Kit for human papilloma virus typing detection
A technology of human papillomavirus and kit, which is applied in the direction of microbe-based methods, microbiological measurement/inspection, microbes, etc., which can solve the problems of low HPV sensitivity, high quality requirements for personnel, and low accuracy, and achieve improved coverage , Improve detection specificity, reduce damage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
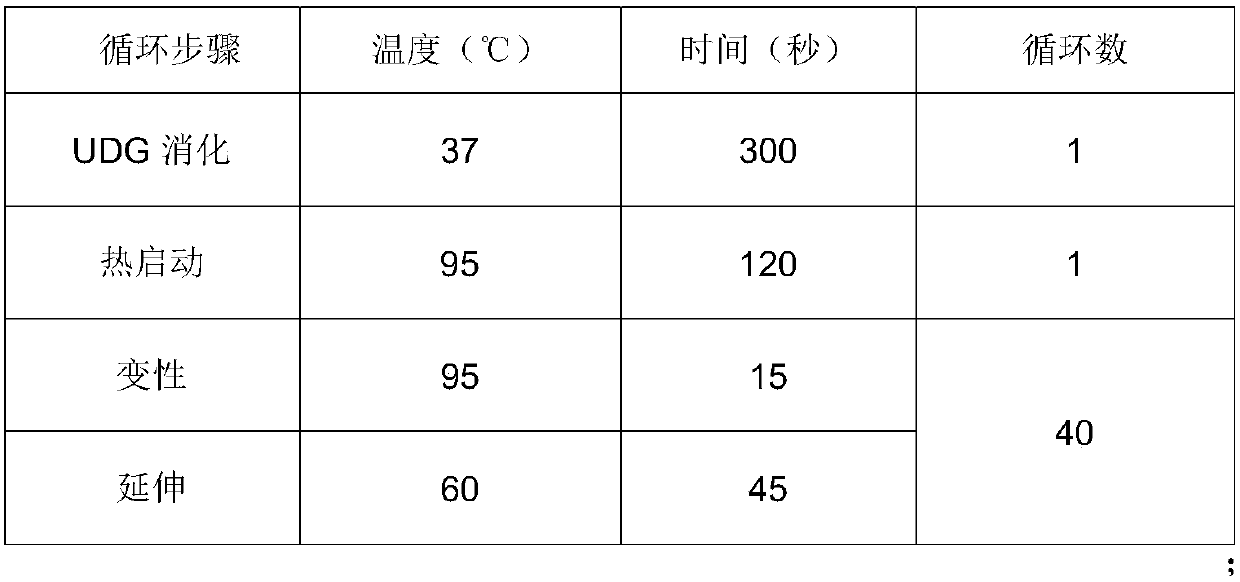
[0066] 1 Main test equipment:
[0067] Qubit 3.0 Fluorescence Quantitative Instrument: Thermo Fisher;
[0068] Centrifuge: Thermo Fisher;
[0069] PCR amplification instrument: our company's independent research and development company;
[0070] Water bath: Changzhou Zhiborui Instrument Manufacturing Co., Ltd.;
[0071] Biological safety cabinet: Haier Group.
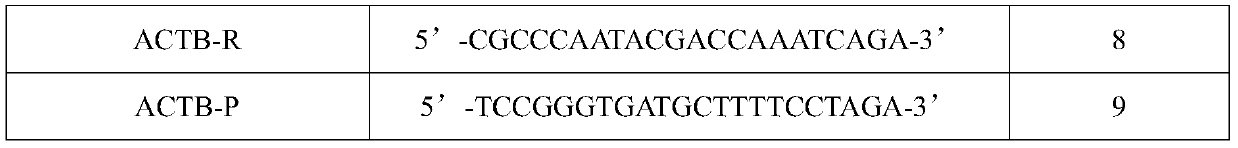
[0072] A kit for detecting human papillomavirus typing, which includes a primer probe premix, a PCR reaction premix, water, a positive quality control, and a negative quality control; the primer probe premix includes detection of 16 , the upstream and downstream primers of HPV types 18, 52, and 58, the fluorescent probes for detecting HPV types 16, 18, 52, and 58, the upstream and downstream primers for detecting human actb gene, and the fluorescent probe for detecting human actb gene; The sequences of each fluorescent probe and upstream and downstream primers in the box are shown in Table 1.
[0073] Table 1 Nucle...
experiment example 1
[0125] Refer to the above detection steps for the standard product, use the sample DNA instead of the plasmid standard product DNA, and detect the HPV infection in the clinical sample; wherein the sample DNA is extracted according to the instructions of the DNA extraction kit used.
[0126] The above kits were used to detect cervical swab samples and urine samples of 50 HPV positive patients. All cervical swab samples had the test results of the qPCR kit as a reference. The test results and analysis results are shown in Table 9 below.
[0127] Table 9
[0128]
[0129]
[0130] The result shows that the kits for qPCR detection of HPV currently used clinically do not provide quantitative detection, but only qualitative typing detection. This is because for cervical swab samples, various factors such as the doctor's sampling method, sampling location, and main infection location will greatly affect the HPV content in the sample. That is to say, the severity of HPV infectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com