Chimeric antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Hybridoma Cell Line CE 25
1.1 Purification of Carcinoembryonic Antigen (CEA)
Colon carcinoma liver metastases obtained from autopsies (within 6 h of death) are extracted with saline. 1 vol. of tissue is first homogenized in 3 vol. of 0.02M phosphate buffer pH 7.4 at 4.degree. C. for 10 min in a Sorvall Omnimixer at 8,000 rpm. The crude homogenate is then centrifuged at 8,000 g for 15 min at 4.degree. C. The clear supernatant is applied to an immunoadsorbent consisting of a pool of the known anti-CEA monoclonal antibodies MAb 35 and MAb 115 (Haskell et al., Cancer Res. 43, 3857, 1983; Buchegger et al., J. Exp. Med. 158, 413, 1983) and MAb 73 (Buchegger et al., Immunol. Letters 5, 85, 1982) coupled to CNBr-activated Sepharose. CEA is eluted with 2M ammonium thiocyanate.
1.2 Immunization of Balb / c Mice
Balb / c mice two months of age are immunized with CEA by injecting intraperitoneally 15 .mu.g of saline-extracted purified CEA with complete Freund's adjuvant. After 4 months, ...
example 2
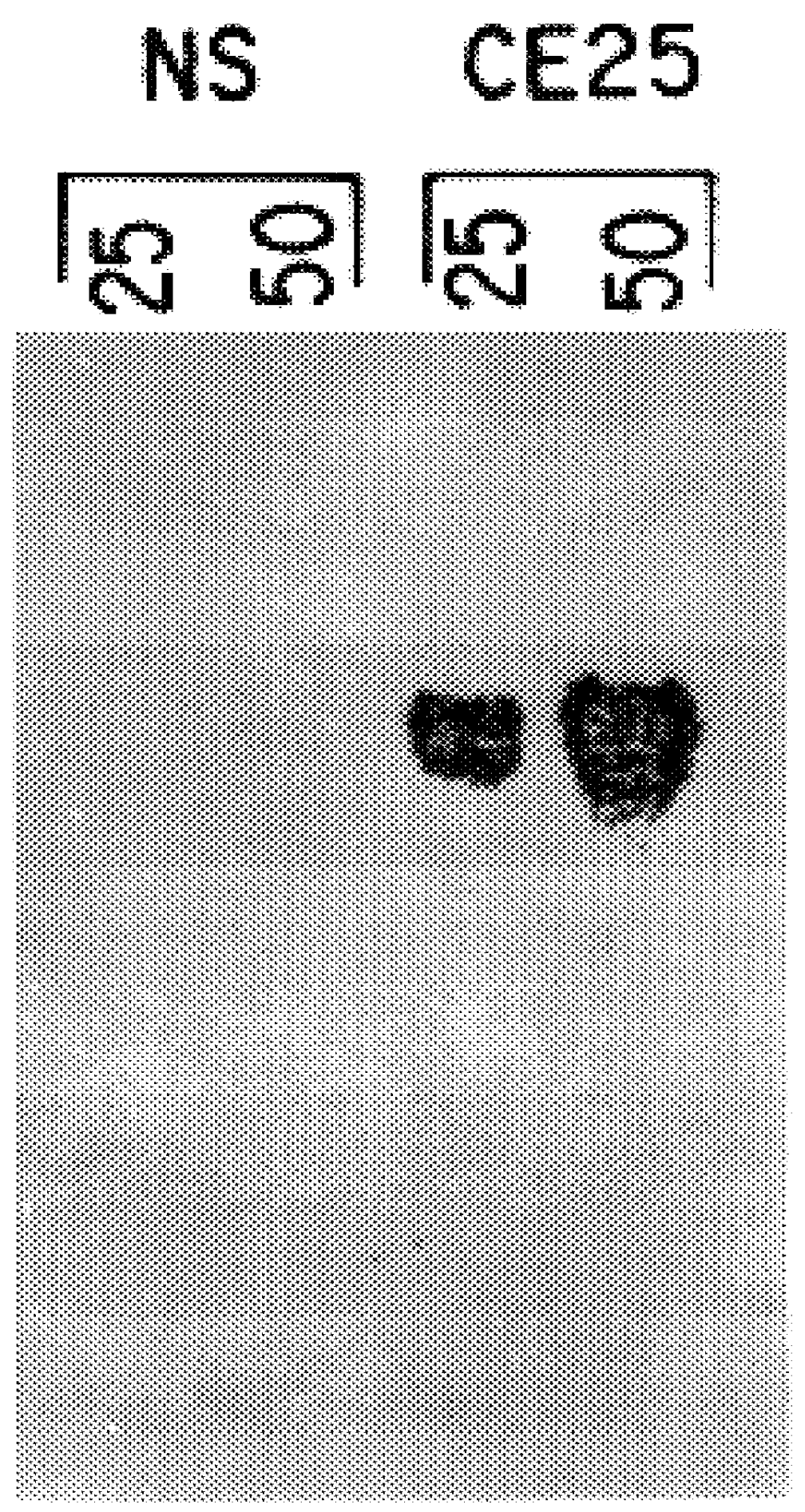
