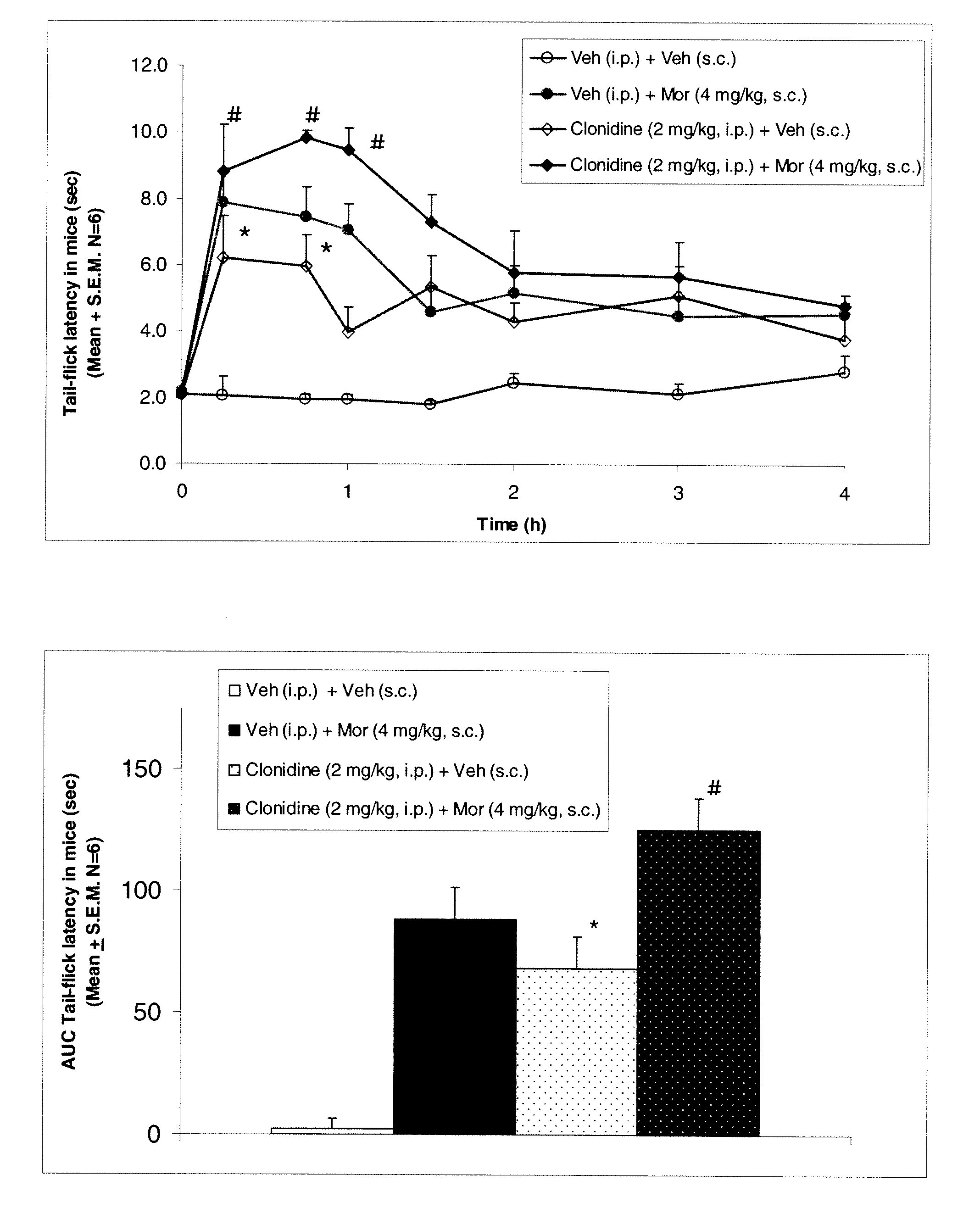
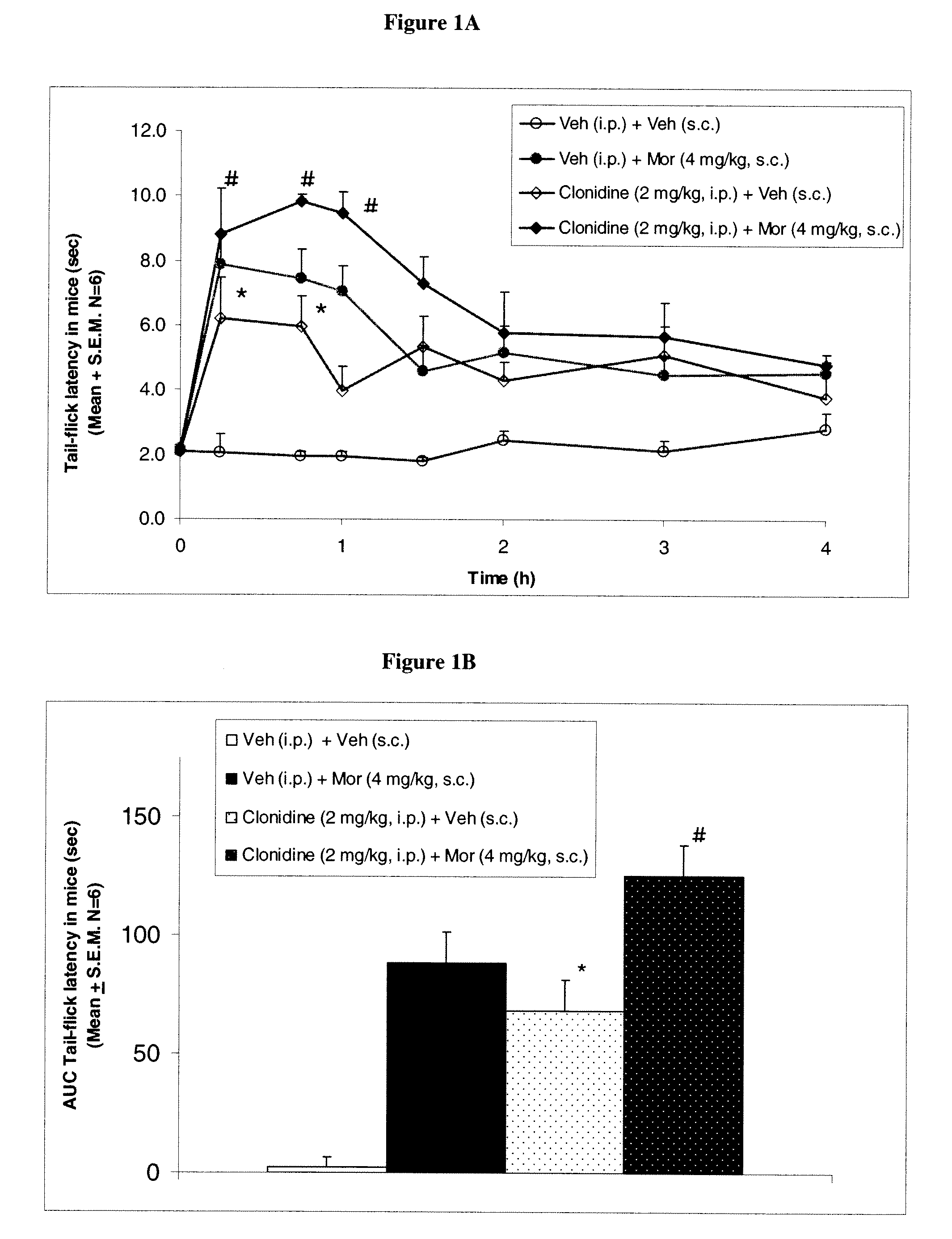
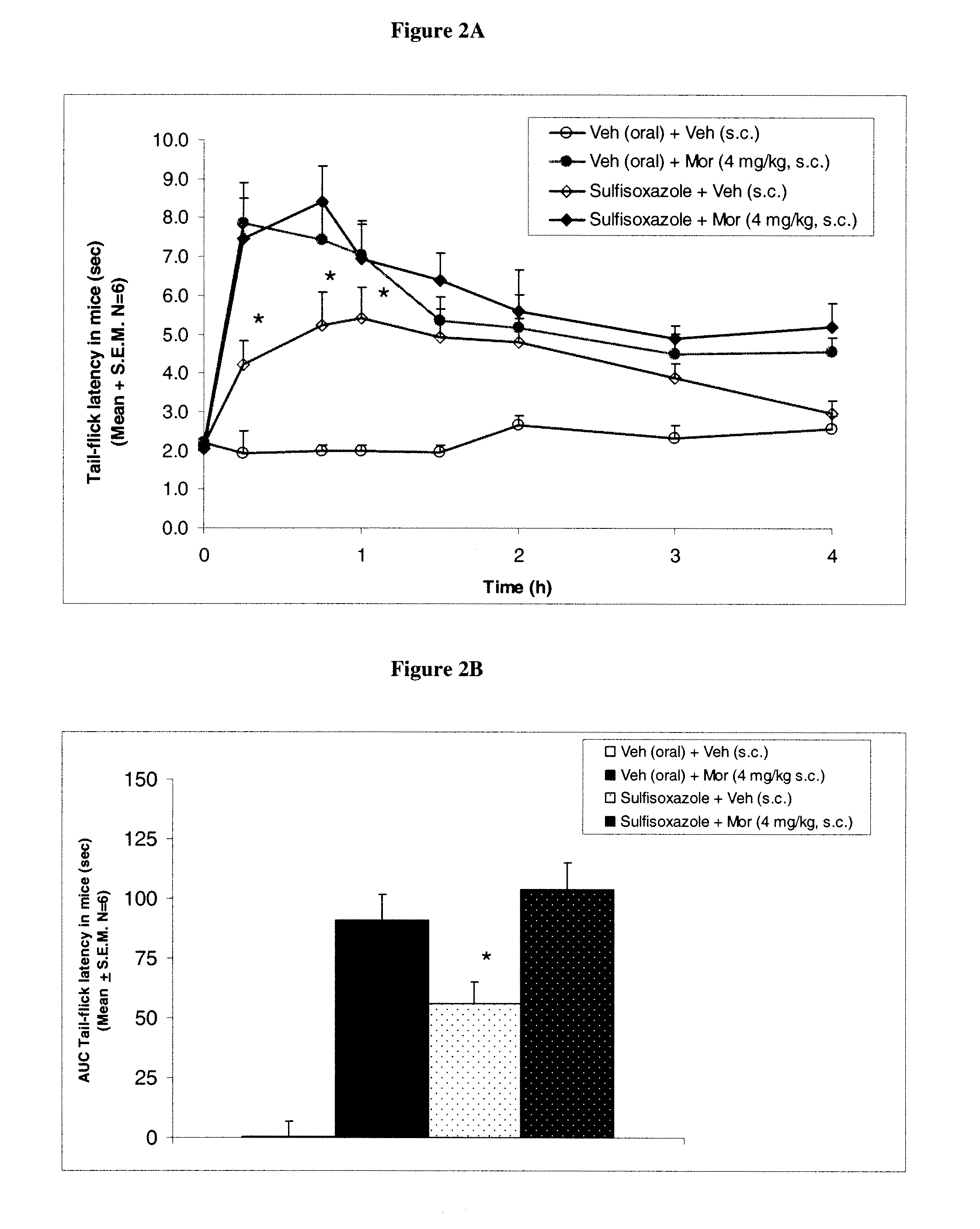
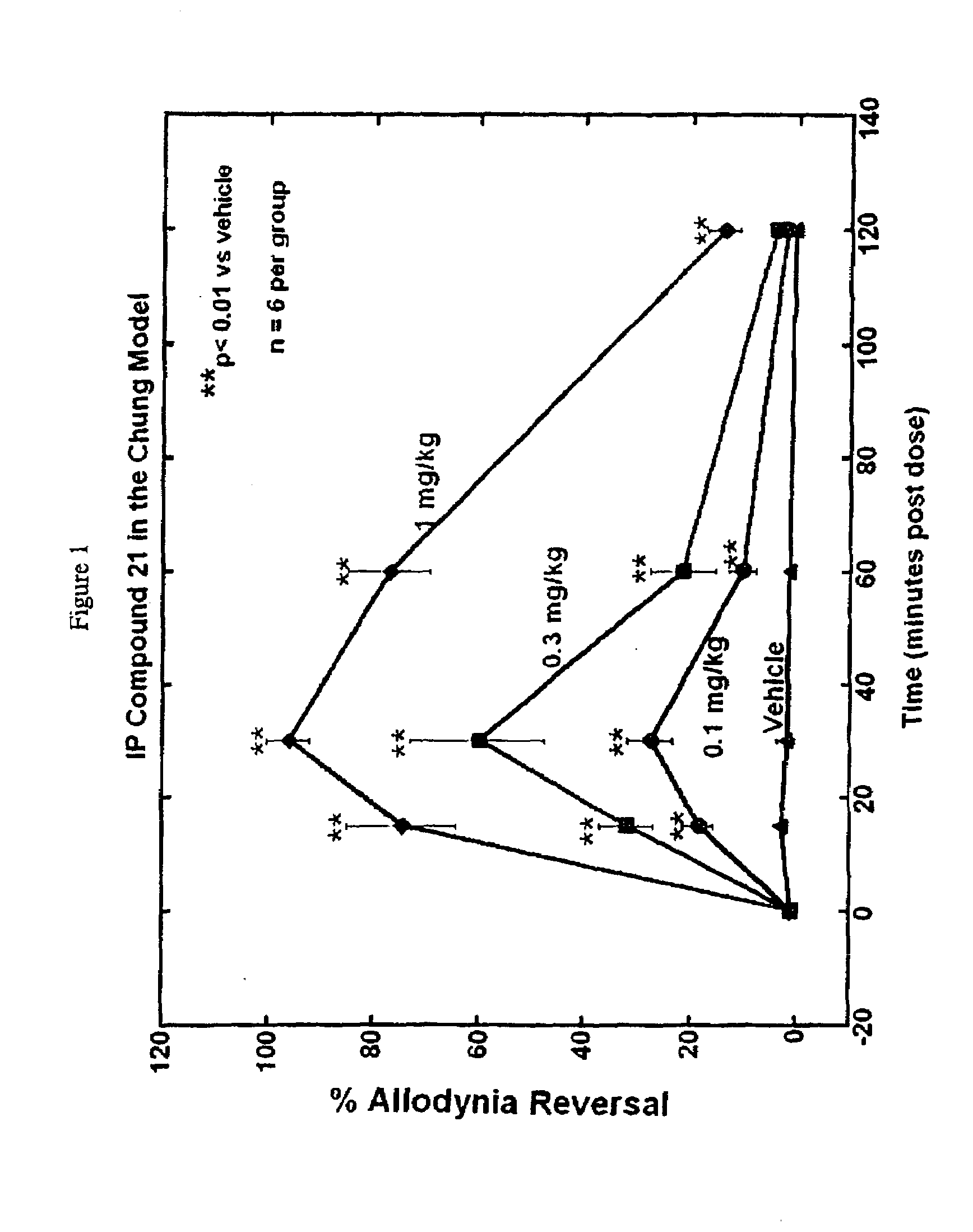
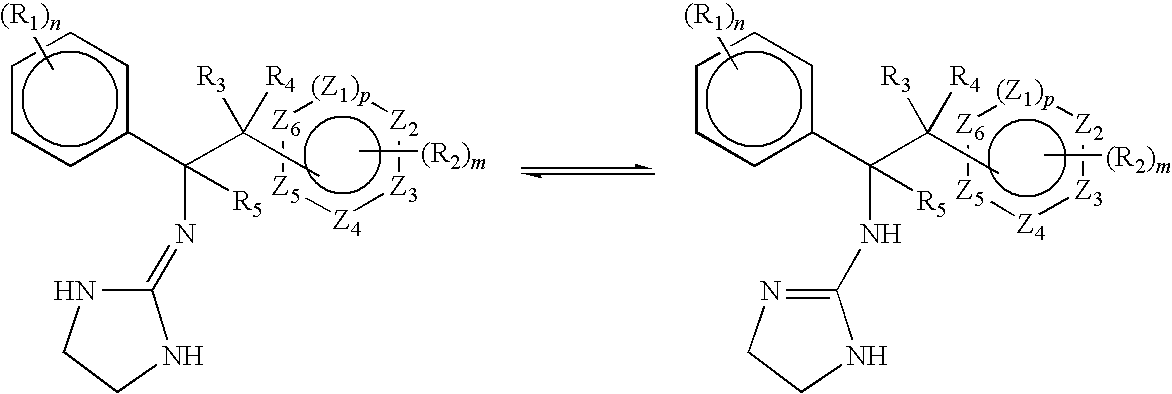Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Alpha 2 adrenergic agonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions having enhanced pharmacokinetic characteristics
InactiveUS7491383B2Reduced activityImprove bioavailabilityBiocideSenses disorderAdrenergicFatty acid
Compositions comprising a therapeutic component and an efficacy enhancing component, that enhances the pharmacokinetic disposition of the therapeutic component, are disclosed. The therapeutic component may include an alpha-2-adrenergic agonist and the efficacy enhancing component may include fatty acids. In one embodiment, the therapeutic component and the efficacy enhancing component form a complex.
Owner:ALLERGAN INC
Prolonged administration of NMDA antagonist and safener drug to alter neuropathic pain condition
