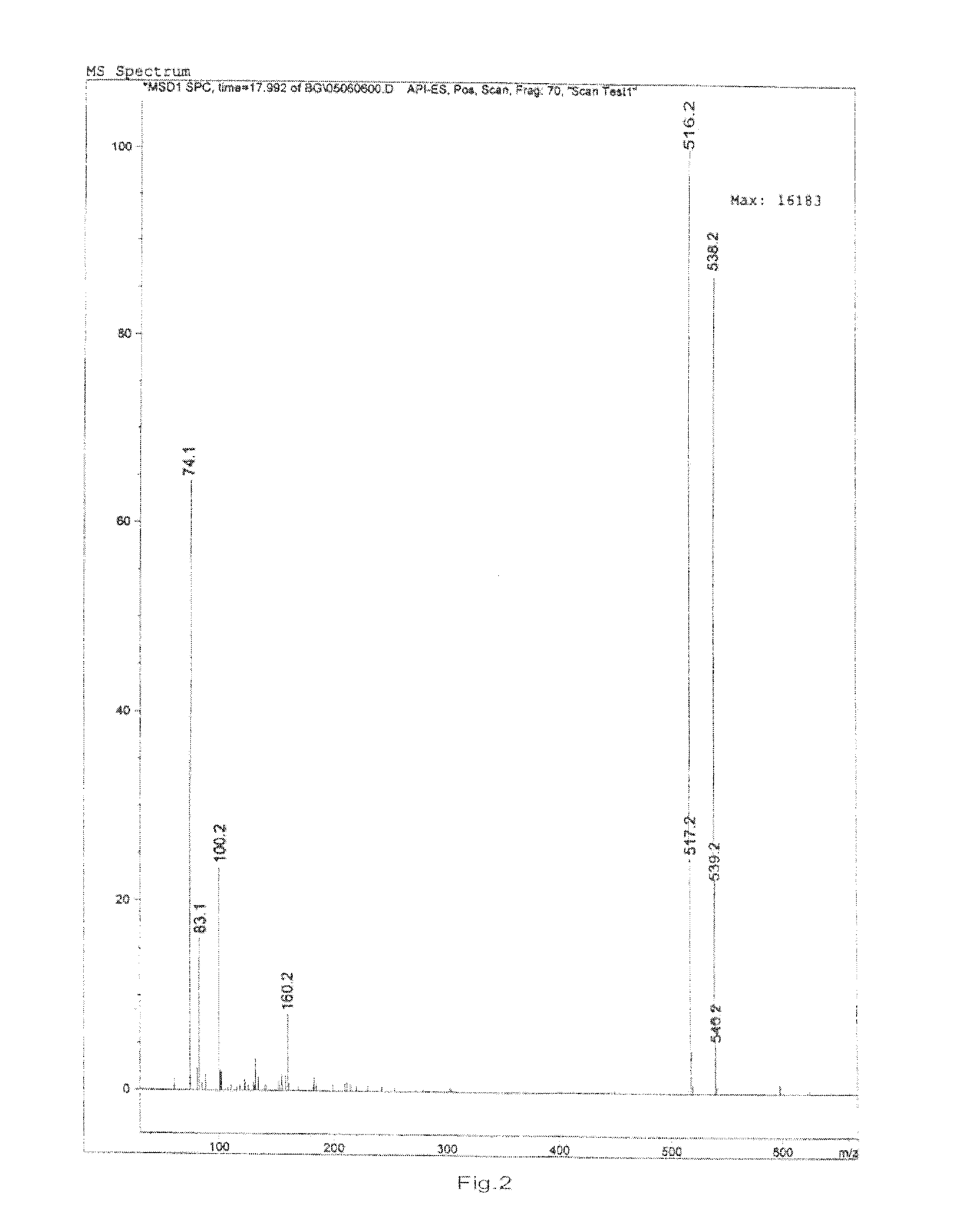
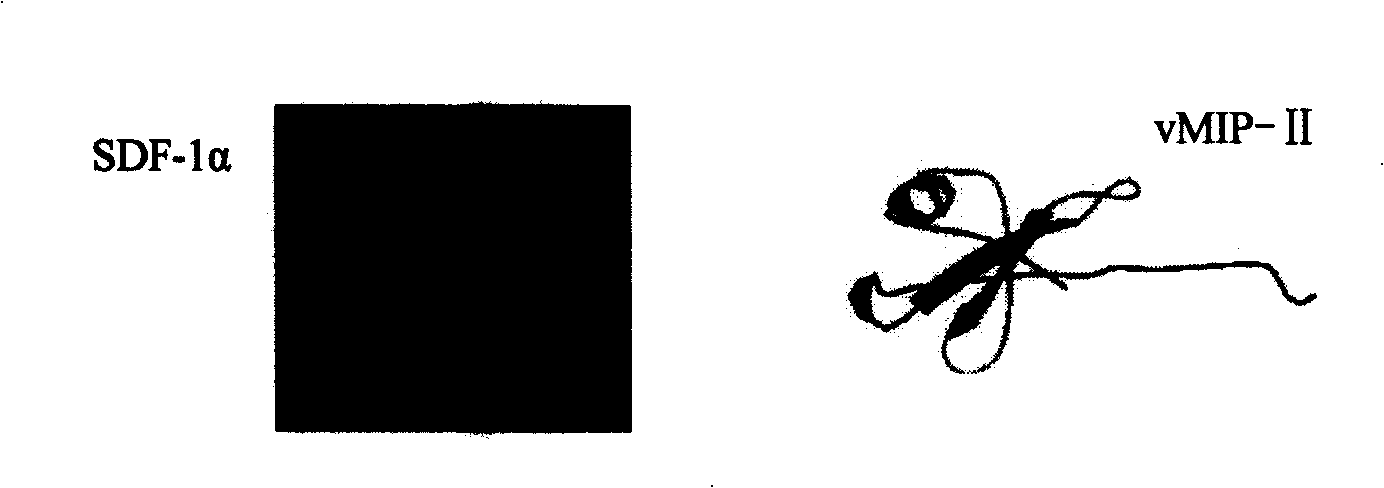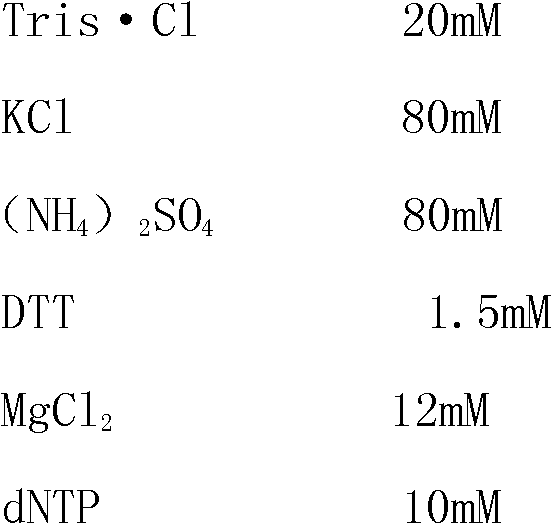Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58 results about "Human herpes virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
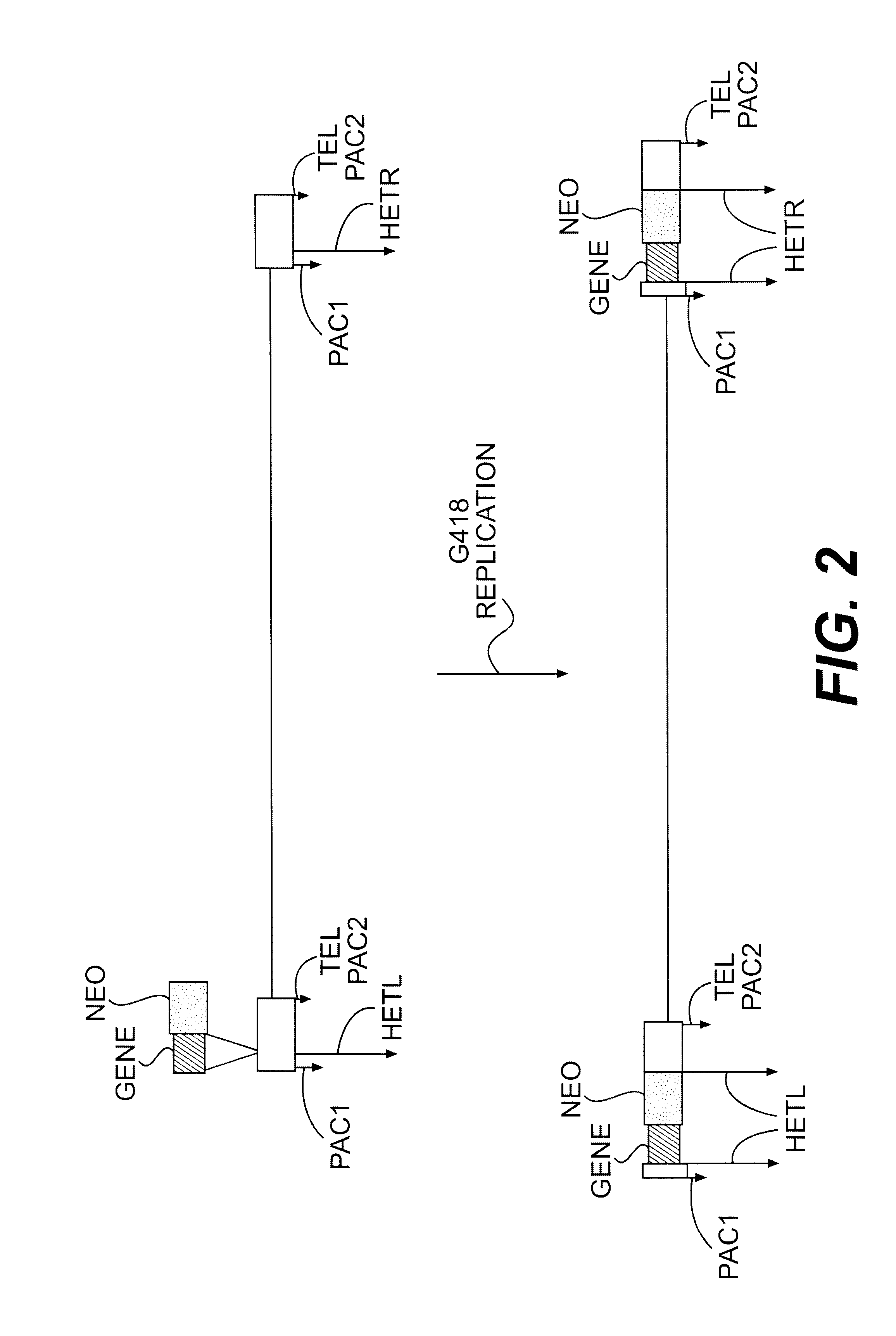
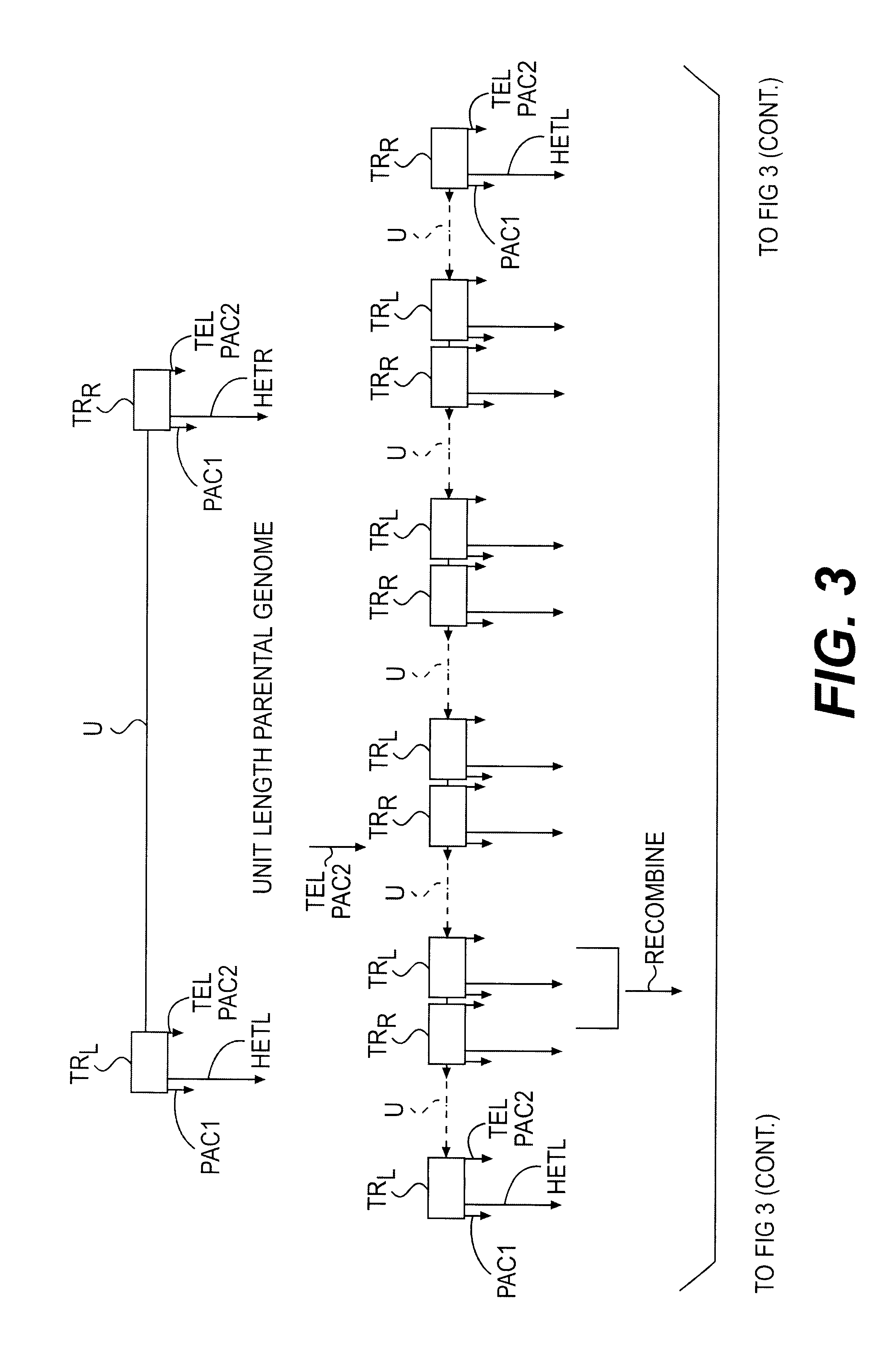
Bioinformatically detectable human herpesvirus 5 regulatory gene
ActiveUS7696334B1Preventing and treating viral diseasesSugar derivativesMicrobiological testing/measurementOperonVirus attack
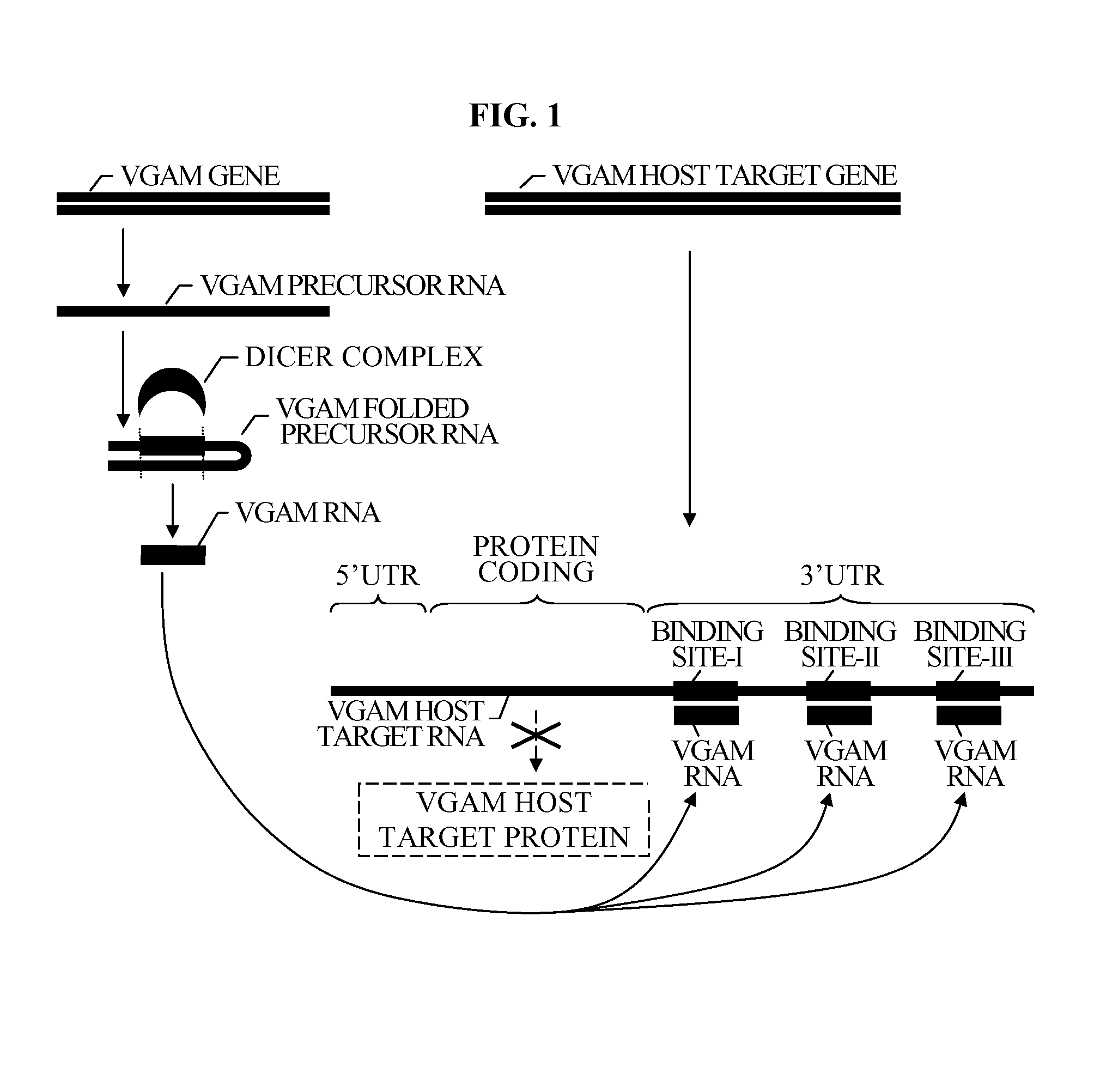
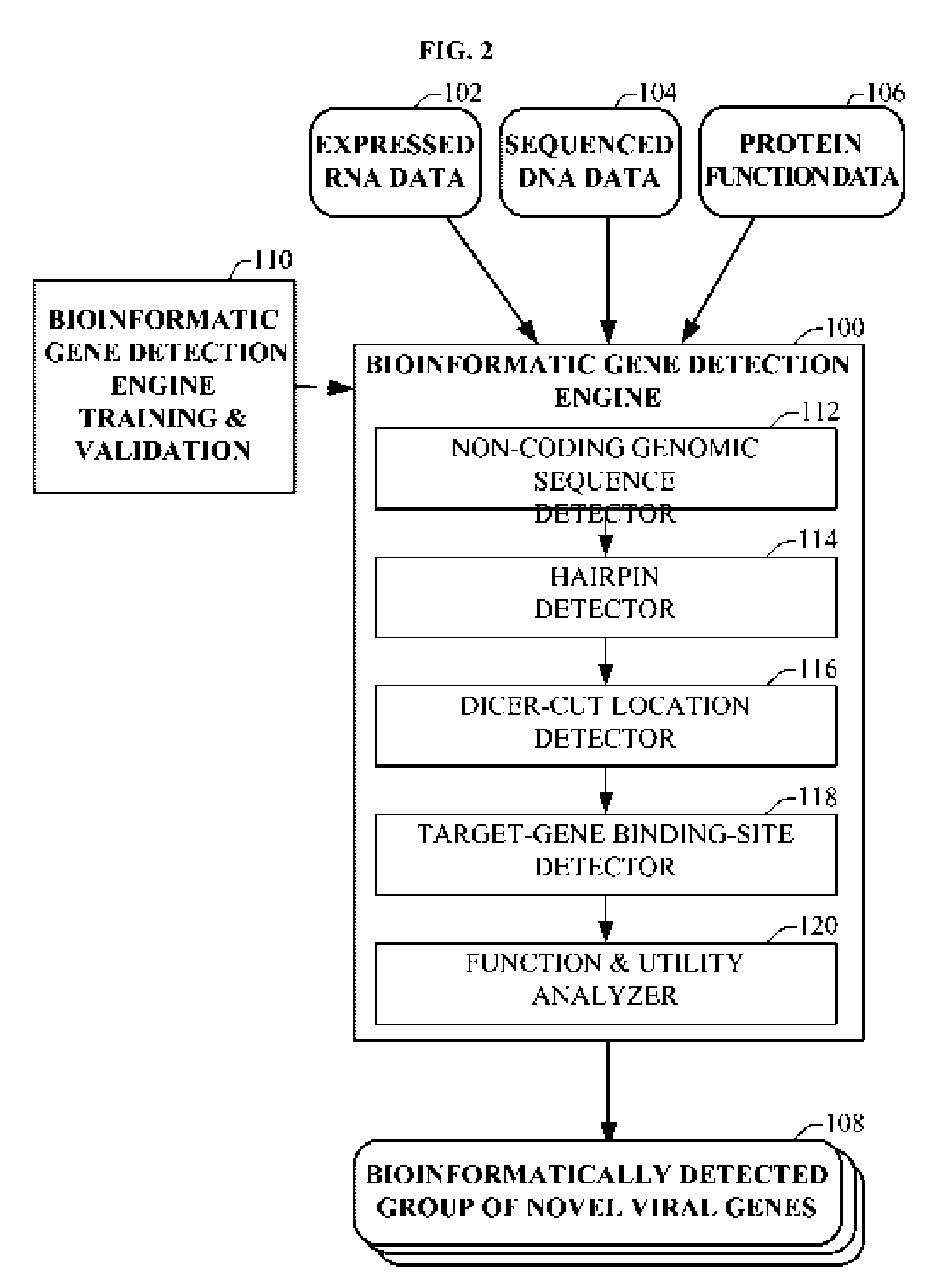
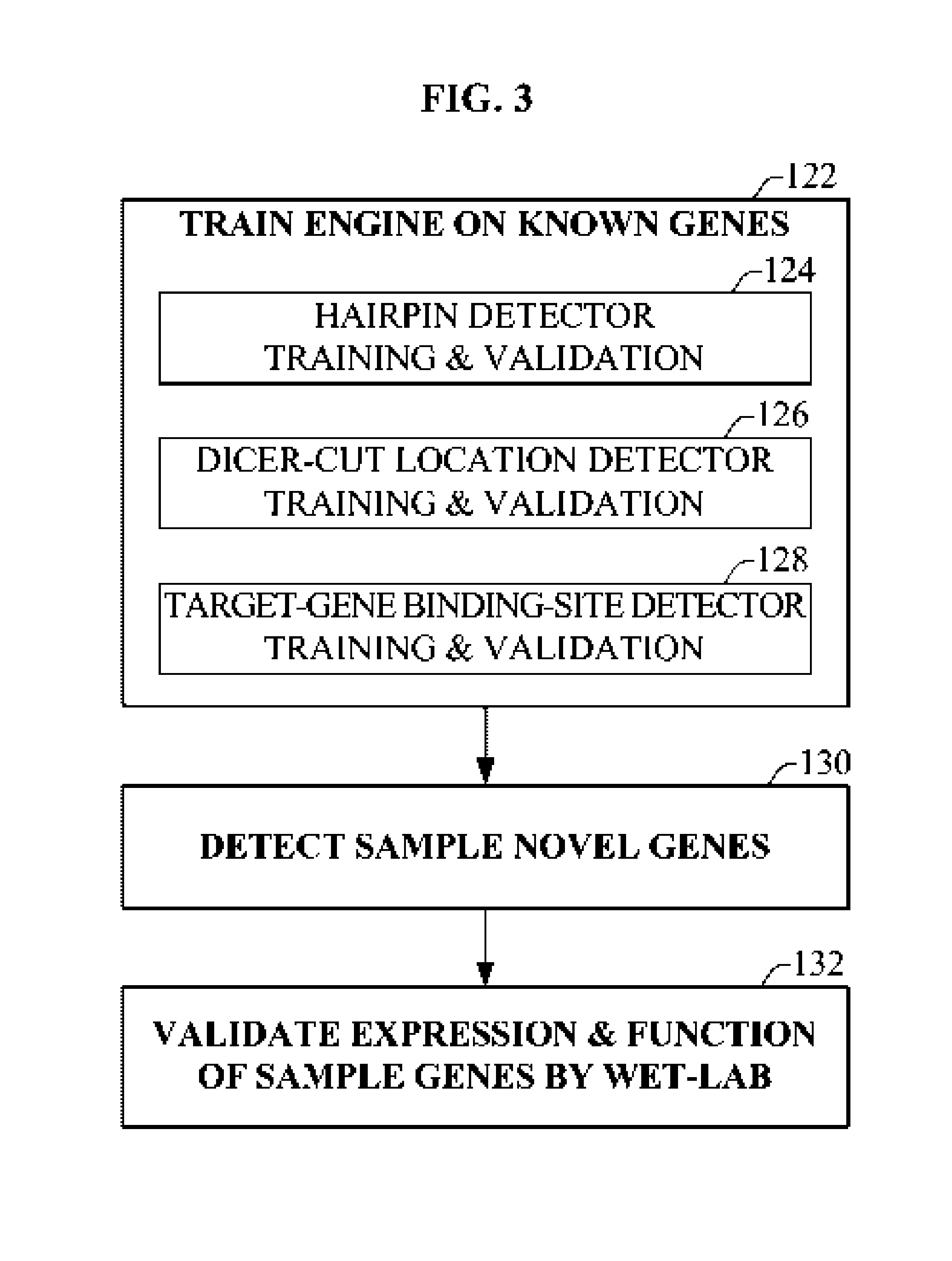
The present invention relates to a group of novel viral RNA regulatory genes, here identified as “viral genomic address messenger genes” or “VGAM genes”, and as “genomic record” or “GR” genes. VGAM genes selectively inhibit translation of known host target genes, and are believed to represent a novel pervasive viral attack mechanism. GR genes encode an operon-like cluster of VGAM genes. VGAM and viral GR genes may therefore be useful in diagnosing, preventing and treating viral disease. Several nucleic acid molecules are provided respectively encoding several VGAM genes, as are vectors and probes, both comprising the nucleic acid molecules, and methods and systems for detecting VGAM genes, and for counteracting their activity.
Owner:ROSETTA GENOMICS
Detection of human herpesviruses
InactiveUS20060252032A1Easy to adaptMicrobiological testing/measurementNucleic acid detectionHuman being
The present invention provides methods, compositions, and kits related to nucleic acid detection assays for detecting human herpes virus. For example, the present invention provides detection assays for detecting human herpes virus subtypes HHV1- through HHV-8.
Owner:THIRD WAVE TECH
Reagent for rapidly detecting and quantifying subtypes of human herpes viruses and kit
ActiveCN106834543AStrong specificityImprove accuracyMicrobiological testing/measurementMicroorganism based processesClinical diagnosisReagent
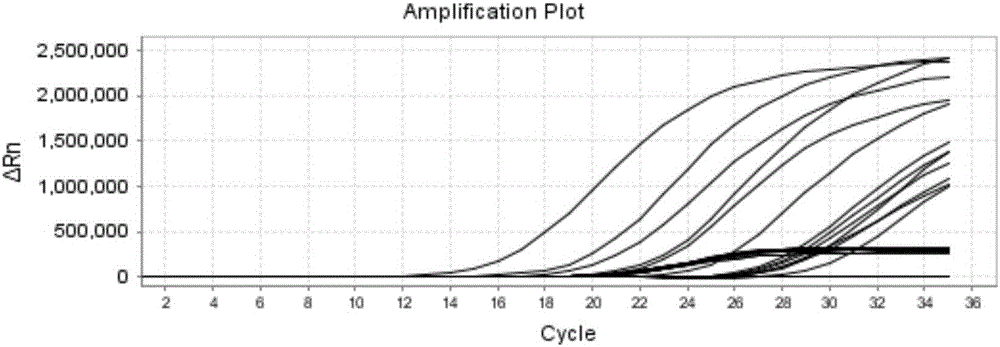
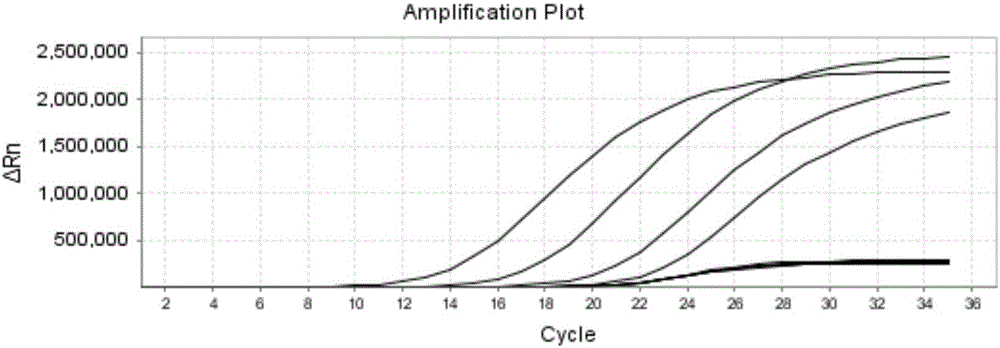
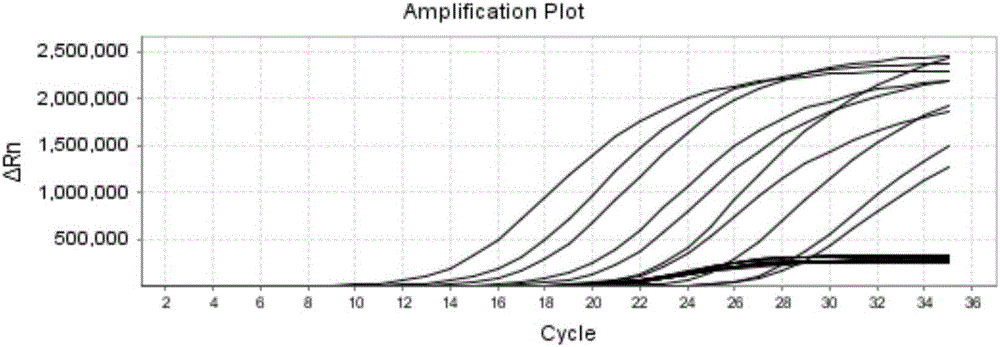
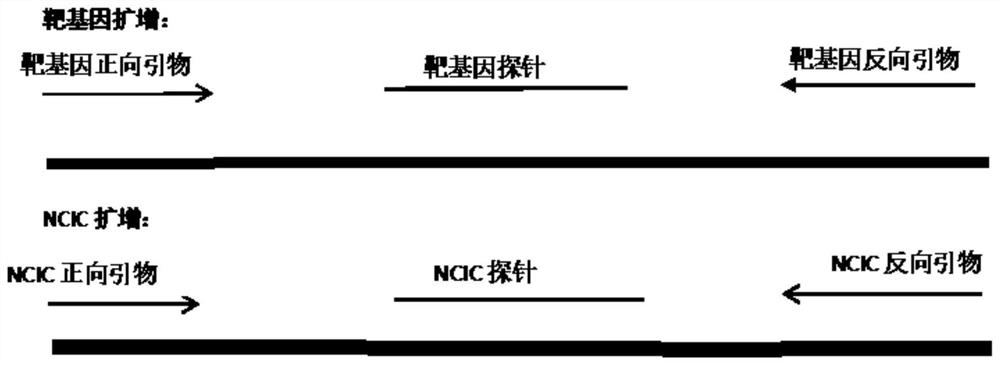
