Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
253results about How to "Stable baseline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
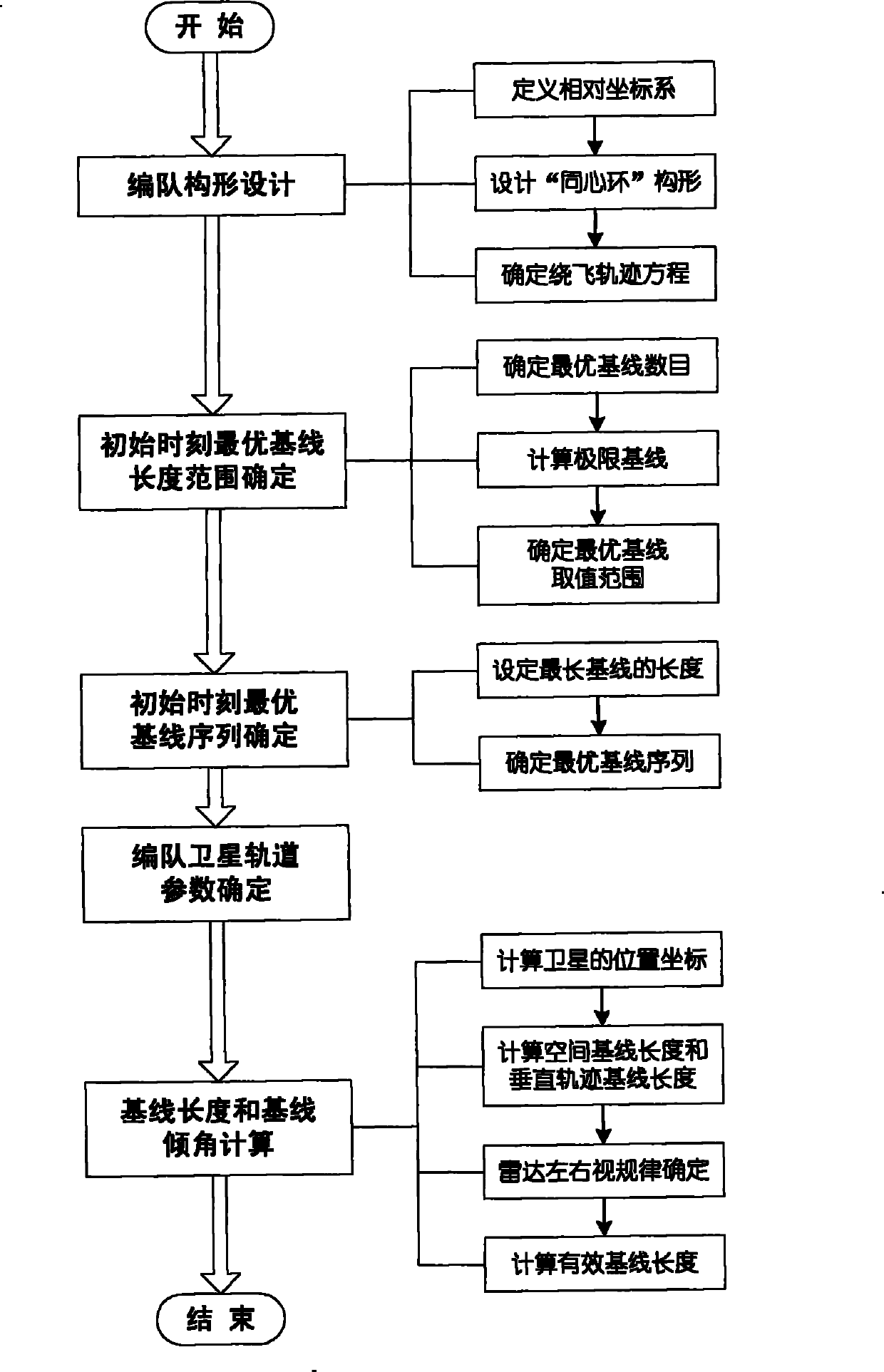
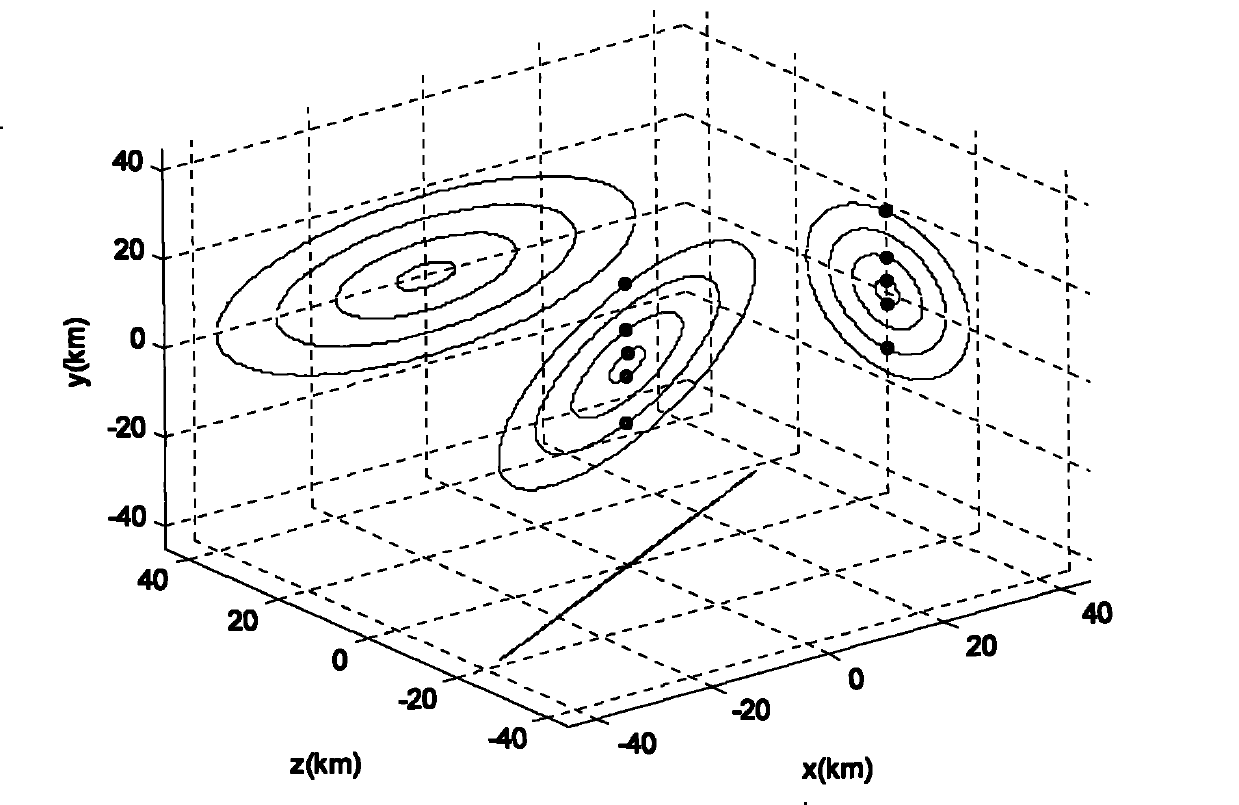
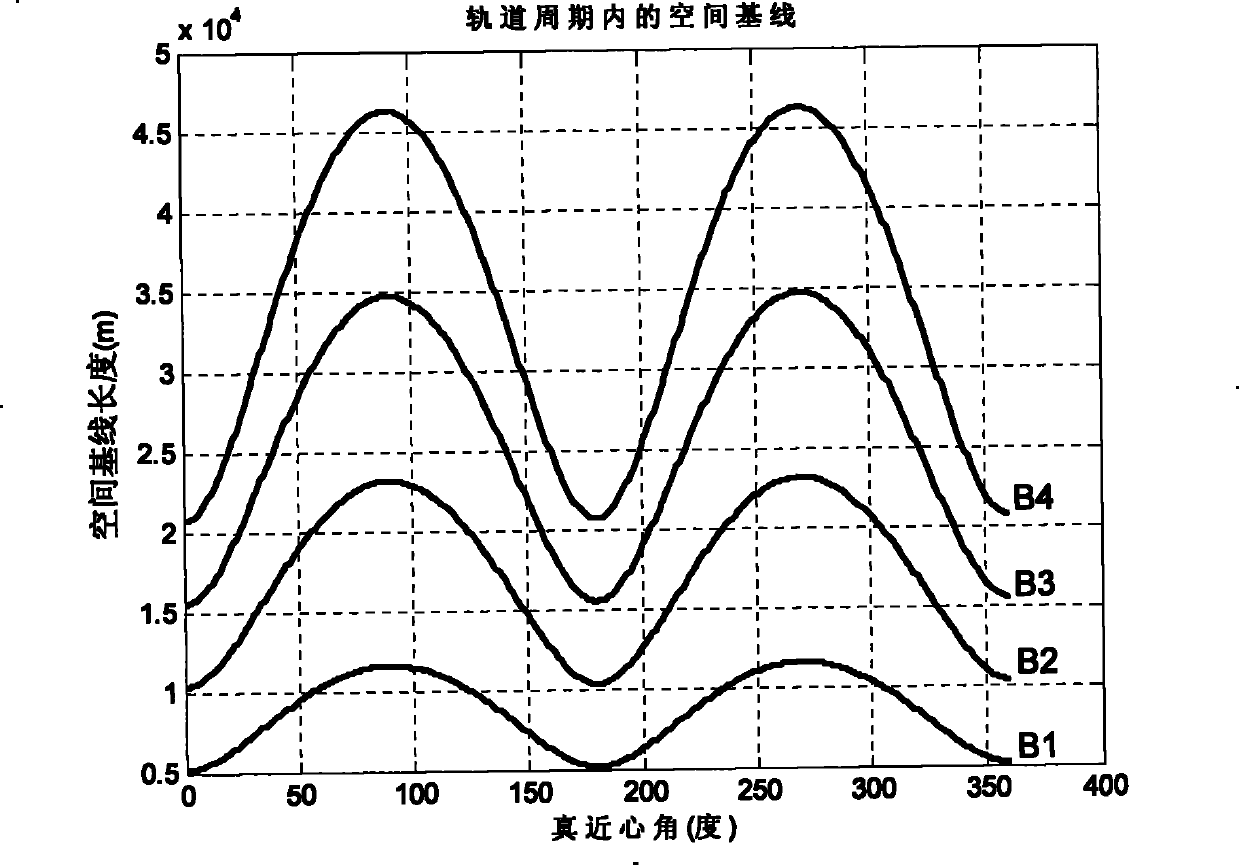
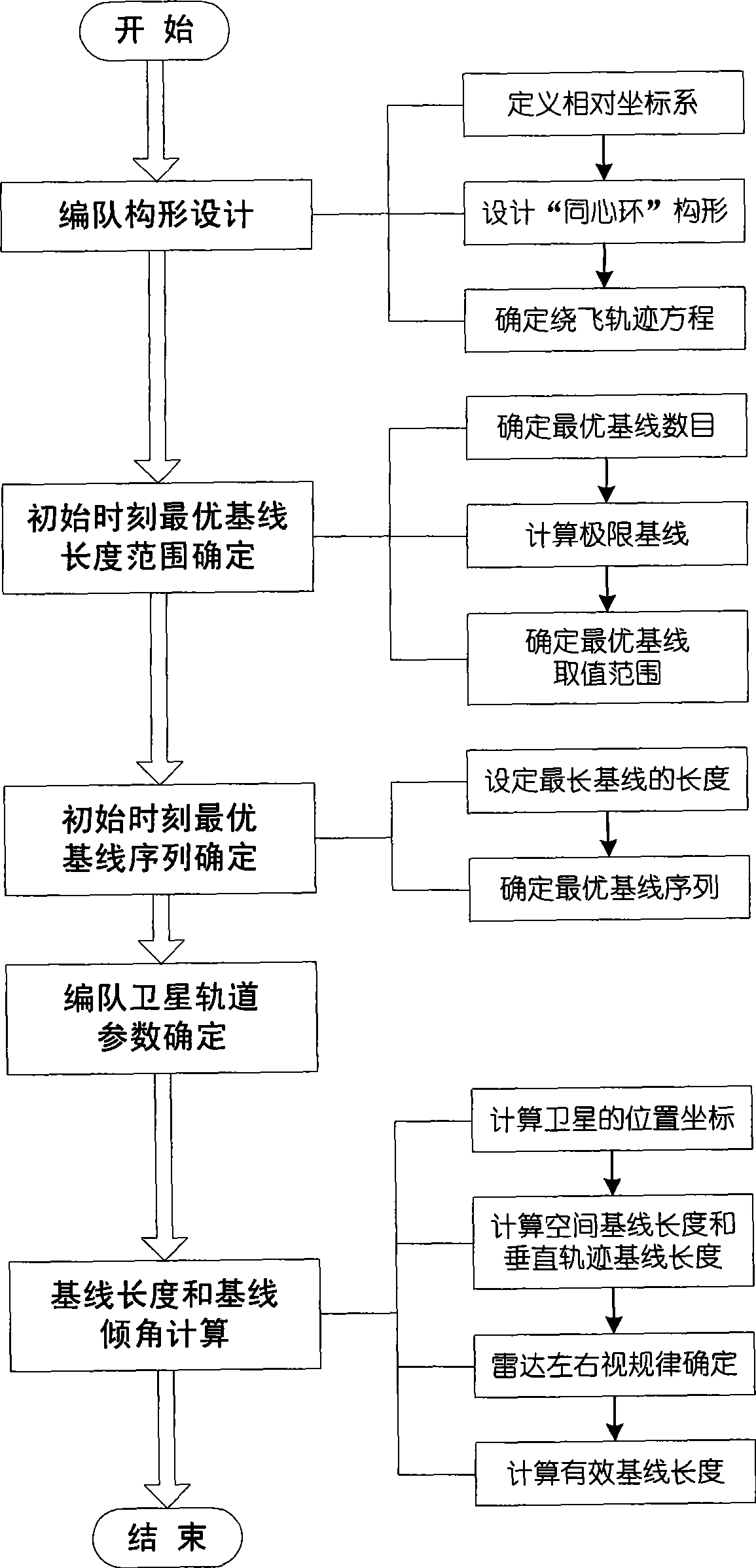
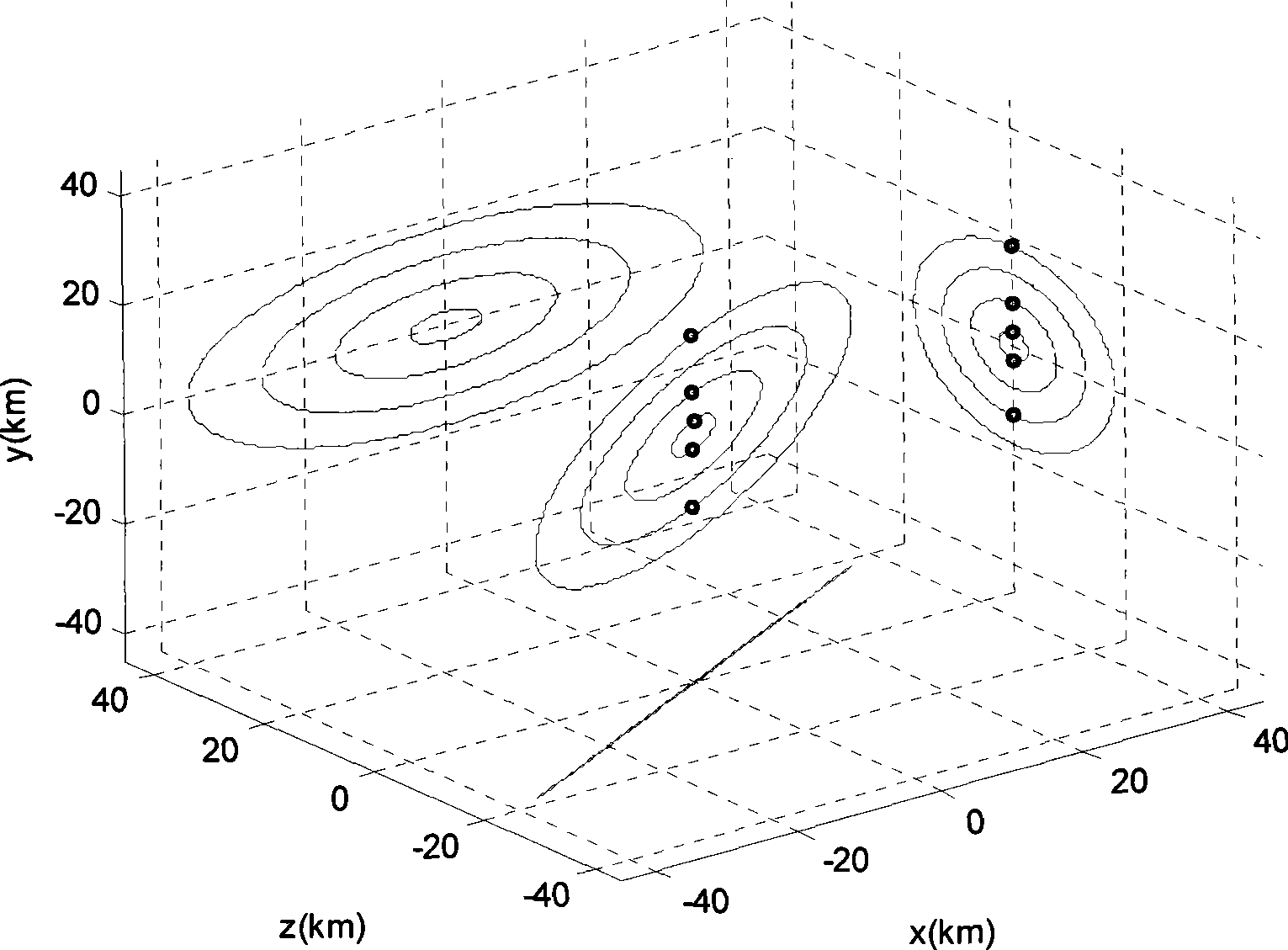
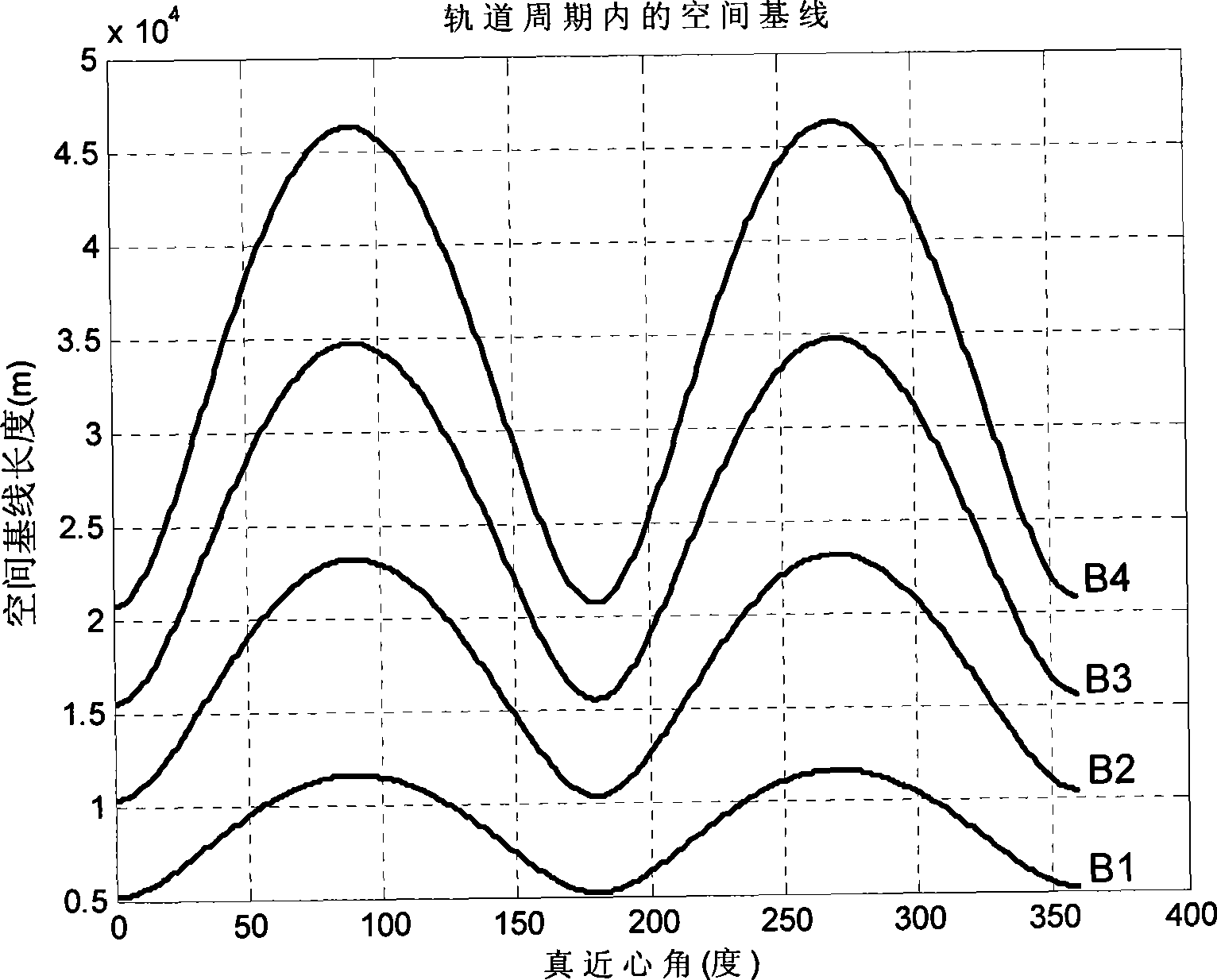
Method for formation configuration of distributed satellites with synthetic aperture radars
InactiveCN101520511AApplicable interference processingStable baselineRadio wave reradiation/reflectionOrbital periodSynthetic aperture sonar
The invention relates to a design method for the formation configuration of distributed satellites with synthetic aperture radars (SAR). The method comprises the following five operation steps: step 1: designing the formation configuration of the distributed satellites and conforming fly-by path equations; step 2: confirming an optimal base line length range of the distributed satellites in start time; step 3: confirming an optimal base line sequence of the distributed satellites in start time; step 4: confirming track parameters of the formed satellites forming a concentric circle configuration; and step 5: calculating effective base lines and vertical path base lines in a track period. The invention provides the concentric circle formation configuration, uses the optimal base line combined constraint conditions of multi-base line interference SARs as design input parameters and designs a control law of radar antenna visual angles to realize that a distributed satellite SAR system can satisfy the design requirements of the optimal base line combined constraint conditions in any time in a track operation period and a basis is provided for the distributed satellite SAR system to obtain high-precision DEM products by a multi-base line interference SAR treatment method.
