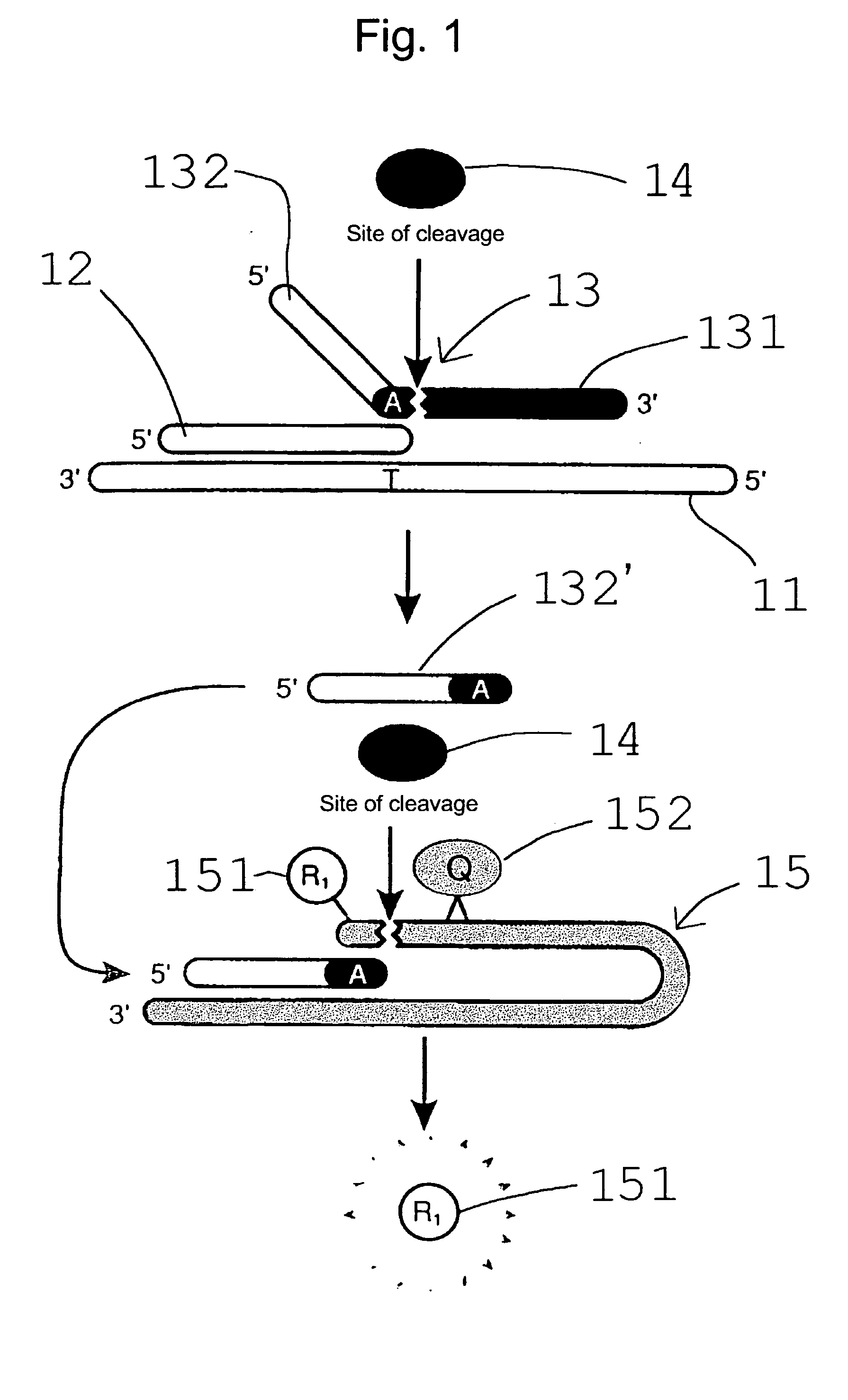
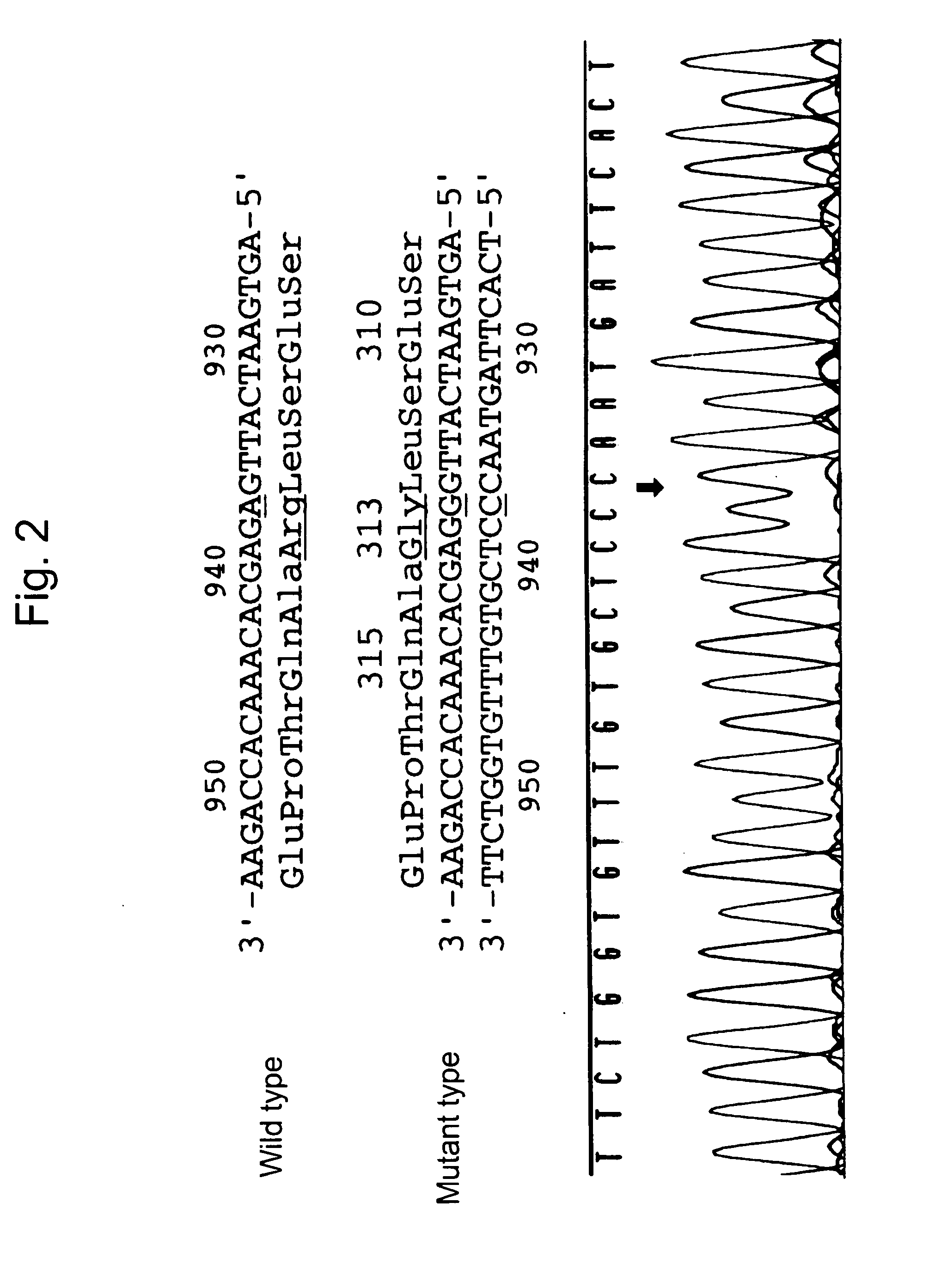
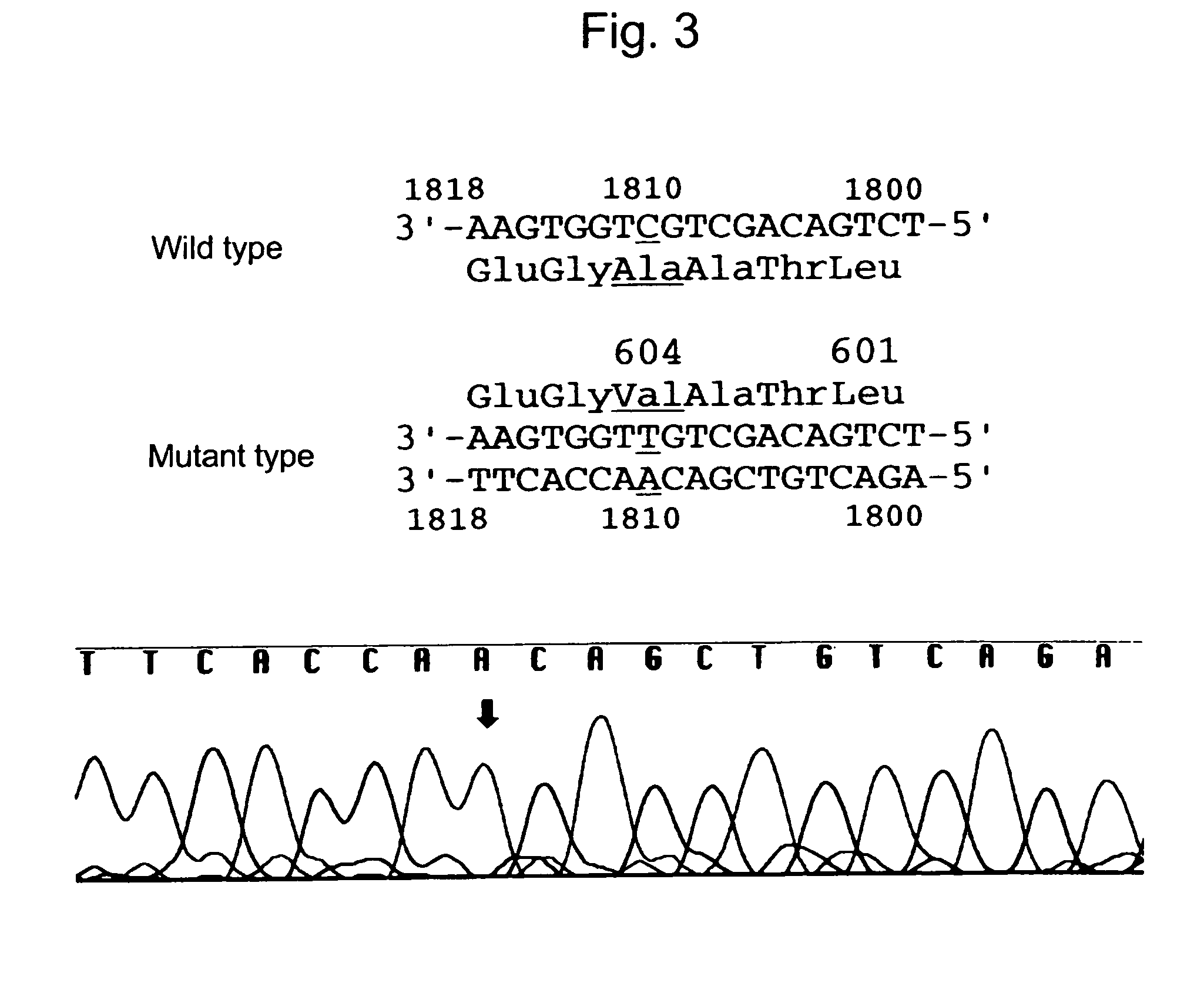
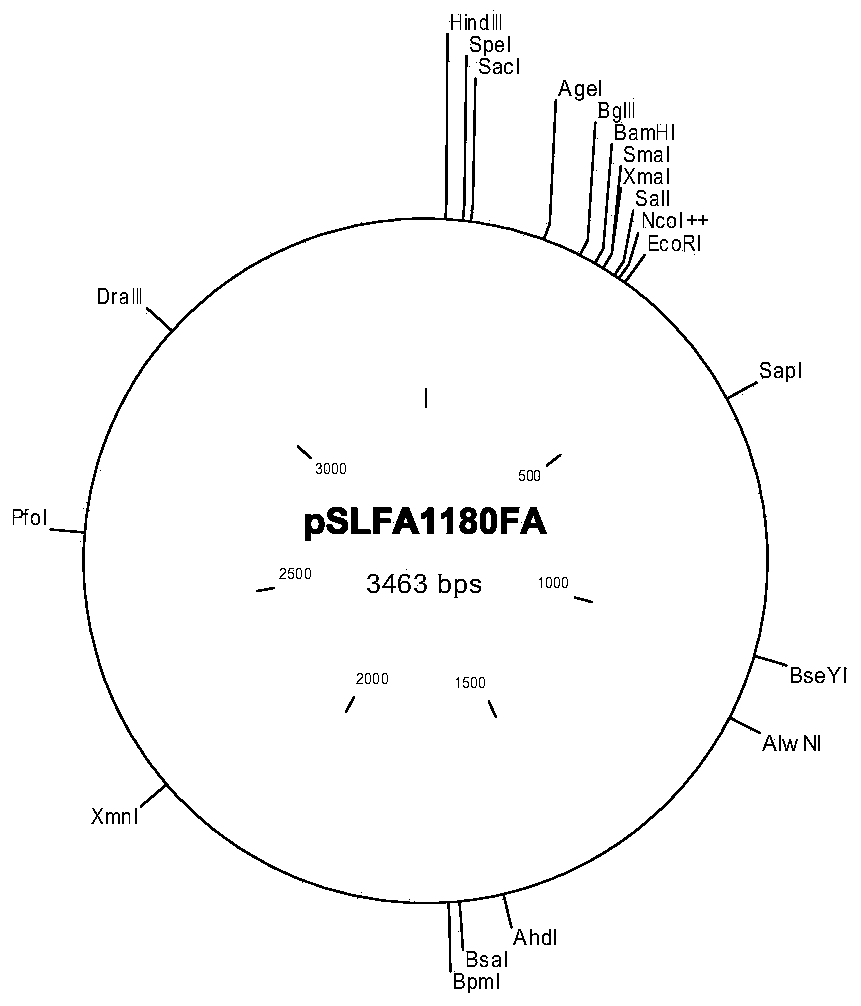
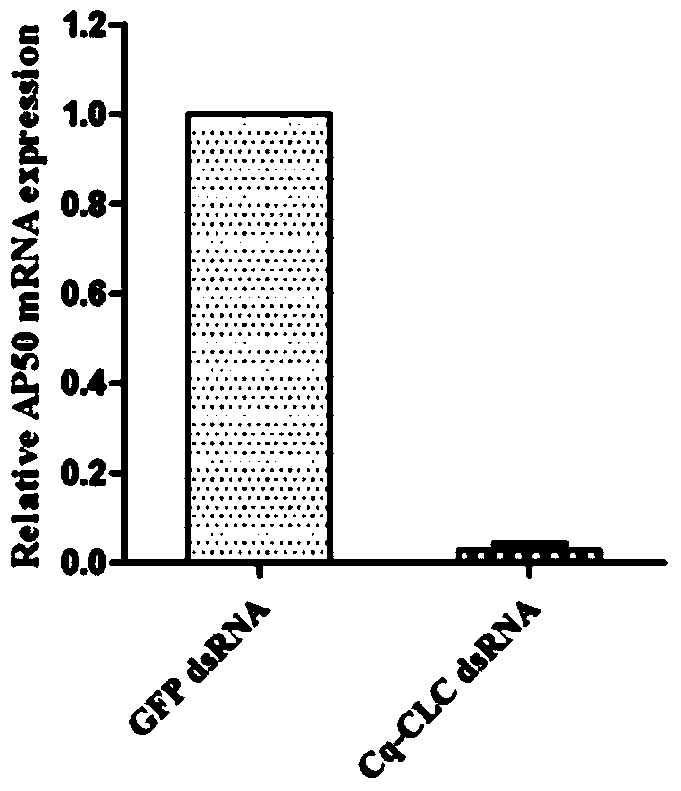Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Chain gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
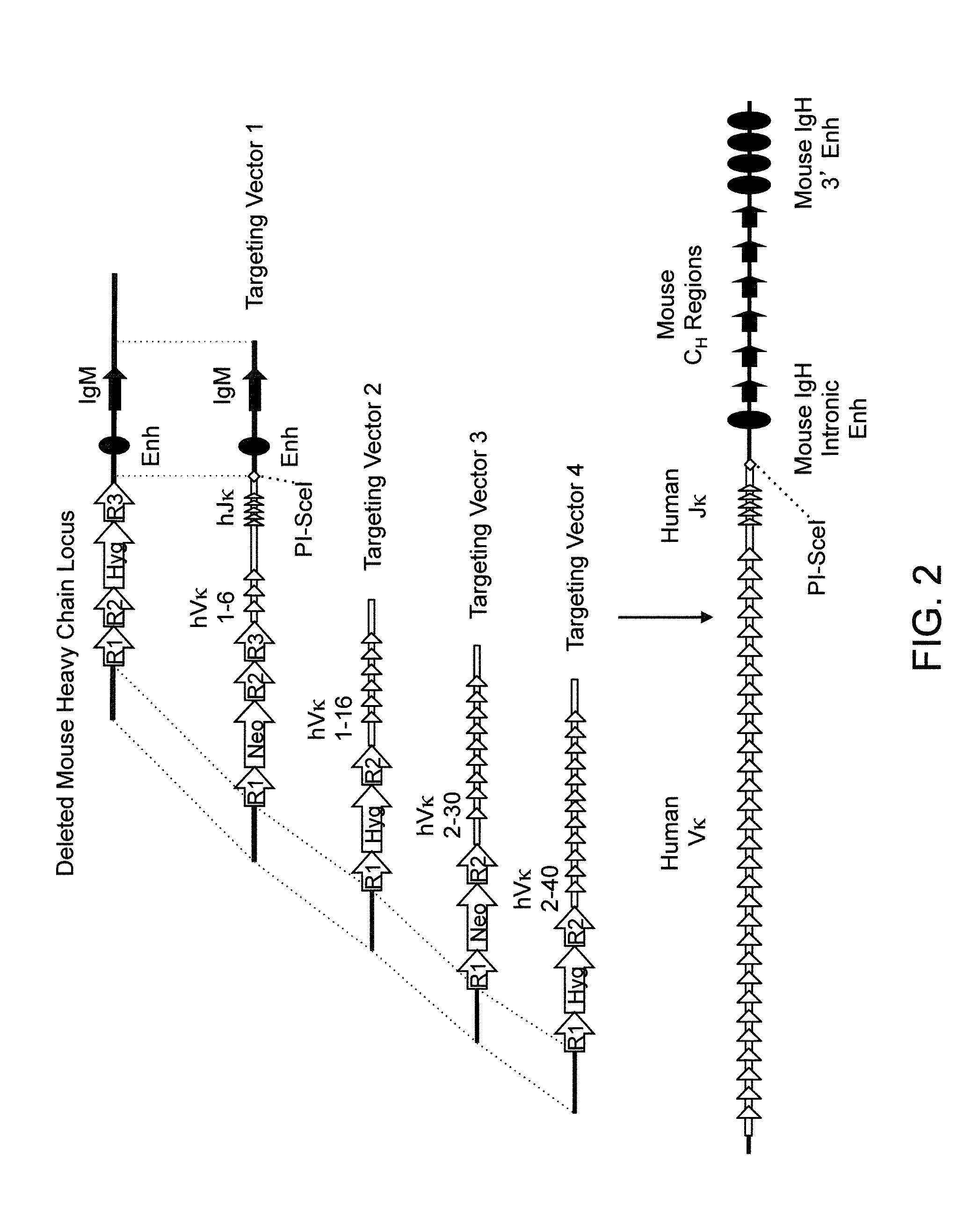
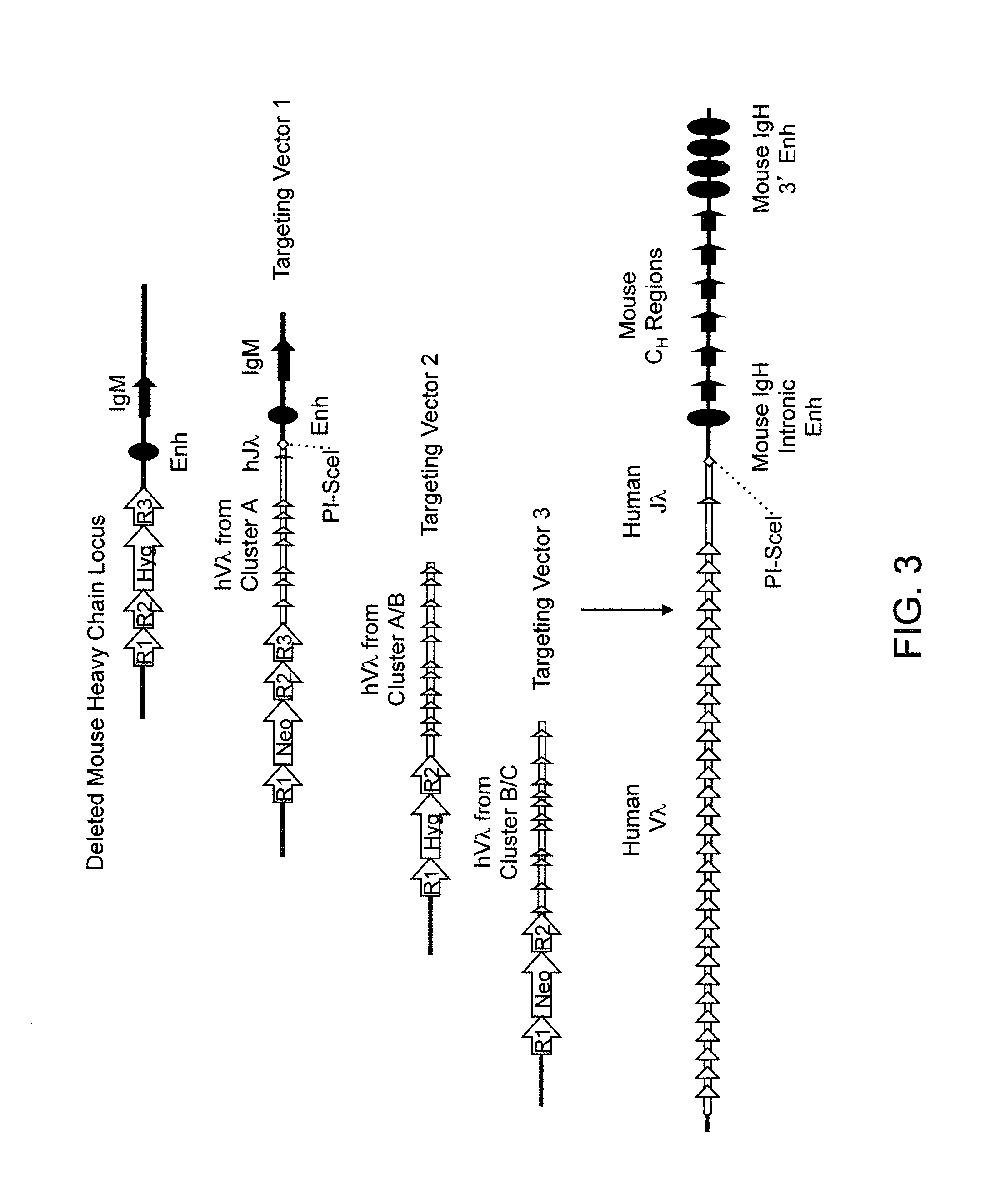
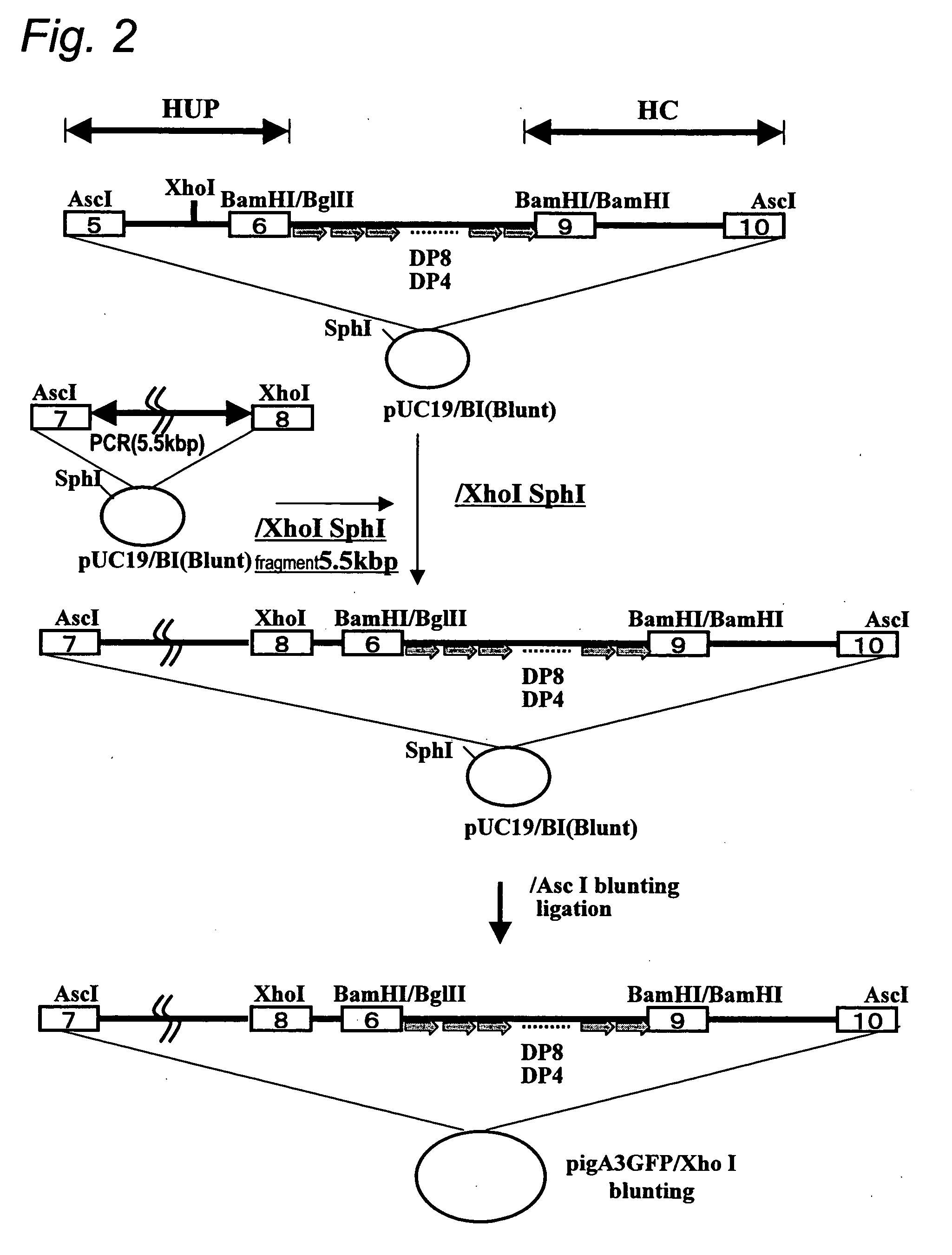
Humanized Rodents that Express Heavy Chain Containing VL Domains
InactiveUS20130212719A1Reduced fertilityImprove fertilityAnimal cellsHybrid immunoglobulinsGenetic MaterialsVariable domain
Non-human animals, tissues, cells, and genetic material are provided that comprise a modification of an endogenous non-human heavy chain immunoglobulin sequence and that comprise an ADAM6 activity functional in a rodent (e.g., a mouse), wherein the non-human animals rearrange human immunoglobulin light chain gene segments in the context of heavy chain constant regions and express immunoglobulin-like molecules comprising human