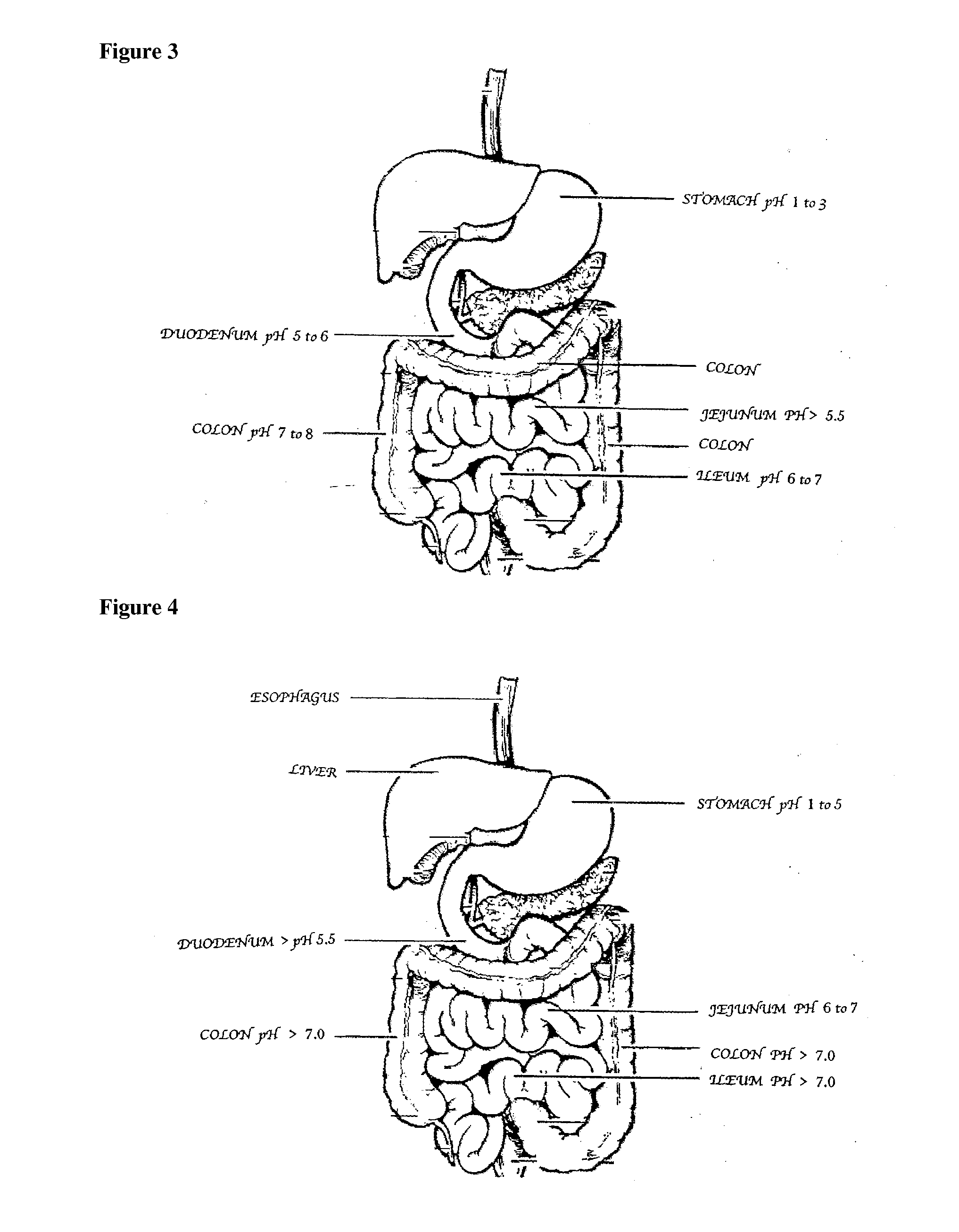
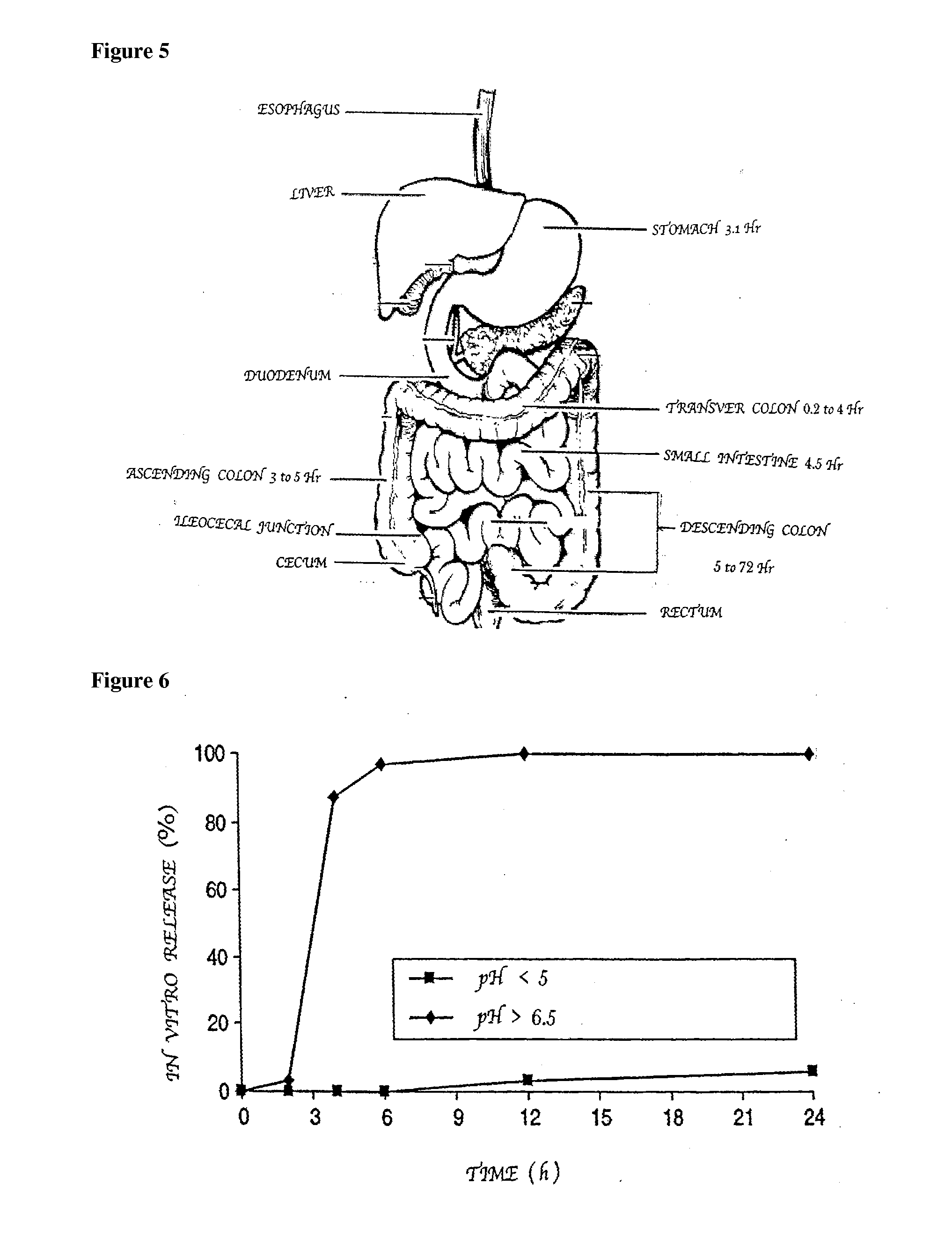
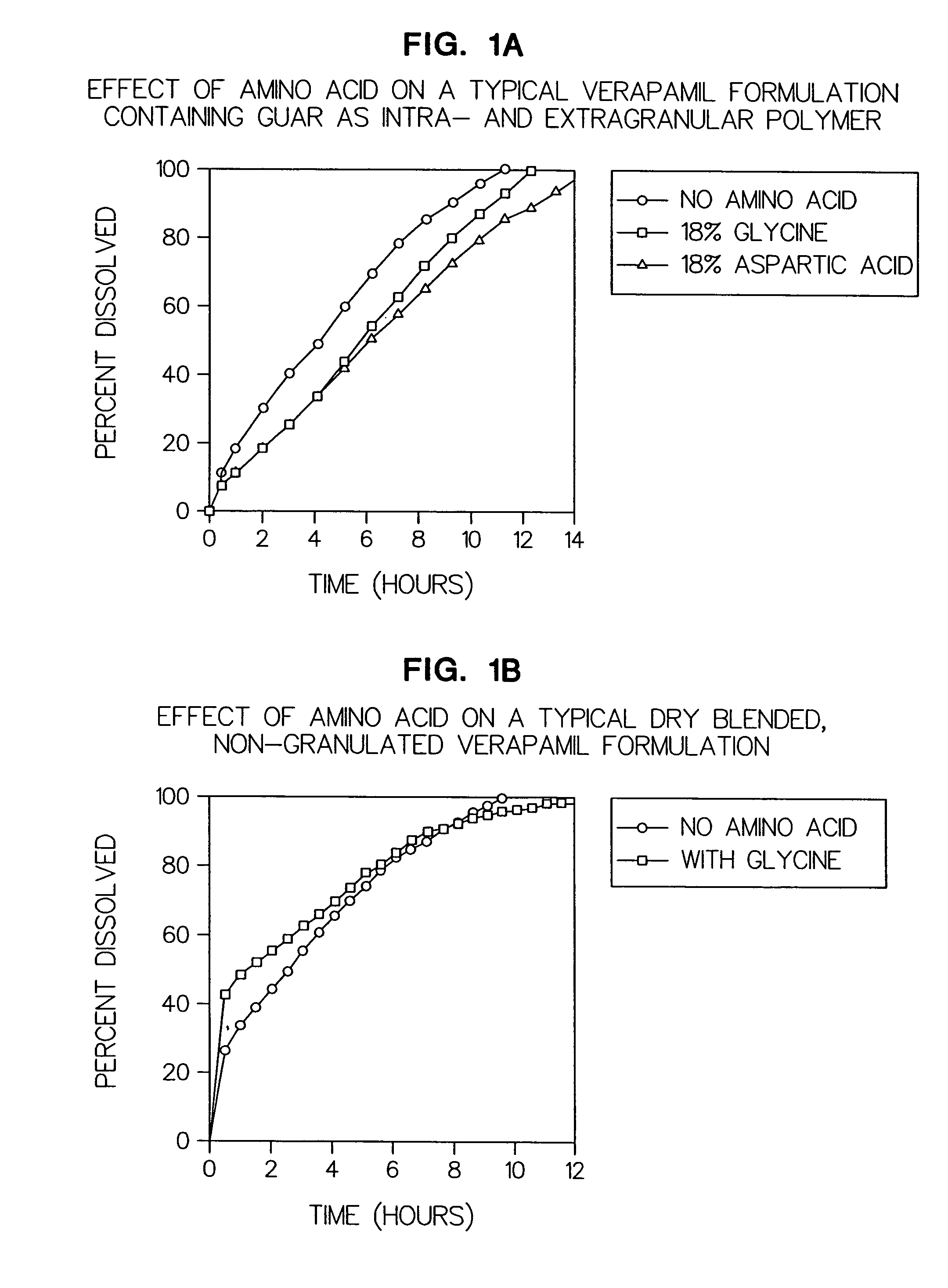
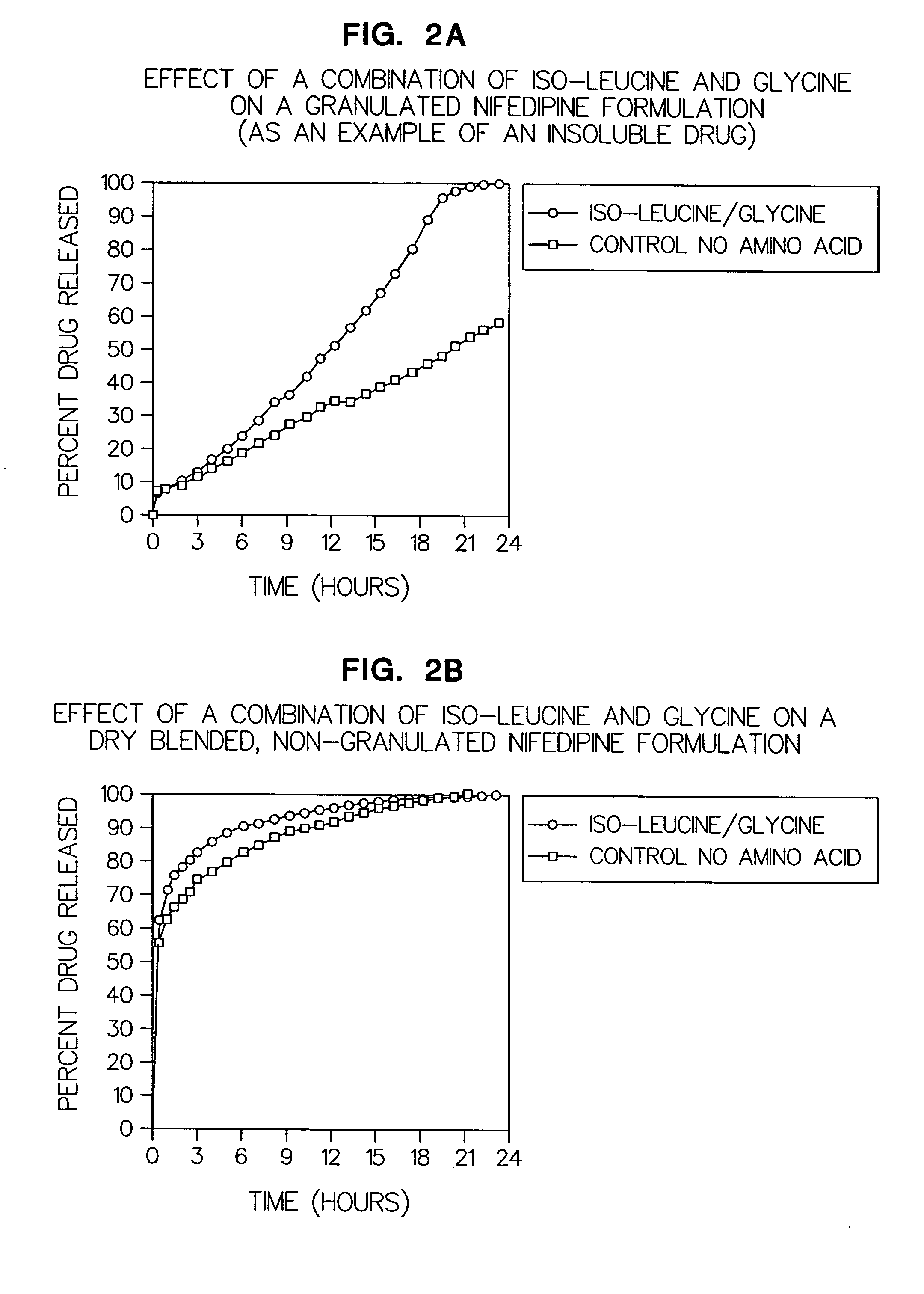
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Extended Release Dosage Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
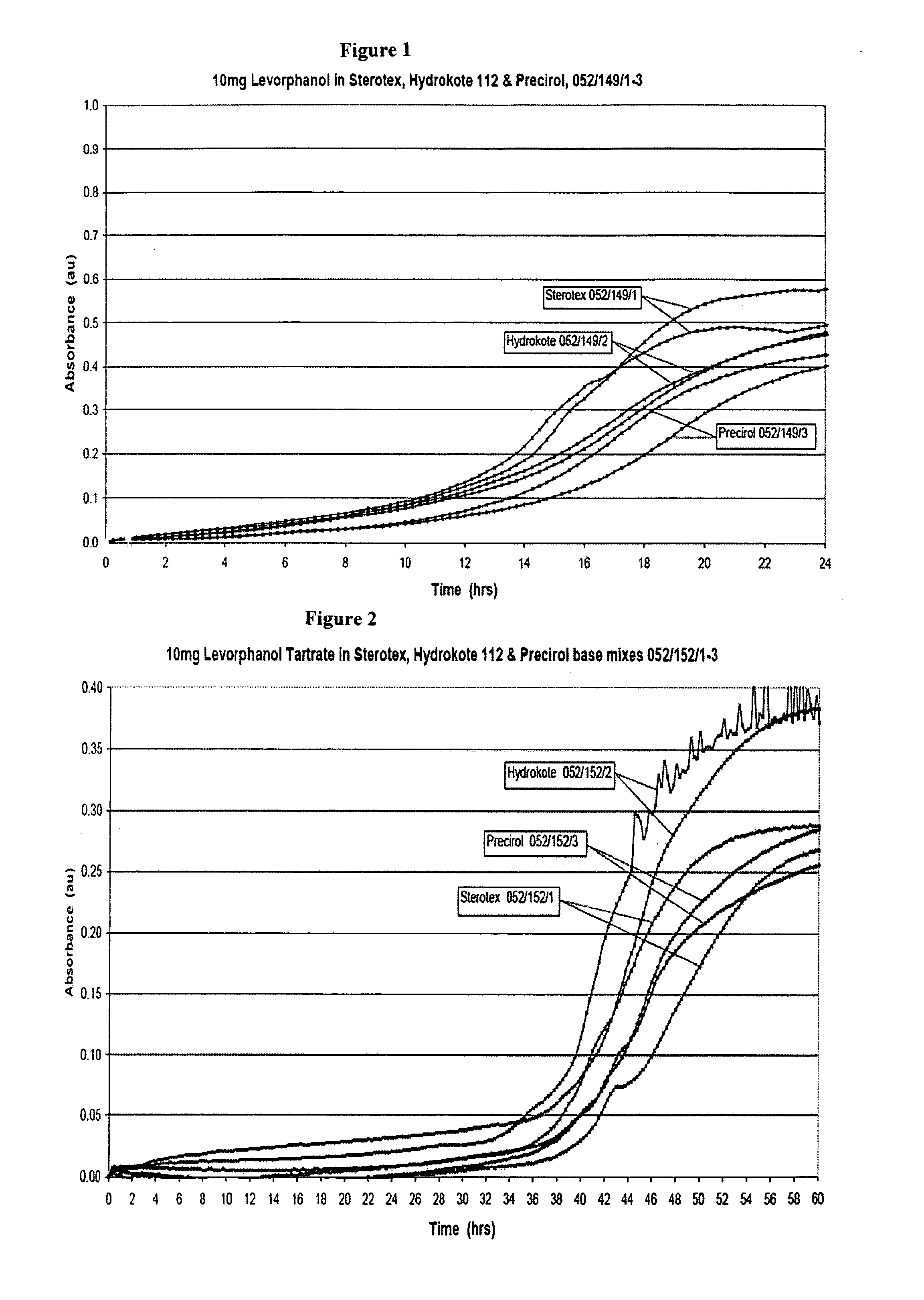
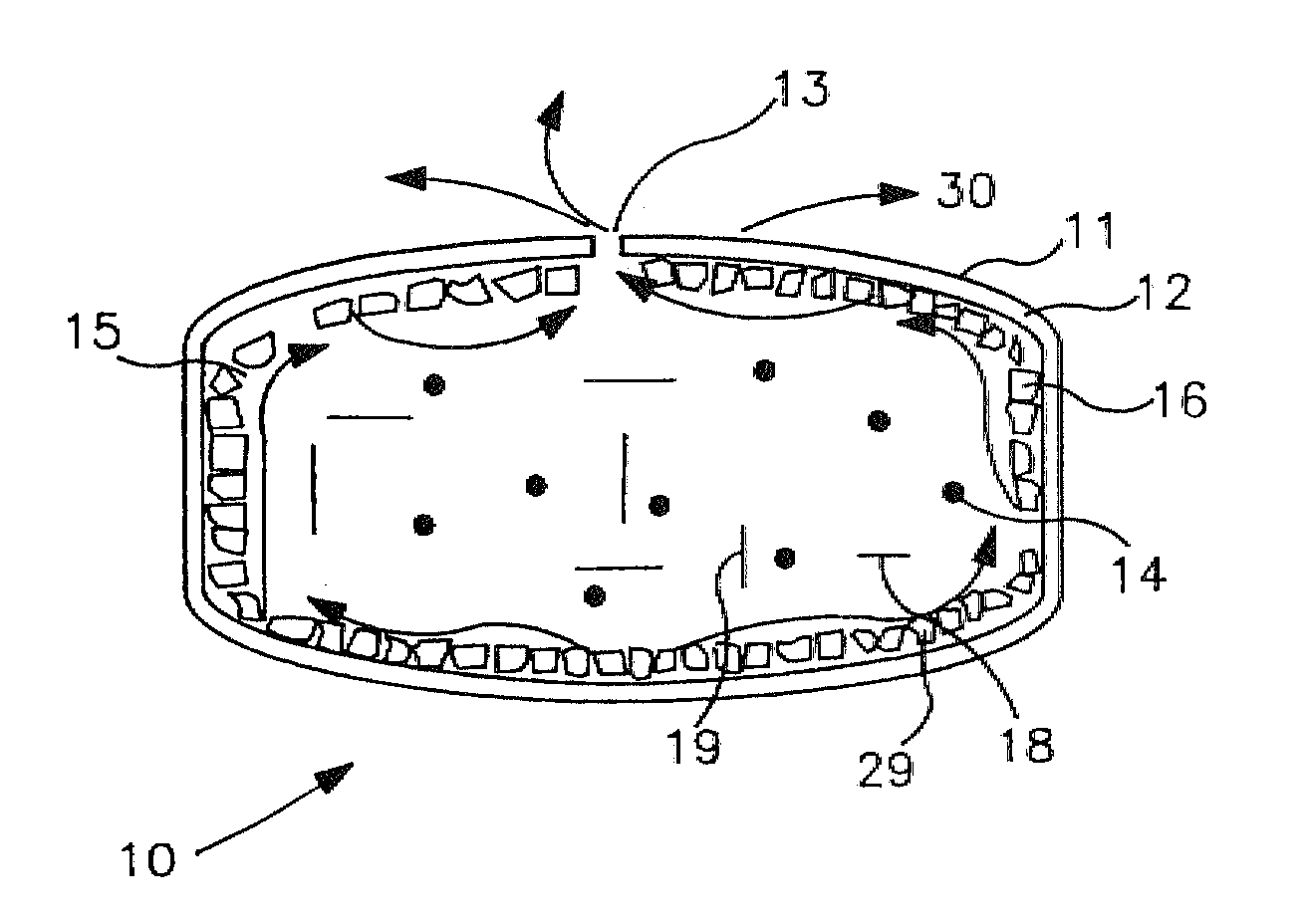
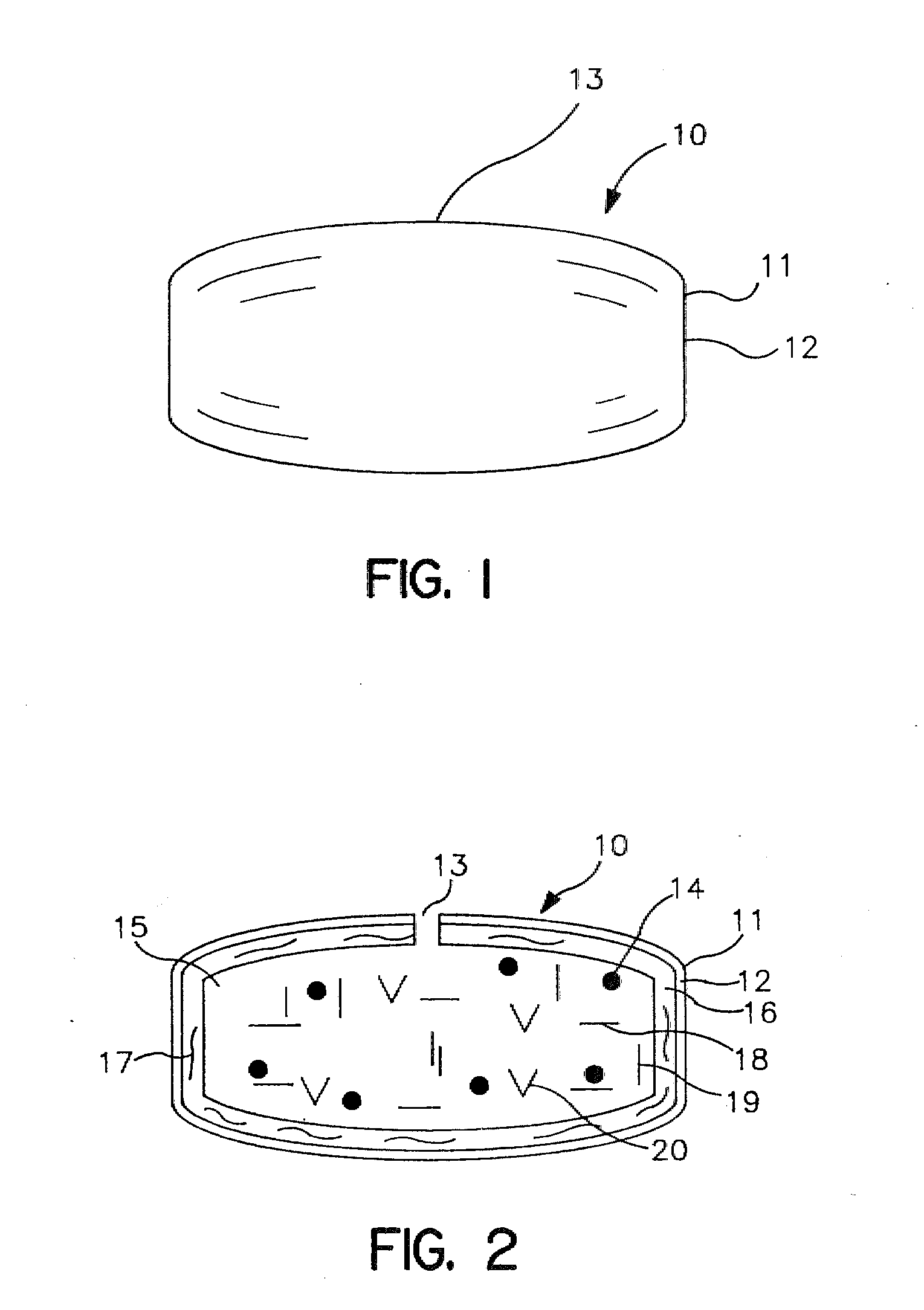
