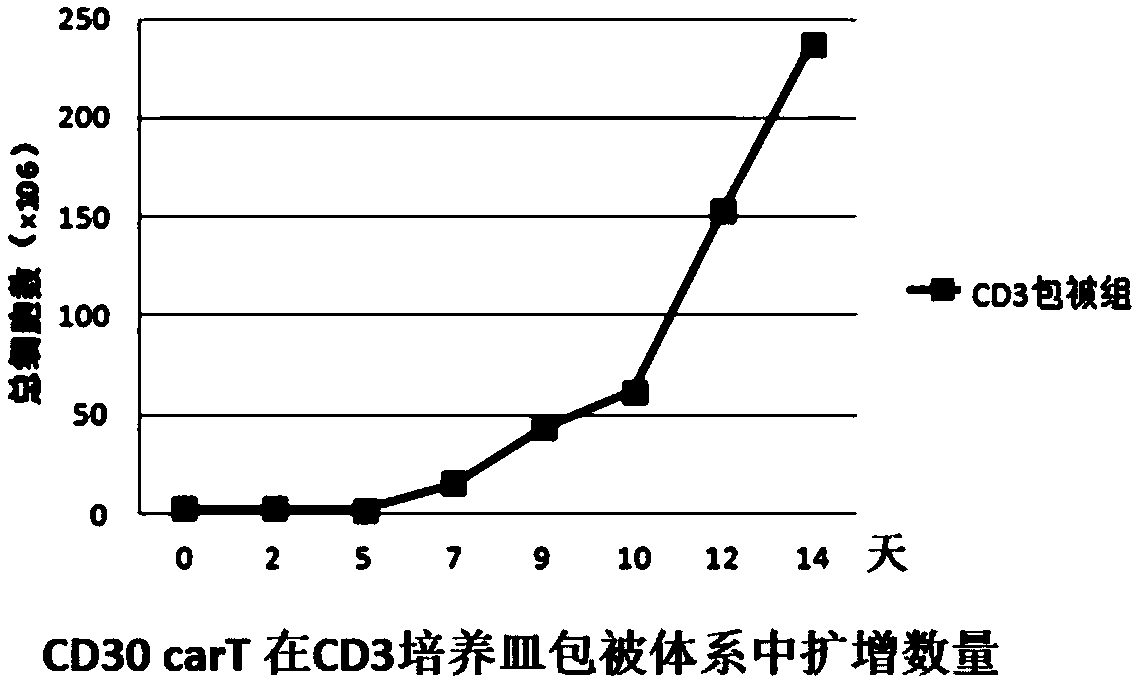
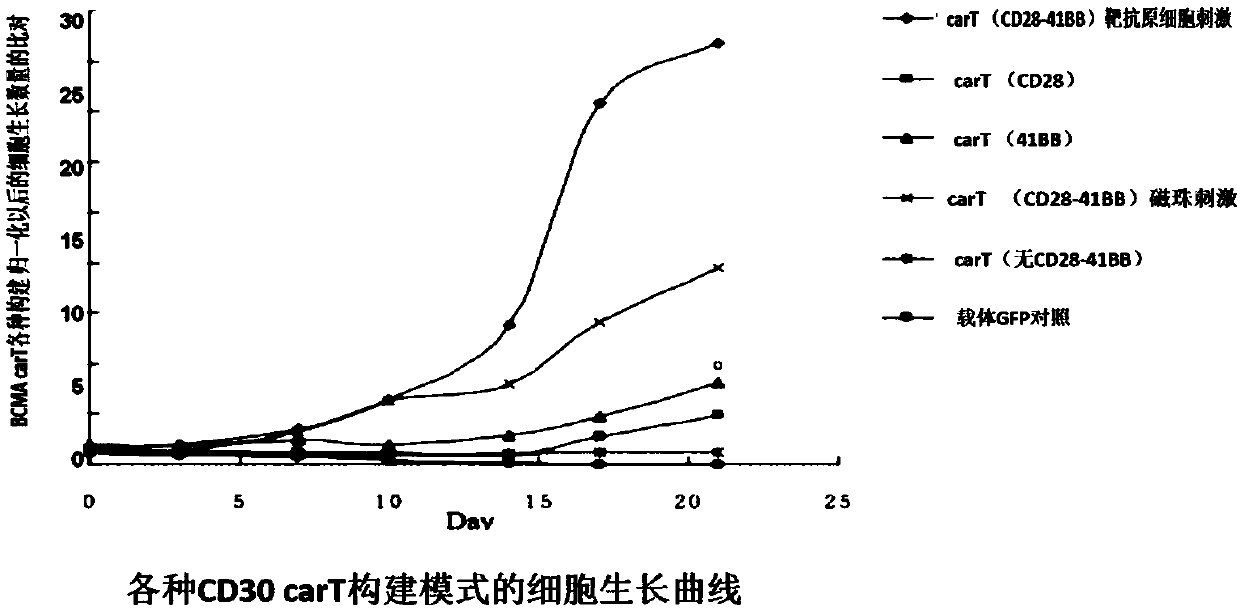
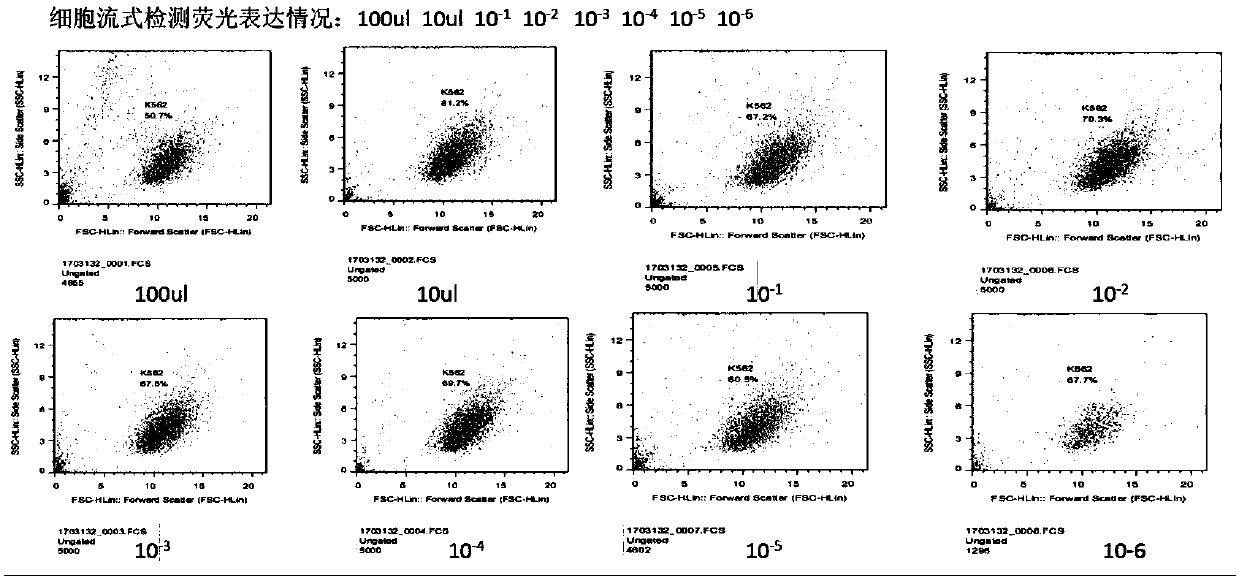
Transgenic T cell of targeted CD30 antigen as well as preparation method and application of transgenic T cell
A transgenic, primitive cell technology, applied in the direction of targeting specific cell fusion, genetically modified cells, receptors/cell surface antigens/cell surface determinants, etc. Problems such as low treatment efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Construction of lentiviral expression vector
[0044] (1) Construction of anti-CD30 AC10 scFv-CD27 Hinge-TM-CD28-41BB-CD3zeta: The construction method is a conventional method.
[0045] (2) Construction of lentiviral expression vector:
[0046] 293T cells were cultured with RPMI1640+10% FBS. After the cell density is 90%, use lipo2000 293T cells. The transfection method is in accordance with the Invitrogen manufacturer's standard operation. The plasmids include: expression plasmid, pMDLg / pRRE, pRSV-Rev, pMD2.G, and mixed plasmid according to 2:1:1:0.5 Ratio of transfected 293T cells. After 24-48 hours, the lentiviral supernatant was harvested twice. After ultracentrifugation at 100,000g for 120min, the supernatant was removed, the lentivirus pellet was obtained, and serum-free T cell culture medium was added. After resuspension, the virus infection titer (IU / ml) was measured by infecting K562 with dilution method.
[0047] Obtain the lentivirus data of a certain ex...
Embodiment 2
[0058] Example 2 Transfection of T cells
[0059] Peripheral blood of healthy people or tumor patients is drawn 50-100ml, or the mononuclear cells are obtained by the Cobra Spectra blood cell separator. After Ficoll separation, they are separated by CD4+ or CD8+ magnetic beads (magnetic beads purchased from Stem CellTechnologies).
