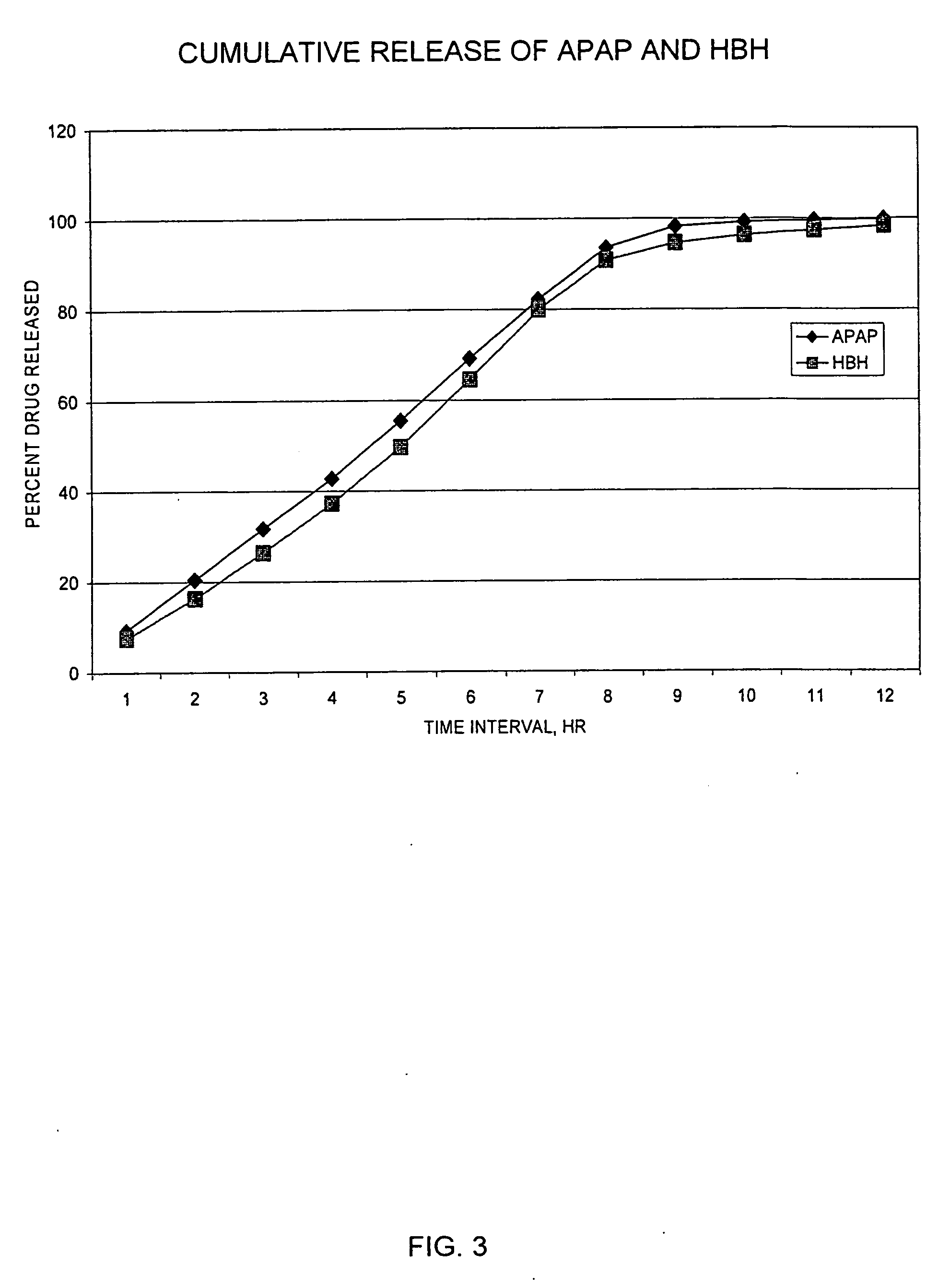
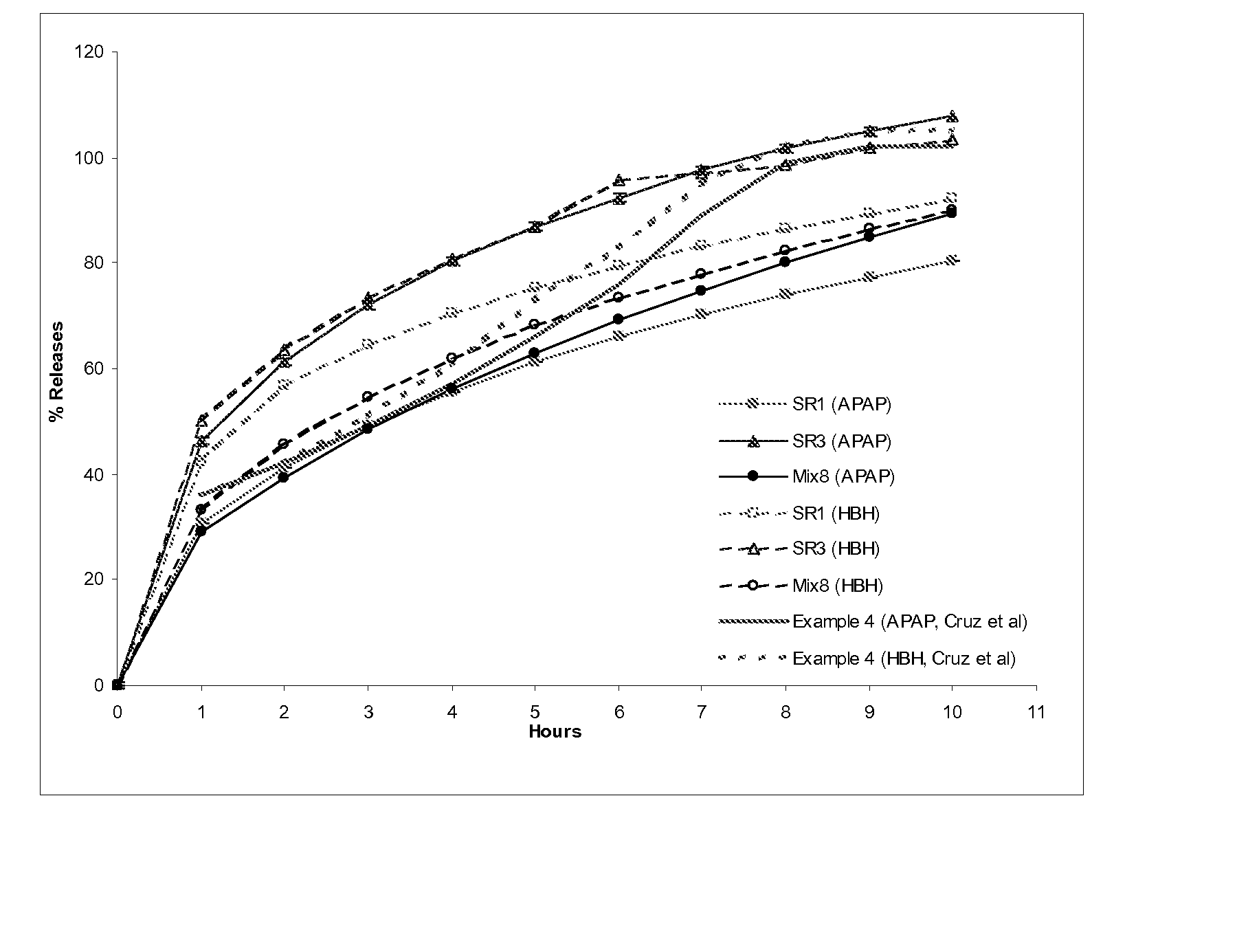
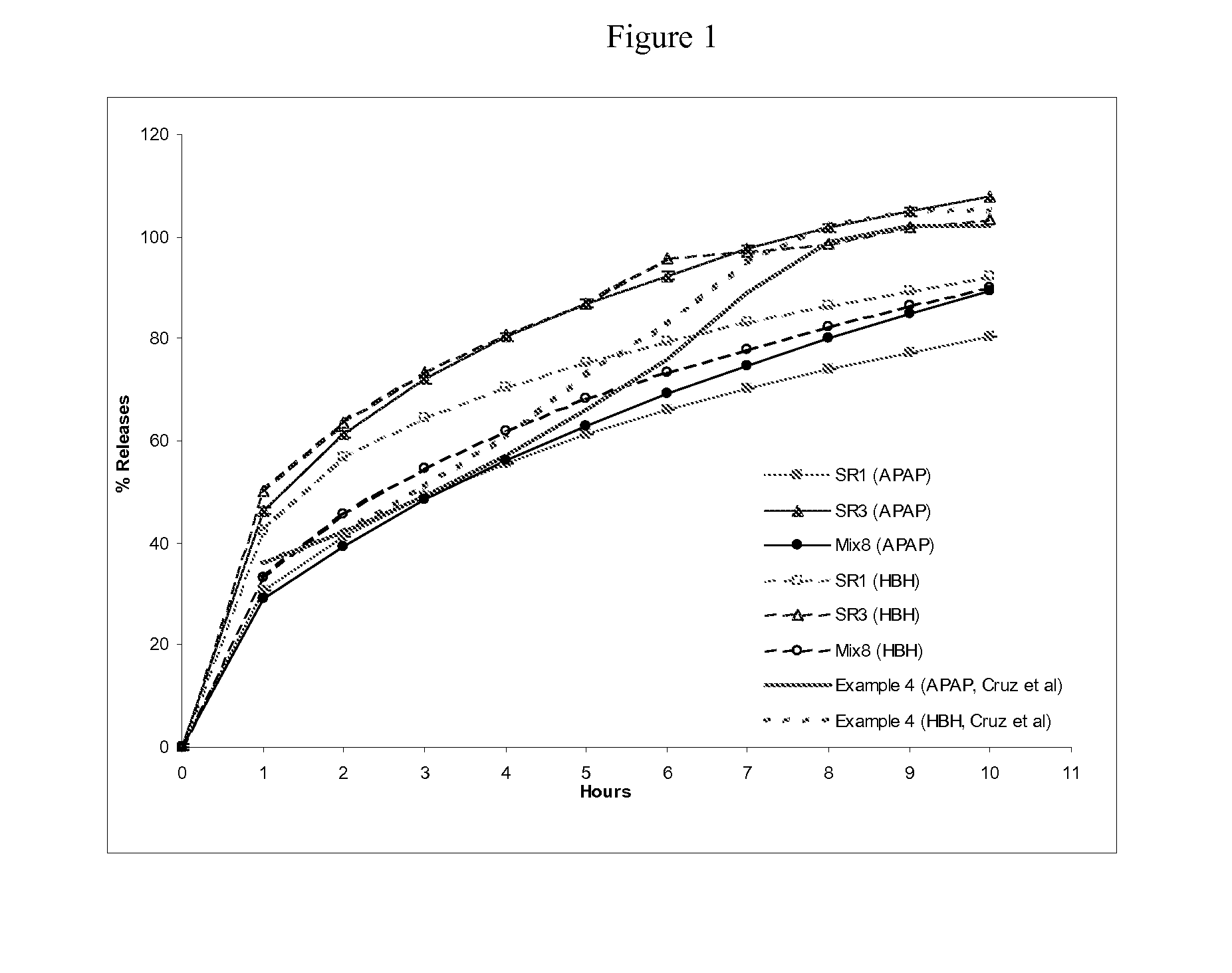
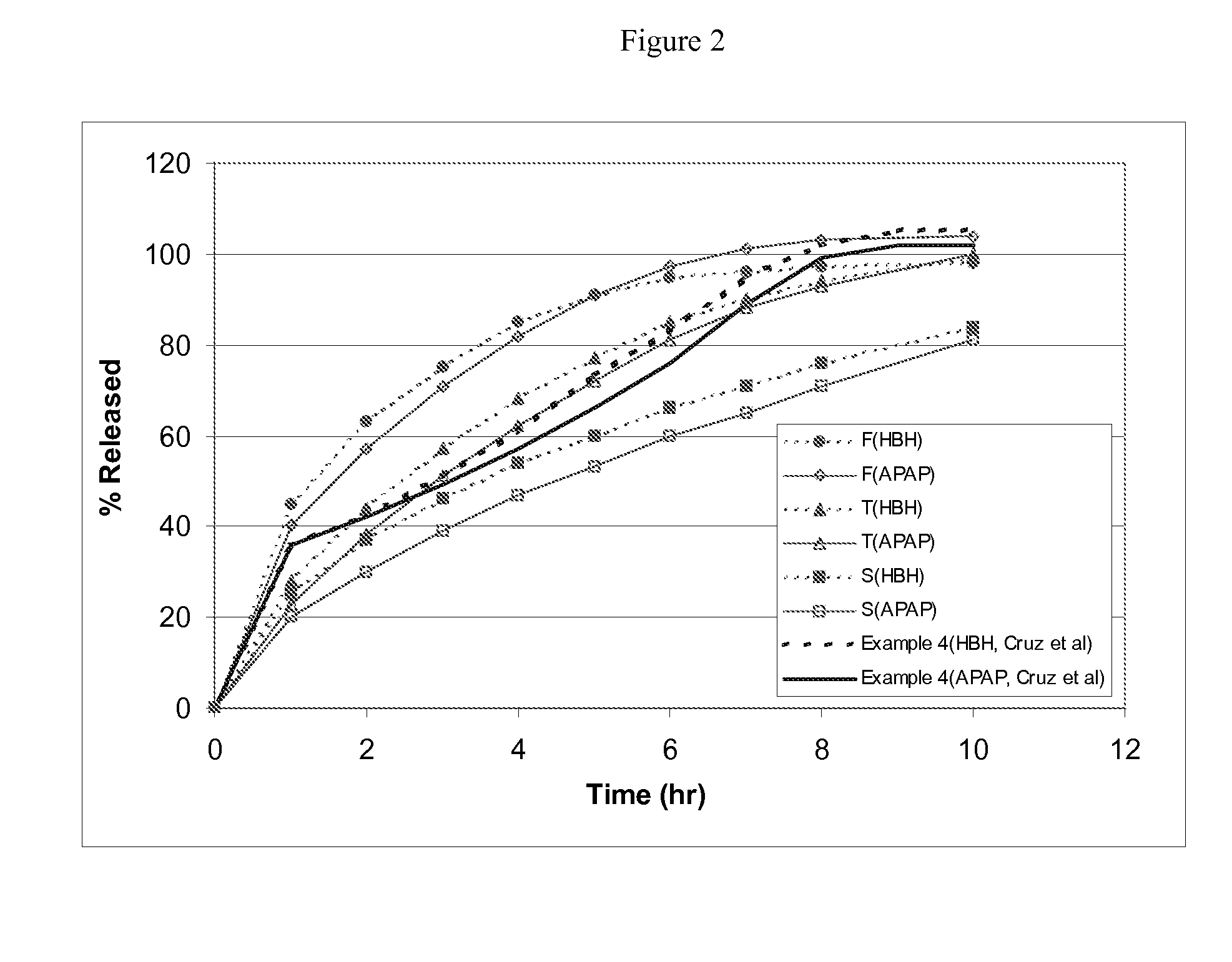
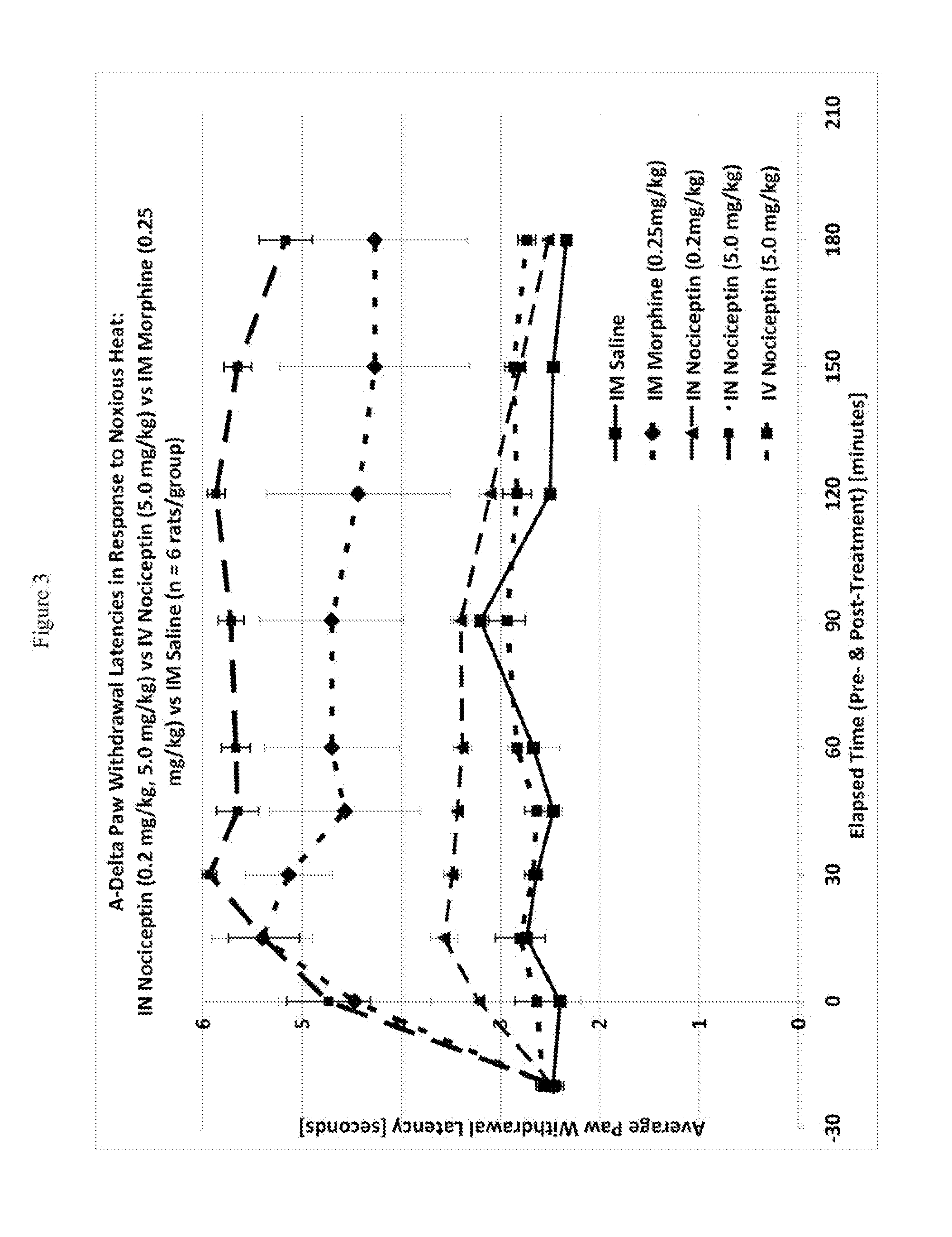
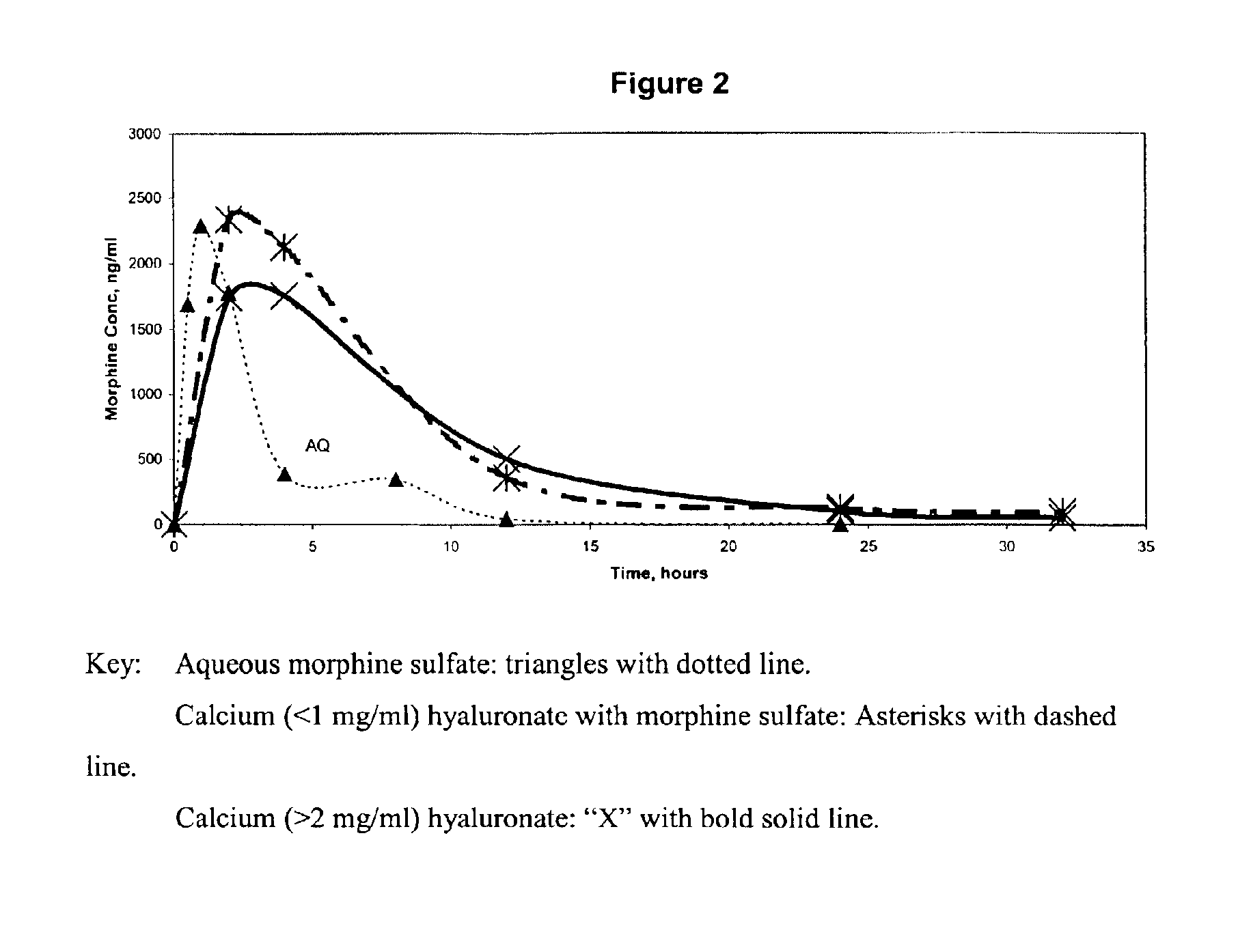
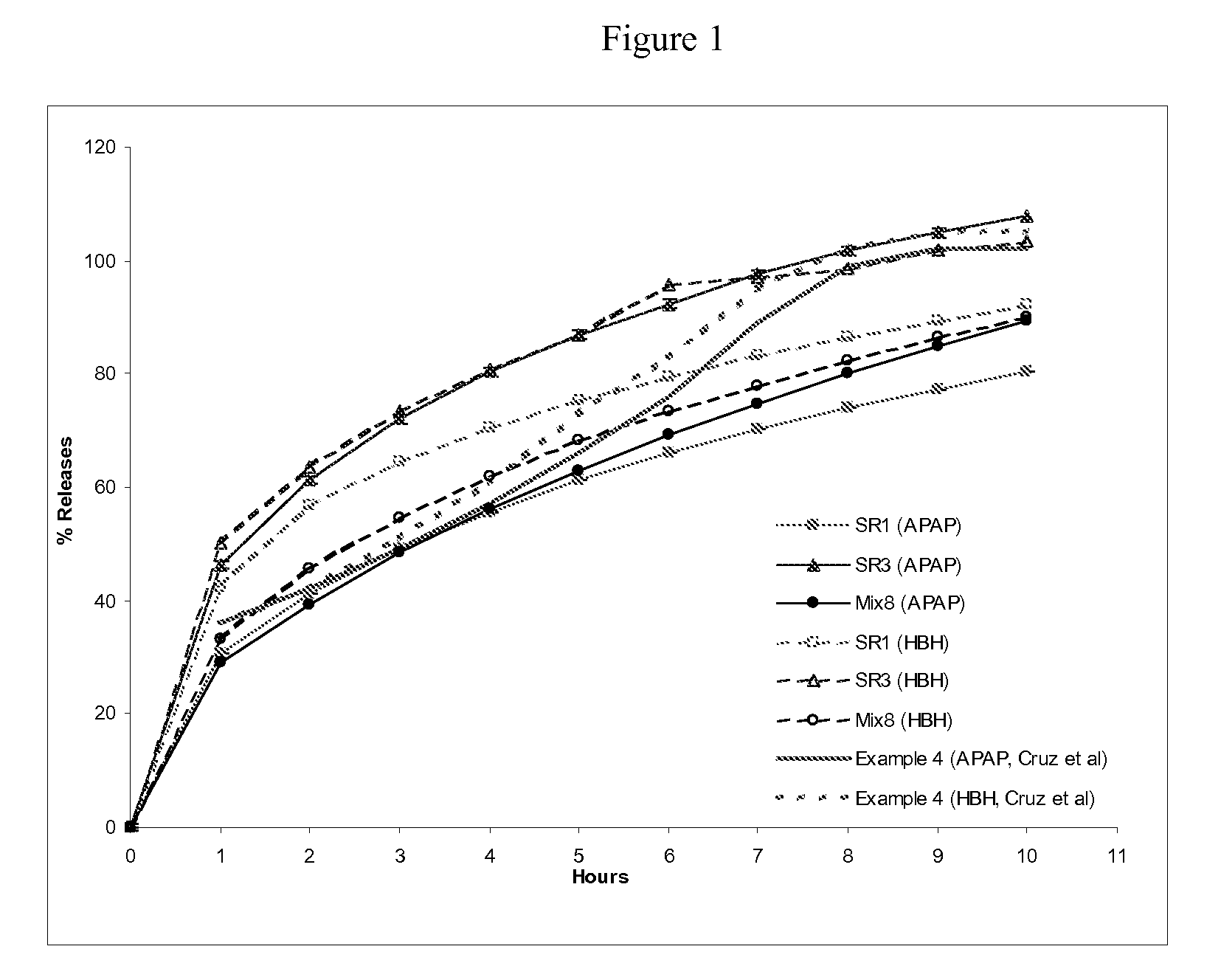
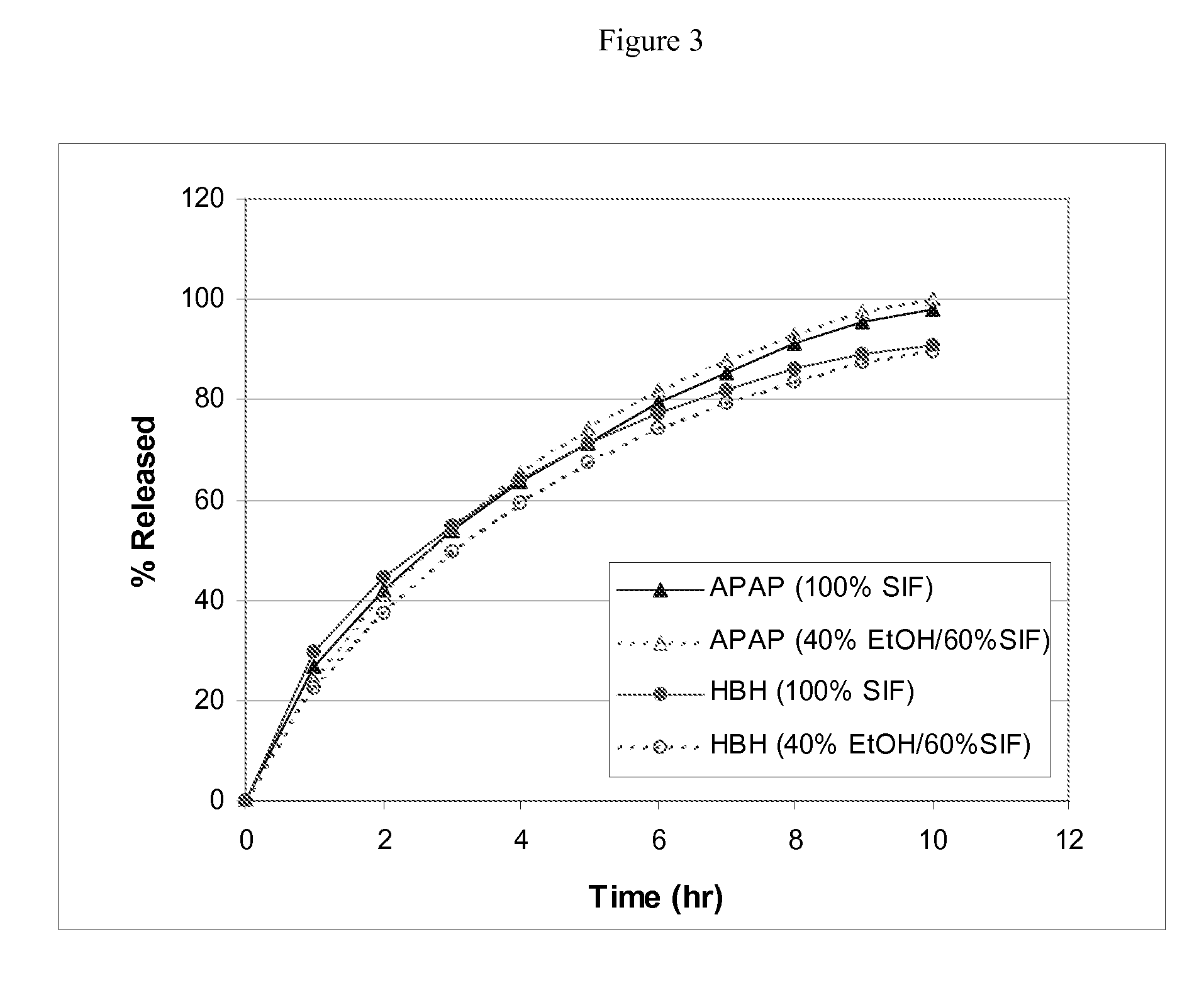
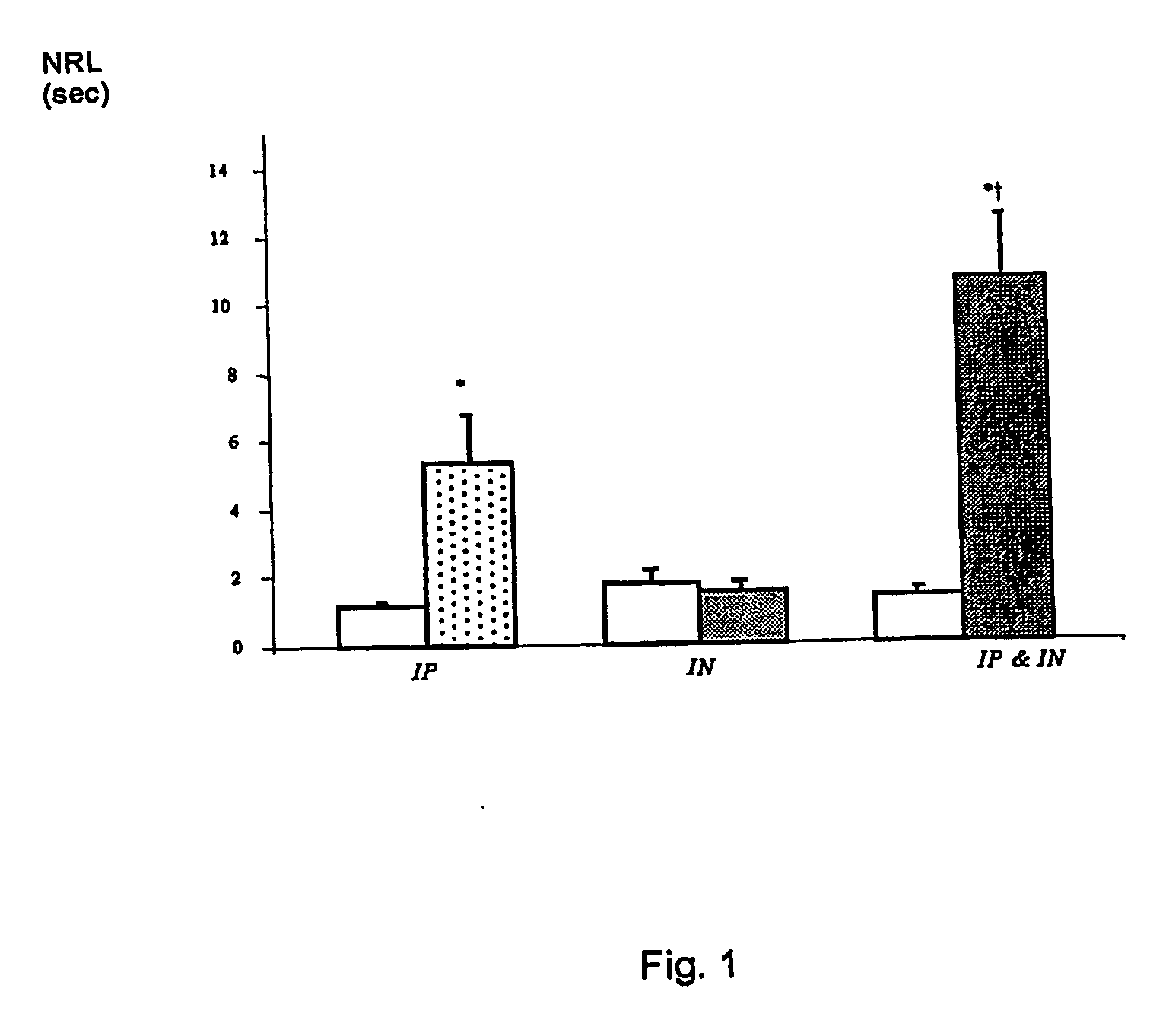
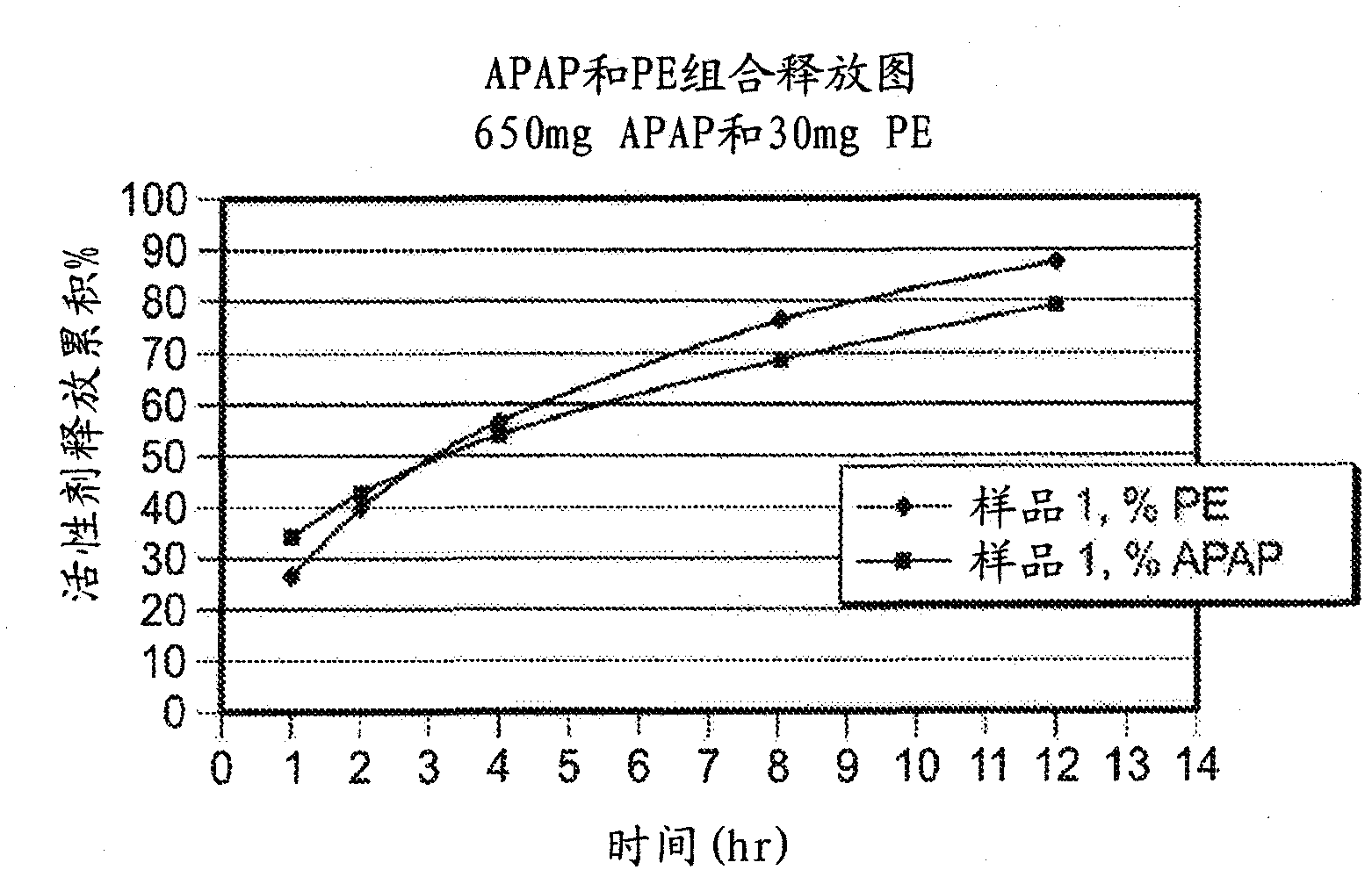
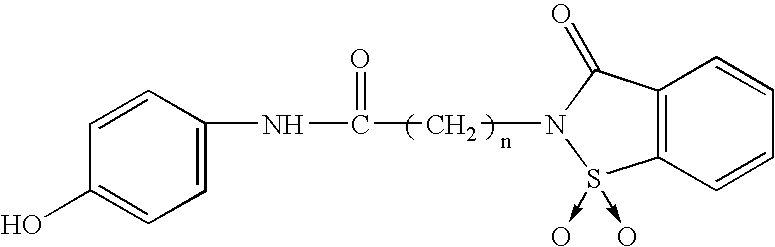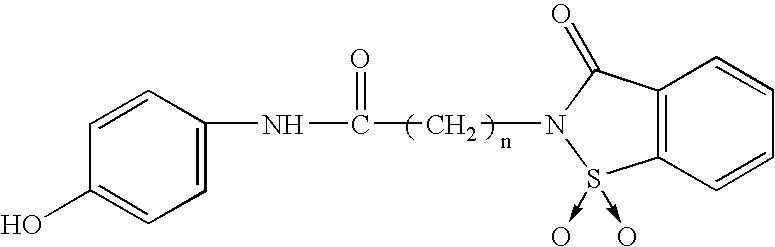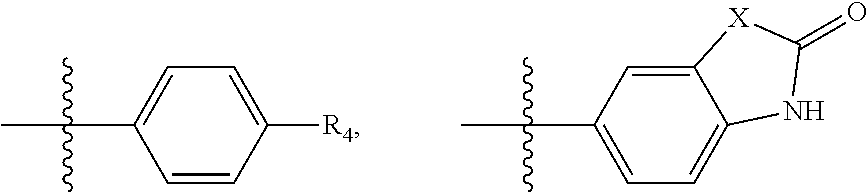Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Non opioid analgesics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non- opioid analgesics are medications that are non-narcotic and are used for the management of mild or moderate pain. Some examples of non-opioid analgesics include acetaminophen; all non-steroidal anti-inflammatory drugs ( NSAIDs) like ibuprofen, ketoprofen, diclofenac, and aspirin; and some drugs called adjuvant analgesics, such as antidepressants, that are used for pain relief despite having a different primary intent.
