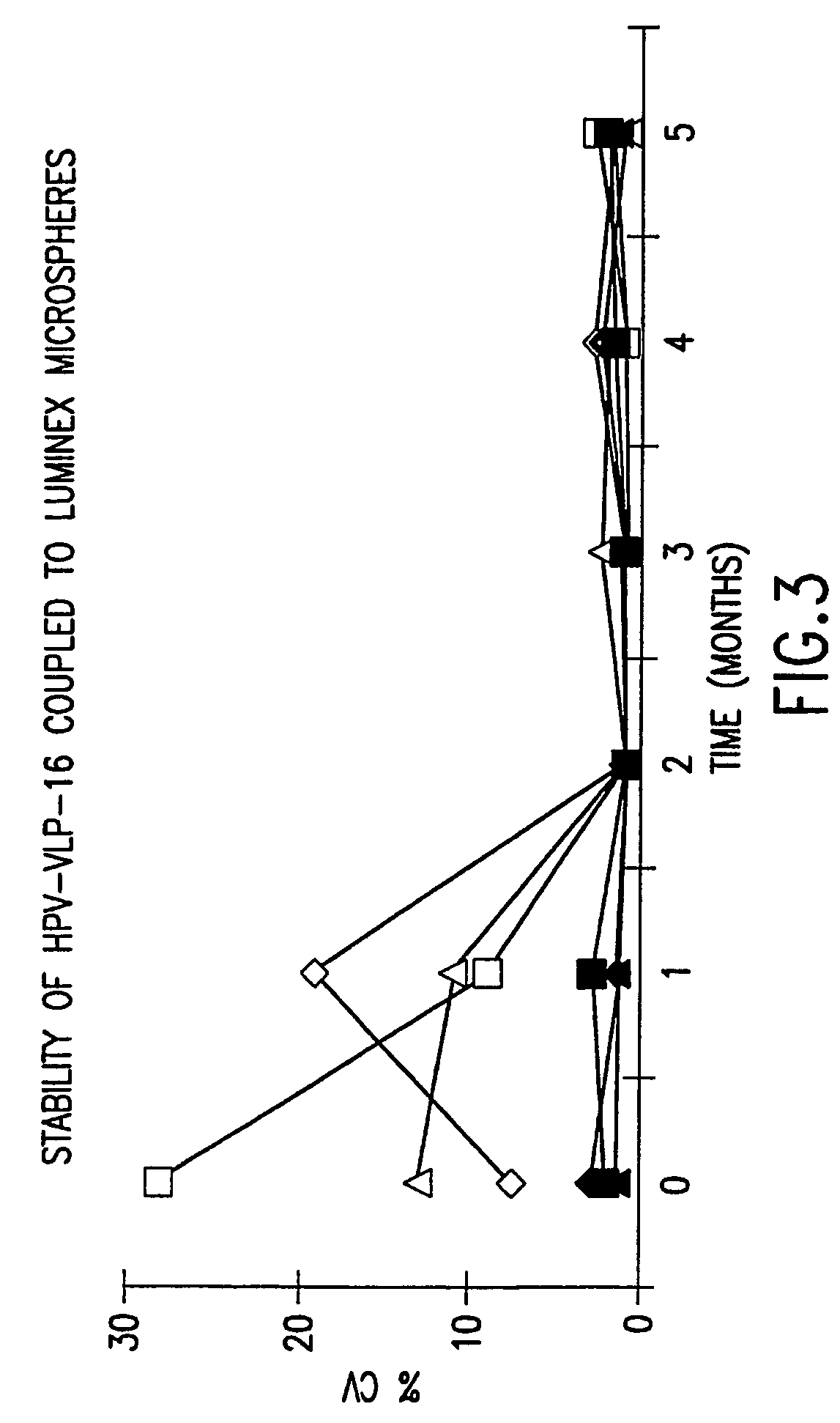
Human papillomavirus multiplexed assay
InactiveUS20050147961A1Inducing high titres of neutralizing antibodiesAnimal cellsHybrid immunoglobulinsMicrosphereFluorescence
The present invention relates to an immunoassay for simultaneously measuring the presence of antibodies to a plurlity of HPV types that utilizes particle-based flow cytometric analysis. The presence and / or titre of neutralizing antibodies in a test sample are determined in a competitive format, where known, type-specific, fluorescently labeled neutralizing monoclonal antibodies compete with antibodies within a test sample for binding to conformationally sensitive, neutralizing epitopes on specific HPV-VLPs. The invention also provides a microsphere complex comprising a microsphere coupled to an HPV VLP.
Owner:MERCK SHARP & DOHME CORP
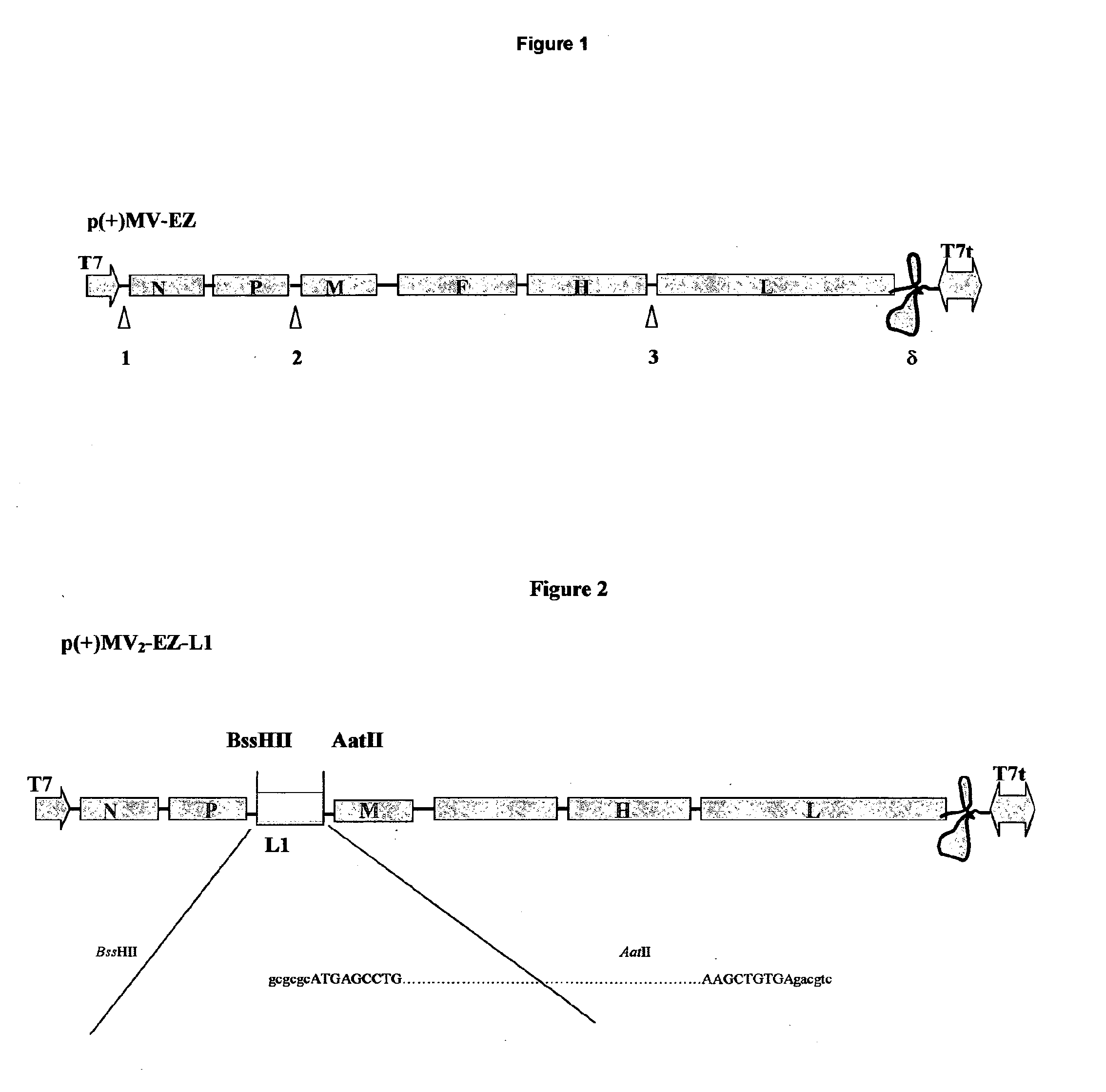
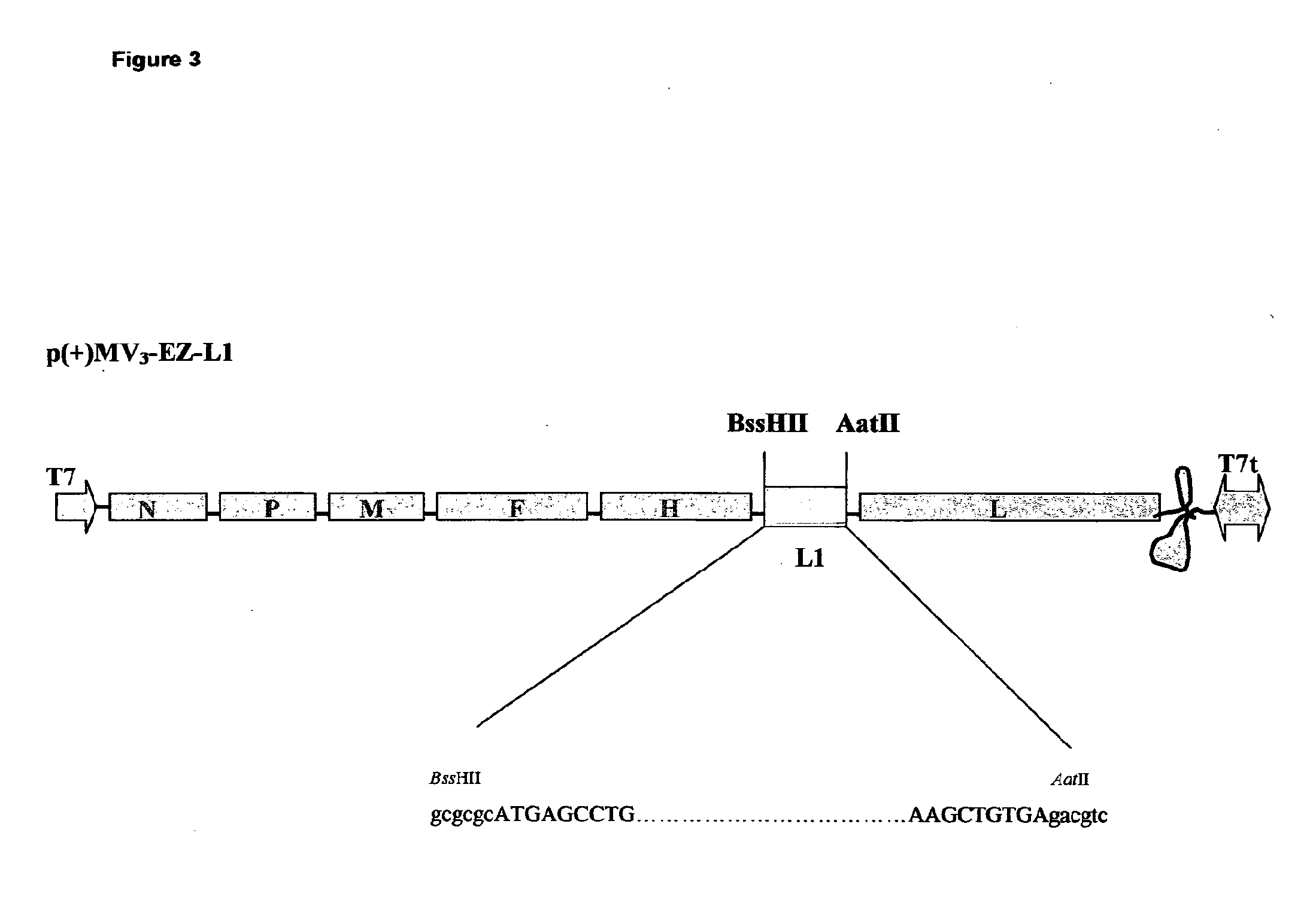
Combined measles-human papilloma vacine
The present invention relates to combined vaccines against measles and human papilloma virus (HPV). In particular, the invention relates to recombinant measles virus vectors containing heterologous nucleic acid encoding single or several antigens derived from HPV, preferably, the major capside antigen L1, the minor capside antigen L2, the early gene E6 and the early gene E7 oncoproteins of HPV type 16, and optionally of types 18, 6 and 11. In a first embodiment, prophylactic vaccines are generated expressing HPV antigens, preferably L1 and / or L2 such that they induce a potent long-lasting immune response in mammals, preferably humans, to protect against HPV and MV infection. In another embodiment, therapeutic vaccines are generated expressing E6 and E7 proteins, and optionally L1 and L2, such that they induced strong immune responses will resolve persistent HPV infections at early or late stages, including HPV-induced cervical carcinoma. In a preferred embodiment, the combined vaccines are easy to produce on a large scale and can be distributed at low cost.
