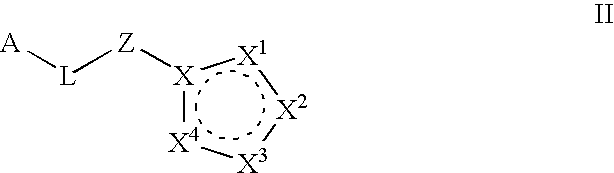Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
115 results about "Thrombo embolism" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of thromboembolism. : the blocking of a blood vessel by a particle that has broken away from a blood clot at its site of formation.
Embolism protection devices
InactiveUS20040093015A1Reduce adverse effectsDilatorsTissue regenerationEmbolic Protection DevicesActive agent
Embolism protection devices can be formed with a biocompatible expandable polymer that can expand upon release within a patient's vessel. Upon release, the structure can be configured to filter flow through the vessel. The material of the embolism protection devices can release one or more biologically active agents, such as a thrombolitic agent, including, for example, tPA. Alternatively or additionally, the embolism protection device can be connected to a tether that elutes one or more biologically active agents.
Owner:MEDTRONIC INC
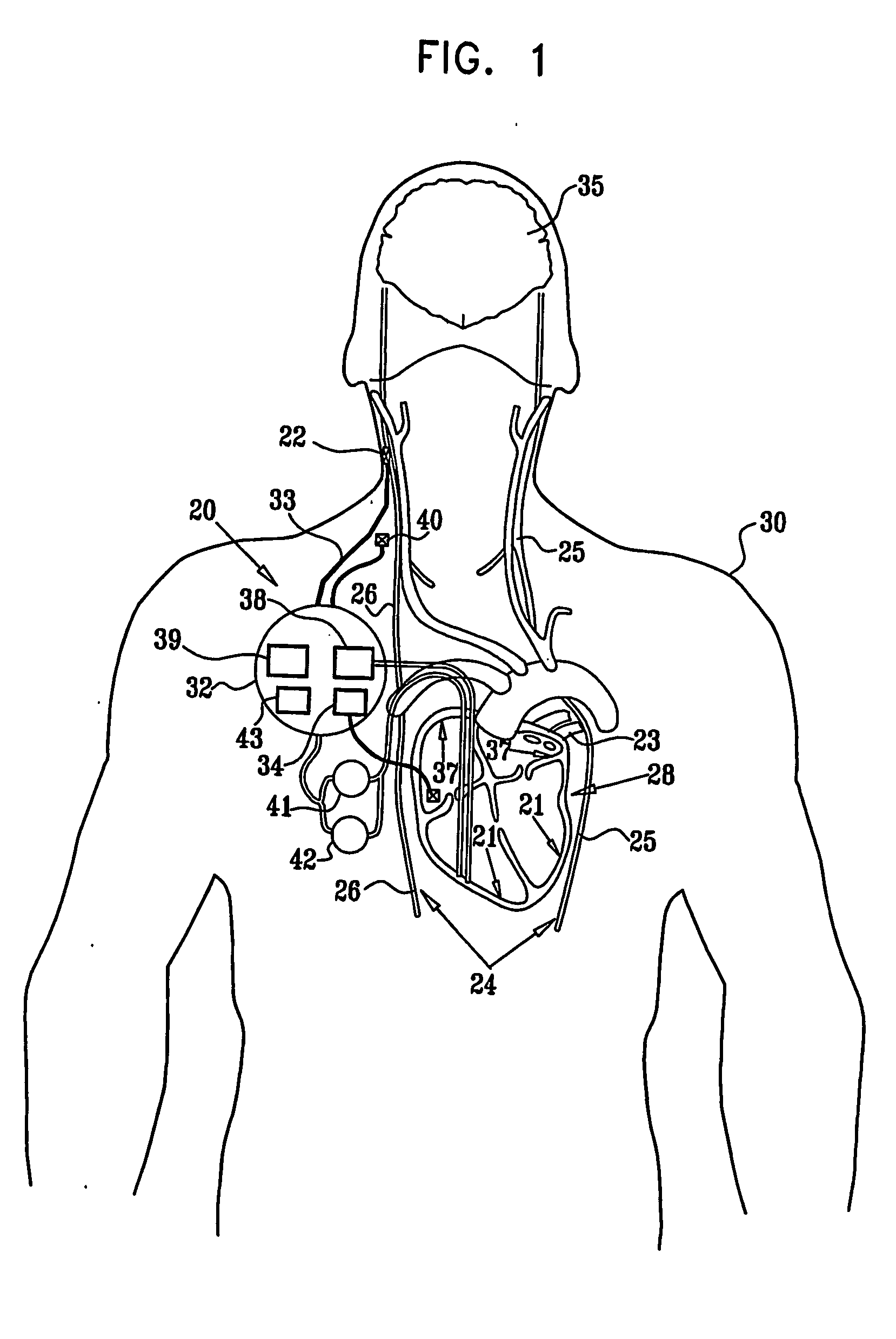
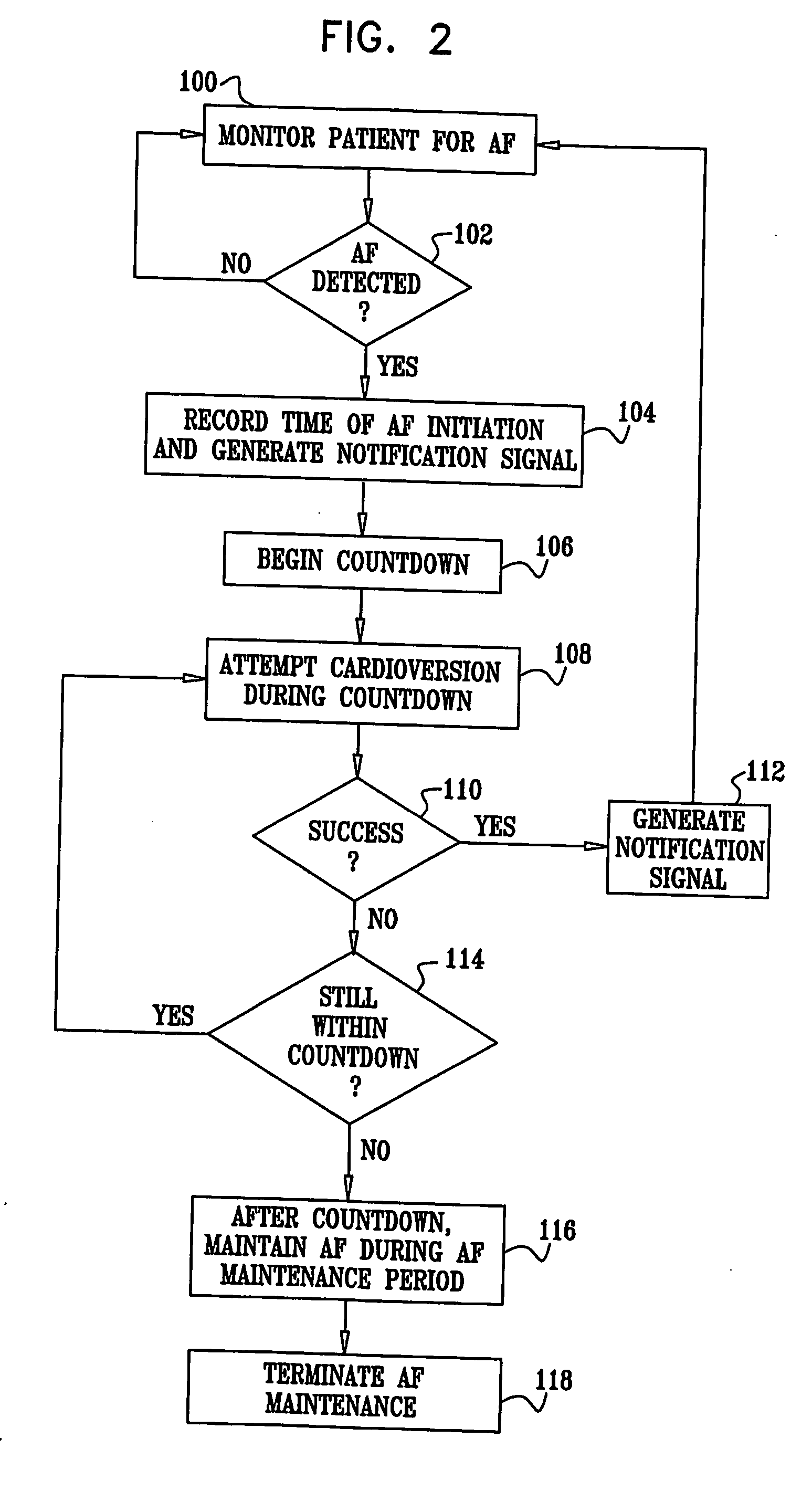
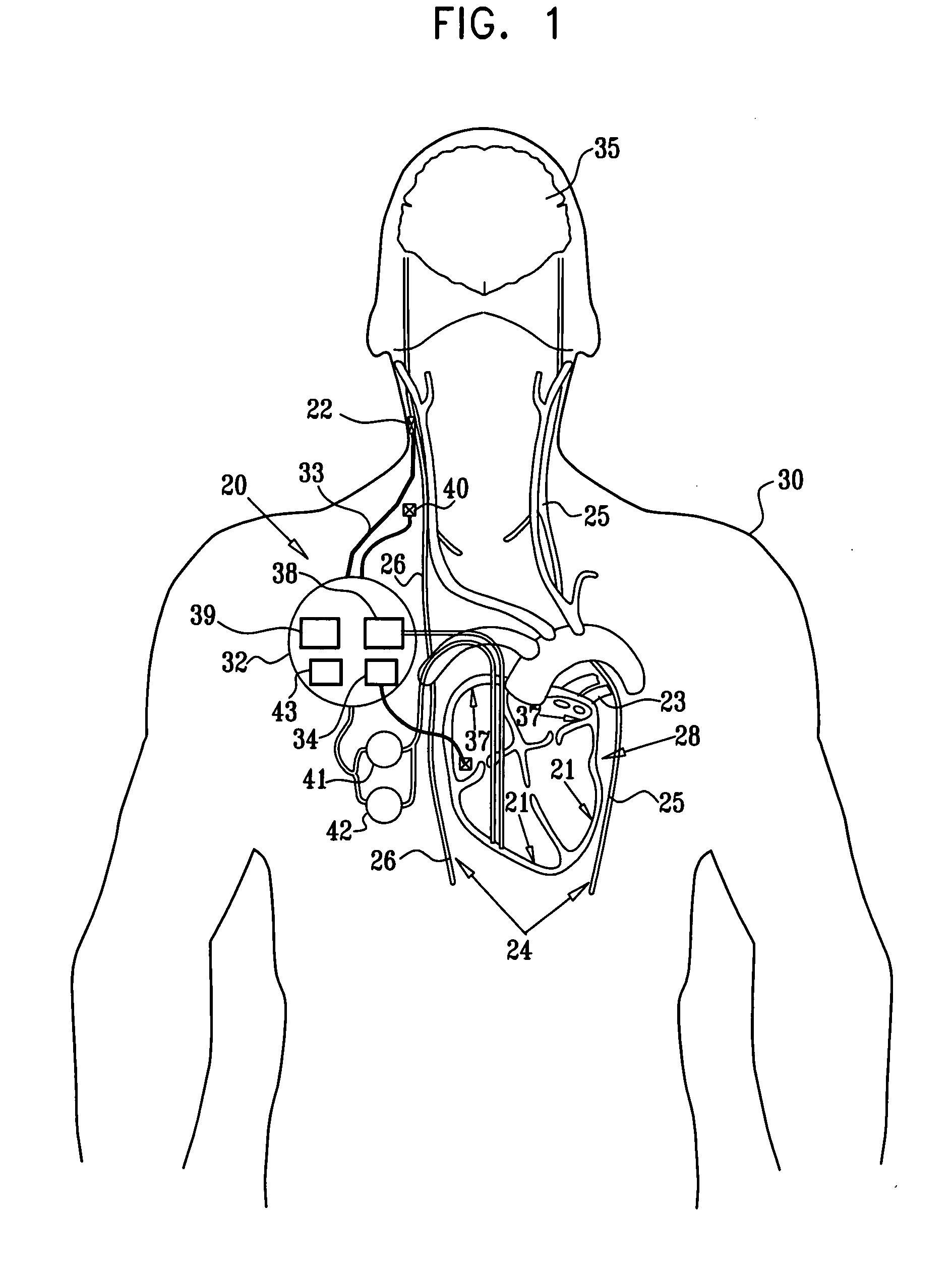
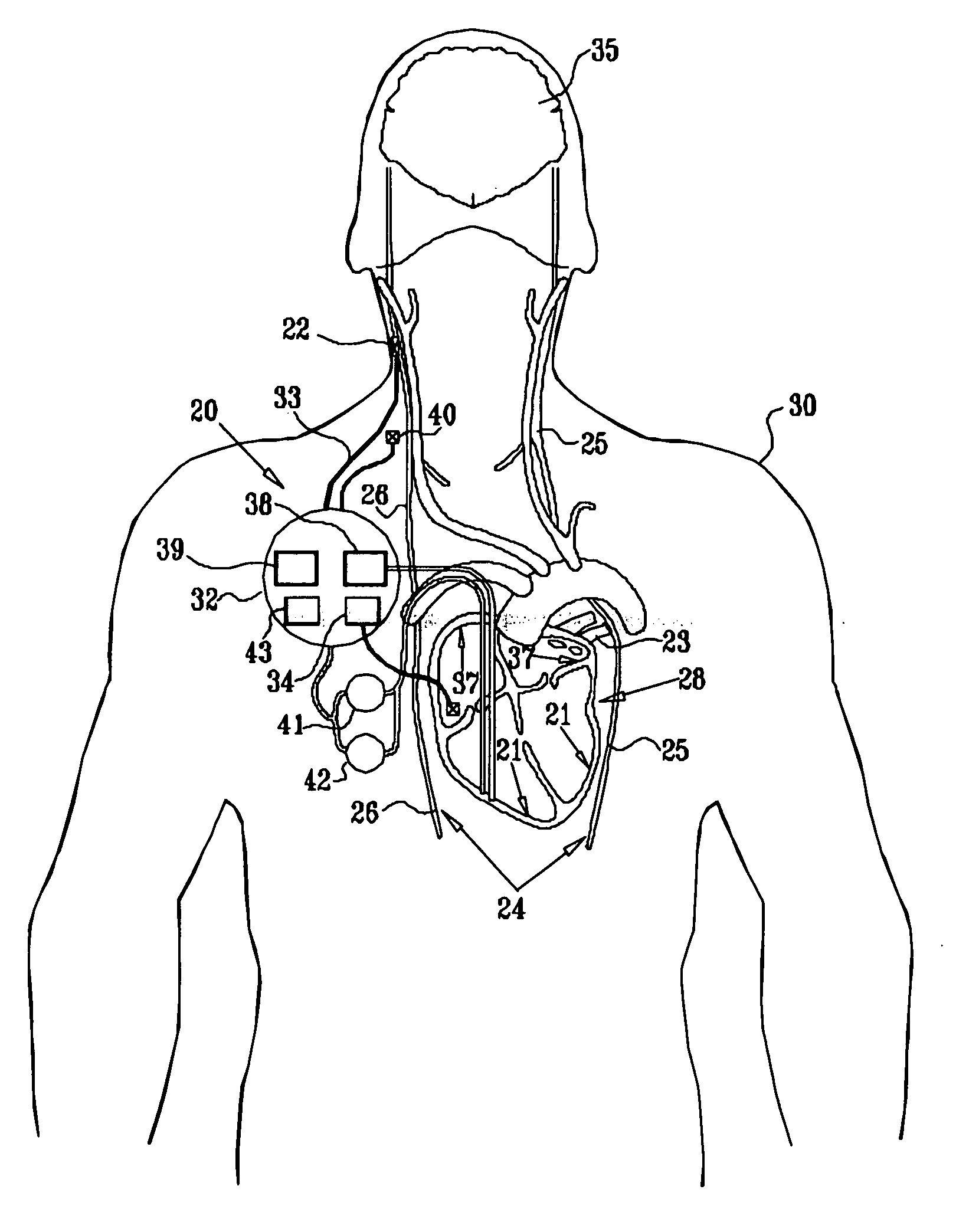
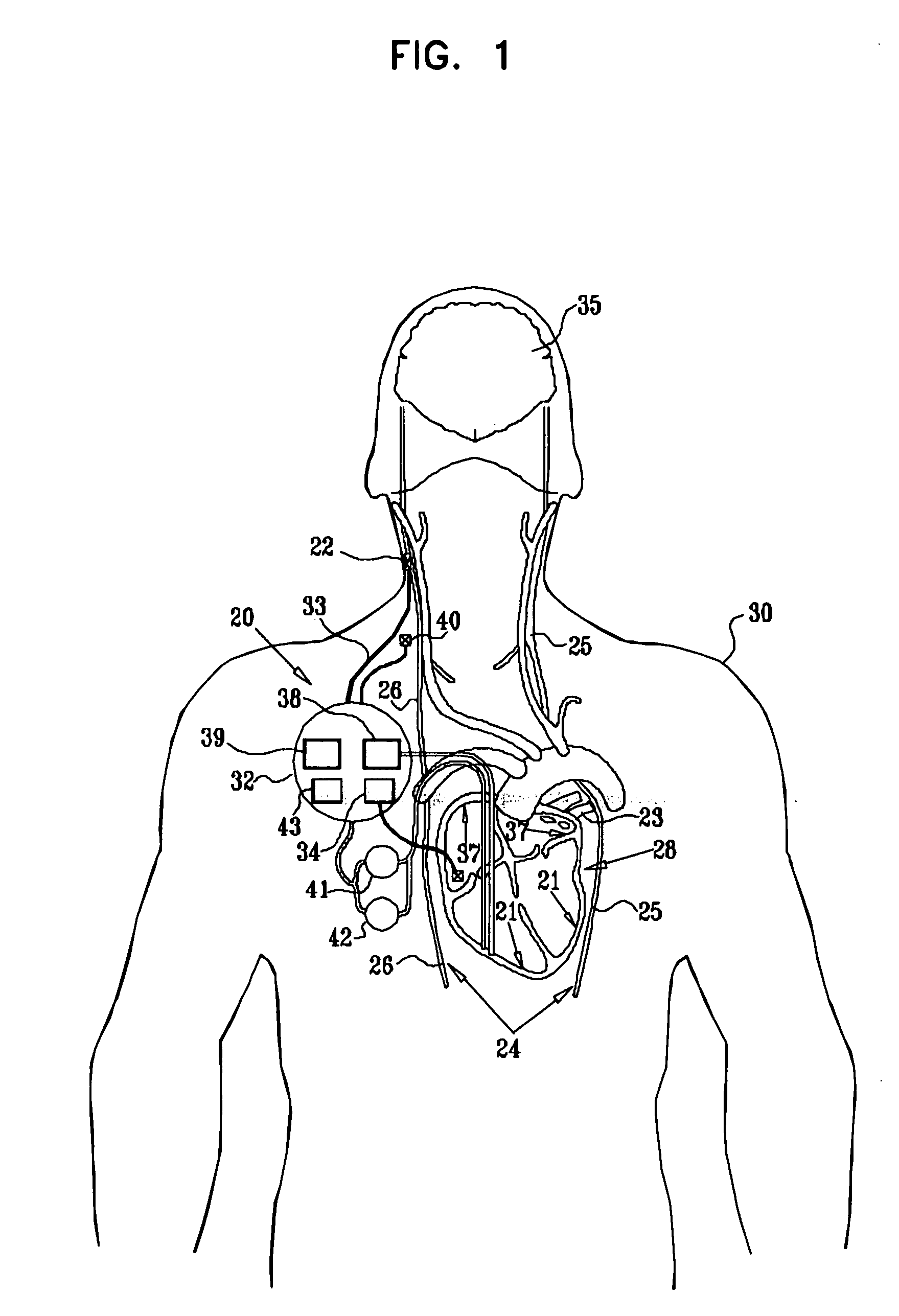
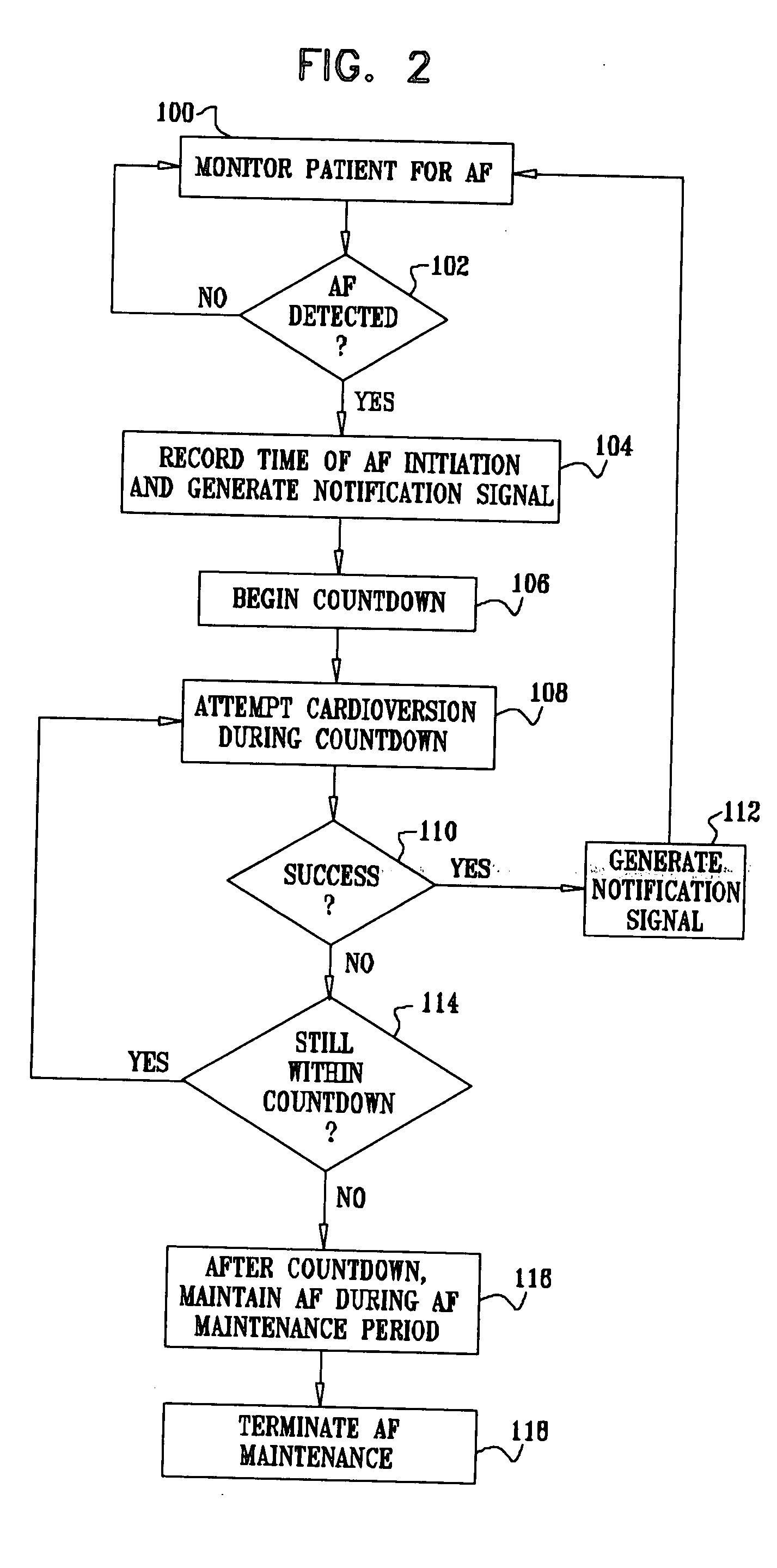
Vagal stimulation for anti-embolic therapy
InactiveUS20060271115A1Increase volumeIncrease stimulationSpinal electrodesHeart defibrillatorsEmbolization TherapyBlood flow
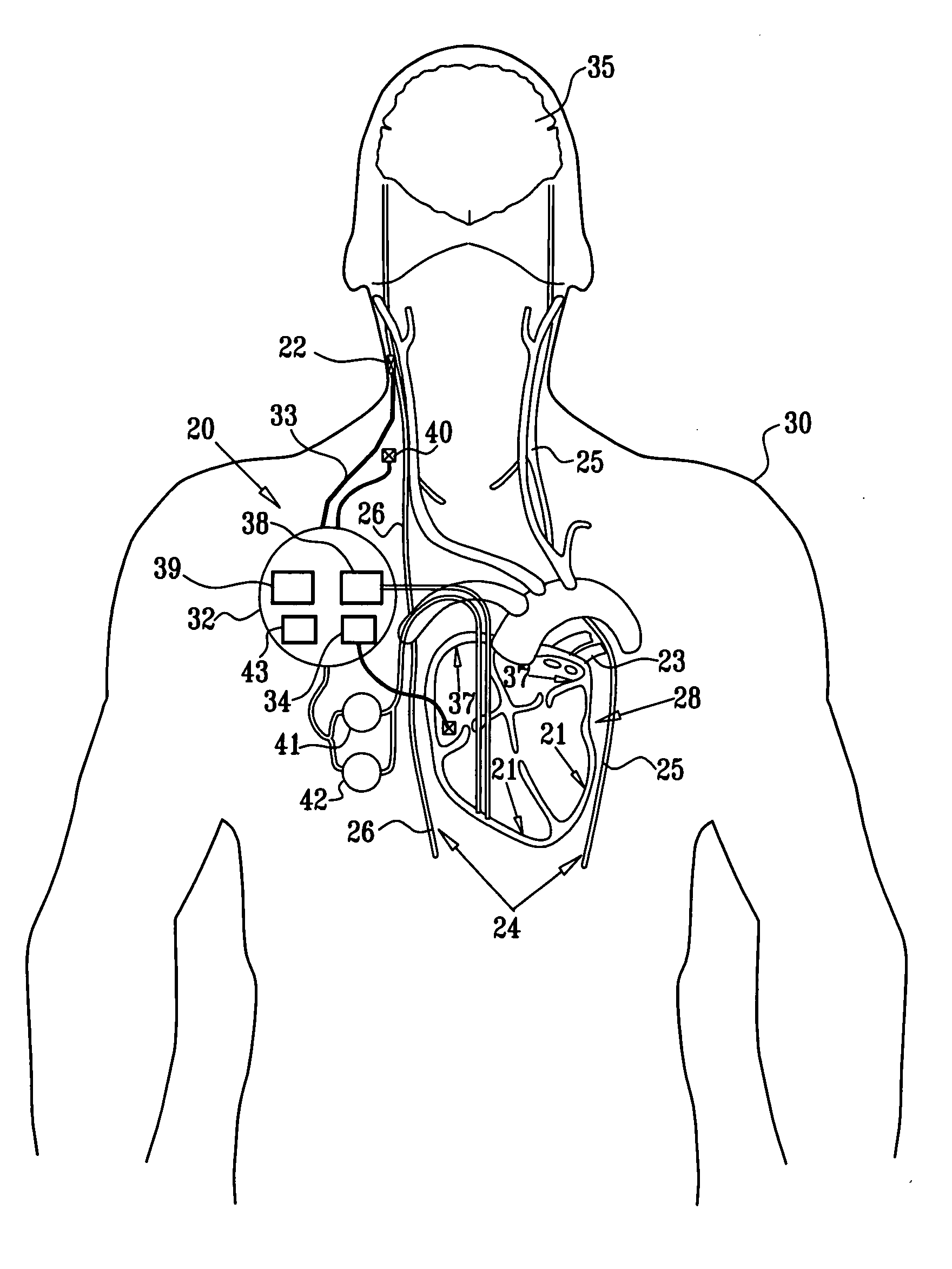