[0060] Lentivirus infects human CD4+T or CD8+T cells: Please refer to TakaraRetronectin instruction manual for lentivirus infection after preparation and concentration. The brief description is as follows:
[0061] Prepare Retronectin with a concentration of 20-100μg / ml, and use a density of 4-20μg / cm for paving 2 After 2 hours at room temperature, aspirate the supernatant for use; add lentivirus to the above plate 125-250μl / cm 2 , 37C warm bath for 4-6 hours; T cells to be infected with a density of 0.5-2.5×10 4 cells / cm 2 Planking. The medium was changed 24 hours after T cell infection.
[0062] T cells cultured for 1 day (medium formula: Lonza X vivo15,...
Embodiment 3
[0064] Example 3 Knock out PD1 gene and CTLA4 gene
[0065] The experiments to knock out the PD1 gene and CTLA4 gene were carried out respectively, and the specific methods are as follows:
[0066] The gRNA, CRISPR-cas9 mRNA, and HDR were mixed and electrotransformed into recombinant T cells (400V, 0.5ms).
[0067] After electroporation, culture the cells for 24 hours and then change the medium (the medium used is: Lonza X vivo15, adult serum 10%, IL2500IU / ml, gentamycin 100ug / ml, IL155ng / ml, IL215ng / ml OKT3antibody 50ng / ml), Pre-coated with OKT monoclonal antibody (10ug / ml, 25C 4hr) in T75flask, continue to culture, count every 2 days, observe the cell morphology and add culture medium, maintain the cell concentration at 1.0-3.0×10 6 / ml between. After the 10th day of culture, T cells were harvested by centrifugation and washed to remove the culture solution.
[0068] The nucleotide sequence of the CRISPR-cas9 mRNA is shown in SEQ ID NO: 7;
[0069] When the PD1 gene is knocked out, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com