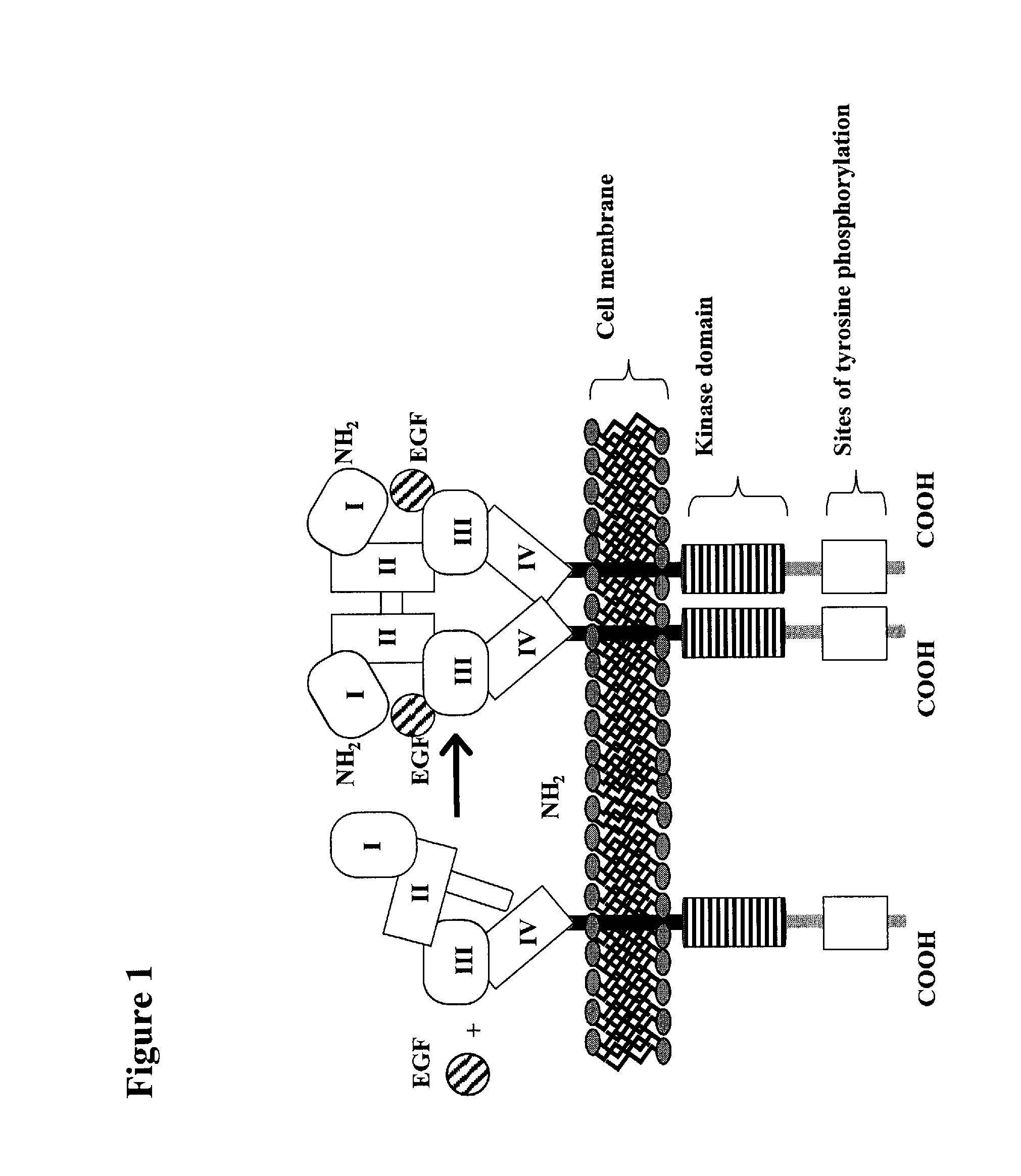
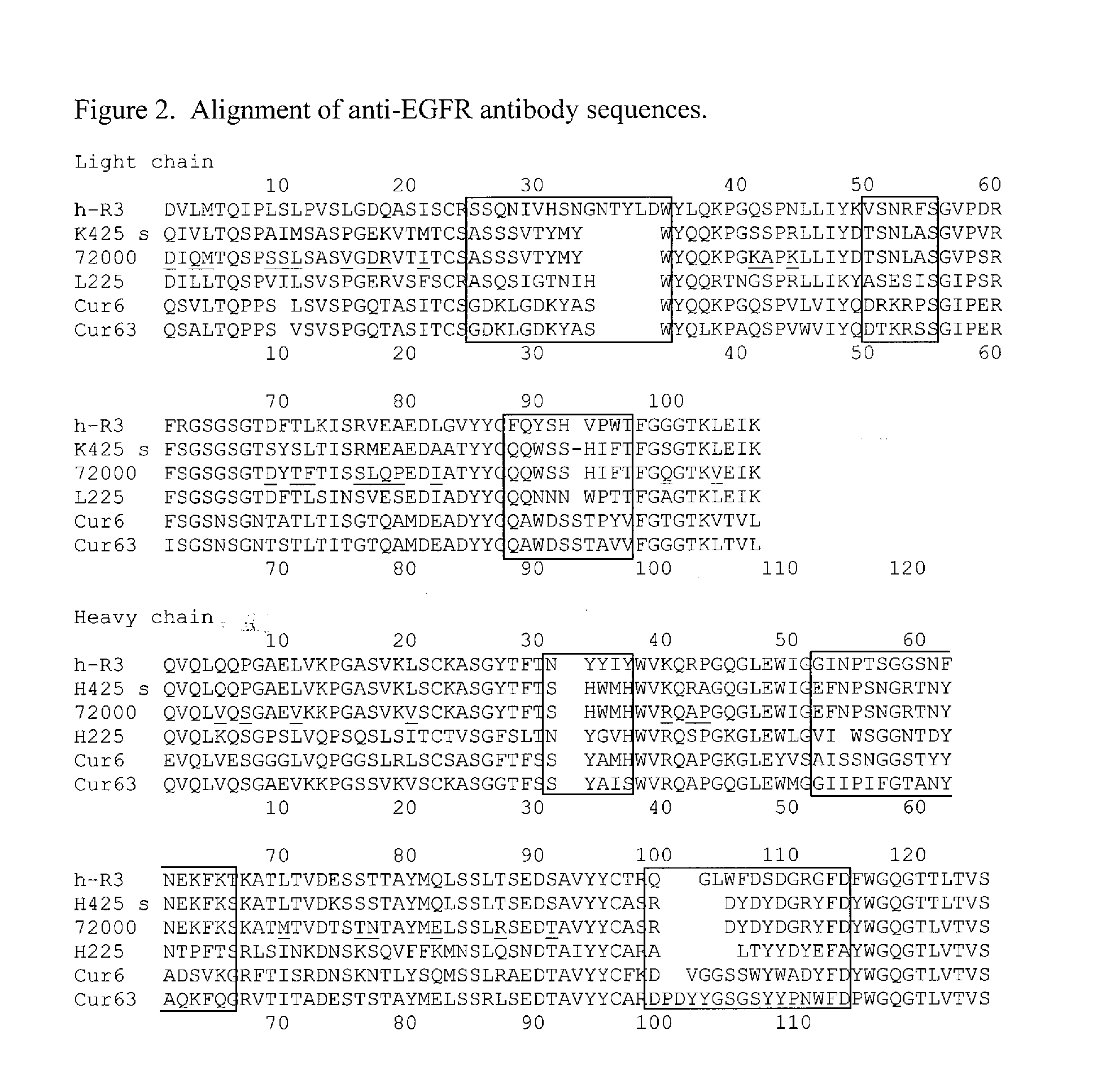
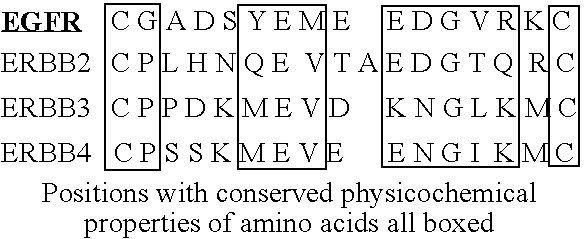
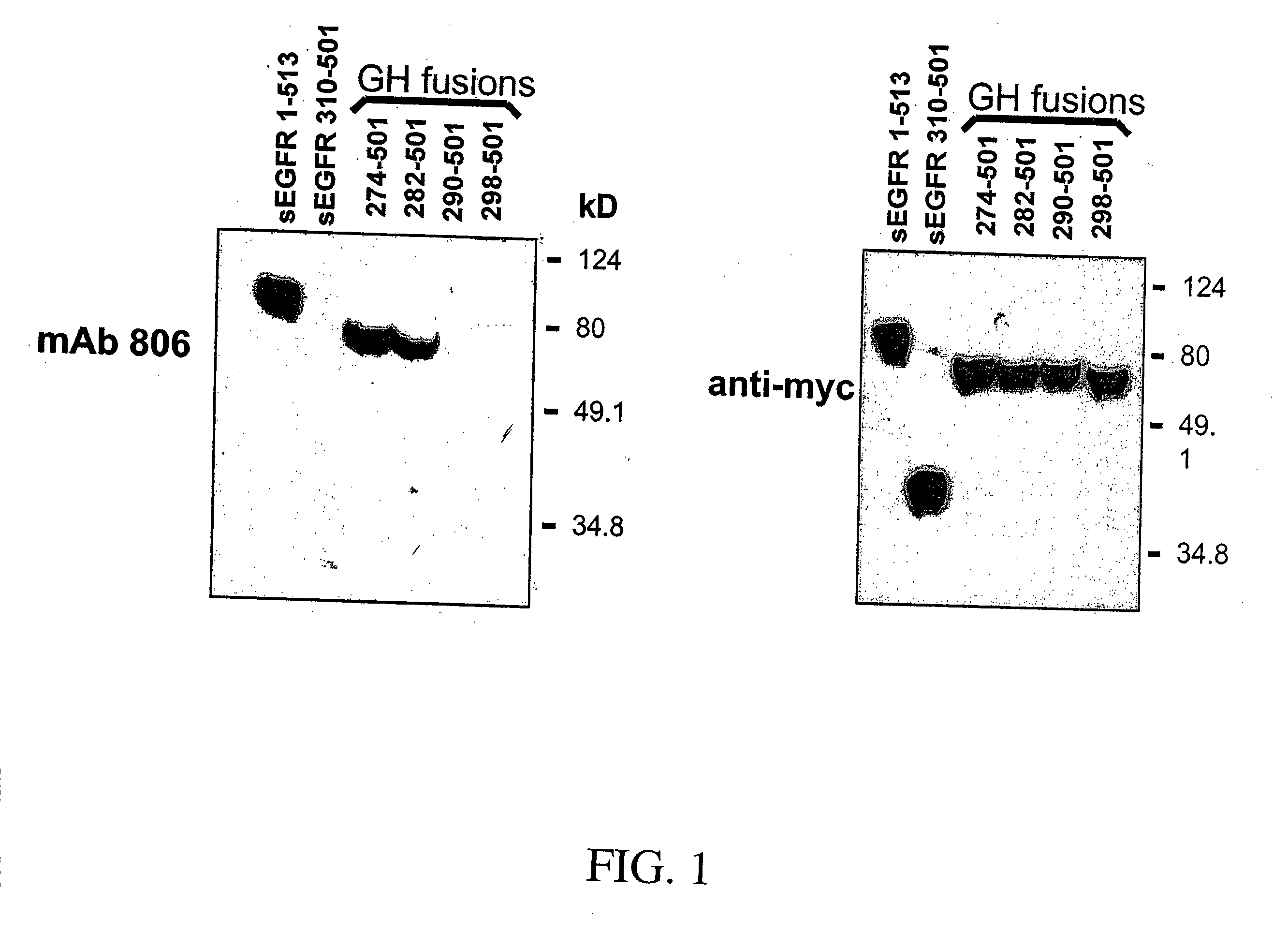
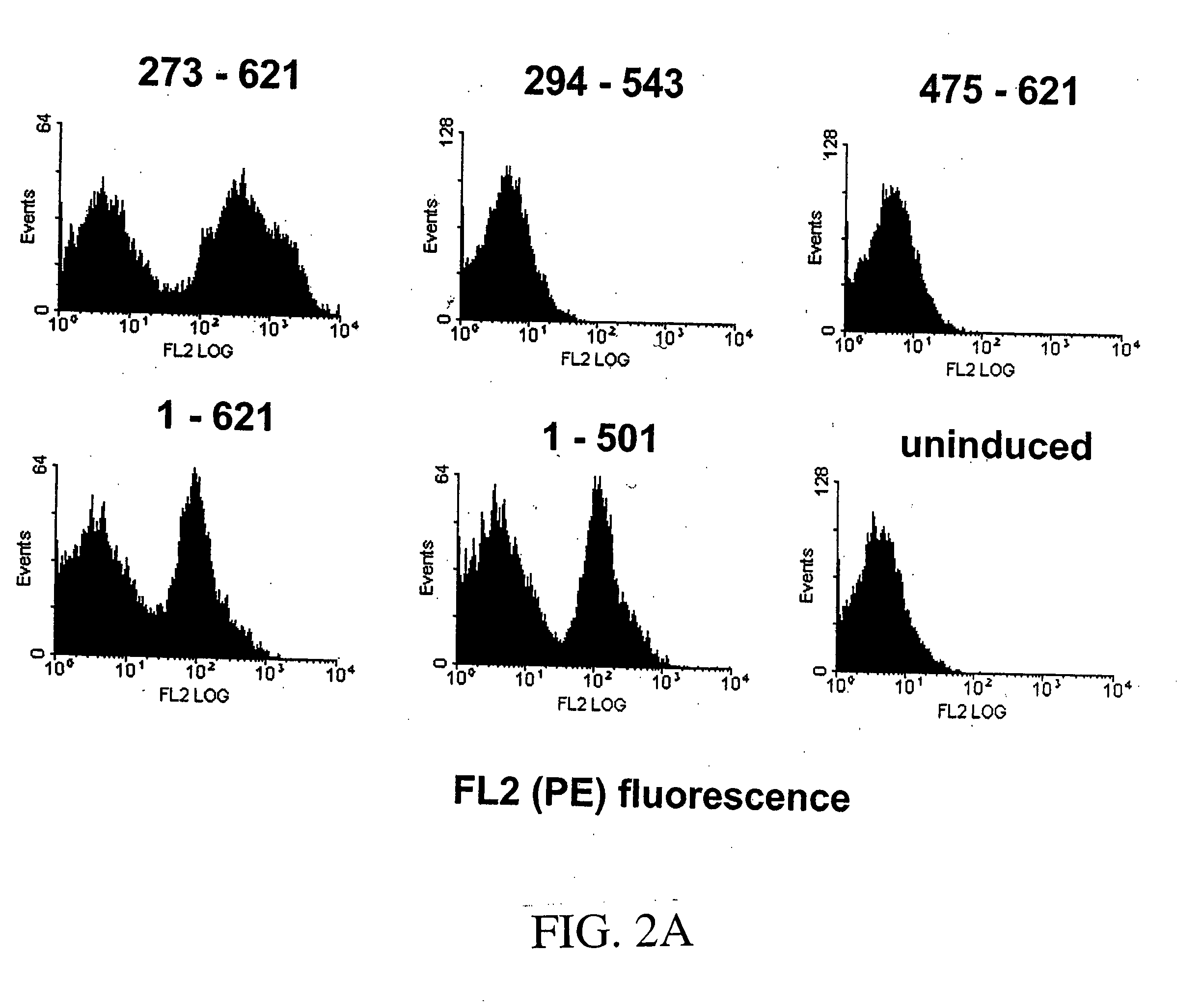
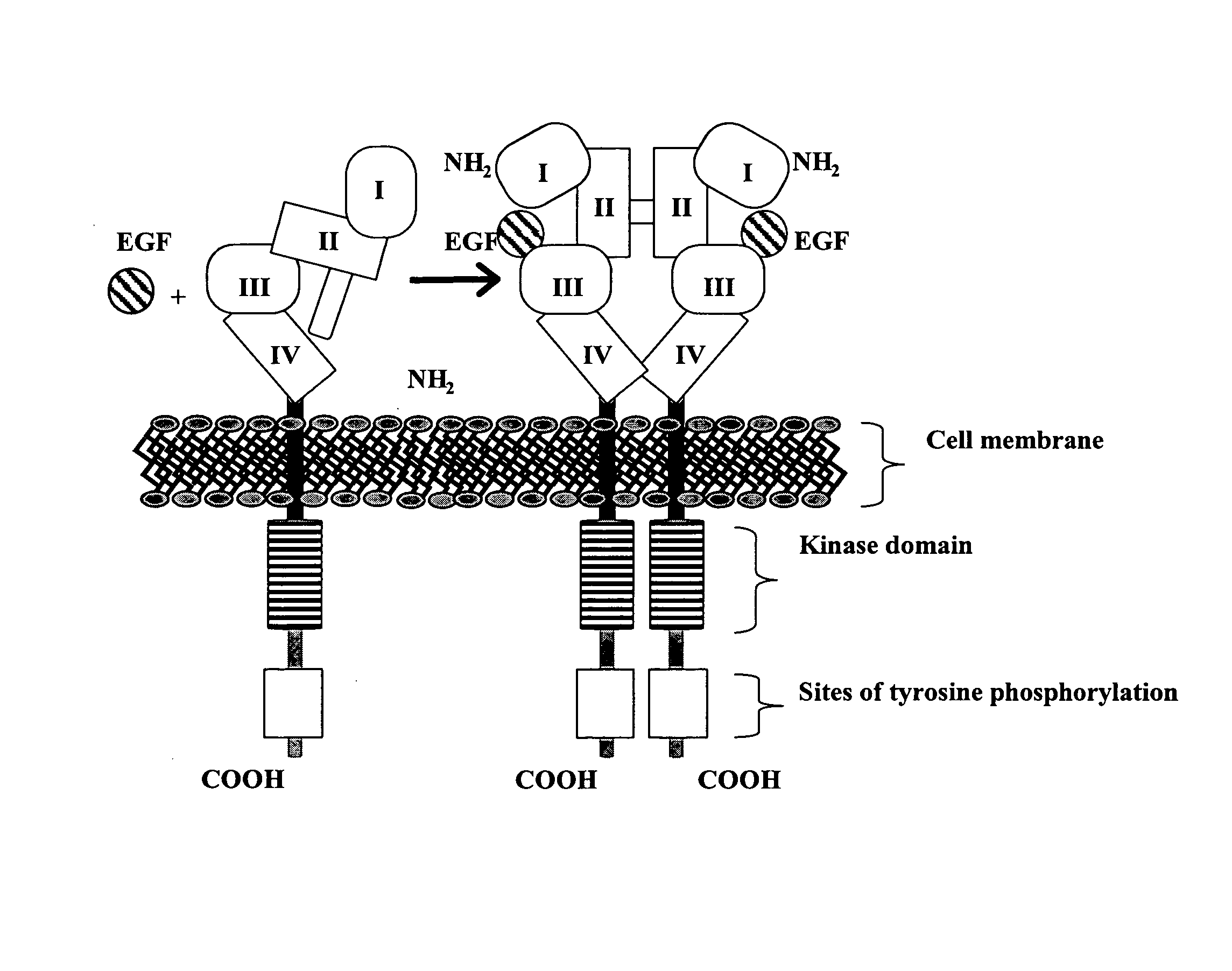
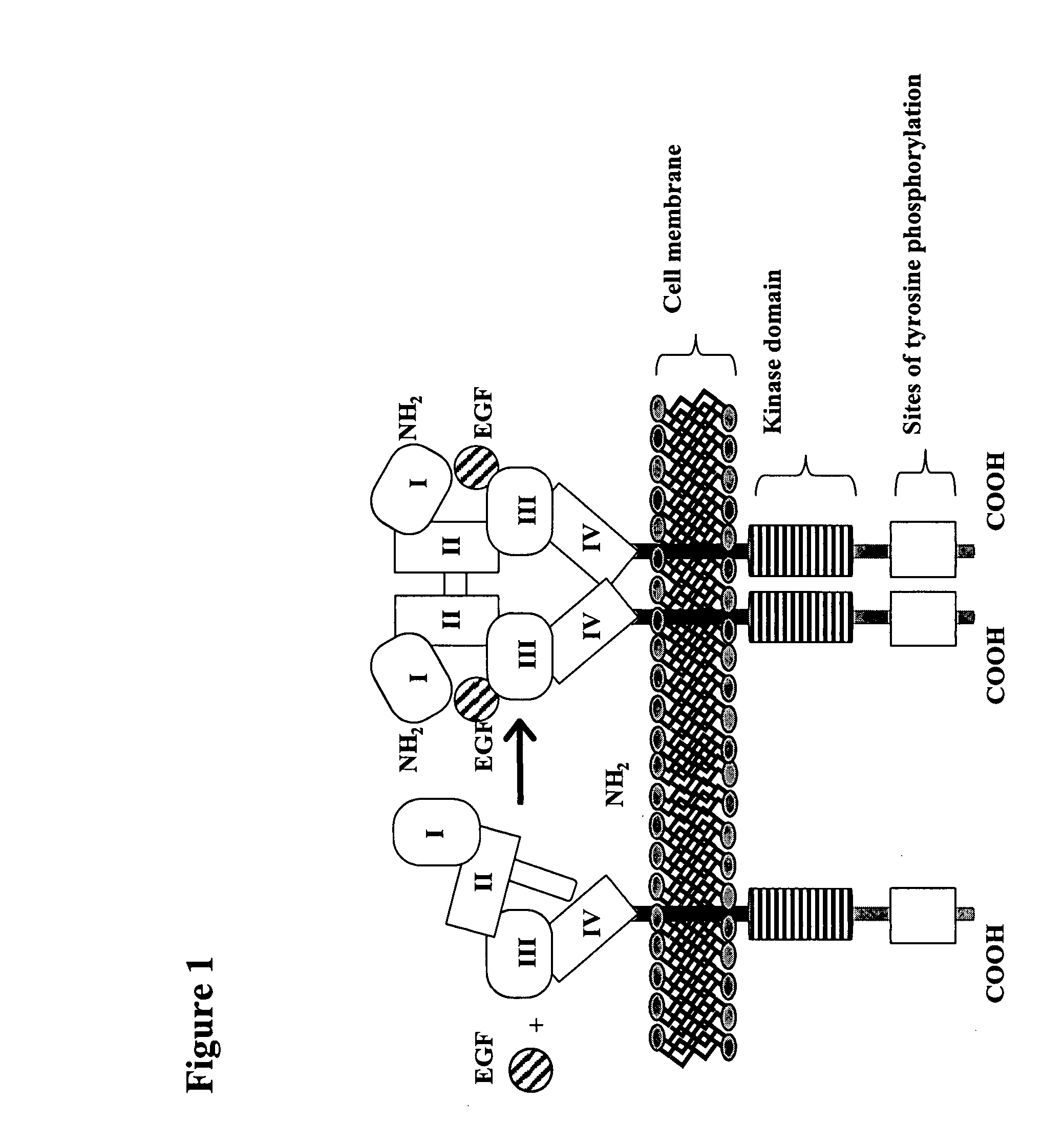
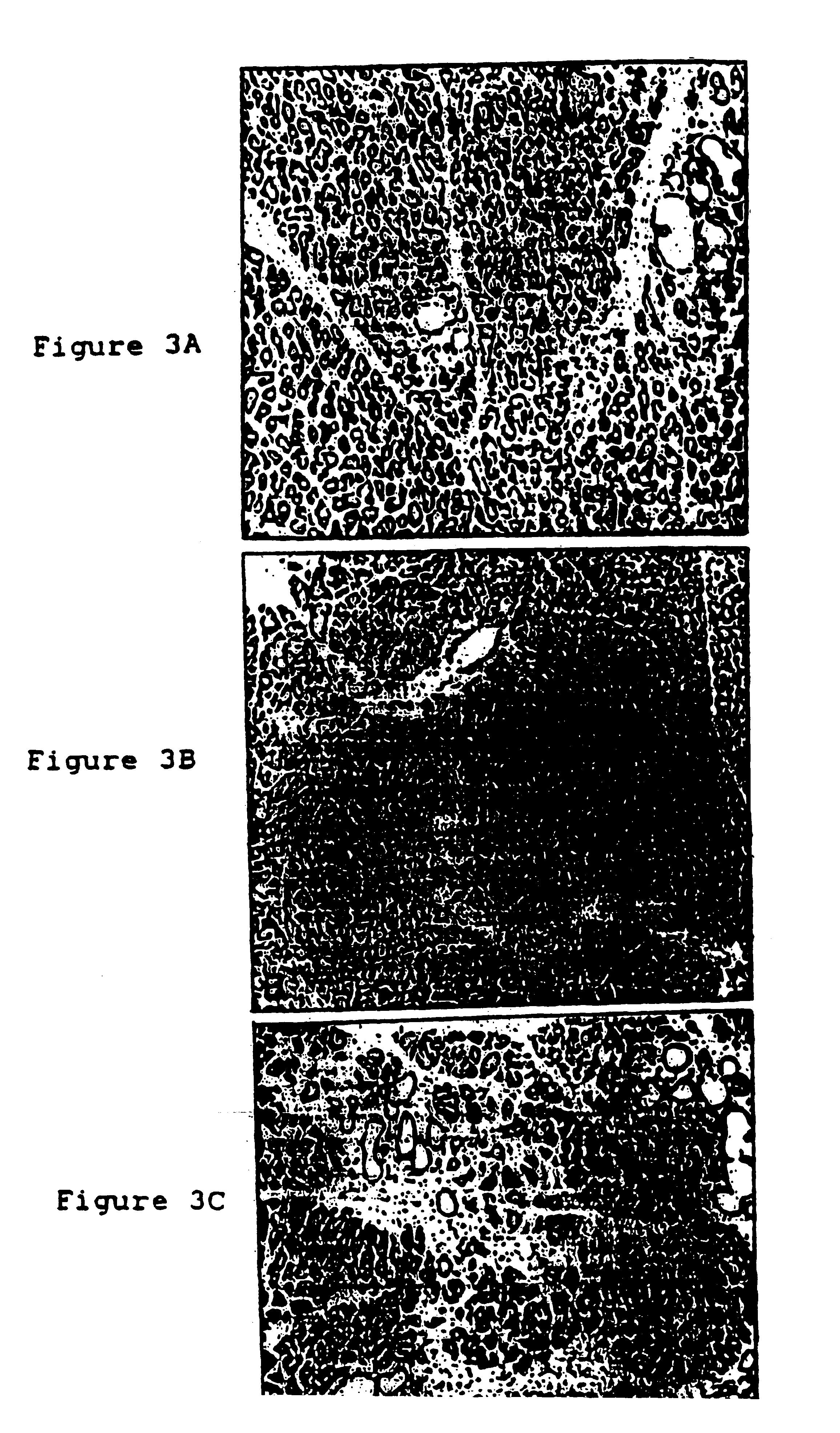
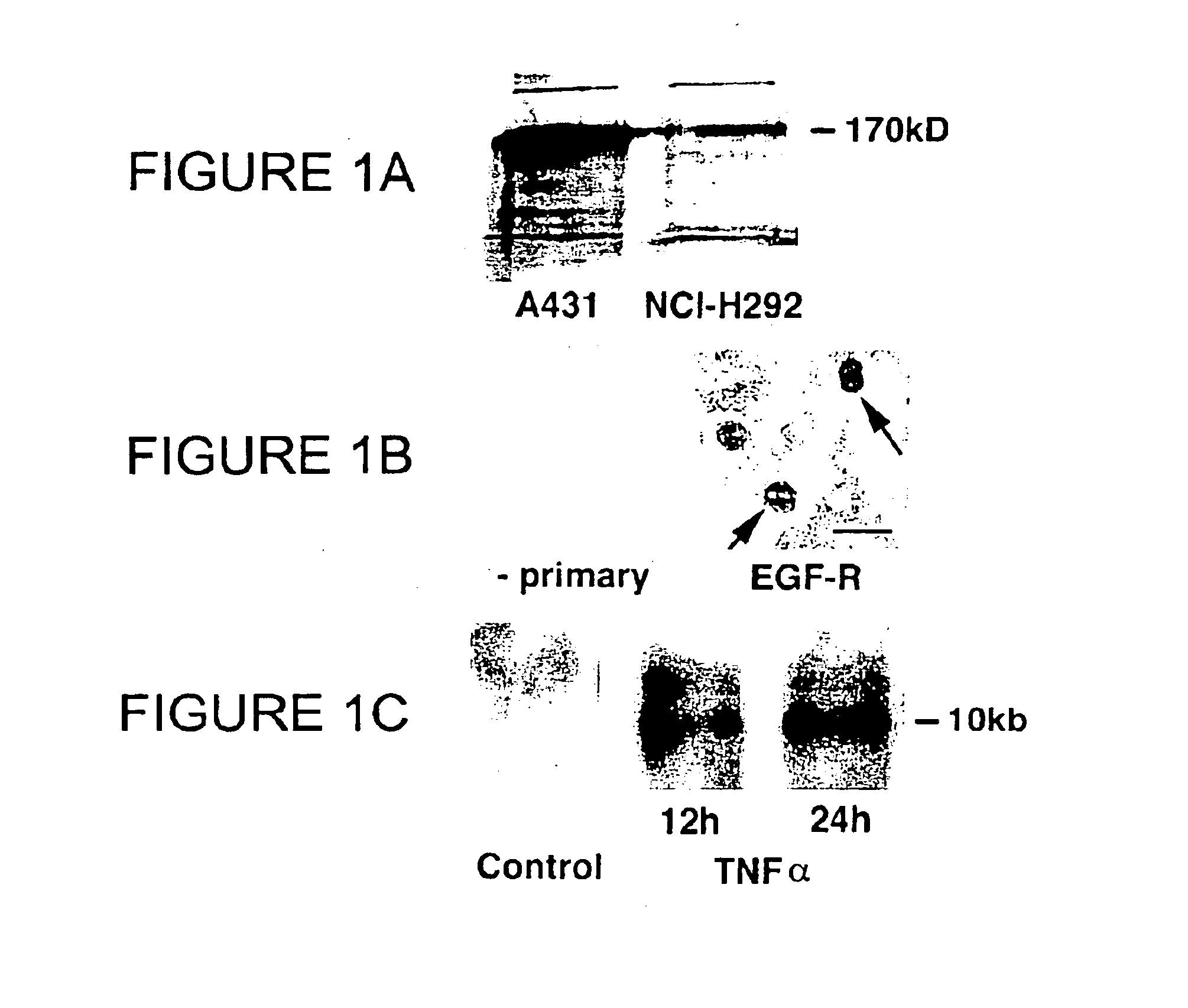
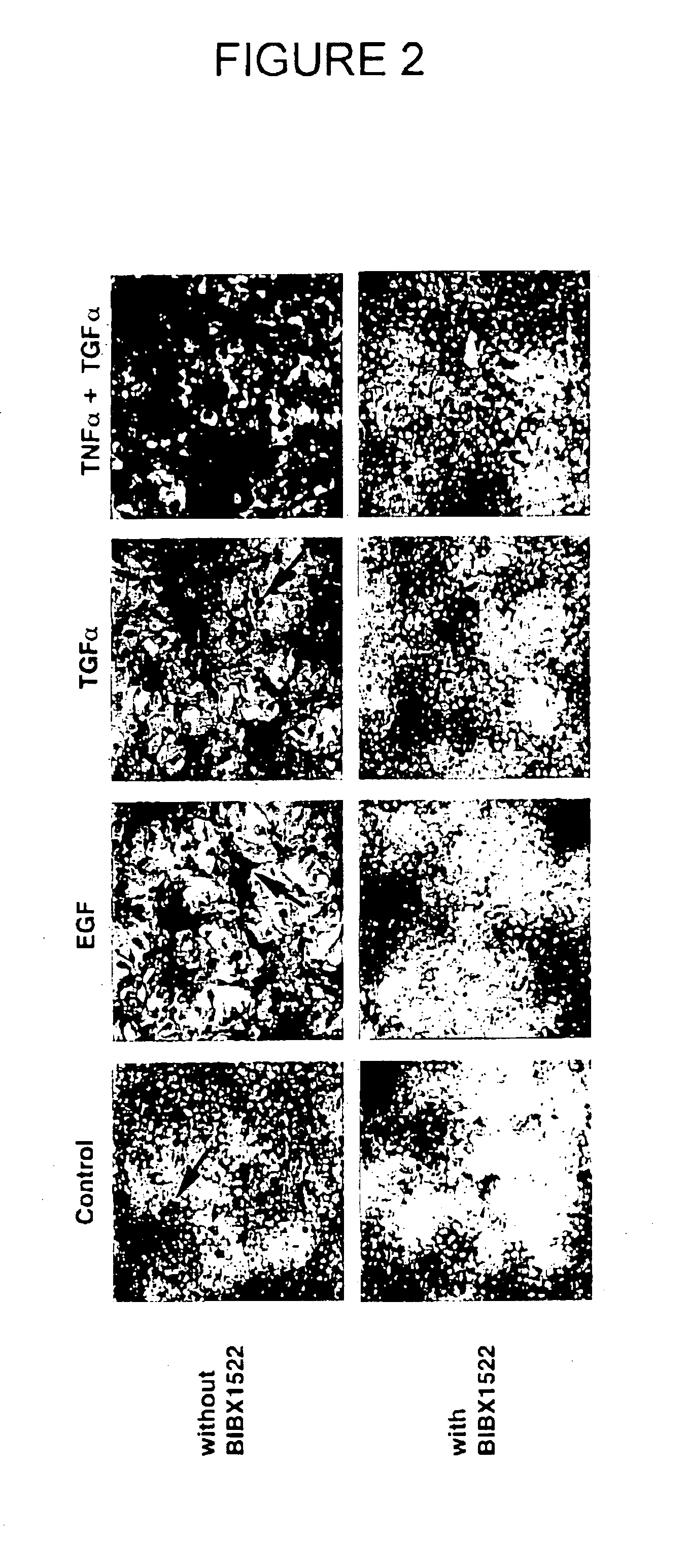
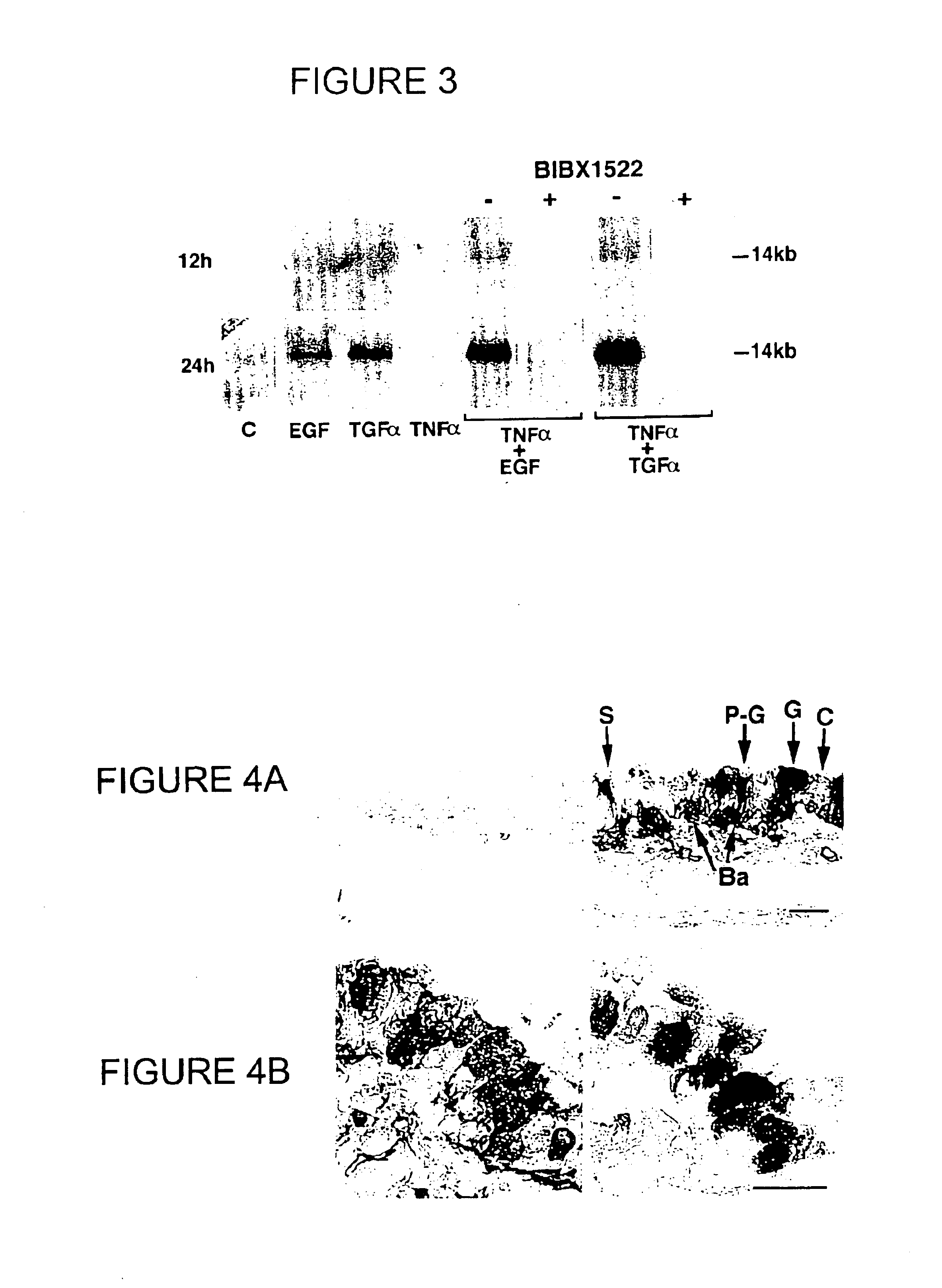
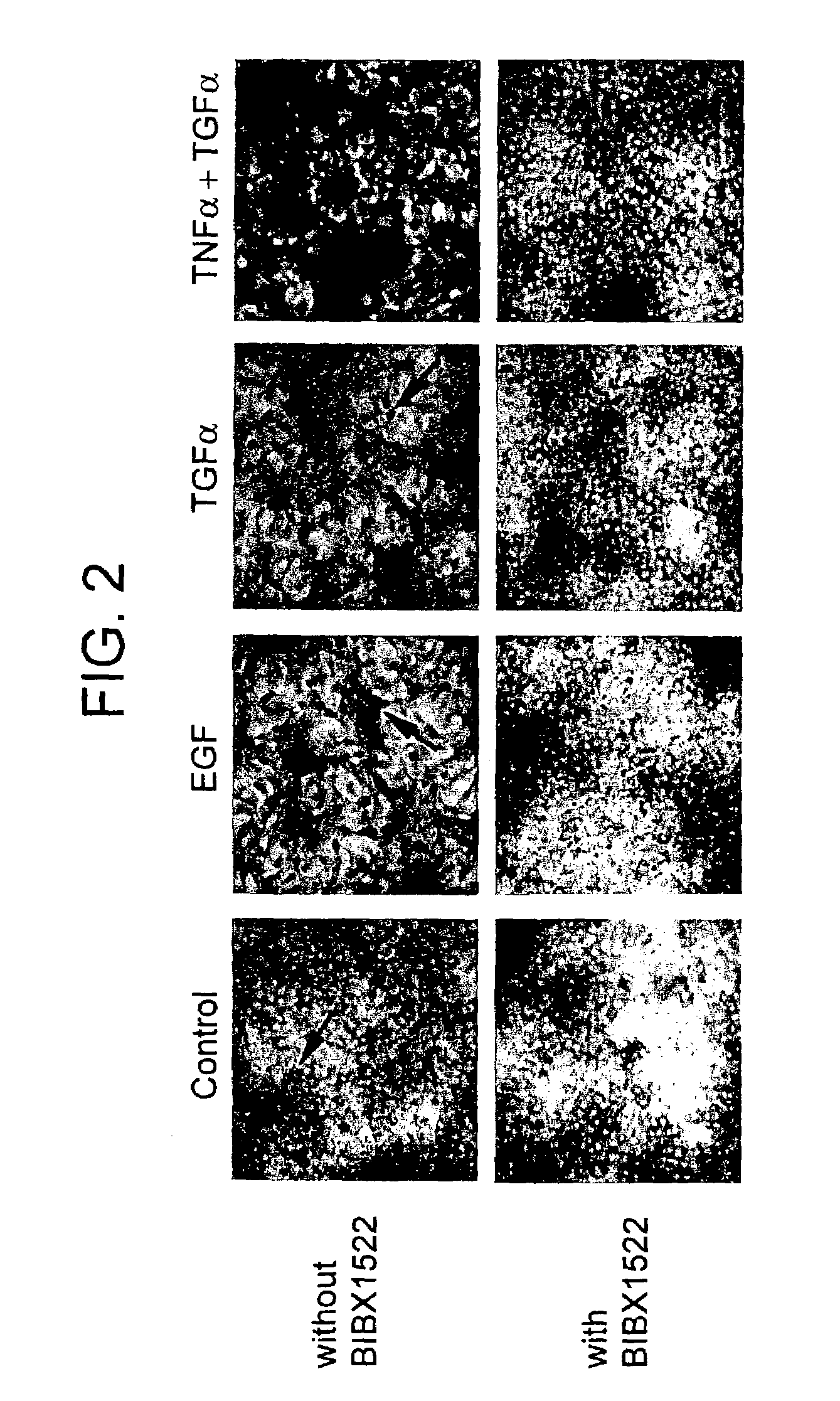
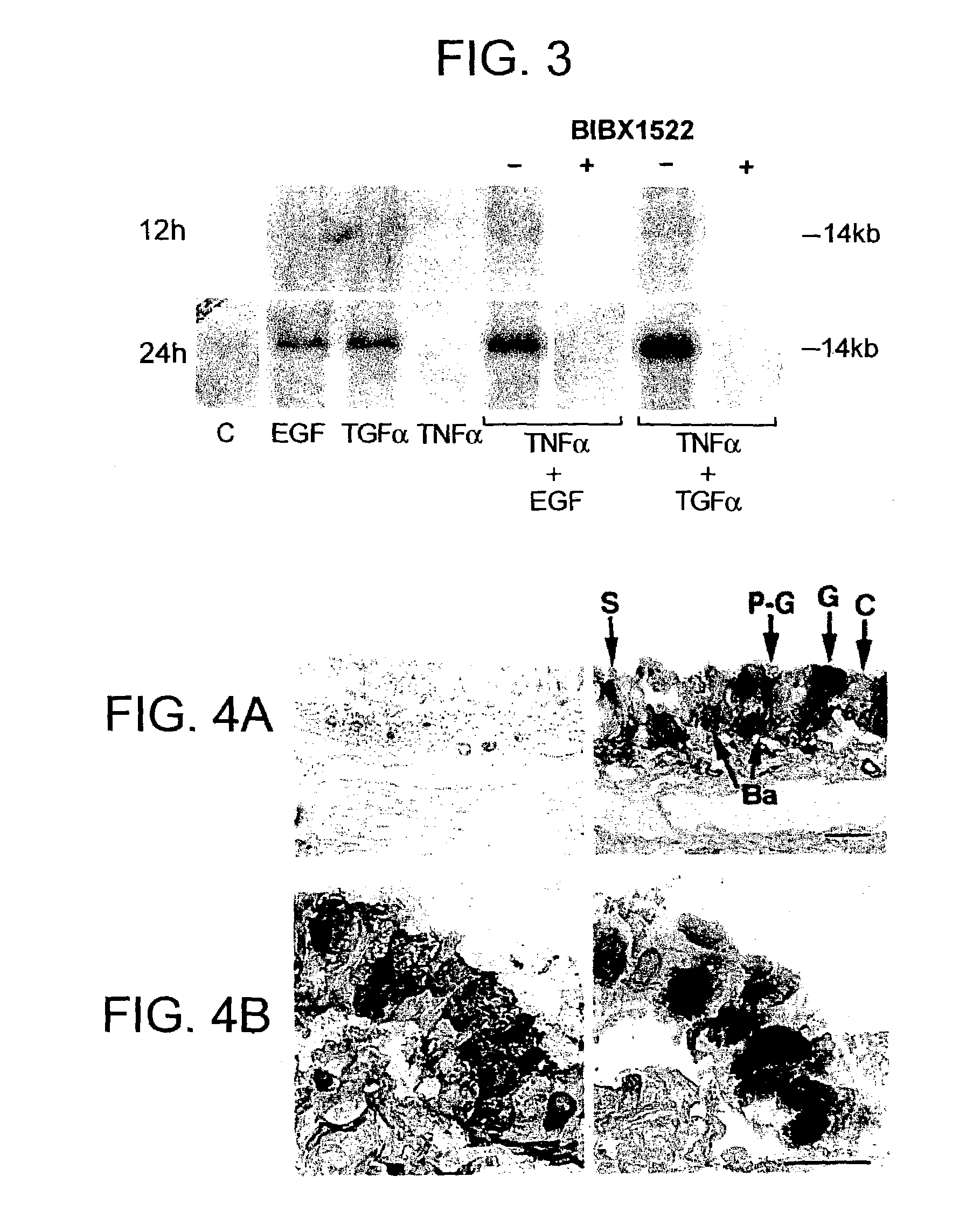
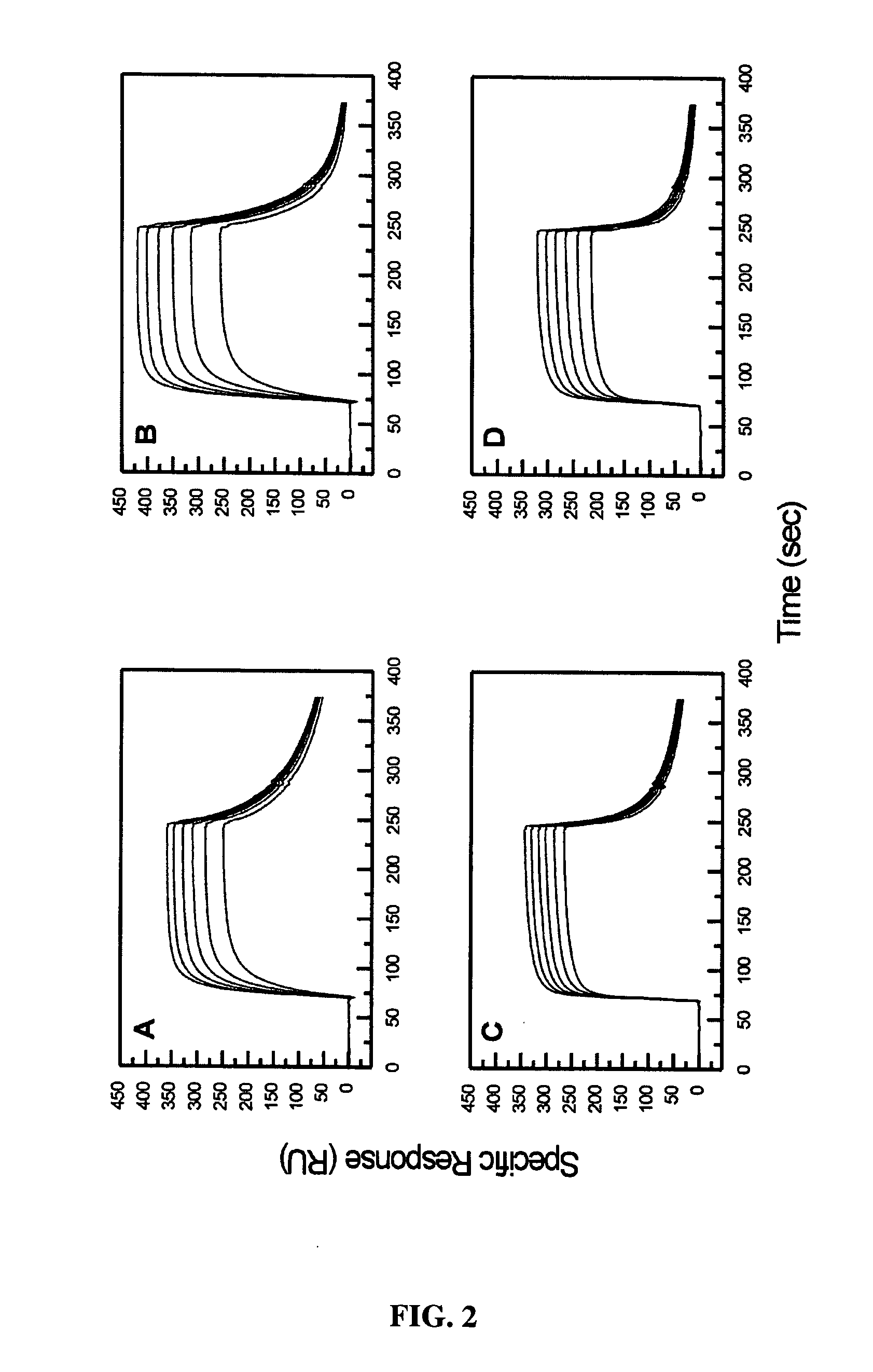
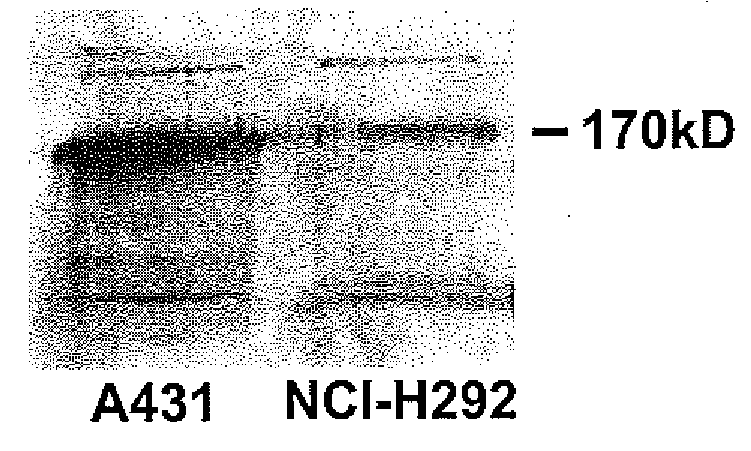
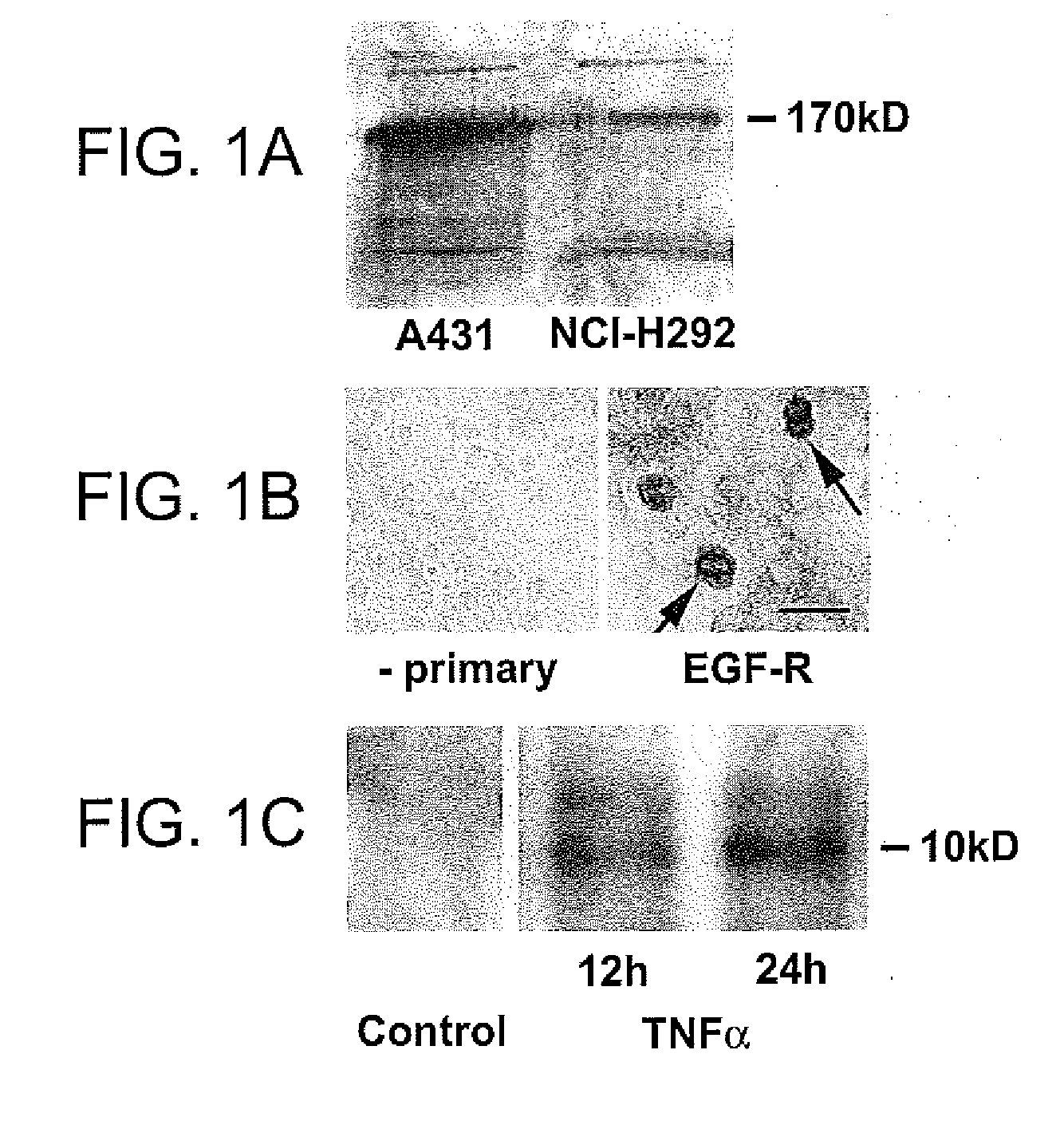
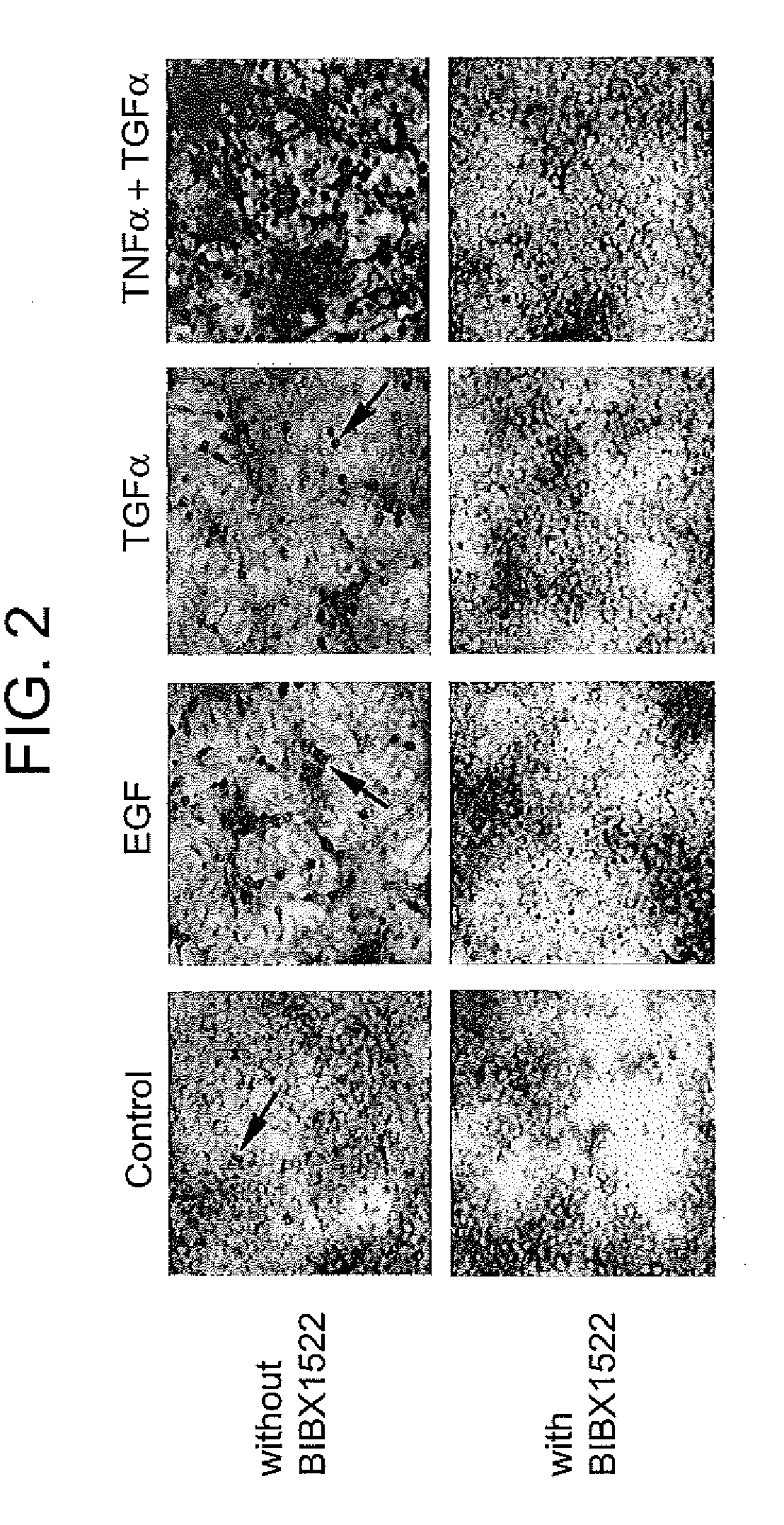