Apparatus (20) for treating a subject (30) suffering from spontaneous atrial fibrillation includes an electrode device (22), adapted to be coupled to a site of the subject (30) selected from the list consisting of: a vagus nerve (24) of the subject (30), an epicardial fat pad of the subject (30), a pulmonary vein of the subject (30), a carotid artery of the subject (30), a carotid sinus of the subject (30), a vena cava vein of the subject (30), and an internal jugular vein of the subject (30), and a control unit (32), adapted to drive the electrode device (22) to apply an electrical current to the site, and to configure the current to maintain the spontaneous AF for at least about 24 hours, so as to modify blood flow within the atria and reduce risk of thromboembolic events.
Owner:MEDTRONIC INC
Embolic filter device and related systems and methods
An embolic filter system is provided that has a bioactive surface, such as locally on the surface itself or via elution into surrounding environs, and such as to debulk its filtered contents or prevent thrombosis or thromboemboli. An engineered wall provides for enhanced porosity for improved combination of blood flow through the filter and size of particulate that may be captured. Manufacturing methods are provided for improved filter assemblies, and a tether system is provided for improved in-situ deployment. A proximal filter assembly is used to debulk contents of a distal embolic filter assembly before it is removed from the patient.
Owner:EMERGE MEDSYST
Embolism protection devices
