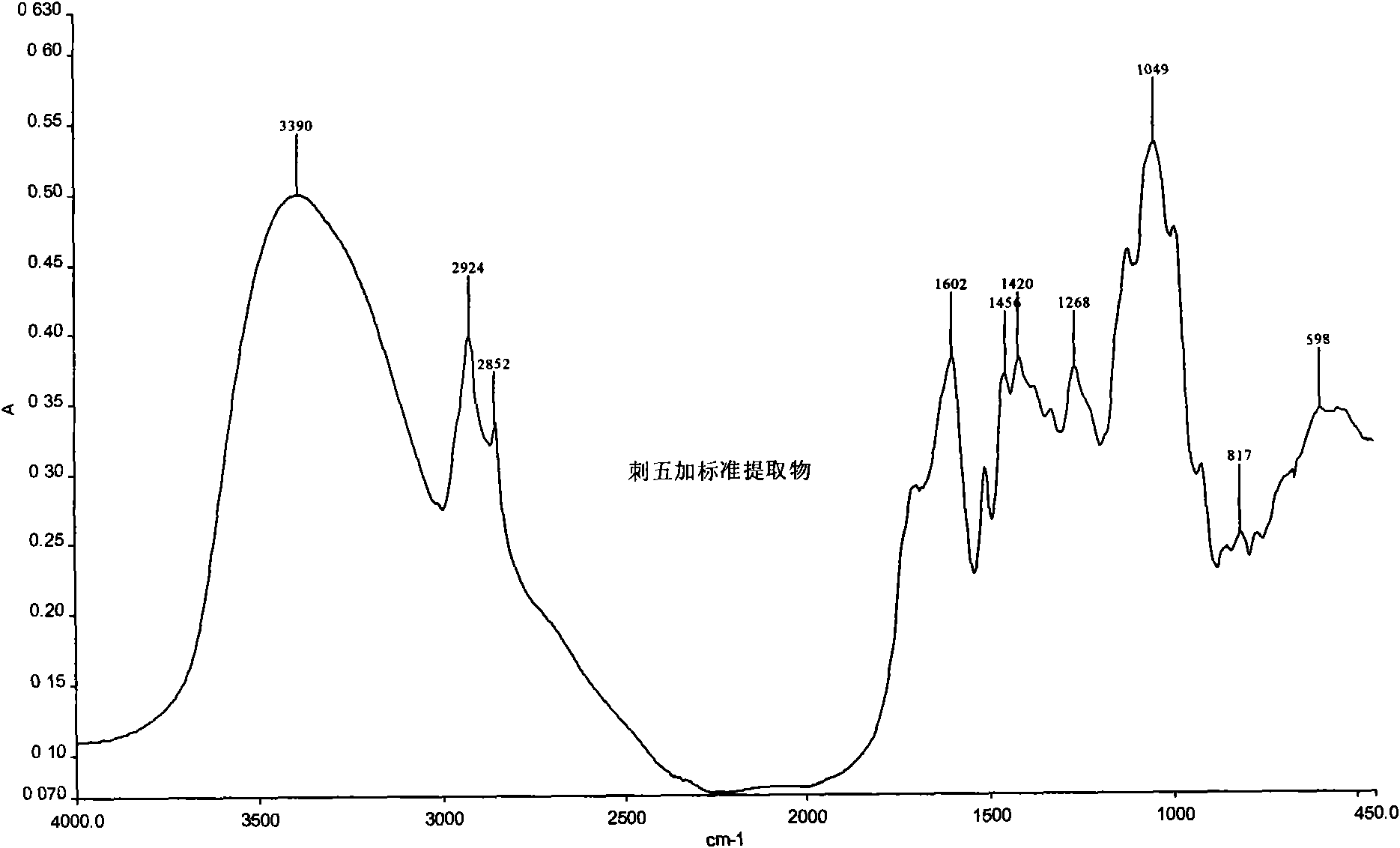
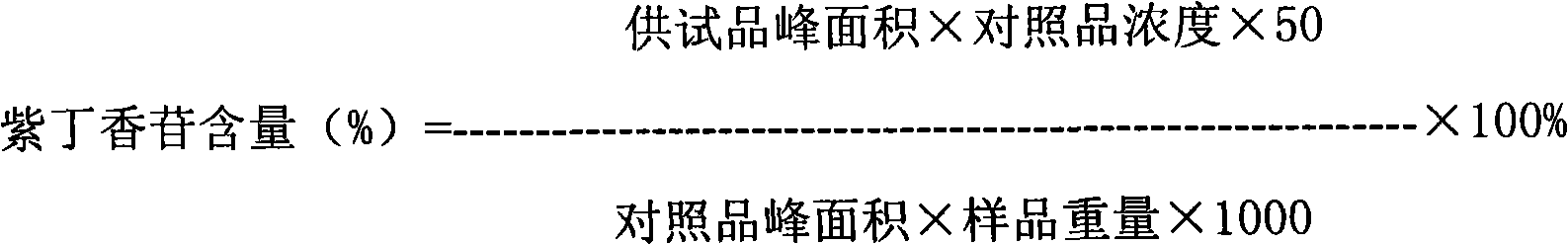
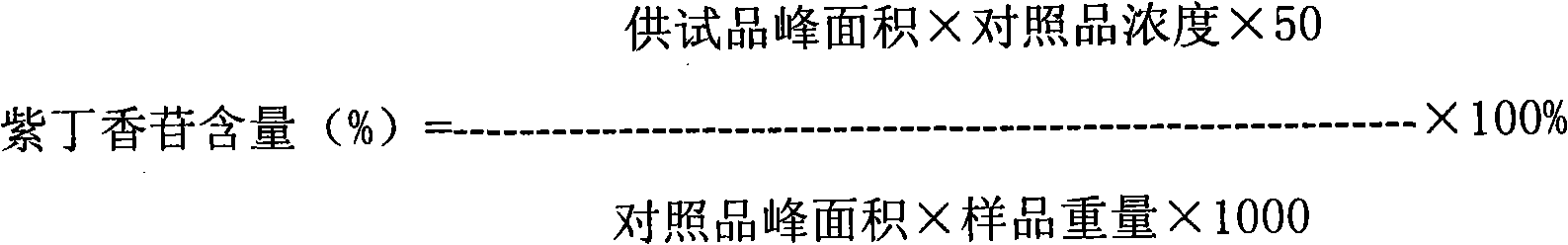
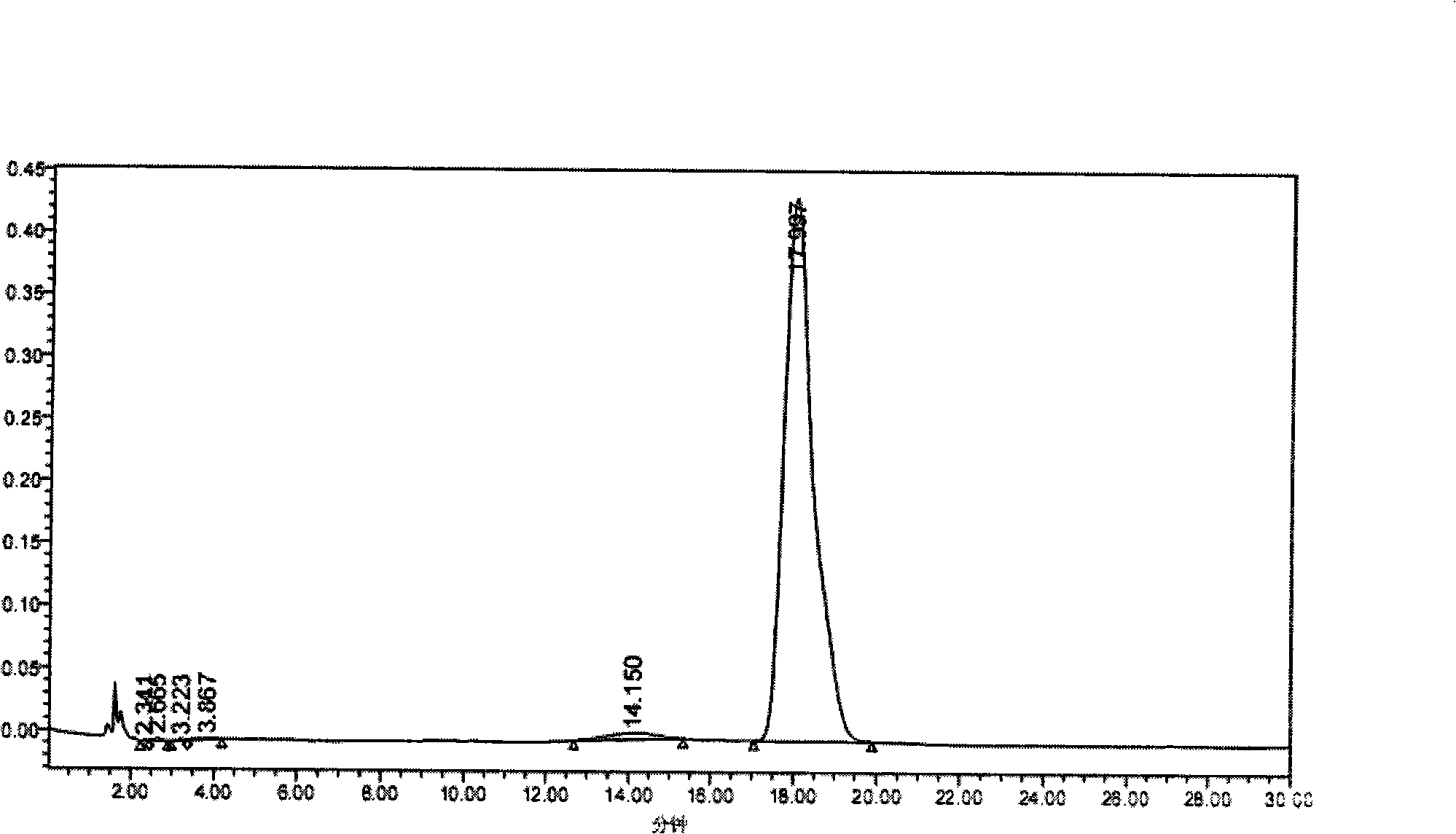
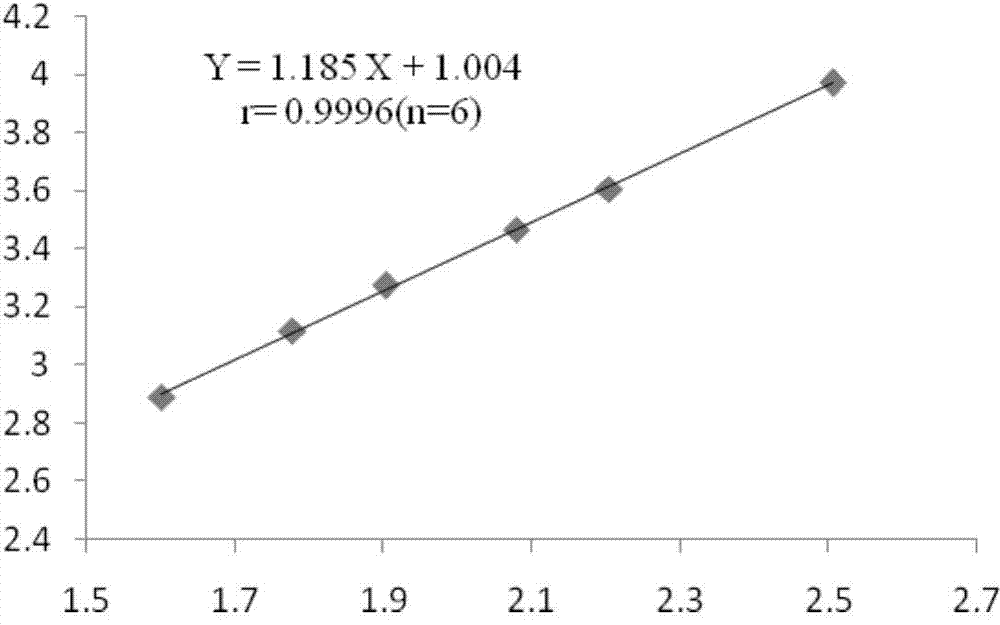
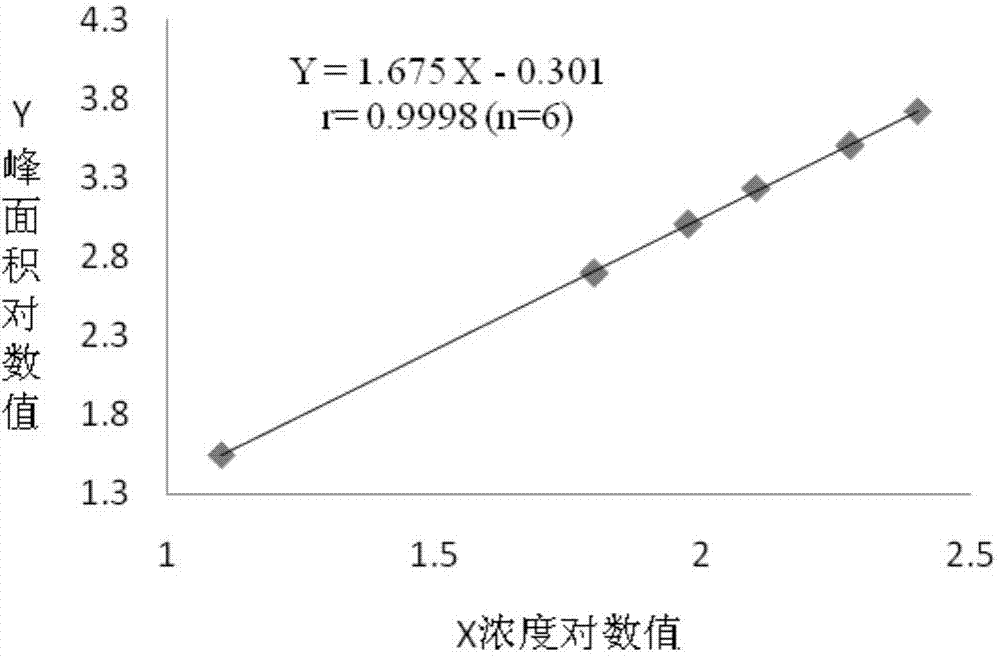
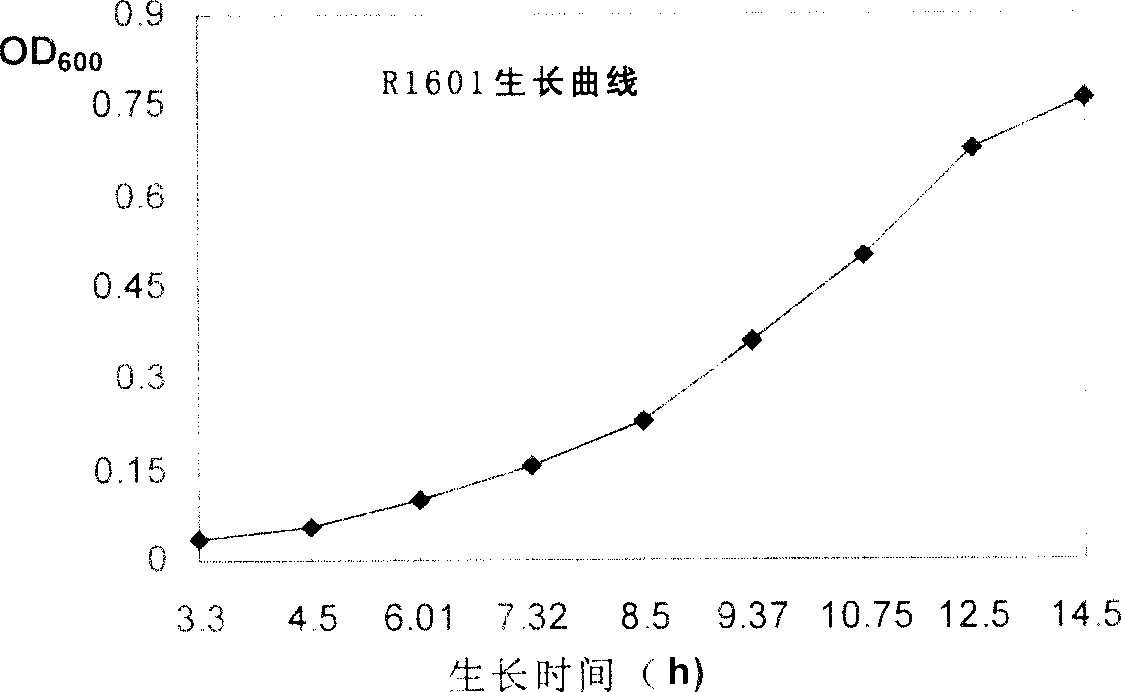
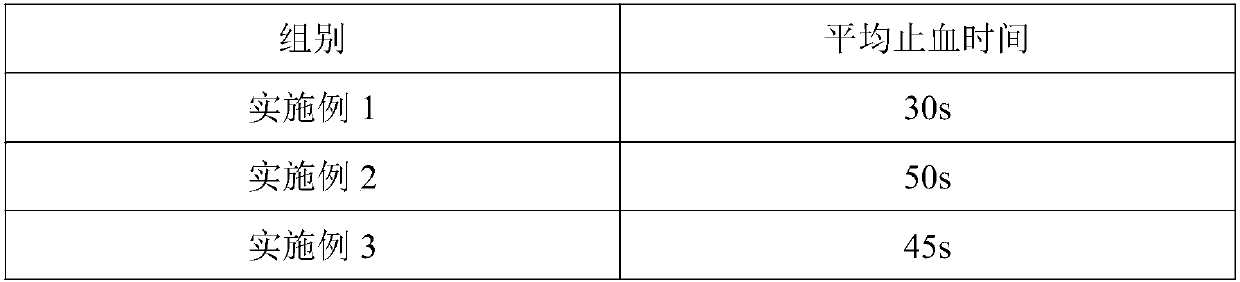
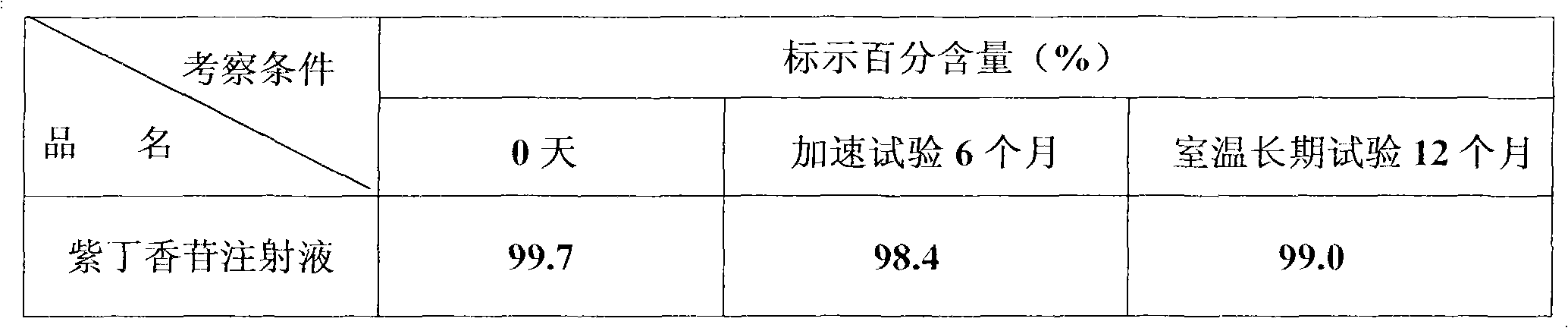
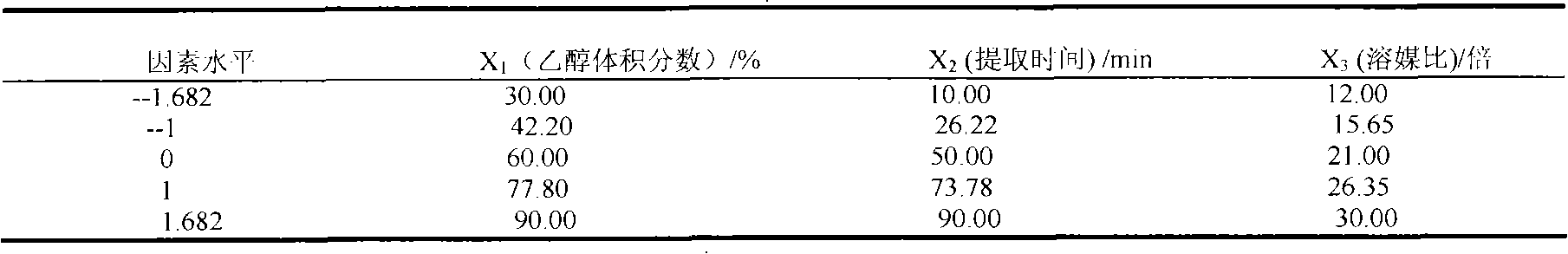
