Application of benzo Alpha-pyrone compound as Gamma-type human herpes virus-resistant medicine
A human herpes virus, pyrone technology, applied in the direction of antiviral agents, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc., can solve problems such as lack of lysis and replication, and achieve high incidence of KS and progression of disease course. Rapid, less toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Virtual screening of compounds
[0043] 1. Compound library preprocessing: The virtual screening compound library is a self-prepared compound database. The compound library is processed as follows: removing ions and complexing water molecules, adding charges, protonating, and generating three-dimensional conformations. These processes are completed in the drug design software package Discovery Studio2.5. The protonation is carried out at pH6.5-8.5. Prepare small molecule libraries for virtual screening.
[0044] 2. Search the database for substructure matching based on the benzoα-pyrone structure to find compounds containing the parent nucleus. Finally, 7 compounds (C18, C19, C28, C30, C33, C34, C35) were selected for cytological experiments. The chemical structures of the compounds are shown in Table 1.
Embodiment 2
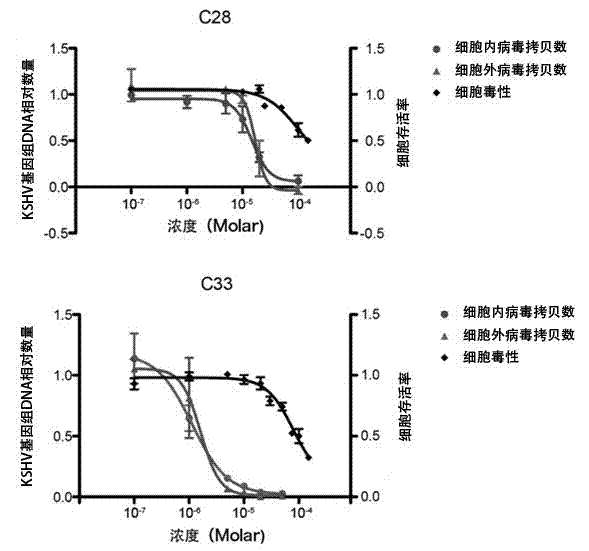
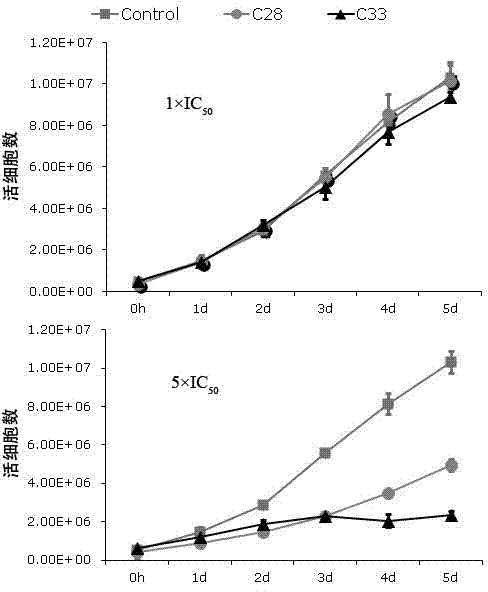
[0045] Example 2: Determination of KSHV split replication inhibitory activity
[0046] 1. Cell culture. BCBL-1 cells (primary effusion lymphoma cell line, containing latent KSHV) were cultured in vitro. The RPMI1640 medium containing 10% fetal bovine serum was used for routine maintenance and passage at 37°C and 5% carbon dioxide concentration.
[0047] 2. Drug intervention. Adjust the logarithmic growth phase BCBL-1 cell density to 3×10 5 cells / ml, use 20ng / ml Tetradecanoyl phorbol acetate (TPA) to induce BCBL-1 cells to enter the lytic replication phase. The compounds to be tested were formulated as drug solutions with different concentrations using DMSO. After BCBL-1 cells were treated with TPA for 3 hours, the cells were treated with different concentrations of compounds (the above 7 compounds), and three parallel wells were set up for each concentration, and a control group without TPA induction and without compound treatment was set up Compare.
[0048] 3. Test met...
Embodiment 3
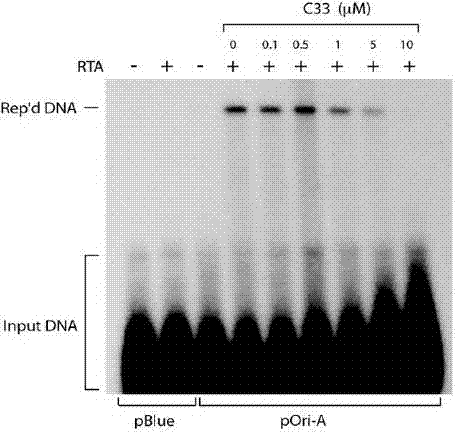
[0052] Example 3 Inhibition of KSHV Virus Particle Release Experiment
[0053] 1. Cell culture. BCBL-1 cells were cultured in vitro. Use RPMI1640 medium containing 10% fetal bovine serum, 100 U / ml penicillin, and 100 μg / ml streptomycin for routine maintenance and passage at 37°C and 5% carbon dioxide concentration.
[0054] 2. Drug intervention. Adjust the logarithmic growth phase BCBL-1 cell density to 3×10 5 cells / ml, use 20ng / ml TPA to induce BCBL-1 cells to enter the lytic replication phase. The compounds to be tested were prepared in different concentrations using DMSO. After BCBL-1 cells were treated with TPA for 3 hours, the cells were treated with different concentrations of compounds (the above 7 compounds), and three parallel wells were set up for each concentration, and a control group without compound treatment and without TPA induction was set up Compare.
[0055] 3. Test method. After the cells were treated with TPA for 5 days, the cell culture solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com