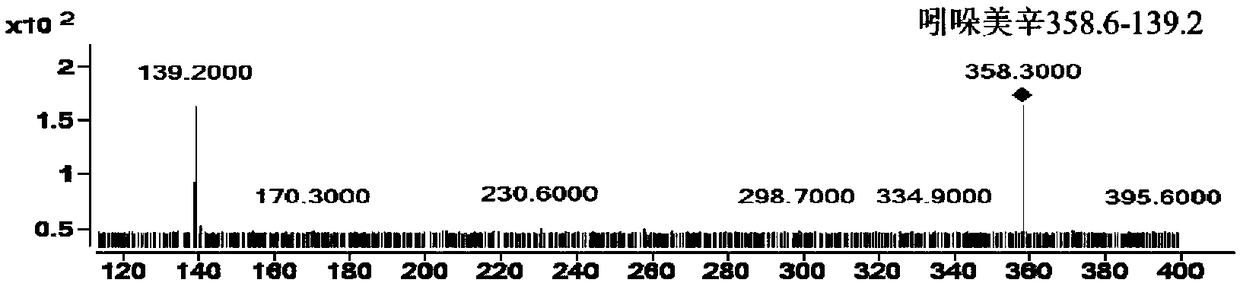
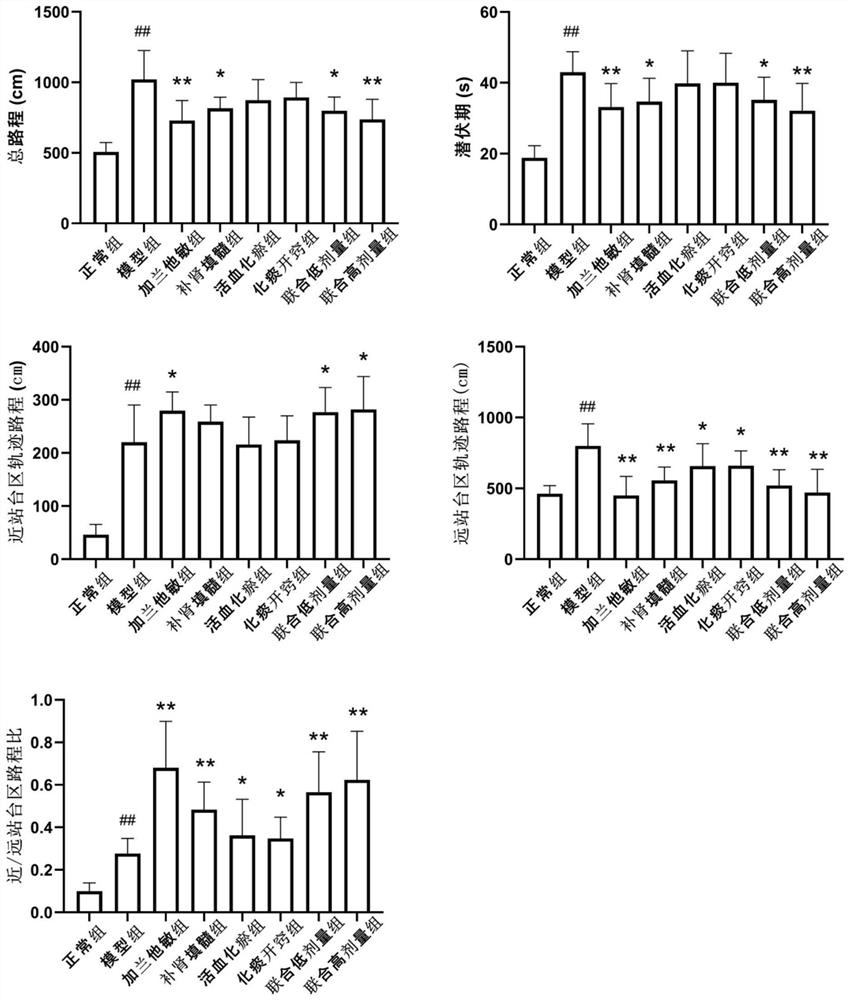
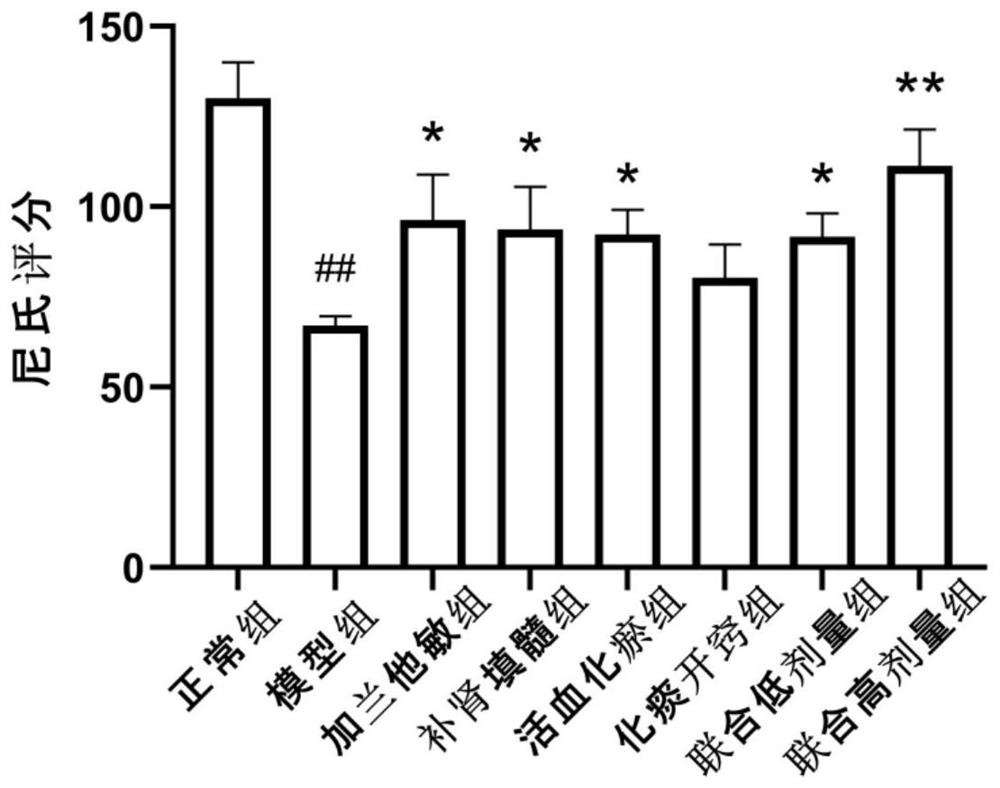
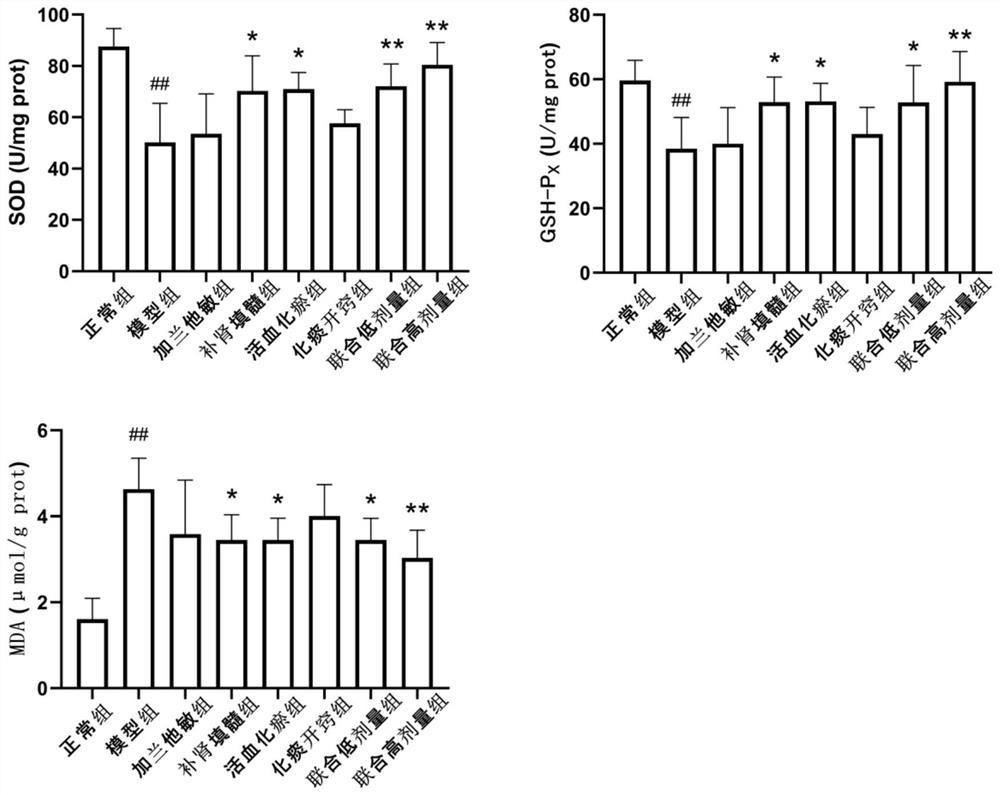
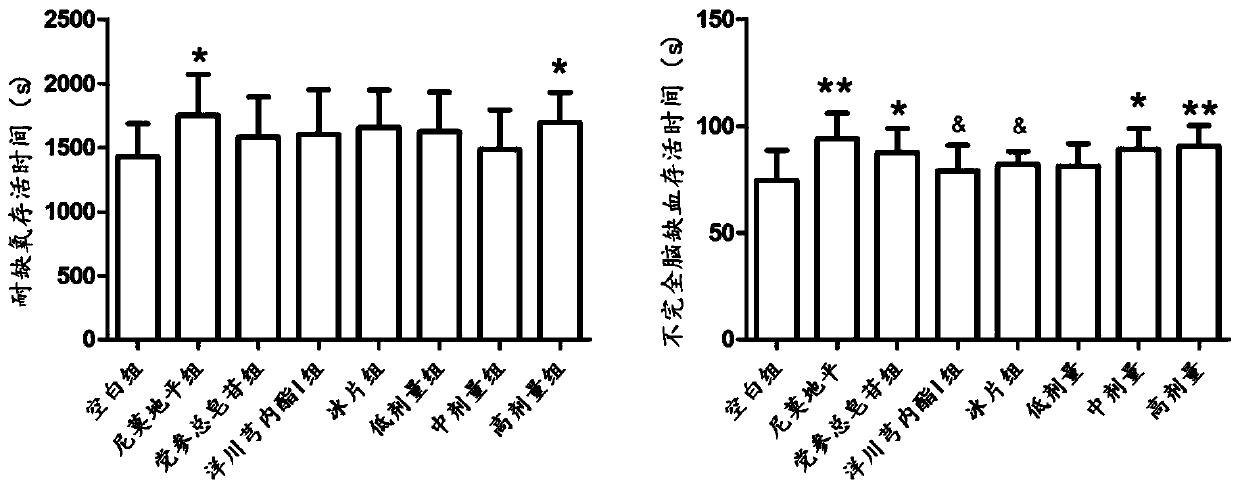
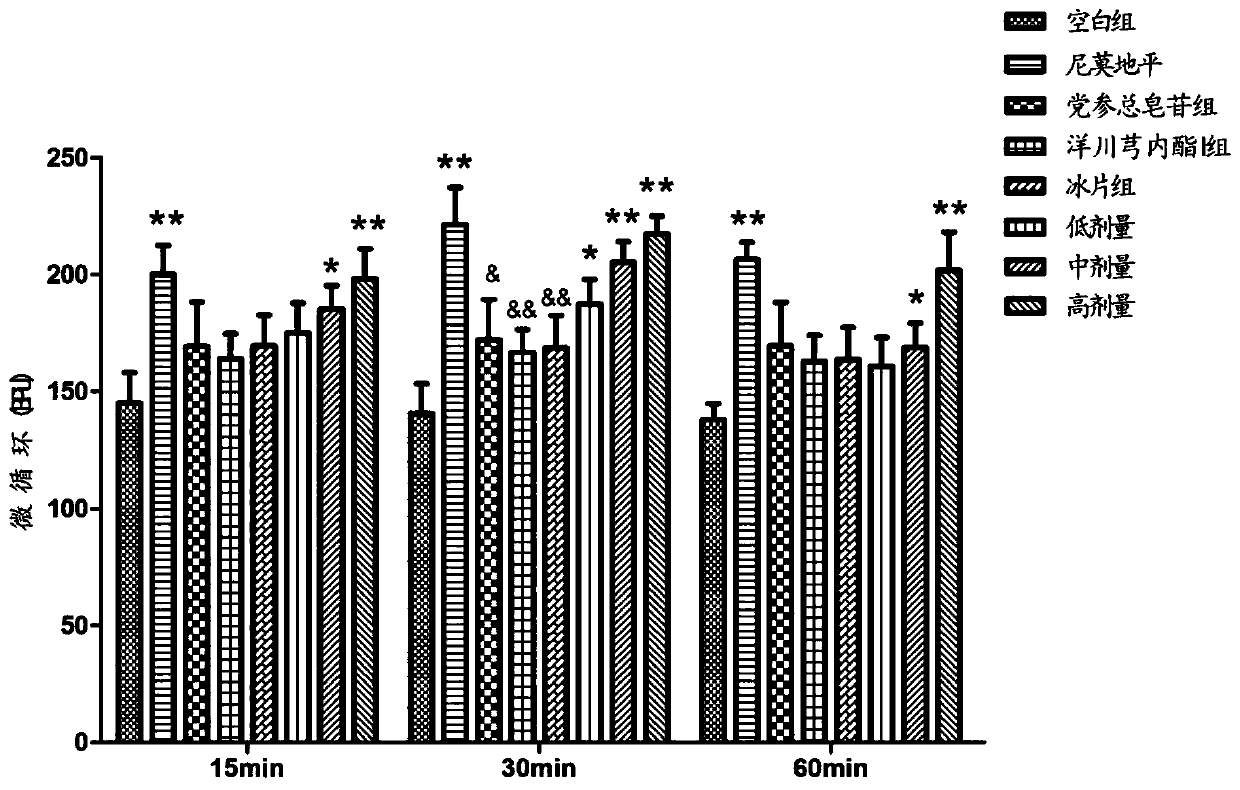
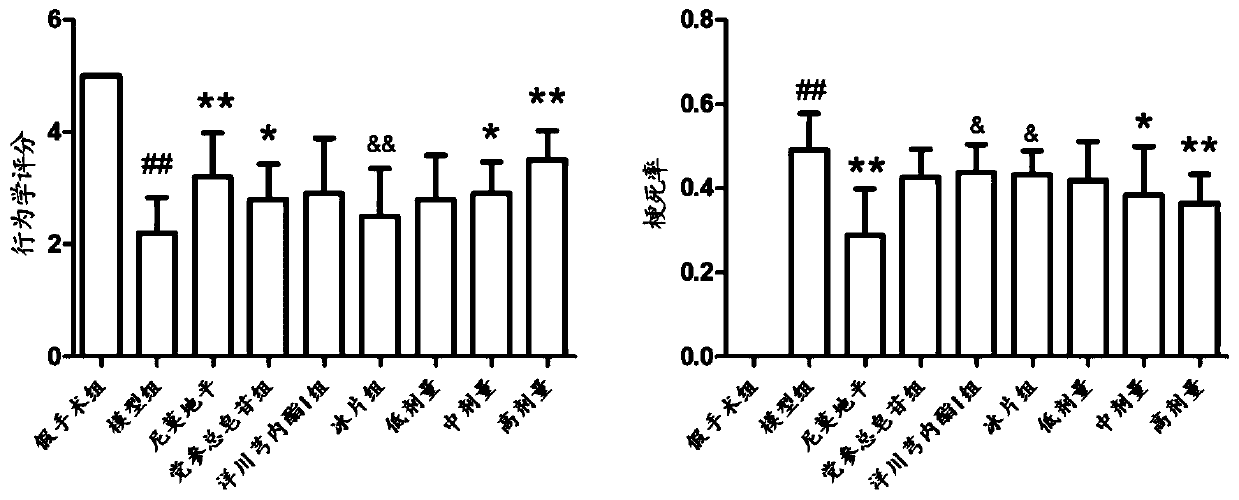
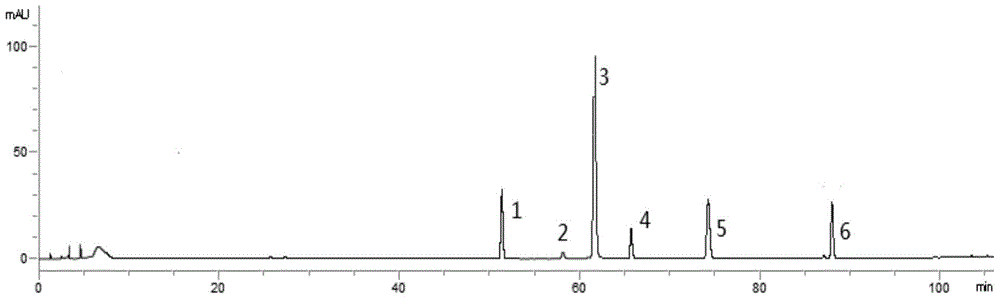
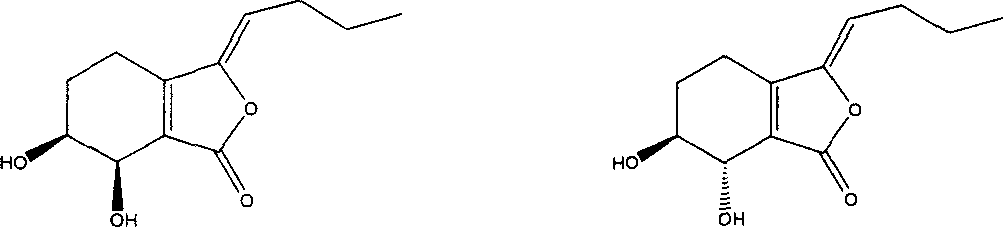Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Senkyunolide I" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quality detection method of traditional Chinese medicine preparation
ActiveCN111044624AReasonable quality inspectionEasy to separateComponent separationBiotechnologyGallic acid ester
The invention relates to a quality detection method of a traditional Chinese medicine preparation. The method comprises the following steps: respectively taking gallic acid, albiflorin, paeoniflorin,ferulic acid, liquiritin, beta-ecdysterone, senkyunolide I, glycyrrhizin, cinnamic acid, cinnamyl aldehyde, paeonol, ammonium glycyrrhizinate and ligustilide as reference substances, and establishinga fingerprint spectrum of the traditional Chinese medicine preparation by adopting high performance liquid chromatography; identifying Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba,moutan bark, ginseng, cassia bark, licorice root and the root of bidentate achyranthes of the traditional Chinese medicine preparation by adopting thin-layer chromatography. The traditional Chinese medicine preparation is prepared by using the following raw materials: Chinese angelica, the rhizome of chuanxiong, radix paeoniae alba, cassia bark, moutan