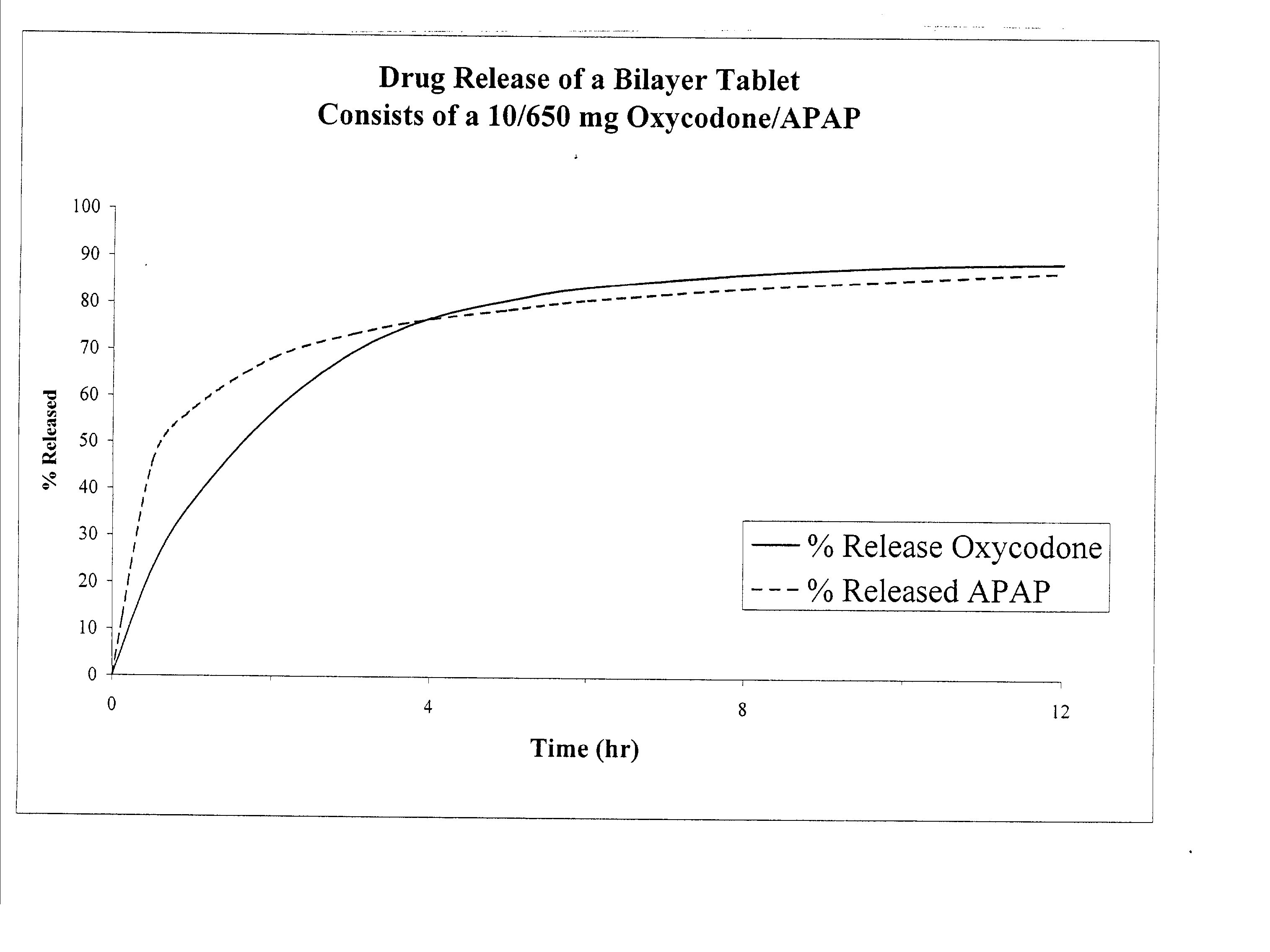
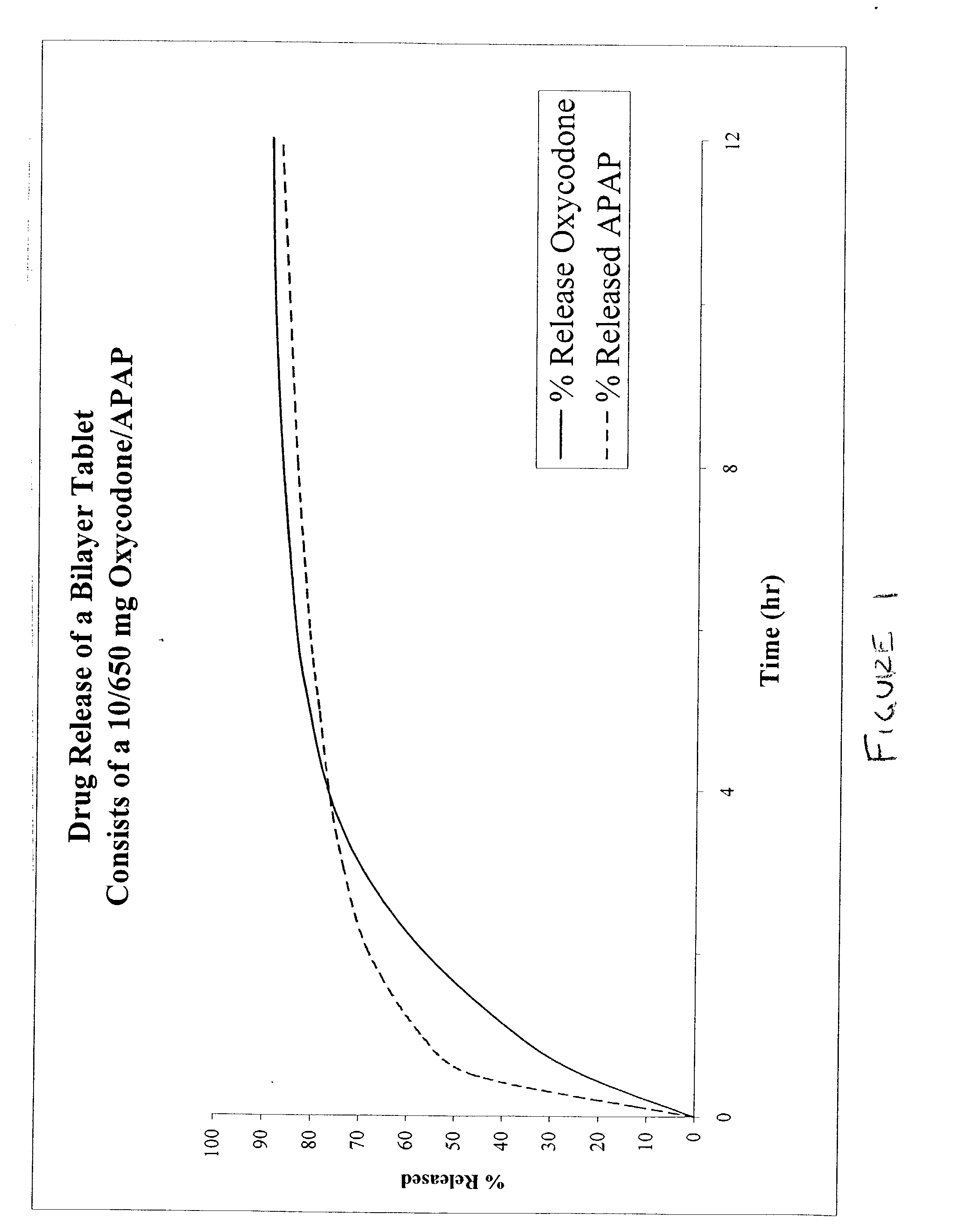
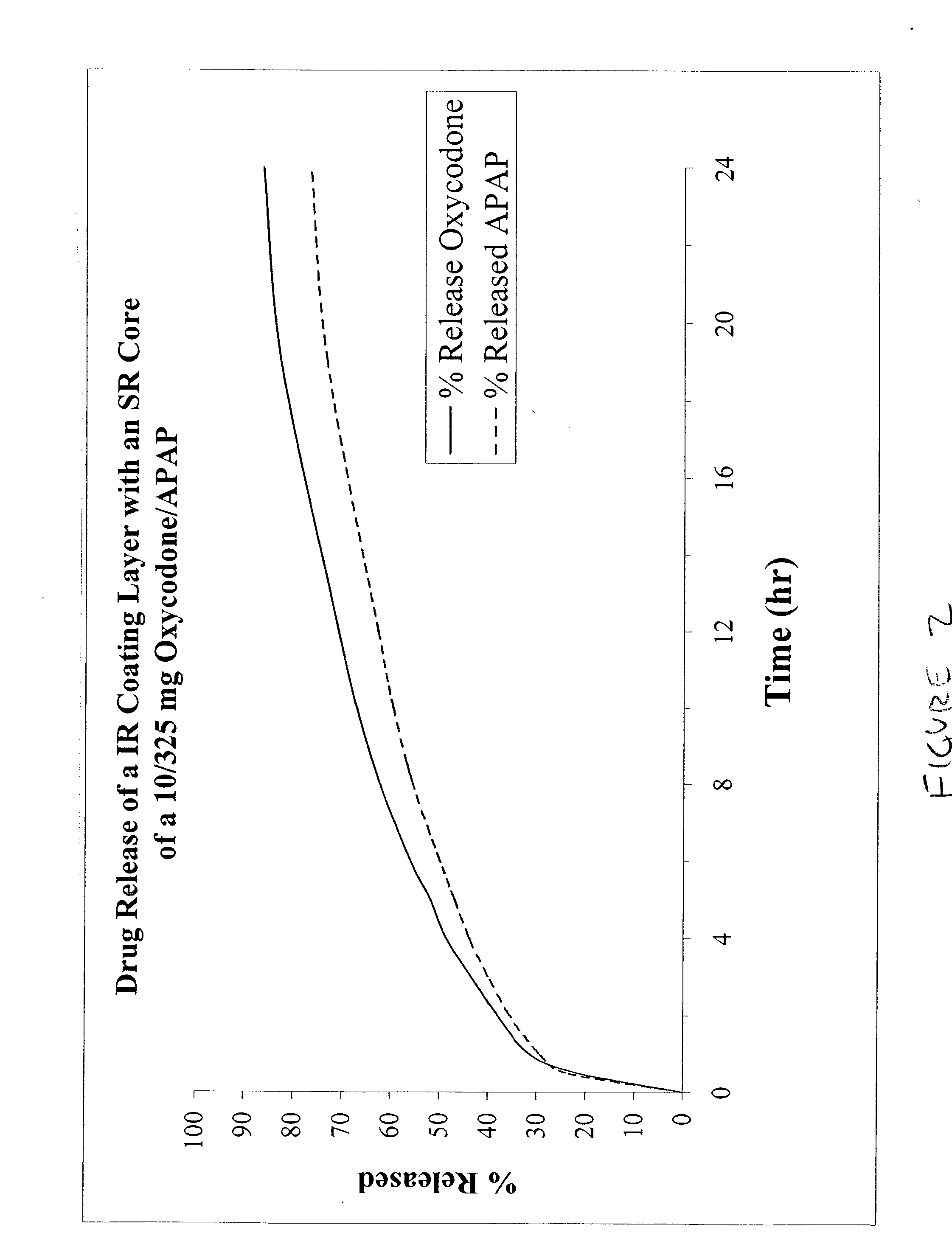
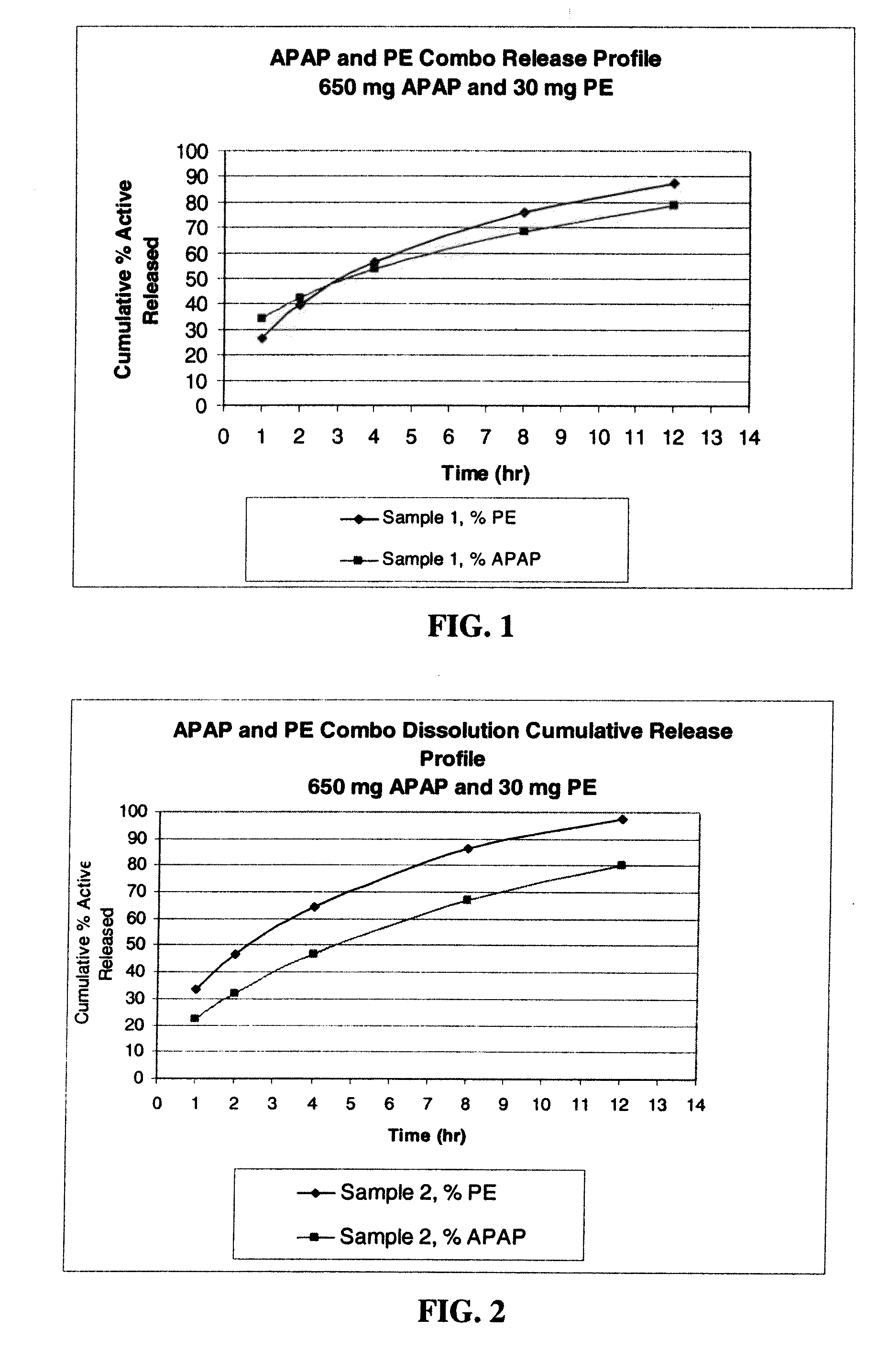
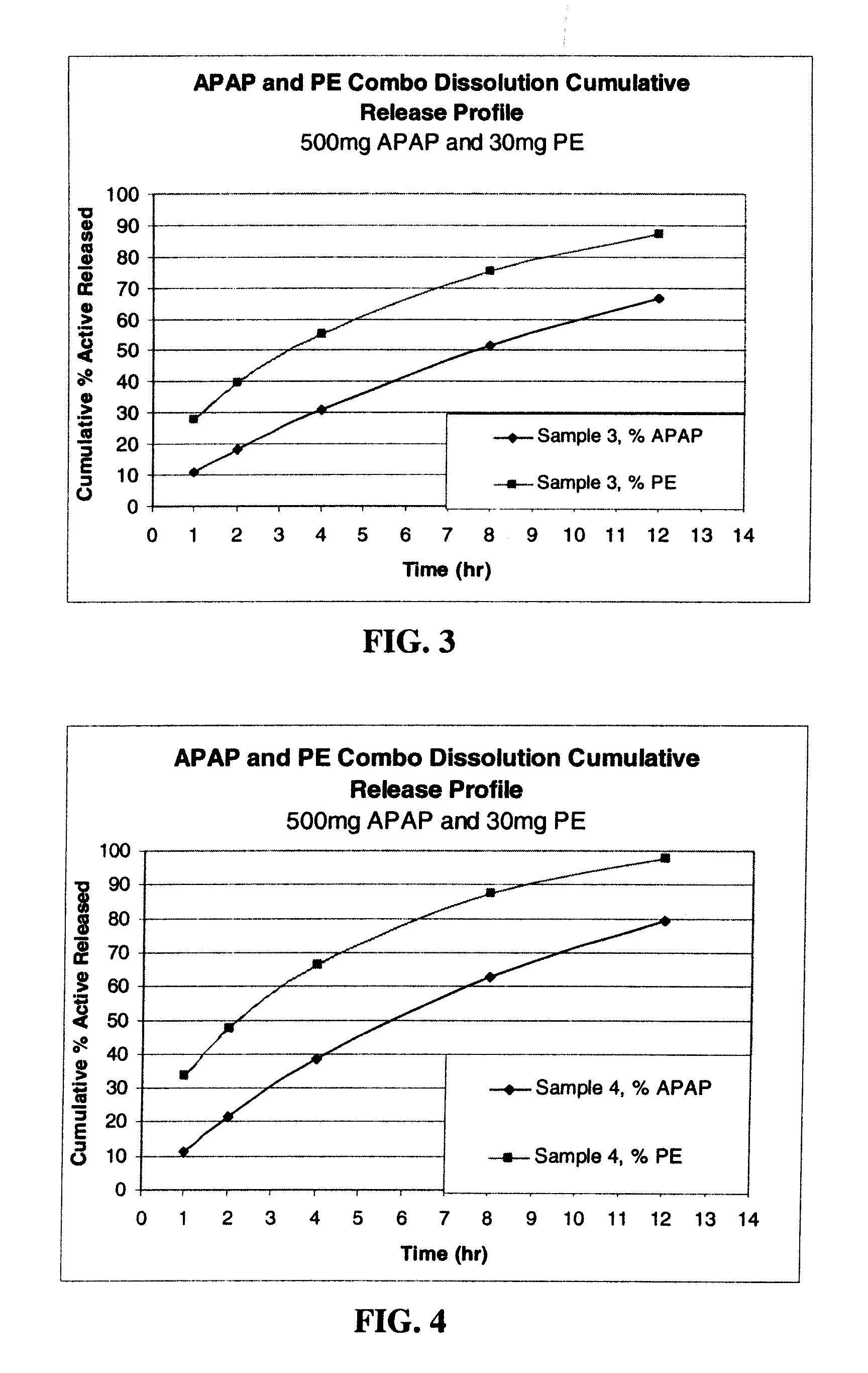
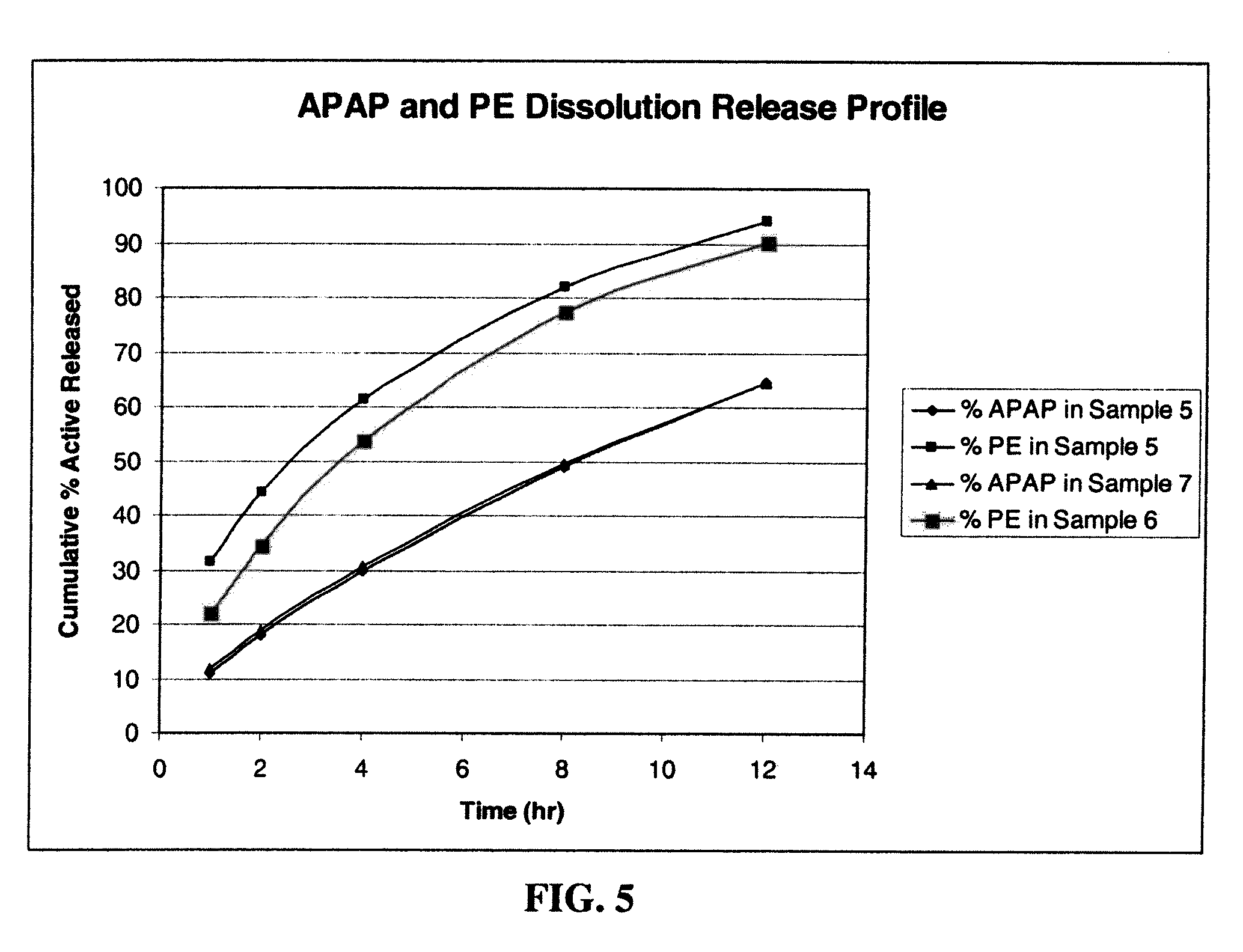
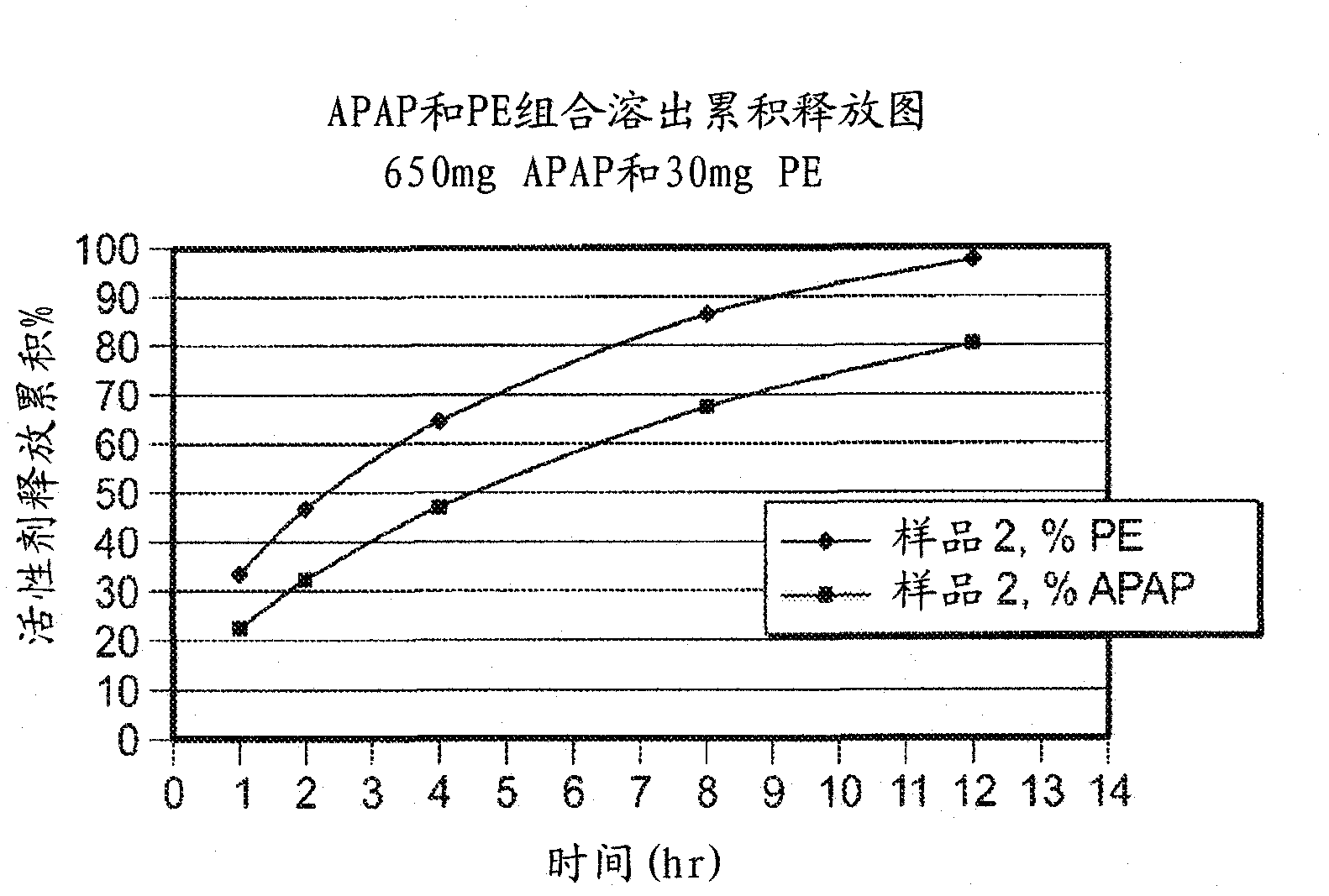
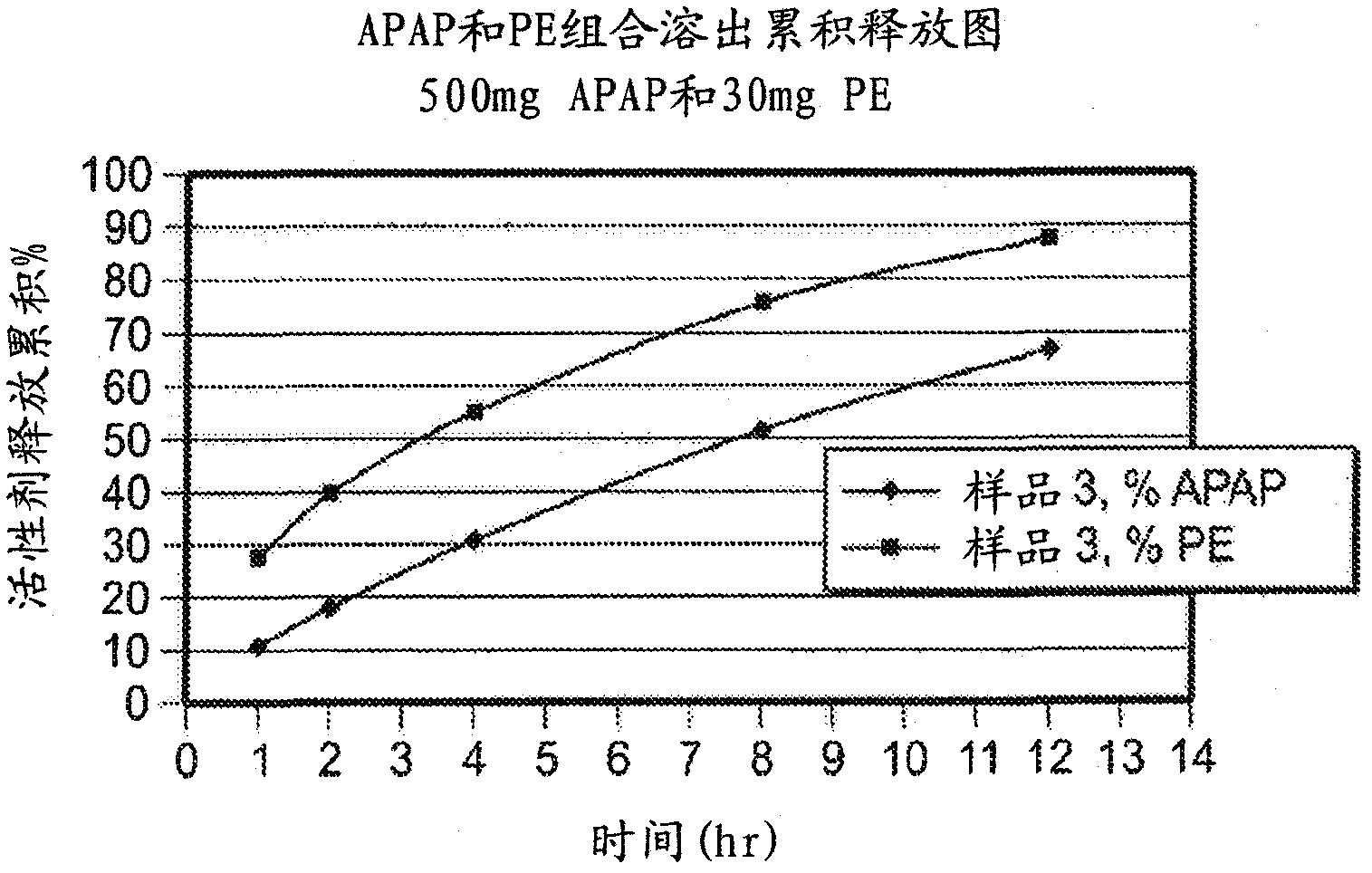
Combination sustained release-immediate release oral dosage forms with an opioid analgesic and a non-opioid analgesic
InactiveUS20030092724A1Long durationConstant plasma levels of opioid and non-opioid analgesicsBiocidePill deliveryImmediate releaseTherapeutic effect
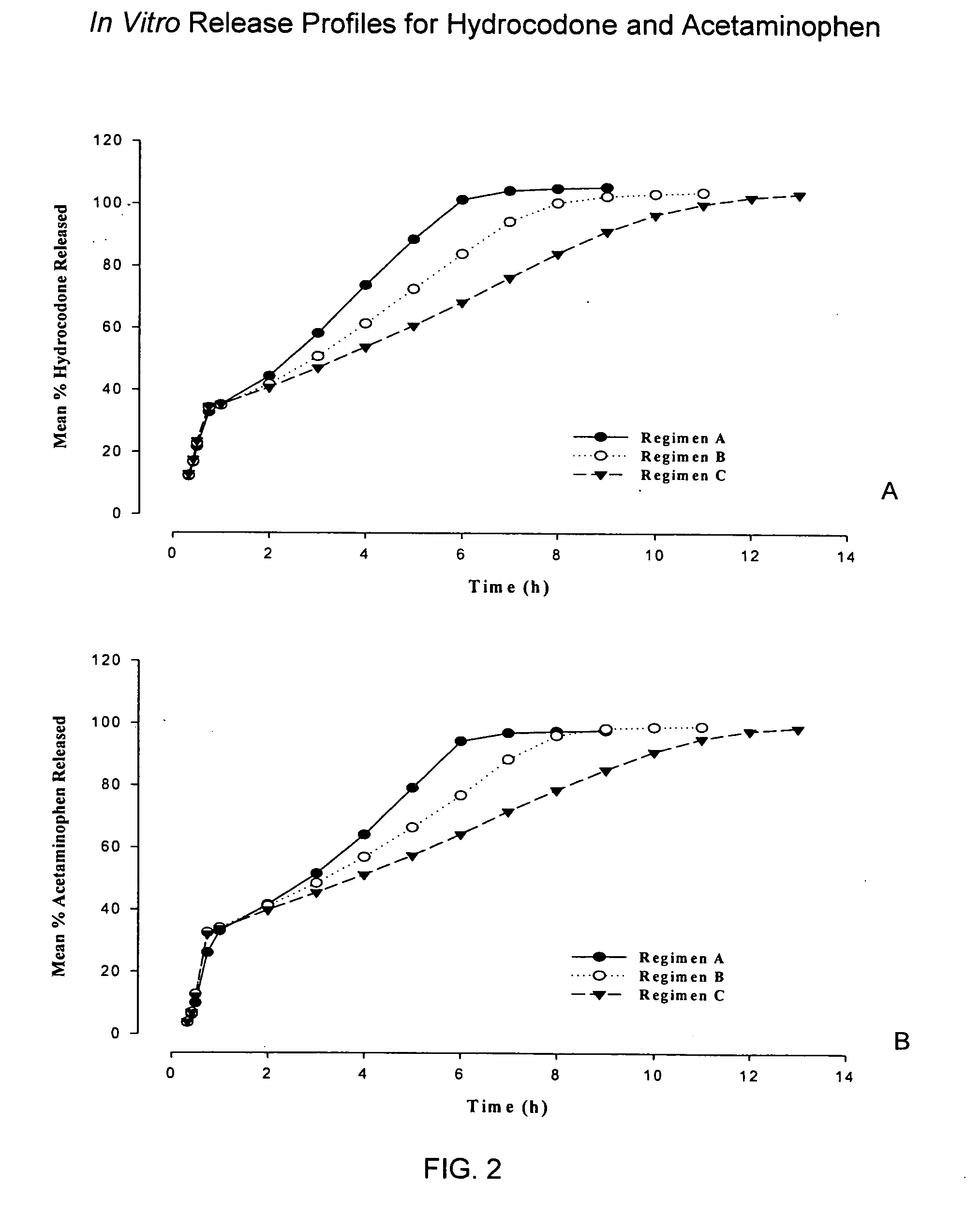
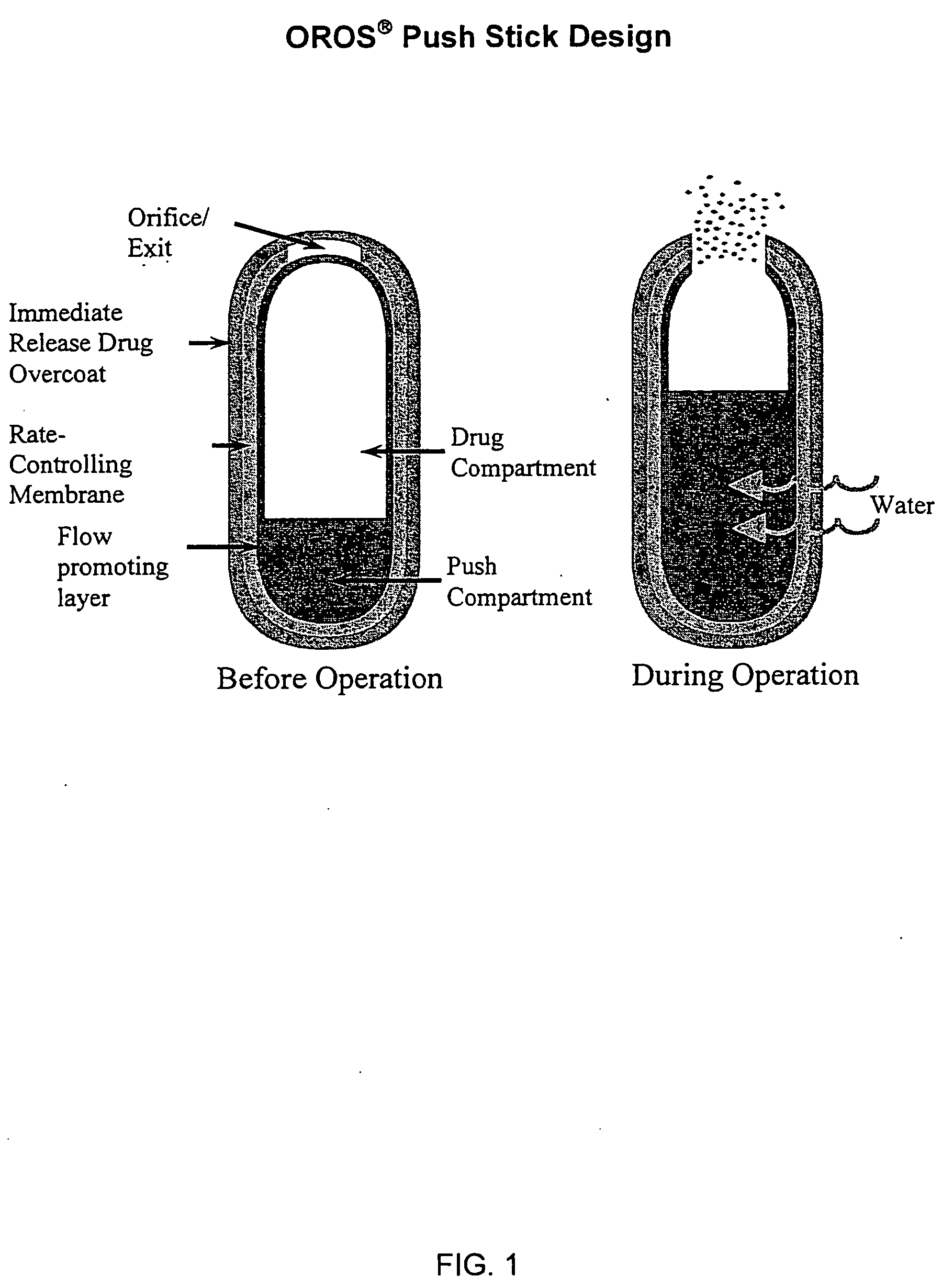
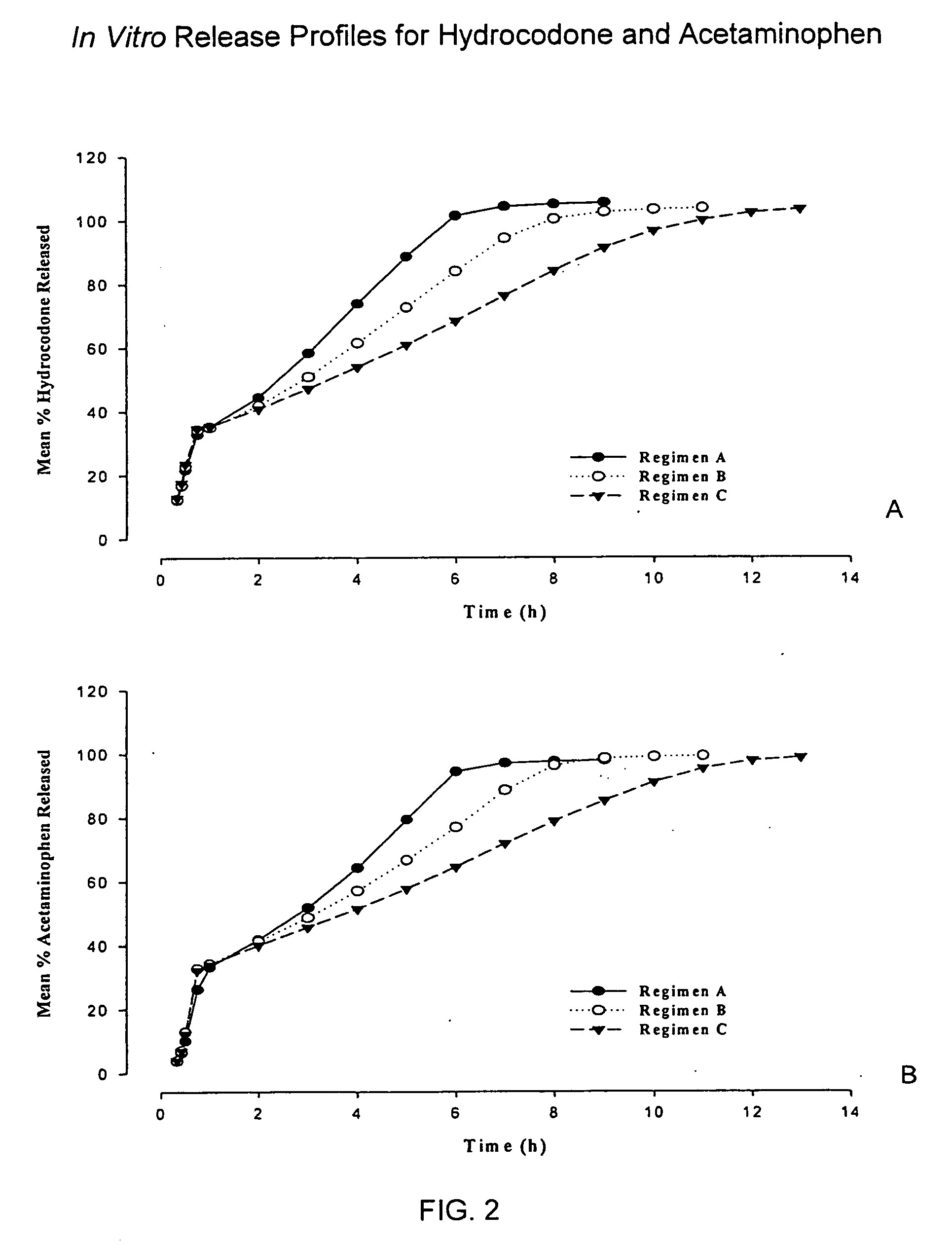
The present invention relates to new and useful oral tablet compositions which include an immediate release portion having an opioid analgesic and a non-opioid analgesic, providing for a rapid onset of therapeutic effect, and a sustained release portion of an opioid analgesic and a non-opioid analgesic, providing for a relatively longer duration of therapeutic effect. A multilayer oral dosage form containing a sustained release layer, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release layer containing the same active ingredients as the sustained release layer, is also disclosed. Also disclosed are oral tablet compositions, containing a sustained release core, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release coating containing the same active ingredients as the sustained release core, are also disclosed. In addition, methods of making and using such oral tablet compositions are disclosed.
