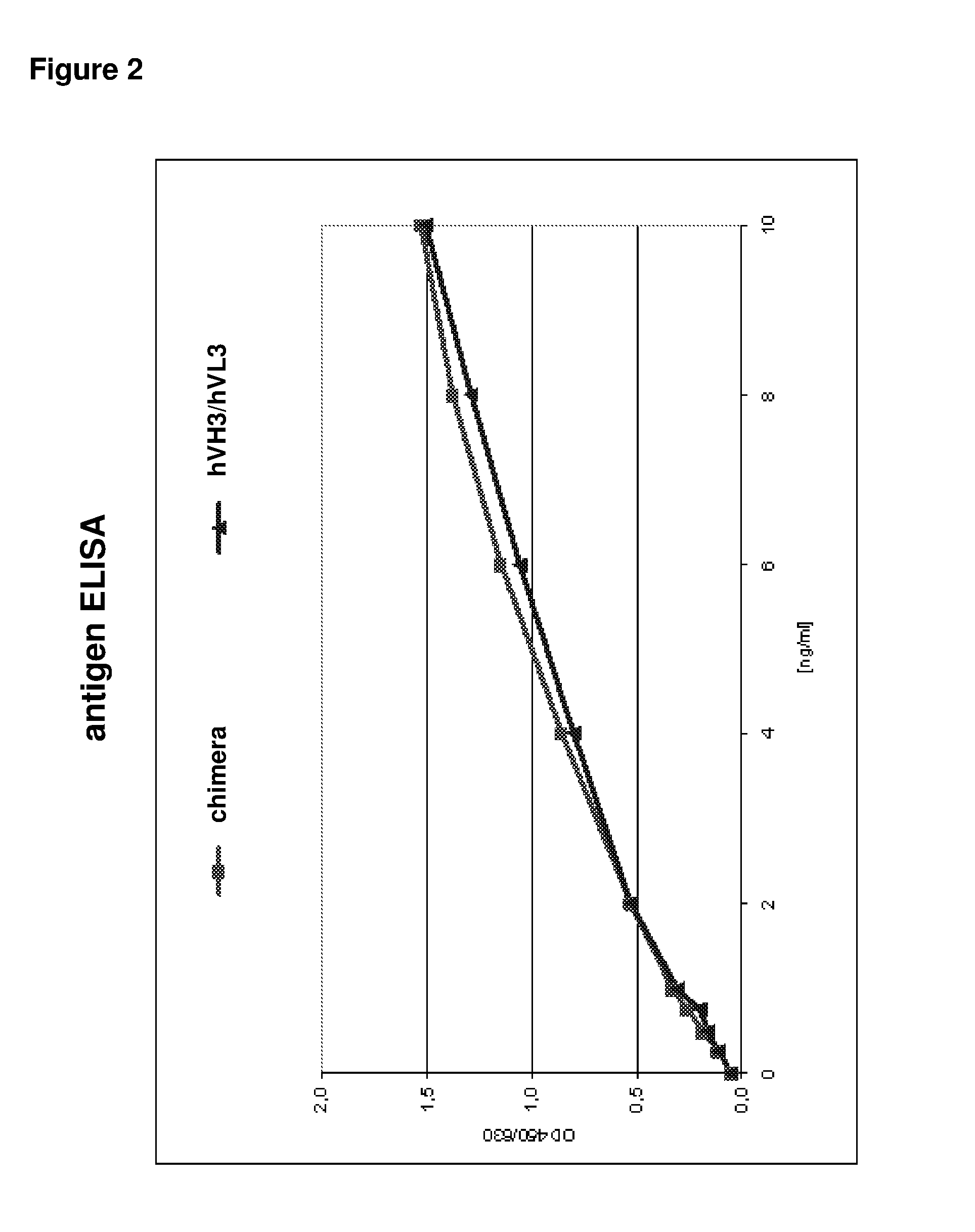
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75 results about "Anti-EGFR Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
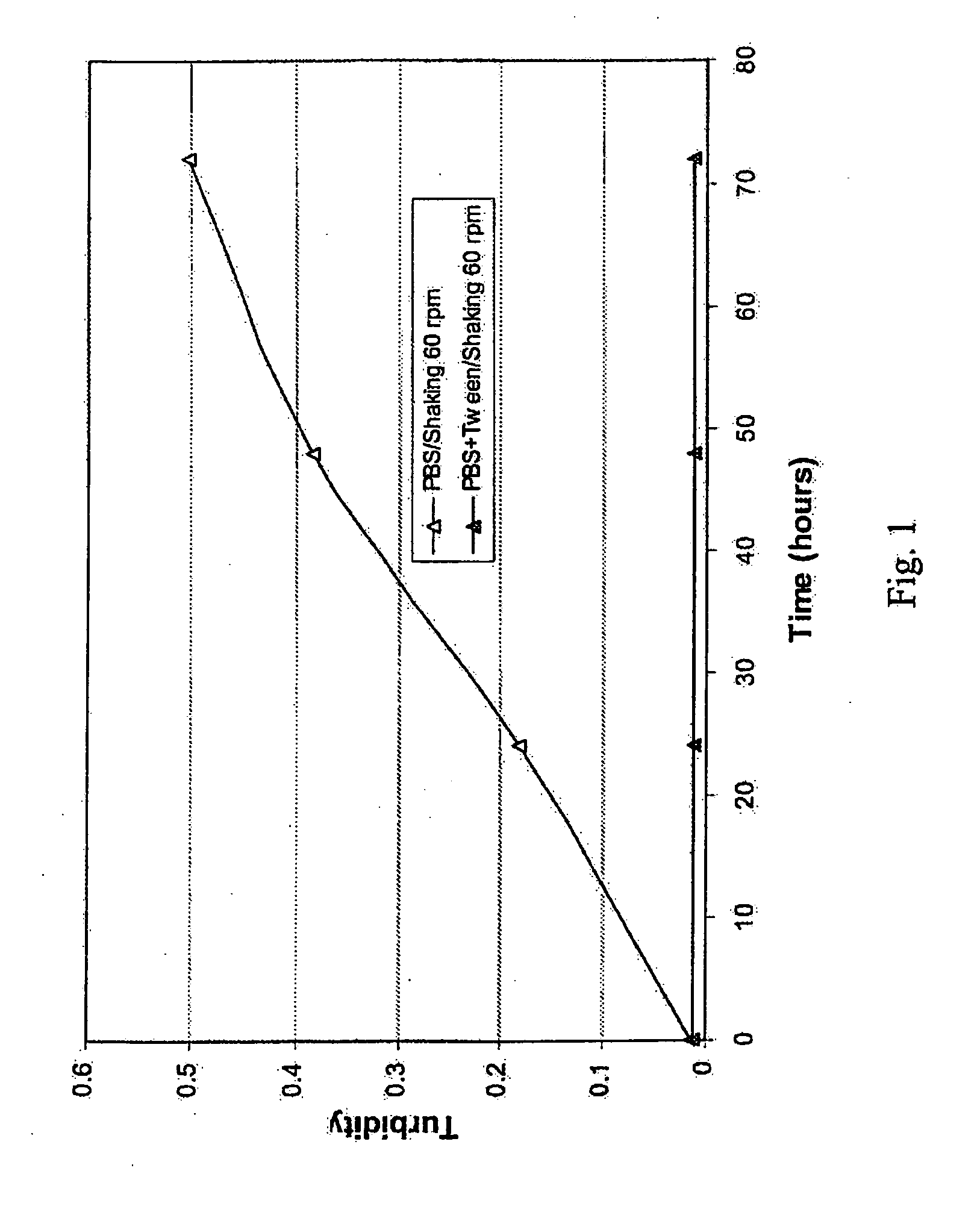
Highly concentrated, liquid formulations of anti-egfr antibodies
InactiveUS20070172475A1Reduce tendency to aggregateLow viscositySenses disorderAntipyreticUltrafiltrationMonoclonal antibody
The invention relates to processes for the preparation of highly concentrated, liquid formulations comprising at least one anti-EGFR antibody and / or one of its variants and / or fragments, in particular monoclonal antibodies against the EGF receptor, particularly preferably of Mab C225 (cetuximab) and Mab h425 (EMD 72000), by ultrafiltration. The invention furthermore relates to highly concentrated, liquid formulations of anti-EGFR antibodies, in particular of monoclonal antibodies against the EGF receptor, particularly preferably Mab C225 (cetuximab) and Mab h425 (EMD 72000) and / or variants and / or fragments thereof, characterised in that the highly concentrated, liquid formulations have a content of anti-EGFR antibodies of 10-250, preferably 50-180 mg / ml, particularly preferably of 100-150 mg / ml, and to the use thereof.
Owner:MERCK PATENT GMBH
Use of Anti-EGFR antibodies in treatment of EGFR mutant mediated disease
ActiveUS20100166744A1Slow tumor growthReducing and inhibiting growth of tumorAntibody ingredientsImmunoglobulinsDiseaseTyrosine-kinase inhibitor
The present invention relates to the treatment of EGFR-mediated disease, particularly cancer, which is resistant to tyrosine kinase inhibitor therapies. Methods for treatment of cancer and reduction of tumor growth in individuals with secondary EGFR mutations, particularly tyrosine kinase domain mutations, resistant to standard therapy are provided. The invention provides methods for the treatment of tyrosine kinase inhibitor resistant cancers with anti-EGFR antibodies. Methods for treatment of recurrent lung cancer, including non-small cell lung carcinoma which is resistant to tyrosine kinase inhibitors, with the antibody anti-EGFR mAb806 are described.
Owner:DANA FARBER CANCER INST INC
Novel anti-IGF-IR antibodies and uses thereof
InactiveUS20080193445A1Stimulate interestPromote secretionMicrobiological testing/measurementImmunoglobulins against growth factorsDiseaseAnticarcinogen
The present invention relates to novel antibodies capable of binding specifically to the human insulin-like growth factor I receptor IGF-IR and / or capable of specifically inhibiting the tyrosine kinase activity of said IGF-IR receptor, especially monoclonal antibodies of murine, chimeric and humanized origin, as well as the amino acid and nucleic acid sequences coding for these antibodies. The invention likewise comprises the use of these antibodies as a medicament for the prophylactic and / or therapeutic treatment of cancers overexpressing IGF-IR or any pathology connected with the overexpression of said receptor as well as in processes or kits for diagnosis of illnesses connected with the overexpression of the IGF-IR receptor. The invention finally comprises products and / or compositions comprising such antibodies in combination with anti-EGFR antibodies and / or compounds and / or anti-cancer agents or agents conjugated with toxins and their use for the prevention and / or the treatment of certain cancers.
Owner:INST DE RECH PIERRE FABRE +1
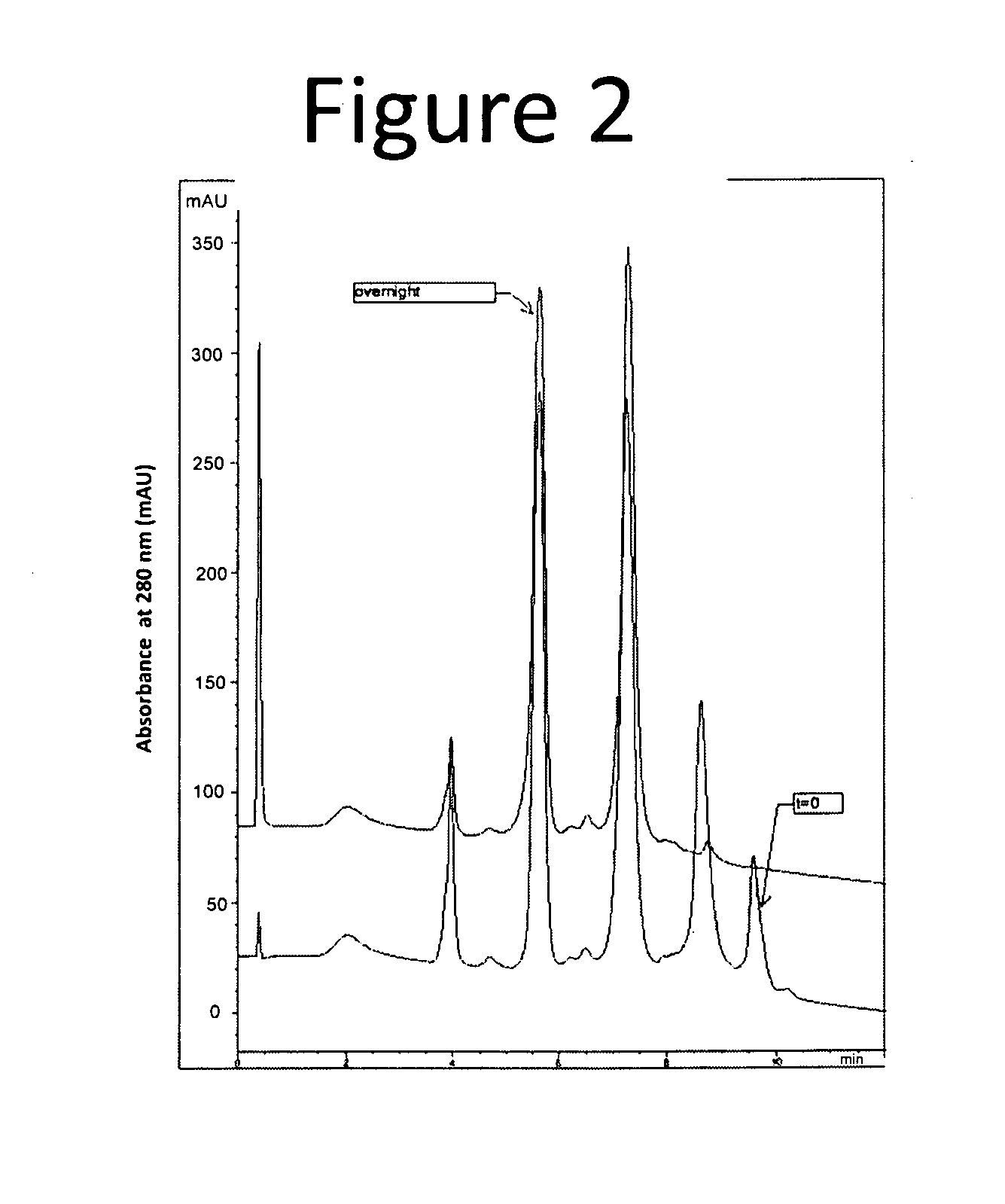
Antibody drug conjugate (ADC) purification
InactiveUS20140286968A1High drug loadingEfficient separationDipeptide ingredientsImmunoglobulinsAnti-EGFR AntibodyAntibody
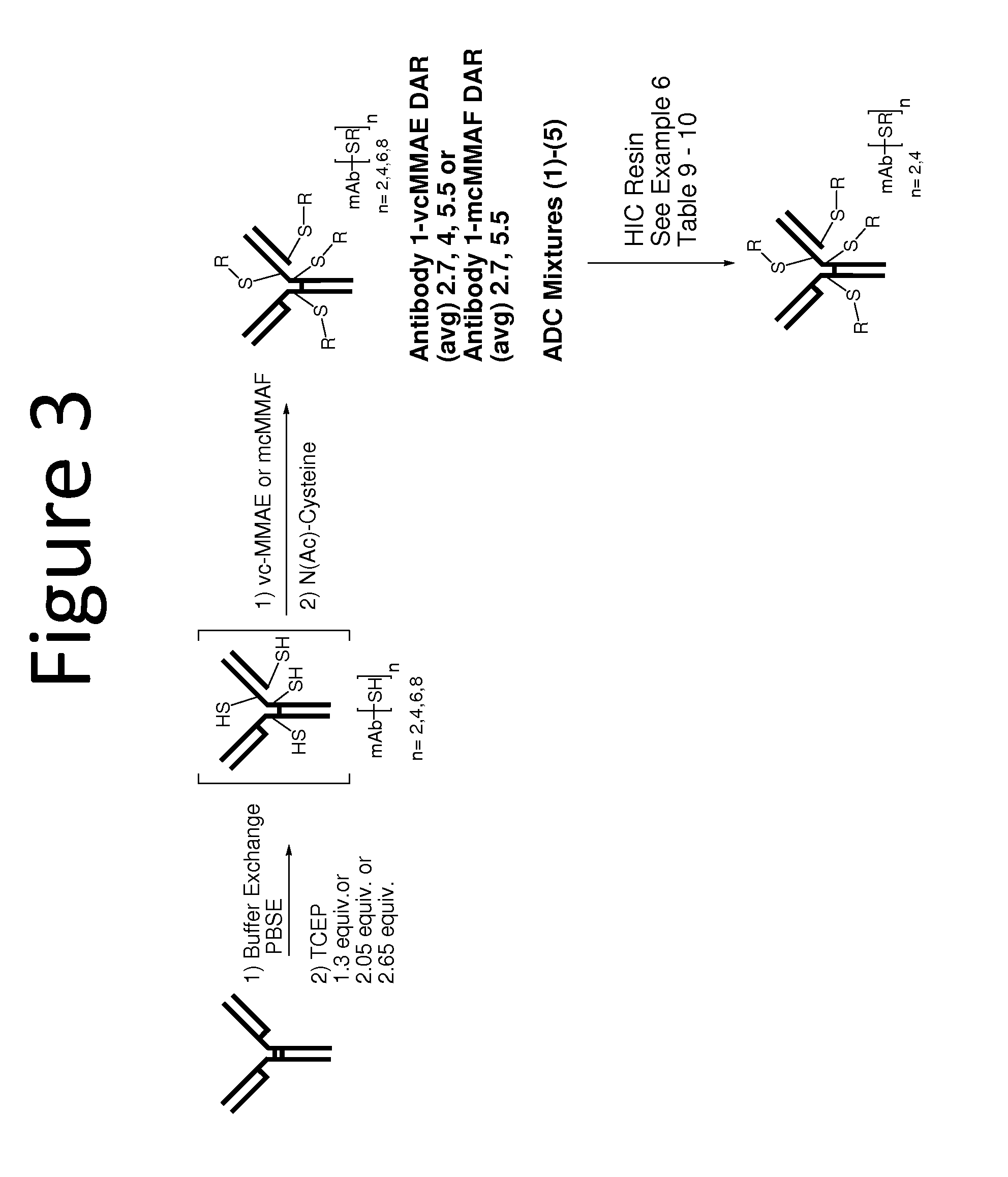
The invention provides methods for obtaining compositions having antibody drug conjugates (ADCs) with specified drug to antibody ratios (DARs). Included in the invention is a method for purifying an ADC mixture having ADCs with a drug loaded species of 6 or more by contacting the mixture with a hydrophobic resin such that a composition comprising less than 15% of the 6 or more drug loaded species is obtained. The invention also provides a composition wherein 70% or more of the ADCs present have a drug loaded species of 4 or less, wherein the ADC comprises an anti-EGFR antibody and an auristatin.
Owner:ABBVIE INC
Solid forms of anti-EGFR antibodies
InactiveUS7960516B2Improve stabilityHigh purityPowder deliveryFrom normal temperature solutionsAdjuvantDissolution
The invention relates to solid forms of antibodies against the EGF receptor, in particular precipitates and crystals of monoclonal antibodies against the EGF receptor, particularly preferably of Mab C225 (cetuximab) and Mab h425 (EMD 72000), which result in biologically active antibody protein through dissolution or suspension in aqueous medium, obtainable by precipitation of the antibody and / or one of its variants and / or fragments dissolved or suspended in aqueous medium by means of a precipitation reagent. The invention furthermore relates to pharmaceutical preparations comprising at least one solid form of above-mentioned antibodies in precipitated non-crystalline, precipitated crystalline or in soluble or suspended form, and optionally excipients and / or adjuvants and / or further pharmaceutical active ingredients, and to a process for the preparation of solid forms of anti-EGFR antibodies according to the invention.