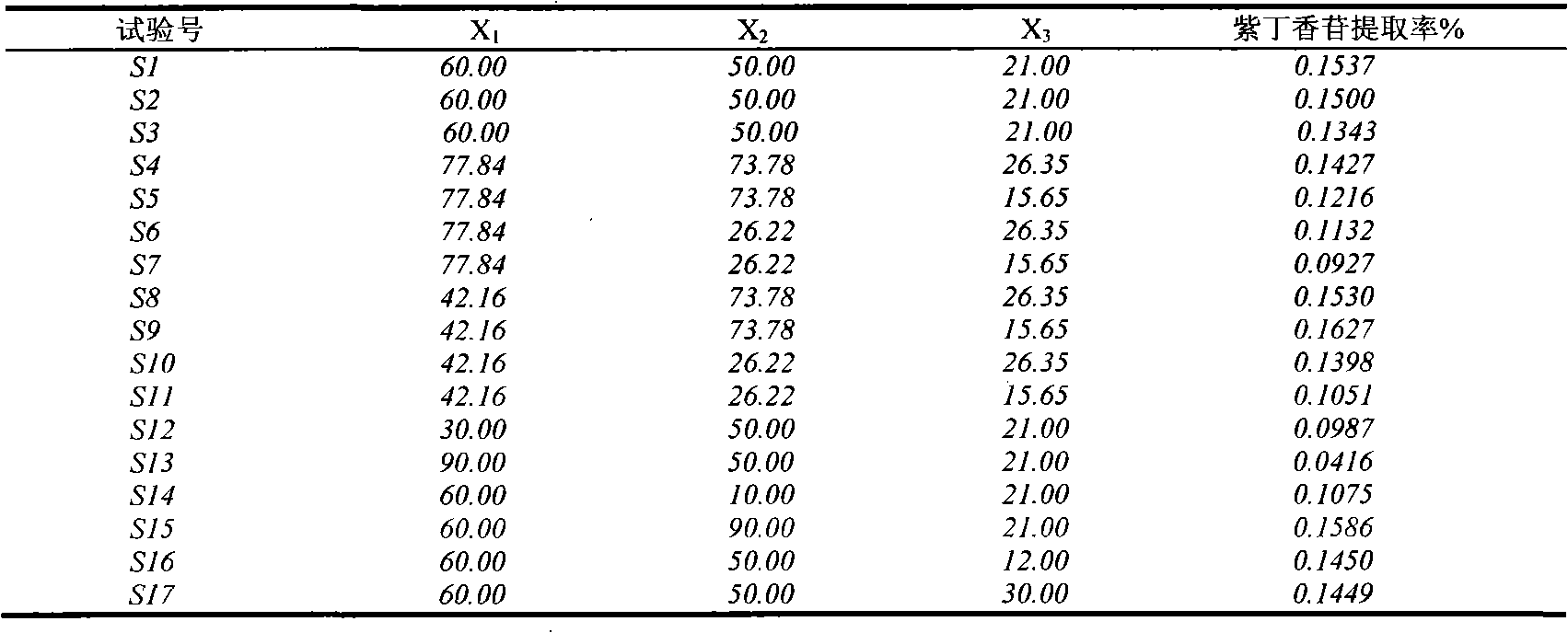
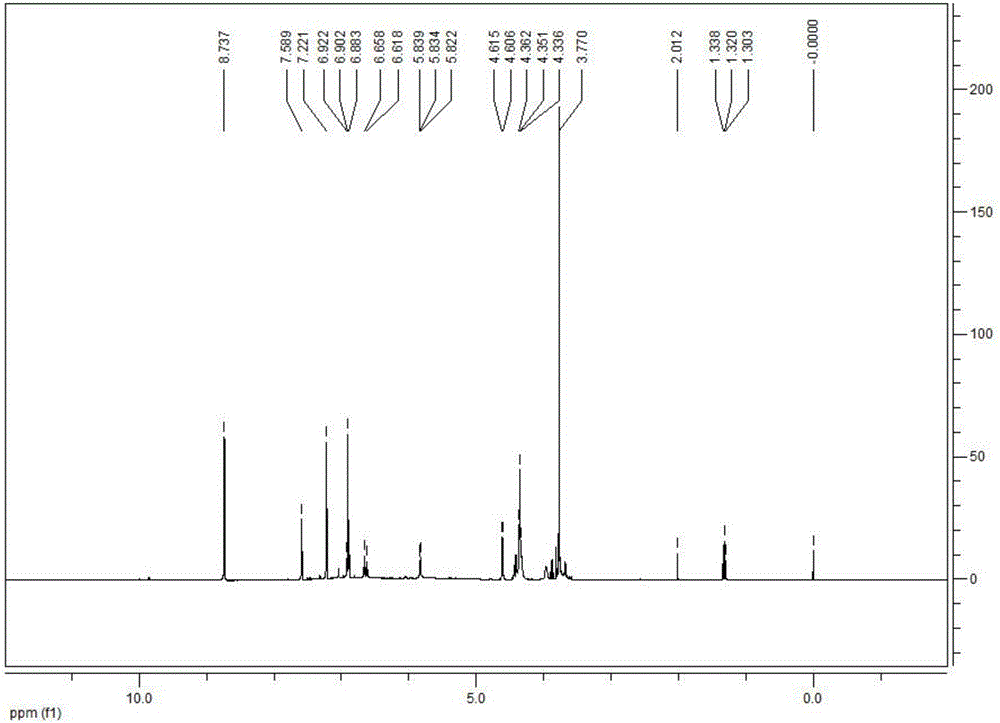
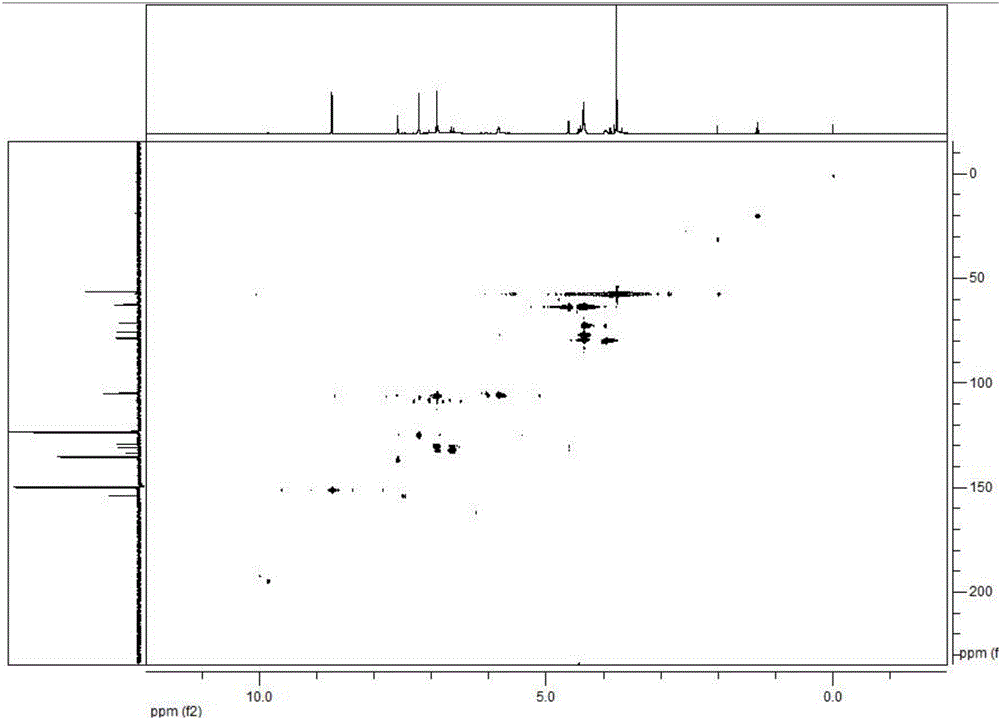
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
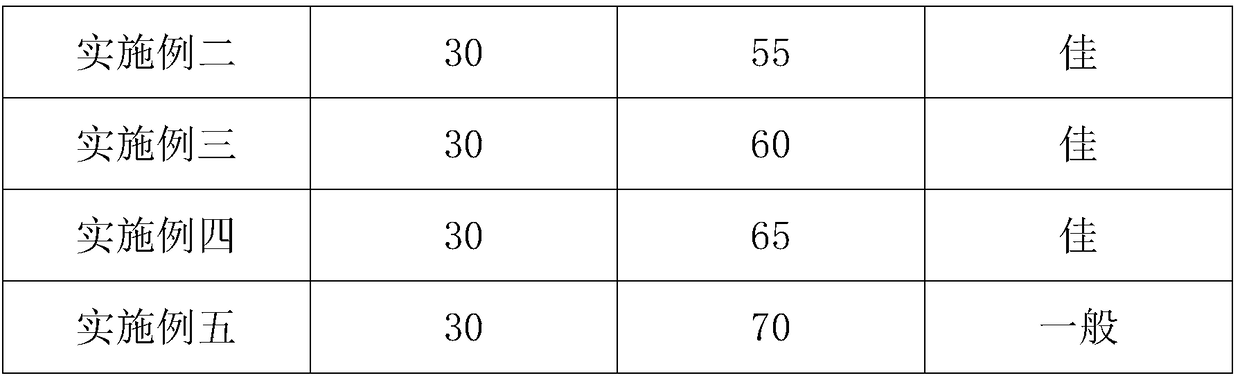
72 results about "Syringin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
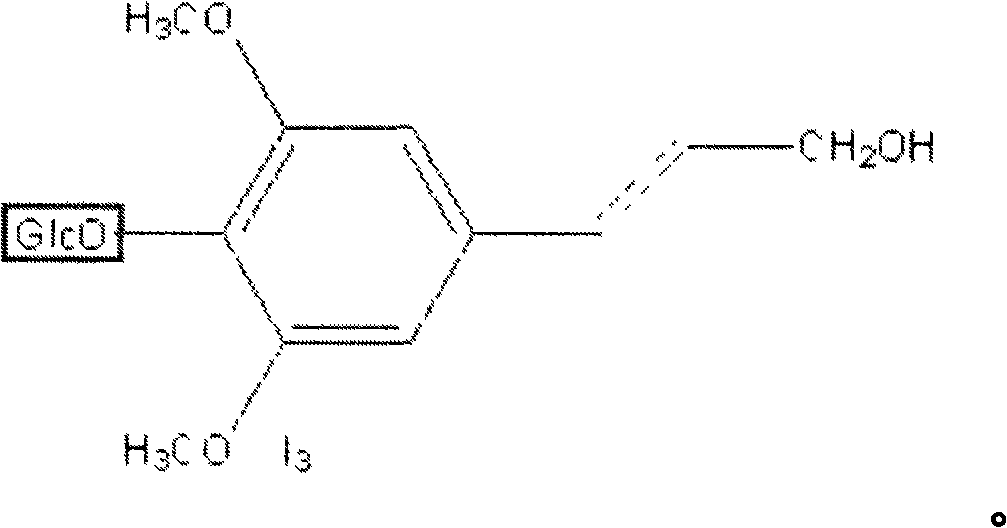
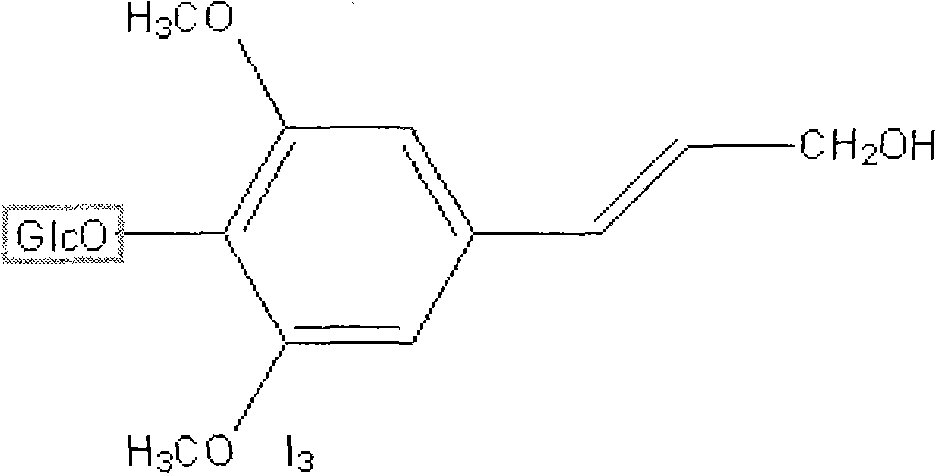
Syringin is a natural chemical compound first isolated from the bark of lilac (Syringa vulgaris) by Meillet in 1841. It has since been found to be distributed widely throughout many types of plants. It is also called eleutheroside B, and is found in Eleutherococcus senticosus (Siberian ginseng). It is also found in dandelion coffee. Syringin may potentially have antidiabetic effects.
