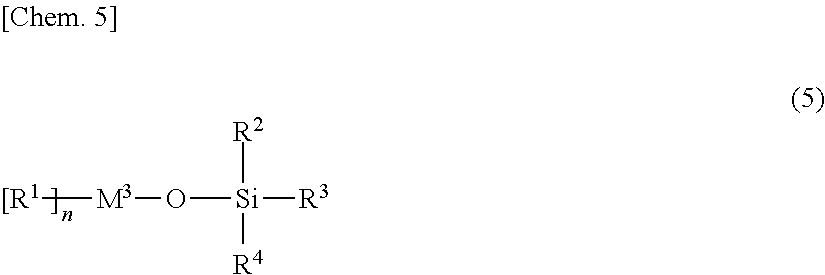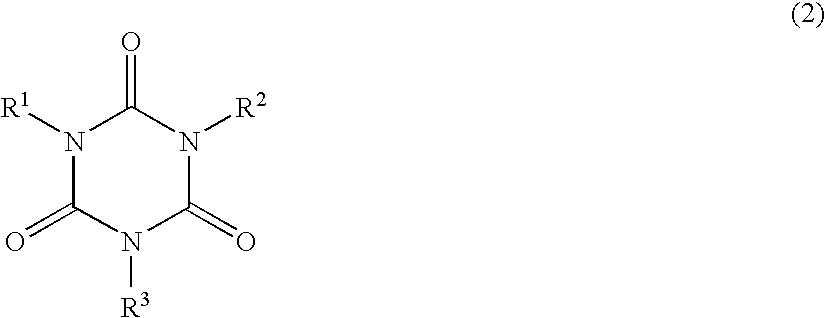Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70results about How to "Sustained effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
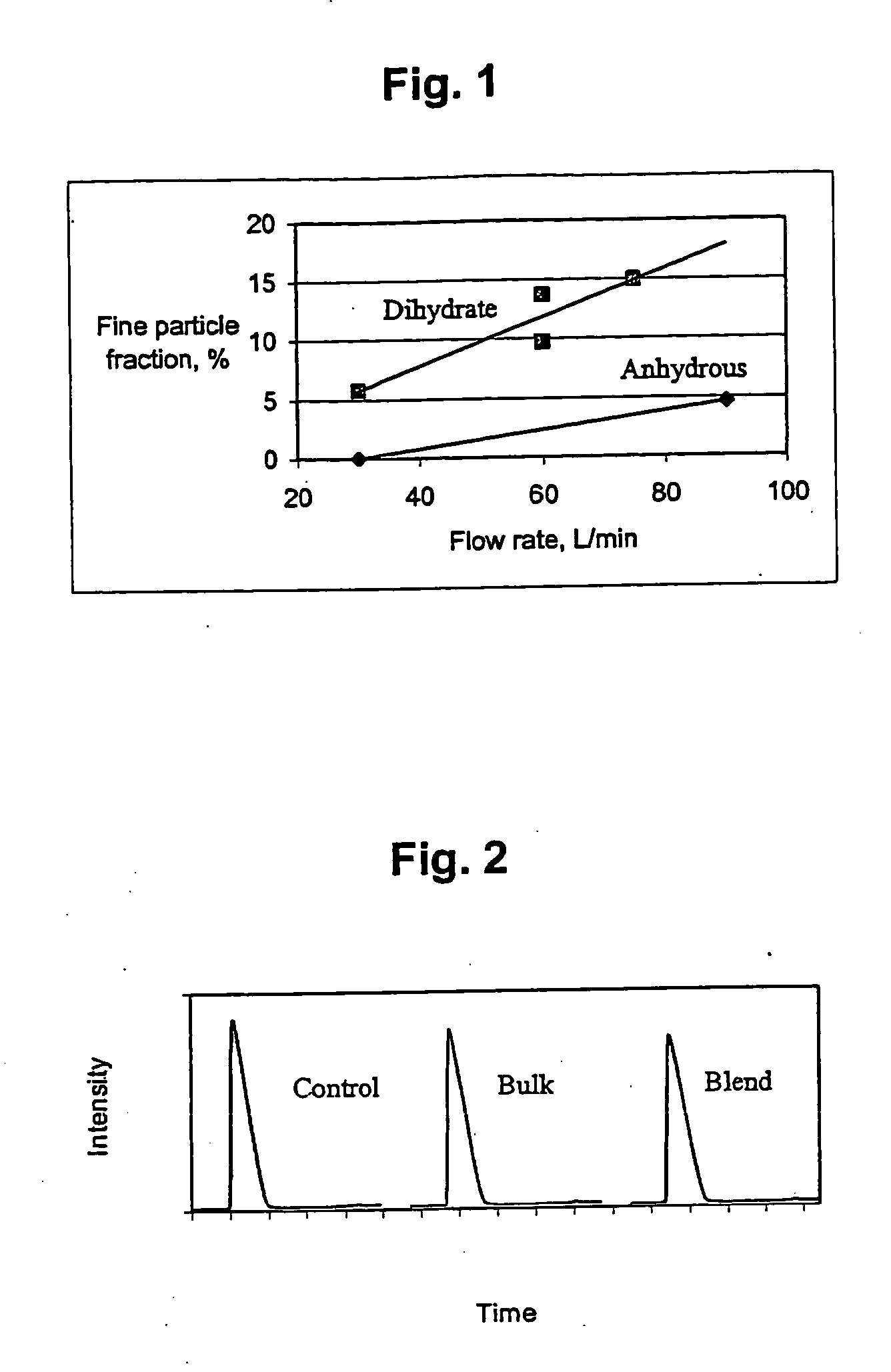
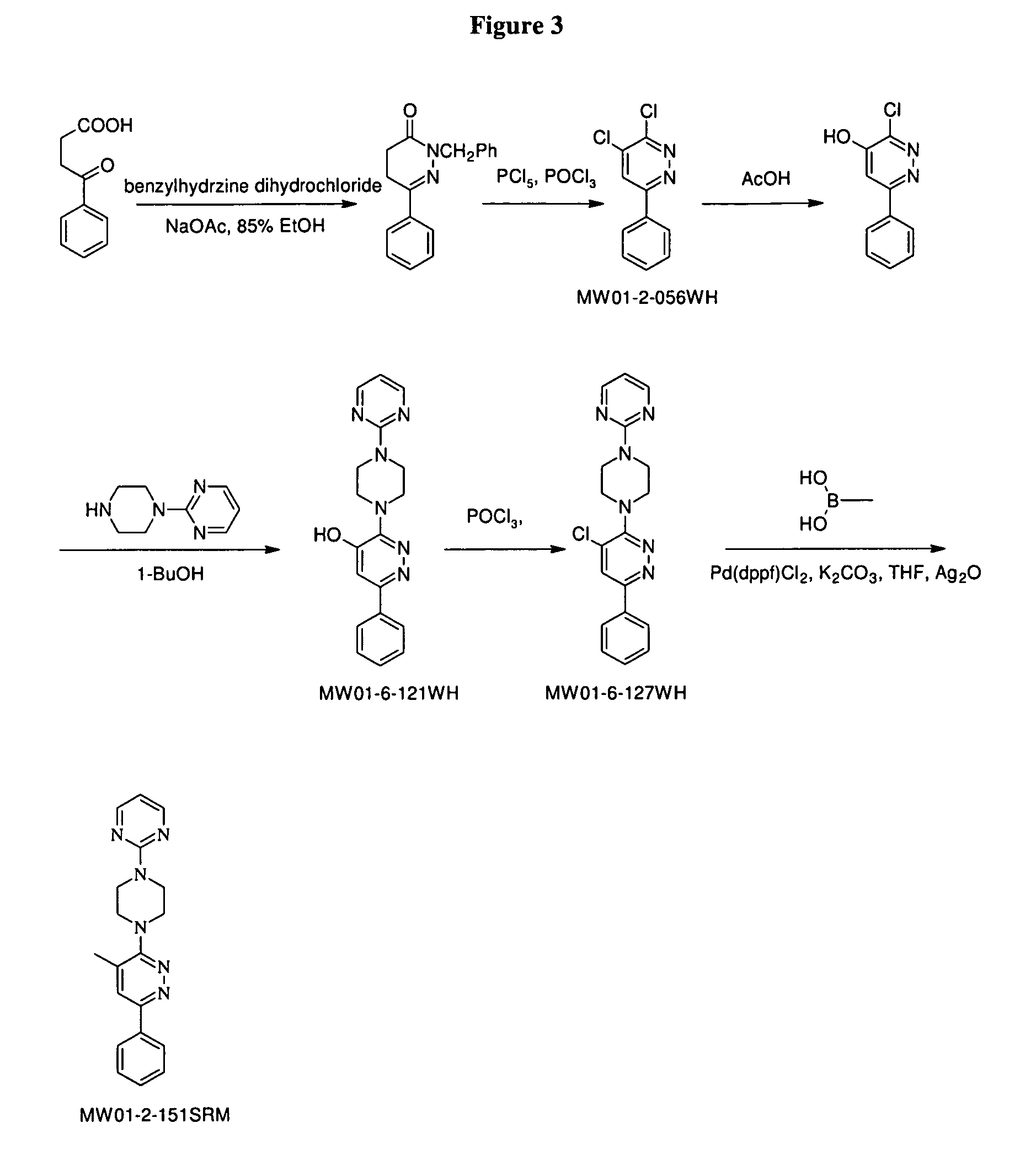
Melt-extruded orally administrable opioid formulations
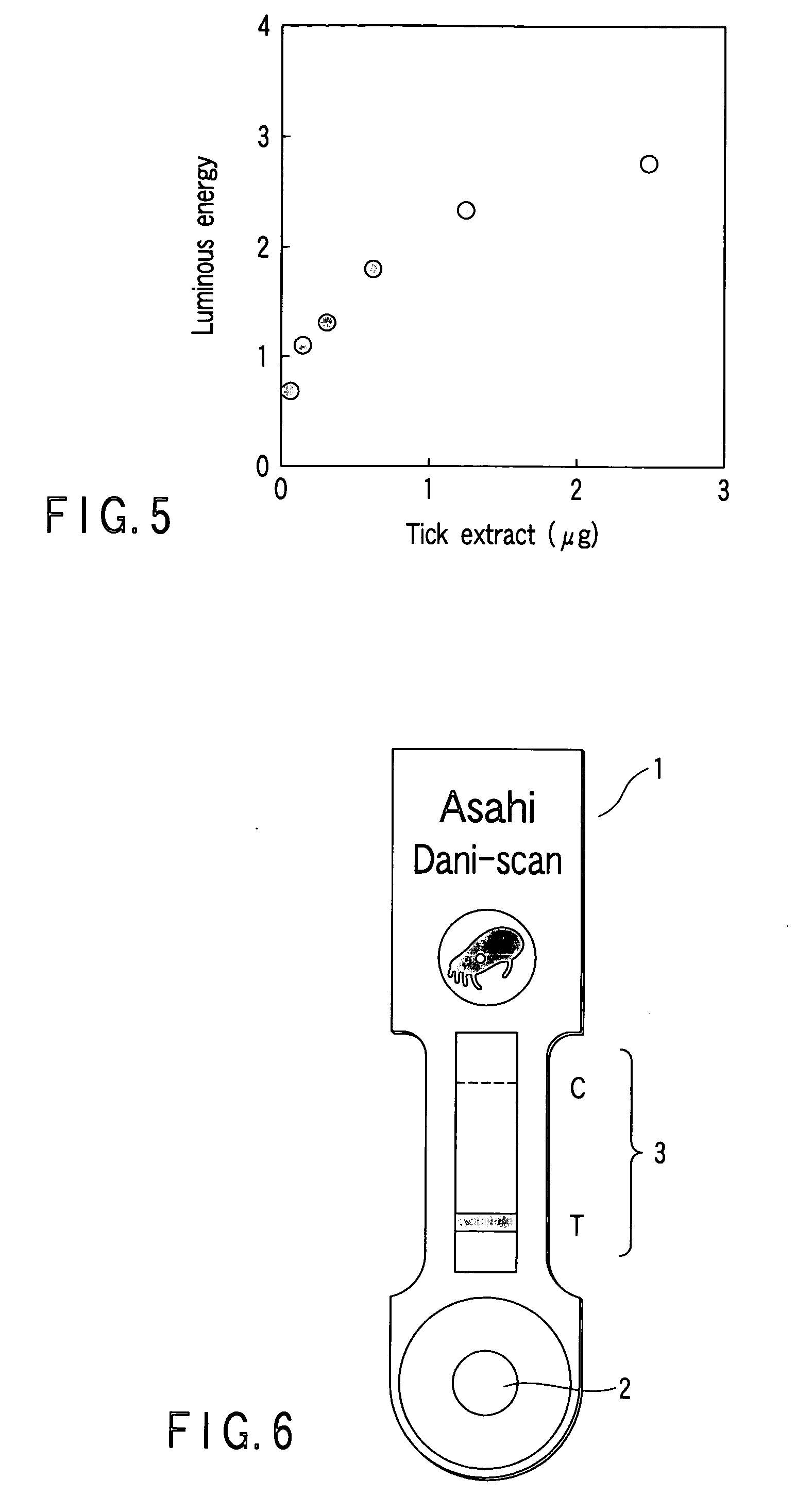
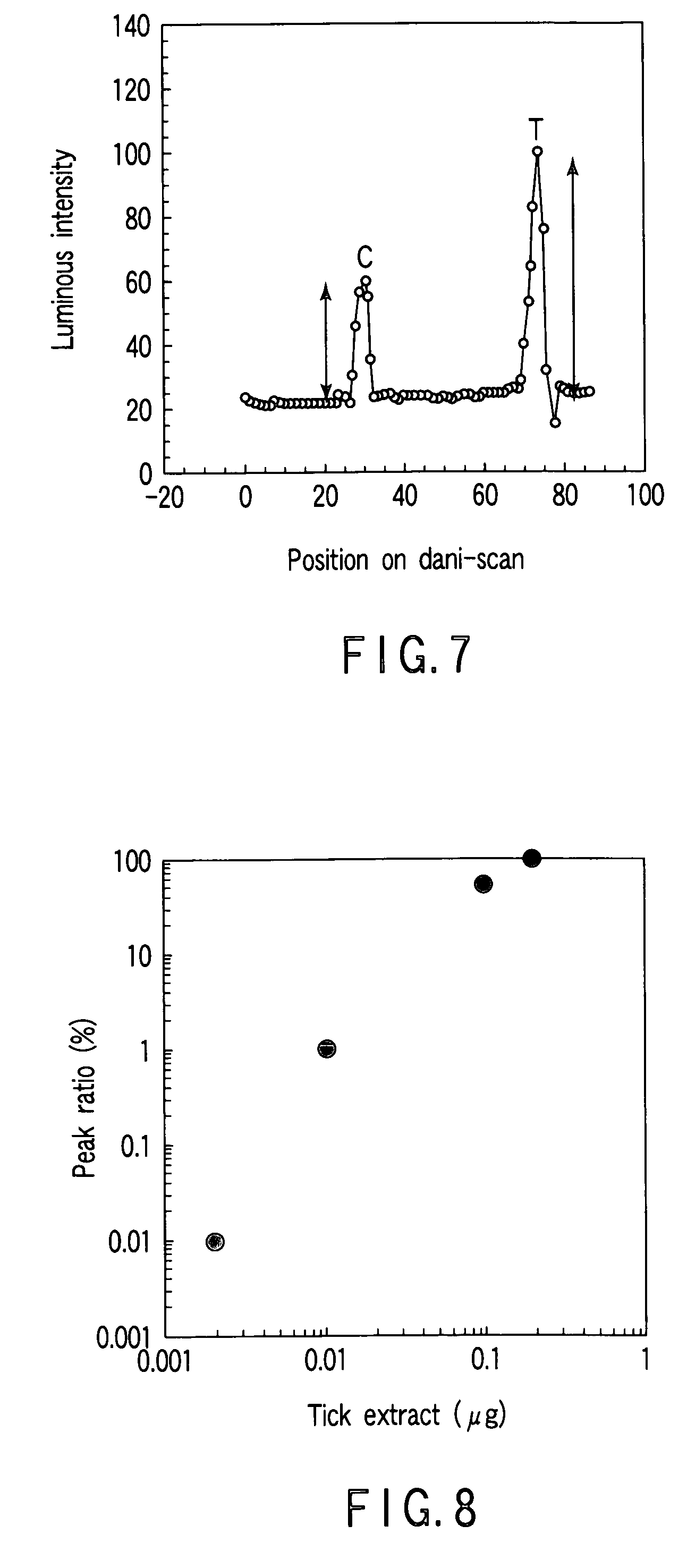
InactiveUS6261599B1Sustained effectSlow and control releaseBiocideOrganic active ingredientsMelt extrusionDosage form
Bioavailable sustained release oral opioid analgesic dosage forms, comprising a plurality of multiparticulates produced via melt extrusion techniques disclosed.