immunoglobulin light chain variable domains fused to heavy chain constant domains that are cognate with human immunoglobulin light chain variable domains fused to light chain constant domains.
Owner:REGENERON PHARM INC
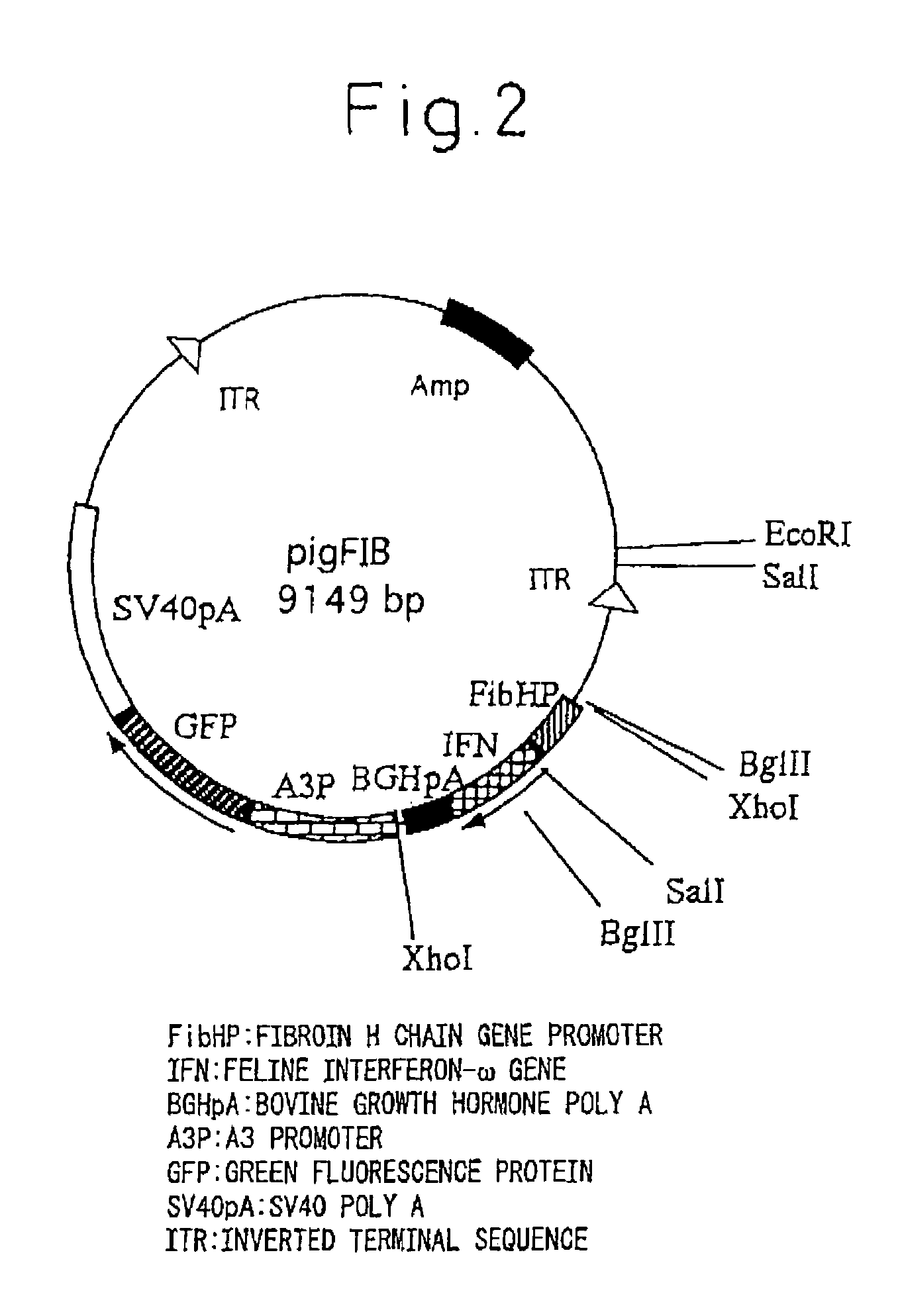
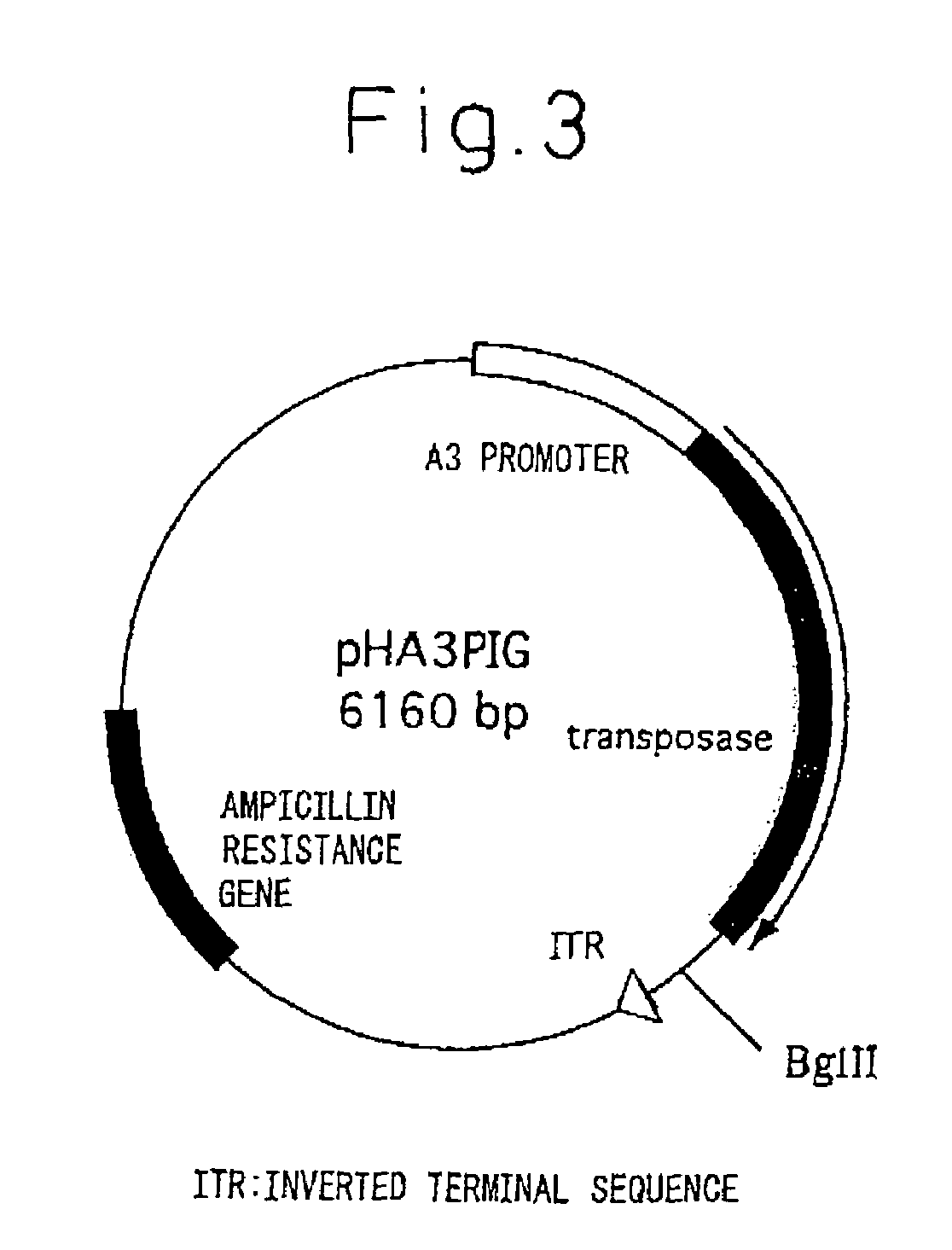
Silk Thread Containing Spider Thread Protein and Silk Worm Producing the Silk Thread
InactiveUS20080287651A1Maintain good propertiesHigh strengthPeptide/protein ingredientsImmunoglobulinsBiotechnologyHigh intensity
A transgenic silkworm having transferred therein a gene which encodes spider thread protein having desired properties of high strength and high elasticity while leaving the silkworm fibroin H chain gene intact, by means of utilizing a transposon function, is used to produce in the transgenic silkworm a spider thread protein having the desired properties without lowering the strength or elasticity of silk thread produced by the transgenic silkworm, thereby providing hybrid silk of spider and silk threads having the desired properties.