The invention discloses a reagent for rapidly detecting various subtypes of human herpes viruses. The reagent comprises forward and reverse primers specifically amplifying HSV-1 RL2, HSV-2 UL28, VZV ORF26, EBV EBNA1, HCMV IE, HHV-6A or HHV-6B U22, HHV-6A or HHV-6B IE, HHV-7 U95 and KSHV ORF72 and a corresponding internal reference standard. The invention also discloses a kit which contains the detection reagent and is used for rapidly detecting various subtypes of the human herpes viruses and also discloses a reagent which contains the detection reagent and is used for rapidly quantifying the various subtypes of the human herpes viruses as well as a detection quantification kit. The reagent disclosed by the invention can rapidly and effectively identify and diagnose the various subtypes on clinical samples in early stage of infection and with different sources, also can conveniently and accurately carry out virus copy number quantitative calculation and has high clinical diagnosis use value.
Owner:FUDAN UNIV
Triple detection kit for human herpes viruses HSV-1, HSV-2 and HCMV
InactiveCN103773898AMeet the needs of clinical diagnosisMeet early infectionMicrobiological testing/measurementFluoProbesBioinformatics
The invention provides a triple detection kit for human herpes viruses HSV-1, HSV-2 and HCMV. The triple detection kit is composed of a quantitative PCR (Polymerase Chain Reaction) reaction liquid, an HSV-1 standard substance, an HSV-2 standard substance, an HCMV standard substance, a negative reference substance, an HSV-1 positive reference substance, an HSV-2 positive reference substance, an HCMV positive reference substance, a specification and a box, wherein the quantitative PCR reaction liquid contains a PCR buffer solution, MgC12, dNTPs (deoxyriboNucleoside Tri-Phosphates), a heat-resisting DNA polymerase, an upstream amplification primer, a downstream amplification primer, an HSV-1 fluorescent probe, an HSV-2 fluorescent probe and an HCMV fluorescent probe. The kit is capable of realizing simultaneous typing detection on the common human herpes viruses HSV-1, HSV-2 and HCMV in a sample by virtue of the real-time quantitative PCR technique and the specific fluorescent probes of three colors; the kit is capable of quantifying positive viruses accurately in real time, so that the production cost and the detection cost can be saved and the detection efficiency can be improved; besides, the kit is capable of meeting diagnosis requirements and helpful for formulating a targeting treatment scheme timely.
Owner:ZHEJIANG UNIV
Lymphotropic agents and vectors
InactiveUS6503752B1Preventing situationHigh sensitivityVectorsSugar derivativesLymphocyteCD4 antigen
Human herpes virus (HHV) 7 is capable of binding to the CD4 antigen and the HHV-7 or a binding protein derived therefrom is thus useful as a CD4-ligand for various therapeutic applications. HHV-6 or HHV-7 are lymphotropic and are thus useful as lymphotropic vectors for delivering DNA into lymphocytes.