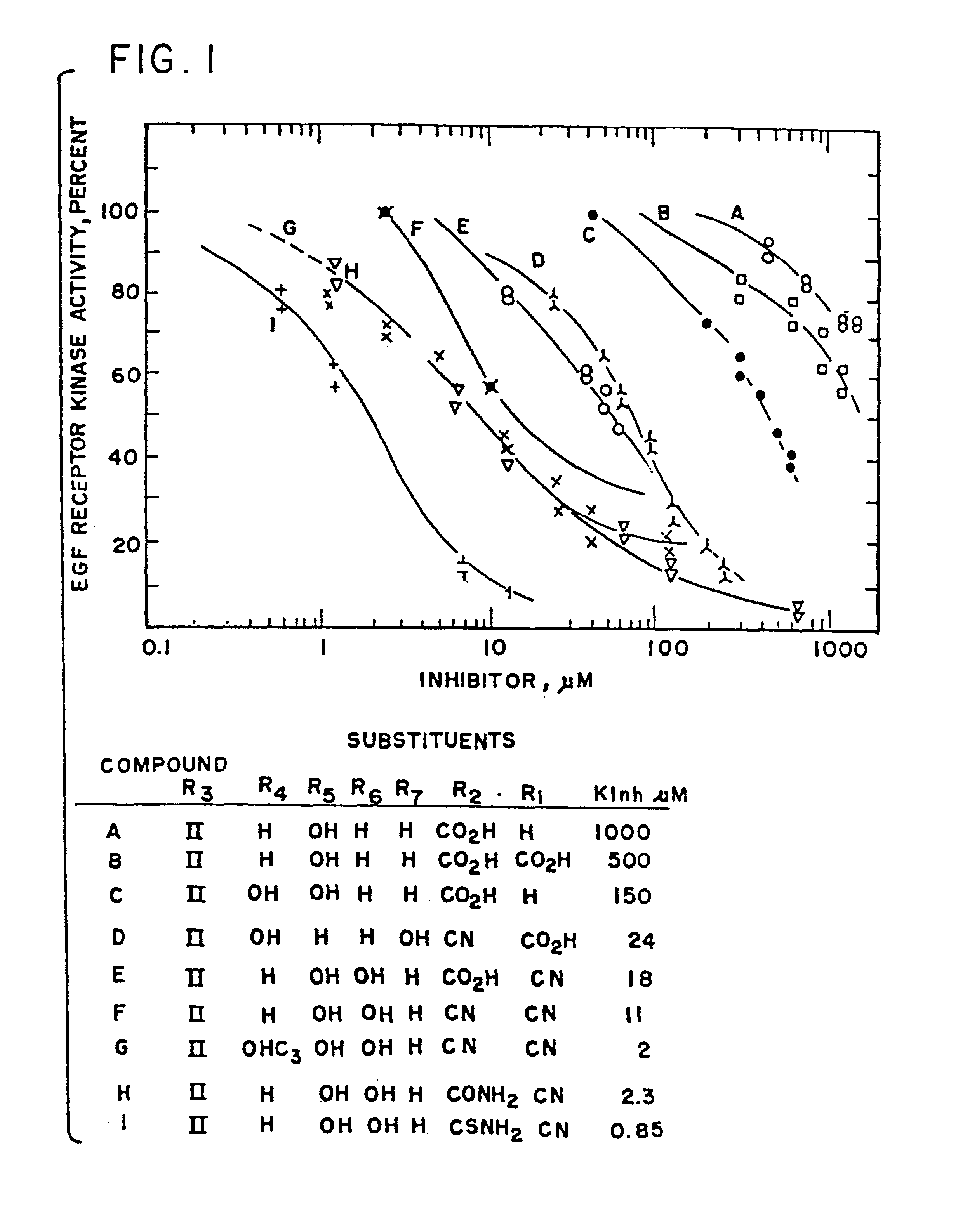
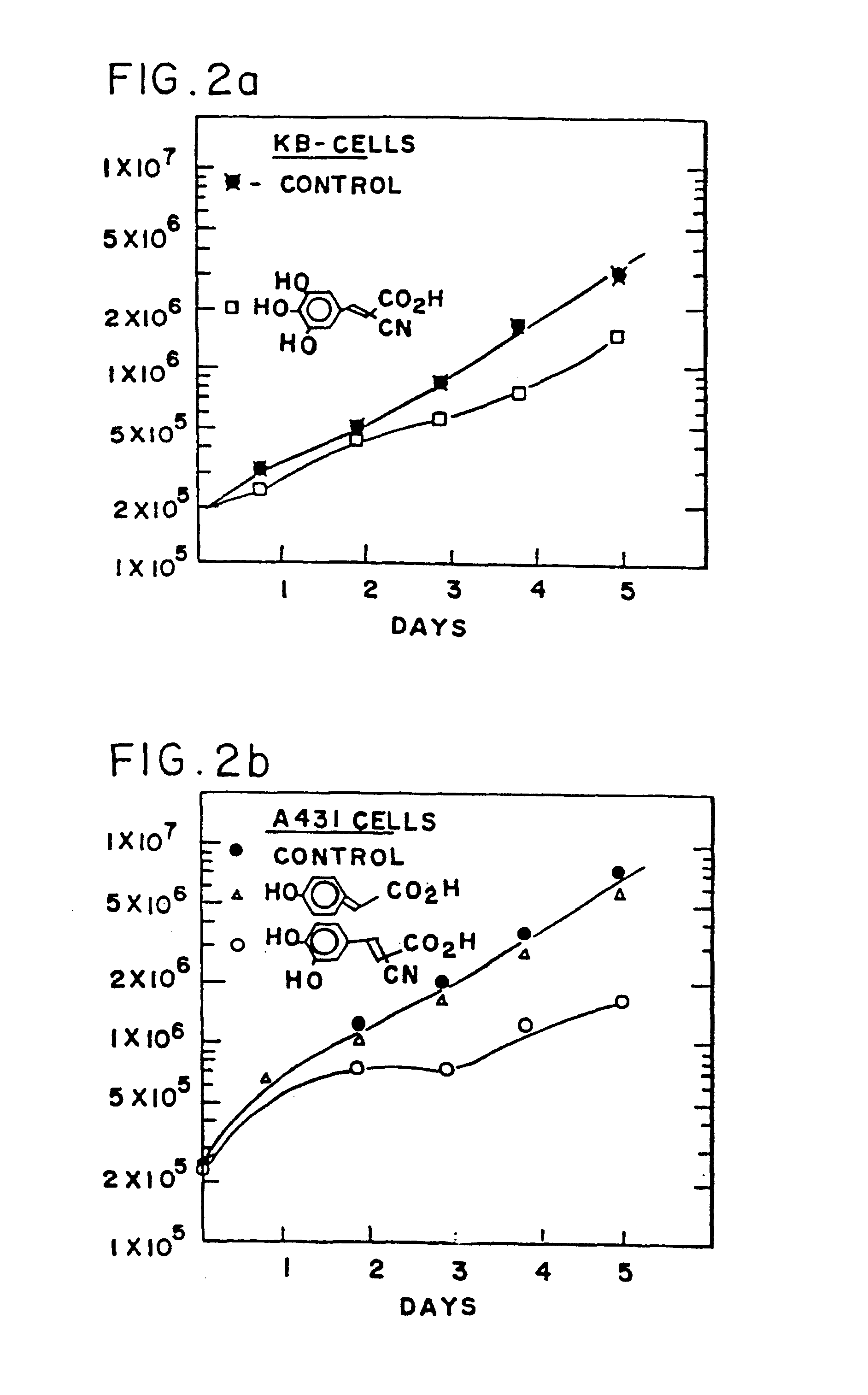
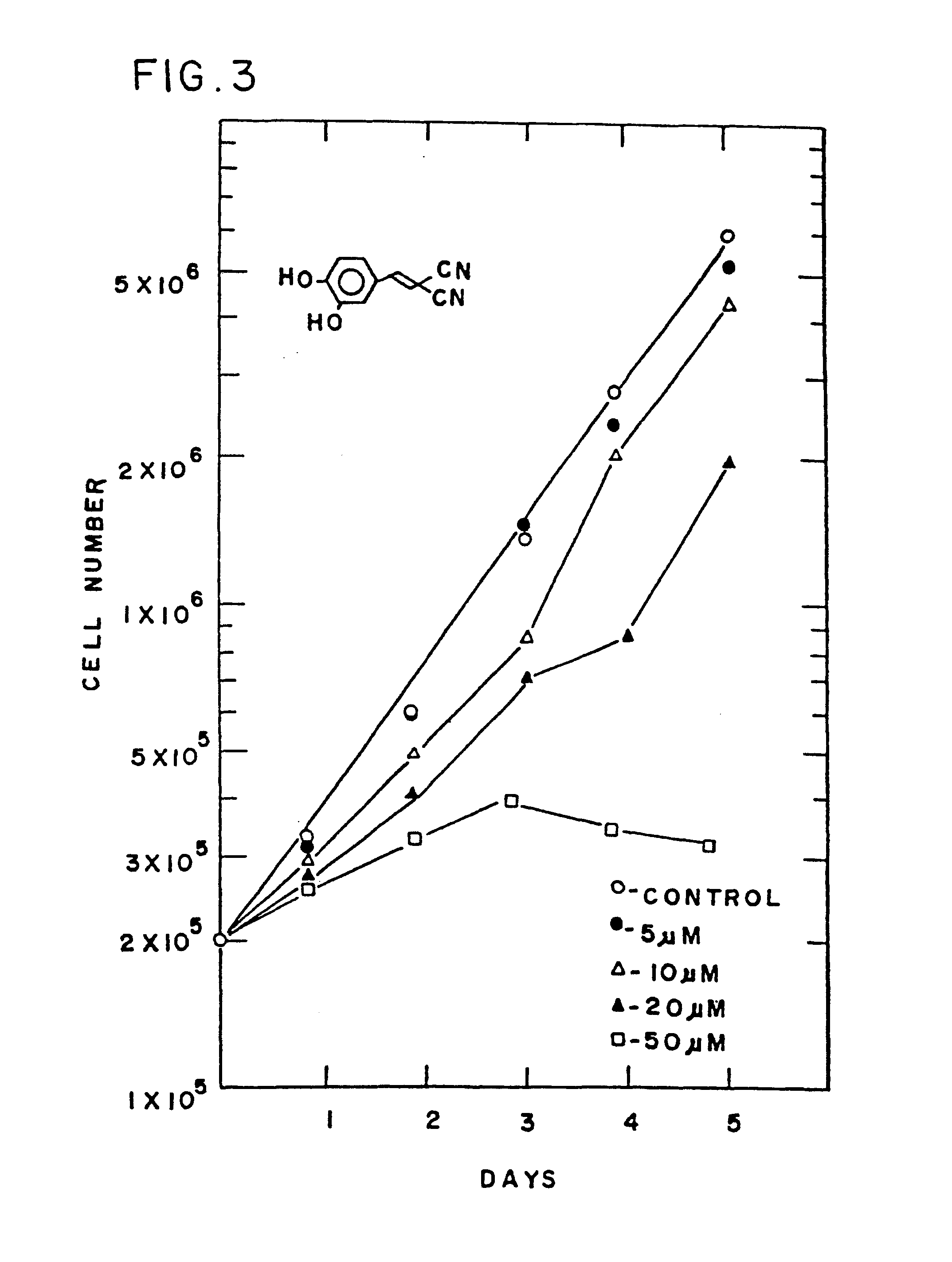
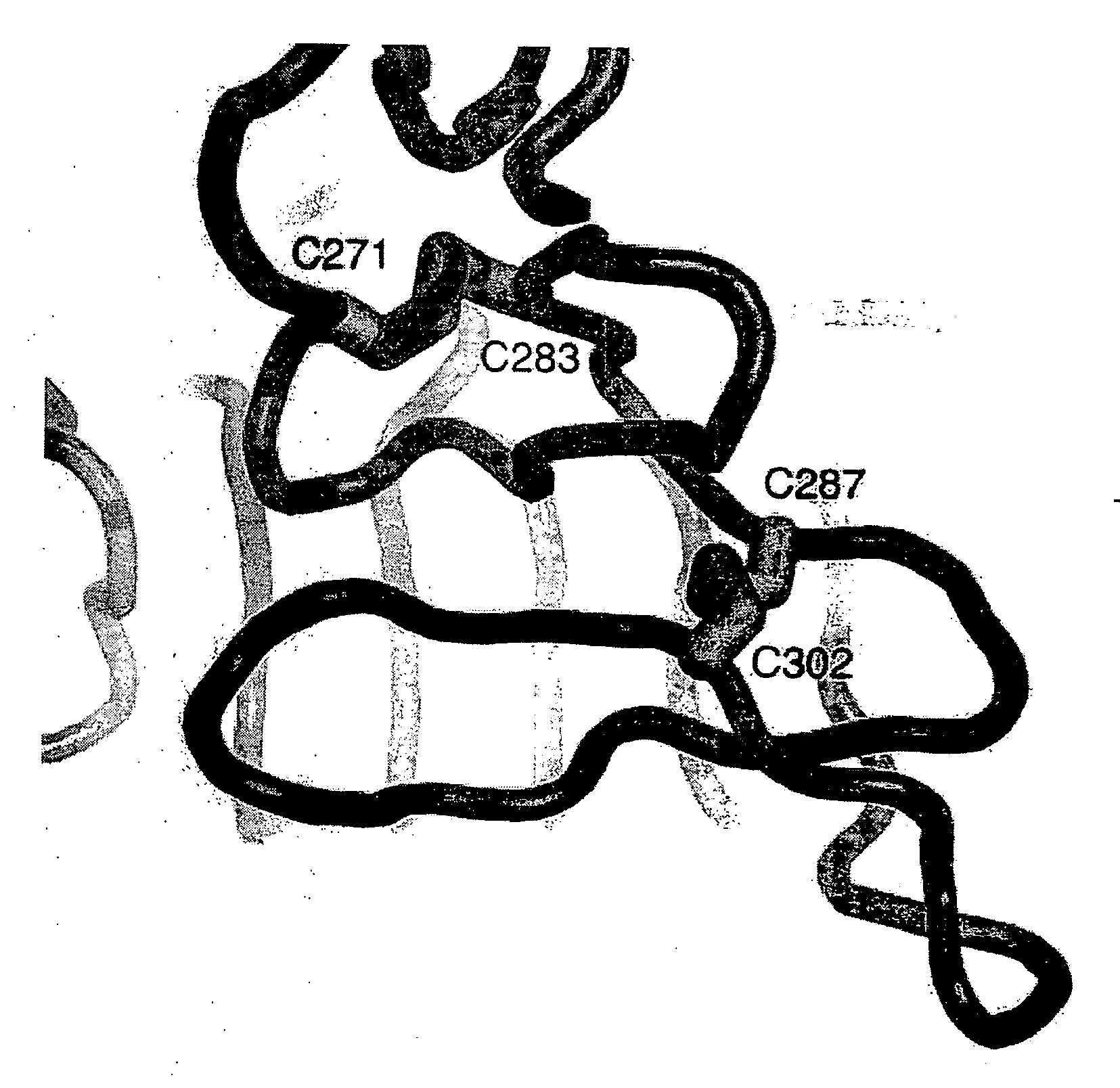
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
90 results about "EGF Receptors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
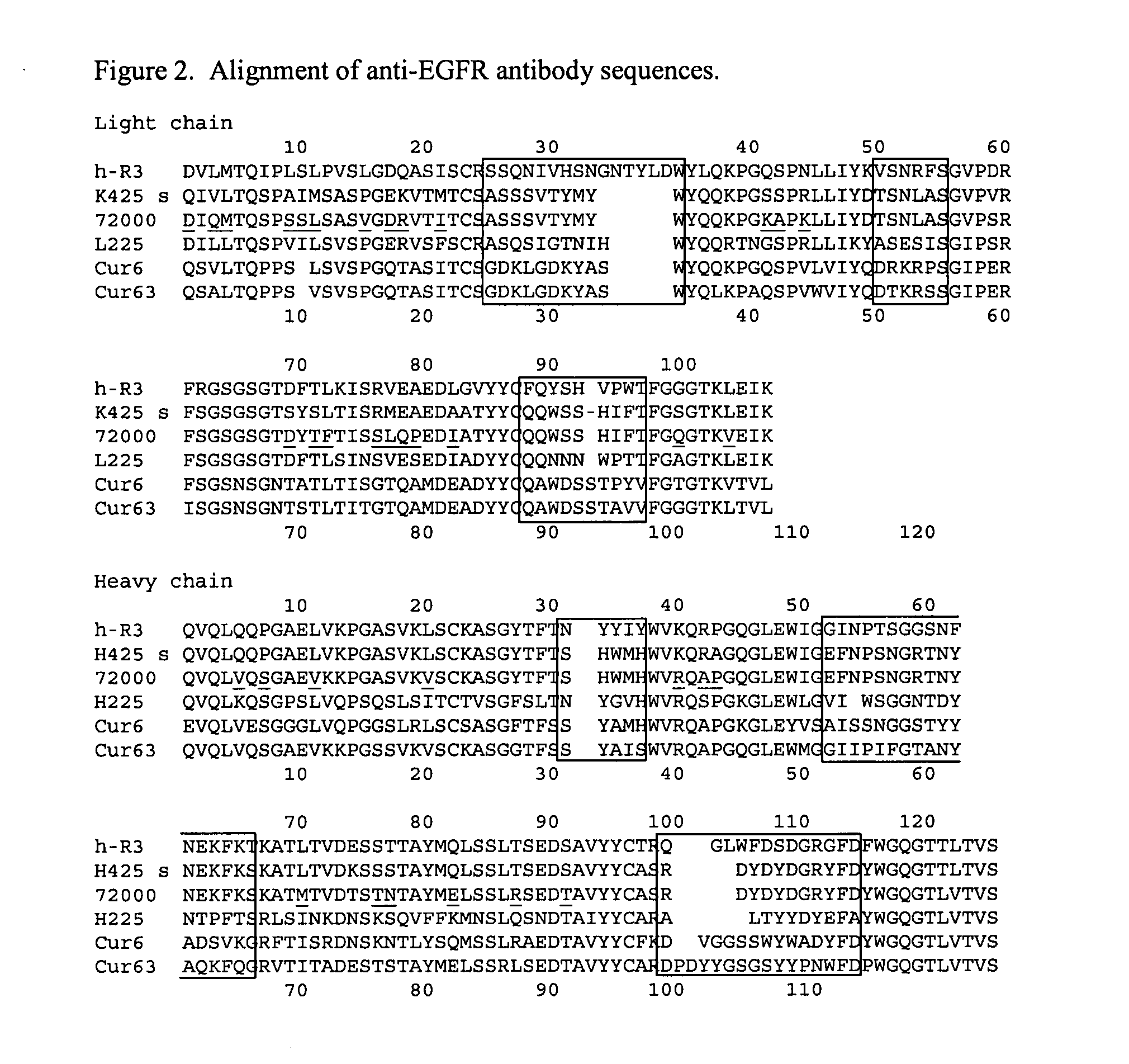
Highly concentrated, liquid formulations of anti-egfr antibodies
InactiveUS20070172475A1Reduce tendency to aggregateLow viscositySenses disorderAntipyreticUltrafiltrationMonoclonal antibody
The invention relates to processes for the preparation of highly concentrated, liquid formulations comprising at least one anti-EGFR antibody and / or one of its variants and / or fragments, in particular monoclonal antibodies against the EGF receptor, particularly preferably of Mab C225 (cetuximab) and Mab h425 (EMD 72000), by ultrafiltration. The invention furthermore relates to highly concentrated, liquid formulations of anti-EGFR antibodies, in particular of monoclonal antibodies against the EGF receptor, particularly preferably Mab C225 (cetuximab) and Mab h425 (EMD 72000) and / or variants and / or fragments thereof, characterised in that the highly concentrated, liquid formulations have a content of anti-EGFR antibodies of 10-250, preferably 50-180 mg / ml, particularly preferably of 100-150 mg / ml, and to the use thereof.
