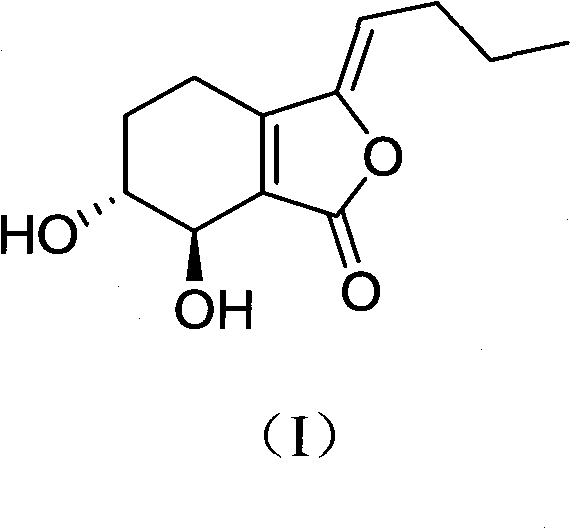
Application of senkyunolide I to medicaments for prevention and treatment of cerebral apoplexy and relevant treatment during convalescence
A technique for yangchuanxiong lactone and cerebral apoplexy, which is applied in the field of new medical use of yangchuanxiong lactone I, and can solve problems such as reducing aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, alcohol extraction method prepares chuanxiong lactone I
[0022] Chuanxiong decoction pieces 1kg, after removing impurities, crushed into powder, 70% ethanol 10L 90 ℃ reflux extraction twice, each time 2 hours, combined extracts, recovered about 8L of solvent to obtain 2L of extract A (equivalent to crude drug 0.5g·ml -1 ). Extract A solution 5000r·min -1 Centrifuge for 15 min, and take the supernatant (the floating oil in the upper layer is discarded). 240g of HPD-100 macroporous resin (equivalent to 80g of dry resin) was wet-packed, the extract A solution was loaded, and the adsorption flow rate was about 0.7L h -1 . After the sample was loaded, it was washed with 3.5 L of distilled water until it was nearly colorless, and then 1.4 L was eluted with 50% 7 alcohol, the alcohol eluate was collected, and the solvent was recovered to obtain extract B. Dissolve extract B in 500ml of distilled water to obtain an aqueous solution of extract B, extract with ...
Embodiment 2
[0023] Embodiment 2. The preparation of Liguscaractone I tablet
[0024] Ingredients Amount per Tablet
[0025] Ligustilide I 100mg
[0026] Microcrystalline Cellulose 37.45mg
[0027] Micronized silica gel 10.5mg
[0028] Stearic acid 17.5mg
[0029] Lactose 10.5mg
[0030] Magnesium Stearate 1.05mg
[0031] According to the prescription quantity, add Ligusticolide I (prepared according to the method of Example 1) into microcrystalline cellulose, micropowder silica gel, stearic acid, lactose and mix evenly to make granules, add magnesium stearate, mix evenly, and press into tablets , That is, (each tablet contains chuanxionglide I 0.1g). The recommended oral dosage for adults is 1-4 tablets / time, 2-3 times a day.
Embodiment 3
[0032] Embodiment 3. The preparation of Yangchuanxiong lactone I capsule
[0033] Ingredients Amount Per Capsule
[0034] Ligustilide I 100mg
[0035] Microcrystalline Cellulose 37.45mg
[0036] Micronized silica gel 10.5mg
[0037] Stearic acid 17.5mg
[0038] Lactose 10.5mg
[0039] Add microcrystalline cellulose, micropowder silica gel, stearic acid, lactose to Ligusticolactone I (prepared by the method of Example 1) according to the prescription amount, and mix evenly, after making granules, fill into No. 0 hard capsule shell, beat Light that is the system (each capsule containing chuanxiong lactone I 0.1g). The recommended oral dosage for adults is 1-4 capsules / time, 2-3 times a day.
[0040] The present invention will be further illustrated by the test example and the pharmacological activity test and results of Ligustilide I below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com