Embolism protection devices can be formed with a biocompatible expandable polymer that can expand upon release within a patient's vessel. Upon release, the structure can be configured to filter flow through the vessel. The material of the embolism protection devices can release one or more biologically active agents, such as a thrombolitic agent, including, for example, tPA. Alternatively or additionally, the embolism protection device can be connected to a tether that elutes one or more biologically active agents.
Owner:MEDTRONIC INC
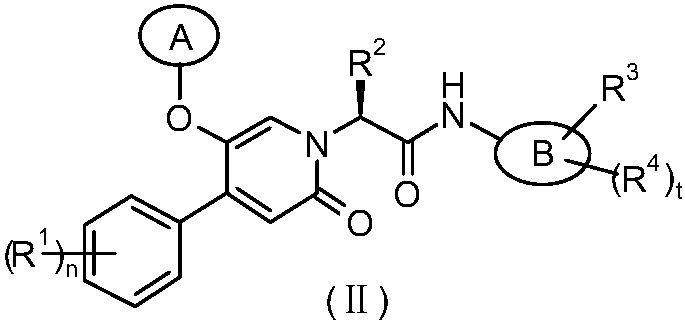
(Hetero)aryl-bicyclic heteroaryl derivatives, their preparation and their use as protease inhibitors
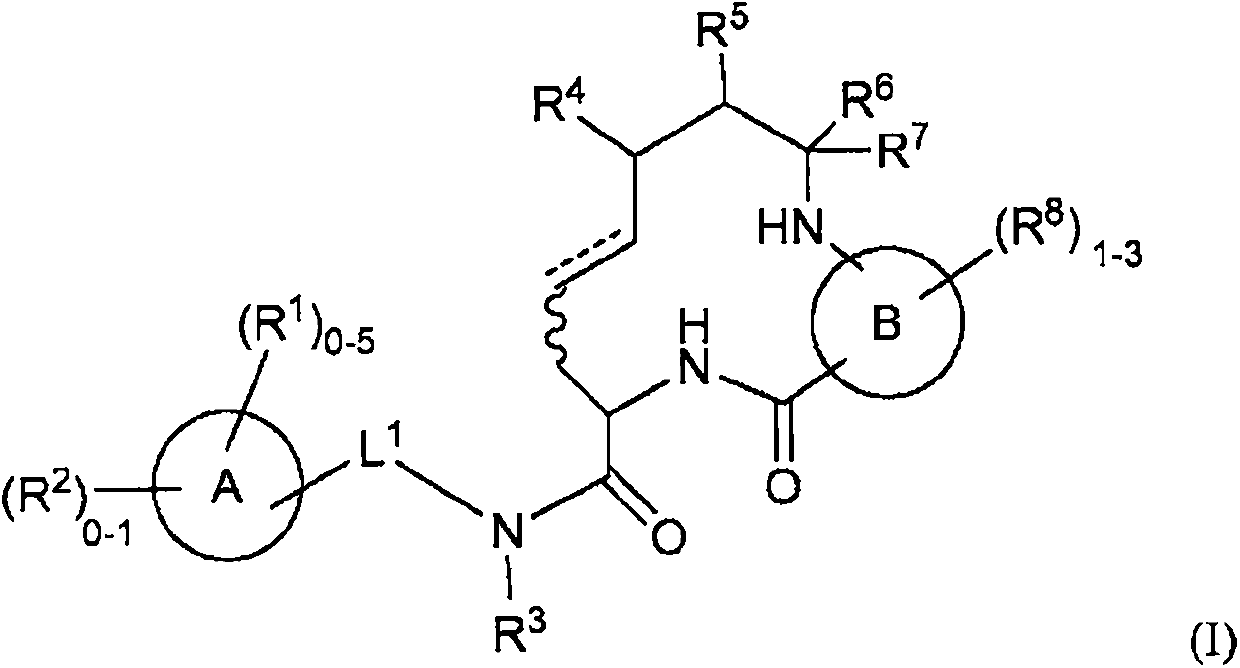
The present invention provides novel compounds of the Formula (I): A-B, its prodrug forms, or pharmaceutically acceptable salts