Owner:CADILA HEALTHCARE LTD
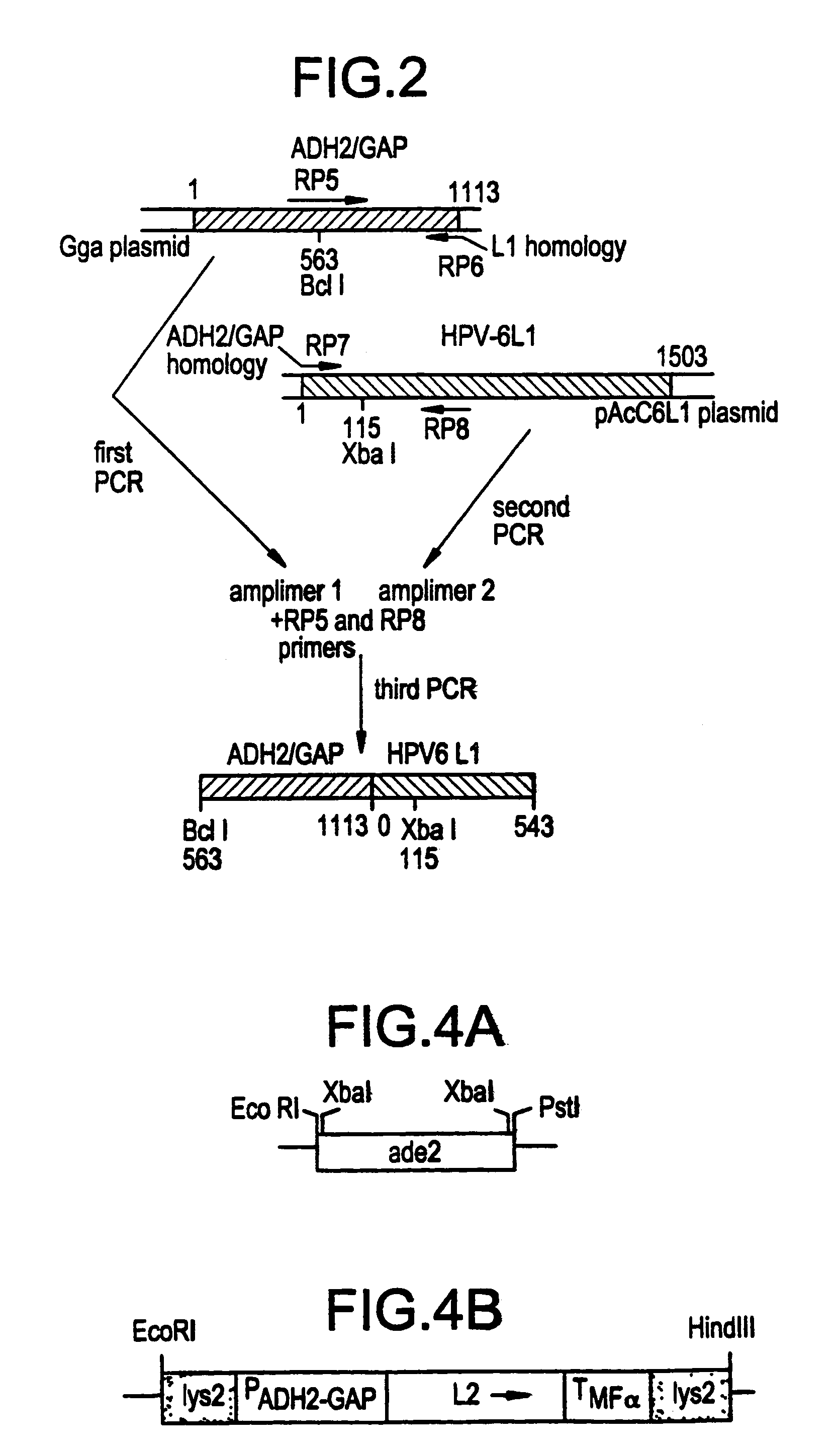
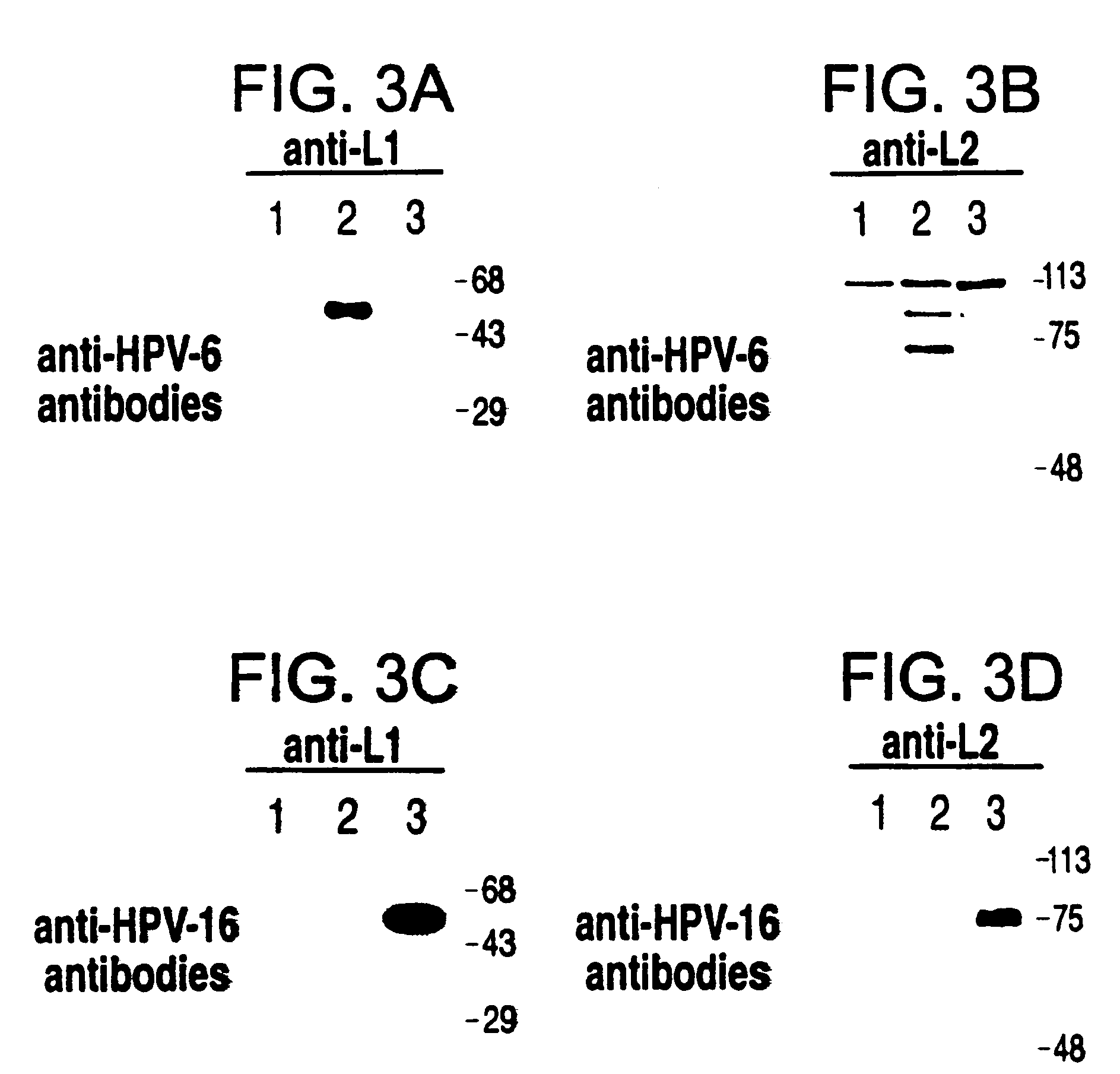
Method for producing yeast expressed HPV types 6 and 16 capsid proteins
Mosaic VLPs of viral capsid proteins from different virus types are described, as are methods of making the same. Specifically, a diploid yeast strain that coexpresses the L1 and L2 capsid proteins of both HPV-6 and HPV-16 as mosaic VLPs is described. The mosaic VLPs induced the production conformational antibodies against both L1 proteins upon administration to mice.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Human papillomavirus multiplexed assay
InactiveUS7067258B2Inducing high titres of neutralizing antibodiesAnimal cellsMicrobiological testing/measurementHuman papillomavirusType specific
The present invention relates to an immunoassay for simultaneously measuring the presence of antibodies to a plurality of HPV types that utilizes particle-based flow cytometric analysis. The presence and / or titre of neutralizing antibodies in a test sample are determined in a competitive format, where known, type-specific, fluorescently labeled neutralizing monoclonal antibodies compete with antibodies within a test sample for binding to conformationally sensitive, neutralizing epitopes on specific HPV-VLPs. The invention also provides a microsphere complex comprising a microsphere coupled to an HPV VLP.