bark, zedoray rhizome, ginseng, licorice root and the root of bidentate achyranthes. With the two detection ways, comprehensive quality evaluation can be conducted on the meridian-warming decoction traditional Chinese medicine preparation. Themethod is simple, convenient, high in accuracy and high in reproducibility; a scientific basis can be provided for quality detection and evaluation of the meridian-warming decoction traditional Chinese medicine preparation, and the product quality is effectively controlled.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
Ligustilide extract and its preparing process and application
InactiveCN101015552AHigh purityHigh yieldOrganic active ingredientsNervous disorderMedicinal herbsCnidium
The invention discloses a preparation method and application of extract of cnidium lactone. The extract of cnidium lactone is extracted from medicinal materials or decoction pieces of chuanxiong rhizome, Cnidium offcinale Makino, Angelica, or Acutelobed angelica, wherein the content of cnidium lactone occupies over 50% of the total weight of extract, total content of ligustilide, Senkyunolide A, Senkyunolide H, and Senkyunolide I occupy over 30% of the total weight of extract. Total lactone content in the cnidium lactone extract is over 30%, total content of lactone in four active constituents is over 30%, thus the product has high purity and no residual solvent. in addition, the preparation method of the invention has the advantages of low toxicity solvent, simple process, high yield, and low cost, thus is suitable for modernize industrial production.