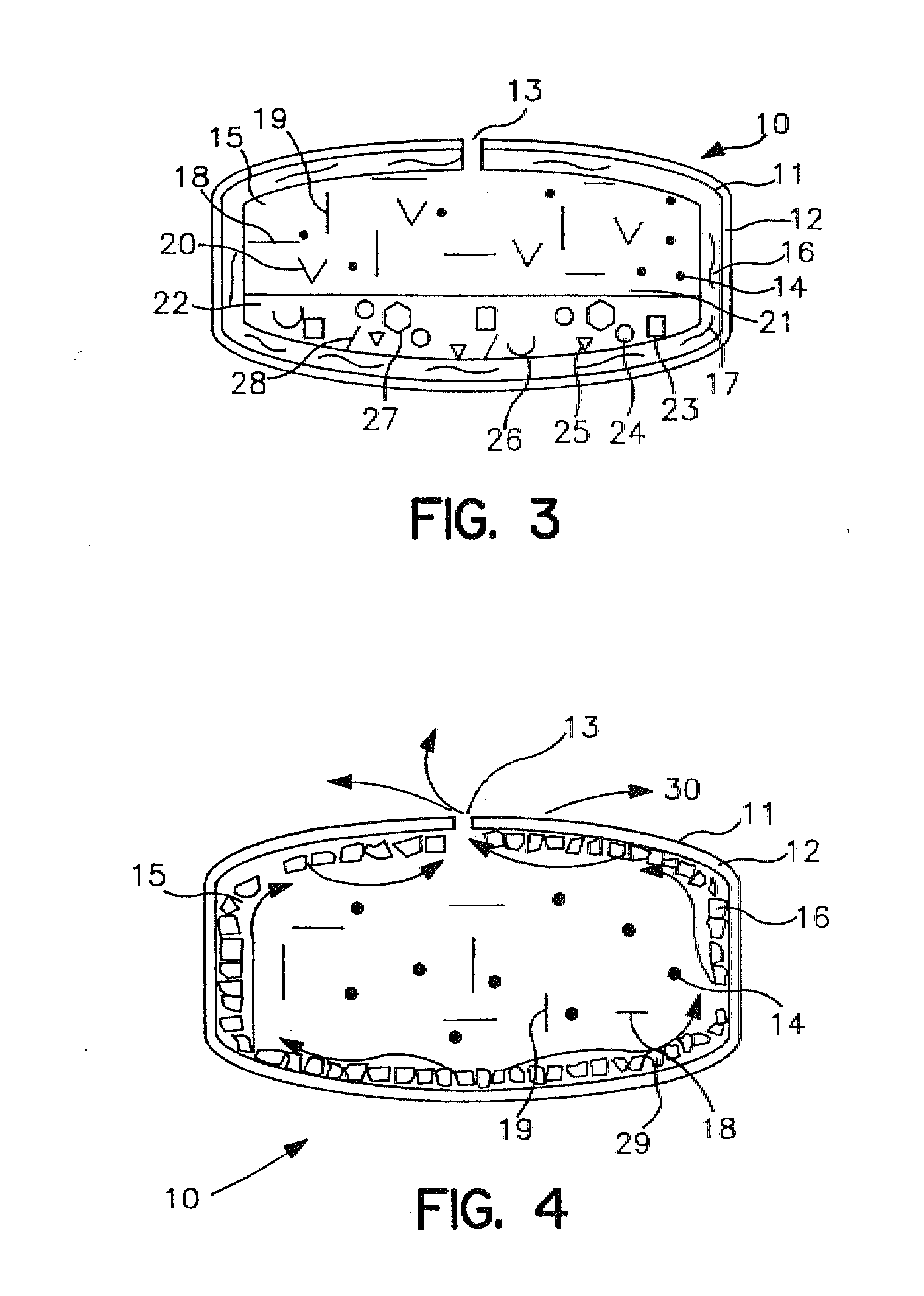
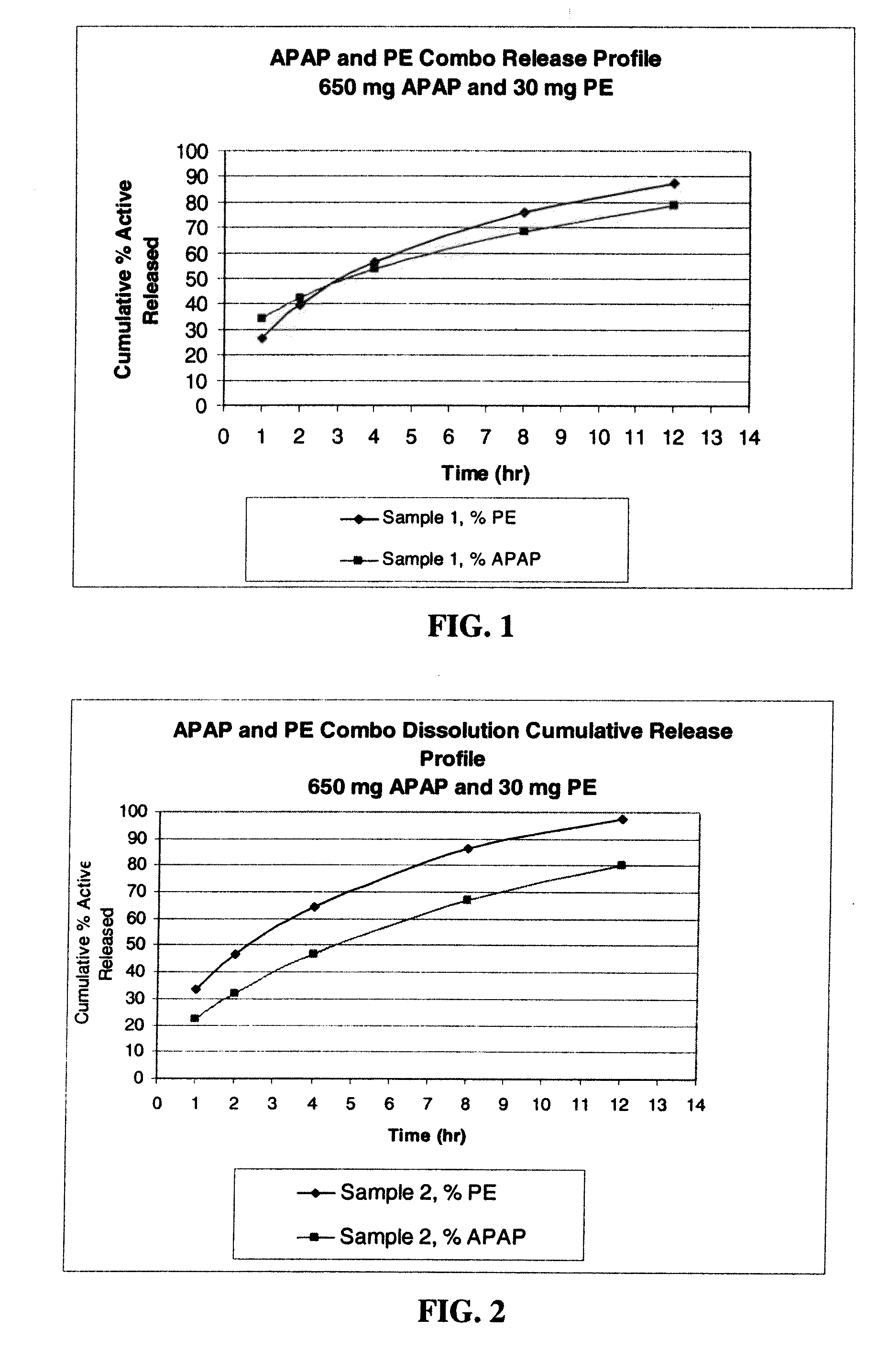
Extended release dosage form
InactiveUS6245357B1Free to changeOrganic active ingredientsNervous disorderExtended Release Dosage Form
A dosage form comprising a composition comprising a drug surrounded by an interior and an exterior wall with an exit for administering the drug to a patient; and a method of using the dosage form are disclosed for an indicated therapy.