Owner:MERCK SHARP & DOHME CORP
HPV CD8+ T-cell epitopes
InactiveUS7153659B2Improving immunogenicitySugar derivativesPeptide/protein ingredientsEpitopeHPV vaccines
The present invention provides means to identify functional CD8+ T-cell epitopes in any protein of interest. The present invention further provides CD8+ T-cell epitopes of various proteins. In additional embodiments, the present invention provides epitopes suitable for use in prophylactic and / or therapeutic vaccines. In particularly preferred embodiments, the present invention provides modified epitopes suitable for use in prophylactic and / or therapeutic vaccines.In some preferred embodiments, the present invention provides means for the development of HPV vaccines, in particular multivalent vaccines for the prevention of infection with high-risk HPV strains. In particular, the present invention provides means to identify CD8+ T-cell epitopes in HPV strains such as HPV 16 and HPV 18. In additional embodiments, the present invention provides means for the development of therapeutic vaccines against high-risk HPV types that prevent the development of benign and / or malignant tumors in infected individuals. The present invention further provides epitopes suitable for use in prophylactic and therapeutic vaccines.
Owner:GENENCOR INT INC
Human papilloma virus constructs
Embodiments of the present invention provide compositions and methods for recombinantly generating tagless constructs of proteins or peptides. In certain embodiments, recombinant proteins or peptides disclosed herein concern human papilloma virus (HPV). Other embodiments concern using these constructs in compositions to elicit immune responses in a subject to one or more HPV types. Therapeutic and prophylactic vaccines for the prevention and treatment of viral infections are also disclosed. Nucleic acids and expression vectors coding for constructs contemplated herein are provided. In certain embodiments, an HPV capsid protein generated is devoid of any fusion tags. In addition, truncated forms of HPV L1 are contemplated.
Owner:UNIV OF COLORADO THE REGENTS OF
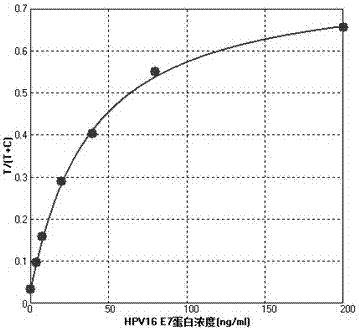
HPV (human papilloma virus) 16/18 detection kit based on digital PCR (polymerase chain reaction) accurate quantitative typing and detection method thereof
ActiveCN105018647ARealize absolute precise quantitative analysisImprove accuracyMicrobiological testing/measurementMicroorganism based processesQuality controlA-DNA
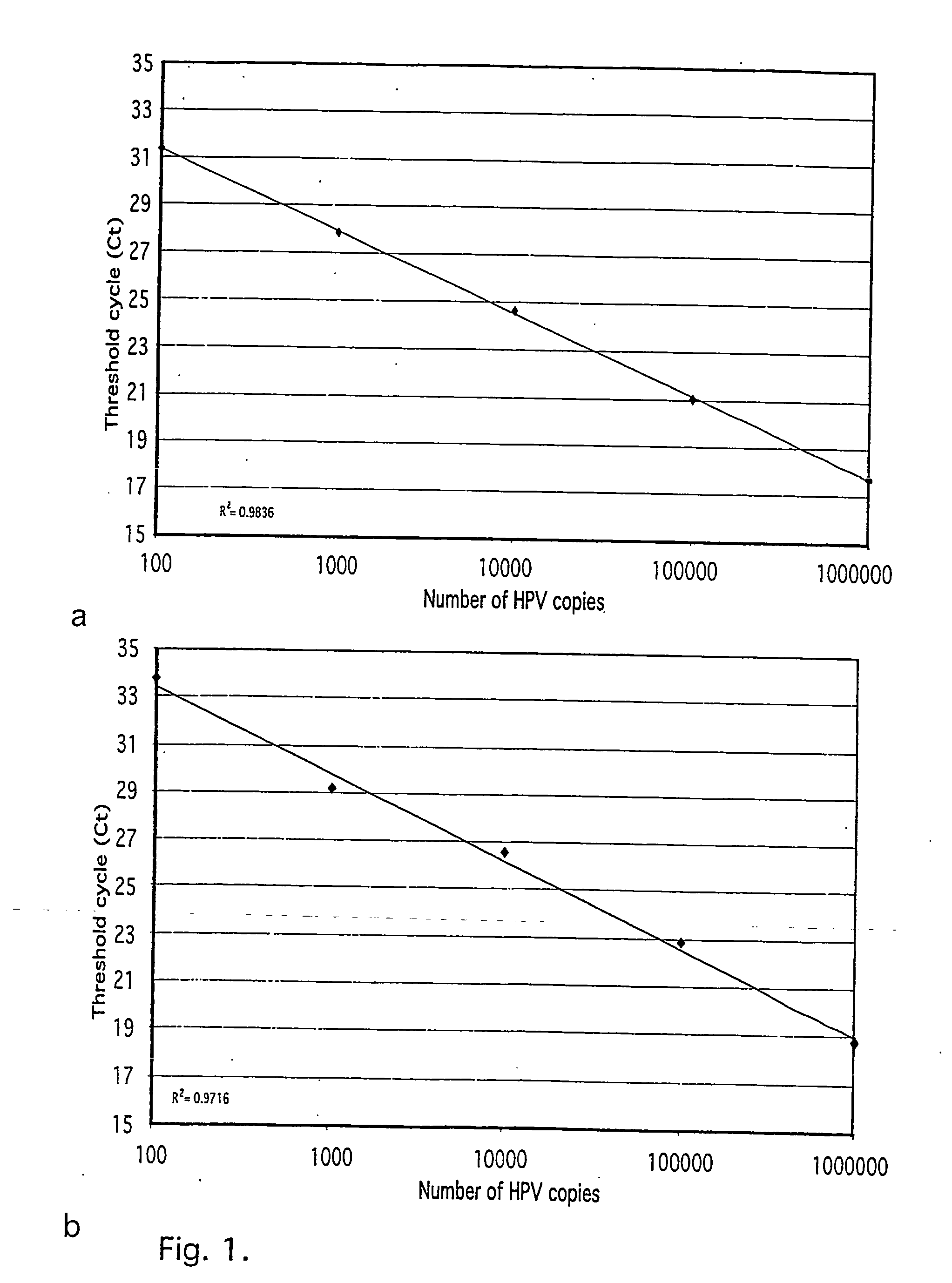
The invention discloses an HPV (human papilloma virus) 16 / 18 detection kit based on digital PCR (polymerase chain reaction) accurate quantitative typing and a detection method thereof. The kit comprises a DNA (deoxyribonucleic acid) extracting solution, a digital PCR reaction buffer solution A, a digital PCR reaction buffer solution B, an HPV 16 virus gene positive quality control, an HPV 18 virus gene positive quality control, a negative quality control and an internal standard solution. The method comprises the following steps: 1) treating a detected sample; 2) treating a quality control product; 3) preparing a digital PCR reaction mixed solution; 4) generating micro-reaction solution drops, and carrying out digital PCR reaction amplification; and 5) reading a fluorescent signal, analyzing the HPV type and calculating the number of copies. The method is independent of the external standard substance and standard curve, is simple to operate, and can directly perform accurate absolute quantitative simultaneous typing on HPV. The method has high sensitivity, and can reduce the interference of the background DNA and matrix and quantify trace HPV; and the detection limit is down to 1 copy.
Owner:ZYBIO INC
Vaccine for Cervical Cancer
ActiveUS20120100169A1Overcome problemsEasy to solveFungiSugar derivativesHuman papillomavirusVirus-like particle
The present invention relates to a pharmaceutical vaccine composition for a human cervical cancer, comprising: (a) (i) a L1 virus-like particle (VLP) of human papillomavirus (HPV) type 16, a L1 VLP of HPV type 18, or a combination thereof; and (ii) a deacylated non-toxic lipooligosaccharide (LOS); and (b) a pharmaceutically acceptable carrier; and a method for preparing a human papillomavirus (HPV) L1 virus-like particle (VLP). The pharmaceutical vaccine composition of the present invention is in both Th1-type immune response (cellular immunity) and Th2-type immune response (humoral immunity) against HPV more excellent than Cervrix™ and Gardasil™, exhibiting a superior efficacy as a vaccine for a human cervical cancer.
Owner:EYEGENE INC
Composition for Preventing or Treating Cervical Cancer Having Human Papillomavirus Plasmodium and Immunity Enhancer
ActiveUS20130195905A1Improving immunogenicityAntibody mimetics/scaffoldsMicroorganismsSequence signalAntigen
A composition for preventing or treating cervical cancer comprising a human papillomavirus plasmodium and an immunity enhancer is provided. A fusion protein including a fusion polypeptide recombined to transform a 3D structure of E6 and E7, which are antigens against types 16 and 18 human papillomavirus (HPV), a signal peptide for secreting the fusion polypeptide outside the cells and an immunity enhancer peptide present in an individual is also provided. The fusion protein may be useful in treating HPV-triggered tumors by inducing an immune response specific to the antigens against the HPV types 16 and 18.
