Pharmaceutical composition for treating neurasthenia and preparation method thereof
A neurasthenia and composition technology, which is applied in the directions of drug combination, pharmaceutical formula, nervous system diseases, etc., can solve the problems of inability to guarantee the efficacy of the drug, the effect is not very obvious, and it is inconvenient for preparation and use, and achieves an ideal comprehensive application effect and a preparation process. Simple and feasible, the effect of reducing resource consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
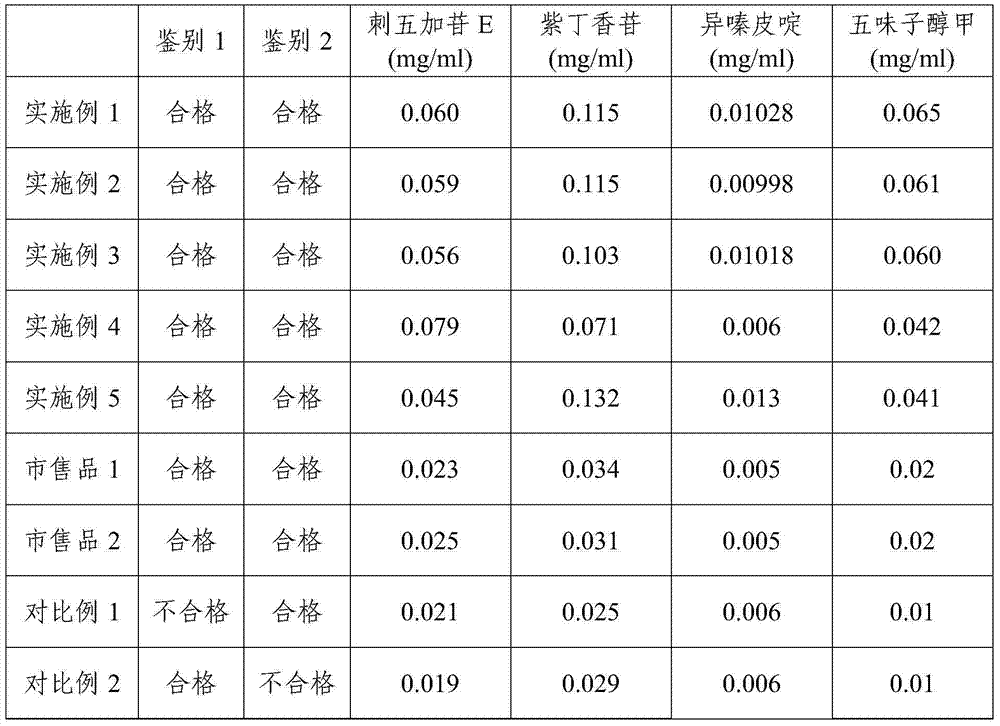
Embodiment 1
[0042] The preparation method of pharmaceutical composition in the present embodiment comprises the following steps:
[0043] (1) Extraction: Soak the Acanthopanax senticosus medicinal material for 30 minutes with 8 times the volume of water, extract 3 times, each time for 1 hour, filter, combine the extracts, concentrate, and obtain the Acanthopanax senticosus water decoction 1.12 (measured at 80°C) ,spare;
[0044](2) Macroporous resin adsorption: take the Acanthopanax senticosus water decoction obtained in step (1), dilute it to a total flavonoid content of 10 mg / ml with purified water, after stirring evenly, the medicinal liquid is filtered through a microporous membrane to clarify, and the filtrate Apply macroporous resin column D101 at a flow rate of 1.2BV / h, then elute with 0.4 times column volume of purified water at the same flow rate, and discard; then elute with 4 times the volume of 50% ethanol, collect the eluate, and adjust The pH is 5.2, and the 50% eluate is c...
Embodiment 2
[0049] Embodiment 2 prepares pharmaceutical composition
[0050] The preparation method of pharmaceutical composition in the present embodiment comprises the following steps:
[0051] (1) Extraction: Soak the Acanthopanax senticosus medicinal material in 12 times the volume of water for 30 minutes, extract twice, 2 hours each time, filter, combine the extracts, concentrate, and obtain Acanthopanax senticosus water decoction 1.10 (measured at 80°C) ,spare;
[0052] (2) Macroporous resin adsorption: take the Acanthopanax senticosus decoction obtained in step (1), dilute it to a total flavonoid content of 12 mg / ml with purified water, stir evenly, and filter the liquid through a microporous membrane to clarify, and the filtrate Apply macroporous resin column HPD100 with a flow rate of 1.5BV / h, then use 0.5 times of column volume purified water to elute with the same flow rate, and discard; then use 6 volume times of 50% ethanol to elute, collect the eluate, adjust The pH is 5.5...
Embodiment 3
[0057] Embodiment 3 prepares pharmaceutical composition
[0058] The preparation method of pharmaceutical composition in the present embodiment comprises the following steps:
[0059] (1) Extraction: Soak the Acanthopanax senticosus medicinal material in 10 times the volume of water for 30 minutes, extract 3 times, each time for 1 hour, filter, combine the extracts, concentrate, and obtain Acanthopanax senticosus water decoction 1.11 (measured at 80°C) ,spare;
[0060] (2) Macroporous resin adsorption: take the Acanthopanax senticosus decoction obtained in step (1), dilute it to a total flavonoid content of 15 mg / ml with purified water, stir evenly, and filter the liquid through a microporous membrane to clarify, and the filtrate Apply macroporous resin column DM130 at a flow rate of 1.2BV / h, then elute with 0.3 times column volume of purified water at the same flow rate, and discard; then elute with 4 volume times of 50% ethanol, collect the eluate, and adjust The pH is 5.6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ld50 | aaaaa | aaaaa |
| Ld50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com