Method for assisting in identifying linear epitope of plant food allergen
A food allergy, plant-based technology, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of high blindness, heavy workload, long time consumption, etc., and achieve the effect of accurate identification results and reduced workload.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
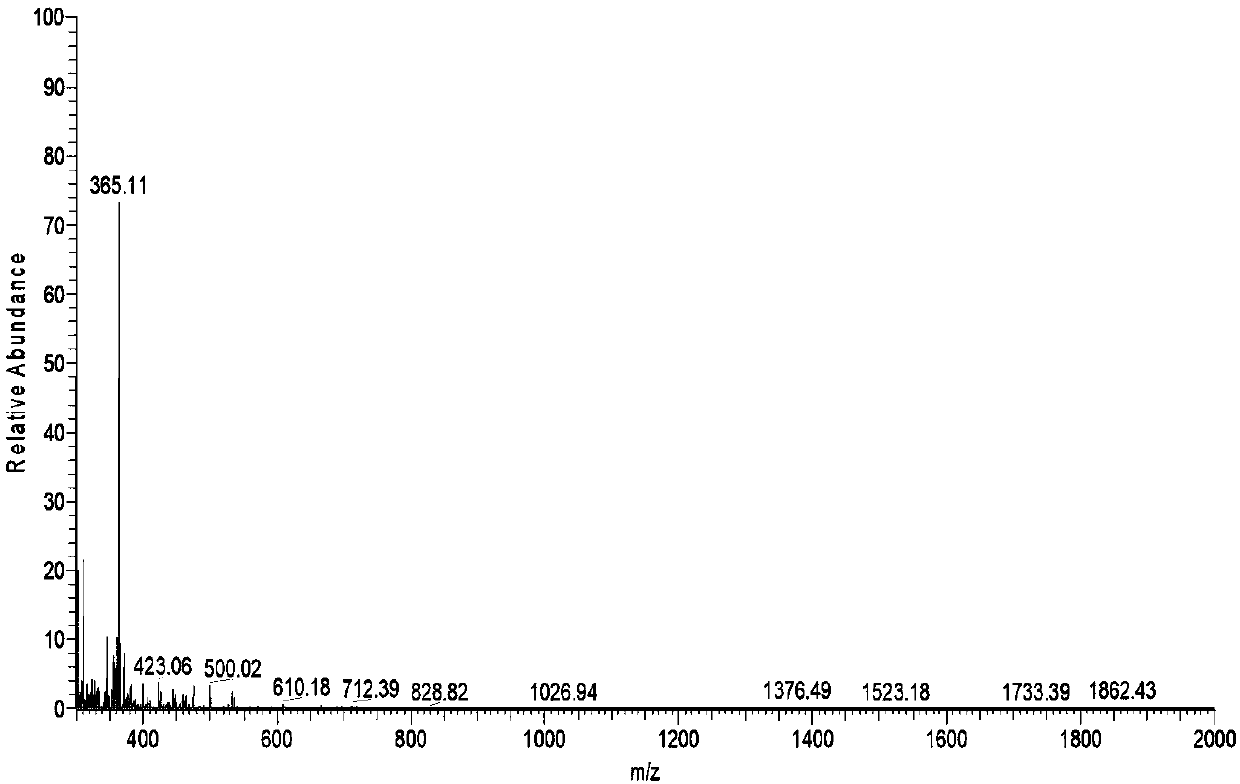
[0034] Peanut allergen Ara h1 is a 7S globulin, which belongs to the Cupin superfamily, and can be recognized by more than 90% of peanut-allergic patients' sera through serological analysis. Because the structural sequence of the Cupin superfamily has the characteristics of high conservation, strong thermal stability, resistance to enzymolysis and indigestibility, Ara h1 is selected as the model allergen in the present invention.
[0035] This embodiment provides a method for assisting in identifying the linear epitope of the peanut allergen Ara h1, comprising the following steps:
[0036] S1. The product of peanut protein digested by pepsin for 60 minutes was used as the sample, and the nano-level HPLC liquid phase system Easy-nLC1000 was used for separation, wherein the buffer solution: solution A was 0.1% formic acid aqueous solution, and solution B was 0.1% formic acid acetonitrile solution; The chromatographic column is balanced with 95% liquid A; the sample is loaded fro...