Owner:GENEXINE INC
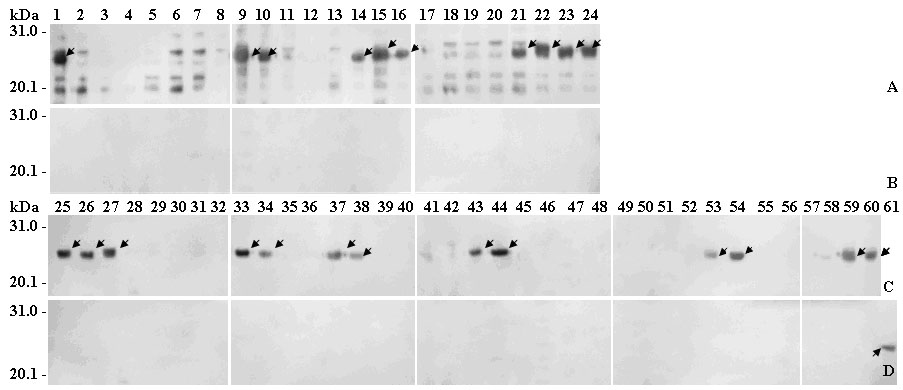
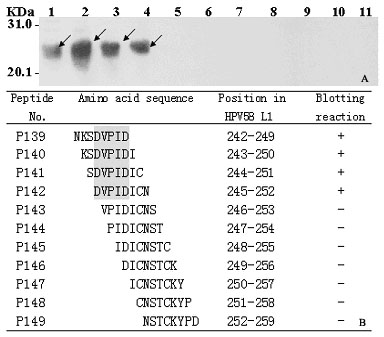
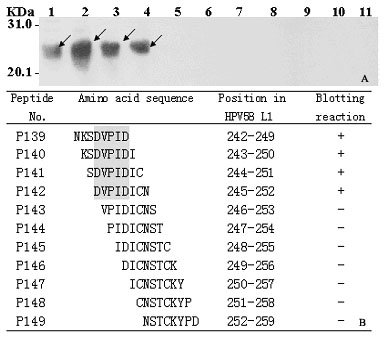
Linear epitope minimum motif peptide of human papilloma virus type 58 L1 protein and application thereof
The invention belongs to the technical field of biomedicine and bioinstrumentation, in particular to an epitope (Bcellepitope) minimum motif peptide of human papilloma virus (HPV) type 58 L1 structural protein and application thereof. The invention provides 16 amino acid sequences containing minimum motif peptide (including 8 peptide sequence capable of cutting off expression of carrier protein such as GST188), and the amino acid sequences can be taken as antigen for independently or combinedly specific detection of blood serum of HPV type 58 virus infection patient and can be used for developing preventive HPV type 58 multi-epitope peptide vaccine capable of simultaneously inducing humoral immunity. The amino acid sequences of HPV type 58 L1 epitope minimum motif peptide and short peptide are shown as SEQIDNo.1-SEQIDNo.32.
Owner:广东南湾医药技术有限公司
Method and system for determining HPV integration site in genome from human cervical carcinoma sample
ActiveCN103320522AAccurately determineLow costBioreactor/fermenter combinationsBiological substance pretreatmentsDNA fragmentationGenomic DNA
The invention discloses a method and a system for determining HPV integration site in genome from a human cervical carcinoma sample. The method provided by the invention comprises the following steps: carrying out first sequencing on genomic DNA from a human cervical carcinoma sample so as to obtain a sequencing result; determining a DNA fragment containing both HPV sequence and human genomic sequence based on the sequencing result; determining an amplification primer pair based on the DNA fragment containing both HPV sequence and human genomic sequence; carrying out PCR amplification on the genomic DNA from the human cervical carcinoma sample by the utilization of the amplification primer pair so as to obtain a PCR product; and carrying out second sequencing on the PCR product so as to determine the HPV integration site in the genome from the human cervical carcinoma sample. The method provided by the invention is simple and easy to operate, requires low cost, and has high efficiency and good repeatability. By the utilization of the method, all HPV types can be detected at a time, and detailed sequence information and the integration site can be determined rapidly and accurately.
Owner:BGI BIOTECH WUHAN CO LTD
Method of simultaneous detection and typing of human papilloma viruses
Owner:PHYSICIAN REFERENCE LAB
Monoclonal antibody for anti-HPV (Human Papillomavirus) 16 type E7 protein, test paper and detection kit
InactiveCN107192830ARealize quantitative detectionAccurate judgmentDisease diagnosisBiological testingHuman papillomavirusBinding site
A monoclonal antibody against HPV16 type E7 protein, a detection test paper and a detection kit. The binding site of monoclonal antibody against HPV16 E7 protein is: amino acid 30-36 of HPV16 E7 protein, amino acid 36-43 of HPV16 E7 protein, amino acid 62-73 of HPV16 E7 protein or HPV16 Amino acids 73-77 of type E7 protein. The detection test paper includes a backing and a sample pad fixed on the backing, a binding pad, an NC film and an absorbent pad; the binding pad is attached and fixed under the sample pad, one end of the NC film is attached and fixed under the binding pad, and the other end Link to each other with water-absorbing pad; NC membrane is sprayed with detection antibody, and detection antibody comprises the monoclonal antibody of the anti-HPV16 type E7 protein of claim 1 and goat anti-mouse secondary antibody, the monoclonal antibody of anti-HPV16 type E7 protein is used as detection line, Goat anti-mouse secondary antibody is used as a quality control line; the binding pad is coated with fluorescent microsphere-labeled antibody. The application can detect HPV type E7 protein simply and quickly.
Owner:ZHUHAI MEIHUA MEDICAL TECH LTD
Kit for human papilloma virus typing detection
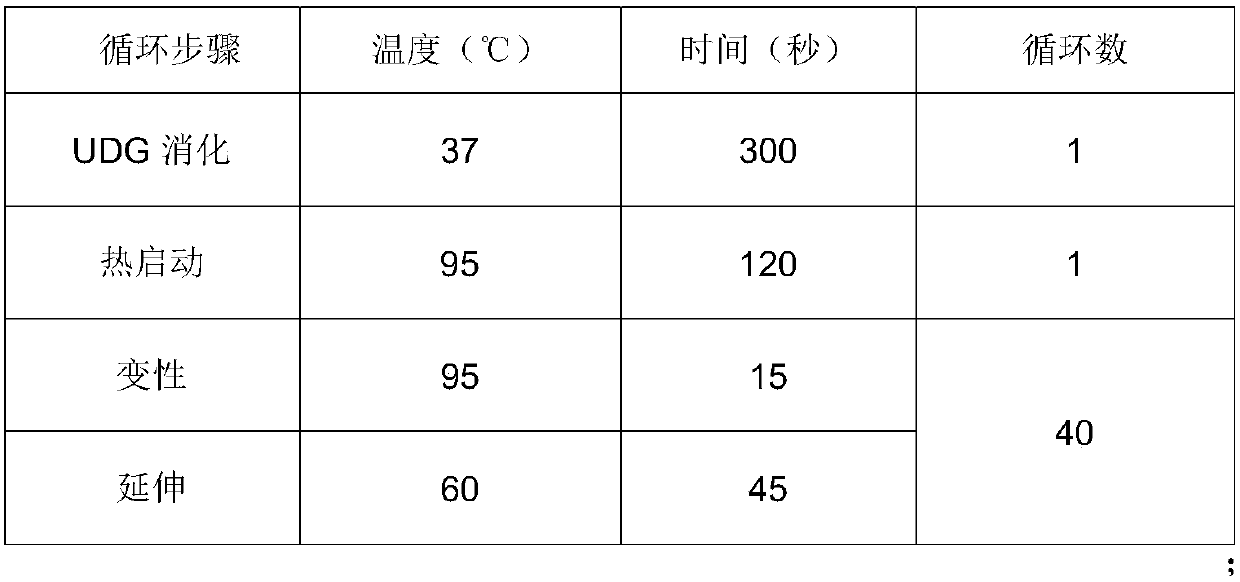
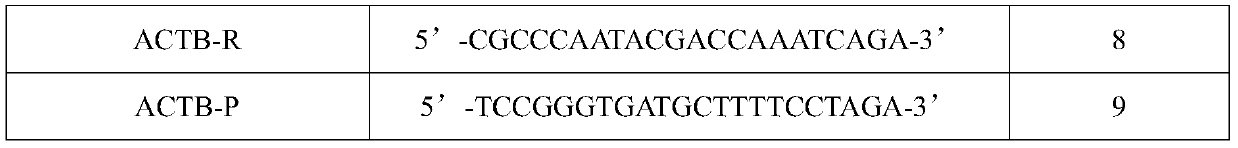
ActiveCN110628953AIncrease coverageHigh detection specificityMicrobiological testing/measurementMicroorganism based processesFluorescenceBiology
The invention belongs to the technical field of diagnostic reagents for human papilloma and particularly relates to a kit for human papilloma virus typing detection and a detection method. The kit comprises a primer probe premixed solution; the primer probe premixed solution comprises upstream and downstream primers for detecting HPV type 16, 18, 52 and 58, fluorescent probes for the HPV type 16,18, 52 and 58 respectively, upstream and downstream primers for detecting human actb genes and fluorescent probes for detecting the human actb genes. For the kit provided by the invention, by adding degenerate bases in a universal primer, the coverage degree of the HPV of different types is increased, and no missed detection is effectively achieved; by adding a design of locked nucleic acid in each fluorescent probe, the detection specificity is improved; on the premise that the reaction is guaranteed to be efficient and accurate, the pore inlet efficiency of a chip is greatly improved; and byusing hot started DNA polymerase and UDG enzyme, the reaction false positive is reduced.