Owner:PURDUE PHARMA LP
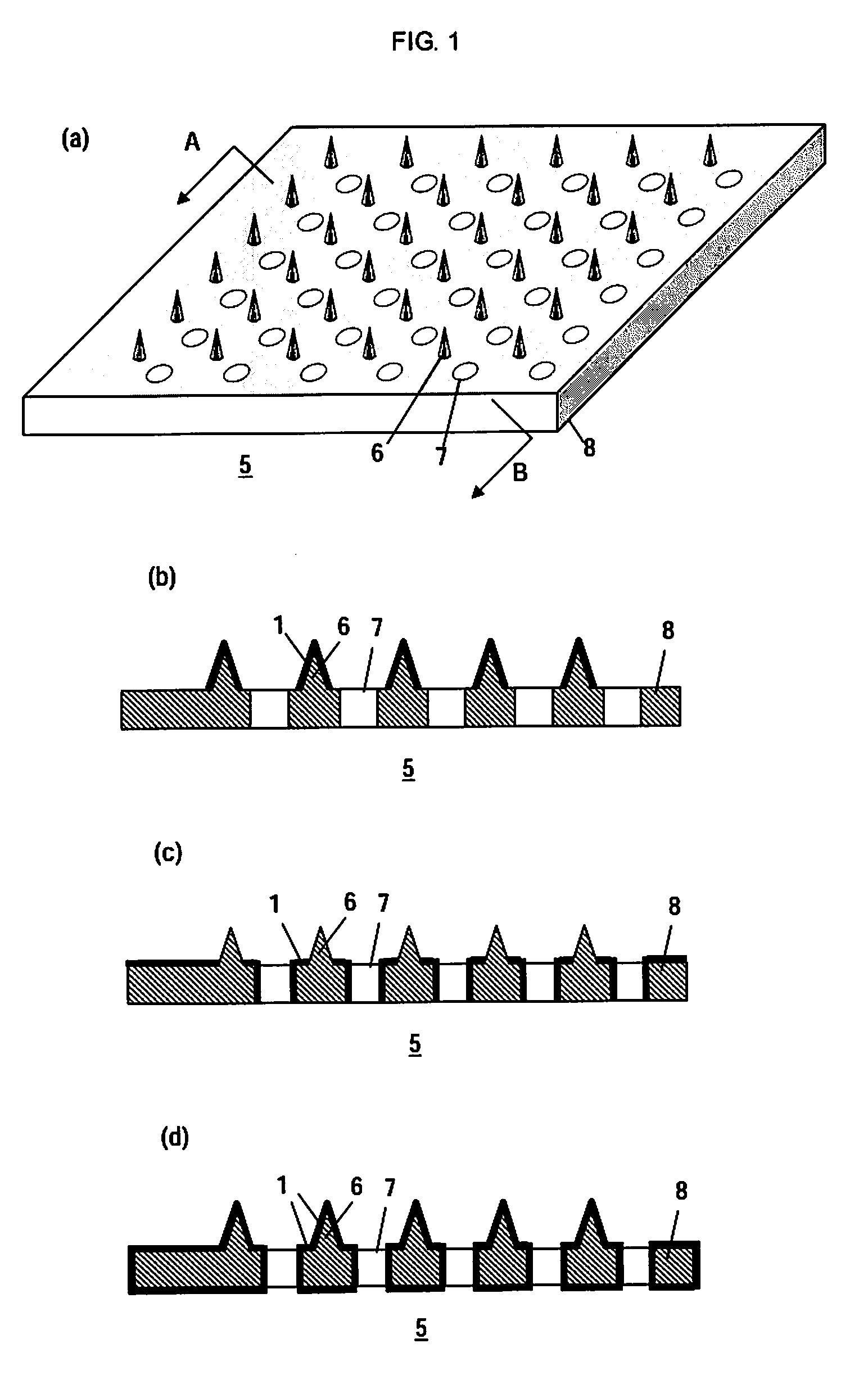
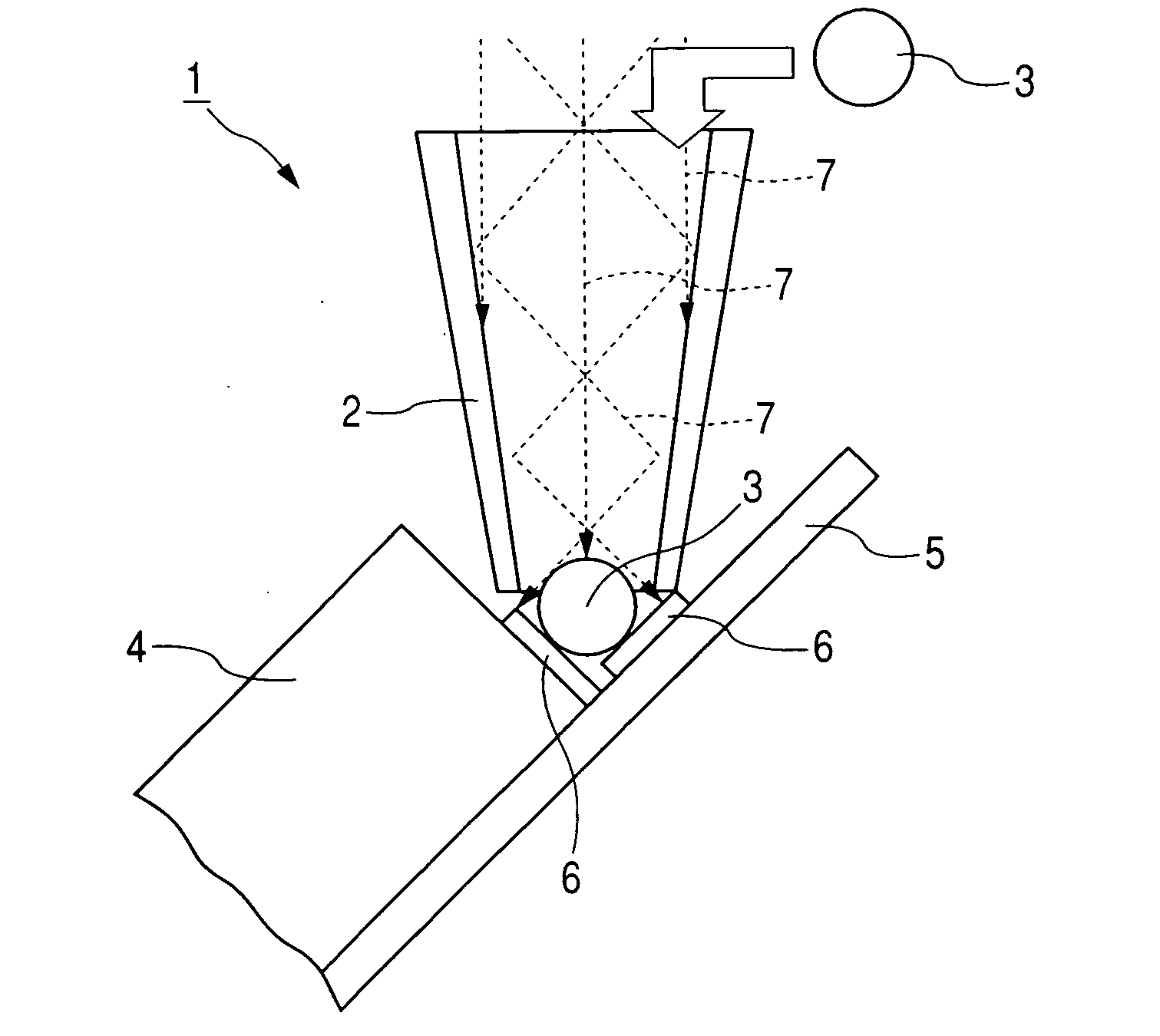
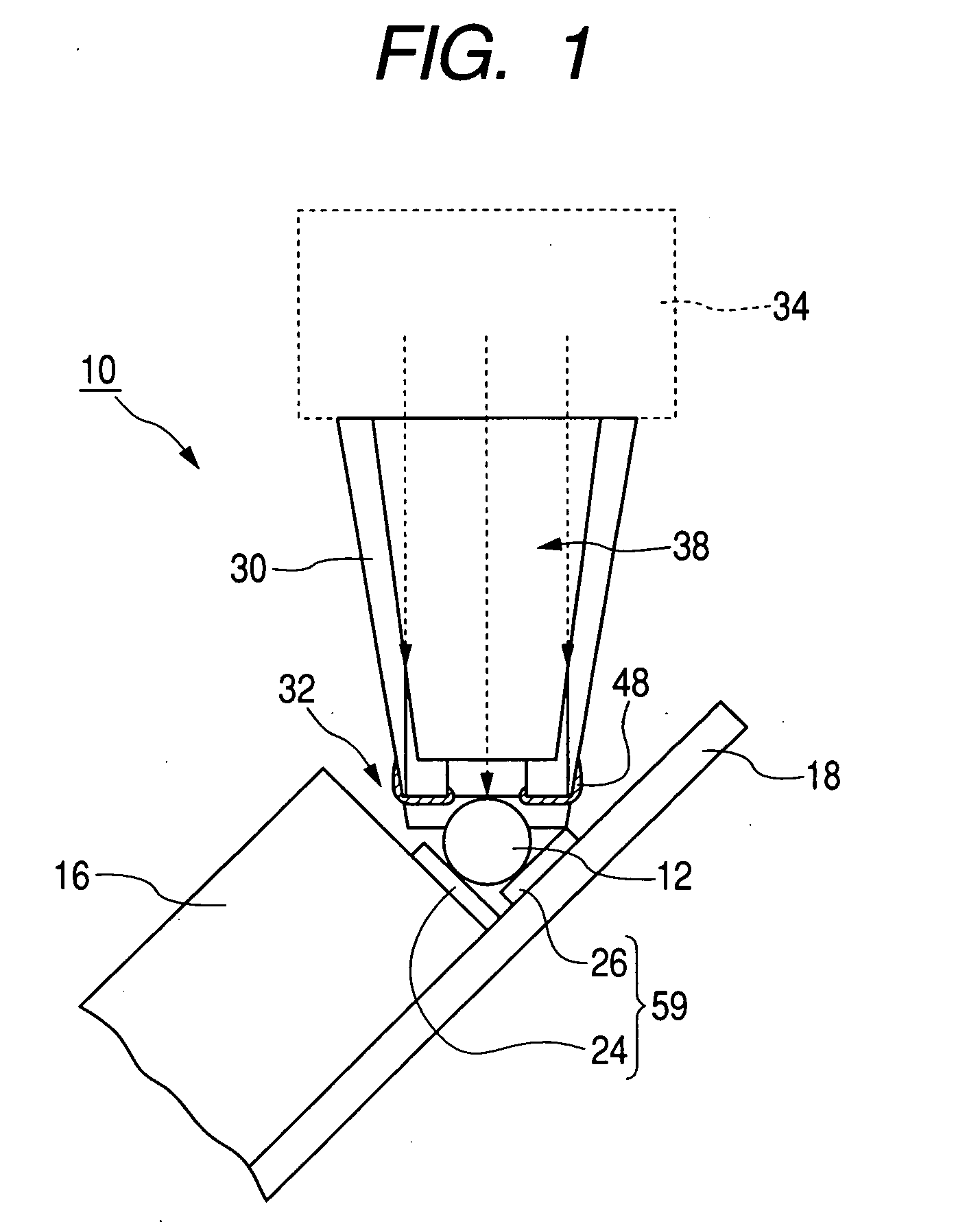
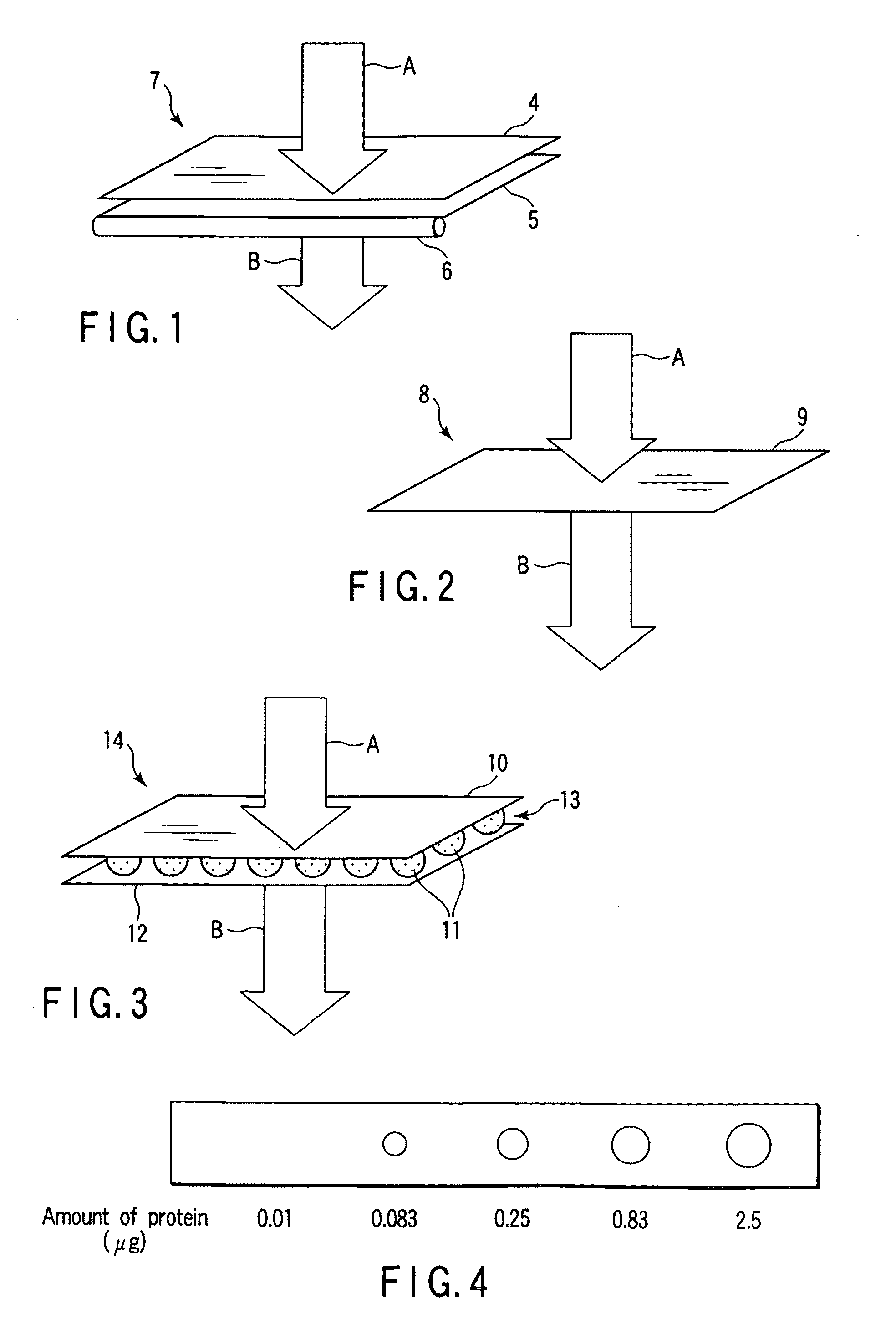
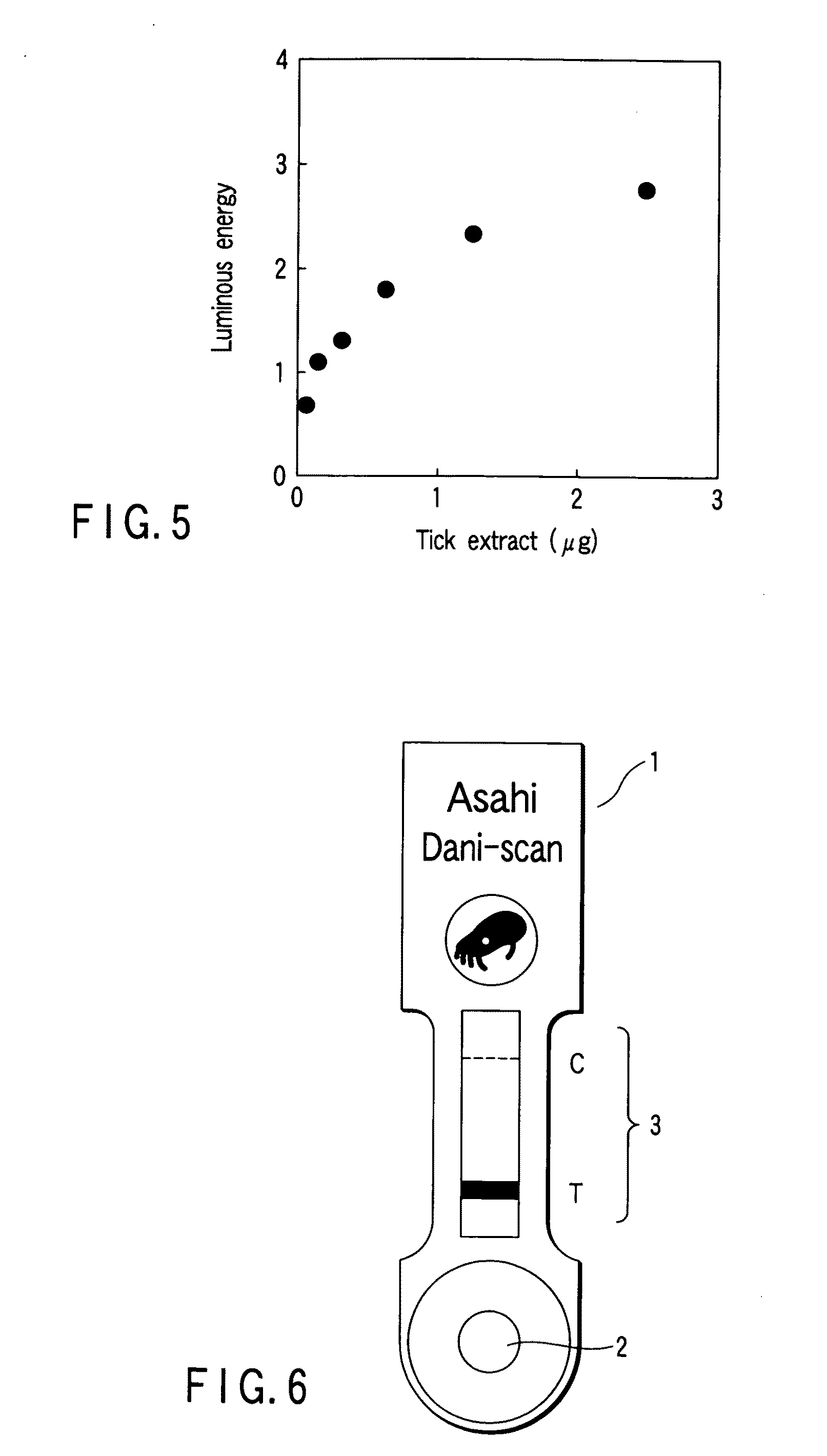
Microneedle Device And Transdermal Administration Device Provided With Microneedles
InactiveUS20090099502A1Good skin permeabilityImprove sustainabilityUltrasound therapyElectrotherapyPolyvinyl alcoholDrug administration
The present invention provides a microneedle device having a coating, which is effective even with a low molecular weight active compound and can sustain the effect of the drug for a long period of time, and a transdermal drug administration apparatus with microneedles. The microneedle device (5) has, on a microneedle substrate (8), a plurality of microneedles (6) that can pierce the skin, wherein the surface of the microneedles (6) and / or the microneedle substrate (8) is partly or entirely coated in fixed state with a coating carrier containing polyvinyl alcohol. The polyvinyl alcohol preferably has a hydrolysis degree of 94.5 mol % or more. Furthermore, the coating carrier can contain a drug.
Owner:HISAMITSU PHARM CO INC
Non-aqueous electrolyte for secondary battery and non-aqueous electrolyte secondary battery employing the same
ActiveUS20130330610A1Improve lithium ion conductivityIncreased durabilityCell electrodesSecondary cellsSolventNon aqueous electrolytes
Provided are: a non-aqueous electrolyte solution for a secondary battery that exhibits both excellent low-temperature discharge characteristics and excellent cycle characteristics on a long-term basis; and a secondary battery.A non-aqueous electrolyte solution for a non-aqueous electrolyte secondary battery that has a positive electrode and a negative electrode capable of the absorbing and releasing of a metal ion, and a separator,the non-aqueous electrolyte solution comprising, in addition to an electrolyte and a non-aqueous solvent, 0.01 mass % or more to less than 3 mass % of a compound having one or more partial structure represented by the following general formula (1) and two or more isocyanate groups in the molecule:(In the general formula (1), R represents hydrogen or a C1-C12 organic group that may contain an isocyanate group and is constituted of atoms selected from the group consisting of hydrogen atom, carbon atom, nitrogen atom, oxygen atom, sulfur atom, phosphorus atom, and halogen atom).
Owner:MU IONIC SOLUTIONS CORP +1
Antibacterial property imparting glass composition, antibacterial fiber, antibacterial twisted yarn and antibacterial cloth
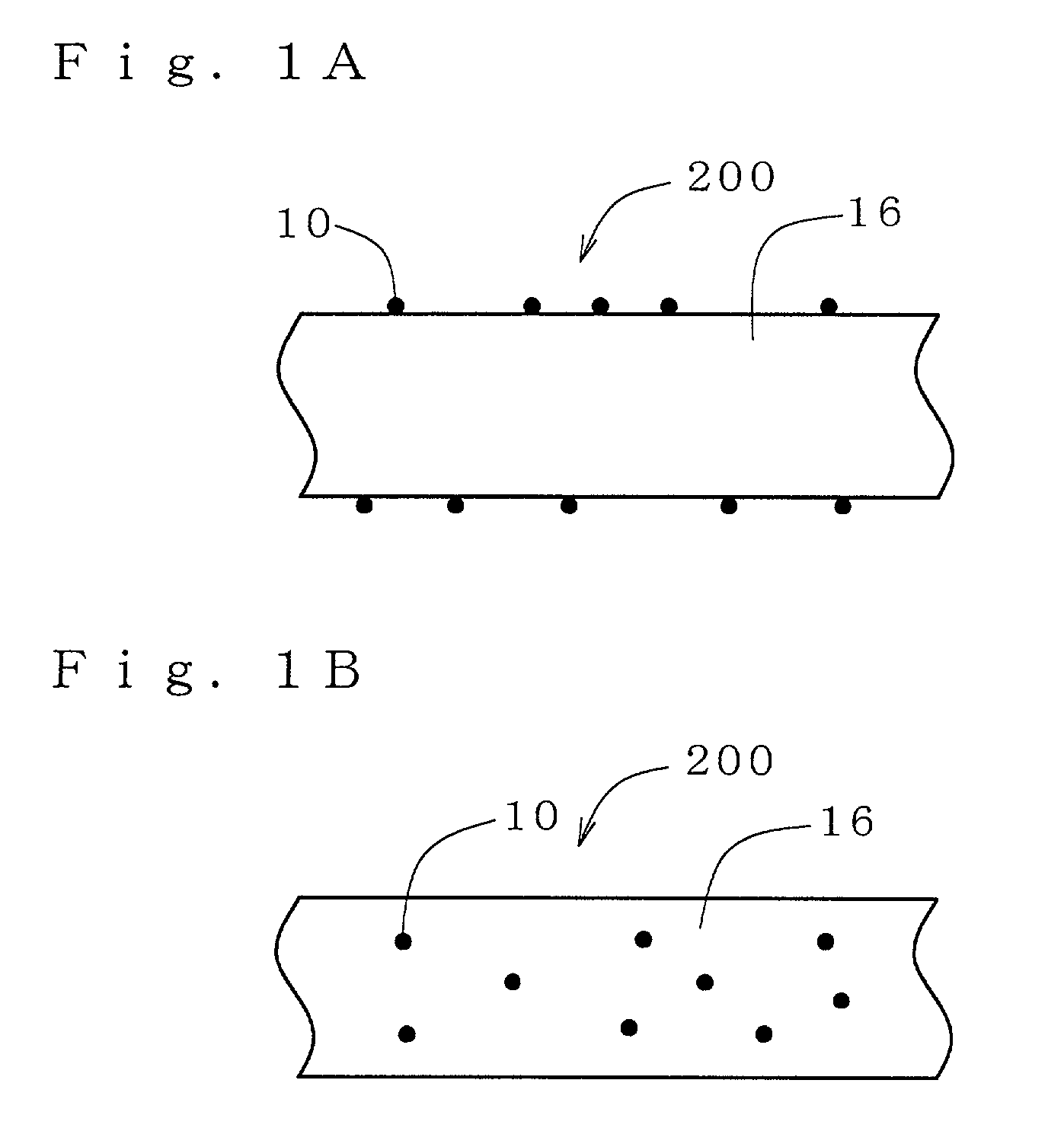
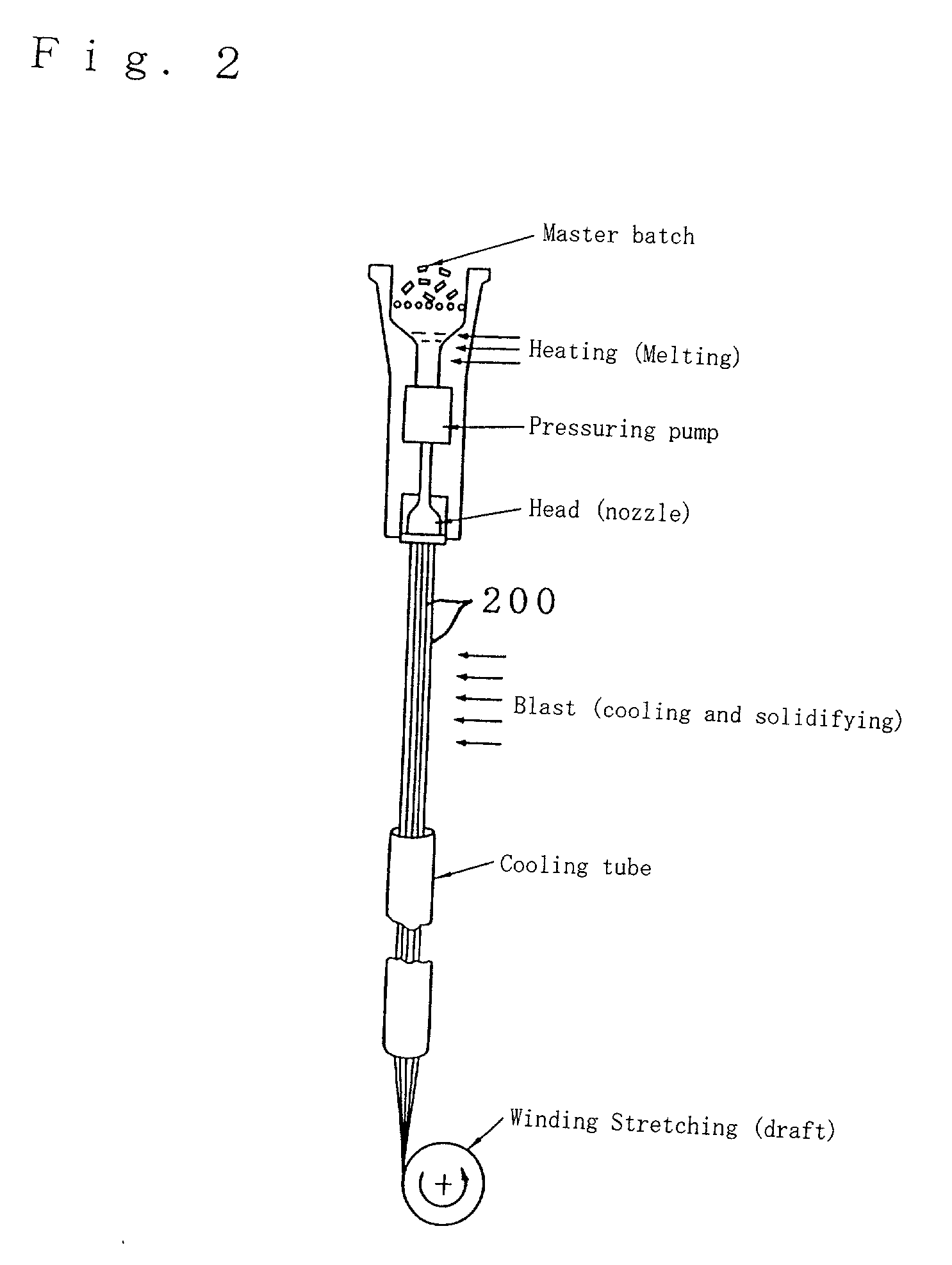
There is provided an antibacterial fiber, an antibacterial twisted yarn and an antibacterial cloth which exhibits high durability to post-processing with water, detergent, staining or the like at a small added amount. In an antibacterial fiber200, an antibacterial property imparting glass composition10 is fixed in a form of, for example, a particle on the surface of a fiber substrate16 and / or dispersed and complexed in the fiber substrate16. An antibacterial property imparting glass composition10 comprises 0.1 to 5.0% by weight of Ag2O in a composition containing 45-67 mol % of P2O5, 5 to 20 mol % of Al2O3, 1 to 40 mol % of 1 or 2 or more selected from MgO, CaO and ZnO, and 20 mol % or less of B2O3. An antibacterial fiber200 containing such the antibacterial property imparting glass composition10 at a ratio of, for example, 0.1 to 5.0% by weight shows high water resistance, acid resistance, alkali resistance and detergent resistance in antibacterial property.