Owner:RAMOT UNIV AUTHORITY FOR APPLIED RES & INDAL DEVMENT
Oligonucleotide therapies for modulating the effects of herpesviruses
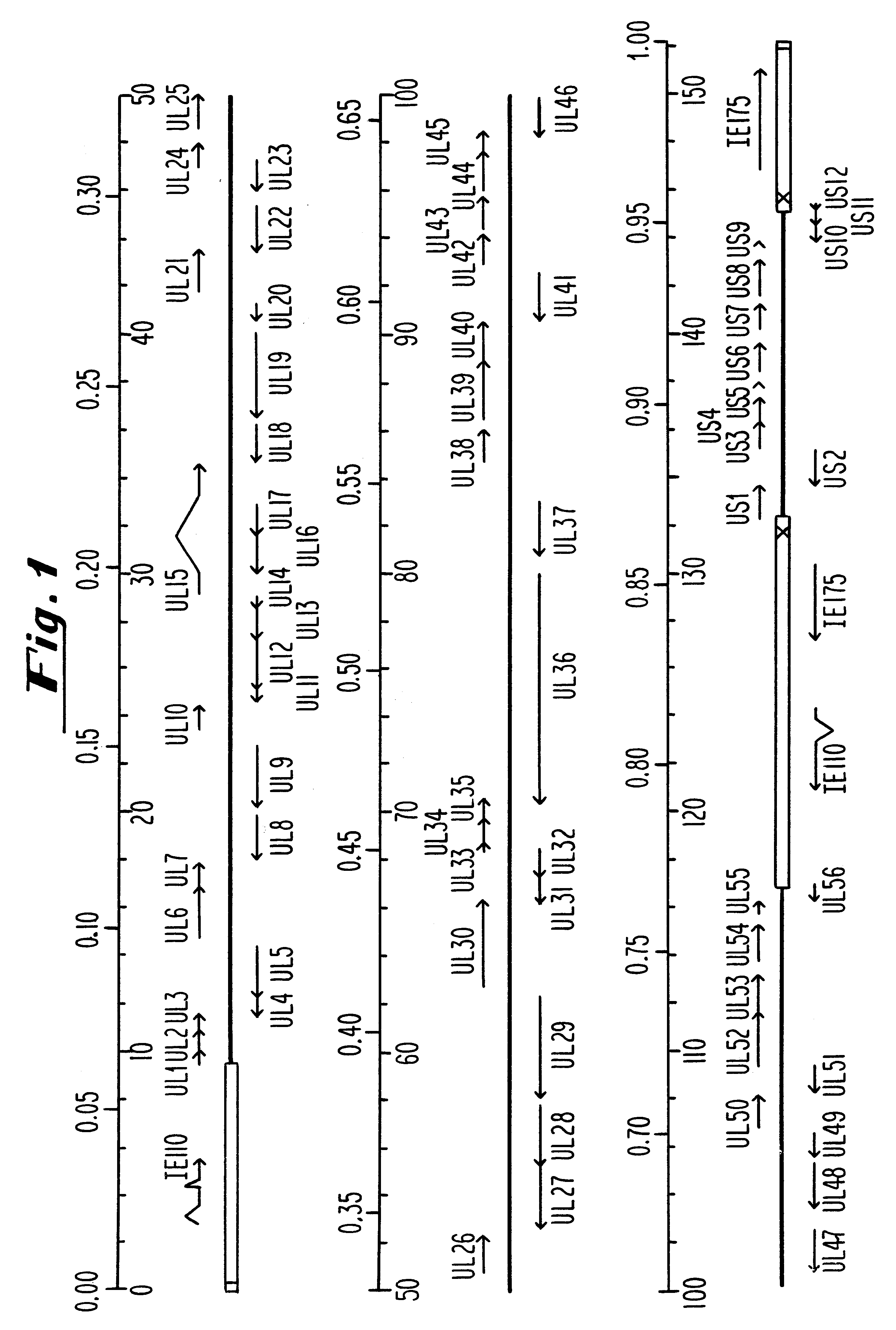
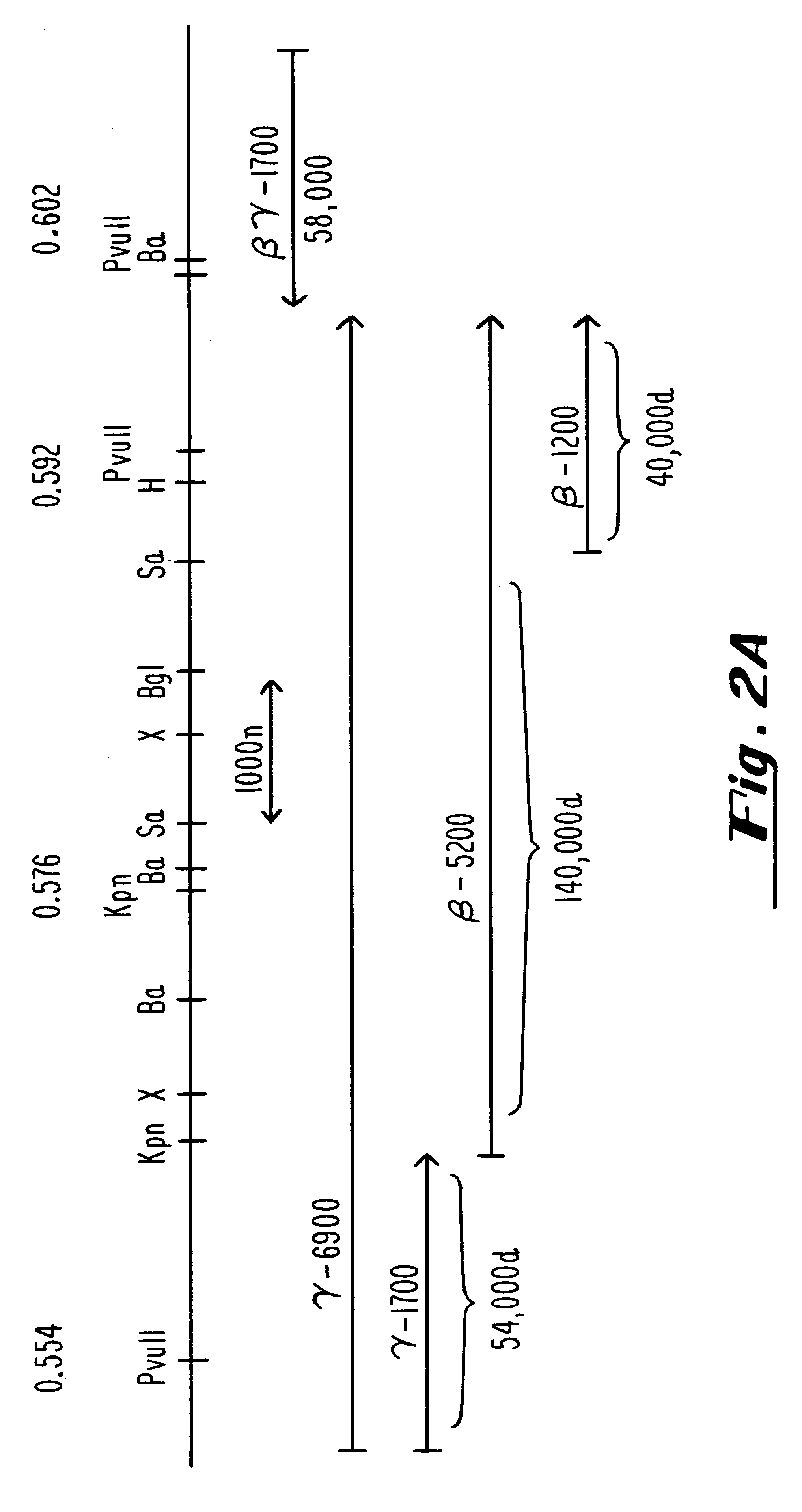
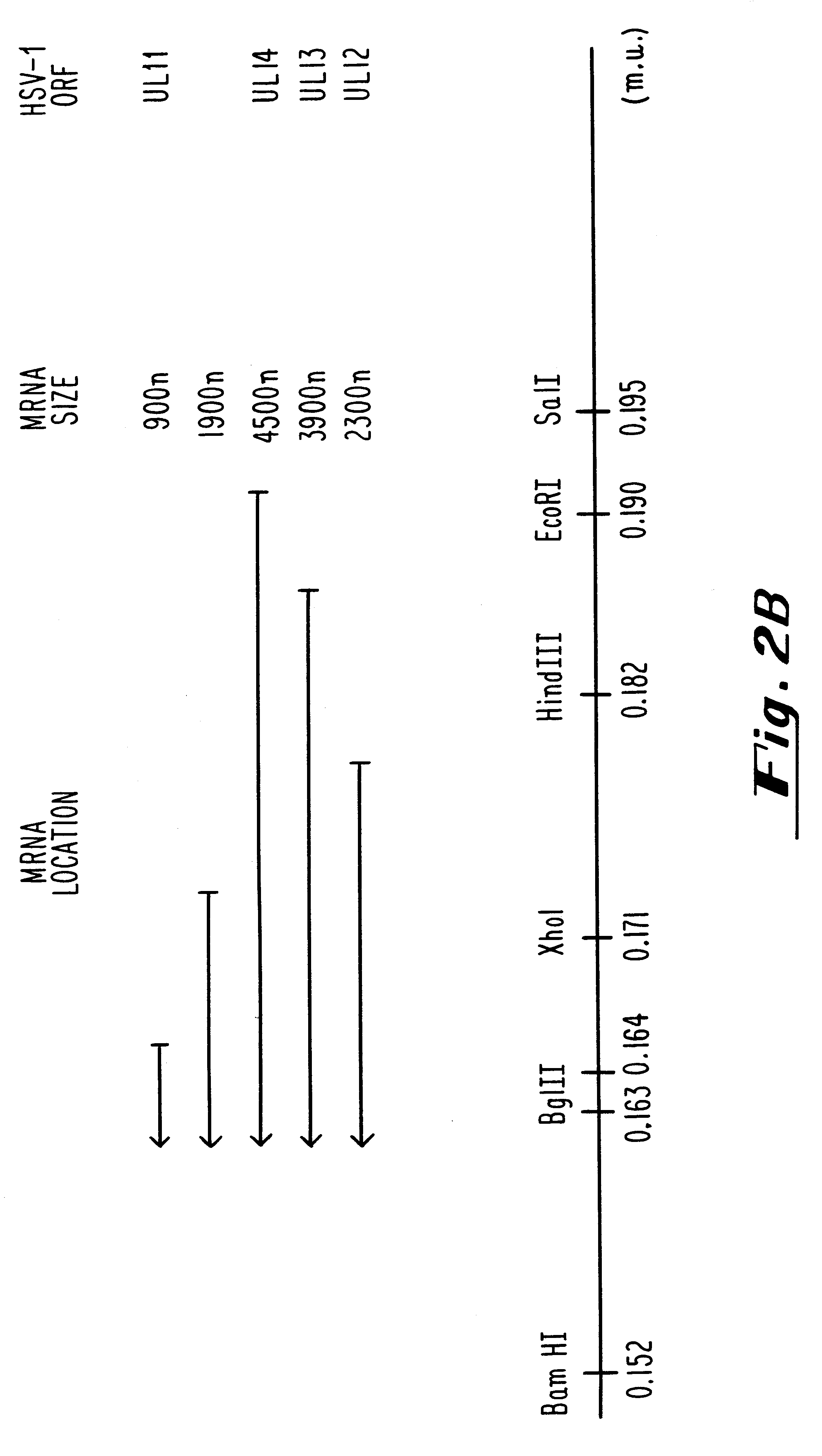
InactiveUS6310044B1Conveniently and desirably presentedFaster replicationPeptide/protein ingredientsGenetic material ingredientsOpen reading frameHerpesvirus infection
Compositions and methods are provided for the treatment and diagnosis of herpesvirus infections. In accordance with preferred embodiments, oligonucleotides are provided which are specifically hybridizable with RNA or DNA deriving from a gene corresponding to one of the open reading frames UL5, UL8, UL9, UL13, UL29, UL30, UL39, UL40, UL42 AND UL52 of herpes simplex virus type 1. The oligonucleotide comprises nucleotide units sufficient in identity and number to effect said specific hybridization. In other preferred embodiments, the oligonucleotides are specifically hybridizable with a translation initiation site; it is also preferred that they comprise the sequence CAT. Methods of treating animals suspected of being infected with herpesvirus comprising contacting the animal with an oligonucleotide specifically hybridizable with RNA or DNA deriving from one of the foregoing genes of the herpesvirus are disclosed. Methods for treatment of infections caused by herpes simplex virus type 1, herpes simplex virus type 2, cytomegalovirus, human herpes virus 6, Epstein Barr virus or varicella zoster virus are disclosed.
Owner:IONIS PHARMA INC
Cosmetic treatment with nitric oxide, device for performing said treatment and manufacturing method therefor
InactiveCN101146556AIncrease supplyDiastolic increaseCosmetic preparationsToilet preparationsVirus wartsNitric oxide
Owner:NOLABS AB
Detection method of brucella, kit and applications of kit
InactiveCN106191286AFull True Reflection ExtractionComprehensive and true reflection of amplificationMicrobiological testing/measurementMicroorganism based processesEscherichia coliSerum samples
The invention discloses a detection method of brucella. The detection method comprises the following steps: acquiring brucella DNA from a serum sample by adopting a centrifugal column method, thus obtaining a to-be-detected sample; carrying out PCR amplification reaction; detecting a reaction result by adopting a fluorescence quantitative PCR instrument, wherein during fluorescence signal collection, fluorescein corresponding to fluorophore at the BKV-FP 5' end of a Taqman fluorescence probe is set, and the fluorescence signals are collected at 60 DEG C; and analyzing the result. The invention further provides a kit for detecting the brucella. The detection method and the kit are high in sensitivity, and the minimal detection can achieve 1000 copies / mL. Meanwhile, the detection method is good in specificity, and the cross reaction with other bacteria, such as escherichia coli, salmonella enteritidis, hepatitis B virus, human herpes virus 4, and human cytomegalovirus can be avoided.
Owner:北京旌准医疗科技有限公司
Screening for CXCR4 receptor antagonism polypeptides for treating breast carcinoma and its uses
InactiveCN101334411AAbility to block transferHigh specific binding activityPeptide/protein ingredientsBiological testingLymphatic SpreadBinding site
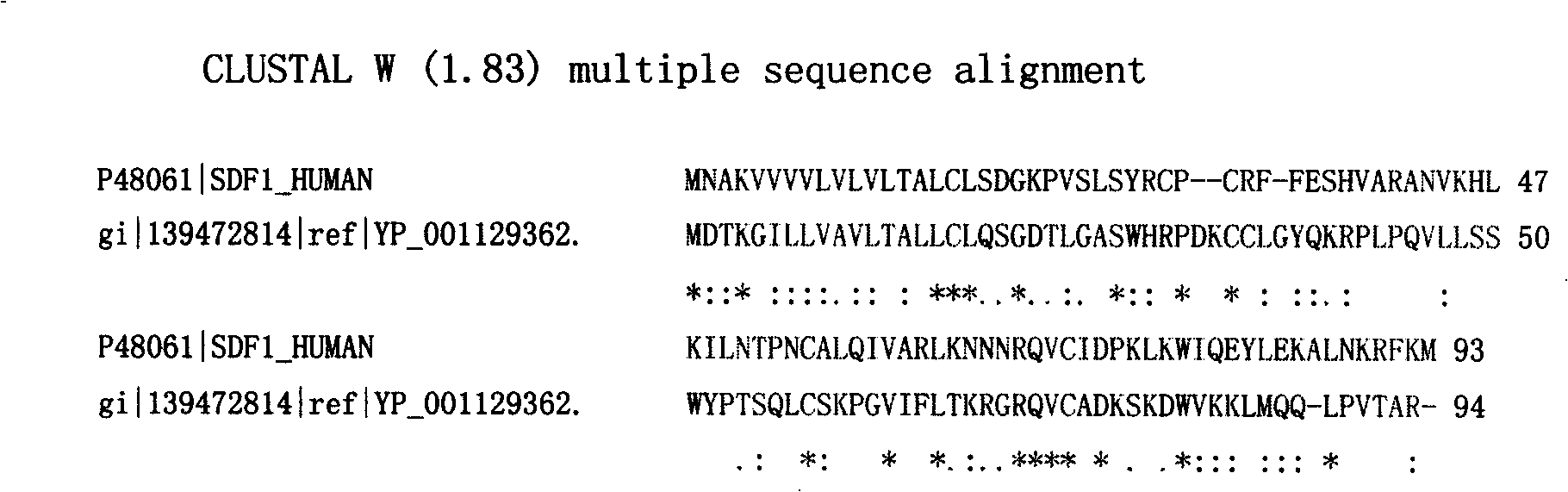
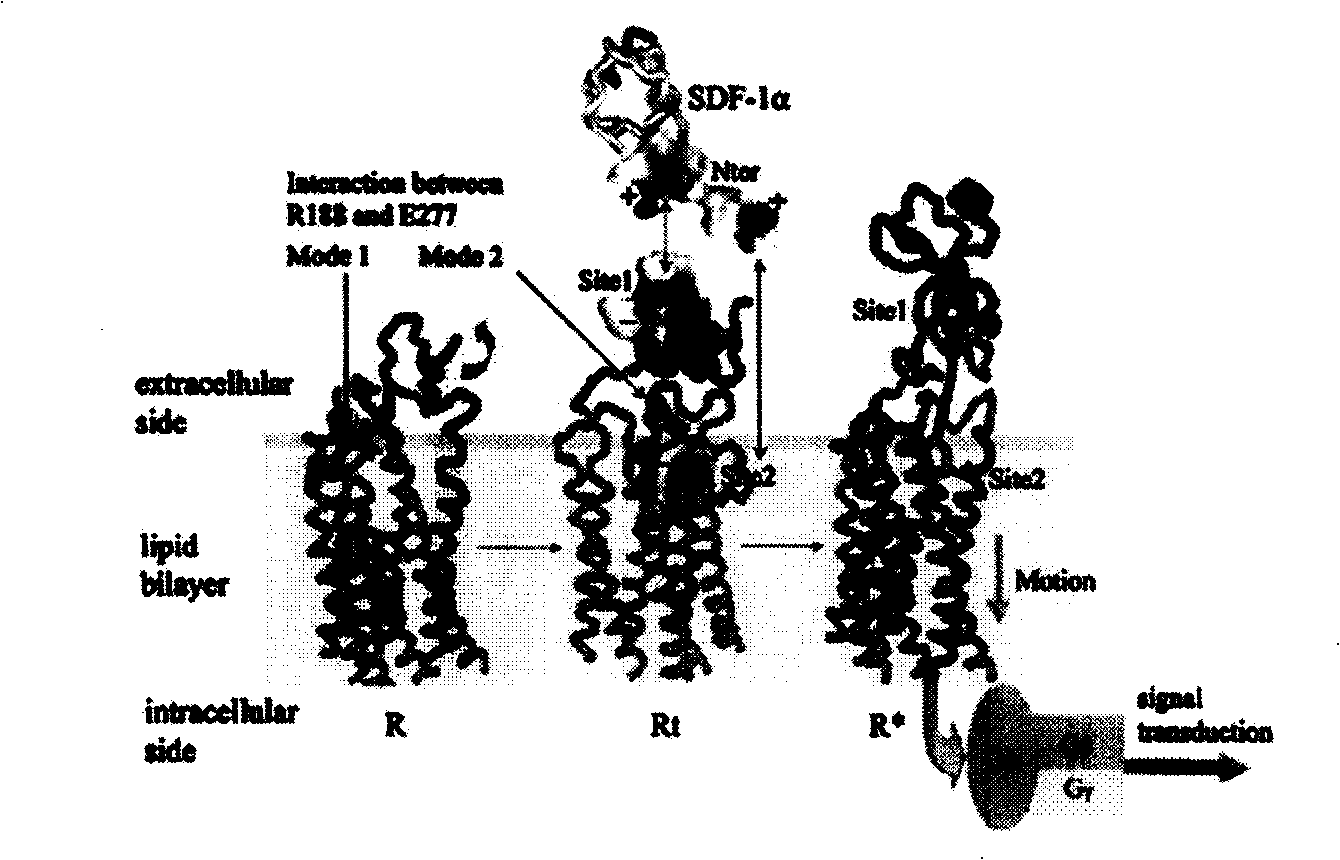
The invention provides a screening and modification method, a marker and an application of polypeptide drugs which are used in the treatment of CXCR4 receptor-mediated breast cancer. A human chemokine SDF-1 Alpha and the homology region of a human herpes virus 8 MIP-II are compared, thereby obtaining a binding site of vMIP-II and CXCR4 receptor and further obtaining a CXCR4 specific binding active peptide. With the role of specific binding CXCR4 and the blocking of the physiological binding of the SDF-1 Alpha and the CXCR4, the CXCR4 specific binding active peptide is a high-efficient and specific chemokine receptor antagonist. The chemotactic activity of wide type SDF-1 Alpha to breast cancer cells and the growth of the breast cancer cells can be inhibited, and the growth and the metastasis of the CXCR4-positive tumor cells are simultaneously inhibited, thereby having the dual-target effect. The invention further provides a marked polypeptide to be targetedly positioned in sentinel lymph node and metastatic site, thereby being used in the detection and the targeted radiotherapy of the metastatic status of the sentinel lymph node before and in the operation of the breast cancer.