Owner:BEIHANG UNIV
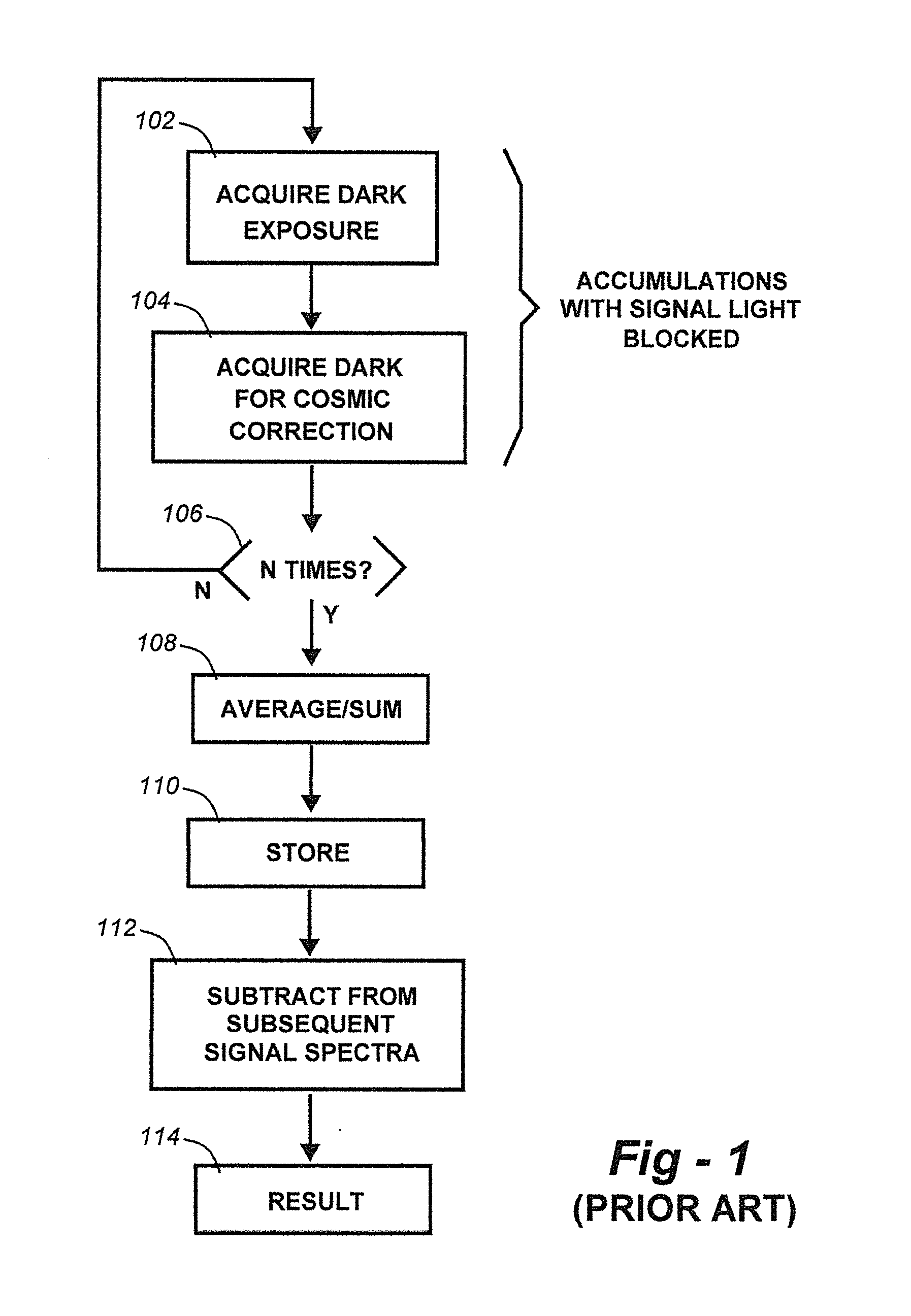
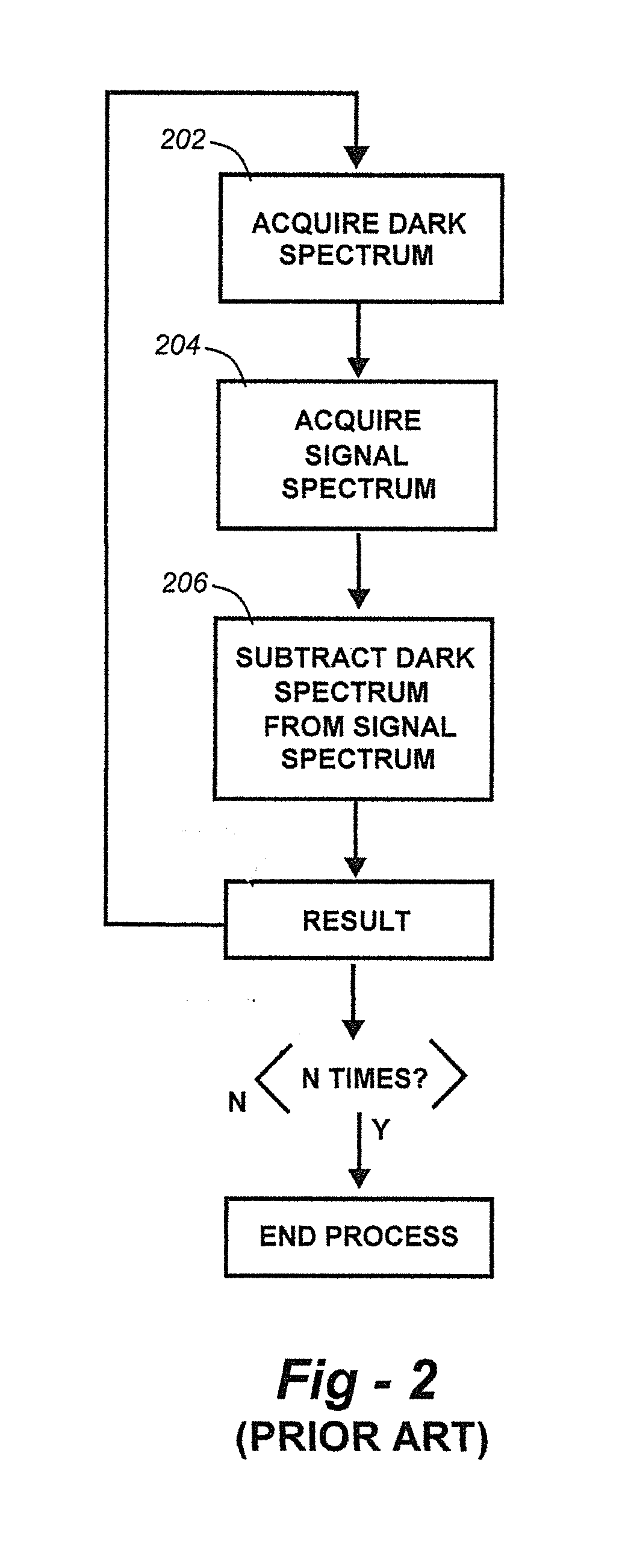
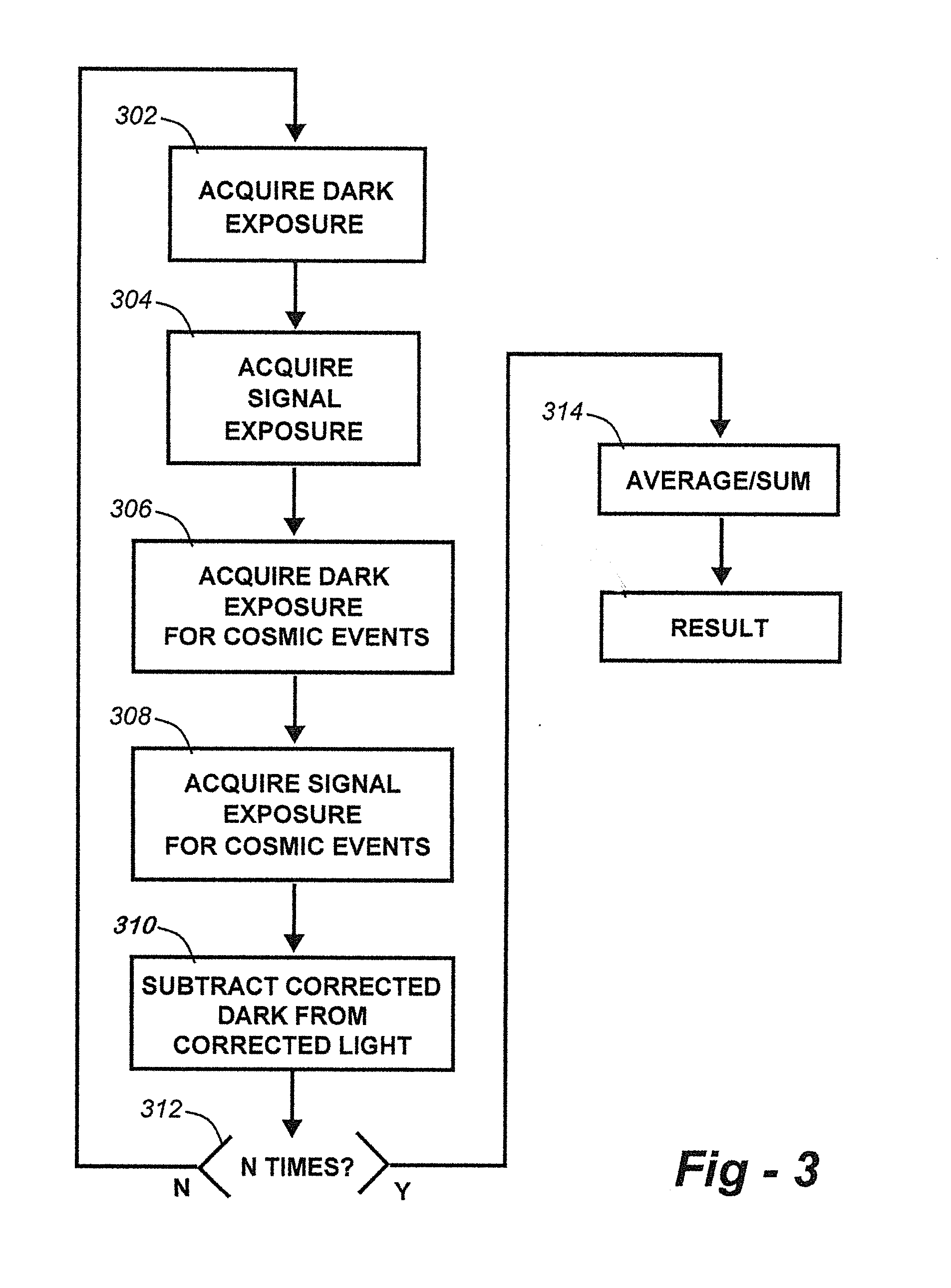
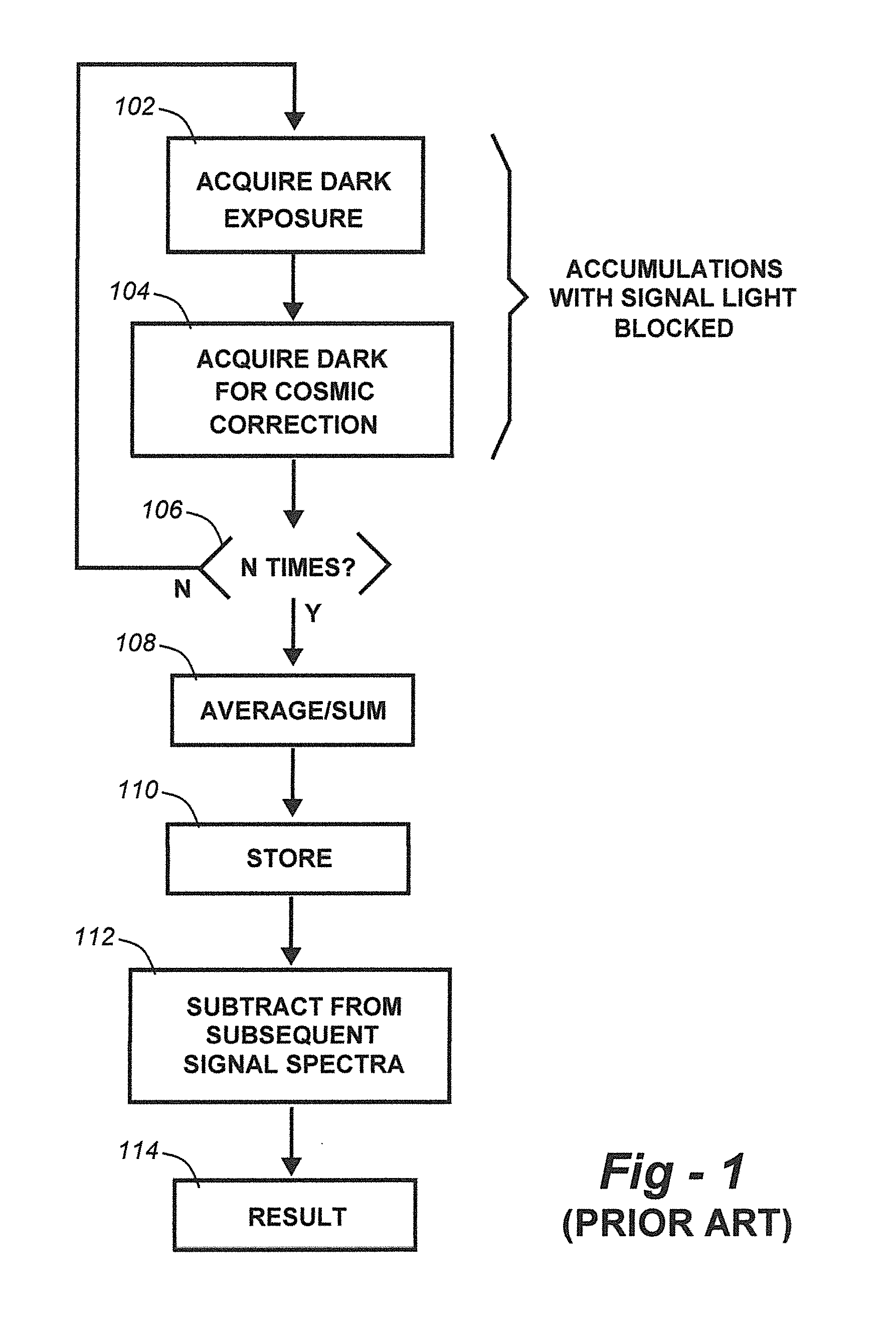
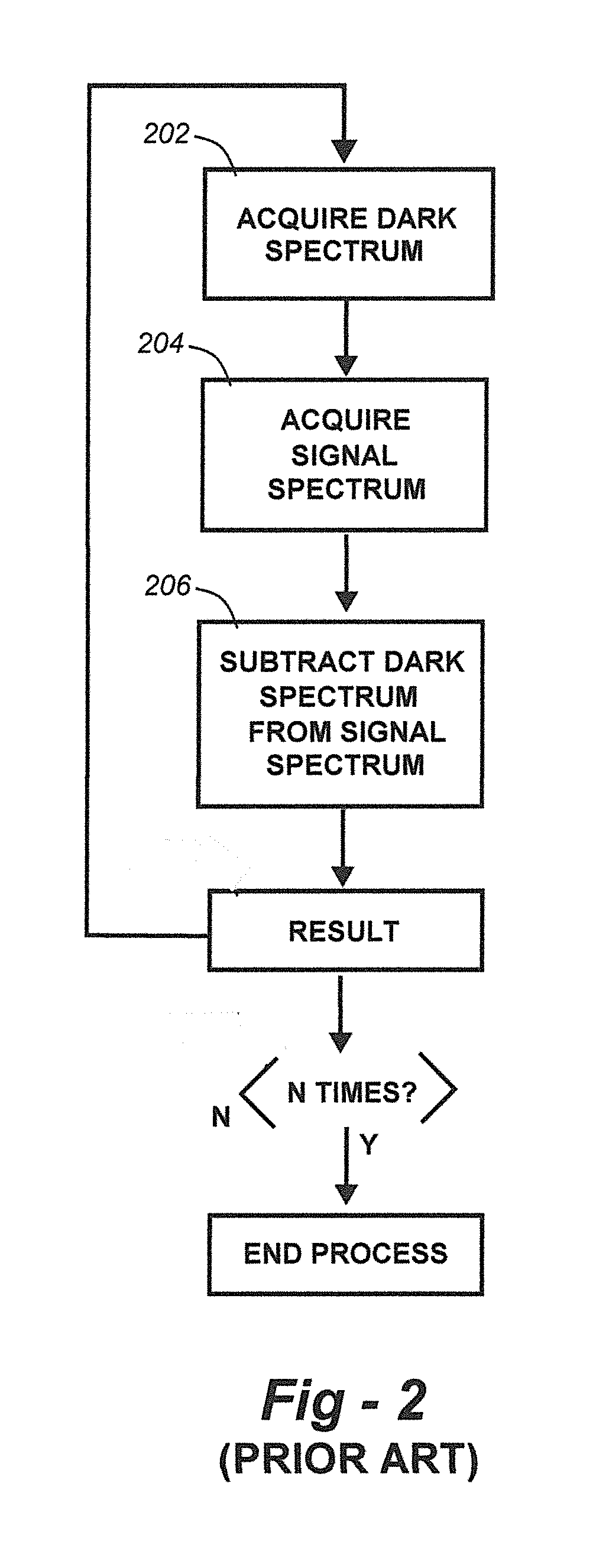
Methods for collection, dark correction, and reporting of spectra from array detector spectrometers
ActiveUS20160356647A1Rapid reporting of dataFast dataRadiation pyrometrySpectrum investigationSpectral responsePower flow
Methods and systems for spectrometer dark correction are described which achieve more stable baselines, especially towards the edges where intensity correction magnifies any non-zero results of dark subtraction, and changes in dark current due to changes in temperature of the camera window frame are typically more pronounced. The resulting induced curvature of the baseline makes quantitation difficult in these regions. Use of the invention may provide metrics for the identification of system failure states such as loss of camera vacuum seal, drift in the temperature stabilization, and light leaks. In system aspects of the invention, a processor receives signals from a light detector in the spectrometer and executes software programs to calculate spectral responses, sum or average results, and perform other operations necessary to carry out the disclosed methods. In most preferred embodiments, the light signals received from a sample are used for Raman analysis.
