Application of senkyunolide I in sepsis lung injury treatment medicine
A technology of chuanxiong lactone and therapeutic drugs, which is applied in the field of medicine and biology, can solve the problems that it is difficult to accurately describe the therapeutic effect of a single molecule and increase the incidence of adverse drug reactions, so as to inhibit platelet activation, reduce adverse drug reactions, and reduce the incidence Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This example provides the application of Liguscaractone I in the treatment of septic lung injury.
[0028] The drug for treating sepsis lung injury includes ligustilide I and a pharmaceutically acceptable carrier.
[0029] The percentage by weight of ligustilide I in the drug for treating sepsis lung injury is 0.1-99.9%.
[0030] The chemical structure of ligustilide I is as follows:
[0031]
[0032] Its molecular formula is C 12 h 16 o 4 , the molecular weight is 224.256.
[0033] The drug dosage forms for the treatment of sepsis lung injury are tablets, sugar-coated tablets, film-coated tablets, enteric-coated tablets, capsules, hard capsules, soft capsules, oral liquids, buccal preparations, granules, granules, Any one of pills, powders, ointments, elixirs, suspensions, powders, solutions, injections, suppositories, ointments, plasters, creams, sprays, drops and patches.
[0034] That is to say, the drug dosage form for the treatment of sepsis lung injury c...
Embodiment 2
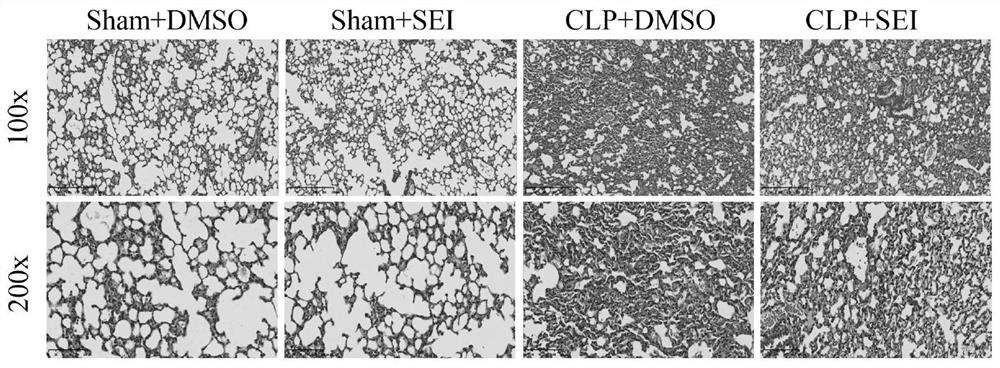
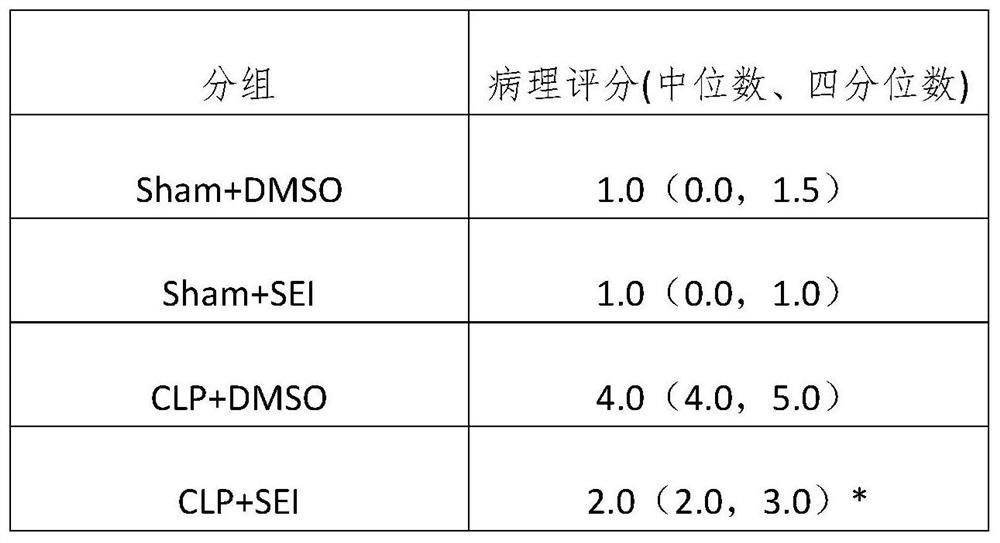
[0047] This example is a pharmacodynamic study on the therapeutic effect of ligustilide I on the sepsis animal model on sepsis lung injury.
