Combination of deramciclane and opoids as analgesics
A technology for opioids and analgesics, applied in the field of combined analgesic drug compositions, which can solve problems such as limiting therapeutic applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
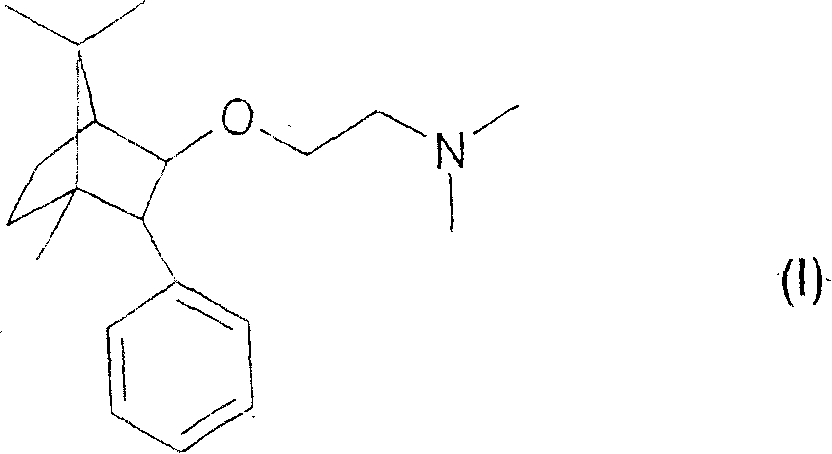
[0019] An analgesic pharmaceutical composition according to the combination of the present invention, comprising as component A) the preferred (1R,2S,4R)-(-)-2-[N,N-(dimethylaminoethoxy)]- 2-Phenyl-1,7,7-trimethylbicyclo[2.2.1]heptane-2(E)-butenedioic acid (1:1).
[0020] The combined analgesic pharmaceutical composition according to the present invention comprises as component A) the particularly preferred (1R, 2S, 4R)-(-)-2-[N,N-(dimethylamino Ethoxy)]-2-phenyl-1,7,7-trimethylbicyclo[2.2.1]heptane or a pharmaceutically acceptable acid addition salt thereof, which contains not more than 0.2% of the general formula
[0021]
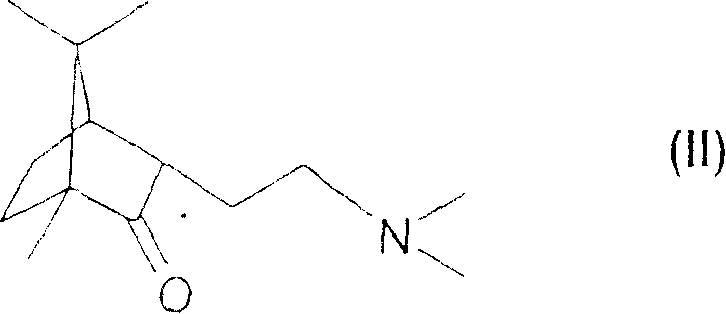
[0022] (1R,3S,4R)-(-)-3-[2-N,N-(Dimethylaminoethyl)]-1,7,7-trimethylbicyclo[2.2.1]heptane -2-ketone or a pharmaceutically acceptable acid addition salt thereof.
[0023] The combined analgesic pharmaceutical composition according to the invention comprises as component A) the particularly preferred (1R,2S,4R)-(-)-2-[N,N-(dimethylaminoethoxy)] - 2-ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com