Owner:ENDRESS + HAUSER OPTICAL ANALYSIS INC
Methods for collection, dark correction, and reporting of spectra from array detector spectrometers
InactiveUS20160356646A1Rapidly reportFast dataRadiation pyrometrySpectrum investigationSystem failureBase line
Methods and systems for spectrometer dark correction are described which achieve more stable baselines, especially towards the edges where intensity correction magnifies any non-zero results of dark subtraction, and changes in dark current due to changes in temperature of the camera window frame are typically more pronounced. The resulting induced curvature of the baseline makes quantitation difficult in these regions. Use of the invention may provide metrics for the identification of system failure states such as loss of camera vacuum seal, drift in the temperature stabilization, and light leaks. In system aspects of the invention, a processor receives signals from a light detector in the spectrometer and executes software programs to calculate spectral responses, sum or average results, and perform other operations necessary to carry out the disclosed methods. In most preferred embodiments, the light signals received from a sample are used for Raman analysis.
Owner:KAISER OPTICAL SYST INC
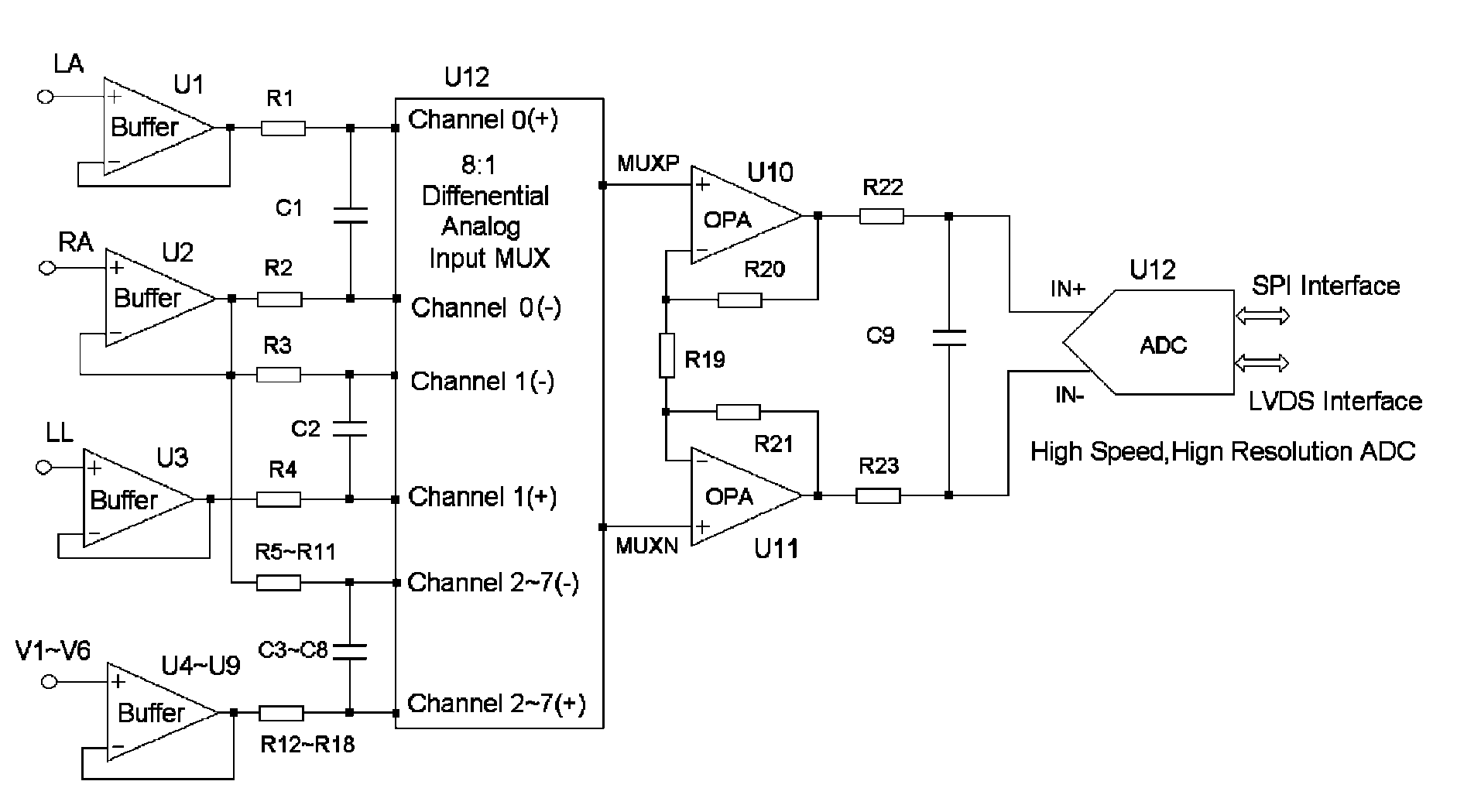
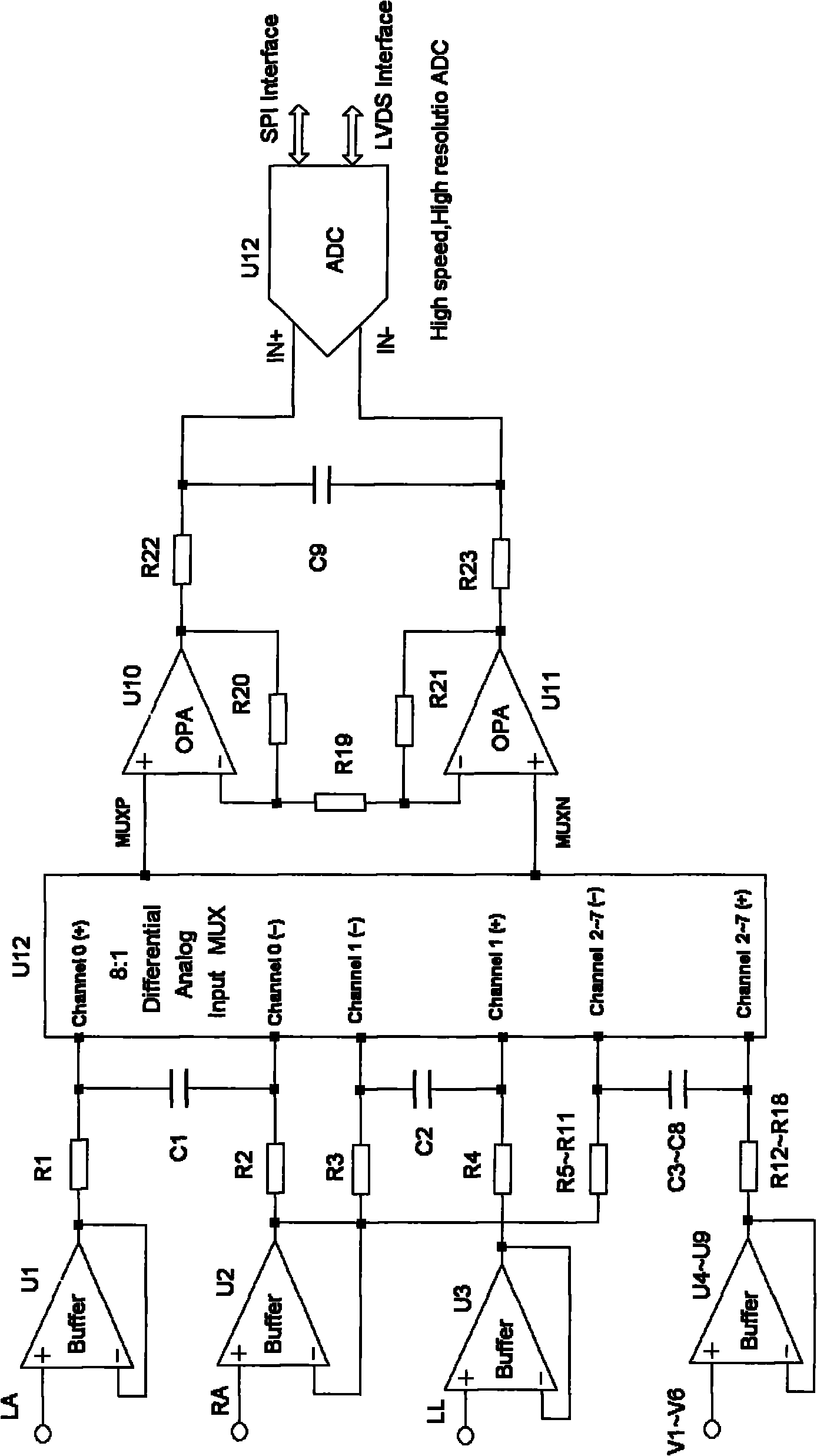
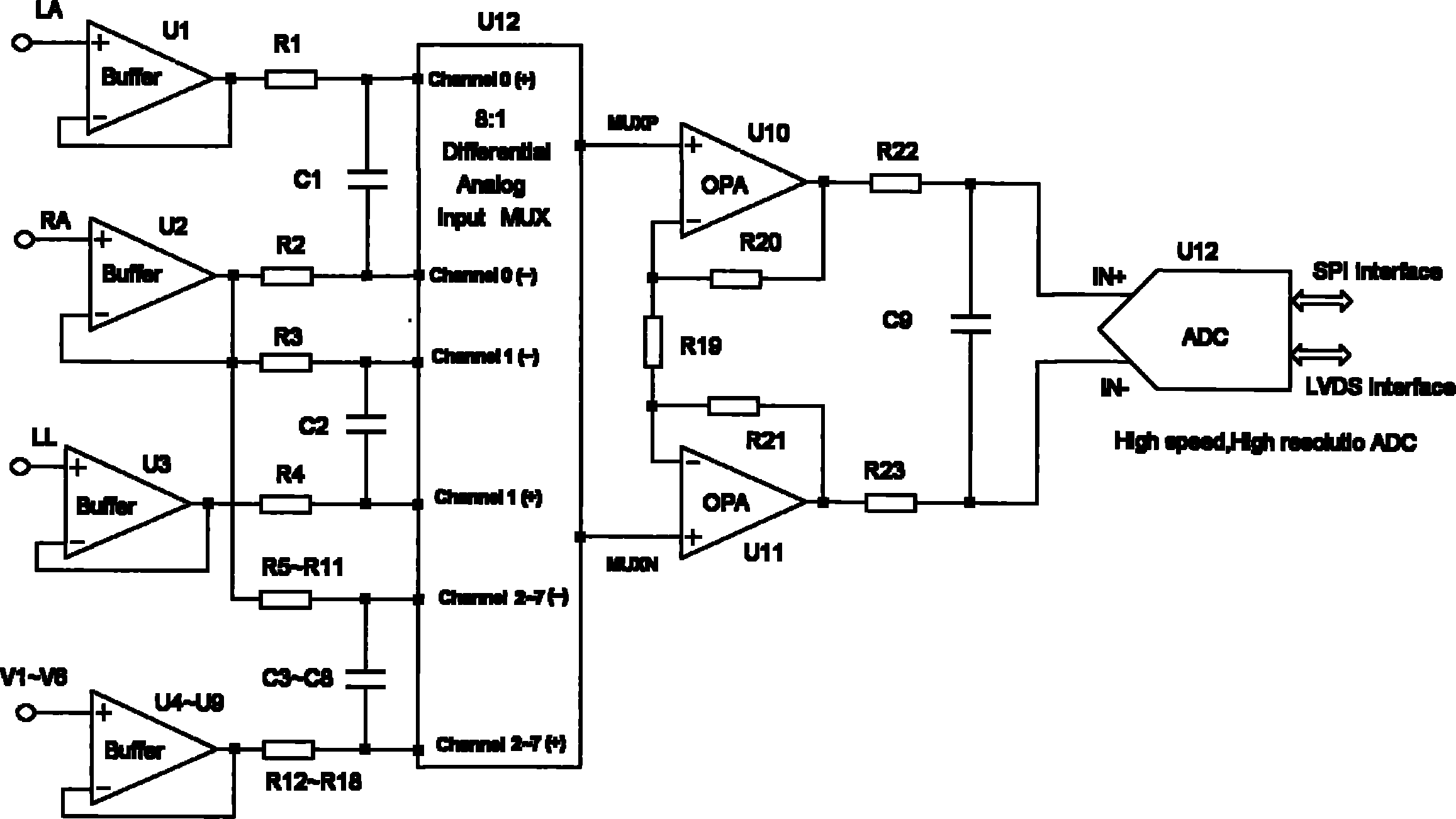
Fully differential non-inverted parallel amplifier for detecting biology electrical signal
ActiveUS7863977B1Low costImprove efficiencyElectrocardiographyAmplifier combinationsLow noiseAnti-aliasing
This invention relates to a fully differential non-inverting parallel amplifier for detecting biology electrical signal, including input buffer circuits, differential filter circuits, data selector, non-inverting parallel amplifying circuits and analog-digital circuits. The biology electrical signal, first impeded and converted by the input buffer circuits, and then low-pass filtered by the differential filter circuits, shall be amplified with its common mode signal rejected by passing through the data selector and non-inverting parallel amplifier circuits. At last, the amplified biology electrical signal is output by analog to digital conversion in the analog-digital circuits after its noises beyond signal high frequency band are filtered by anti-aliasing filter net. This invention, with low noise and high common mode rejection ratio, stable baseline, large signal input dynamic range, is reliable and not easy to be saturated. Furthermore, it can support mature PACE Detecting with a low cost. It is notable in social and economical benefits for its simple electrical circuits and easy use in any biology electrical testing equipments and controlling system.
Owner:EDAN INSTR
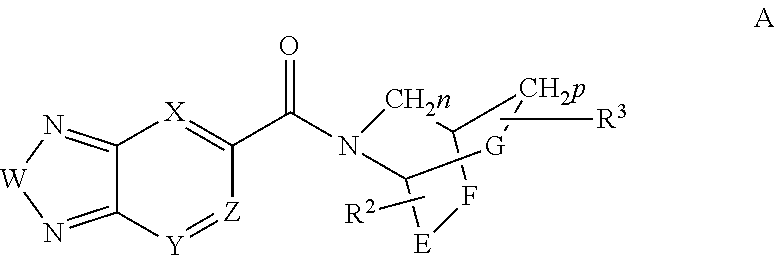
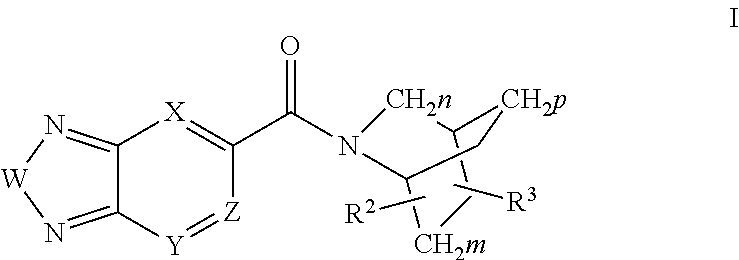
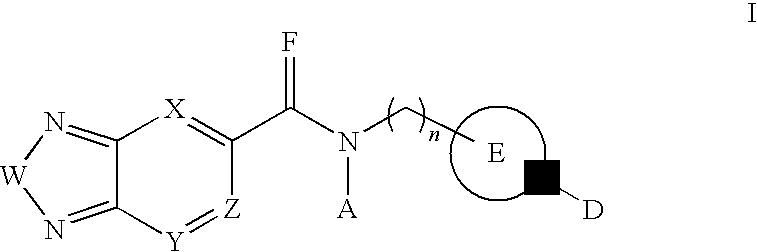
Di-substituted amides for enhancing glutamatergic synaptic responses
InactiveUS8013003B2Stable baselineIncrease amplitudeBiocideNervous disorderSIDS - Sudden infant death syndromeCentral sleep apnea
This invention relates to compounds, pharmaceutical compositions and methods for use in the prevention and treatment of cerebral insufficiency, including enhancement of receptor functioning in synapses in brain networks responsible for basic and higher order behaviors. These brain networks, which are involved in regulation of breathing, and cognitive abilities related to memory impairment, such as is observed in a variety of dementias, in imbalances in neuronal activity between different brain regions, as is suggested in disorders such as Parkinson's disease, schizophrenia, respiratory depression, sleep apneas, attention deficit hyperactivity disorder and affective or mood disorders, and in disorders wherein a deficiency in neurotrophic factors is implicated, as well as in disorders of respiration such as overdose of an alcohol, an opiate, an opioid, a barbiturate, an anesthetic, or a nerve toxin, or where the respiratory depression results form a medical condition such as central sleep apnea, stroke-induced central sleep apnea, obstructive sleep apnea, congenital hypoventilation syndrome, obesity hypoventilation syndrome, sudden infant death syndrome, Rett syndrome, spinal cord injury, traumatic brain injury, Cheney-Stokes respiration, Ondines curse, Prader-Willi's syndrome and drowning. In a particular aspect, the invention relates to compounds useful for treatment of such conditions, and methods of using these compounds for such treatment.
Owner:CORTEX PHARMA INC
Method for establishing lonicerae and forsythiae powder UPLC fingerprint spectrum
ActiveCN105806975AEasy to separateGood peak shapeComponent separationChemical compositionRepeatability
The invention discloses a method for establishing a lonicerae and forsythiae powder UPLC fingerprint spectrum.The method can quickly and accurately identify authenticity and merits of a product and has the advantages of simplicity, convenience, stability, high precision, good repeatability and the like.By means of the method, kinds and quantity of chemical components contained in lonicerae and forsythiae powder can be comprehensively reflected, global description and evaluation are conducted on the quality of the lonicerae and forsythiae powder, the quality and the medicine effects of the lonicerae and forsythiae powder are truly combined, which is conductive to illuminating the function mechanism of the lonicerae and forsythiae powder, and a basis is provided for technical promotion and deep development of the lonicerae and forsythiae powder.By means of the method, the number of the chemical components detected in the lonicerae and forsythiae powder fingerprint spectrum is relatively large, characteristic peak height proportions are moderate, a base line is stable, and the separation degree, peak shapes and column efficiency are good.