Owner:MERCK PATENT GMBH
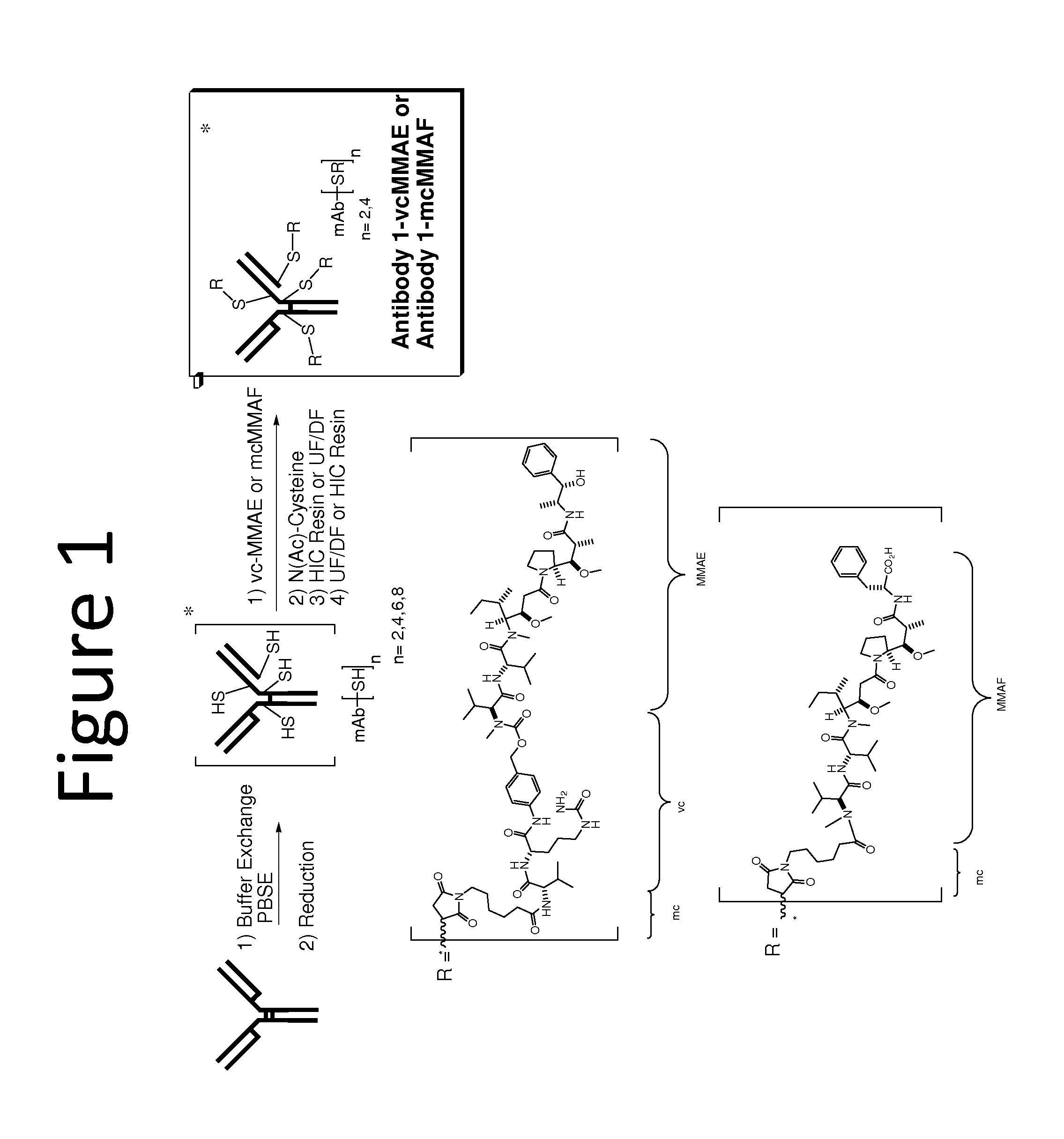
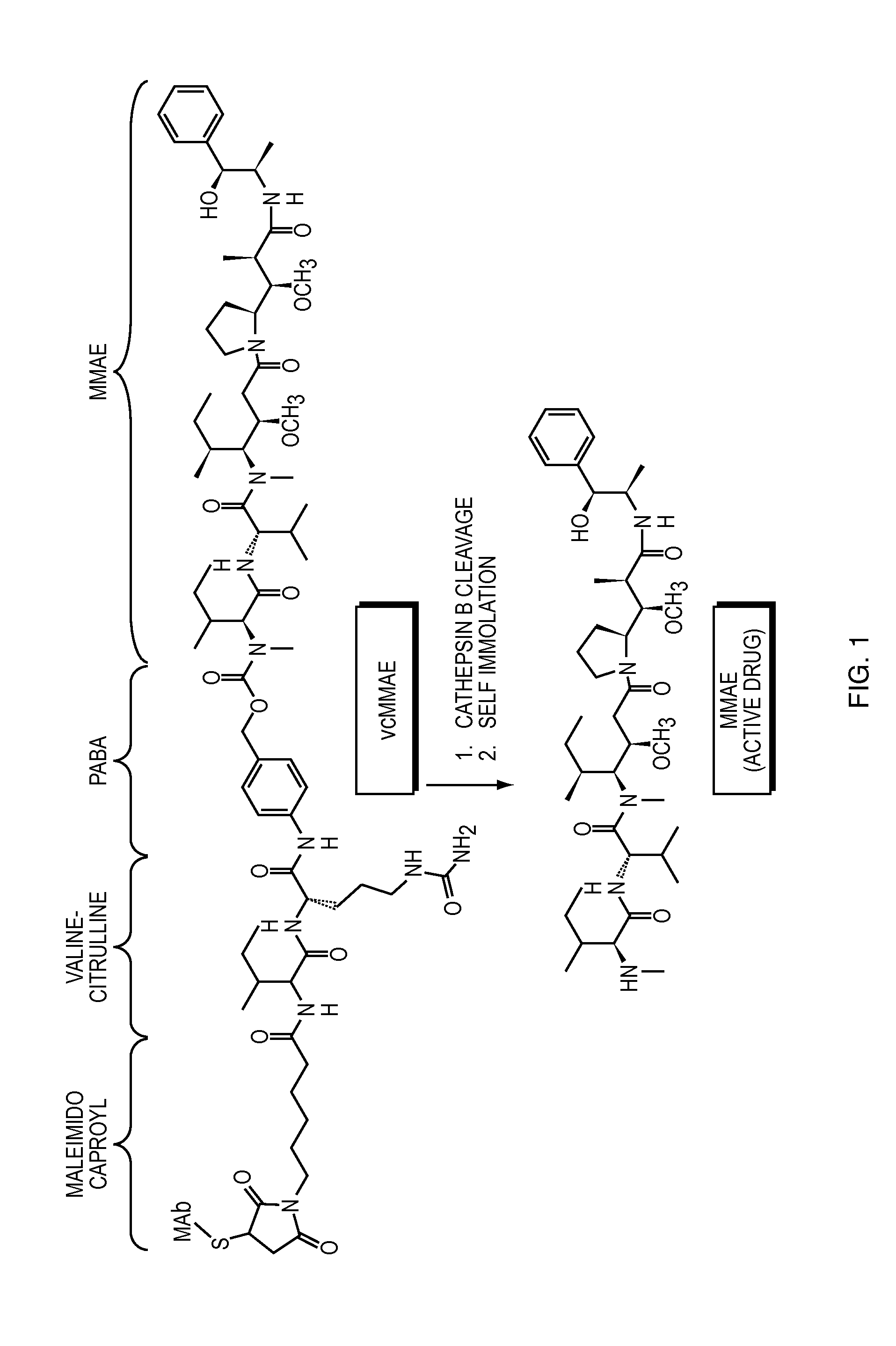
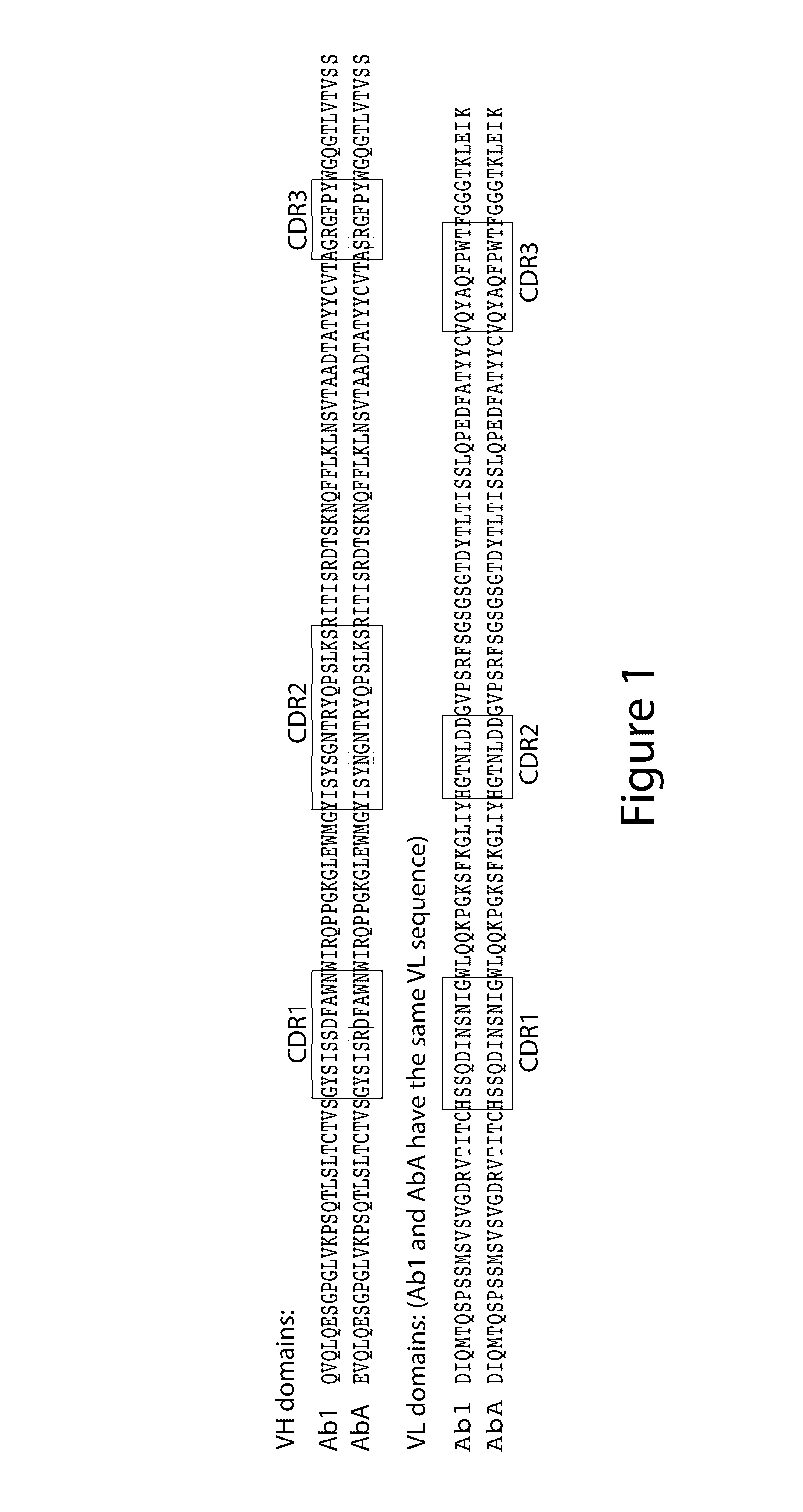
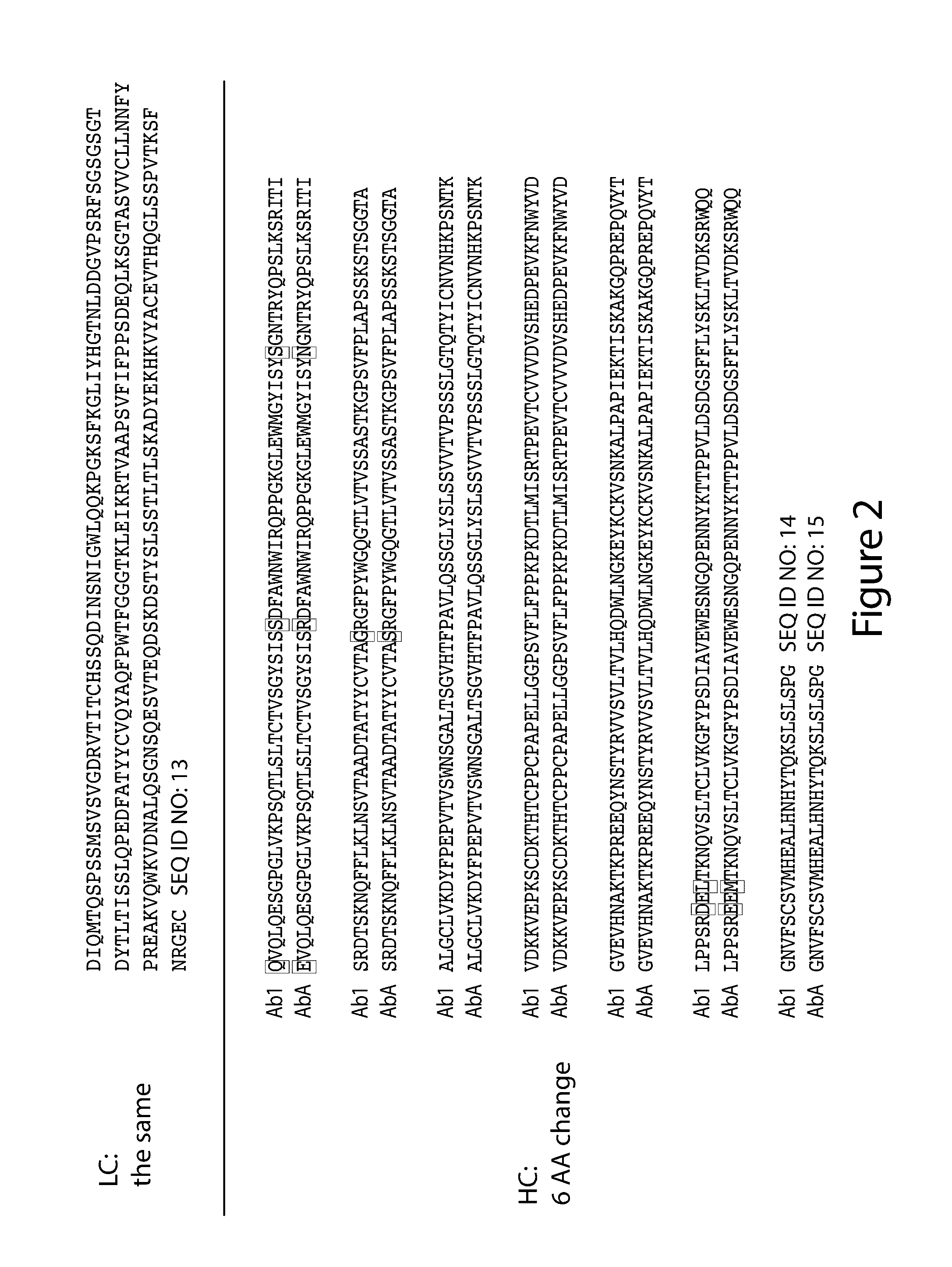
Anti-egfr antibodies and antibody drug conjugates
The invention relates to anti-epidermal growth factor (EGFR) antibodies and antibody drug conjugates (ADCs), including compositions and methods of using said antibodies and ADCs.
Owner:ABBVIE INC
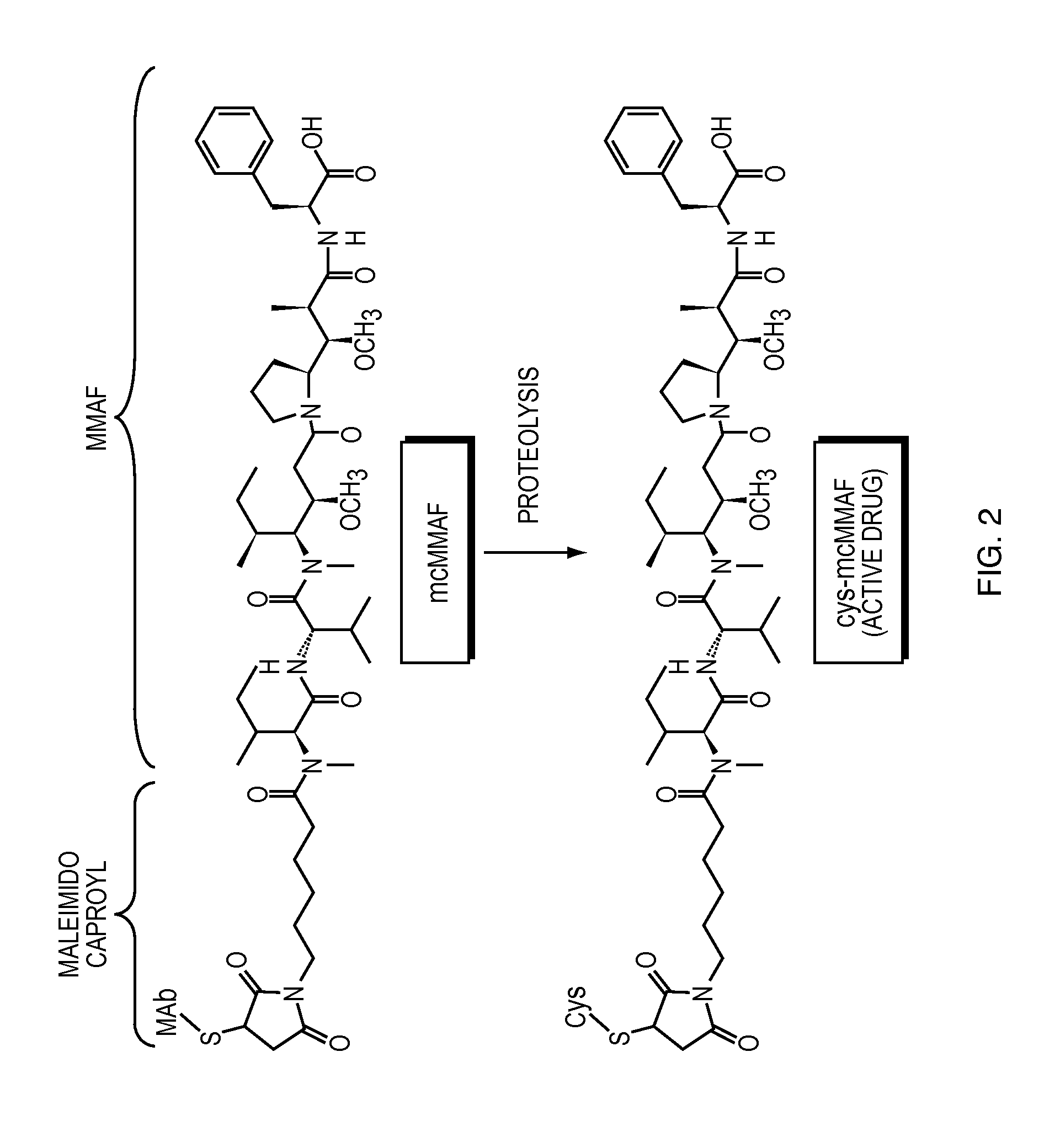
Anti-egfr antibody drug conjugate formulations
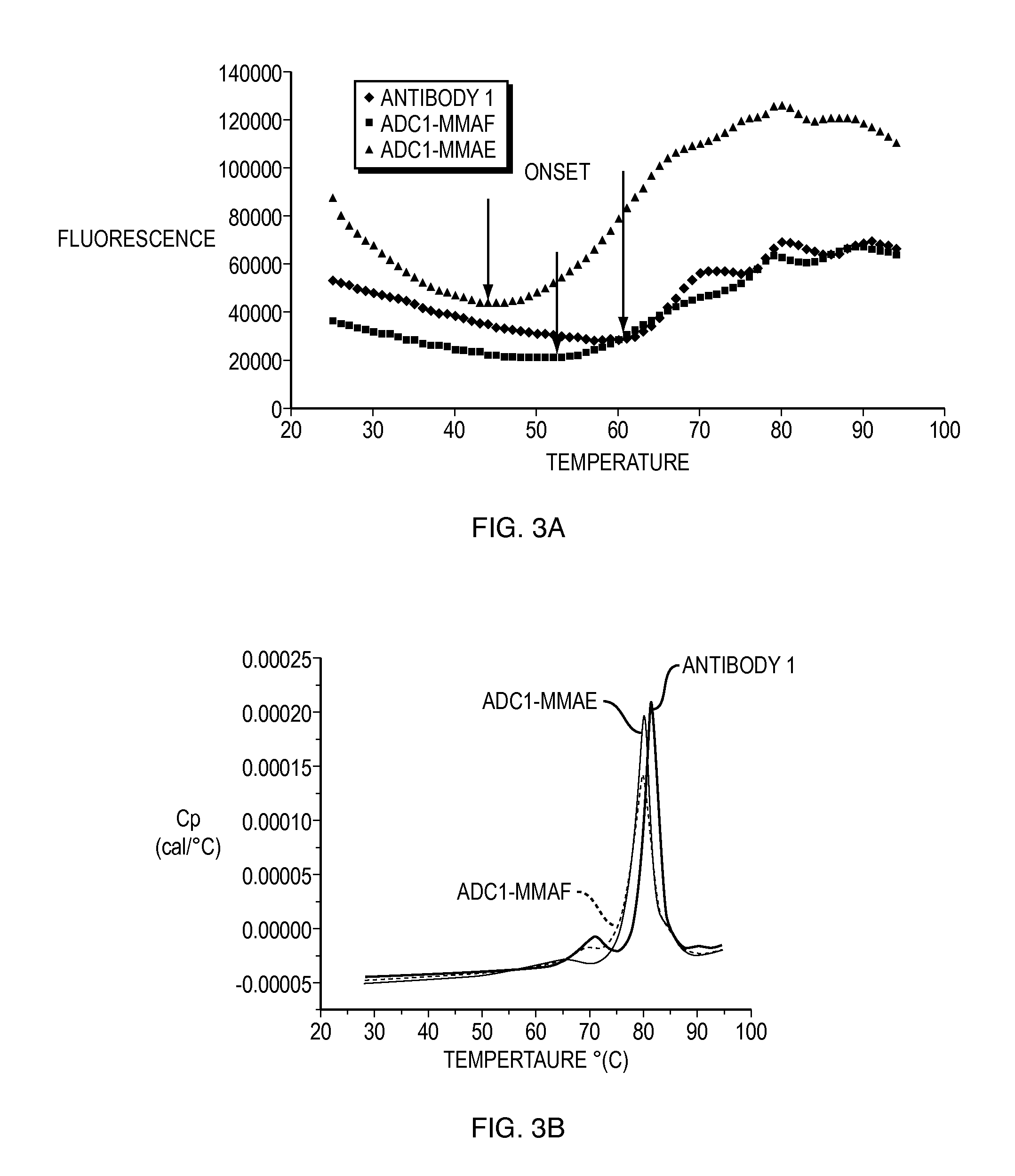
The invention provides a stable formulation comprising an anti-EGFR antibody drug conjugate (ADC), including an anti-EGFR antibody, e.g., antibody 1, conjugated to an auristatin, e.g., MMAF, histidine, a sugar, and a surfactant.
