A hplc-qqq/ms method for determining the content of active ingredients in Longshengzhi Capsules
A detection method and technology for active ingredients, applied in the field of chemical analysis and detection of Chinese patent medicines, can solve problems such as unclear material basis and inability to comprehensively reflect the quality of Chinese medicines, and achieve the effect of rapid detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
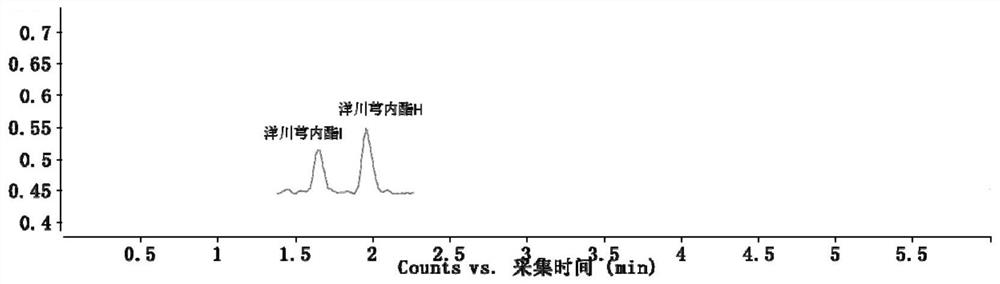
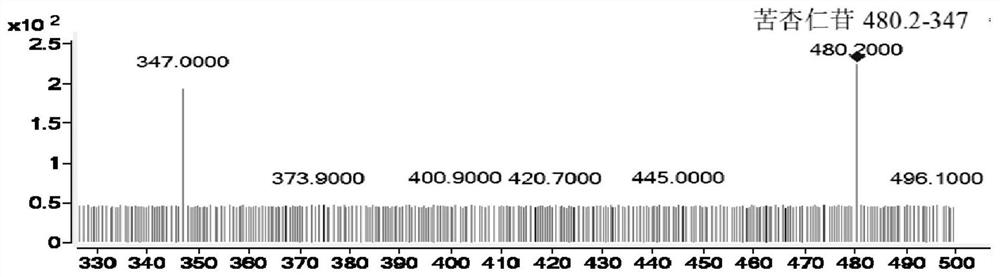
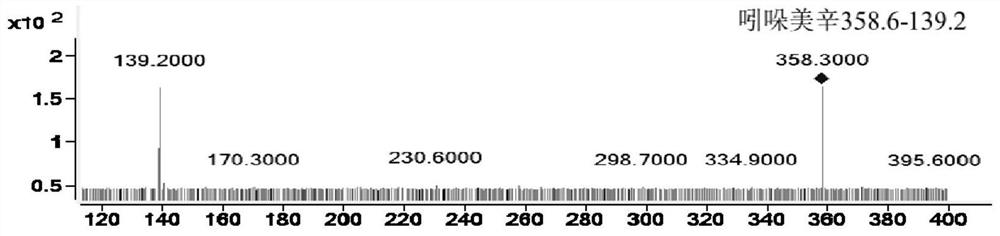
[0071] Example 1: The HPLC-QQQ / MS method established by the present invention is used to determine the content of active ingredients in Longshengzhi Capsules. Detection method 1 Instruments and reagents
[0072] 1.1 Instrument
[0073] Agilent1260 high performance liquid chromatography, Agilent6470 triple quadrupole tandem mass spectrometer (Agilent, USA); analytical balance (Mettler, Switzerland); 3-18KS centrifuge (Sigma, Germany); KQ-500E ultrasonic cleaning machine (Kunshan City Ultrasonic Instruments Ltd).
[0074] 1.2 Reagent
[0075] Reference substances astragaloside IV (H-013-170614), hydroxysafflower yellow A (Q-008-170303), acteosin glucoside (M-020-170929), acteosin (M-021-170517), bitter Amygdalin (K-001-161216), Ferulic Acid (A-002-161216), Dehydrocostalactone (Q-016-170816), Syringin (C-009-161217), Eleutheroside E (C-008-171229), amygdalin (K-001-161216), paeoniflorin (S-010-170214), oxidized paeoniflorin (Q-019-180315), benzoyl paeoniflorin (B-024 -171216), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com