InactiveUS20050148673A1Reduce neurotoxic side effectInherent activityBiocideOrganic active ingredientsNR1 NMDA receptorSide effect
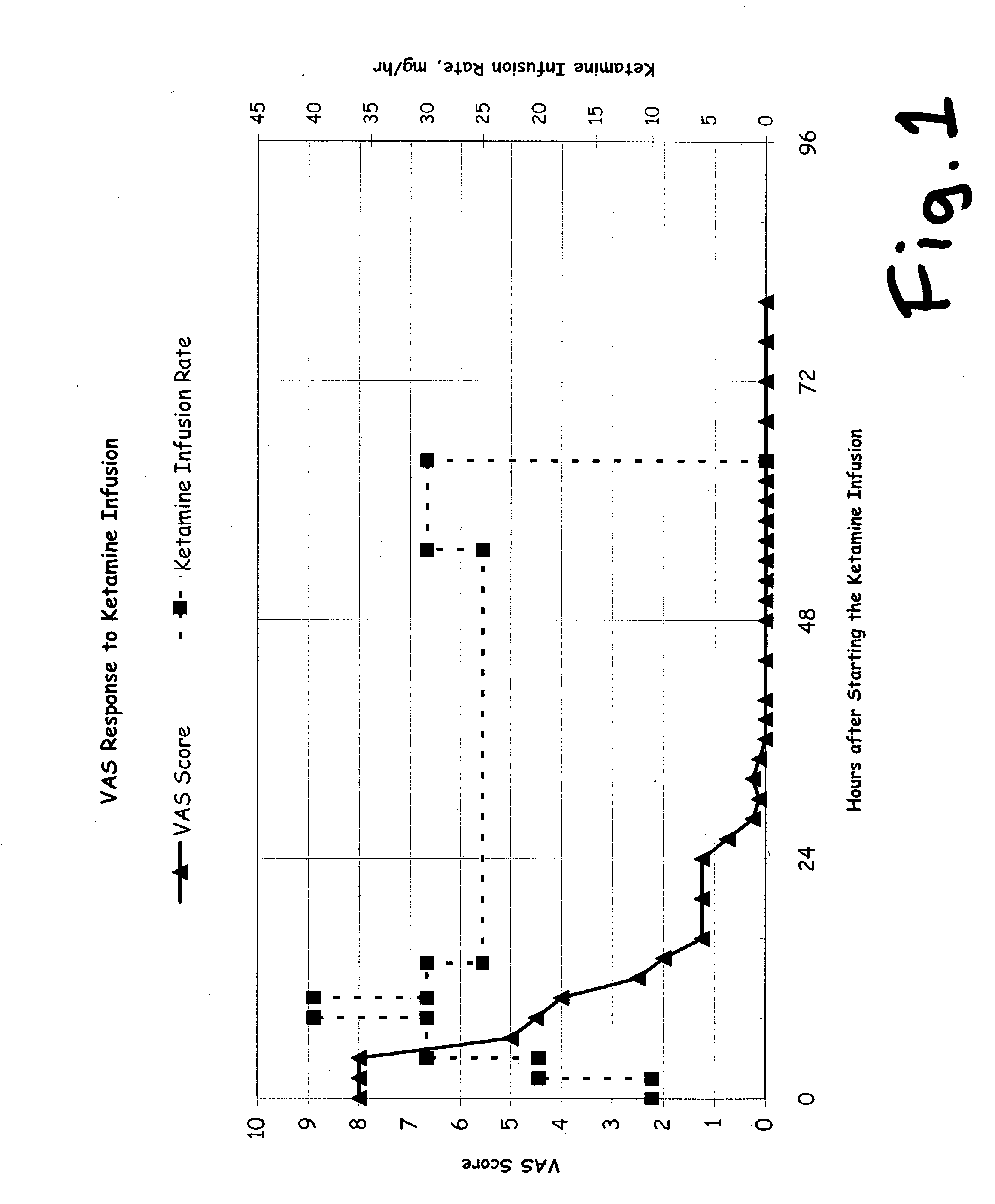
A drug that inhibits NMDA receptors (such as ketamine, a surgical anesthetic) is continuously administered to patients suffering from neuropathic pain. Unless the NMDA antagonist drug has inherent safening activity, this treatment requires a “safener” drug to prevent the neurotoxic side effects of NMDA antagonists. One class of safener drugs that increase the efficacy of the treatment include alpha-2 adrenergic agonists, such as clonidine. The treatment lasts for several days and nights, continuously. A maximum tolerated dosage is titered for each patient, such as by observing slurring of speech, and the patient does not lose consciousness except during normal sleep. Magnesium and / or drugs that inhibit ketamine-degrading enzymes can also be used. Patients who suffered for years from chronic intractable pain emerged from this treatment with apparently permanent relief, or with lasting reductions in their levels of pain.