Owner:ABBVIE INC +1
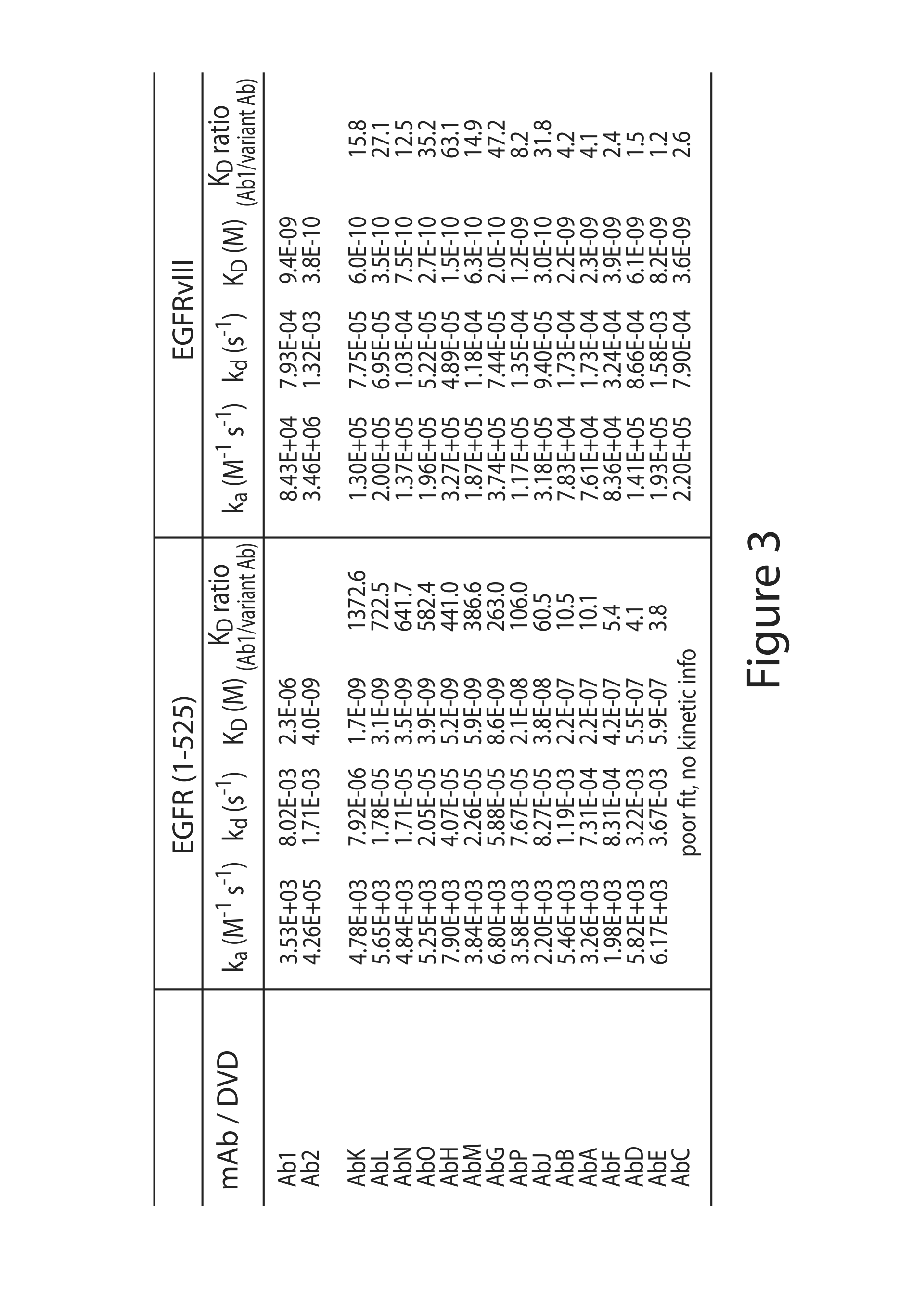
Anti-EGFR antibodies and antibody drug conjugates
ActiveUS9493568B2In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsAnti-EGFR AntibodyAntibody
Owner:ABBVIE INC
Solid forms of anti-egfr antibodies
InactiveUS20070122411A1Improve stabilityHigh purityPowder deliveryFrom normal temperature solutionsAdjuvantDissolution
The invention relates to solid forms of antibodies against the EGF receptor, in particular precipitates and crystals of monoclonal antibodies against the EGF receptor, particularly preferably of Mab C225 (cetuximab) and Mab h425 (EMD 72000), which result in biologically active antibody protein through dissolution or suspension in aqueous medium, obtainable by precipitation of the antibody and / or one of its variants and / or fragments dissolved or suspended in aqueous medium by means of a precipitation reagent. The invention furthermore relates to pharmaceutical preparations comprising at least one solid form of above-mentioned antibodies in precipitated non-crystalline, precipitated crystalline or in soluble or suspended form, and optionally excipients and / or adjuvants and / or further pharmaceutical active ingredients, and to a process for the preparation of solid forms of anti-EGFR antibodies according to the invention.
Owner:MERCK PATENT GMBH
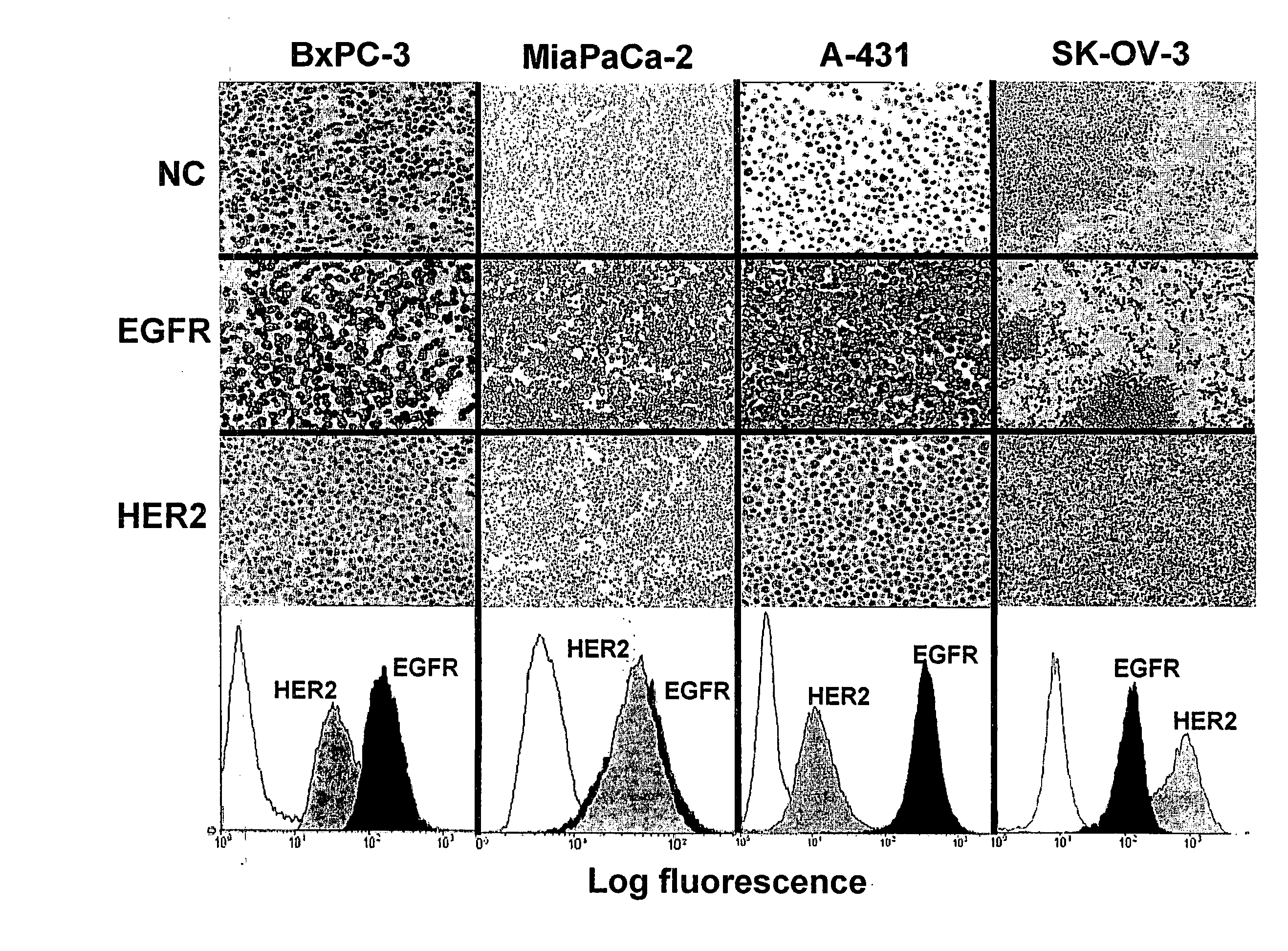
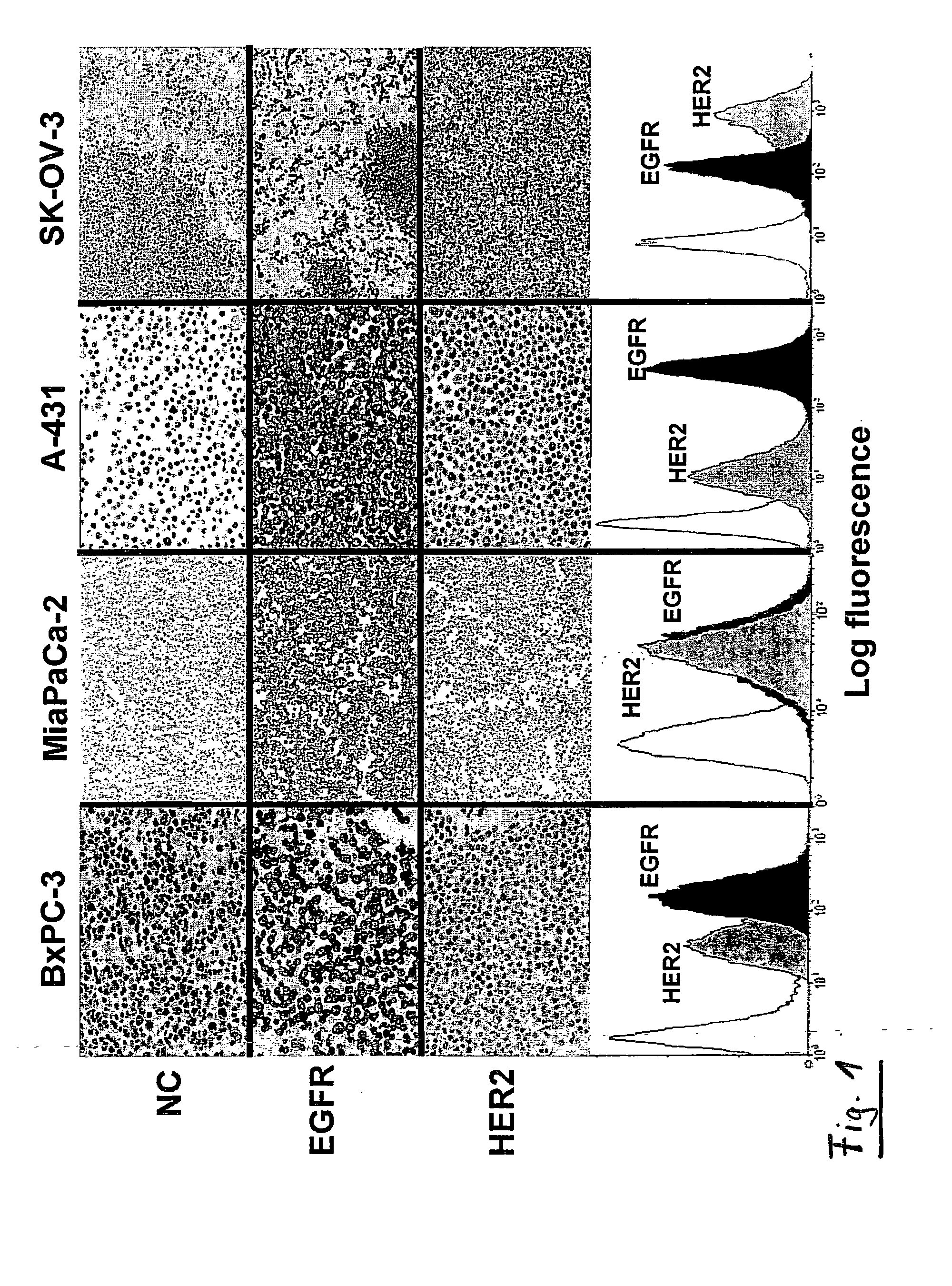
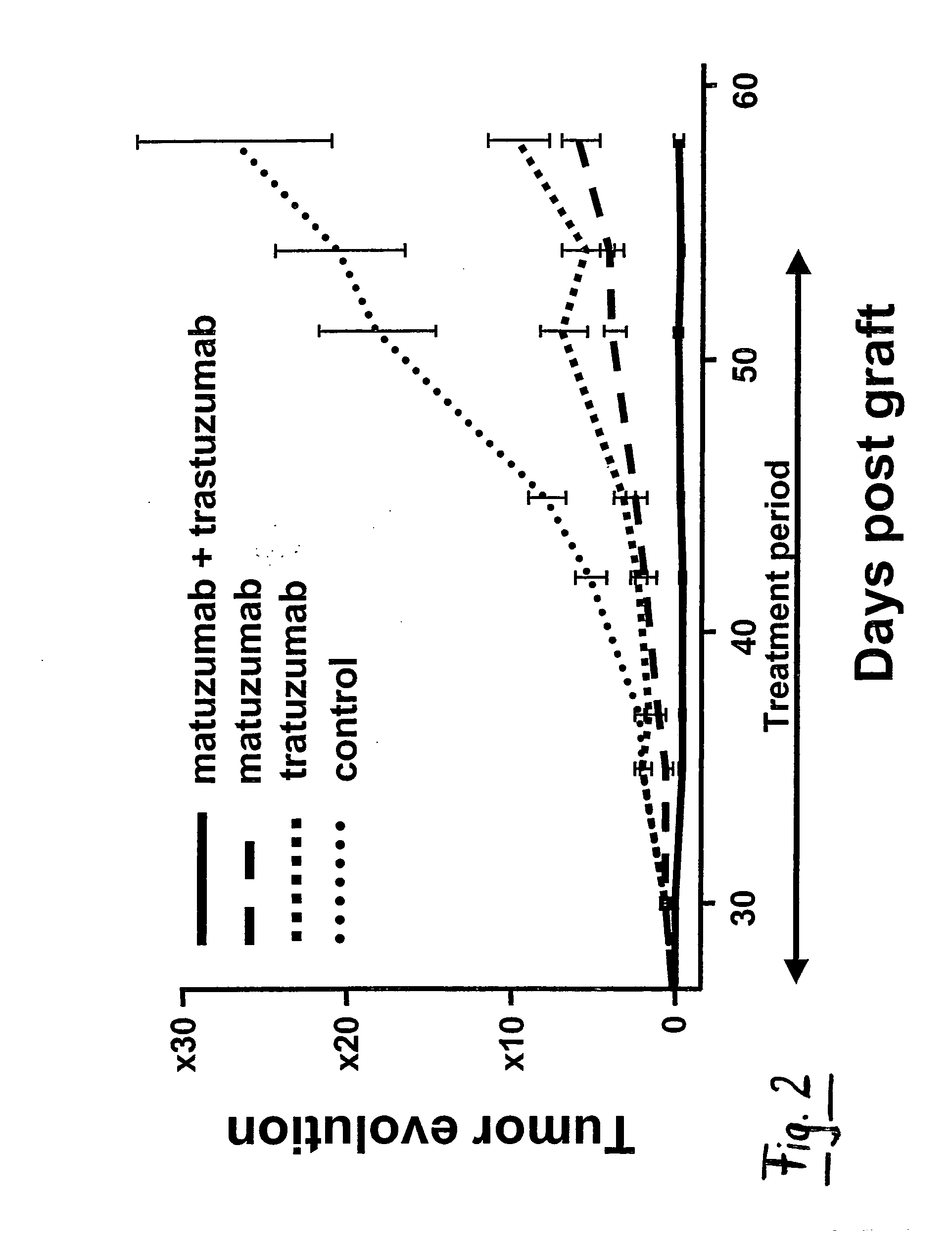
Combination Therapy Using Anti-EGFR and Anti-HER2 Antibodies
InactiveUS20090214541A1Good curative effectPrevent dimerizationAntibody ingredientsImmunoglobulinsEGFR AntibodyAnti her2
The invention relates to the combined use of anti-EGFR antibodies and anti-Her2 antibodies for the treatment of cancer, especially suitable for cancer expressing high levels of the EGFR type and low levels of HER2. The invention refers in particular monoclonal antibody “trastuzumab” (HERCEPTIN®) directed against the HER2 receptors the efficacy of which can be significantly increased in vivo when combined with monoclonal antibody “matuzumab” (hmAB 425, EMD 72000) directed against EGF receptors. The combination treatment is suitable for patients suffering from cancer having said receptor profile, preferably pancreatic cancer.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
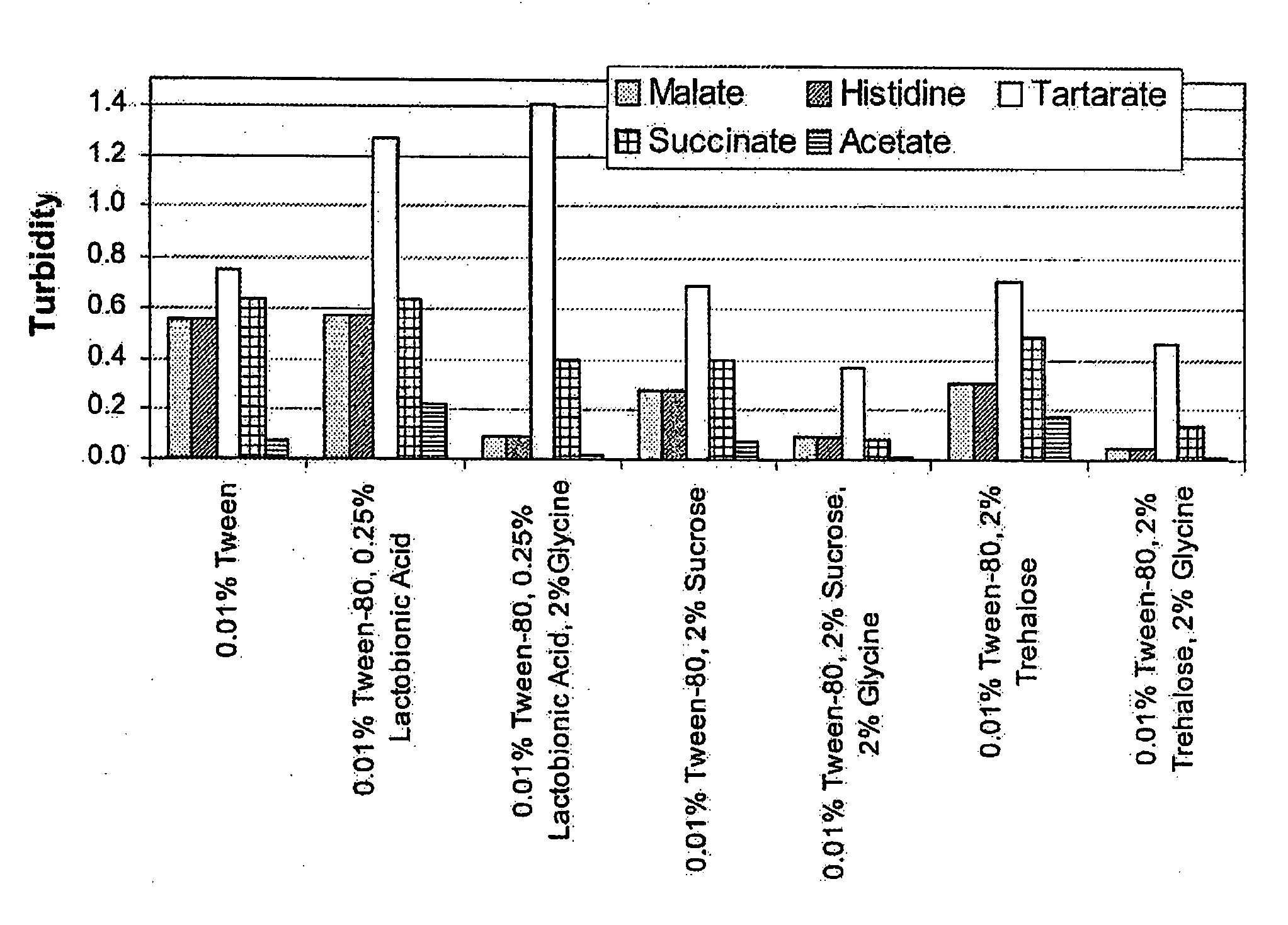
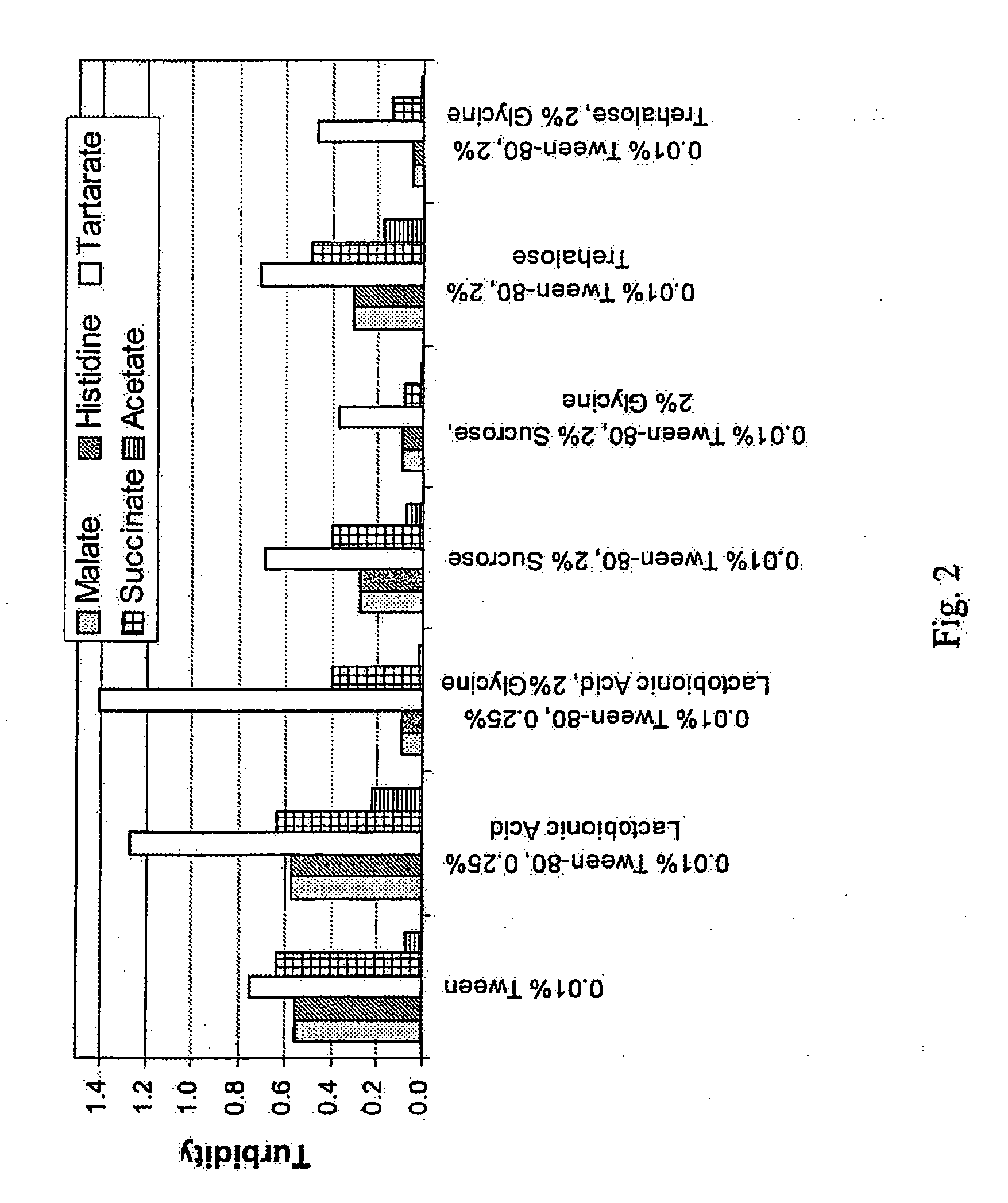
Lyophilized formulations of anti-egfr antibodies