Owner:ENDO PHARMA INC
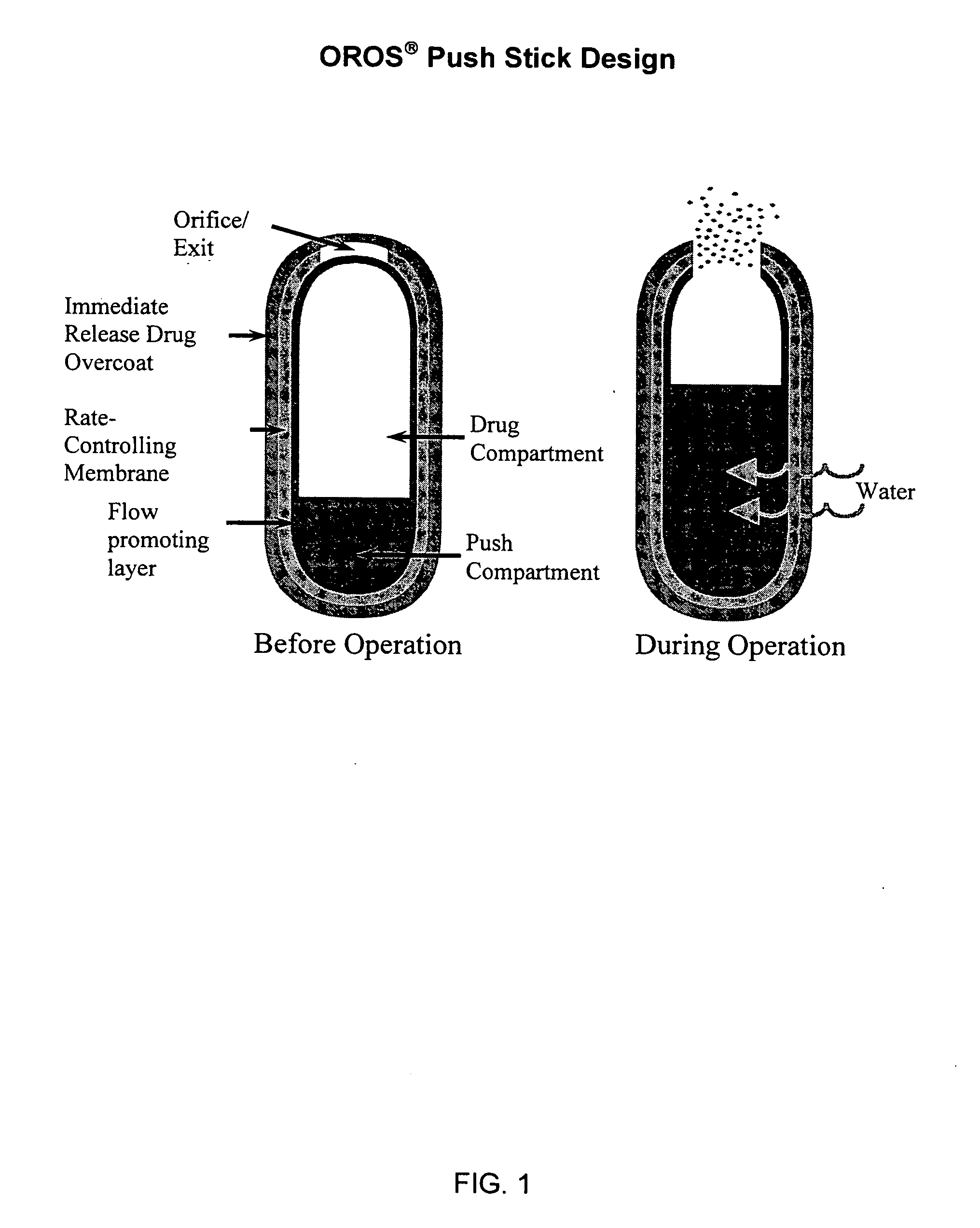
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Sustained release monoeximic formulations of opioid and nonopioid analgesics
InactiveUS20080031901A1Improve abilitiesSimple compositionBiocideAntipyreticHydrocodoneAnalgesic agents
Owner:ABBOTT LAB INC
Methods and compositions for treating distress dysfunction and enhancing safety and efficacy of specific medications
InactiveUS20110159048A1Good treatment effectEliminate side effectsBiocideNervous disorderDiseaseNeurotransmitter systems
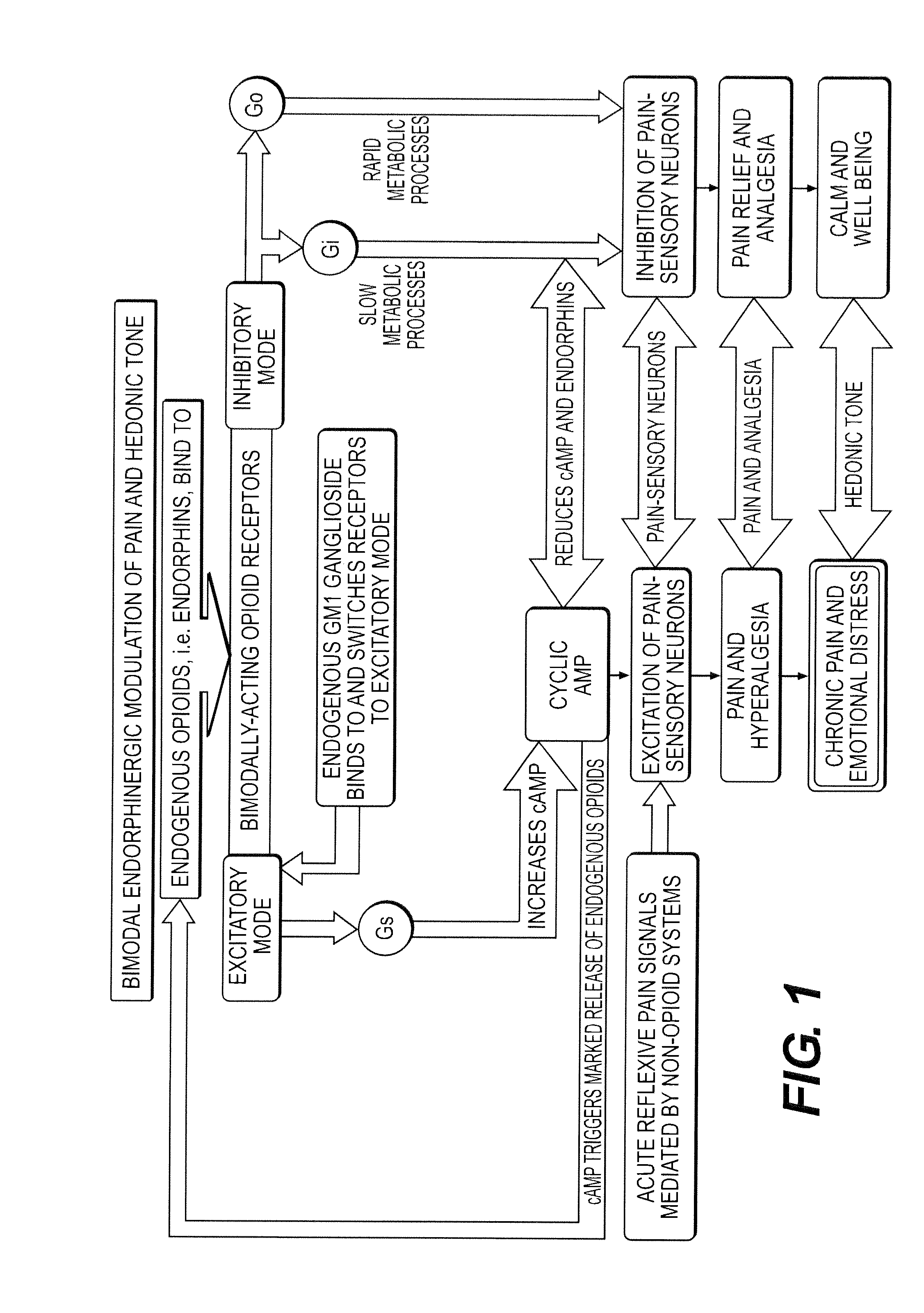
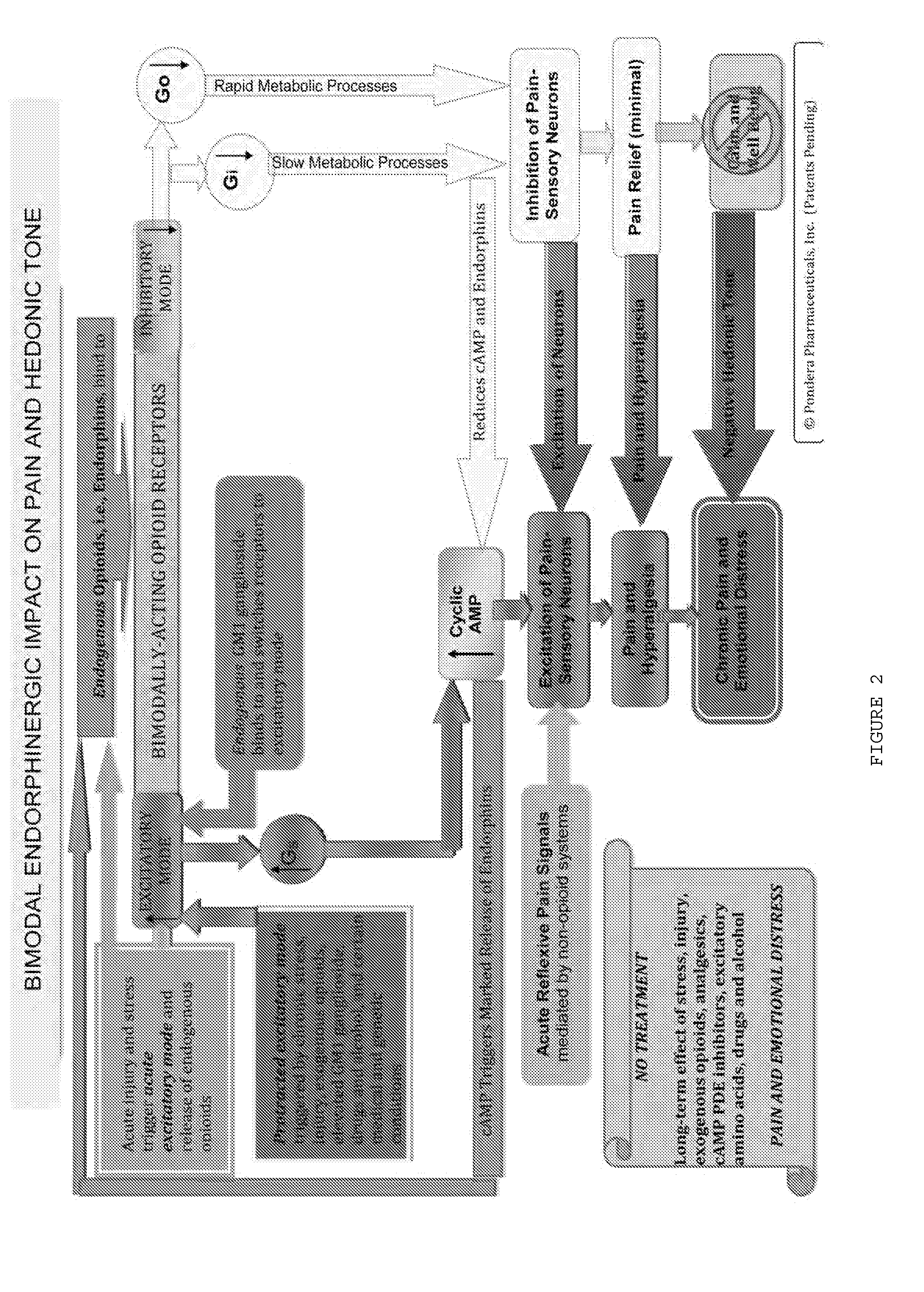
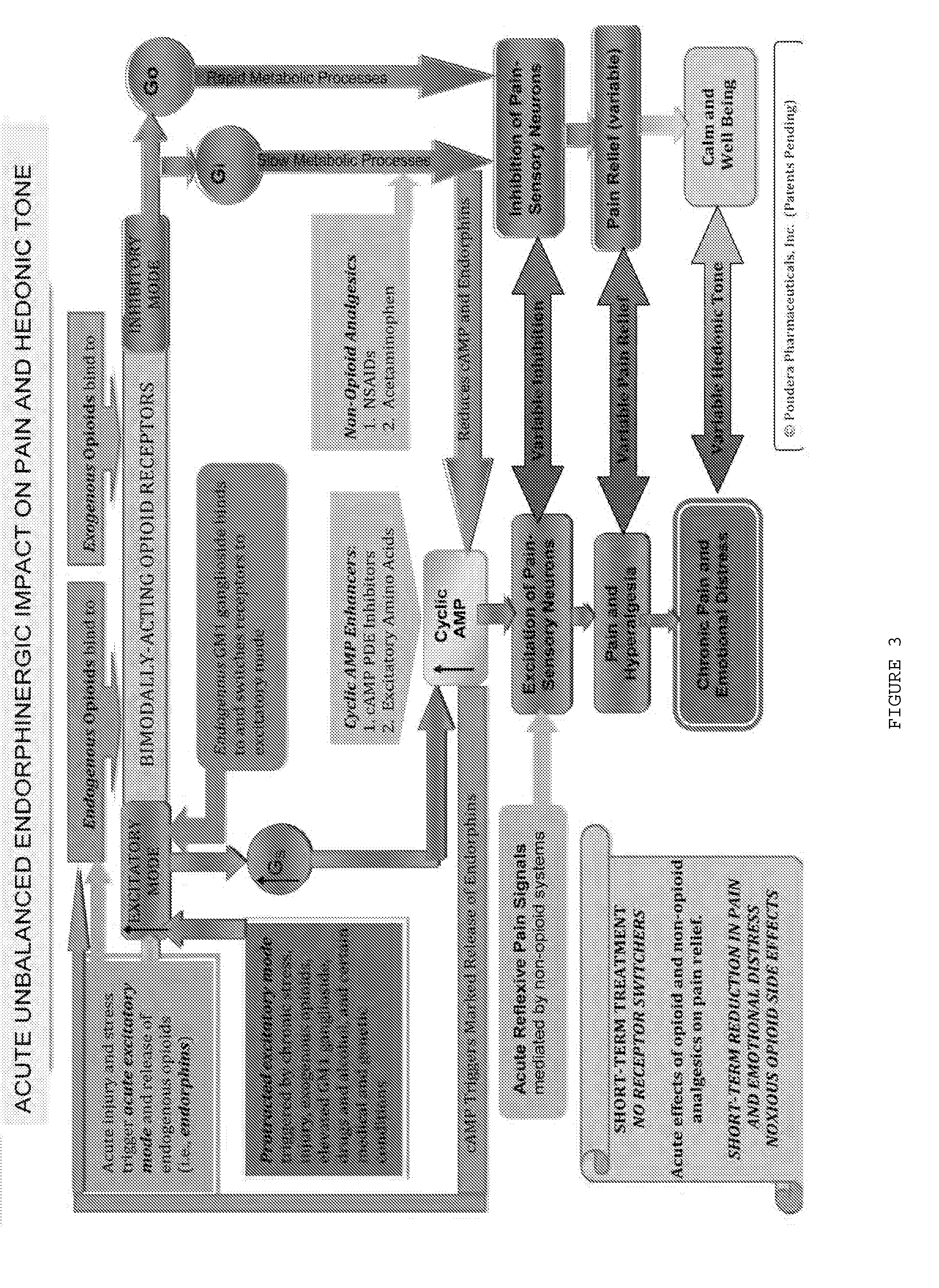
The present invention relates to methods and compositions for reducing Distress Dysfunction by restoring and maintaining homeostatic balance in the neurotransmitter systems underlying the Stress Response and the experience of distress and hedonic tone. Distress Dysfunction refers to the experience of dysfunctional emotional and physical distress that interferes with the individual's quality of life and functioning. A novel understanding of the bimodal opioid modulation of pain, and its impact, through serotonergic, dopaminergic, epinephrinergic, and norepinephrinergic processes, on hedonic tone, leads directly to new generation pharmaceutical formulations that are remarkably safe and effective for the treatment of a wide variety of Distress Dysfunctions, including anxiety, depression, anger, insomnia, mood disorders, eating disorders, sexual problems, pain, substance and behavioral addictions, gastrointestinal disorders, autistic spectrum disorders, attention-deficit and hyperactivity disorders, and other emotional and physical distress disorders. The foundation of this discovery is the power of Receptor Switchers, such as ultra-low-dose and very-low-dose opioid antagonists and GM1 ganglioside attenuators, in blocking acute and protracted excitatory opioid receptor signaling. Co-administration of Receptor Switchers with Endorphin Enhancers, such as specific cAMP PDE inhibitors and excitatory amino acids, is an excellent formulation for restoring healthy homeostatic balance to the endogenous opioid system, using the body's endorphins to reduce emotional and physical distress, and through synergistic and homeostatic processes, restoring positive hedonic tone. The addition of Synergistic Enhancers, such as amino acids, SSRI and SNRI agents, and non-opioid analgesics, as well as Exogenous Opioids, enhances and prolongs these therapeutic benefits. The novel principles discovered by this invention also teach a new generation of safe and effective formulations for the treatment of respiratory conditions, neuropathy, and nociceptive pain.
Owner:PONDERA BIOTECH
Sustained release formulations of opioid and nonopioid analgesics
ActiveUS20070281018A1Improve abilitiesSimple compositionBiocideAnimal repellantsHydrocodoneSustained Release Formulations
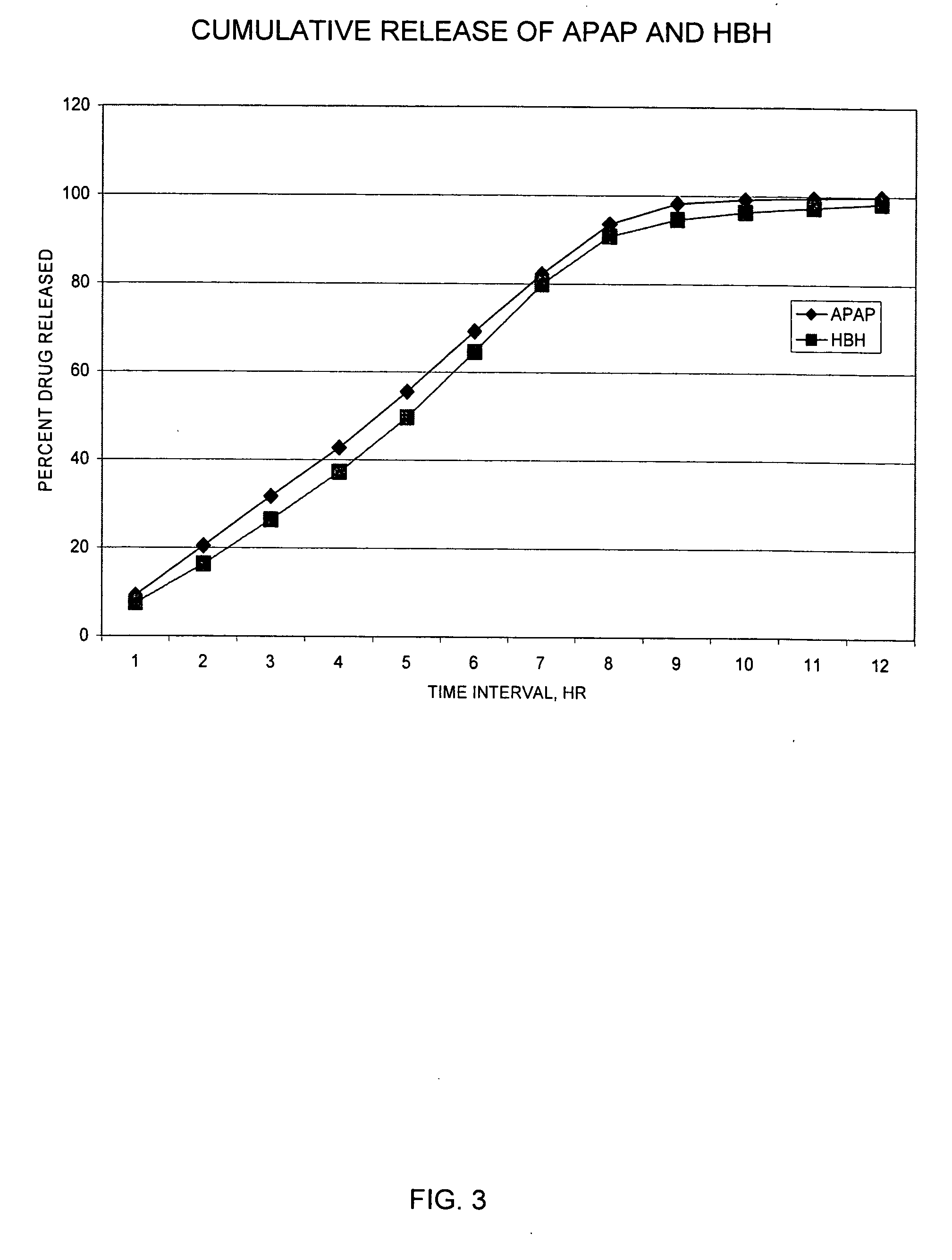
The present invention relates to SRSR solid dosage forms for administering pharmaceutical agents, particularly Hydrocodone and acetaminophen, methods for preparing said dosage forms, and methods for providing therapeutic agents to patients in need of treatment.
