Linear epitope minimum motif peptide of human papilloma virus type 58 L1 protein and application thereof
A technology of L1 protein and epitope peptide, which can be applied in the directions of viral antigen components, antiviral agents, and medical preparations containing active ingredients, etc., which can solve the problem of inability to identify linear antigenic epitopes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
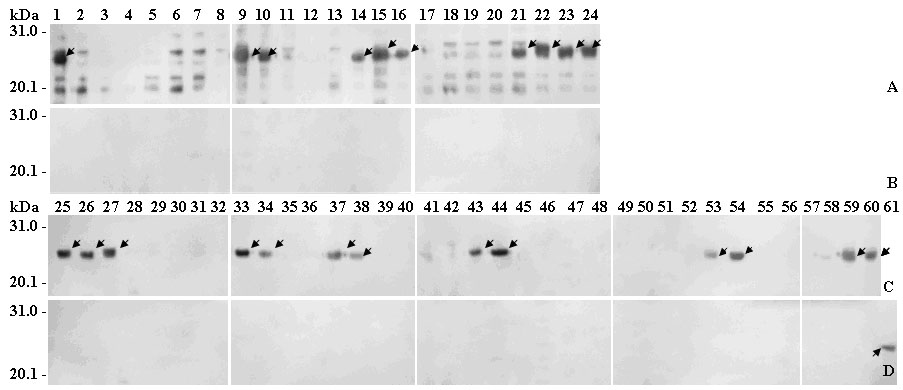
[0033] Example 1: Linear BCE scanning mapping of human papillomavirus type 58 (HPV58) L1 protein
[0034] Materials and methods:
[0035] 1. HPV58 gene clone (pLink322 / HPV58 plasmid) is commercially available. The pRSET C plasmid was purchased from Invitrogen, USA. The heat-inducible expression plasmid pXXGST-1 was constructed by the inventor of this patent (patent application number: 200710173305.2). Escherichia coli BL21 (DE3) strain was commercially available.
[0036] 2. Restriction enzymes EcoR I, BamH I, Sal I, Taq enzyme and T4 DNA ligase were purchased from Japan TaKaRa Biotechnology Company, pre-stained protein molecular weight standards and horseradish peroxidase-labeled goat anti-rabbit secondary antibody (IgG / HRP) Shanghai Sangon Biotechnology Service Company. ECL chemiluminescent color development kit was purchased from GE Healthcare UK. A 0.2 μm nitrocellulose membrane was purchased from Whatman Gmbh Hahnestr. 6 × His monoclonal antibody was purchased from...
Embodiment 2
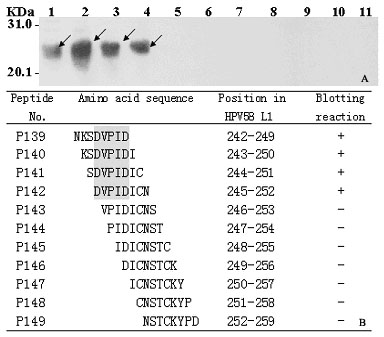
[0048] Embodiment 2: HPV58 viral L1C protein P27 epitope peptide minimum motif identification
[0049] Materials and methods:
[0050] See the corresponding part of Example 1.
[0051] The specific steps for identifying the minimal motif of the P27 epitope peptide are as follows:
[0052] 1. According to the results of scanning and positioning the epitope of the L1C protein antigen in Example 1, the minimal motif identification of the P27 epitope peptide was carried out as an example. Design a series of 8 peptides (P139-P149) that span the 18 peptide sequences of the P27 epitope peptide and overlap each other by 7 amino acid residues (P139-P149) to encode the positive and negative strands of DNA (5'-end plus 5'-gatcc, 3'-end plus taag-3'; minus-strand 5'-end plus 5'-tcgactta, 3'-end plus g-3'), send out DNA synthesis.
[0053] The 2-4 operation steps are the same as the 2-4 steps in the embodiment 1.
[0054] 5. The blotted membrane was repeatedly washed four times with P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com