Method for detecting pathogens infected by cerebrospinal fluid based on PCR and nanopore sequencing
A pathogen and sequencing data technology, applied in the field of bioinformatics, can solve problems such as inability to culture viruses, inability to fully meet clinical needs, long cycle, etc., and achieve high clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
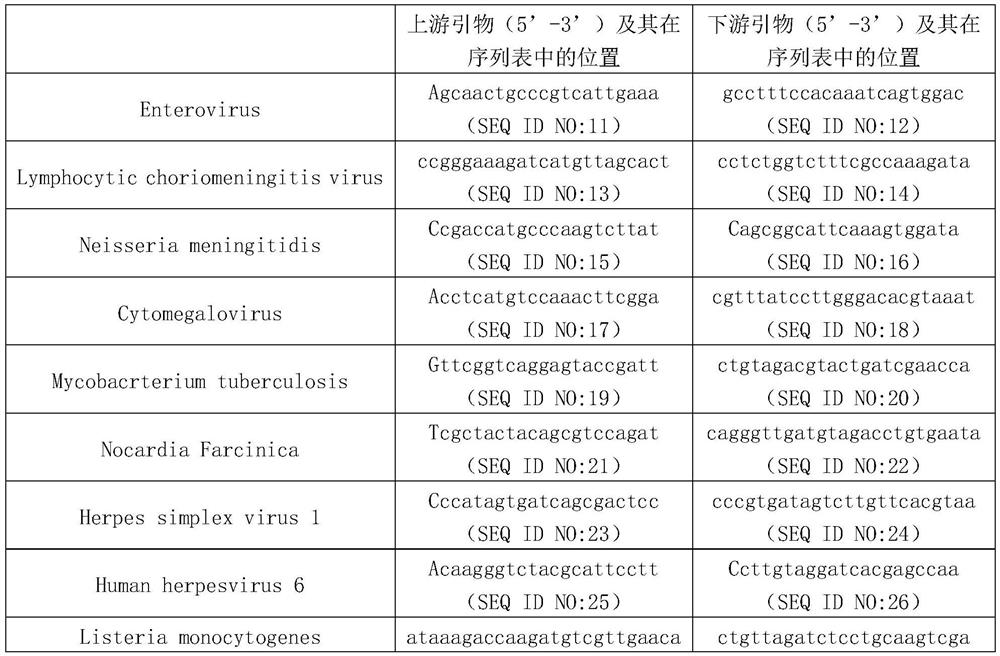
[0035] Example 1. Establishment of a method for high-throughput simultaneous detection of which pathogens are infected in multiple cerebrospinal fluid samples
[0036] 1. Obtaining RNA from cerebrospinal fluid
[0037] 1. Collect cerebrospinal fluid (avoid contamination during collection), and store in DNA / RNA Shield (Zymo, Catalog Code R1100-50).
[0038] 2. After completing step 1, cut the cerebrospinal fluid into small pieces to obtain the target sample.
[0039] 3. Take the Glass PowerBead Tube, add 200 μL of the target sample obtained in step 2 and 600 μL of PM1 / β-ME, and place it in a Qiagen homogenizer for 10 minutes at maximum speed; centrifuge at 13,000 g for 1 minute at room temperature, and collect the supernatant.
[0040] 4. Take a 2ml collection tube, add the supernatant collected in step 3 and 150 μL IRS liquid, vortex and mix, incubate at 4°C for 5 minutes; then centrifuge at 13000g for 1 minute, and collect the supernatant (avoid contact with the precipitate)...
Embodiment 2
[0112] Embodiment 2, the method accuracy that detection embodiment 1 establishes
[0113] Sample 1 to be tested is the cerebrospinal fluid of patient 1 who has been clinically confirmed to be infected with Neisseria meningitidis.
[0114] Sample 2 to be tested is the cerebrospinal fluid of patient 2 who has been clinically confirmed to be infected with Cytomegalovirus.
[0115] Sample 3 to be tested is the cerebrospinal fluid of patient 3 who has been clinically confirmed to be infected with Listeria monocytogenes.
[0116] The sample 4 to be tested is the cerebrospinal fluid of the healthy person 1 .
[0117] The sample 5 to be tested is the cerebrospinal fluid of the healthy person 2 .
[0118] Test sample 1-test sample 5 according to the method established in Example 1. The results showed that test sample 1 was infected with Neisseria meningitidis, test sample 2 was infected with Cytomegalovirus, test sample 3 was infected with Listeriamonocytogenes, test sample 4 and test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com