Owner:贵阳德昌祥药业有限公司 +1
Quality analysis method of compound Ganmaoling tablets
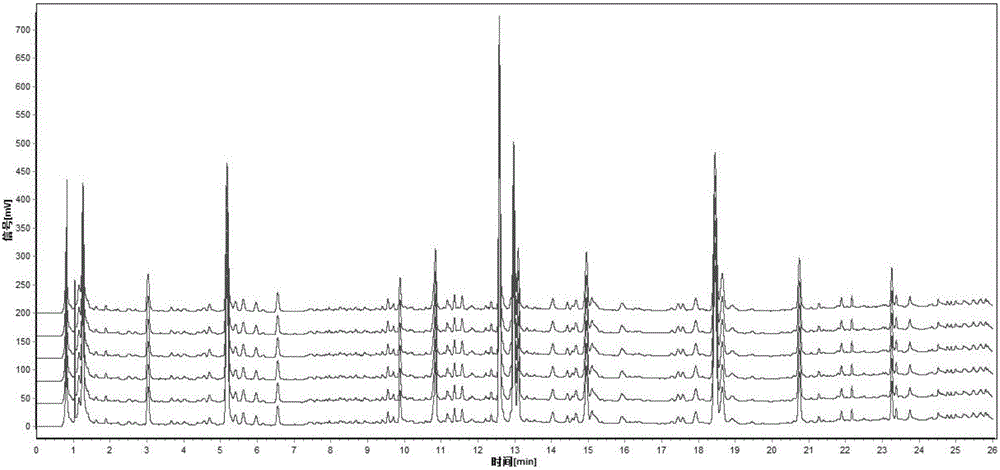
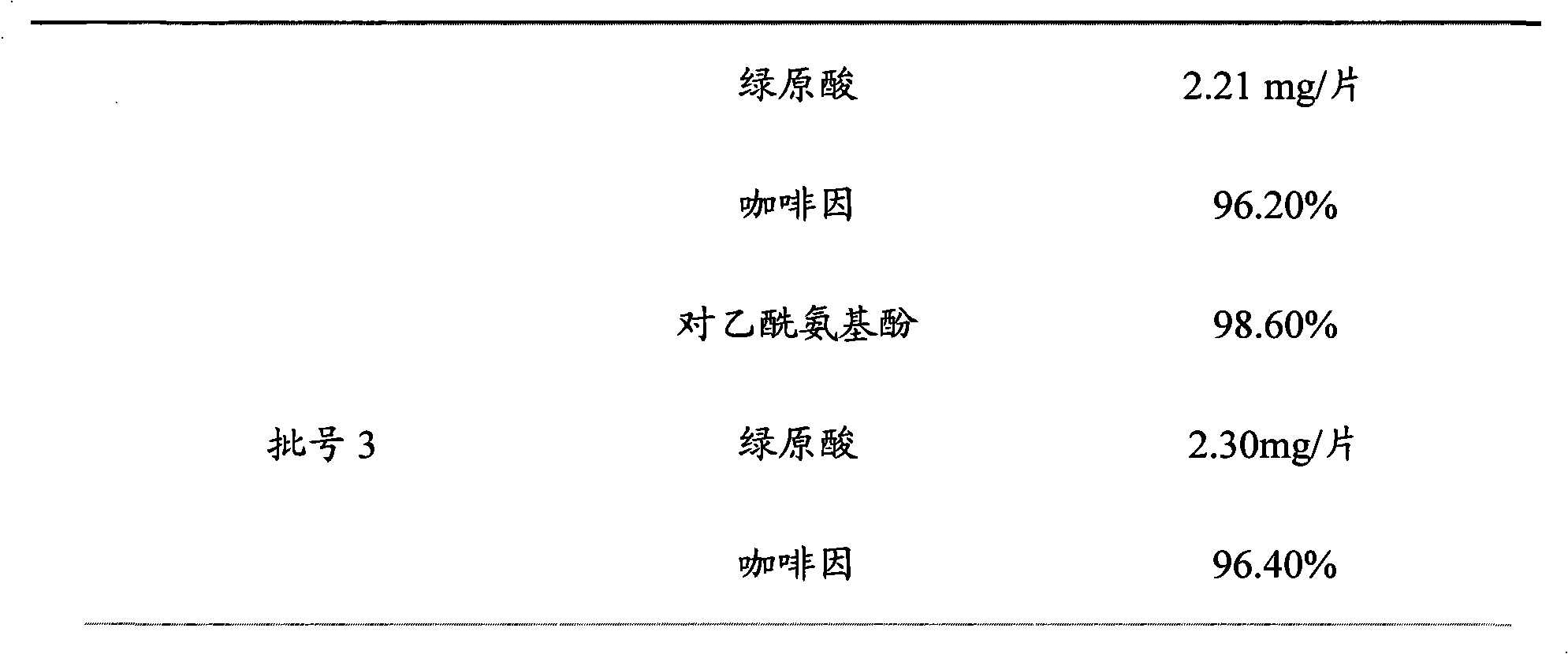
ActiveCN101961430ASave inspection timeSave inspection costComponent separationAntiinfectivesChlorogenic acidReference product
The patent refers to the field of 'pharmaceutical preparations'. The invention discloses a quality analysis method of compound Ganmaoling tablets. In the method, high-performance liquid chromatography (HLPC) is used for measure the chlorogenic acid content, acetaminophen content and caffeine content of the compound Ganmaoling tablets, test product solution is prepared and reference product solution is prepared and measured. The quality analysis method of the invention can detect the acetaminophen, chlorogenic acid and caffeine components under the same color spectrum condition at the same time, so the test time and cost are saved and the working efficiency is improved; the test method has high sensitivity, high separating degree; the basic line is stable; a negative reference is not affected; and the accuracy, repeatability, linearity and stability of the test method meet scientific research and production requirements, and the detection method is suitable for promotion and application.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
Method for controlling quality of corydalis tuber and preparation thereof and drug effect thereof by using finger print
InactiveCN101288699AEffective representation qualitySuitable for useComponent separationCardiovascular disorderPalmatineCorydalis cava
The invention relates to a fingerprint map for controlling the quality and efficacy of rhizoma corydalis and preparation of the rhizoma corydalis and a preparation method of the fingerprint map. The invention discloses that the fingerprint map is one of anti-myocardial ischemic active site in the rhizoma corydalis, and the active site mainly comprises quaternary ammonium base components, wherein, columbamine, 13-methyldehydrocorydalmine, dehydrocorybulbine, palmatine, dehydrocorydaline are the main five components of quaternary ammonium base. The fingerprint map can identify the authenticity of samples and evaluate the quality and efficacy of the rhizoma corydalis or the preparation thereof, so that the quality of the rhizoma corydalis and the preparation thereof have real controllable standards, so as to ensure the product quality to be stable. The method has the advantages of being simple to operate and being accurate and reliable, which is applicable to controlling the quality and efficacy of the rhizoma corydalis and the preparation thereof.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Full-differential same-phase parallel amplifying device for acquiring bioelectric signal
ActiveCN101997515AStable baselineFast signalOne-way transmission networksDifferential amplifiersLarge dynamic rangeInstrumentation
The invention discloses a full-differential same-phase parallel amplifying device for acquiring a bioelectric signal. The amplifying device comprises an input buffer circuit, a differential filtering circuit, a data selector, a same-phase parallel amplifying circuit and an analog-digital conversion circuit which are connected sequentially. The input buffer circuit performs impedance conversion on the bioelectric signal first; the bioelectric signal subjected to low-pass filtering by the differential filtering circuit passes through the data selector and the same-phase parallel amplifying circuit; the bioelectric signal