Owner:TORAY IND INC +1
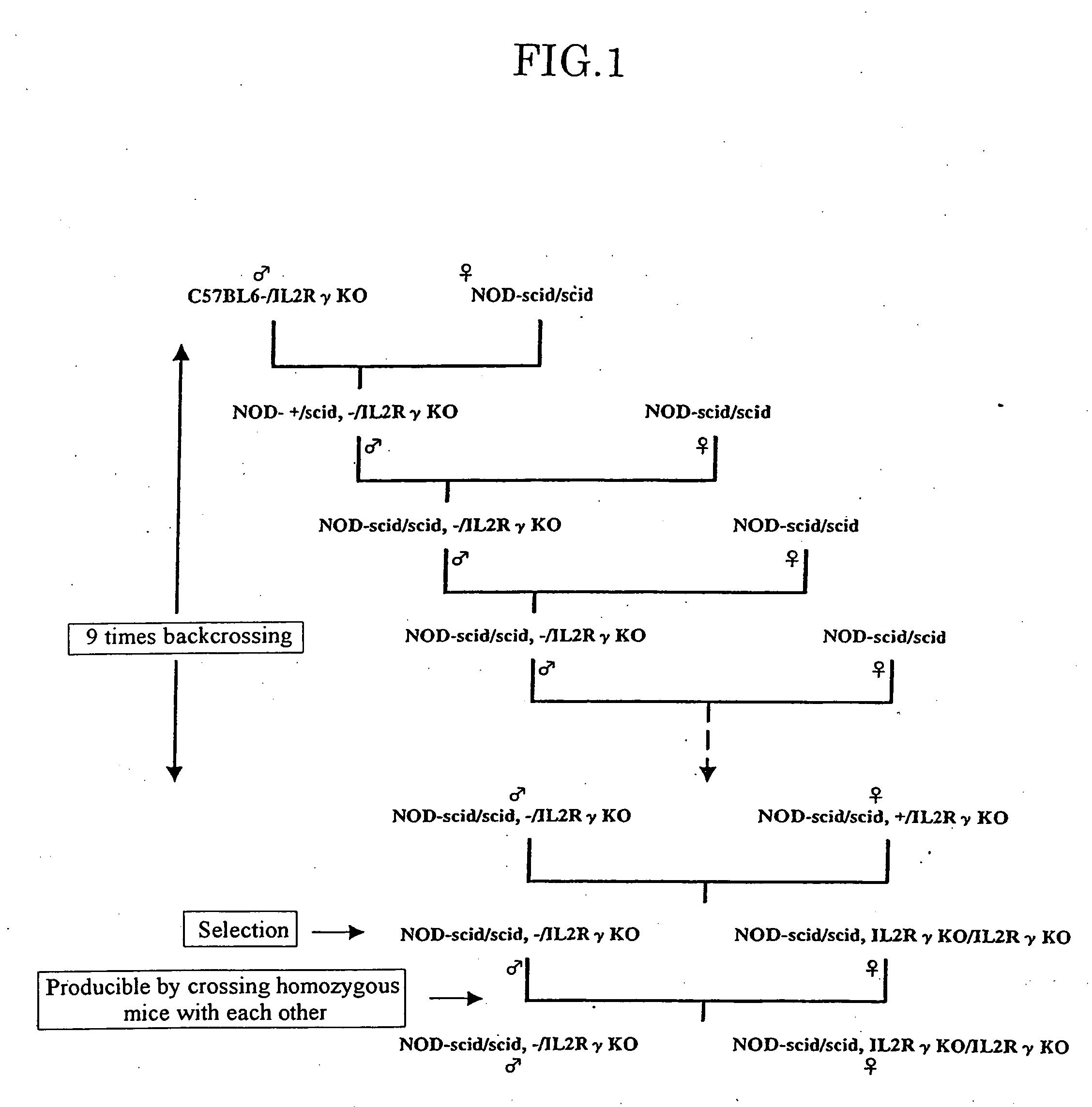
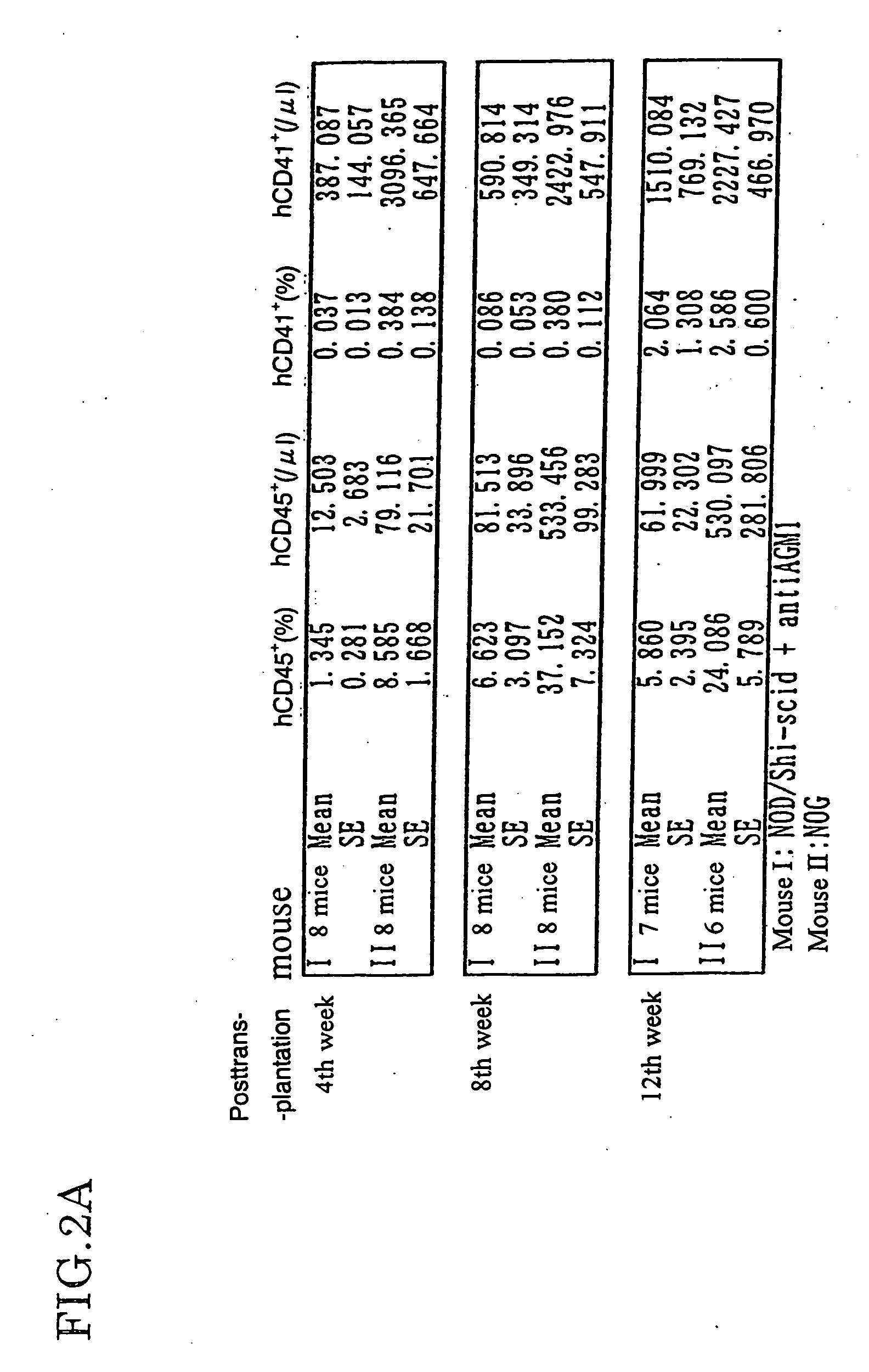
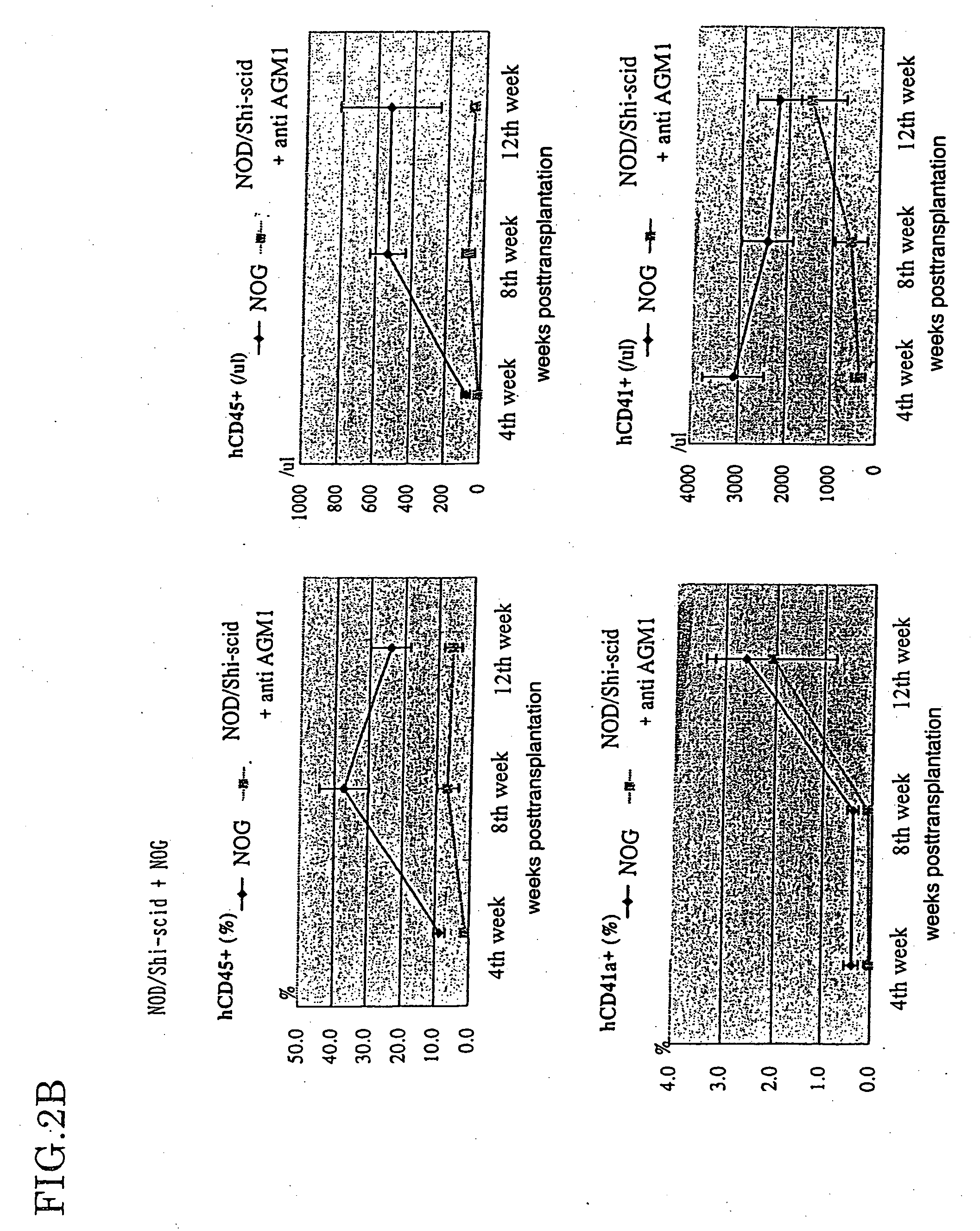
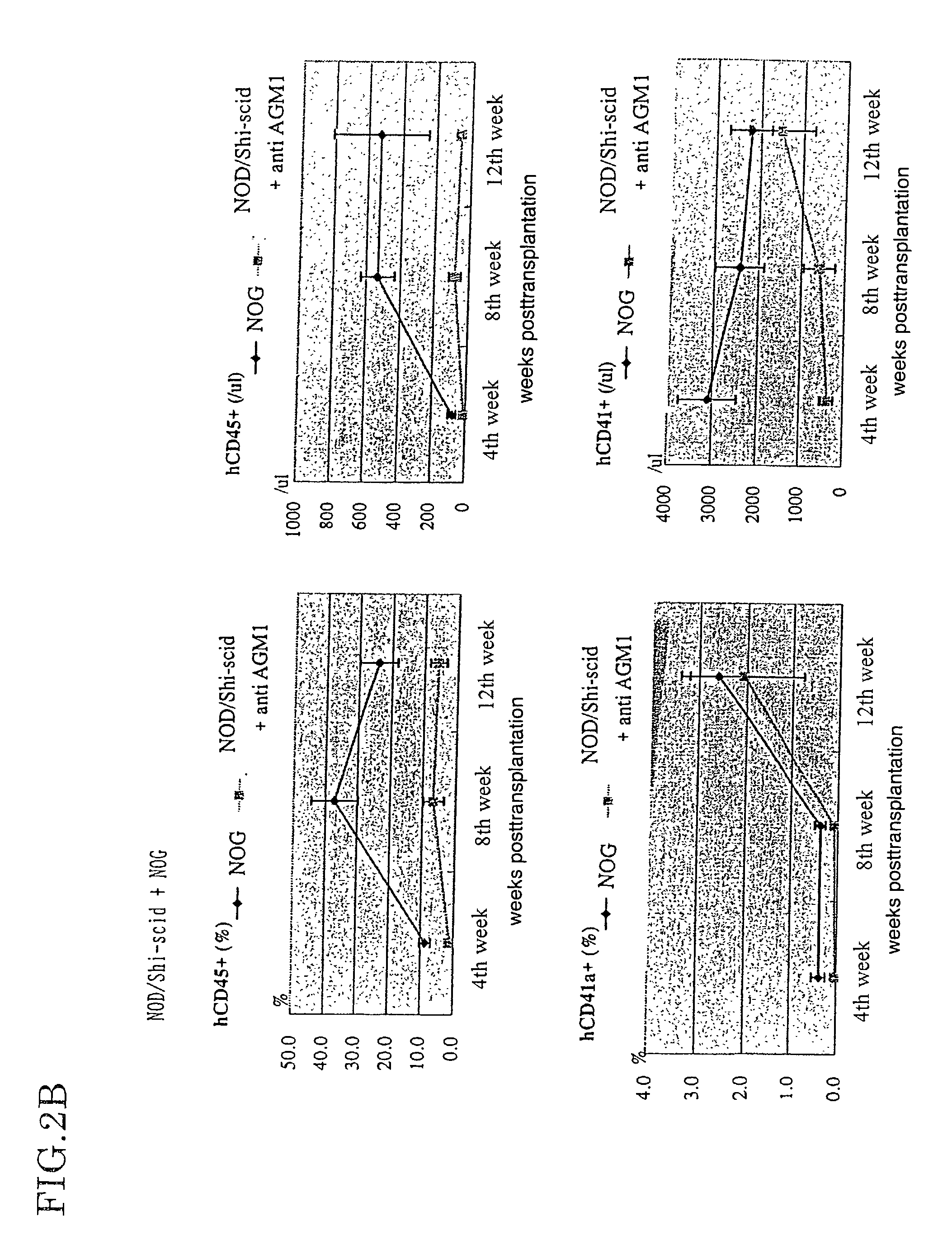
Method of producing a mouse suitable for engraftment, differentiation and proliferation of heterologous cells, mouse produced by this method and use of the mouse
InactiveUS20070011753A1Excellent heterologous cell engraftment capacityImmunoglobulins against animals/humansArtificially induced pluripotent cellsHeterologousAbnormal tissue growth
The present invention provides an immunodeficient mouse (NOG mouse) suitable for engraftment, differentiation and proliferation of heterologous cells, and a method of producing such a mouse. This mouse is obtained by backcrossing a C.B-17-scid mouse with an NOD / Shi mouse, and further backcrossing an interleukin 2-receptor γ-chain gene-knockout mouse with the thus backcrossed mouse. It is usable for producing a human antibody and establishing a stem cell assay system, a tumor model and a virus-infection model.
Owner:ITO MAMORU +9
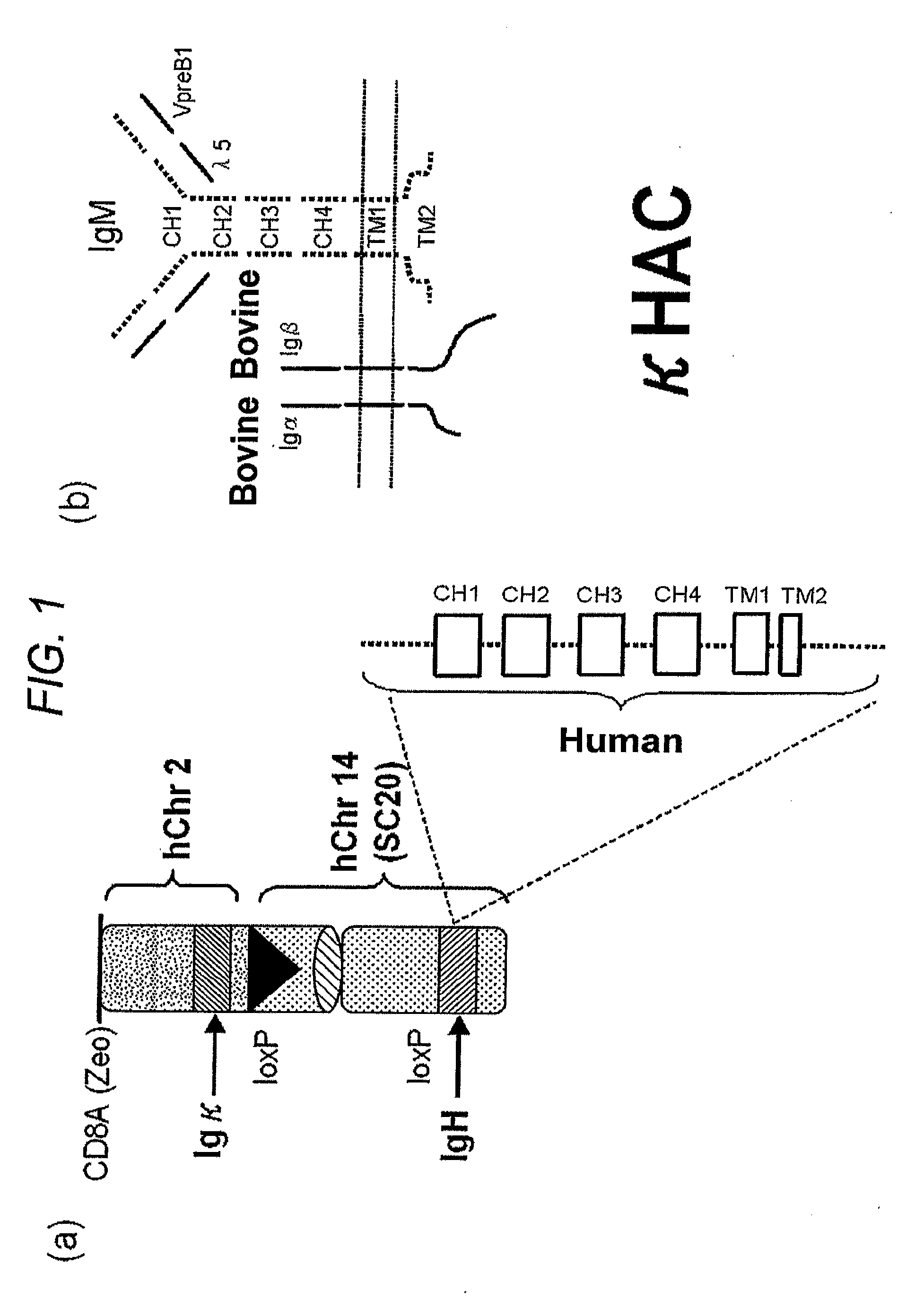
Human artificial chromosome vector
InactiveUS20120233715A1Efficient productionImprove efficiencyNucleic acid vectorImmunoglobulinsHuman artificial chromosomeGene
A human artificial chromosome vector comprising a human antibody heavy chain gene, a human antibody light chain gene, and a human antibody surrogate light chain gene.
Owner:SANFORD APPLIED BIOSCI
Method of producing a mouse suitable for the engraftment, differentiation and proliferation of heterologous cells, mouse produced by this method and use of the mouse
InactiveUS7145055B2Excellent heterologous cell engraftment capacityImmunoglobulins against animals/humansMutant preparationAbnormal tissue growthHeterologous
The present invention provides an immunodeficient mouse (NOG mouse) suitable for engraftment, differentiation and proliferation of heterologous cells, and a method of producing such a mouse. This mouse is obtained by backcrossing a C.B-17-scid mouse with an NOD / Shi mouse, and further backcrossing an interleukin 2-receptor γ-chain gene-knockout mouse with the thus backcrossed mouse. It is usable for producing a human antibody and establishing a stem cell assay system, a tumor model and a virus-infection model.