Owner:BENGBU MEDICAL COLLEGE
Application of benzo Alpha-pyrone compound as Gamma-type human herpes virus-resistant medicine
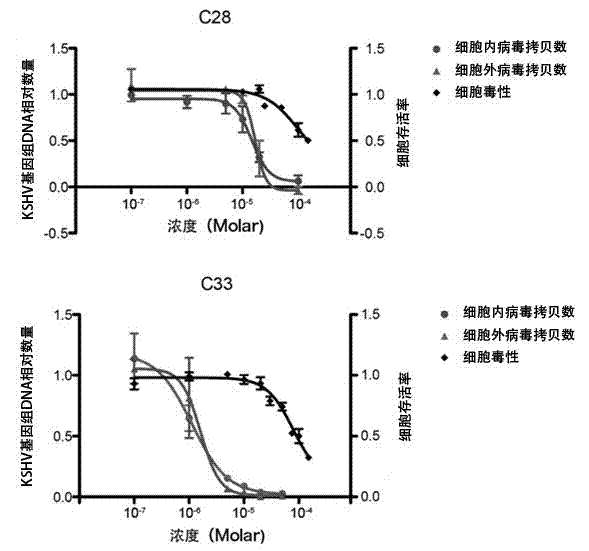
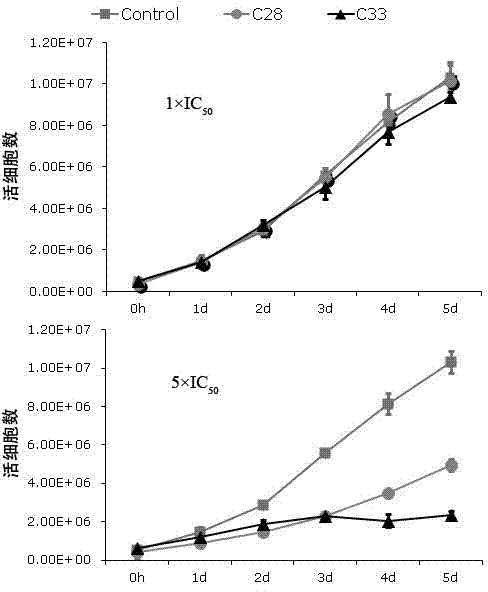
ActiveCN103070858AStrong inhibitory activityLow toxicityAntiviralsHeterocyclic compound active ingredientsDiseaseNovobiocin
The invention discloses an application of a benzo Alpha-pyrone compound as a Gamma-type human herpes virus-resistant medicine. According to the invention, the compound resistant to Gamma-type human herpes virus infection is higher in activity as compared with a novobiocin which is a marketed medicine, and is less in toxicity based on a cytoxicity test. The compound can be used for curing or preventing related diseases caused by KSHV infection.
Owner:SUN YAT SEN UNIV
Recombinant protein vaccine, recombinant expression vector containing genes for coding recombinant protein vaccine and application of recombinant protein vaccine
ActiveCN104707135AInhibition of activationActivation blockBacteriaMicroorganism based processesDiseaseTreatment effect
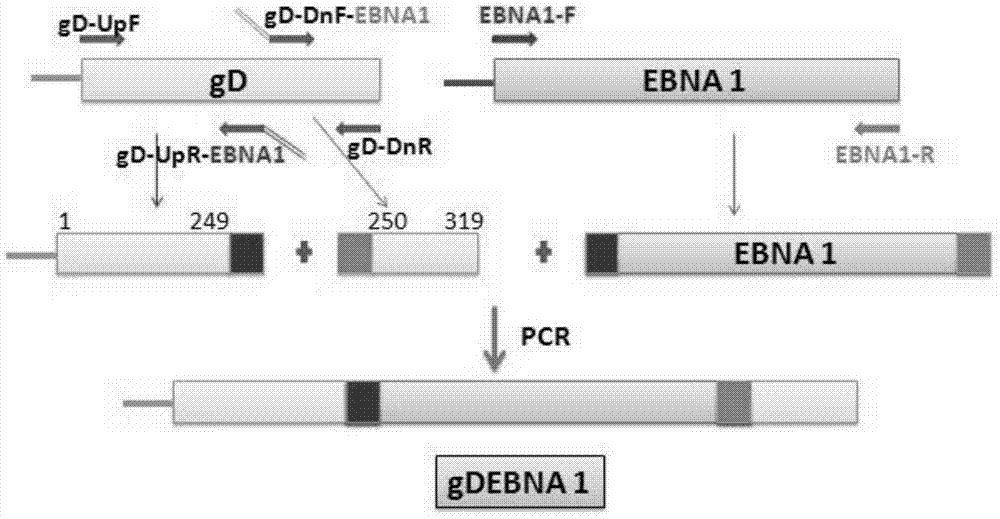
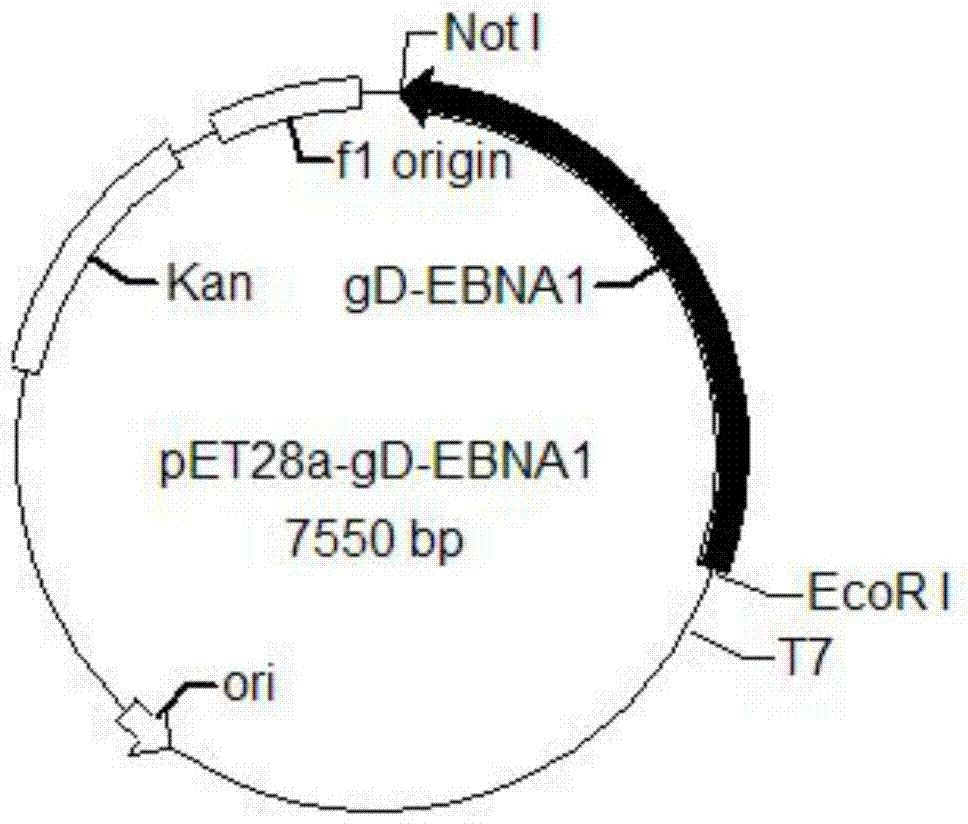
The invention provides a recombinant protein vaccine. The recombinant protein vaccine contains an epitope of EB virus antigen 1 (EBNA1) and an epitope of human herpes virus glycoprotein; fusion proteins can effectively activate a specific cell toxic T lymphocyte (CTL) reaction, directly restrain the expression EBNA1, and give play to treatment effects on EB virus-associated tumors; an immunosuppression path can be directly blocked through herpes virus glycoprotein, and the activity of regulatory T cells is improved. In this way, by means of the recombinant protein vaccine, EB virus infections and diseases related to the EB virus infections and tumors related to the EB virus infections can be prevented or treated in a multi-way manner more comprehensively. The invention further provides a recombinant expression vector containing genes for coding the recombinant protein vaccine and application of the recombinant protein vaccine.
Owner:SHENZHEN INST OF ADVANCED TECH
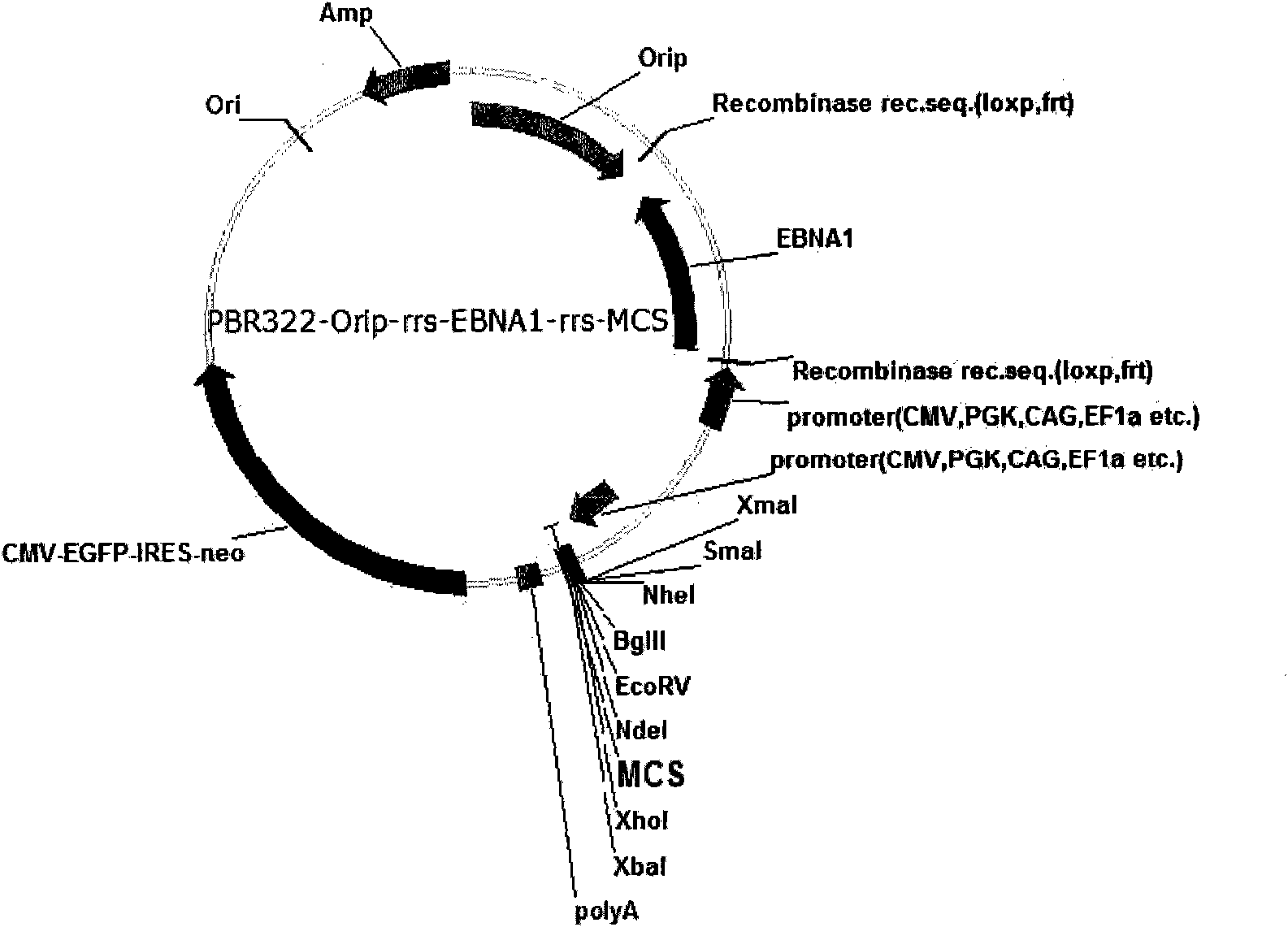
Expression system for nonintegrated, long-time and erasable expression vector
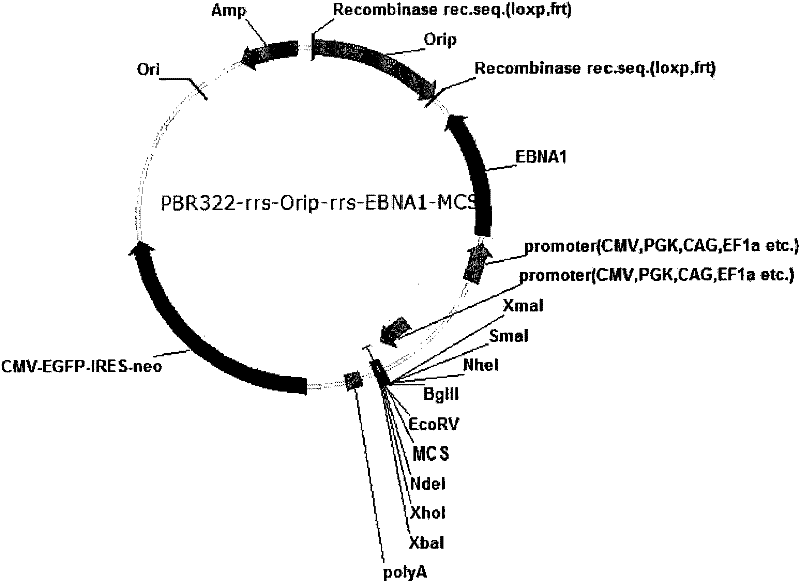
ActiveCN101955977AStable expressionControlled deletionFermentationGenetic engineeringReprogrammingRecognition sequence
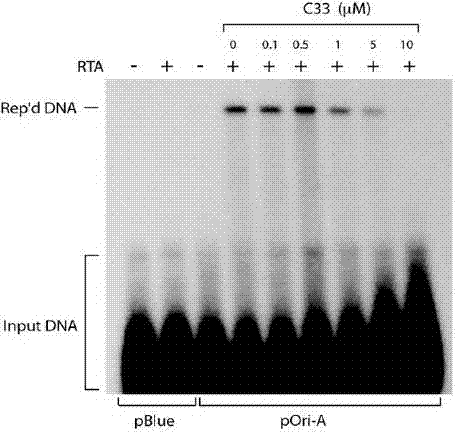
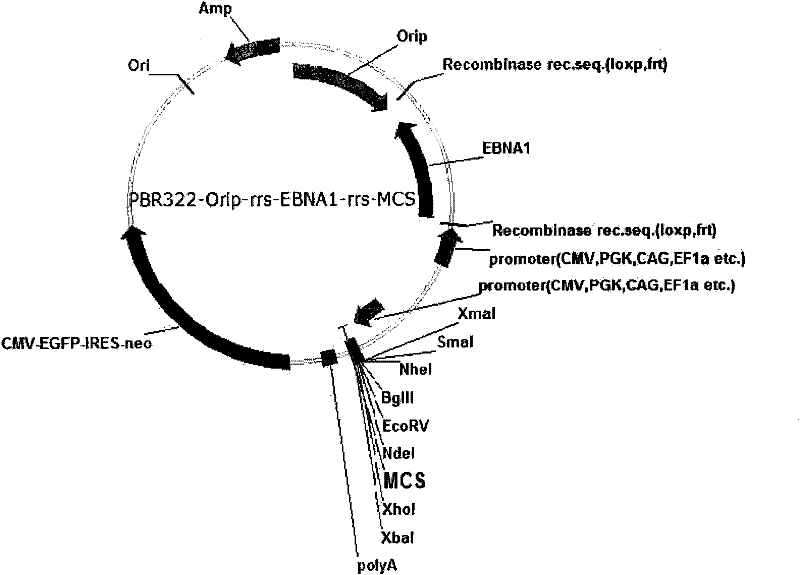
The invention provides an expression system for a nonintegrated, long-time and erasable expression vector. The expression vector of the expression system adopts human herpes virus replication origin (Orip), an EBNA-1 expression box is introduced, and both ends of the Orip, both end of EBN-1 or both ends of Orip-EBNA-1 are provided with recombinase recognition sequences. The expression vector is not integrated in a cell genome, can stably express for a long time, can controllably erase vector plasmid by instantaneously inducing (or expressing) recombinase from a hose cell, can be applied to most kinds of cells by using a strong initiator and can distinguish a cell with the vector plasmid from a cell without the vector plasmid by using a real-time marker. The expression system can widely applied to mammal cell expression and biomedicine engineering for realizing cell reprogramming by expressing a foreign gene.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Application of 2-furan acrylaldehyde compound to preparation of medicine for resisting human gamma-herpes virus
ActiveCN106727489AInhibition of lytic replicationHigh chance of KSOrganic active ingredientsAntiviralsFuranEbola virus
The invention discloses application of a 2-furan acrylaldehyde compound to preparation of a medicine for resisting human gamma-herpes virus. The 2-furan acrylaldehyde compound provided by the invention can be used for inhibiting lytic replication of the human gamma-herpes virus; compared with a marketing drug, namely acyclovir, the 2-furan acrylaldehyde compound has relatively small toxicity and the activity is equivalent. Therefore, the compound has a good application prospect in treatment of related diseases caused by KSHV (Kaposi's Sarcoma-associated Herpesvirus) and EBV (Ebola Virus) infection.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