Owner:ABBVIE INC
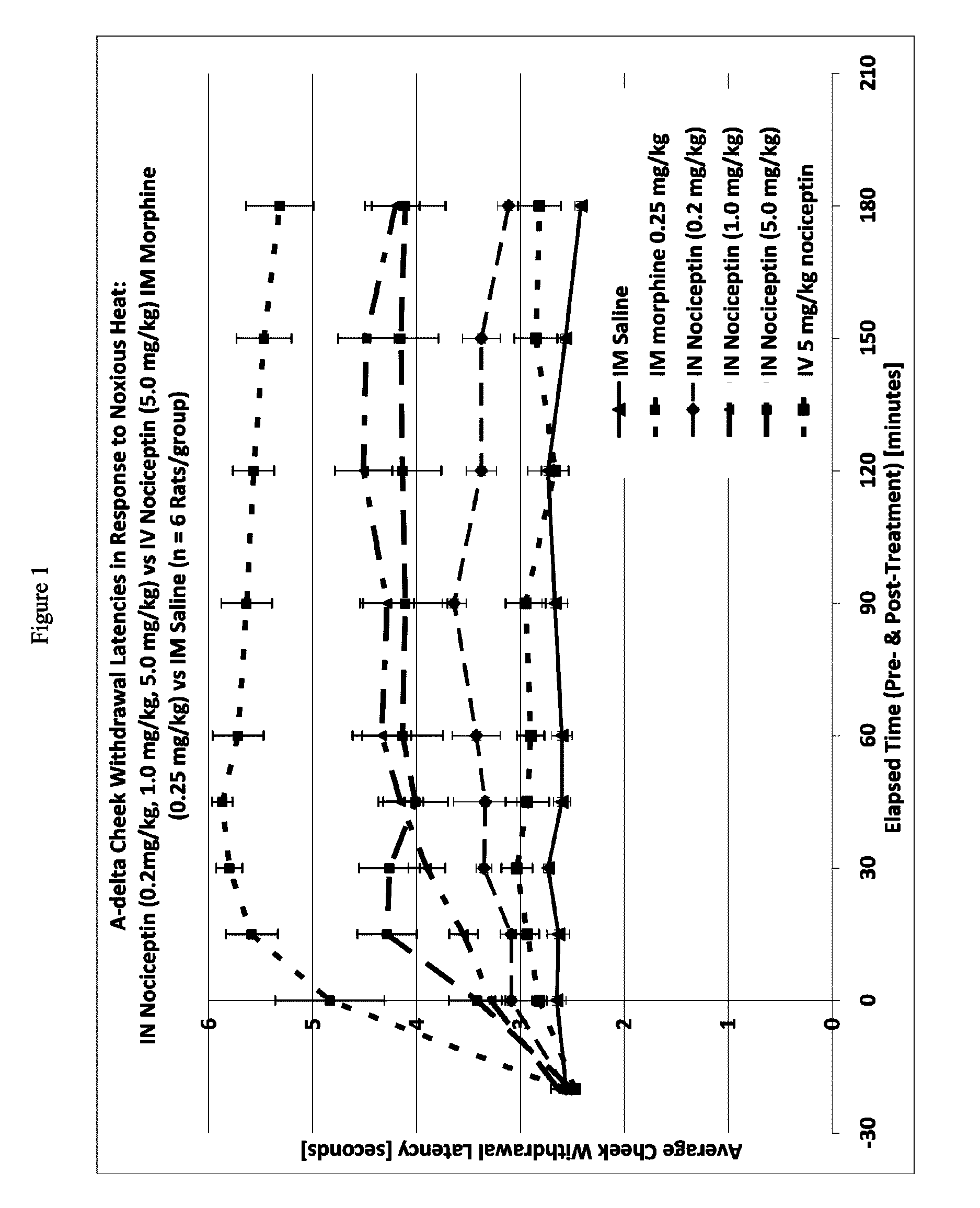
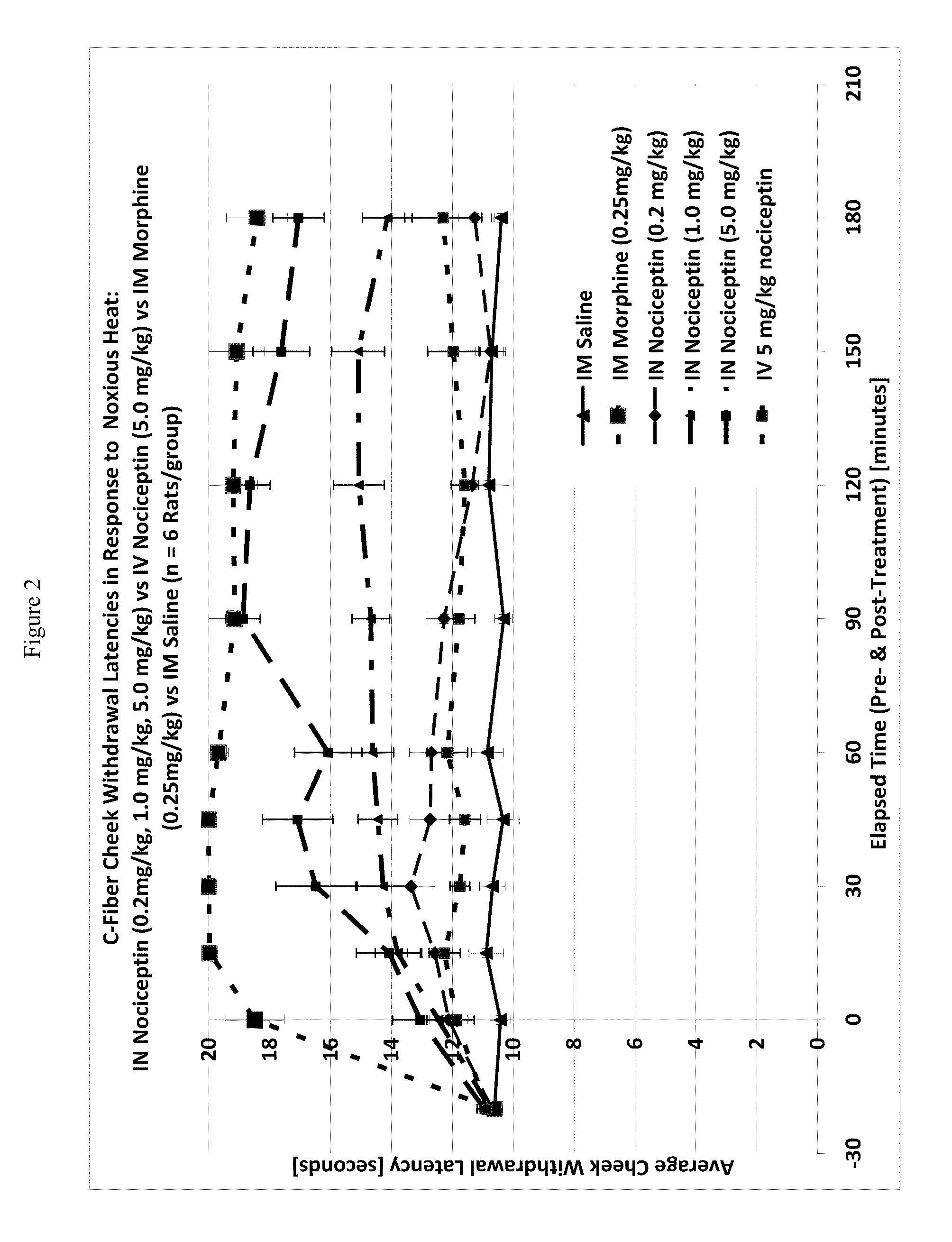
Methods for treatment of pain
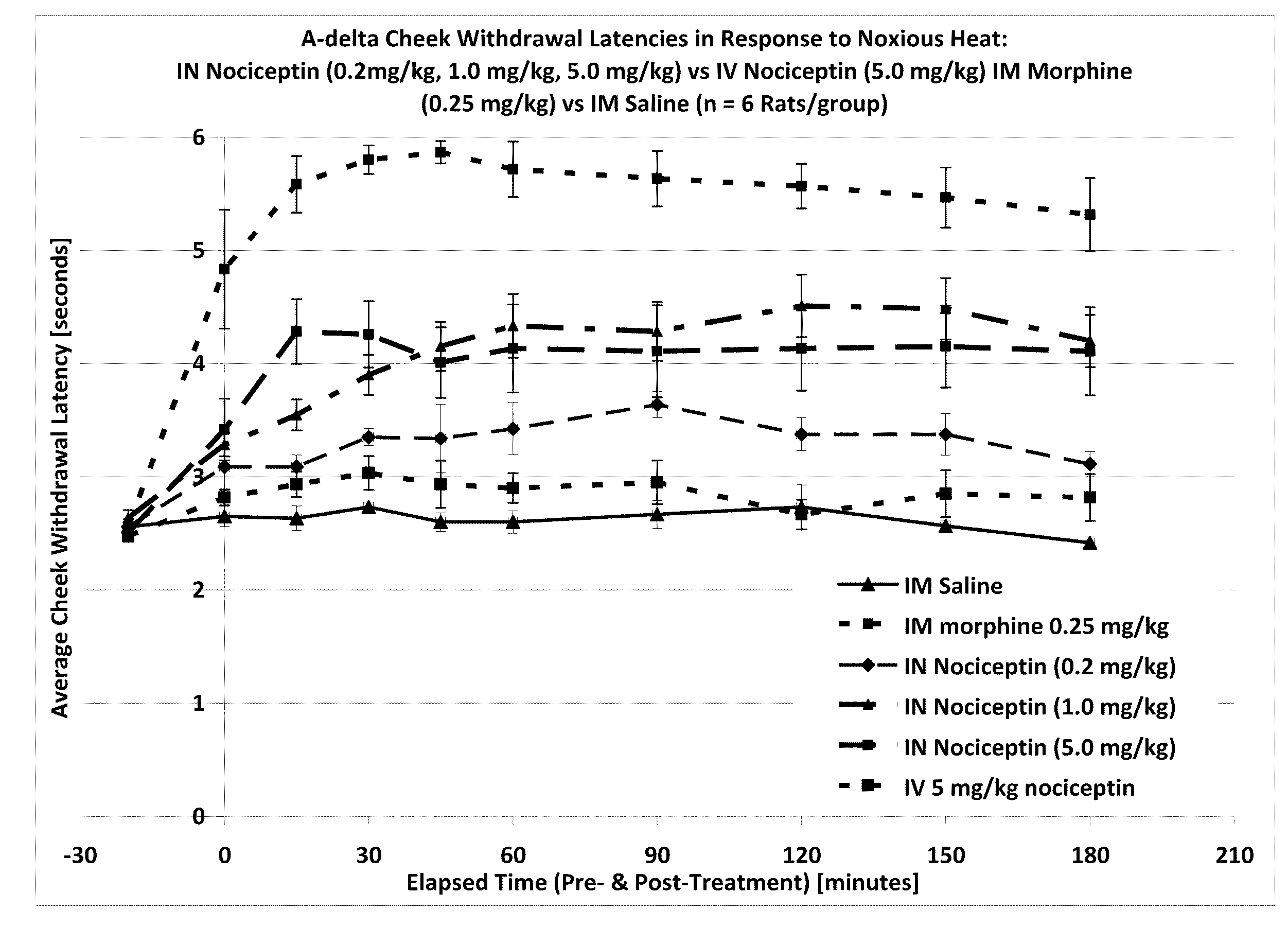
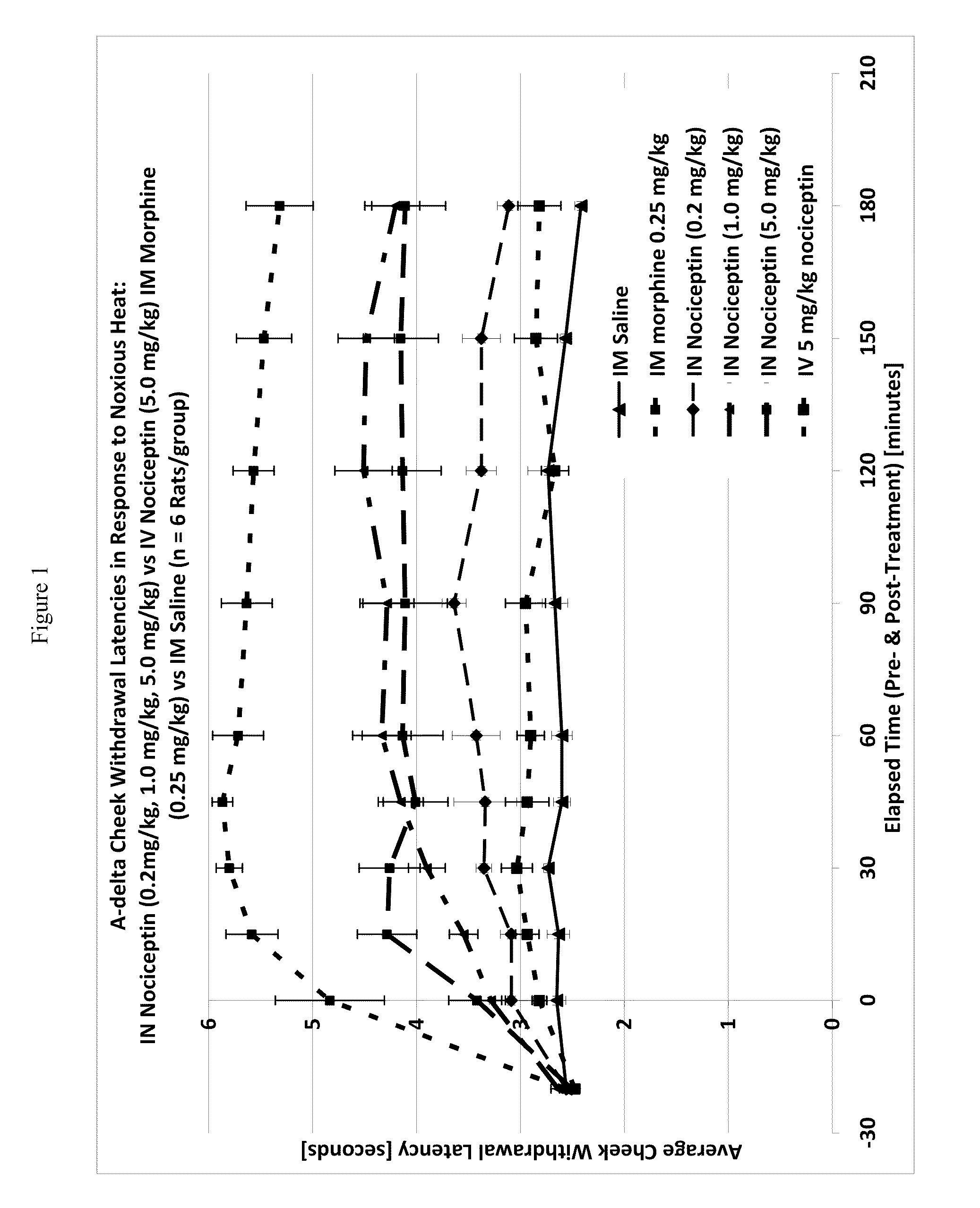
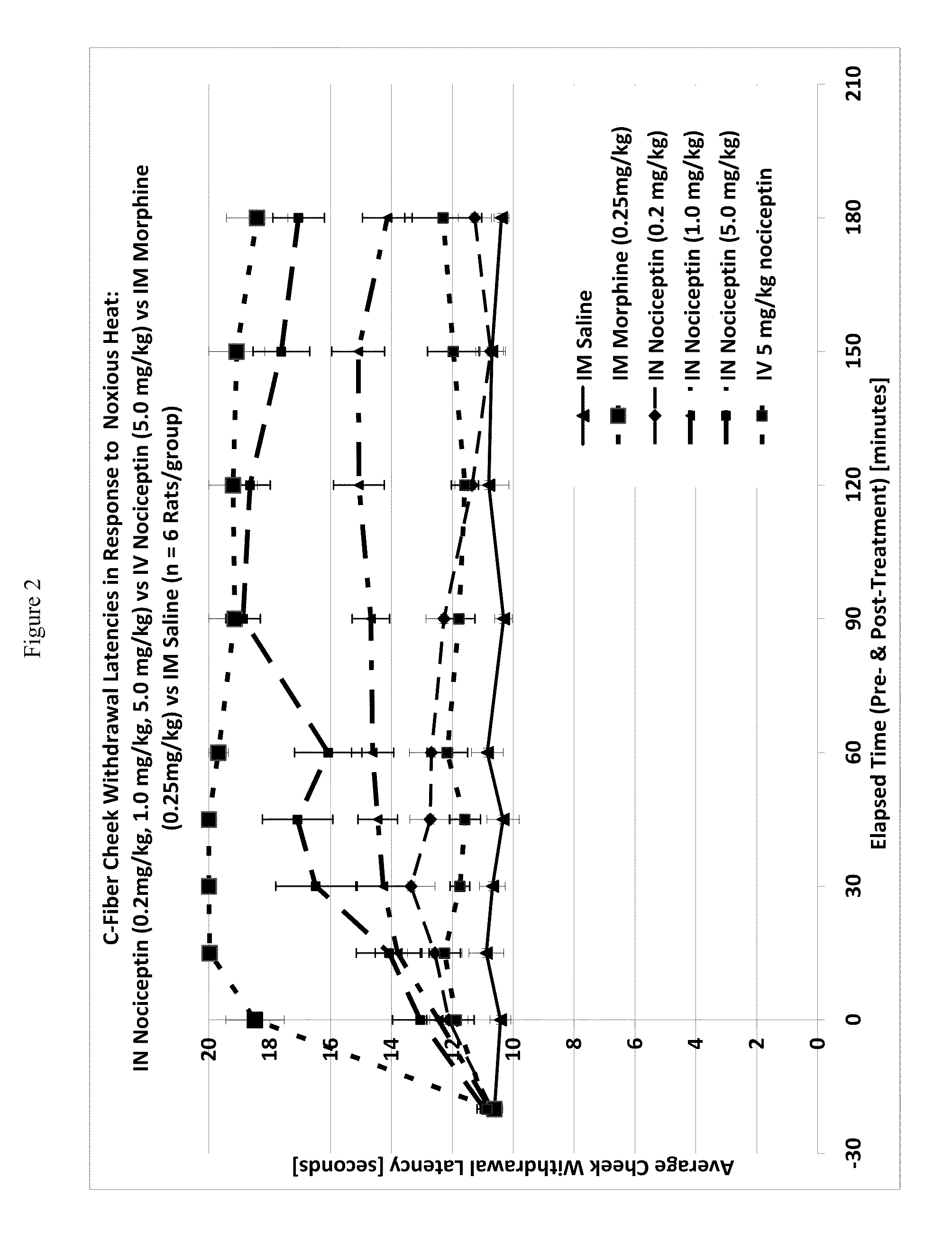
The invention provides for methods and compositions for treatment of pain via craniofacial mucosal administration of an analgesic compound (e.g. a non-opioid analgesic peptide, an NOP agonist or N / OFQ). Intranasal administration of certain analgesic peptides such as N / OFQ results in global analgesic effects.