In one embodiment, the present invention provides a stable lyophilized formulation comprising an anti-EGFR antibody, preferably cetuximab; lactobionic acid; and a buffer, preferably histidine. In one preferred embodiment, the present invention provides a stable lyophilized formulation comprising about 50 mg / mL to about 140 mg / mL of ERBITUX?, about 0.125% lactobionic acid, about 25 mM histidine buffer at a pH of about 6.0, about 0.005% Tween 80, and about 1.875% glycine.
Owner:IMCLONE SYSTEMS
Combination therapy using anti-EGFR antibodies and anti-hormonal agents
InactiveUS20070202101A1Reduce morbidityReduction in severity and frequencyOrganic active ingredientsBiocideAbnormal tissue growthLymphatic Spread
The invention relates to a combination therapy for the treatment of tumors and tumor metastases, preferably breast and prostate tumors, comprising administration of anti-EGFR (Her1) antibodies and anti-hormonal agents, optionally together with cytotoxic / chemotherapeutic agent. The method and the pharmaceutical compositions comprising said agents can result in a synergistic potentiation of the tumor cell proliferation inhibition effect of each individual therapeutic agent, yielding more effective treatment than found by administering an individual component alone.
Owner:MERCK PATENT GMBH
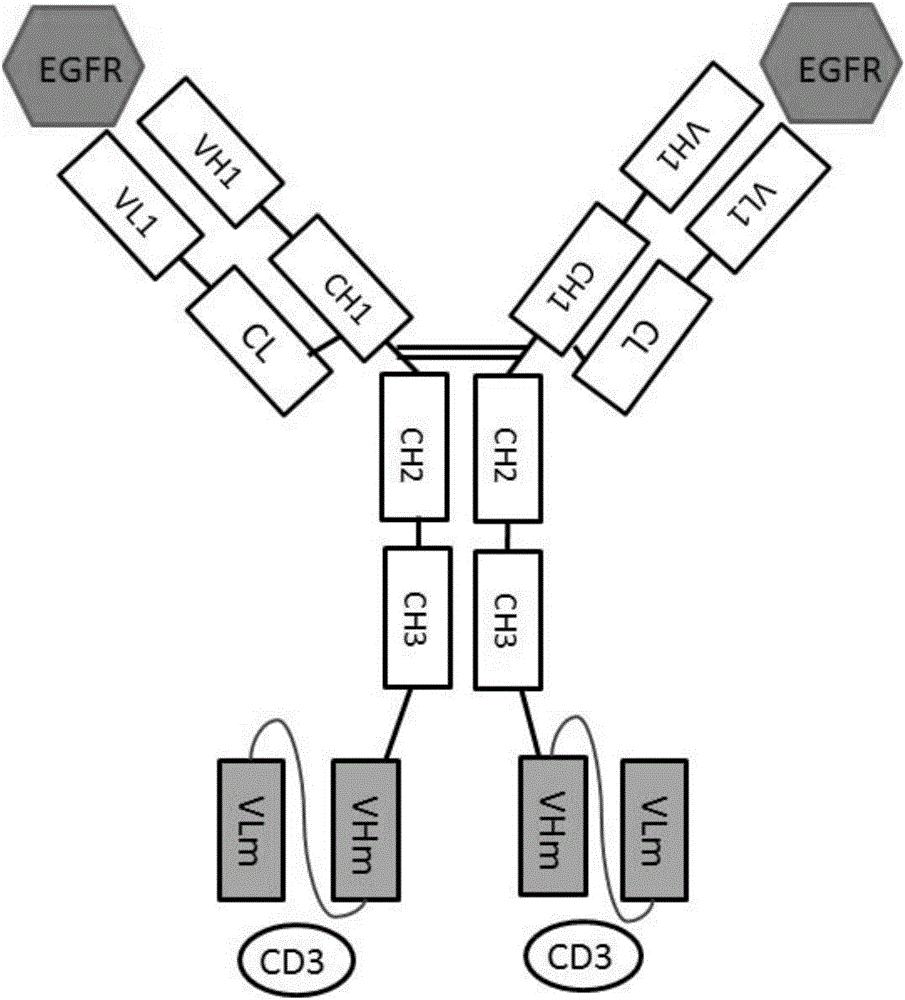
Anti-EGFR (epidermal growth factor receptor) and anti-CD3 (cluster of differentiation 3) bispecific antibody and application thereof
ActiveCN106632681AConducive to biological functionsConducive to biological functionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntigen
Owner:BEIJING DONGFANG BIOTECH +1
Paclitaxel immune nano liposome and preparation method and application thereof
InactiveCN102379848AOrganic active ingredientsMacromolecular non-active ingredientsCancer cellMedicine
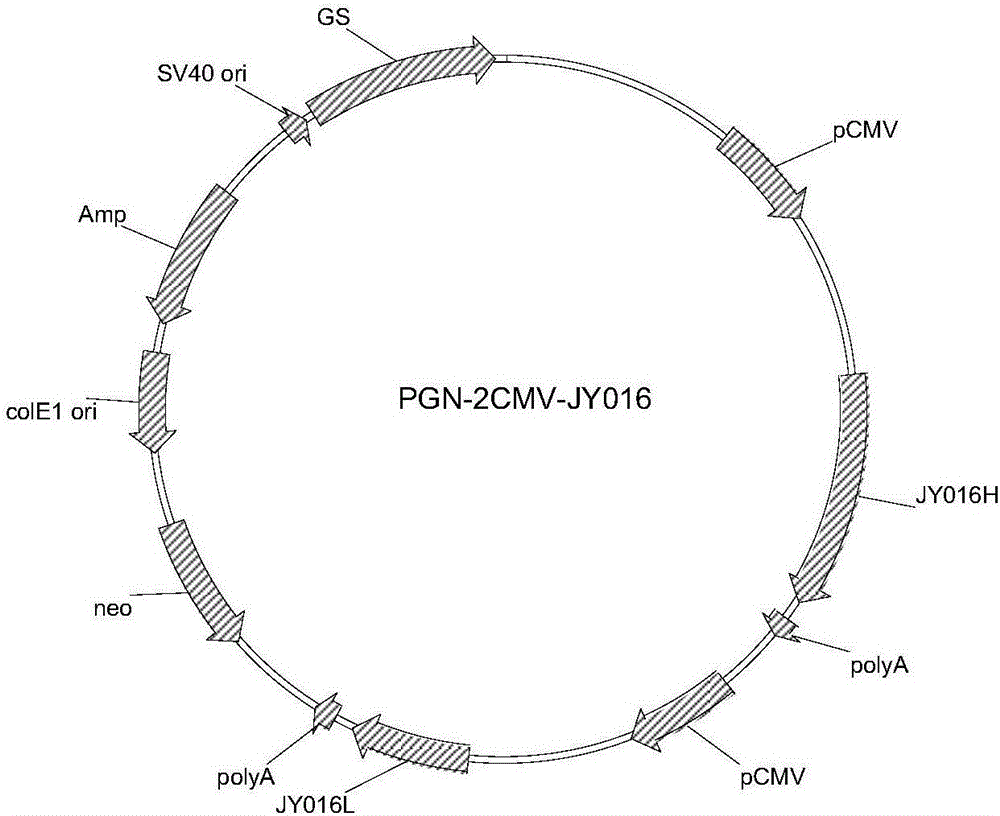
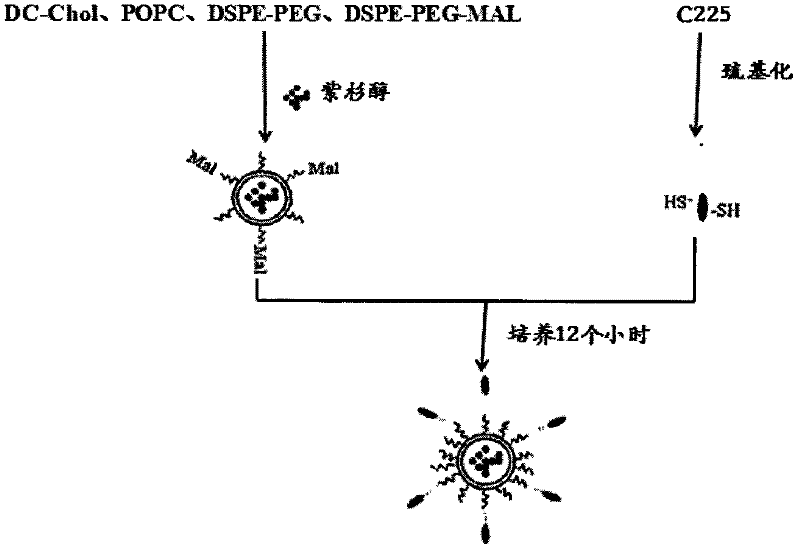
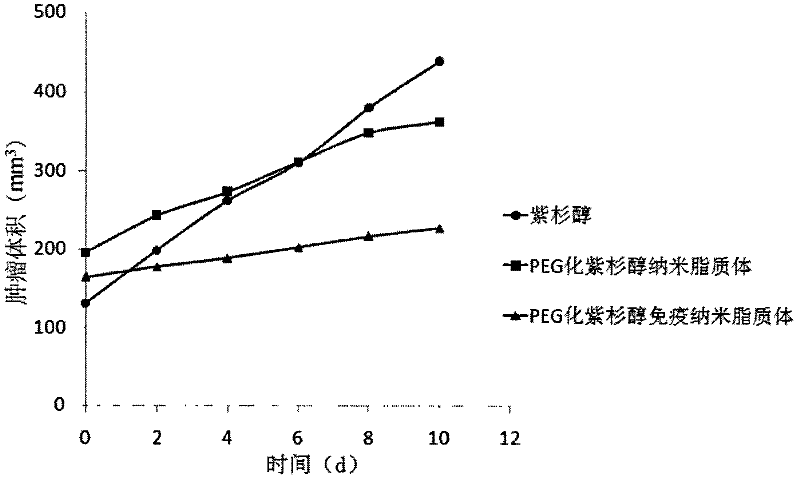
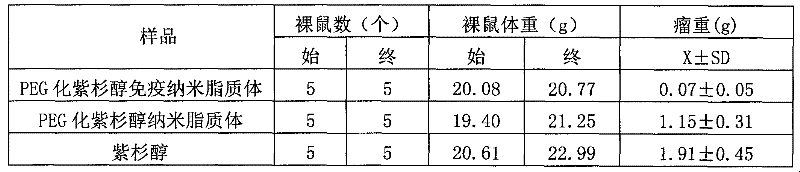
The invention belongs to the field of biomedicine, and particularly discloses a PEG (polyethylene glycol) gylation paclitaxel immune nano liposome and a preparation method and application thereof. Paclitaxel is wrapped in the liposome, and the outside of the liposome is connected with an anti-EGFR (epidermal growth factor receptor) antibody. The paclitaxel immune nano liposome wrapped with the paclitaxel inside and externally connected with the anti-EGFR antibody has the remarkable advantages of fine biological target property and remarkable cancer cell suppressing effect, and can be used for treating EGFR positive tumors.
