EGFR-homing double-stranded rna vector for systemic cancer treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1. PolyIC / MPPE induces the expression of immunocompetent cytokines in A431 and MDA-MB-468 cells. Expression of IFN-α, IP-10 and Gro-α in glioblastoma cells (U87MGwtEGFR), but not in cells with low levels of EGFR (U87MG). These data support the notion that cells produce these cytokines only when a certain threshold level of dsRNA is internalized, a dose that is only reached in cells that overexpress EGFR. In this study, we extended the analysis to two additional EGFR overexpressing cancer cell lines: A431 (vulvar cancer) and MDA-MB-468 (breast cancer). When these cells were transfected with PolyIC / MPPE (2.5 μg / ml), we detected up to 5.1 pg / ml of IFN-β; 148 pg / ml of Gro-α and 188 pg / ml of IP-10 (Table 2). Gro-α and IP-10 are chemokines responsible for the recruitment of T cells to areas where they are expressed. Thus, after challenge with PolyIC / MPPE, A431 and MDA-MB-468 cells, such as U87MGwtEGFR cells, secreted cytokines into the medium.
[0093] Table 2. Cyt...
Embodiment 2
[0095] Example 2. In vitro activation of human immune cells.
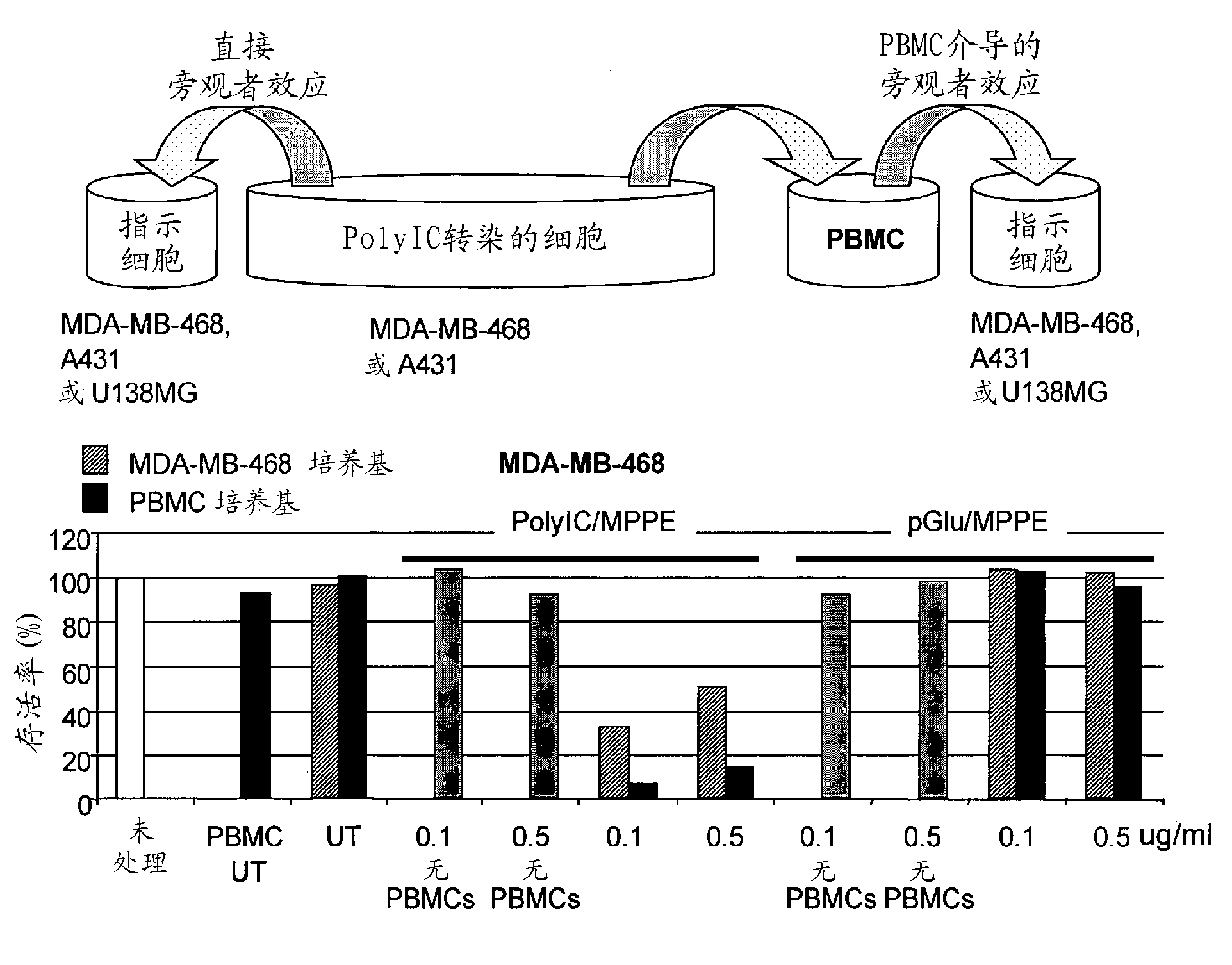
[0096] Considering the above results, we hypothesized that cytokine-enriched media from A431 and MDA-MB-468 cells treated with PolyIC / MPPE would stimulate the immune system. We checked whether this was the case by testing the effect of culture medium from PolyIC-transfected cancer cells on healthy human peripheral blood mononuclear cells (PBMC). PBMCs are composed of several types of immune cells (NK, T cells, NK-T cells, macrophages). When activated, these cells produce toxic cytokines, eg, IFN-γ and TNF-α, known to be effective against various cancer cells. PBMC also interact, causing a synergistic, highly antiproliferative effect. For example, activated T cells and NK cells produce IFN-γ, which activates macrophages and stimulates TNF-α production. The release of IL-2 into the medium is directly related to PBMC activation and can be conveniently quantified by ELISA. PBMCs are thus a convenient system to stud...
Embodiment 3
[0098] Example 3. Activation of PBMCs in vivo.
[0099] Selective expression of these cytokines in tumors overexpressing EGFR was also demonstrated in vivo (Table 3). SCID-NOD mice bearing subcutaneous tumors overexpressing EGFR in the right flank and U138MG tumors in the left flank were treated intravenously with 4 consecutive daily injections of PolyIC / MPPE, and finally a single peritoneal dose of four million PBMCs Injection. Expression, at much higher concentrations, in tumors overexpressing EGFR (Table 3). These cytokines are expected to selectively attract PBMCs to EGFR-overexpressing tumors, where PBMCs will be activated.
[0100] Table 3 Cytokine expression patterns in vivo.
[0101]
[0102] We then asked whether the concentration of cytokines on vector-transfected EGFR-overexpressing tumors was high enough to actually attract and activate immune cells. In separate experiments, cells were injected s.c. into the right flank (A431 or MDA-MB-468) and left flank (U...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com