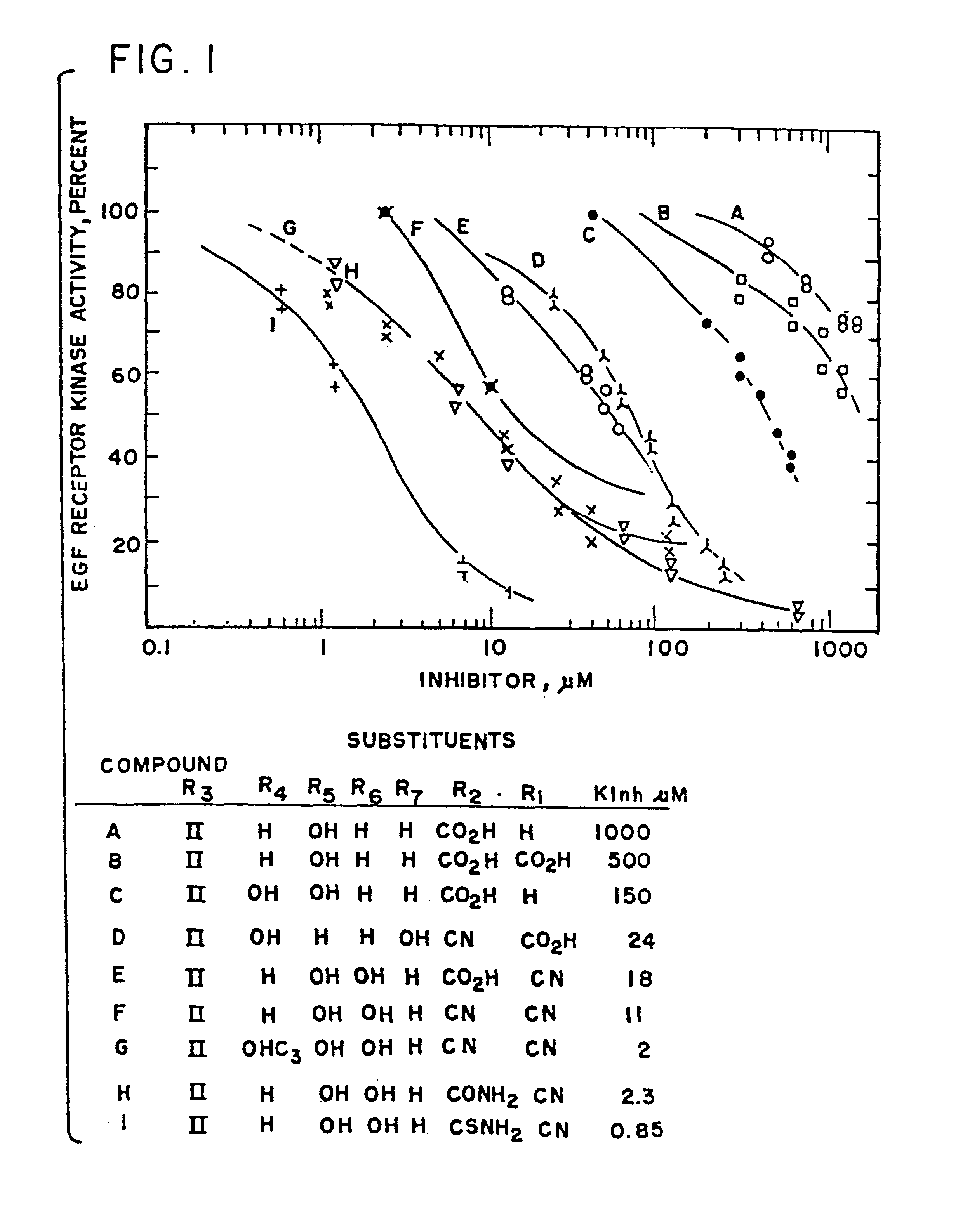
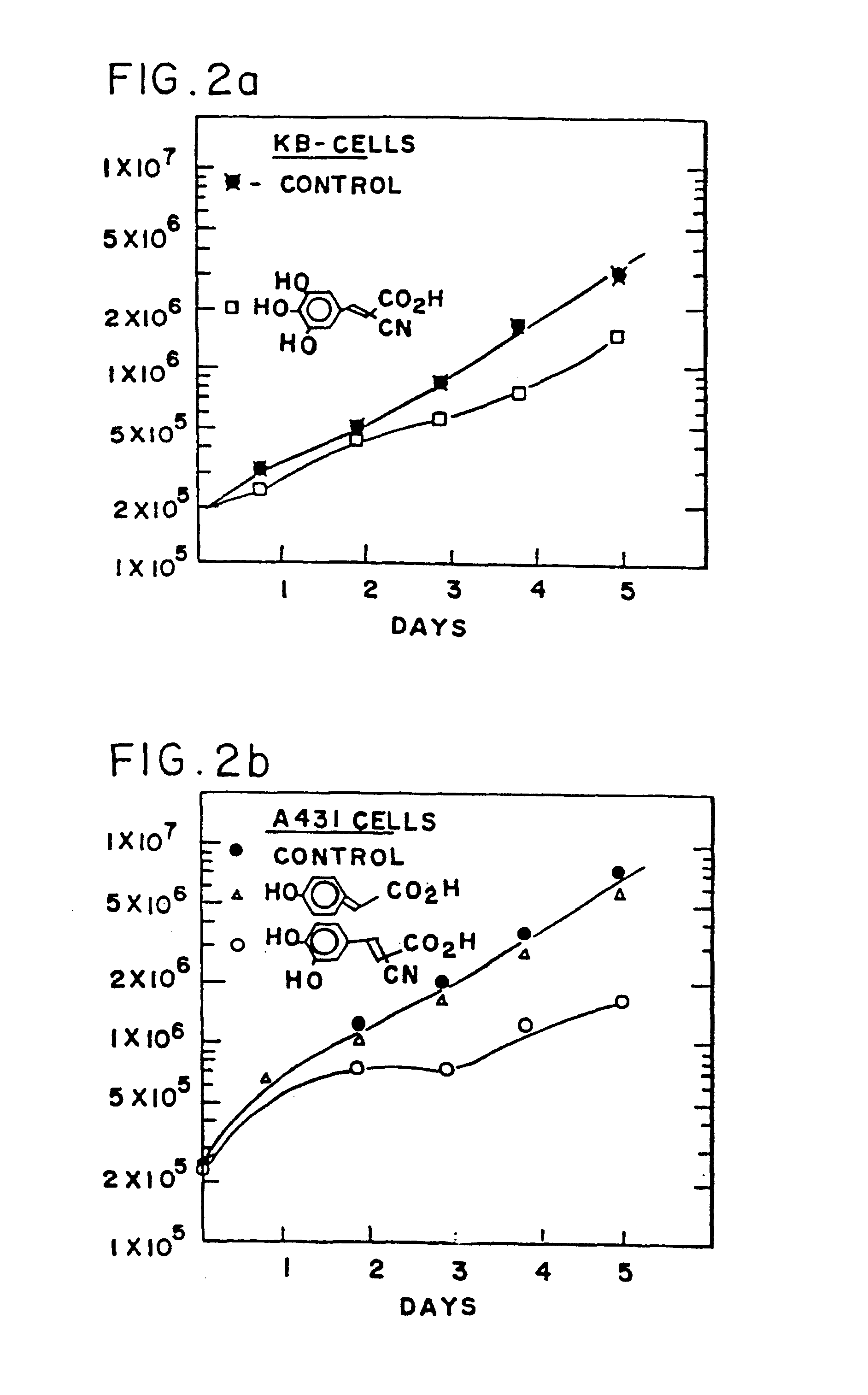
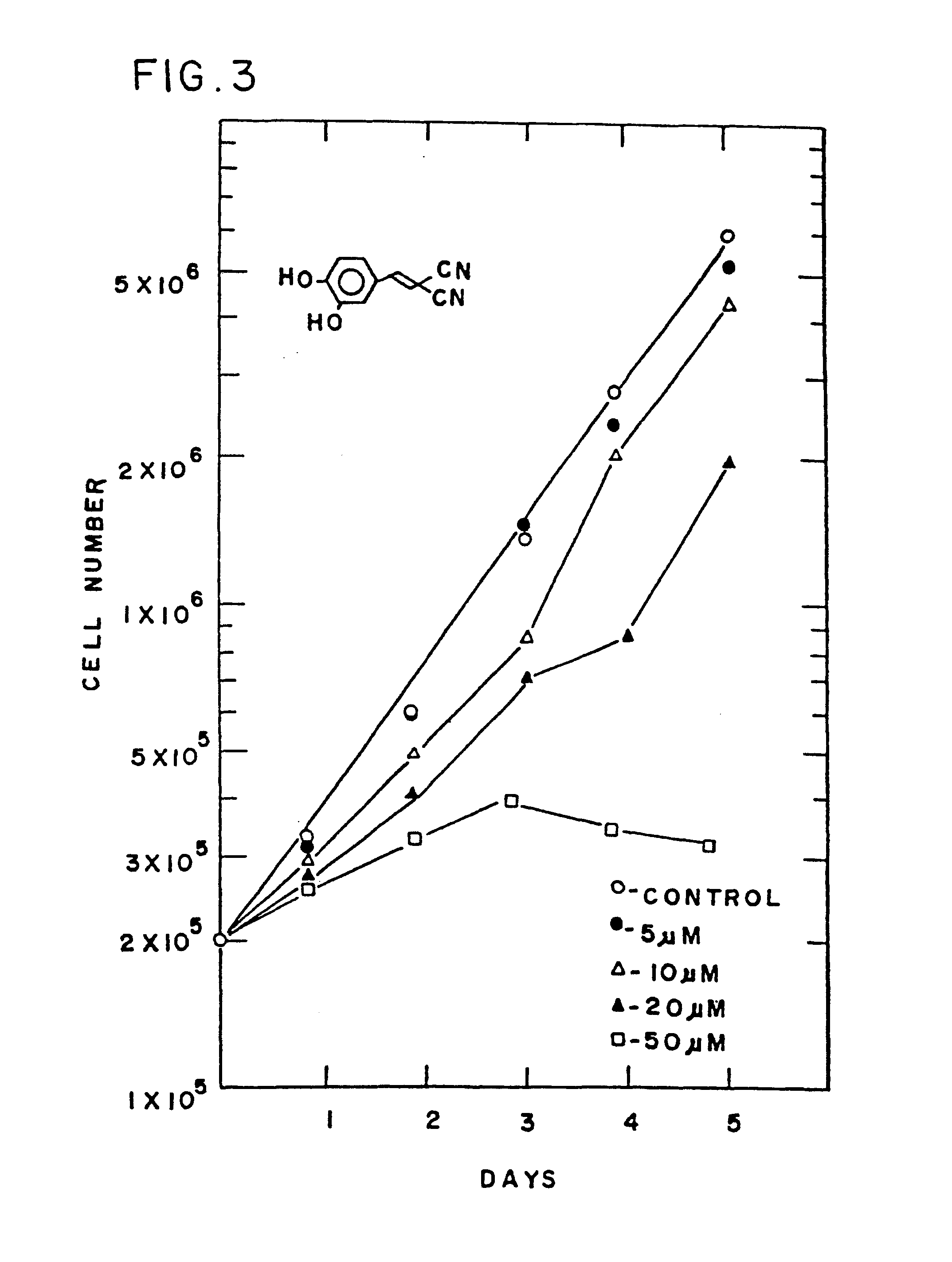
Styryl compounds which inhibit EGF receptor protein tyrosine kinase
a technology of egf receptor and tyrosine kinase, which is applied in the field of egf receptor protein tyrosine kinase inhibition, can solve the problems of high toxicity of compounds, unsatisfactory, and tumor cells are more readily attacked, and achieve the effect of inhibiting egf receptor kinase and effectively inhibiting egf-dependent autophosphorylation of receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3,4-Dihydroxybenzylidene malononitrile