Owner:MERCK PATENT GMBH
Treatment Of Tumors Expressing Mutant EGF Receptors
InactiveUS20090311803A1Reduces and prevents signalingShrink tumorAntibody ingredientsImmunoglobulinsAntibodyEGF Receptors
The invention discloses methods for identifying antibodies that reduce or prevent signaling by intact epidermal growth factor receptor (EGFR), or mutant EGFRs, such as EGFRvIII.
Owner:WAY JEFFREY C +4
EGF receptor epitope peptides and uses thereof
ActiveUS20050255555A1Peptide/protein ingredientsGenetic material ingredientsEpitopeAbnormal tissue growth
The present invention relates generally to growth factor receptor epitope peptides, particularly EGF family receptor epitope peptides. The invention also relates to the use of the receptor peptides in generating antibodies which have anti-tumor or anti-cancer activity or in stimulating an immunological response. The invention further relates to antibodies specifically directed against the receptor peptides. Methods for generating an immune response and for treatment of tumors and cancer are also provided.
Owner:COMMONWEALTH SCI & IND RES ORG +1
Treatment of tumors expressing mutant EGF receptors
InactiveUS20070274991A1Shrink tumorSlowing and preventing in size of tumorAntibody ingredientsImmunoglobulinsEpitopeWilms' tumor
The invention discloses methods of treatment of tumors that express oncogenic forms of the epidermal growth factor receptor (EGFR), such as EGFRvIII. The methods include testing of cancer patients for expression of EGFRvIII in their tumors, followed by treatment with a protein that contains antibody variable regions that recognize specific epitopes on the surface of the EGFR.
Owner:MASSACHUSETTS INST OF TECH +1
Use of inhibitors of the EGFR-mediated signal transduction for the treatment of benign prostatic hyperplasia (BPH)/prostatic hypertrophy
The present invention relates to the use of specific EGF-receptor antagonists for preparing a pharmaceutical composition for the prevention and / or treatment of benign prostatic hyperplasia and / or prostatic hypertrophy, a method for the treatment or prevention of benign prostatic hyperplasia / prostatic hypertrophy comprising administering an EGF-receptor antagonist of groups (A), (B) or (C), described herein optionally in combination with known compounds for the treatment of benign prostatic hyperplasia / prostatic hypertrophy, as well as associated pharmaceutical compositions.
Owner:BOEHRINGER INGELHEIM PHARM KG
Solid forms of anti-EGFR antibodies
InactiveUS7960516B2Improve stabilityHigh purityPowder deliveryFrom normal temperature solutionsAdjuvantDissolution
The invention relates to solid forms of antibodies against the EGF receptor, in particular precipitates and crystals of monoclonal antibodies against the EGF receptor, particularly preferably of Mab C225 (cetuximab) and Mab h425 (EMD 72000), which result in biologically active antibody protein through dissolution or suspension in aqueous medium, obtainable by precipitation of the antibody and / or one of its variants and / or fragments dissolved or suspended in aqueous medium by means of a precipitation reagent. The invention furthermore relates to pharmaceutical preparations comprising at least one solid form of above-mentioned antibodies in precipitated non-crystalline, precipitated crystalline or in soluble or suspended form, and optionally excipients and / or adjuvants and / or further pharmaceutical active ingredients, and to a process for the preparation of solid forms of anti-EGFR antibodies according to the invention.
Owner:MERCK PATENT GMBH
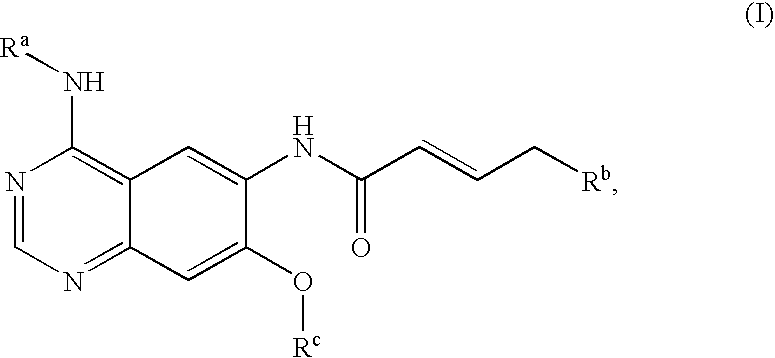
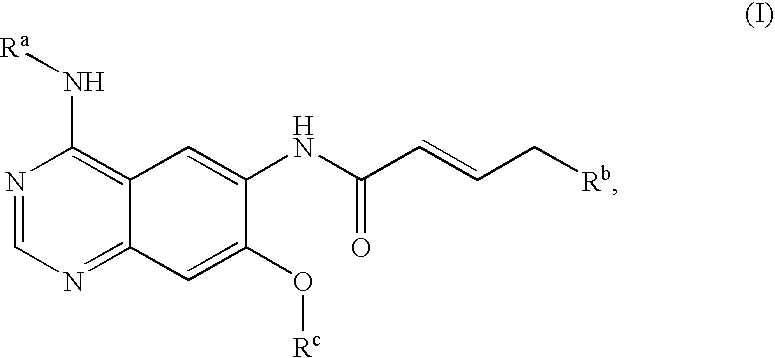
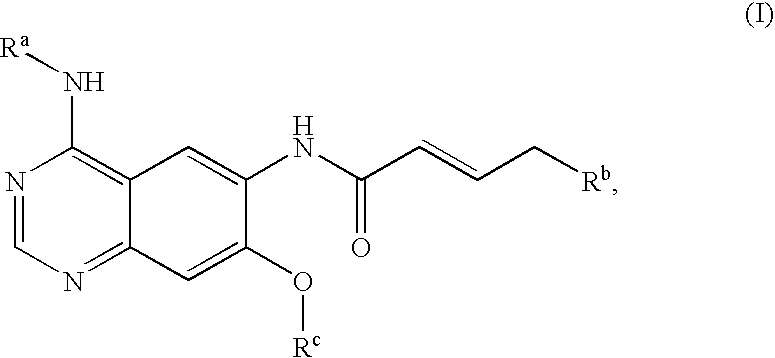
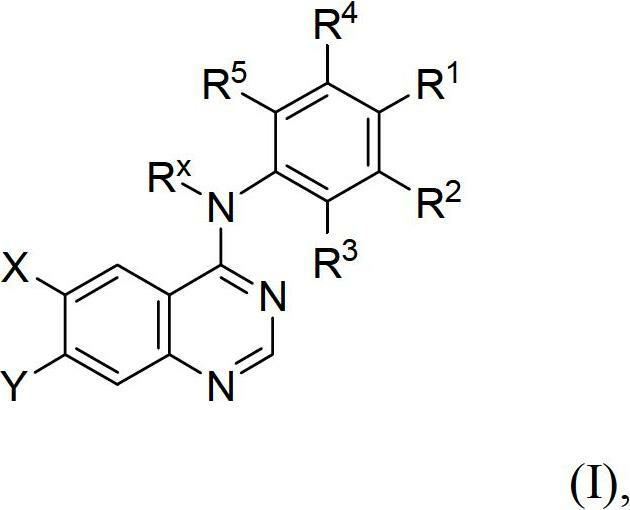
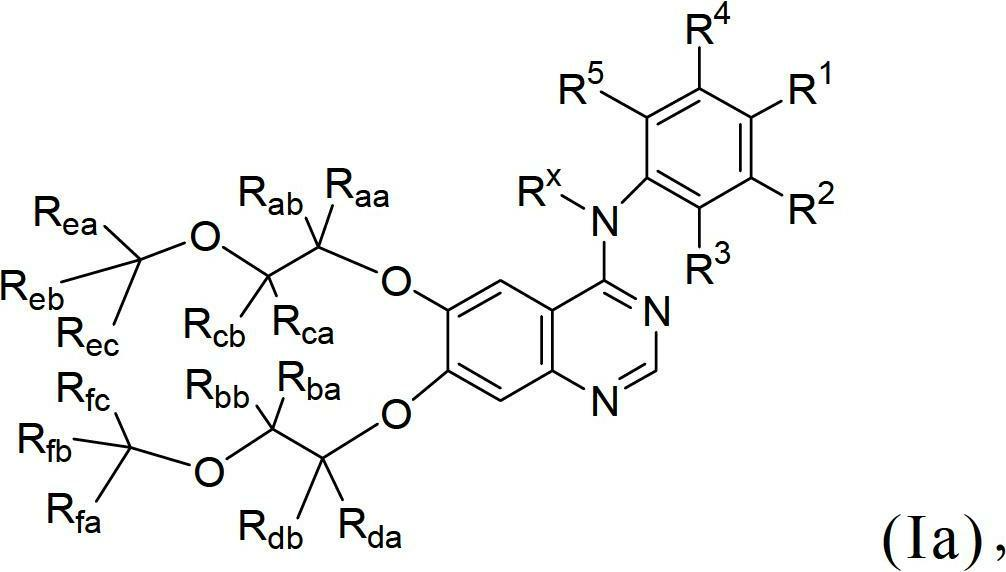
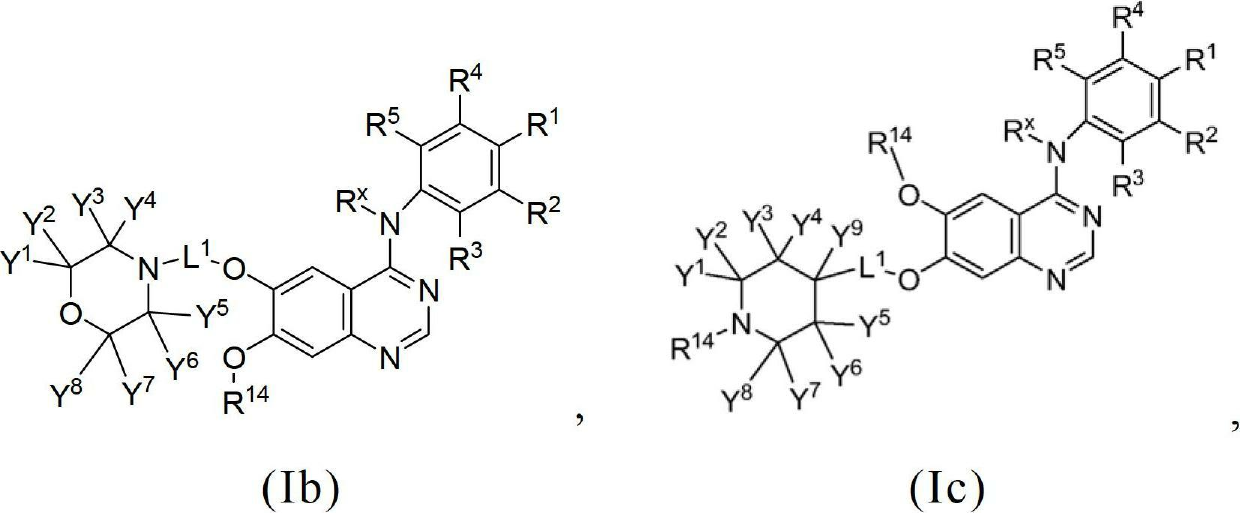
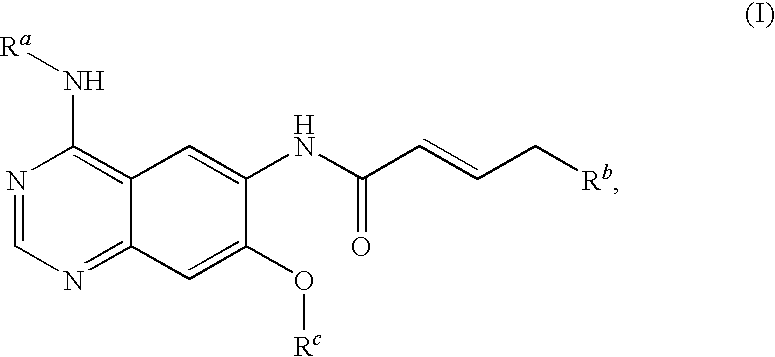
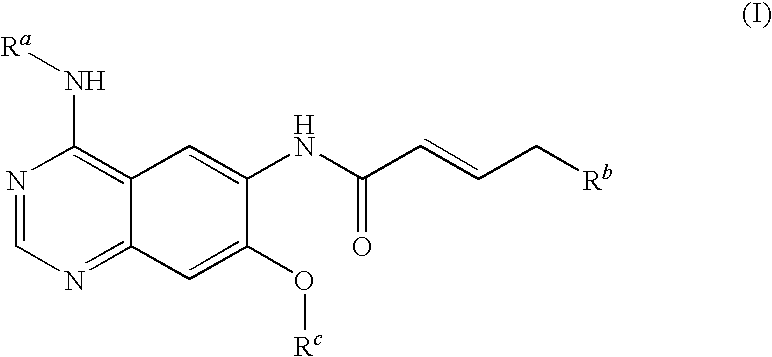
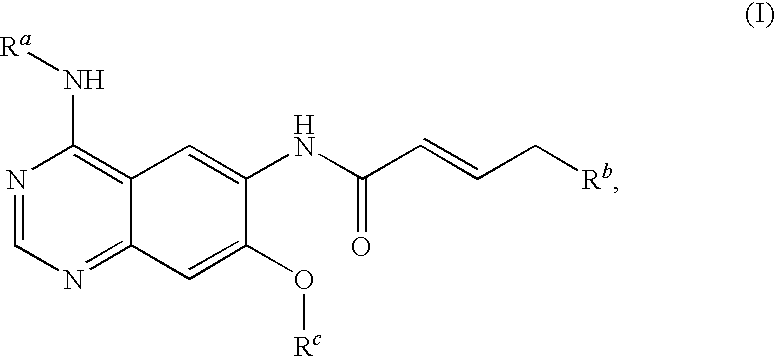
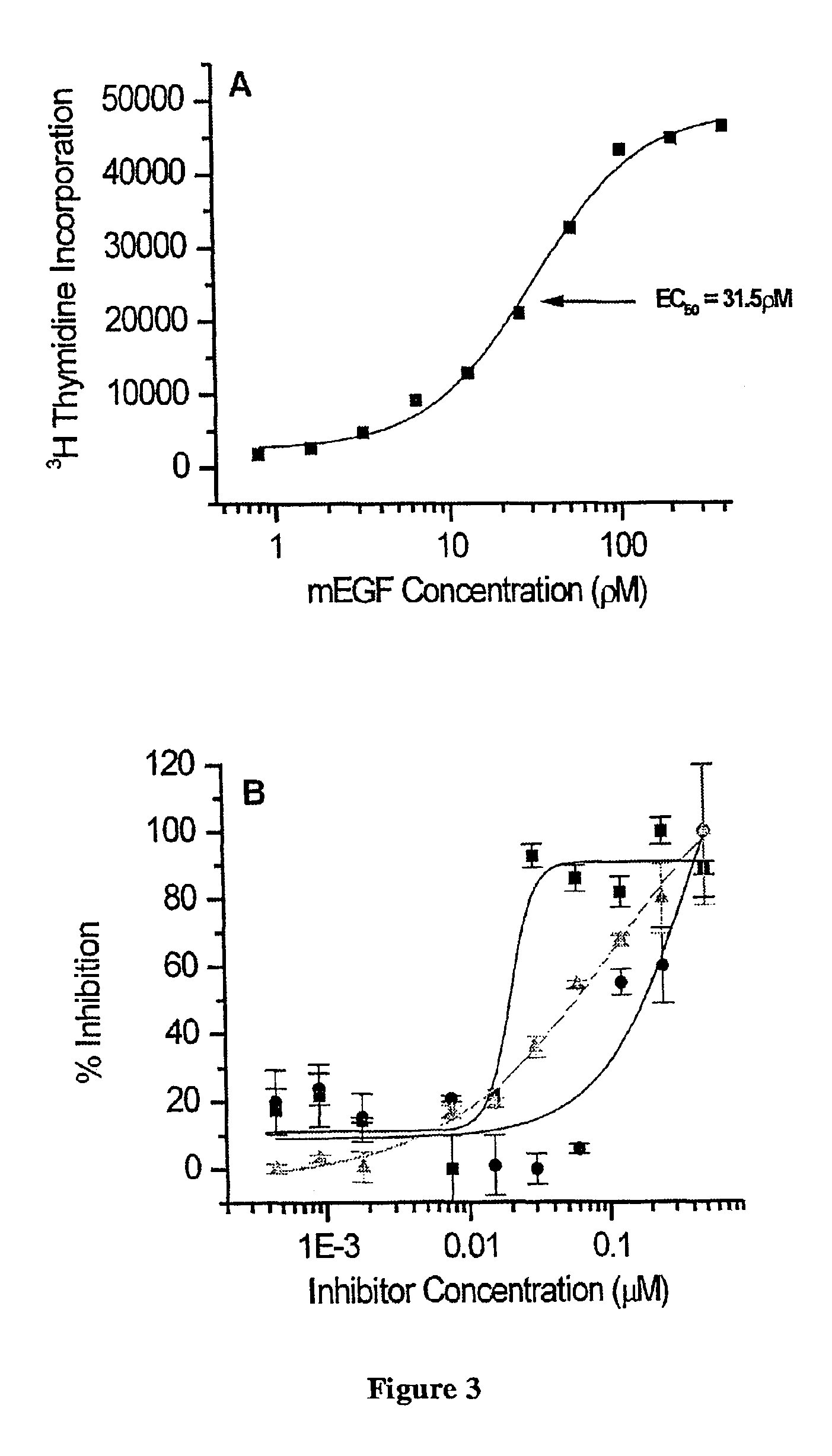
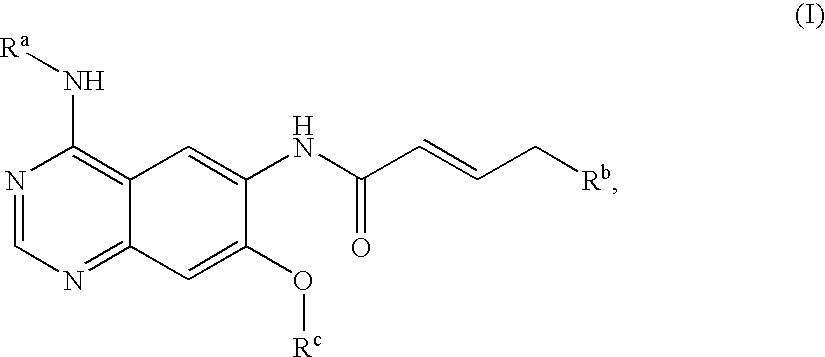
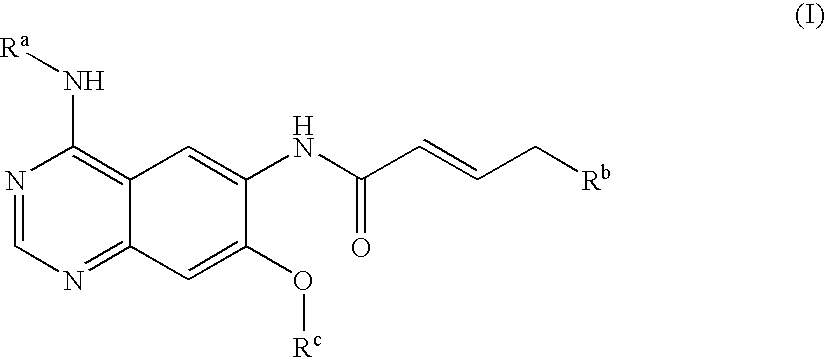
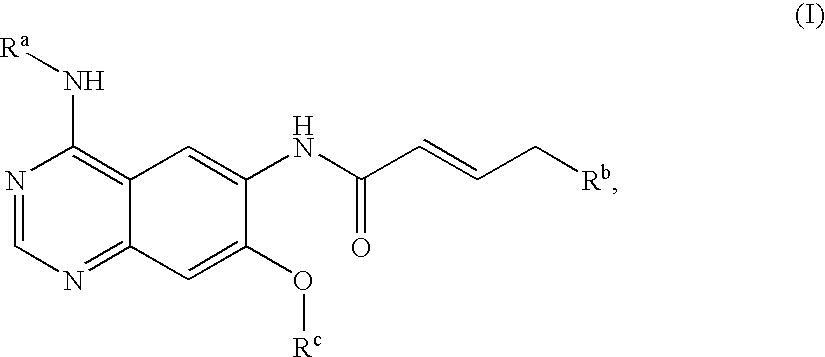
Novel quinazoline derivatives
This disclosure concerns novel quinazoline compounds of Formula (I) as defined in the specification and compositions comprising such novel compounds. These compounds are useful anticancer agents, especially in inhibiting the function of the EGF receptor tyrosine kinases, HERl tyrosine kinase, and HER2 tyrosine kinase. Thus, the disclosure also concerns a method of treating hyperproliferative diseases or conditions, such as various cancers and benign prostate hyperplasia (BPH), by use of these novel compounds or a composition comprising such novel compounds.