is amplified and a common-mode signal is suppressed; the noise of the bioelectric signal outside a signal high-frequency band is filtered through an anti-aliasing filtering network; and the amplified signal is subjected to analog-digital conversion by the analog-digital conversion circuit and then is output. The noise and common-mode suppression ratio can reach a high index, a base line is stable, a dynamic signal input range is wide, signals are difficult to saturate, and the device has high in reliability and can support perfect PACE detection. Meanwhile, the circuits are simple and are low in cost, and the device can be used for various bioelectric detection instruments and systems and has obvious economic benefit.
Owner:EDAN INSTR
Establishment method for UPLC fingerprint spectrum of gynecological reconstruction pill
The invention discloses an establishment method for a UPLC fingerprint spectrum of a gynecological reconstruction pill. According to the method, the authenticity and merits of the product can be quickly and accurately identified, and the advantages of being easy and convenient to use, stable, high in precision, good in reproducibility and the like are achieved; in addition, the variety and number of chemical components contained in the gynecological reconstruction pill can be comprehensively reflected, then the quality of the gynecological reconstruction pill is integrally described and evaluated, therefore, the quality and the medicine effect of the gynecological reconstruction pill can be truly combined together, illumination of the action mechanism is promoted, and a basis is provided for technology improvement and deep development of the gynecological reconstruction pill. Furthermore, the fingerprint spectrum, detected through the method, of the gynecological reconstruction pill contains relatively more chemical components and is moderate in height proportion of all characteristic peaks, stable in base line and good in separation degree, peak shape and column efficiency.
Owner:GUIYANG COLLEGE OF TRADITIONAL CHINESE MEDICINE
Canzhiling oral solution fingerprint map building method, fingerprint map and application thereof
ActiveCN104849364AImprove stabilityGood reproducibilityComponent separationSystems analysisChemical composition
The invention discloses a canzhiling oral solution fingerprint map building method. The map includes the following steps of preparation of a test solution, preparation of a reference solution, testing through a high performance liquid chromatograph and processing of data and a map. The invention further discloses a Canzhiling oral solution fingerprint map and a method for utilizing the fingerprint map to control quality of a Canzhiling oral solution. The Canzhiling oral solution fingerprint map building method is simple in operation, stable, reliable, high in accuracy and high in separation degree, the fingerprint map is high in stability and reproducibility and large in information quantity, and the fingerprint map is adopted as a quality control means for the Canzhiling oral solution, so that one-sidedness caused by judging of overall quality of a preparation by testing one or two chemical ingredients is avoided, and probability of artificial processing in order to enabling quality to be up to standards is lowered; samples of multiple batches are analyzed systematically, so that quality of the Canzhiling oral solution can be evaluated more comprehensively and scientifically, and product quality and efficacy are guaranteed.
Owner:SHANDONG UNIV