Owner:ISHIZUKA GARASU
Antibacterial glass composition and antibacterial polymer composition using the same
ActiveUS6939820B2Improve water resistanceHigh and sufficiently sustained antibacterial performanceBiocideInorganic phosphorous active ingredientsAntimicrobial polymerPhotochemistry
The present invention provides an antibacterial glass composition exhibiting high antibacterial performance with sufficiently sustaining antibacterial performance by adding a small amount of the antibacterial component, and an antibacterial polymer composition using the antibacterial glass composition. The present invention provides an antibacterial glass composition containing 0.1 to 5.0% by weight of Ag2O in a glass composition containing 30 to 60 mol % of P2O5, 1 to 15 mol % of one or more compounds selected from the group consisting of K2O, Na2O and Li2O, 35 to 55 mol % of one or more compounds selected from the group consisting of MgO, CaO and ZnO, and 0.01 to 3 mol % of one or more compounds selected from the group consisting of La2O3 and Y2O3.
Owner:ISHIZUKA GARASU
IAP bir domain binding compounds
InactiveUS20080069812A1Improve effectivenessPharmaceutically acceptable stabilityPeptide/protein ingredientsAntipyreticEnantiomerTautomer
Disclosed is an isomer, enantiomer, diastereoisomer or tautomer of a compound represented by Formula I or II or a salt thereof, in which R1, R2, R3, R100, R200, R300, A, A1, BG, Q and Q1 are substituents described herein. Also disclosed is the use of compounds of Formula I and II to treat proliferative disorders such as cancer.
Owner:PHARMASCIENCE INC
Method for manufacturing a bonded soi wafer and bonded soi wafer
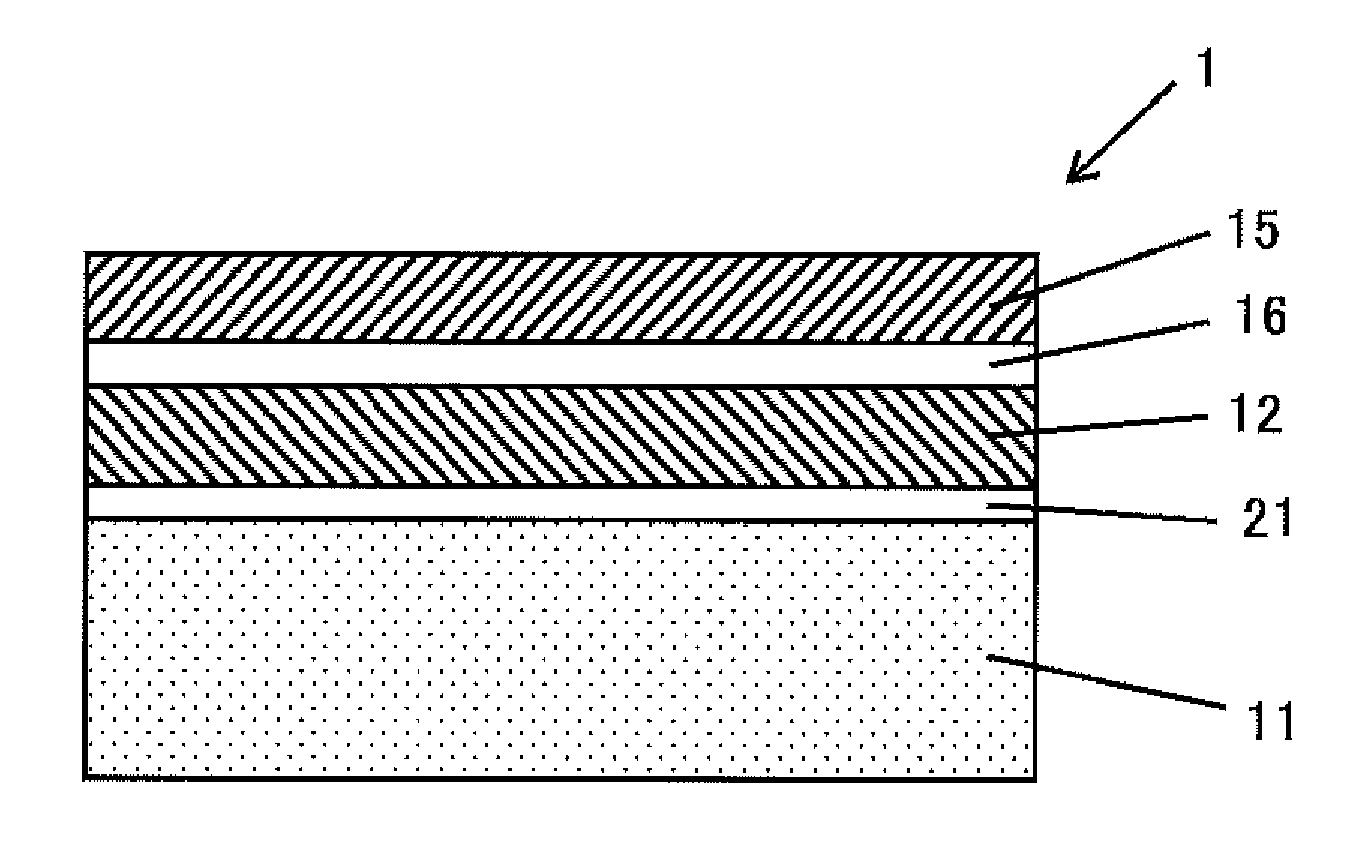
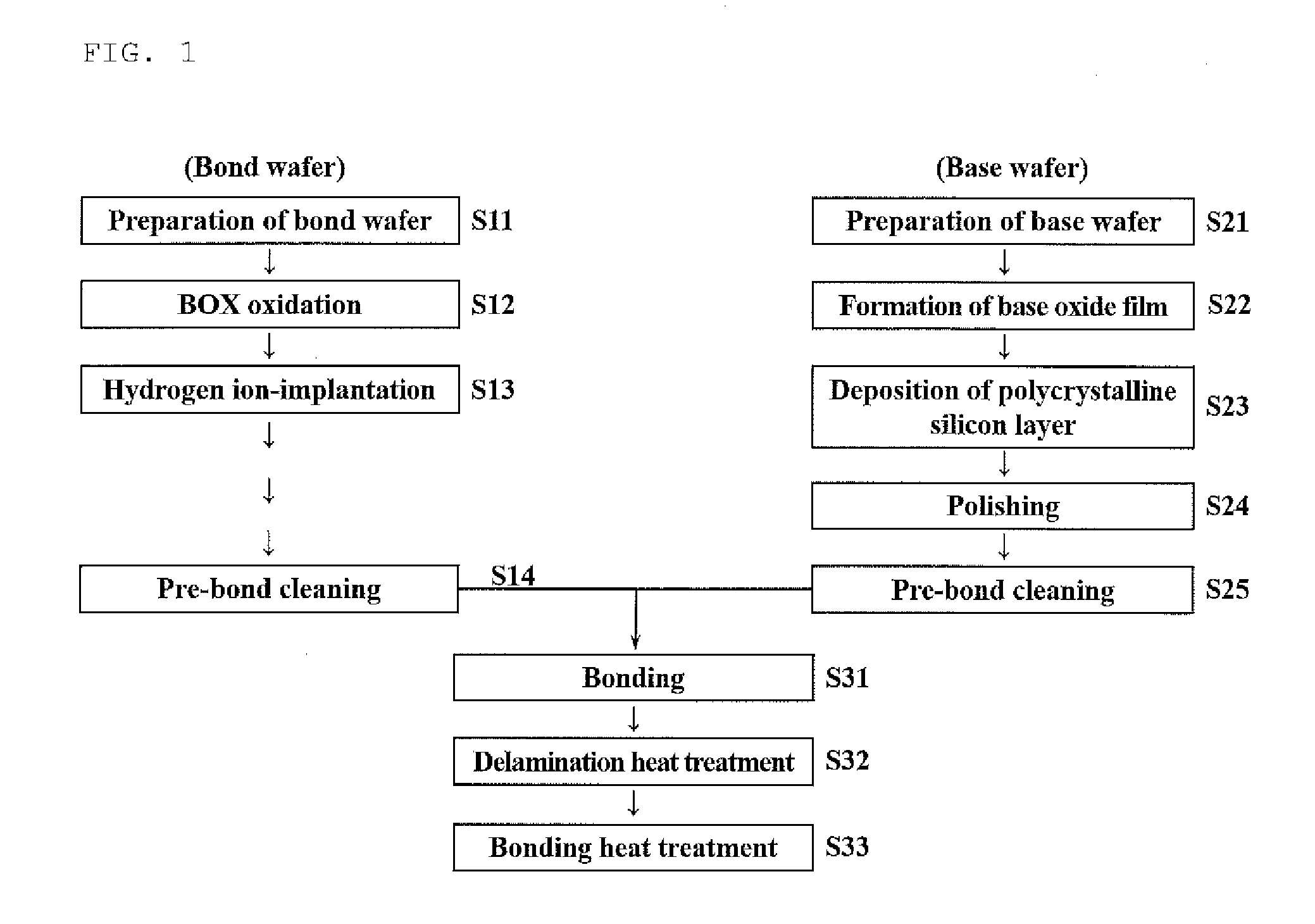
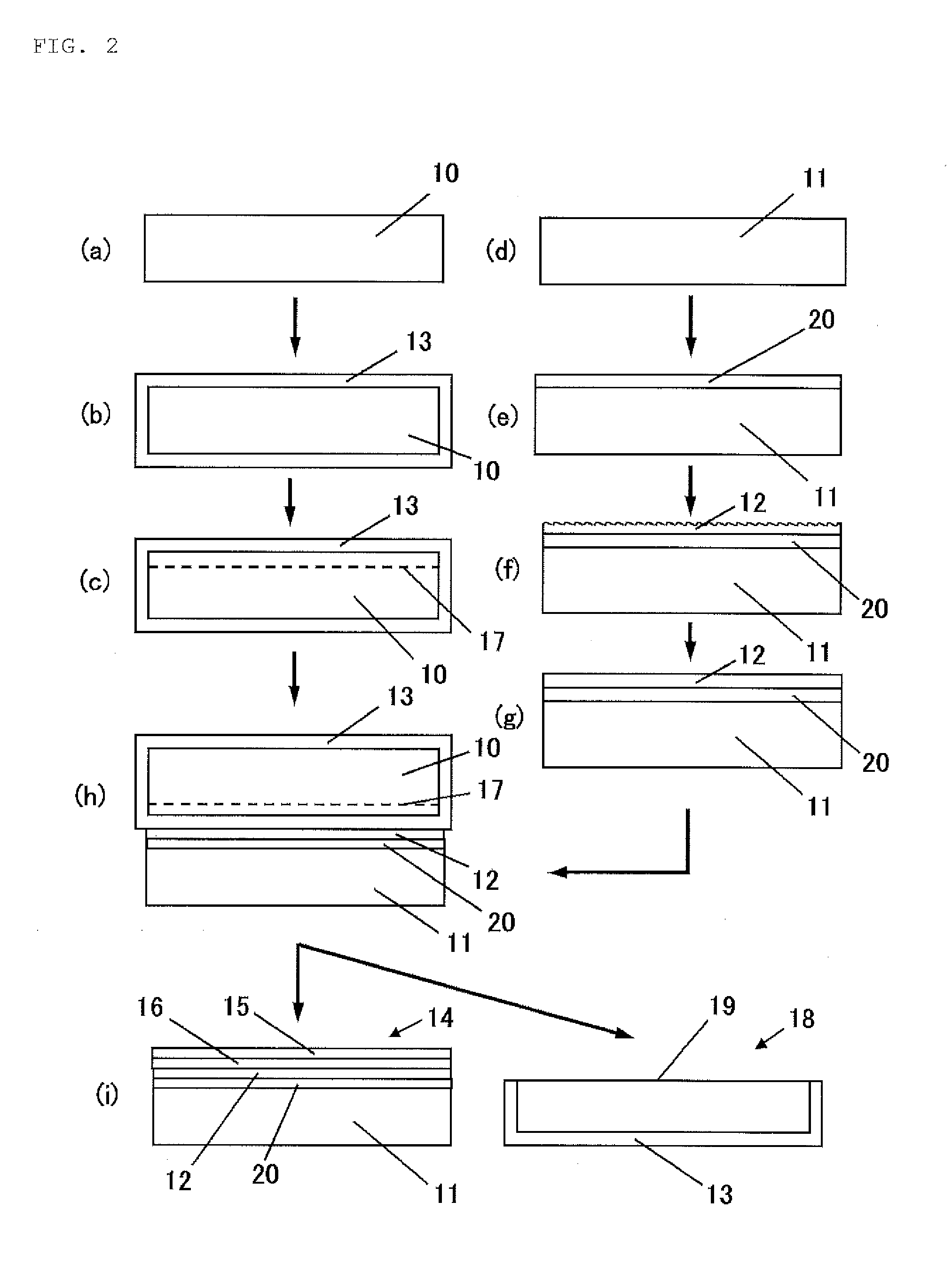
ActiveUS20170033002A1Sustained effectAvoid grain growthLayered productsSolid-state devicesPolycrystalline siliconSingle crystal
A bonded SOI wafer is manufactured by bonding a bond and a base wafer, each composed of a silicon single crystal, via an insulator film, depositing a polycrystalline silicon layer on the bonding surface side of the base wafer, polishing a surface of the polycrystalline silicon layer, forming the insulator film on the bonding surface of the bond wafer, bonding the polished surface of the polycrystalline silicon layer and the bond wafer via the insulator film, and thinning the bonded bond wafer to form an SOI layer; wherein, the base wafer is a silicon single crystal wafer having a resistivity of 100 Ω·cm or more, depositing the polycrystalline silicon layer further includes a stage for previously forming an oxide film on the surface of the base wafer on which the polycrystalline silicon layer is deposited, and the polycrystalline silicon layer is deposited at a temperature of 900° C. or more.
Owner:SHIN-ETSU HANDOTAI CO LTD
Pharmaceutical compositions releasing their active agents from a buccal or sublingual location to overcome an absorption window problem
InactiveUS20040202693A1Improve bioavailabilityReduced bioavailabilityAntibacterial agentsBiocideActive agentSublingual location
Disclosed are controlled release dosage forms of pharmaceutical or nutritional agents that are intended for retention in a buccal or sublingual location for administration. The dosage forms are particularly useful for the sustained release administration of drugs that have a limited window of absorption in the gastrointestinal tract and that are minimally, if at all, absorbed mucosally.
Owner:SUPERNUS PHARM INC
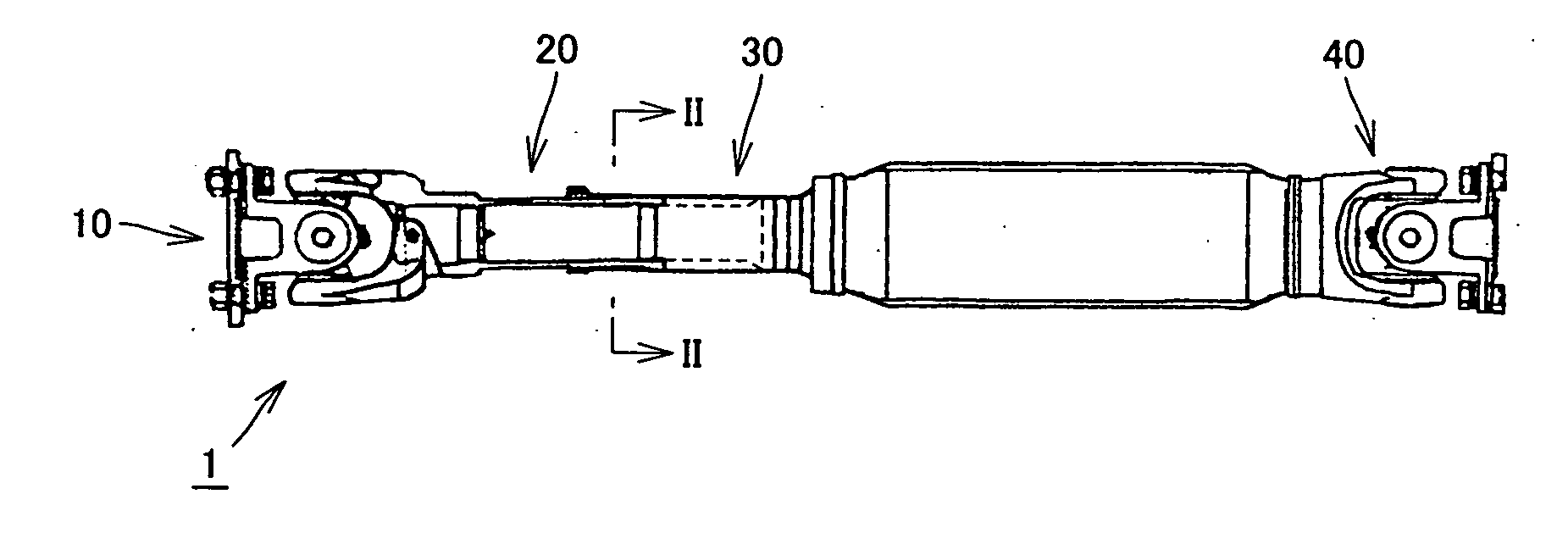
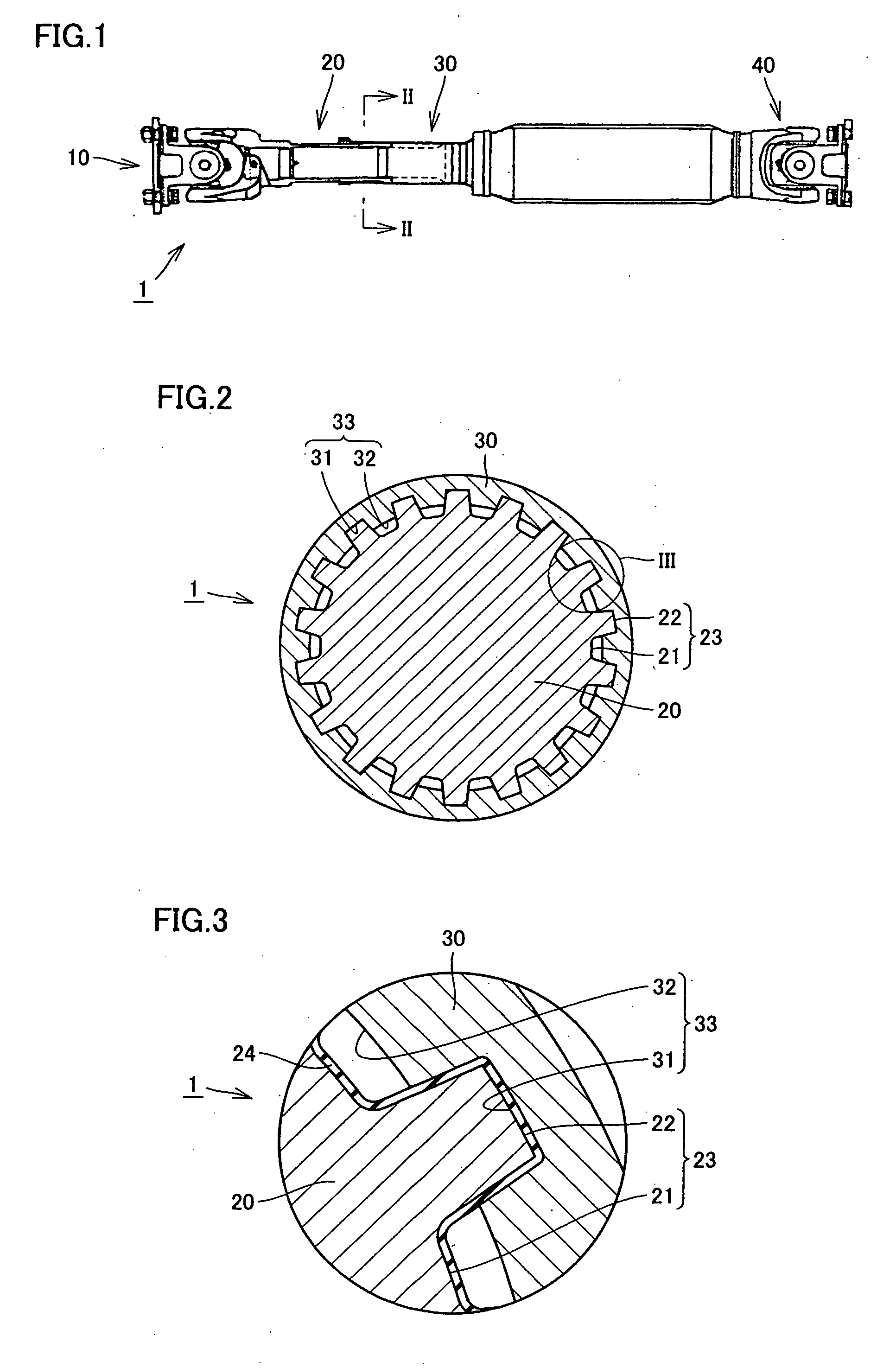
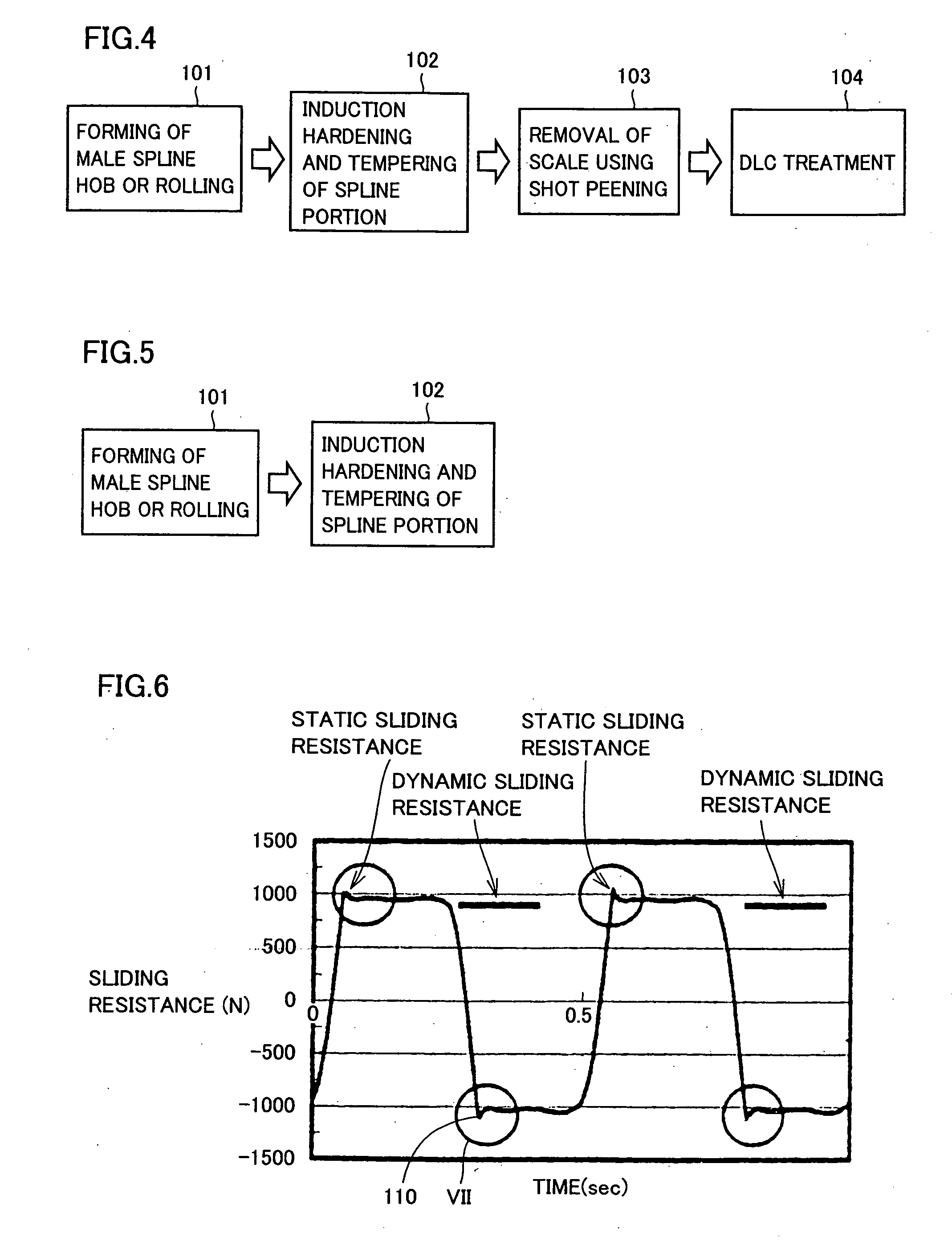
Propeller shaft
InactiveUS20070149299A1Inhibit wearImprove wear resistanceClutchesYielding couplingDrive shaftPropeller
A propeller shaft having high wear resistance is provided. The propeller shaft has a spline shaft and a spline sleeve meshing with each other, and a DLC coating is applied to any one of the spline shaft and the spline sleeve. The DLC coating has surface roughness Ra of 0.5 μm or smaller.