Owner:张强 +1
Use of inhibitors of the egfr-mediated signal transduction for the treatment of benign prostatic hyperplasia (BPH) / prostatic hypertrophy
The present invention relates to the use of specific EGF-receptor antagonists for preparing a pharmaceutical composition for the prevention and / or treatment of benign prostatic hyperplasia and / or prostatic hypertrophy, a method for the treatment or prevention of benign prostatic hyperplasia / prostatic hypertrophy comprising administering an EGF-receptor antagonist of groups (A), (B) or (C), described herein optionally in combination with known compounds for the treatment of benign prostatic hyperplasia / prostatic hypertrophy, as well as associated pharmaceutical compositions.
Owner:BOEHRINGER INGELHEIM PHARM KG
Truncated EGF receptor
InactiveUS6946543B2High sensitivityTherapeutic potentialFungiBacteriaEGF Receptor LigandsEGF Receptors
The present invention relates to truncated EGF receptor molecules that exhibit increased binding affinities for EGFR ligands such as EGF and TGF-α. The present invention also relates to methods of screening for EGF receptor ligands and methods of treatment which involve the use of these molecules.
Owner:COMMONWEALTH SCI & IND RES ORG +1
Use of inhibitors of the EGFR-mediated signal transduction for the treatment of benign prostatic hyperplasia (BPH) / prostatic hypertrophy
The present invention relates to the use of specific EGF-receptor antagonists for preparing a pharmaceutical composition for the prevention and / or treatment of benign prostatic hyperplasia and / or prostatic hypertrophy, a method for the treatment or prevention of benign prostatic hyperplasia / prostatic hypertrophy comprising administering an EGF-receptor antagonist of groups (A), (B) or (C), described herein optionally in combination with known compounds for the treatment of benign prostatic hyperplasia / prostatic hypertrophy, as well as associated pharmaceutical compositions.
Owner:BOEHRINGER INGELHEIM PHARMA KG
Antibodies to EGF receptor epitope peptides
The present invention relates generally to growth factor receptor epitope peptides, particularly EGF family receptor epitope peptides. The invention also relates to the use of the receptor peptides in generating antibodies which have anti-tumor or anti-cancer activity or in stimulating an immunological response. The invention further relates to antibodies specifically directed against the receptor peptides. Methods for generating an immune response and for treatment of tumors and cancer are also provided.
Owner:COMMONWEALTH SCI & IND RES ORG +1
Pharmaceutical Compounds
InactiveUS20100004232A1Reduce morbidityPatient compliance is goodBiocideSenses disorderDiseaseImatinib resistant
The use of a compound for the manufacture of a medicament for the prophylaxis or treatment of: A. a disease state or condition mediated by a kinase which is BCR-abl, VEGFR, PDGFR, EGFR, Flt3, JAK (e.g. JAK2 or JAK3), C-abl, PDK1, Chk (e.g. Cbk1 or Chk2), FGFR (e.g. FGFR3), Ret, Eph (e.g. EphB2 or EphB4), or Src (e.g. cSrc); or B. a cancer in which the cancer cells thereof contain a drug resistant kinase mutation which is: (a) a threonine gatekeeper mutation; or (b) a drug-resistant gatekeeper mutation; or (c) an imatinib resistant mutation; or (d) a nilotinib resistant mutation; or (e) a dasatinib resistant mutation; or (f) a T670I mutation in KIT; or (g) a T674I mutation in PDGFR; or (h) T790M mutation in EGFR; or (i) a T315I mutation in abl; or C. a cancer which expresses a mutated molecular target which is a mutated form of BCRabl, c-kit, PDGF, EGF receptor or ErbB2; or D. a disease mediated by a kinase containing a mutation in a region of the protein that binds to or interacts with other cancer agents but does not bind to or interact with the compounds of formula (I) or (I′), for example a mutated kinase selected from c-abl, c-kit, PDGFR including PDGFR-beta and PDGFR-alpha, and ErbB family members such as EGFR (ErbB1), HER2 (ErbB2), ErbB3, and ErbB4, members of the Ephrin receptor family including EphA1, EphA2, EphA3, EphA4, EphA5, EphA8, EphA10, EphB1, EphB2, EphB3, EphB5, EphB6, c-Src and kinases of the JAK family such as TYK2; wherein the compound is a compound of the formula (I or I′): or a salt, solvate, tautomer or N-oxide thereof wherein R0′, R1, R1′, R2′, R3′, R4′, A′, X′, E, A and M are as defined in the claims.