Owner:HARBUT RONALD E +2
Methods and Compositions for Treating Dry Eye Disease and Other Eye Disorders
ActiveUS20160243116A1Enhanced effect is goodMaintain good propertiesOrganic active ingredientsSenses disorderDiseaseAdrenergic
The present invention discloses pharmaceutical preparations for treatment of eye disorders containing an alpha 2 adrenergic agonist, to processes for producing the pharmaceutical preparations and methods for treatment of various eye disorders including dry eye and Meibomian gland dysfunction and a medicinal applicator for topical application of an alpha 2 adrenergic agonist to a subject, a package assembly for the medicinal applicator and methods of using the medicinal applicator to treat eye disorders.
Owner:OCUGEN INC +1
Anaesthetic formulation comprising an NMDA-antagoinst and an alpha-2 adrenergic agonist
InactiveUS6562855B1Good curative effectReduce the possibilityOrganic active ingredientsBiocideAdrenergicAntagonist
A pharmaceutical composition is described. The composition comprises a formulation. The formulation comprises an NMDA antagonist and an alpha-2 adrenergic agonist. In a preferred aspect, there is provided an improved anaesthetic comprising an NMDA antagonist and an alpha-2 adrenergic agonist.
Owner:IMPERIAL INNOVATIONS LTD
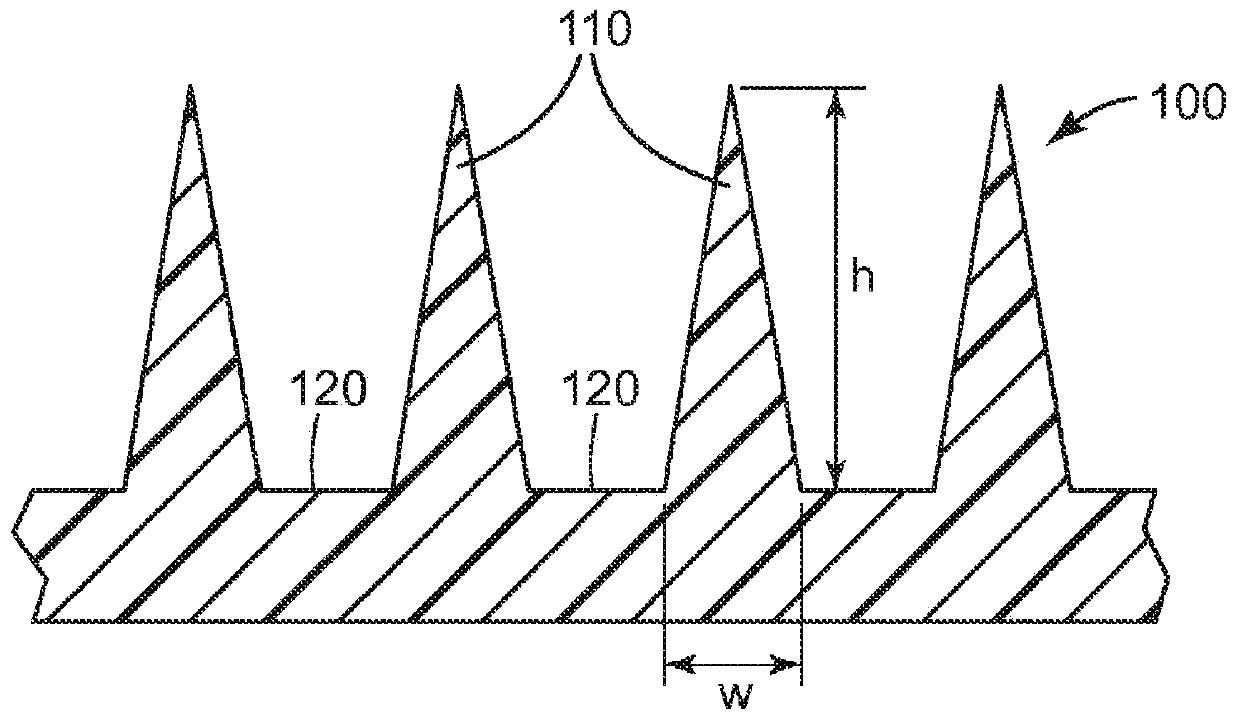
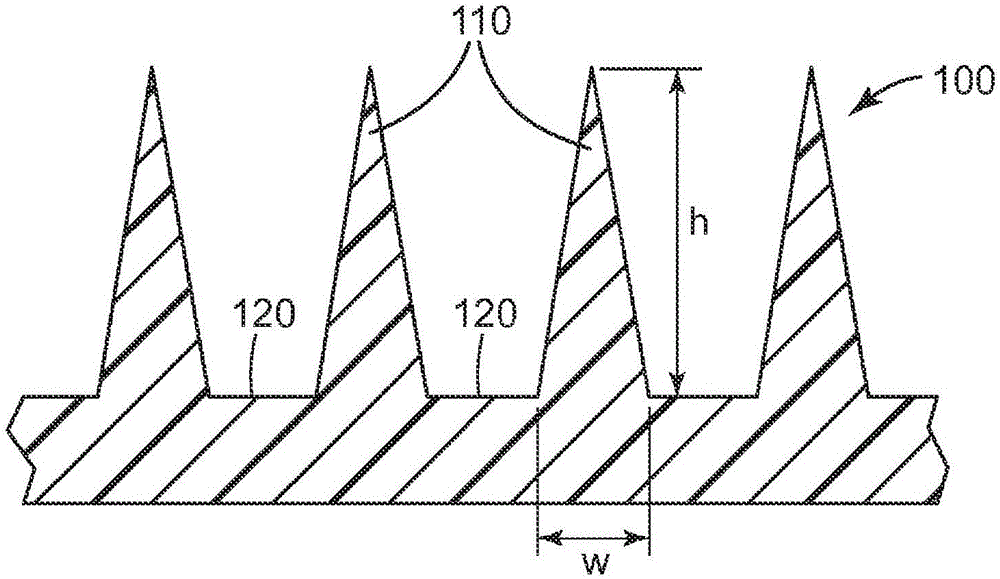
Microneedle devices and methods
A medical device, comprising: an array of microneedles, and a coating disposed on the microneedles, wherein the coating comprises: a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of alpha 1 adrenergic agonists, alpha 2 adrenergic agonists, and a combination thereof; wherein the local anesthetic is present in an amount of at least 1 wt-% based upon total weight of solids in the coating, and wherein the dose-extending component / local anesthetic weight ratio is at least 0.0001; a medical device, comprising an array of dissolvable microneedles, the microneedles comprising: a dissolvable matrix material; at least 1 wt-% of a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of alpha 1 adrenergic agonists, alpha 2 adrenergic agonists, and a combination thereof; wherein the dose-extending component / local anesthetic weight ratio is at least 0.0001, and wherein wt-% is based upon total weight of solids in all portions of the dissolvable microneedles which contain the local anesthetic; a method of extending a topically delivered local anesthetic dose in mammalian tissue using the devices; and methods of making the devices are provided.
Owner:3M INNOVATIVE PROPERTIES CO
Treatment of insulin resistance
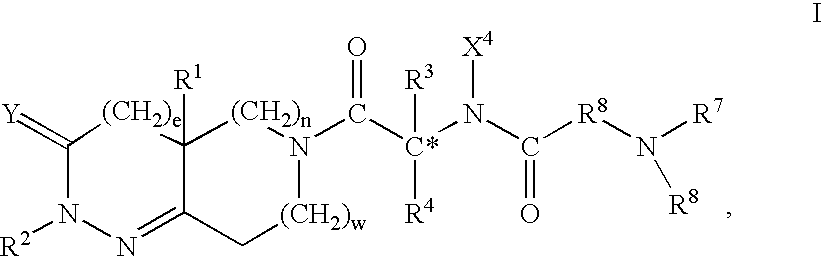
This invention is directed to methods of treating insulin resistance in a mammal which comprise administering an effective amount of a compound of formula I, where the variables are defined in the specification, or the stereoisomeric mixtures, diastereomerically enriched, diastereomerically pure, enantiomerically enriched or enantiomerically pure isomers, or the pharmaceutically acceptable salts and prodrugs thereof to said mammal. The compounds of formula I are growth hormone secretagogues and as such are useful for increasing the level of endogenous growth hormone. In another aspect this invention provides certain intermediates which are useful in the synthesis of the foregoing compounds and certain processes useful for the synthesis of said intermediates and th compounds of formula I. This invention is further directed to methods comprising administering to a human or other animal a combination of a functional somatostatin antagonist such as an alpha-2 adrenergic agonist and a compound of formula I.
Owner:PFIZER INC
Methods and compositions for treating dry eye disease and other eye disorders
ActiveUS9597328B2Good effectMaintain good propertiesPowder deliveryOrganic active ingredientsDiseaseEye disease
Owner:OCUGEN INC +1
Treatment of autistic spectrum disorder
A method for treating a patient having ASD is disclosed. The method includes administering to the patient a therapeutically effective amount of an alpha-2 adrenergic agonist in an extended release dosage form in combination with a therapeutically effective amount of inositol.
Owner:PRICE RICHARD LOUIS
Pharmaceutical compositions comprising alpha-2-adrenergics and trefoil factor family peptides
Disclosed herein are dosage forms comprising an alpha-2-adrenergic agonist and a trefoil factor family peptide. Related to these dosage forms are methods of treating glaucoma or reducing intraocular pressure and methods of treating gastrointestinal disorders.