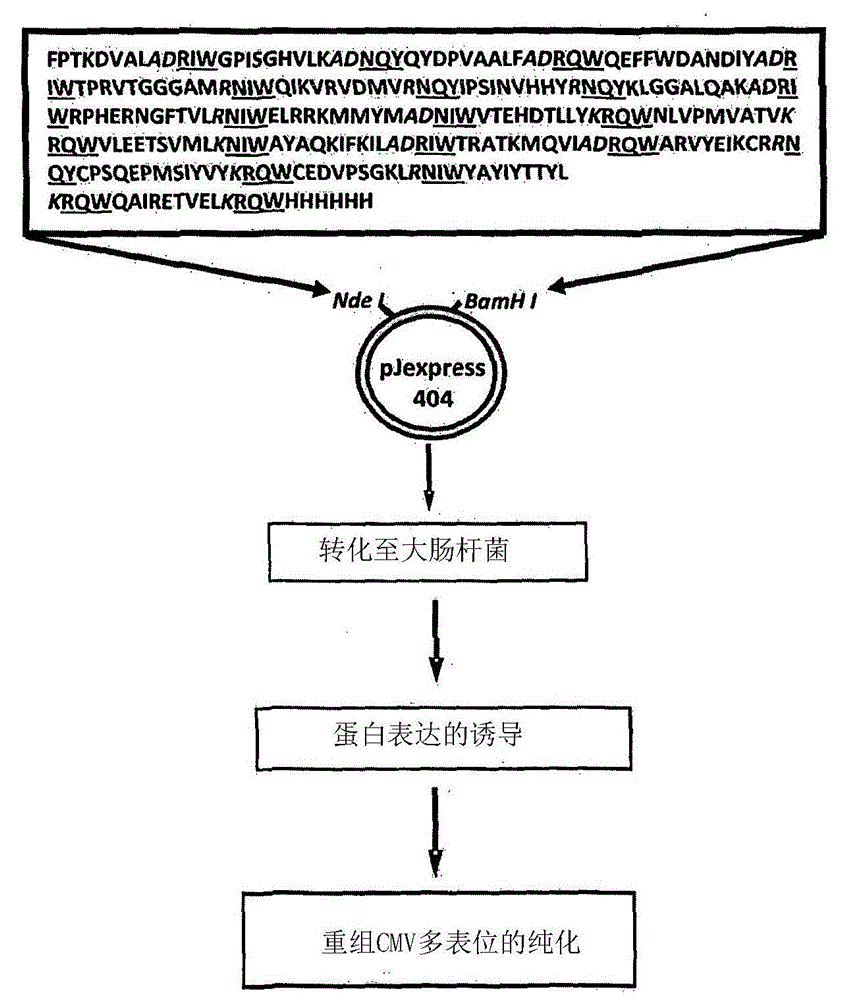
Improved human herpesvirus immunotherapy
An isolated protein comprises respective amino acid sequences of each of a plurality of CTL epitopes from two or more different herpesvirus antigens and further comprises an intervening amino acid or amino acid sequence between at least two of said CTL epitopes comprising proteasome liberation amino acids or amino acid sequences and, optionally, Transporter Associated with Antigen Processing recognition motifs. The isolated protein is capable of rapidly expanding human cytotoxic T lymphocytes (CTL) in vitro and eliciting a CTL immune response in vivo upon administration to an animal as an exogenous protein. Typically, the isolated protein comprises no more than twenty (20) CTL epitopes derived from cytomegalovirus and / or Epstein-Barr virus antigens.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
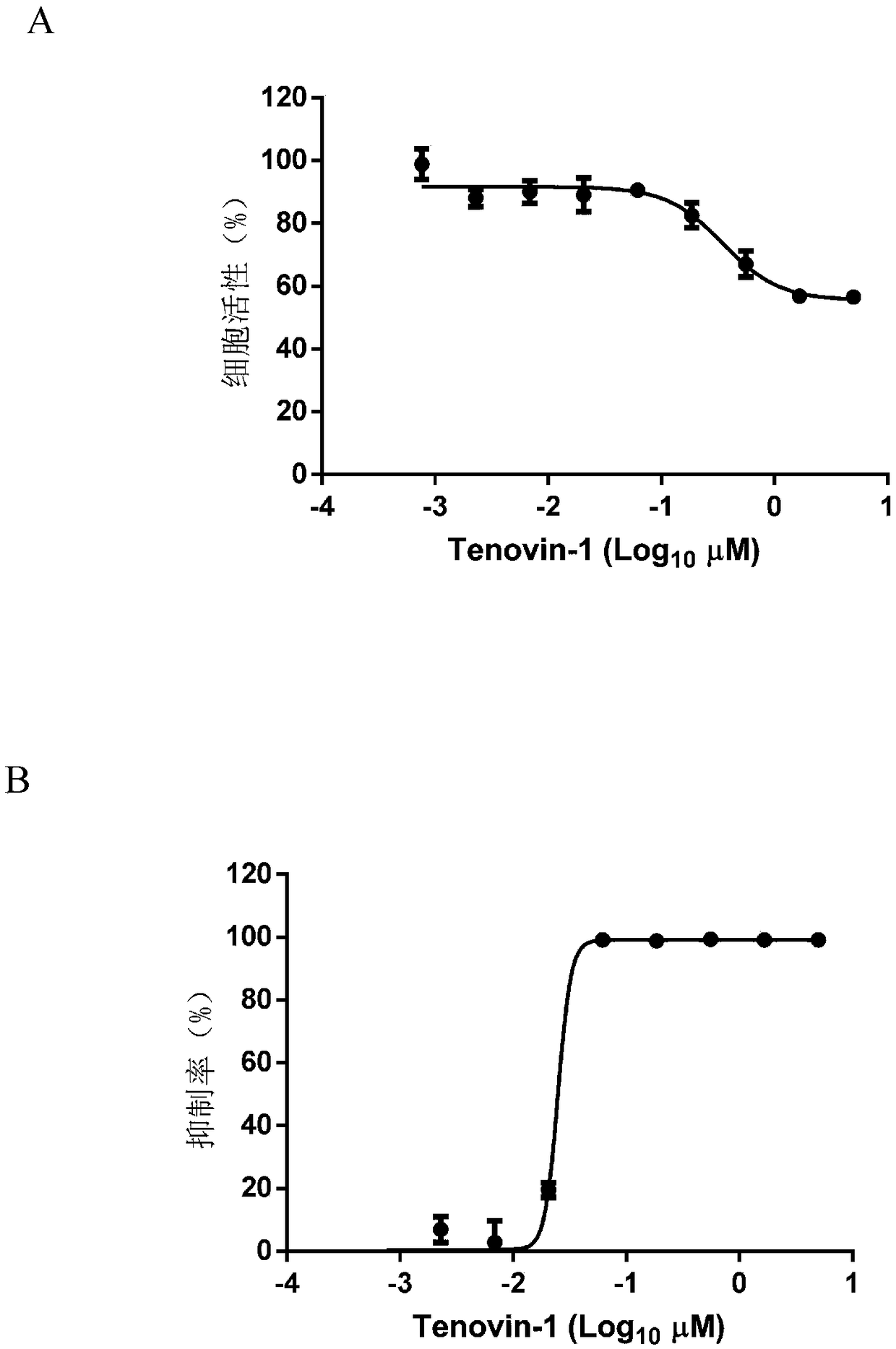
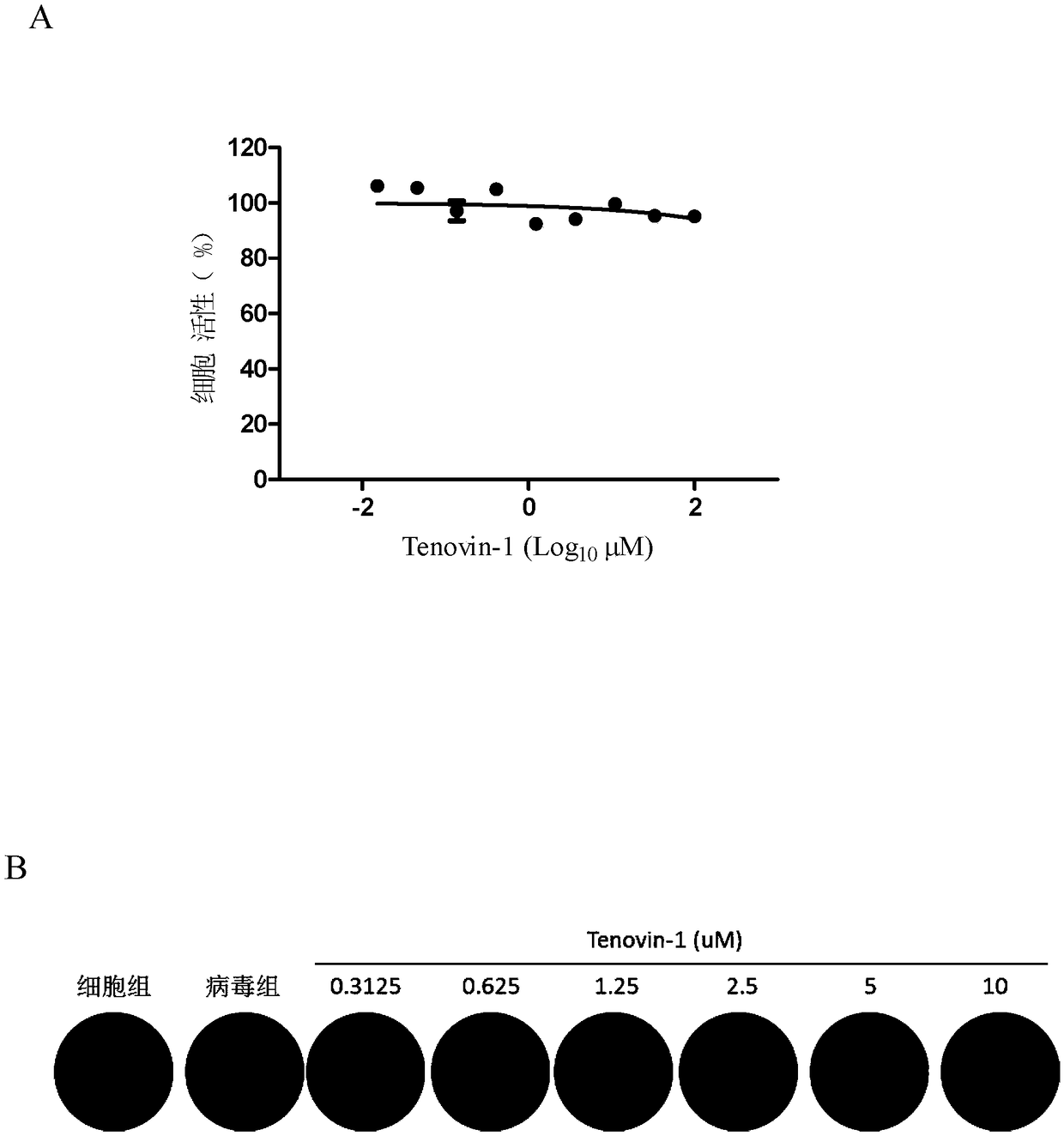
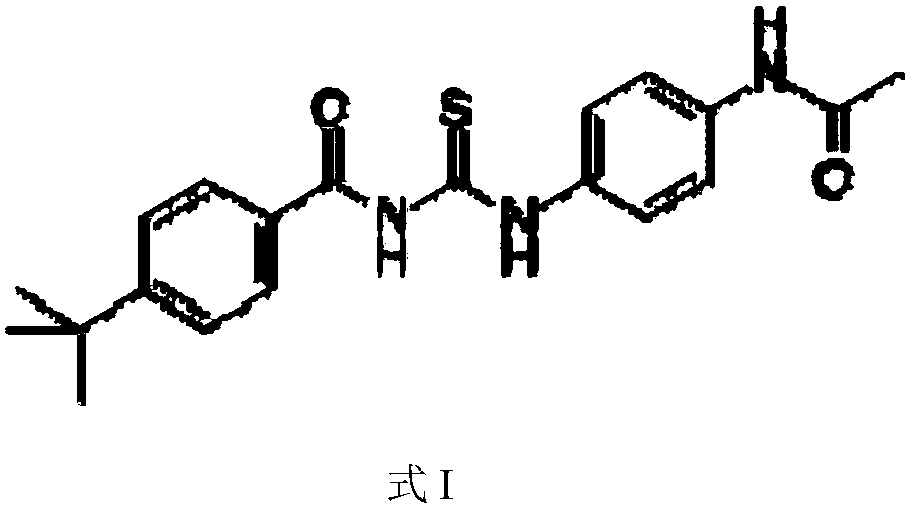
Application of Tenovin-1 in preparing drug for preventing and controlling human herpes virus infection
InactiveCN108619123AAvoid infectionGood inhibitory effectAntiviralsAmide active ingredientsHerpes simplex virus DNAInfection disease
The invention provides a use of Tenovin-1 in preparing a drug. The drug is used for treating or preventing herpes virus infection. According to the embodiment of the invention, the Tenovin-1 can be effectively used for treating or preventing herpes virus infection. Detection on herpes viruses in different subtypes shows that, the Tenovin-1 has the activity of broad-spectrum anti-herpes viruses, ishigh in selection index, can be developed into a drug for treating herpes virus infection diseases and has a wide application prospect.
Owner:武汉威立得生物医药有限公司
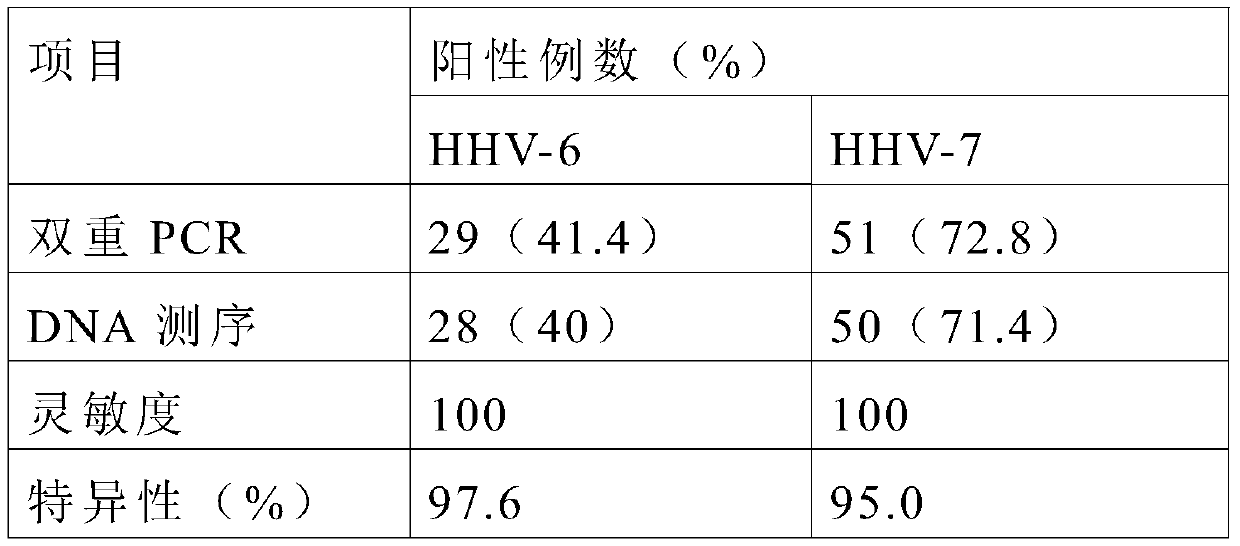
Primer, probe and kit for synchronously detecting human herpes virus types 6, 7 and 8
ActiveCN106119424ALittle interactionStrong specificityMicrobiological testing/measurementMicroorganism based processesVirus typeSynchronous detection
The invention discloses a primer, a probe and a kit for synchronously detecting human herpes virus types 6, 7 and 8. By selecting a new target gene, redesigning a primer and a kit, and optimizing a reaction system, the false positive rate of clinical synchronous detection on human herpes viruses is greatly reduced, the primer, the probe and the kit are good in sensitivity, good in specificity and short in detection time, and the technique can be applied to clinical application and popularization.
Owner:湖南光谷创新医疗器械公共服务平台有限公司
Kit for detecting human herpes virus infection and detection method thereof
PendingCN111500788AEasy to detectQuick checkMicrobiological testing/measurementDNA/RNA fragmentationInfected patientNucleotide
Owner:领航医学科技(深圳)有限公司
Human herpes virus six-type real-time fluorescence quantitative PCR (polymerase chain reaction) kit
InactiveCN102154515AShort detection cycleImprove efficiencyMicrobiological testing/measurementMicroorganism based processesQualitative analysisBioinformatics
The invention provides a human herpes virus six-type real-time fluorescence quantitative PCR (polymerase chain reaction) kit, and relates to a PCR kit in the technical field of the biology. The kit comprises a pair of specific primers, a specific fluorescent probe, a positive control, a negative control, a 5*PCR Buffer and an Enzyme Mix, wherein the specific primers are designed to be: F: 5'-ACAAAGCGAAATTATCCAGAGCGT-3', and R: 5'-GCGCTAGGTTGAGGATGATCGC-3'; and the specific probe is designed to be: FP: 5'-FAM-ACACCAGACGTCACACCCGAAGGAATT-TAMARA3'. The kit is short in detection period, high in efficiency, high in viral specificity detection, high in accuracy, qualitative and quantitative in virus analysis, higher in the sensitivity compared with the common PCR and immunology detection method, simple in operation, easy to popularize, and good in the repeatability of the experimental result.