Owner:TIANJIN GOALGEN BIOTECH
Anti-EGFR antibody therapy based on an increased copy number of the EGFR gene in tumor tissues
InactiveUS20090269344A1Survival predictionHigh gene copy numberMicrobiological testing/measurementAntibody ingredientsAnti-EGFR AntibodyFhit gene
The invention relates to an indidualized and personalized diagnosis and therapy of cancer based on specific molecular alterations which occur in specific tumor tissue of specific tumor patient populations. The therapy and diagnostic is based on the findings that proliferation and tumor growth of specific EGFR bearing tumor tissue expressing an amplified EGFR gene copy number may be abolished by anti-EGFR antibodies, while other individual molecular alterations such as mutations occurring in tumor tissues are unaffected by the same anti-EGFR antibody treatment.
Owner:MERCK PATENT GMBH
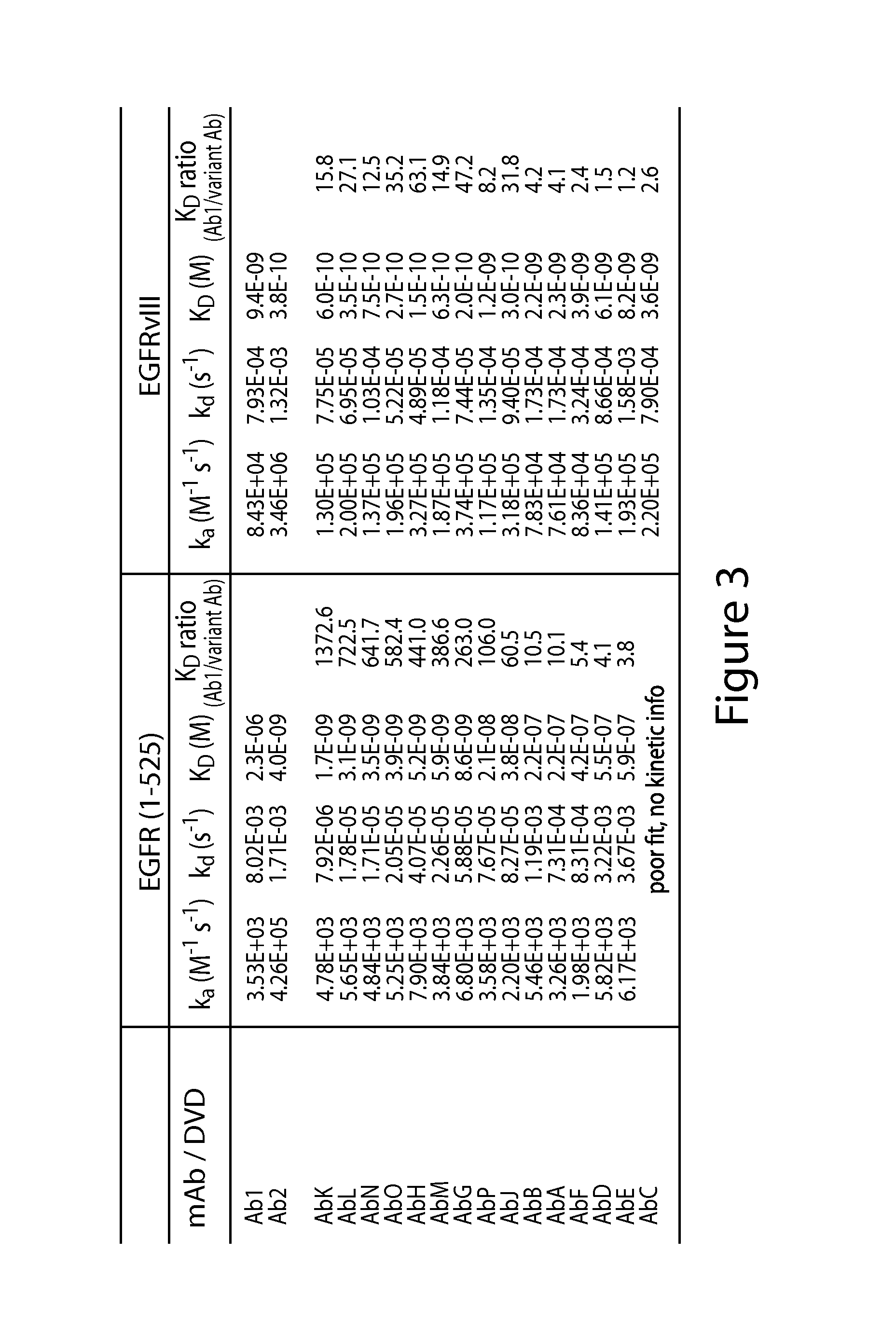
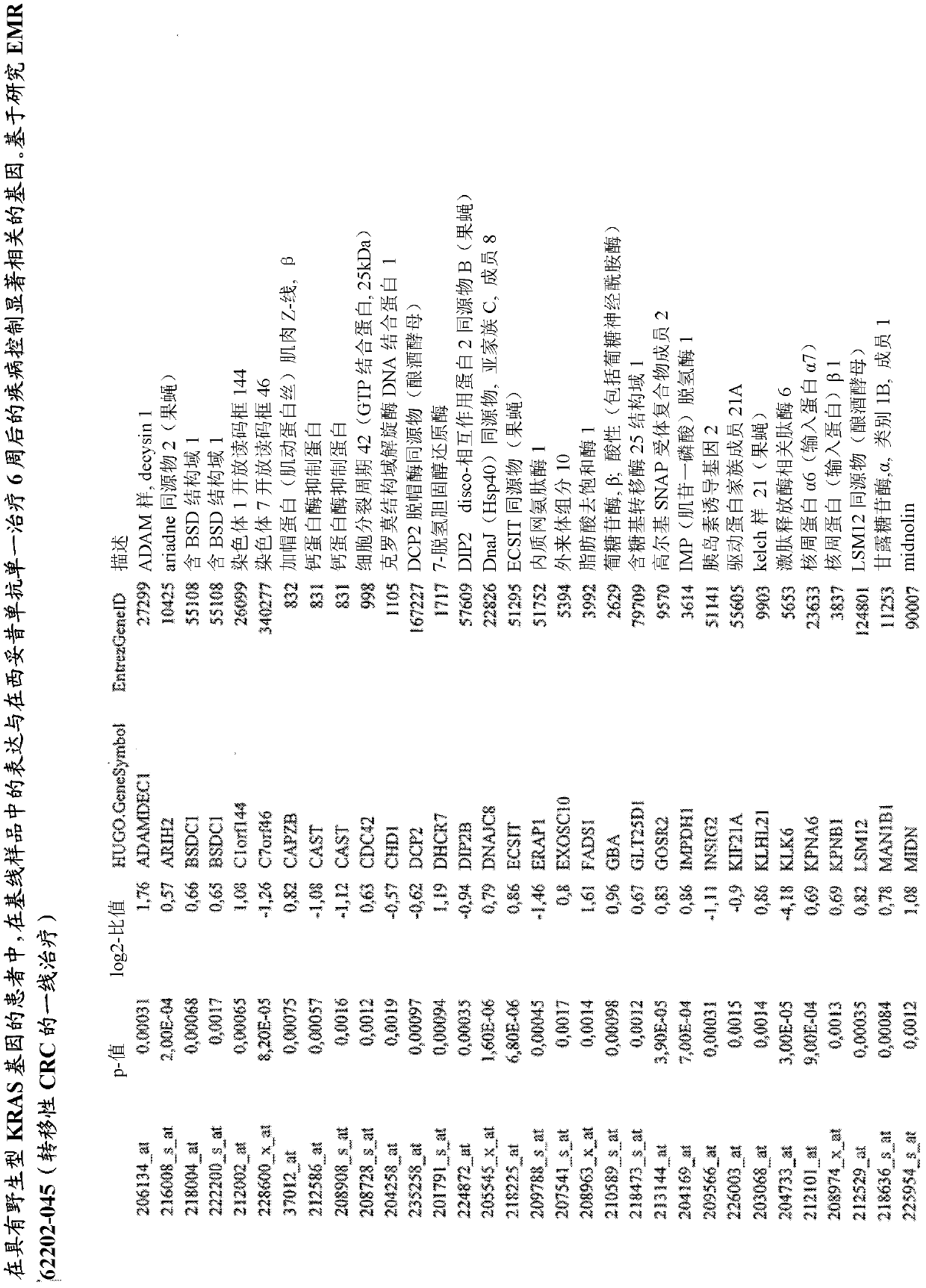
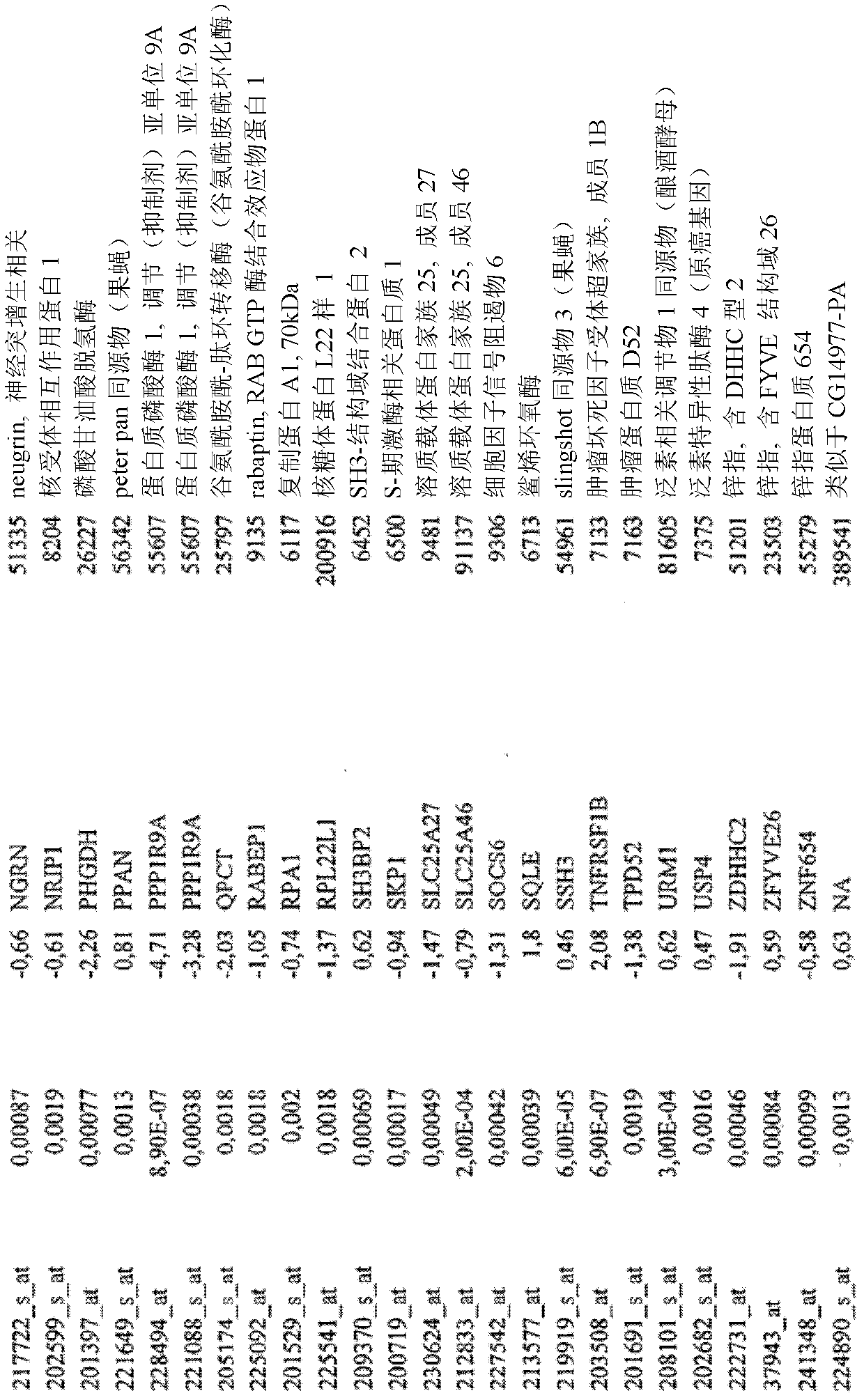
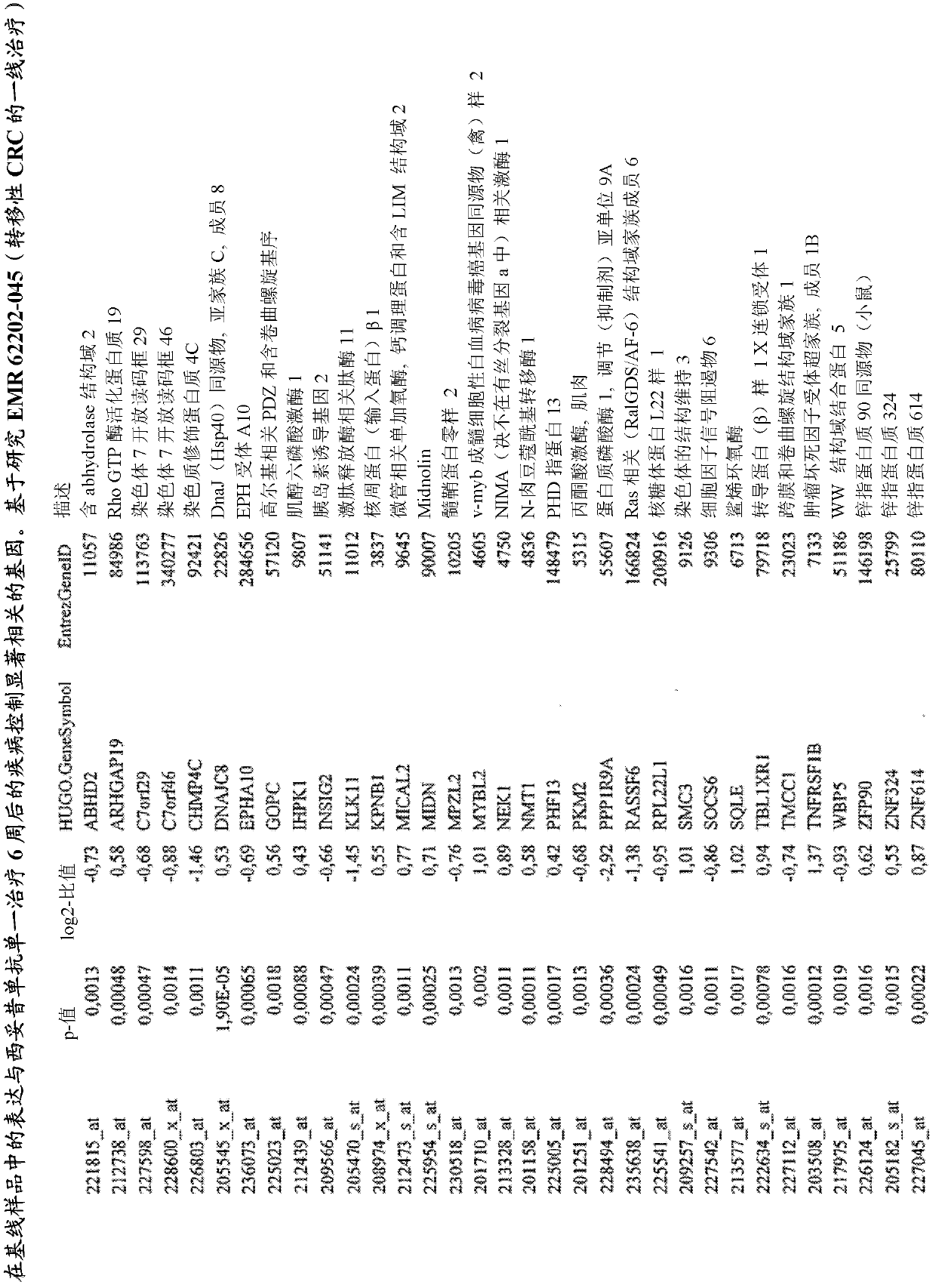
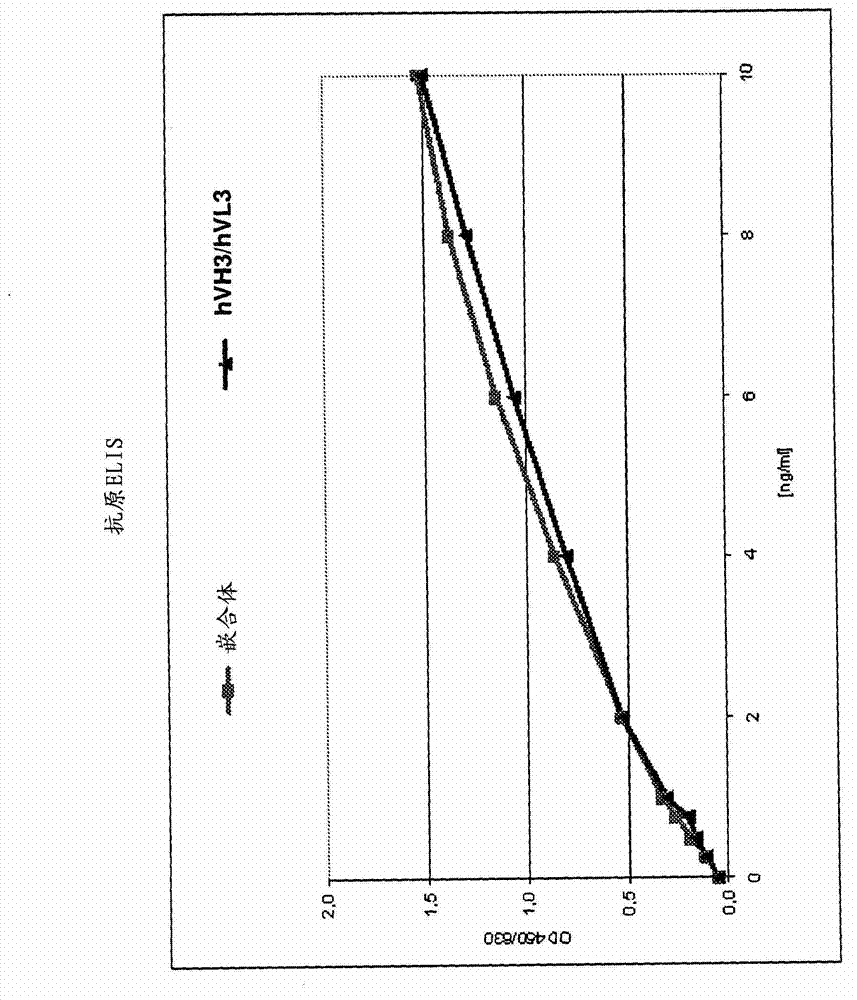
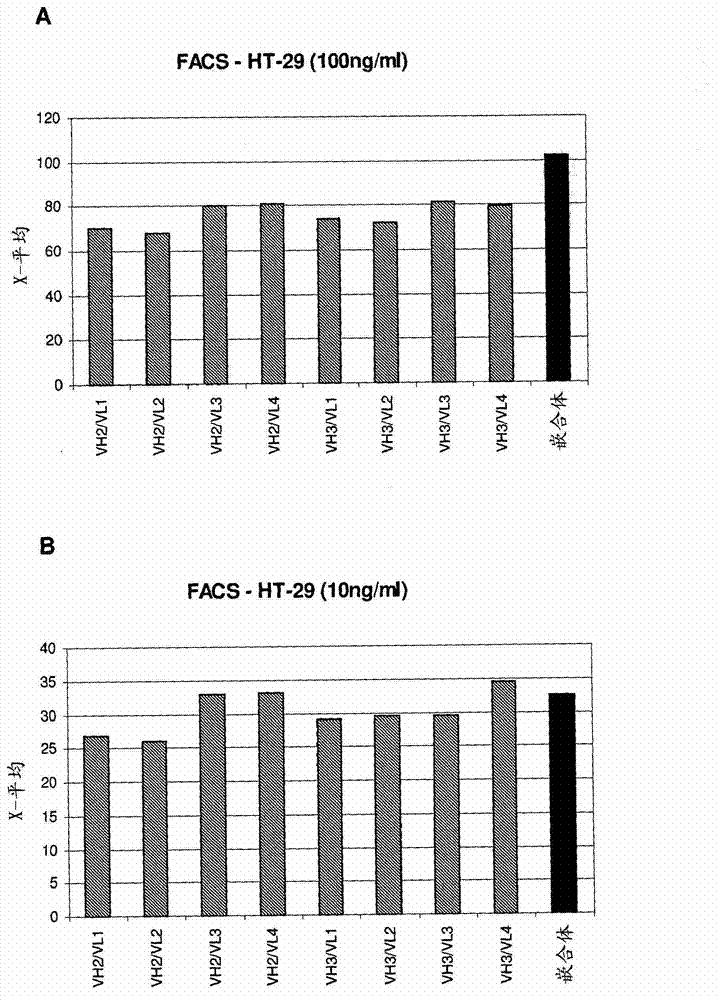
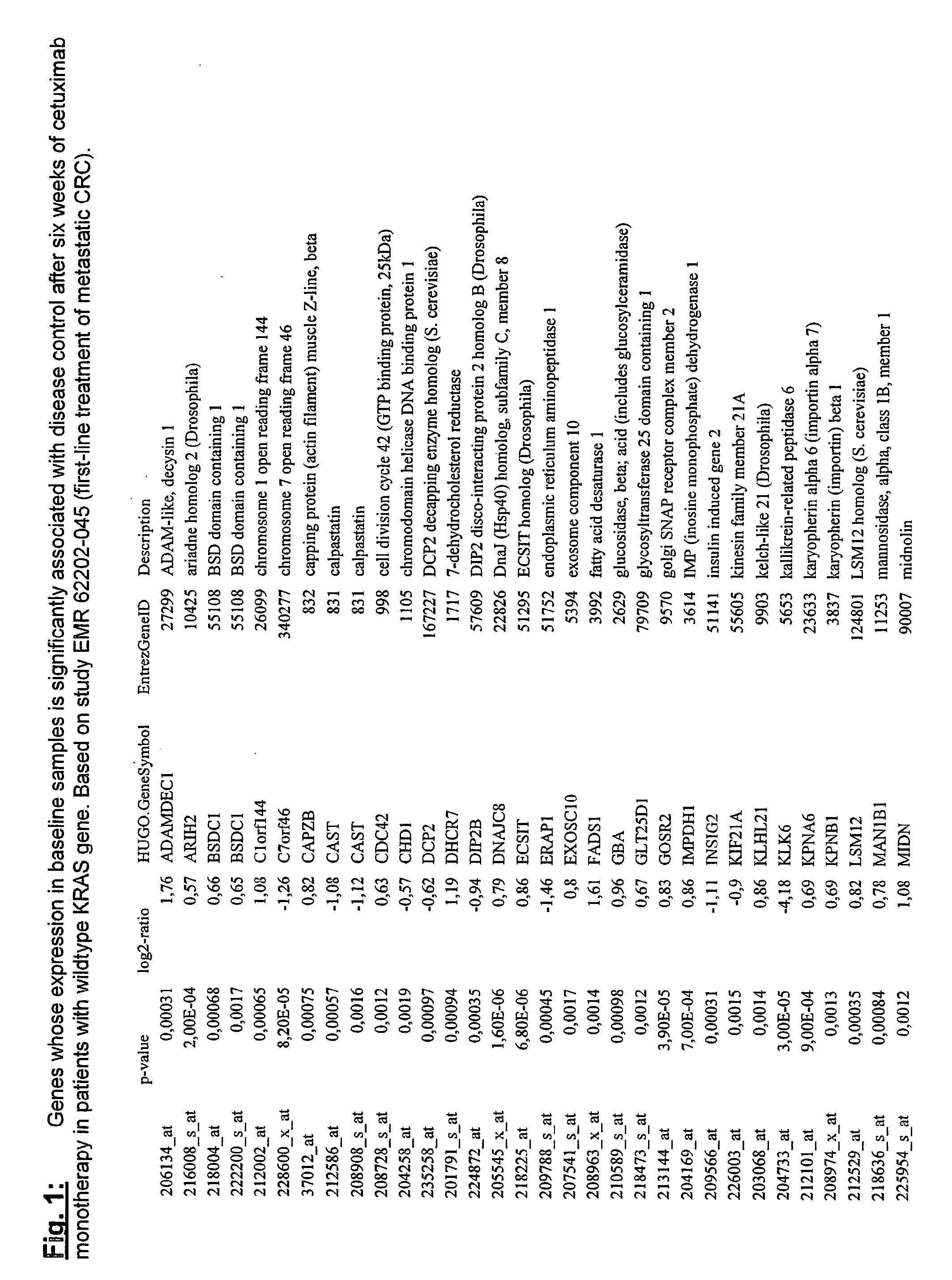
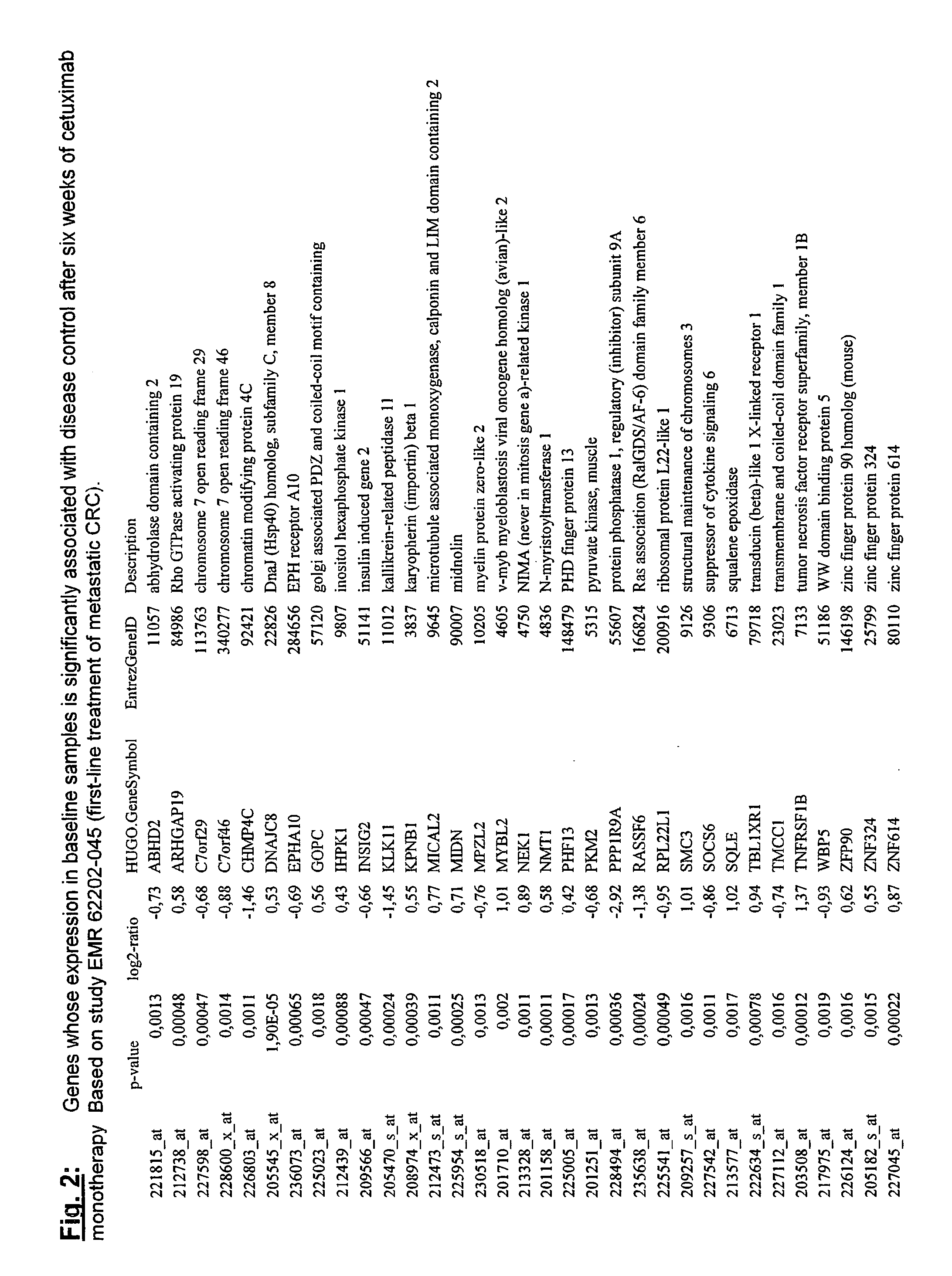
Biomarkers and methods for determining efficacy of anti-egfr antibodies in cancer therapy
The invention relates to biomarkers based on gene expression products and methods for determining the efficacy of anti-EGFR antibodies in the treatment of EGFR expressing cancer. The invention is further related to the prediction of sensitivity or resistance of a patient suffering from EGFR expressing cancer to the treatment of said patient with a specific anti- EGFR antibody. The invention is preferably related to the identification of respective biomarkers that allow a better prediction of the clinical outcome of the treatment with anti- EGFR antibodies in patients with KRAS wild-type tumors. In this context, the invention especially relates to anti-EFGR antibody c225 / cetuximab (Erbitux TM ) and its use in patients suffering from colorectal Cancer (CRC).
Owner:MERCK PATENT GMBH
Anti-cancer treatments with Anti-egfr antibodies having a low fucosylation
InactiveUS20160068609A1Good curative effectEffective treatmentAntibody ingredientsPharmaceutical non-active ingredientsFucosylationSide effect