Owner:ALLERGAN INC
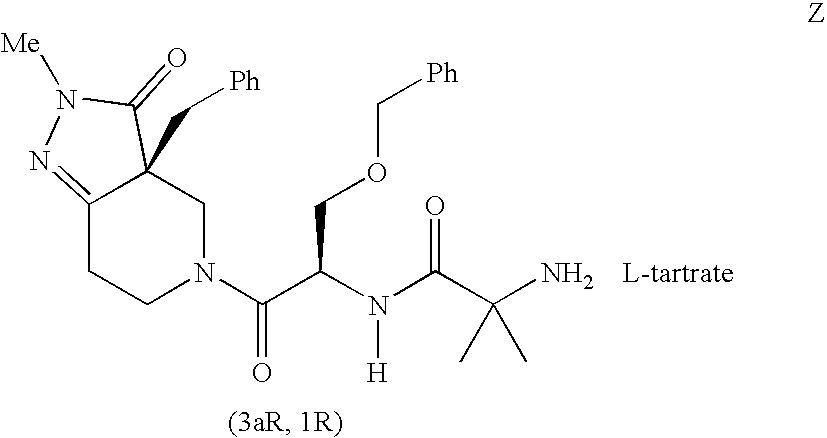
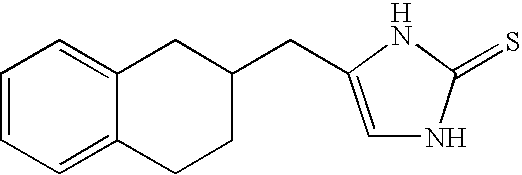
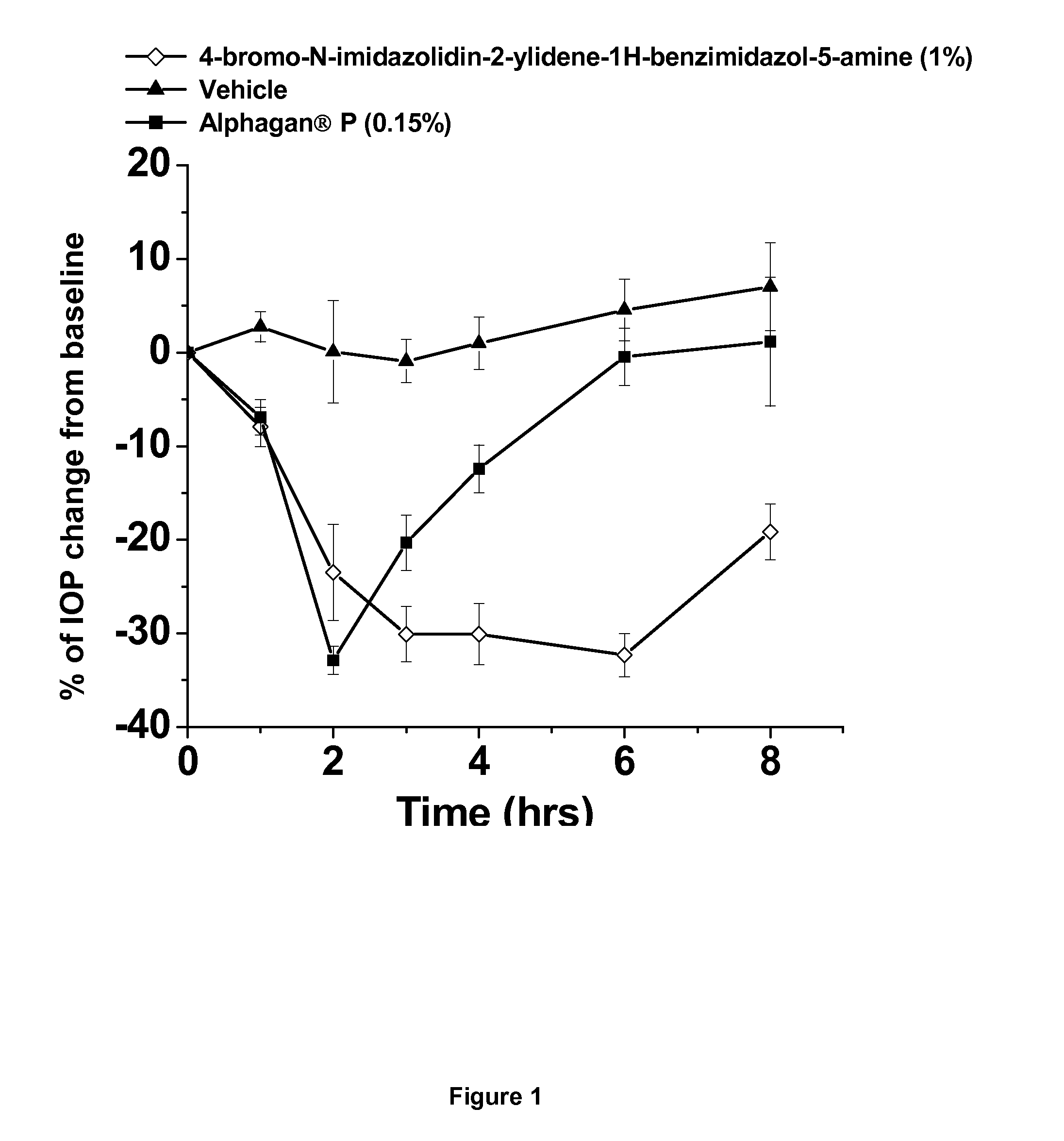
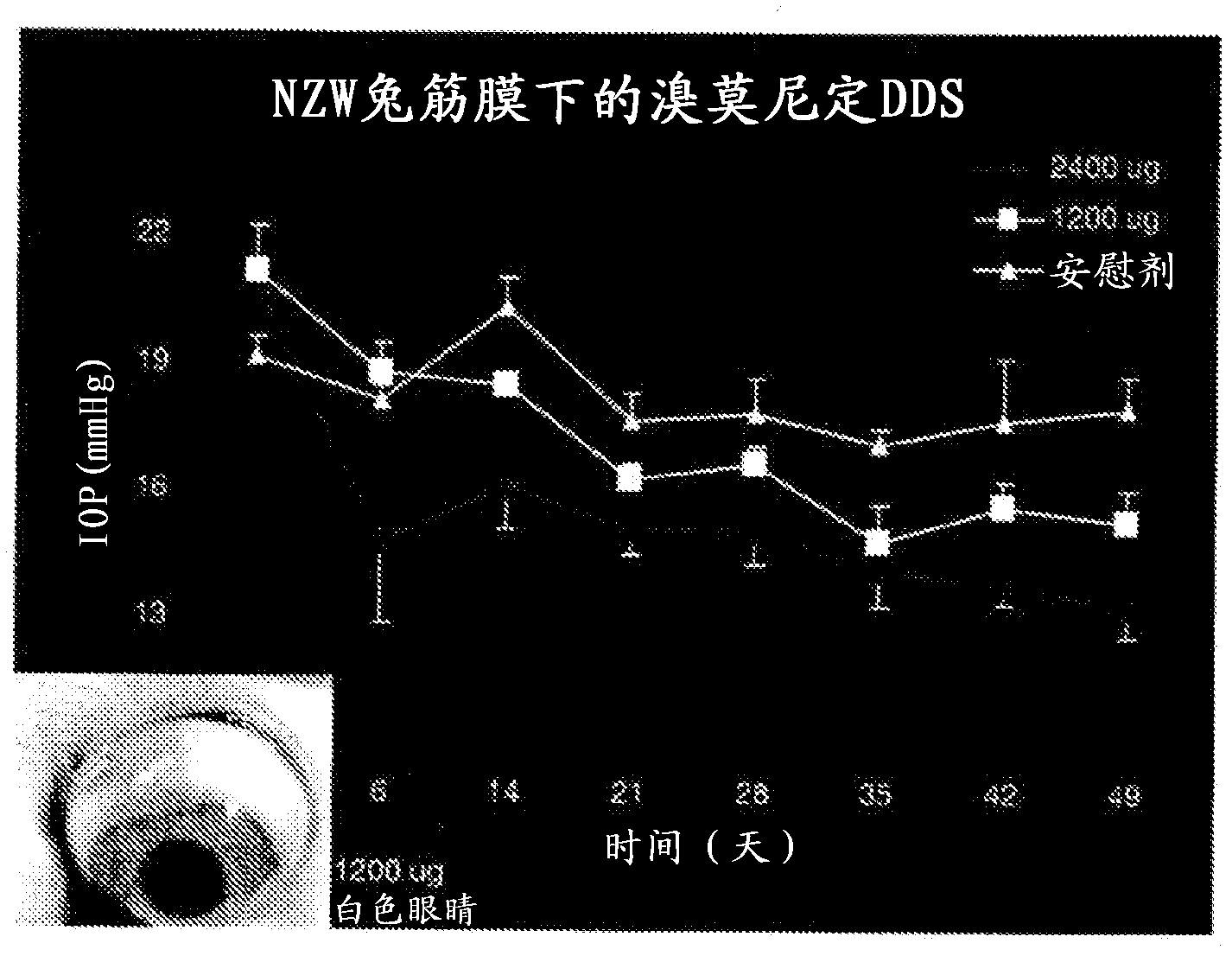
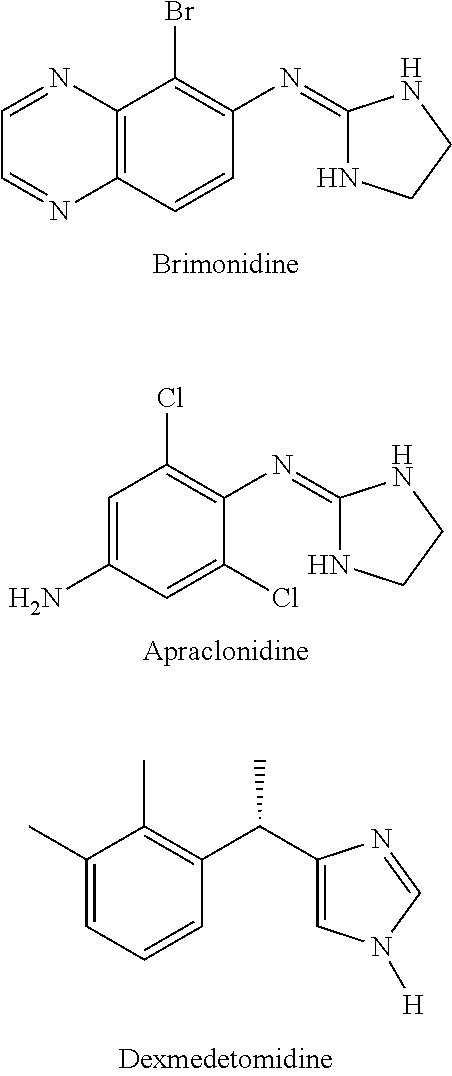
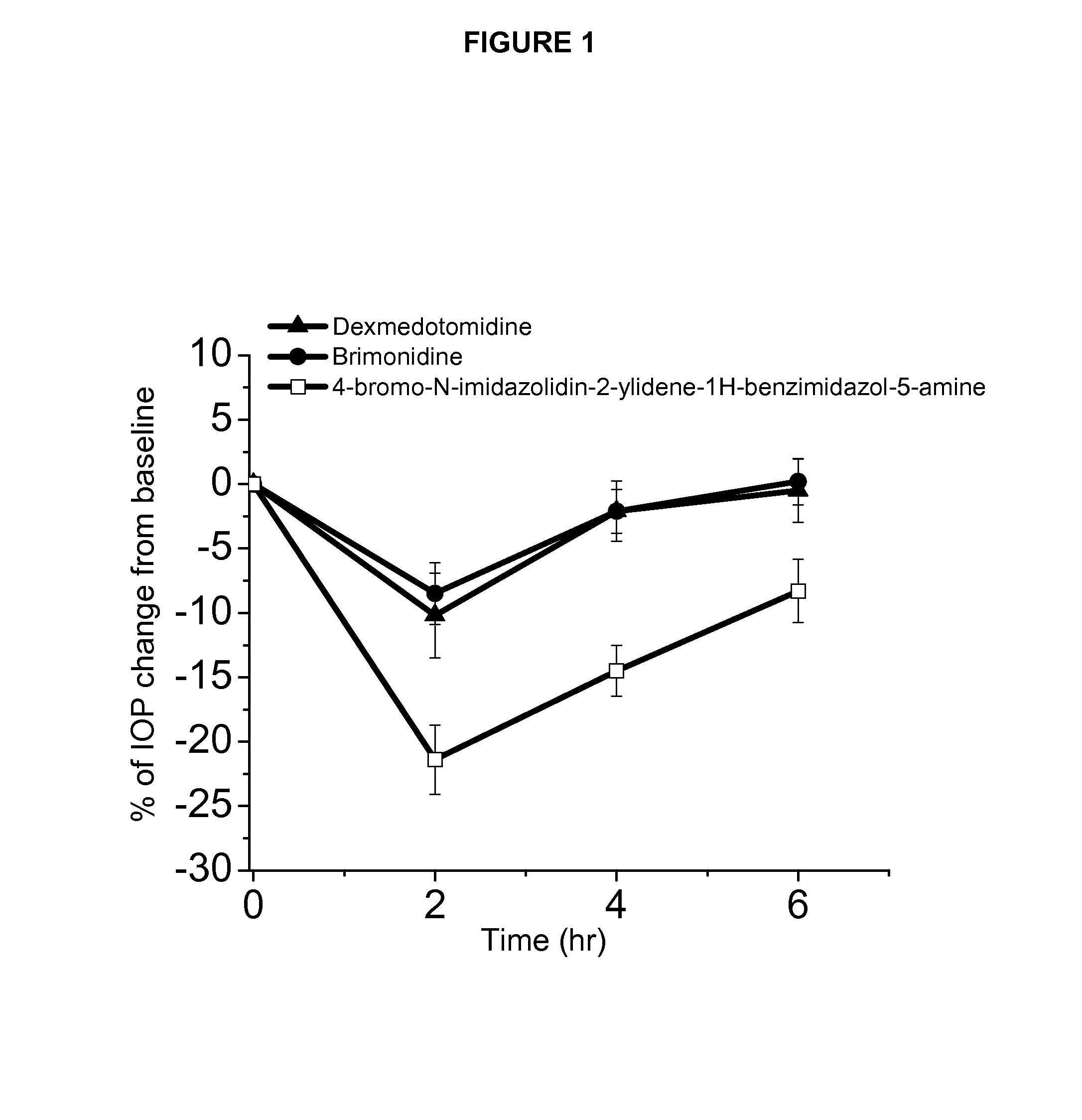
Alpha-2 adrenergic agonist having long duration ofintraocular pressure-lowering effect
ActiveUS20110178145A1Facilitated releaseLower eye pressureBiocideOrganic active ingredientsAdrenergic2-Imidazoline
The present invention provides a method of lowering intraocular pressure which comprises administering a therapeutically effective amount of a pharmaceutical composition comprising 4-bromo-5-(2-imidazolin-2-ylamino)benzimidazole, or a salt thereof to the affected eye of a patient, as a single dose, wherein the affected eye has an intraocular pressure less than the baseline intraocular pressure for at least eight (8) hours.