Owner:TOYOTA JIDOSHA KK
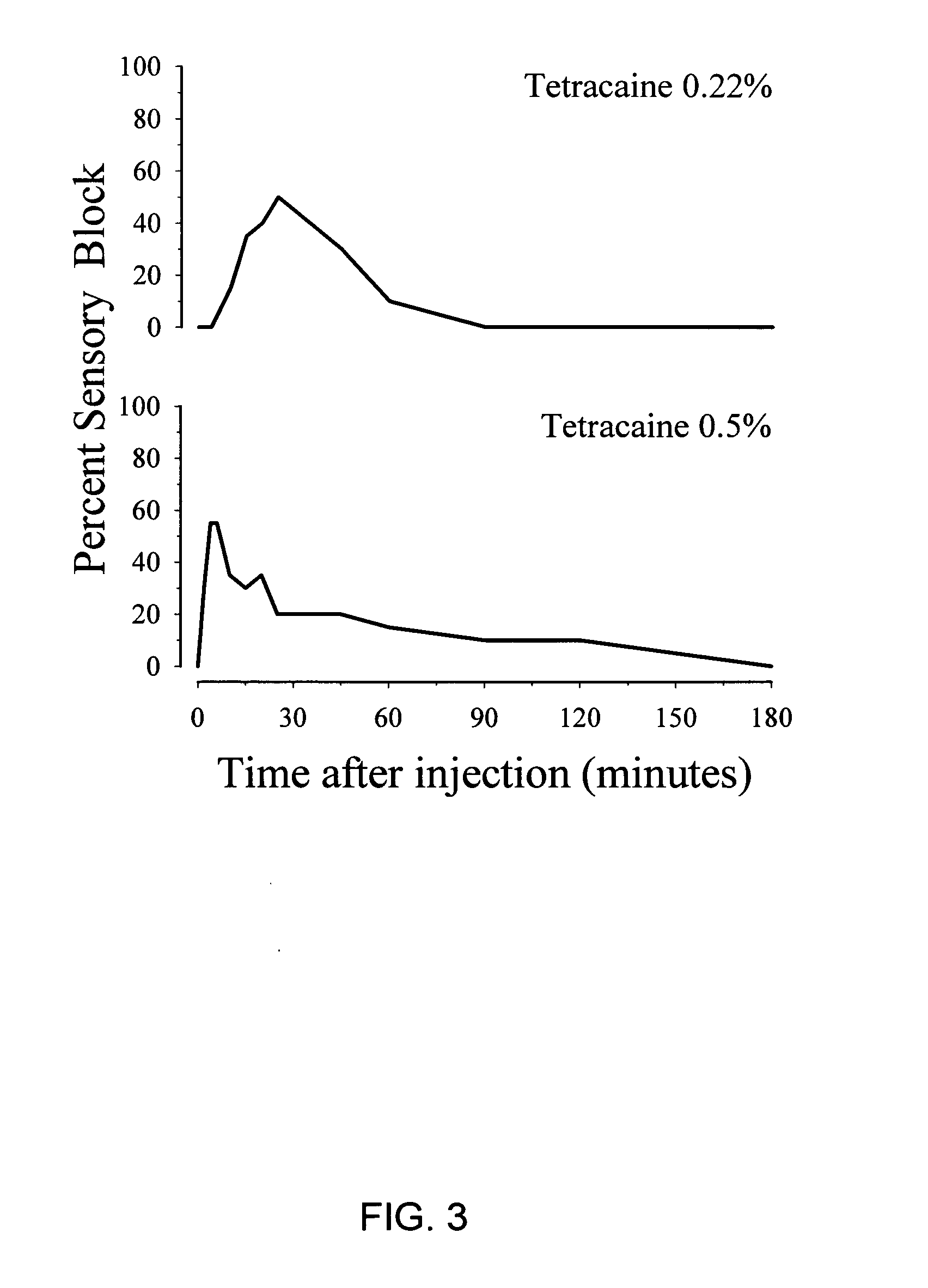
Ester combination local anesthetic
InactiveUS20050137177A1Low risk of toxicityRapid actionBiocideAnimal repellantsPain durationChemical toxicity
The present invention provides compositions and methods for improved local anesthesia and / or analgesia, in which onset of action is rapid, the risk of toxicity is low, and the effect is sustained. More particularly, the present invention provides a combination of at least two ester anesthetics for administration to a subject, where at least one ester anesthetic provides a rapid onset of action and at least one ester anesthetic provides sustained activity. The compositions of the present invention are useful for the production of analgesia and / or anesthesia and are particularly useful for the prophylaxis and / or treatment of pain.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Ophthalmic percutaneously absorbed preparation containing muscarinic receptor agonist
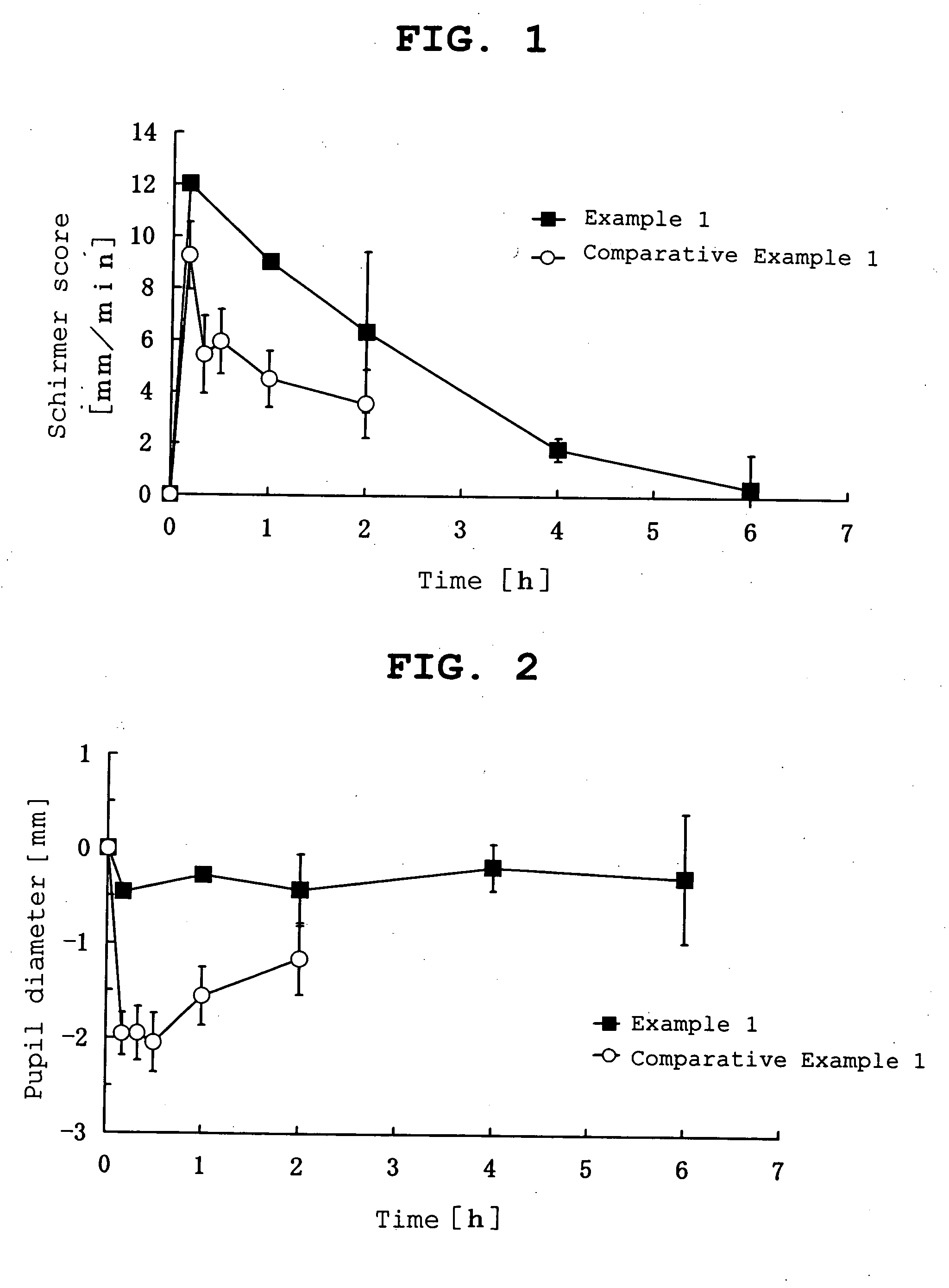
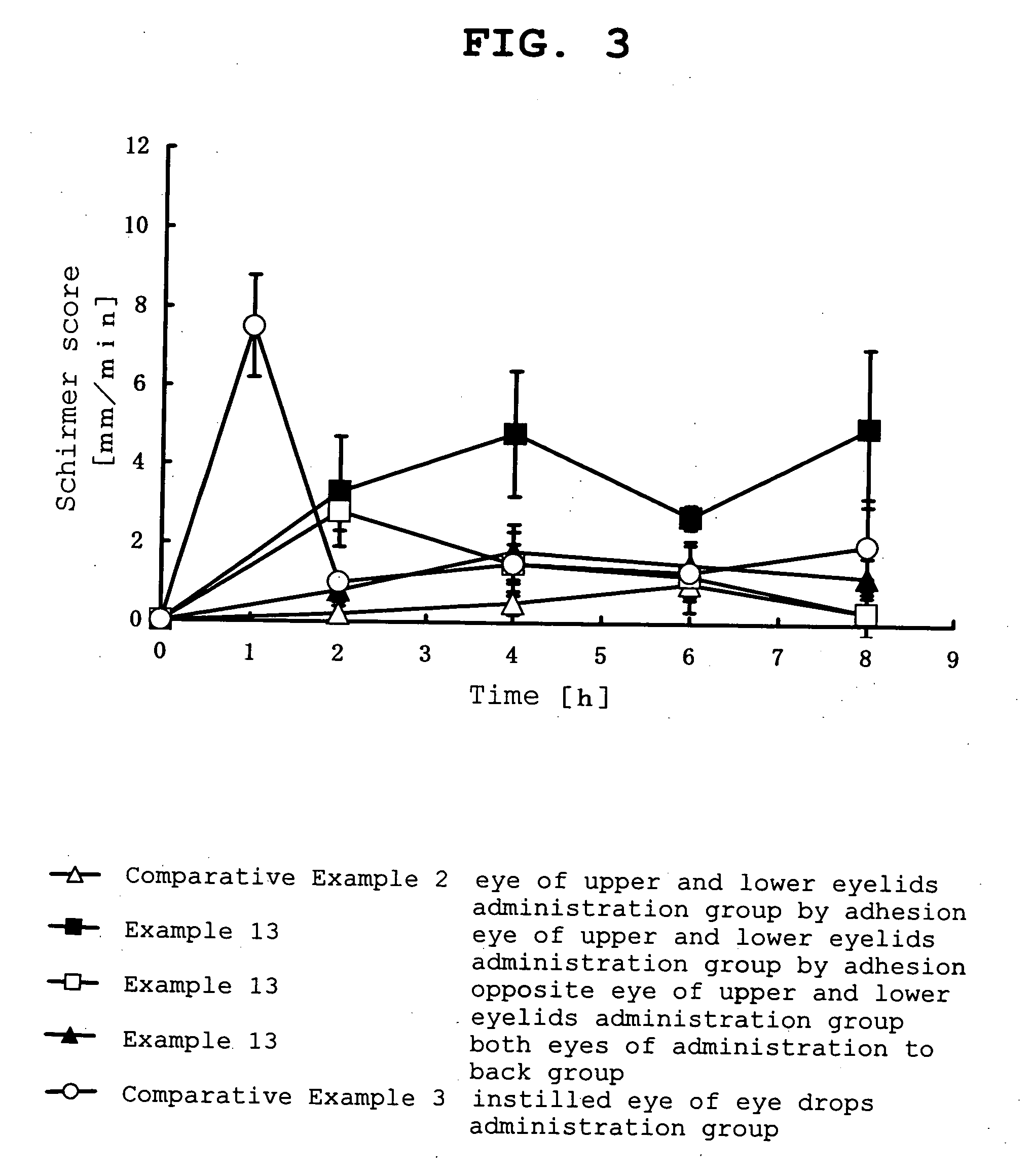
InactiveUS20070053964A1Promote secretionSustained effectOrganic active ingredientsBiocideEyelidSide effect
A percutaneous absorption type ophthalmic preparation comprising muscarinic receptor agonist is prepared, which can promote lacrimal fluid secretion by administration to the skin surface of the eyelid, and which causes fewer side effects such as miosis, thereby to provide a percutaneous absorption type ophthalmic preparation capable of maintaining a therapeutically effective concentration of a muscarinic receptor agonist for promoting lacrimal fluid secretion, which is associated with fewer side effects such as miosis, and a method of promoting lacrimal fluid secretion by administering a percutaneous absorption type ophthalmic preparation containing a muscarinic receptor agonist to the skin surface of the eyelid.
Owner:SENJU PHARMA CO LTD
Absorbable epsilon-caprolactone copolymers
InactiveUS6703035B2Increases rate of autocatalytic hydrolysisImprove toughnessSuture equipmentsPowder deliveryNitrogenCopolyester
This invention deals with crystalline, nitrogen copolyester lubricant coating devices comprising sutures, wherein said lubricant comprises a triaxial copolyester chain with a central nitrogenous base or a copolyester with more than one carboxylic group ionically linked to a basic amino acid.
Owner:POLY MED
Allergen inactivating method, allergen inactivating filter, air treating apparatus, virus inactivating agent, virus inactivating method, virus inactivating filter, air conditoning unit and air conditioner
InactiveUS20050042716A1Easy to integratePractical and convenientBiocideMechanical apparatusVirus inactivationEnzyme
Owner:MITSUBISHI HEAVY IND LTD
Bonding apparatus using conductive material
InactiveUS20060065642A1Avoid stickingStablyPrinted circuit assemblingSolid-state devicesMetallurgyConductive materials
The present invention provides a bonding apparatus in which solder is conveyed onto a predetermined electrode, and the solder is melted by irradiation of a laser beam and is bonded to the electrode and has an object to prevent the solder from adhering to a solder conveying system of the apparatus. To achieve the above object, in the bonding apparatus, a film having low wettability to the solder and having a predetermined thickness such as a DLC film is coated on a region and a neighborhood of a solder holding and conveying member, which is contacted with the solder.