Application of Saussurea involucrata culture as new raw material in daily chemicals
InactiveCN101897656AProtect resourcesMeet needsCosmetic preparationsHair cosmeticsChlorogenic acidActive matter
The invention relates to an application of Saussurea involucrata culture as a new raw material in daily chemicals, belonging to an application of pure vegetal raw material in the field of daily chemicals. The Saussurea involucrata culture is characterized by comprising the following active ingredients: flavone, polyose, chlorogenic acid, Syringin, trace elements, terpene and the like. The invention does not have damage to the hair and skin of human bodies, has strong practical applicability, wide application range and no pollution, and can not damage the natural resources of the Saussurea involucrata, can be used as a vegetal active matter to be added into the daily chemicals, and has remarkable effect in the aspects of eliminating the inflammation, preventing oxidation, delaying senility and blocking ultraviolet rays. The advised additive amount of the Saussurea involucrata culture is 0.01-10%.
Owner:DALIAN PRACTICAL BIOTECH
Content detection method for determining effective components in longshengzhi capsule by HPLC-QQQ/MS method
ActiveCN109307721AEasy to separateQuick analysisComponent separationBenzoylpaeoniflorinGradient elution
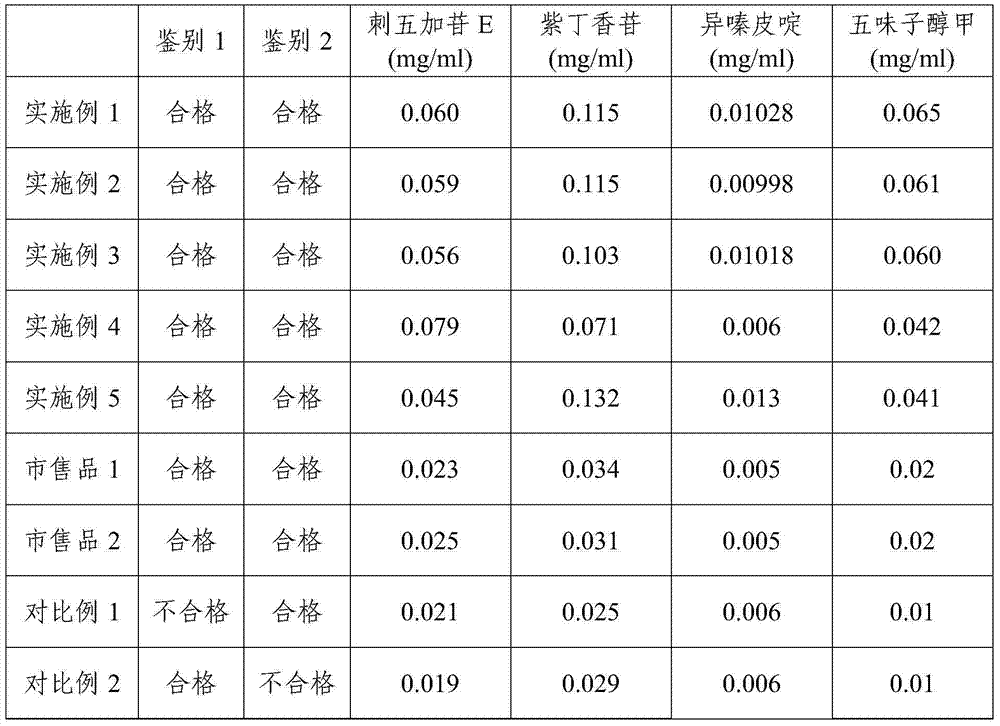
The invention provides a content detection method for determining effective components in a longshengzhi capsule by an HPLC-QQQ / MS method. The chromatograph condition is that the chromatographic column takes a octadecyl-bonded silica gel column as a filling agent, 0.1% formic acid-water as a mobile phase A, 0.1% formic acid-acetonitrile as a mobile phase B, and the volume ratio of the mobile phaseA and the mobile phase B is 80% to 0%:20 to 100%, so that gradient elution is performed. According to the content detection method for determining the effective components in the longshengzhi capsuleby the HPLC-QQQ / MS method, the effective substance components of traditional Chinese medicines such as astragaloside A, hydroxysafflor yellow A, calycosin 7-o-glucoside, calycosin, ferulic Acid, syringin, n-butylphthalide, ligustilide, senkyunolide A, senkyunolide I, senkyunolide H, ligustrazine, isofraxidin, dehydrocostus lactone, amygdalin, syringin E, paeoniflorin, oxypaeoniflora, benzoylpaeoniflorin, etc., in the longshengzhi capsule can be determined. The method has the advantages of being simple in operation method, high in sensitivity and accurate in content.
Owner:SHAANXI BUCHANG PHARMA
Callus culture method of snow lotus herb
InactiveCN101773071AIncrease contentReduce manufacturing costPlant tissue cultureHorticulture methods6-benzyladenineBULK ACTIVE INGREDIENT
The invention discloses a callus culture method of snow lotus herb, comprising the steps of selection and asepsis of plants, induction and culture of callus and large-scale culture. The core technology of the method is selection of the following large-scale culture medium: MS+0-50g / L of cane sugar+0-10g / L of agar+0.1-5.0mg / L of alpha-naphthylacetic acid+0.1-5.0mg / L of 6-benzyladenine. During large-scale culture, an abiotic elicitor, 40-60mu mol / L of SNP+40-60mu mol / L of MJ+40-60mu mol / L of SA is added. The method is easy to operate, and the active ingredient is high in content and low in production cost, does not pollute environment and can reach the level of industrialization. Syringin produced by the culture technology in the invention is invariant, high in content, low in cost and short in cycle and is very competitive in the markets.
Owner:烟台汇鹏生物科技有限公司
Panax root extract detection method
InactiveCN102048777AImprove accuracyHigh sensitivityNervous disorderDigestive systemReference productSyringin
The invention relates to a medicament detection method, in particular to a panax root extract detection method. The detection method comprises: measuring the content of a syringin component; and comparing the intermediate infrared one-dimensional spectrum of the panax root extract and the intermediate infrared one-dimensional spectrum of a reference product.
Owner:TIANJIN TASLY MORDEN TCM RESOURCES
Extraction and separation method of syringin
InactiveCN101328198ALow costSimple and fast operationSugar derivativesSugar derivatives preparationSyringa oblataChromatographic separation
The invention relates to a method for extracting and separating ligustrin, which is to extract the ligustrin by the decoction method through crushing of excrementum passeris (Syringa oblata Lindl.var. alba Hort.ex Rehd.) branches. A ligustrin extract is eluted by 20 to 60 percent alcohol solution through an XDA-1 or AB-8 macroporous absorption resin column, and undergoes chromatographic separation through a silica gel column, and then ligustrin crystals with the purity over 97 percent can be obtained. The method comprises the following techniques: extraction of the ligustrin from the excrementum passeris, separation of macroporous resins and the silica gel column, crystallization and so on. The method has the advantages of low cost, simple and convenient operation, convenient industrialized production and so on. Both the stability and the reproducibility of the techniques are good; the purity of products obtained is more than 97 percent; and the method is suitable for extraction in mass production.
Owner:SHAANXI UNIV OF SCI & TECH
Low-toxicity acanthopanax injection and preparation method thereof
ActiveCN104306417AAdd other ionsLow in potassiumPharmaceutical delivery mechanismPlant ingredientsSyringinPotassium ions
The invention relates to a low-toxicity acanthopanax injection and a preparation method thereof. The acanthopanax injection is prepared from the following active ingredients: 1.8-5.5mg / ml of total flavonoids, 0.13-0.70mg / ml of syringin, 0.06-0.34mg / ml of eleutheroside E and less than or equal to 800micro g / ml of potassium ions. The acanthopanax injection is low in content of potassium ions, which is reduced from less than or equal to 1000 micro g / ml in the national standard to lower than 800 micro g / ml (or counting at 800micro g / 5mg total flavonoids). The injection has the advantages of small abnormal toxicity and high safety. Animal experiments indicate that the tolerated concentration of a study subject is increased from 5mg / ml in the national standard to 18mg / ml. The abnormal toxicity inspection result on the low-toxicity acanthopanax injection provided by the invention is superior to the existing commercially available products.
