Antibody blocking cetuximab and egfr binding, its kit and hybridoma cell
A technology of cetuximab and kits, applied in chemical instruments and methods, methods based on microorganisms, biochemical equipment and methods, etc., capable of solving problems such as acute allergic reactions and effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] In order to make the technical means, creative features, goals and effects achieved by the present invention easy to understand, the present invention will be further described below in conjunction with specific illustrations.
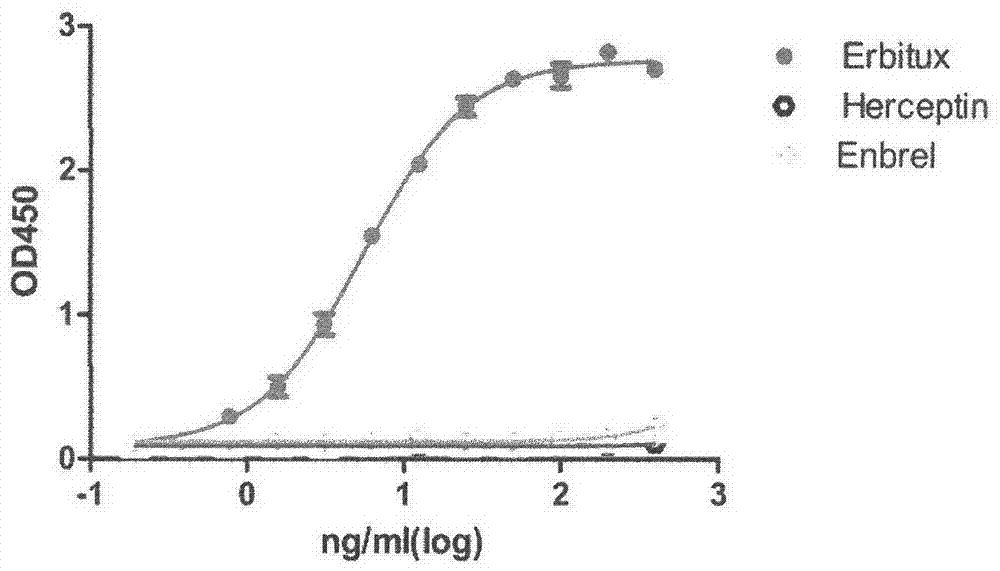
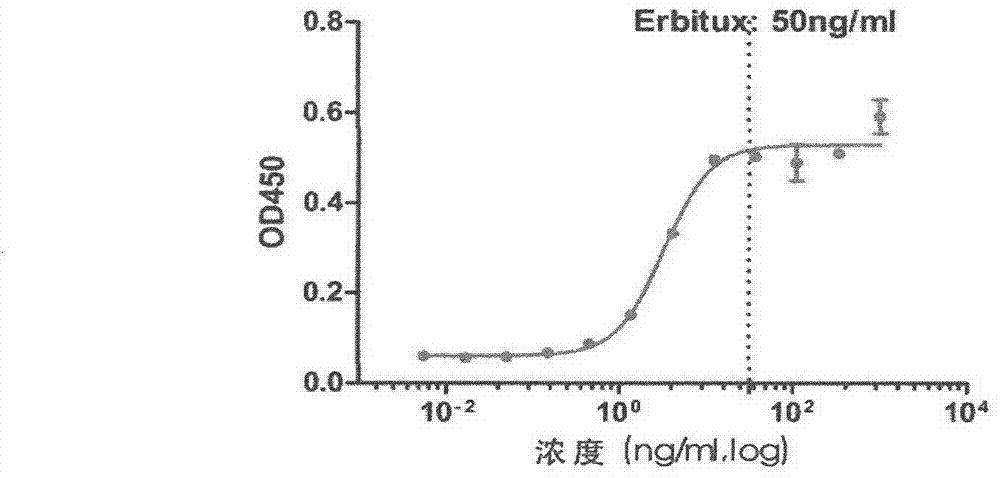
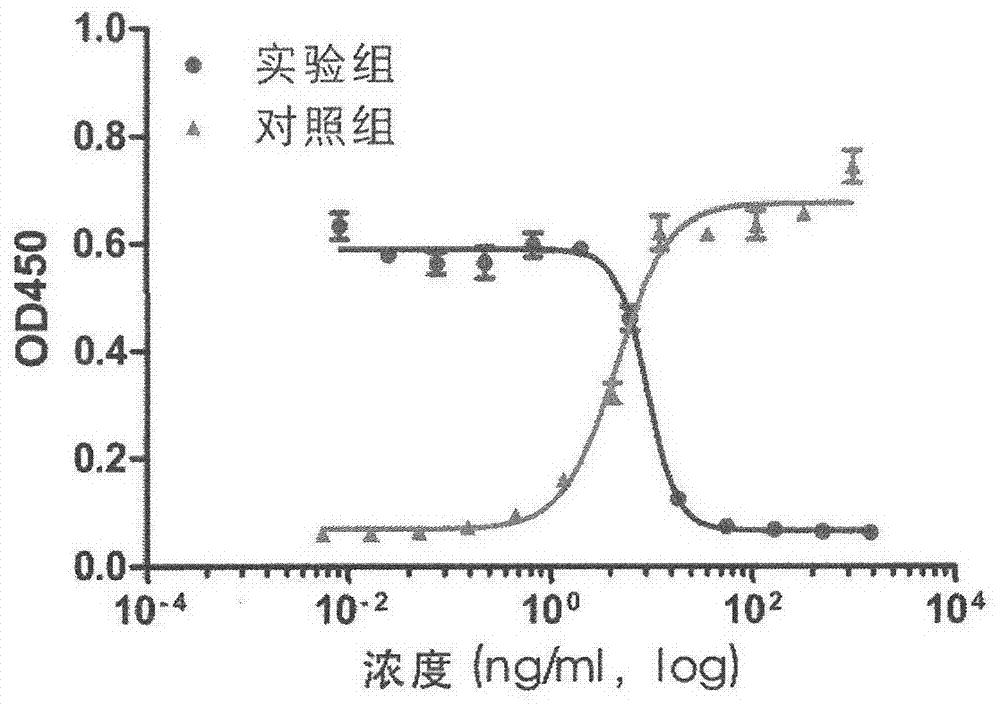
[0018] Specifically, four aspects are involved: first, the preparation process of monoclonal antibody 15-B2-4-1; second, the specificity of binding of monoclonal antibody 15-B2-4-1 to cetuximab; third, Neutralizing properties of mAb 15-B2-4-1; IV, Amino acid sequence of mAb 15-B2-4-1.
[0019] 1. Preparation of monoclonal antibody by hybridoma technology
[0020] The main steps include: animal immunization, cell fusion, screening of hybridoma cells, cloning of hybridoma cells, preparation of monoclonal antibodies, etc.
[0021] (1) Animal immunization: 6-week-old BALB / C female mice were used. The antigen was commercialized cetuximab Erbitux. Conventional immunization method, the immunization route is subcutaneous injection. Mix equal volumes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com