[0146]To 11 g (80 mmol) of 3,4-dihydroxybenzaldehyde and 5.5 g (83 mmol) of malononitrile in 40 ml of ethanol, 7 drops of piperidine were added and the mixture was heated at 70° C. for 0.5-1 hour and then poured into water. The resulting solid precipitate was separated by filtration to give 12.7 g (86% yield) of a yellow solid, m.p. 225° C.
examples 2-4
[0147]Following the same procedure as set forth for Example 1 above, the following were prepared: Example 2, 3-methoxy-4,5-dihydroxybenzylidene malononitrile, m.p. 235° C.; Example 3, 3,5-di-tert butyl-4-hydroxybenzylidene malononitrile, m.p. 135° C.; and Example 4, 3,5-di-tert butylbenzylidene malononitrile, m.p. 95°-98° C.
example 5
α-Hydroxy-3,4,5-trihydroxybenzylidene malononitrile
[0148]To 2 g (30 mmol) of malononitrile and 4 ml (40 mmol) of triethylamine in 100 ml of CH2Cl2, triacetyl galloyl chloride (prepared from 7 g (24 mmol) of triacetyl gallic acid and thionyl chloride) in 50 ml CH2Cl2 was added. The resulting mixture was then stirred for two hours at room temperature, poured into 50 ml water and hydrolyzed by heating for 2 minutes at 80° C. with a solution of 2.5 g NaOH in 30 ml ethanol. The mixture was extracted with ethyl acetate and the organic extract was further worked up by washing with water, drying, filtering and evaporation. Chromatography on silica gel gave 1.5 g (29% yield) of the product as an oily solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell proliferation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com