Owner:SAE MAGNETICS H K LTD 50
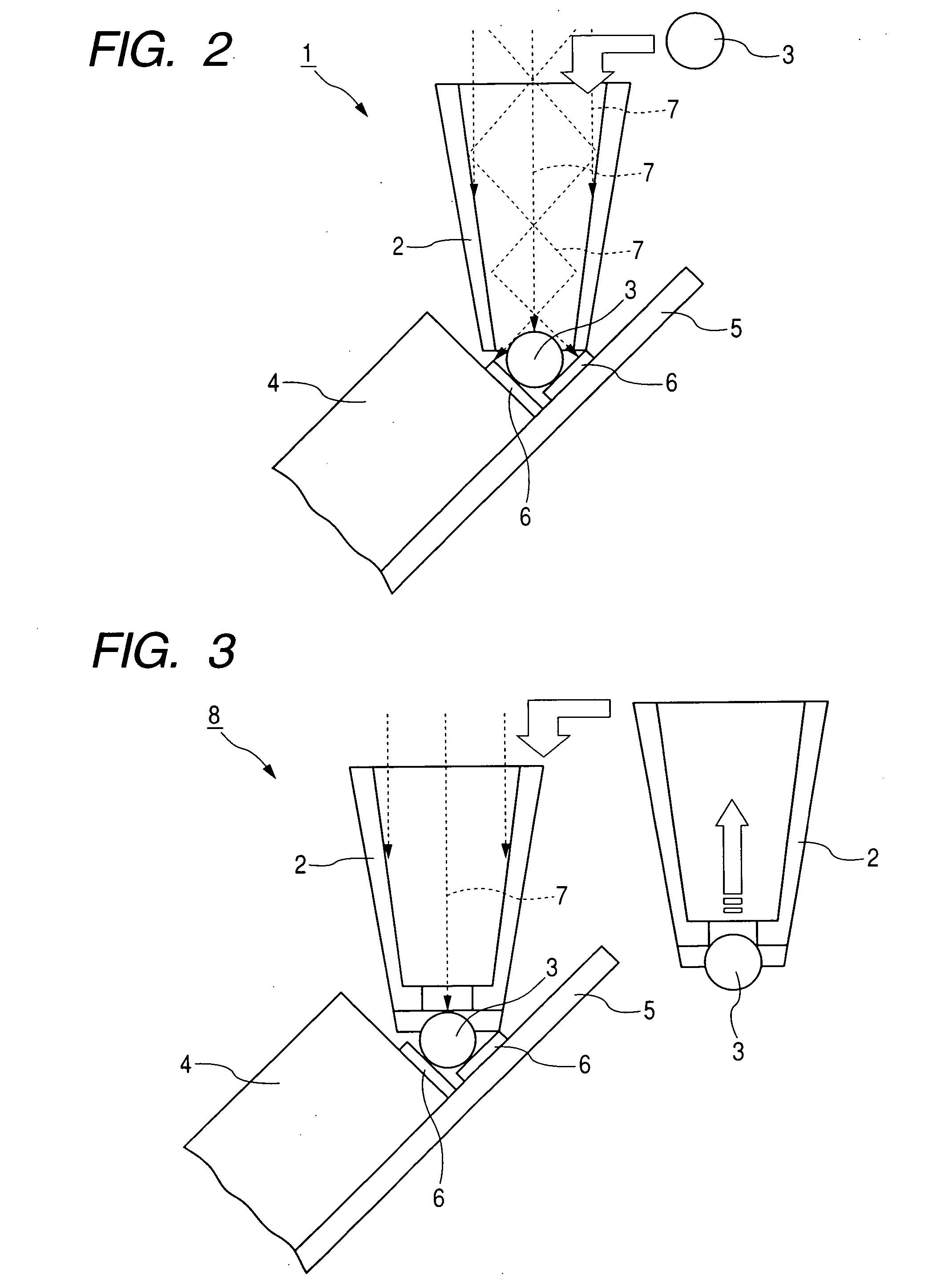
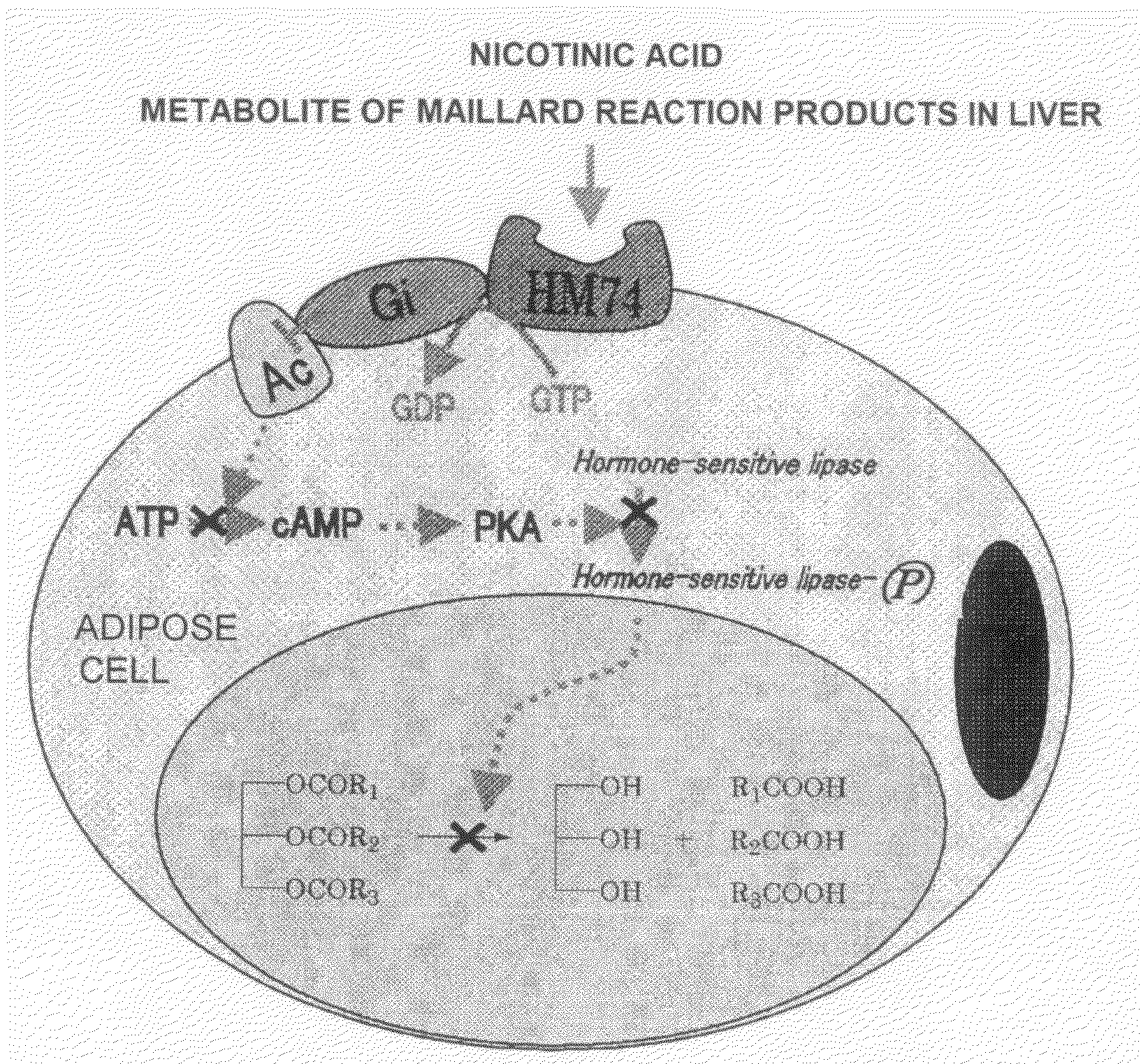
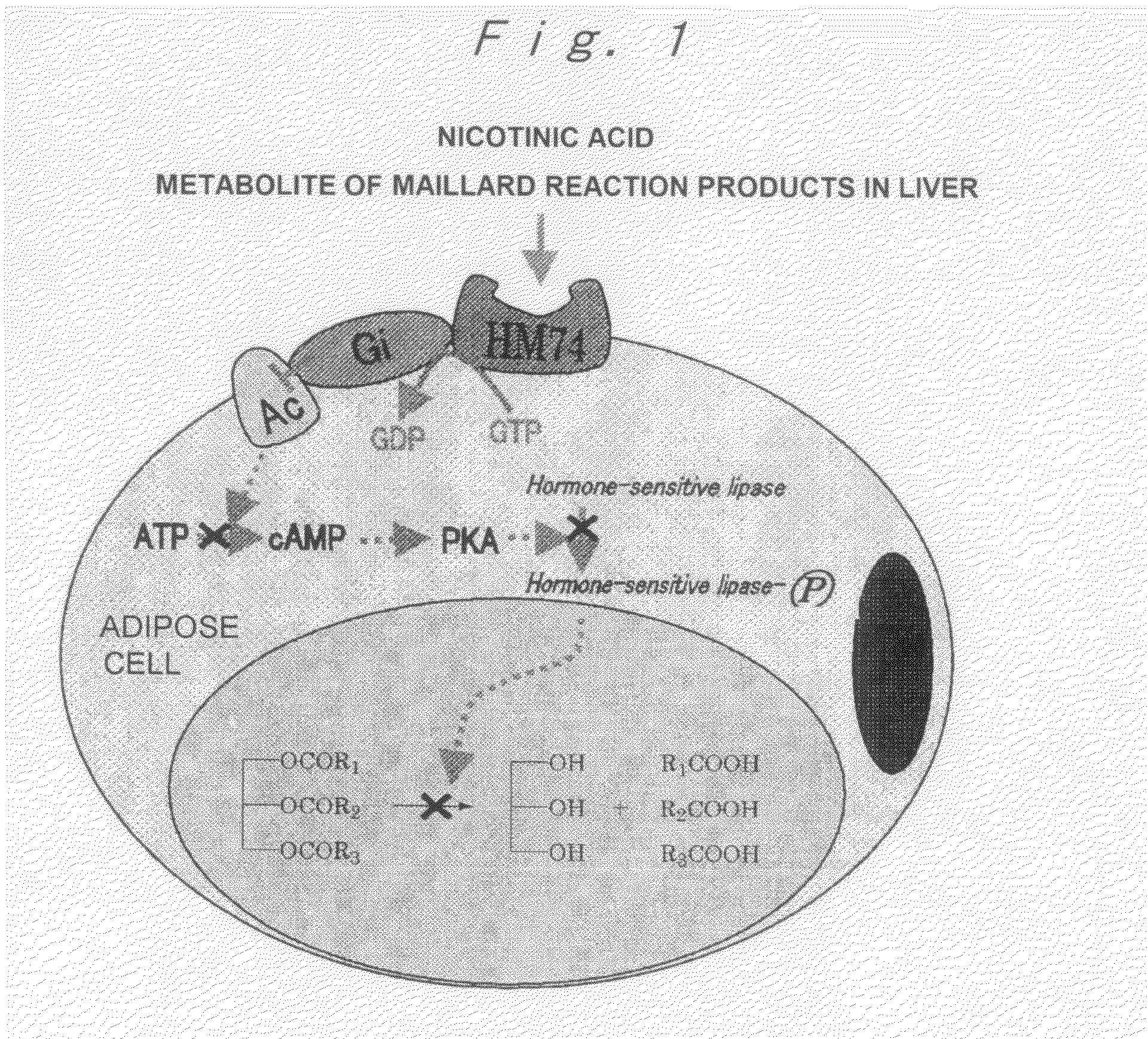
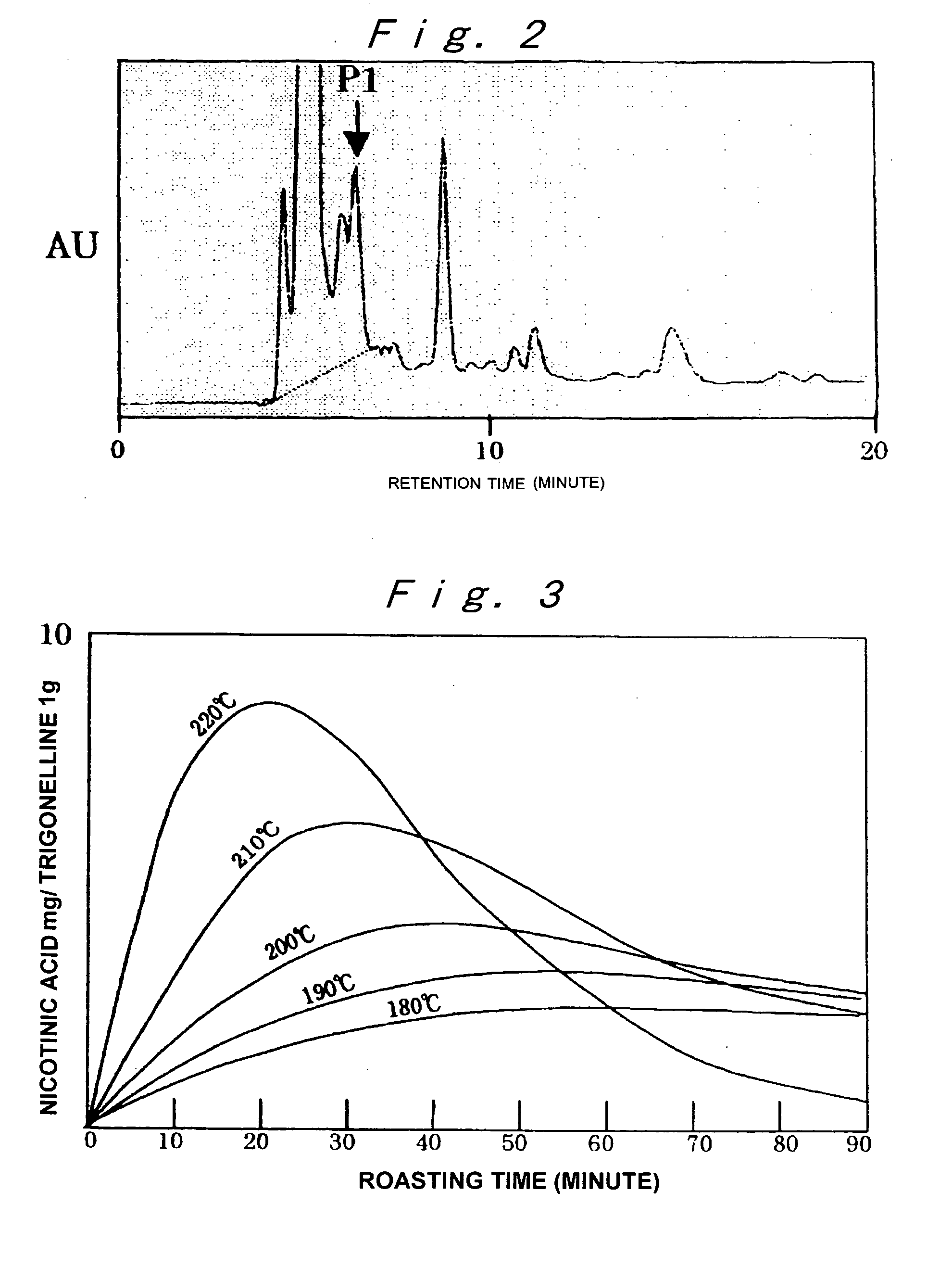
Modified Coffee, Method of Roasting Coffee Bean, Coffee-Like Supplement and Auxiliary Food
InactiveUS20080227832A1Reduce the amount requiredRaise the ratioBiocideMetabolism disorderMaillard reactionMetabolite
A modified coffee containing 3 mg or more of a nicotinic acid compound and 10 mg or more of a Maillard reaction product per 10 g of roasted coffee beans. A coffee-like supplement containing at least one nicotinic acid compound, at least one Maillard reaction product, and / or at least one metabolite of the Maillard reaction product.
Owner:TAMA TLO LTD
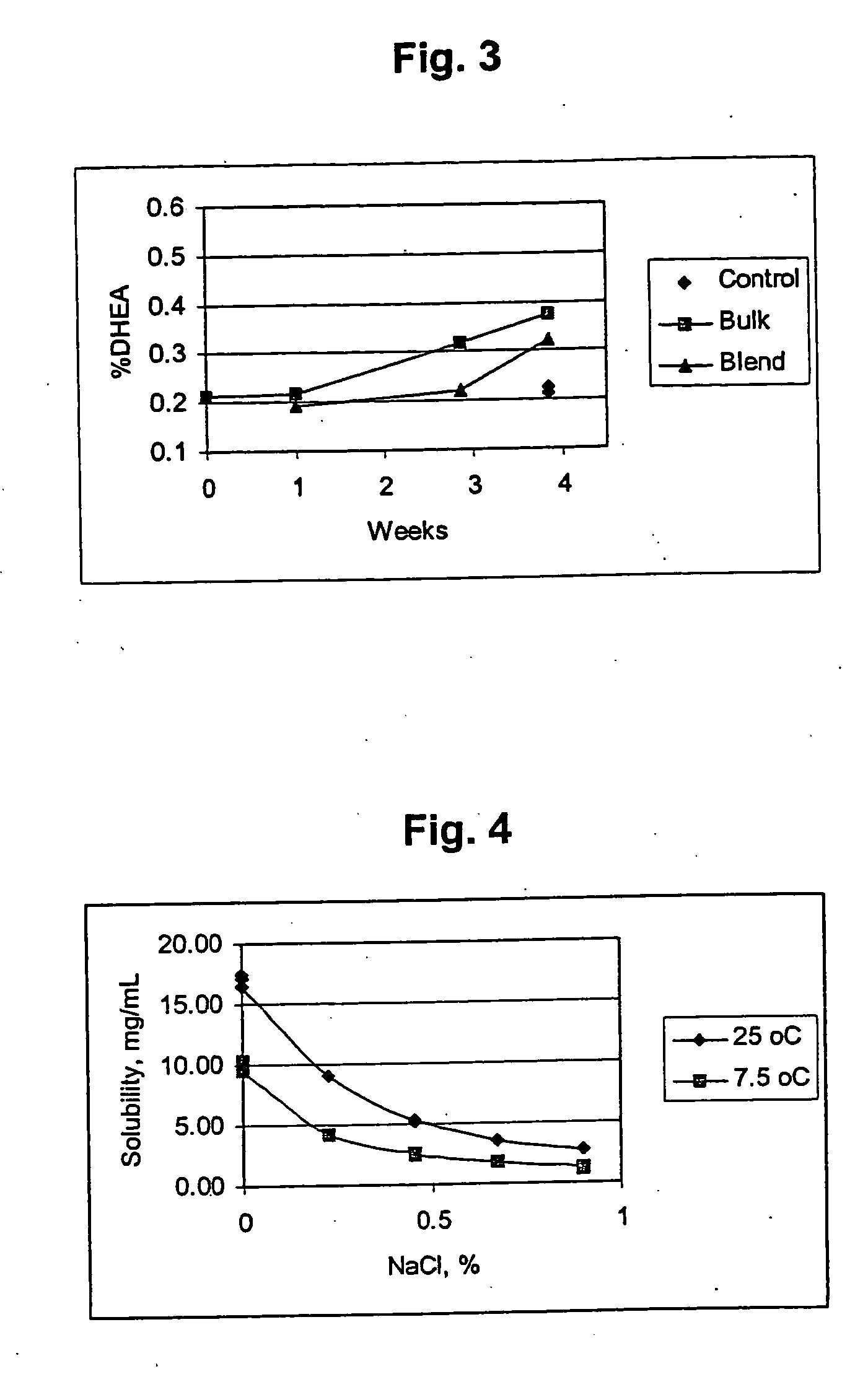
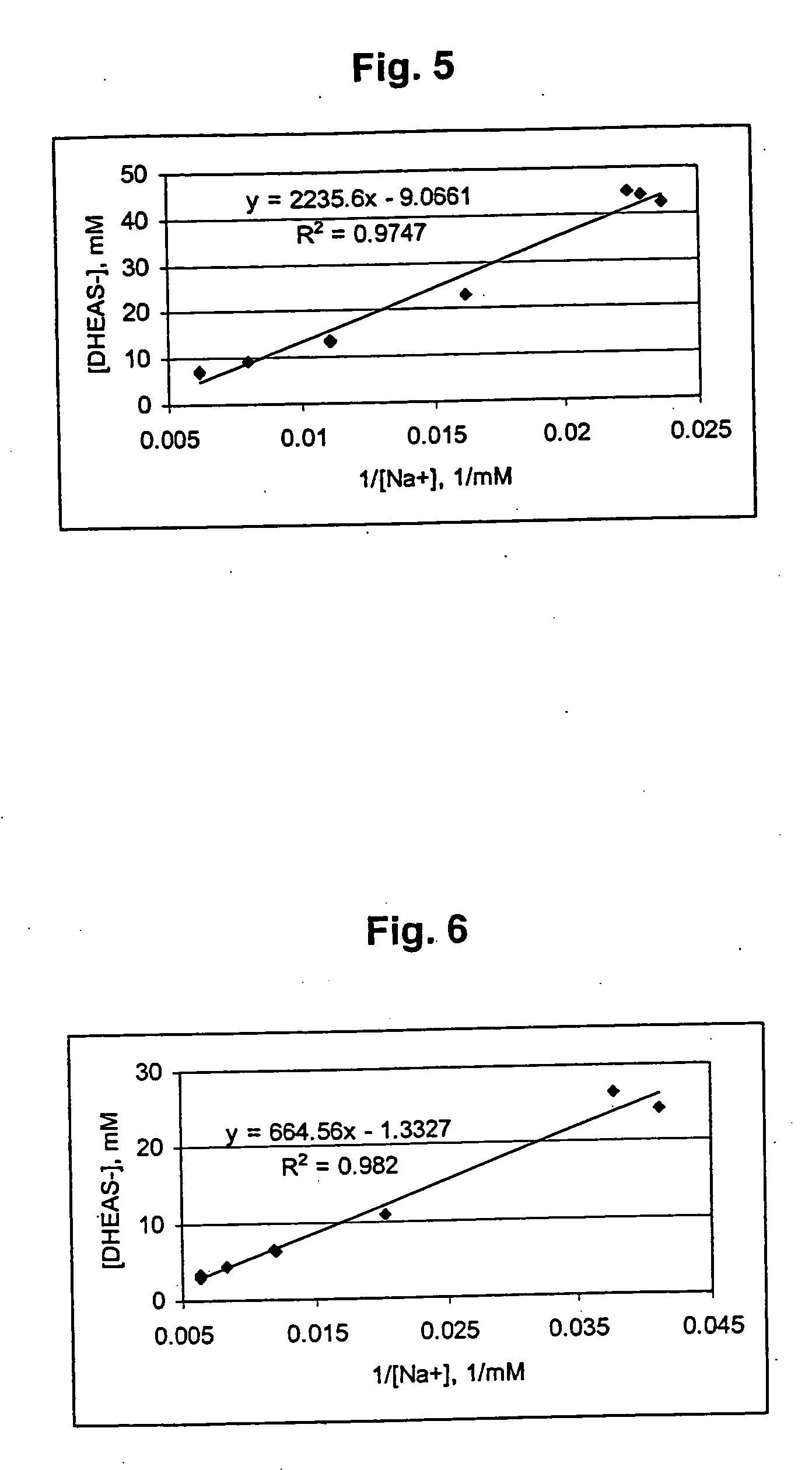
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with an antihistamine for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026890A1Alleviate different aspectConvenient treatmentBiocideOrganic active ingredientsDiseaseActive agent
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising an antihistamine for the treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease.