Owner:ALZA CORP
Extended Release Oral Pharmaceutical Compositions of 3-Hydroxy-N-Methylmorphinan and Method of Use
InactiveUS20120065221A1Increased CmaxSufficient bioavailabilityBiocideNervous disorder3-Hydroxy-N-methylmorphinanExtended Release Dosage Form
The present invention is directed to oral, therapeutically effective extended release pharmaceutical compositions of 3-hydroxy-N-methylmorphinan, including delayed onset, extended release dosage forms and the use thereof.
Owner:RELMADA THERAPEUTICS
Oral pharmaceutical extended release dosage form
An enteric coated pharmaceutical extended release dosage form of a H+, K+-ATPase inhibitor giving an extended plasma concentration profile of a H+, K+-ATPase inhibitor. The extended plasma profile is obtained by a pharmaceutical composition which comprises a core material of a hydrophilic or hydrophobic matrix, and the H+, K+-ATPase inhibitor and optionally pharmaceutically acceptable excipients. The dosage form may be administered once daily.
Owner:ASTRAZENECA AB
Extended Release Dosage Form
InactiveUS20070128279A1Satisfies needEnhances fluid fluxBiocideAntipyreticOsmotic pumpBiomedical engineering
A membrane system comprising an interior wall, a fluid-permeable exterior wall surrounding the interior wall and an internal compartment defined by the membrane system, wherein fluid permeability of the interior wall is responsive to osmolarity of an osmotic core within the internal compartment are disclosed. A controlled release dosage form comprising the membrane system and a process for delivering an osmotically active formulation from an osmotic pump over an extended period of time are also disclosed.
Owner:ALZA CORP
Methods and compositions for the treatment of viral infections
Compositions for treating flu comprise an M2 inhibitor, and optionally a neuraminidase inhibitor, wherein at least one of said M2 inhibitor or said neuraminidase inhibitor is provided in an extended release dosage form.
Owner:NEUROMOLECULAR PHARMA
Extended release dosage form
InactiveUS20110229533A1Enhances fluid fluxImprove permeabilityBiocideSalicyclic acid active ingredientsOsmotic pumpBiomedical engineering
A membrane system comprising an interior wall, a fluid-permeable exterior wall surrounding the interior wall and an internal compartment defined by the membrane system, wherein fluid permeability of the interior wall is responsive to osmolarity of an osmotic core within the internal compartment are disclosed. A controlled release dosage form comprising the membrane system and a process for delivering an osmotically active formulation from an osmotic pump over an extended period of time are also disclosed.
Owner:ALZA CORP
Gastric Retentive Extended-Release Dosage Forms Comprising Combinations of a Non-Opioid Analgesic and an Opioid Analgesic
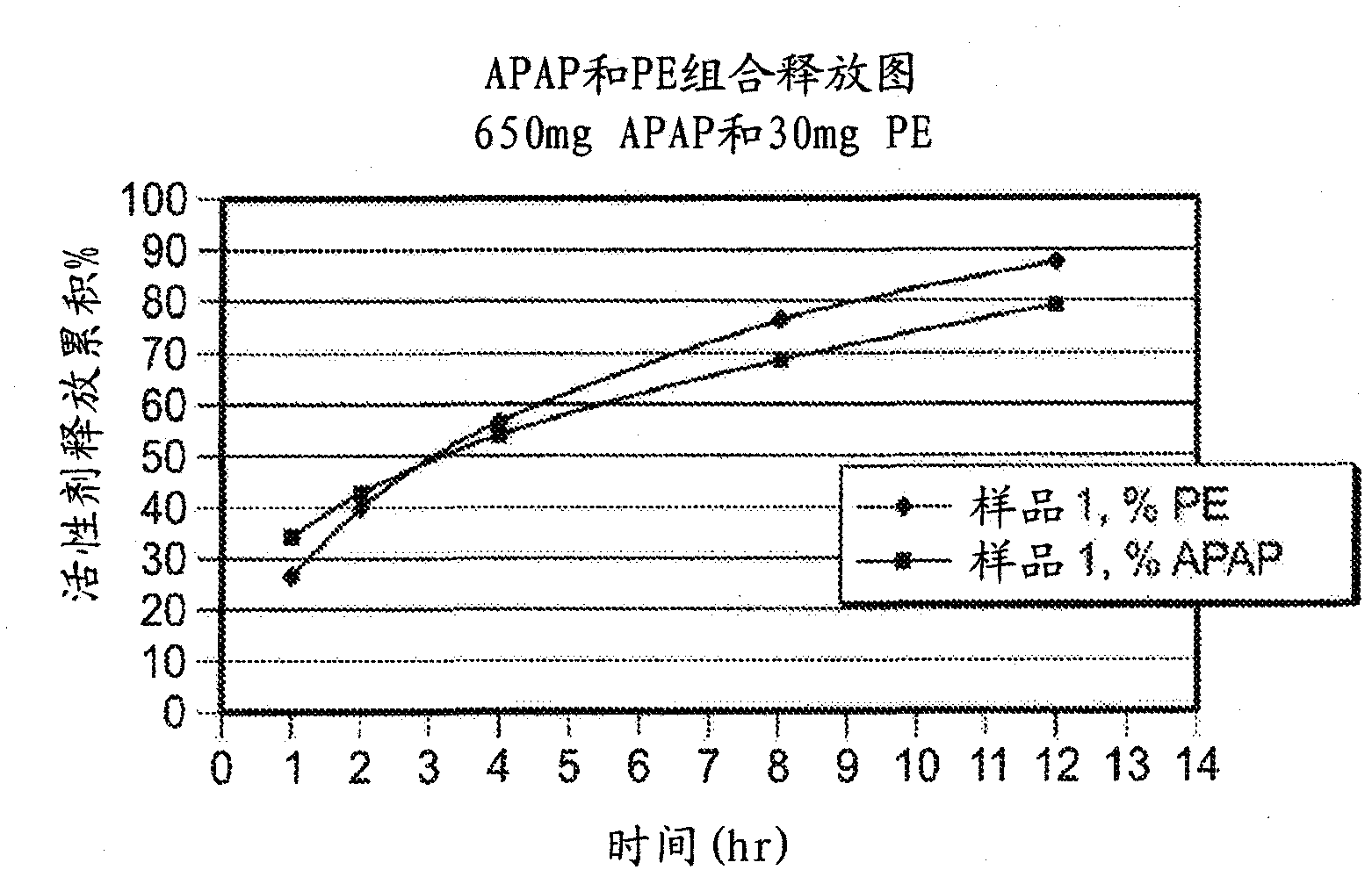
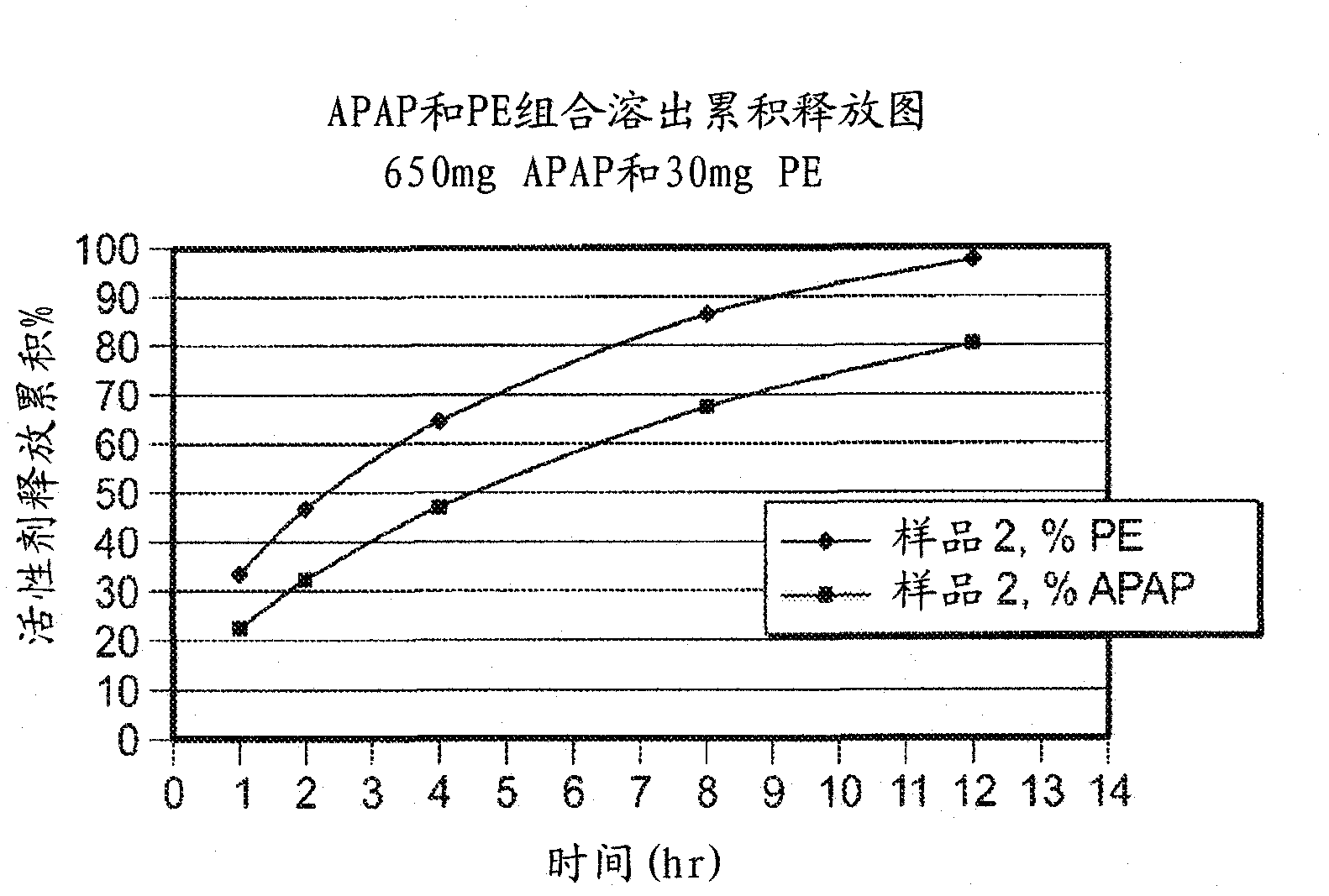
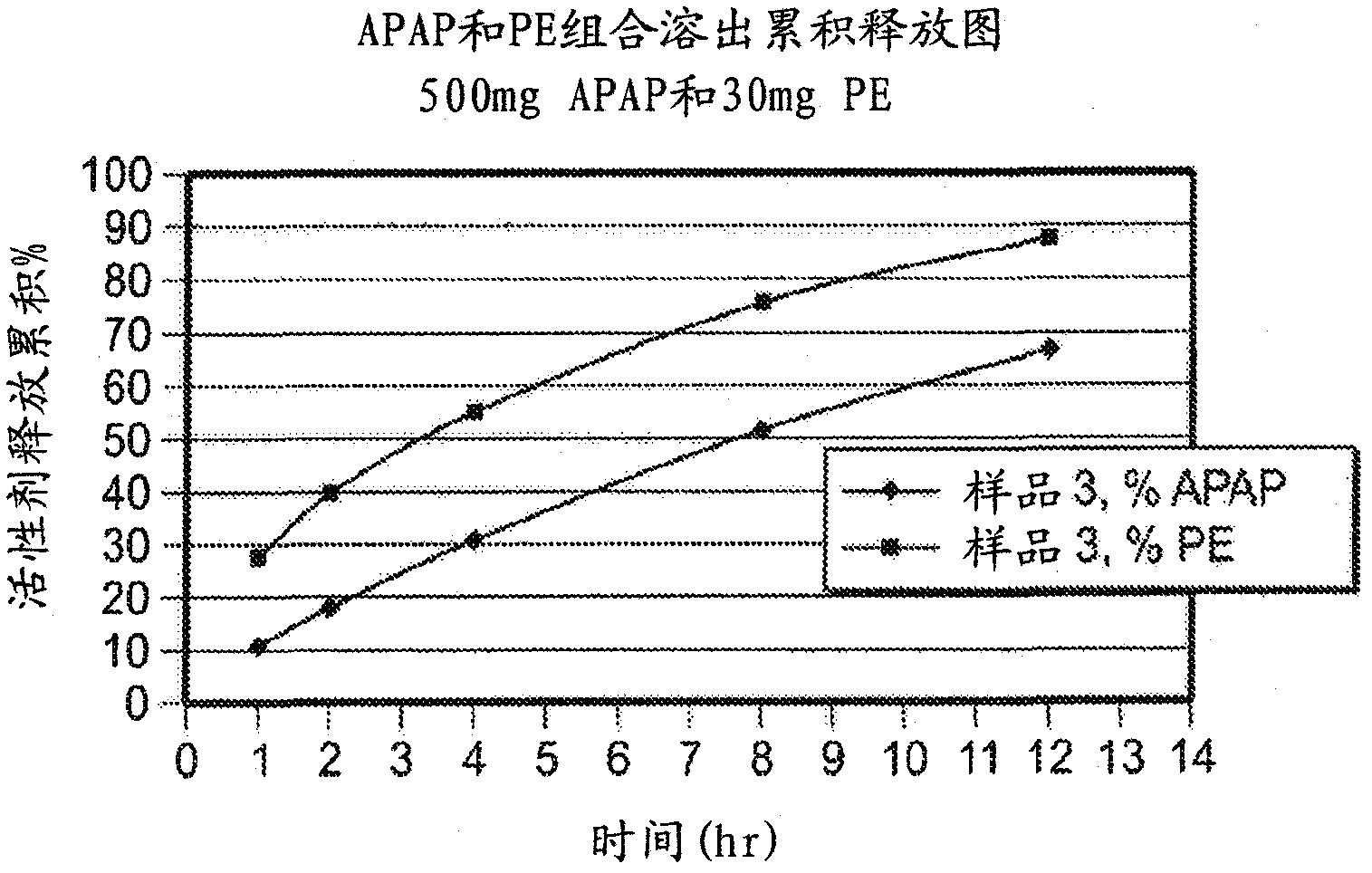
Compositions and methods for the treatment of pain in a mammal are described. More specifically, a dosage form designed for release of acetaminophen and an opioid is described, wherein the dosage form provides delivery of the drugs to the upper gastrointestinal tract (“GI”) of a mammal for an extended period of time.