Di-substituted amides for enhancing glutamatergic synaptic responses
InactiveUS20100120764A1Stable baselineIncrease amplitudeBiocideNervous disorderSIDS - Sudden infant death syndromeCentral sleep apnea
This invention relates to compounds, pharmaceutical compositions and methods for use in the prevention and treatment of cerebral insufficiency, including enhancement of receptor functioning in synapses in brain networks responsible for basic and higher order behaviors. These brain networks, which are involved in regulation of breathing, and cognitive abilities related to memory impairment, such as is observed in a variety of dementias, in imbalances in neuronal activity between different brain regions, as is suggested in disorders such as Parkinson's disease, schizophrenia, respiratory depression, sleep apneas, attention deficit hyperactivity disorder and affective or mood disorders, and in disorders wherein a deficiency in neurotrophic factors is implicated, as well as in disorders of respiration such as overdose of an alcohol, an opiate, an opioid, a barbiturate, an anesthetic, or a nerve toxin, or where the respiratory depression results form a medical condition such as central sleep apnea, stroke-induced central sleep apnea, obstructive sleep apnea, congenital hypoventilation syndrome, obesity hypoventilation syndrome, sudden infant death syndrome, Rett syndrome, spinal cord injury, traumatic brain injury, Cheney-Stokes respiration, Ondines curse, Prader-Willi's syndrome and drowning. In a particular aspect, the invention relates to compounds useful for treatment of such conditions, and methods of using these compounds for such treatment.
Owner:CORTEX PHARMA
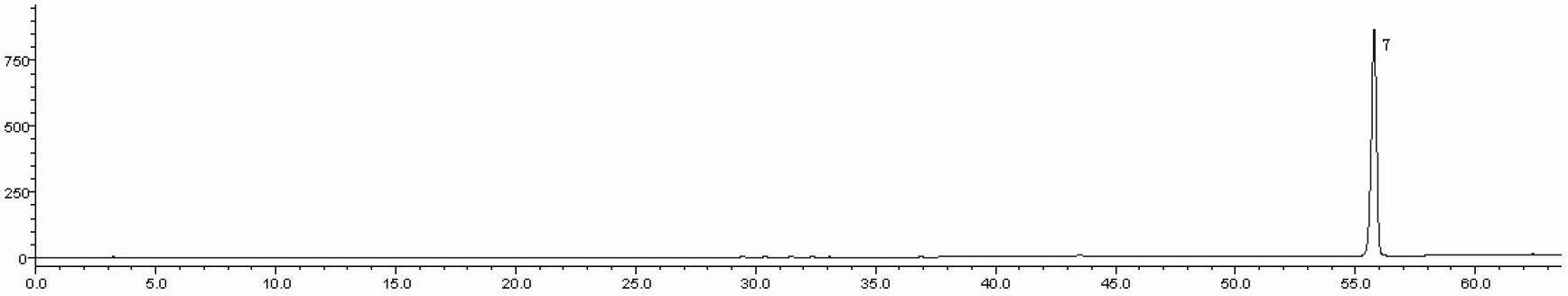
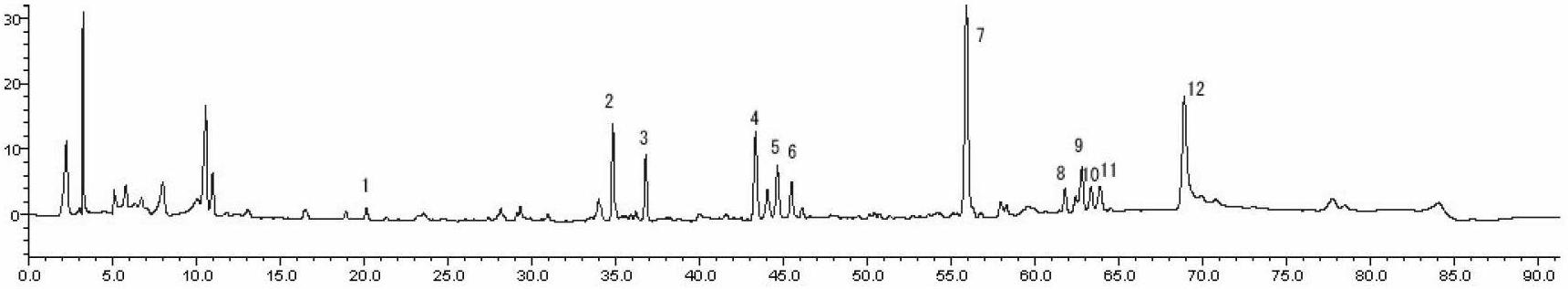
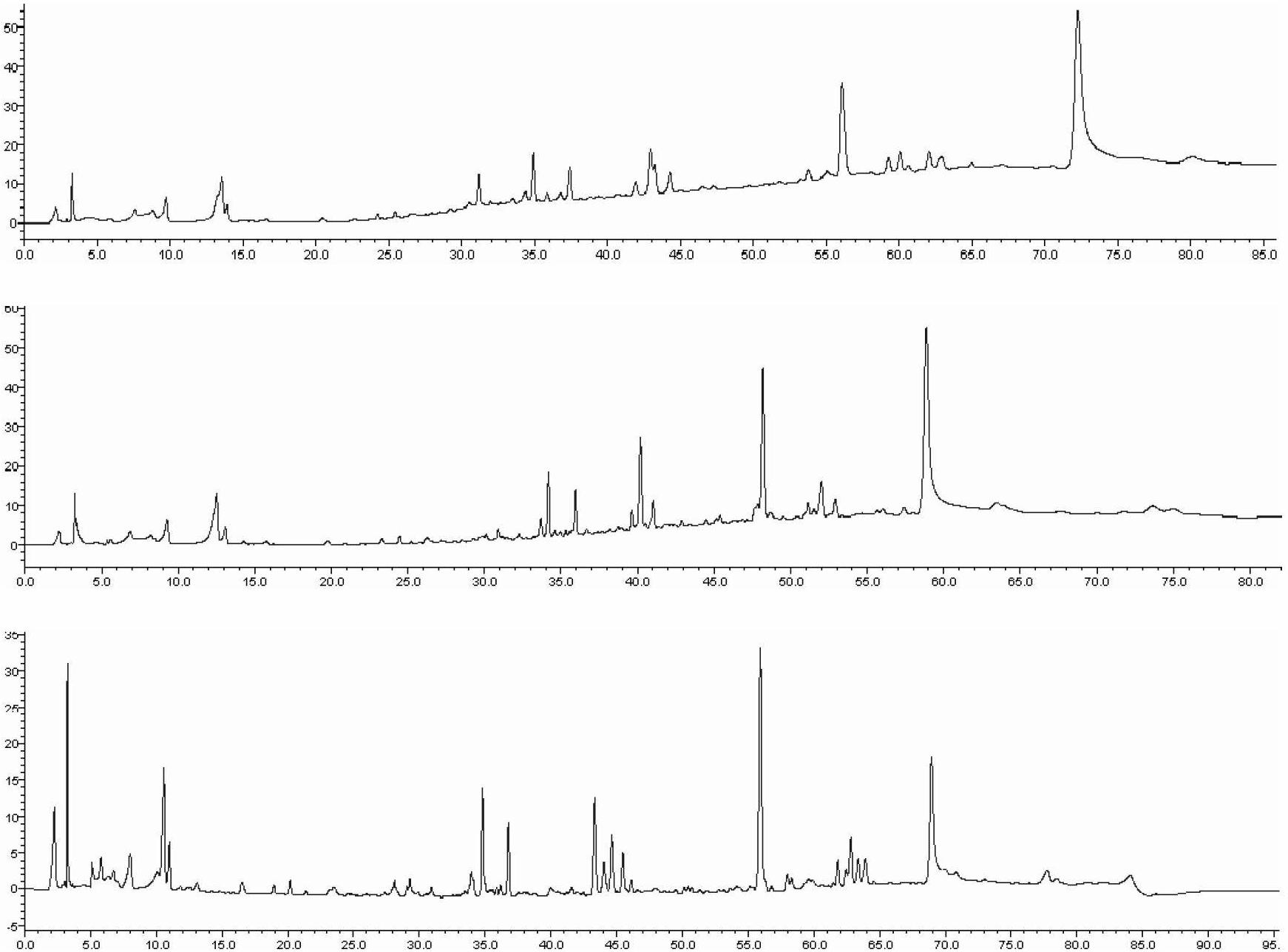
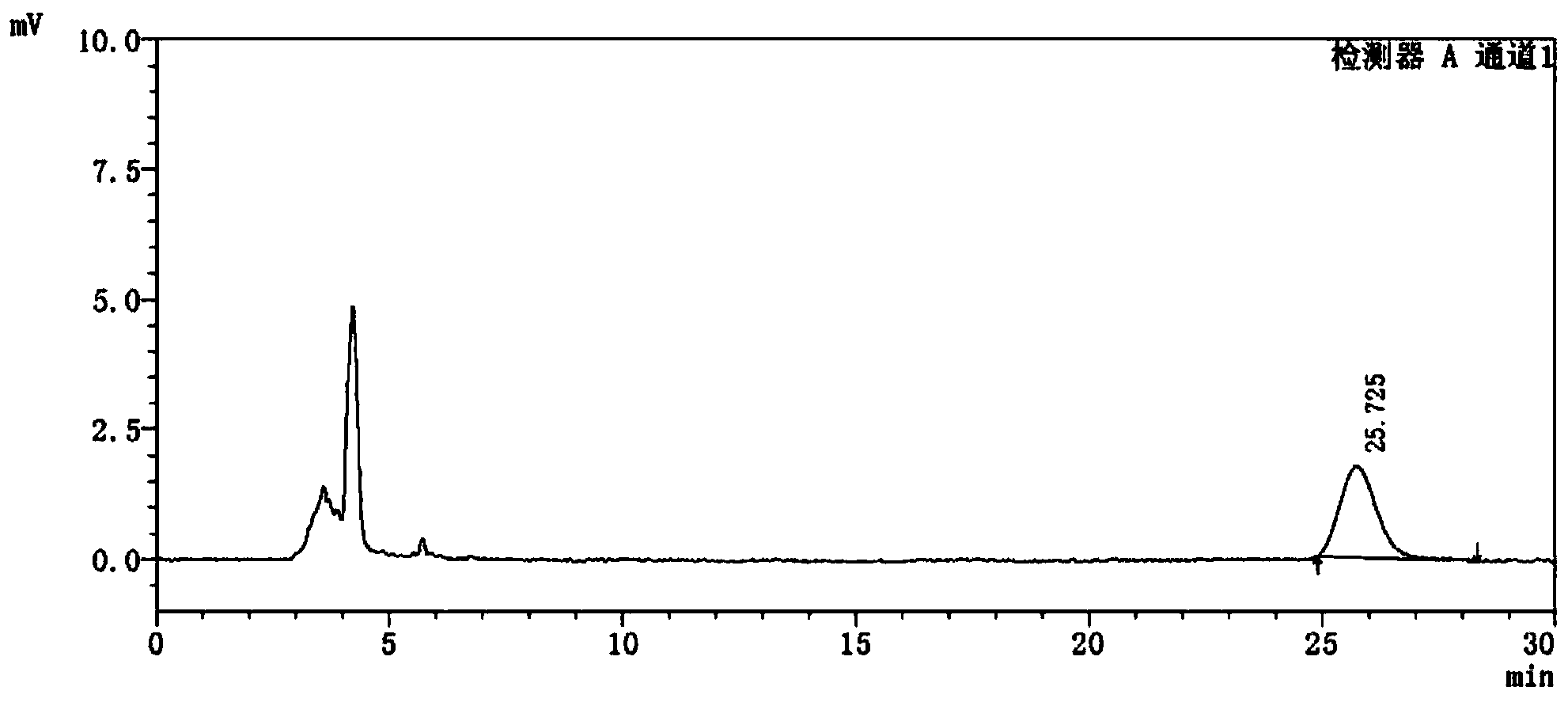
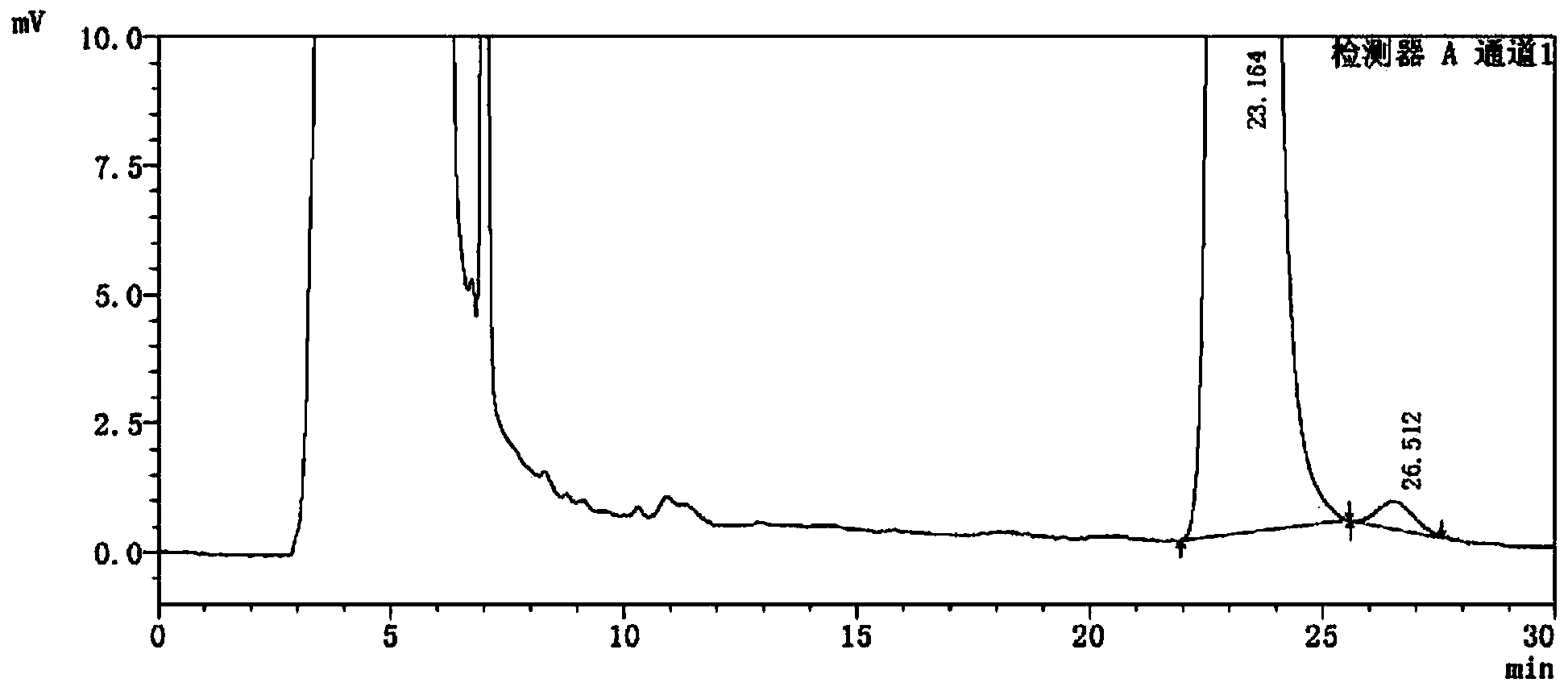
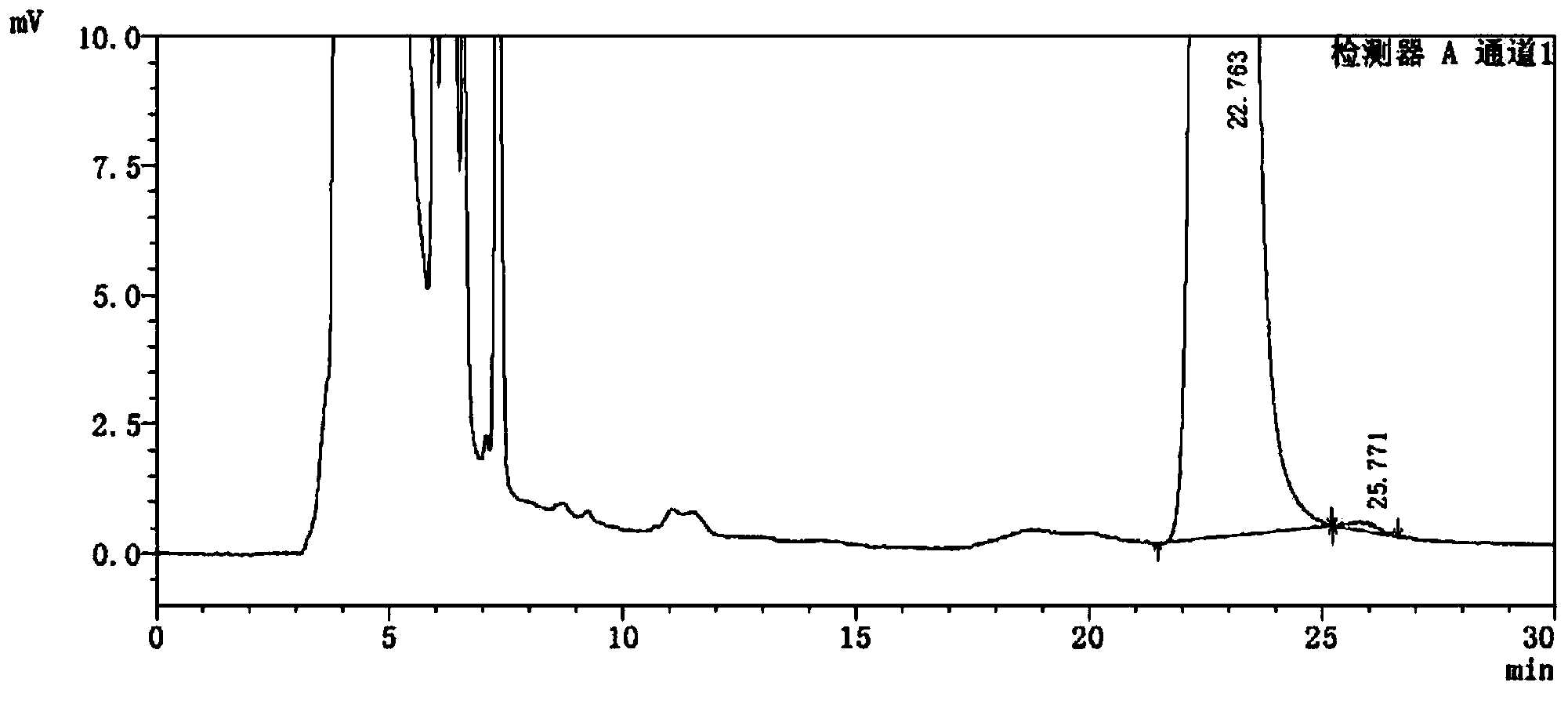
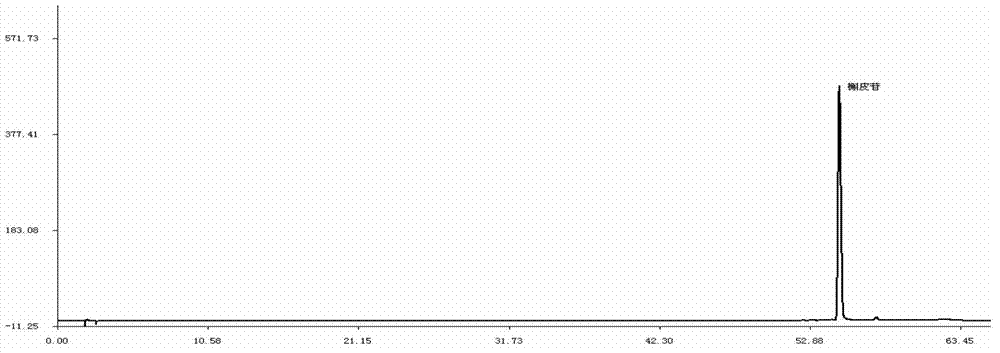
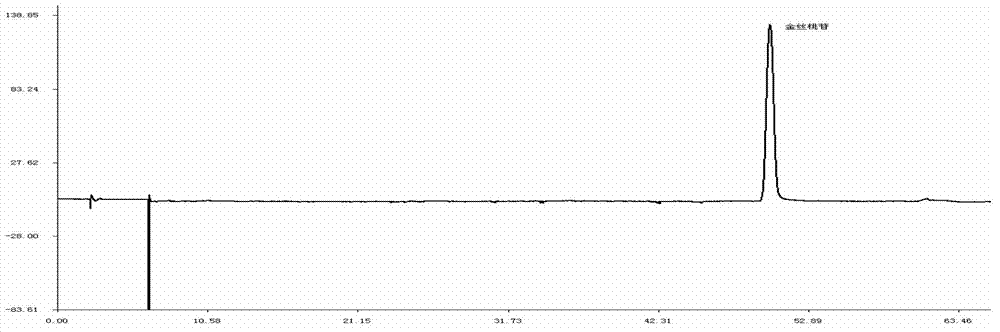
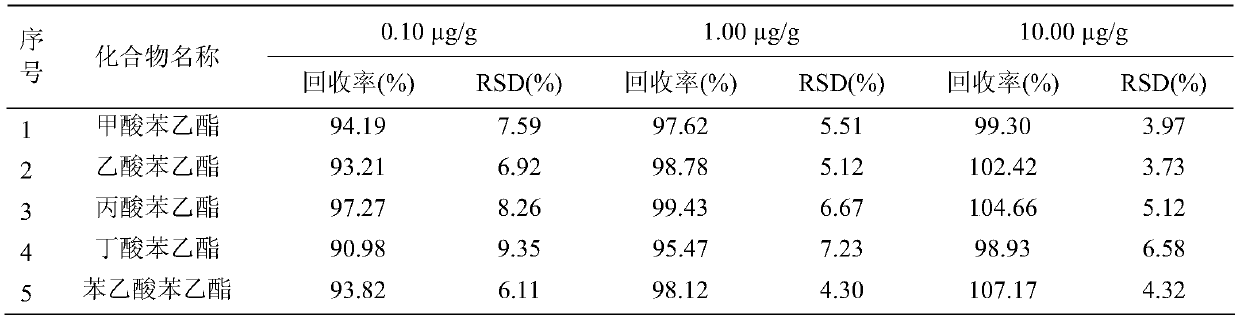
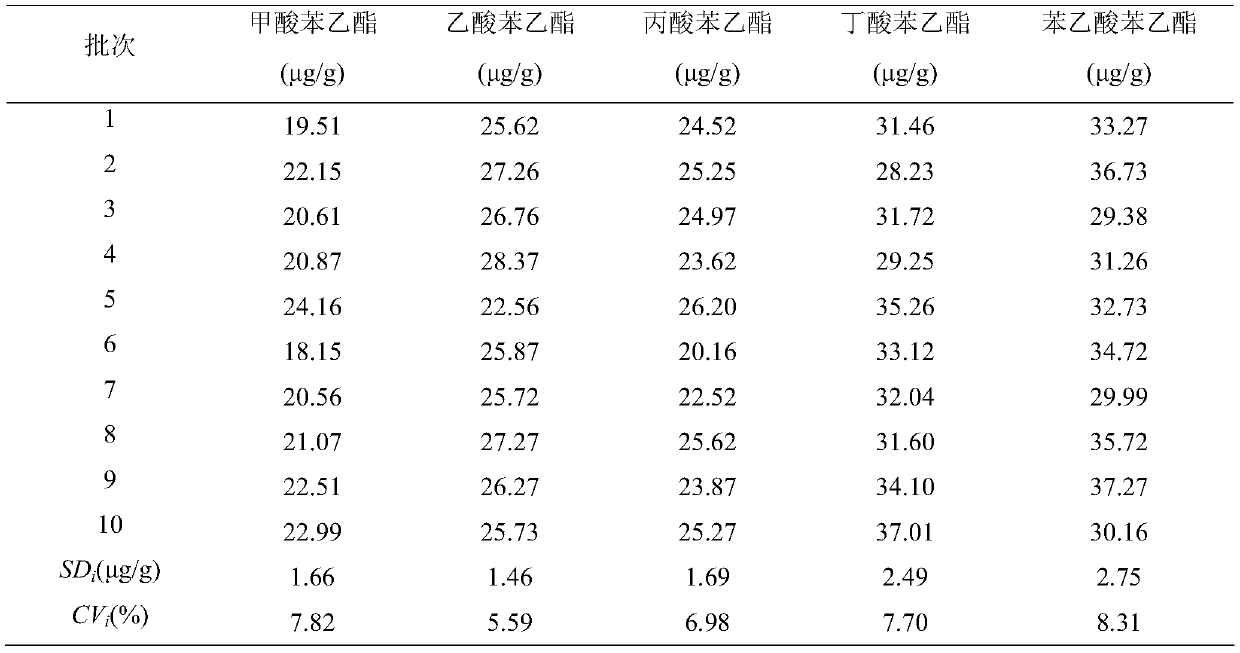
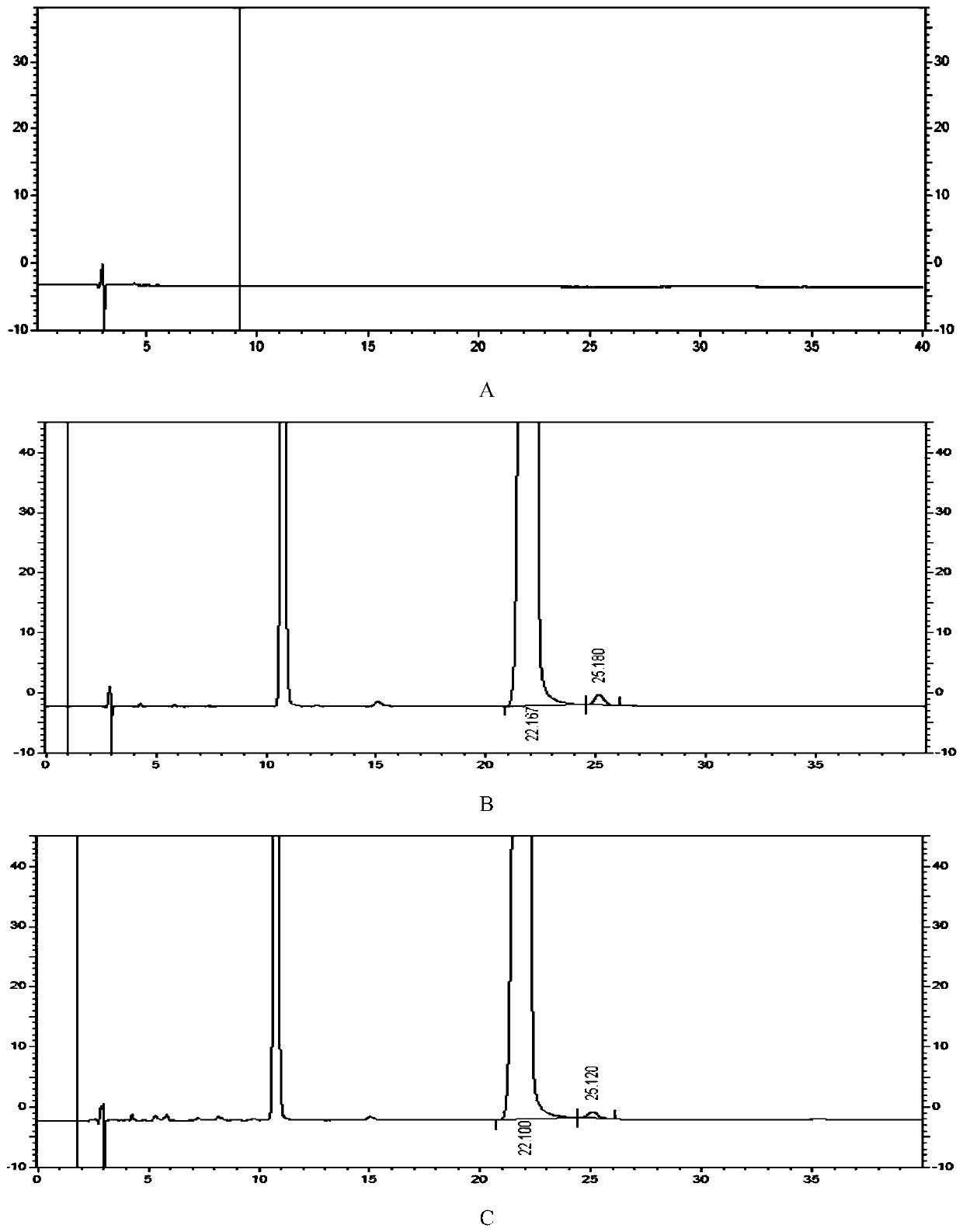
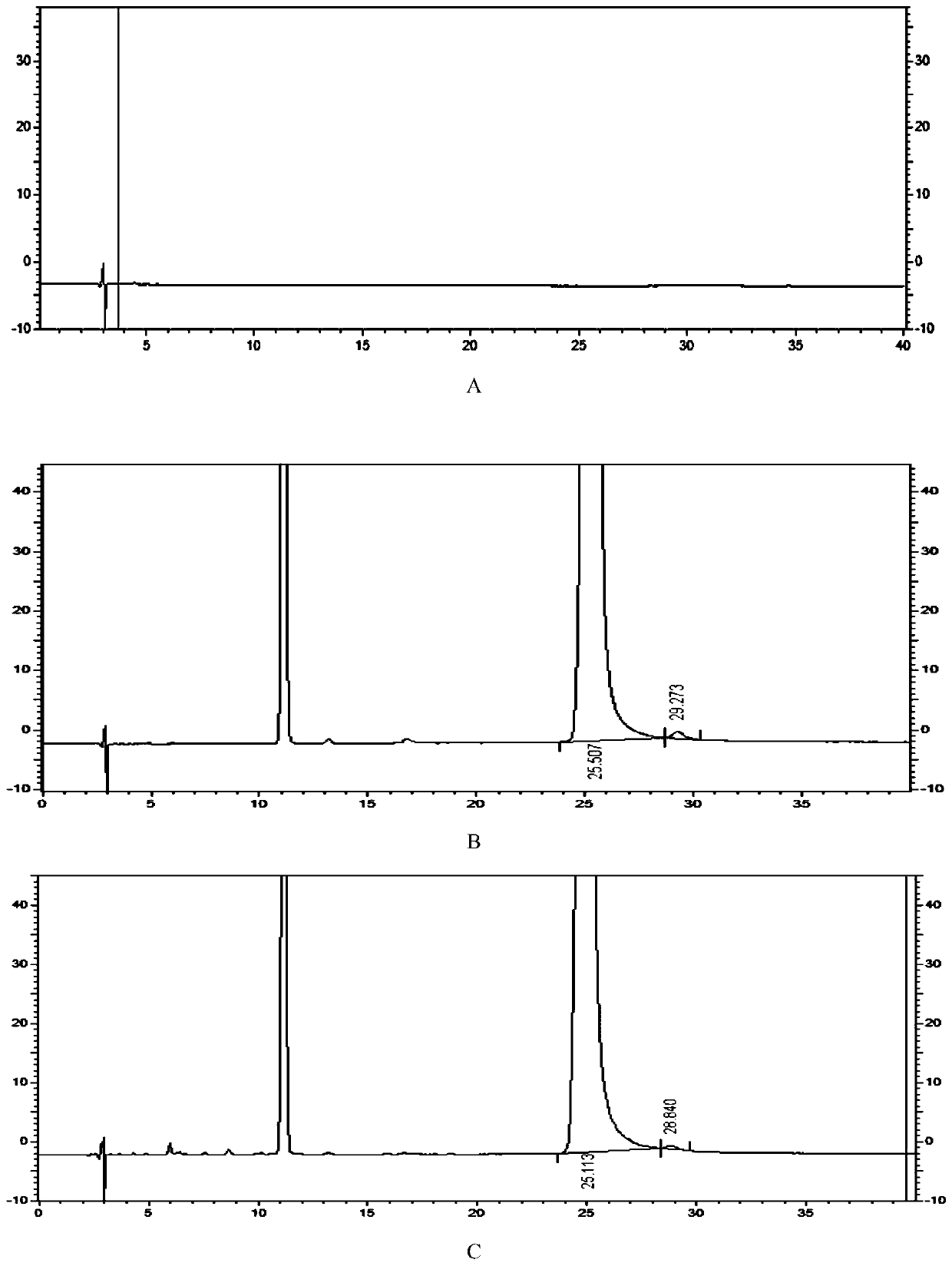
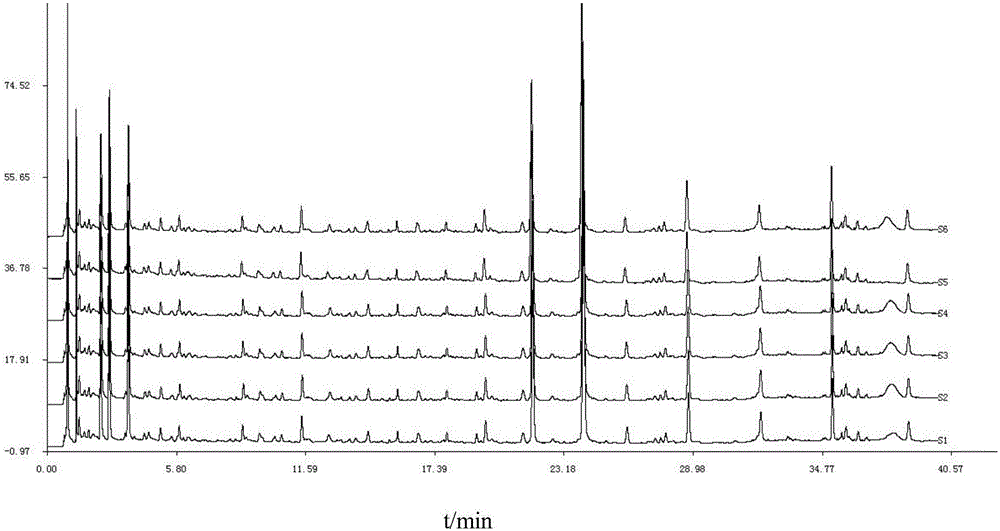
Detection method of 1-[2-(2, 4-dimethylphenylthioalkyl)phenyl]piperazine or salt thereof
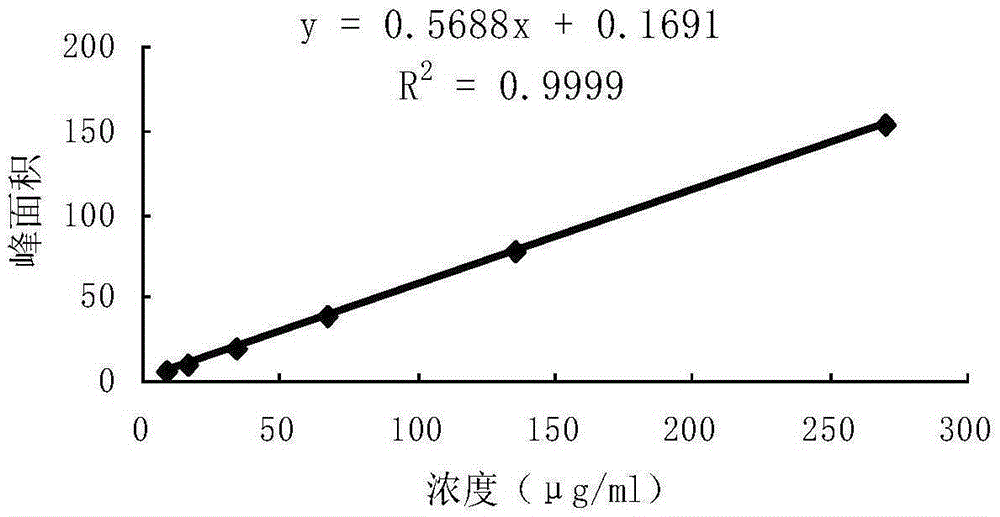
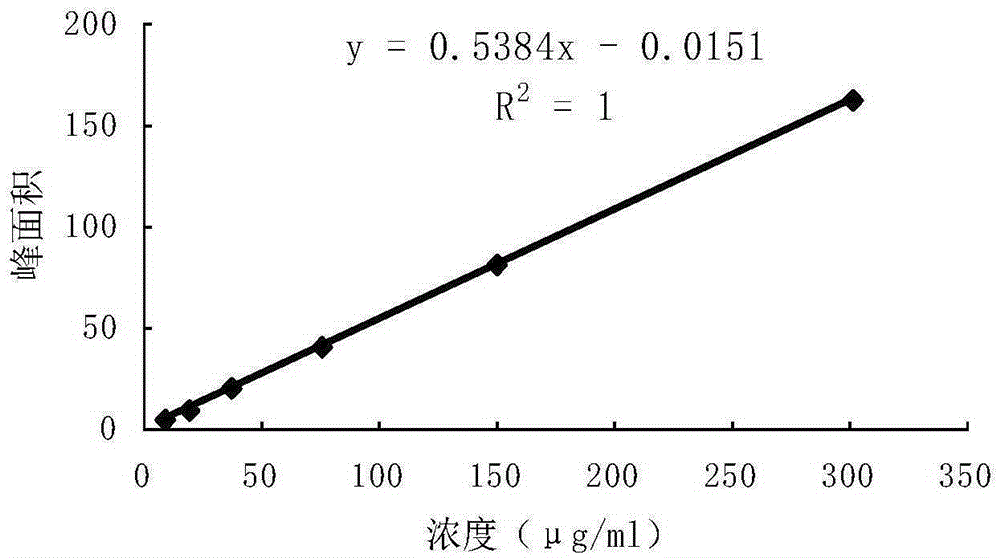
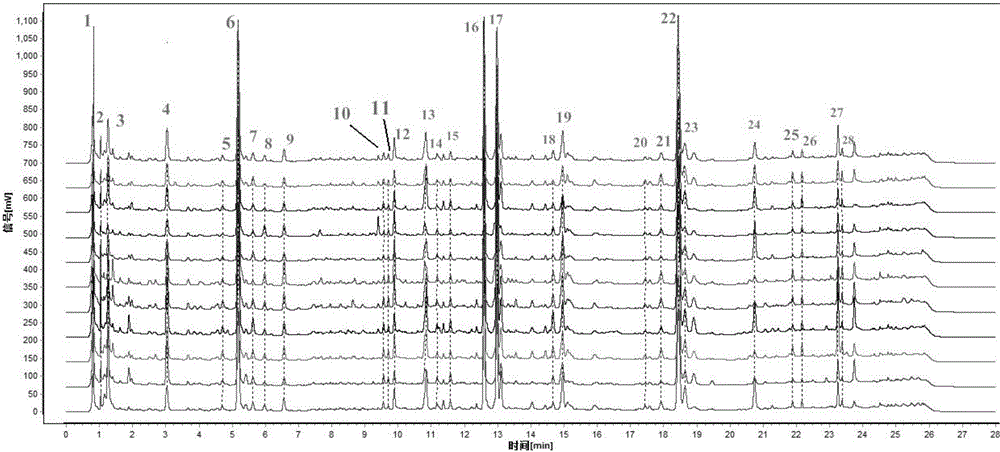
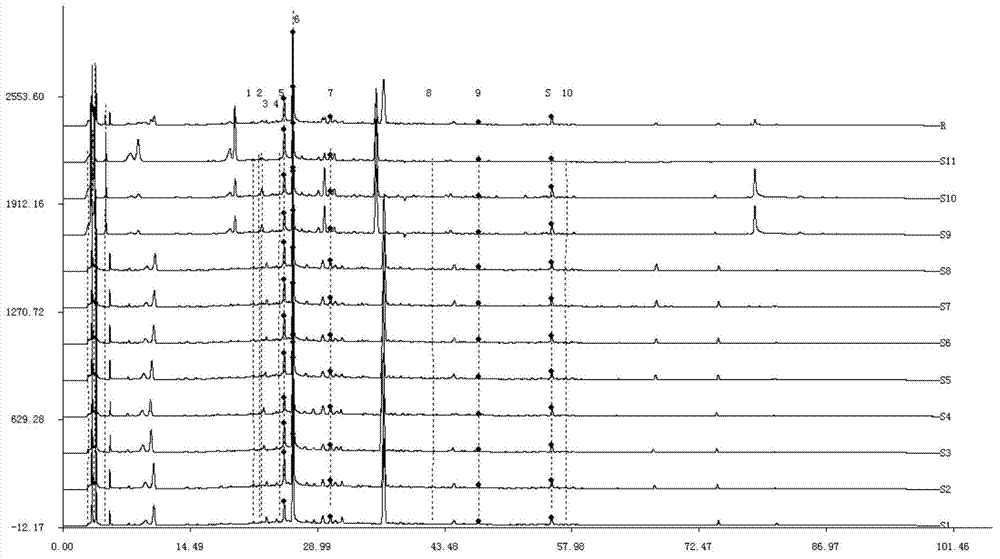
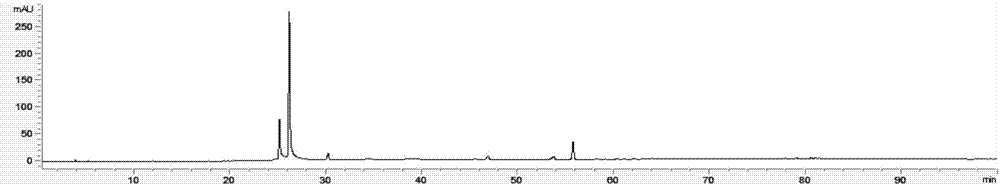
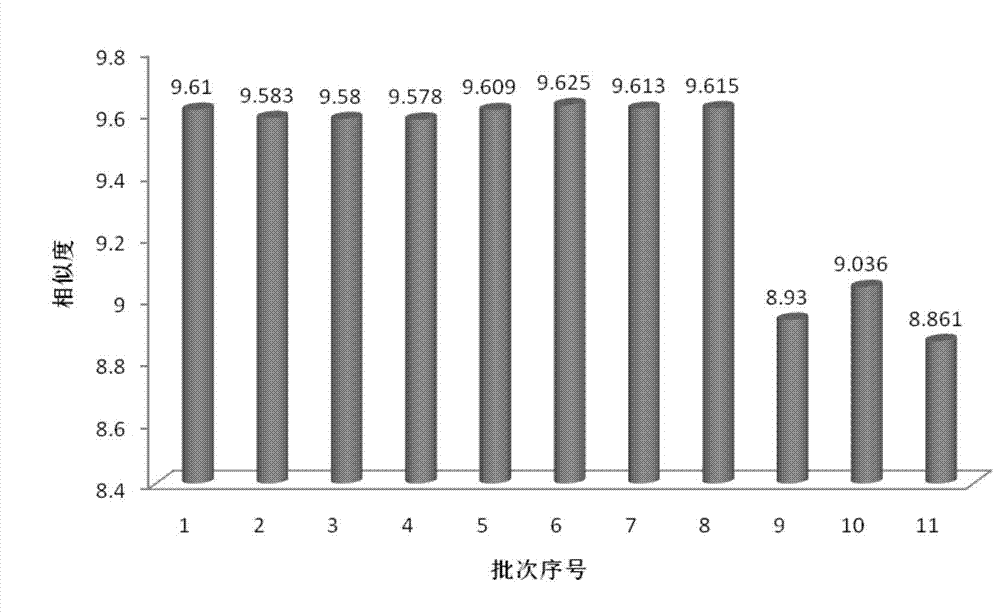
ActiveCN106556663AEasy to separateStable baselineComponent separationPhenylpiperazineGradient elution
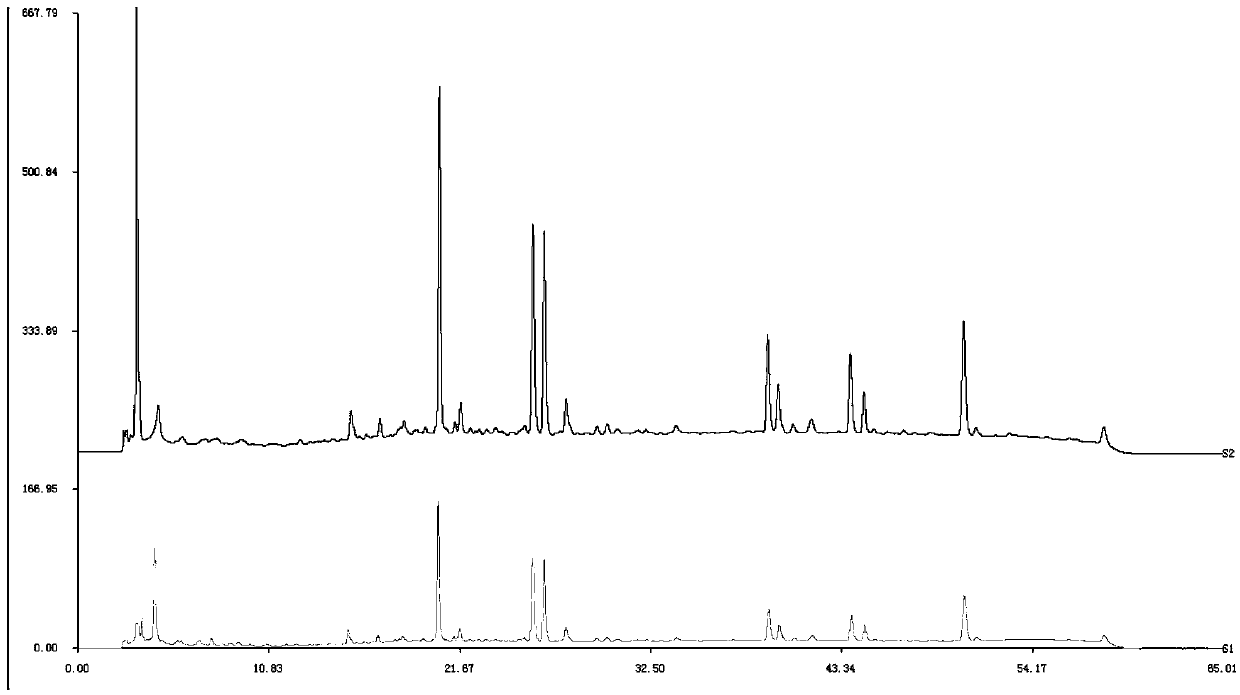
The invention provides a detection method of 1-[2-(2, 4-dimethylphenylthioalkyl)phenyl]piperazine or salt thereof. The method includes the steps of: taking a methanol solution containing 1-[2-(2, 4-dimethylphenylthioalkyl)phenyl]piperazine or salt thereof as a test solution, and performing detection according to high performance liquid chromatography conditions, which specifically includes: use of pentafluorophenyl as the filler; employment of a methanol-0.02mol.L<-1> ammonium acetate buffer solution as the mobile phase A, and taking of acetonitrile as the mobile phase B; and conducting gradient elution. The detection method has the advantages of good separation degree of active components and impurities, stable baseline, and good repeatability and stability of a sample and impurities, thus being more beneficial to quality control.