Owner:CENT INST FOR EXPERIMENTAL ANIMALS
Silkworm fibroin heavy-chain gene mutation sequence and mutation method and application
ActiveCN102358902AHigh expressionHigh purityFermentationVector-based foreign material introductionGeneticsSilk gland
The invention relates to a silkworm fibroin heavy-chain gene mutation method, which specifically comprises the step of: acting mRNA (Messenger Ribonucleic Acid) of a coded zinc-finger nuclease sequence on loci 1325-1362 of a silkworm fibroin heavy-chain gene shown as SEQ ID NO:1 to form target positions for recognizing zinc-finger nuclease, thereby obtaining a series of silkworm fibroin heavy-chain mutated genes; and the mutated sequence can be applied to preparation of sericin and extrinsic proteins. Mutants provided by the invention have the following advantages that: (1) a posterior division of silkgland of a fibroin heavy-chain gene mutant provided by the invention serious degrades, a cocoon shell only contains the sericin synthesized and secreted by a middle division of silkgland, if the mutated strains are utilized to transgenically express the extrinsic proteins, the expressed amount and the purity of the extrinsic proteins can be greatly increased, and thus, a brand-new useful genetic material is provided for the development of a silkworm fibroin bioreactor; and (2) the cocoon shell of the mutated strain provided by the invention only contains the sericin, and thus, a new source is provided for the large-scale production of the sericin.
Owner:SOUTHWEST UNIV
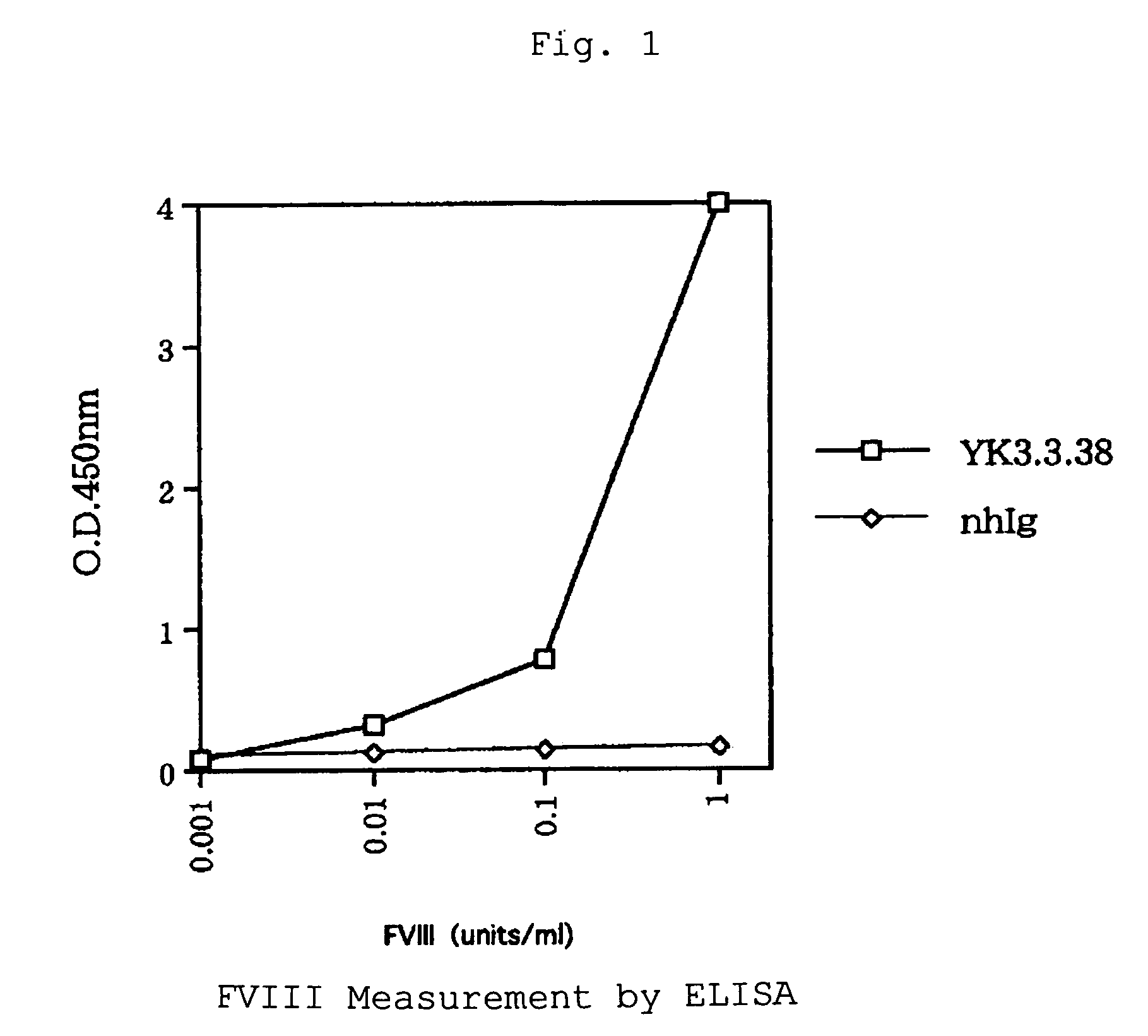
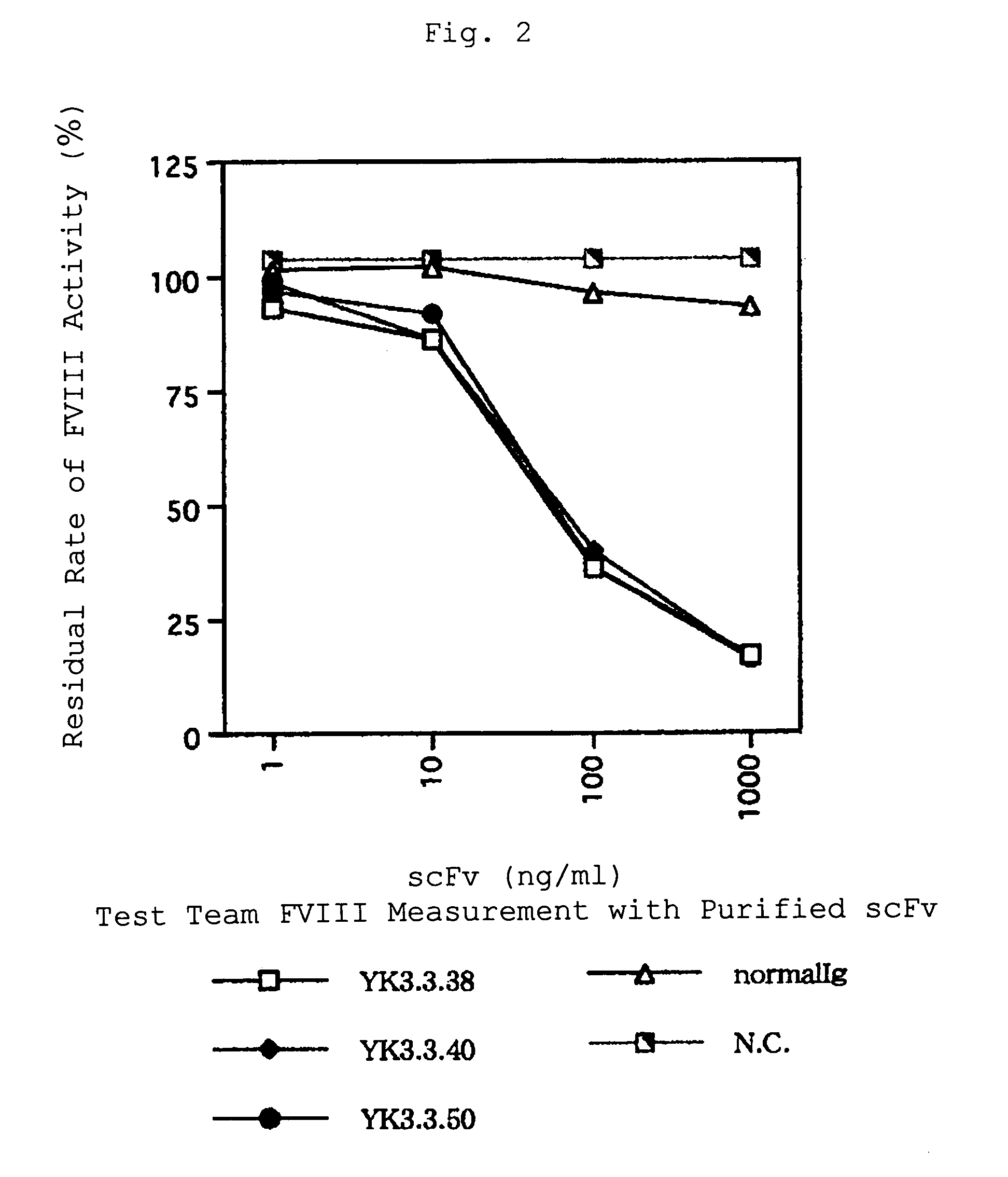
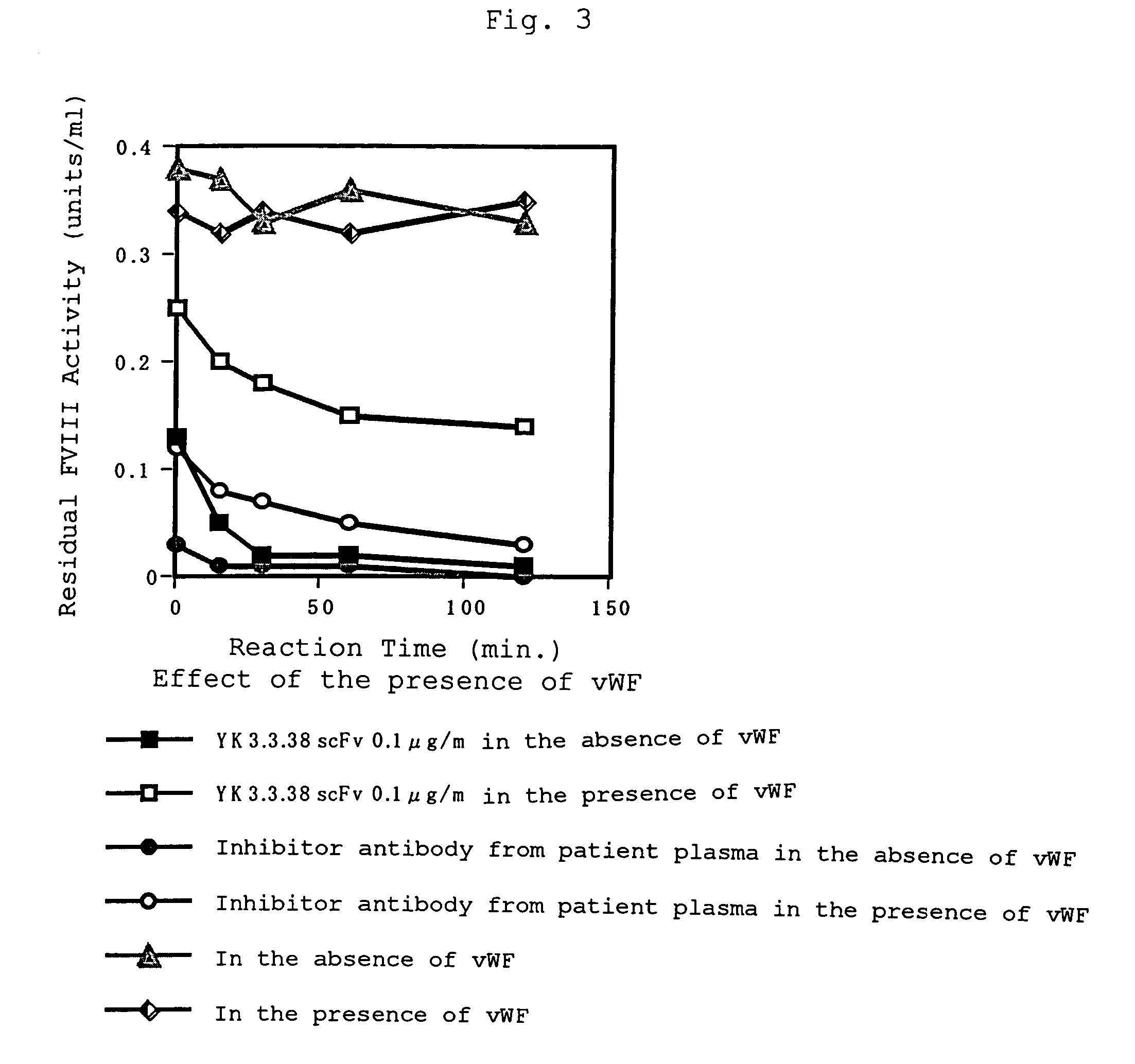
Human-type anti-blood coagulation factor VIII antibody
InactiveUS7214785B2Immunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIRandom combination
The present invention provides a human antibody against blood coagulation factor VIII (hereinafter also referred to as “FVIII”) and an antibody fragment that binds to human FVIII and specifically inhibits the coagulation activity of human FVIII. ScFv display phage libraries, prepared by using scFv DNAs constructed by random combinations of immunoglobulin VH chain genes and VL chain genes from lymphocytes from hemophilia A patients, is reacted with FVIII immobilized to a solid phase via anti-FVIII monoclonal antibody, and scFv clones capable of binding to FVIII are cloned to reveal VH and VL chains of FVIII-specific antibody.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Human source Fab antibody for anti recombined alkalescent fibroblast growth factor and application
InactiveCN101092457AHigh affinityImprove featuresImmunoglobulins against growth factorsAntibody ingredientsSurface displayFibrosis
This invention discloses anti-recombinant alkaline fibroblast growth factor human-derived Fab antibody and its application. The antibody segment genome comprises: heavy chain Fd chain and light chain kappa chain. This invention utilizes phage surface display technique to successfully construct kappa chain and Fd chain genes of human-derived Fab antibody that can neutralize recombinant human bFGF or FGF-2. Functional neutralizing antibody that can specifically bind bFGF or FGF-2 is obtained in prokaryotic cells, yeast cells, insect cells and eukaryotic cells, which can be used to prepare antibody drugs that can diagnose and treat tumors, and inhibit kidney, liver and lung fibrosis. The bFGF or FGF-2 human-derived Fab antibody has high affinity and specificity, and can be directly used to develop antibody drugs.
Owner:JINAN UNIVERSITY
Process for producing physiologically active protein using genetically modified silkworm
InactiveUS20050177877A1Improve purification effectEasy to aimAnimal cellsDepsipeptidesProtein targetActive protein
The present invention provides a genetic engineering material for insects that enables a target protein to be purified easily, without requiring the use of recombinant baculovirus, while simultaneously providing a process for producing exogenous protein using that genetic engineering material. A gene recombinant silkworm is obtained by inserting an exogenous protein gene such as a cytokine gene coupled to a promoter that functions in silk glands into a silkworm chromosome. An exogenous protein such as a cytokine is then extracted and purified from the silk glands or cocoon of that silkworm or its offspring. A large amount of exogenous protein can be produced within silk gland cells, outside silk gland cells or in silk thread or a cocoon by inserting an expression gene cassette, in which the DNA sequence of the 3′ terminal portion and the DNA sequence of the 5′ terminal portion of fibroin H chain gene are fused to the exogenous protein gene, into silk gland cells and so forth.