Owner:DEPOMED SYST INC
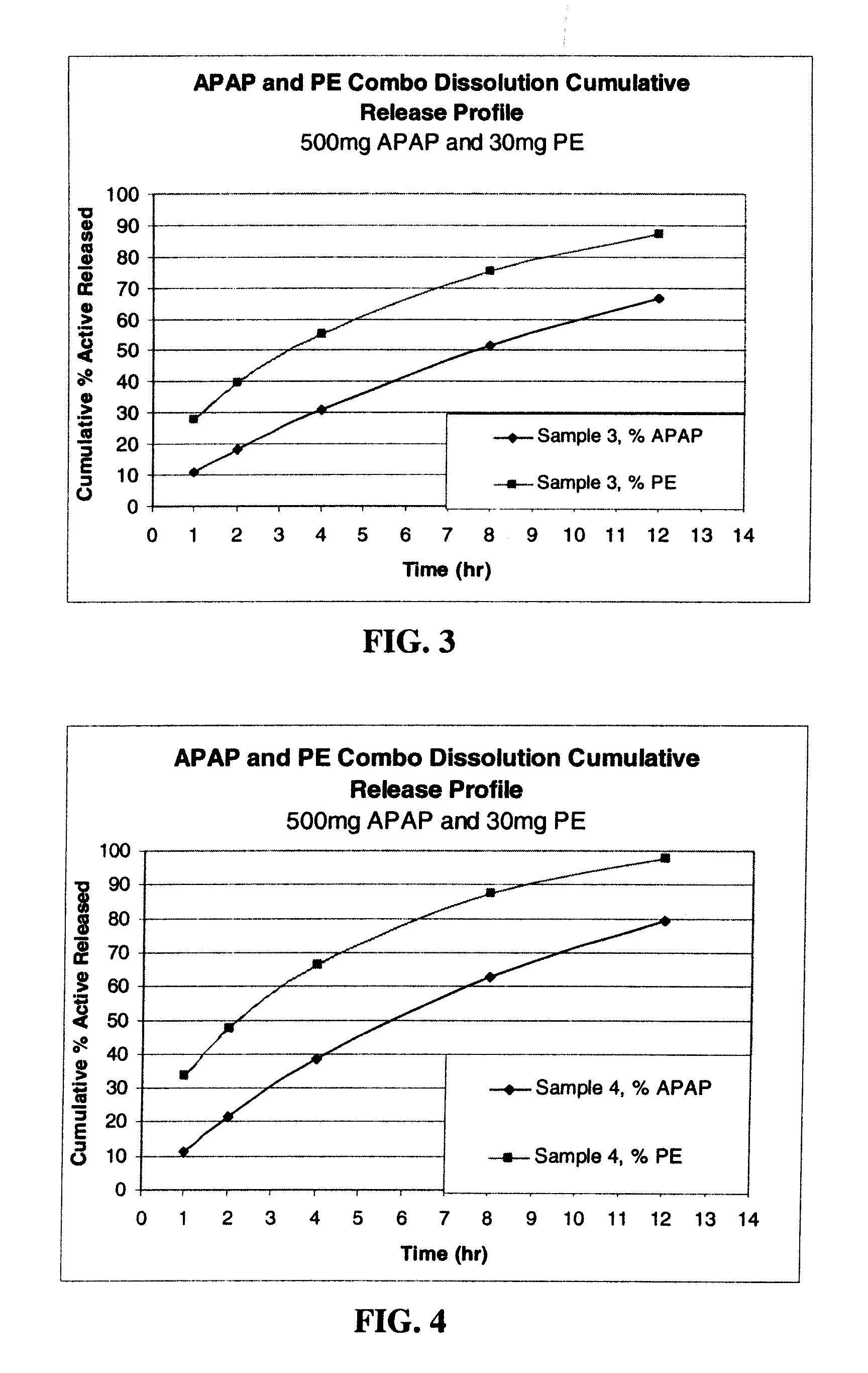
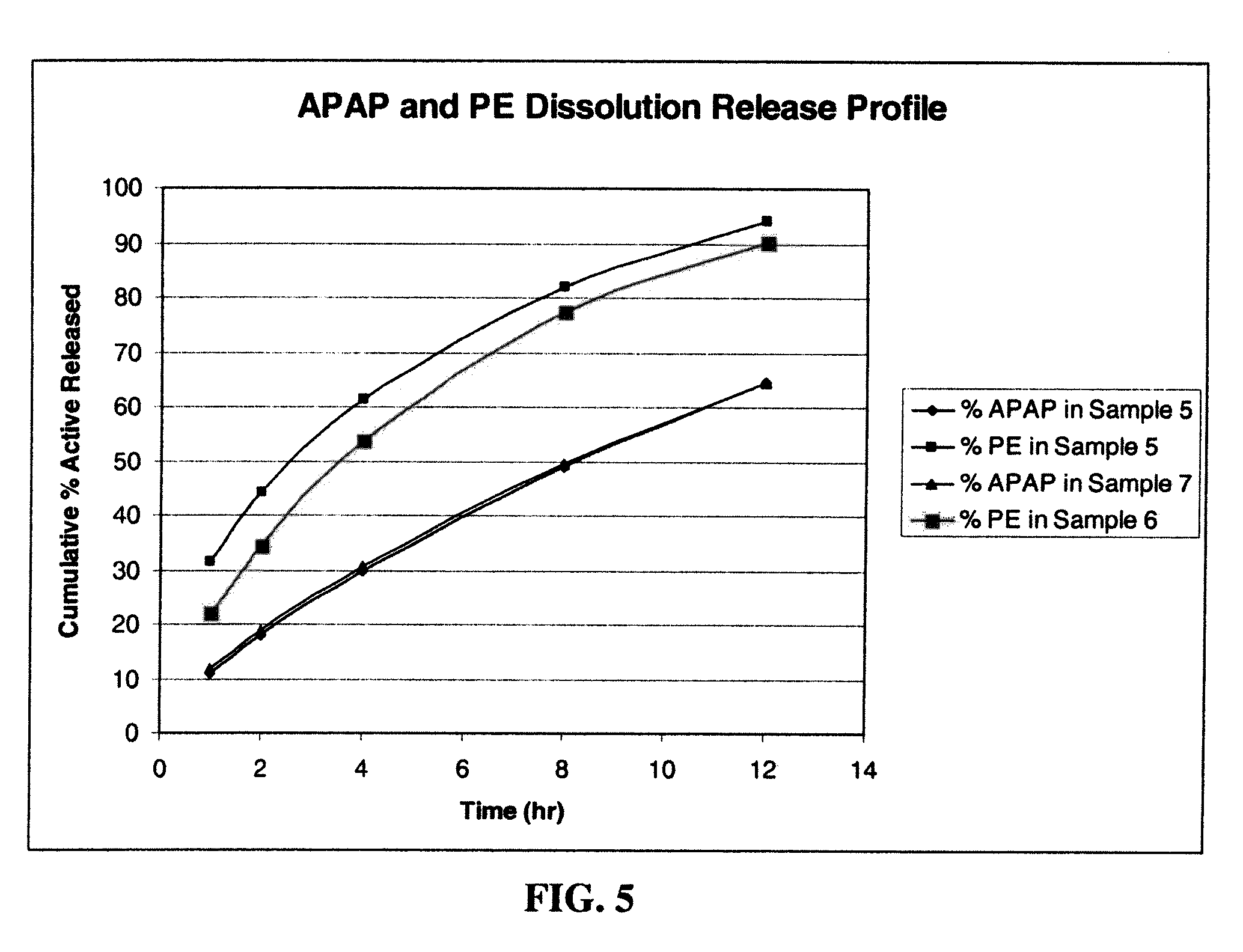
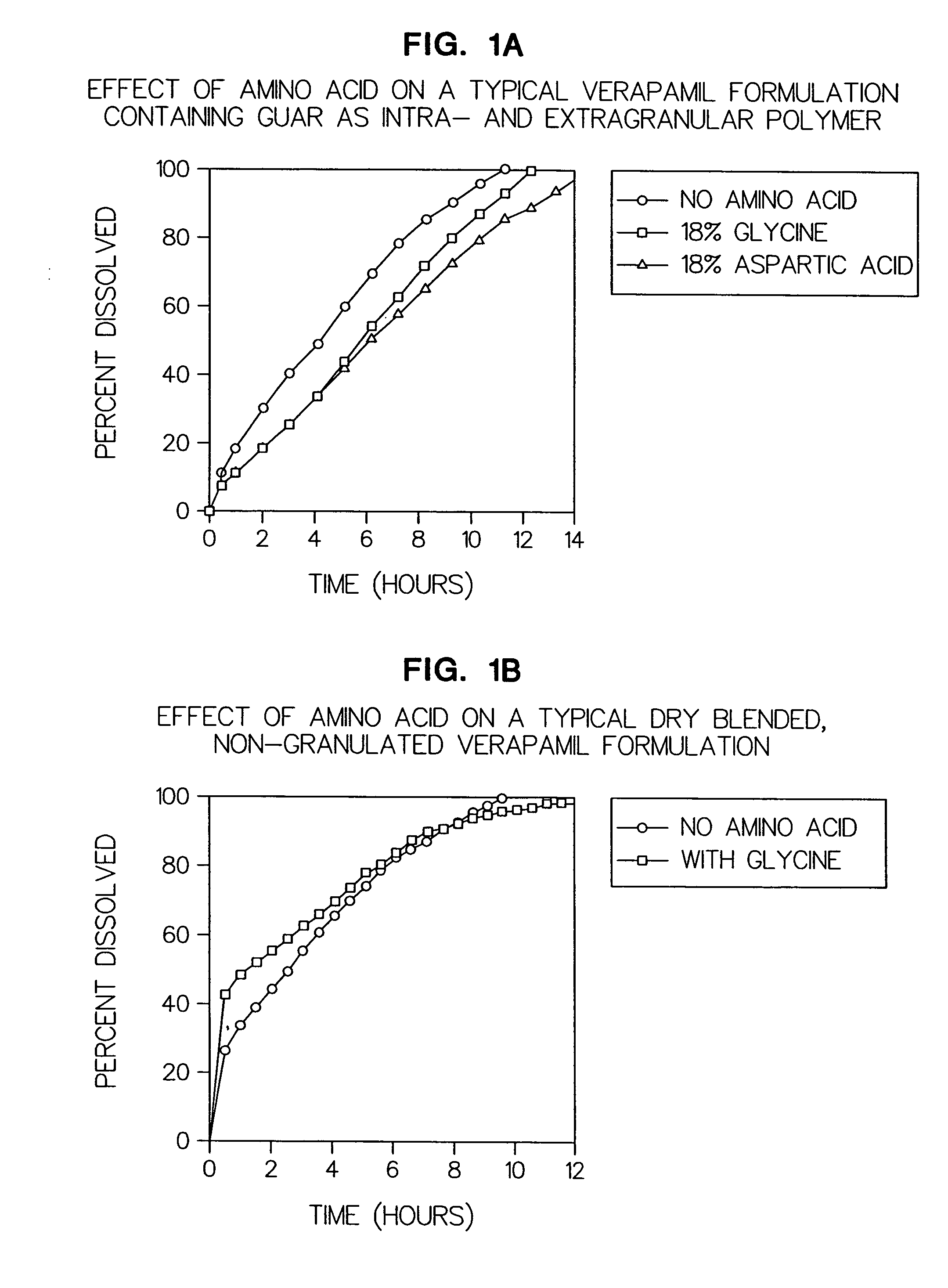
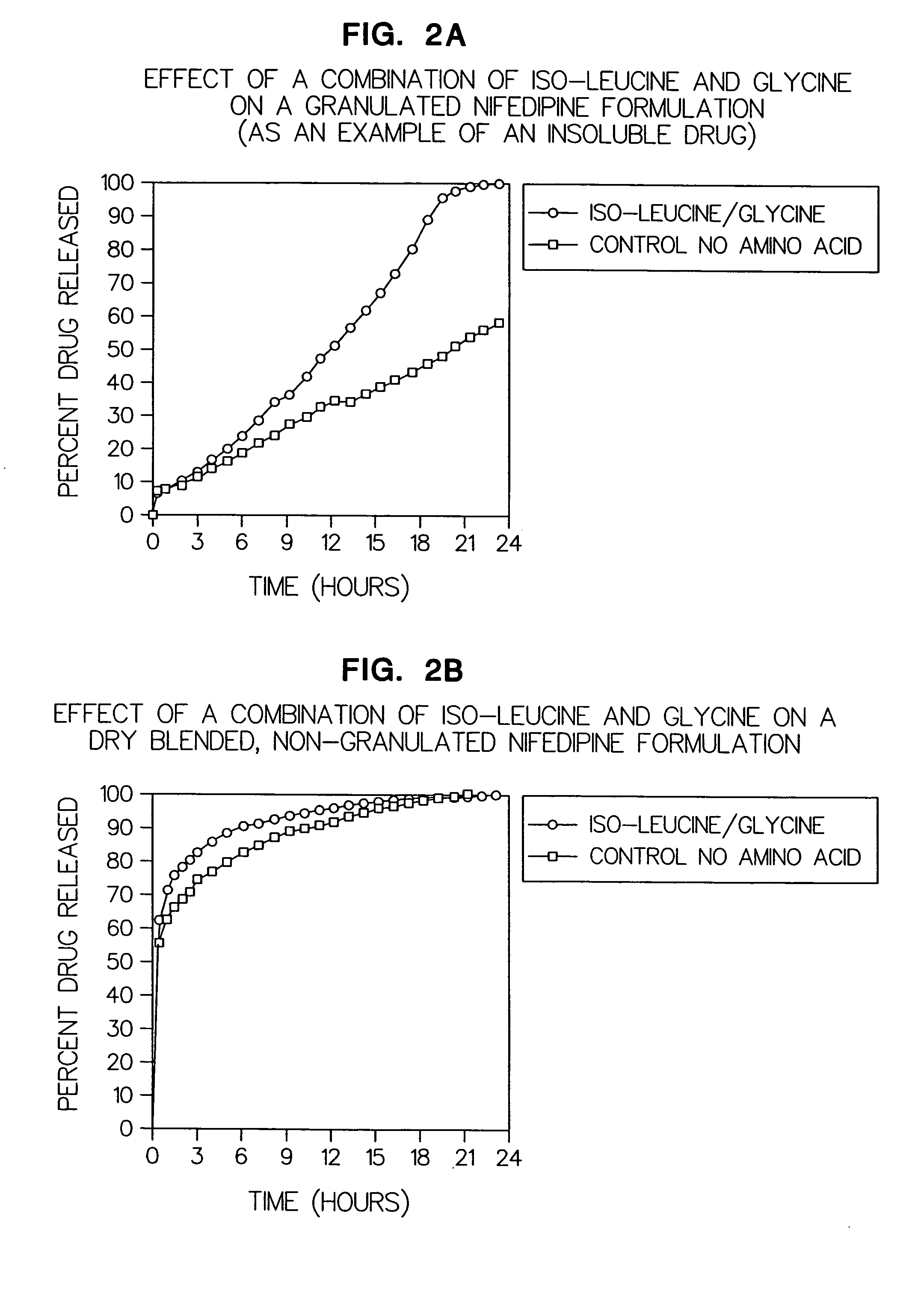
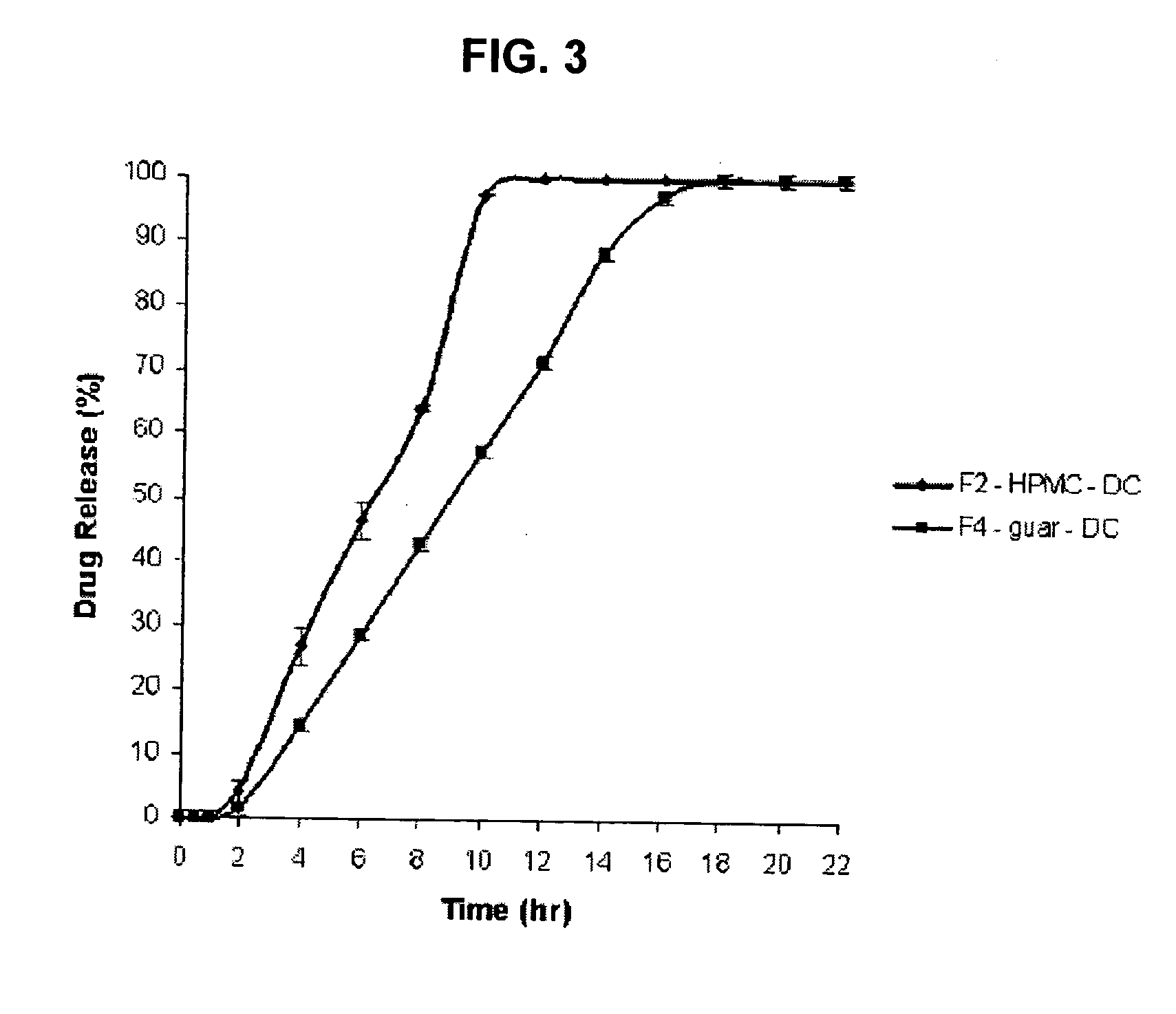
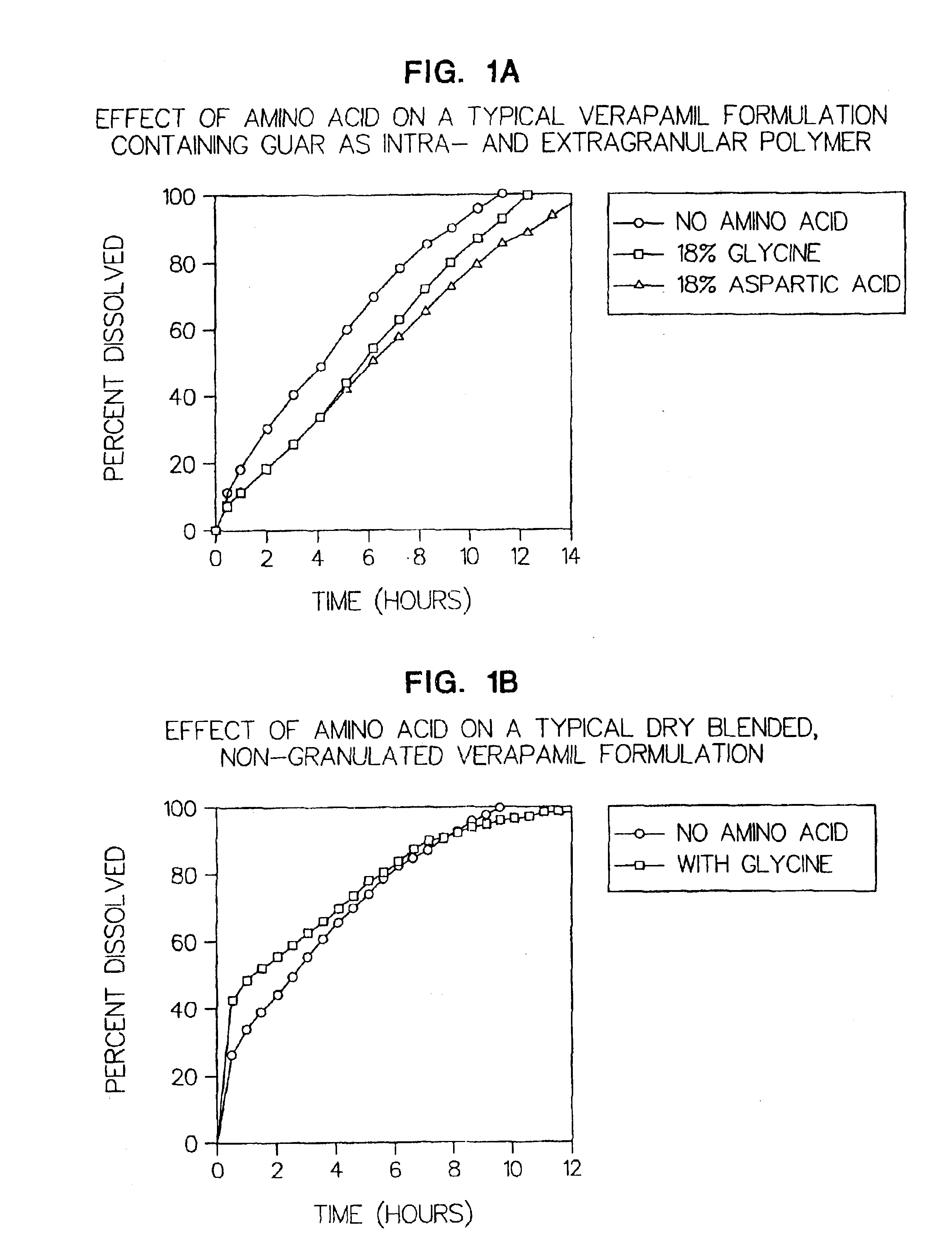
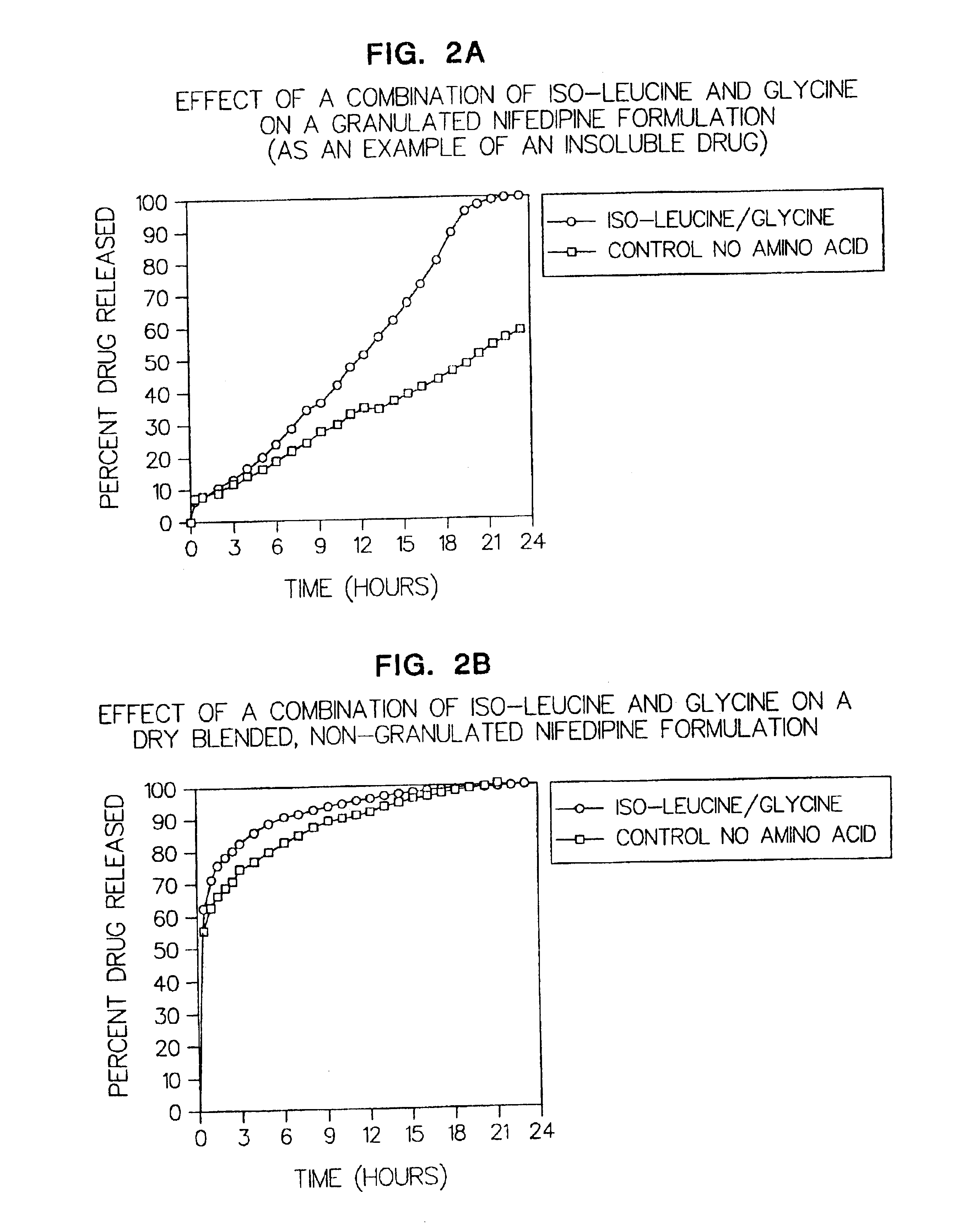
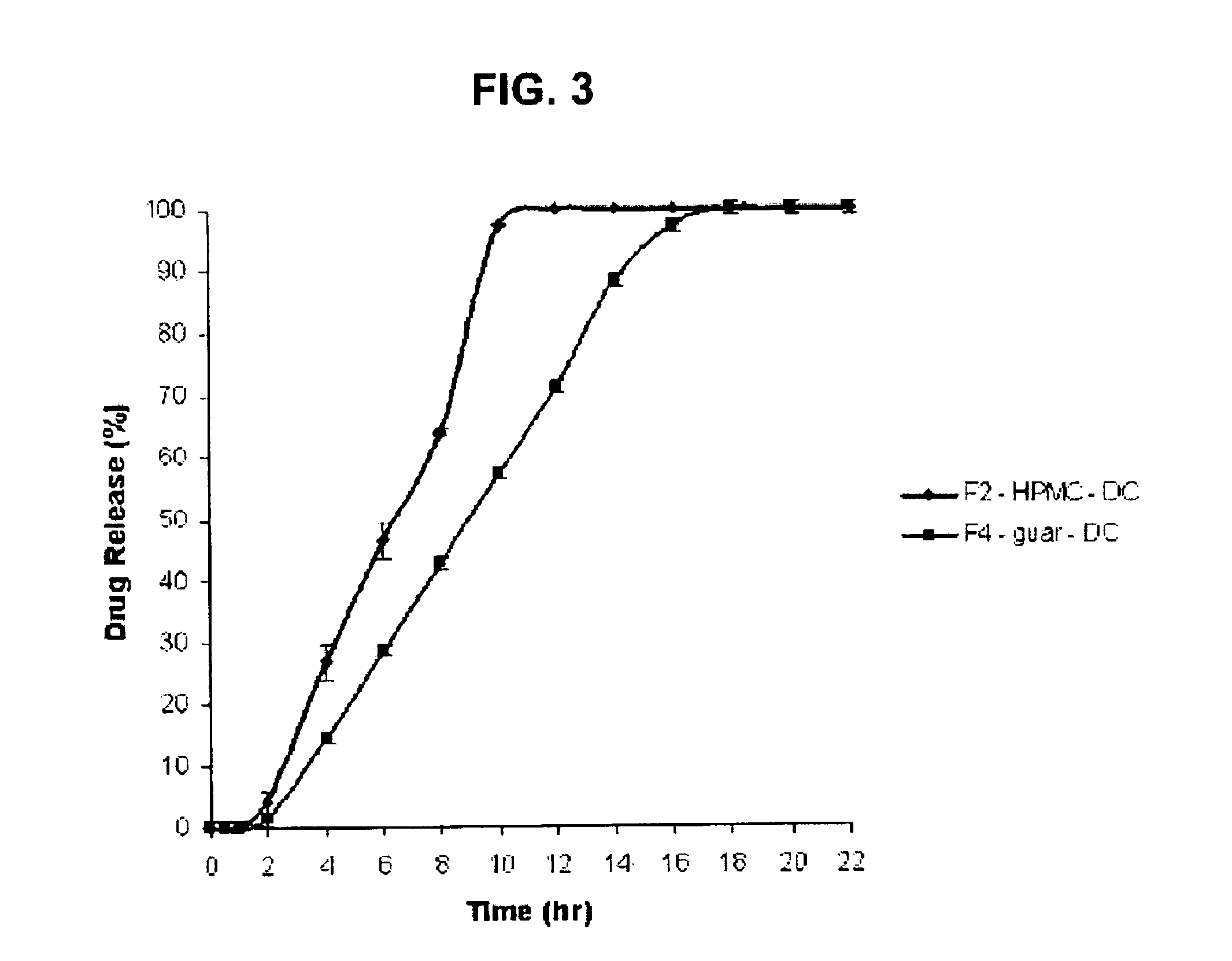
Amino acid modulated extended release dosage form
Disclosed herein is a oral extended release dosage form comprising a plurality of granules of an effective amount of a pharmaceutically active compound, at least one amino acid, and an intragranular polymer in which the granule is dispersed within a hydrophilic extragranular polymer matrix which is more rapidly hydrating than the intragranular polymer. The amino acid is selected for hydropathy characteristics depending on solubility characteristics of the active compound.
Owner:SCOLR PHARMA
Amino acid modulated extended release dosage form
InactiveUS6936275B2Minimizing complicationReduce manufacturing costPowder deliveryBiocideSolubilityPolymer
Disclosed herein is a oral extended release dosage form comprising a plurality of granules of an effective amount of a pharmaceutically active compound, at least one amino acid, and an intragranular polymer in which the granule is dispersed within a hydrophilic extragranular polymer matrix which is more rapidly hydrating than the intragranular polymer. The amino acid is selected for hydropathy characteristics depending on solubility characteristics of the active compound.