Owner:TIANJIN UNIV
Method for determining fingerprint chromatography of radix astragali and ligusticum wallichii extract products
The invention relates to a method for determining fingerprint chromatography of radix astragali and ligusticum wallichii extract products, which comprises the following steps: 1)taking Radix Astragali and Ligusticum wallichii extract product fine powder, preciously weighing, adding methanol, performing ultrasonic extraction, filtering and drying a filtrate, dissolving residue by ethanol and metering volume, filtering by a filter membrane, taking a subsequent filtrate to obtain a tested object solution; 2)taking a ferulic acid reference substance, a senkyunolide I reference substance, a calycosin glucoside reference substance, an ononin reference substance, a calycosin reference substance and a fermlononetin reference substance, preciously weighing, respectively adding methanol to prepare a reference substance solution; and 3)respectively and preciously absorbing the tested object solution and the reference substance solution, and injecting a high efficiency liquid chromatography for determining to obtain the fingerprint chromatography of radix astragali and ligusticum wallichii extract products. The method has active effect for guiding clinical medication and effective feeding guidance to the bulk drugs during the production process, and ensuring the reliable quality; the method has the advantages of convenient and fast operation, so that similarity result can be obtained, the quality of the traditional Chinese medicinal materials radix astragali and ligusticum wallichii extract products can be evaluated, and the result is objective and accurate.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV +1
Content detection method for determining effective components in longshengzhi capsule by HPLC-QQQ/MS method
ActiveCN109307721AEasy to separateQuick analysisComponent separationBenzoylpaeoniflorinGradient elution
The invention provides a content detection method for determining effective components in a longshengzhi capsule by an HPLC-QQQ / MS method. The chromatograph condition is that the chromatographic column takes a octadecyl-bonded silica gel column as a filling agent, 0.1% formic acid-water as a mobile phase A, 0.1% formic acid-acetonitrile as a mobile phase B, and the volume ratio of the mobile phaseA and the mobile phase B is 80% to 0%:20 to 100%, so that gradient elution is performed. According to the content detection method for determining the effective components in the longshengzhi capsuleby the HPLC-QQQ / MS method, the effective substance components of traditional Chinese medicines such as astragaloside A, hydroxysafflor yellow A, calycosin 7-o-glucoside, calycosin, ferulic Acid, syringin, n-butylphthalide, ligustilide, senkyunolide A, senkyunolide I, senkyunolide H, ligustrazine, isofraxidin, dehydrocostus lactone, amygdalin, syringin E, paeoniflorin, oxypaeoniflora, benzoylpaeoniflorin, etc., in the longshengzhi capsule can be determined. The method has the advantages of being simple in operation method, high in sensitivity and accurate in content.
Owner:SHAANXI BUCHANG PHARMA
Separation and preparation method of ligustilide
ActiveCN104003963AEasy to operateImprove efficiencyOrganic chemistryAcetic acidCountercurrent chromatography
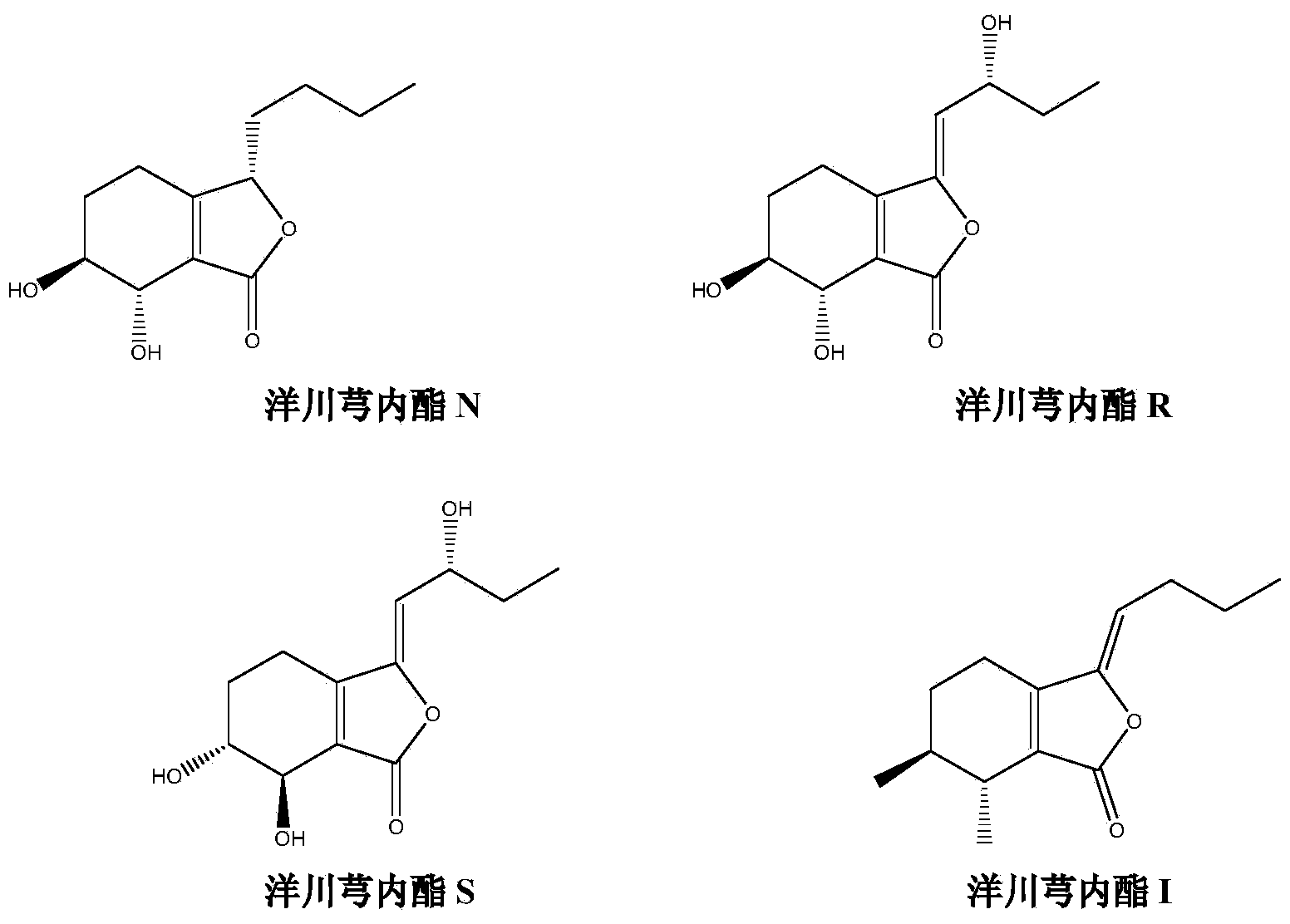
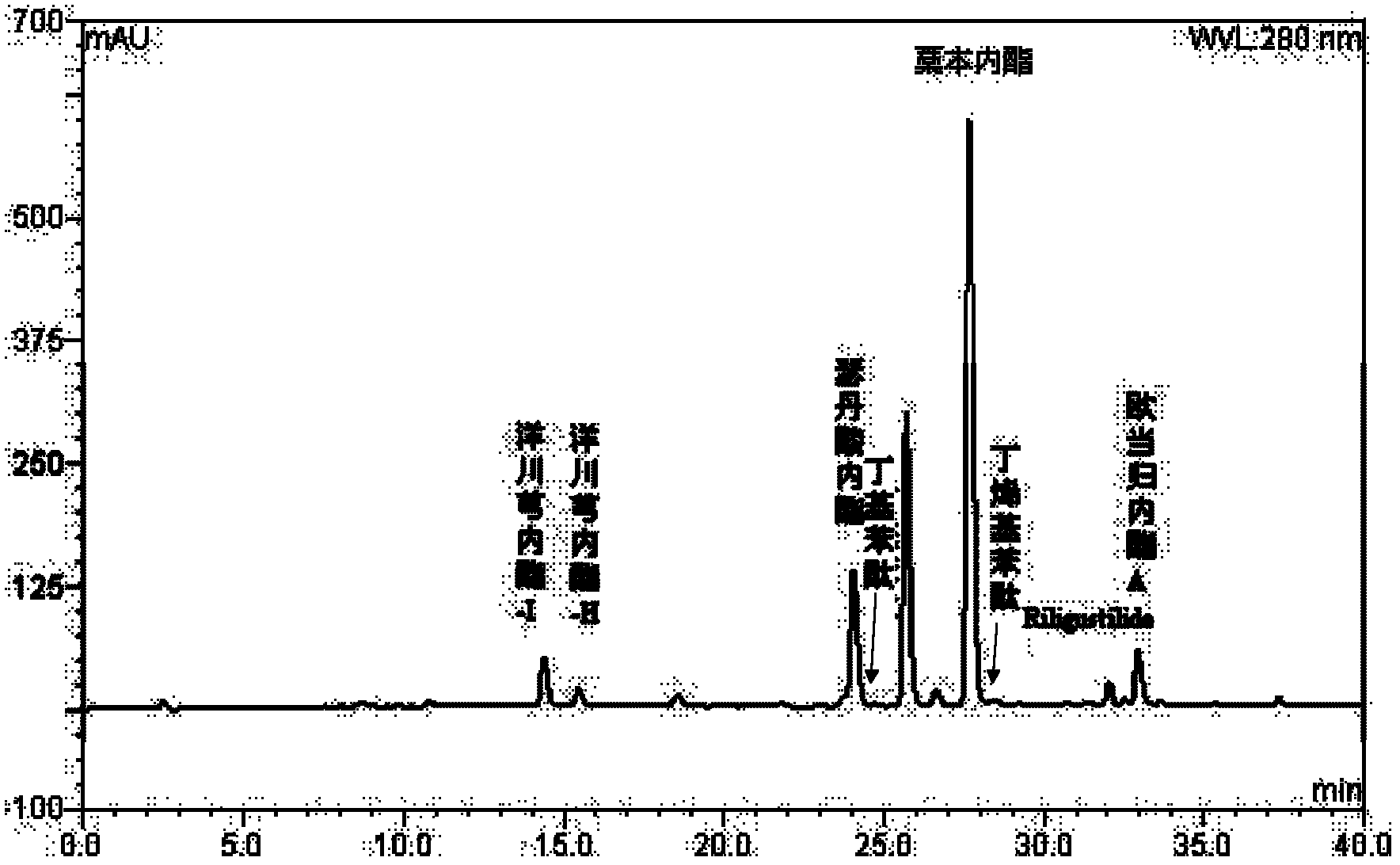
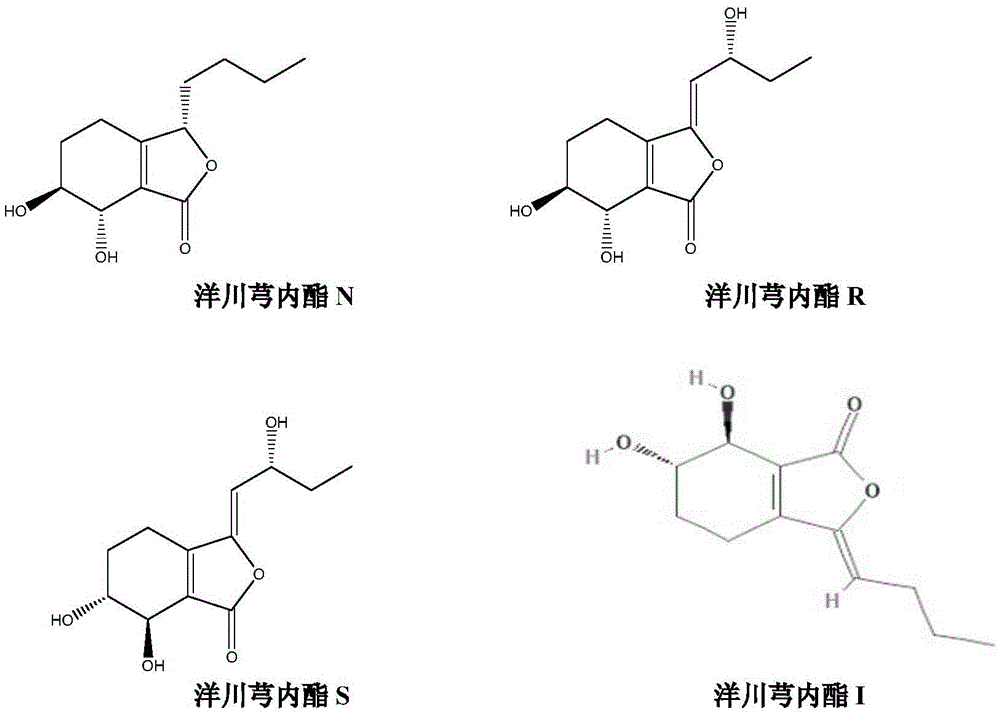
The invention discloses a separation and preparation method of ligustilide. The method comprises the following steps by the technology of high-speed countercurrent chromatography. The method comprises the following steps: extracting ligusticum wallichii by a solution to obtain a ligusticum wallichii extract; extracting the ligusticum wallichii extract by ethyl acetate to obtain an ethyl acetate part; separating the ethyl acetate parts by high-speed countercurrent chromatography at the same time to obtain a plurality of ligustilide compounds which are respectively senkyunolide N, senkyunolide R, senkyunolide S and senkyunolide I through structural identification, wherein the purity is over 98%. The separation and preparation method of ligustilide disclosed by the invention has the advantages of simplicity in operation, few use level of solvent, no sample adsorption, loss and pollution, high efficiency and the like.