Owner:宁波胤瑞生物医学仪器有限责任公司
In vitro diagnostic kit for identification of human papillomavirus in clinical samples
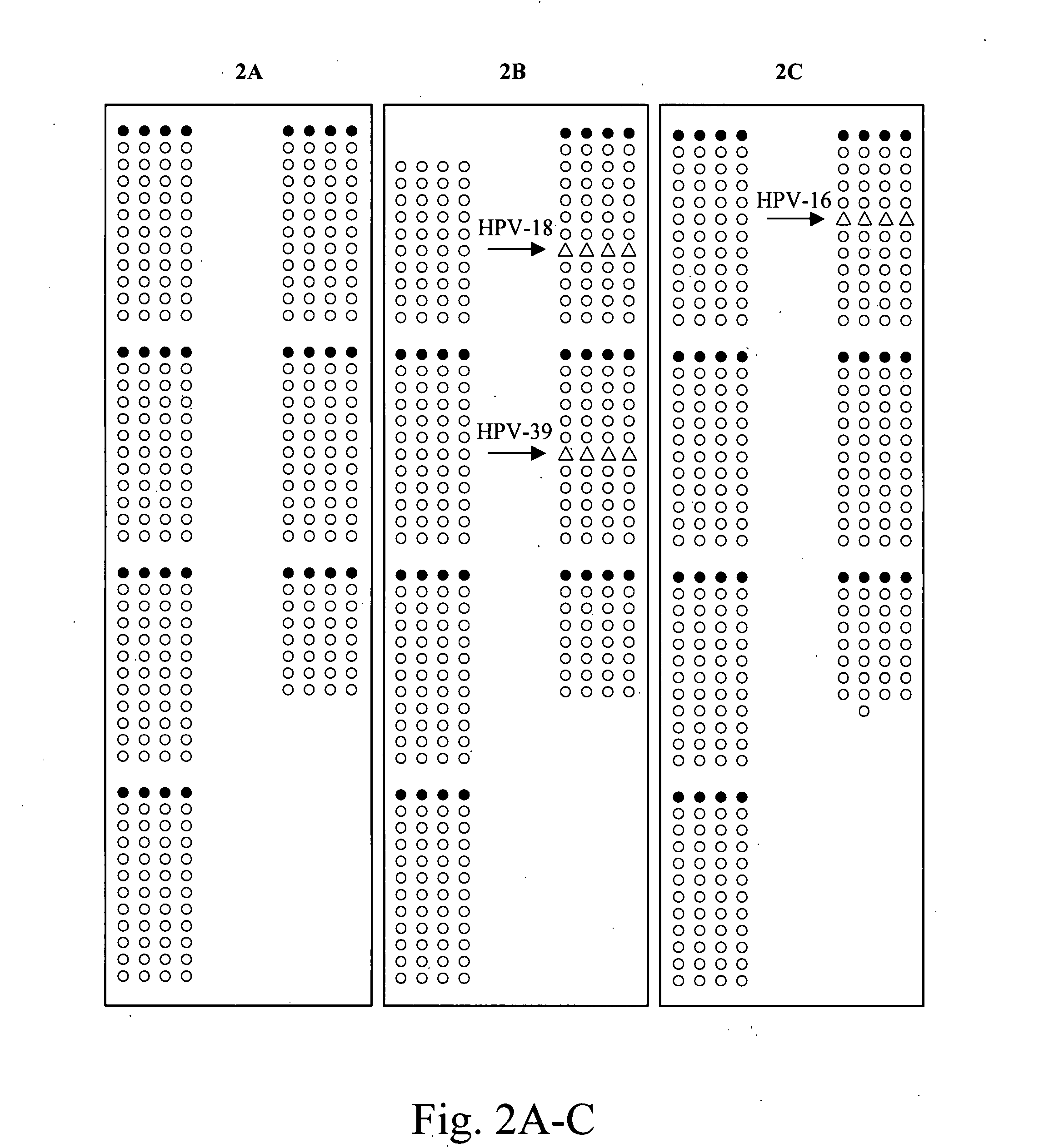
A method and kit for detection and typing of HPV in a sample are described, as is a reaction vessel for use in the method. Universal HPV primers are used to amplify a sample by PCR; the amplified sample is then hybridised to an array of HPV type-specific probes to determine the HPV type.
Owner:GENOMICA SAU
Nucleic acid composition for detecting HPV (Human Papilloma Virus) types as well as application and kit thereof
InactiveCN106755593AIncrease the number ofHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesEpidemiologyBiology
The invention discloses a nucleic acid composition for detecting HPV (Human Papilloma Virus) types as well as application and a kit thereof and belongs to the technical field of gene detection. The nucleic acid composition for detecting HPV types comprises a first nucleic acid composition used for detecting HPV16 subtypes and a second nucleic acid composition used for detecting HPV18 subtypes, wherein the first nucleic acid composition comprises first primer pair expressed as SEQ ID NO.1-2 and a first capture probe expressed as SEQ ID NO. 3; the second nucleic acid composition comprises a second primer pair expressed as SEQ ID NO.4-5 and a second capture probe expressed as SEQ ID NO. 6. The nucleic acid composition takes PCR and EFIRM technologies as base, is capable of detecting classes of the HPV subtypes in the samples with high sensitivity and high specificity, has the characteristics of short detection time and low detection cost, and can be applied to a large amount of clinical detection and large-scale epidemiology screening.
Owner:北京易活生物科技有限公司
Fluorescence PCR method for diagnosis of human papilloma viral infection
PendingCN101251469ALow efficiencyReduce complexityMicrobiological testing/measurementMaterial analysis by optical meansForward primerHuman papilloma virus infection
Owner:GENETEL PHARMA SHENZHEN
HPV CD8+ T-cell epitopes
InactiveUS20050181458A1Improving immunogenicityPeptide/protein ingredientsVirus peptidesEpitopeHPV vaccines
The present invention provides means to identify functional CD8+ T-cell epitopes in any protein of interest. The present invention further provides CD8+ T-cell epitopes of various proteins. In additional embodiments, the present invention provides epitopes suitable for use in prophylactic and / or therapeutic vaccines. In particularly preferred embodiments, the present invention provides modified epitopes suitable for use in prophylactic and / or therapeutic vaccines. In some preferred embodiments, the present invention provides means for the development of HPV vaccines, in particular multivalent vaccines for the prevention