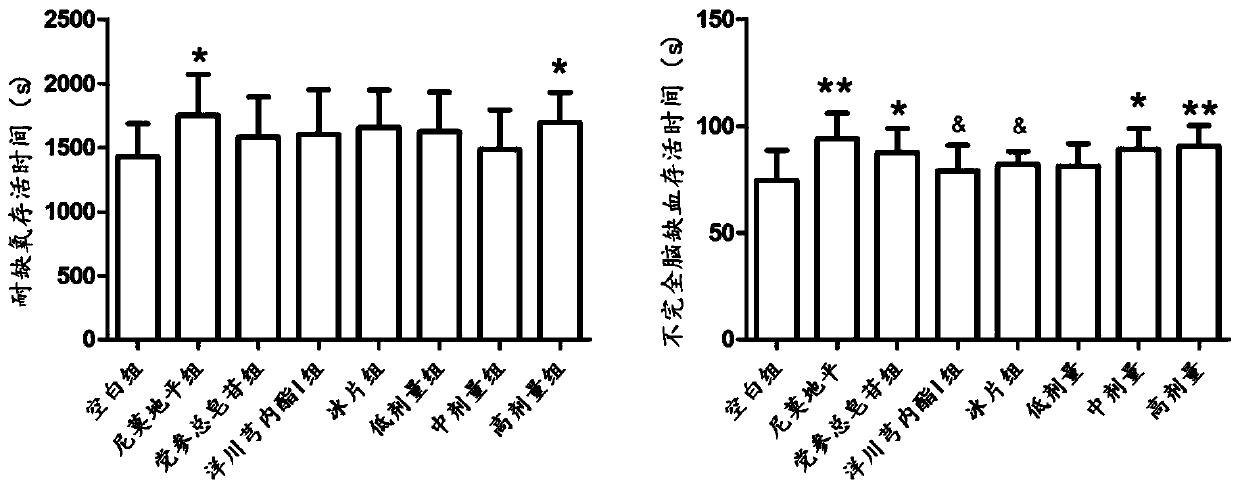
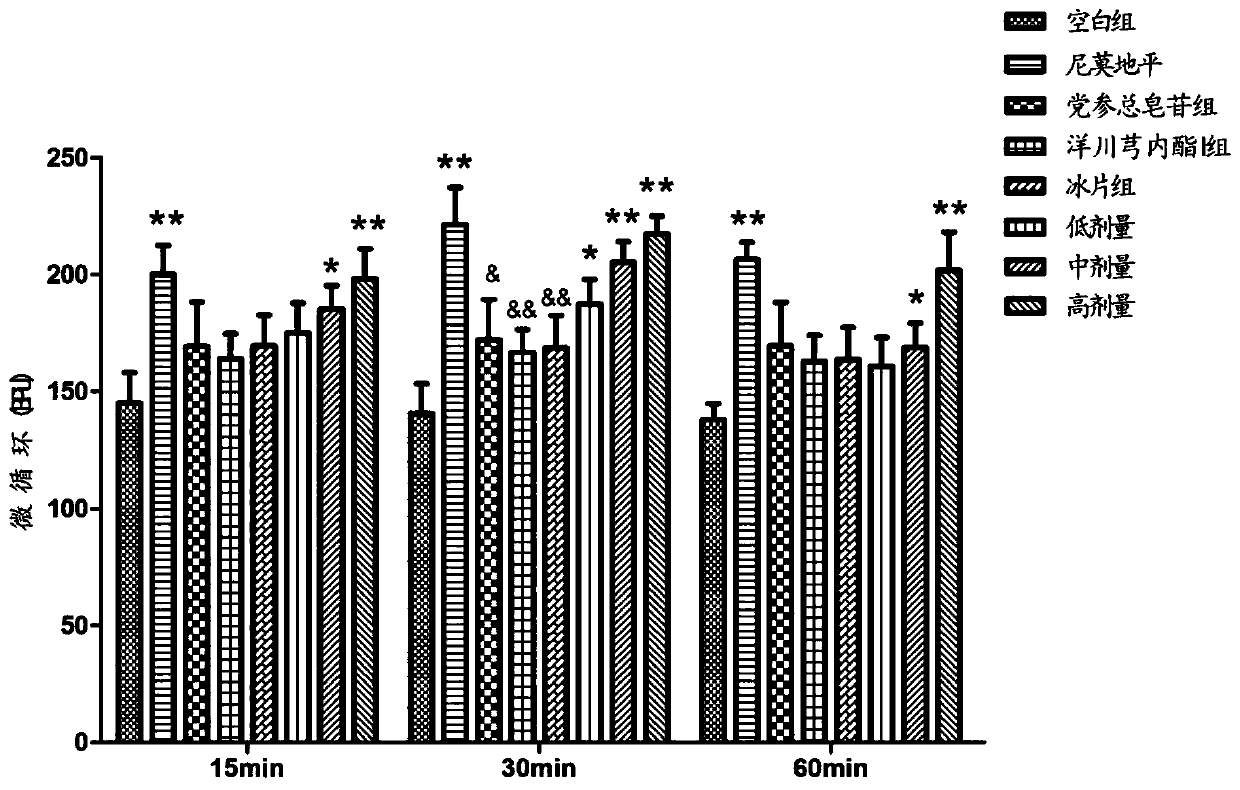
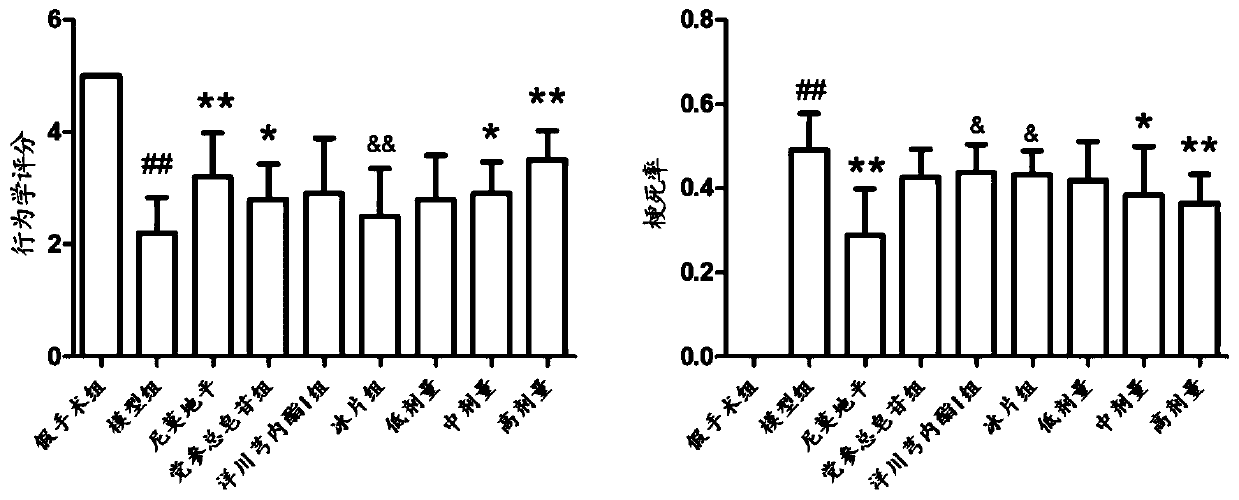
Pharmaceutical composition for treating cerebral arterial thrombosis and application thereof
A technology for ischemic stroke and a composition, applied in the field of medicine, can solve the problems of insufficient drugs, many adverse reactions, poor curative effect, etc. effects of stroke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The sources of the components are as follows.
[0033] The preparation method of the total saponins of Codonopsis Codonopsis: Codonopsis Codonopsis medicinal material is crushed, passed through a 40-mesh sieve, added ethanol with a volume fraction of 70% (according to 1g of powder to add 30mL ratio), soaked for 24h, ultrasonicated at 40°C for 30min, suction filtered, and the filtrate was decompressed to recover the solvent to obtain Codonopsis total saponins extract. The yield of total saponins of Codonopsis pilosula was 1.522%.
[0034] Ligusticolide I reference substance was purchased from Chengdu Croma Biotechnology Co., Ltd., batch number 140827. Borneol was purchased from Beijing Tongrentang Pharmacy, produced by Beijing Sanhe Pharmaceutical Co., Ltd., batch number 77820501.
Embodiment 2
[0036] Preparation of a pharmaceutical composition for treating ischemic cerebral apoplexy: take 4 parts of Codonopsis total saponins, 5 parts of Ligusticolactone I and 11 parts of Borneol. After mixing with 1.5 parts of starch, sieve and dry, add 0.1 part of magnesium stearate and 0.1 part of talcum powder, and press into tablets.
Embodiment 3
[0038] Preparation of a pharmaceutical composition for treating ischemic cerebral apoplexy: 5 parts of Codonopsis total saponins, 6 parts of Ligusticolactone I and 12 parts of Borneol are weighed. After mixing with 1.7 parts of starch, sieve and dry, then add 0.11 parts of magnesium stearate and 0.11 parts of talcum powder, and tablet it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com