Owner:EPIGENESIS PHARMA LLC
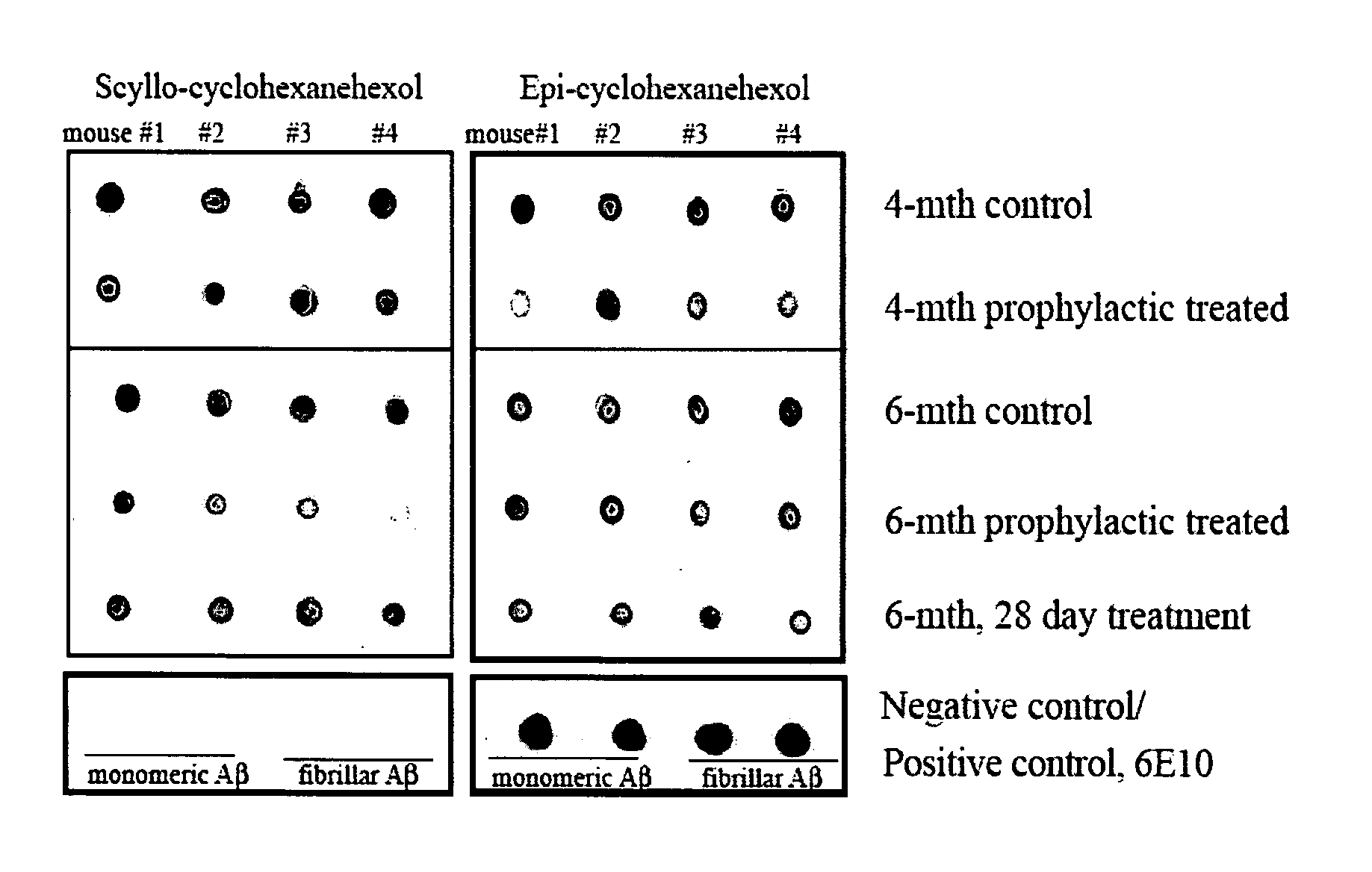
Compositions and methods for treatment of disorders of protein aggregation
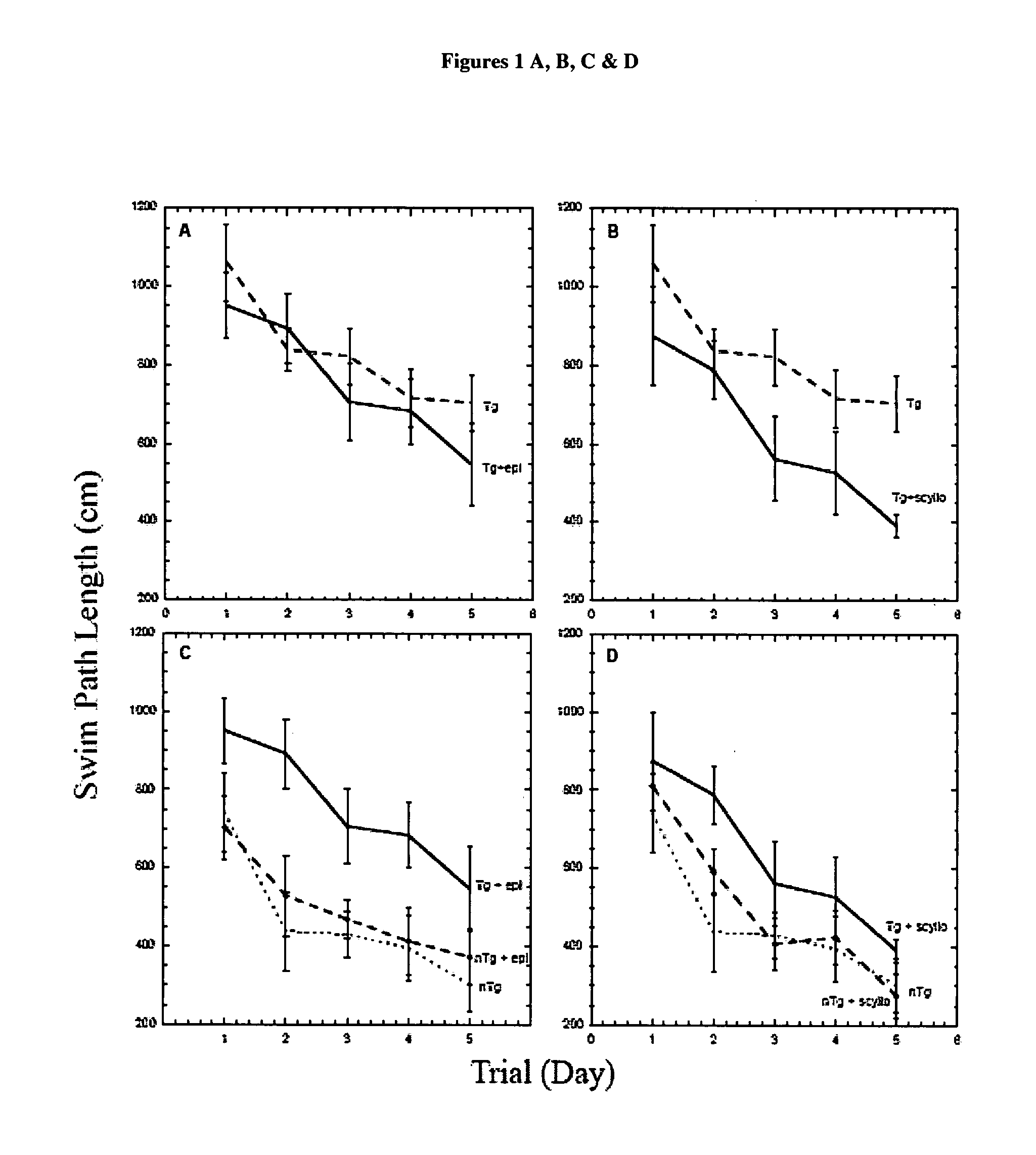
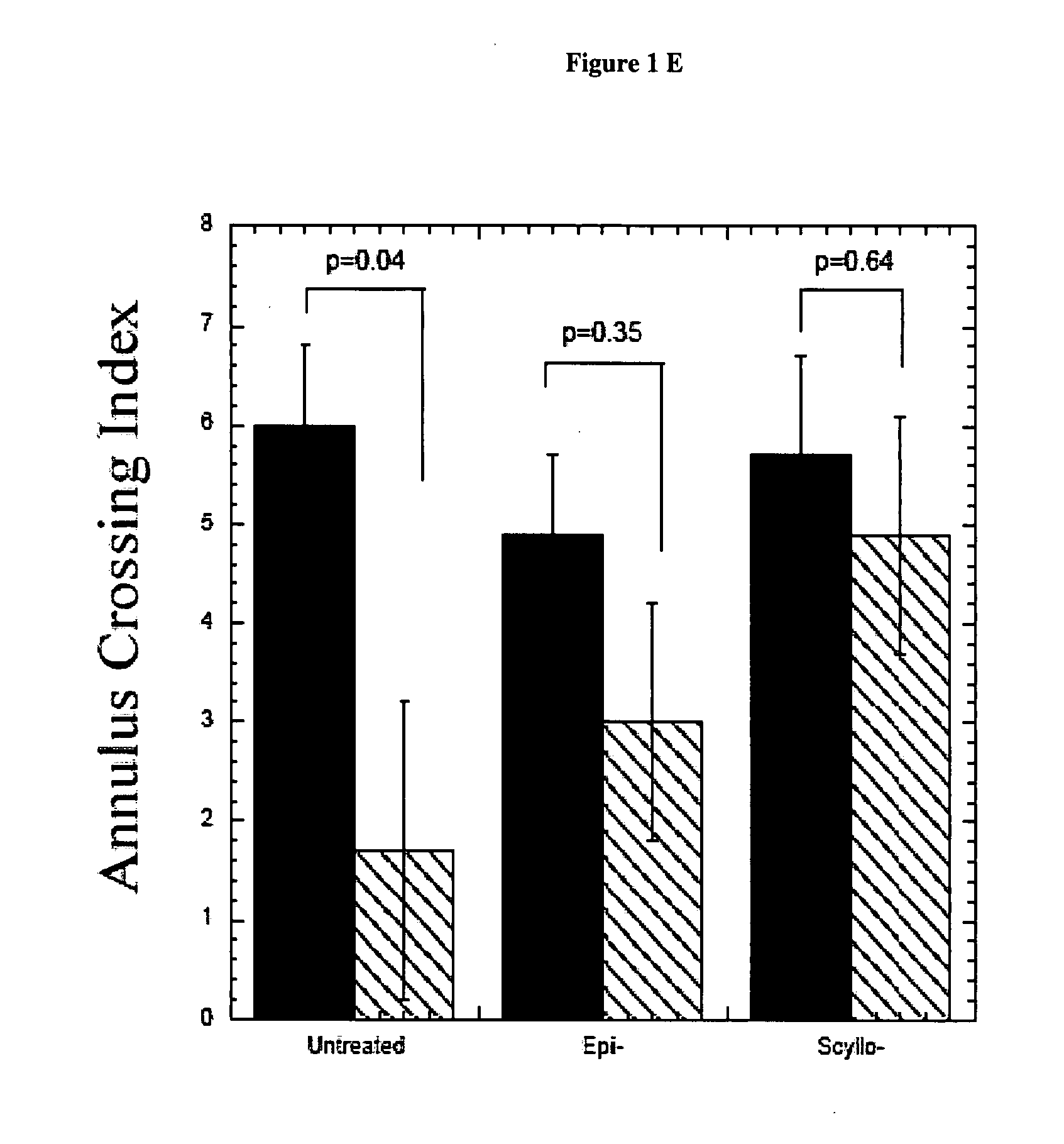
InactiveUS20070197453A1Sustained beneficial effectIncreased and restored long-term potentiationBiocideNervous disorderCell AggregationsProtein aggregation
The invention relates to compositions, methods and uses comprising an epi-inositol compound that provides beneficial effects in the treatment of a disorder and / or disease including a disorder in protein folding and / or aggregation, and / or amyloid formation, deposition, accumulation, or persistence. In aspects of the invention, the epi-inositol compounds provide beneficial effects in the treatment of Alzheimer's disease, dementia, and mild cognitive impairment.
Owner:MCLAURIN JOANNE
Sustained improver of muscular fatigue
A long-acting improver of muscular fatigue characterized by comprising 4 kinds of amino acids made up of leucine, isoleucine, valine and glutamine, and a whey protein component (whey protein and / or decomposition product of whey protein). At least one of a whey protein isolate (WPI), a whey protein concentrate (WPC), β-lactoglobulin, and α-lactalbumin is used as the whey protein. Novel food or drink, and pharmaceuticals which exhibit sustained recovery effects on muscular fatigue are provided.
Owner:KYOWA HAKKO BIO CO LTD
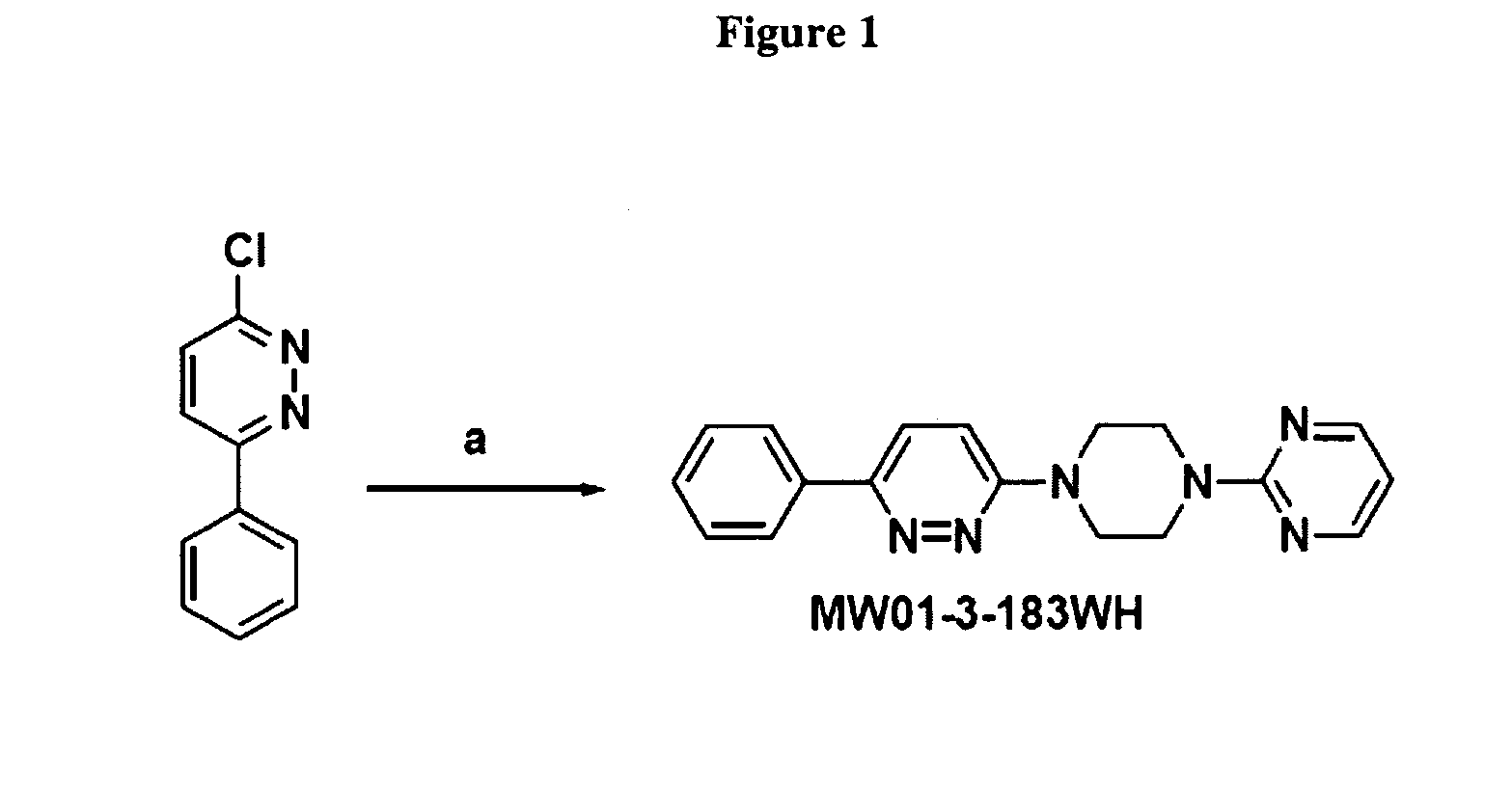
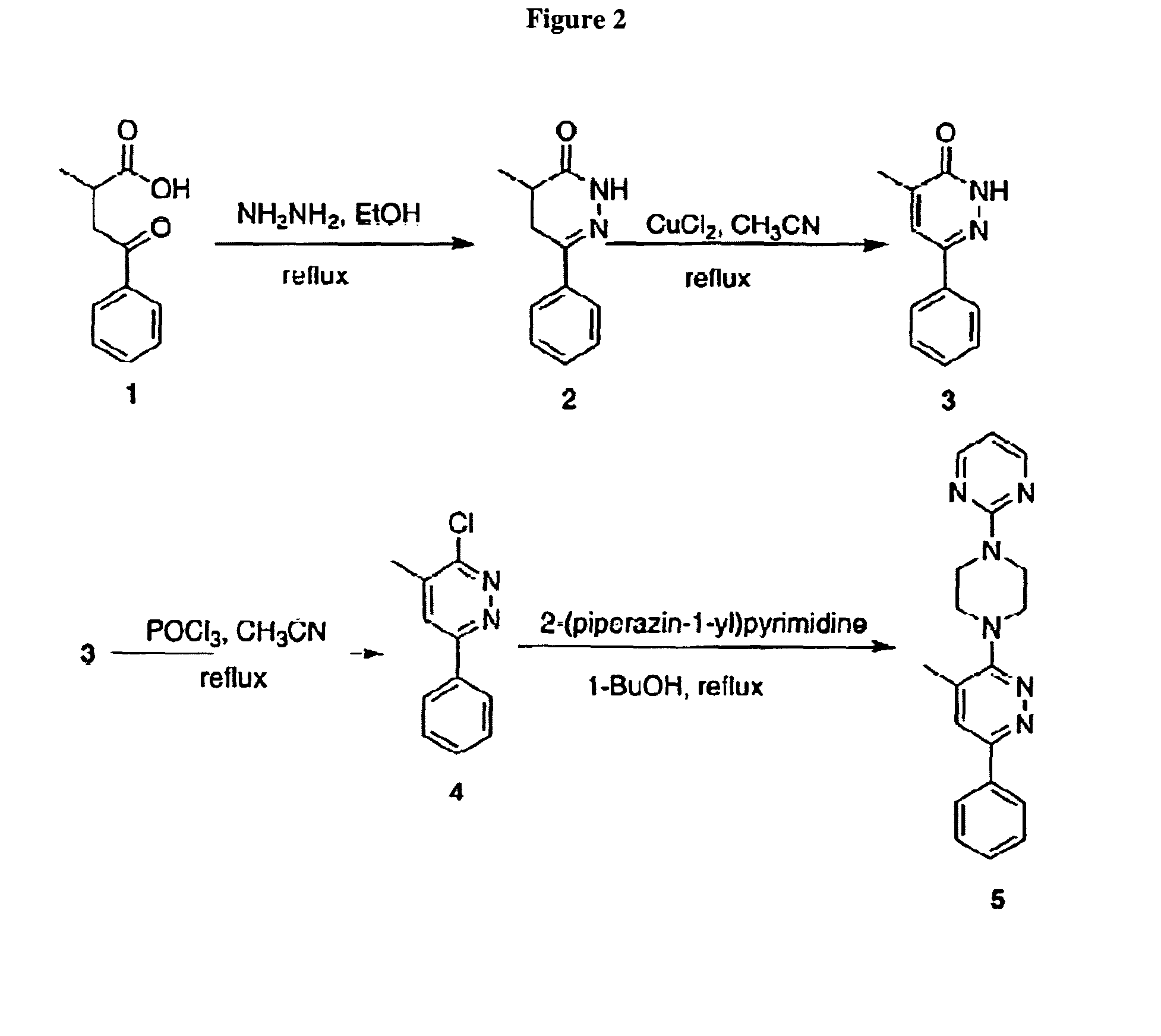
Compositions and treatments using pyridazine compounds and cholinesterase inhibitors
ActiveUS8158627B2Improve toleranceReduce adverse effectsNervous disorderAntipyreticDiseaseCellular pathways
The invention relates to compositions, conjugates and methods comprising pyridazine compounds and cholinesterase inhibitors for modulation of cellular pathways (e.g., signal transduction pathways), for treatment or prevention of inflammatory diseases (e.g., Alzheimer's disease), for research, drug screening, and therapeutic applications.
Owner:NORTHWESTERN UNIV
Allergen inactivating method, allergen inactivating filter, air treating apparatus, virus inactivating agent, virus inactivating method, virus inactivating filter, air conditioning unit and air conditioner
InactiveUS20090269249A1Easy to integratePractical and convenientBioreactor/fermenter combinationsBiological substance pretreatmentsVirus inactivationAir conditioning
Owner:MITSUBISHI HEAVY IND LTD
Antibacterial glass composition and antibacterial polymer composition using the same
ActiveUS20040170700A1Improve water resistanceHigh and sufficiently sustained antibacterial performanceBiocideInorganic phosphorous active ingredientsAntimicrobial polymerPhotochemistry
The present invention provides an antibacterial glass composition exhibiting high antibacterial performance with sufficiently sustaining antibacterial performance by adding a small amount of the antibacterial component, and an antibacterial polymer composition using the antibacterial glass composition. The present invention provides an antibacterial glass composition containing 0.1 to 5.0% by weight of Ag2O in a glass composition containing 30 to 60 mol % of P2O5, 1 to 15 mol % of one or more compounds selected from the group consisting of K2O, Na2O and Li2O, 35 to 55 mol % of one or more compounds selected from the group consisting of MgO, CaO and ZnO, and 0.01 to 3 mol % of one or more compounds selected from the group consisting of La2O3 and Y2O3.