Owner:ASTEX THERAPEUTICS LTD
Treatment for diabetes
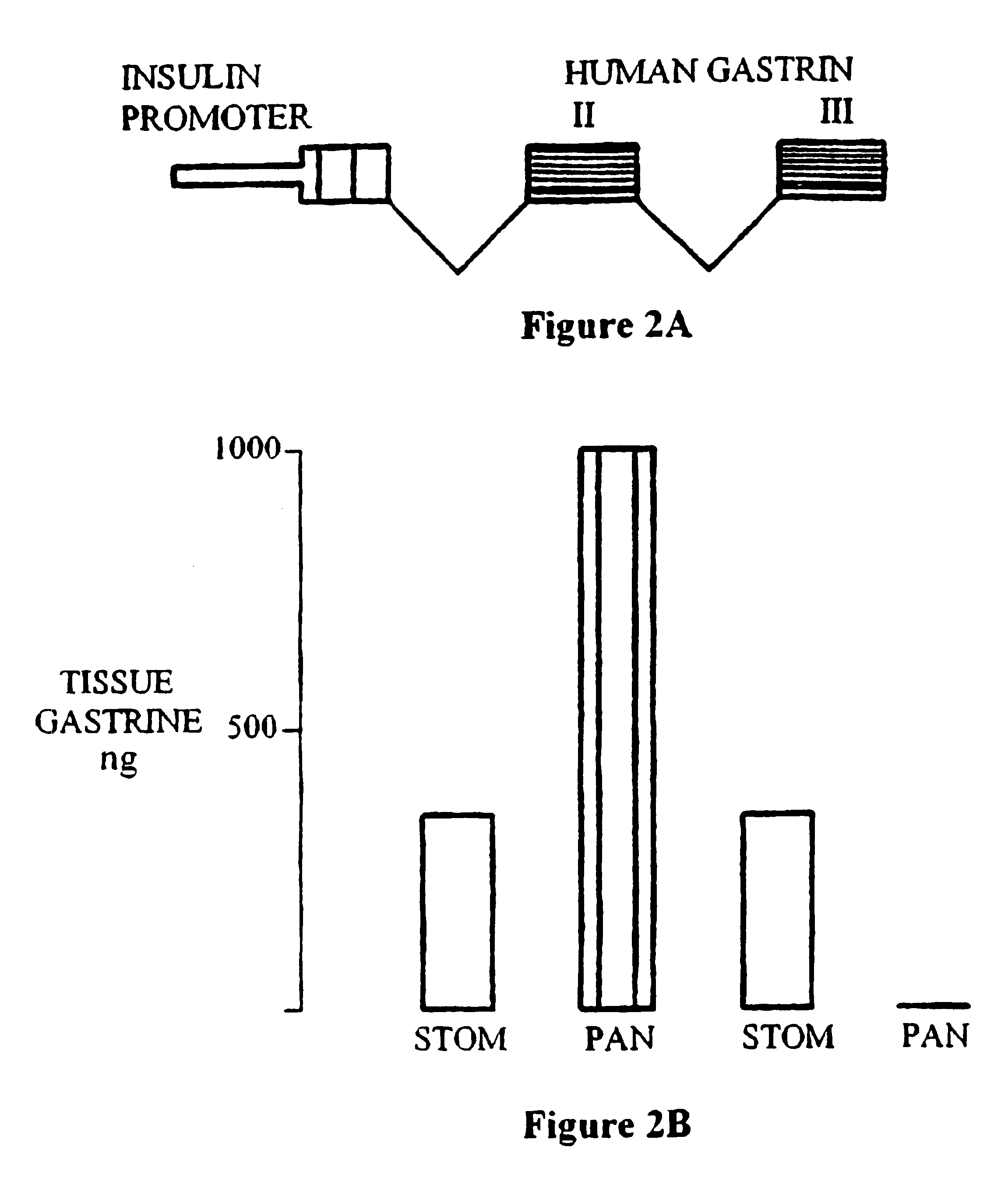
InactiveUS6558952B1Not prevent and decrease progressionMore complicatedOrganic active ingredientsPeptide/protein ingredientsInsulin Secreting CellEGF Receptors
Methods and compositions for treating diabetes mellitus in a patient in need thereof are provided. The methods include administering to a patient a composition providing a gastrin / CCK receptor ligand, e.g., a gastrin, and / or an epidermal growth factor (EGF) receptor ligand, e.g., TGF-alpha, in an amount sufficient to effect differentiation of pancreatic islet precursor cells to mature insulin-secreting cells. The composition can be administered systemically or expressed in situ by cells transgenically supplemented with one or both of a gastrin / CCK receptor ligand gene, e.g., a preprogastrin peptide precursor gene and an EGF receptor ligand gene, e.g., a TGF-alpha gene. The methods also include transplanting into a patient cultured pancreatic islets in which mature insulin-secreting beta cells are proliferated by exposure to a gastrin / CCK receptor ligand and an EGF receptor ligand.
Owner:THE GENERAL HOSPITAL CORP +1
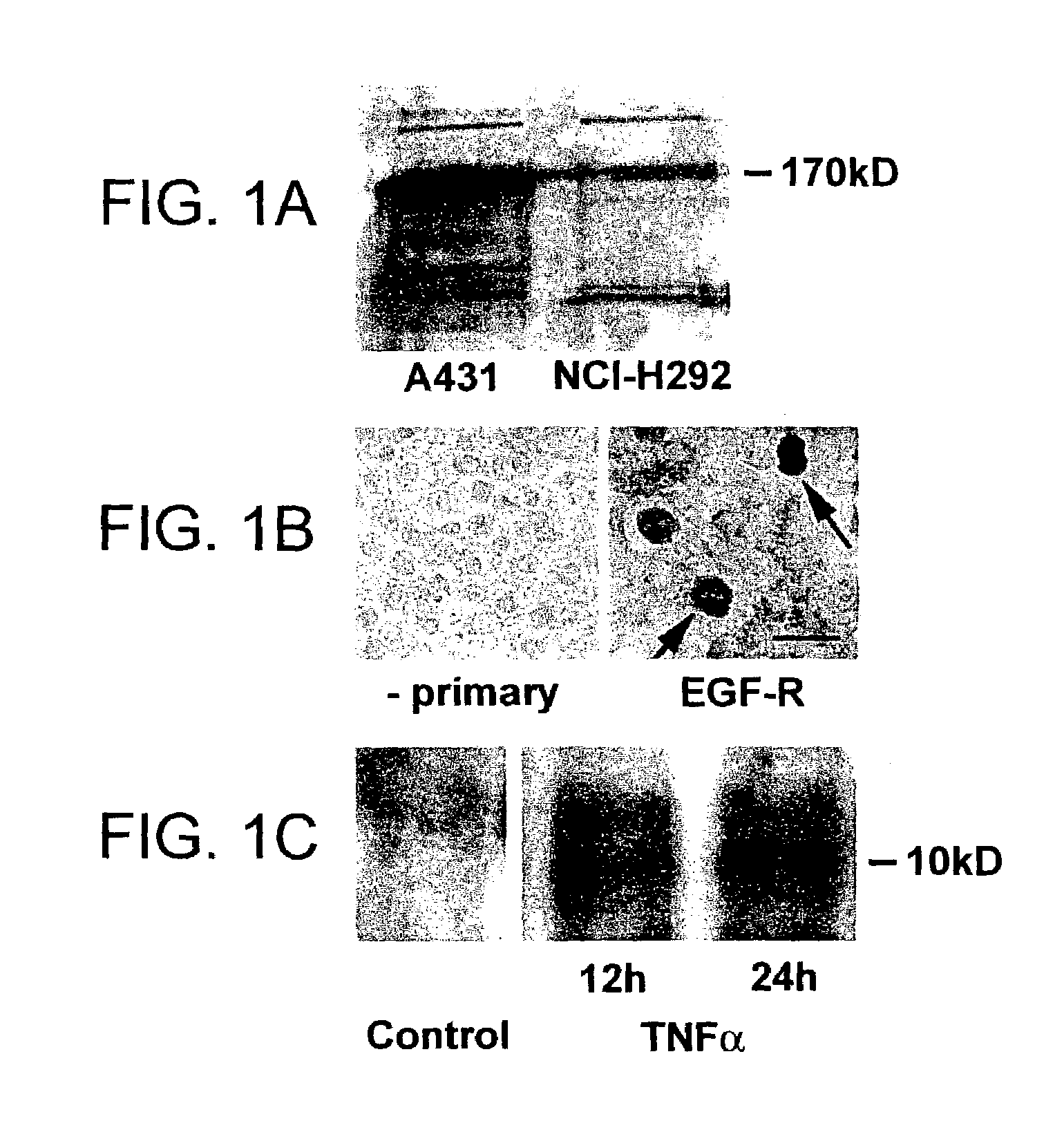
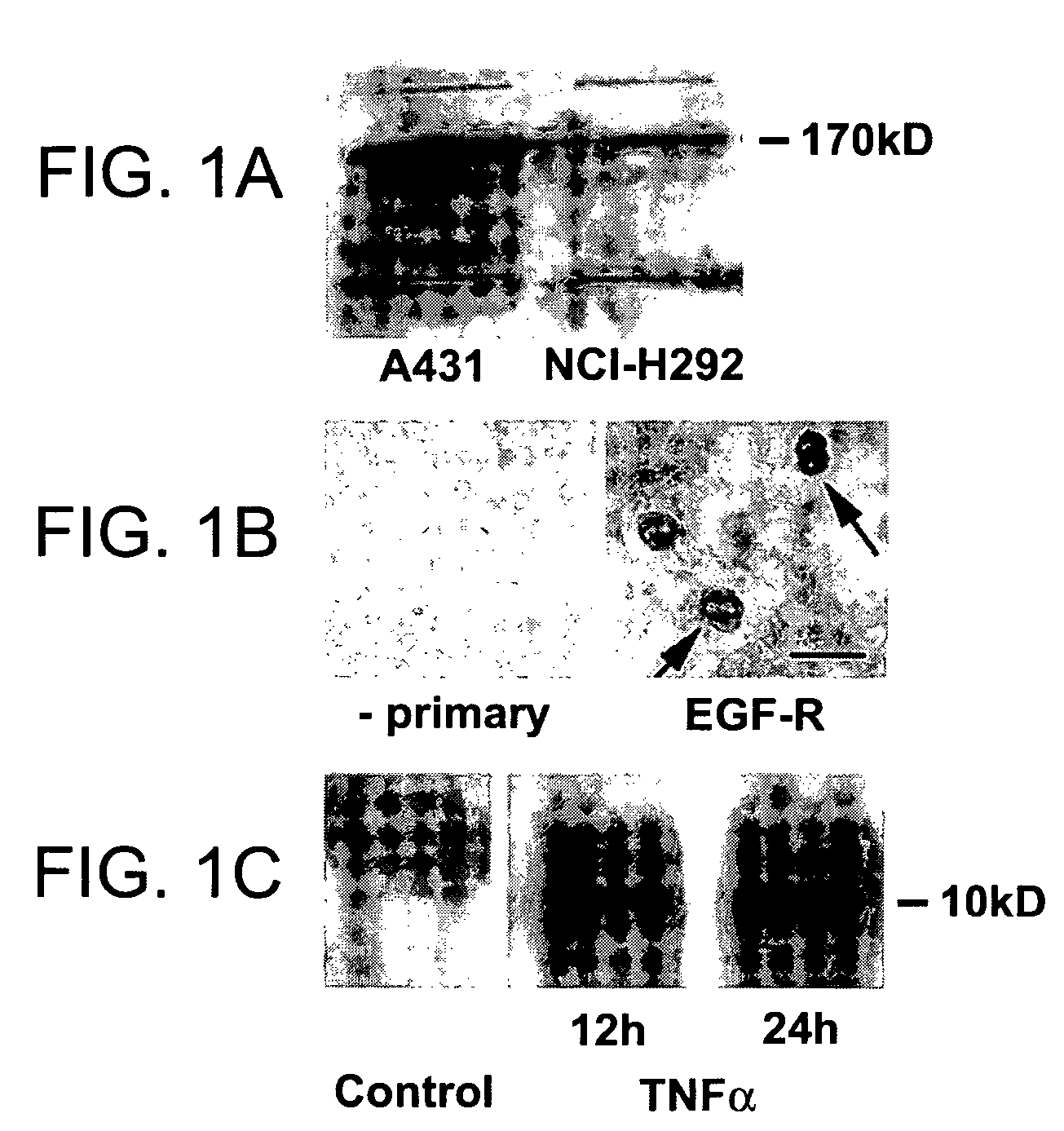
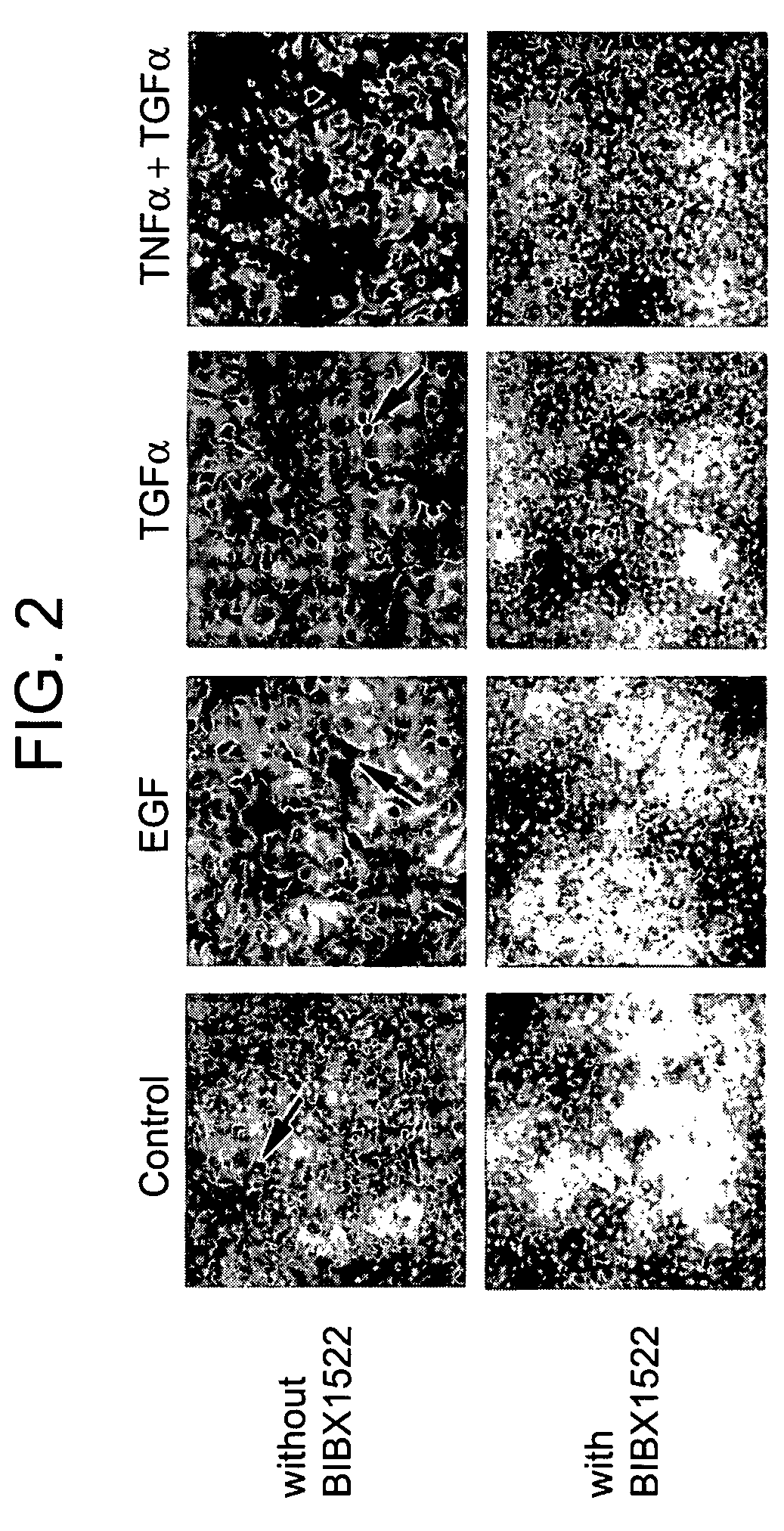
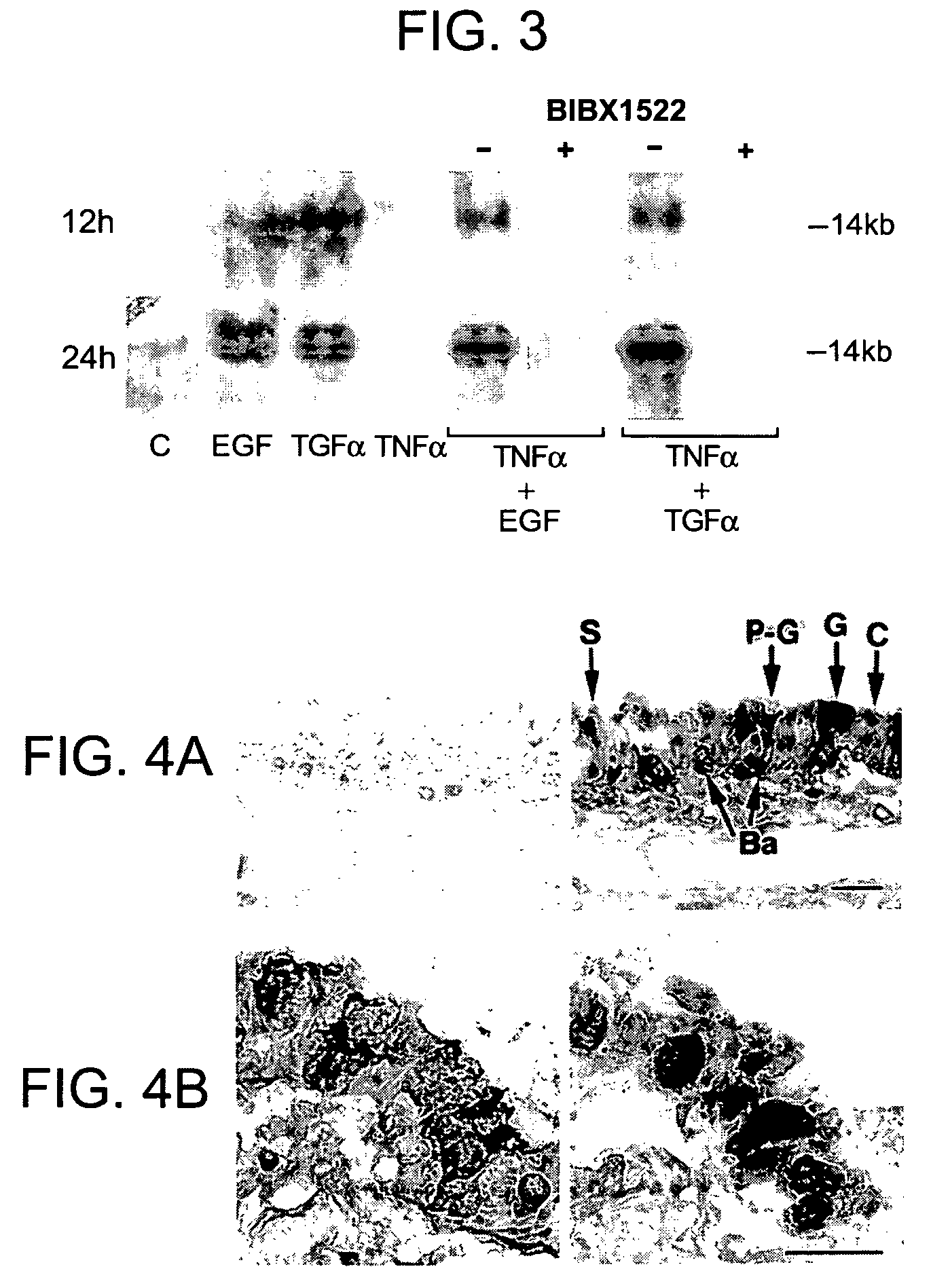
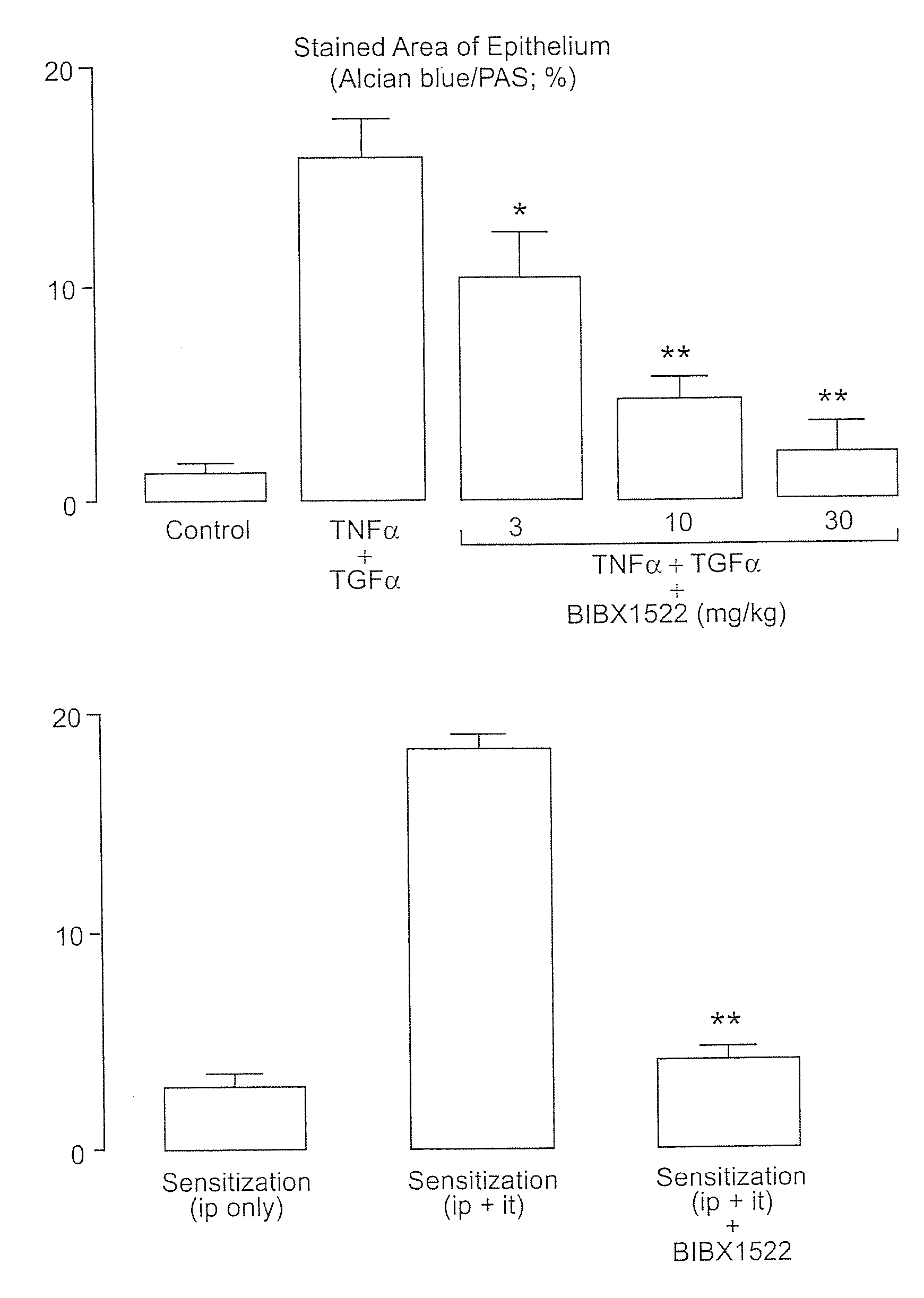
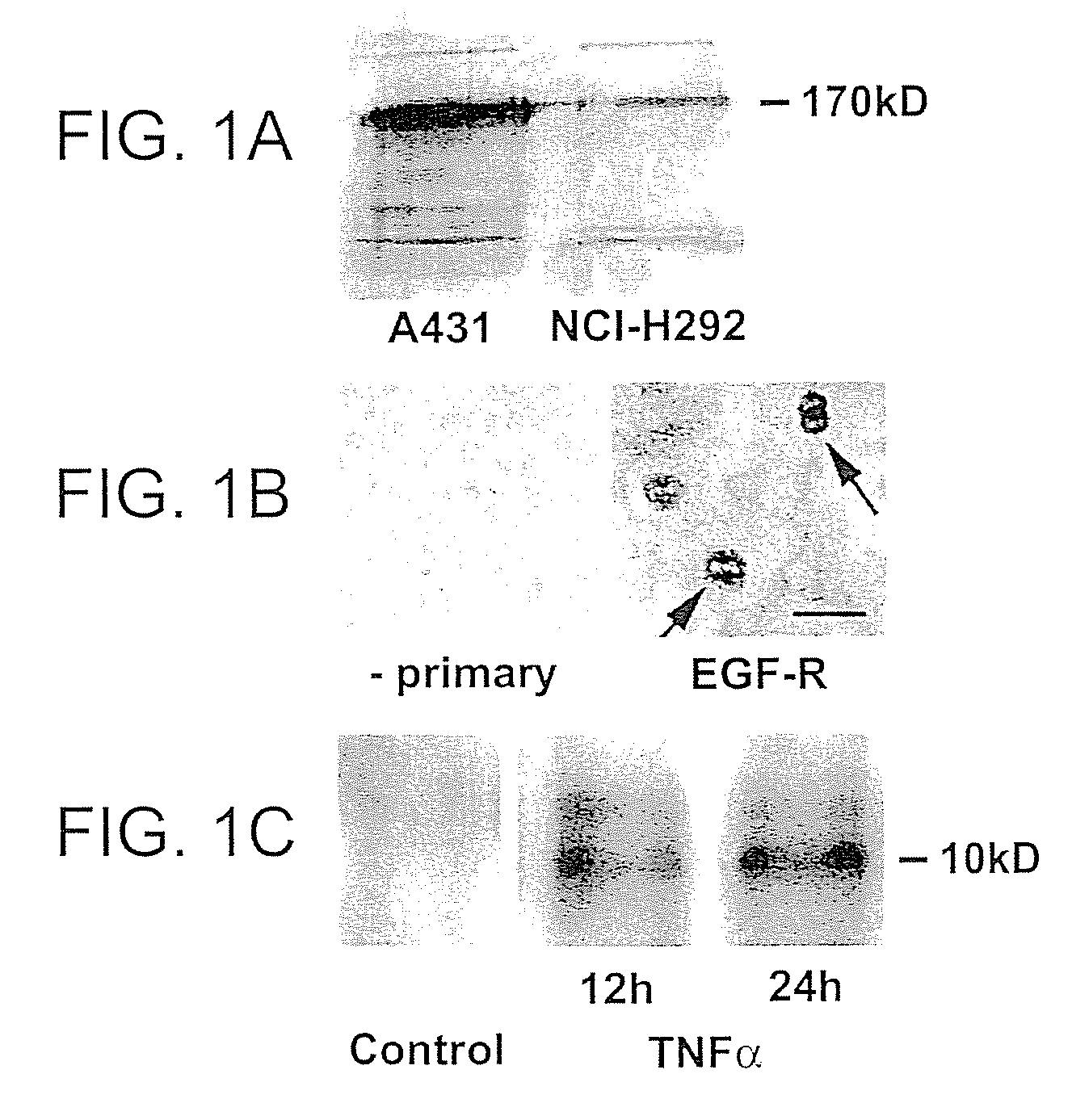
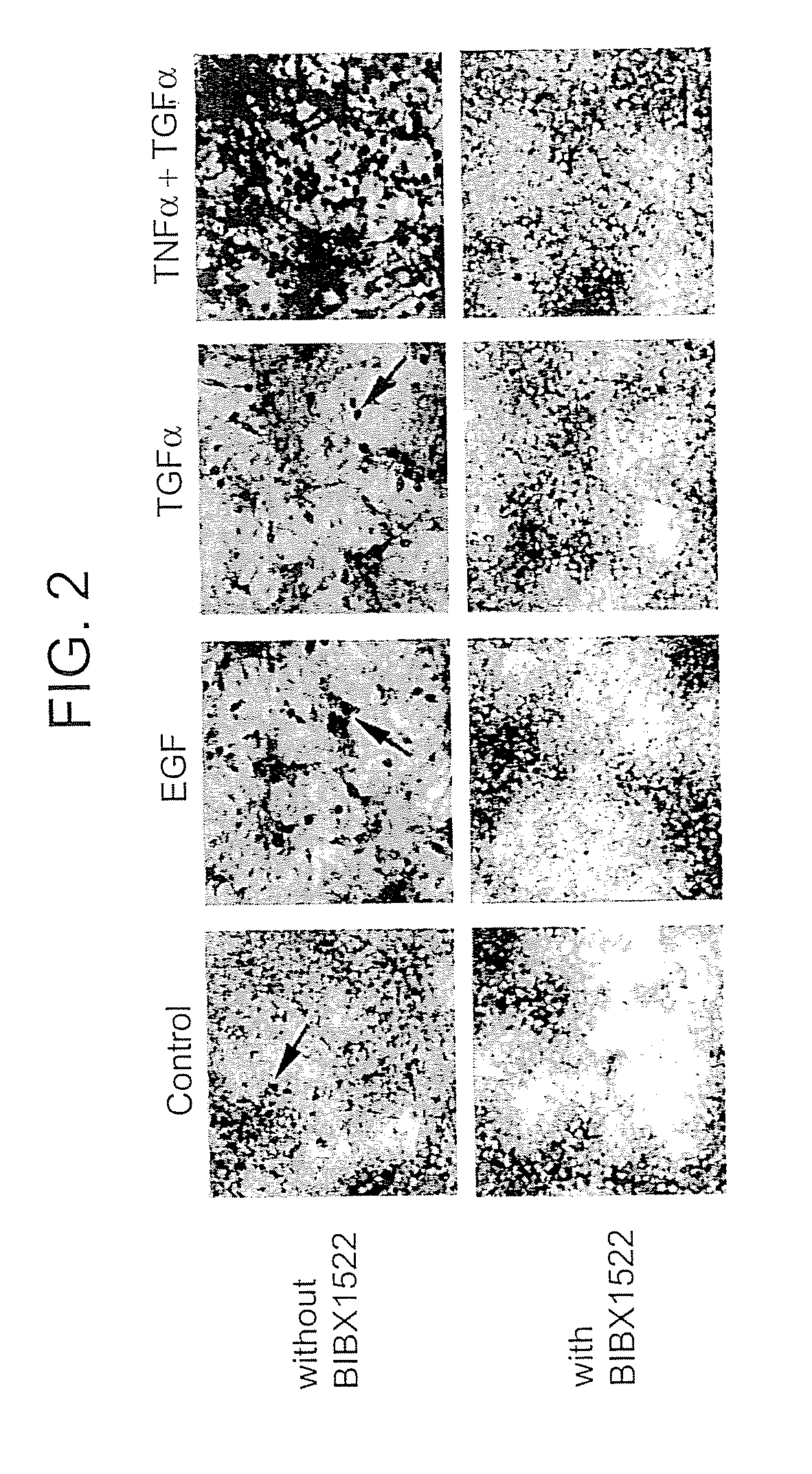
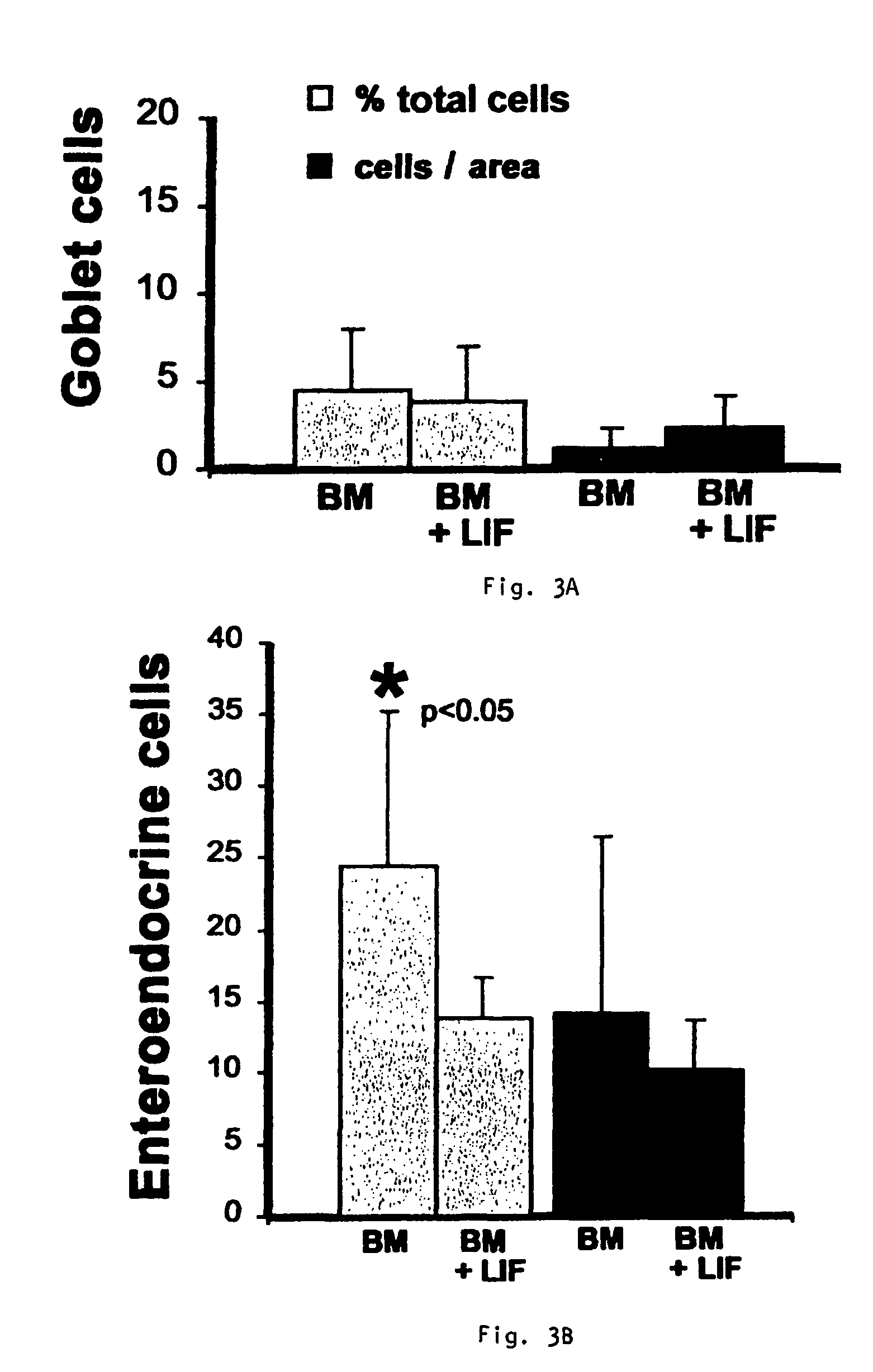
Preventing airway mucus production by administration of EGF-R antagonists
InactiveUS6846799B1Preventing excessive formationReduce formationOrganic active ingredientsBiocideGoblet cellMucus production
Hypersecretion of mucus in the lungs is inhibited by the administration of an epidermal growth factor receptor (EGF-R) antagonist. The EGF-R antagonist may be in the form of a small organic molecule, an antibody, or portion of an antibody that binds to and blocks the EGF receptor. The EGF-R antagonist is preferably administered by injection in an amount sufficient to inhibit formation of goblet cells in pulmonary airways. The degranulation of goblet cells that results in airway mucus production is thereby inhibited. Assays for screening candidate agents that inhibit goblet cell proliferation are also provided.
Owner:RGT UNIV OF CALIFORNIA
Solid forms of anti-egfr antibodies
InactiveUS20070122411A1Improve stabilityHigh purityPowder deliveryFrom normal temperature solutionsAdjuvantDissolution
The invention relates to solid forms of antibodies against the EGF receptor, in particular precipitates and crystals of monoclonal antibodies against the EGF receptor, particularly preferably of Mab C225 (cetuximab) and Mab h425 (EMD 72000), which result in biologically active antibody protein through dissolution or suspension in aqueous medium, obtainable by precipitation of the antibody and / or one of its variants and / or fragments dissolved or suspended in aqueous medium by means of a precipitation reagent. The invention furthermore relates to pharmaceutical preparations comprising at least one solid form of above-mentioned antibodies in precipitated non-crystalline, precipitated crystalline or in soluble or suspended form, and optionally excipients and / or adjuvants and / or further pharmaceutical active ingredients, and to a process for the preparation of solid forms of anti-EGFR antibodies according to the invention.
