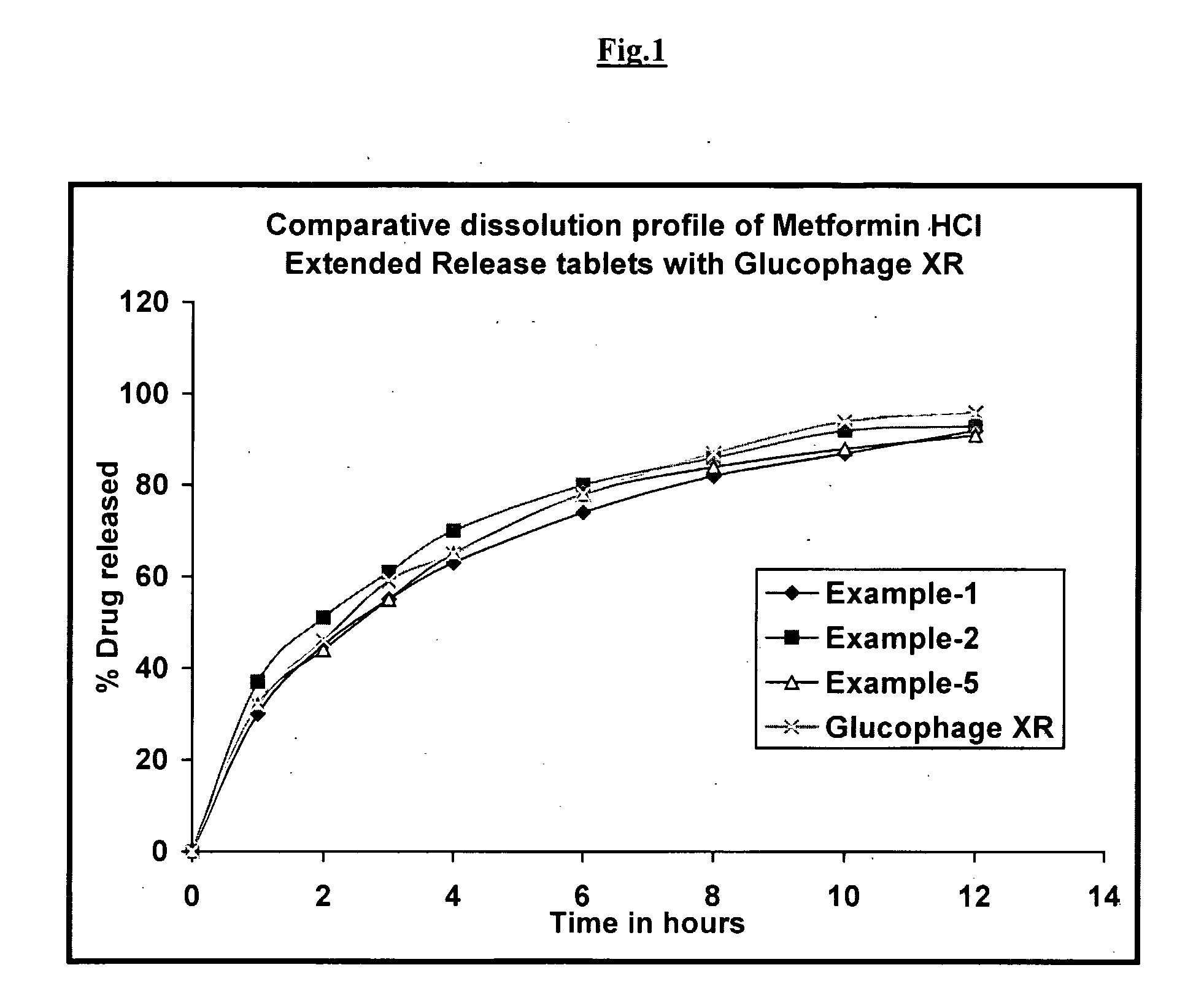
Pharmaceutical Compositions of Metformin
a technology of metformin and composition, which is applied in the field of extended release dosage, can solve the problems of slow release rate, two difficulties are further compounded, and the formulation is difficult to provide a slow release rate, and achieves good reproducibility and adequate hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0049]
IngredientsQuantityMetformin HCl750 mg Magnesium Aluminum Silicate240 mg Xanthan gum60 mgCarbopol 971 P96 mgHydroxypropyl cellulose14 mgMagnesium stearate10 mgIPAq.s.Tablet Weight1170 mg
example 2
[0050]
IngredientsQuantityMetformin HCl750 mg Magnesium Aluminum Silicate150 mg Xanthan gum60 mgCarbopol 971 P96 mgHydroxypropyl cellulose14 mgMagnesium stearate10 mgIPAq.s.Tablet Weight1080 mg
[0051]The processing steps that are involved in examples 1 and 2 are[0052]i) sifted metformin HCl, magnesium aluminum silicate, xanthan gum, and carbopol 971 P through #40 mesh,[0053]ii) loaded the material of step (i) in RMG and mixed for 15 minutes,[0054]iii) dissolved hydroxypropyl cellulose in sufficient quantity of IPA:water[0055]iv) added the binder solution of step (iii) to dry mix of step (ii) and continued mixing until granules of uniform consistency were obtained,[0056]v) granules were dried, milled and lubricated,[0057]vi) granules of step (v) were compressed to form extended release tablets of metformin.
example 3
[0058]
IngredientsQuantityMetformin HCl750 mg Magnesium Aluminum Silicate240 mg Xanthan gum60 mgCarbapol 971 P96 mgPVP K9014 mgMagnesium stearate10 mgIPAq.s.Tablet Weight1170 mg
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com