The present invention pertains to the field of cancer therapy using anti-cancer antibodies. The medical use of anti-EGFR antibodies having improved glycosylation characteristics, in particular a reduced fucosylation, is provided which show anti-cancer efficacy and an improved adverse side effect profile.
Owner:GLYCOTOPE GMBH
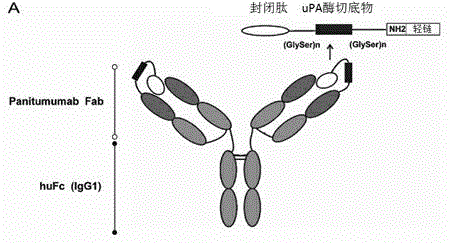
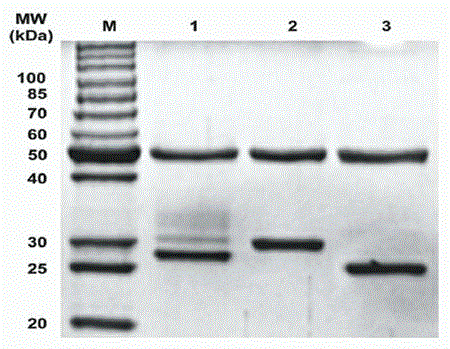
Novel anti-epidermal growth factor receptor antibody, preparation method and application thereof
InactiveCN104910275AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsBinding siteEGFR Antibody
The invention discloses a novel anti-epidermal growth factor receptor EGFR antibody, a preparation method and application thereof. The novel whole human source anti-EGFR monoclonal antibody Pan-P provided by the invention is IgG1 type antibody, and has ADCC effect. Also, the antigen-binding site of the antibody provided by the invention contains a section of blocking peptide with tumor-specific protease cleavage site. The antibody is an enzyme-mediated specific binding antibody, has specific binding effect to tumors, and can reduce skin toxicity caused by general anti-EGFR antibodies.
Owner:TAIZHOU MABTECH PHARM CO LTD
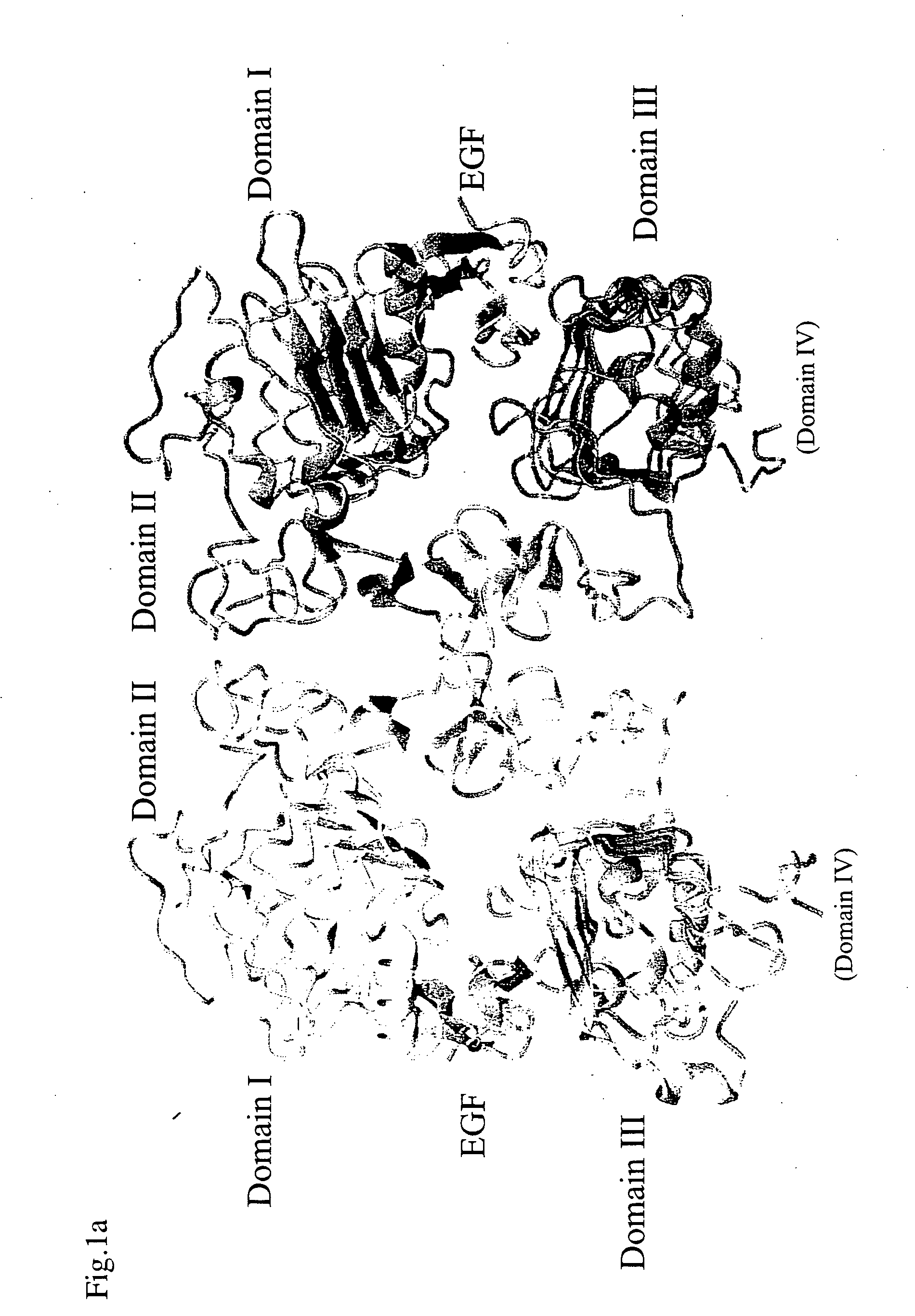
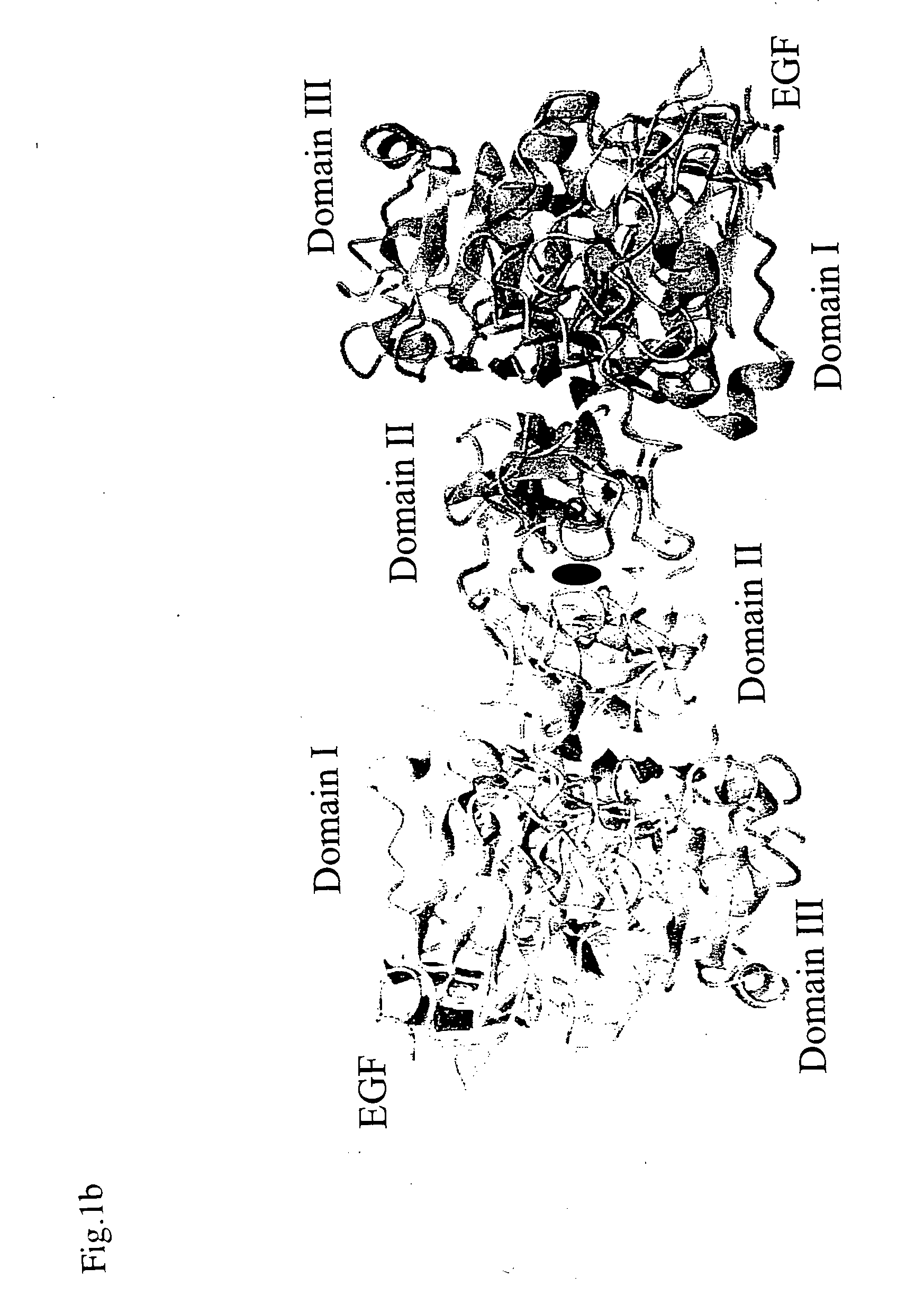
Egf/egfr complex
InactiveUS20070032637A1Efficient productionQuality improvementPeptide/protein ingredientsAnalogue computers for chemical processesEpitopeAnti-EGFR Antibody
The present invention relates to a crystal of a complex of an epidermal growth factor (EGF) and an epidermal growth factor receptor (EGFR), a crystal of a complex of EGFR and a substance regulating EGFR activity, structure coordinates of these crystals, a method for screening for the substance regulating EGFR activity, a method for designing the substance regulating EGFR activity, a method for designing an EGFR variant or an EGF variant, a method for producing an EGFR variant or an EGF variant and an EGF variant or an EGFR variant obtainable by such method, a method for designing an epitope using the structure coordinates of the EGF-EGFR complex, a method for producing an anti-EGFR antibody or an anti-EGF antibody and an antibody obtainable by such method, a polypeptide or a salt thereof comprising a region that forms an EGFR dimerization site, and the like.