Owner:四川弘远药业有限公司
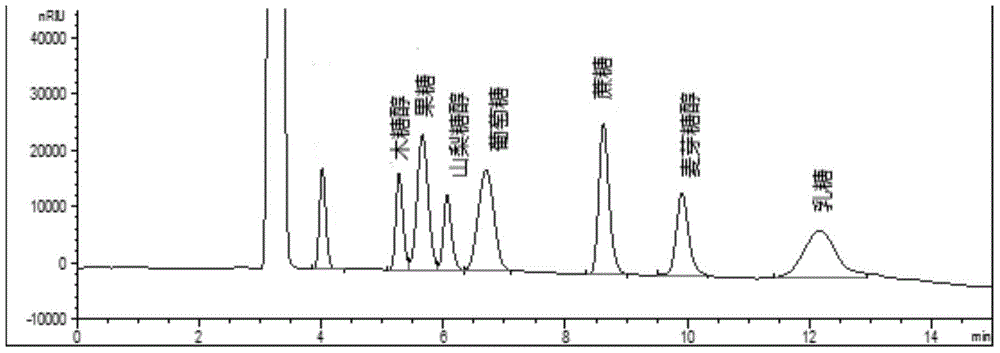
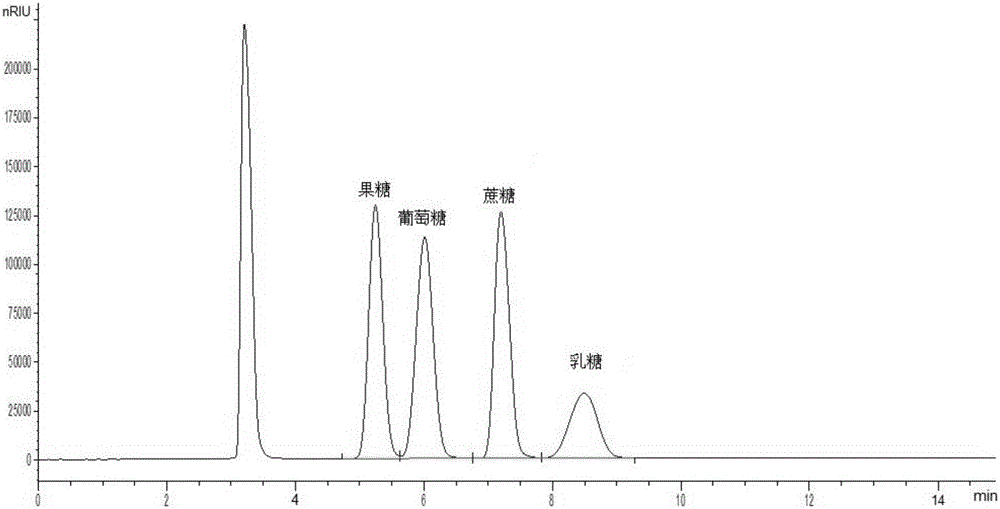
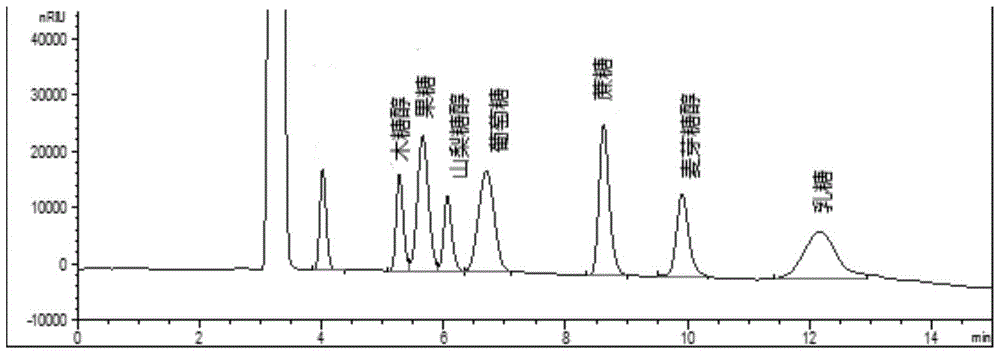
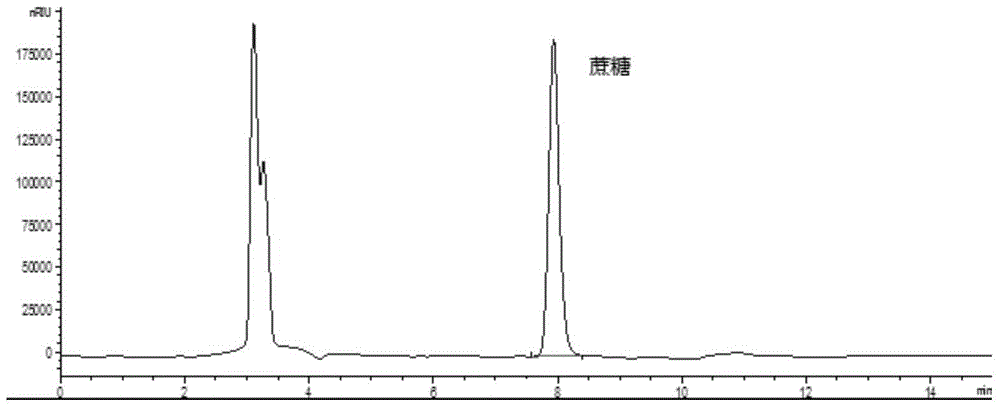
Method for simultaneously detecting multiple sugars and sugar alcohols in food
InactiveCN105717207AReduce churnImprove toleranceComponent separationAlcohol sugarsColumn temperature
The invention relates to a method for simultaneously detecting multiple sugars and sugar alcohols in food. An HPLC-differential refraction detection method is adopted as the method; the method is characterized by comprising the HPLC chromatographic conditions that an amide-bonded column is adopted as a chromatographic column, single mobile phases of acetone, water and triethylamine are adopted as mobile phases, isocratic elution is performed, the column temperature is 70 DEG C, the flowing velocity is 0.8 ml / min, and the sample injection amount is 10 milliliters. The detection method can be suitable for the food such as honey, wine and baijiu; detection can be performed only by simply processing a sample, the method is rapid, high in recovery rate, wide in linear range, good in correlation coefficient and low in detection limit, and the blank of simultaneous detection on the sugars and sugar alcohols in the food in China is filled up.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Detection method for atractylodes macrocephala koidz medicinal materials
InactiveCN102680631ATrue reflection of qualityEnsure medication safetyComponent separationHplc fingerprintMedicine
The invention discloses a detection method for atractylodes macrocephala koidz medicinal materials. The method adopts HPLC (high performance liquid chromatography) fingerprint spectrum for detection and includes the operation steps of: (1) preparing sample solution; (2) preparing reference solution; and (3) respectively and precisely absorbing the sample solution and the reference solution to inject into a liquid chromatograph so as to elute by taking an acetonitrile-water system as a flowing phase and detect at wavelengths of 248+ / -5nm. After the atractylodes macrocephala koidz medicinal materials are decocted with water, and then the HPLC fingerprint spectrum is applied for detection, so that quality of the atractylodes macrocephala koidz medicinal materials can be reflected more truly. Moreover, chromatographic conditions such as the flowing phase are selected specifically, so that chromatogram baselines are stable and convenient to integrate, resolution of characteristic peaks is good, and similarity among different medicinal materials is high. The detection method for the atractylodes macrocephala koidz medicinal materials can be effectively used for quality control of atractylodes macrocephala koidz and provides a guarantee of medication security of the atractylodes macrocephala koidz medicinal materials.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Method for detecting drug and enantiomer impurities of drug
The invention relates to the technical field of chemical analysis, in particular to a method for detecting a drug and enantiomer impurities of the drug. The method is characterized in that a sample to be detected, organic amine and acyl chloride are subjected to derivatization reaction, a reaction resultant is separated through a chirality chromatographic column by the adoption of high performance liquid chromatography and detected through an ultraviolet detector, and then the content is obtained through calculation. The method is stable, practicable and easy, convenient and rapid to implement.
Owner:HEFEI COSOURCE PHARMA CO LTD
Methods for the treatment of respiratory depression
InactiveUS20110003835A1Stable baselineIncrease amplitudeBiocideNervous disorderSIDS - Sudden infant death syndromeCentral sleep apnea
This invention relates to compounds, pharmaceutical compositions and methods for use in the prevention and treatment of cerebral insufficiency, including enhancement of receptor functioning in synapses in brain networks responsible for basic and higher order behaviors. These brain networks, which are involved in regulation of breathing, and cognitive abilities related to memory impairment, such as is observed in a variety of dementias, in imbalances in neuronal activity between different brain regions, as is suggested in disorders such as Parkinson's disease, schizophrenia, respiratory depression, sleep apneas, attention deficit hyperactivity disorder and affective or mood disorders, and in disorders wherein a deficiency in neurotrophic factors is implicated, as well as in disorders of respiration such as overdose of an alcohol, an opiate, an opioid, a barbiturate, an anesthetic, or a nerve toxin, or where the respiratory depression results form a medical condition such as central sleep apnea, stoke-induced central sleep apnea, obstructive sleep apnea, congenital hypoventilation syndrome, obesity hypoventilation syndrome, sudden infant death syndrome, Rett syndrome, spinal cord injury, traumatic brain injury, Cheney-Stokes respiration, Ondines curse, Prader-Willi's syndrome and drowning, hi a particular aspect, the invention relates to bicyclic amide compounds useful for treatment of such conditions, and methods of using these compounds for such treatment.
Owner:CORTEX PHARMA
Fingerprint spectrum detection method for meridian warming decoction
ActiveCN110907553AImprove stabilityGood reproducibilityComponent separationLigusticum chuanxiongGinseng
The invention relates to a fingerprint spectrum detection method for meridian warming decoction. The method comprises the following steps: 1) preparing a test solution; weighing 1-2g of angelica sinensis, 1-2g of ligusticum wallichii, 1-2g of radix paeoniae alba, 1-2g of cinnamon, 1-2g of moutan bark, 1-2g of curcuma zedoary powder, weighing 3-4g of ginseng powder, 3-4g of liquorice powder and 3-4g of radix achyranthis bidentatae powder; adding 250-350mL of water, uniformly mixing, heating to boil with strong fire, slowly decocting with slow fire, filtering while the decoction is about 150-170mL, and adding water into the filtrate to dilute to 240-260mL; precisely sucking 8-12mL of the standard decoction of the meridian warming decoction, adding methanol to dilute to 40-60mL, sealing, weighing the mass, carrying out ultrasonic (the power is 180-220W and the frequency is 40-60kHz) treatment for 8-12min, standing overnight, weighing the mass again, supplementing the lost mass with methanol, uniformly shaking, filtering, and taking the subsequent filtrate, thereby obtaining the test solution; 2) detecting: taking and injecting 5-15 [mu]L of the test solution obtained in the previous step into a high performance liquid chromatograph, and obtaining a chromatogram; and 3) performing result judgment: comparing the chromatogram obtained in the previous step with a standard control fingerprint, and if the similarity is greater than 90%, the sample being qualified.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
Herba houttuyniae aboveground part extract and detection method thereof
ActiveCN102809617AImprove quality controlMaterial basis is clearComponent separationOrganic acidHplc fingerprint
The invention provides an aboveground part extract and a detection method thereof. The extract is detected via an HPLC (High Performance Liquid Chromatography) fingerprint method. The herba houttuyniae aboveground part extract covers multi-class active components, is unambiguous is material basis, can serve as a new standard control for medicinal material quality detection, and more facilitates quality control to the herba houttuyniae aboveground part. According to the invention, the optimal detection method of the herba houttuyniae aboveground part is achieved via a comprehensively surveying sample solution preparation method and color spectrum conditions. When the detection method is applied, a base line is stable, chromatographic peak shape and resolution are favorable, and meanwhile, flavonoid and organic acid chemical compounds of the herba houttuyniae can be synchronously detected, provides a reliable method for quality detection of the herba houttuyniae aboveground part, and ensures medicinal material quality of the herba houttuyniae aboveground.
Owner:ZHEJIANG GUOJING PHARM CO LTD
Fingerprint detection method for Shenshuaining granule
ActiveCN105486790AEasy to separatePromote absorptionComponent separationPhosphoric acidGradient elution
The invention relates to a fingerprint detection method for Shenshuaining granule. The detection method employs HPLC for detection, and comprises the following steps: a, determining chromatographic condition; b, preparing a tested object solution; and c, establishing fingerprint. The chromatographic conditions in the step comprise that the chromatographic column is C18 chromatographic column; the mobile phase comprises a mobile phase A and a mobile phase B with the ratio of 5%:95%-100%:0%, the mobile A is acetonitrile or methanol, and the mobile phase B is water, a phosphoric acid aqueous solution with the concentration of 0.01%-0.5% or a formic acid aqueous solution with the concentration of 0.01%-0.5%; the detection wave length is 190-450 nm; the column temperature is 20-40 DEG C; the volume flow is 0.8-1.2 mL / min; the sample size is 5-100 mu L; and the elution program employs gradient elution and the elution time is 80-100 min. The detection method is simple, rapid, easy to operate, high in precision, good in stability and good in reappearance, possesses many characteristic peaks, and can well provide quality control basis for production of Shenshuaining granule.
Owner:山西德元堂药业有限公司
Method for formation configuration of distributed satellites with synthetic aperture radars
InactiveCN101520511BApplicable interference processingStable baselineRadio wave reradiation/reflectionOrbital periodStart time
Owner:BEIHANG UNIV
Cigarette flavoring uniformity detection method based on additional marker
ActiveCN110487924AUniformity is scientifically evaluatedUniformity evaluationComponent separationChemistryAccelerated solvent extraction
The invention provides a cigarette flavoring uniformity detection method based on an additional marker. The method comprises the following steps: adding a phenylethanolate compound serving as an additional marker into a tobacco essence, wherein the tobacco essence containing the additional marker is added to tobacco shreds through a flavoring process, balancing, grinding and sieving different batches of flavored cut tobaccos, performing accelerated solvent extraction to respectively obtain extract liquor of different batches of cut tobaccos, performing gas chromatography-tandem mass spectrometry analysis on the extract liquor to obtain the content of additional markers in a plurality of extract liquor, and further conducting calculation to obtain the flavoring uniformity of cigarettes. According to the cigarette flavoring uniformity method based on the additional marker, compounds except essence and tobacco shred components are selected as the additional marker, the uniformity of the cigarette flavoring process can be evaluated more objectively, scientifically and accurately in a quantified mode, and the method has very important significance in evaluating the tobacco shred makingprocess and stabilizing the product quality.
Owner:SHANGHAI TOBACCO GRP CO LTD
Method for detecting impurities in formoterol fumarate or related preparations thereof
The present invention provides a method for detecting impurities in formoterol fumarate or related preparations thereof. The method comprises a step of detecting diastereoisomer impurities (impuritiesI) in the formoterol fumarate or related preparations by using a high performance liquid chromatography method, wherein an eluent used in the high performance liquid chromatography is a system consisting of a liquid A and a liquid B, the liquid A is an ion pair reagent buffer, the liquid B is acetonitrile, and the elution mode is isocratic elution. The method of the invention has the advantages of good tolerance, long service life, the good reproducibility of a detection result, and multiple types of optional columns, and the reduction of the application cost. A measurement result obtained bythe method of the invention is accurate, and the method can be simultaneously applied to the detection of the content of diastereoisomer impurities in a formoterol fumarate crude drug and related preparations.
Owner:GUANGZHOU JOINCARE RESPIRATORY DRUG ENG TECH CO LTD
Method for simultaneously detecting multiple sugars and sugar alcohols in dairy products
InactiveCN105675758AReduce churnImprove toleranceComponent separationCorrelation coefficientIsocratic elution
The invention relates to a method for simultaneously detecting multiple sugars and sugar alcohols in dairy products. The method is an HPLC-RID (high performance liquid chromatography-refractive index detection) method and is characterized in that the chromatographic condition of HPLC is as follows: an amido bond column is adopted as a chromatographic column, a single mobile phase of acetone, water and trithylamine is adopted, and isocratic elution is performed; the column temperature is 70 DEG C; the flow velocity is 0.8 ml / min; the sample size is 10 mu L. The detection method is applicable to the dairy products such as milk, cakes, candy, milk powder and the like. Detection can be performed after samples are treated simply. The method is quick, high in recovery rate, wide in linear range, good in correlation coefficient and low in detection limit and fills up the blank of simultaneous detection of the sugars and the sugar alcohols in the dairy products.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
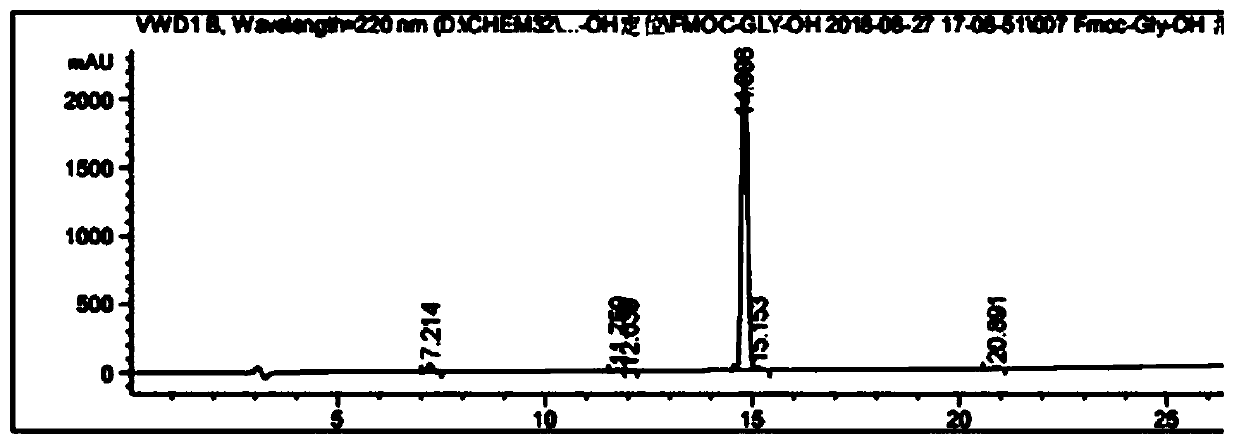
Fmoc-protected amino acid purity and related substance analysis method
ActiveCN111505160AStrong elution abilityAvoid decompositionComponent separationBiochemical engineeringCombinatorial chemistry
The invention discloses an Fmoc-protected amino acid purity and related substance analysis method. The method comprises the following steps of S1, preparing an Fmoc-Osu impurity control solution, andenabling the ratio of Fmoc-Osu to Fmoc-protected amino acid to be (0.1-1.0): 100 to obtain an Fmoc-Osu impurity control solution; S2, preparing a related impurity control solution, enabling the ratioof single related impurity to Fmoc-protected amino acids to be 0.1-1.0: 1 100, obtaining a plurality of reference substance solutions, etc. According to the invention, a mobile phase, an impurity control solution and the gradient elution conditions are comprehensively designed and optimized, the analysis method is applied to the operation process of the system, the baseline is smooth, no interference peak basically exists in a blank test, the impurity separation effect is prominent, and the specificity is good. The test data shows that the detection limit can achieve 0.002%, the sensitivity isextremely high, the detection result can provide the reliable and effective information for the research, the production and the purification of the product, the solid foundation is established for the protected amino acid quality control standard, and the positive effect is provided for the quality control of the final product.
Owner:成都市科隆化学品有限公司
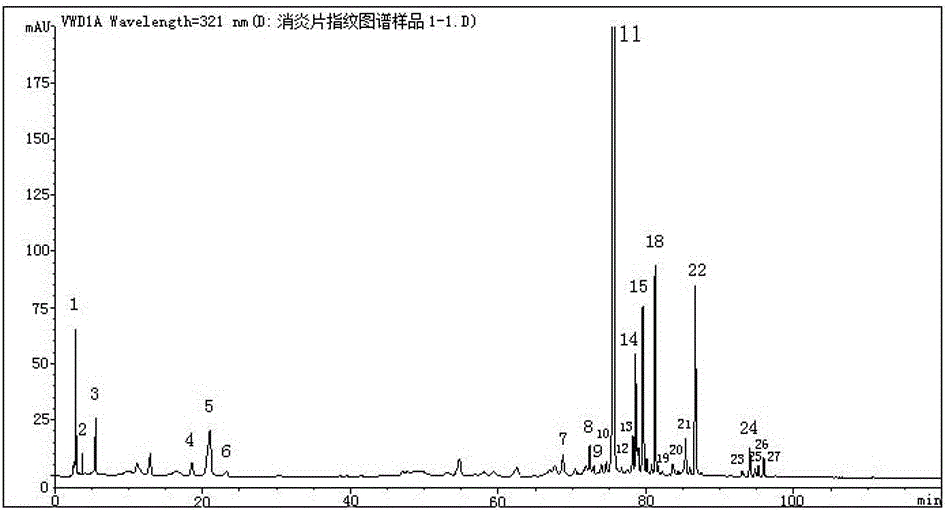
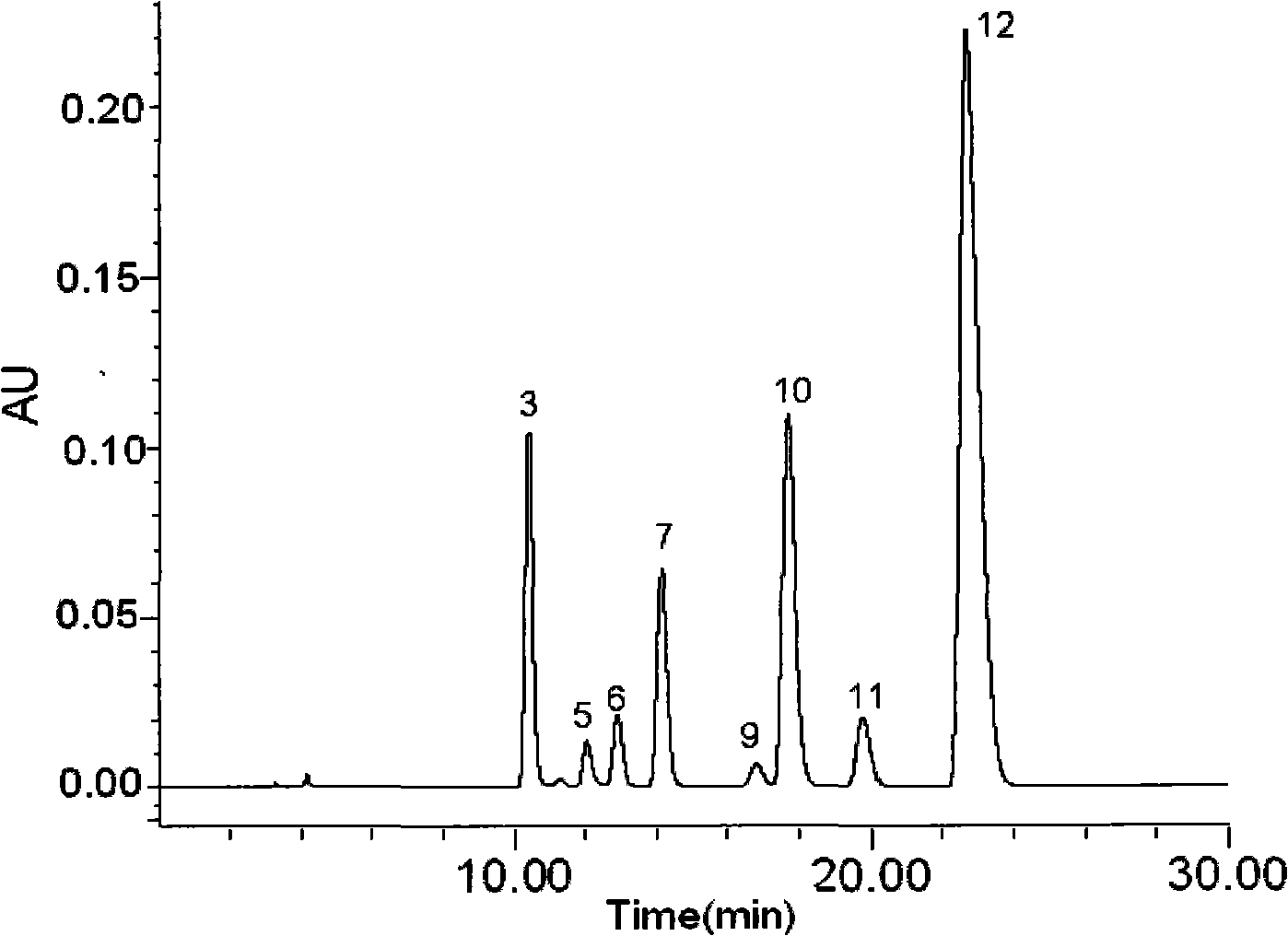
Anti-inflammatory tablet HPLC fingerprint construction method
An anti-inflammatory tablet HPLC fingerprint construction method comprises the following steps: 1, preparing a test solution: grinding an anti-inflammatory tablet, weighing the ground tablet, placing the ground tablet in an extractor, adding petroleum ether, carrying out hot reflux extraction, removing the petroleum ether, adding methanol, carrying out ultrasonic treatment, supplementing methanol, and filtering the obtained solution; 2, preparing a reference solution: taking a chlorogenic acid reference substance, an aesculetin reference substance, a scutelloside reference substance, a linarin reference substance, a baicalein reference substance and a wogonin reference substance; and 3, determining: respectively taking the reference solution and the test solution, respectively injecting the reference solution and the test solution to a liquid chromatograph, recording the chromatogram in 120min, and processing the chromatogram through using fingerprint software to obtain the fingerprint of the anti-inflammatory tablet. The method has the advantages of establishing the common mode of the HPLC characteristic fingerprint of the anti-inflammatory tablet, calibration of 27 common peaks, effective characterization of the quality of the anti-inflammatory tablet, overcoming of unicity and one-sidedness of original quality control methods, and high application values.