Owner:NAT INST OF AGROBIOLOGICAL SCI
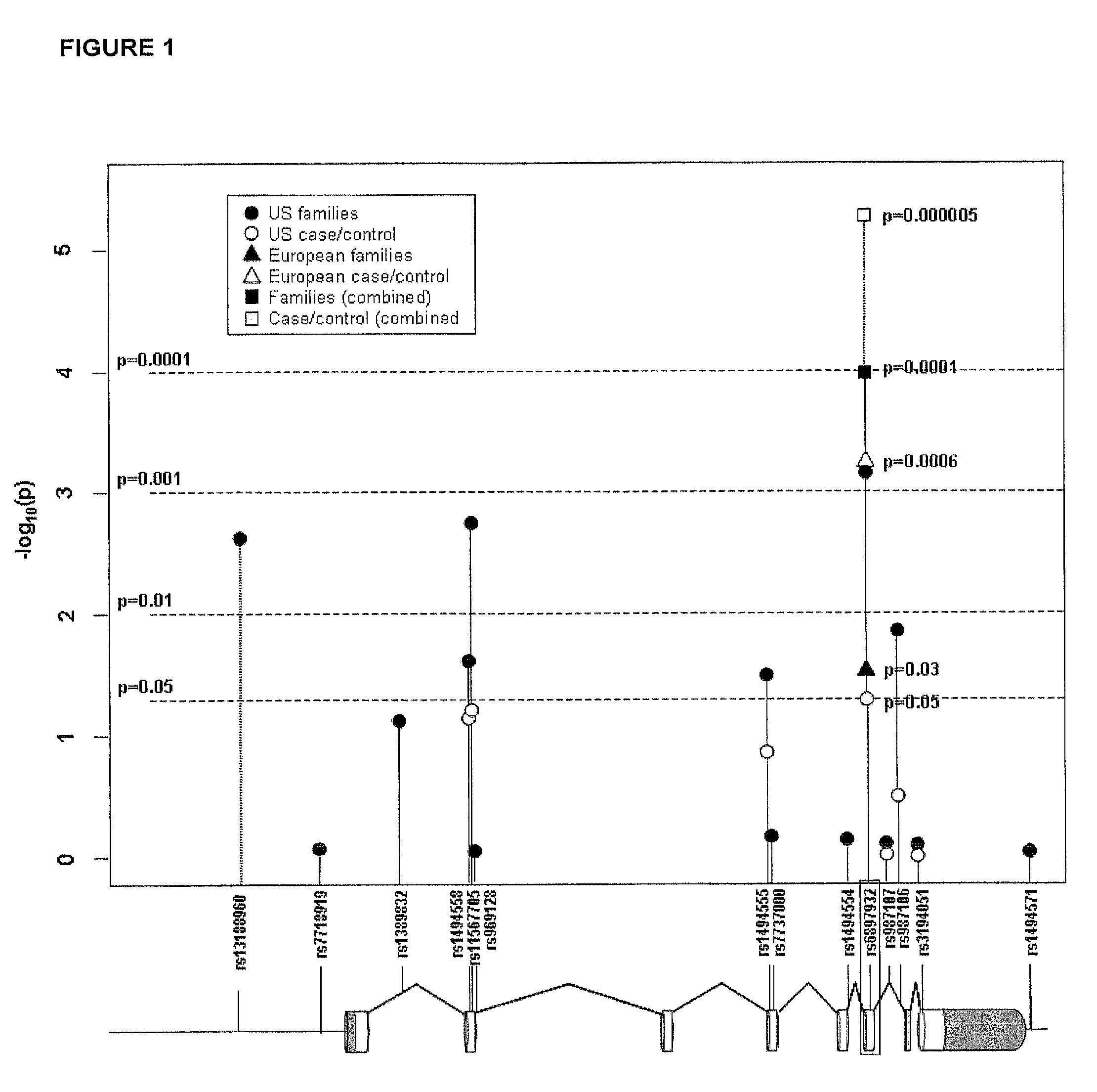
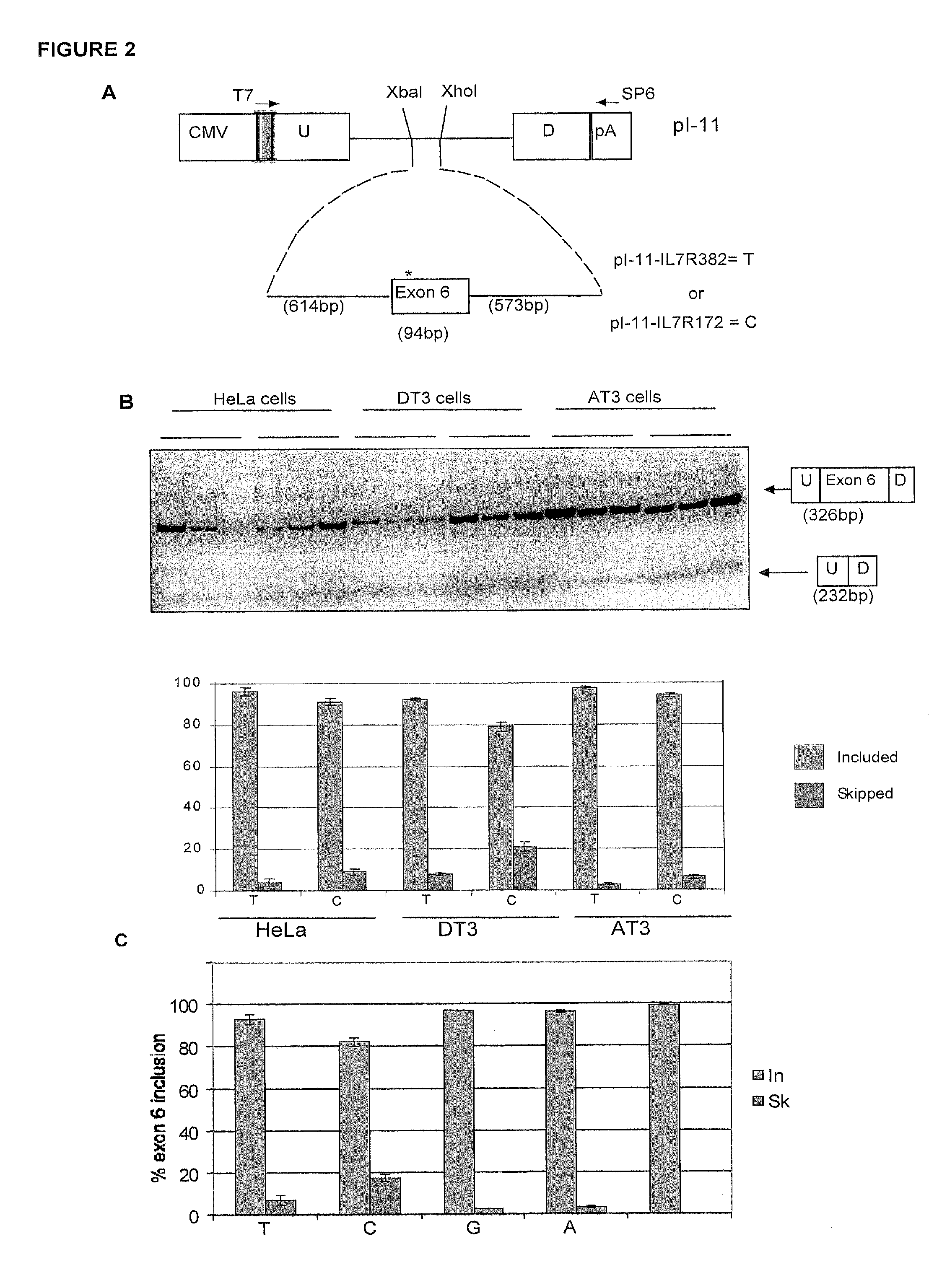
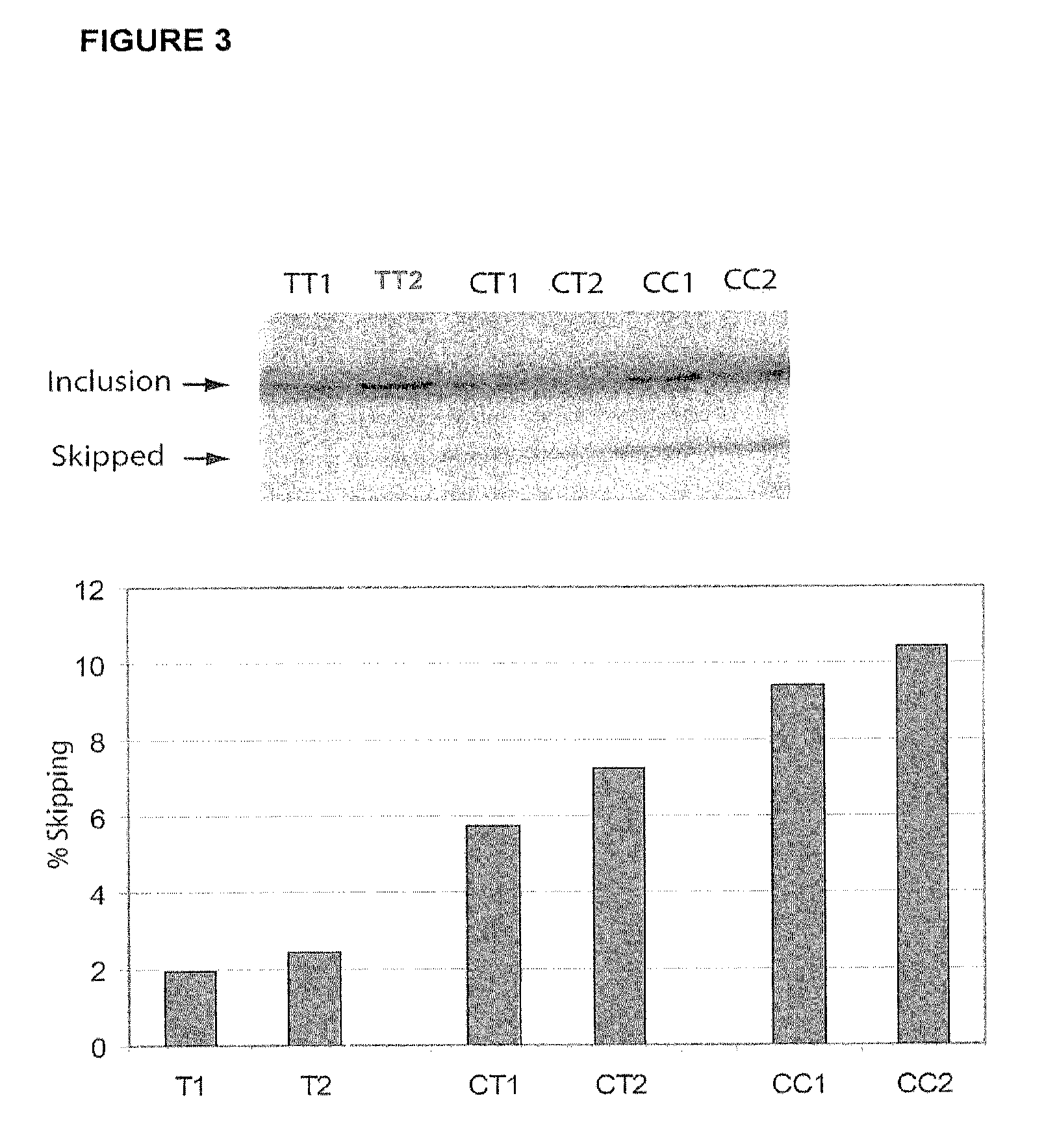
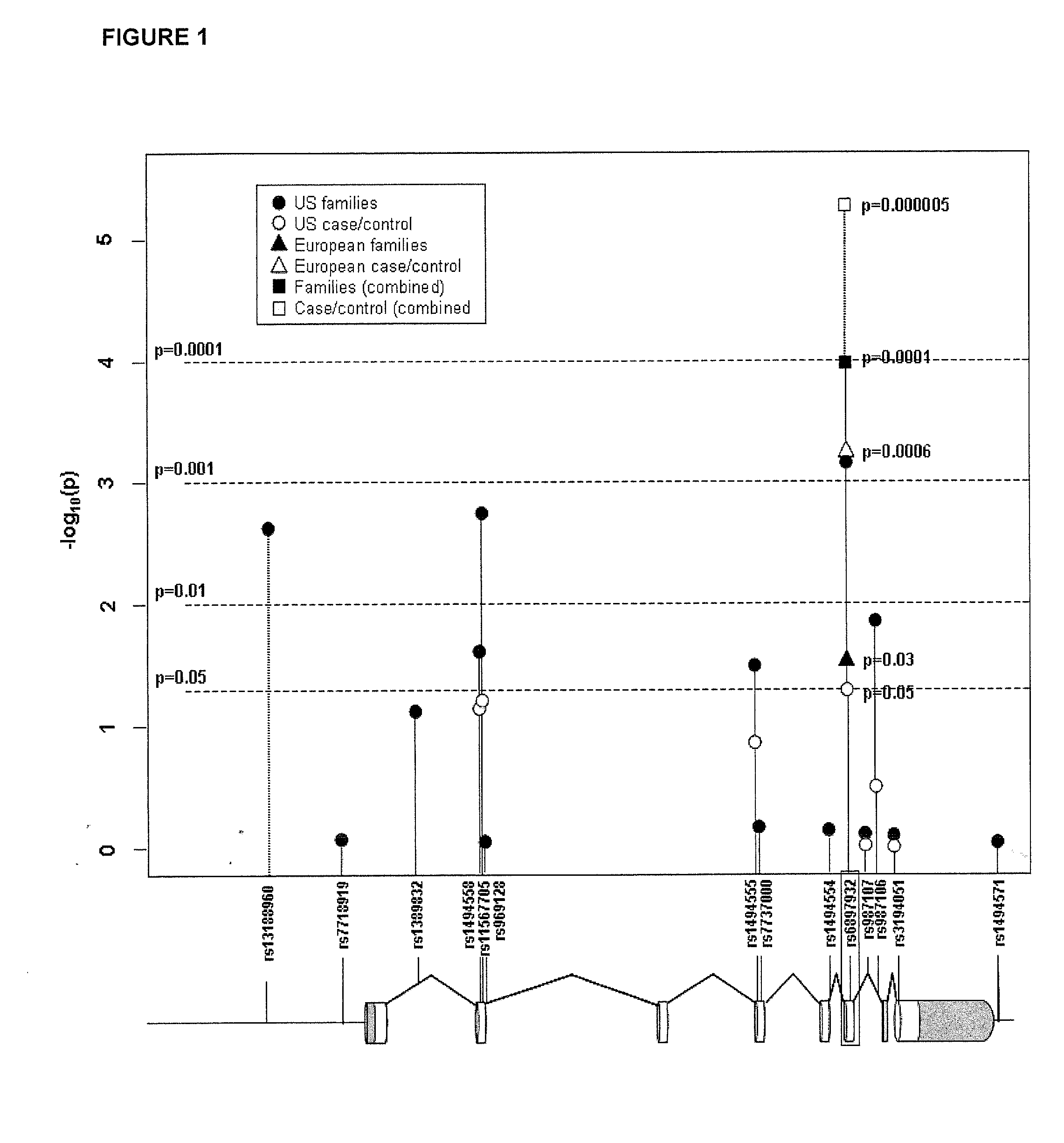
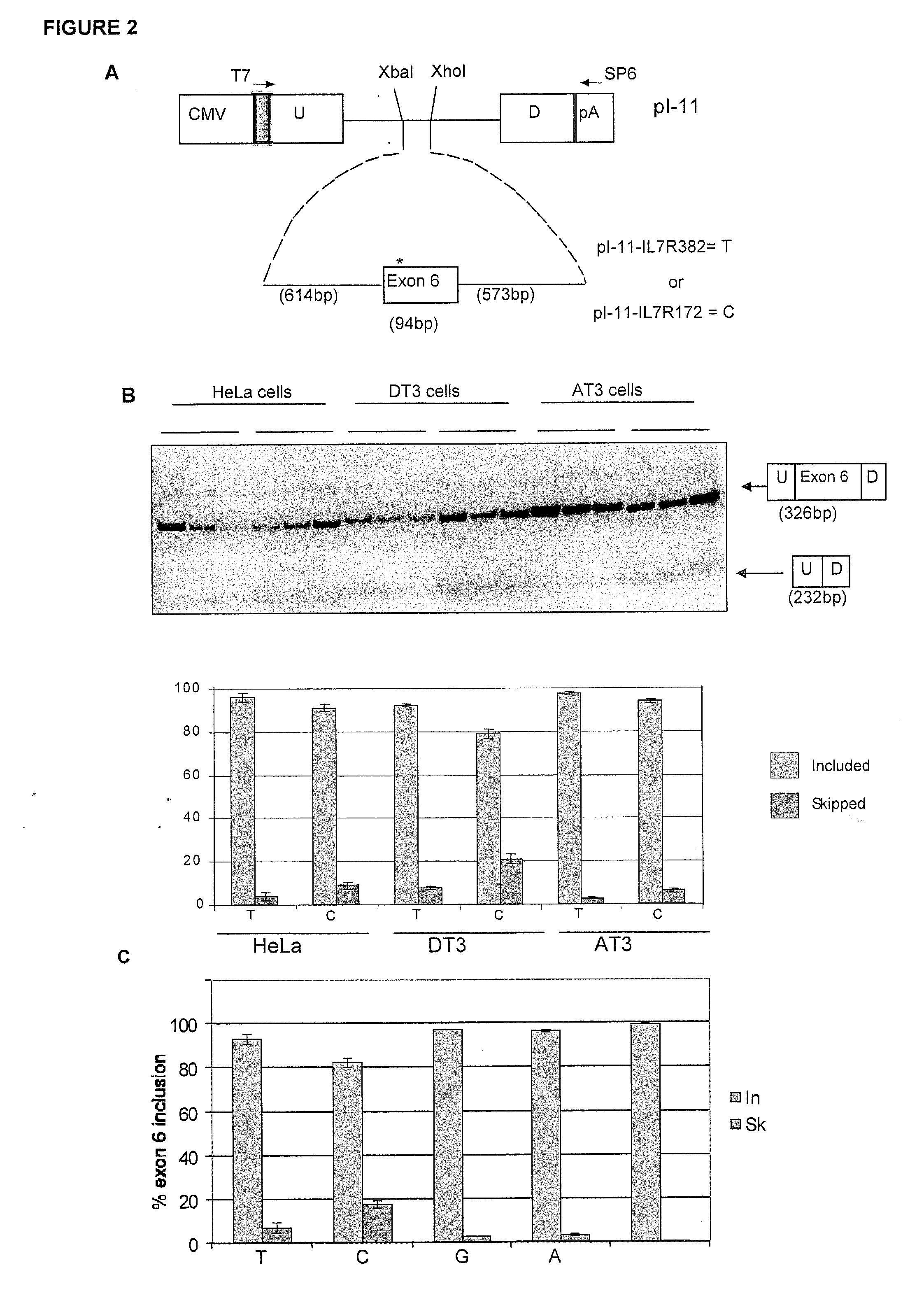
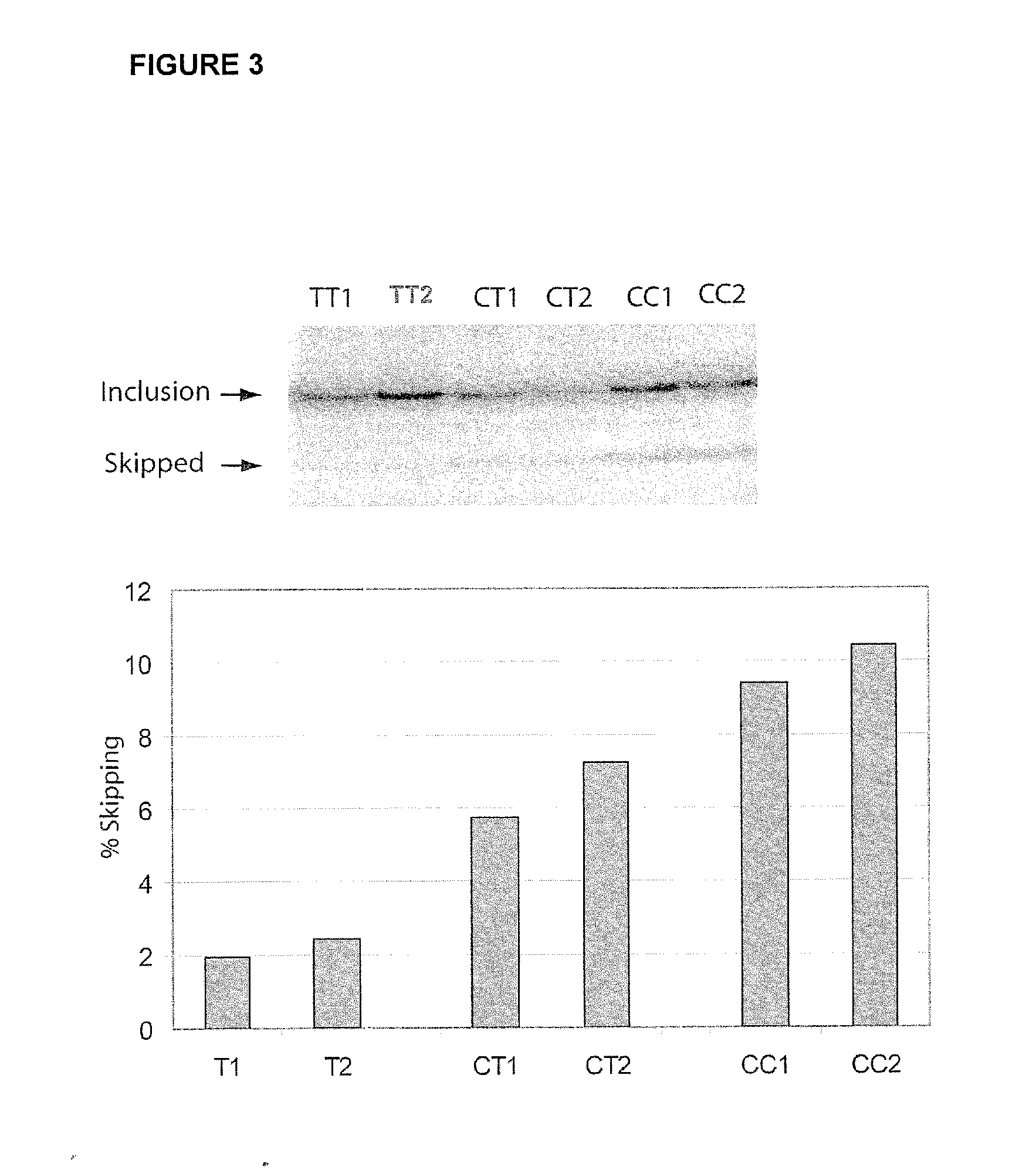
Methods and compositions for correlating genetic markers with multiple sclerosis
InactiveUS8158344B2Reduce riskReduce the amount requiredSugar derivativesMicrobiological testing/measurementIncreased riskInterleukin 7 Receptor Alpha
Owner:DUKE UNIV +1
Process for producing physiologically active protein using genetically modified silkworm
InactiveUS7659112B2Improve purification effectEasy to aimAnimal cellsDepsipeptidesProtein targetActive protein
The present invention provides a genetic engineering material for insects that enables a target protein to be purified easily, without requiring the use of recombinant baculovirus, while simultaneously providing a process for producing exogenous protein using that genetic engineering material. A gene recombinant silkworm is obtained by inserting an exogenous protein gene such as a cytokine gene coupled to a promoter that functions in silk glands into a silkworm chromosome. An exogenous protein such as a cytokine is then extracted and purified from the silk glands or cocoon of that silkworm or its offspring. A large amount of exogenous protein can be produced within silk gland cells, outside silk gland cells or in silk thread or a cocoon by inserting an expression gene cassette, in which the DNA sequence of the 3′ terminal portion and the DNA sequence of the 5′ terminal portion of fibroin H chain gene are fused to the exogenous protein gene, into silk gland cells and so forth.