Owner:HARBIN ZHENBAO PHARMA
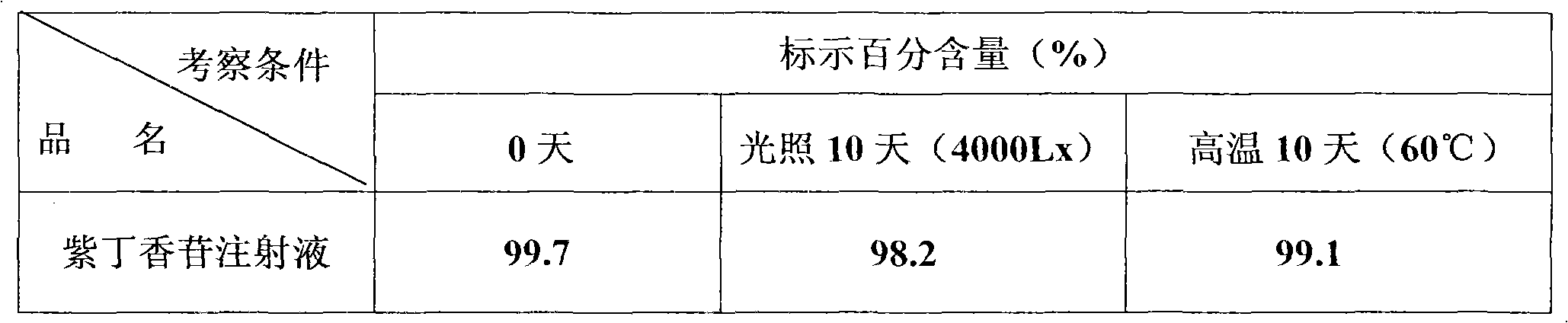
Syringin for injection, preparation method and application thereof
InactiveCN101554370AEasy to useMeet preparation requirementsPowder deliveryOrganic active ingredientsSyringinExcipient
The invention relates to a syringin for injection, a preparation method and the application thereof. The invention can provide an injection that is clinically and safely used and has the pharmacological actions of resisting fatigue, tranquilizing and allaying excitement, expanding blood vessel, reducing blood viscosity, stanching, and the like. The injection contains syringin, pharmacology-permissible pH regulating substance, a stabilizing agent and an excipient. 50-2800 mg dry substance in each injection contains 5-2000 mg of syringin, 0.04-0.8 g of excipient and 0.0001-0.1g of stabilizing agent. Of the above substance, the pH regulating substance has an adding amount for regulating the weight fraction pH value to 3.5-7.5. The product of the invention is used for preparing medicaments for resisting fatigue, tranquilizing and allaying excitement, expanding blood vessel, reducing blood viscosity, stanching, resisting dumps.
Owner:苑立超
Method for extracting syringin from ovate leaf holly bark
InactiveCN102558253AIncrease contentNo need to control residuesSugar derivativesSugar derivatives preparationActivated carbonAlcohol
The invention relates to a method for extracting syringin from ovate leaf holly bark. The method comprises the following steps of: crushing the ovate leaf holly bark medicine into ovate leaf holly bark with 40-80 meshes, adding the crushed ovate leaf holly bark into an extraction kettle, collecting syringin extract by using supercritical CO2 gradient extraction, dissolving the syringin extract by using 20-40% alcohol solution, filtering, adding a polyamide resin column into filtrate to adsorb impurities, carrying out concentration under reduced pressure on over-column liquid, standing for crystallization, filtering crystals, dissolving the crystals in hot water, adding activated carbon for decolorization, carrying out concentration under reduced pressure on decolorized liquid, and then standing for crystallization, drying crystals to obtain a product. The method has the advantages of low energy consumption and less pollution and is an environmentally-friendly extraction and separation method.
Owner:苏州宝泽堂医药科技有限公司
Method for simultaneously separating and preparing syringin and oleuropein from syringa oblate lindl.
InactiveCN104693249ASimple production processReduce energy consumptionSugar derivativesSugar derivatives preparationSyringinSmall branch
The invention discloses a method for simultaneously separating and preparing syringin and oleuropein from syringa oblate lindl. The method comprises the following steps: extracting by taking small branches of syringa oblate lindl. as raw materials by virtue of a homogenization process, carrying out macroporous resin column adsorption, implementing gradient elution with two ethanol solutions different in concentration, respectively separating eluents through column chromatography on silica gel and recrystallizing so as to obtain syringin crystal and oleuropein crystal. The method disclosed by the invention includes processes of syringin and oleuropein extraction from syringa oblate lindl., macroporous resin and silica gel column separation, and the like, and the method has the advantages of low raw material cost, simple process, recoverable adopted solvent and resin, and the like; two products prepared by the method are higher than 95% in purity; therefore, the method is applicable to industrial production on a large scale.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Saussurea involucrata extract and and producing method thereof
ActiveCN101209274BEasy to operateAntagonize painAntipyreticAnalgesicsChlorogenic acidBiocompatibility Testing
The invention relates to the technical field of medicine, and provides a snow lotus extract, a snow lotus cataplasm and a preparation method thereof. The snow lotus extract contains syringin, chlorogenic acid and rutin, the contents of which are respectively 6 times, 16 times and 200 times of the snow lotus injection. The snow lotus cataplasm forms snow lotus extract, adhesive, humectant, filler,thickening agent, cross linker and transdermal enhancer according to weight proportions of the raw materials. The results of the pharmacodynamic test of the invention indicate that the snow lotus cataplasm of the invention can obviously antagonize chemical pains, inhibit injury reaction and have anti-inflammatory effect; the invention has good performance on layer residue, skin tracing ability, using comfortableness, skin uncomfortable symptoms and tearing pain sense, can be torn and pasted repeatedly, has good biocompatibility, affinity, air permeability and perspiration resistance to the skin, and is not easy to cause allergy.
Owner:XINJIANG BIOCHEM PHARMA CO LTD
Quantitative analysis method of six chemical components in Chinese herbal medicine compound preparation using rhizoma dioscoreae nipponicae and acanthopanax roots as Chinese herbal medicines
ActiveCN104502485AImprove quality controlImprove accuracyComponent separationChlorogenic acidEleutherococcus senticosus
The invention discloses a quantitative analysis method of six chemical components in a Chinese herbal medicine compound preparation using rhizoma dioscoreae nipponicae and acanthopanax roots as Chinese herbal medicines. The quantitative analysis method comprises the following steps: (1) preparing a mixed control solution; (2) preparing a first test sample solution and a second test sample solution; (3) determining contents, namely, respectively taking the mixed control solution, the first test sample solution and the second test sample solution, performing gradient elution under high performance liquid chromatography detection conditions of using C18 bonded silica gel as a chromatographic column of a filling agent and acetonitrile-formic acid solution as a mobile phase, performing high performance liquid chromatography detection by combining a diode array detector and an evaporative light-scattering detector of detectors to obtain high performance liquid chromatograms of the mixed control solution, the first test sample solution and the second test sample solution, and determining the contents of dioscin, pseudoprotodioscin, protodioscin, chlorogenic acid, syringin and isofraxidin in a test sample by using an external standard method. The quantitative analysis method is simple, convenient and rapid to operate, high in accuracy and excellent in repeatability, and can be used for improving the quality control level of a product.
Owner:TIANJIN UNIV +1
Method for culturing xinjiang saussurea involucrata fuzzy root and regenerated seeding and method for producing lilac saponin
InactiveCN1742562ASolve resource problemsAvoid destructionPlant phenotype modificationRoot growthCataphyll
The method for culturing hairy root and regenerative seedling of saussurea involucrata and producing syringin includes the following steps: placing the green seed leaf of saussurea involucrata germinated seed on the induced comatus seedling culture medium to make culture to obtain comatus bud, placing the comatus bud on the seedling growth culture medium to make single plant culture, placing the single plant on the root-inducing culture medium to make culture to obtain the regenerative seedling whose lower end has root from seedling, cutting explant of seedling with root from seedling, placing the obtained leaf segment, leaf stalk segment and root segment on the preculture culture medium to make dark culture, using root-growing agrobacterium mediation to make conversion, culturing and inducing explant to promote root growth, placing the obtained hairy root on the screening culture medium to make subculture, and making liquid amplification culture of root tip to obtain regenerative seedling, drying hairy root and its regenerative seedling culture, pulverizing, separating by using organic solvent, extracting to obtain its syringin.
Owner:INST OF BOTANY CHINESE ACAD OF SCI
Biological membrane preparation for promoting wound healing and preparation method of biological membrane preparation
InactiveCN107583101AHeal fastHealing biofilm preparations have rapid hemostasisBandagesSide effectChemistry
The invention belongs to the field of medicine products and particularly relates to a biological membrane preparation for promoting wound healing and a preparation method of the biological membrane preparation. The biological membrane preparation is prepared from components in parts by weight as follows: 0.20-0.75 parts of saccharomycopsis fibuligera polysaccharide extract, 10-25 parts of calciumalginate, 2-6 parts of syringin, 100-150 parts of chitosan and 50-80 parts of silk fibroin. Compared with the prior art, the biological membrane preparation for promoting wound healing has functions of rapid hemostasis and rapid healing; the components are safe and non-irritant and have the effect of repairing scars. The biological membrane preparation is low in cost, good in effect and free of side effects and belongs to natural products.
Owner:GUANGZHOU RAINHOME PHARM&TECH CO LTD
Fingerprint map and high performance liquid chromatography identification method for Xuezhitong capsule
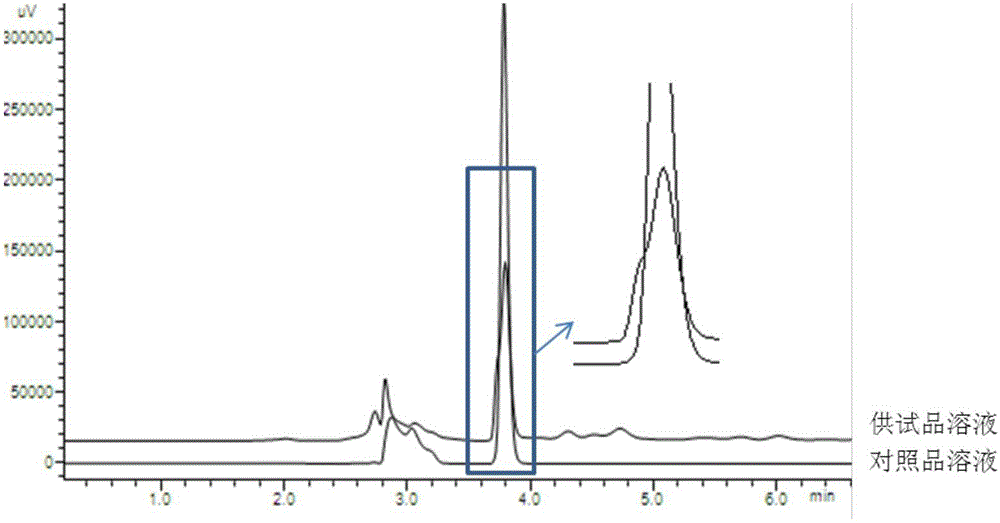
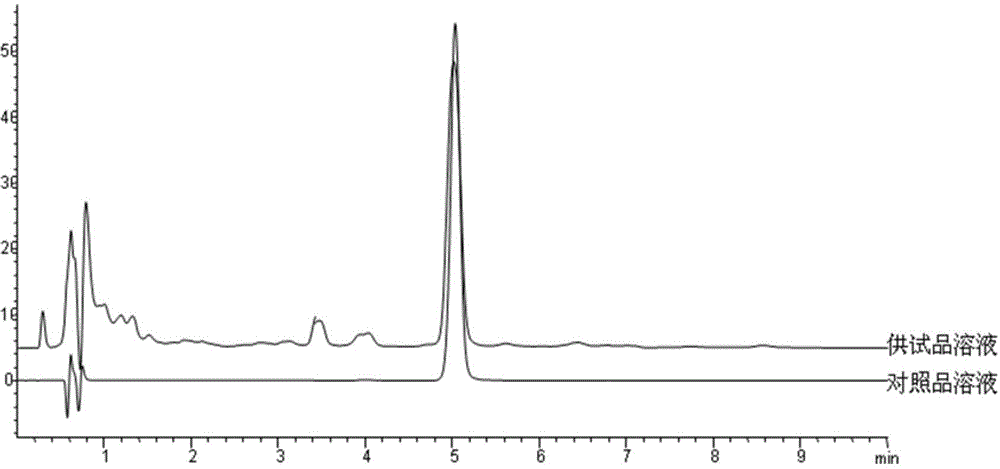
The invention discloses a fingerprint map and high performance liquid chromatography identification method for a Xuezhitong capsule. The invention firstly discloses an establishing method of a Xuezhitong capsule fingerprint map. The establishing method comprises the following steps: (1) adding a Xuezhitong capsule content in an extraction solvent for extraction to obtain an extracting solution, and purifying the extracting solution to obtain a test sample solution; (2) performing high performance liquid chromatographic analysis on the test sample solution to acquire a high performance liquid chromatogram; and (3) selecting a common peak from chromatographic peaks in the high performance liquid chromatogram to obtain the Xuezhitong capsule fingerprint map. The invention further discloses the Xuezhitong capsule fingerprint map established by the method. The invention also discloses a Xuezhitong capsule high performance liquid chromatography identification method. The method can accurately identify adenosine and syringin in the Xuezhitong capsule and is good in accuracy, stability and repeatability. The fingerprint map and high performance liquid chromatography identification method for the Xuezhitong capsule, established by the invention, has an application prospect in quality control of the Xuezhitong capsule.