Owner:吉林修正药业新药开发有限公司 +1
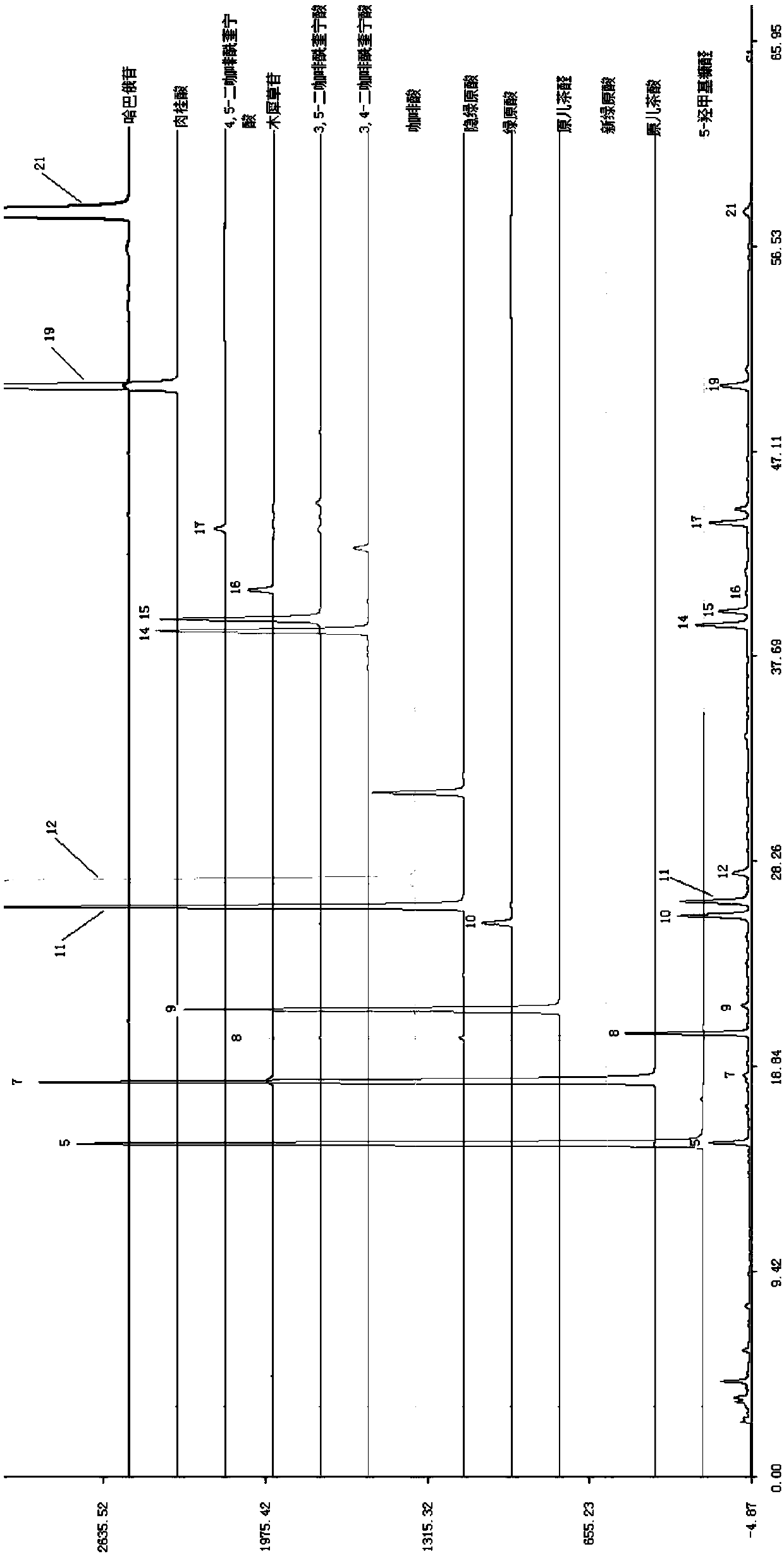
Method for detecting ingredients of Mailuoning oral liquid for clearing heat, nourishing yin, activating blood circulation and removing blood stasis
ActiveCN109596751AEasy to separateSuitable for establishmentComponent separationAdditive ingredientColumn temperature
The invention discloses a method for detecting ingredients of Mailuoning oral liquid for clearing heat, nourishing yin, activating blood circulation and removing blood stasis. The method comprises thefollowing steps: performing HPLC detection on a Mailuoning oral liquid test solution and a standard solution, wherein the chromatographic conditions are as follows: a C18 chromatographic column, anda column temperature of 25-40 DEG C; using methanol as a mobile phase A, using an aqueous acid solution as a mobile phase B, performing gradient elution, recording a chromatogram, calculating the similarity of a test sample by using similarity software with a fingerprint of the Mailuoning oral liquid as the reference, wherein the fingerprint of the test sample should be similar with a standard fingerprint; and calculating the contents of the ingredients in the Mailuoning oral liquid by using an external standard one point method. The method disclosed by the invention has good precision, linearrelationship, stability, repeatability, high recovery rate and good durability; the method disclosed by the invention has the advantages of good degree of separation and reproducibility of the fingerprint of the Mailuoning oral liquid, and comprehensive information, 21 common peaks are marked in total, the similarity of each batch of samples is above 0.95, and the quality of the Mailuoning oral liquid can be evaluated comprehensively, objectively and scientifically.
Owner:JINLING PHARMA
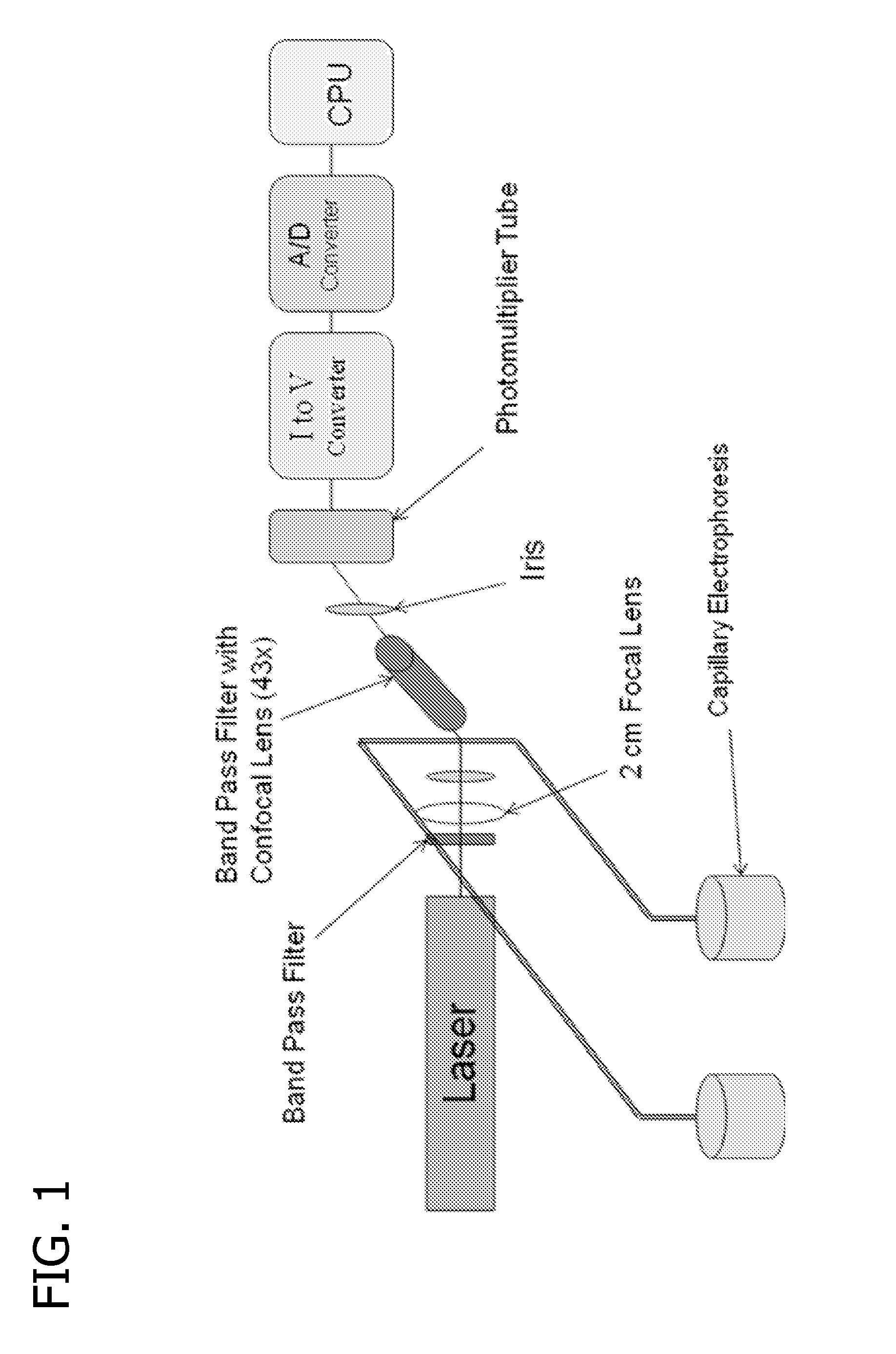
Method and apparatus for cancer screening
ActiveUS20110147217A1Improve stabilityGood reproducibilitySludge treatmentVolume/mass flow measurementBacteriuriaPteridine synthesis
A method and apparatus for detection of pteridine levels in a biological sample using CE-LIF which is useful for early cancer screening involving fully oxidizing pteridine compounds in a sample such as a urine sample, subjecting to CE-LIF to assess compound concentration, and compare to expected levels in for healthy or cancer-bearing patients.
Owner:UNIVERSITY OF MISSOURI
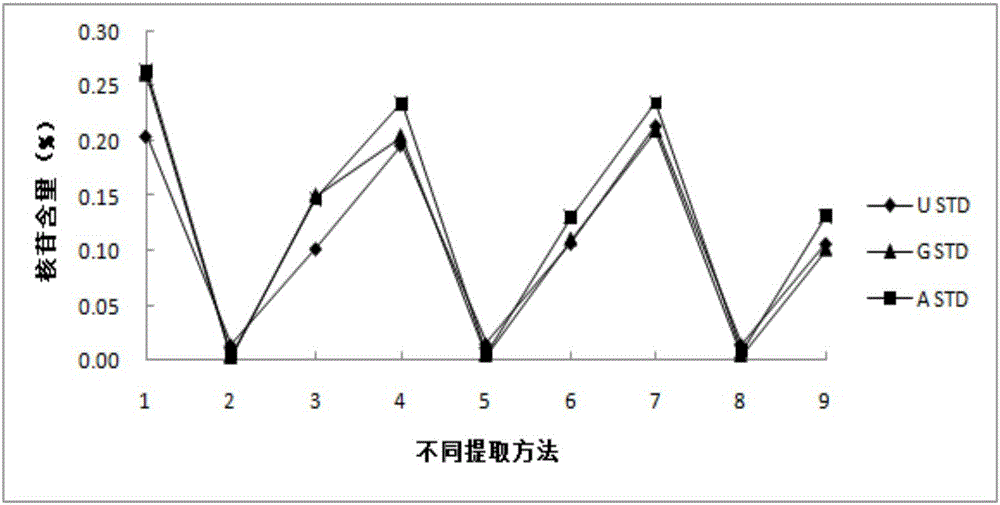
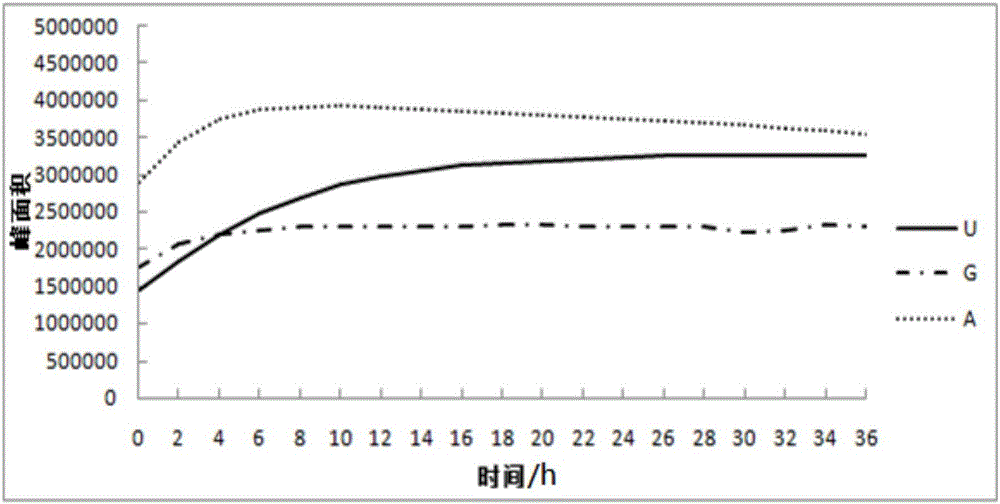
Method for detecting nucleoside components in Morchella esculenta
The invention belongs to the field of medical domestic fungus analysis and detection and relates to a method for simultaneous detection of guanosine, uridine and adenosine in Morchella esculenta by HPLC. The method comprises adding water into Morchella esculenta powder, carrying out constant temperature ultrasonic extraction, carrying out full shaking, taking the supernatant, filtering the supernatant through a water filter head, carrying out standing to obtain a sample solution to be detected, preparing a nucleoside reference solution and detecting nucleoside components in Morchella esculenta through HPLC. The method for detecting nucleoside components in Morchella esculenta has high stability, good repeatability and a good precision, produces a reliable and convenient result and can provide scientific basis for quality control of Morchella esculenta.
Owner:东莞东阳光保健品研发有限公司
Nine-element wind-extinguishing particle component quantitative detection method and fingerprint construction method
ActiveCN105301136AEasy to separateObjective assessment of qualityComponent separationChemical compositionTest sample
The present invention relates to the technical field of analytical chemistry, and particularly relates to a nine-element wind-extinguishing particle component quantitative detection method and a fingerprint construction method. The detection method comprises taking a nine-element wind-extinguishing particle to-be-tested sample and a standard substance for HPLC detection, and obtaining nine-element wind-extinguishing particle components and contents according to HPLC detection results; wherein HPLC detection chromatographic conditions are as follows: a C18 chromatographic column is used, methanol is used as a mobile phase A, and triethylamine is used as a mobile phase B for gradient elution. The fingerprint measured by the detection method can more fully reflect nine-element wind-extinguishing particle chemical components, chromatographic peak separation is good, baseline is steady, peak forms are good, reproducibility is good, the method has good linear relationship, precision, stability and recovery rate, and can accurately detect the nine-element wind-extinguishing particle chemical component content, and the obtained control fingerprint can objectively assess the quality of the sample.
Owner:JIANGSU KANION PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



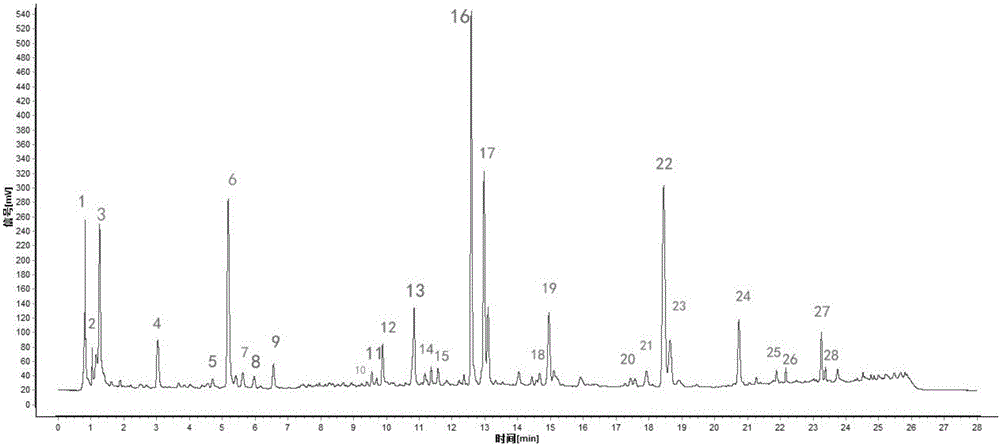


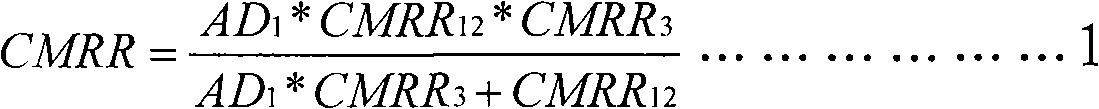


![Detection method of 1-[2-(2, 4-dimethylphenylthioalkyl)phenyl]piperazine or salt thereof Detection method of 1-[2-(2, 4-dimethylphenylthioalkyl)phenyl]piperazine or salt thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e7d43c42-507c-48f9-bde6-61b308cd2563/HDA0000903848940000011.png)
![Detection method of 1-[2-(2, 4-dimethylphenylthioalkyl)phenyl]piperazine or salt thereof Detection method of 1-[2-(2, 4-dimethylphenylthioalkyl)phenyl]piperazine or salt thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e7d43c42-507c-48f9-bde6-61b308cd2563/HDA0000903848940000012.png)
![Detection method of 1-[2-(2, 4-dimethylphenylthioalkyl)phenyl]piperazine or salt thereof Detection method of 1-[2-(2, 4-dimethylphenylthioalkyl)phenyl]piperazine or salt thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e7d43c42-507c-48f9-bde6-61b308cd2563/HDA0000903848940000021.png)