Owner:JAPAN SCI & TECH CORP +2
Anti-egfr antibody therapy based on an increased copy number of the egfr gene in tumor tissues
InactiveCN101155932AMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsAnti-EGFR AntibodyWilms' tumor
The invention relates to an individualized and personalized diagnosis and therapy of cancer based on specific molecular alterations which occur in specific tumor tissue of specific tumor patient populations. The therapy and diagnostic is based on the findings that proliferation and tumor growth of specific EGFR bearing tumor tissue expressing an amplified EGFR gene copy number may be abolished by anti-EGFR antibodies, while other individual molecular alterations such as mutations occurring in tumor tissues are unaffected by the same anti-EGFR antibody treatment.
Owner:MERCK PATENT GMBH
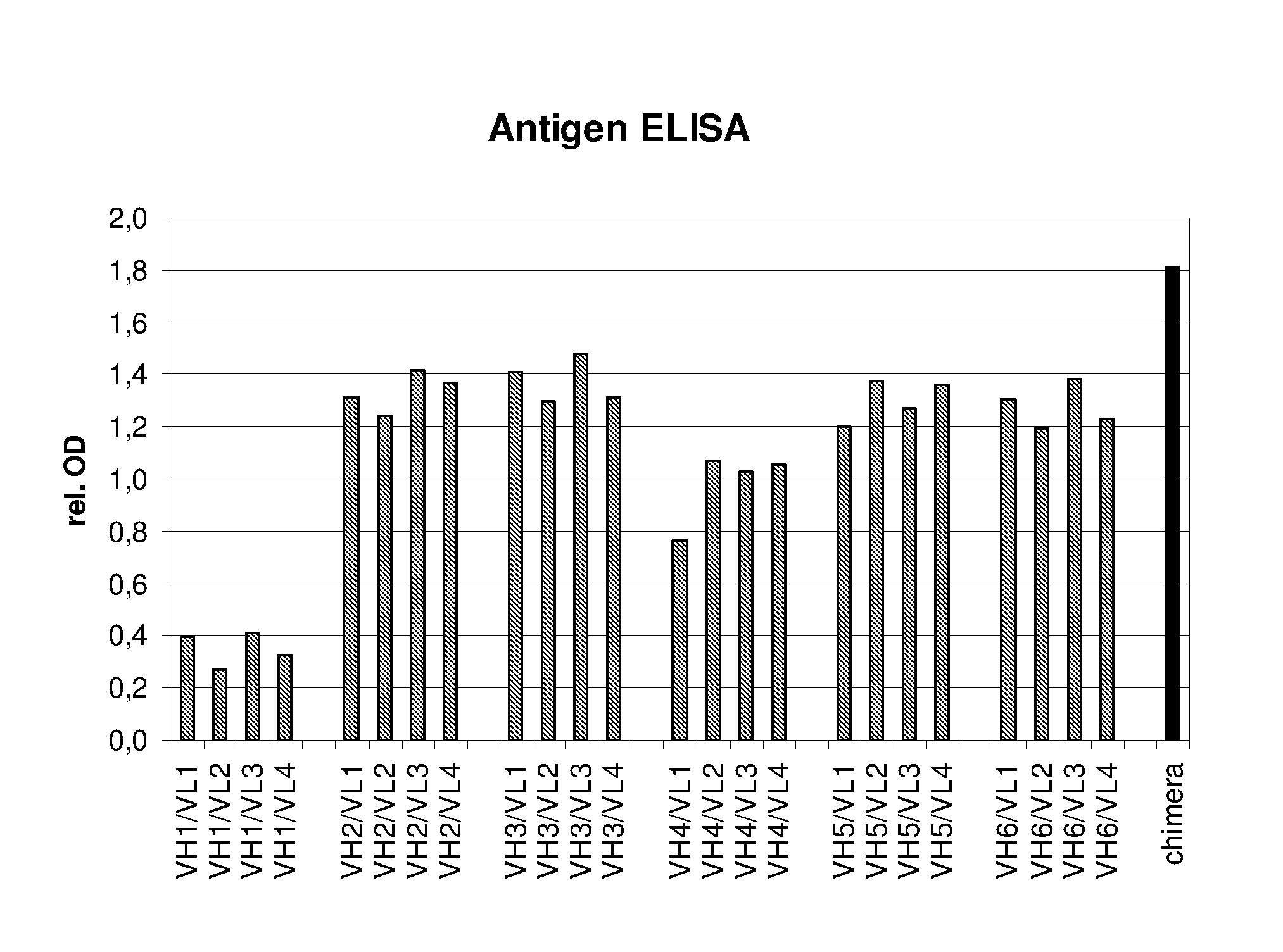
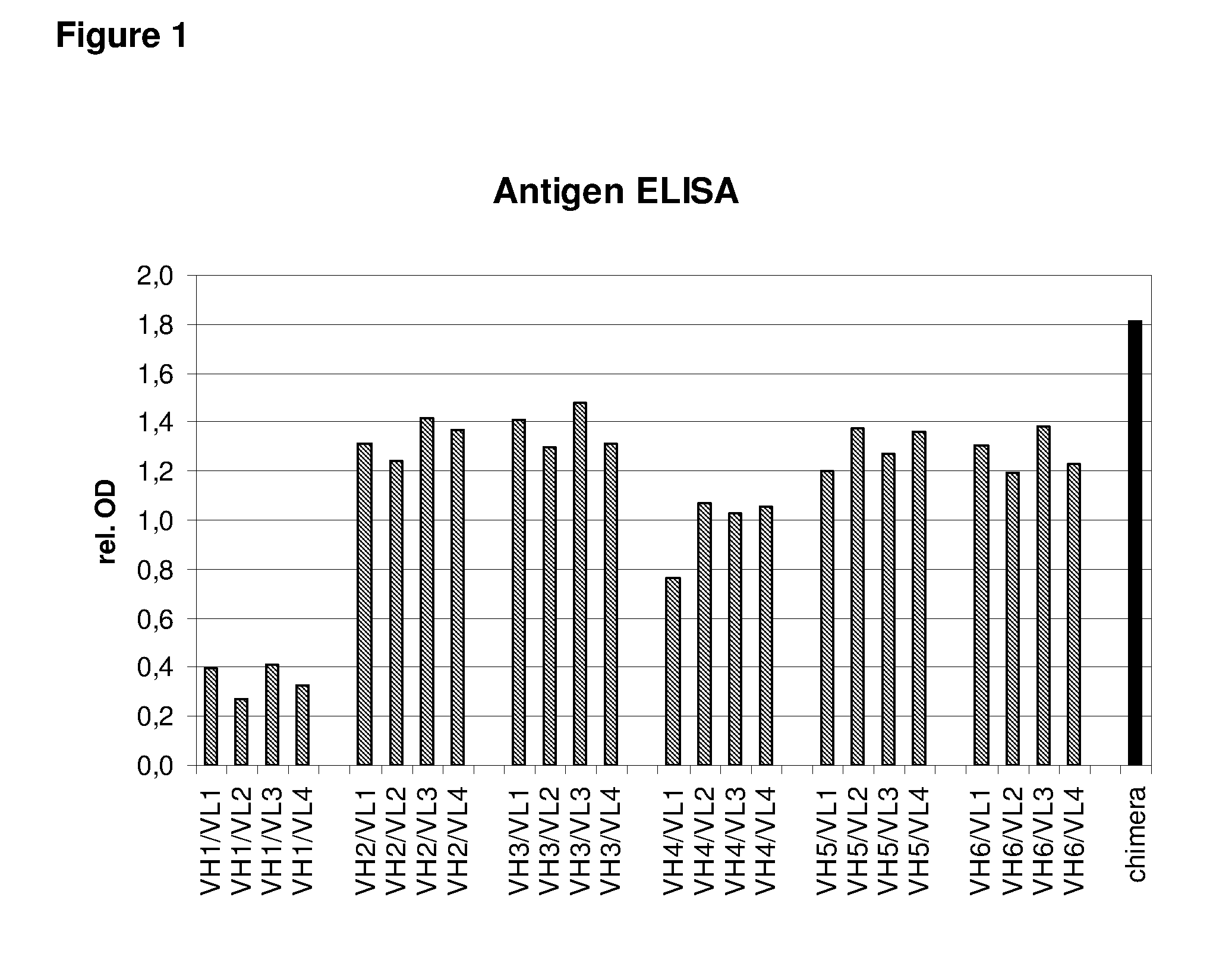
Humanized EGFR antibodies
InactiveCN103025760AEliminate side effectsHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsEGFR AntibodyAntigen binding
The present invention pertains to humanized anti-EGFR antibodies having antigen binding properties similar to those of the murine or chimeric anti-EGFR antibody from which they are derived. In particular, the present invention is directed to humanized anti-EGFR antibodies which are useful in the treatment of cancer.
Owner:GLYCOTOPE GMBH
Biomarkers and methods for determining efficacy of Anti-egfr antibodies in cancer therapy
The invention relates to biomarkers based on gene expression products and methods for determining the efficacy of anti-EGFR antibodies in the treatment of EGFR expressing cancer. The invention is further related to the prediction of sensitivity or resistance of a patient suffering from EGFR expressing cancer to the treatment of said patient with a specific anti-EGFR antibody. The invention is preferably related to the identification of respective biomarkers that allow a better prediction of the clinical outcome of the treatment with anti-EGFR antibodies in patients with KRAS wild-type tumors. In this context, the invention especially relates to anti-EFGR antibody c225 / cetuximab (Erbitux®) and its use in patients suffering from colorectal Cancer (CRC).
Owner:MERCK PATENT GMBH
Methods using recombinant anti-epidermal growth factor receptor antibody compositions
ActiveUS8663640B2Rapid reduction in size of tumourLow efficacyImmunoglobulins against cell receptors/antigens/surface-determinantsVertebrate cellsAbnormal tissue growthHuman cancer
The invention relates to the field of recombinant antibodies for use in human cancer therapy. More specifically the invention provides the use of an antibody composition with two distinct non-overlapping binding specificities to human EGFR. The antibody composition is effecting in treating cancer following treatment with other anti-EGFR antibodies, whether the cancer shows progression during or following the prior treatment or not. The antibody composition can also be used for repeated treatment of recurrent tumors following first-line therapy with the antibody composition of the invention, as the composition does not lead to selection of resistant tumors. A further therapeutic use is the use of an antibody composition of the invention for treatment of cancer that is resistant to known anti-EGFR antibodies.
Owner:LES LAB SERVIER
Genetic Markers for Predicting Responsiveness to Combination Therapy
InactiveUS20120020984A1Microbiological testing/measurementDigestive systemAnti vegf antibodyTopoisomerase inhibitor
The invention provides compositions and methods for determining the likelihood of successful treatment with an effective amount of an anti-VEGF antibody or equivalent thereof, in combination with anti-EGFR antibody or equivalent thereof, and, in some aspects in combination with a topoisomerase inhibitor. The methods comprise determining the identity of a gene of interest in a patient sample and correlating the patient's genotype with the predictive response. Patients identified as responsive are then treated with the appropriate therapy.
Owner:UNIV OF SOUTHERN CALIFORNIA
Therapeutic agent for lung cancer that has acquired egfr-tki resistance
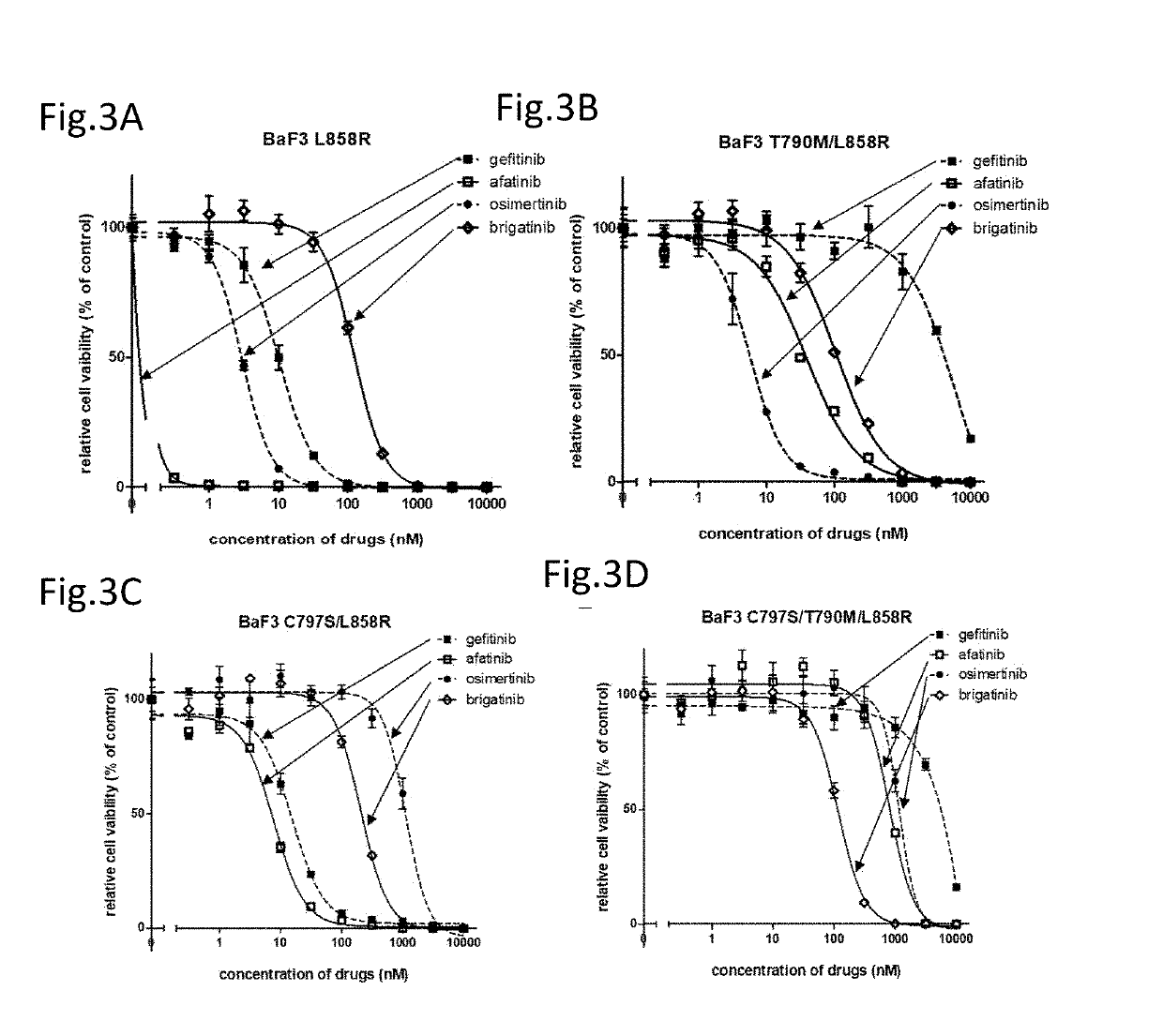
ActiveUS20190160066A1Good treatment effectGood effectOrganic active ingredientsMicrobiological testing/measurementFirst generationBULK ACTIVE INGREDIENT
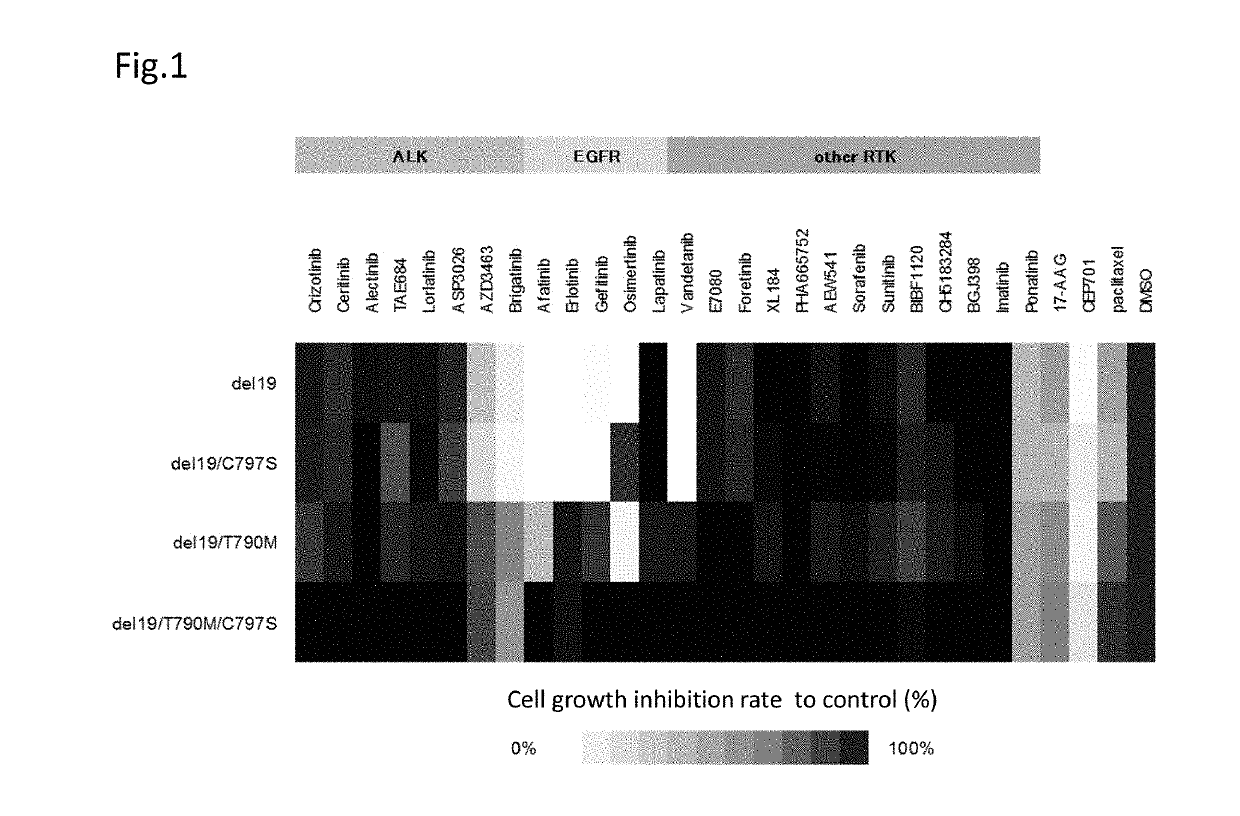
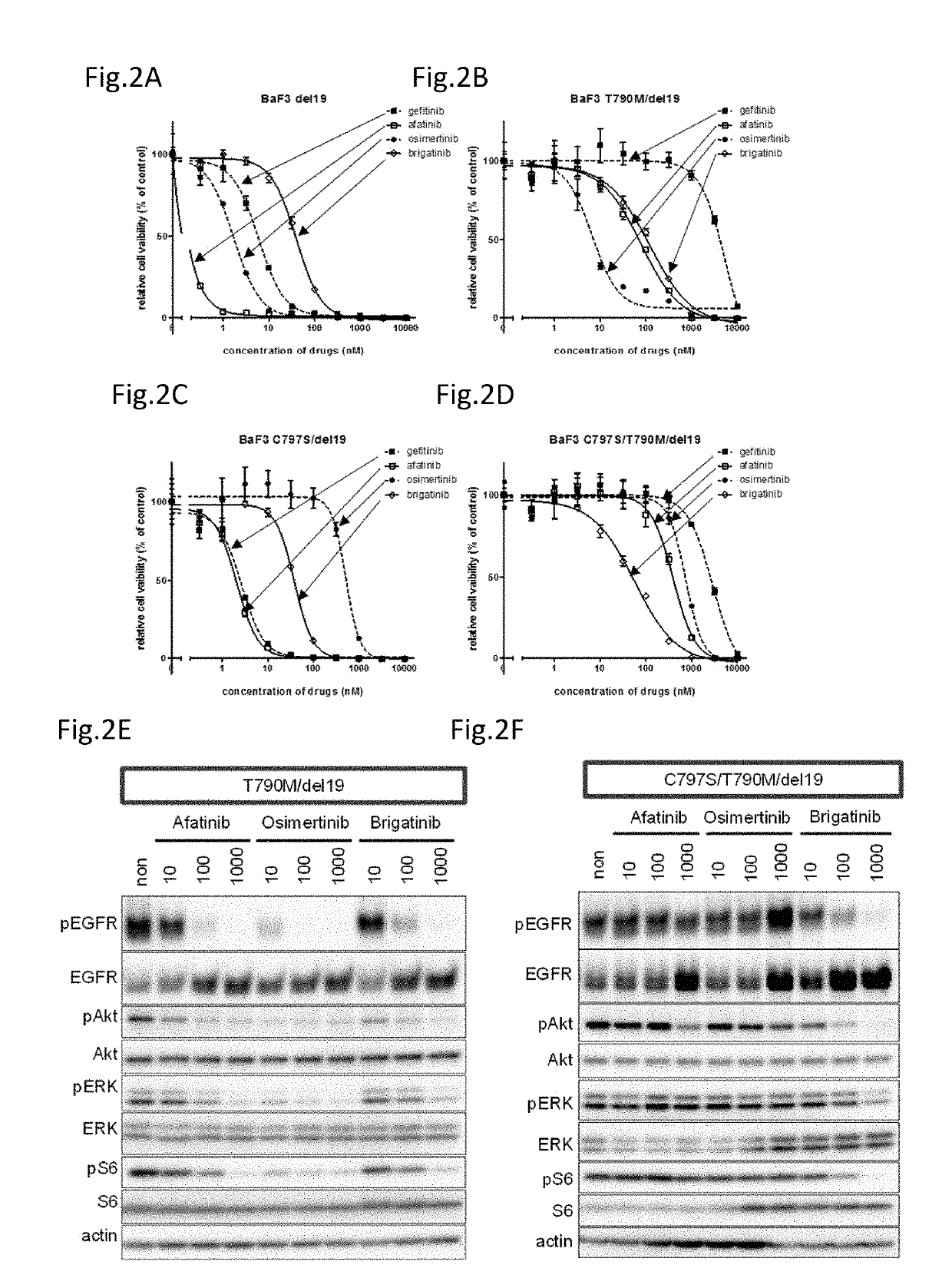
A drug containing, as an active ingredient, a compound represented by ALK inhibitors such as brigatinib, AP26113-analog, and AZD3463 has been found to be effective against a non-small cell lung cancer having a point mutation at C797S in EGFR which has acquired a resistance to chemotherapy agents. Further, the drug used in combination with an anti-EGFR antibody demonstrates a notable suppression effect on the tumor growth. The drug has a potential to be a therapeutic agent effective against a non-small cell lung cancer which is resistant to gefitinib, a first generation therapeutic agent and osimertinib, a third generation therapeutic agent.