Owner:CHANGSHA BROAD OCEAN BIO SCI & TECHN CO LTD
Preparation containing ligustilide type component for treating cardio-cerebrovascular disease and preparation method thereof
InactiveCN102631387APrevent escapeImprove bioavailabilityCardiovascular disorderPlant ingredientsDiseaseWater vapor
The invention discloses a preparation containing a ligustilide type component and a preparation method thereof. The ligustilide type component comprises the following components by weight percent: ligustilide 14%-42%, sedanolide 3%-20%, butylidenephthalide 0.1%-5%, butylphthalide 0.1%-3%, senkyunolide-H 0.2%-3%, senkyunolide-I 0.4%-5%, levistilide A 0.5%-1.5%, and riligustilide 0.4%-1.2%. The preparation method prevents the ligustilide type component from being destroyed by the wet distillation method and the solvent extraction method, and the quality of the ligustilide type component is more ensured without solvent residue. The preparation containing the ligustilide type component is safe and controllable on the effective part. Drop pills and sublingual tablets containing the ligustilide type component can avoid the defects of the oral preparation after administration such as liver first pass effect and gastrointestinal reaction, and an injection containing the ligustilide type component can also avoid the possible situations during using the injections such as acute poisoning reaction and allergic reaction. The preparation containing the ligustilide type component is safer and more effective, and has good economic benefit and social benefit.
Owner:TIANJIN UNIV
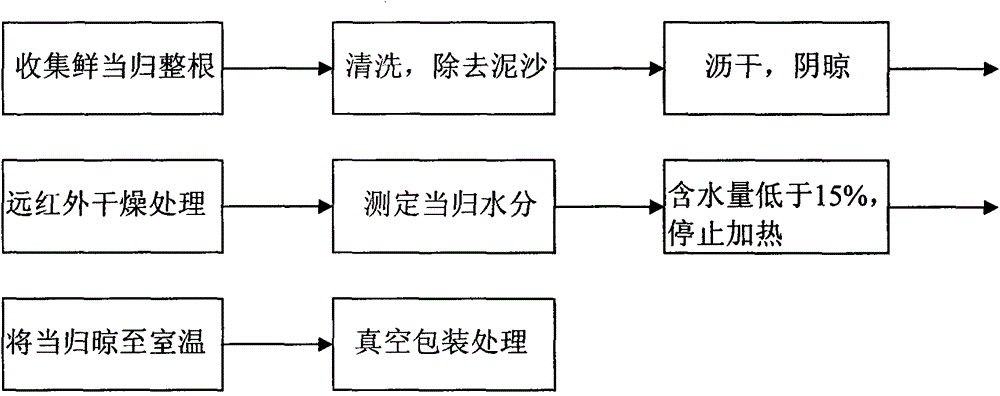
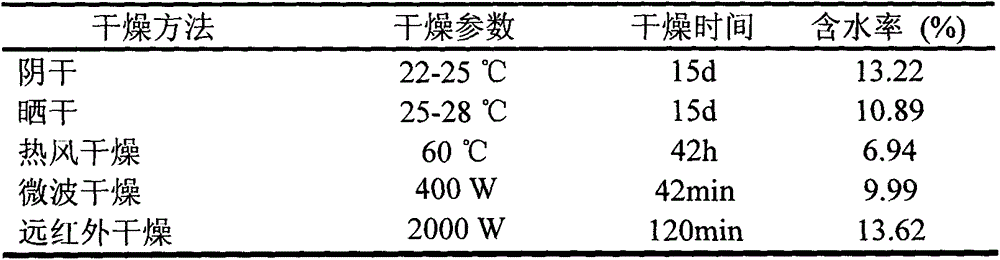
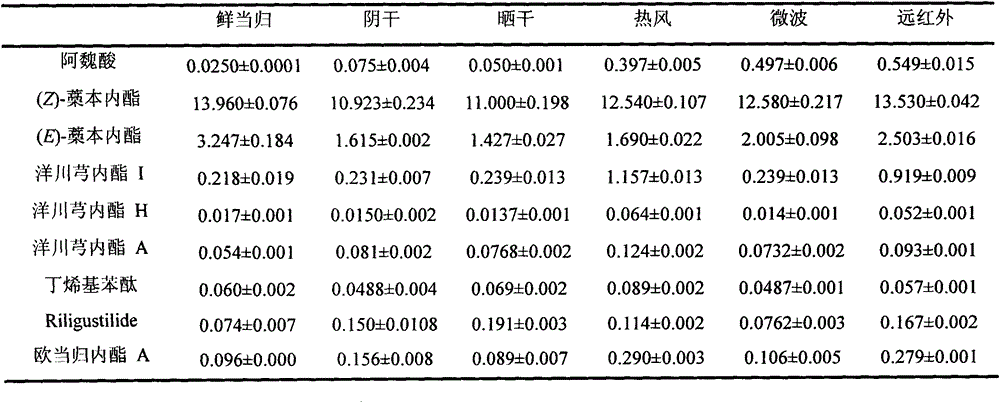
Method for rapidly drying fresh whole angelica roots
InactiveCN104940260AAppearance traits are completeEasy to keepPlant ingredientsAlternative technologyDrying time
The invention discloses a method for rapidly drying fresh whole angelica roots. The method comprises the following steps: collecting fresh whole angelica roots; cleaning to remove silt; draining and airing; drying in a far infrared manner; measuring the water content; and vacuumizing and packaging. Compared with the traditional drying method, the method disclosed by the invention has the advantages that the drying time is greatly shortened; the drying efficiency is increased; simultaneously, effective components in angelica, such as ferulic acid, ligustilide, senkyunolide I, senkyunolide H and butylidene phthalide, can be retained; in addition, the drying technology adopted in the invention is green and free from pollution; the whole angelica roots can be effectively prevented from being mildewed; long-term storage can be easily realized; and the drying technology can be used as the substitute technology for processing whole angelica roots through sulphur fumigation.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Preparation method of senkyunolide I
ActiveCN107619401AIncrease contentImprove the problem of low content and difficulty in mass productionOrganic chemistryChromatographic separationAlkaline water
The invention discloses a preparation method of senkyunolide I, and belongs to the technical field of traditional Chinese medicine component preparation. The preparation method is characterized in that extract containing ligustilide is taken as a raw material, and reaction liquor is subjected to extraction and column chromatography isolation to obtain a senkyunolide I pure product after the raw material and potassium hydrogen persulfate composite salt perform heating reaction in an alkaline water-containing solvent. According to the preparation method disclosed by the invention, the extract containing ligustilide is taken as the raw material, and senkyunolide I is prepared through a method combining chemical conversion and chromatographic separation. The preparation method has the characteristics of simple and convenient process, less solvent consumption and high yield, and is suitable for preparing high-purity senkyunolide I on a large scale.