of infection with high-risk HPV strains. In particular, the present invention provides means to identify CD8+ T-cell epitopes in HPV strains such as HPV 16 and HPV 18. In additional embodiments, the present invention provides means for the development of therapeutic vaccines against high-risk HPV types that prevent the development of benign and / or malignant tumors in infected individuals. The present invention further provides epitopes suitable for use in prophylactic and therapeutic vaccines.
Owner:GENENCOR INT INC
Vaccine for HPV infection and/or hepatitis b comprising HPV/hbs chimeric protein as active ingredient
InactiveUS20150344529A1Improve productivityQuality improvementBacteriaAntibody mimetics/scaffoldsAntiendomysial antibodiesImmunocompetence
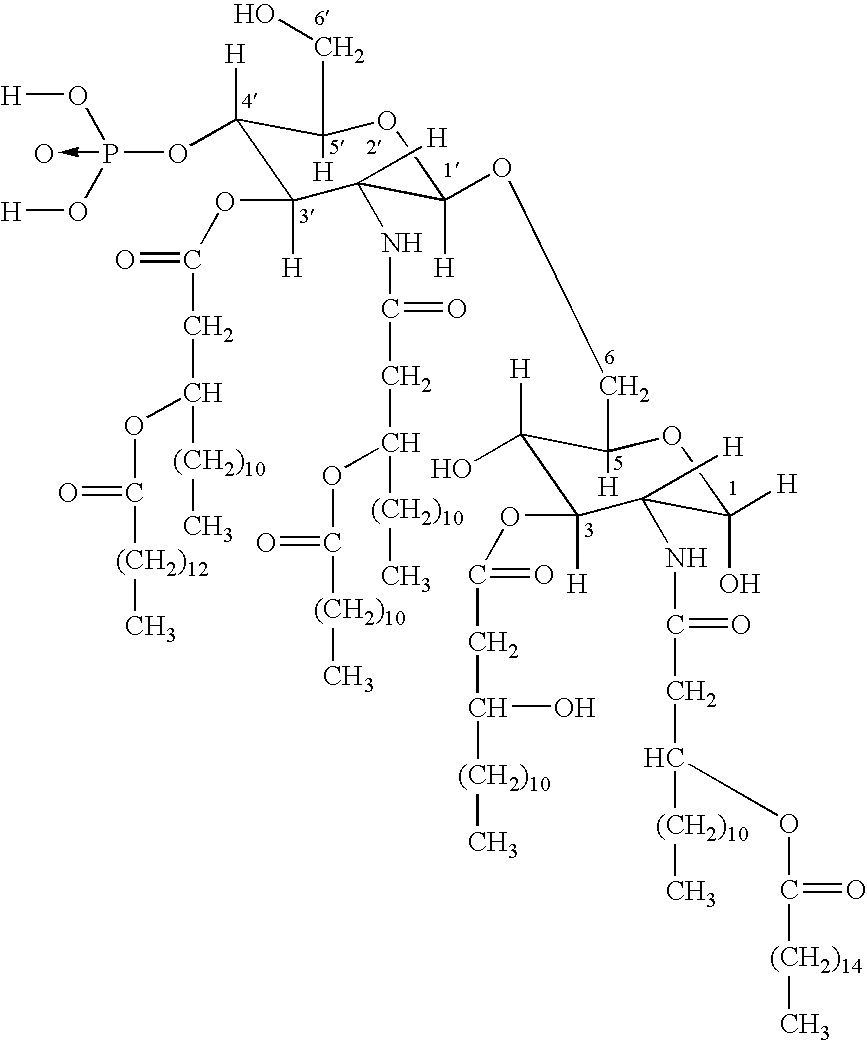
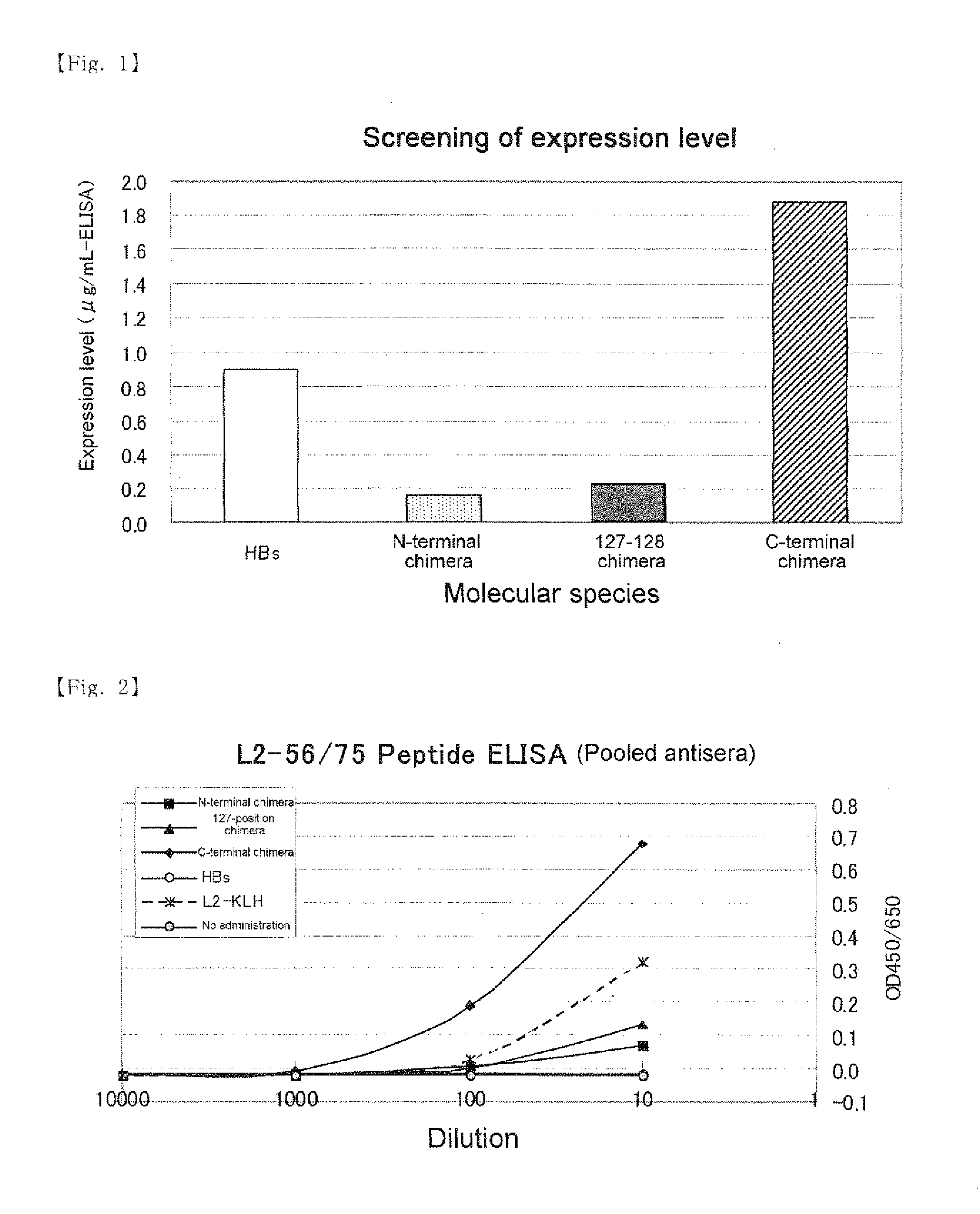
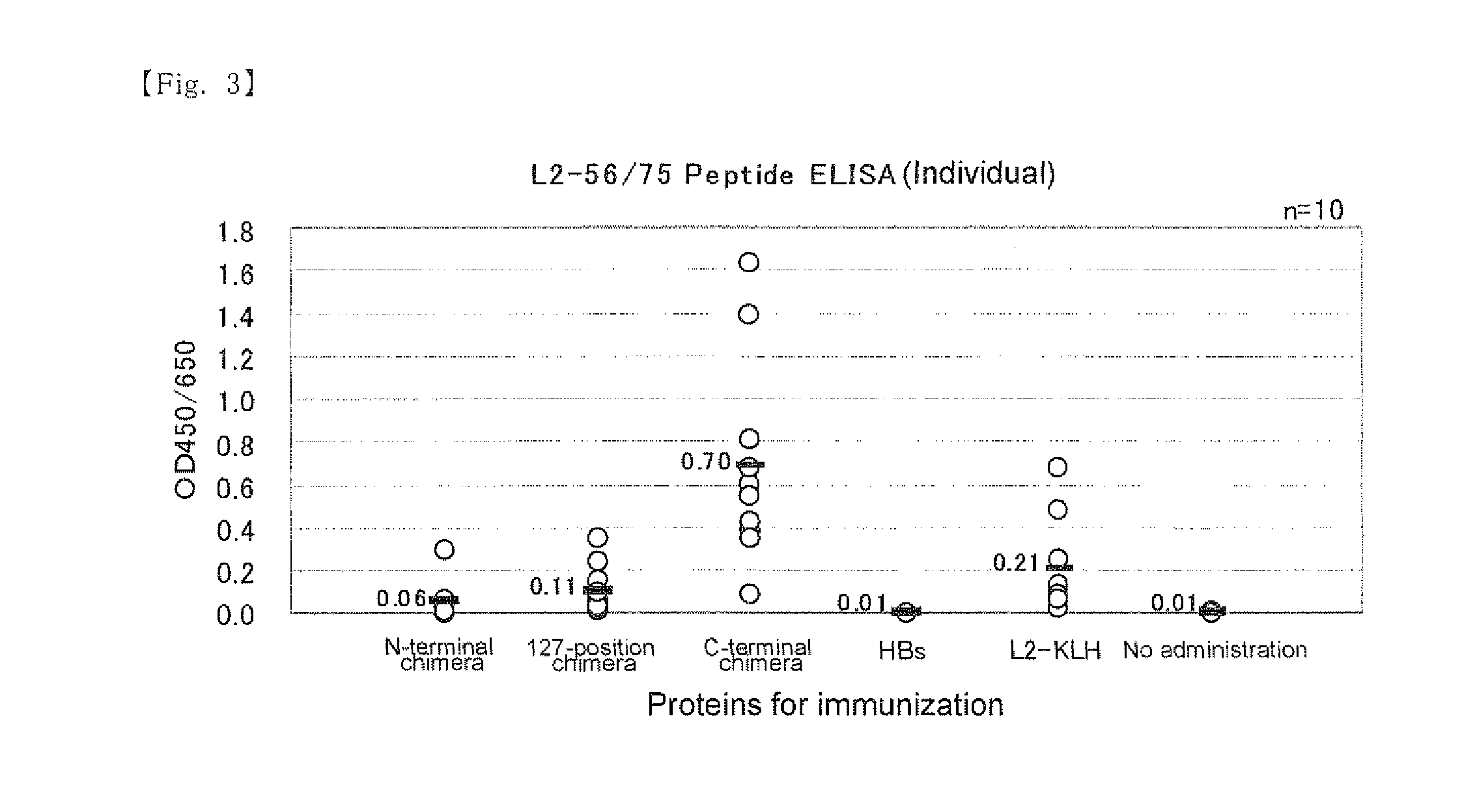
A chimeric protein of HPV-L2 peptide and HBs protein wherein the HPV-L2 peptide is (1) a peptide consisting of the core sequence region of 20 amino acid residues, (2) a peptide inside the core sequence region comprising 6 amino acid residues Gly-Gly-Leu-Gly-Ile-Gly or (3) a peptide consisting of 70 or less amino acid residues obtained by adding an amino acid sequence derived from HPV L2 protein at the N-terminal and / or the C-terminal of the core sequence region; and a vaccine for HPV infection and / or hepatitis B comprising the chimeric protein as an active ingredient. A vaccine comprising the chimeric protein of the present invention is the one that has an increased expression level in a host, an enhanced immune ability (early antibody induction) and a broader spectrum effective for a number of HPV types.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST +1
Human papilloma virus sample particle vaccines
ActiveCN101116745AImprove the effectiveness of preventionAvoid infectionAntibody medical ingredientsAntineoplastic agentsHuman papilloma virus infectionEpidemiology
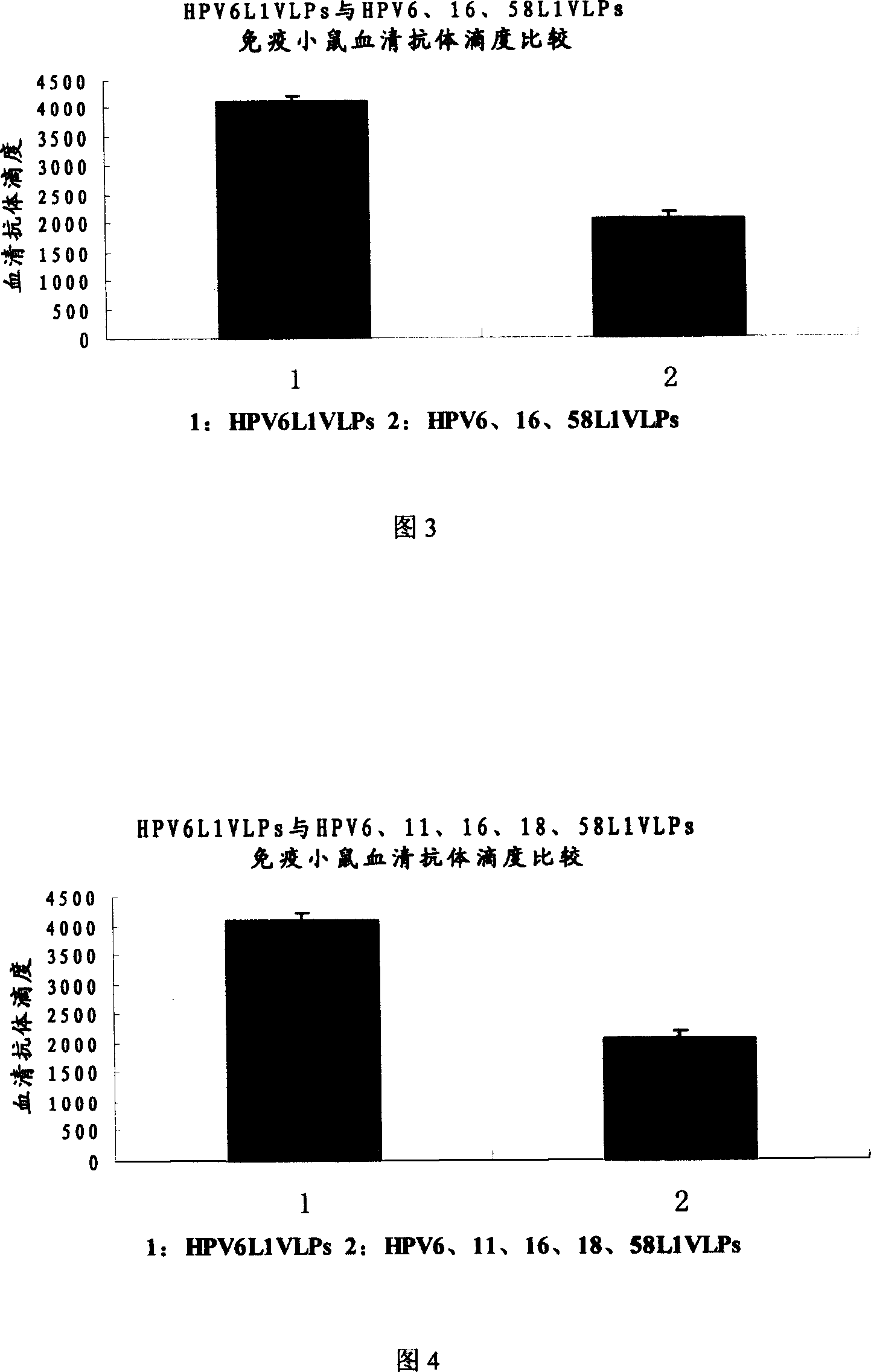
The invention relates to a virus-like particle vaccine against human papilloma virus, belonging to the biotechnology field. One milliliter vaccine contains the following components: HPV6L1VLPs: 10-60 ug, HPV16L1VLPs: 5-40 ug and HPV58L1VLPs10-60 ug. The invention has the advantages that HPV6L1, HPV16L1 and HPV58L1 proteins are secreted and expressed respectively, and the L1 proteins can be self-assembled to be VLPs. According to the infection epidemiology investigation result of human papilloma virus in some placed in Asia and China, the vaccine prepared by VLPs which is obtained by L1 proteins of HPV6, HPV16 and HPV58 can prevent 60 percent of the inventions resulting from HPV. The additional HPV type can further increases preventive effects of the vaccine.