Owner:NAT INST OF AGROBIOLOGICAL SCI
Recombinant immune cytokine and application thereof
InactiveCN107915777AImprove anti-tumor activityPromote secretionPeptide/protein ingredientsAntibody mimetics/scaffoldsMonoclonal antibodyLymphocyte
The invention relates to a recombinant immune cytokine targeting PD-L1 and IL-2 receptors and an application of the recombinant immune cytokine. By means of a whole-genome synthesis technology, an Fcheavy-chain gene of an IgG1 subclass antibody of a PD-L1 monoclonal antibody is fused with a mutated IL-2 gene through linker, and the novel recombinant immune cytokine is obtained. The recombinant immune cytokine has better tumor-inhibiting activity, especially for tumor types with PD-L1 weak expression, and achieves effects of activating in-vivo lymphocytes and promoting cytokine production.
Owner:TAIZHOU MABTECH PHARM CO LTD
Anti-PD-L1 monoclonal antibody, and application thereof in preparation of anticancer drugs
ActiveCN111018989AInhibit bindingHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsPhage antibodiesAntiendomysial antibodies
The invention discloses an anti-PD-L1 monoclonal antibody. A mouse is immunized with a PD-L1 protein, the spleen of the mouse is separated after successful immunization is determined, total RNA is extracted, reverse transcription is carried out to obtain cDNA, PCR amplification is performed to obtain antibody light-chain and heavy-chain genes, a single-chain antibody phage library is constructed,and screening is performed to obtain the target antibody. The anti-PD-L1 monoclonal antibody screened from the phage antibody library has good affinity to PD-L1, and can inhibit the combination of PD-L1 and PD-1. The anti-PD-L1 monoclonal antibody is used as an immune checkpoint inhibitor after being further humanized, and can be used for treating cancers and infectious diseases.
Owner:冠科生物技术(中山)有限公司
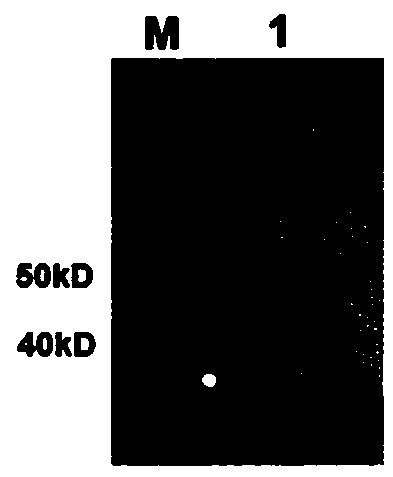
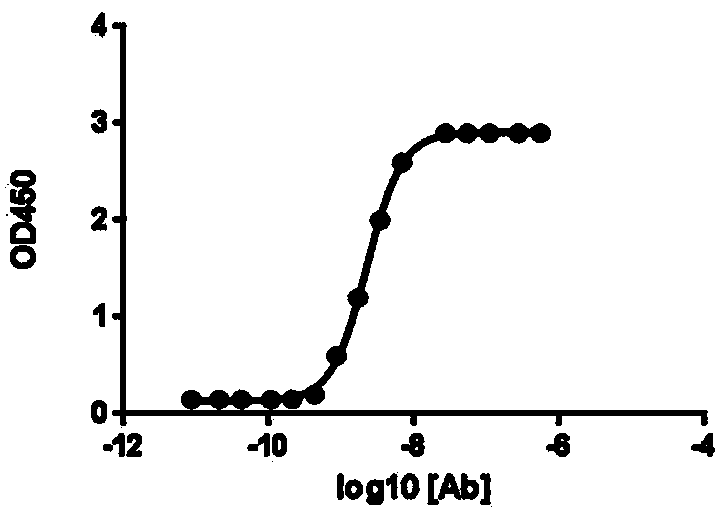
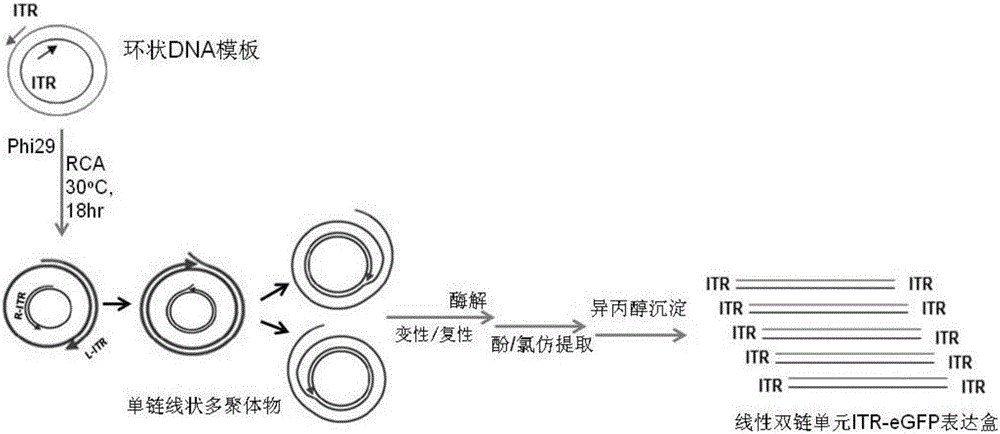
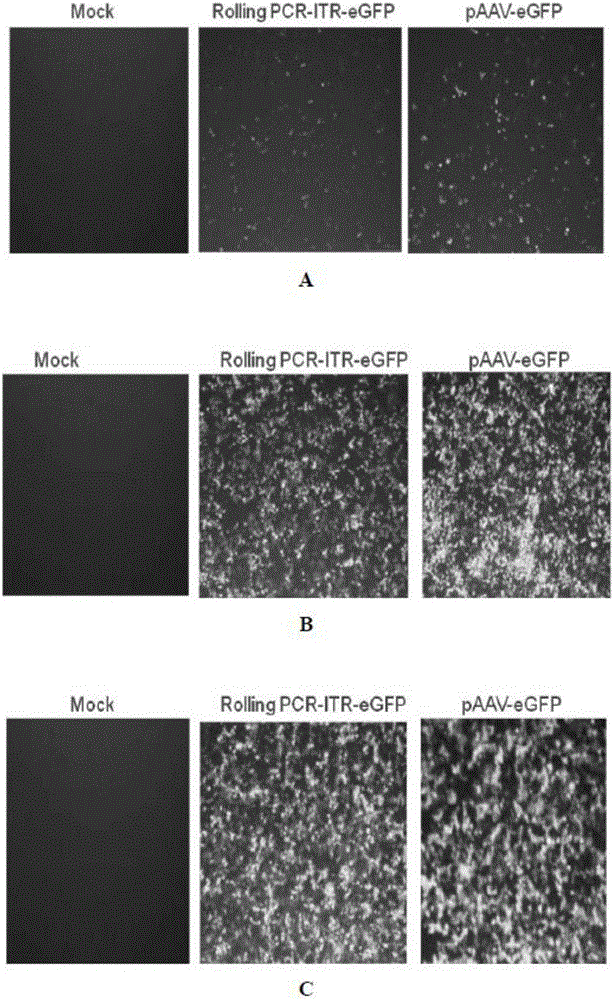
In-vitro preparation method of linear double-chain adeno-associated virus genome
InactiveCN106086012AHigh purityEasy to produceOrganic active ingredientsGenetic therapy composition manufactureGene expressionAdeno associate virus
The invention discloses a linear double-chain adeno-associated virus genome. The linear double-chain adeno-associated virus genome comprises an adeno-associated virus left inverted terminal repeat L-ITR, a nucleic acid polymerase promoter sequence, a target gene sequence, a polyadenylic acid signal sequence and an adeno-associated virus right inverted terminal repeat R-ITR and does not contain virus replication protein genes Rep, virus structural protein genes Cap, a bacterial plasmid DNA sequence and bacterial DNA methylated modification. The linear double-chain adeno-associated virus genome has the advantages that the limitation that a single-chain adeno-associated virus genome needs a rate-limiting step for converting the single-chain genome into a double-chain genome during gene expression is broke through, and the toxic and side effect of triggering congenital immunity response is avoided. The invention further discloses an in-vitro preparation method of the linear double-chain adeno-associated virus genome.
Owner:BIOMICS BIOTECH
Methods and compositions for correlating genetic markers with multiple sclerosis
InactiveUS20090035778A1Potent consequenceDecreased exon skippingSugar derivativesMicrobiological testing/measurementIncreased riskInterleukin 7 Receptor Alpha
The present invention provides, in certain aspects, a method of identifying a subject as having an increased risk of developing multiple sclerosis, comprising detecting in the subject the presence of a nucleotide variant in the interleukin 7 receptor alpha chain gene, whereby the presence of said variant identifies the subject as having an increased risk of developing multiple sclerosis.
Owner:DUKE UNIV +1
Regulation of expression of high-affinity immunoglobulin e (ige) receptor beta-chain
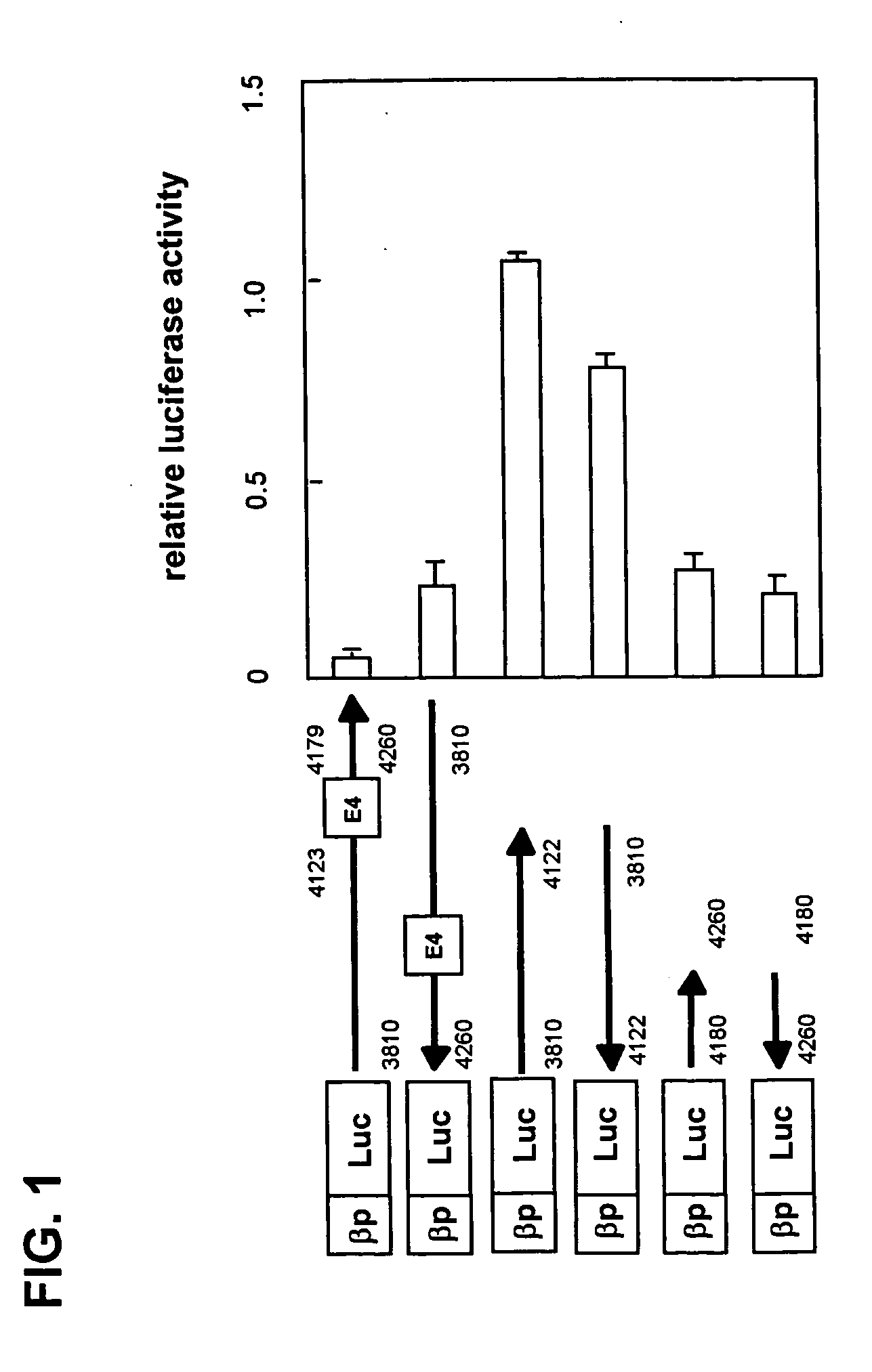
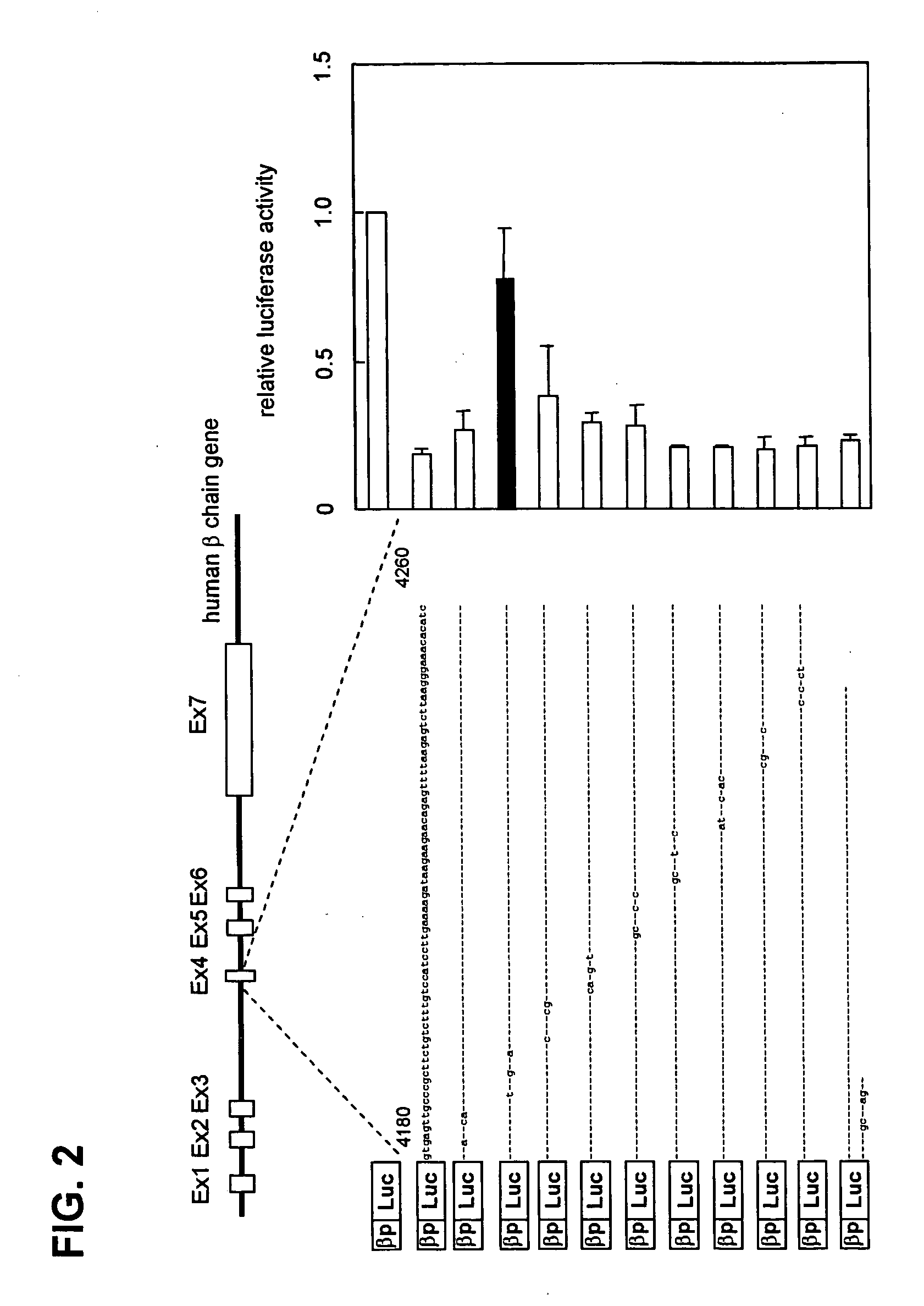
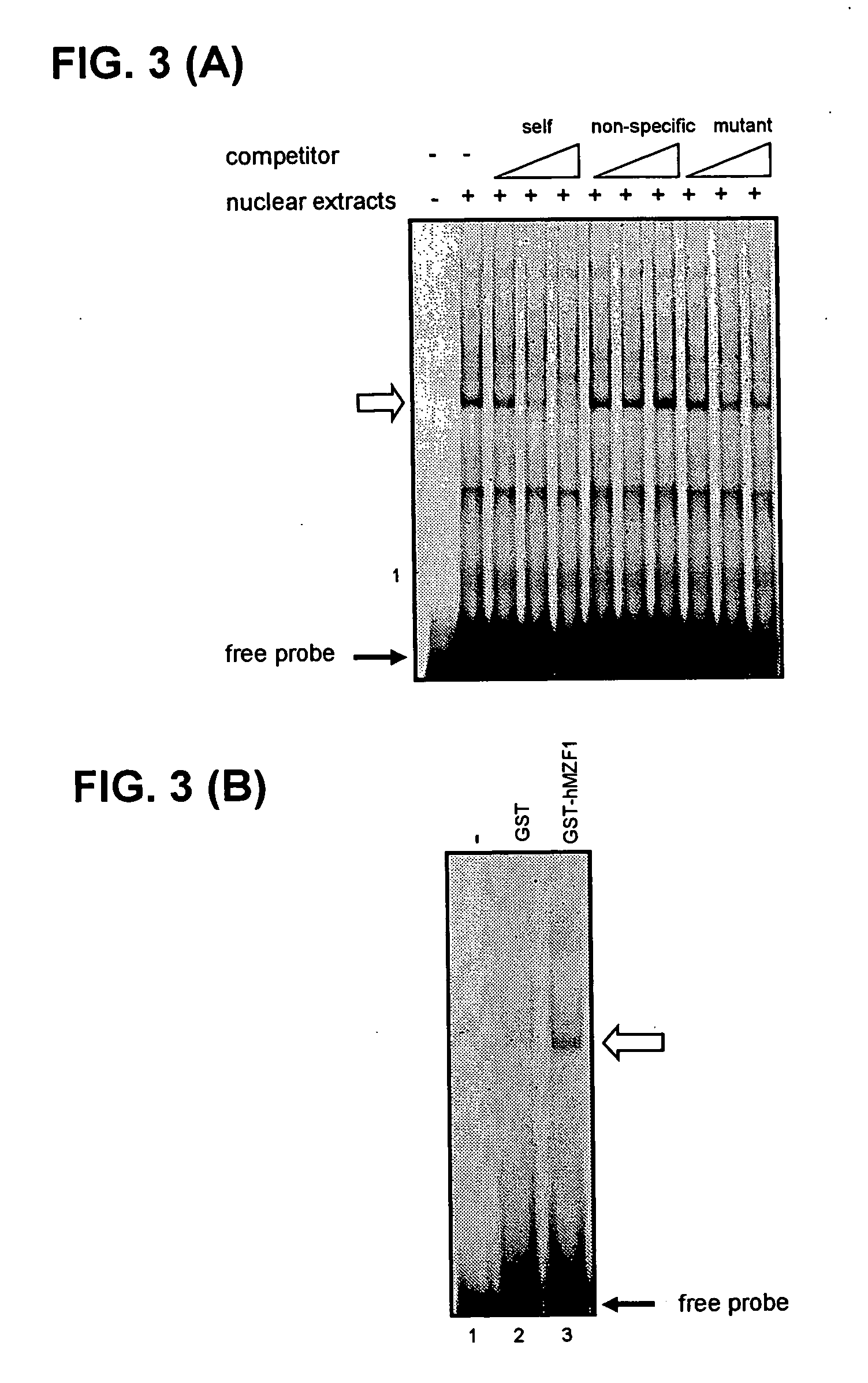
The present invention provides a polynucleotide comprising all or a part of the nucleotide sequence identified as SEQ ID NO:1 that regulates transcription of the human high-affinity IgE receptor (FcεRl) β-chain gene, and a method for regulating transcription of the FcεRl β-chain gene which comprises the step of promoting binding between a transcription regulatory complex containing MZF-1 and the transcription regulatory region of the FcεRl β-chain gene that contains the nucleotide sequence identified as SEQ ID NO: 1. In addition, the present invention provides a screening method and a kit therefor that utilizes the method for regulating transcription of the FcεRl β-chain gene having the step of promoting binding between a transcription regulatory complex containing MZF-1 and the transcription regulatory region of the FcεRl β-chain gene that contains the nucleotide sequence identified as SEQ ID NO:1, thereby enabling development of a novel agent for the prevention and treatment of allergic diseases.