Owner:SICHUAN ACAD OF CHINESE MEDICINE SCI
Preparation of senkyunolide I and senkyunolide H from ligusticum wallichii extract via high-speed countercurrent chromatography
InactiveCN103896892ASimple manufacturing processHigh yieldOrganic chemistryN-Butyl AlcoholChromatography column
The invention discloses preparation of senkyunolide I and senkyunolide H from ligusticum wallichii extract via high-speed countercurrent chromatography, and relates to a method used for preparing senkyunolide I and senkyunolide H from ligusticum wallichii extract via high-speed countercurrent chromatography and preparative liquid chromatography. According to the method, n-butyl alcohol, acetic acid, and water are mixed, and a layered mixture is obtained; an upper phase is collected, a high-speed countercurrent chromatographic column is filled with the upper phase, a host machine is rotated, a lower phase is pumped as a mobile phase, and the ligusticum wallichii extract is dissolved in the lower phase for sampling through a sampling valve; a SP-200 ultraviolet detector is used for detecting; and eluent containing senkyunolide I and senkyunolide H are collected. The method is simple in operation; preparation period is short; yield is high; senkyunolide I and senkyunolide H products with a total content more than 90% can be obtained; and continuous production can be realized.
Owner:SHANGHAI ZHANGJIANG ENG RES CENT OF MODERN PREPARATION TECH OF TRADITIONAL CHINESE MEDICINE
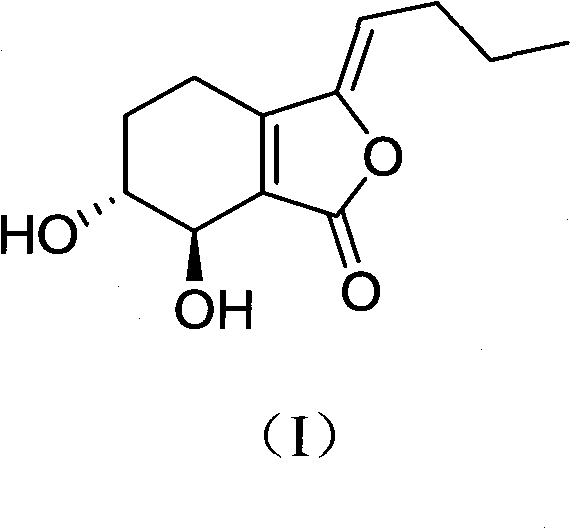
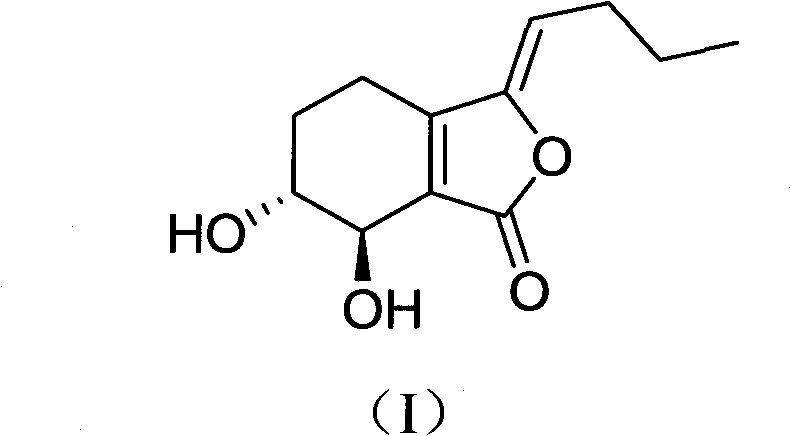
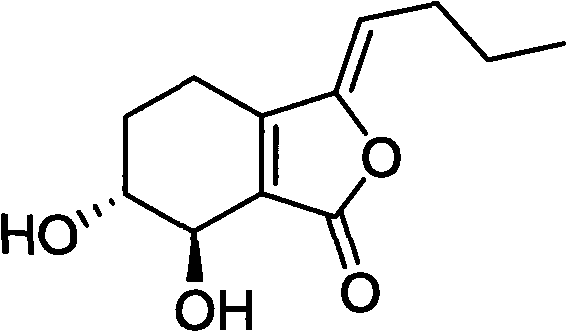
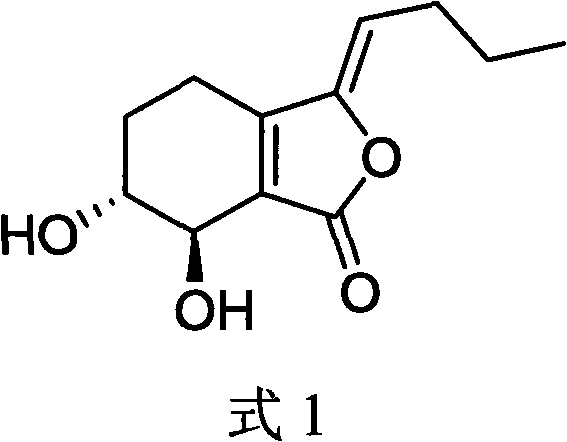
Application of senkyunolide I to medicaments for prevention and treatment of cerebral apoplexy and relevant treatment during convalescence
InactiveCN102144998AIncrease cerebral blood flowOrganic active ingredientsCardiovascular disorderArteriolar VasoconstrictionCortical spreading depression
The invention discloses new medicinal application of senkyunolide I shown in a structural formula (I). In the application, the influence of the senkyunolide I on the cerebral blood flow change of rats in a reperfusion model of local cerebral ischemia caused by middle cerebral artery occlusion (MCAO) of the rats and a kalium chloratum (KCL) induced rat cortical spreading depression model is studied by adopting an animal model, and pharmacological results prove that the senkyunolide I can improve the cerebral blood flow of the rats after MCAO molding obviously and can restore and exceed the original cerebral blood flow formed before modeling quickly after reperfusion so as to play a certain role in cerebral protection. In addition, the senkyunolide I can resist the condition that cerebral blood flow descends due to KCL-induced rat local cerebral vasoconstriction, so the senkyunolide I can be used for preparing medicaments for the prevention and treatment of cerebral apoplexy and relevant treatment during convalescence.
Owner:SHANGHAI ZHANGJIANG ENG RES CENT OF MODERN PREPARATION TECH OF TRADITIONAL CHINESE MEDICINE
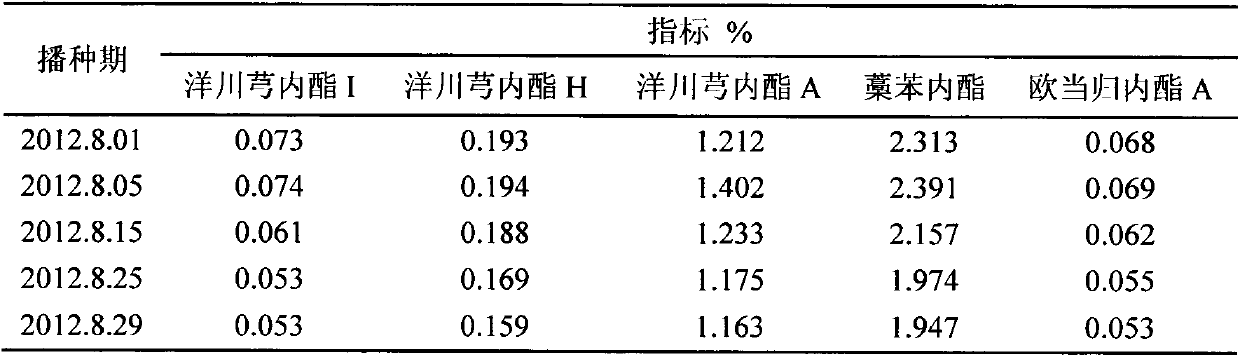
Method for improving lactone substance content in ligusticum wallichii medicinal materials by utilizing seeding time
InactiveCN103609325AIncrease the content of lactonesImprove qualityHorticultureMedicinal herbsMedicine
The invention discloses a method for improving the lactone substance content in ligusticum wallichii medicinal materials by utilizing the seeding time, and belongs to the field of cultivation technical research of medicinal plants. According to the method, it is determined that the seeding time ranges from August 1st to August 5th (before the beginning of autumn) every year, and is 10-20 days earlier than the seeding time (from August 10th to August 20th, namely from the beginning of autumn to the limit of heat) in traditional production, and therefore the content of five lactone substances in the ligusticum wallichii medicinal materials can be improved, wherein the five lactone substances are senkyunolide I, senkyunolide H, senkyunolide A, ligustilide and levistilide A.