Owner:CHANGCHUN BCHT BIOTECH
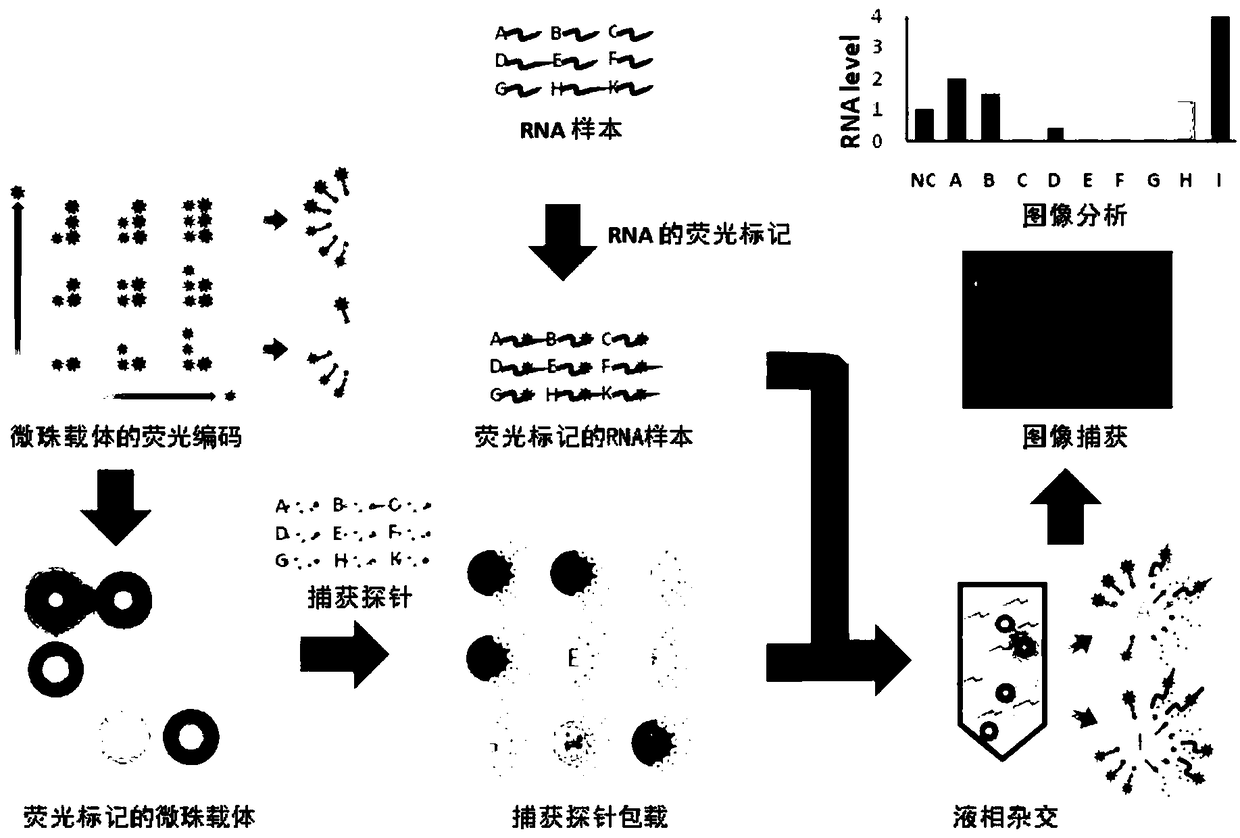
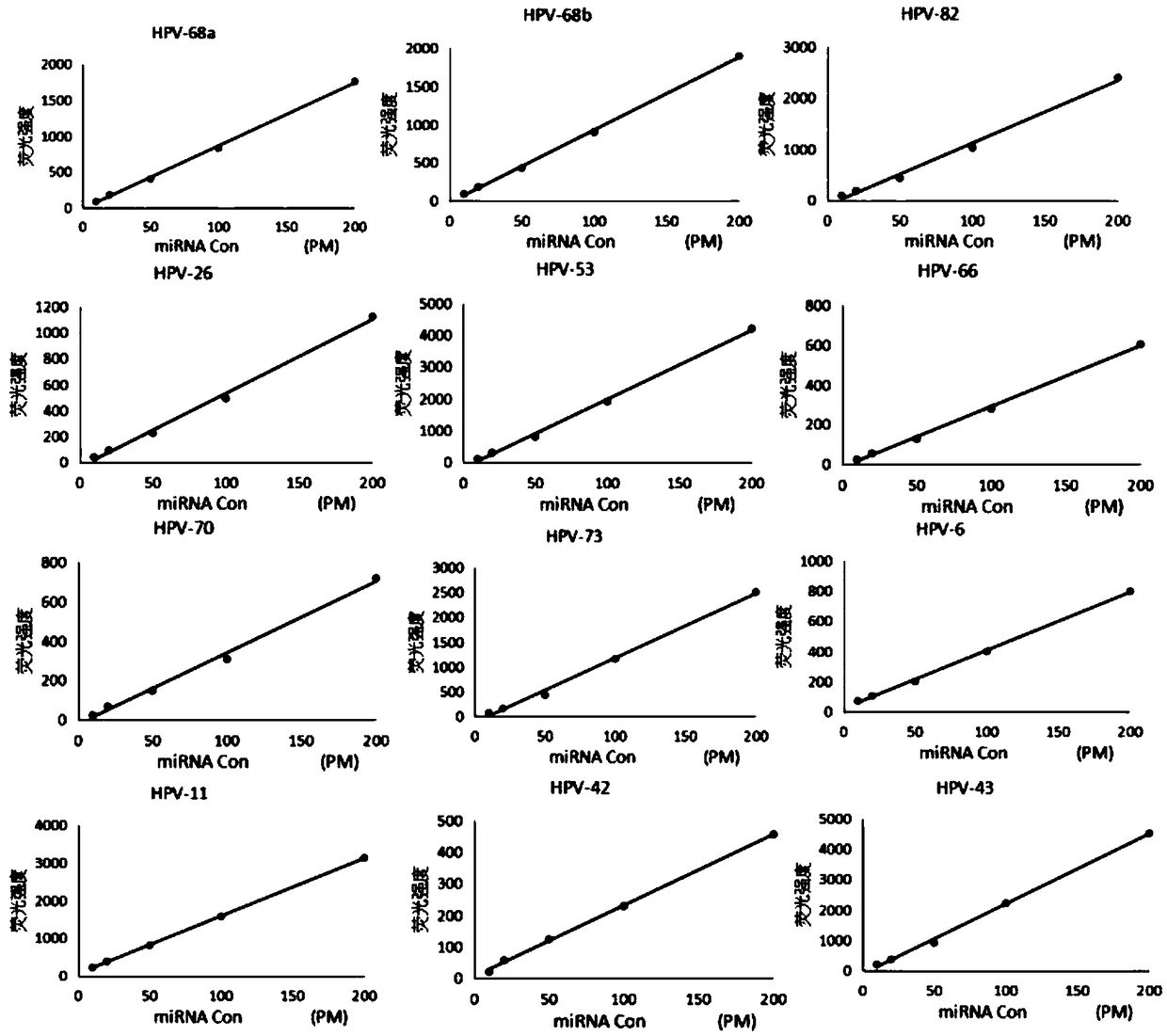
Human papillomavirus quick detection method, liquid-phase chip and kit
ActiveCN108374039AAchieve precise quantitative labelingImprove stabilityNucleotide librariesMicrobiological testing/measurementHuman papillomavirusMicrosphere
The invention discloses a human papillomavirus quick detection method, a liquid-phase chip and a kit. The liquid-phase chip comprises microsphere carriers, labeled molecules and nucleic acid capture probes aiming at different HPV types, the microsphere carriers are connected with coupling groups, the labeled molecules are available for independent detection, each capture probe comprises a specificnucleotide sequence and at least one coupling group, and each specific nucleotide sequence and target nucleic acid molecules coded by different types of HPV viruses form complementary pairs. By adoption of the method, accurate quantitative marking of the microsphere carriers can be realized, and stability and distinguishability in marking of the same type of microsphere carriers are remarkably improved. On the basis of the chip, the invention further provides a HPV detection method and a kit, 24 common HPV types can be detected in one time, the defect of detection omission in application of serology and immunohistochemical methods can be overcome while HPV detection efficiency is greatly improved, and early detection of the HPV viruses is realized.