Owner:WUHAN UNIV
Human herpes virus hominis pyrolysis, reproduction and activation host marker and purpose thereof
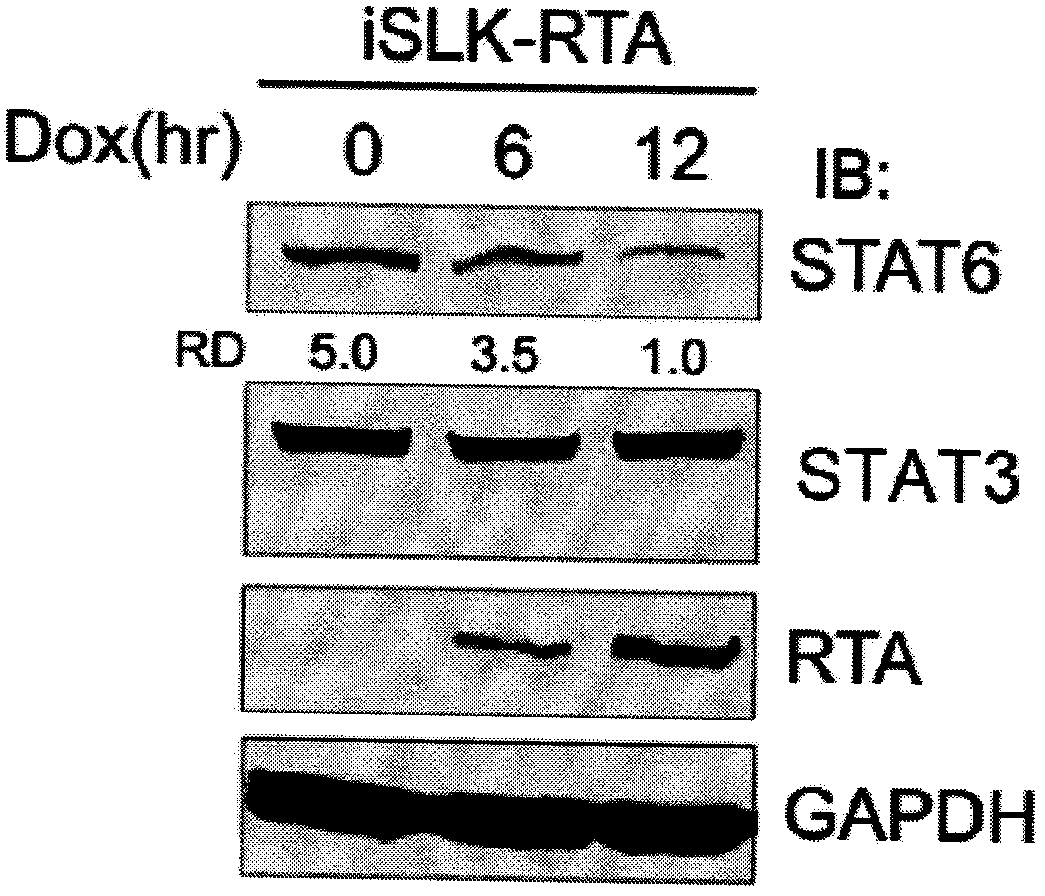
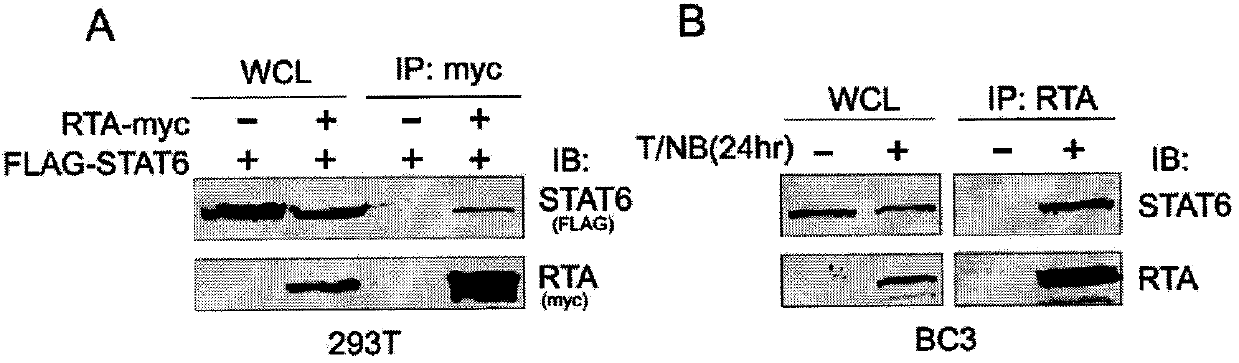
The invention belongs to the technical field of biomedicine, and relates to a human herpes virus hominis pyrolysis, reproduction and activation detection marker, in particular to the purpose of a signal transducer and activator of transcription 6 (Signal transducer and activator of transcription 6,STAT6) as the human herpes virus hominis pyrolysis, reproduction and activation detection marker. Target protein-STAT6 which is consistently regulated during human herpes virus pyrolysis and reproduction is obtained from the host angle, the experiment result shows that the STAT6 protein is significantly reduced in the infection course of Kaposi sarcoma herpesvirus (KSHV), and the STAT6 protein can be used as the marker for detecting pyrolysis, reproduction and activation of various herpes viruses, can be further used as a common target for diagnosing and treating herpes virus relevant tumor diseases, and can be further used for preparing products for preventing, detecting and treating human herpes virus hominis infection.
Owner:FUDAN UNIV
Treatment of human herpesviruses using hyperthermia
InactiveCN1383375AEasy to explainOrganic active ingredientsPeptide/protein ingredientsVirologyHyperthermia
The present invention provides a method for treating patients with human herpes virus infection, comprising: raising the patient's core body temperature at least once and then returning to normal, the core body temperature is raised to a temperature and duration sufficient to reduce or eliminate the patient's human herpes virus load.
Owner:FIRST CIRCLE MEDICAL
Expression system for nonintegrated, long-time and erasable expression vector
ActiveCN101955977BStable expressionControlled deletionGenetic engineeringFermentationRecombinaseBiomedicine
The invention provides an expression system for a nonintegrated, long-time and erasable expression vector. The expression vector of the expression system adopts human herpes virus replication origin (Orip), an EBNA-1 expression box is introduced, and both ends of the Orip, both end of EBN-1 or both ends of Orip-EBNA-1 are provided with recombinase recognition sequences. The expression vector is not integrated in a cell genome, can stably express for a long time, can controllably erase vector plasmid by instantaneously inducing (or expressing) recombinase from a hose cell, can be applied to most kinds of cells by using a strong initiator and can distinguish a cell with the vector plasmid from a cell without the vector plasmid by using a real-time marker. The expression system can widely applied to mammal cell expression and biomedicine engineering for realizing cell reprogramming by expressing a foreign gene.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Virus titration method
InactiveCN101983244AQuantitatively accurateMicrobiological testing/measurementBiological material analysisContinuous evaluationBody fluid
Provided is a method for counting human herpes virus (HHV) harvested from body fluids and a kit for performing said method. In the past, skilled technique was required to accurately count HHV from body fluids. Provided is a novel titration method that allows simpler, and more accurate and efficient measurement of HHV counts in body fluids. Since the method can tolerate continuous evaluation of HHV counts in body fluids, it can also be used for quantitative evaluation of accumulated fatigue.
Owner:JAPAN TOBACCO INC
Primer probe combination and kit for detecting pathogen nucleic acids of human herpes virus and application of primer probe combination and kit
ActiveCN113122660AMicrobiological testing/measurementDNA/RNA fragmentationNucleic acid amplification techniqueMicroorganism
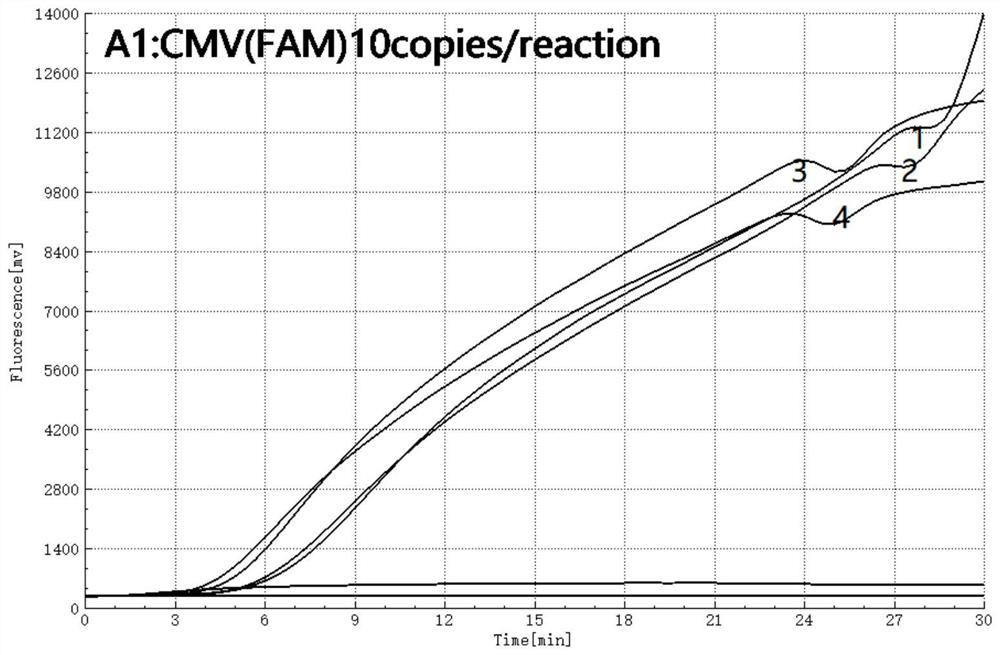