Owner:MERCK PATENT GMBH
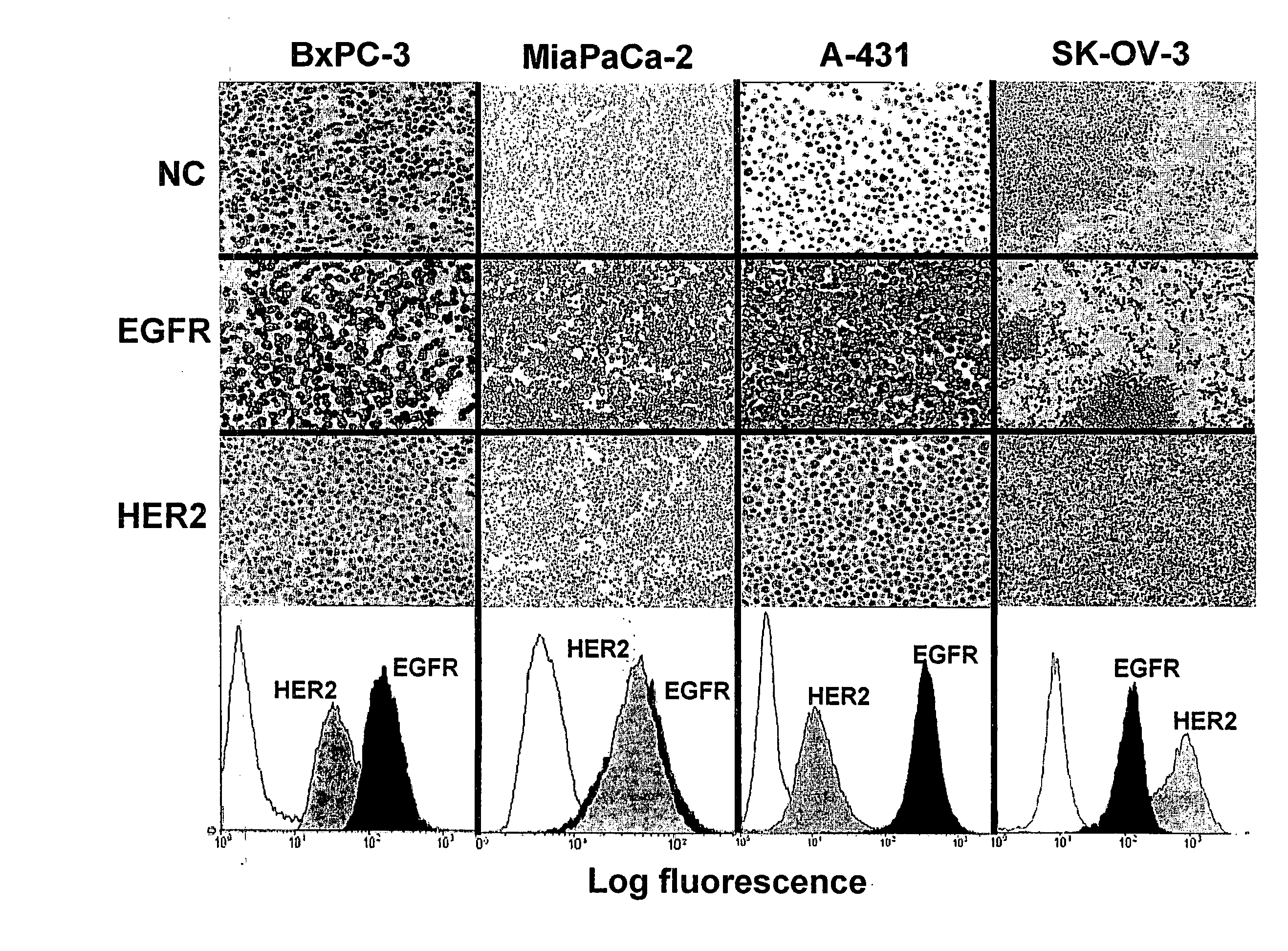
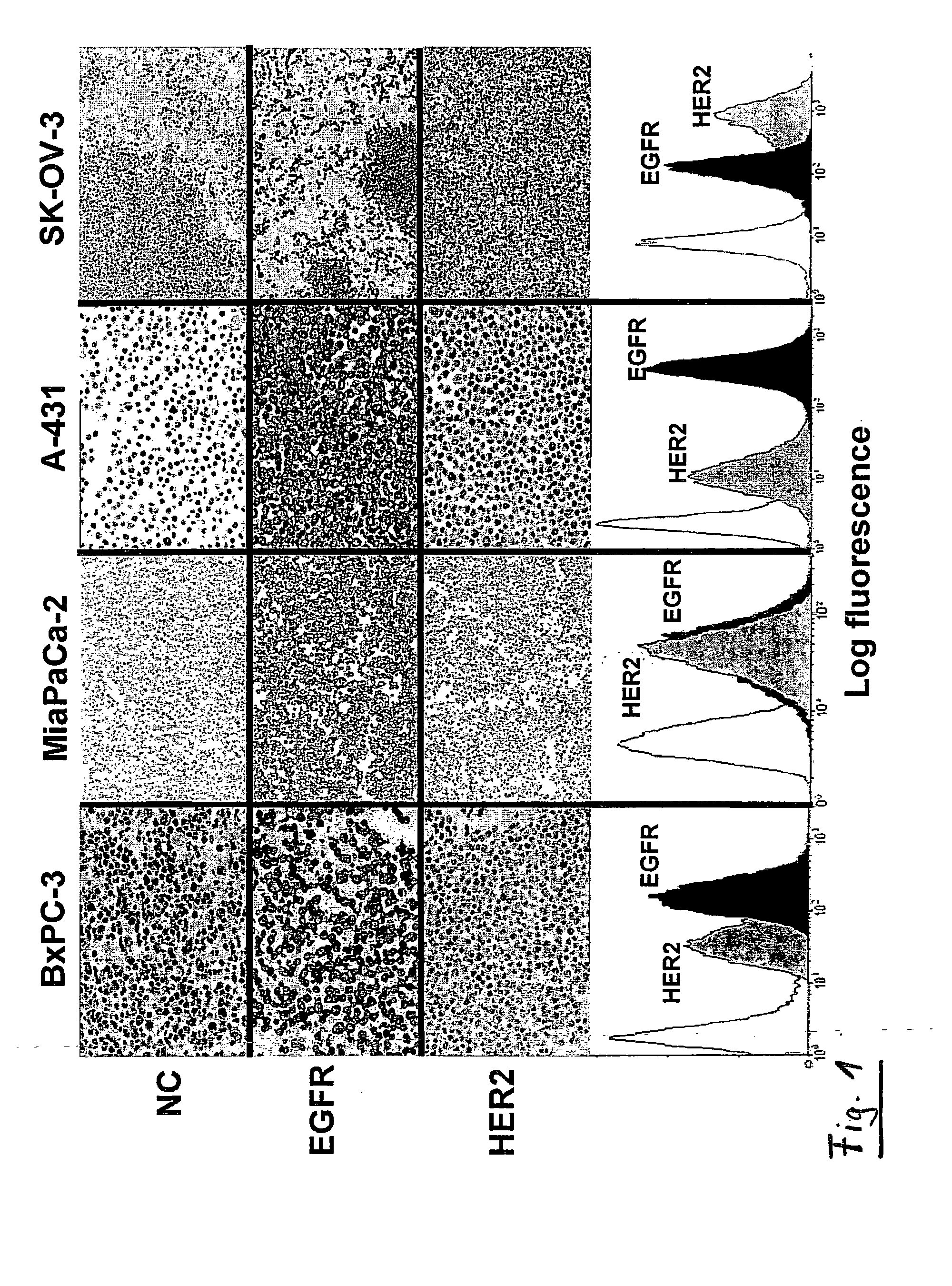
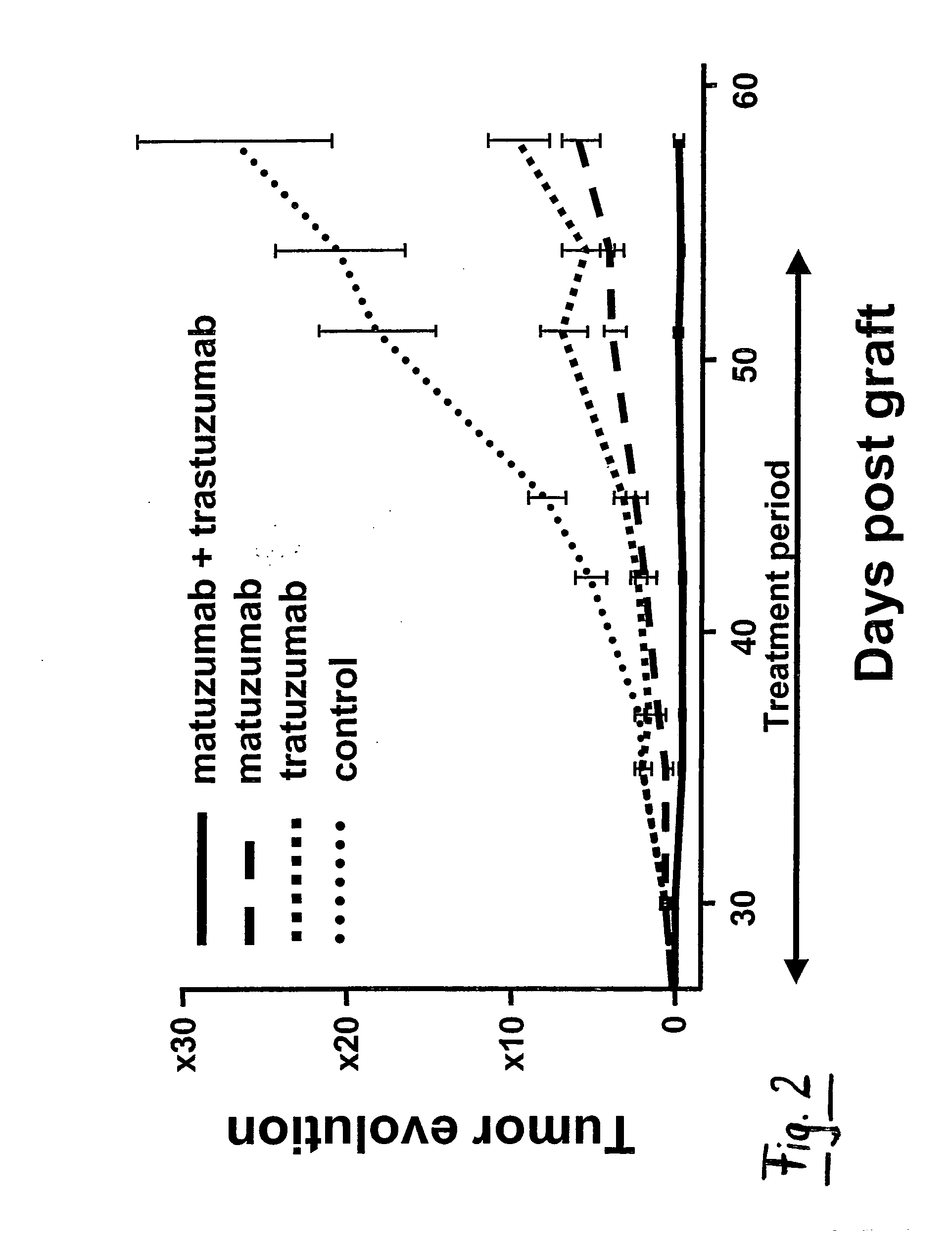
Combination Therapy Using Anti-EGFR and Anti-HER2 Antibodies
InactiveUS20090214541A1Good curative effectPrevent dimerizationAntibody ingredientsImmunoglobulinsEGFR AntibodyAnti her2
The invention relates to the combined use of anti-EGFR antibodies and anti-Her2 antibodies for the treatment of cancer, especially suitable for cancer expressing high levels of the EGFR type and low levels of HER2. The invention refers in particular monoclonal antibody “trastuzumab” (HERCEPTIN®) directed against the HER2 receptors the efficacy of which can be significantly increased in vivo when combined with monoclonal antibody “matuzumab” (hmAB 425, EMD 72000) directed against EGF receptors. The combination treatment is suitable for patients suffering from cancer having said receptor profile, preferably pancreatic cancer.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Preventing airway mucus production by administration of EGF-R antagonists
InactiveUS7358222B2Preventing excessive formationReduce formationOrganic active ingredientsPeptide/protein ingredientsGoblet cellMucus production
Hypersecretion of mucus in the lungs is inhibited by the administration of an epidermal growth factor receptor (EGF-R) antagonist. The EGF-R antagonist may be in the form of a small organic molecule, an antibody, or portion of an antibody that binds to and blocks the EGF receptor. The EGF-R antagonist is preferably administered by injection in an amount sufficient to inhibit formation of goblet cells in pulmonary airways. The degranulation of goblet cells that results in airway mucus production is thereby inhibited. Assays for screening candidate agents that inhibit goblet cell proliferation are also provided.
Owner:RGT UNIV OF CALIFORNIA
Predictors of patient response to treatment with egf receptor inhibitors
ActiveUS20090298701A1Increased normalized expression levelImprove responseNucleotide librariesMicrobiological testing/measurementGene productOncology
The present invention provides methods and compositions to facilitate determining whether an EGFR-expressing cancer in an individual is an EGFR inhibitor-responsive cancer, as well as methods for determining the likelihood that a patient having an EGFR-expressing cancer will exhibit a beneficial response to an EGFR inhibitor therapy. The methods generally involve determining a normalized expression level of a gene product that correlates with EGFR inhibitor responsiveness.
Owner:GENOMIC HEALTH INC +1
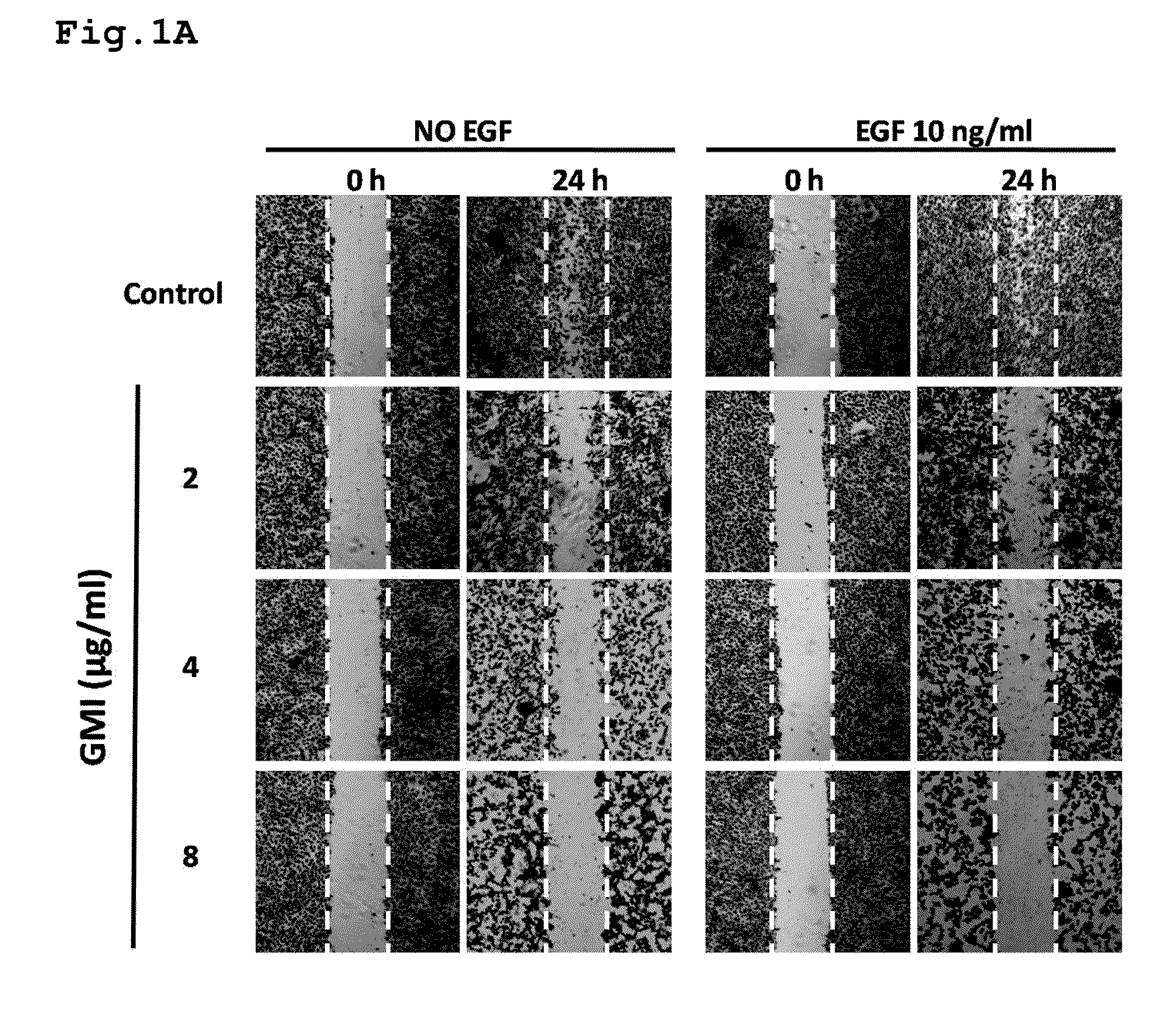
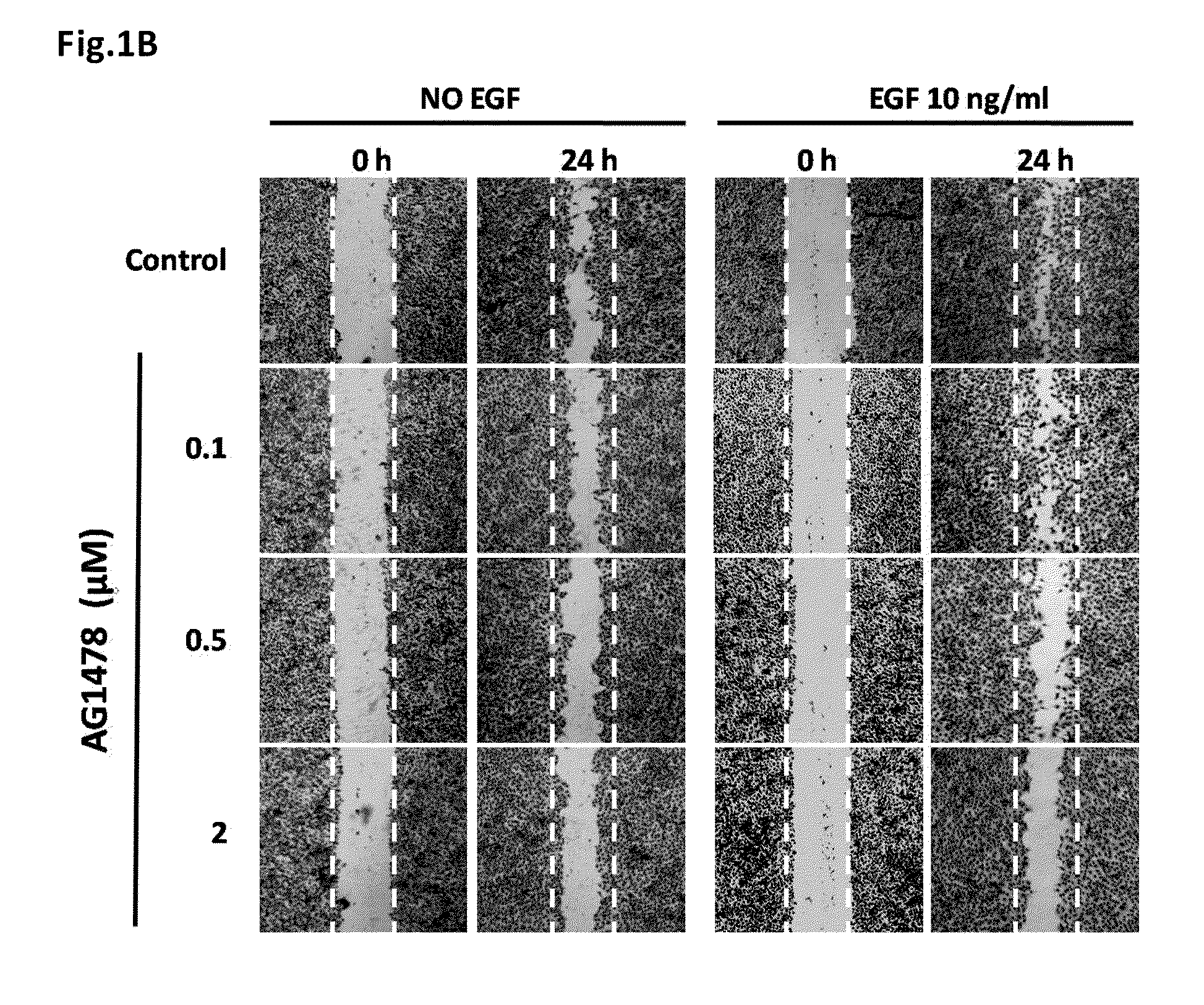
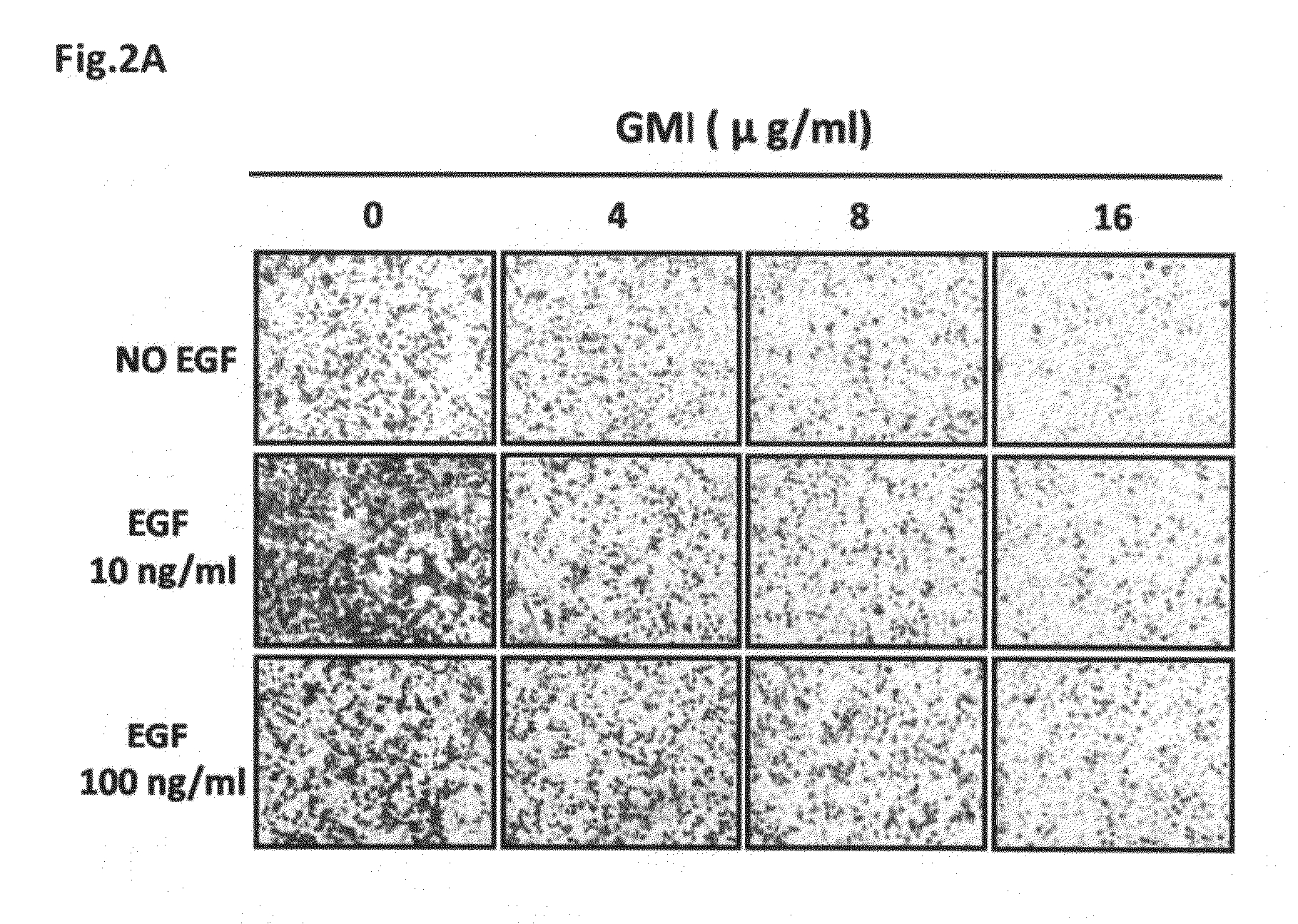
Uses of an Immunomodulatory Protein (GMI) from Ganoderma Microsporum
ActiveUS20110318429A1Inhibitory activityHeavy metal active ingredientsOrganic active ingredientsLymphatic SpreadCancer cell
The invention provides a method for inhibiting EGF receptor activity comprising contacting an EGF receptor with an immunomodulatory protein (GMI) from Ganoderma microsporum, or a recombinant thereof. Also provided is a method for treating invasion and metastasis of cancer cells, comprising administering an effective amount of an immunomodulatory protein (GMI) from Ganoderma microsporum, or a recombinant thereof, to a subject in need of such treatment.
Owner:MYCOMAGIC BIOTECH
Preventing airway mucus production by administration of EGF-R antagonists
InactiveUS7354894B2Preventing excessive formationReduce formationOrganic active ingredientsBiocideGoblet cellMucus production
Hypersecretion of mucus in the lungs is inhibited by the administration of an epidermal growth factor receptor (EGF-R) antagonist. The EGF-R antagonist may be in the form of a small organic molecule, an antibody, or portion of an antibody that binds to and blocks the EGF receptor. The EGF-R antagonist is preferably administered by injection in an amount sufficient to inhibit formation of goblet cells in pulmonary airways. The degranulation of goblet cells that results in airway mucus production is thereby inhibited. Assays for screening candidate agents that inhibit goblet cell proliferation are also provided.
Owner:RGT UNIV OF CALIFORNIA
Truncated EGF receptor
InactiveUS20060234343A1High sensitivityTherapeutic potentialFungiBacteriaEGF Receptor LigandsEGF Receptors
The present invention relates to truncated EGF receptor molecules that exhibit increased binding affinities for EGFR ligands such as EGF and TGF1. The present invention also relates to methods of screening for EGF receptor ligands and methods of treatment which involve the use of these molecules.
Owner:COMMONWEALTH SCI & IND RES ORG +1
Preventing airway mucus production by administration of egf-r antagonists
InactiveUS20080175797A1Preventing excessive formationReduce formationBiocideOrganic active ingredientsGoblet cellMucus production
Owner:RGT UNIV OF CALIFORNIA
Preventing airway mucus production by administration of EGF-r antagonists
InactiveUS20070270330A1Preventing excessive formationReduce formationPowder deliveryOrganic active ingredientsGoblet cellMucus production
Hypersecretion of mucus in the lungs is inhibited by the administration of an epidermal growth factor receptor (EGF-R) antagonist. The EGF-R antagonist may be in the form of a small organic molecule, an antibody, or portion of an antibody that binds to and blocks the EGF receptor. The ESGF-R antagonist is preferably administered by injection in an amount sufficient to inhibit formation of goblet cells in pulmonary airways. The degranulation of goblet cells that results in airway mucus production is thereby inhibited. Assays for screening candidate agents that inhibit goblet cell proliferation are also provided.