thereof, wherein A represents a saturated, unsaturated, or a partially unsaturated bicyclic heterocyclic ring structure, and B represents an aryl or a heteroaryl group. Preferred compounds of the present invention comprise a benzimidazole or indole nucleus. The compounds of this invention are inhibitors of serine proteases, Urokinase (uPA), Factor Xa (FXa), and / or Factor VIIa (FVIIa), and have utility as anti cancer agents and / or as anticoagulants for the treatment or prevention of thromboembolic disorders in mammals.
Owner:AXYX PHARMA INC
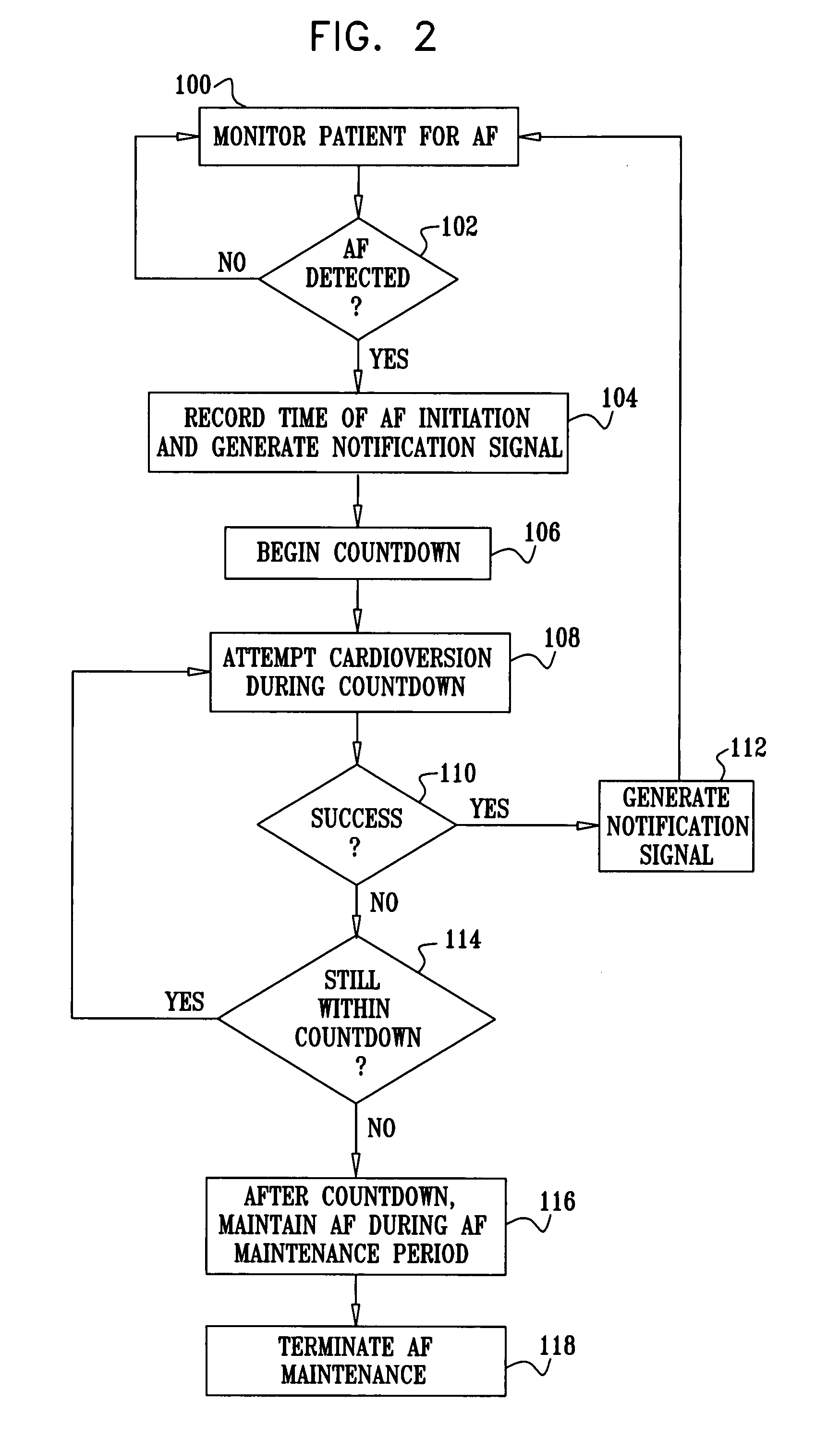
Vagal stimulation for cardioversion of atrial fibrillation
InactiveUS20080091241A1Reduce frequencyIncreased riskSpinal electrodesHeart defibrillatorsBlood flowThrombus
Apparatus (20) for treating a subject (30) suffering from spontaneous atrial fibrillation includes an electrode device (22), adapted to be coupled to a site of the subject (30) selected from the list consisting of: a vagus nerve (24) of the subject (30), an epicardial fat pad of the subject (30), a pulmonary vein of the subject (30), a carotid artery of the subject (30), a carotid sinus of the subject (30), a vena cava vein of the subject (30), and an internal jugular vein of the subject (30), and a control unit (32), adapted to drive the electrode device (22) to apply an electrical current to the site, and to configure the current to maintain the spontaneous AF for at least about 24 hours, so as to modify blood flow within the atria and reduce risk of thromboembolic events.