Owner:TONIX PHARMA HLDG LTD
Synergistic combinations including N-acylated 4-hydroxyphenylamine derivatives
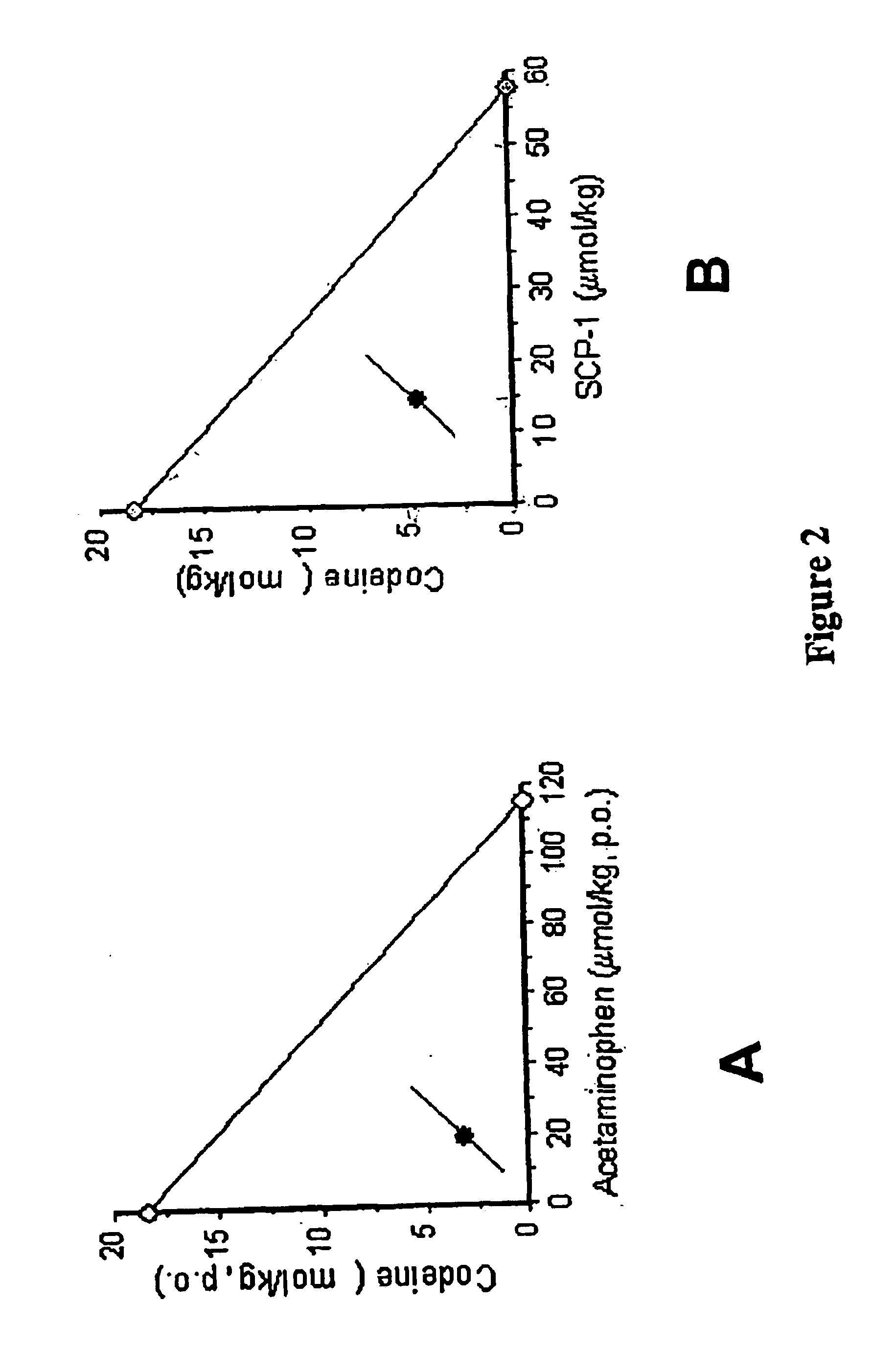
The present invention relates to pharmaceutical combinations of opioid and non-opioid analgesics in an intimate admixture with an analgesic from a series of N-acylated 4-hydroxyphenylamine derivatives, linked via an alkylene bridge to the nitrogen atom of a 1,2-benzisothiazol-3(2H)-one 1,1-dioxide group and methods for their use to alleviate pain in mammals. The analgesic combinations exhibit enhanced analgesic potency, do not suppress blood coagulation, and have little hepatotoxic effect.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
Gastric Retentive Extended-Release Dosage Forms Comprising Combinations of a Non-Opioid Analgesic and an Opioid Analgesic
Compositions and methods for the treatment of pain in a mammal are described. More specifically, a dosage form designed for release of acetaminophen and an opioid is described, wherein the dosage form provides delivery of the drugs to the upper gastrointestinal tract (“GI”) of a mammal for an extended period of time.
Owner:DEPOMED SYST INC
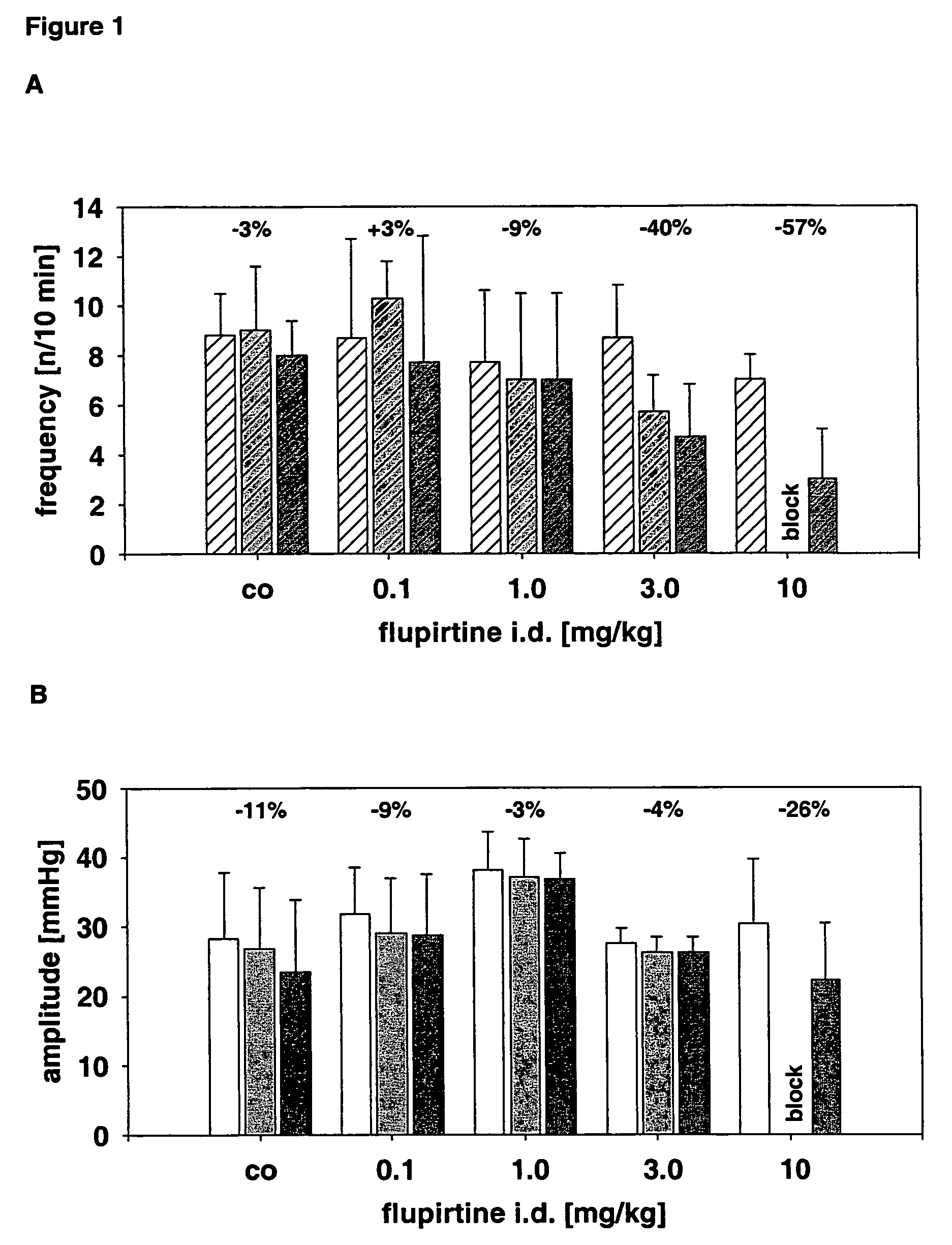
Use of the non-opiate analgesic drug flupirtine for the treatment of overactive bladder and associated diseases including urge incontinence, urinary flow problems as a result of prostate hyperplasia and irritable bowel syndrome
InactiveUS7309713B2Reduce concentrationReduce maintenanceBiocideDigestive systemAnalgesics drugsFecal incontinence
The present invention is directed to the prevention, reversal and medical treatment of lower urinary tract dysfunction including bladder instability and other related diseases as described below including urinary flow problems, urgency and incontinence as a result of prostate hyperplasia (BPH) and to the prevention, reversal and medical treatment of irritable bowl syndrome (IBS) with special focus on the diarrhea-predominant and mixed diarrhea-constipation type IBS, both in human beings and animals.
Owner:RUNDFELDT DR CHRISTIAN
Methods for treatment of pain
The invention provides for methods and compositions for treatment of pain via craniofacial mucosal administration of an analgesic compound (e.g. a non-opioid analgesic peptide, an NOP agonist or N / OFQ). Intranasal administration of certain analgesic peptides such as N / OFQ results in global analgesic effects.
Owner:TONIX PHARMA HLDG LTD
Extended release analgesic for pain control
InactiveUS6939538B2Electrostatic shieldingAvoid accessSalicyclic acid active ingredientsBiocideControlled releaseAbdominal cavity
An extended release analgesic for controlling pain comprised of an opioid or non-opioid analgesic drug ionically bound to hyaluronic acid, poly-γ-glutamic acid or other ionic polymers, and injected into a body either subcutaneously, intramuscularly or intraperitoneally, utilizing counter-ions of different valences to control the rate of release into the body.
Owner:BIOMEDICAL RES MODELS
Synergistic combinations including N-acylated 4-Hydroxyphenylamine derivatives and caffeine
The present invention relates to pharmaceutical combinations of opioid and non-opioid analgesics in an intimate admixture with caffeine and an analgesic from a series of N-acylated 4-hydroxyphenylamine derivatives, linked via an alkylene bridge to the nitrogen atom of a 1,2-benzisothiazol-3(2H)-one 1,1-dioxide group and methods for their use to alleviate pain in mammals. The analgesic combinations exhibit enhanced analgesic potency and are free from antipyretic activity, do not suppress blood coagulation, and have little hepatotoxic effect.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Sustained release formulations of opioid and nonopioid analgesics
ActiveUS8541026B2Improve abilitiesSimple compositionBiocideAnimal repellantsHydrocodoneSustained Release Formulations
The present invention relates to SRSR solid dosage forms for administering pharmaceutical agents, particularly Hydrocodone and acetaminophen, methods for preparing the dosage forms, and methods for providing therapeutic agents to patients in need of treatment.
Owner:ABBVIE INC
Medicament for treating amphetamine type stimulant dependency and mixed dependency of amphetamine type stimulants and opiates substances
InactiveCN103495172AGood effectControl withdrawal symptomsOrganic active ingredientsNervous disorderBenzodiazepineDrug withdrawal symptoms
The invention discloses a medicament for treating amphetamine type stimulant dependency and mixed dependency of amphetamine type stimulants and opiates substances. The medicament is prepared from the following components by weight percent: 2-95% of antipsychotics, 0.001-5% of alpha2 adrenergic agonists, 0-5% of anticholinergic agent, 0-80% of nonopioid analgesic, and 0-10% of benzodiazepine. The pharmaceutical composition disclosed by the invention achieves an ideal effect when the symptoms of a sufferer abusing stimulants such as benzedrine, or abusing the opiates substances in a merging manner are treated; the withdrawal symptom can be rapidly and obviously controlled; the detoxification recovery rate can be up to over 90%.
Owner:卢正堂 +1
Use of non-opiates for the potentation of opiates
A non-opioid analgesic is used for the treatment of intermittent or episodic pain experienced by a patient undergoing chronic pain treatment with an opioid analgesic.
Owner:SOSEI R&D LIMITED
Gastric retentive extended-release dosage forms comprising combinations of a non-opioid analgesic and an opioid analgesic
InactiveCN102105136AImprove complianceOrganic active ingredientsNervous disorderUpper gastrointestinalNon opioid analgesics
Compositions and methods for the treatment of pain in a mammal are described. More specifically, a dosage form designed for release of acetaminophen and an opioid is described, wherein the dosage form provides delivery of the drugs to the upper gastrointestinal tract ('Gl') of a mammal for an extended period of time.
Owner:蒂宝制药公司
Methods for treatment of pain
The invention provides for methods and compositions for treatment of pain via craniofacial mucosal administration of an analgesic compound (e.g. a non-opioid analgesic peptide, an NOP agonist or N / OFQ). Intranasal administration of certain analgesic peptides such as N / OFQ results in global analgesic effects.
Owner:TONIX PHARMA HLDG LTD
Methods and compositions comprising sequential administration opioid receptor agonists
Owner:QRXPHARMA
Therapeutic agent for pain
InactiveCN102026987ALong-term useImprove drug deliveryOrganic active ingredientsNervous disorderBenzodiazepinePharmaceutical medicine
Disclosed is a therapeutic and / or prophylactic agent for cancer pain, which can be administered continuously for a long period between the early stage and the final stage of the cancer pain therapy in place of conventional non-opioid or opioid analgesic agents. The therapeutic and / or prophylactic agent for cancer pain comprises a 1,5-benzodiazepine derivative represented by general formula (1) [wherein R1 represents a C1-6 alkyl group; R2 represents a phenyl group or a cyclohexyl group; and Y represents a single bond or a C1-4 alkylene group] or a pharmaceutically acceptable salt thereof as an active ingredient.
Owner:ZERIA PHARMA
Method For Treating Pruritus
Benzomorphan compounds are found to be useful for treating, ameliorating or preventing pruritus, and in particular pruritus associated with (including induced by) the administration of opioids. Antipruritic activity is believed to be mediated through the dual action of the compounds as mu opioid receptor antagonists and kappa opioid receptor agonists. Pharmaceutical compositions contain therapeutically effective amounts of these useful compounds, optionally in combination with second therapeutic agents, such as opioid or non-opioid analgesics or other compounds.
Owner:EURO-CELTIQUE SA
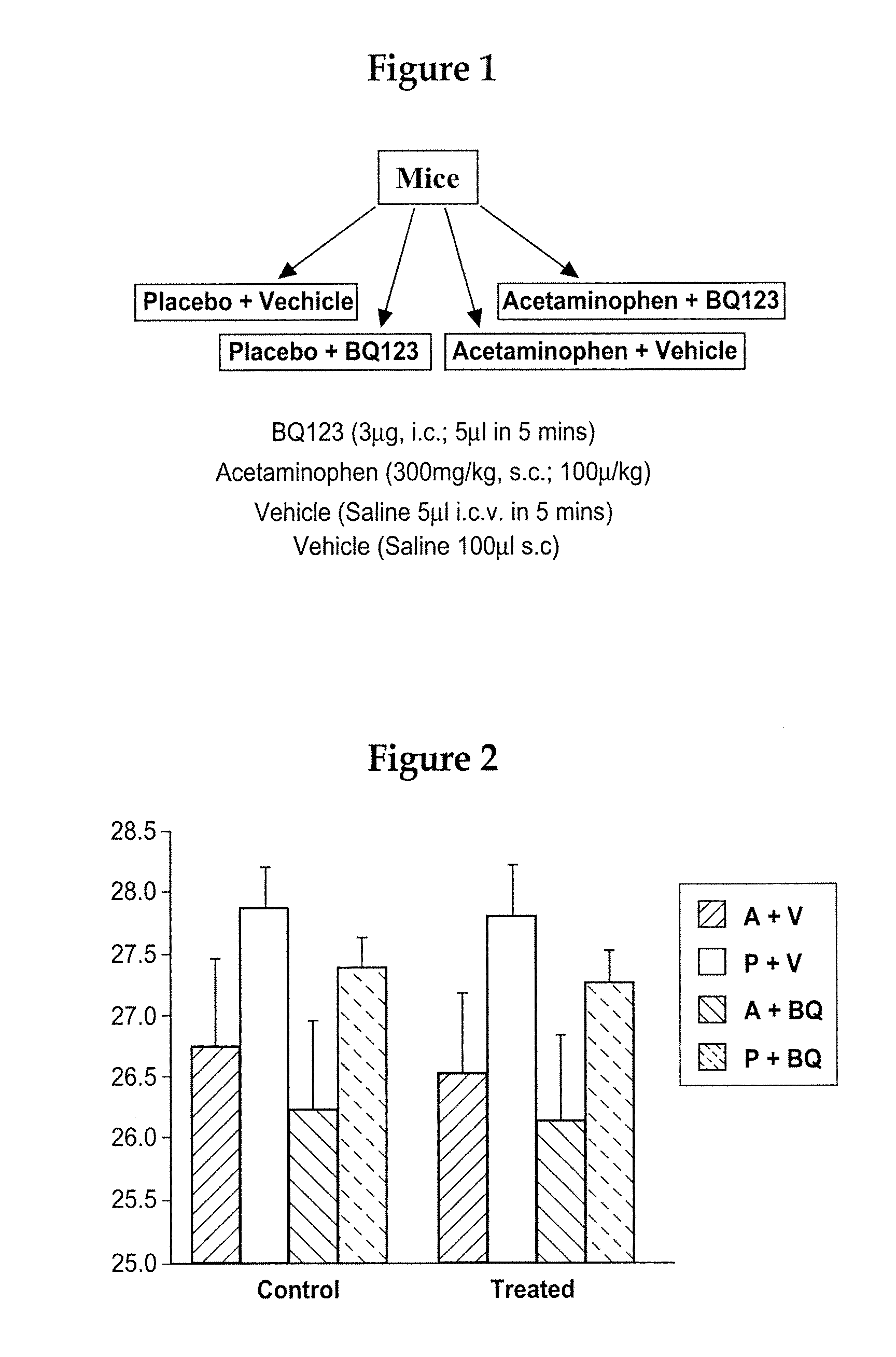
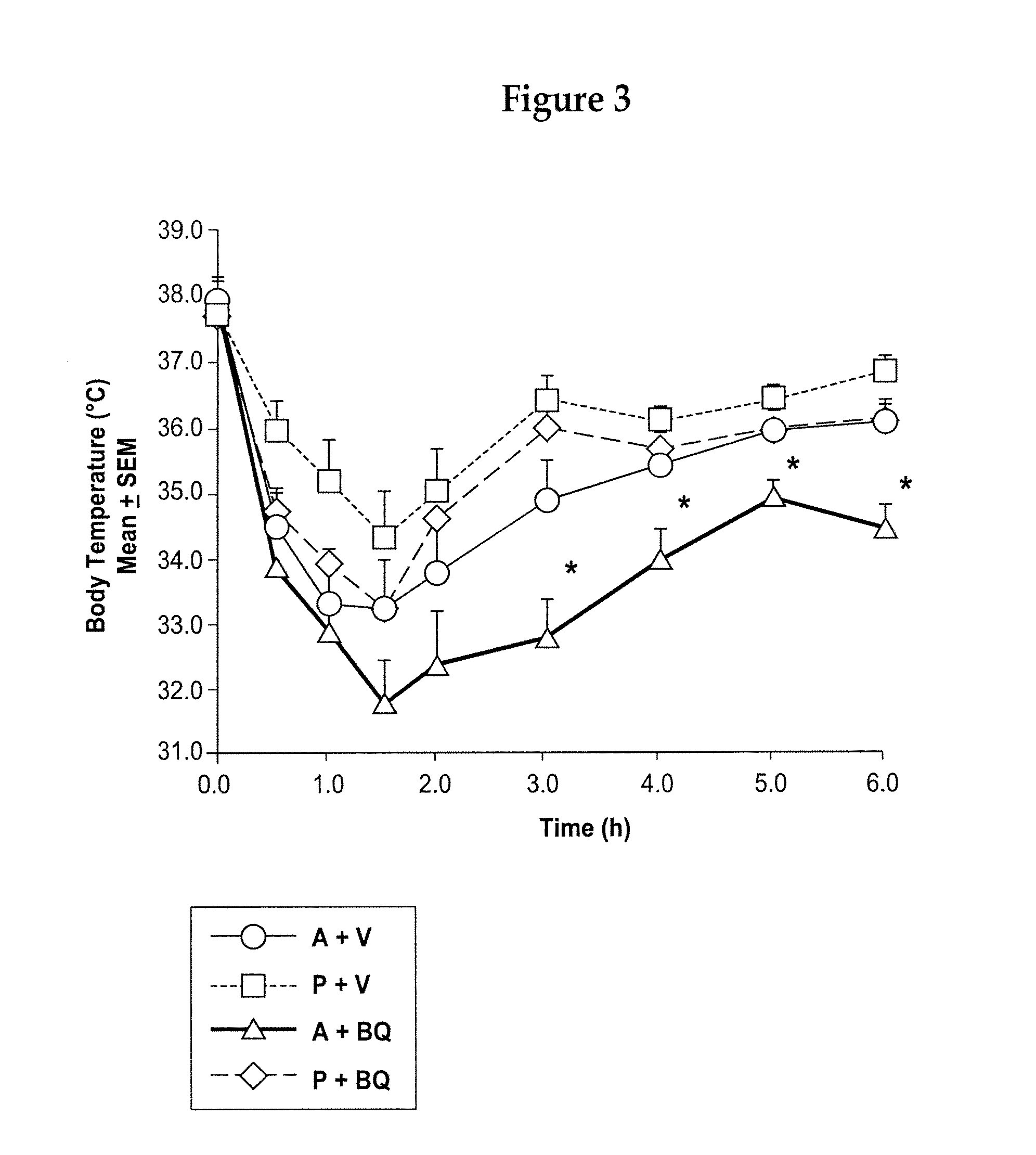
Method and Composition for Potentiating the Antipyretic Action of a Nonopiod Analgesic
InactiveUS20080207763A1Affect propertyImprove heat dissipation abilityBiocideSalicyclic acid active ingredientsActive agentTreatment fever
A composition and method of treating fever, and optionally treating pain, is disclosed. The composition and method utilize a nonopioid analgesic and an endothelin antagonist as active agents to treat fever in mammals, including humans. The composition also is useful in the prevention and treatment of stroke and other cardiovascular disorders, like myocardial infarction.