Owner:RGT UNIV OF CALIFORNIA
Antibody and antibody fragments for inhibiting the growth of tumors
InactiveUS20070116707A1Heavy metal active ingredientsPeptide/protein ingredientsAntibody fragmentsWilms' tumor
Owner:GOLDSTEIN NEIL I +3
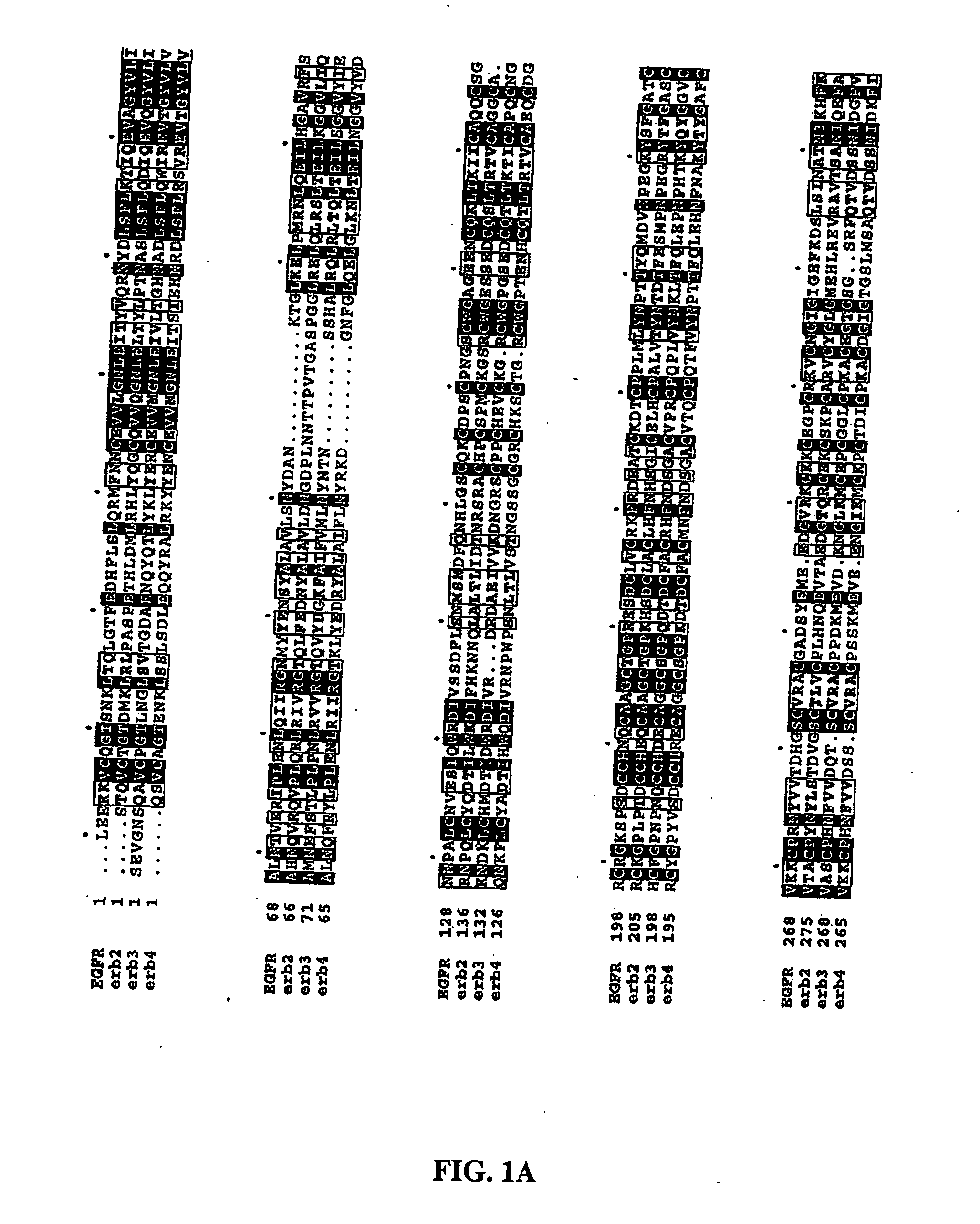
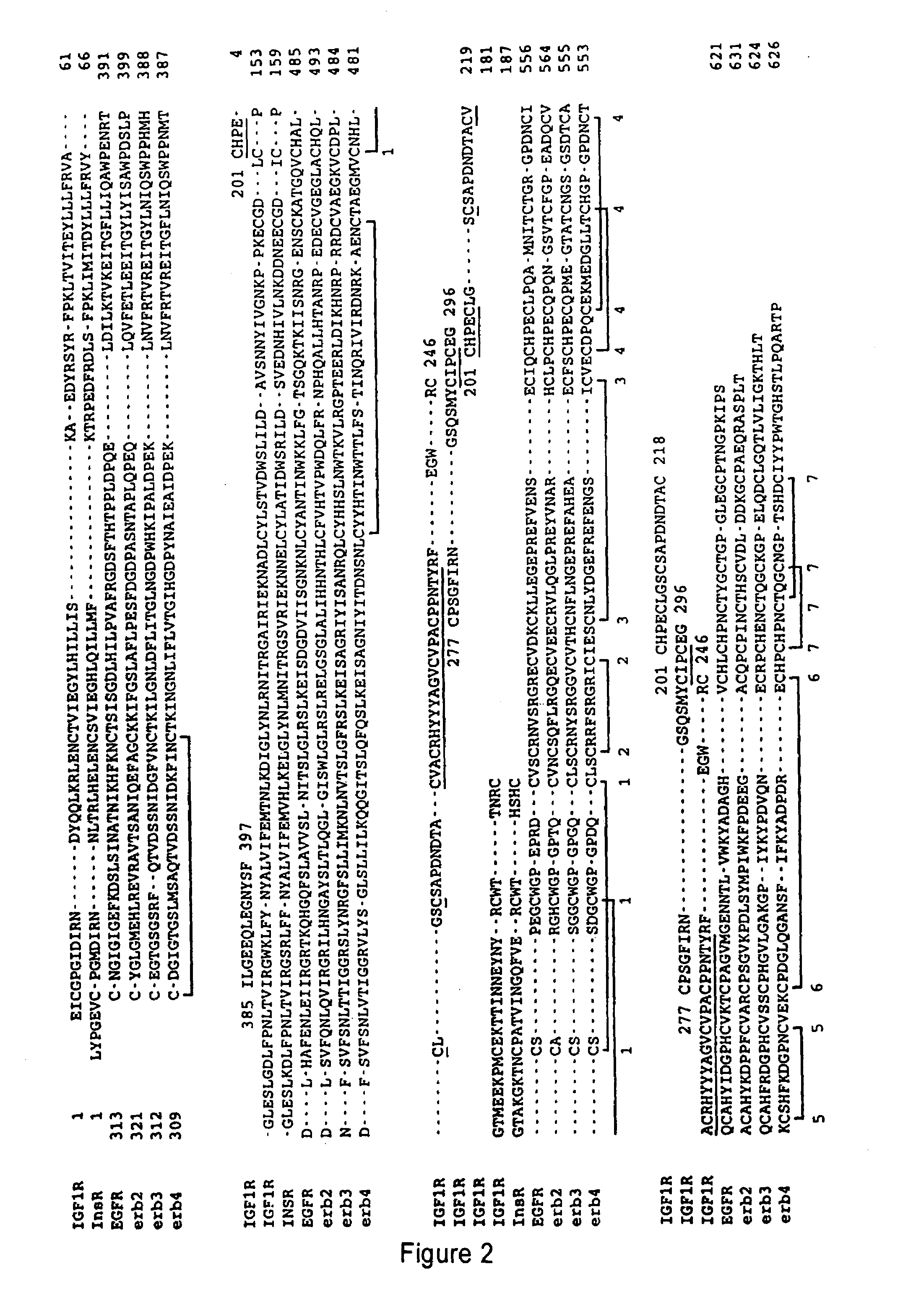
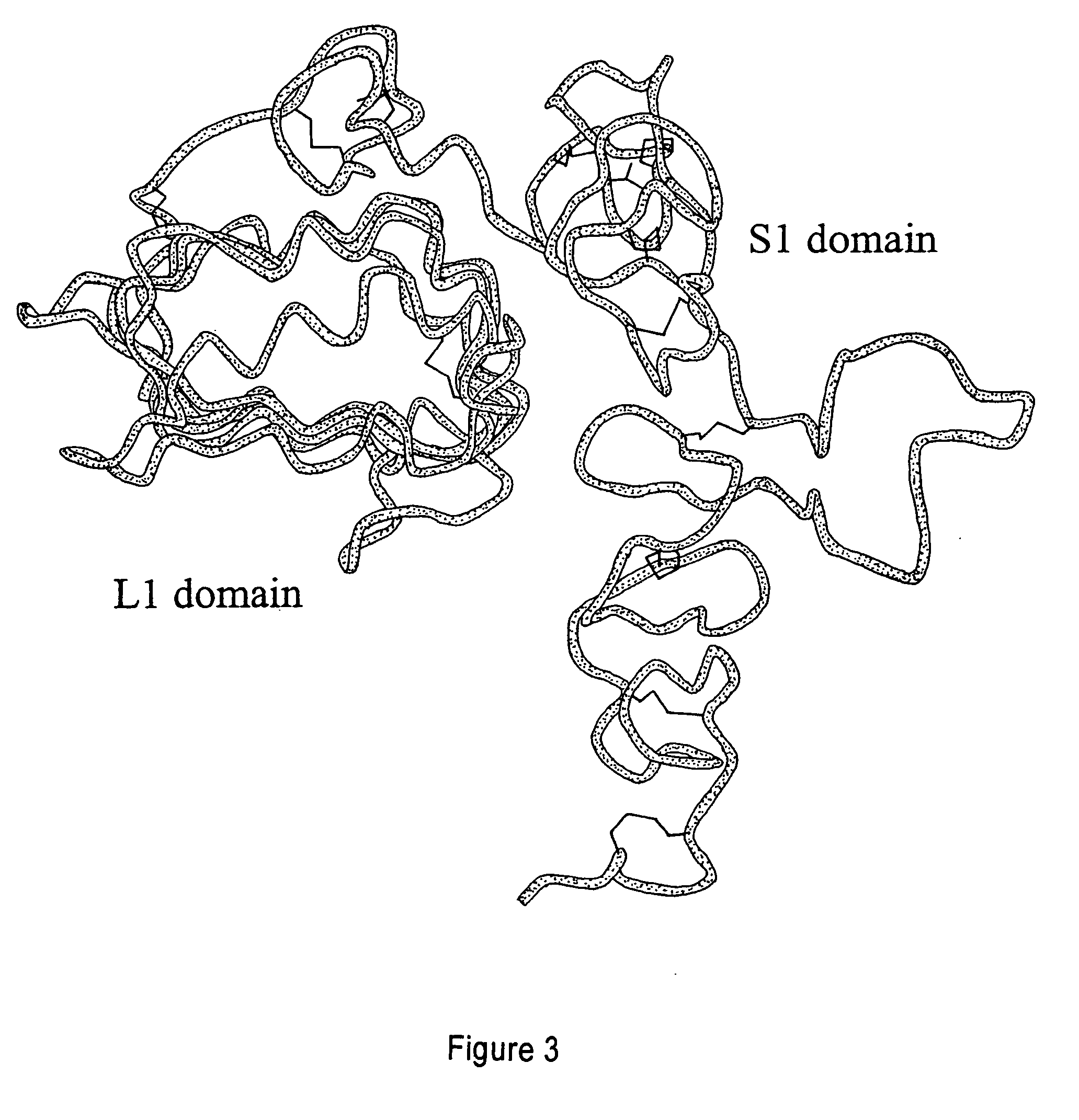
Methods of screening based on the EGF receptor crystal structure
InactiveUS20080025983A1High binding affinityImprove bindingMolecular designImmunoglobulins against growth factorsCrystal structureBiology
This invention relates to the structure of members of the epidermal growth factor (EGF) receptor family and to receptor / ligand interactions. In particular, it relates to the field of using the EGF receptor family structure to select and screen for compounds that inhibit the formation of active receptor dimers.
Owner:COMMONWEALTH SCI & IND RES ORG +2
Styryl compounds which inhibit EGF receptor protein tyrosine kinase
InactiveUSRE38761E1Effectively inhibit EGF-dependent autophosphorylation of the receptorHalogenated hydrocarbon active ingredientsBiocideProtein-Tyrosine KinasesMedicine
A method of inhibiting cell proliferation in a patient suffering from such disorder comprising administering to said patient an effective amount of a composition comprising, in admixture with a pharmaceutically acceptable carrier, a compound, or a pharmaceutically acceptable salt thereof, which is a substituted styrene compound which can also be a naphthalene, an indane or a benzoxazine; including nitrile and molononitrile compounds, and pharmaceutical compositions comprising, in admixture with a pharmaceutically acceptable carrier, a pharmaceutically-effective amount of such compound.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
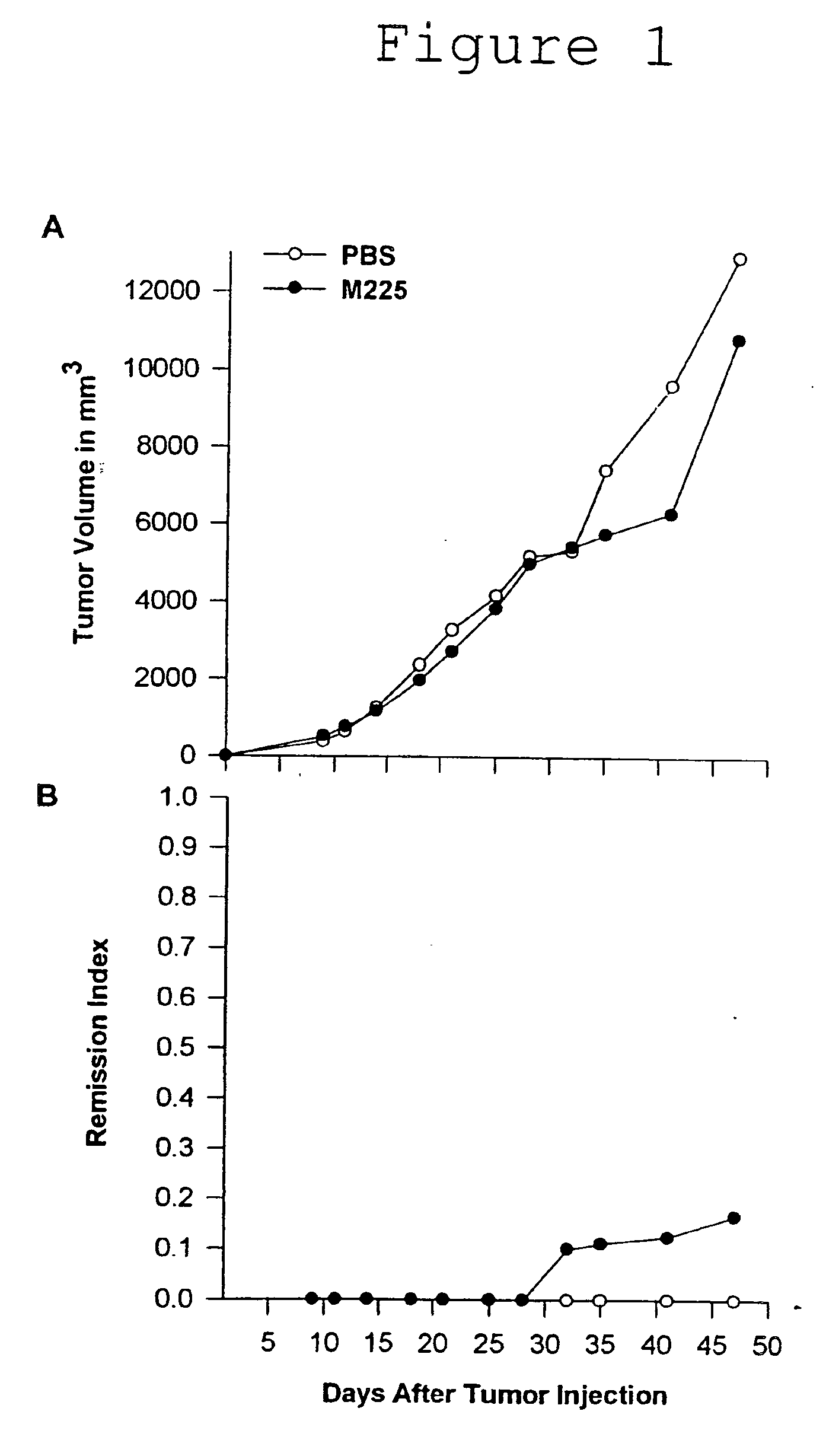
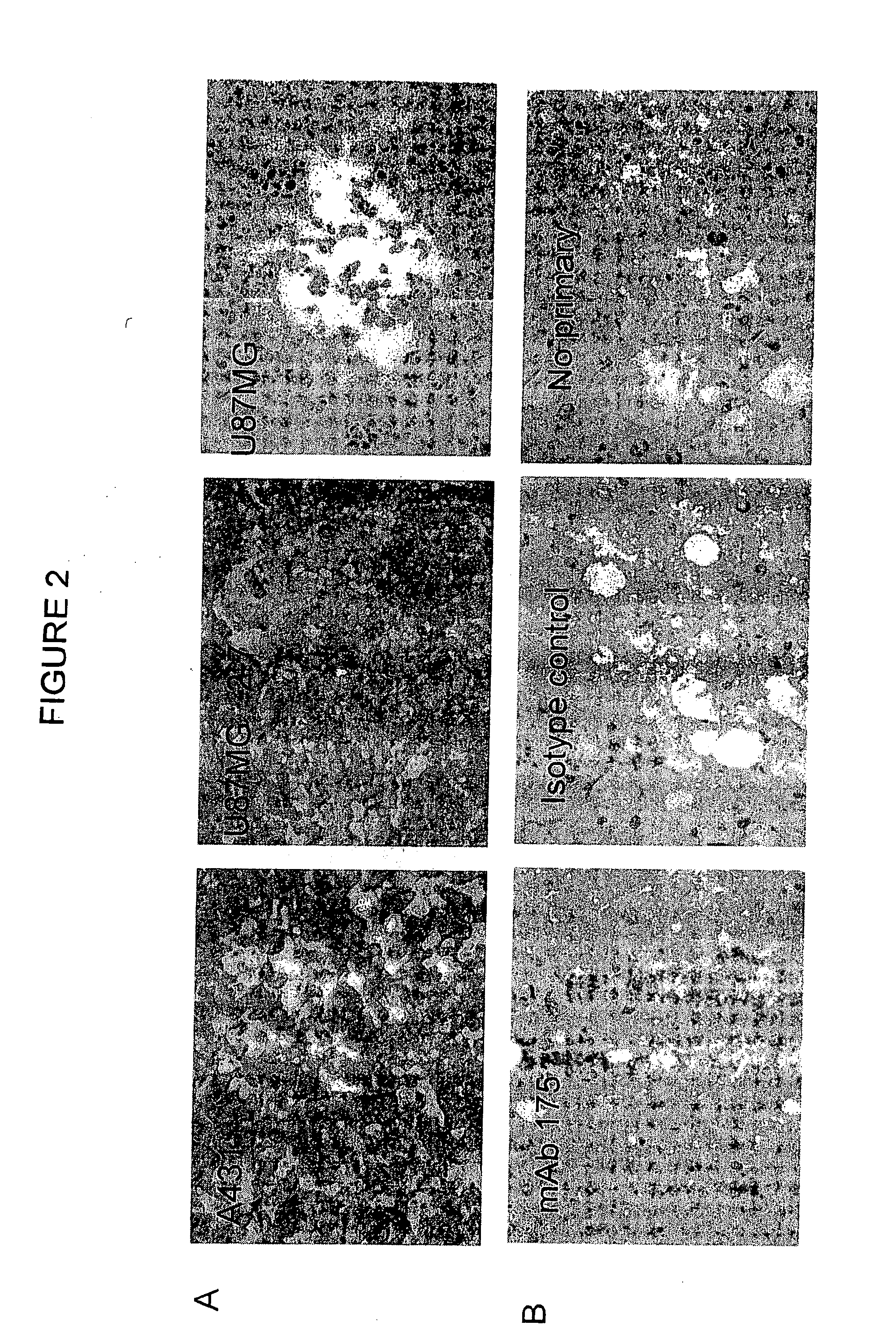
Monoclonal antibody 175 tageting the egf receptor and derivatives and uses thereof
The present invention relates to antibodies, particularly antibody 175, and fragments thereof or antibodies derived therefrom, which bind to the EGF receptor, particularly to amplified or overexpressed epidermal growth factor receptor (EGFR) and to the de2-7 EGFR truncation of the EGFR. These antibodies are useful in the diagnosis and treatment of cancer. Recombinant or hybrid antibodies having the variable region heavy or light chain sequence(s) of antibody 175 are also provided. The antibodies of the present invention may also be used in therapy in combination with chemotherapeutics or anti-cancer agents and / or with other antibodies or fragments thereof.
Owner:LUDWIG INST FOR CANCER RES
Methods of treating colorectal cancer with anti-epidermal growth factor antibodies
InactiveUS20080008704A1Inhibiting EGF receptor-mediated signalingInhibit bindingAntibody ingredientsImmunoglobulinsCuticleCancer therapy
A method for treating colorectal cancer is disclosed and consists of administering to a patient in need of such treatment a pharmaceutically effective dose of an agent capable of blocking the binding of EGF-receptor to its natural ligand(s) and / or inhibiting EGF-receptor-mediated signaling. Also disclosed is a method of increasing a colorectal cancer patient response to anti-cancer therapy which consists of administering to said patient a pharmaceutically effective dose of an agent capable of blocking the binding of EGF-receptor to its natural ligand(s) and / or inhibiting EGF-receptor-mediated signaling.
Owner:IMCLONE SYSTEMS
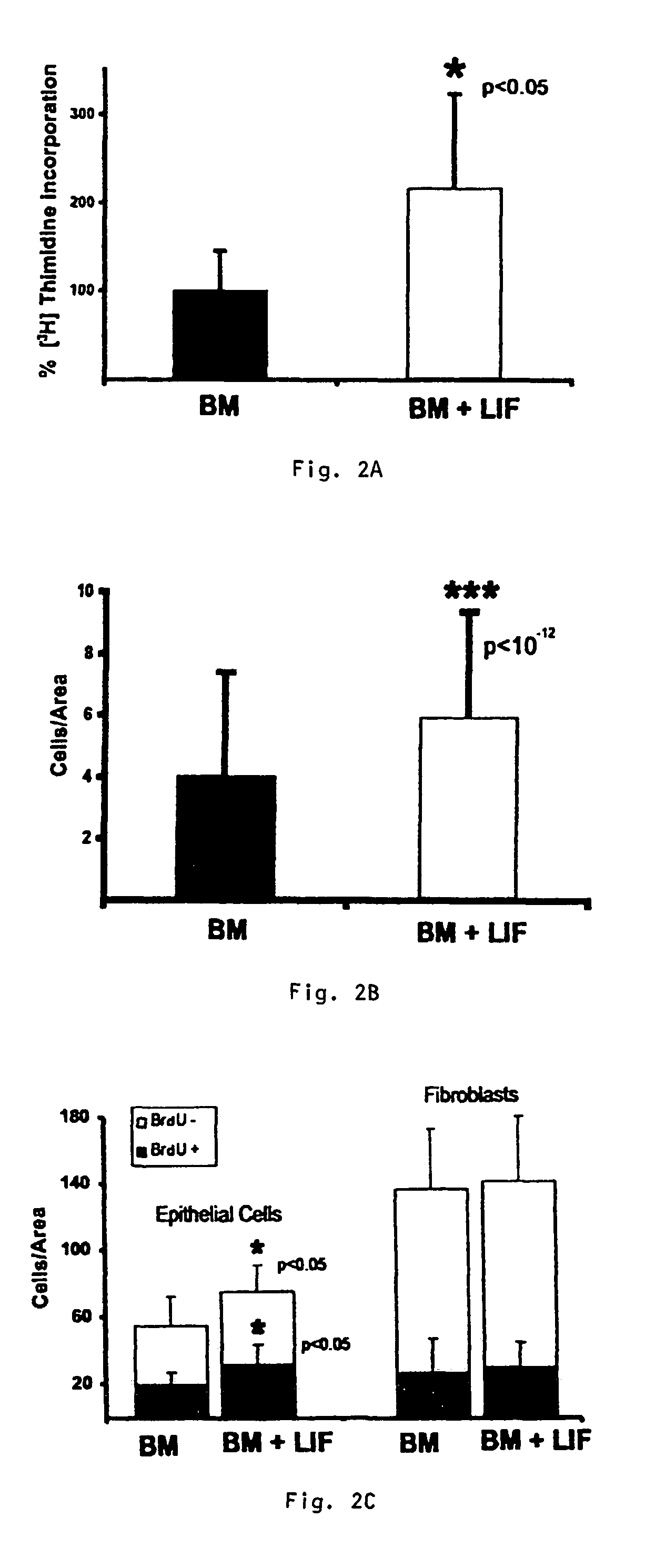
Organotypic intestinal culture and methods of use thereof
InactiveUS7217570B2Promote repairBiocideGastrointestinal cellsIntestinal structureCell culture media
An organotypic culture comprises an artificial stroma overlayed with epithelial cells isolated from a human colon or intestine. The stroma comprises a mixture of collagen and human fibroblasts isolated from a human colon or intestine. The culture contains a factor that binds the IGF-1 receptor, a factor that binds the EGF receptor, and a factor that binds the LIF receptor. These factors may be added exogenously to the culture via medium or may be expressed by various recombinantly engineered cell types in the culture. The organotypic culture can result in growth that is in situ-like or emphasizes other physiological or morphological states, depending on the balance of factors in the growth media. The organotypic culture may be used in methods for screening of therapeutic, carcinogenic, or growth enhancement factors, or for treating intestinal injuries by applying to the site of an injury the intact culture or the components thereof.
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY
Method of designing agonists and antagonists to EGF receptor family
InactiveUS6941229B1Inhibit bindingPromote desolvationPeptide/protein ingredientsDrug compositionsAgonistReceptor molecule
Owner:COMMONWEALTH SCI & IND RES ORG +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com