Owner:SCOLR PHARMA
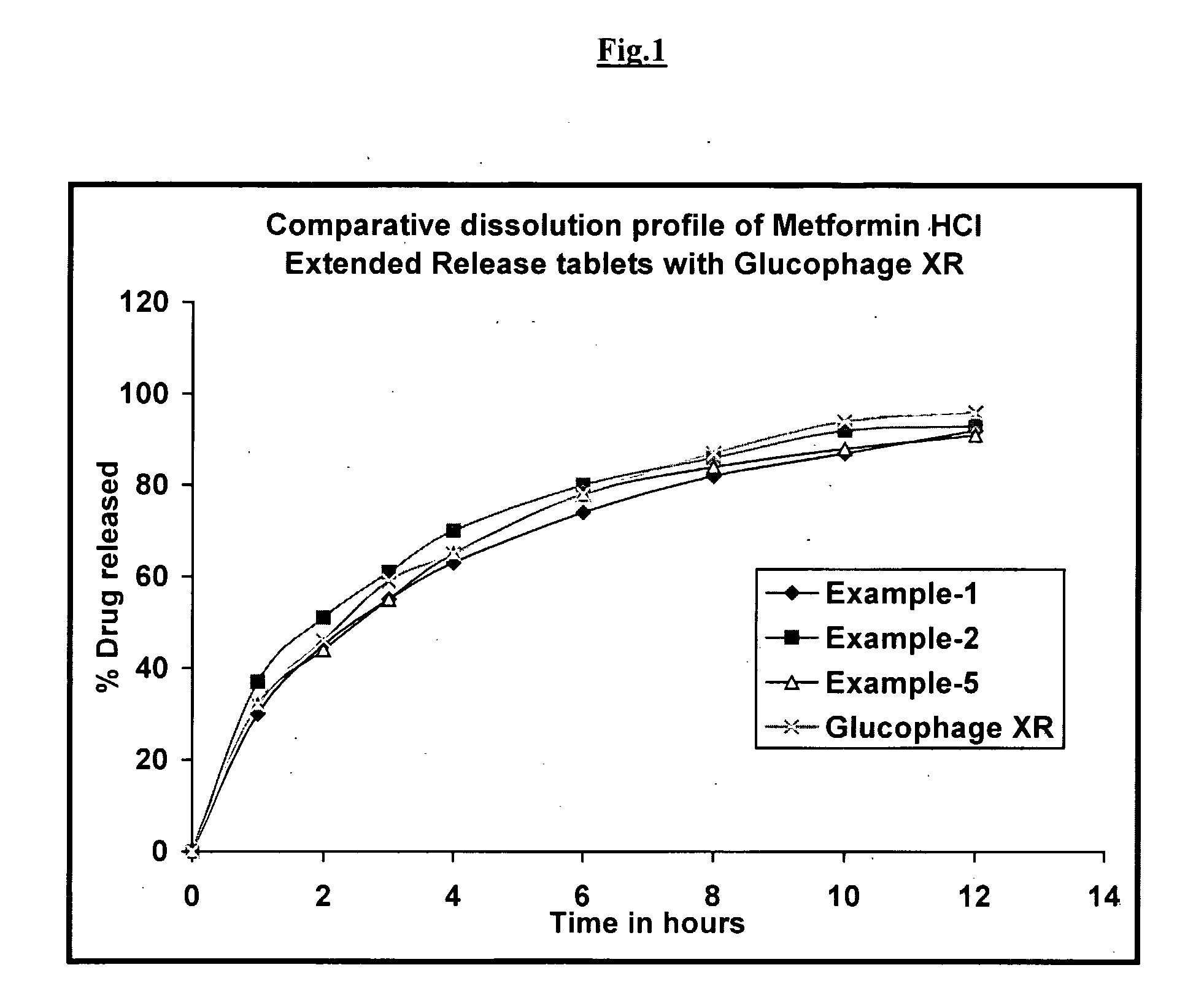
Pharmaceutical Compositions of Metformin
InactiveUS20090124702A1Solve the lack of hardnessGood reproducibilityOrganic active ingredientsBiocideDiabrezideWater soluble
The present invention relates to an extended release dosage form of highly water-soluble antidiabetic drug metformin or its pharmaceutically acceptable salts. This invention also relates to methods for preparing the extended release dosage forms of metformin or its pharmaceutically acceptable salts.
Owner:SIVA SATYA KRISHNA BABU PECHETTI +3
Compositions of (-)-17-(cyclobutylmethyl)morphinan-3,14-diol
ActiveUS9364430B2Increase rangeSlow onsetOrganic active ingredientsGranular deliveryModified Release Dosage FormMorphinan
The present invention is directed to oral, therapeutically effective modified release pharmaceutical compositions of (−)-17-(cyclobutylmethyl)morphinan-3,14-diol and it pharmaceutically acceptable salts and the use thereof, including delayed onset and extended release dosage forms. The present invention is also directed at modified release dosage forms of oral (−)-17-(cyclobutylmethyl)morphinan-3,14-diol which provide robust efficacy and reduced potential for abuse and misuse.
Owner:RELMADA THERAPEUTICS
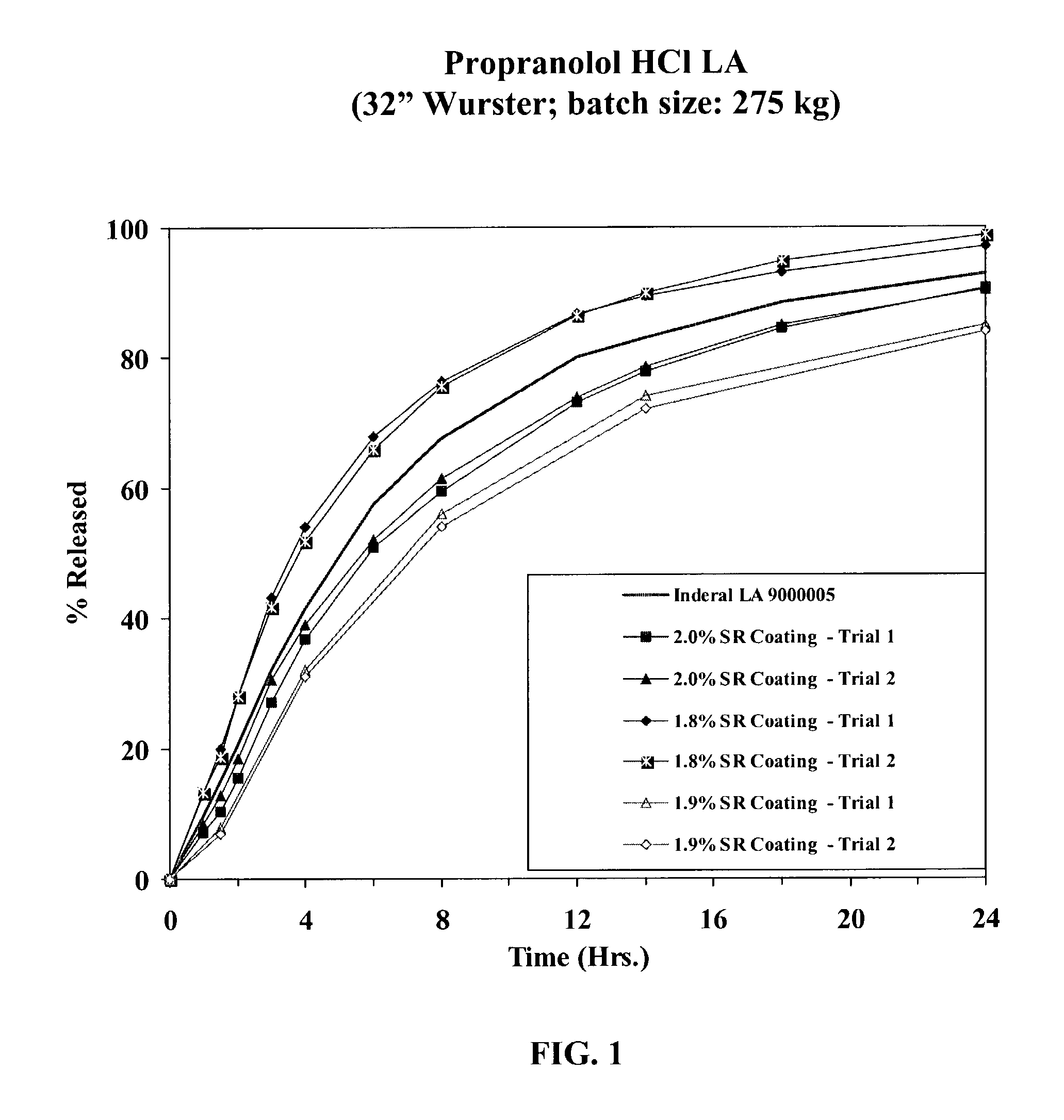
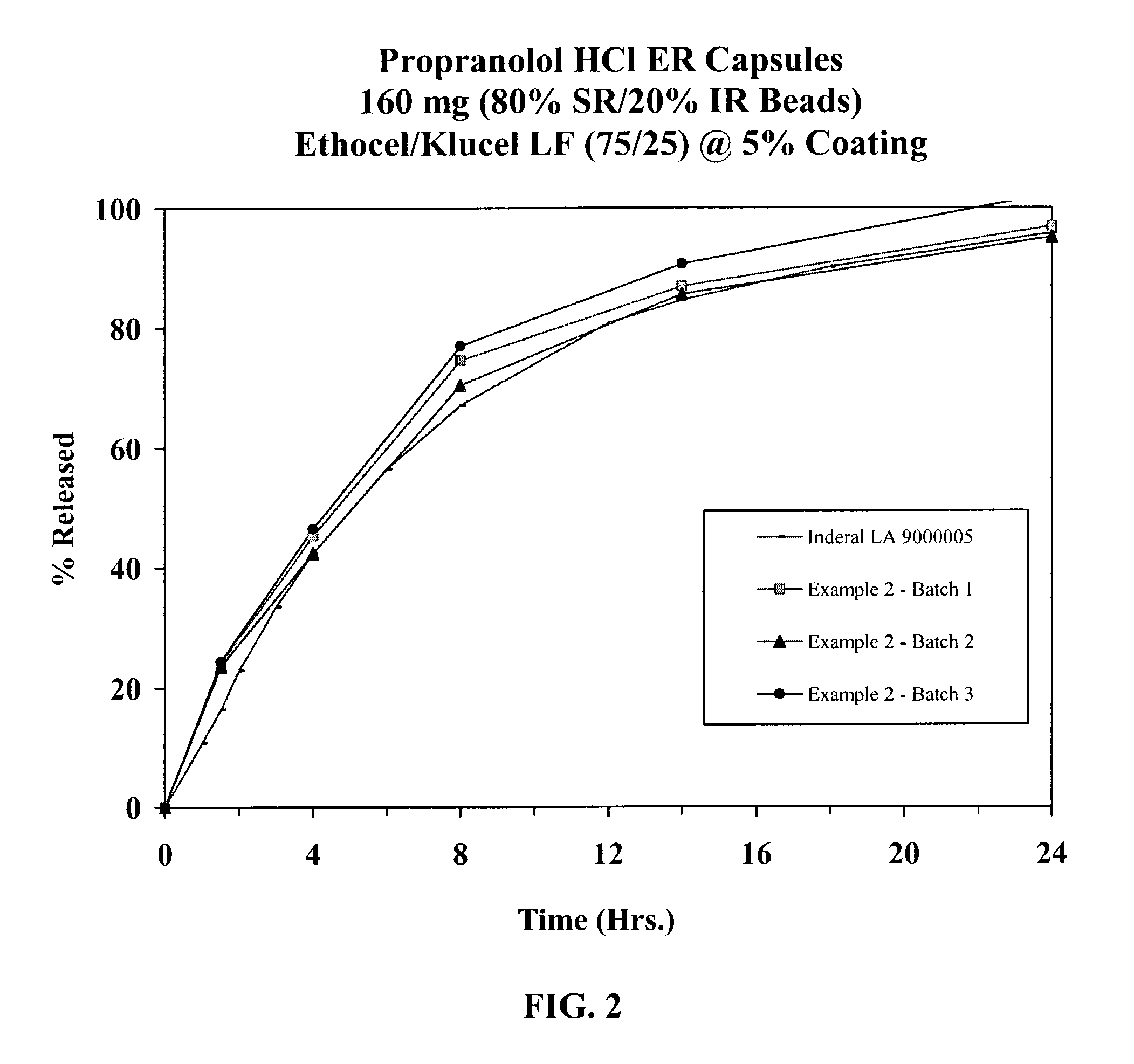
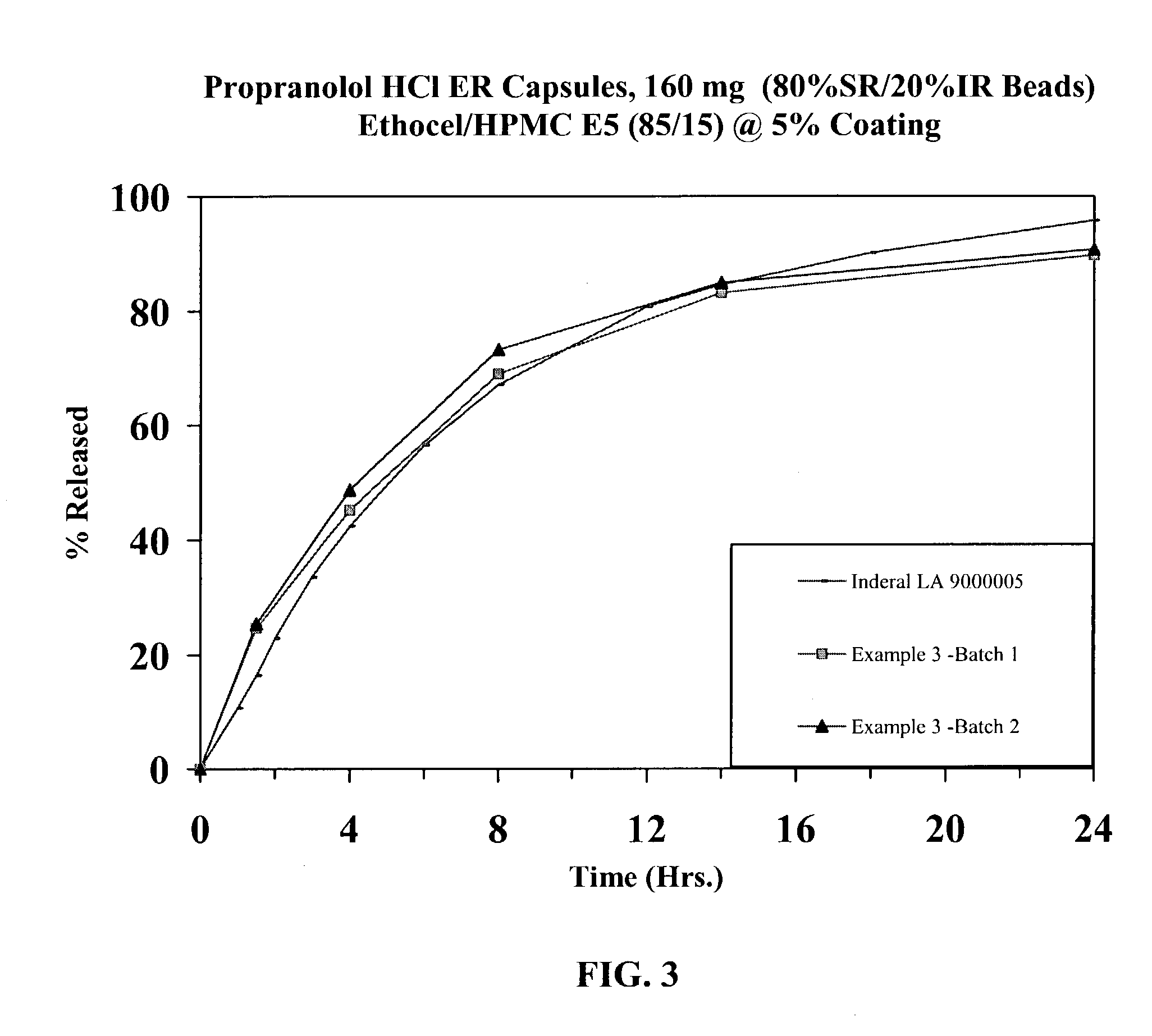
Extended release dosage forms of propranolol hydrochloride
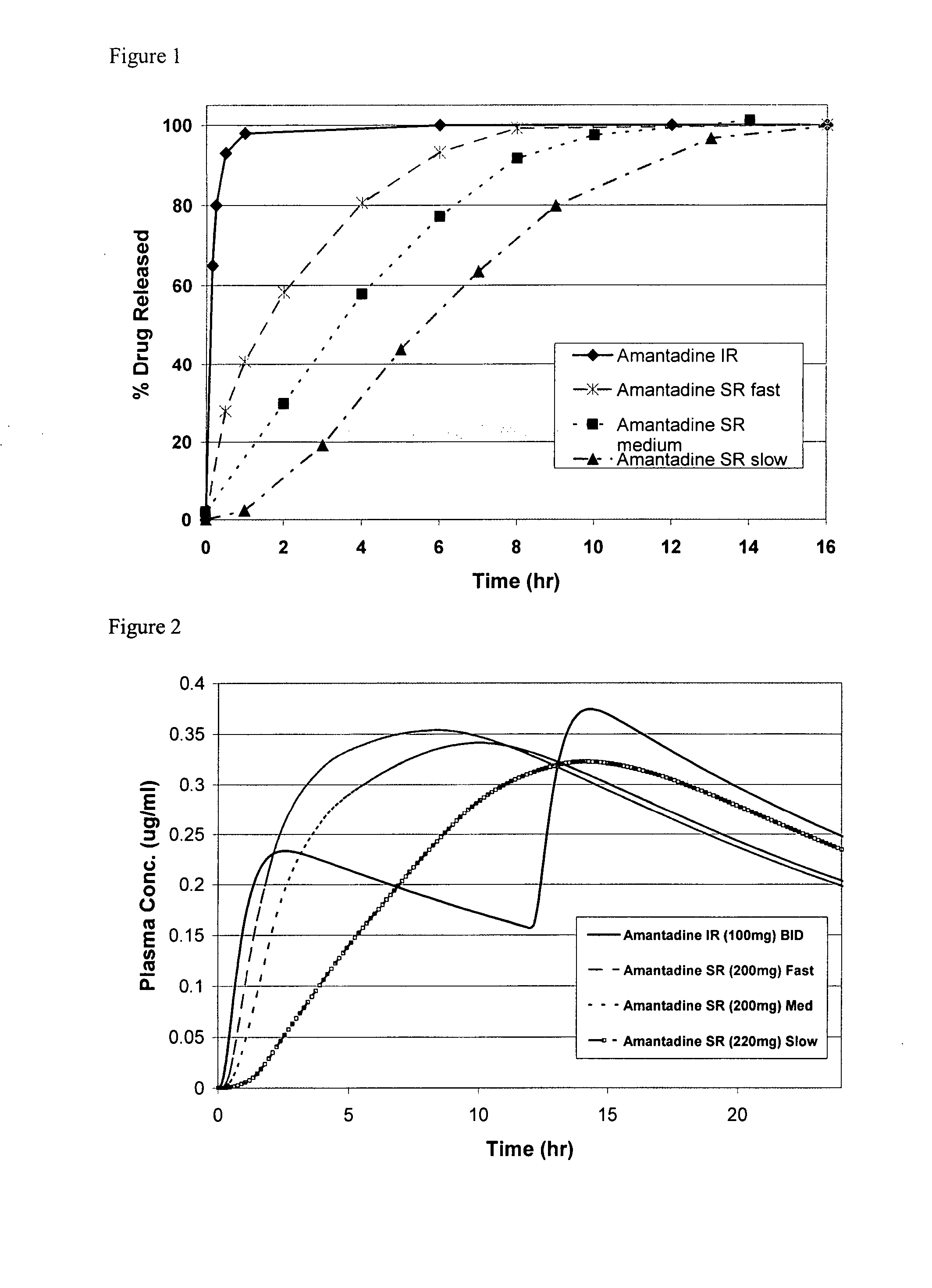
A unit dosage form, such as a capsule or the like for delivering drugs into the body in a sustained release fashion similar to that produced by INDERAL® LA indicated for the treatment of cardiovascular diseases, comprises two populations of propranolol-containing particles (beads, pellets, granules, etc.). Each bead population exhibits a pre-designed rapid release profile (i.e., substantially complete release within 60 minutes) or sustained release profile over a period of 24 hours. Such a cardiovascular drug delivery system is designed by combining immediate release (IR) beads and sustained release (SR) beads. SR beads may be obtained by membrane coating IR beads with a water-insoluble polymer such as ethylcellulose or a mixture of a water insoluble polymer and a water-soluble polymer such as hydroxypropylcellulose at a ratio of from about 65 / 35 to 95 / 5.
Owner:ADARE PHARM INC
Compositions of (-)-17-(Cyclobutylmethyl) Morphinan-3,14-Diol
ActiveUS20140200237A1Increase rangeSlow onsetBiocideGranular deliveryModified Release Dosage FormDiol
The present invention is directed to oral, therapeutically effective modified release pharmaceutical compositions of (−)-17-(cyclobutylmethyl)morphinan-3,14-diol and it pharmaceutically acceptable salts and the use thereof, including delayed onset and extended release dosage forms. The present invention is also directed at modified release dosage forms of oral (−)-17-(cyclobutylmethyl)morphinan-3,14-diol which provide robust efficacy and reduced potential for abuse and misuse.
Owner:RELMADA THERAPEUTICS
Extended release dosage forms of quetiapine
Owner:RANBAXY LAB LTD
Amino acid modulated extended release dosage form
Disclosed herein is a oral extended release dosage form comprising a plurality of granules of an effective amount of a pharmaceutically active compound, at least one amino acid, and an intragranular polymer in which the granule is dispersed within a hydrophilic extragranular polymer matrix which is more rapidly hydrating than the intragranular polymer. The amino acid is selected for hydropathy characteristics depending on solubility characteristics of the active compound.
Owner:SCOLR PHARMA
Extended-release dosage form
Provided are pharmaceutical formulations comprising sustained release particles each having an inner core bead comprising an active pharmaceutical ingredient an intermediate coating substantially surrounding the inner core bead, and an outer coating substantially surrounding the intermediate coating comprising a pH independent polymer. Also provided is a pharmaceutical formulation comprising two bead populations wherein each of the first and second bead populations have a different drug release profile. Also provided is a method of preparing an extended release dosage composition comprising one or more bead populations.
Owner:MYLAN PHARMA INC
Pharmaceutical compositions comprising hydromorphone and naloxone
InactiveUS20150283091A1Improve stability and dissolution propertyImproved stability and dissolution propertyBiocideNervous disorderAcetic acidSodium metabisulfite