The invention relates to the field of detection of microorganisms and nucleic acid genomes, in particular to a primer combination and a probe for detecting nucleic acids of a clinically common human herpes virus (cytomegalovirus) by using an isothermal nucleic acid amplification technology. The primer combination and the probe provided by the invention are good in specificity and high in sensitivity.
Owner:冯志山
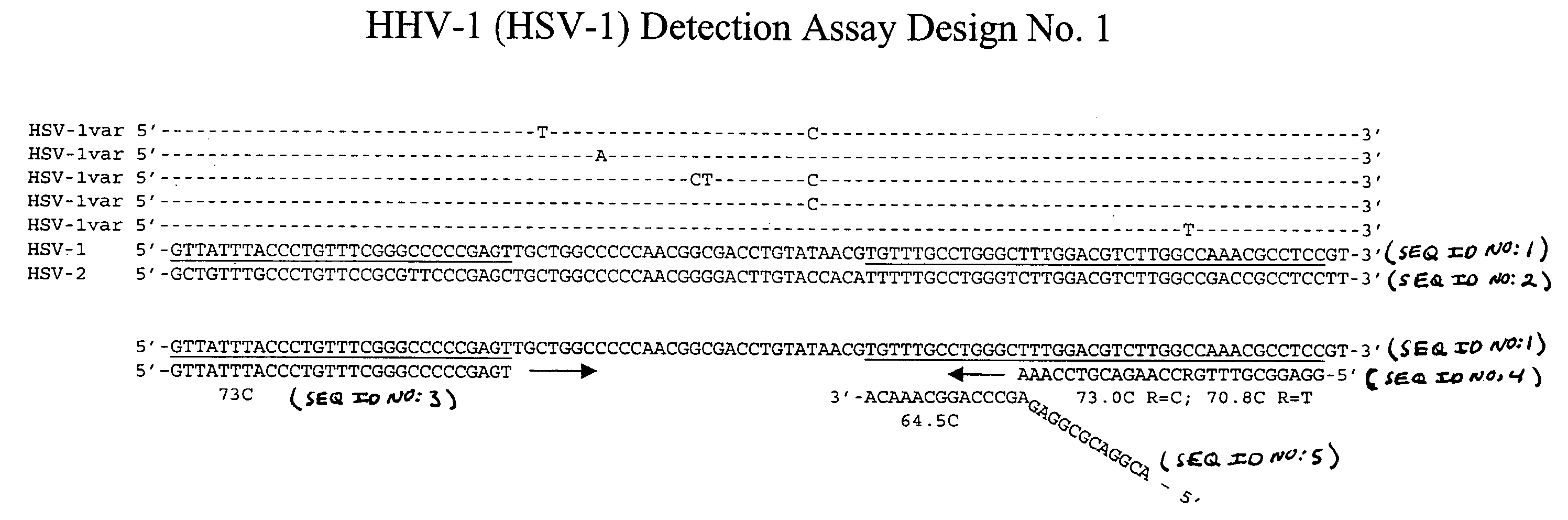
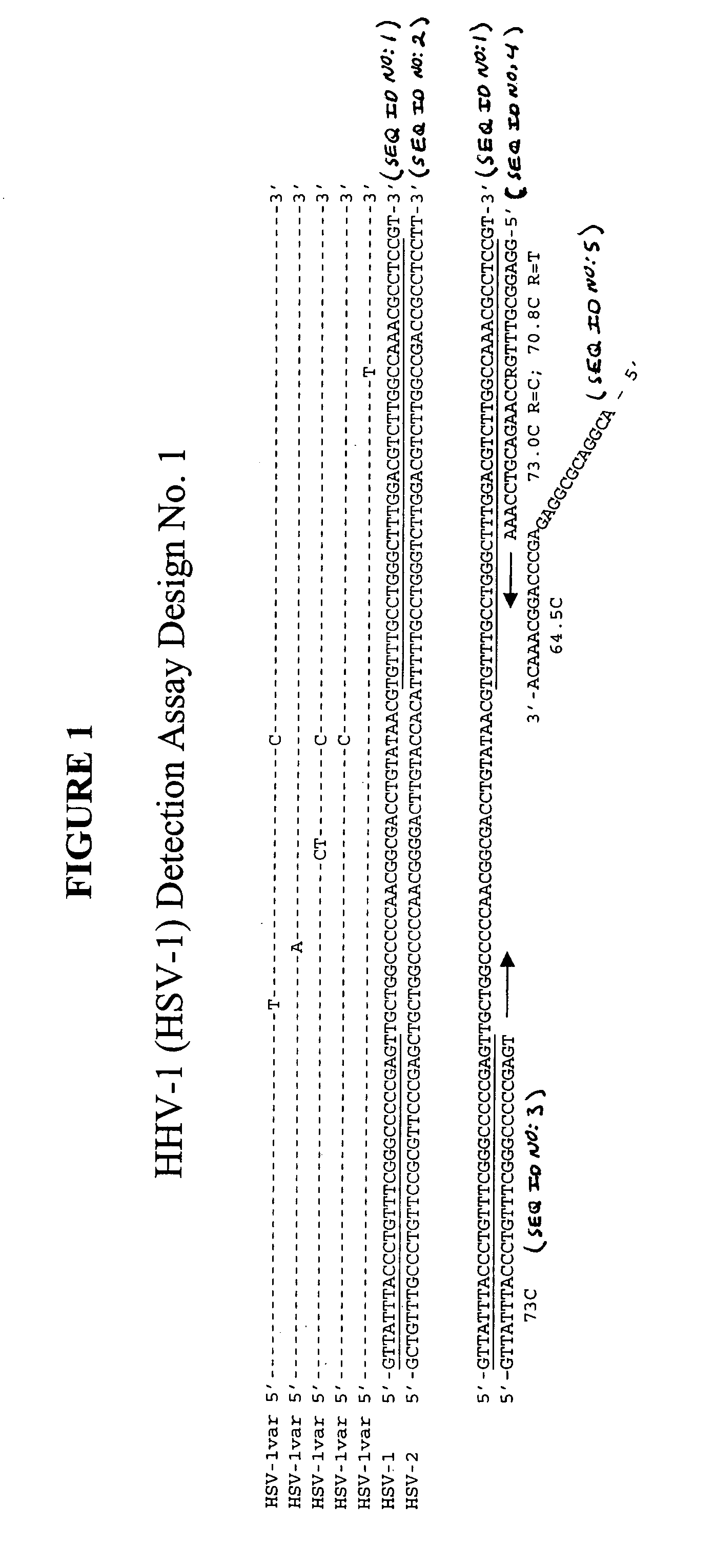
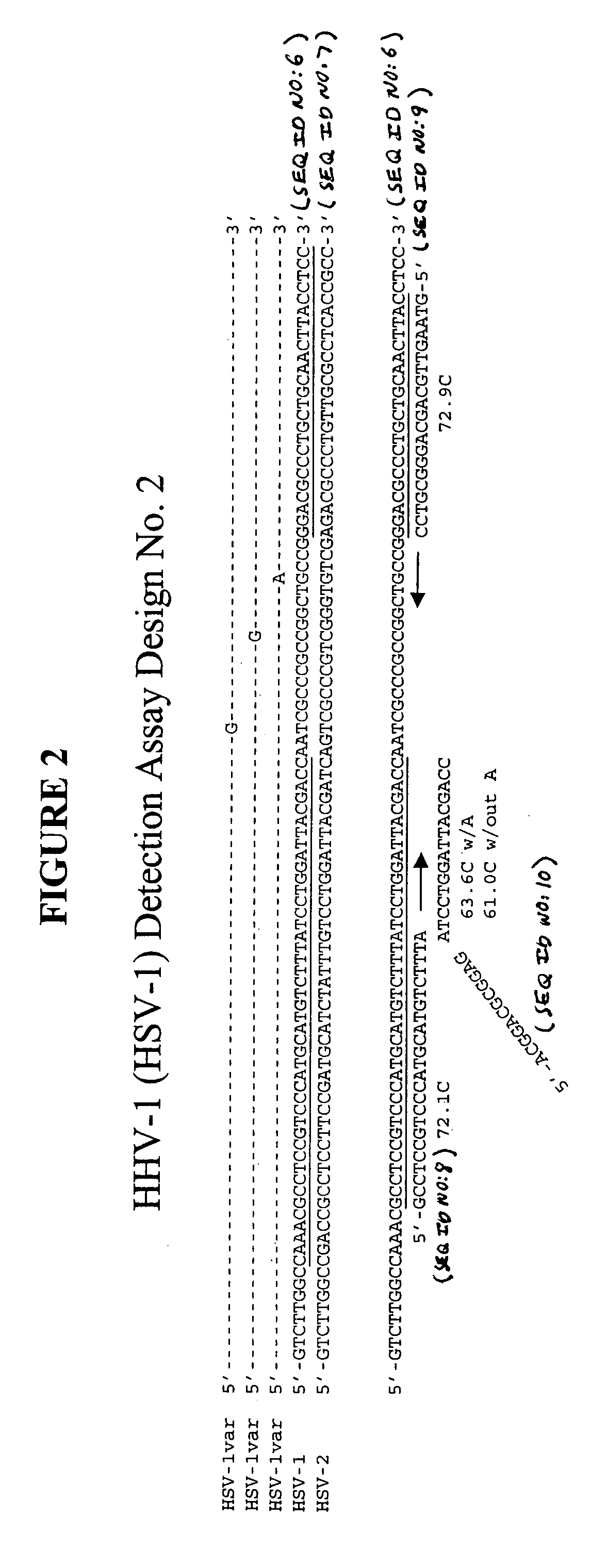
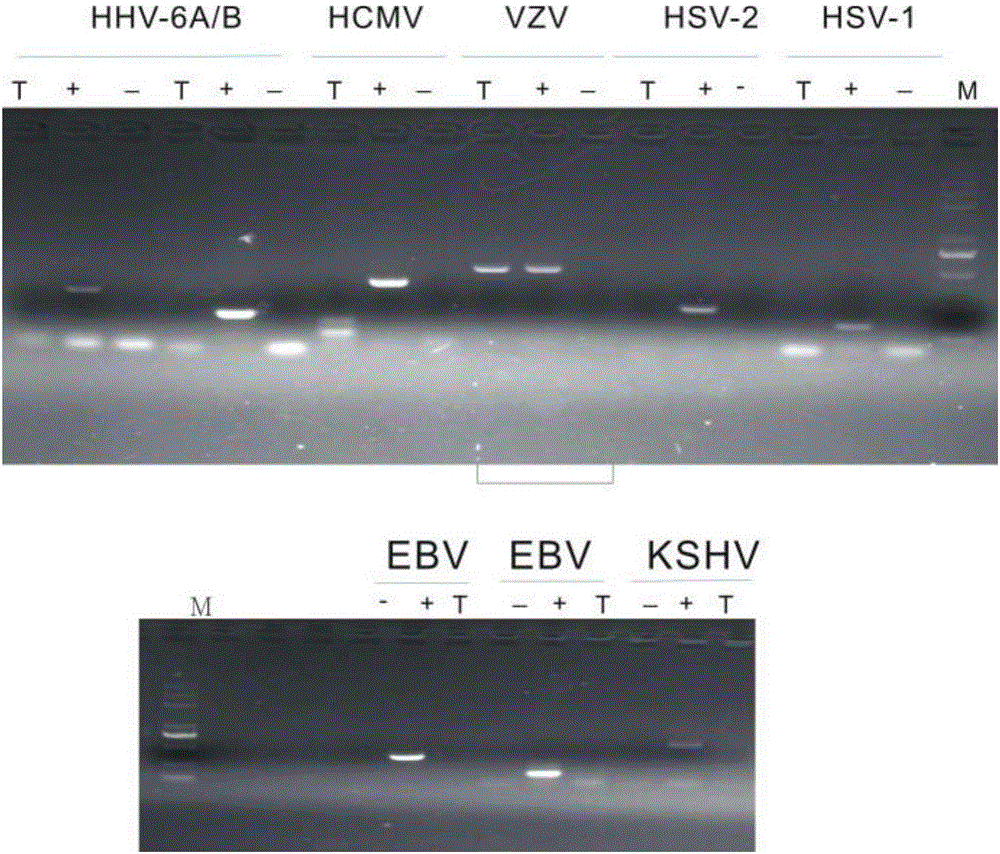
Clinical assays for the detection and typing of human herpesviruses
InactiveUS7348145B2Quick checkTest cycle time superiorSugar derivativesMicrobiological testing/measurementHeterologousHeteroduplex
The present invention provides methods of unambiguously identifying a human herpesvirus in a sample. The assays, which allow for the detection and typing of all ten human herpesviruses, involve multiplex PCR assays using consensus primers to amplify conserved regions of the herpesvirus DNA. A dot blot / chemiluminescence assay and real time PCR assay ideal for clinical setting were disclosed. A heteroduplex mobility assay suitable for uses in research laboratory was also presented.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Nucleotide Analogue Prodrug and the Preparation Thereof
InactiveUS20120010171A1Better activity profileBetter anti-viral activityBiocideOrganic active ingredientsPurification methodsNucleotide
The present invention provides: 1) Derivative solid form of 9-[2-(R)-[bis[pivaloyloxymethoxy]-phosphinylmethoxy]propyl]adenine (bis-POM PMPA, abbreviated as TD hereinafter), including crystalline form A and form B of TD, TD fumarate salts and cyclodextrin inclusion complex of TD; 2) Synthesis and purification methods of TD and Solidification method of TD oil, including converting TD oil to crystalline TD in Form A and Form B, solid TD salts and cyclodextrin inclusion complex of TD; 3) Stable pharmaceutical compositions containing TD derivatives and their preparation; 4) The use of the above TD derivatives in the antiviral treatments, especially in the treatment of HIV, HBV, CMV, HSV-1, HSV-2 and human Herpes virus infections.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +1
Method for detecting pathogens infected by cerebrospinal fluid based on PCR and nanopore sequencing
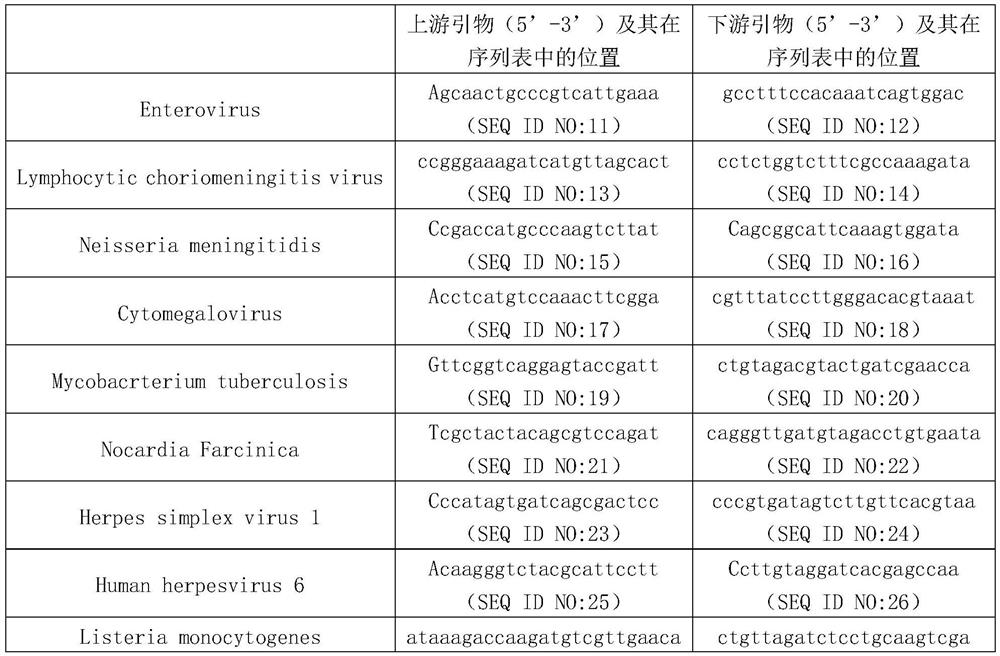
ActiveCN113201599AAccurate and rapid etiological diagnosisHigh clinical application valueMicrobiological testing/measurementAgainst vector-borne diseasesEnterovirusListeria monocytogenes
The invention discloses a method for detecting pathogens infected by cerebrospinal fluid based on PCR (polymerase chain reaction) and nanopore sequencing. Experiments prove that the method provided by the invention is used for detecting the cerebrospinal fluid, and the method can be used for accurately judging which one or more of pathogens for infecting the central nervous system is or are selected from the group consisting of Enterovirus, Lymphotic virus, Neisseria meningiosis, Cytomegalovirus, Mycobacterium tubers, Nocardia Farcina, Herpes simplex virus 1, Human herpes virus 6, Listeria monocytogenes and Herpes simplex virus 2. The invention provides accurate and rapid etiological diagnosis for central nervous system infection, and has very high clinical application value.
Owner:PEOPLES HOSPITAL PEKING UNIV
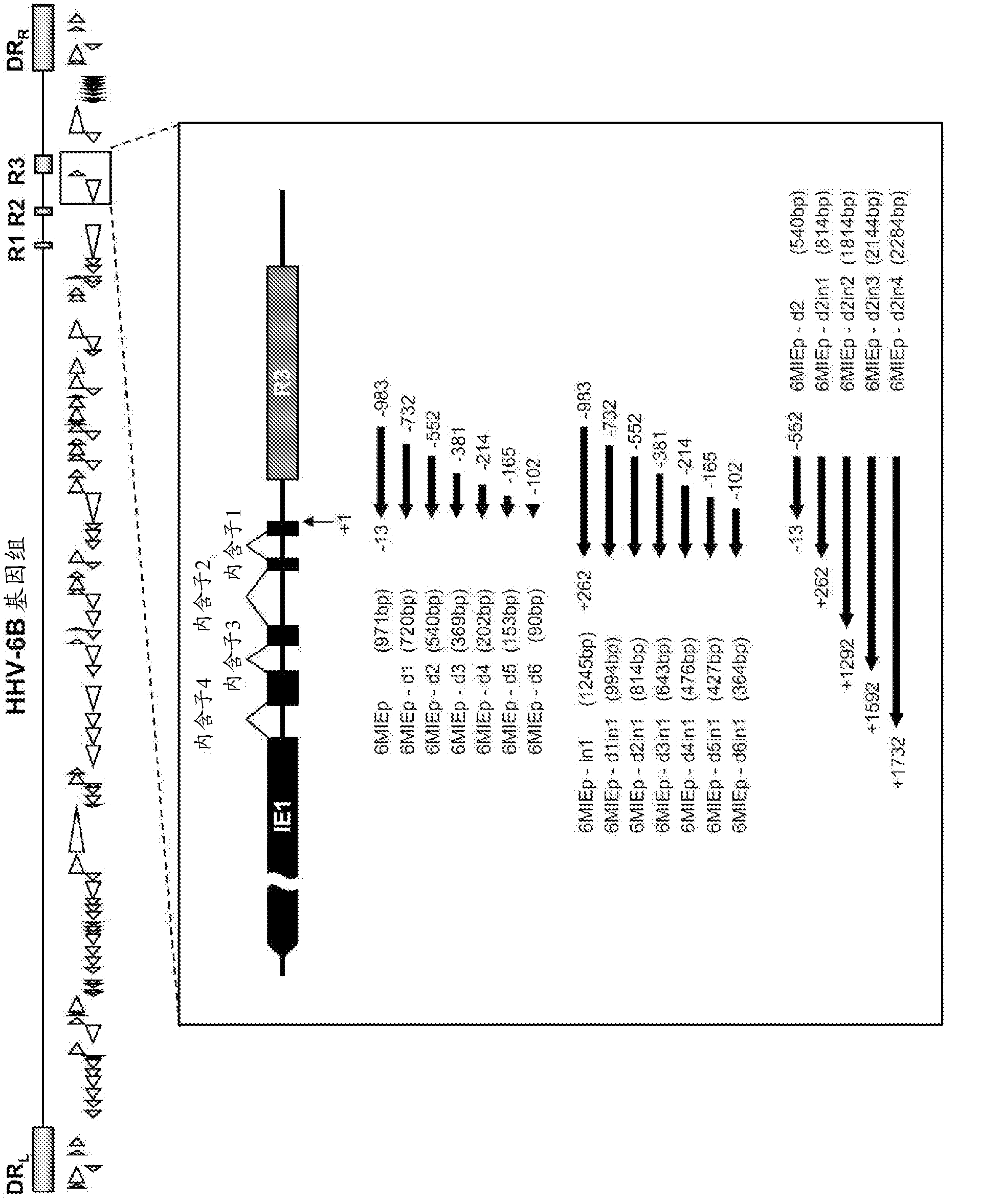
Enhancer for promoter, and use thereof
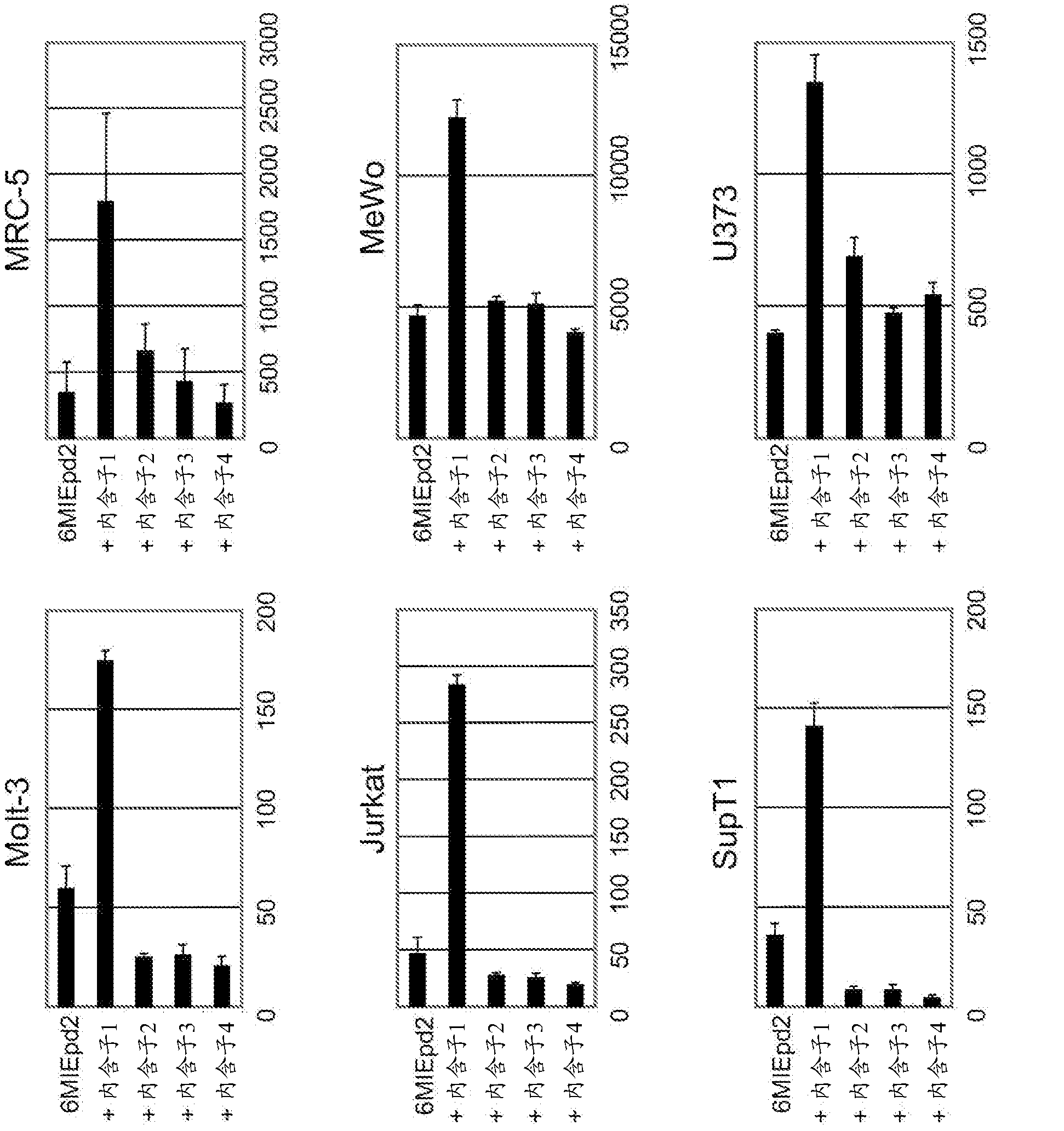
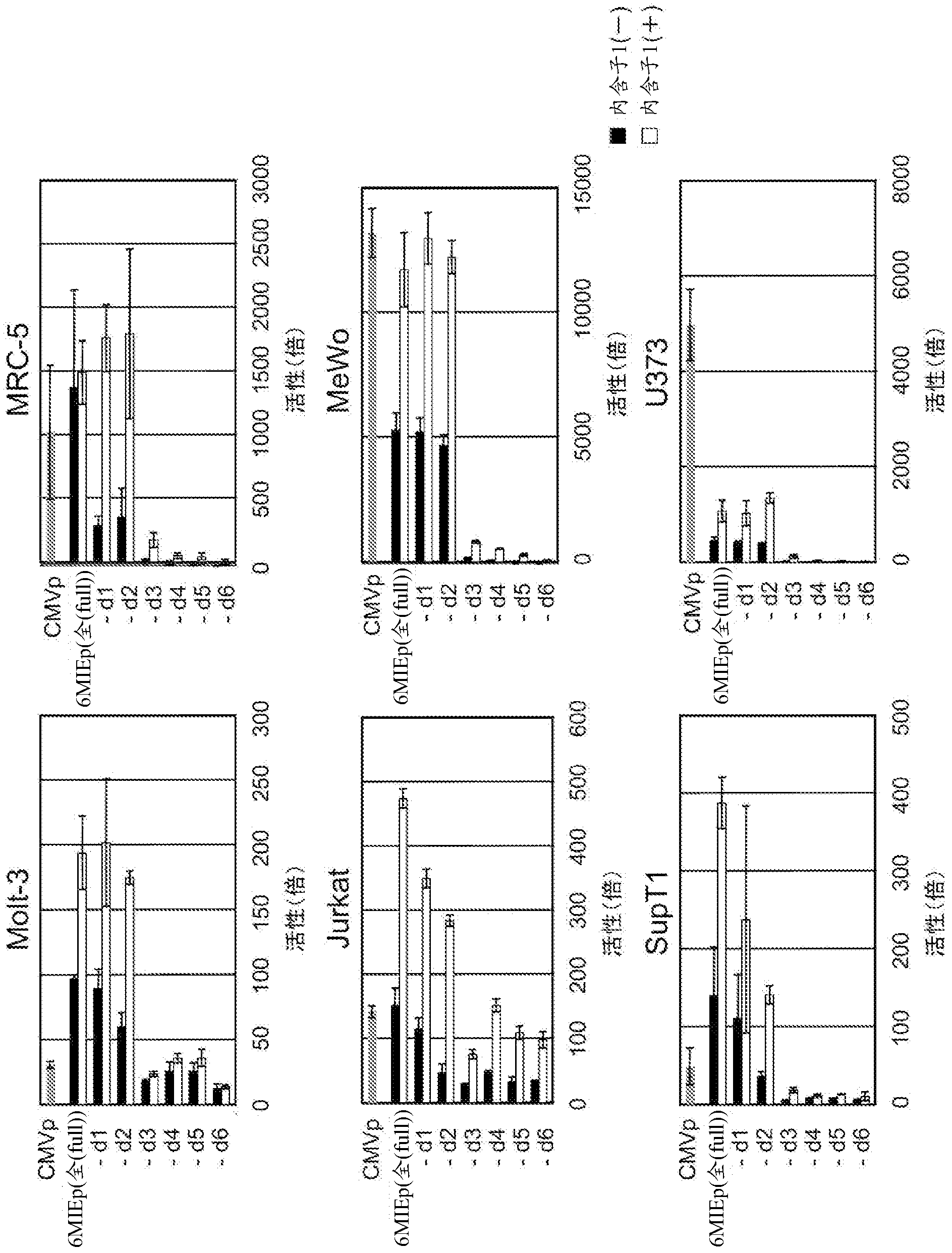
Disclosed is an enhancer for a viral promoter such as a promoter that can induce expression selectively and strongly in immunocompetent cells (e.g., lymphocytes) or blood cells. It is found unexpectedly that an intron has the above-mentioned enhancer activity. Thus, it is found that an enhancer for a promoter, which comprises an intron sequence for a major immediate early gene (MIE) of human herpes virus-6 (HHV-6) (particularly HHV-6B) or a fragment of the intron sequence, has a potent promoter activity.