Owner:ISHIZUKA GARASU
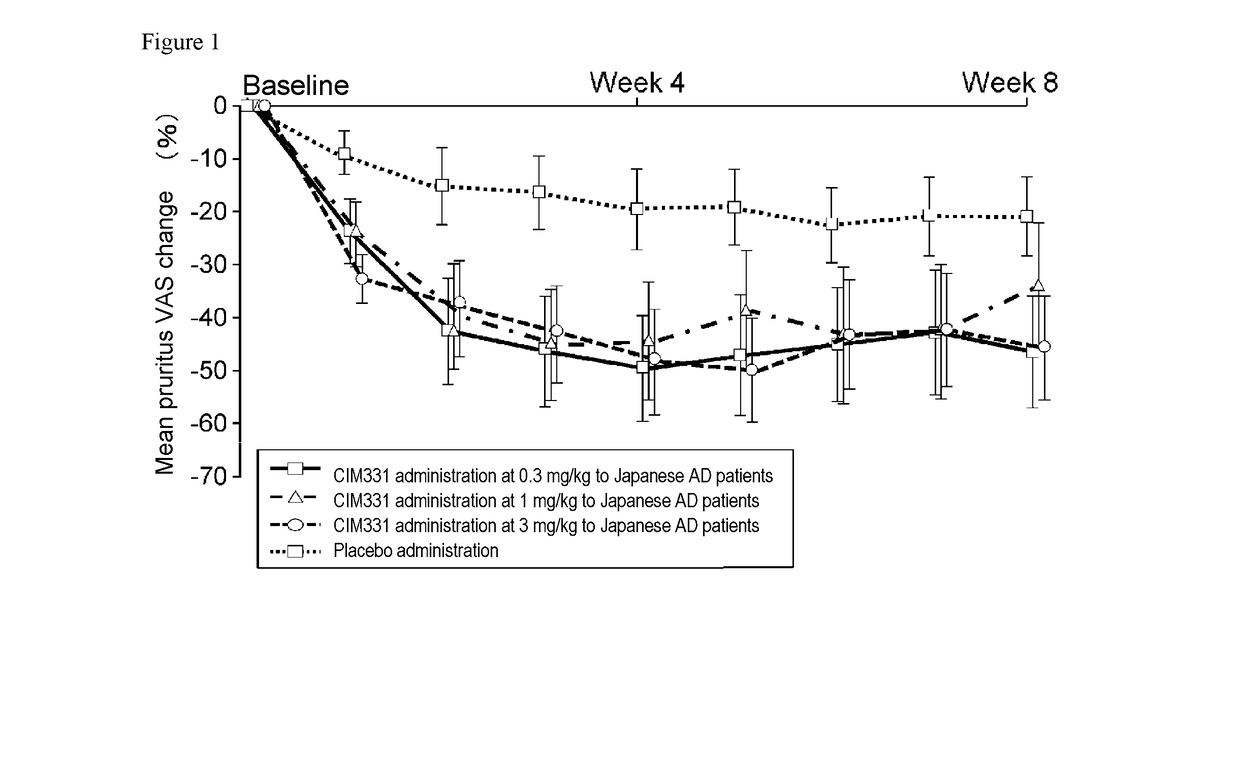
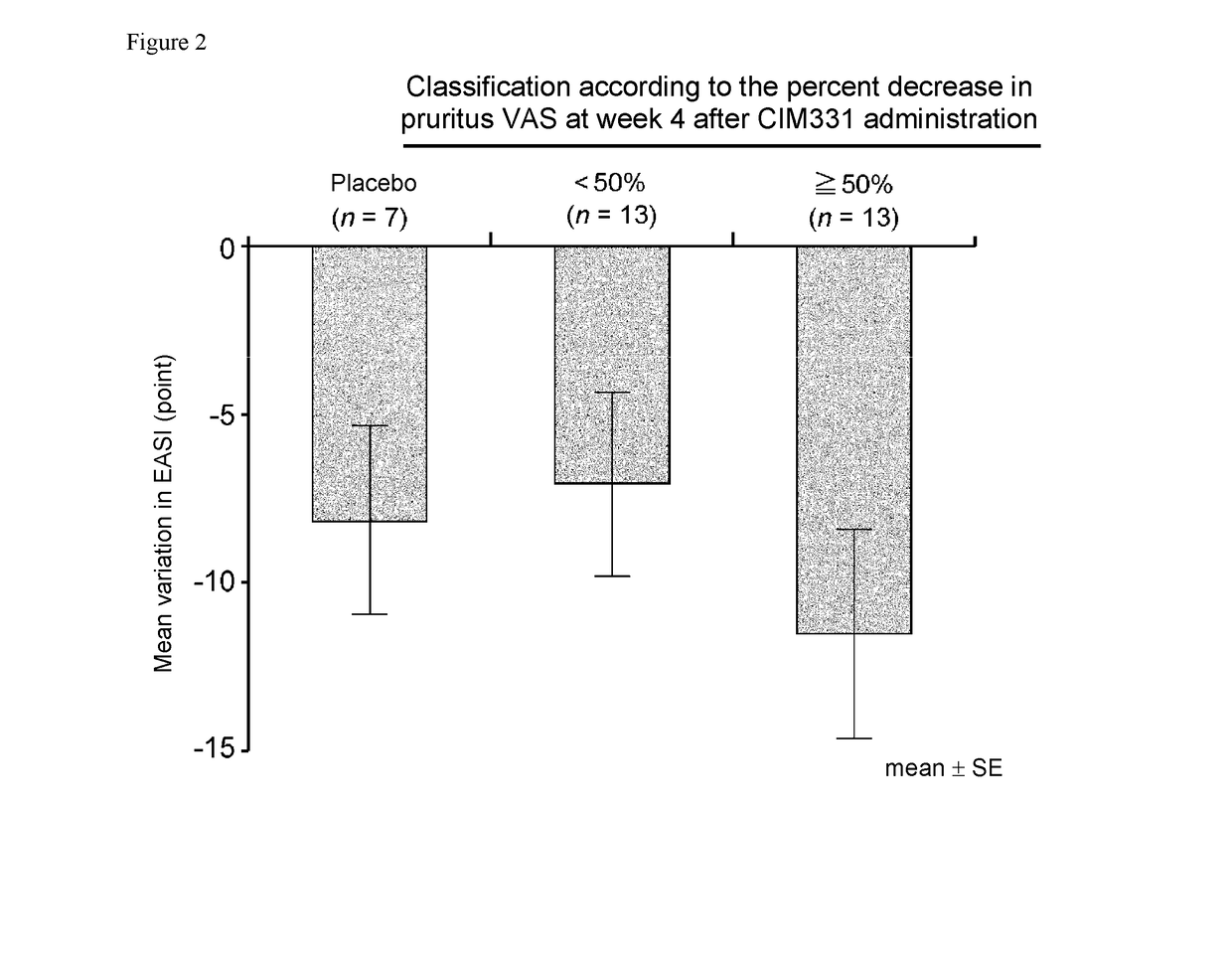
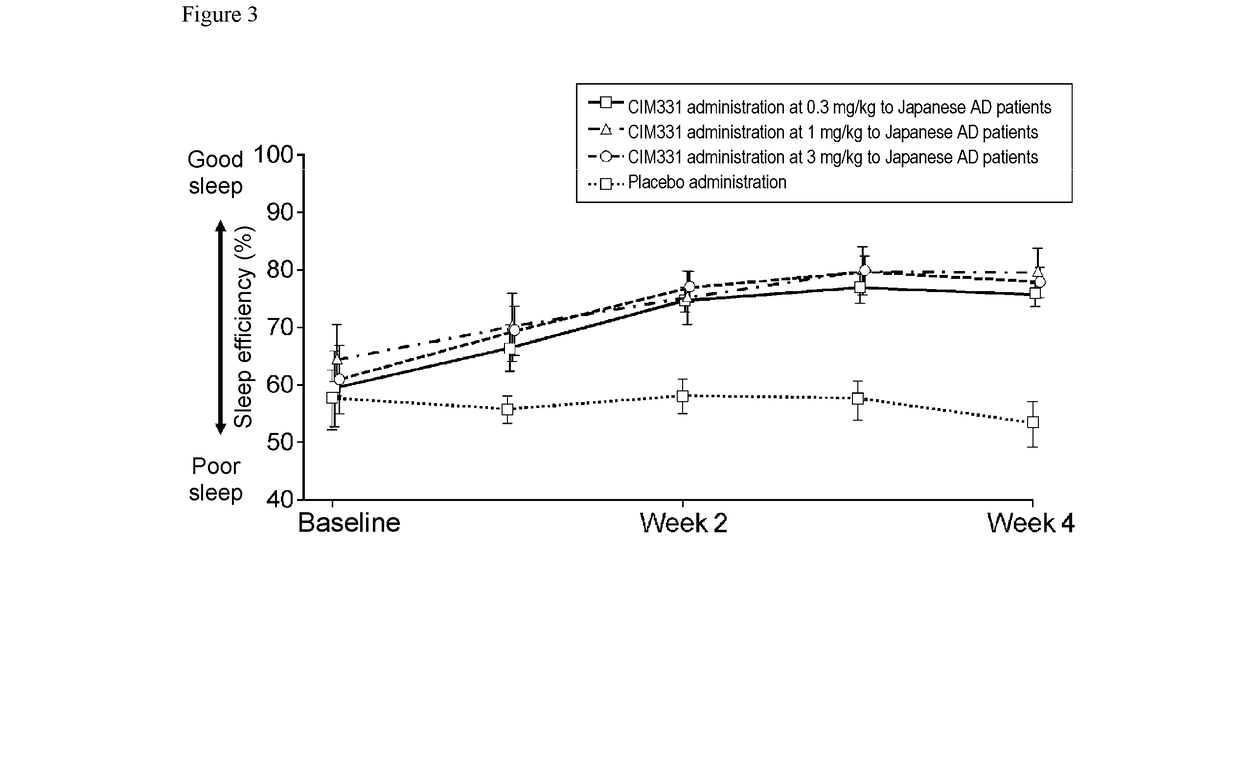
Pharmaceutical composition for prevention and/or treatment of atopic dermatitis comprising il-31 antagonist as active ingredient
ActiveUS20180079817A1Increased timeDecrease sleep onset latencyNervous disorderImmunoglobulins against cytokines/lymphokines/interferonsDose intervalAD - Atopic dermatitis
In a non-limiting embodiment, there is provided a pharmaceutical composition for prevention and / or treatment of atopic dermatitis comprising an IL-31 antagonist as an active ingredient, wherein the IL-31 antagonist is repeatedly administered in equal amounts at the same dosing interval to a subject with or potentially with atopic dermatitis, at 0.1 to 1000 mg / body / 1 day to 12 weeks, preferably at 0.1 to 1000 mg / body / 2 weeks, 0.1 to 1000 mg / body / 4 weeks, or 0.1 to 1000 mg / body / 8 weeks.
Owner:CHUGAI PHARMA CO LTD
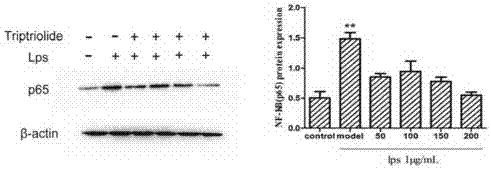
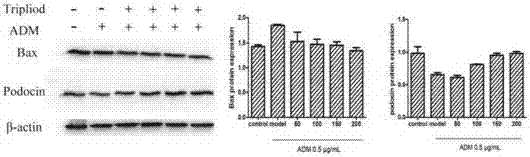
Novel low-toxicity tripterygium polyglycoside, and preparation method and applications thereof
ActiveCN106860500ALow toxicityReduce pathological damageAntipyreticAnalgesicsMedicineCurative effect
The invention discloses a novel low-toxicity tripterygium polyglycoside. The novel low-toxicity tripterygium polyglycoside is obtained via chemical processing and adding of triptolide. The invention also discloses applications of the novel low-toxicity tripterygium polyglycoside in the field of medicine. The novel low-toxicity tripterygium polyglycoside is capable of relieving nephrotic syndromes such as kidney pathological injury and proteinuria, improving body inflammation level, possesses obvious curative effect on nephrotic syndromes, is low in toxicity, and effect lasts long.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Absorbable epsilon-caprolactone copolymers
InactiveUS20020114840A1Increases rate of autocatalytic hydrolysisImprove toughnessSuture equipmentsPowder deliveryIonNitrogenous base
This invention deals with crystalline, nitrogen copolyester lubricant coating devices comprising sutures, wherein said lubricant comprises a triaxial copolyester chain with a central nitrogenous base or a copolyester with more than one carboxylic group ionically linked to a basic amino acid.
Owner:POLY MED
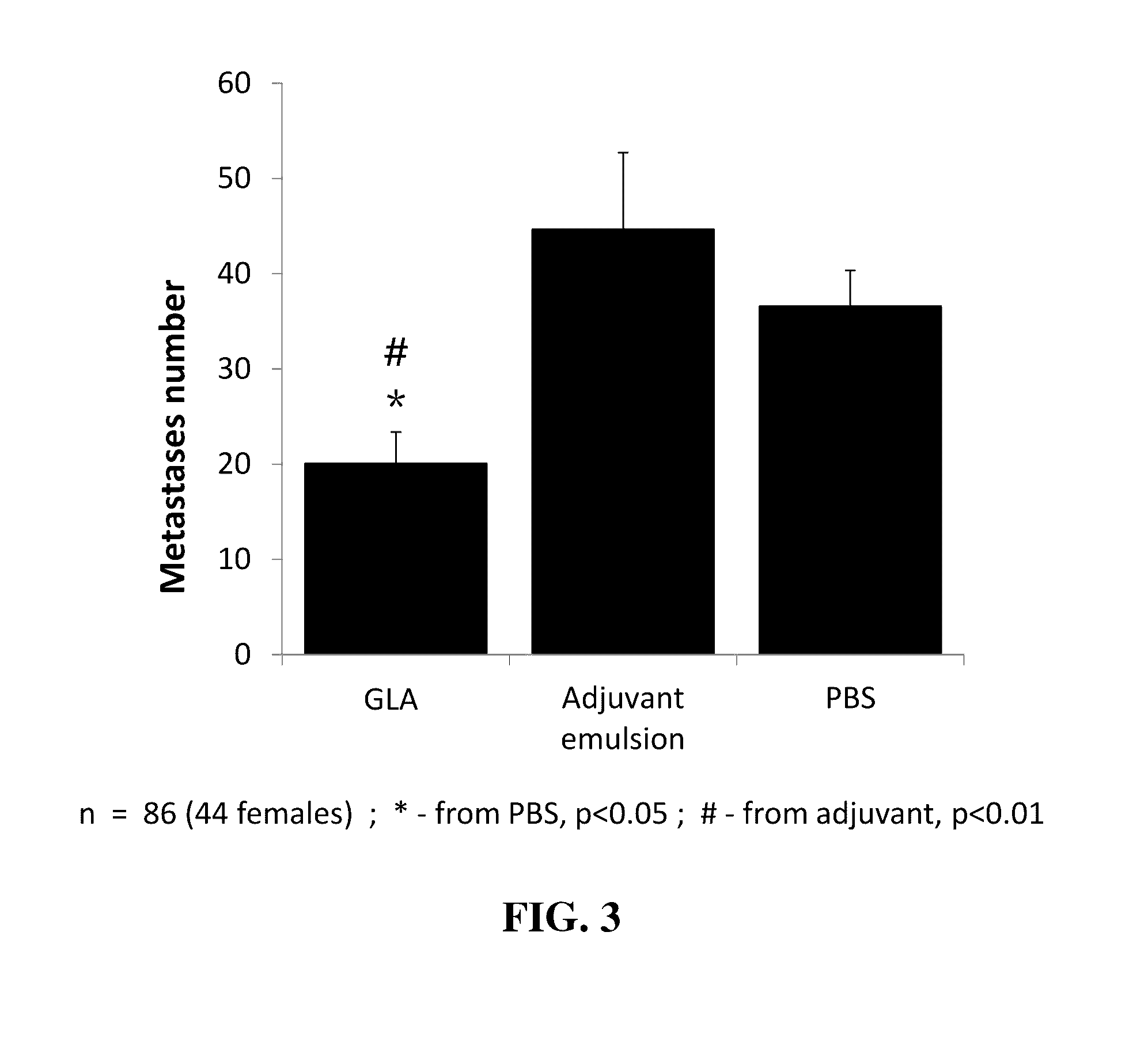
Compositions and methods for reducing or preventing metastasis
ActiveUS9463198B2Body dosingImprove treatment outcomesOrganic active ingredientsBiocideLipid formationAdjuvant
Compositions comprising glucopyranosyl lipid adjuvant (GLA) and methods for reducing or preventing the formation of cancer metastasis utilizing same are provided. The compositions may be formulated for local-regional delivery. The compositions may be substantially devoid of a cancer antigen. The treatment with GLA may be combined with treatment with a COX2 inhibitor and a beta-adrenergic blocker.
Owner:ACCESS TO ADVANCED HEALTH INST
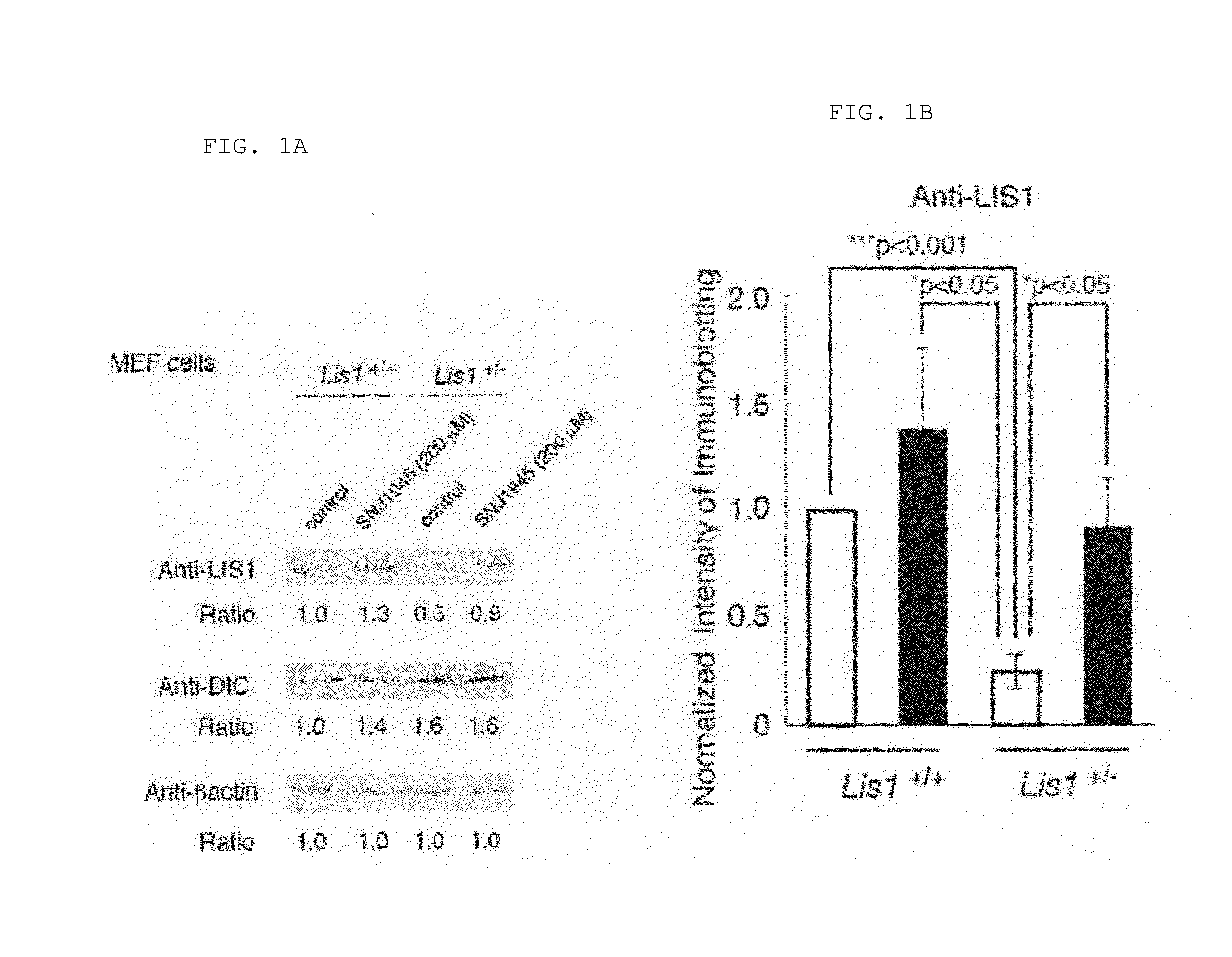
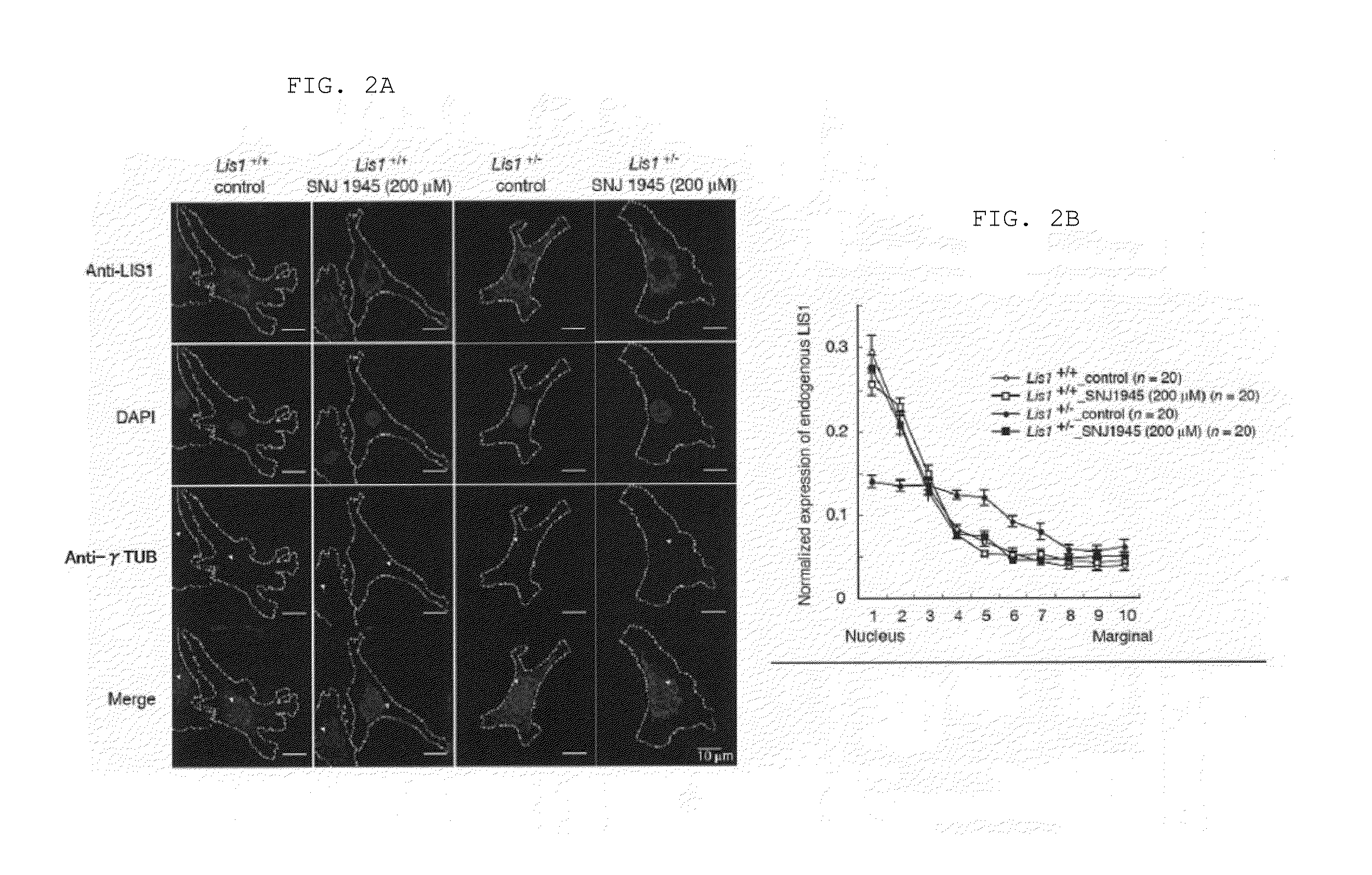
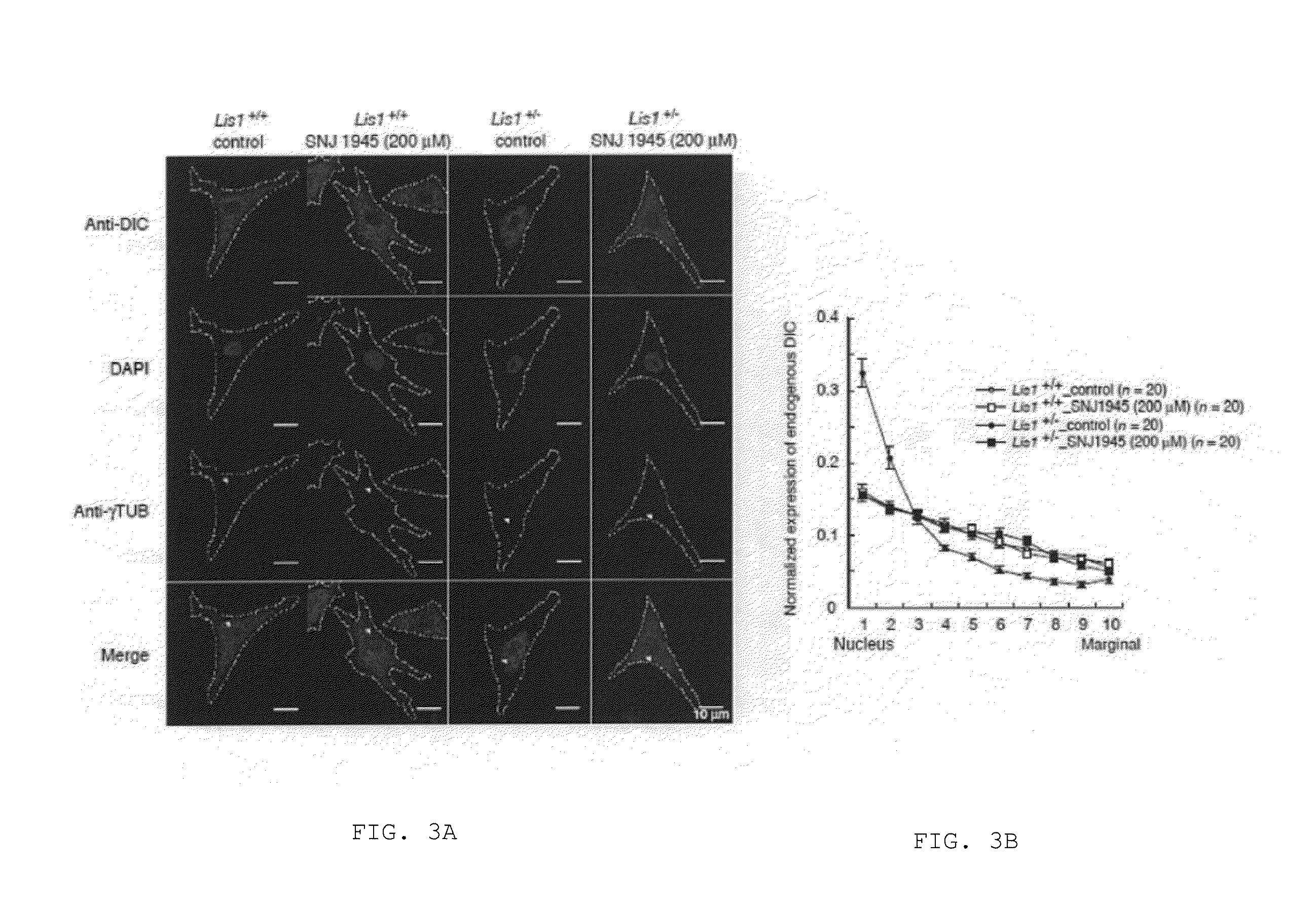
Lissencephaly therapeutic agent
ActiveUS20160250276A1Symptoms improvedAvoid developmentOrganic active ingredientsDipeptide ingredientsHalogenCompound (substance)
An object of the present invention is to provide a medicament and method for treating lissencephaly patients. The present invention provides a lissencephaly therapeutic or preventive agent comprising a compound represented by the general formula (I):wherein R1 is lower alkyl substituted with lower alkoxy, lower alkyl substituted with a heterocyclic group, a heterocyclic group, or a group represented by the formula (IIa):wherein R4 is lower alkyl, R3 is lower alkylene, and m is an integer of 1 to 6;R2 is lower alkyl optionally substituted with phenyl; andR3 is lower alkyl optionally substituted with halogen, lower alkoxy, or phenyl; condensed polycyclic hydrocarbon; or hydrogen.