There is described a prolonged release pharmaceutical dosage form comprising a plurality of coated beads, each of the coated beads comprising: (a) a granule; (b) a first layer coated on the granule, the first layer comprising: (i) hydromorphone or a pharmaceutically acceptable salt thereof, (ii) naloxone or a pharmaceutically acceptable salt thereof, (iii) an antioxidant compound, and (iii) a chelating compound; and (c) a second layer coated on the first layer, the second layer comprising a prolonged release agent. The dosage form has improved stability and dissolution properties. Another aspect of the invention relates to use of a combination of an antioxidant (such as sodium metabisulfite) and a chelating agent (such as ethylenedinitrotetraacetic acid disodium salt dihydrate) to improve the stability and / or dissolution properties of a prolonged release dosage form comprising (i) hydromorphone or a pharmaceutically acceptable salt thereof and (ii) naloxone or a pharmaceutically acceptable salt thereof.
Owner:PURDUE PHARMA LP
Treatment of autistic spectrum disorder
A method for treating a patient having ASD is disclosed. The method includes administering to the patient a therapeutically effective amount of an alpha-2 adrenergic agonist in an extended release dosage form in combination with a therapeutically effective amount of inositol.
Owner:PRICE RICHARD LOUIS
Extended release dosage form of paliperidone
The present invention relates to an extended release solid oral pharmaceutical composition comprising Paliperidone or its pharmaceutically acceptable salts and process for preparing the same.
Owner:TORRENT PHARMA LTD
Carbamazepine extended release dosage form
InactiveUS20090169619A1Efficient processingBiocideNervous disorderOral medicationBlood concentration
Extended release pharmaceutical dosage forms of carbamazepine for oral administration to maintain a patient's blood concentration for at least a 12 hour period, methods of administering dosage forms and processes for the preparation of such dosage form.
Owner:COREPHARMA
Gastric retentive extended-release dosage forms comprising combinations of a non-opioid analgesic and an opioid analgesic
InactiveCN102105136AImprove complianceOrganic active ingredientsNervous disorderUpper gastrointestinalNon opioid analgesics
Compositions and methods for the treatment of pain in a mammal are described. More specifically, a dosage form designed for release of acetaminophen and an opioid is described, wherein the dosage form provides delivery of the drugs to the upper gastrointestinal tract ('Gl') of a mammal for an extended period of time.
Owner:蒂宝制药公司
Extended-release dosage form
Provided are pharmaceutical formulations comprising sustained release particles each having an inner core bead comprising an active pharmaceutical ingredient an intermediate coating substantially surrounding the inner core bead, and an outer coating substantially surrounding the intermediate coating comprising a pH independent polymer. Also provided is a pharmaceutical formulation comprising two bead populations wherein each of the first and second bead populations have a different drug release profile. Also provided is a method of preparing an extended release dosage composition comprising one or more bead populations.
Owner:MYLAN PHARMA INC
Diagnosis and treatment of a form of autistic spectrum disorder
A method for treating a patient having P.R.I.C.E. Syndrome is disclosed. The method includes administering to the patient a therapeutically effective amount of an alpha-2 adrenergic agonist in an extended release dosage form, optionally in combination with a therapeutically effective amount of inositol and / or NAC.
Owner:PRICE RICHARD LOUIS
Methods and composition for treatment of cardiovascular conditions
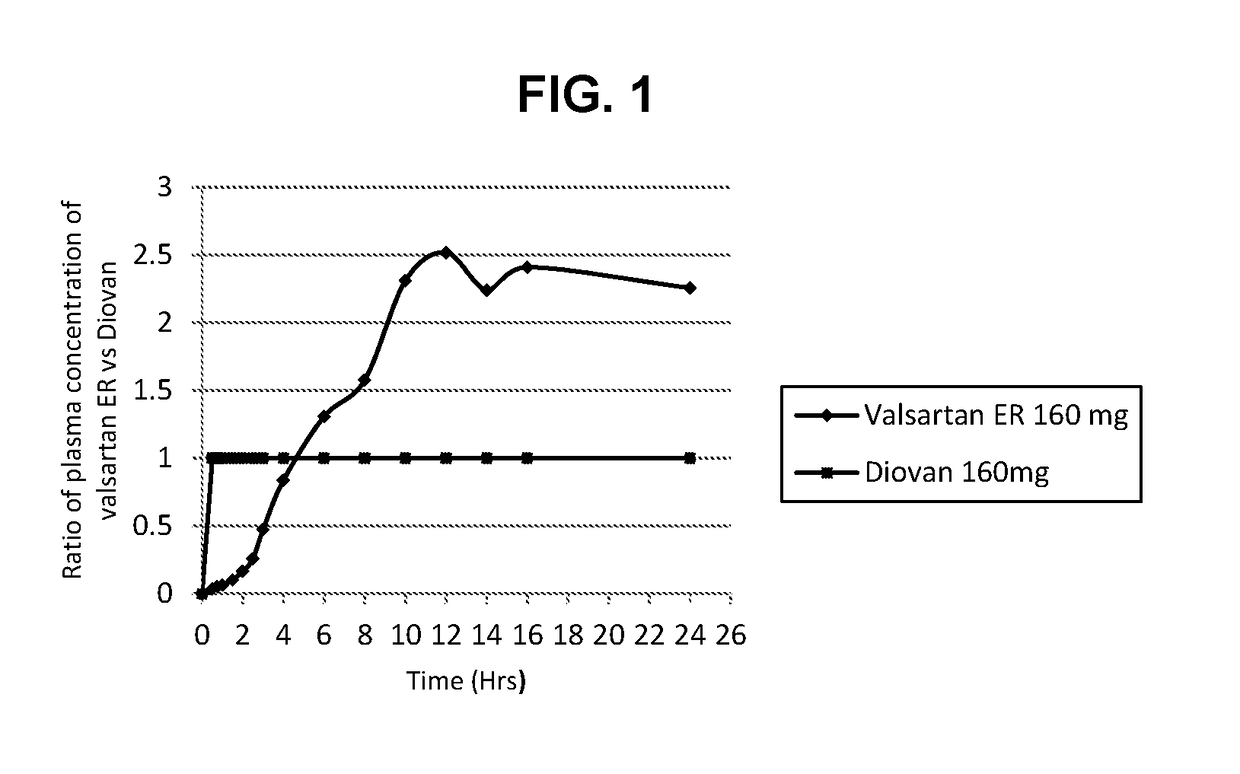
The present invention relates to methods for treatment of cardiovascular conditions selected from high blood pressure, heart failure, or heart attack. The present invention further relates to a method of treating cardiovascular conditions comprising orally administering once a day to a human subject in need thereof the compound valsartan in an extended release dosage form, wherein the ratio of mean plasma concentration of valsartan provided by the extended release dosage form to the mean plasma concentration of valsartan provided by an immediate release dosage form of valsartan over 8 hour to 24 hour period after administration is greater than 1 in a single dose human pharmacokinetic study.
Owner:EZRA PHARMA LLC +1
Diagnosis and treatment of p.r.i.c.e. syndrome
A method for treating a patient having P.R.I.C.E. Syndrome is disclosed. The method includes administering to the patient a therapeutically effective amount of an alpha-2 adrenergic agonist in an extended release dosage form.