[0048] 2.1 Experimental materials
[0049] 48 male clean-grade C57BL / 6 mice were divided into 4 groups, 12 in each group, 22-25g, ligustonin I (purchased from Shanghai Zheyan Biotechnology Co., Ltd.), sevoflurane, dimethyl sulfoxide , hematoxylin and eosin stain, BCA kit, MPO fluorescent antibody.
[0050] 2.2 Experimental method
[0051] After the mice were anesthetized with sevoflurane, they were laparotomed through a midline abdominal incision, the cecum was exposed, ligated at 1 / 2 of the cecum, and pierced with a 22G needle. A small amount of intestinal content was squeezed out and the abdomen was closed layer by layer. In the control group, the cecum was exposed in the same manner but the cecum was not ligated or perforated. The experimental mice were divided into sham operation dimethyl sulfoxide group, sham operation ligustonin...
Embodiment 3
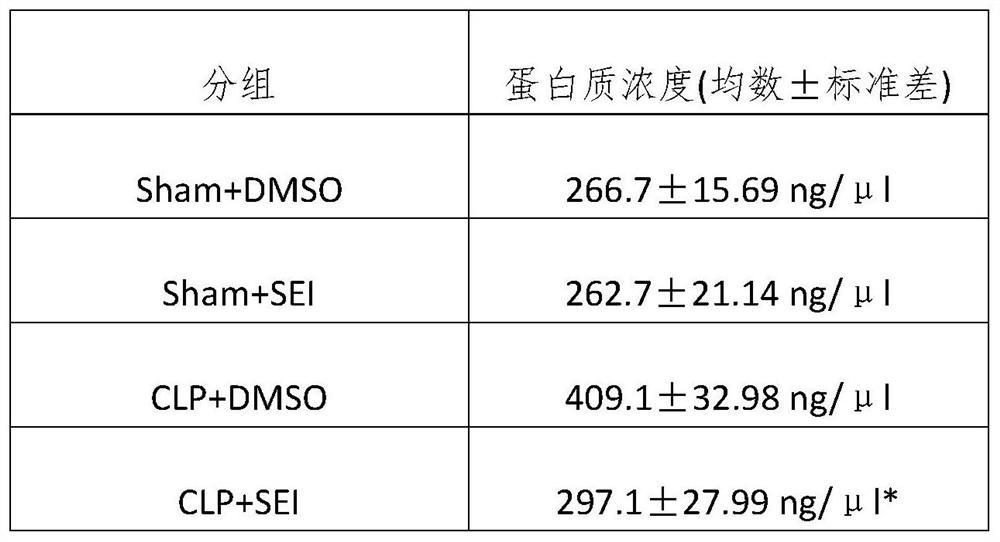
[0061] This example is a pharmacodynamic study on the inhibitory effect of ligustilide I on lung tissue inflammatory factors and oxidative stress levels in a sepsis animal model.
[0062] 3.1 Experimental materials
[0063] 24 male clean-grade C57BL / 6 mice were divided into 4 groups, 6 mice in each group, 22-25g, ligustonin I (purchased from Shanghai Zheyan Biotechnology Co., Ltd.), sevoflurane, dimethyl sulfoxide , TNF-α, IL-1β, IL-6 ELISA kit, MDA kit.
[0064] 3.2 Experimental method
[0065] After the mice were anesthetized with sevoflurane, they were laparotomed through a midline abdominal incision, the cecum was exposed, ligated at 1 / 2 of the cecum, and pierced with a 22G needle. A small amount of intestinal content was squeezed out and the abdomen was closed layer by layer. In the control group, the cecum was exposed in the same manner but the cecum was not ligated or perforated. The experimental mice were divided into sham operation dimethyl sulfoxide group, sham op...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com