Isolation of DNA from the Hybridoma Cell Lines CE 25, P3-NS2 / 1Ag4 and Balb / c Mouse Kidney Cells
CE 25 hybridoma cells (5.times.10.sup.7) are grown in suspension culture at 37.degree. C. in DMEM (Seromed)+10% FCS (Seromed), 1 mM sodium pyruvate (Seromed), 2 mM glutamine (Seromed), 50 .mu.M 2 mercaptoethanol and 100 .mu.g / ml of gentamycin (Seromed) in a humidified atmosphere of air and 7.5% CO.sub.2, in 175 cm.sup.3 tissue culture flasks (Falcon 3028). Cells are harvested by centrifugation, flash-frozen in liquid nitrogen and kept frozen as a pellet at -80.degree. C. in a clean, sterile plastic capped tube.
The frozen cells are resuspended in 10 ml of PBS to which is added 90 ml of 0.3 M sucrose, 5 mM MgCl.sub.2, 0.1% (w / v) Triton-X100, 10 mM Tris-HCl, pH 7.5, at 4.degree. C. in a clean, sterile 100 ml plastic beaker. Cells are lysed by mixing, and nuclei collected by centrifugation (10 min, 10,000 rpm, 4.degree. C., Sorvall RC-5 centrifuge, SS-34 rotor). The supernatant is removed and ...
example 3
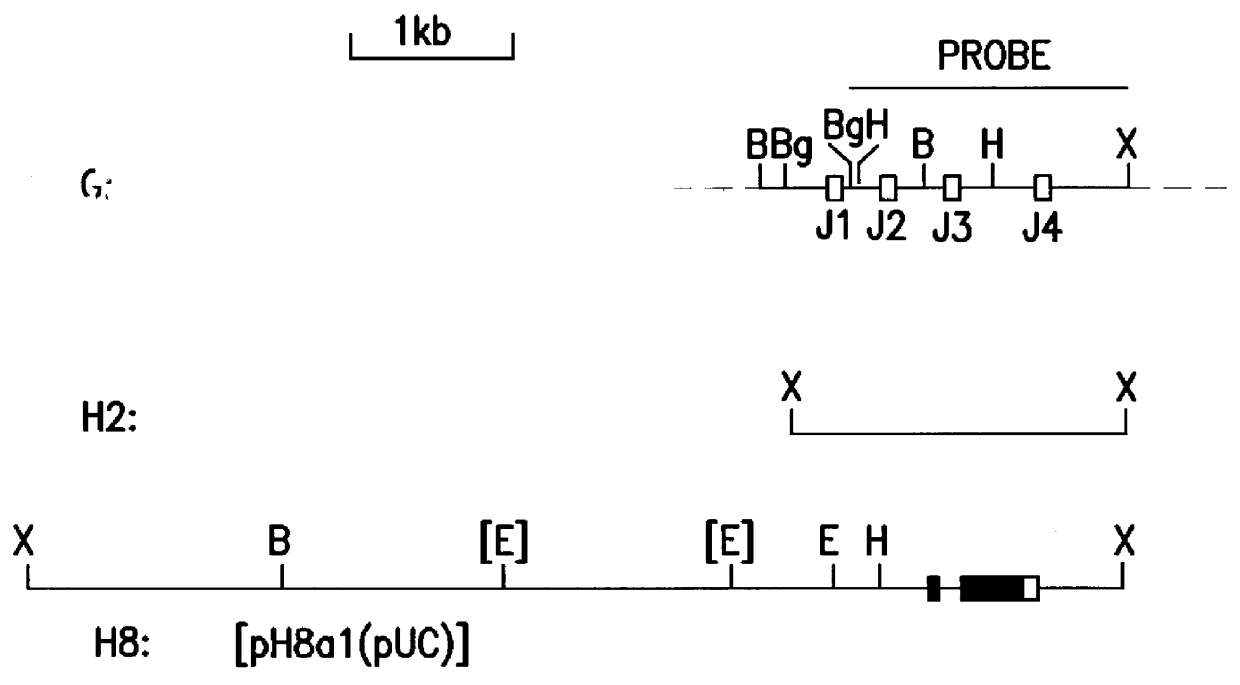
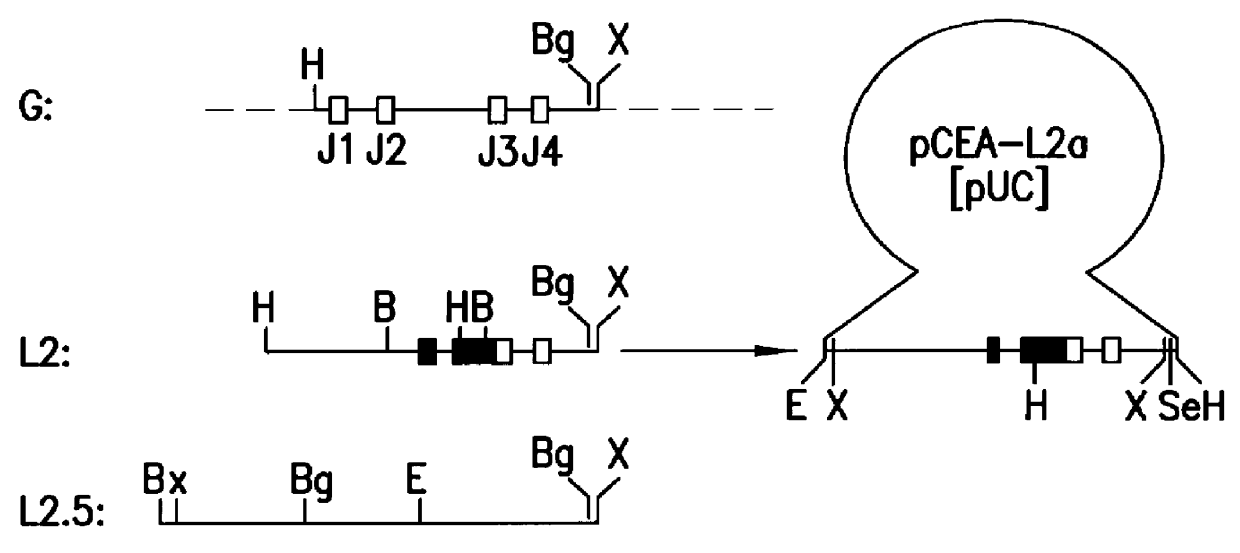
Analysis of Rearranged Ig H- and L-chain Gene Loci in CE 25 Cells
Hybridoma CE 25 contains H- and L-chain Ig gene loci derived from the P3-NS2 / 1Ag4 cell used as fusion partner for the generation of the hybridoma. The P3-NS2 / 1Ag4 cell line is derived from the MOPC-21 myeloma (Storb et al., Nucleic Acids Res. 8, 4681, 1980). These `endogenous` rearranged loci are distinguished from CE 25-specific rearranged genes by the following procedures:
3.1 Source and Preparation of Probe DNA Fragments
The probe DNA segment used for the detection of the Balb / c mouse germline H-chain J-region DNA segment is an approximately 1750 bp BglII / XbaI segment of Balb / c mouse liver DNA, corresponding to nucleotide positions 1130-2881 of the published germline H-chain Ig locus (Newell et al., Science 209, 1128, 1980; EMBL data base entry MUSIGCDO7).
The probe DNA segment used for the detection of the Balb / c mouse germline L-chain J-region segment is an approximately 2240 bp HindIII / XbaI segment of Balb / c mouse l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Digital information | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com