Owner:PRICE RICHARD LOUIS
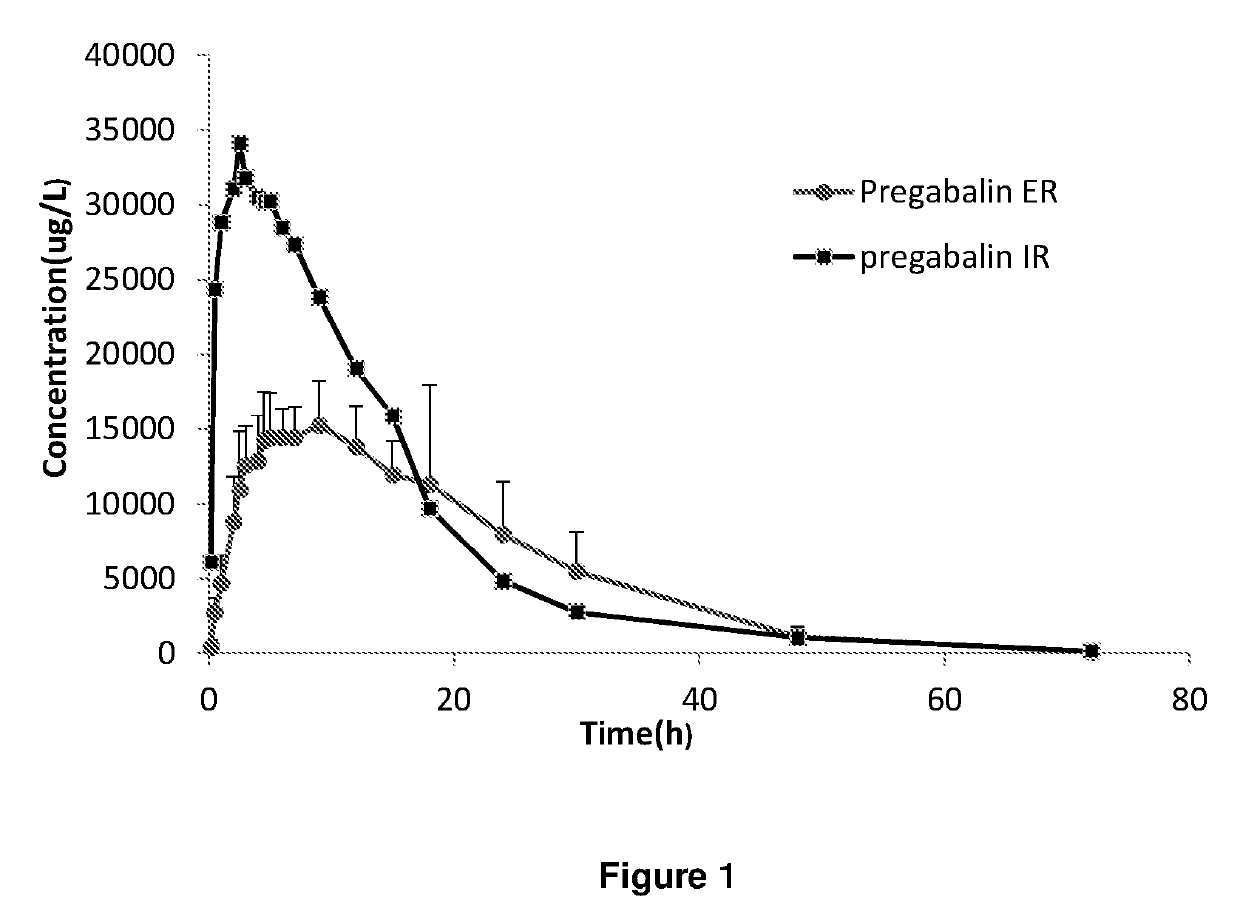
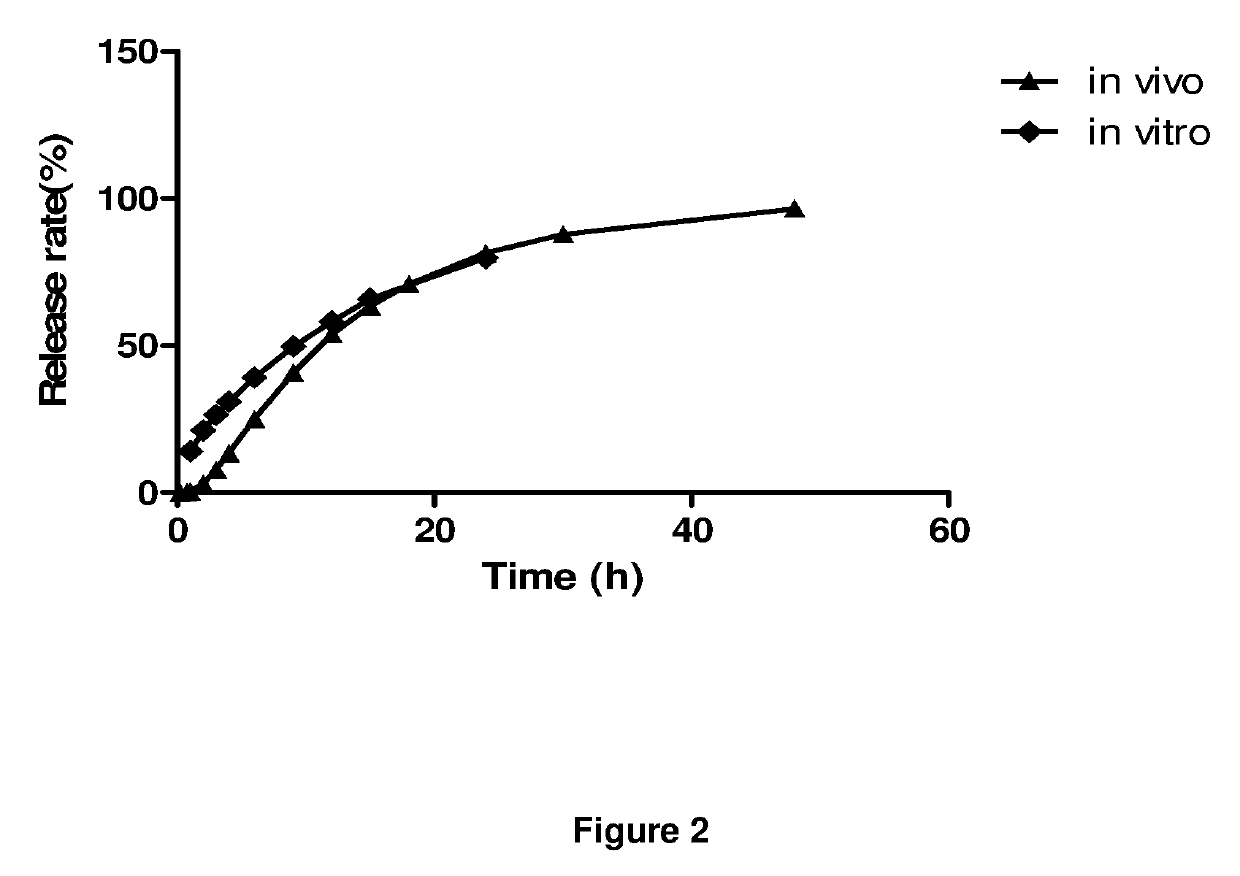
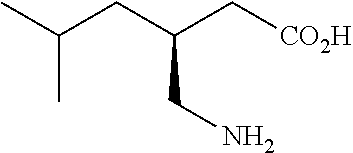
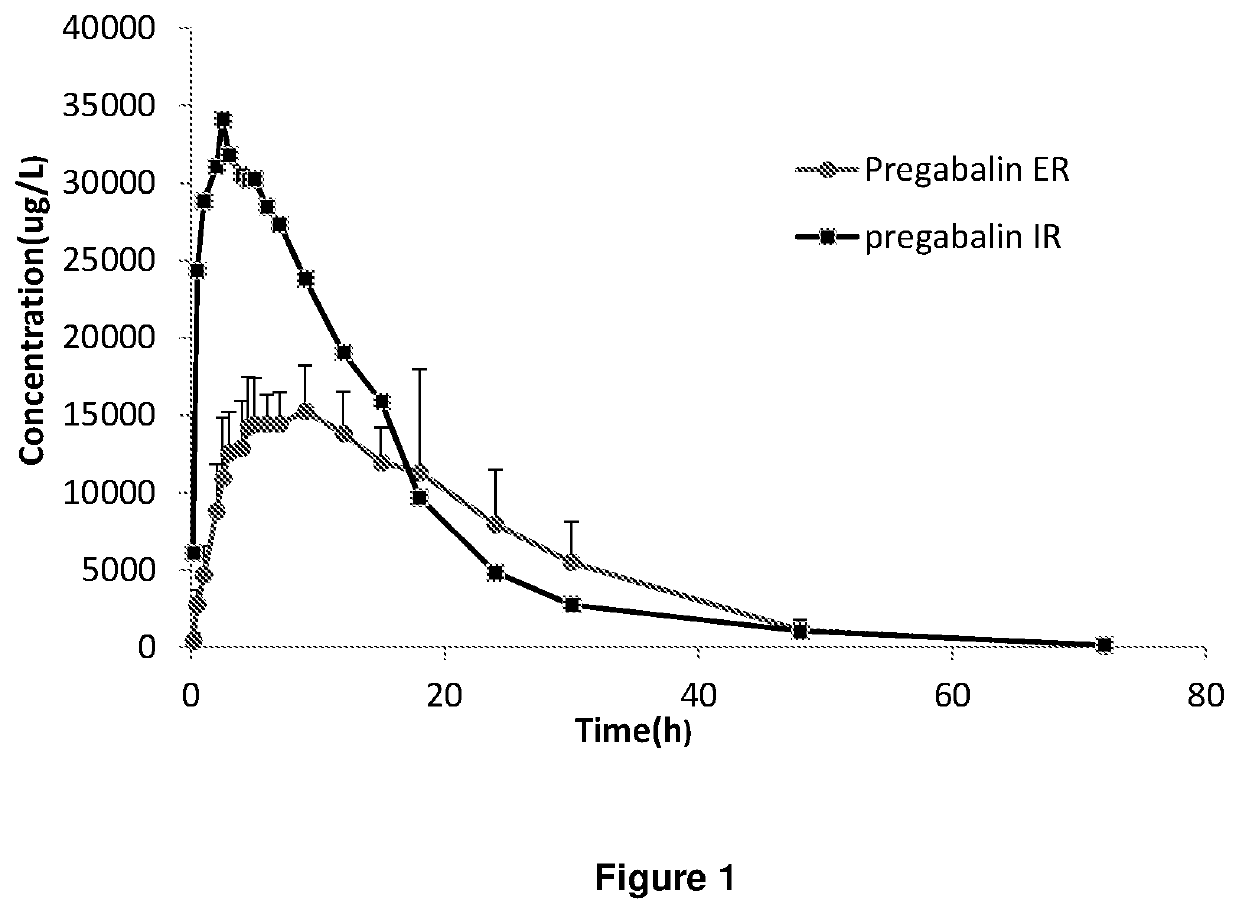
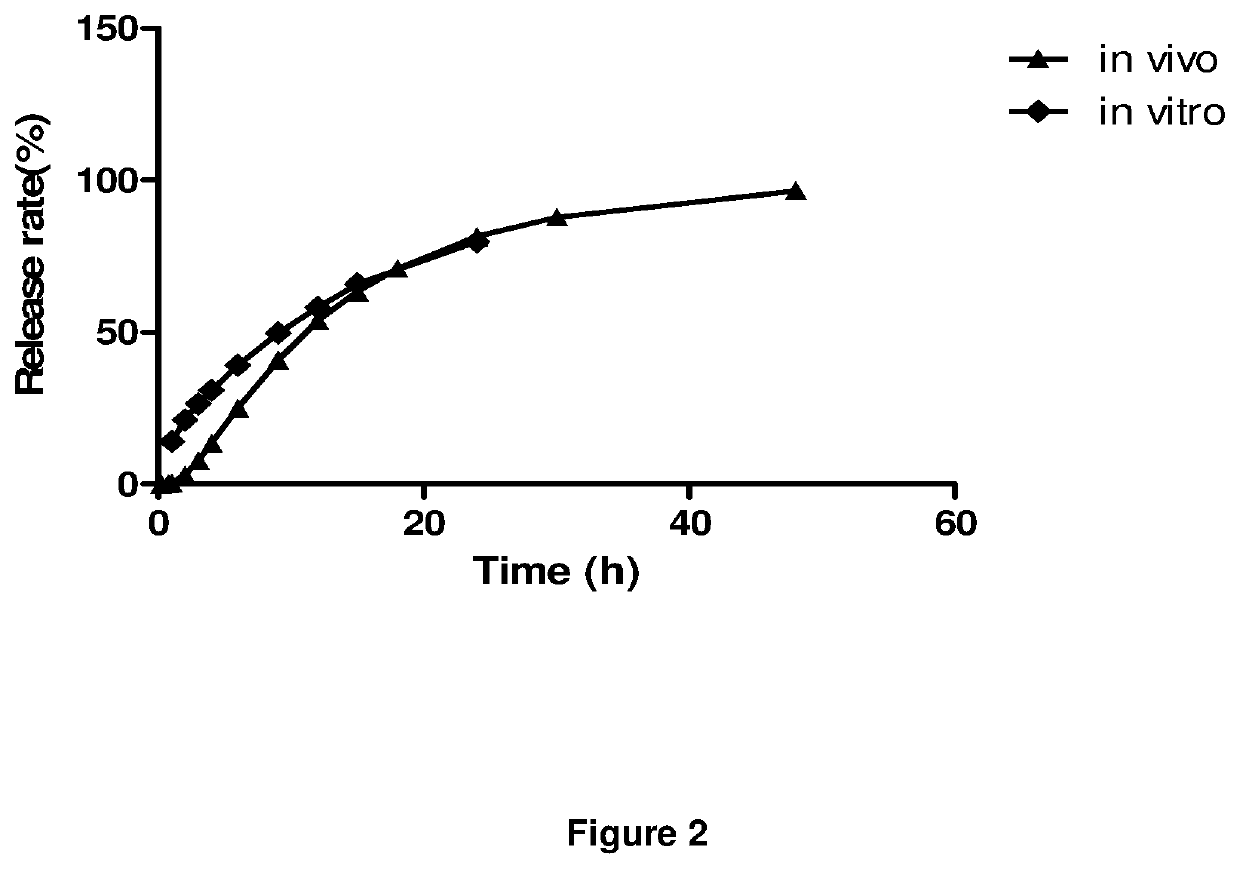
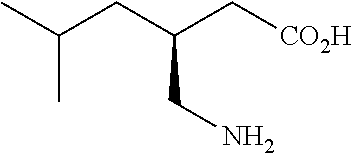
Extended release dosage forms of pregabalin
ActiveUS20190298675A1Small indexLow profileOrganic active ingredientsHydrocarbonsDiseaseDual release
The present invention relates to extended-release pharmaceutical compositions comprising pregabalin or a salt thereof, which are adapted to release the pregabalin active ingredient according to a dual release profile. The formulations comprise two components, the first (fast ER) providing extended-release of the active ingredient in a short controlled manner lasting from about 4 to about 6 hours, and the second (slow ER or maintenance) providing extended release of the active ingredient over a period of 24 hours. The proportion of each component in the formulation may be adjusted to achieve the desired AUC and therapeutic effect following oral administration to a subject. The invention further relates to methods of using the pharmaceutical compositions for treating conditions and disorders which are responsive to pregabalin treatment, such as neuropathic pain associated with diabetic peripheral neuropathy (DPN), post herpetic neuralgia (PHN), epilepsy, seizures and fibromyalgia.
Owner:MAPI PHARMA
Treatment of autistic spectrum disorder
A method for treating a patient having ASD is disclosed. The method includes administering to the patient a therapeutically effective amount of an alpha-2 adrenergic agonist in an extended release dosage form in combination with a therapeutically effective amount of inositol.
Owner:PRICE RICHARD LOUIS
Extended release dosage forms of pregabalin
ActiveUS11026908B2Small indexLow profileOrganic active ingredientsHydrocarbonsDual releaseTherapeutic effect
The present invention relates to extended-release pharmaceutical compositions comprising pregabalin or a salt thereof, which are adapted to release the pregabalin active ingredient according to a dual release profile. The formulations comprise two components, the first (fast ER) providing extended-release of the active ingredient in a short controlled manner lasting from about 4 to about 6 hours, and the second (slow ER or maintenance) providing extended release of the active ingredient over a period of 24 hours. The proportion of each component in the formulation may be adjusted to achieve the desired AUC and therapeutic effect following oral administration to a subject. The invention further relates to methods of using the pharmaceutical compositions for treating conditions and disorders which are responsive to pregabalin treatment, such as neuropathic pain associated with diabetic peripheral neuropathy (DPN), post herpetic neuralgia (PHN), epilepsy, seizures and fibromyalgia.
Owner:MAPI PHARMA
Extended Release Dosage Form Comprising Cyclobenzaprine Hydrochloride
InactiveUS20170056342A1Simplified and convenient manufacturing processAvoid problemsOrganic active ingredientsPharmaceutical product form changeExtended Release CapsuleBiomedical engineering
An improved extended release capsule of cyclobenzaprine, and in particular, cyclobenzaprine hydrochloride, is provided. The improved capsule, comprising one or more matrix-type tablets providing extended release of the cyclobenzaprine, provides a dosage form that is bioequivalent to the currently marketed AMRIX® capsules while providing a simplified manufacturing process. Also provided is a method for the preparation of the improved extended release capsule of cyclobenzaprine.
Owner:APOTEX TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com