Owner:ALLERGAN INC
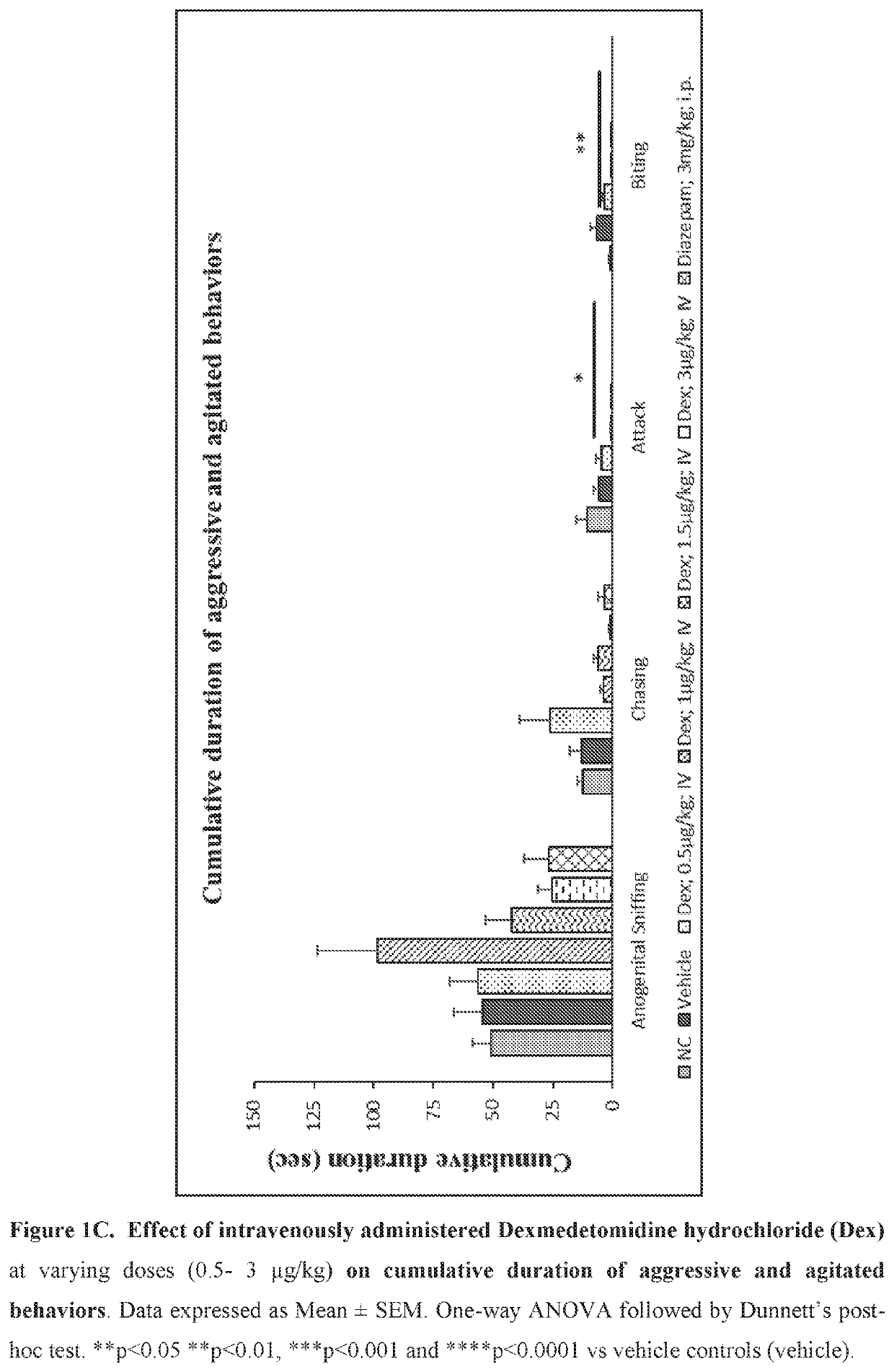
Use of sublingual dexmedetomidine for the treatment of agitation
PendingUS20190365715A1Preventing and reducing signOrganic active ingredientsNervous disorderNeuropsychiatric diseaseSublingual administration
The present invention discloses a method of treating agitation or the signs of agitation in a subject comprising the sublingual administration of an effective amount of an alpha-2 adrenergic agonist, more particularly Dexmedetomidine, or a pharmaceutically acceptable salt thereof. The method is particularly suitable for the treatment of agitation associated with neurodegenerative and / or neuropsychiatric diseases. The present invention also discloses the sublingual administration of an alpha-2 adrenergic agonist, more particularly Dexmedetomidine or a pharmaceutically acceptable salt thereof at a dose that is effective to treat agitation or the signs of agitation in a subject, but does not cause significant sedation.
Owner:BIOXCEL THERAPEUTICS INC
Compositions containing alpha-2-adrenergic agonist components
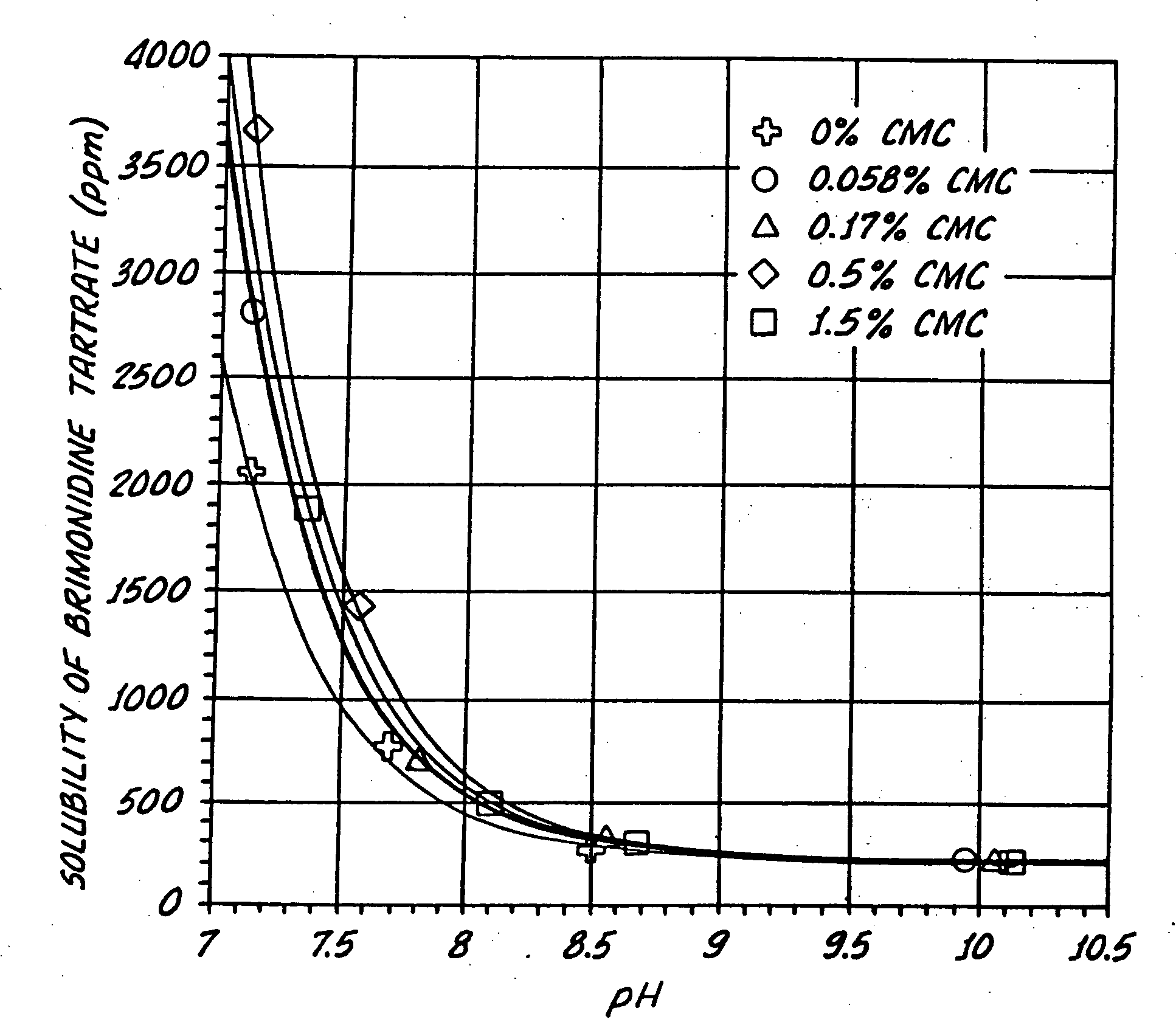
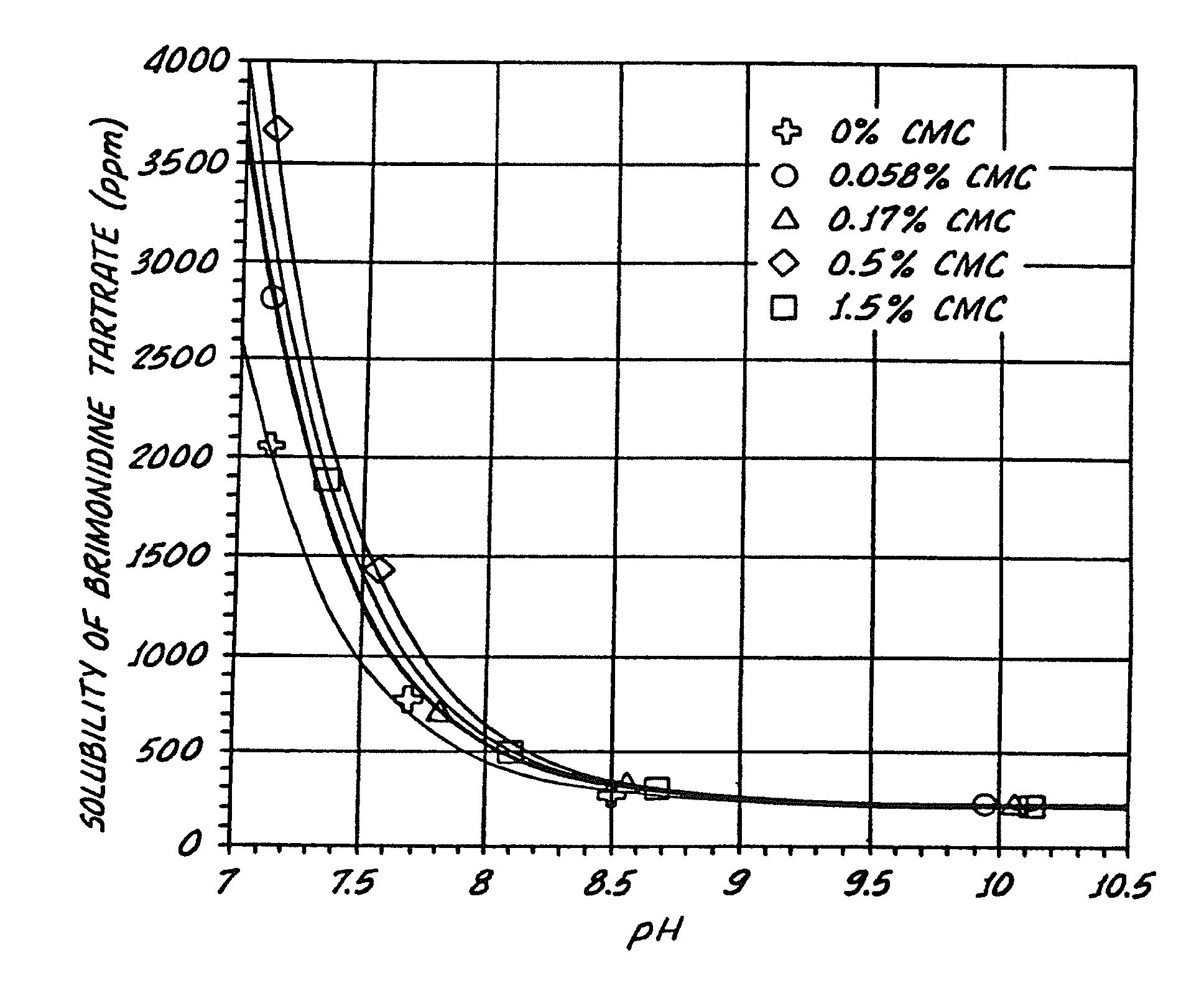
InactiveUS20050026924A1Reduce adverse reactionsImprove anti-corrosion performanceBiocidePowder deliverySolubilityGreek letter alpha
Compositions useful for improving effectiveness of alpha-2-adrenergic agonist components include carrier components, alpha-2-adrenergic agonist components, solubility enhancing components which aid in solubilizing the alpha-2-adrenergic agonist components. In one embodiment, the alpha-2-adrenergic agonist components include alpha-2-adrenergic agonists. In another embodiment, the solubility enhancing components include carboxymethylcellulose.
Owner:ALLERGAN INC
Methods to Treat Pain Using an Alpha-2 Adrenergic Agonist and an Endothelin Antagonist
The present invention relates, in general to treatment of pain comprising administering an alpha-2 adrenergic agonist and an endothelin antagonist, wherein administration of the agents acts as an analgesic and ameliorates pain in a subject.
Owner:ENDOGENX
Compositions containing alpha-2-adrenergic agonist components
Compositions useful for improving effectiveness of alpha-2-adrenergic agonist components include carrier components, alpha-2-adrenergic agonist components, solubility enhancing components which aid in solubilizing the alpha-2-adrenergic agonist components. In one embodiment, the alpha-2-adrenergic agonist components include alpha-2-adrenergic agonists. In another embodiment, the solubility enhancing components include carboxymethylcellulose.
Owner:ALLERGAN INC
Diagnosis and treatment of a form of autistic spectrum disorder
A method for treating a patient having P.R.I.C.E. Syndrome is disclosed. The method includes administering to the patient a therapeutically effective amount of an alpha-2 adrenergic agonist in an extended release dosage form, optionally in combination with a therapeutically effective amount of inositol and / or NAC.
Owner:PRICE RICHARD LOUIS
Methods to treat pain using an alpha-2 adrenergic agonist and an endothelin antagonist
InactiveUS20110263542A1Good analgesic effectProduce analgesiaBiocideNervous disorderEndothelin receptor antagonistEndothelin Antagonists