Owner:SICHUAN AGRI UNIV
Pharmaceutical compositions for treating sepsis and application of pharmaceutical compositions
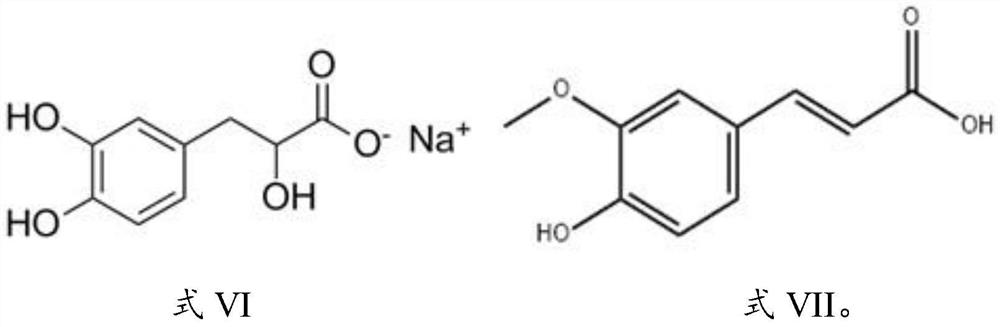
The invention provides pharmaceutical compositions for treating sepsis and application of the pharmaceutical compositions, and belongs to the technical field of chemical drugs. The invention provides a pharmaceutical composition containing three components, namely hydroxysafflor yellow A, paeoniflorin and albiflorin, and further provides a pharmaceutical composition containing seven components, namely hydroxysafflor yellow A, paeoniflorin, albiflorin, oxypaeoniflorin, senkyunolide I, salvianic acid A sodium and ferulic acid. The cell level and the animal level prove that the two pharmaceutical compositions have the efficacy of effectively treating sepsis, and meanwhile, the drug toxicity experiment shows that the drug has relatively high safety. The pharmaceutical compositions provided by the invention are medicinal components of Xuebijing injection and can be used for clinically treating sepsis.
Owner:TIANJIN CHASE SUN PHARM CO LTD
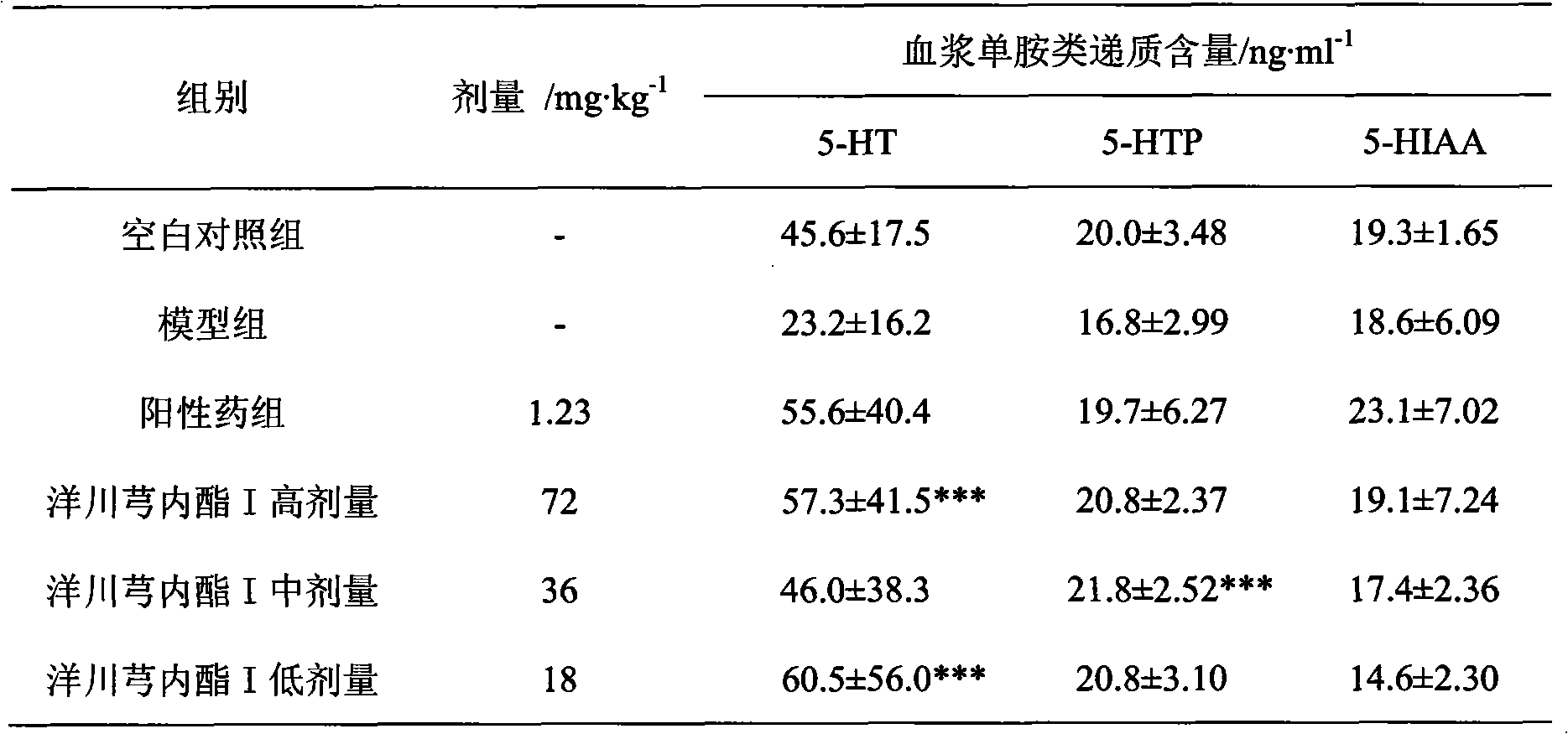
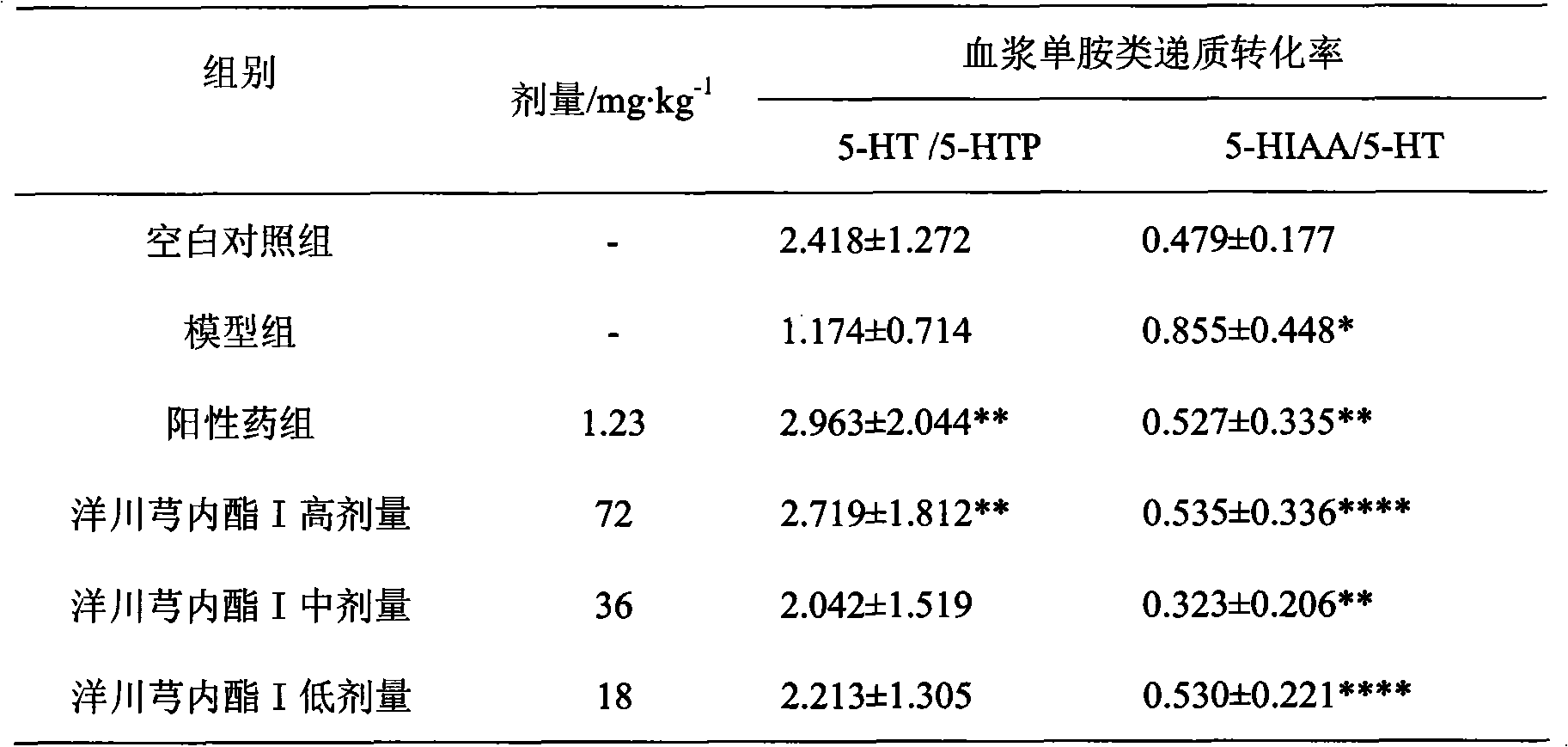
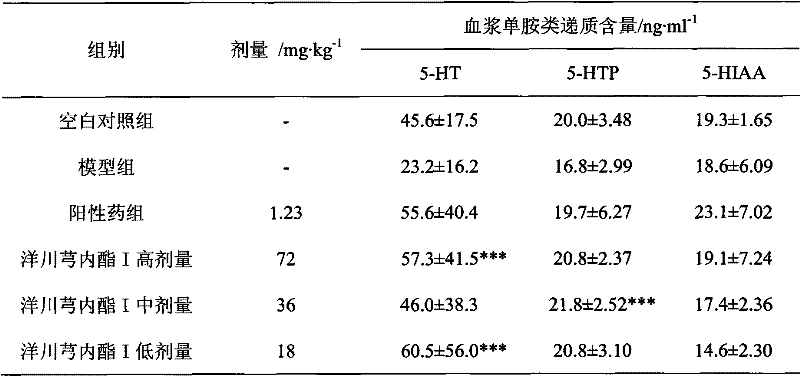
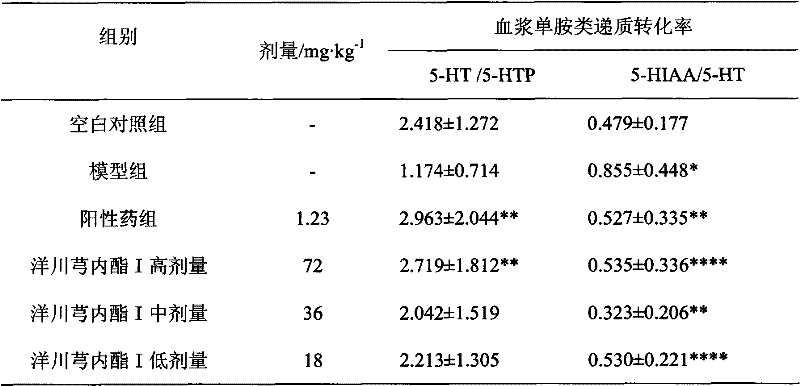
Application of senkyunolide I in preparation of antidepressant medicament, migraine medicament and medicaments for other diseases relevant to 5-hydroxytryptamine system
The invention discloses new medical application of senkyunolide I. Based on animal models, the influence of the senkyunolide I on the plasma and brain tissue monoamine transmitters of rats, the influence of the senkyunolide I on the plasma and brain tissue NO of rats and the influence of the senkyunolide I on the pain threshold of mice are researched in the invention, and the subsequent embodiment and the pharmacological result can confirm that the senkyunolide I can adjust the content of monoamine transmitters, especially 5-hydroxytryptamine (5-HT) in the plasma and brain tissues. Thus, the senkyunolide I can be used for preparing medicaments for preventing and curing depression, migraine disease and other diseases relevant to a 5-hydroxytryptamine system.