Owner:吉林省东方制药有限公司
Syringin imprinted monolithic column preparation method
ActiveCN112275265AIncreased durabilityReduce blotting costsOther chemical processesFunctional monomerTetrafluoroborate
The invention relates to a syringin imprinted monolithic column preparation method, which comprises: adopting syringin as a template, adding polyethylene glycol 400, isopropanol and methanol to dissolve the template, adopting eutectic solvent choline chloride itaconic acid as a functional monomer, adding cross-linking agent ethylene glycol dimethacrylate, porogenic agent 1-butyl -3 -methyl imidazole tetrafluoroborate and initiator azo diisobutyronitrile, and carrying out a polymerization reaction to obtain the syringin molecularly imprinted monolithic column with selectivity. According to themethod, the eutectic solvent choline chloride itaconic acid is used as the functional monomer, compared with use of a traditional functional monomer 4-vinylpyridine, the method is green and environmentally friendly, meanwhile, the syringin imprinting cost is reduced, the syringin imprinting monolithic column and a blank control column are successfully synthesized in a stainless steel tubular column, and chromatographic condition optimization is conducted on the syringin imprinting monolithic column. Experimental results show that the maximum imprinting factor can reach 3.11. The method is simple in preparation, the syringin imprinted monolithic column is good in durability, and a cost-saving method is provided for separation and purification of syringin.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Radix astragali and acanthopanax gracilistylus soluble powder for poultry and preparation method thereof
InactiveCN104083429ASimple preparation processHigh drug contentPowder deliveryAntinoxious agentsRadix Astragali seu HedysariEleutherococcus gracilistylus
The invention discloses radix astragali and acanthopanax gracilistylus soluble powder for poultry and a preparation method thereof. The soluble powder prepared by adding glucose as an auxiliary material on the basis of a formula which comprises radix astragali and acanthopanax senticosus and changing a dosage form and a process can be used for effectively treating the diseases, namely chicken immunodeficiency, anti-stress and the like. According to the radix astragali and acanthopanax gracilistylus soluble powder, the dosage form is changed on the basis of a human medicine radix astragali and acanthopanax gracilistylus tablet, so that the medicine can be used for poultry animals; an extraction preparation process is also changed, so that the yield of syringin extracted from the acanthopanax senticosus is higher; glucose is added as the auxiliary material, so that the prepared soluble powder has good formability, hygroscopicity, solubility and mouthfeel.
Owner:江西中成中药原料有限公司
Ginseng-acanthopanax oral liquid and production process thereof
ActiveCN103040889AGuaranteed safetyNo "dryness"Antinoxious agentsPharmaceutical delivery mechanismBiotechnologySyringin
The invention provides a ginseng-acanthopanax oral liquid and a production process thereof. The following raw materials are adopted in parts by weight: 1 part of ginseng, 10-20 parts of acanthopanax and 15-40 parts of purified water. The following index system is adopted to strictly control product quality: the content of syringin is more than or equal to 2.0mg / 100ml, the pH value is 4.0-6.0, and the soluble solid content is more than or equal to 1.0%. The production process of the ginseng-acanthopanax oral liquid, provided by the invention, omits a step of alcohol precipitation, and the loss of ginseng polysaccharides is avoided, so that good anti-fatigue effect of the oral liquid is guaranteed; and the whole technological process adopts sterile operation, high temperature sterilization and suitable packing material, so that the microbiological safety of a product is guaranteed, the product is stable, no antiseptic substance is required to be added, and a food is safe and reliable.
Owner:四川合泰新光医药有限公司
Acanthopanax senticosus detection method
InactiveCN105806972AStable baselineEasy to separateComponent separationEleutherococcus senticosusSyringin
The invention provides an acanthopanax senticosus detection method; a liquid chromatography method is used for detecting a test sample solution and a reference solution, and liquid chromatography determination conditions comprise that octadecylsilane bonded silica gel is used as a filler; an acetonitrile-water mixture is used as a mobile phase, and an external standard method is used for detecting the content of syringin in a test sample. The detection method has the advantages of smooth baseline and good separating degree, can effectively detect and quantify the effective compound syringin in acanthopanax senticosus.
Owner:CHENGDU KANGHONG PHARMA GRP
Preparation method of syringin
InactiveCN108912186AIncrease contentEasy extractionSugar derivativesSugar derivatives preparationAlcoholSyringin
The invention discloses a preparation method of syringin. The method specifically includes the steps of: (1) drying, crushing, sieving the Chinese herbal material ilex rotunda, and loading the productinto an extraction container; (2) conducting refluxing extraction with a solvent, combining the extracted liquid, and conducting concentration to an appropriate amount; (3) adding an alcohol solventinto the extracted liquid, performing heating to boiling, and conducting decolorization; (4) filtering impurities out of the decolorized solution, adjusting the solution to neutral, and concentratingthe filtrate; and (5) carrying out crystallization refining treatment. The preparation method of syringin disclosed by the invention has the advantages of high syringin content, convenient extraction,environmental protection and cost reduction. At the same time, the method is simple and fast, has high separation efficiency and achieves a good effect.
Owner:湖州展舒生物科技有限公司
Syringin pharmaceutical composition and preparation method and application thereof
InactiveCN102038694AEasy to useMeet preparation requirementsOrganic active ingredientsNervous disorderSyringinSolvent
A syringin pharmaceutical composition and preparation method and application thereof. Only acanthopanax injection and other Chinese herbal medicine compound preparations are related with the syringin containing injections on the market, and no syringin injection is available for clinical use. The syringin pharmaceutical composition comprises syringin with a purity of above 98%, a pharmacologically allowable pH adjusting substance, a cosolvent, and the balance of water, wherein the concentration of the syringin is 2mg / ml-250mg / ml and the concentration of the cosolvent in the composition is 0.1ml / ml-0.65ml / ml, and the pH adjusting substance, by weight, is added in an amount sufficient to adjust the pH in a range of 3.5-7.5. The invention provides a clinically safe injection with pharmacological effects of fatigue prevention, tranquilization, vessel dilation, and hemostasis.
Owner:王洪涛
Pharmaceutical composition for treating diabetic retinopathy and preparation method thereof
InactiveCN105727271AImprove immunityAlleviate symptoms of lesionsSenses disorderPeptide/protein ingredientsDiabetic retinopathyFriedelin
The invention discloses a pharmaceutical composition for treating diabetic retinopathy and a preparation method thereof. The pharmaceutical composition for treating diabetic retinopathy consists of the following components in parts by weight: coarse grain mixed powder, pseudolaric acid, p-chlorophenoxy isobutyric acid, syringin, phycocyanobilin, friedelin, stigmasterol, formononetin, L-alpha-aminoadipic acid, pancreatin, L-malic acid, vitamin and L-glutamic acid. The pharmaceutical composition disclosed by the invention realizes the effects of controlling blood glucose and treating retinopathy by improving glycometabolism, focusing on blood glucose reduction assisted by the medicinal components for nourishing yin and clearing heat and resolving stasis and by improving the human immunity to enhance the tolerance and resistance against lesion; and moreover, the pharmaceutical composition is simple in technology, realizes a good curative effect and can be promoted for application.
Owner:孔德华
Method for preparing snowdrop syringin
This invention relates to a cultivation method for cells of Xuelian lilac aglycons including: applying Xuelian explant or seeds to bourgeon young plants to induce calli on the 1 / 2 cultivation medium, said callus cells are used to produce Xuelian lilac aglycons, MS cultivation medium is the solid for generating the Xuelian lilac aglycon cells or for suspending cultivation, 70% alcohol or n-butyl alcohol is used in separating, extracting and preparing the aglycons and HPLC is used for testing its content. This invented content of gross lilac compound is 3-4% of the dry weight of cells in this invented cell cultivating material and its productivity is 400-900mg / L.
Owner:INST OF BOTANY CHINESE ACAD OF SCI
Syringin extracting process
The invention belongs to the technical field of research and development of medicinal products. The invention relates to a syringin extracting process, which comprises: grinding syringa amurensis branches to 40 meshes, soaking the ground syringa amurensis branches in 54 to 60 percent ethanol in an amount which is 17 to 21 times that of ground syringa amurensis branches for 1 to 2 hours, performing ultrasonic extraction for 80 to 90 minutes at 30 DEG C under a ultrasonic power of 80w, filtering and obtaining ethanol extract; recovering ethanol solvent to obtain concentrated solution; adding water into the concentrated solution to dilute the concentrated solution, filtering to obtain supernate; loading the supernate onto a D101 macroporous adsorption resin column for absorption; eluting by using distilled water, 10 percent ethanol respectively to obtain 10-percent ethanol eluent, subjecting the 10-percent ethanol eluent to JTY-1 reverse-phase resin column chromatography, collecting eluent, recovering solvent to obtain coarse crystals, and recrystallizing in a methanol solvent to obtain syringing. The product of the invention has anti-inflammatory, pain-relieving and anti-cancer activities and a liver-protecting function. The product of the process is suitable for industrial production.