The present invention relates, in general to treatment of pain comprising administering an alpha-2 adrenergic agonist and an endothelin antagonist, wherein administration of the agents acts as an analgesic and ameliorates pain in a subject.
Owner:ENDOGENX
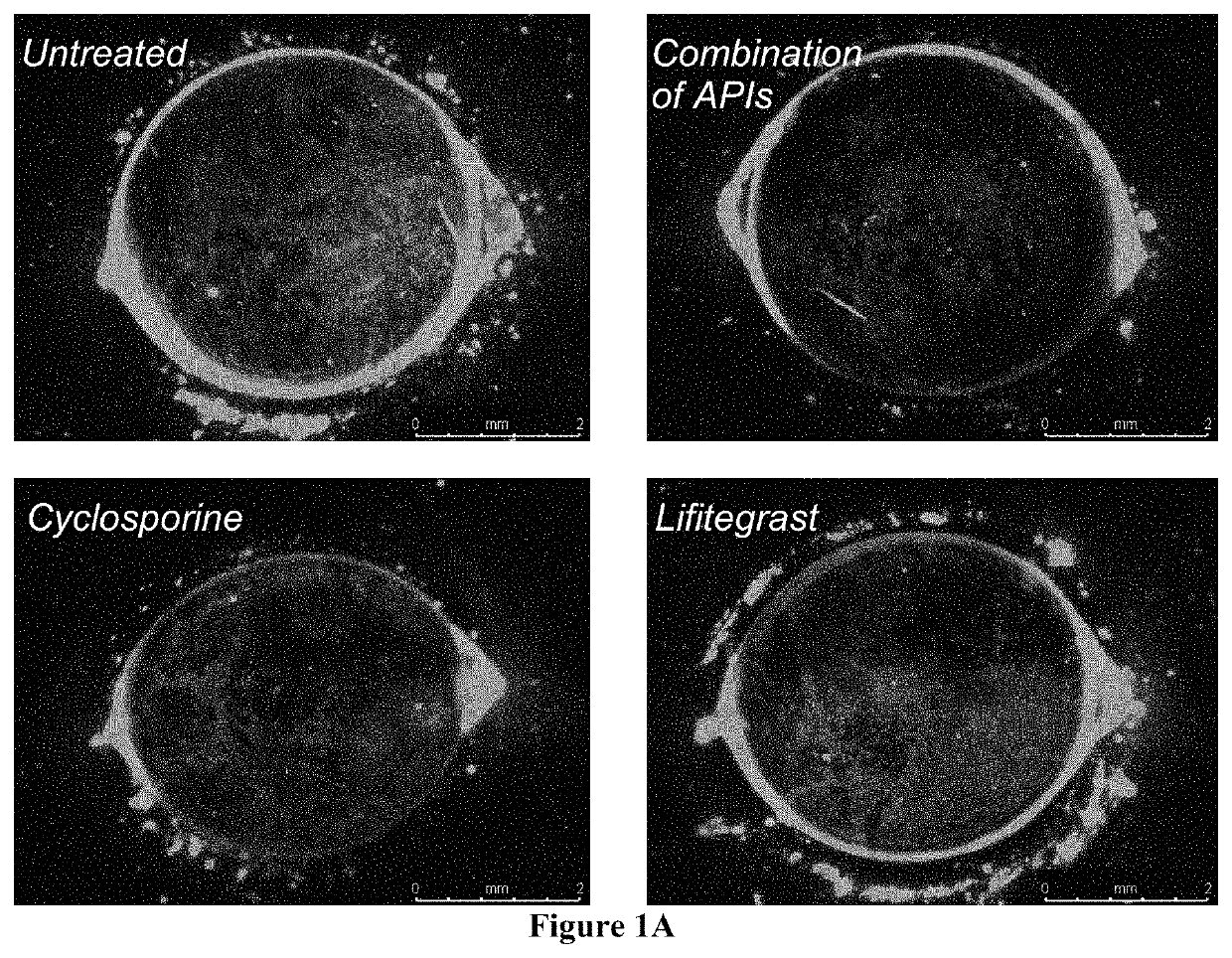
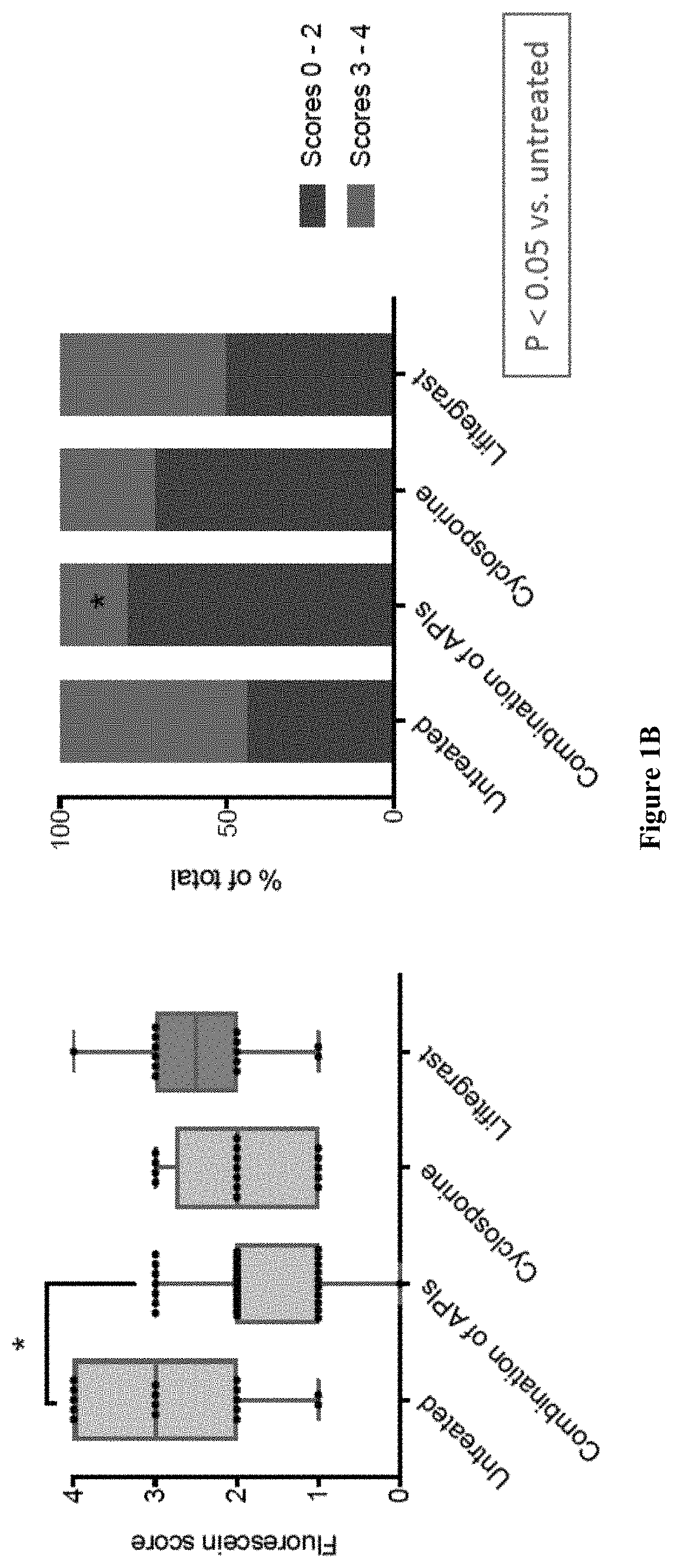
Ophthalmic compositions and methods of use
ActiveUS20180333414A1Small amountEliminate side effectsOrganic active ingredientsSenses disorderAntigenHerpes zoster keratitis
The present invention provides a composition comprising two or more of the following pharmaceutically active compounds: (i) an alpha 2 adrenergic agonist; (ii) a corticosteroid; (iii) a lymphocyte function-associated antigen antagonist; (iv) a non-steroidal anti-inflammatory drug (NSAID); (v) a sodium channel blocker; and (vi) an antibiotic, provided at least one of the pharmaceutically active compound is selected from the group consisting of (i) alpha 2 adrenergic agonist and (ii) corticosteroid. The present invention also provides a method for using such composition to treat an eye disorder such as a dry eye syndrome; ocular graft-versus-host-disease; ocular rosacea; allergic conjunctivitis; autoimmune ocular surface disease; thygeson's superficial punctuate keratopathy; herpes zoster keratitis; Stevens-Johnson syndrome; keratitis; conjunctivitis; blepharitis; blepharochalasis; conjunctivochalasis; blepharoconjunctivitis; blepharokeratoconjunctivitis; post-operative inflammation or pain from ocular surgery; scleritis; episcleritis; anterior uveitis; iritis; cyclitis; ocular surface vascular disorder; ulcerative keratitis; photokeratitis; dacryocystitis; eyelid disorder; congenital alacrima; xerophthalmia; dacryoadenitis; vernal keratoconjunctivitis; pinguecula; and / or ocular surface disorder induced by chemical burns, thermal burns, or physical insult to the ocular surface.
Owner:OCUGEN INC
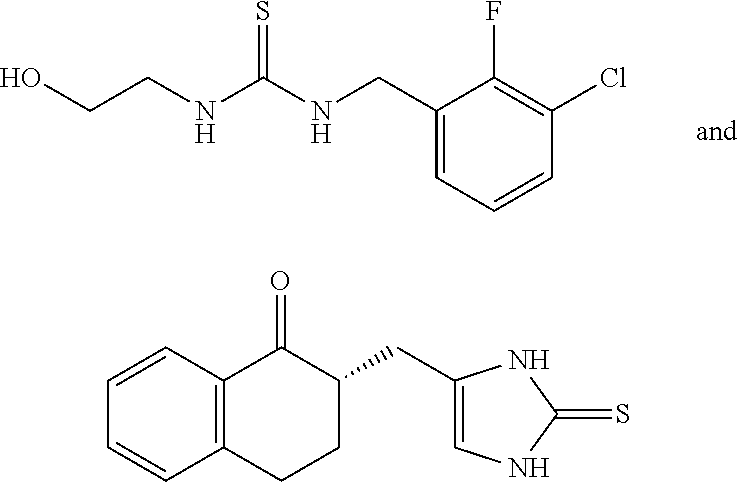
Selective subtype alpha 2 adrenergic agents and methods for use thereof
The invention provides well-defined heterocyclic compounds that are useful as subtype selective alpha 2 adrenergic agonists. As such, the compounds described herein are useful in treating a wide variety of disorders associated with selective subtype modulation of alpha 2 adrenergic receptors.
Owner:ALLERGAN INC
Treatment of insulin resistance with growth hormone secretagogues
This invention is directed to methods of treating insulin resistance in a mammal which comprise administering an effective amount of a compound of formula I, where the variables are defined in the specification, or the stereoisomeric mixtures, diastereomerically enriched, diastereomerically pure, enantiomerically enriched or enantiomerically pure isomers, or the pharmaceutically acceptable salts and prodrugs thereof to said mammal. The compounds of formula I are growth hormone secretagogues and as such are useful for increasing the level of endogenous growth hormone. In another aspect this invention provides certain intermediates which are useful in the synthesis of the foregoing compounds and certain processes useful for the synthesis of said intermediates and the compounds of formula I. This invention is further directed to methods comprising administering to a human or other animal a combination of a functional somatostatin antagonist such as an alpha-2 adrenergic agonist and a compound of formula I.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Ophthalmic compositions and methods of use