Owner:NAT INST OF BIOMEDICAL INNOVATION HEALTH & NUTRITION +1
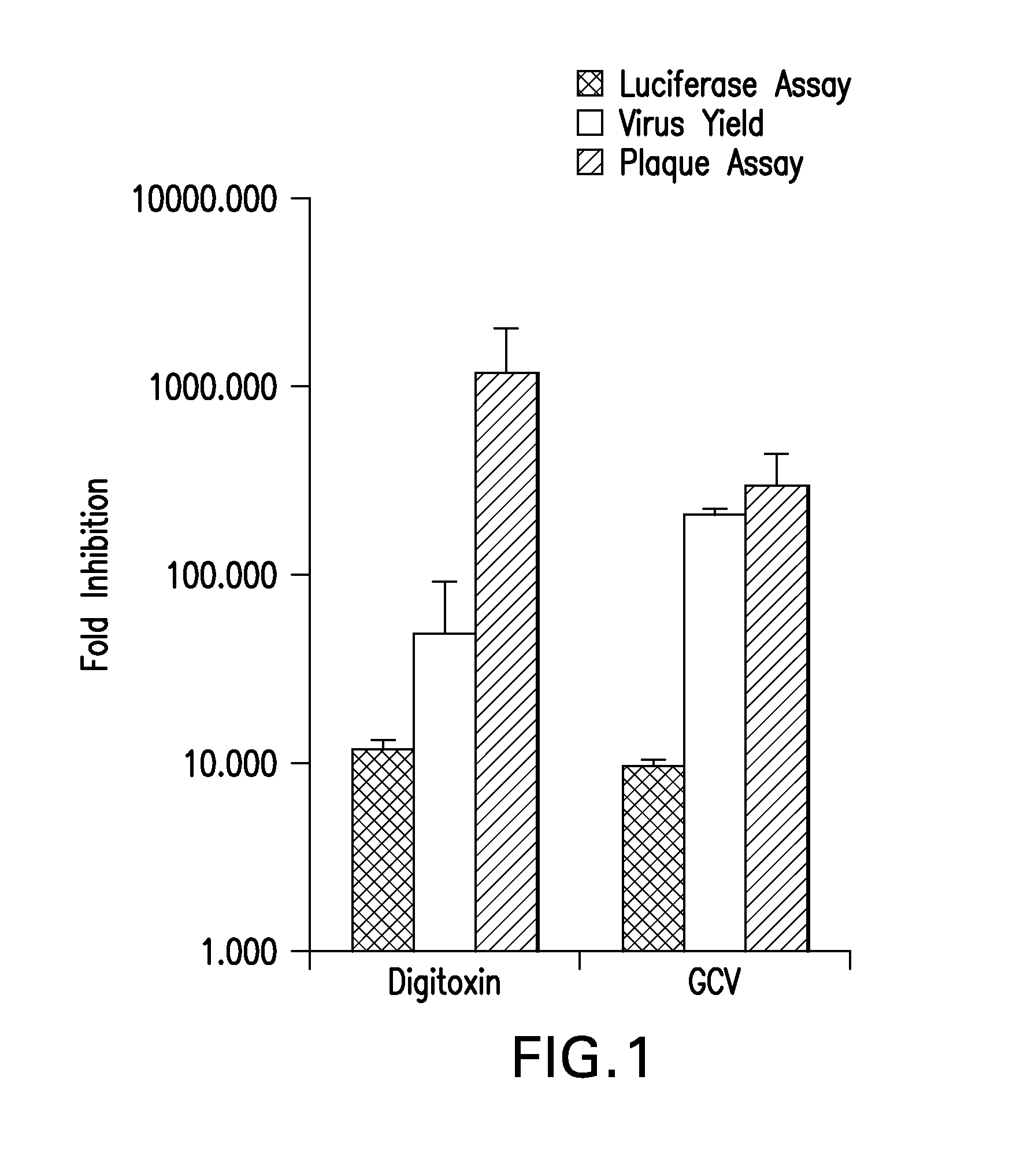
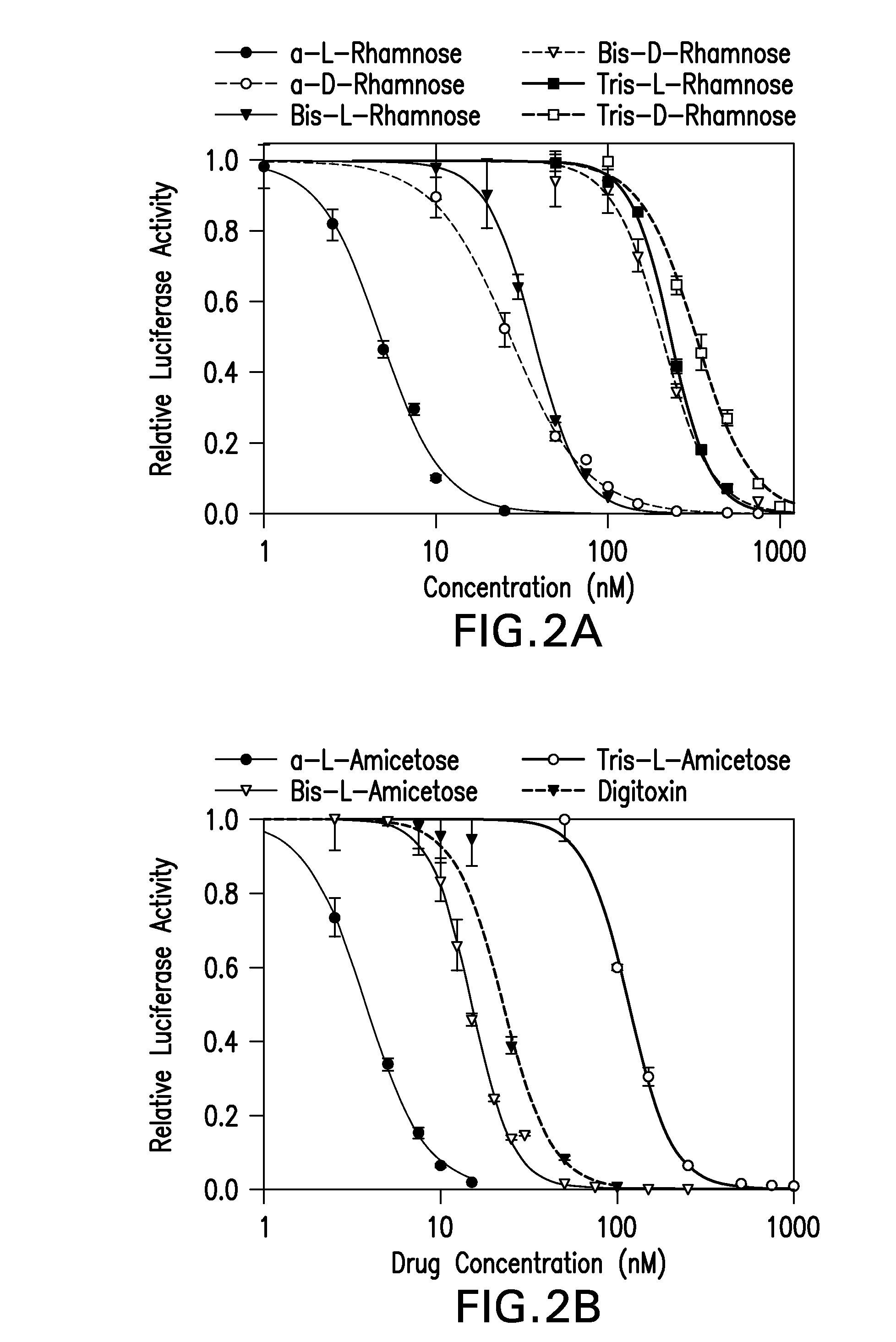
Cardiac glycoside analogs and their use in methods for inhibition of viral infection
The present invention provides methods for inhibition of human herpes virus replication in a subject comprising administering to the subject a therapeutically effective amount of a pharmaceutically acceptable composition comprising a cardiac glycoside analog, including for example, a digitoxin analog and pharmaceutically acceptable carrier. Other methods of the present invention include administering a digitoxin analog along with at least one other biologically active compound and pharmaceutically acceptable carrier. Methods for inhibition of the α3 subtype of the Na / K ATPase in a subject comprising administering to the subject a therapeutically effective amount of a pharmaceutically acceptable composition comprising a digitoxin analog are also provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Recombinant protein vaccine, recombinant expression vector containing gene encoding the recombinant protein vaccine and application thereof
ActiveCN104707135BHigh activityEfficient use ofBacteriaMicroorganism based processesDiseaseRegulatory T cell
The invention provides a recombinant protein vaccine, which contains the epitope of Epstein-Barr virus nuclear antigen 1 (EBNA1) and the epitope of human herpes virus glycoprotein, and the fusion protein can not only effectively activate specific cytotoxicity T lymphocyte (CTL) reaction, directly inhibits the expression of EBNA1, exerts the therapeutic effect of Epstein-Barr virus-related tumors; it can also directly block the immunosuppressive pathway through the herpes virus glycoprotein, and improve the activity of regulatory T cells; therefore, the present invention provides The recombinant protein vaccine can prevent or treat Epstein-Barr virus infection and related diseases and tumors more comprehensively and in multiple ways; the invention also provides a recombinant expression vector containing the gene encoding the recombinant protein vaccine and the application of the recombinant protein vaccine.
Owner:SHENZHEN INST OF ADVANCED TECH
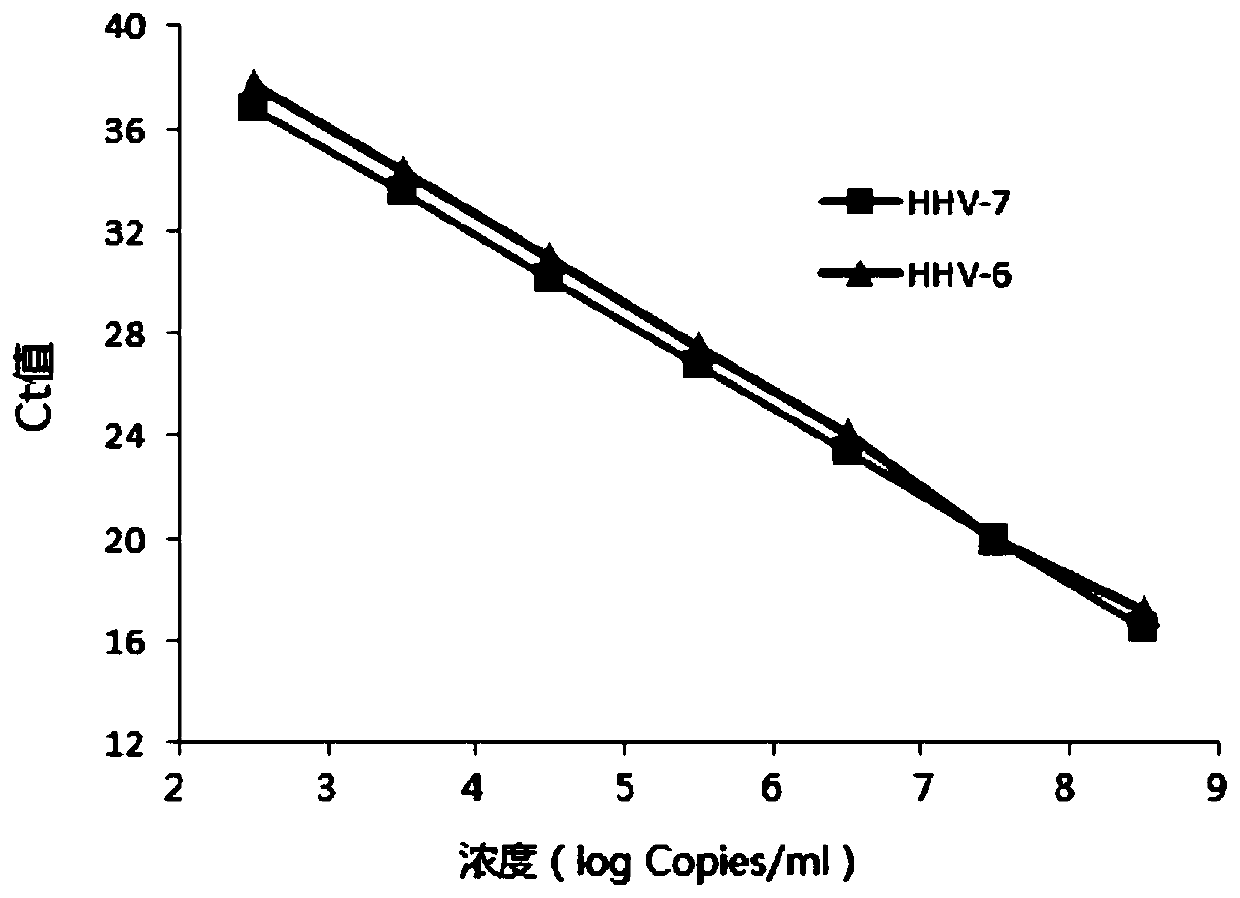
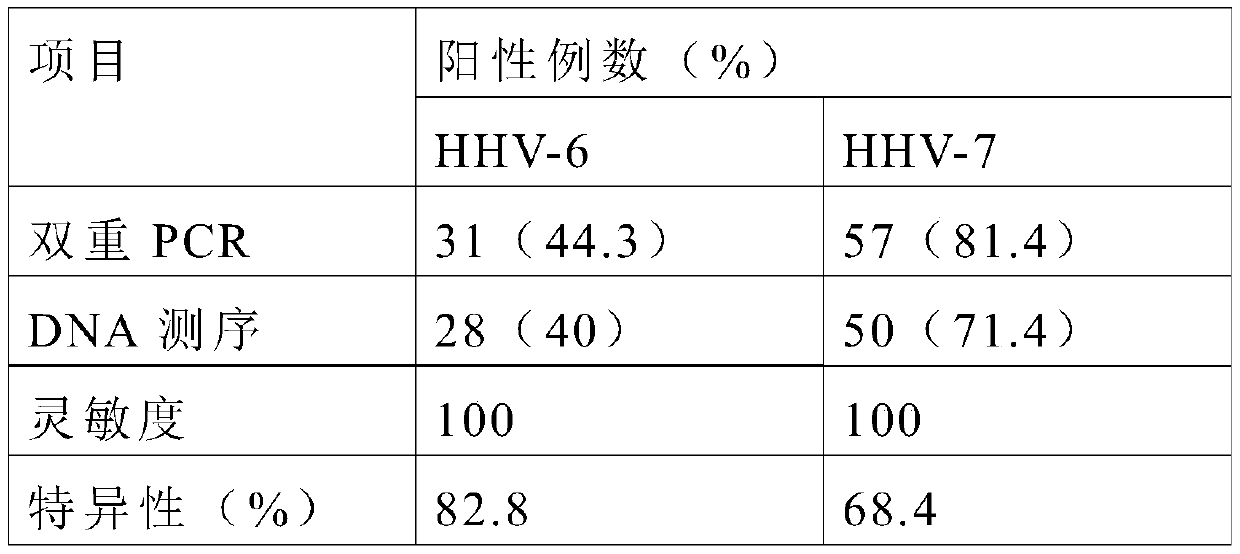
Primers, probes and kits for simultaneous detection of human herpesvirus types 6 and 7
ActiveCN106119425BReduce false positive rateHigh sensitivityMicrobiological testing/measurementMicroorganism based processesReaction systemVirology
The invention discloses primers, probes and kit for synchronously detecting human herpesvirus-6 and human herpesvirus-7. According to the primers, the probes and the kit, through selecting a novel target gene and redesigning the primers and the probes, the reaction system is optimized, the false positive incidence of clinical synchronous detection on human herpesviruses is substantially lowered, the sensitivity is high, the specificity is good, and the time for detection is shorter, so that the clinical application and popularization of the technology can be achieved.
Owner:武汉光谷创鑫医药孵化服务有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com