Owner:NIHON UNIVERSITY
Human artificial chromosome containing human antibody lambda light chain
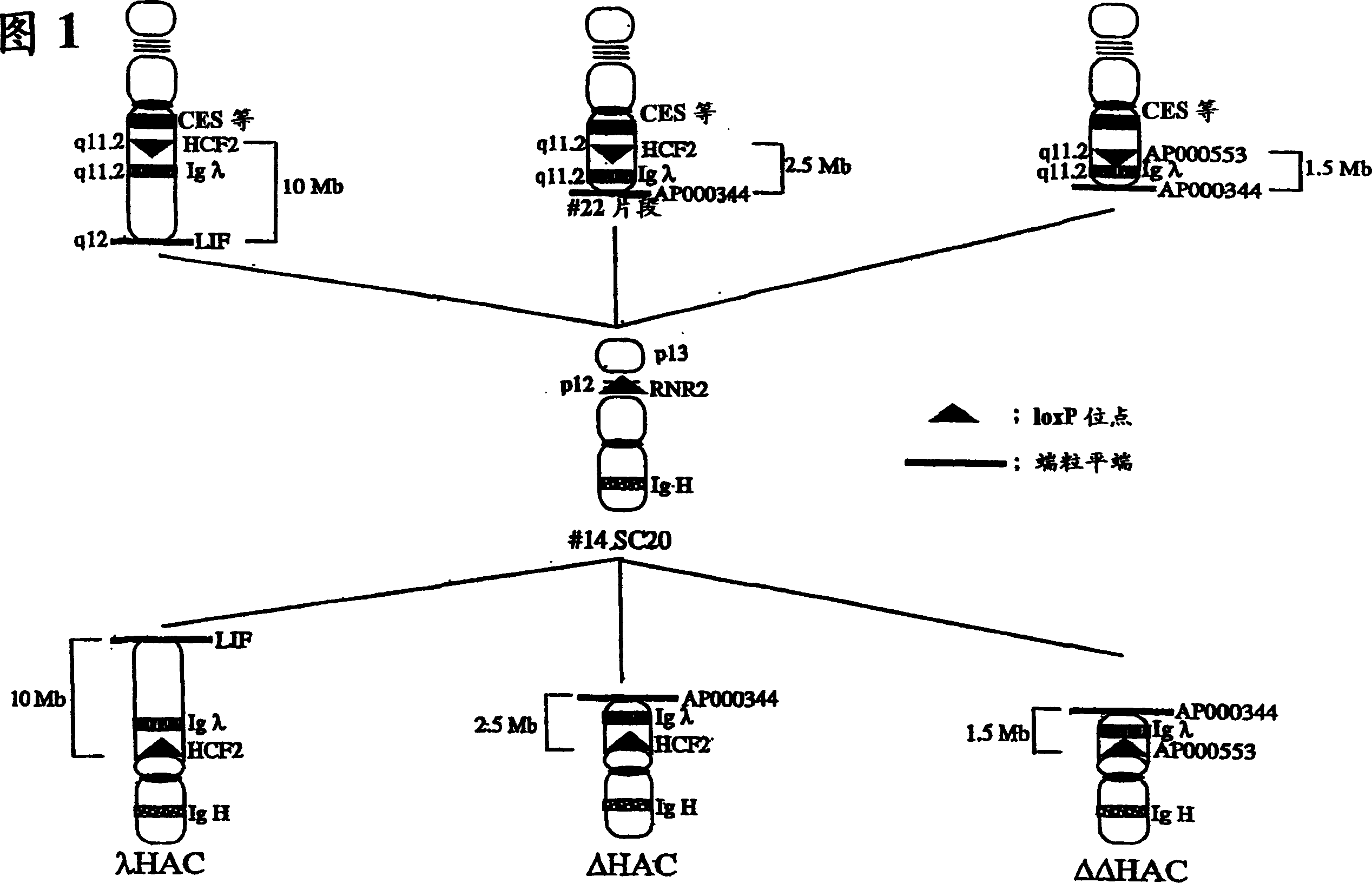
InactiveCN1789416AGenetic material ingredientsImmunoglobulins against cytokines/lymphokines/interferonsHuman animalHuman artificial chromosome
The present invention relates to a human artificial chromosome which is genetically transmissible to the next generation with high efficiency and the method for using the same. More specifically, the present invention relates to: a human artificial chromosome in which an about 3.5 Mb to about 1 Mb region containing an antibody » light chain gene derived from human chromosome 22 is bound to a chromosome fragment which is transmissible to a progeny through a germ line of a non-human animal, said chromosome fragment is derived from another human chromosome; a non-human animal carrying the human artificial chromosome and an offspring thereof; a method for producing the non-human animal; a method for producing a human antibody using the non-human animal or an offspring thereof; and a human antibody-producing mouse carrying the human artificial chromosome.
Owner:KYOWA HAKKO KIRIN CO LTD +1
Method for detecting gene specifying allergic predisposition
A method of detecting genes which comprises detecting gene polymorphisms in allergy-predisposition-related genes; i.e., interleukin 12 receptor IL-1 β2 chain and β1 chain genes, interleukin 18 receptor α chain gene, interferon γ receptor 1 chain gene and interleukin 12.p40 subunit gene, to thereby detect allergic predisposition. Use of the present method enables contribution to the prevention of onset of allergic diseases and to the treatment of allergic diseases.
Owner:BML INC +1
Silk protein peptide fragment for identifying novel natural color silkworm and preparation method of silk protein peptide fragment
InactiveCN104232665ANo mismatchSimple methodPeptide preparation methodsHybrid peptidesBombyx moriPeptide fragment
The invention discloses a silk protein peptide fragment for identifying a novel natural color silkworm. A gene sequence of the silk protein peptide fragment comprises domestic silkworm silk H chain amorphous region genes, recombination genes of an H chain crystalline region and an H chain amorphous region, tussah H chain genes and natural silkworm silk H chain genes. The invention further discloses a preparation method of the silk protein peptide fragment. The preparation method comprises the following steps of cloning of the silkworm silk amorphous region genes of the domestic silkworm variety and the establishment and expression of an expression vector; cloning of the recombination genes of the silkworm silk crystalline region and the silkworm silk amorphous region of the domestic silkworm variety and the establishment and expression of an expression vector; and cloning of the silkworm silk genes of the natural color cocoon variety and the establishment and expression of an expression vector. The method is simple and easy to implement, and ensures that the sequence is accurate and errorless, and the gene mutation or the gene deletion of each established expression vector is avoided, so that the triple-coded mispairing or the exceptional amino acid errors of expression products cannot be generated, and thus an obtained product can be further prepared into a silkworm silk antibody with high precision. At the present, the report related to the preparation of the peptide fragment from the white domestic silkworm silk variety (silk protein amorphous regions and crystalline regions / amorphous region units) and the yellow tussah silk variety or green natural silkworm silk variety is not provided.
Owner:HUNAN NANXUN HENGYUE AGRI SCI & TECH CO LTD
Preparation method for comprehensively amplifying humanTCR beta chain library by adopting few degenerate primers
PendingCN114107287AReduce in quantityImprove throughputMicrobiological testing/measurementLibrary creationMultiplexDimer
The invention provides a preparation method for comprehensively amplifying a humanTCR beta chain library by adopting a small amount of degenerate primers, and relates to the technical field of amplification preparation of an immune repertoire sequencing library. Lymphocyte lysis and total RNA (Ribonucleic Acid) extraction: step one, preprocessing a sample, and then extracting RNA; step 2, reverse transcription: carrying out reverse transcription on the obtained total RNA of the lymphocyte to obtain cDNA (complementary deoxyribonucleic acid); step 3, multiplex PCR: carrying out a first round of PCR and a second round of PCR on the cDNA, and carrying out specific amplification on the TCR beta chain gene of the cDNA; 4, a sequencing linker sequence and index are added, the constructed library is short but contains a complete CDR3 segment, higher flux and lower price can be obtained, the adding amount of each specific primer and an amplification system are determined after a large number of experiments are optimized, the number of primer dimers can be greatly reduced, and the high-quality library is obtained.
Owner:四川芸释新医学检验实验室有限公司
ChIFNGR1 gene and its coding protein and application
The invention provides a chick IFN-gamma receptor alpha chain (IFNGR1) gene which comprises a nucleotide sequence shown in SEQ ID No.1 or a nucleotide sequence which is obtained after changing, adding and / or deleting one or a plurality of nucleotides in the prior nucleotide sequence and has the same functions. The invention also provides a protein chick IFNGR1 coded by the gene, and proteins have functions of ligand synthesis and transmission of IFN-gamma stimulus cue. Chick IFNGR1 extracellular fused proteins have antivirus activity and are a potential antivirus biological agent.
Owner:CHINA AGRI UNIV
Recombinant transgenic vector carrying human insulin-like growth factor-I and use thereof
InactiveCN102250935AClear efficacyHigh biosecurityPeptide/protein ingredientsMetabolism disorderInsulin-like growth factorGrowth Factor Gene
The invention discloses a recombinant transgenic vector carrying a human insulin-like growth factor-I and a use thereof. The recombinant transgenic vector comprises an exogenous human insulin-like growth factor(IGF)-I gene, a promoter and an enhancer element; a transposons for constructing the recombinant transgenic vector is a piggyback transposons; and the promoter is fibroin heavy chain protein promoter. The recombinant transgenic vector also comprises corresponding DNA sequence of the downstream signal peptide of the fibroin heavy chain protein promoter and the sequence of a bombyx mori fibroin light-chain gene fib-LpolyA signal site. Transgenic bombyx mori producing IGF-I, which is prepared by using the recombinant transgenic vector, has a higher IGF-I expression level and can ensurethe safety of the exogenous gene and rhabdovirus.
Owner:SUZHOU UNIV
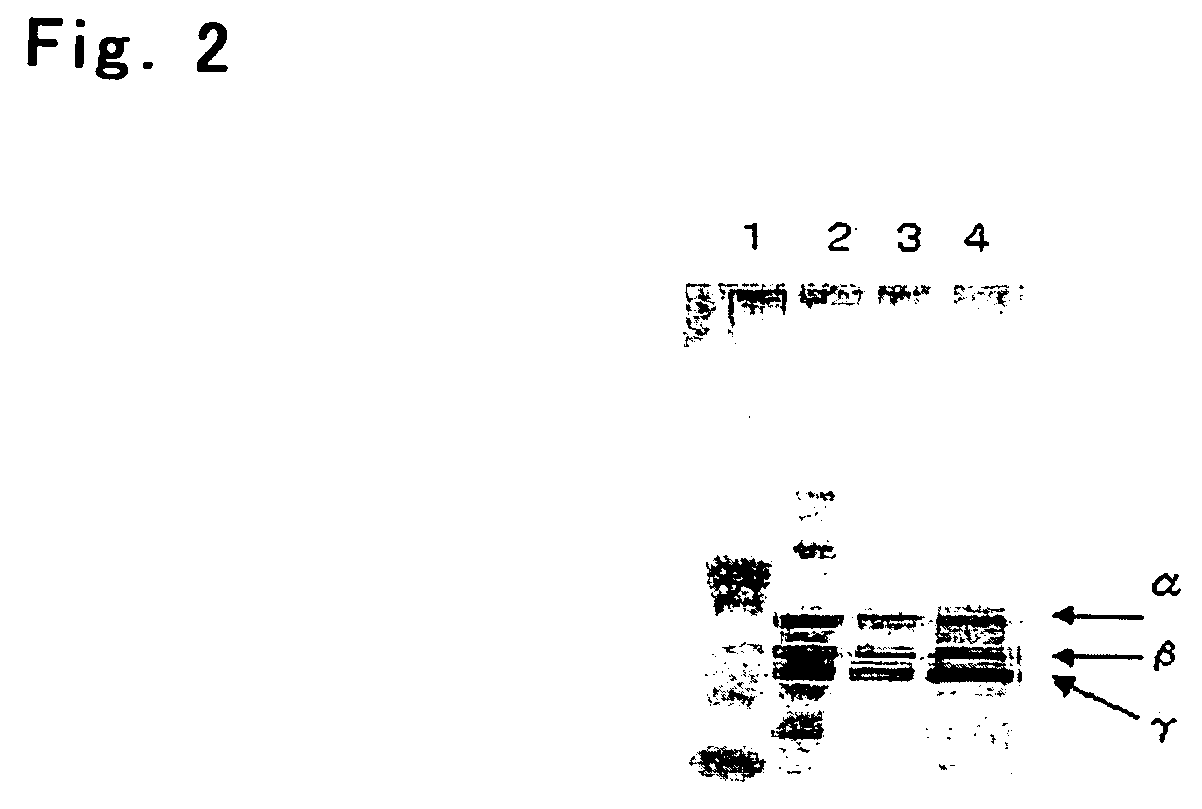
Process For Producing Recombinant Fibrinogen Highly Producing Cell and Highly Producing Cell
When genes encoding three kinds of proteins constituting fibrinogen, an α chain (or variant of α chain), a β chain and a γ chain (or variant of γ chain) are incorporated into an animal cell, a constitutional ratio of respective genes is such that a γ chain (and / or variant of γ chain) gene is an equal amount to a 1000-fold amount relative to an α chain (and / or variant of α chain) gene and a β chain gene and, further, by using a baculovirus P35 gene, a recombinant fibrinogen highly producing cell is prepared.
Owner:KM BIOLOGICS CO LTD
ChIFNGR1 gene and its coding protein and application
The invention provides a chick IFN-gamma receptor alpha chain (IFNGR1) gene which comprises a nucleotide sequence shown in SEQ ID No.1 or a nucleotide sequence which is obtained after changing, adding and / or deleting one or a plurality of nucleotides in the prior nucleotide sequence and has the same functions. The invention also provides a protein chick IFNGR1 coded by the gene, and proteins havefunctions of ligand synthesis and transmission of IFN-gamma stimulus cue. Chick IFNGR1 extracellular fused proteins have antivirus activity and are a potential antivirus biological agent.
Owner:CHINA AGRI UNIV
Method for amplifying mouse monoclonal antibody heavy light chain gene sequence, primers thereof and method for screening primers
PendingCN112500476AEasy to operateReduce in quantityImmunoglobulinsFermentationForward primerMouse monoclonal antibody
The invention discloses a method for amplifying a mouse monoclonal antibody heavy light chain gene sequence, primers thereof and a method for screening the primers. Forward primers used by all composite primers are all compositions, the composite primers are obtained by screening primer pairs capable of amplifying non-functional gene sequences in advance, and moreover, the primers for amplifying aSp2 / 0 cell non-functional light chain gene are removed from each group of the forward primers, PCR amplification is performed by using the composite primers, the obtained primers do not require TA cloning, the purity requirement of sequencing can be met, further, the number of the amplification tubes can be reduced by using the composite primers, the amplification operation can be simplified, thedetection sensitivity can be improved, and the PCR amplification operation is simpler and more convenient.