Owner:SHANDONG UNIV AT WEIHAI
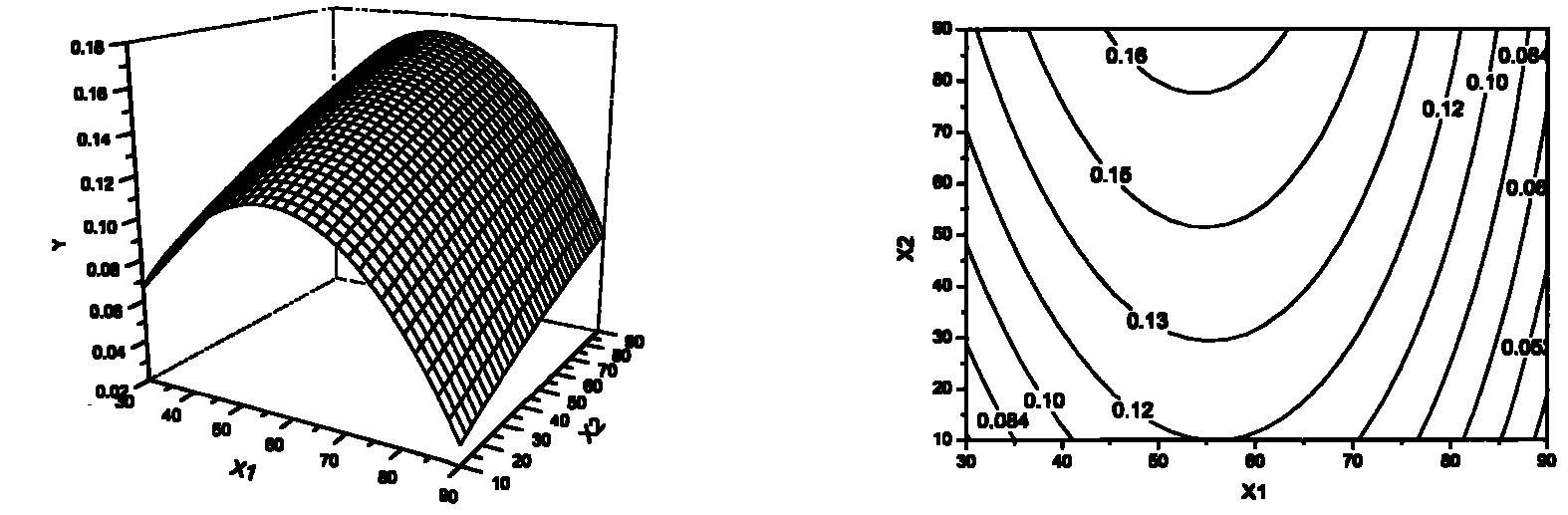
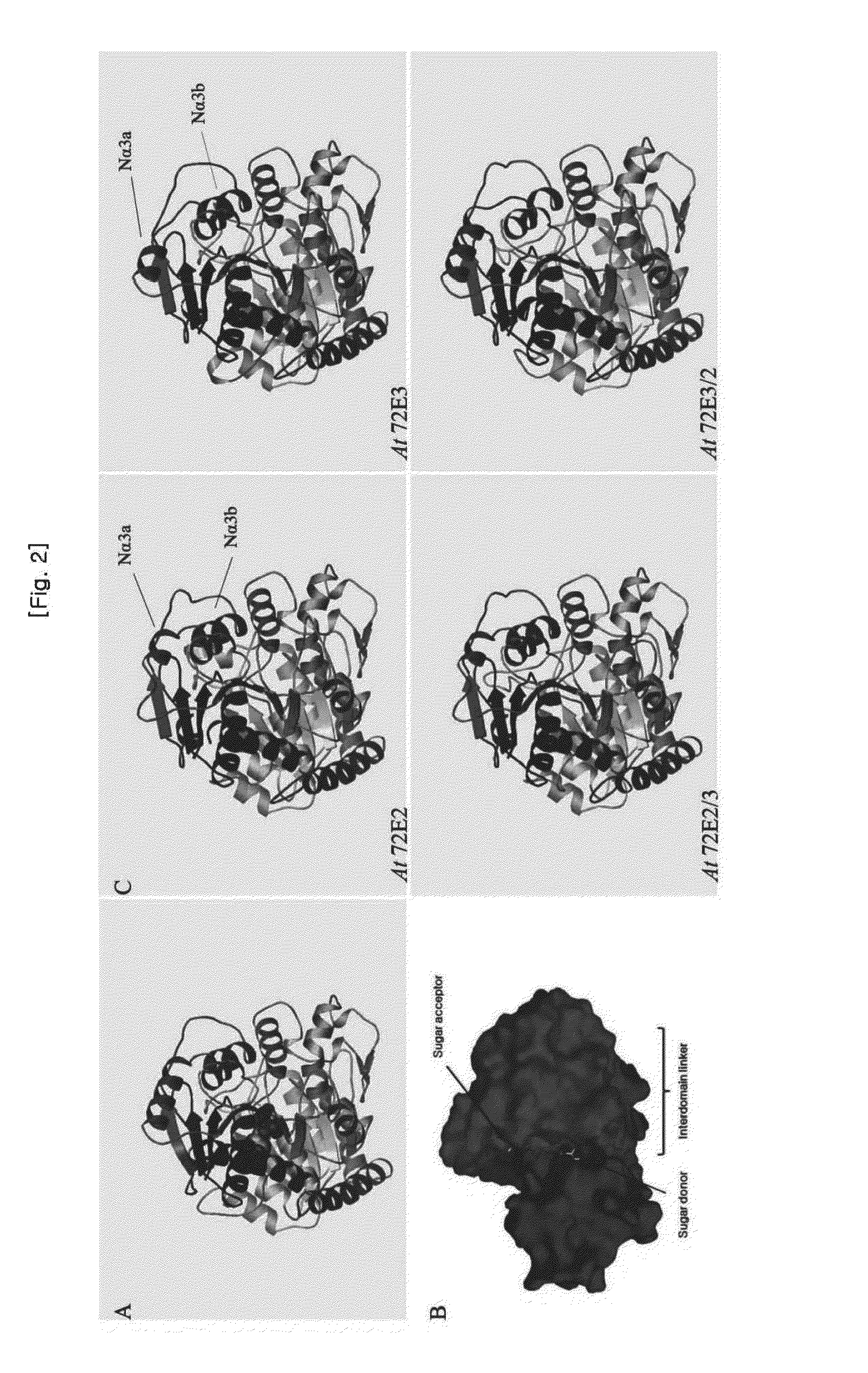
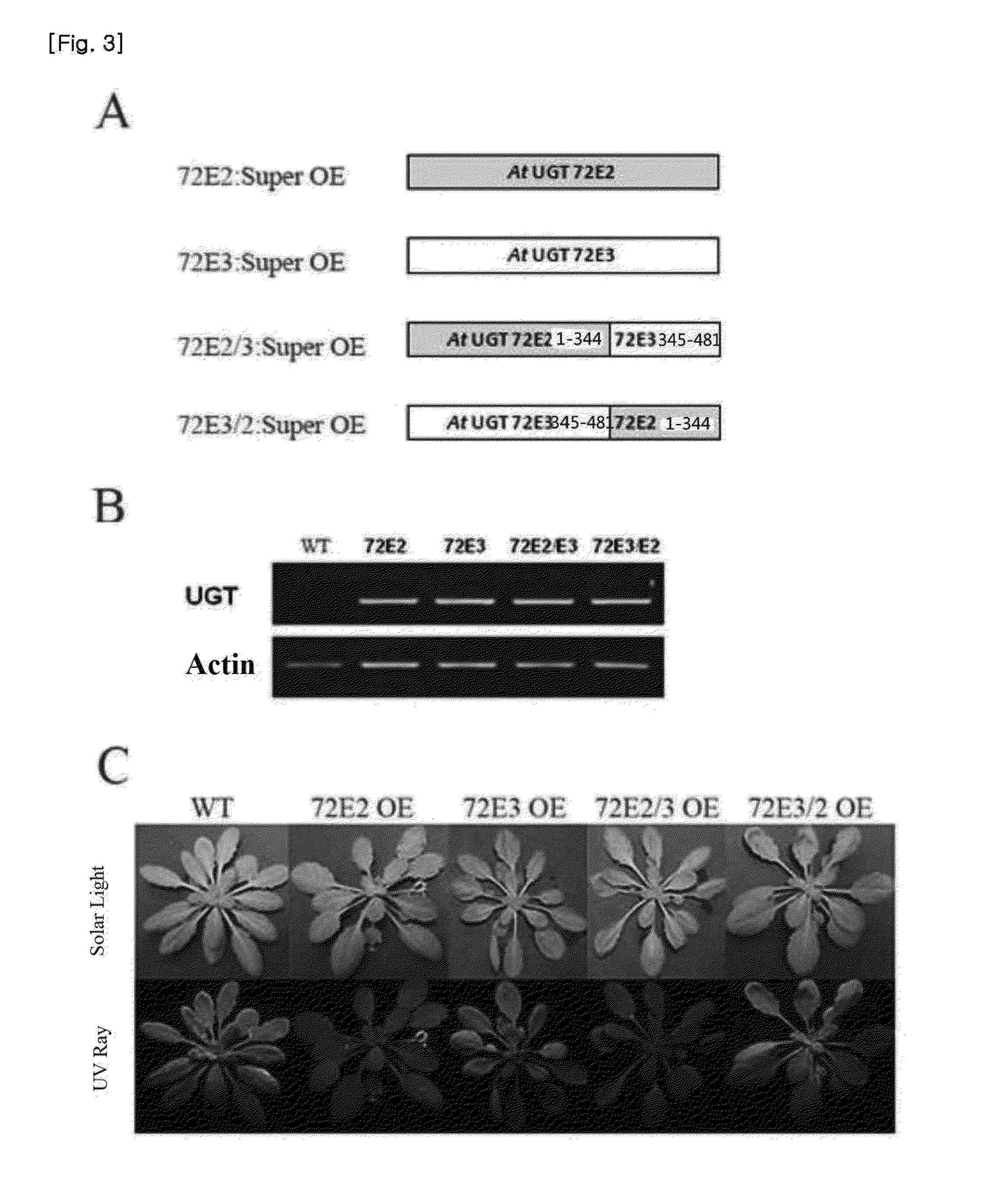
Method for producing transgenic plant with increased syringin production and plant produced by using the same
ActiveUS20150252338A1Effective massRaise transfer toSugar derivativesTransferasesPhenylpropanoidSyringin
The present invention relates to recombinant glycosyl transferase UGT72E3 / 2 gene having an excellent syringin synthesis ability based on remarkable enzyme specificity for sinapyl alcohol, a method for producing a transgenic plant with increased syringin production based on a metabolic process which uses F5H and CHS genes that are involved with the phenylpropanoid biosynthesis pathway and Myb58 gene as a transcription factor for positive regulation of the gene that is involved with lignin biosynthesis pathway, and a plant obtained by the method. According to the present invention, syringin with various pharmaceutical applications can be effectively produced in a large amount in a plant, and thus it is expected to allow the development of an industry relating to agrobiological materials that are highly valuable as foods or pharmaceuticals.