ActiveUS10555947B2Small amountEliminate side effectsOrganic active ingredientsSenses disorderHerpes zoster keratitisPostoperative inflammation
The present invention provides a composition comprising two or more of the following pharmaceutically active compounds: (i) an alpha 2 adrenergic agonist; (ii) a corticosteroid; (iii) a lymphocyte function-associated antigen antagonist; (iv) a non-steroidal anti-inflammatory drug (NSAID); (v) a sodium channel blocker; and (vi) an antibiotic, provided at least one of the pharmaceutically active compound is selected from the group consisting of (i) alpha 2 adrenergic agonist and (ii) corticosteroid. The present invention also provides a method for using such composition to treat an eye disorder such as a dry eye syndrome; ocular graft-versus-host-disease; ocular rosacea; allergic conjunctivitis; autoimmune ocular surface disease; thygeson's superficial punctuate keratopathy; herpes zoster keratitis; Stevens-Johnson syndrome; keratitis; conjunctivitis; blepharitis; blepharochalasis; conjunctivochalasis; blepharoconjunctivitis; blepharokeratoconjunctivitis; post-operative inflammation or pain from ocular surgery; scleritis; episcleritis; anterior uveitis; iritis; cyclitis; ocular surface vascular disorder; ulcerative keratitis; photokeratitis; dacryocystitis; eyelid disorder; congenital alacrima; xerophthalmia; dacryoadenitis; vernal keratoconjunctivitis; pinguecula; and / or ocular surface disorder induced by chemical burns, thermal burns, or physical insult to the ocular surface.
Owner:OCUGEN INC
Diagnosis and treatment of p.r.i.c.e. syndrome
A method for treating a patient having P.R.I.C.E. Syndrome is disclosed. The method includes administering to the patient a therapeutically effective amount of an alpha-2 adrenergic agonist in an extended release dosage form.
Owner:PRICE RICHARD LOUIS
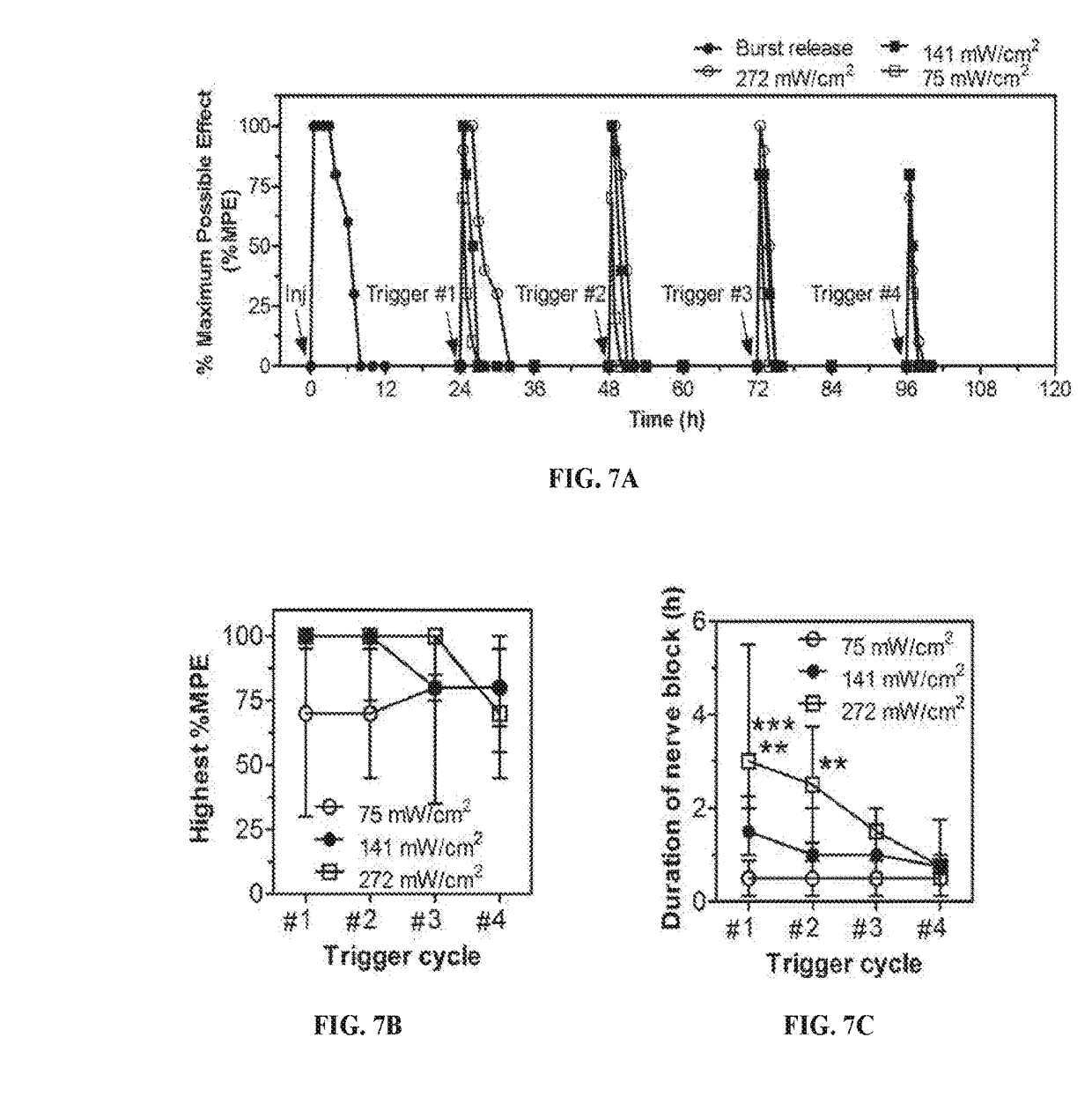
Compositions and Methods for On-Demand High-Efficiency Triggerable Anesthesia
ActiveUS20190070115A1Extended durationIncrease the number ofOrganic active ingredientsAnaestheticsAnesthetic AgentSingle injection
Compositions and methods for administration of local anesthetics that are delivered by a single injection and enable repeated on-demand or high influx analgesia over extended periods have been developed. Pharmaceutical compositions including an effective amount of one or more sodium channel blockers including site 1 sodium channel blockers, optionally one or more alpha-2-adrenergic agonists, which are optionally encapsulated in liposomes, particles or microbubbles, and one or more triggerable elements are provided. The triggerable elements allow delivery of the encapsulated anesthetic drugs when an appropriate triggering stimuli are applied. Exemplary triggering agents or stimuli include near-infrared irradiation, UV- and visible light, ultrasound and magnetic field. In one embodiment, ultrasound is used to trigger a burst of microbubbles to enhance penetration of local anesthetic.