Embodiment 2
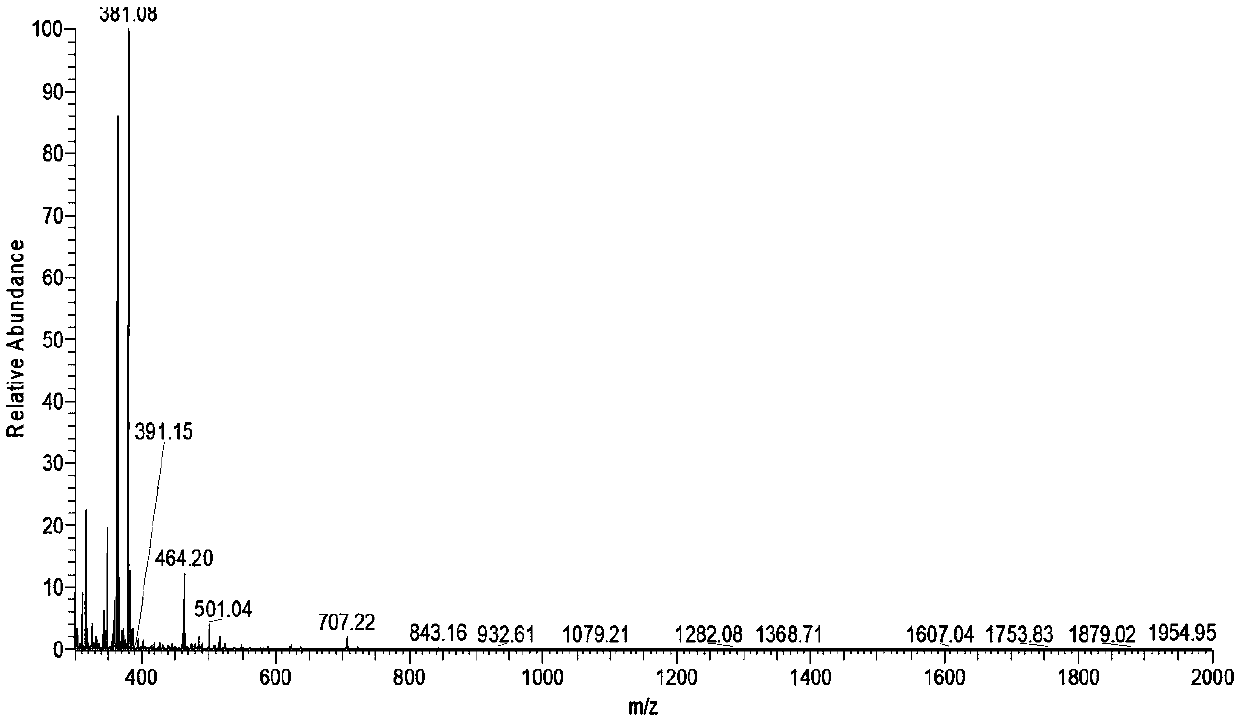
[0050] The walnut allergen Jug r 2 belongs to the 7S vicilin family, which is the same family as the peanut allergen Ara h1. Its primary sequence consists of 593 amino acids and its molecular weight is about 44kDa. It exists in the form of trimeric oligomers under natural conditions.
[0051] This embodiment provides a method for assisting in identifying the linear epitope of the walnut allergen Jug r 2, comprising the following steps:
[0052] S1. The product of walnut protein digested by pepsin for 60 minutes was used as a sample, and the nano-level HPLC liquid phase system Easy-nLC1000 was used for separation, wherein the buffer solution: solution A was 0.1% formic acid aqueous solution, and solution B was 0.1% formic acid acetonitrile solution; The chromatographic column is balanced with 95% liquid A; the sample is loaded from the autosampler to the pre-column C18trap column (3mm, 0.10×20mm), and then separated by the analytical column C18column (1.9mm, 0.15×120mm), with a ...
Embodiment 3
[0067] Wheat allergen CM16 is a protein with a molecular weight of 17kDa and composed of 143 amino acids. It belongs to the gliadin superfamily and contains multiple cysteine residues, which can form intramolecular disulfide bonds to ensure the structural stability of the protein and It is heat resistant.
[0068] This embodiment provides a method for assisting in identifying the linear epitope of wheat allergen CM16, comprising the following steps:
[0069] Using the product of wheat protein digested by trypsin for 60 minutes as a sample, a total of 6753 peptides were detected by using the steps S1-S3 in Example 1, and a search database was constructed according to the amino acid sequence of wheat allergens provided by the Uniprot database, and searched with pFind The engine detected a total of 23 peptides belonging to wheat allergen proteins, most of which were derived from α-amylase / trypsin inhibitor subtypes and high molecular weight glutenin subunits, and 3 of them matc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com