Owner:UNIV OF ILLINOIS CHICAGO
Carbuncle eliminating cream for treating cancer pain and preparation method of carbuncle eliminating cream
InactiveCN111110785ATo achieve the purpose of treating cancer painConvenient for long-term medicationNervous disorderAntipyreticAconitum carmichaeliOncology
The invention relates to a carbuncle eliminating cream for treating cancer pain and a preparation method of the carbuncle eliminating cream. The carbuncle eliminating cream comprises 5-15 parts of frankincense, 5-15 parts of myrrh, 5-15 parts of peach kernels, 5-15 parts of common monkshood roots, 5-15 parts of radix angelicae, 5-15 parts of radix clematidis, 5-15 parts of fennel, 5-15 parts of radix aucklandiae, 5-15 parts of edible tulip, 5-15 parts of scorpion, 5-15 parts of rhizoma corydalis, 5-15 parts of notopterygium roots and 5-15 parts of rhizoma chuanxiong. The preparation method comprises the following steps: crushing and decocting the above raw materials, and carrying out anaerobic fermentation. The carbuncle eliminating cream is externally applied by adopting the theory of traditional Chinese medicine syndrome differentiation and treatment, can effectively treat cancer pain, has a treatment effective rate of 91.57%, presents obviously superior effect compared with non-opioid analgesics and opioid analgesics, and protects patients from dependence and addiction.
Owner:北京中西联盟肿瘤医学研究院(普通合伙)
Use of the non-opiate analgesic drug flupirtine of the treatment of overactive bladder and associated diseases including urge incontinence, urinary flow problems as a result of prostate hyperplasia and irritable bowl syndrome
InactiveUS20060173052A1Reduce concentrationReduce maintenanceBiocideDigestive systemAnalgesics drugsDisease
The present invention is directed to the prevention, reversal and medical treatment of lower urinary tract dysfunction including bladder instability and other related diseases as described below including urinary flow problems, urgency and incontinence as a result of prostate hyperplasia (BPH) and to the prevention, reversal and medical treatment of irritable bowl syndrome (IBS) with special focus on the diarrhea-predominant and mixed diarrhea-constipation type IBS, both in human beings and animals.
Owner:RUNDFELDT DR CHRISTIAN
Methods and compositions comprising sequential administration of opioid receptor agonists
InactiveUS20120309779A1Effective analgesiaReducing and eliminating undesired side effectBiocideNervous disorderSide effectNon opioid analgesics
Methods and compositions for the alleviation of pain in a patient. The methods and compositions sequentially administer a therapeutically effective amount of first compound having opioid receptor agonist activity, followed by a therapeutic effective amount of a second or subsequent compound(s) having opioid receptor agonist activity, one or more non-opioid analgesic compounds or one or more hybrid opioid compounds, or mixtures thereof. The methods and compositions effectively alleviate pain with a lower incidence of opioid-induced side effects.
Owner:QRXPHARMA
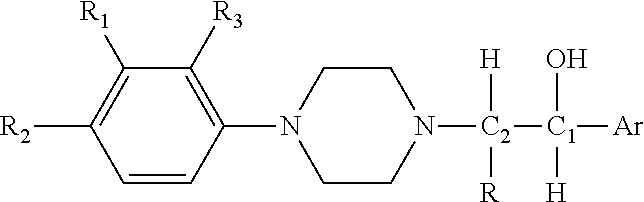
Substituted Phenylpiperazinyl Aralkylalcohol Derivatives, Pharmaceutical Compositions Containing Such Derivatives and Uses Thereof
ActiveUS20110294822A1Decreased gastric motilityOvercome side effectsOrganic active ingredientsNervous disorderPhenylpiperazineAnalgesics effects
The invention relates to a substituted phenylpiperazine aryl alkanol derivative represented by the following general formula and its salt and hydrate,wherein C1 and C2 represent chiral carbon atoms, and the compound is one of the six isomers: (1RS, 2SR), (1RS, 2RS), (1R, 2S), (1S, 2S), (1R, 2R) or (1S, 2R); and R, R1, R2, R3 and Ar are as defined in the specification. The derivative is non-opioid analgesic, has good analgesic effect and relatively small side effects. The invention also relates to a composition comprising the derivative and its use.
Owner:NHWA PHARMA CORPORATION
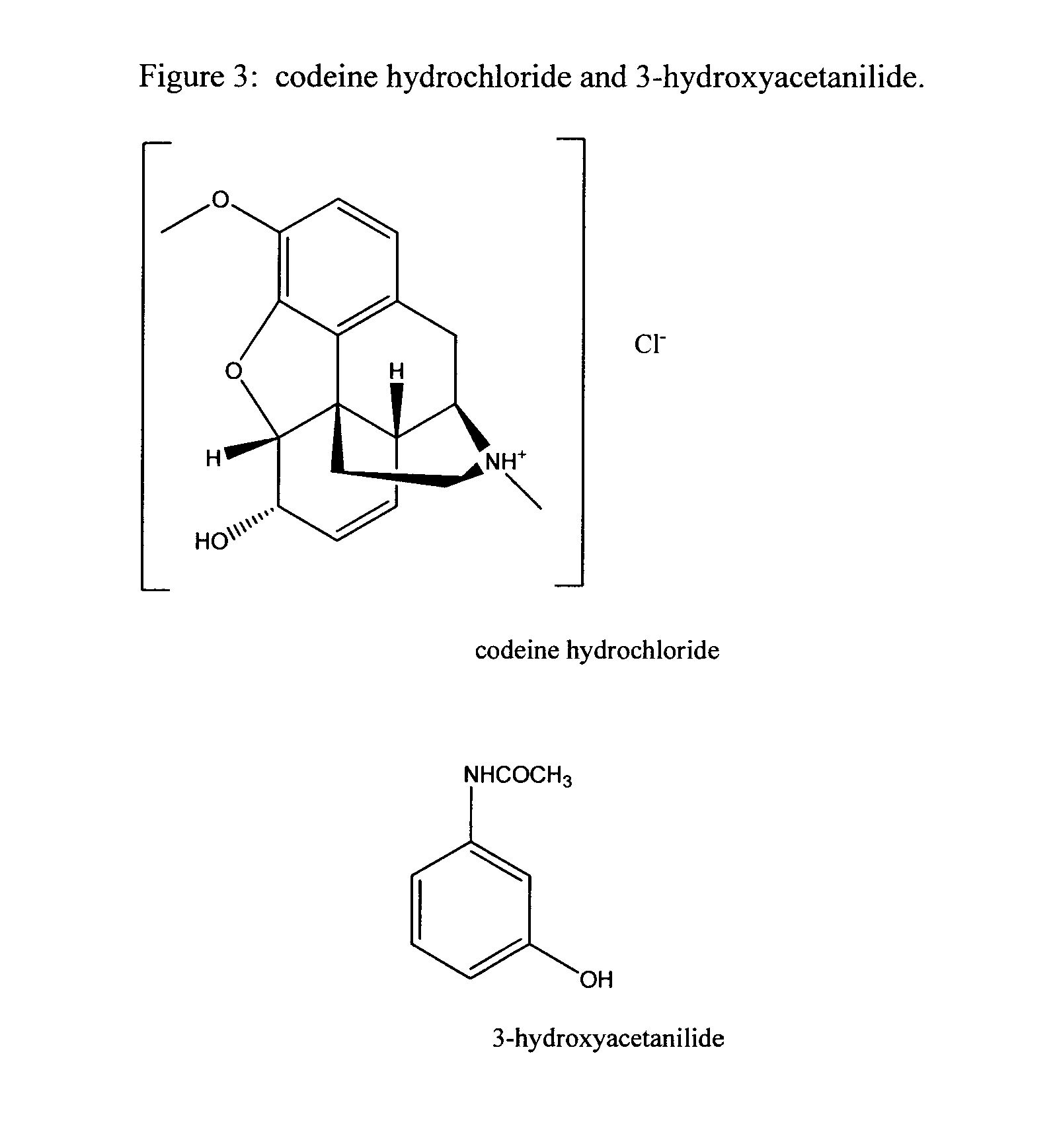
Opioid analgesics and 3-hydroxyacetanilide for treating pain
InactiveUS20140378496A1Less side effectsEffective pain reliefBiocideAnimal repellantsLiver toxicityPain controlling
Pharmaceutical combinations of opioid analgesics and analgesics that act through non-opioid mechanisms are commonly used to provide pain relief. An example of this pharmaceutical combination is the product Vicodin™, where the opioid analgesic is hydrocodone and the non-opioid is acetaminophen. However, liver toxicity from the acetaminophen component is common. The invention provides an improvement over the opioid and acetaminophen pharmaceutical combinations for the management of pain by the concomitant administration of an opioid analgesics and the non-opioid analgesic 3-hydroxyacetanilide. This combination has been found to exhibit unexpectedly enhanced analgesic activity when dosed orally in a mammal.
Owner:SLX PHARMA
Therapeutic agent for pain
To provide a cancer pain therapeutic and / or prophylactic agent which can be administered to a patient for a long period of time from the early stage to the final stage of the cancer pain therapy, instead of conventional non-opioid analgesic agents or opioid analgesic agents.The cancer pain therapeutic and / or prophylactic agent containing, as an active ingredient, a 1,5-benzodiazepine derivative represented by formula (1):(wherein R1 represents a C1-6 alkyl group, R2 represents a phenyl group or a cyclohexyl group, and Y represents a single bond or a C1-4 an alkylene group) or a pharmaceutically acceptable salt thereof.
Owner:ZERIA PHARMA
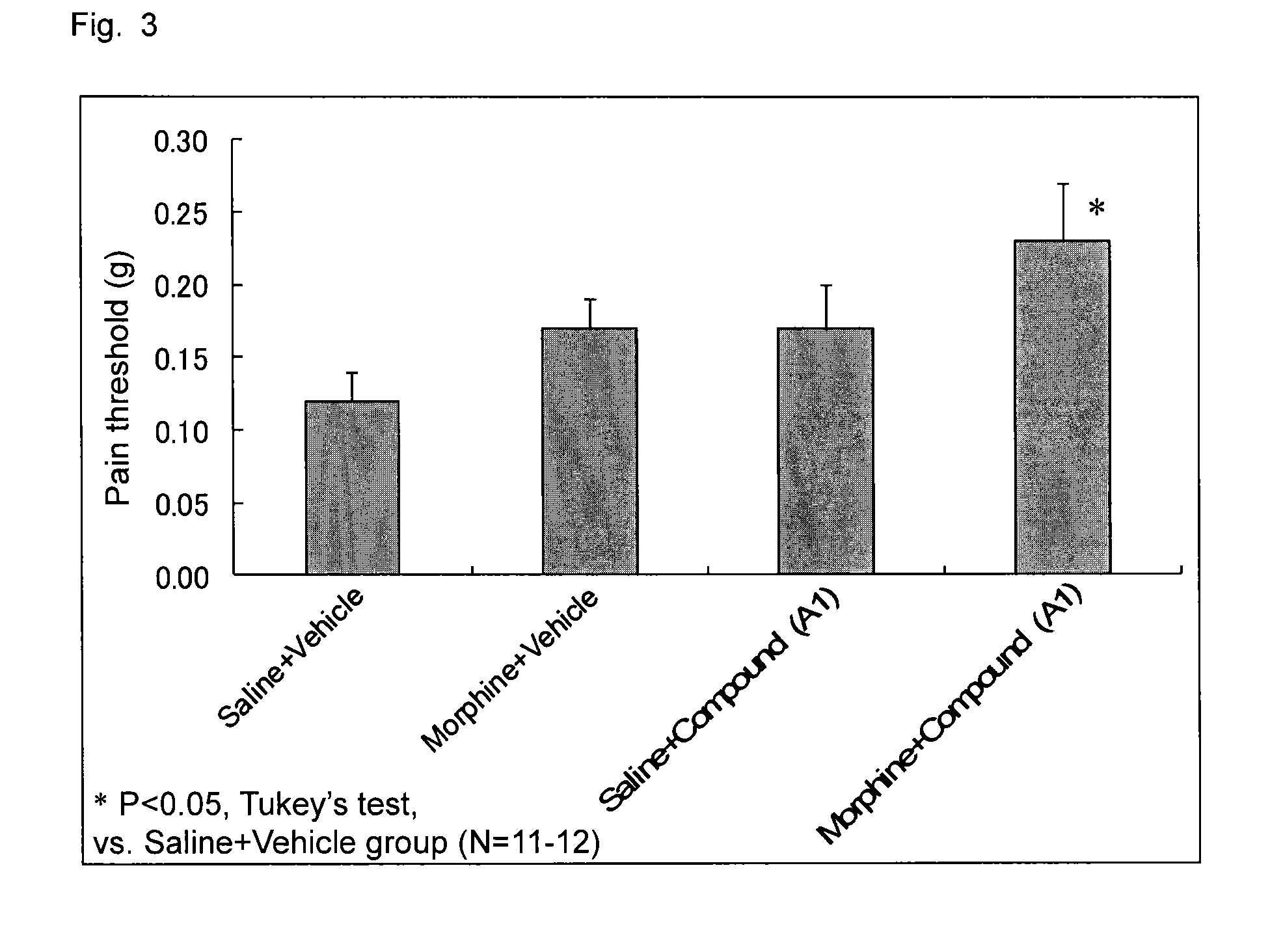
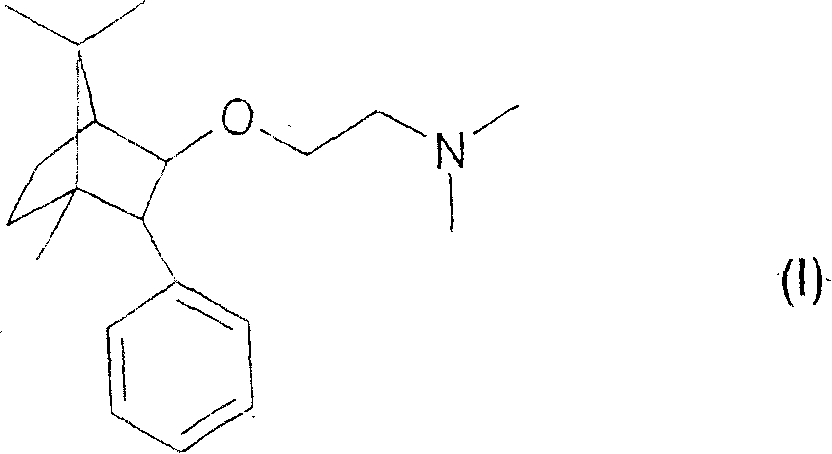
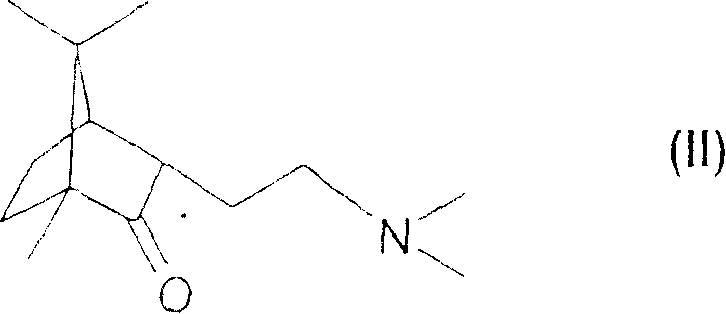
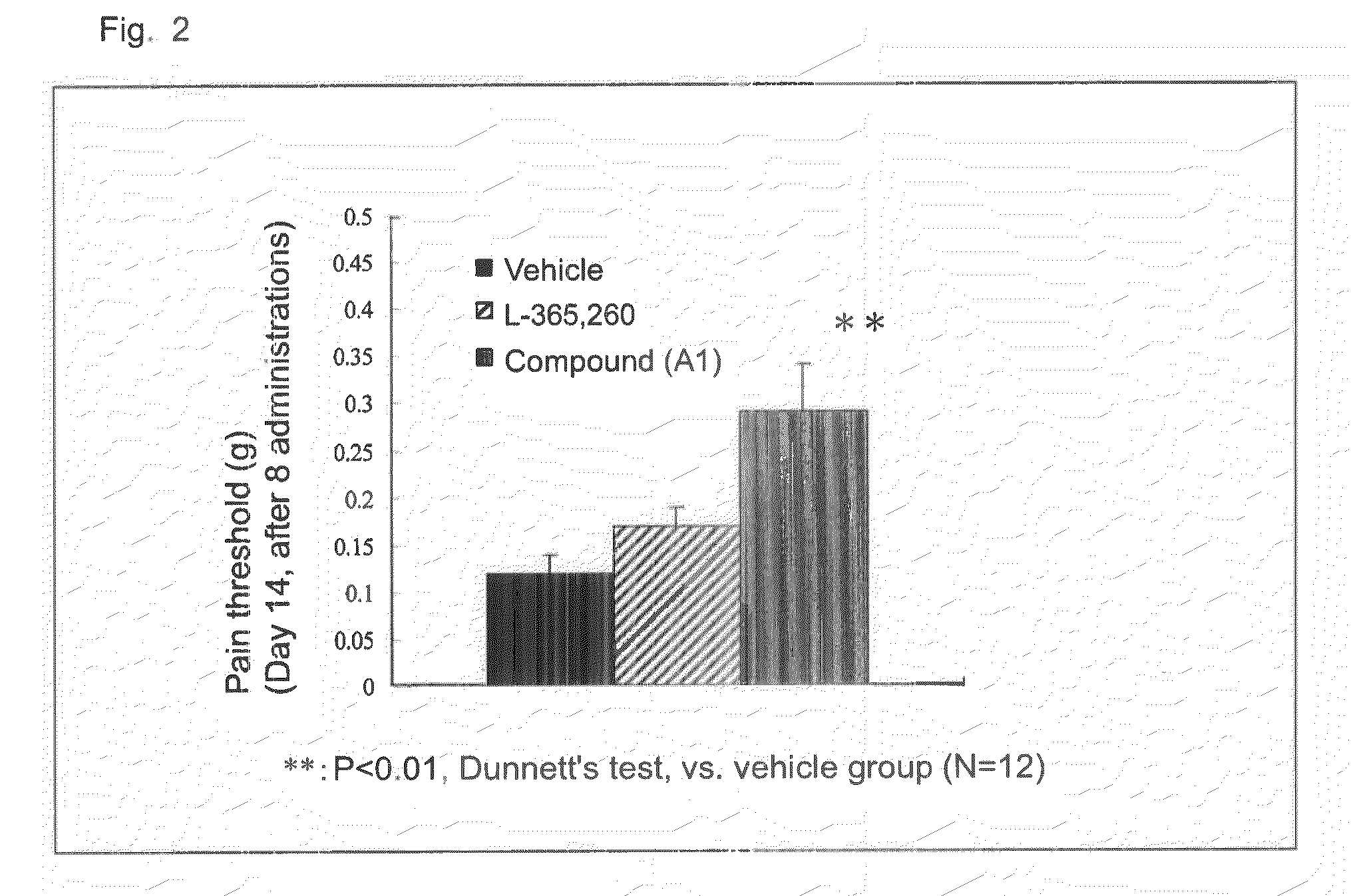
Combination of deramciclane and opoids as analgesics
The invention relates to a combined analgesic pharmaceutical composition which comprises as component: A) (1R,2S,4R)-(-)-2-[N,N-(dimethylaminoethoxy)]-2-phenyl-1,7-7-trimethylbicyclo[2.2.1]heptane or a pharmaceutically acceptable acid addition salt thereof and as component B) morphine, an opioide type analgesic and / or a non-opioide type analgesic in admixture with suitable pharmaceutical carriers and / or auxiliary agents. Deramciclane increases the analgesic effect of morphine.
Owner:EGIS GYOGYSZERGYAR NYILVANOSAN MUKODO RESZVENY TARSASAG
Therapeutic agent for pain
To provide a cancer pain therapeutic and / or prophylactic agent which can be administered to a patient for a long period of time from the early stage to the final stage of the cancer pain therapy, instead of conventional non-opioid analgesic agents or opioid analgesic agents.The cancer pain therapeutic and / or prophylactic agent containing, as an active ingredient, a 1,5-benzodiazepine derivative represented by formula (1):(wherein R1 represents a C1-6 alkyl group, R2 represents a phenyl group or a cyclohexyl group, and Y represents a single bond or a C1-4 an alkylene group) or a pharmaceutically acceptable salt thereof.
Owner:ZERIA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com