Owner:SHANGHAI ZHANGJIANG ENG RES CENT OF MODERN PREPARATION TECH OF TRADITIONAL CHINESE MEDICINE +1
Microwave-assisted extraction method of three lactone compounds in rhizoma chuanxiong
ActiveCN107308684ASmall temperature fluctuationsImprove extraction efficiencyEnergy based chemical/physical/physico-chemical processesSolid solvent extractionPropanoic acidDeep eutectic solvent
Owner:DONGGUAN UNIV OF TECH
Specific chromatogram construction method of compound composition containing ligusticum wallichii, borneol and artificial musk and pharmaceutical preparation
ActiveCN114813987ARapid identificationAccurate identificationComponent separationAgainst vector-borne diseasesMedicinal herbsMusk ketone
The invention provides a specific chromatogram construction method of a compound composition containing ligusticum wallichii, borneol and artificial musk and a pharmaceutical preparation, which comprises the following steps: A) dissolving a raw material to be detected by using a solvent, and extracting to obtain a liquid to be detected; the method comprises the following steps: respectively taking one or more of borneol, musk ketone, ligustilide, senkyunolide A, senkyunolide I and butylidenephthalide as reference substances, and dissolving with a solvent to obtain a reference substance solution; and B) determining the to-be-detected solution and the reference substance solution by adopting a gas chromatographic method to obtain the GC characteristic chromatogram of the compound composition containing ligusticum wallichii, borneol and artificial musk and the pharmaceutical preparation. According to the method, a characteristic spectrum method is established for the composition containing volatile components of ligusticum wallichii, borneol and artificial musk for the first time, 25 common characteristic peaks are confirmed, the relative retention time of the composition is stipulated, and a contrast characteristic spectrum is established, so that whether the composition and a compound preparation contain ligusticum wallichii, borneol and artificial musk medicinal materials or not can be quickly and accurately identified; and high-efficiency, simple, convenient and rapid detection capability is realized.
Owner:SICHUAN NEO GREEN PHARMA TECH DEV
Fingerprint spectrum detection method for ethyl acetate part of ligusticum wallichii
ActiveCN110907555AImprove the quality evaluation systemGuaranteed clinical efficacyComponent separationClinical efficacyEthyl acetate
The invention discloses a fingerprint spectrum detection method for an ethyl acetate part of ligusticum wallichii. The fingerprint spectrum detection method adopts high performance liquid chromatography for detection. Through the detection method provided by the invention, the senkyunolide I, the senkyunolide H, the senkyunolide A and the ligustilide in the ligusticum wallichii can be measured atthe same time, the quality of effective parts with the analgesic effect can be controlled, the method is simple, convenient and reliable, a quality evaluation system of the ligusticum wallichii is perfected, the clinical curative effect of the ligusticum wallichii is guaranteed, and practical application and popularization value is achieved.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
A kind of separation and preparation method of Ligusticum lactone
ActiveCN104003963BEasy to operateImprove efficiencyOrganic chemistryAcetic acidCountercurrent chromatography
Owner:CHANGSHA BROAD OCEAN BIO SCI & TECHN CO LTD
Application of Ligusticolide I in the preparation of antidepressant drugs, migraine drugs and other serotonergic system-related disease drugs
The invention discloses a new medical application of senkyunolide I. Inventor adopts animal model, has studied the effect of chuanxionglide I on rat plasma and brain tissue monoamine transmitter, the influence of chuanxionglide I on rat plasma and brain tissue NO and the effect of chuanxionglide I on rat plasma and brain tissue NO. The impact of mouse pain threshold, subsequent examples and pharmacological results thereof will confirm that Ligustin I has the ability to regulate monoamine transmitters, especially the content of 5-hydroxytryptamine (5-HT) in blood plasma and brain tissue, so Ligustilide I can be used to prepare medicines for preventing and treating depression, migraine, and other diseases related to serotonergic system.
Owner:SHANGHAI ZHANGJIANG ENG RES CENT OF MODERN PREPARATION TECH OF TRADITIONAL CHINESE MEDICINE +1
Method for preparing senkyunolide I from extract of Chinese angelica
The invention discloses a method for preparing senkyunolide I from extract of Chinese angelica, which comprises the following steps: (1) purifying by using macroporous resin; (2) purifying by extraction; and (3) purifying by chromatography. In the invention, the preparation process of the senkyunolide I is simplified, and the yield is increased to a great extent. In addition, the method prepares the senkyunolide I by using the extract of Chinese angelica which is a traditional Chinese medicine instead of Szechuan lovage rhizome which is an original source plant. Therefore, the method can be used in the industrial preparation of senkyunolide I.
Owner:SHANGHAI ZHANGJIANG ENG RES CENT OF MODERN PREPARATION TECH OF TRADITIONAL CHINESE MEDICINE
Pharmaceutical composition for treating vascular dementia and application thereof
PendingCN114288352AThe composition formula is simpleCompatibility is reasonableOrganic active ingredientsNervous disorderFormularyDisease
The invention is applicable to the technical field of medicines, and provides a pharmaceutical composition for treating vascular dementia and related diseases, which comprises 1-6 parts of stibene glucoside, 0.2-4 parts of catalpol, 0.5-4 parts of salvianic acid A sodium, 3-8 parts of senkyunolide I, 3-8 parts of rhizoma acori graminei volatile oil and 0.2-5 parts of tenuifolin. The pharmaceutical composition provided by the invention is simple in formula, economical and practical, complementary to each other, reasonable in compatibility and capable of effectively treating vascular dementia. Moreover, the components are all from natural products, so that the composition has no adverse reaction and toxic and side effects after long-term use and is worthy of popularization and application. The invention also provides a preparation method of the medicinal composition, the medicinal preparation is prepared by uniformly mixing the components according to the formula ratio and mixing with pharmaceutically acceptable auxiliary materials, and the method is simple, convenient to operate and suitable for large-scale production.
Owner:NANJING XIAOZHUANG UNIV +1
Skyunolide I compound and application thereof in treating myocardial hypertrophy disease
The invention relates to the technical field of medicine, in particular to a senkyunolide I compound and application thereof to treatment of myocardial hypertrophy diseases, the compound comprises senkyunolide I and salvianolic acid B according to the mass ratio of 1-5: 4, and the compound has the advantage of being good in myocardial hypertrophy treatment effect.
Owner:CHIATAI QINGCHUNBAO PHARMA
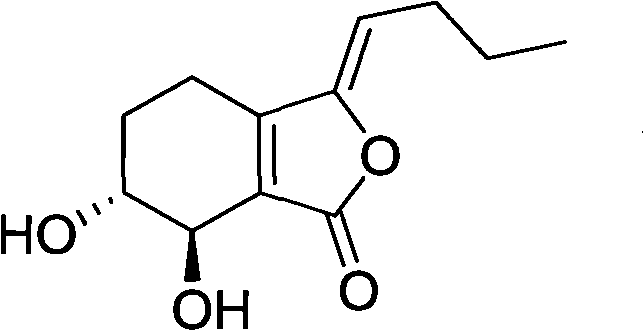
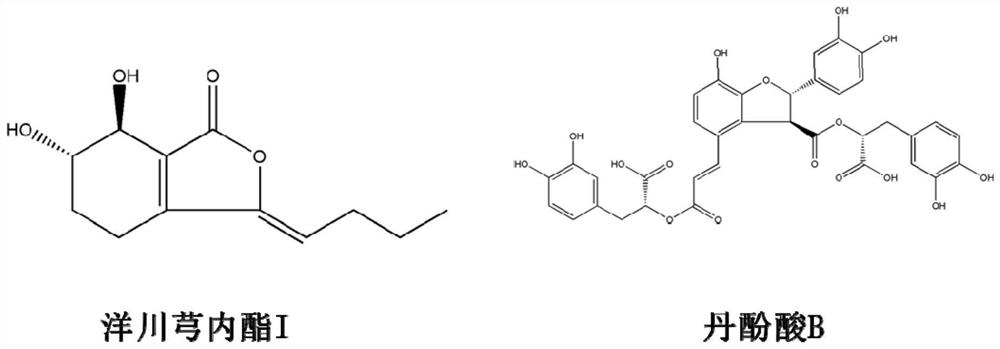
A kind of preparation method of Yangchuanxiongolide I
Owner:SICHUAN ACAD OF CHINESE MEDICINE SCI
A microwave-assisted extraction method of three lactone compounds in Rhizoma Chuanxiong
ActiveCN107308684BImprove extraction efficiencyHigh selectivityEnergy based chemical/physical/physico-chemical processesSolid solvent extractionPropanoic acidDeep eutectic solvent
The invention relates to a microwave-assisted extraction method of three lactone compounds in rhizoma chuanxiong and belongs to the field of extraction separation. The microwave-assisted extraction method includes: allowing an extracting agent to contact with rhizoma chuanxiong powder, wherein the extracting agent is a deep eutectic solvent formed by choline chloride and acetic acid, propionic acid or ethylene glycol according to the mole ratio of 1:1-1:5. The microwave-assisted extraction method has the advantages that ligustilide, senkyunolide I and senkyunolide H in the rhizoma chuanxiong powder can be extracted, the extraction efficiency of the lactone compounds can reach 180mg / L, and the method is high in extraction efficiency, high in selectivity, mild in operation condition and environmentally friendly.