Owner:DONG A UNIV RES FOUND FOR IND ACAD COOP
Saussurea involucrata extract and application thereof to preparation of cosmetics
InactiveCN109157448AGuaranteed uptimeHigh degree of automationCosmetic preparationsAntipyreticChlorogenic acidRutin
The invention discloses a saussurea involucrata extract. The saussurea involucrata extract contains 0.5-1.2% of syringin, 7-17% of chlorogenic acid and 8-25% of rutin in percentage by weight. The saussurea involucrata extract is obtained according to the following steps: S1, crushing saussurea involucrata, then putting into an extraction tank, extracting with ethanol, and combining the extracts; S2, filtering, cooling and centrifuging the extract to obtain a supernatant; S3, concentrating the supernatant to obtain a concentrate; S4, adding macroporous resin to the concentrate for purification,then eluting with ethanol, fractionally collecting the effluent, concentrating the effluent into thick paste or making the effluent into dry paste powder, thereby obtaining the saussurea involucrataextract. The supernatant is concentrated by using an MVR evaporator, so that the operation is stable, the degree of automation is high, the power consumption is low, and the operating cost is low; inaddition, the process is simple, the practicability is strong, and partial load operation characteristics are excellent; and the saussurea involucrata extract has the functions of absorbing ultraviolet rays, resisting radiation, resisting inflammation, easing pains, resisting ageing and the like in cosmetics.
Owner:GUANGZHOU JINSME FINE CHEM CO LTD
Pharmaceutical composition for treating neurasthenia and preparation method thereof
ActiveCN104490904ASignificantly calms the mind and calms the mindComprehensive application effect is idealNervous disorderDigestive systemSyringinCurative effect
The invention relates to a pharmaceutical composition for treating neurasthenia and a preparation method thereof. The pharmaceutical composition comprises the following active ingredients: 0.03-0.5mg / ml eleutheroside E, 0.05-0.5mg / ml syringin, 0.005-0.05mg / ml isofraxidin and 0.03-0.5mg / ml schizandrin; preferably, 0.05-0.2mg / ml eleutheroside E, 0.1-0.3mg / ml syringin, 0.01-0.03mg / ml isofraxidin and 0.05-0.2mg / ml schizandrin. The pharmaceutical composition provided by the invention has the advantages that the preparation process is simple and feasible, the properties of the traditional Chinese medicine components are fully considered in the preparation process, excessive impurities are removed by macroporous adsorption resin to improve the concentration of active ingredients in the preparation and play the effects of the traditional Chinese medicine components; meanwhile, the used resin can be repeatedly used, so that resource consumption can be reduced. The traditional Chinese medicine composition prepared in the invention has a remarkable curative effect.
Owner:安徽九洲方圆制药有限公司
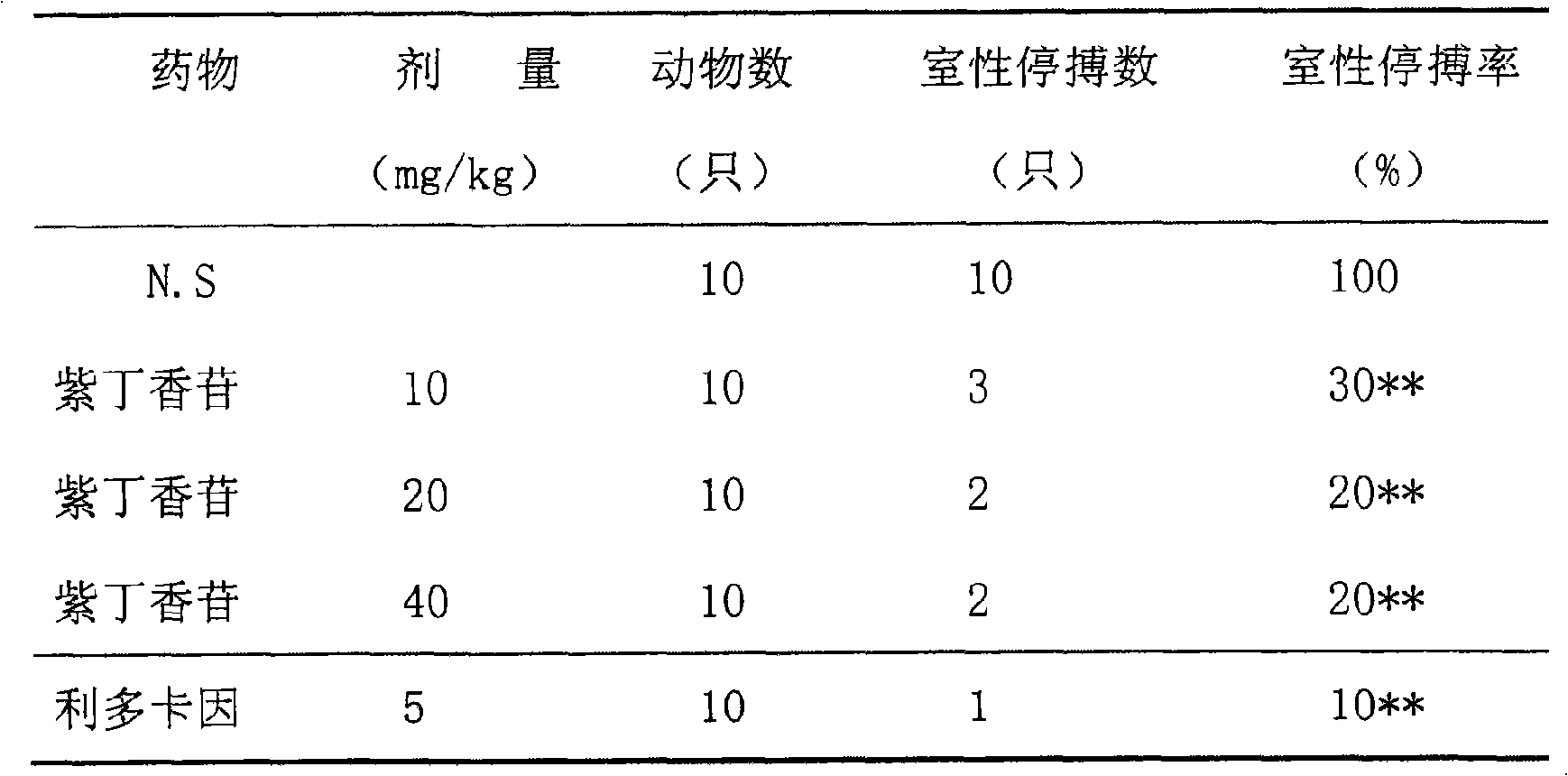
Application of syringin in preparing drugs for treating cardiovascular and cerebrovascular diseases
InactiveCN101596203AEnhance pharmacological effectsGood effectOrganic active ingredientsMetabolism disorderDiseaseSide effect
The invention relates to application of syringin in preparing drug combination for treating cardiovascular and cerebrovascular diseases. The syringin preparation is safe and nontoxic and has strong pharmacological action and good drug effect, thereby indicating bright prospect in drugs; the syringin preparation is simple in preparing process, has no toxic side effects and can be in oral forms such as tablets, pills, capsules and the like, or in injectable forms such as powder-injection, instillations and the like; the drugs prepared by the syringin can evidently lessen the degree and scope of myocardial ischemia caused by the anterior descending branch of canine coronary artery, reduce the myocardial oxygen consumption index, obviously withstand barium chloride induced arrhythmia in rats and reduce the cerebral infarct area of focal cerebral ischemia in rats; the above effects show that the drugs have obvious effects against arrhythmia, myocardial ischemia and cerebral ischemia. The drugs can be clinically used for preventing and treating coronary diseases, angina, stroke and cerebral infarct, etc.
Owner:潘书洋 +1
Preparation process and quality control method of Eleutherococcus senticosus reference extract
PendingCN112763639AGuaranteed stabilityReasonable control of powder collection rateComponent separationSyringa oblataSyringin
The invention discloses a preparation process and a quality control method of an Eleutherococcus senticosus reference extract, the Eleutherococcus senticosus reference extract is prepared by decocting and extracting Eleutherococcus senticosus decoction pieces as a raw material, in the preparation process, the powder yield and the content of syringin which is an effective substance can be effectively controlled by reasonably controlling the decoction times, the water addition amount, the decoction time and the like, the stability of the radix acanthopanacis senticosi reference extract component is ensured, and the process is stable in operation. The quality control method can realize the stability and reliability of the preparation process of the Eleutherococcus senticosus reference extract.
Owner:SICHUAN NEO GREEN PHARMA TECH DEV
Method for measuring effective content of acanthopanax in medicine
InactiveCN101368934AOvercome the disadvantages that the content of active ingredients is not easy to control and affect clinical applicationGuarantee product qualityComponent separationTheoretical plateMethanol water
The invention relates to a method for measuring the effective content of acanthopanax root in medicine. Based on the chromatographic conditions of high-performance liquid chromatography measurement, the method uses octadecyl bonded silica as the filler, and takes methanol-water as the mobile phase, with the detection wavelength reaching 265nm; the theoretical plate number is not less than 3000 in accordance with syringin wave peak calculation; firstly, the reference substance solution with 0.02mg of syringin in each 1ml of the solution is prepared; then 1g of the measured medicine is put into a 50ml-measuring flask and diluted to 50ml scale with methanol; 5ml of subsequent filtrate is processed through decompression and drying and then added to a treated C18 solid extraction column; 4ml of 30% methanol eluate and 30% methanol solution are collected and diluted to 5ml scale so as to prepare the sample solution; 10ul of the reference substance solution and 10ul of the sample solution are respectively extracted and injected into the liquid chromatography to measure the acanthopanax root content based on syringin. The method is convenient and fast and has accurate measurement standards to ensure the product quality and efficacy of the medicine containing acanthopanax root.
Owner:山东仙河药业有限公司
Method for extracting syringin compound from tobaccos
The invention discloses a method for extracting a syringin compound from tobaccos. The method is characterized by comprising the following steps: preparing a tobacco extract; dissolving the tobacco extract into water, and extracting; eluting an extraction solution with ethanol in a macroporous resin column; collecting eluate, performing reduced pressure concentration, performing capillary sampling thin-layer chromatography, and combining eluate containing the similar components; performing reduced pressure concentration till a solid state to obtain a polar tobacco extract; performing thin-layer chromatography, combining samples with amaranth spots, and performing reduced pressure concentration to obtain a crude extract; uniformly stirring the crude extract and silica gel, separating by using a normal phase silica gel chromatographic column, and eluting; through the chromatographic column, combining fragrance-forming substances according to different Rf values and different polarities to obtain a fine extract; performing normal phase column chromatography on the fine extract, and eluting, segmenting into 5 parts A-E during TLC detection, performing gel column chromatography on a segment B, feeding the segment B into a semi-preparative chromatography system, and separating; under chromatographic conditions, retaining for 14.32 minutes, and separating to obtain the monomer compound.
Owner:GUIZHOU TOBACCO SCI RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com