Owner:OSAKA CITY UNIVERSITY
Lithographic printing plate precursor and plate making method thereof
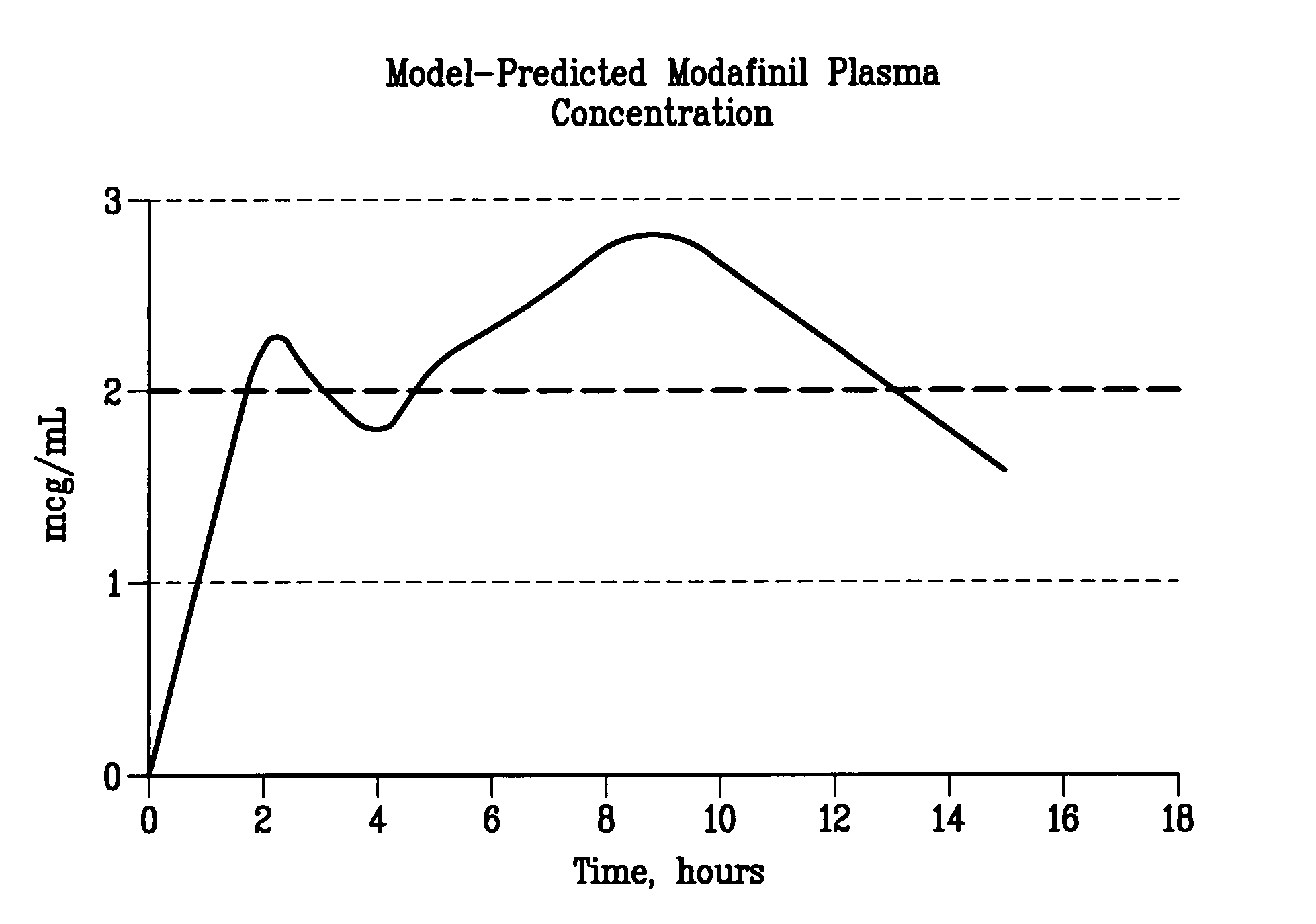
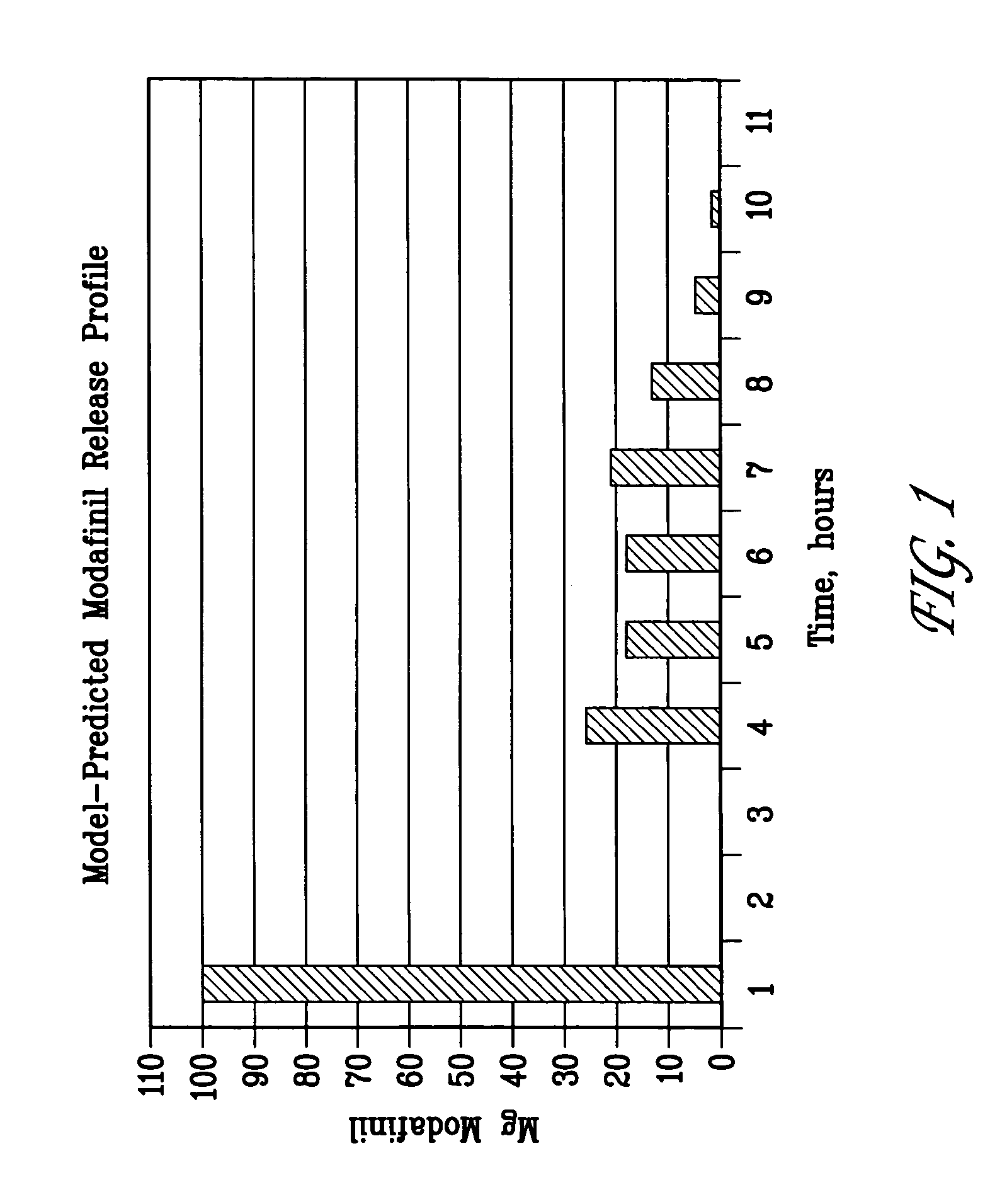
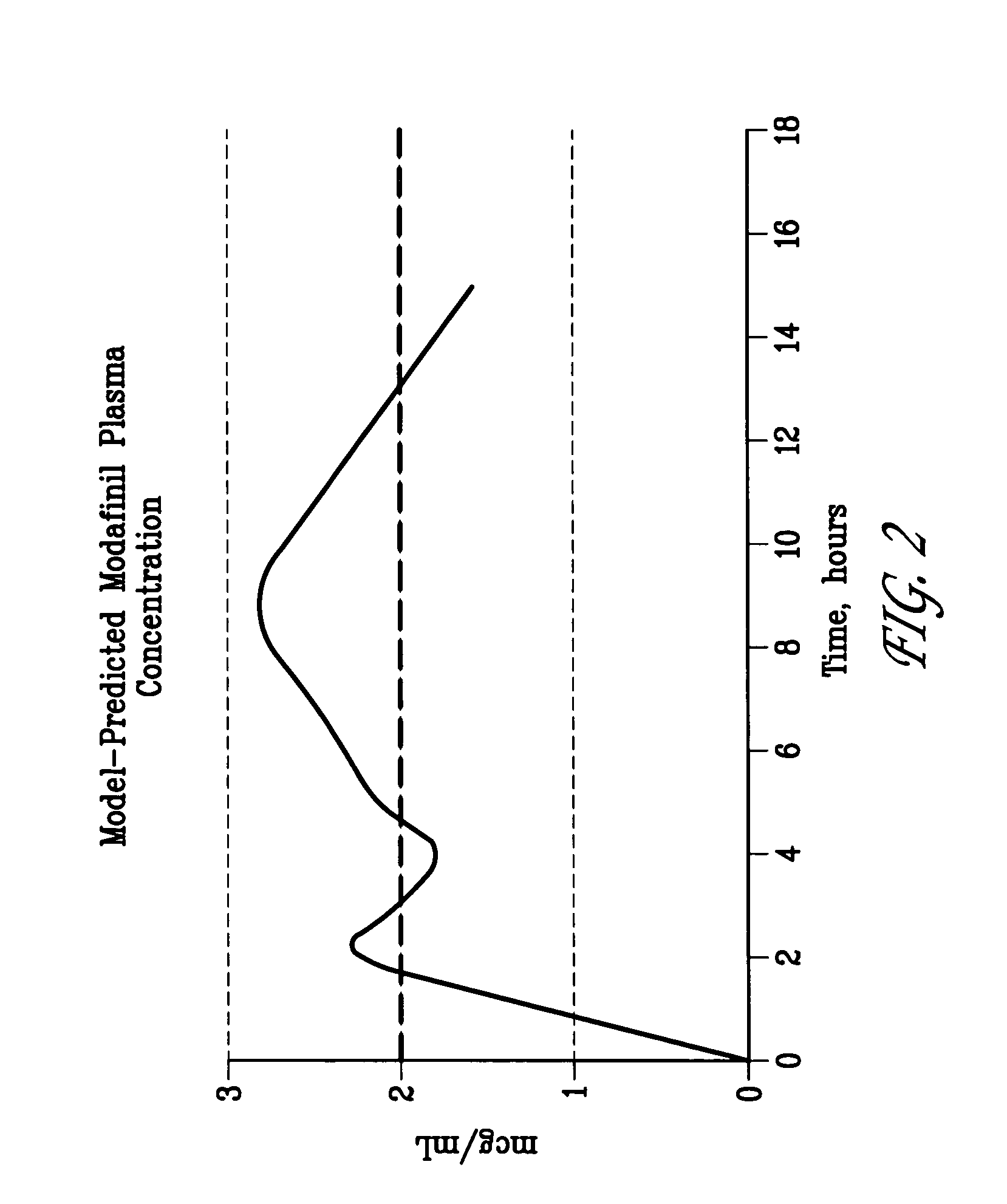
ActiveUS20100075252A1Reduce molecular weightSustained effectPhotosensitive materialsRadiation applicationsChemical compoundImage recording
A lithographic printing plate precursor includes: a support; and an image-recording layer containing (A) an infrared absorbing agent, (B) a radical polymerization initiator, (C) a polymerizable compound and (D) an epoxy compound having a molecular weight of 1,000 or less.
Owner:FUJIFILM CORP
Compositions and methods for treating myocardial infarction
InactiveUS20090105148A1Increasing cell proliferationReduce apoptosisPeptide/protein ingredientsVertebrate cellsDiseaseMuscle tissue
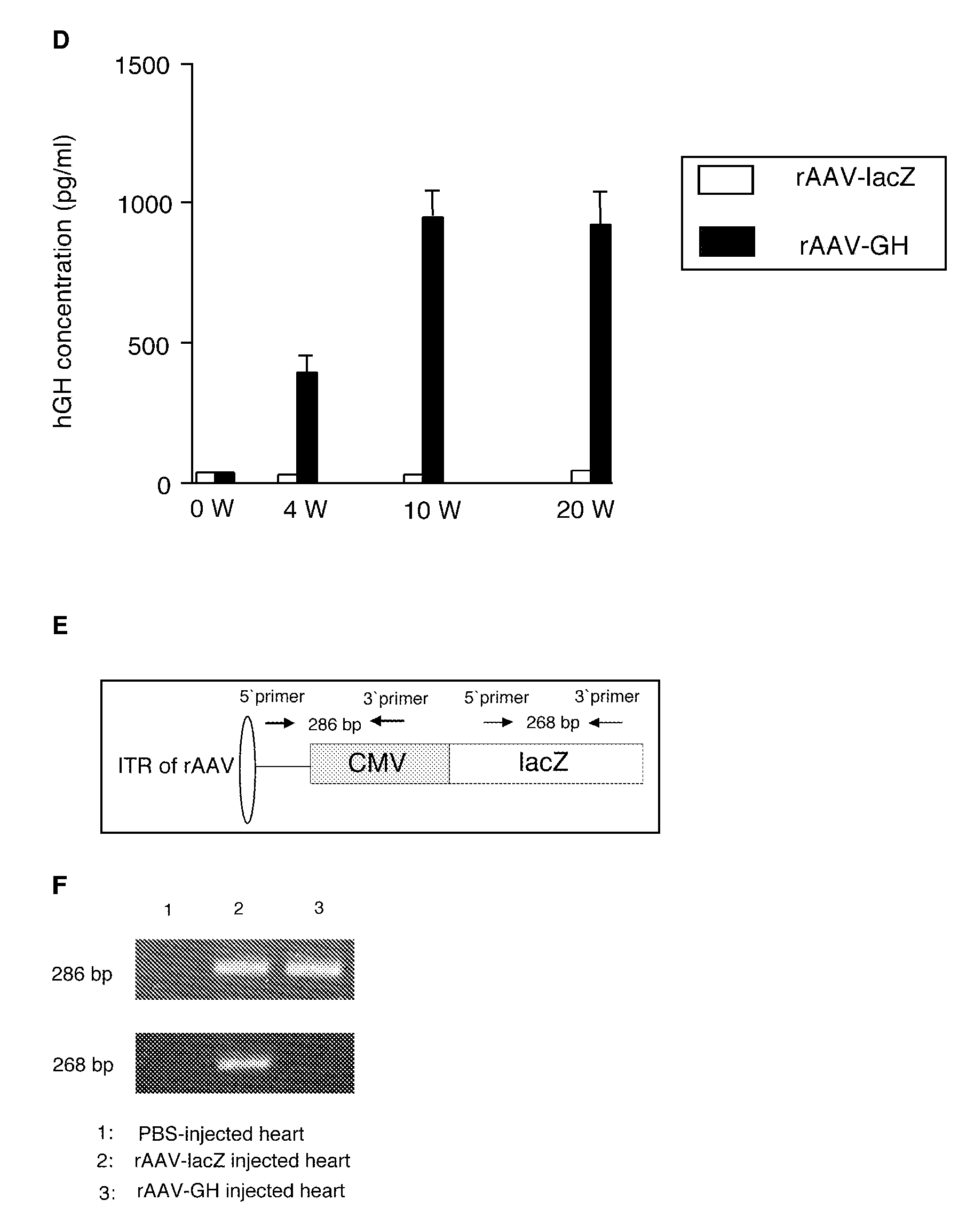
The invention features compositions and methods that are useful for preventing or treating a cardiac disease or for promoting cardiac health following a myocardial infarction. The invention further features compositions and methods for promoting angiogenesis, cell proliferation, and / or decreasing apoptosis in muscle tissue, such as cardiac tissue. The invention provides for the expression of human growth hormone in cardiac muscle following a myocardial infarction.
Owner:STEWARD RES & SPECIALTY PROJECTS
Microprotein-inactivating ultrafine metal particles
ActiveUS8106228B2Efficient inactivationSustained effectBiocidePowder deliveryOrganic acidMetal particle
Microprotein-inactivating ultrafine metal particles comprising ultrafine metal particles having a bond between an organic acid component and a metal, having an infrared absorption peak ascribable to the bond between the organic acid and the metal near 1518 cm−1, and capable of efficiently inactivating microproteins without impairing properties of the resin or formability thereof. When used in the form of a resin composition or a coating agent, the microprotein-inactivating ultrafine metal particles are capable of exhibiting an effect for inactivating microproteins.
Owner:TOYO SEIKAN KAISHA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com