Owner:杜权
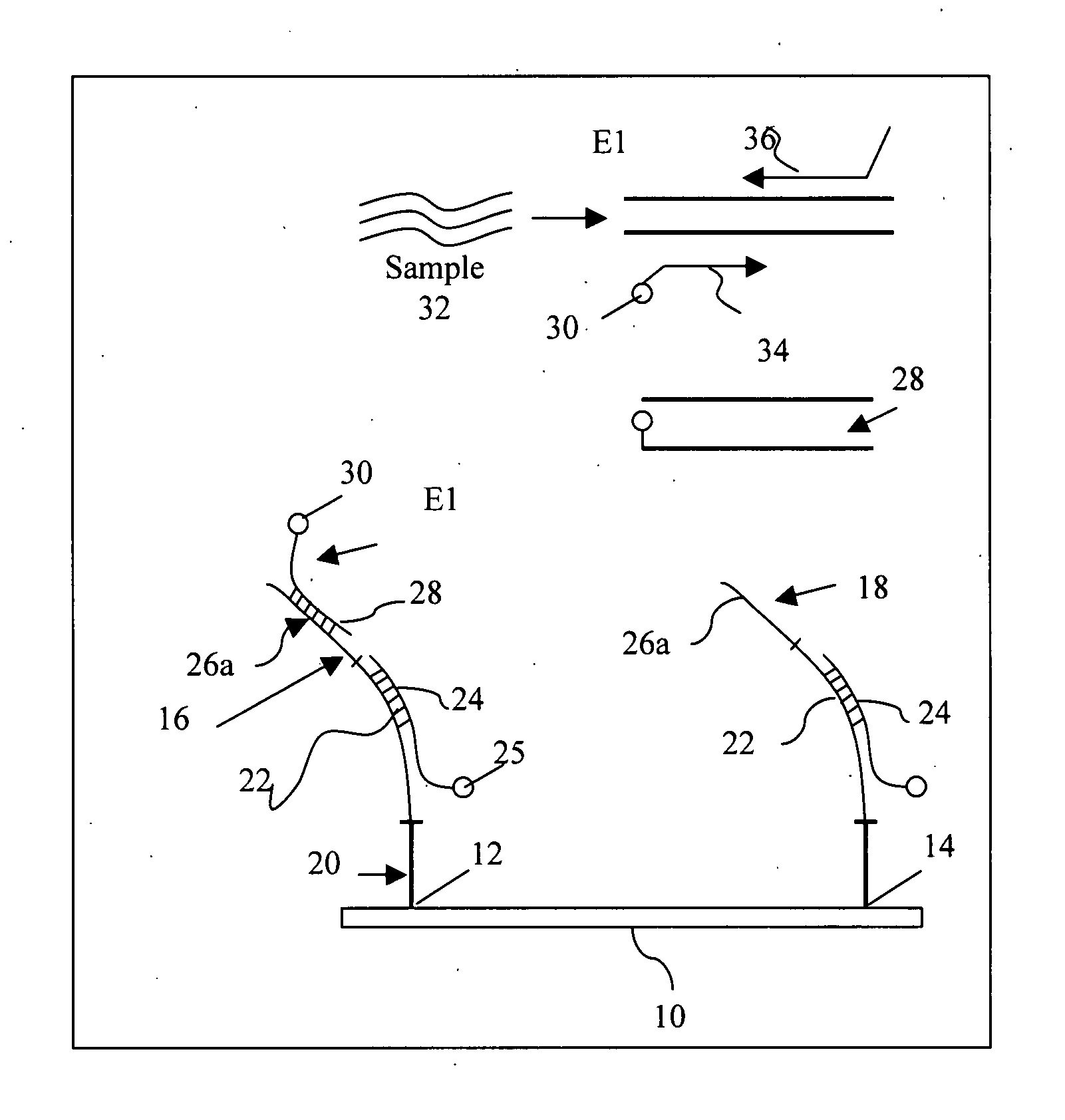
Multiplexed assay and probes for identification of HPV types
InactiveUS20070207456A1Bioreactor/fermenter combinationsBiological substance pretreatmentsA-DNABiology
A DNA microarray, preferably in the form of a chip, contains probes which hybridize to generate primers capable of amplifying approximately 89 HPV types. These target the E1 region of the gene. The design of the chip allows for the detection of any known HPV type, based on a unique probe sequence derived from the HPV E1 region. The present assay utilizes a number of primers that can amplify from about one to six different types of HPV. A large number of primers can be used together. After amplification, the amplicons are contacted with specific probes that are unique for each HPV type. The array further employs a control sequence, which normalizes variability due to sample size.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
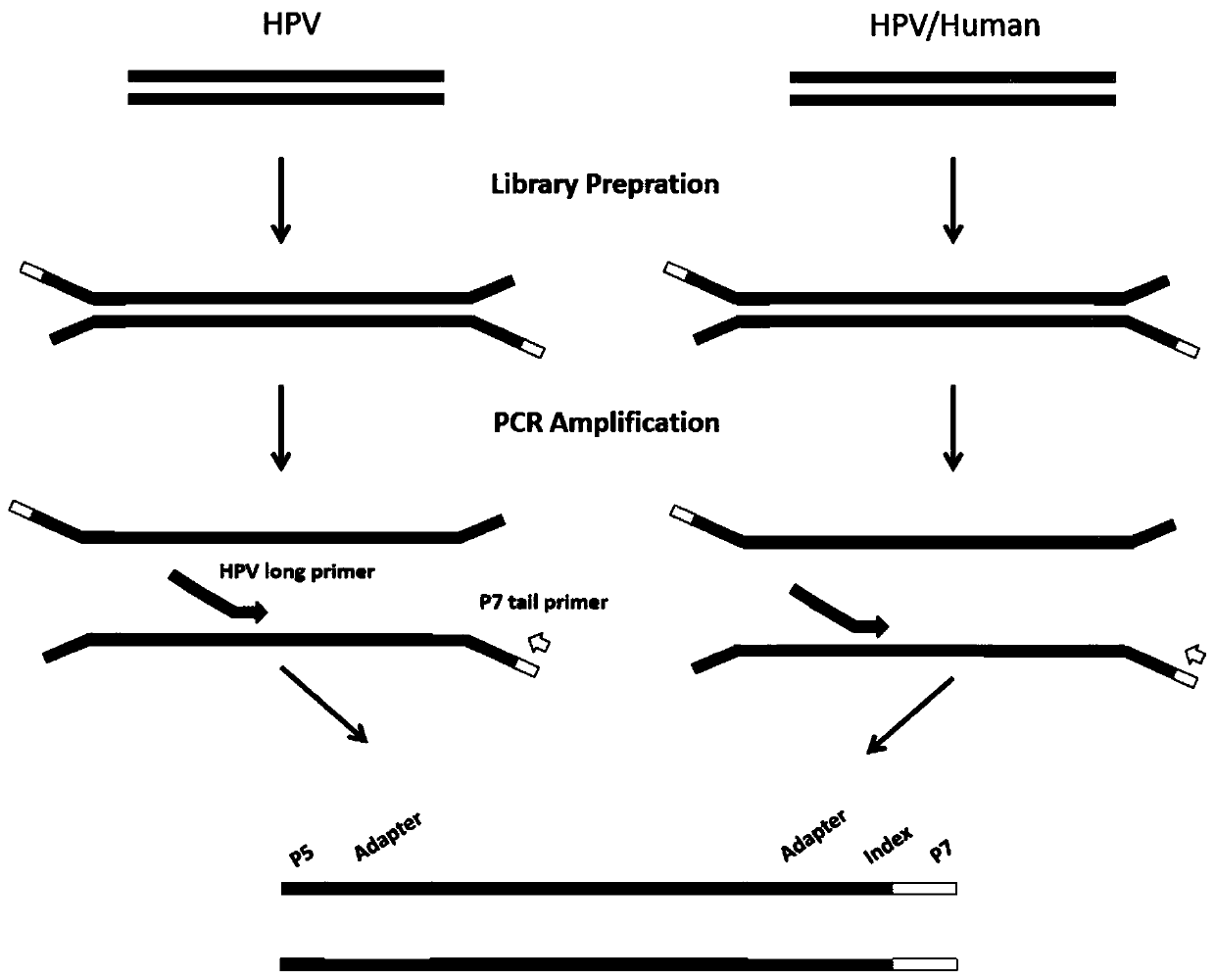
Amplification and sequencing method for identification of various HPV types and genomic integration analysis
PendingCN110093454AMicrobiological testing/measurementMicroorganism based processesDiseaseBuilding product
The invention relates to an amplification and sequencing method for identification of various HPV types and genomic integration analysis. The method comprises the steps: firstly, DNA is interrupted, then the interrupted DNA is screened and purified by magnetic beads, and fragments having the main peak of about 350 bp are retained; then, normal terminal repairing is carried out and a joint is connected, and purification is carried out once by the magnetic beads; and finally, PCR amplification and enrichment are carried out, a library building product of the previous step is used as a template,a mixed solution of 29 primers and P7 terminal primers are amplified, a sequencing library is obtained after purification is carried out once by the magnetic beads, and sequencing is carried out withcooperation of an illumina sequencing platform. The method can simultaneously obtain two results of the HPV types and the human genomic integration sites by one-time sequencing; the number of reads generated by sequencing can be used as a basis for quantitative analysis to further judge the development of disease; and the result information of the HPV types obtained by sequencing is more accurateand comprehensive than that by qPCR.
Owner:NANJING GEZHI GEMONICS CO LTD
Anti-HPV type vaginal temperature-sensitive gel, preparation method and application thereof.
InactiveCN110743003AGood killing effectEasy to useAntibacterial agentsOrganic active ingredientsDiseaseHuman papillomavirus
The invention provides anti-HPV type vaginal temperature-sensitive gel, a preparation method and application thereof. The temperature-sensitive gel includes poloxamer, chitosan, chlorhexidine gluconate, giant salamander oligosaccharide peptides, recombinant human interferons and cell growth factors at the specific ratio, and on this basis, antiviral drugs, antibacterial drugs, antimycotic drugs, antifungal drugs, antimycoplasma drugs and other functional components can be further added. The temperature-sensitive gel is a sustained release preparation with a pH value consistent with the vaginalenvironment, when the temperature is lower than 28 DEG C, the temperature-sensitive gel is in a liquid state, can penetrate into deep folds of the uterus and the vagina and kill pathogens, gel is formed after making contact with the body temperature for 3-5 minutes to cover the disease part, and is not prone to overflowing, and action time and action concentration of the drug and the drug receiving part are guaranteed to the maximum extent; meanwhile, the gel can block the protein synthesis of human papillomavirus (HPV) E6 and E7, and inhibit the proliferation of HPV; and meanwhile, high bactericidal effects on gram-positive bacteria, gram-negative bacteria and fungi are achieved.
Owner:刘志鹏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com