Owner:CHILDRENS MEDICAL CENT CORP
Preservative free ocular compositions and methods for using the same for treating dry eye disease and other eye disorders
ActiveUS20180153885A1Reduce needOrganic active ingredientsSenses disorderDiseaseBULK ACTIVE INGREDIENT
The present invention provides a preservative free ophthalmic formulation. In particular, the ophthalmic formulations of the invention are aqueous formulations comprising nanoemulsion of oil. The present invention also provides a method for treating an eye disorder. In one particular embodiment, the invention provides methods for treating dry eye syndrome using an preservative free formulation comprising a nanoemulsion of oil and alpha 2 adrenergic agonist, pharmaceutically acceptable salt thereof or a mixture thereof. In particular, the alpha 2 adrenergic agonist of the invention has a higher alpha 2A agonist activity compared to alpha 2B agonist activity. This invention also provides a preservative free ophthalmic composition comprising a nanoemulsion of oil, a therapeutically effective amount of an alpha 2 adrenergic agonist, a pharmaceutically acceptable salt thereof or a combination thereof as an active ingredient for treating a dry eye syndrome.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Intraocular drug delivery systems
Provided is intraocular drug delivery systems. The invention refers to biodegradable implants sized and suitable for implantation in an ocular region or site and methods for treating ocular conditions. The implants provide an extended release of an active agent at a therapeutically effective amount for a period of time between 10 days and one year or longer. The active agents are selected from estradiol such as 2-methoxyl estradiol or alpha 2-adrenergic agonist such as brimonidine or derivatives thereof or prostaglandin analogues.
Owner:ALLERGAN INC
Treatment of autistic spectrum disorder
A method for treating a patient having ASD is disclosed. The method includes administering to the patient a therapeutically effective amount of an alpha-2 adrenergic agonist in an extended release dosage form in combination with a therapeutically effective amount of inositol.
Owner:PRICE RICHARD LOUIS
Compositions containing alpha-2-adrenergic agonist components
Compositions useful for improving effectiveness of alpha-2-adrenergic agonist components include carrier components, alpha-2-adrenergic agonist components, solubility enhancing components which aid in solubilizing the alpha-2-adrenergic agonist components. In one embodiment, the alpha-2-adrenergic agonist components include alpha-2-adrenergic agonists. In another embodiment, the solubility enhancing components include carboxymethylcellulose.
Owner:ALLERGAN INC
Alpha-2 adrenergic agonist for treating intraocular pressure and ocular diseases through intravitreal and intracameral routes
InactiveUS20140275197A1Facilitated releaseHigh structural similarityBiocideOrganic active ingredientsDiseaseIntracameral route
The present invention provides a method of lowering intraocular pressure which comprises administering a therapeutically effective amount of a pharmaceutical composition comprising 4-bromo-5-(2-imidazolin-2-ylamino)benzimidazole, or a salt thereof to the affected eye of a patient, as a single dose, wherein the affected eye has an intraocular pressure less than the baseline.
Owner:ALLERGAN INC
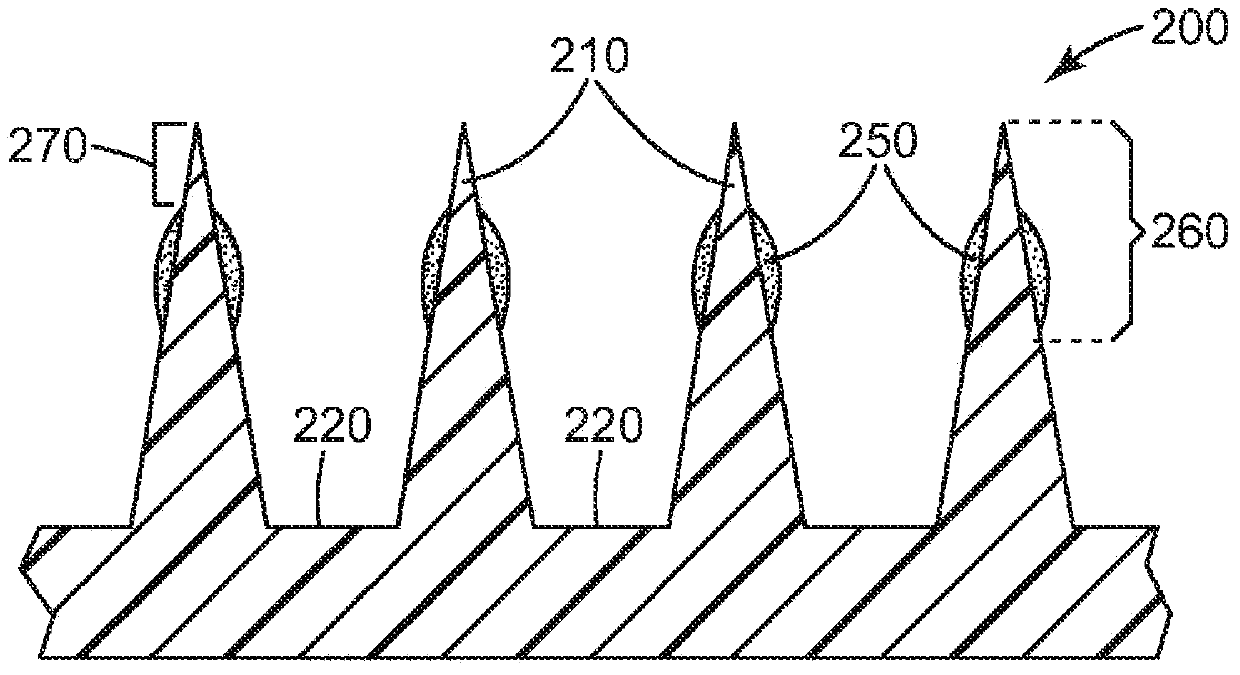
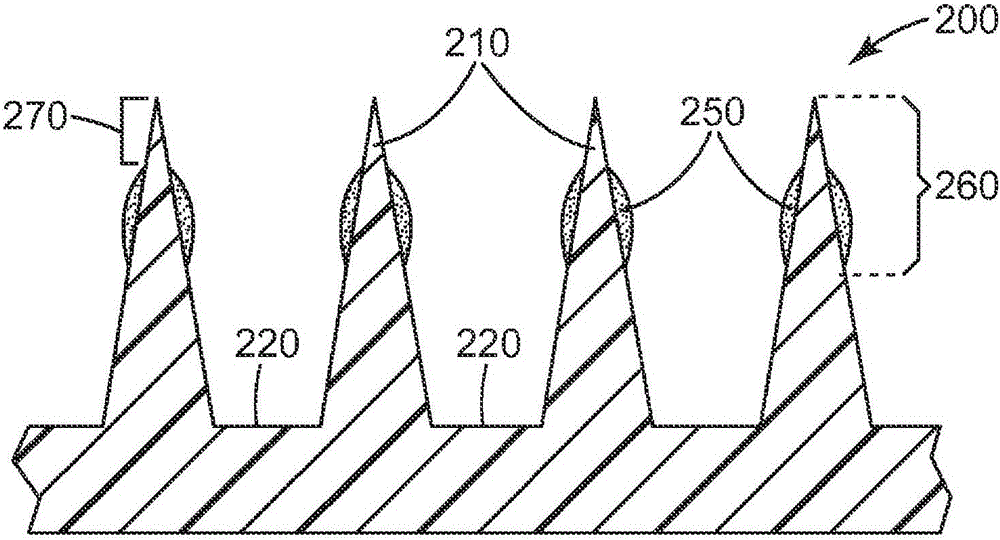
Microneedle devices and methods
The present invention provides a medical device, which comprises: a microneedle array, and a coating disposed on the microneedles, wherein the coating comprises: a local anesthetic selected from lidocaine, Prilocaine and combinations thereof; and a local anesthetic dose sustained release component selected from the group consisting of alpha 1 adrenergic agonists, alpha 2 adrenergic agonists and combinations thereof; wherein based on the coating The total weight of solids, the local anesthetic is present in an amount of at least 1% by weight, and wherein the dose-extending component / local anesthetic weight ratio is at least 0.0001; a medical device comprising An array of dissolving microneedles comprising: a dissolvable matrix material; at least 1% by weight of a local anesthetic selected from the group consisting of lidocaine, prilocaine, and combinations thereof; and a local anesthetic dose sustained release component , the local anesthetic dose-sustaining release component is selected from α1 adrenergic agonist, α2 adrenergic agonist and combinations thereof; wherein the weight ratio of the dose-sustaining component / local anesthetic is at least 0.0001, and wherein the weight % based on the total weight of solids in all portions of the dissolvable microneedles containing the local anesthetic; a method of using the device to provide sustained release of a locally delivered dose of local anesthetic in mammalian tissue; and a method of manufacturing method of the device.
Owner:3M INNOVATIVE PROPERTIES CO
Diagnosis and treatment of P.R.I.C.E. syndrome
A method for treating a patient having P.R.I.C.E. Syndrome is disclosed. The method includes administering to the patient a therapeutically effective amount of an alpha-2 adrenergic agonist in an extended release dosage form.
Owner:PRICE RICHARD LOUIS
Alpha-2 adrenoceptor and sigma receptor ligand combinations
The invention refers to a combination comprising a Sigma ligand of general formula (I) and alpha-2-adrenergic agonist compound, a medicament comprising said active substance combination, and the use of said active substance combination for the manufacture of a medicament, particularly for the prophylaxis and / or treatment of pain.
Owner:LAB DEL DR ESTEVE SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com