Owner:南京基蛋生物医药有限公司
antigen-specific helper t cell receptor gene
InactiveCN104797711BPeptide/protein ingredientsMammal material medical ingredientsNucleotideT lymphocyte
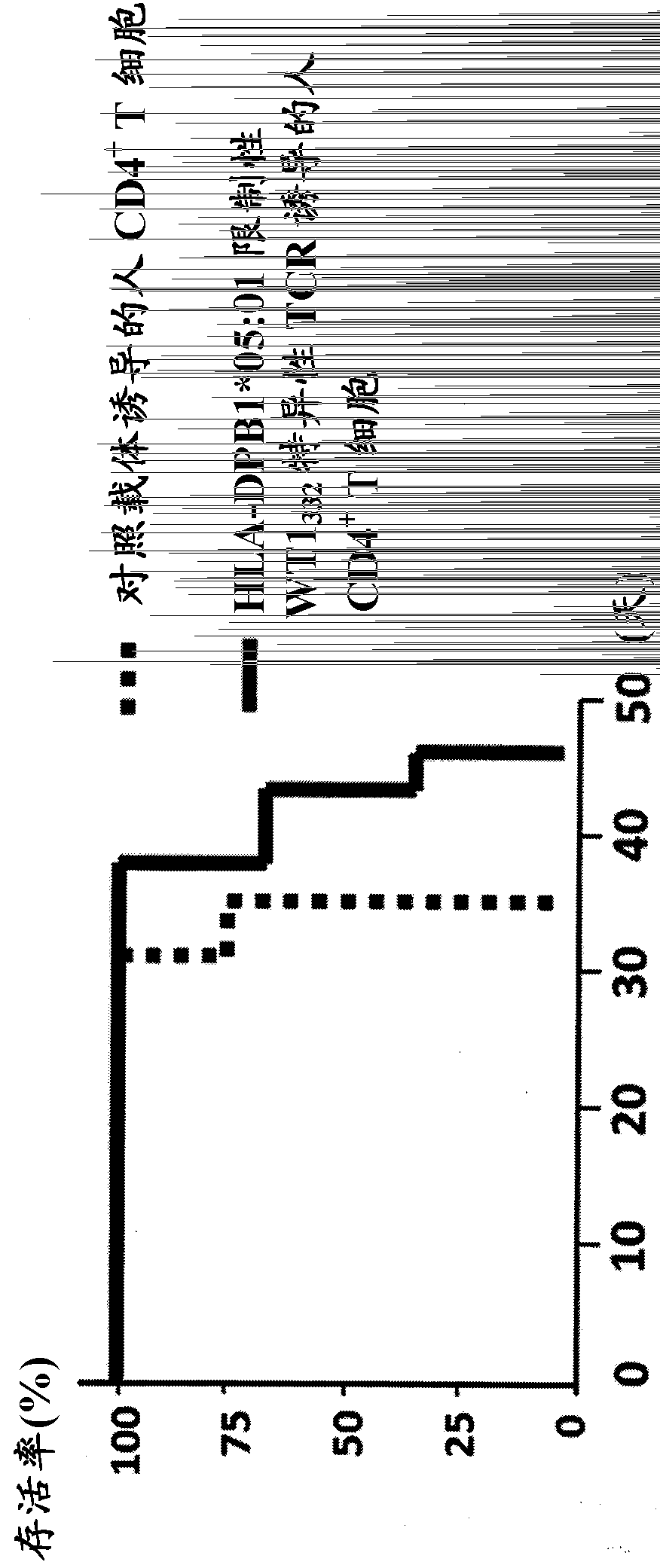
The present invention relates to a polynucleotide encoding CDR3 in the TCR-α chain and TCR-β chain genes of CD4+ helper T cells specific for a WT1 helper peptide having the amino acid sequence of SEQ ID NO: 123 . The invention also relates to the peptides encoded by said polynucleotides. The present invention also relates to CD4+T cells into which the TCR gene containing the polynucleotide has been introduced, the use of CD4+T cells to induce WT1-specific cytotoxic T-lymphocytes (CTL), cancer treatment, and the like.
Owner:INT INST OF CANCER IMMUNOLOGY INC
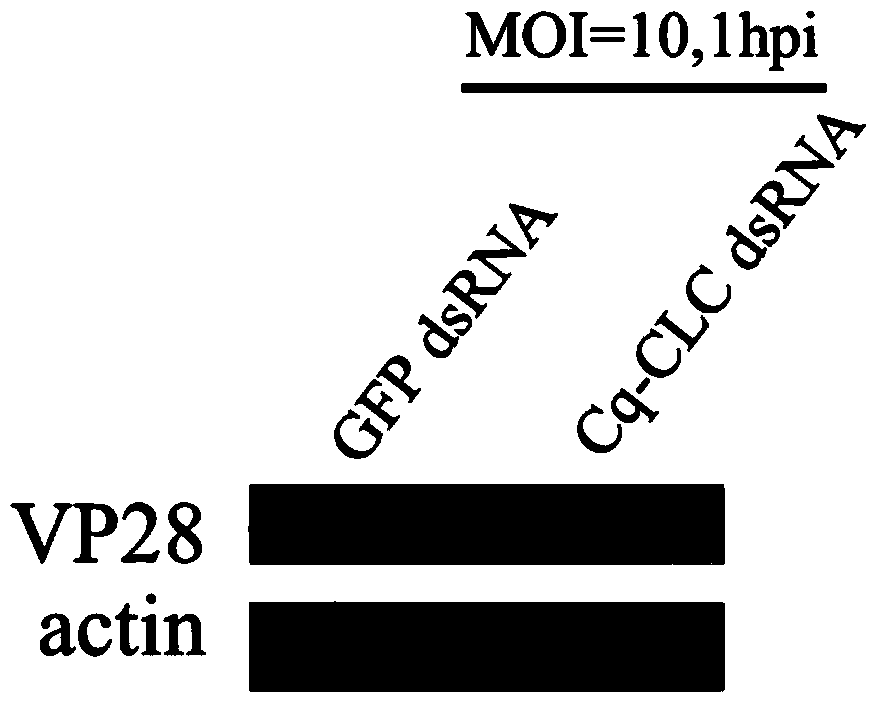
Preparation method and applications of shrimp clathrin light chain gene and targeted dsRNA thereof
InactiveCN104059923ABlock entry pathwayInhibitionFermentationPlant genotype modificationShrimpBioinformatics
The invention provides a preparation method and applications of a shrimp clathrin light chain gene and a targeted dsRNA thereof, relating to a clathrin light chain gene. The clathrin light chain gene originates from C. quadricarinatus, and is named as Cq-CLC. The dsRNA targeted gene of the targeted shrimp clathrin light chain gene is Cq-CLC, and is named as Cq-CLC dsRNA. The preparation method comprises the following steps: 1) designing a primer and preparing dsRNA referring to a kit; 2) transfecting C. quadricarinatus Hpt cells by the dsRNA obtained in the step 1), and detecting the knock-out efficiency on the targeted gene Cq-CLC; and 3) knocking out the Cq-CLC according to the step 2), infecting WSSV, detecting endocytosis amount of WSSV in the Hpt cells knocked-out by the Cq-CLC, and comparing a control group. The Cq-CLC can be used as an important target spot of developing an anti-WSSV class new drug.
Owner:XIAMEN UNIV
Integrated gene recombined process, recombined gene and coding protein obtained thereby
InactiveCN1861789BAchieve reorganizationSimple processRecombinant DNA-technologyGel electrophoresisUltrapure water
Owner:SHENYANG INST OF APPL ECOLOGY CHINESE ACAD OF SCI
Flexible and high-throughput sequencing of targeted genomic regions
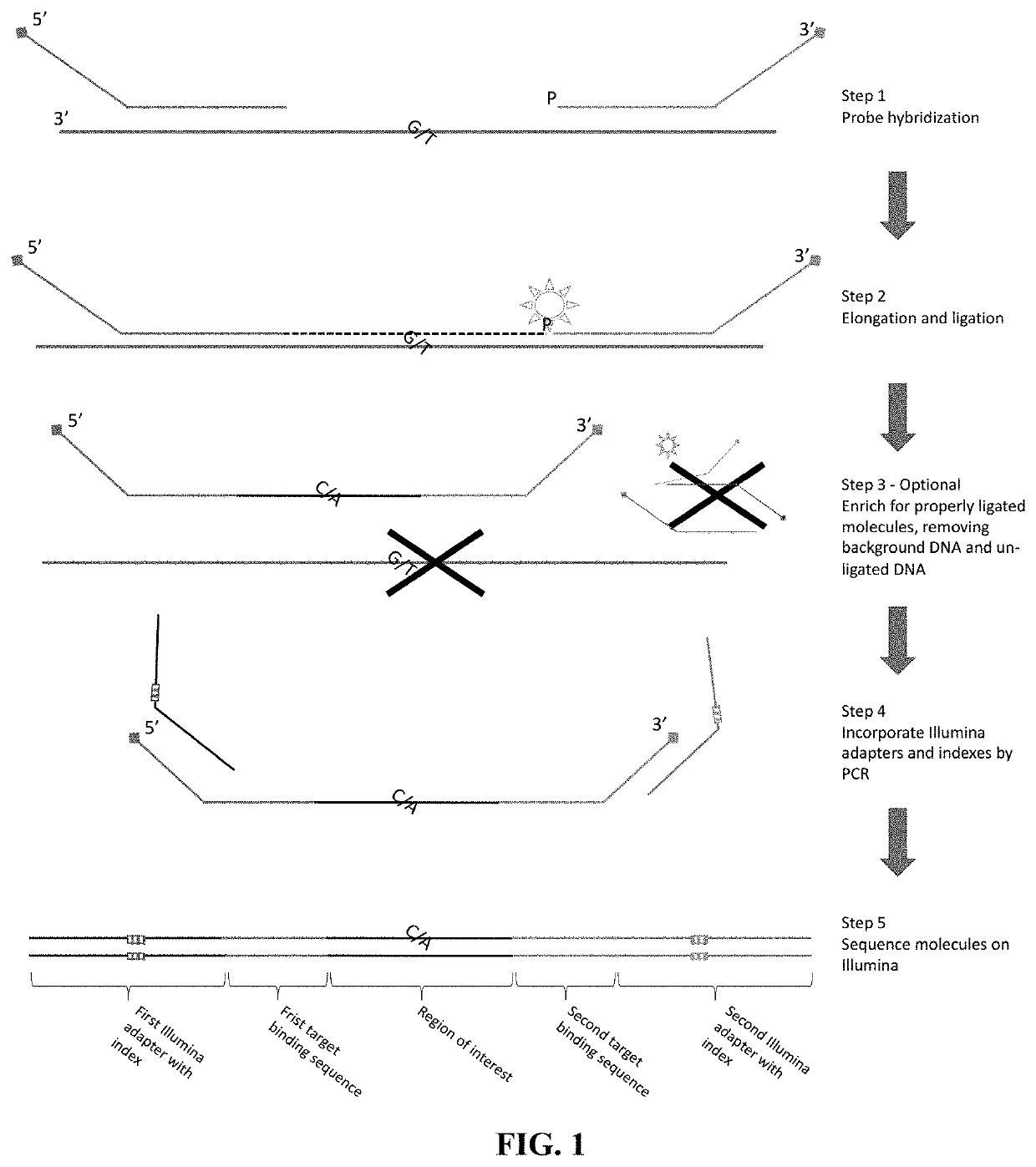
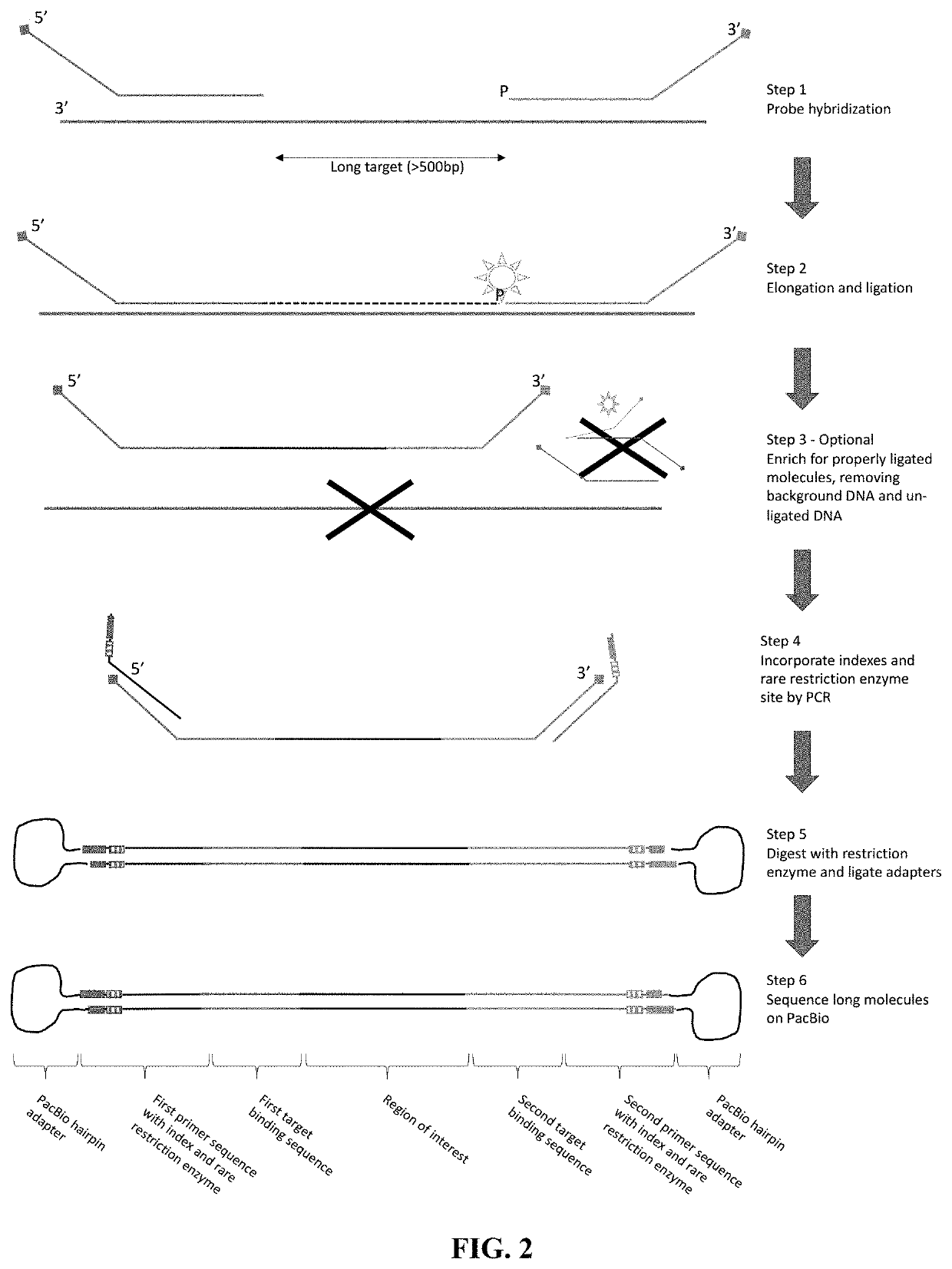
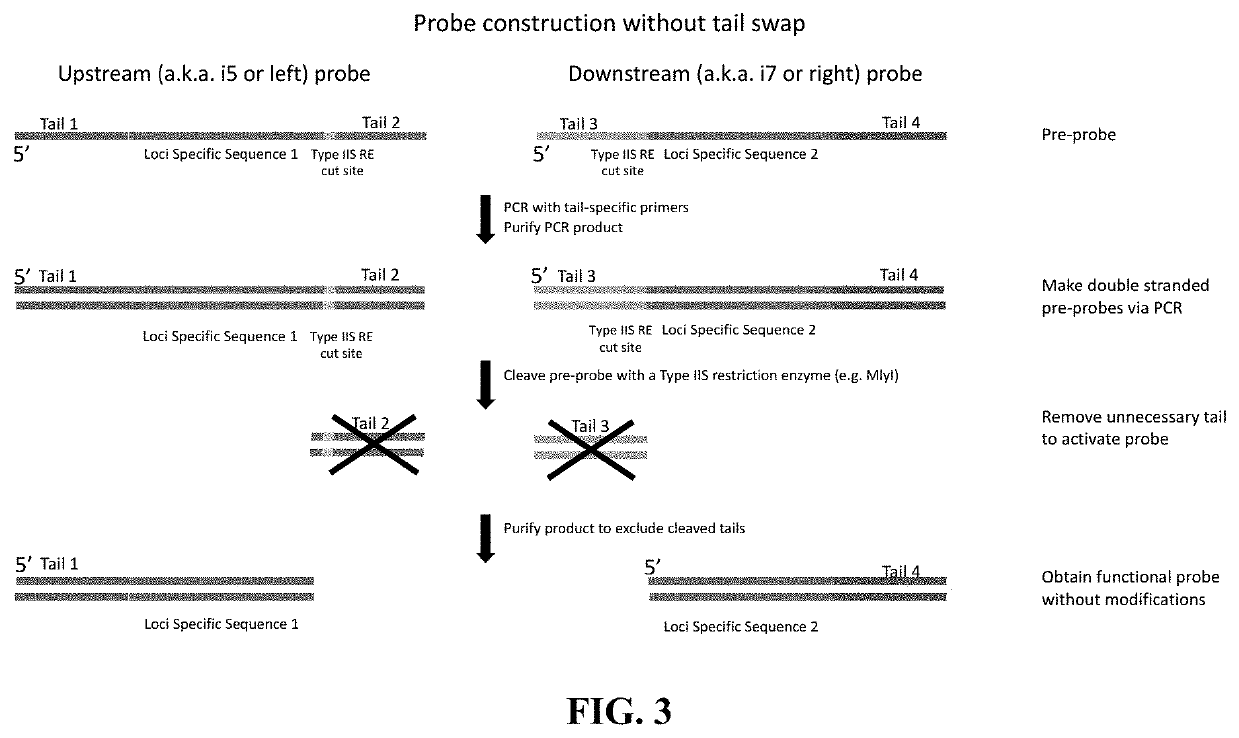
The disclosure pertains to materials and methods for capturing a target genomic region, comprising hybridizing an extension probe and a ligation probe to target sequences that flank the target genomic region; elongating the 3′ end of the extension probe until the 3′ end of the elongated extension probe is adjacent to the 5′ end of the ligation probe; and ligating the 3′ end of the elongated extension probe with the 5′ end of the ligation probe to produce a ligated probe. The ligated probe can be PCR amplified to produce copies of the target genomic region that can be detected or sequenced. Certain embodiments of the invention also provide methods of producing double stranded probes suitable for capturing and analyzing both strands of a target genomic region in a double stranded genomic DNA. The invention also provides kits for performing the methods disclosed herein.
Owner:RAPID GENOMICS LLC
CAR-NK with safety switch and preparation method thereof
InactiveCN112080512AImprove targetingImprove accuracyPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsAntigen receptorNatural Killer Cell Inhibitory Receptors
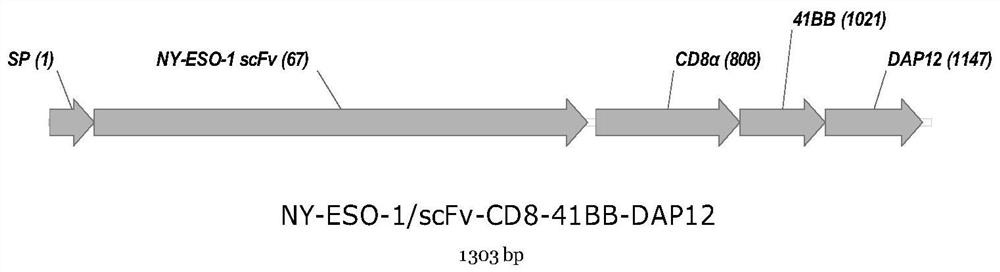
The invention provides a gene segment for expressing a chimeric antigen receptor with a safety switch. The gene segment for expressing the chimeric antigen receptor with the safety switch comprises anNY-ESO-1 single-chain gene segment and a modified epidermal growth factor receptor gene segment which are connected in sequence. The modified epidermal growth factor receptor gene segment comprises an extracellular structural domain III base sequence, an extracellular structural domain IV base sequence and a transmembrane structural domain base sequence of an epidermal growth factor receptor. Onthe one hand, the antigen receptor has improved targeting ability to kill malignant tumor cells. On the other hand, after the CAR-NK cells generate corresponding side effects in the treatment process,the CAR-NK cells can be rapidly removed through the ADCC effect of the NK cells.
Owner:SHENZHEN LUOHU PEOPLELS HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com