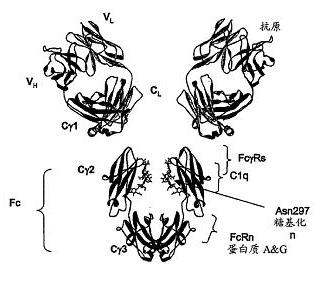
Fusion protein for treating diabetes and preparation method for fusion protein
A fusion protein and diabetes technology, applied in the field of diabetes treatment drugs, can solve the problems of adverse reactions, large effective doses, and high production costs, and achieve the effects of prolonging the half-life of drugs, reducing the number of administrations, and avoiding adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
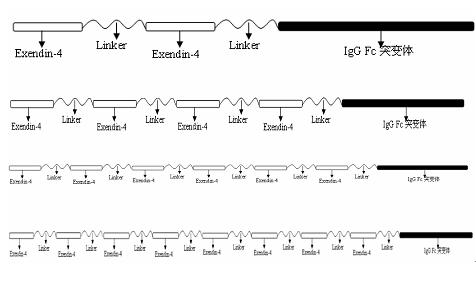
[0082] like figure 2 As shown, in this example, the fusion protein used to treat diabetes is a fusion protein composed of two Exendin-4-Linkers and human IgG Fc mutants; the Linker flexible peptide is (Gly 4 Ser) n, n=1-5; human IgG Fc mutant is IgG1 Fc mutant, and the amino acid mutation position of IgG1 Fc mutant is Glu233Pro / Leu234Val / Leu235Ala / ΔGly236; combined mutation position of Ala327Gly / Ala330Ser / Pro331Ser, wherein Mutation positions are numbered according to the EU index.
[0083] Among them, the Linker connected to Exendin-4 at both ends is a flexible peptide segment (Gly 4 Ser) n, n=3;
[0084] Among them, the Linker whose one end is connected to Exendin-4 and the other end is connected to the human IgG1 Fc Fc mutant is a flexible peptide (Gly 4 Ser) n, n=1.
Embodiment 2
[0086] like figure 2 As shown, in this example, the fusion protein used to treat diabetes is a fusion protein composed of two Exendin-4-Linkers and human IgG Fc mutants; the Linker flexible peptide is (Gly 4 Ser) n, n=1-5; the human IgG Fc mutant is an IgG1 mutant, and the amino acid mutation position of the IgG1 Fc mutant is Glu233Pro / Leu234Val / Leu235Ala / ΔGly236; the combined mutation position of Ala327Gly / Ala330Ser / Pro331Ser, wherein the mutation Position numbers are according to the EU index.
[0087]Among them, the Linker connected to Exendin-4 at both ends is a flexible peptide segment (Gly 4 Ser) n, n=4;
[0088] Among them, the Linker whose one end is connected to Exendin-4 and the other end is connected to the human IgG1 Fc mutant is a flexible peptide (Gly 4 Ser) n, n=2.
Embodiment 3
[0090] like figure 2 As shown, in this example, the fusion protein used to treat diabetes is a fusion protein composed of two Exendin-4-Linkers and human IgG Fc mutants; the Linker flexible peptide is (Gly4Ser)n, n=1-5; Human IgG Fc mutants are IgG1 mutants, and the amino acid mutation positions of IgG1 Fc mutants are the combined mutation positions of Glu233Pro / Leu234Val / Leu235Ala / ΔGly236; Ala327Gly / Ala330Ser / Pro331Ser, wherein the mutation positions are numbered according to the EU index.
[0091] Among them, the Linker connected to Exendin-4 at both ends is a flexible peptide segment (Gly 4 Ser) n, n=3;
[0092] Among them, the Linker whose one end is connected to Exendin-4 and the other end is connected to the human IgG1 Fc mutant is a flexible peptide (Gly 4 Ser) n, n=3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com