Owner:DONGGUAN UNIV OF TECH
Pharmaceutical composition for treating cerebral arterial thrombosis and application thereof
ActiveCN111000886AThe composition formula is simpleCompatibility is reasonableHydroxy compound active ingredientsCardiovascular disorderFormularyDisease
Being applicable to the technical field of medicine, the invention provides a pharmaceutical composition for treating cerebral arterial thrombosis related diseases. The pharmaceutical composition comprises: 3-7 parts of total saponins of codonopsis pilosula, 4-8 parts of senkyunolide I and 10-15 parts of borneol. The pharmaceutical composition provided by the invention is simple in formula, economical and practical, complementary, and reasonable in compatibility, and can effectively treat cerebral arterial thrombosis. Moreover, the components are all from natural products, and no adverse reaction, toxic or side effect is generated after long-term use, therefore the pharmaceutical composition is worthy of popularization and application. According to the preparation method of the pharmaceutical composition provided by the invention, the components in the formula ratio are uniformly mixed and then are mixed with pharmaceutically acceptable auxiliary materials so as to obtain the pharmaceutical preparation. The method is simple, convenient to operate, and is suitable for large-scale production.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE +1
A method for determining the fingerprint of the extract of Radix Astragali and Chuanxiong
ActiveCN104713956BEnsure identificationEasy to operateComponent separationTest objectActive ingredient
The invention relates to a method for determining fingerprint chromatography of radix astragali and ligusticum wallichii extract products, which comprises the following steps: 1)taking Radix Astragali and Ligusticum wallichii extract product fine powder, preciously weighing, adding methanol, performing ultrasonic extraction, filtering and drying a filtrate, dissolving residue by ethanol and metering volume, filtering by a filter membrane, taking a subsequent filtrate to obtain a tested object solution; 2)taking a ferulic acid reference substance, a senkyunolide I reference substance, a calycosin glucoside reference substance, an ononin reference substance, a calycosin reference substance and a fermlononetin reference substance, preciously weighing, respectively adding methanol to prepare a reference substance solution; and 3)respectively and preciously absorbing the tested object solution and the reference substance solution, and injecting a high efficiency liquid chromatography for determining to obtain the fingerprint chromatography of radix astragali and ligusticum wallichii extract products. The method has active effect for guiding clinical medication and effective feeding guidance to the bulk drugs during the production process, and ensuring the reliable quality; the method has the advantages of convenient and fast operation, so that similarity result can be obtained, the quality of the traditional Chinese medicinal materials radix astragali and ligusticum wallichii extract products can be evaluated, and the result is objective and accurate.
Owner:上海百洋制药股份有限公司 +1
Application of senkyunolide I in sepsis lung injury treatment medicine
InactiveCN113413379APrecision therapyEfficient therapeutic effectAntibacterial agentsOrganic active ingredientsOxidative stressPharmaceutical drug
The invention provides application of senkyunolide I in a sepsis lung injury treatment medicine, and belongs to the technical field of medical biology. The senkyunolide I has the effects of inhibiting inflammatory cytokines and oxidative stress level in the medicine for treating the sepsis lung injury, and has the effect of inhibiting platelet activation in the medicine for treating the sepsis lung injury. The senkyunolide I has the effect of inhibiting the formation of a neutrophil extracellular capture net in the sepsis lung injury treatment medicine.
Owner:中国人民解放军海军军医大学第一附属医院
Composition and pharmaceutical preparation thereof
The invention relates to a composition and a pharmaceutical preparation thereof, the composition contains ferulic acid and ligustilide, the weight ratio of ferulic acid to Z-ligustilide is 1: (4-10), or ferulic acid to Z-ligustilide is 1: 4, or ferulic acid to Z-ligustilide is 1: 10, or ferulic acid to Z-ligustilide is 1: 9.43, and the composition can also contain senkyunolide I, (-)-apigenyl lactide, or (-)-apigenyl lactide, or (-)-apigenyl lactide, or (-)-apigenyl lactide, or (-)-apigenyl lactide, or (-)-apigenyl lactide, or (-)-apigenyl lactide. And senkyunolide H. The composition is prepared from the following components in percentage by weight: 1-10
Owner:津药达仁堂集团股份有限公司第六中药厂
A hplc-qqq/ms method for determining the content of active ingredients in Longshengzhi Capsules
ActiveCN109307721BHigh sensitivityEasy to operateComponent separationEleutherosideBenzoylpaeoniflorin
The invention provides a content detection method for determining effective components in a longshengzhi capsule by an HPLC-QQQ / MS method. The chromatograph condition is that the chromatographic column takes a octadecyl-bonded silica gel column as a filling agent, 0.1% formic acid-water as a mobile phase A, 0.1% formic acid-acetonitrile as a mobile phase B, and the volume ratio of the mobile phaseA and the mobile phase B is 80% to 0%:20 to 100%, so that gradient elution is performed. According to the content detection method for determining the effective components in the longshengzhi capsuleby the HPLC-QQQ / MS method, the effective substance components of traditional Chinese medicines such as astragaloside A, hydroxysafflor yellow A, calycosin 7-o-glucoside, calycosin, ferulic Acid, syringin, n-butylphthalide, ligustilide, senkyunolide A, senkyunolide I, senkyunolide H, ligustrazine, isofraxidin, dehydrocostus lactone, amygdalin, syringin E, paeoniflorin, oxypaeoniflora, benzoylpaeoniflorin, etc., in the longshengzhi capsule can be determined. The method has the advantages of being simple in operation method, high in sensitivity and accurate in content.
Owner:SHAANXI BUCHANG PHARMA
Application of senkyunolide I in treating diseases
The invention provides an application of an absorption component of ligusticum wallichii senkyunolide I in treating functional dyspepsia, depression and depression-based dyspepsia. The senkyunolide I is characterized by effectively treating functional dyspepsia, depression and depression-based dyspepsia.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
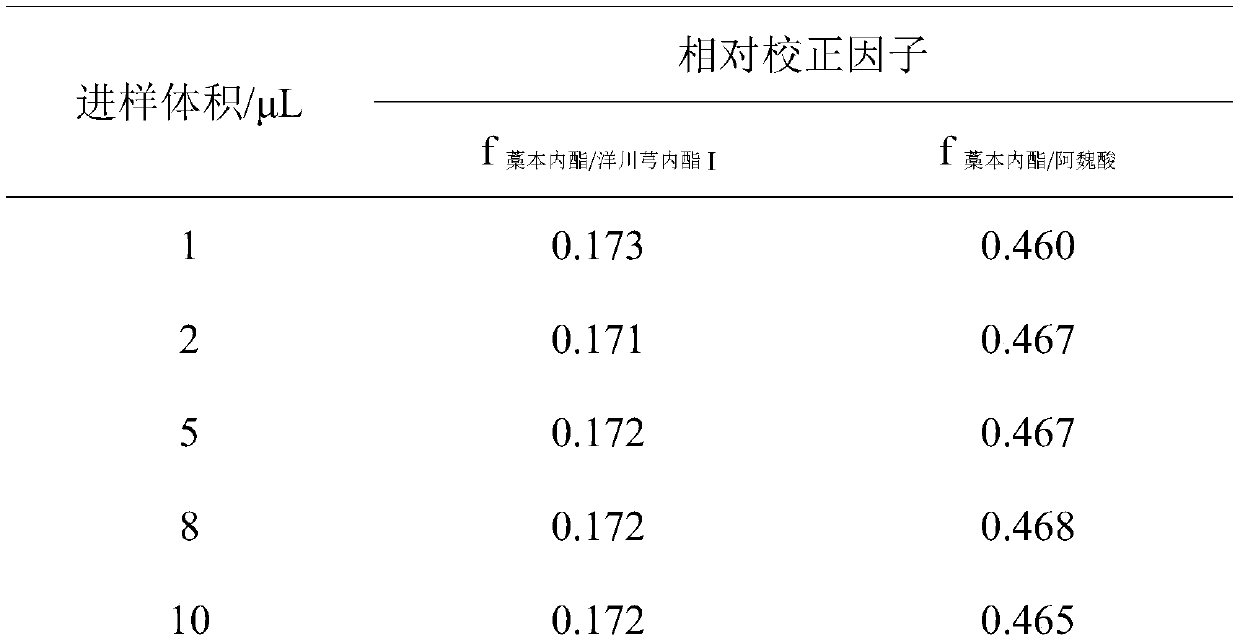
Method for determining components of angelica sinensis pills (concentrated pills) by using quantitative analysis of multiple components by single marker
The invention discloses a method for determining components of angelica sinensis pills (concentrated pills) by adopting a quantitative analysis of multiple components by single marker. According to the method, ligustilide is used as an internal reference substance to perform quantitative analysis of multiple components by single marker, a relative correction factor (f) between ligustilide and ferulic acid and senkyunolide I is established, the content of ferulic acid and senkyunolide I is calculated through the relative correction factor (f), and a liquid chromatography method is used for determination. The method is high in practicability, simple to operate, rapid and accurate in testing, cost-saving, good in repeatability, good in stability and good in durability, and provides a basis for establishing quality evaluation of the angelica sinensis pills (concentrated pills).
Owner:河南省食品药品检验所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com