Owner:JAPANESE FOUND FOR CANCER RES
Anti-egfr antibodies and their uses
InactiveCN102753580AImmunoglobulins against cell receptors/antigens/surface-determinantsAntineoplastic agentsAnti-EGFR AntibodyAntibody
The present invention relates to antibodies directed to EGFR and uses of such antibodies, for example, to treat diseases associated with the activity and / or overproduction of EGFR.
Owner:ABBVIE BIOTHERAPEUTICS
Antibodies for the treatment of cancers
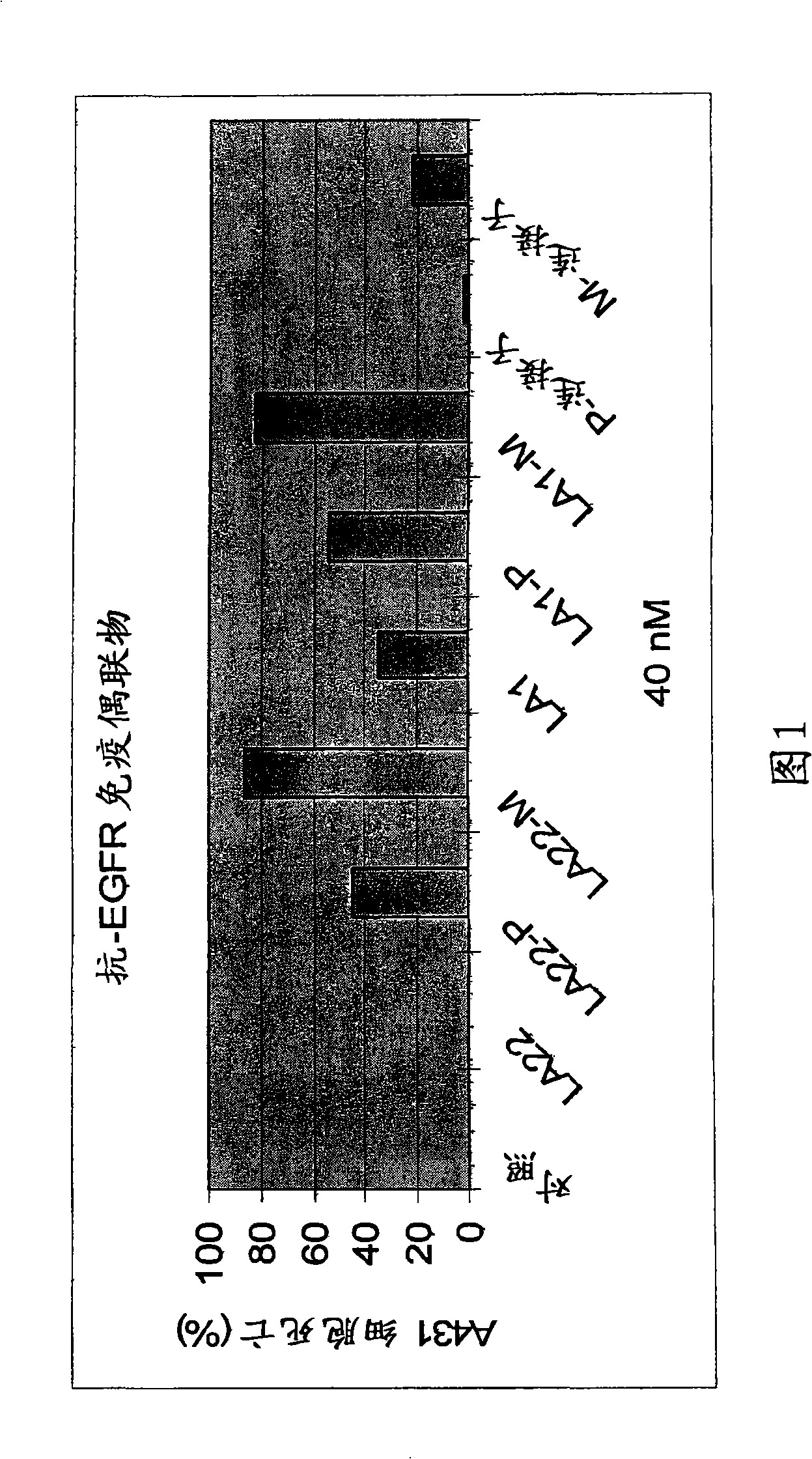
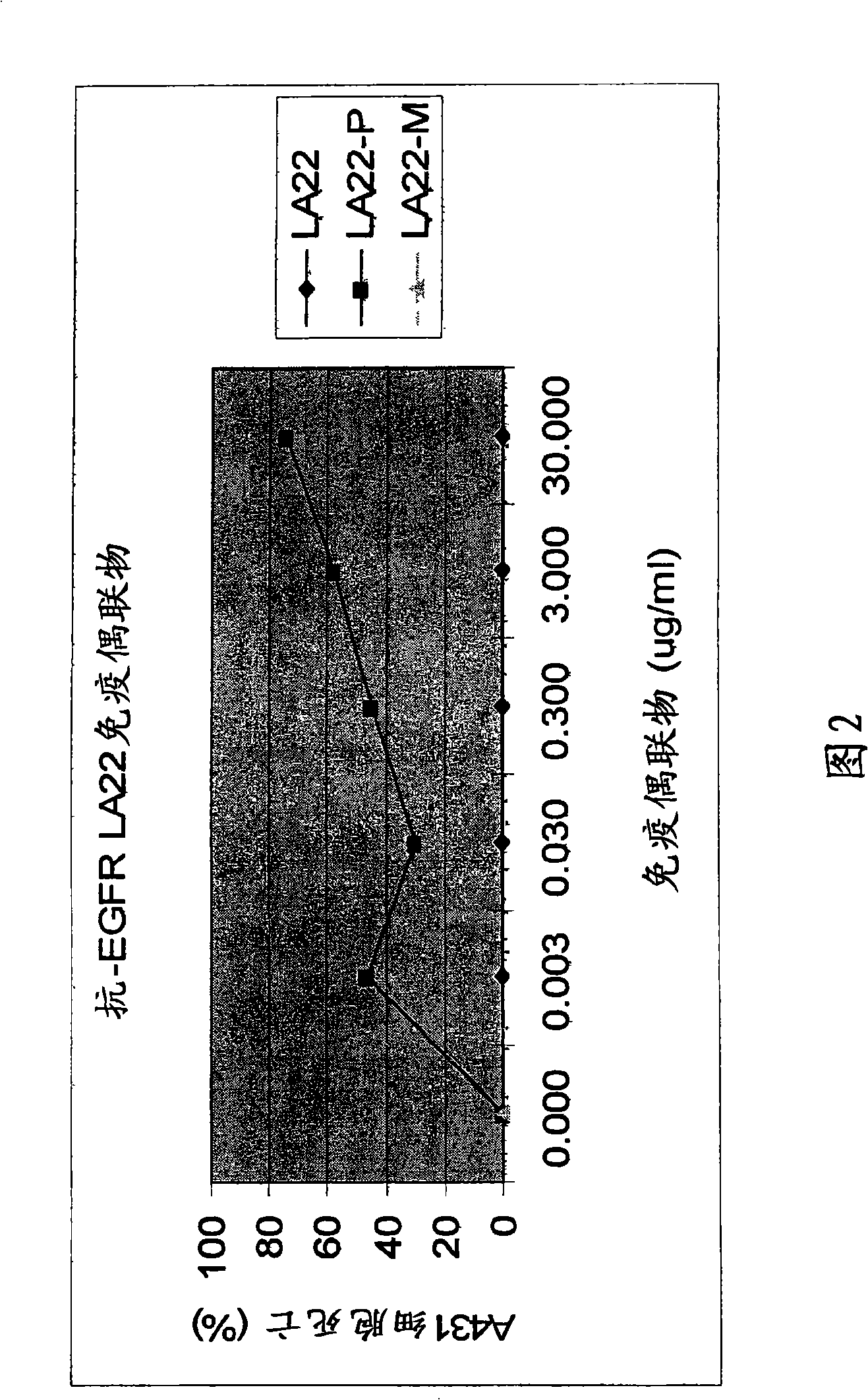
The present invention features monoclonal antibodies LA1 or LA22 conjugated with mitomycin C, pingyangmycin or other anti-cellular agents. The present invention also features other anti-EGFR antibodies conjugated with mitomycin C or pingyangmycin. The antibodies of the present invention can be used to treat cancers, including but not limited to, those of epithelial origin, such as glioblastoma or cancer of the lung, breast, head and neck, and bladder.
Owner:WELSON PHARMA
Humanized EGFR antibodies
InactiveUS9051370B2Impair antigen bindingLong half-lifeAnimal cellsSugar derivativesEGFR AntibodyAntigen binding
The present invention pertains to humanized anti-EGFR antibodies having antigen binding properties similar to those of the murine or chimeric anti-EGFR antibody from which they are derived. In particular, the present invention is directed to humanized anti-EGFR antibodies which are useful in the treatment of cancer.
Owner:GLYCOTOPE GMBH
Solid forms of anti-EGFR-antibodies.
The invention relates to solid forms of antibodies against the EGF receptor, especially precipitates and crystals of monoclonal antibodies against the EGF receptor, especially preferably Mab C225 (Cetuximab) and Mab h425 (EMD 72000), that create biologically active antibody proteins by dissolution or suspension in an aqueous medium. Said proteins can be obtained by precipitation of the antibody dissolved or suspended in the aqueous medium and / or one of the variants or fragments thereof by means of a precipitation reagent. The invention also relates to pharmaceutical preparations containing at least one solid form of the cited antibody in a precipitated non-crystalline form, a precipitated crystalline form or a soluble or suspended form, and optionally carrier and / or auxiliary substances and / or other pharmaceutical active ingredients, and to a method for producing inventive solid forms of anti-EGFR-antibodies.
Owner:MERCK PATENT GMBH
Stable antibody preparation
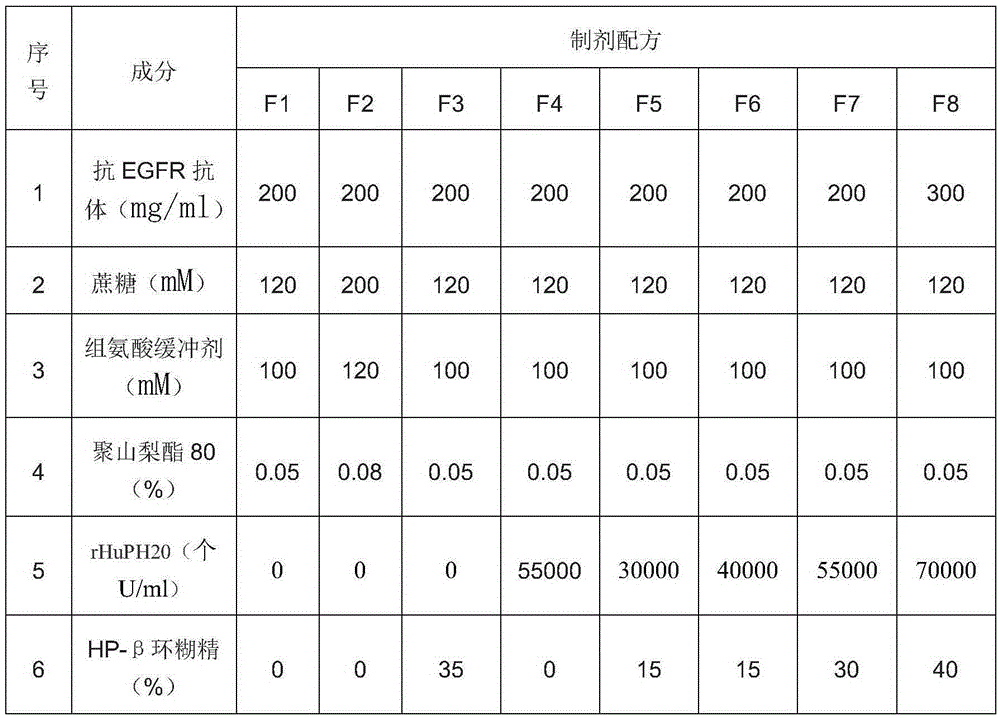
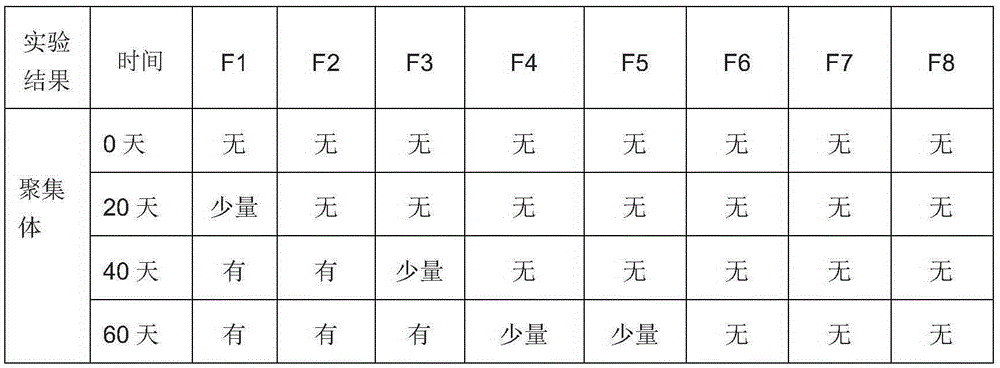
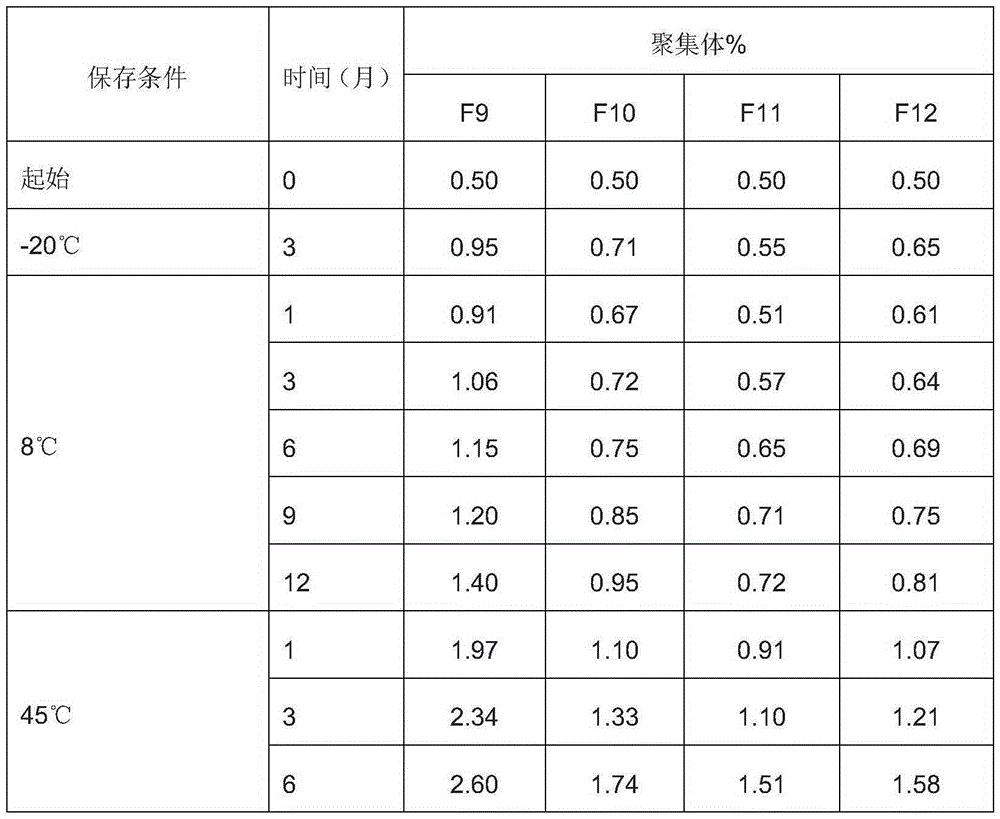
InactiveCN106620690AImprove stabilityReasonable shelf lifeAntibody ingredientsMacromolecular non-active ingredientsHigh concentrationDisease
The present invention relates to the technical field of biology, discloses a stable antibody preparation, specifically a high-concentration anti-EGFR antibody preparation containing hyaluronidase and cyclodextrin, wherein the preparation has good stability, is particularly suitable for subcutaneous injection, and can be used for treating human head and neck squamous cell carcinoma, colorectal cancer, pancreatic cancer, non-small cell lung cancer, bladder cancer, and other diseases.
Owner:SHANGHAI NAT ENG RES CENT OF ANTIBODY MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com