Owner:MEDTRONIC INC
Combined parasympathetic stimulation and cardiac pacing
InactiveUS20080091245A1Reduce frequencyIncreased riskHeart defibrillatorsHeart stimulatorsThrombusControl cell
Apparatus for treating a subject suffering from spontaneous atrial fibrillation includes an electrode device, adapted to be coupled to a vagus nerve of the subject, and a control unit, adapted to drive the electrode device to apply an electrical current to the vagus nerve, and to configure the current to maintain the spontaneous AF for at least about 24 hours, so as to modify blood flow within the atria and reduce risk of thromboembolic events.
Owner:MEDTRONIC INC
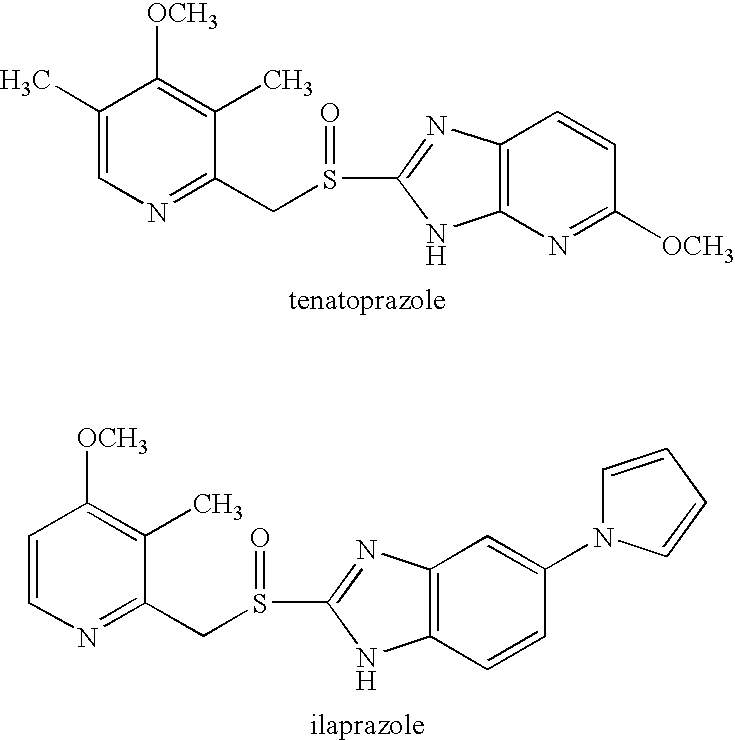
New Combination Dosage Form
InactiveUS20070122470A1Salicyclic acid active ingredientsBiocideGastrointestinal complicationsSalicylic acid
The present invention relates to an oral pharmaceutical preparation for use in the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid. The present preparation comprises a fixed oral dosage form comprising a proton pump inhibitor in combination with acetyl salicylic acid. Furthermore, the present invention refers to a method for the manufacture thereof and the use thereof in medicine. The present invention also relates to a specific combination comprising esomeprazole, or an alkaline salt thereof or a hydrated form of any one of them, and acetyl salicylic acid for use as a medicament for the prevention of thromboembolic vascular events, such as myocardial infarction or stroke, and for the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid.
Owner:ASTRAZENECA AB
Novel combination of selective factor VIIa and/or factor XIa inhibitors and selective plasma kallikrein inhibitors
The present invention relates to a novel pharmaceutical combination for treating thromboembolic and / or inflammatory diseases, wherein the combination has: (a) a first therapeutic agent independently selected from the group consisting of a selective Factor VIIa inhibitor, a selective Factor XIa inhibitor, a combination of the selective Factor VIIa and XIa inhibitors, or pharmaceutically acceptable salt forms thereof; and (b) a second therapeutic agent comprising a selective plasma kallikrein inhibitor or a pharmaceutically acceptable salt form thereof. The instant invention is also directed to a method and composition suitable for treating thromboembolic and / or inflammatory diseases using the novel combinations.
Owner:BRISTOL MYERS SQUIBB CO
Fused azole-pyrimidine derivatives
The present invention relates to hovel fused azolepyrimidine derivatives, processes for preparing them and pharmaceutical preparations containing them. The fused azolepyrimidine derivatives of the present invention exhibit enhanced potency for phosphotidylinositol-3-kinase (PI3K) inhibition, especially for PI3K-γ inhibition and can be used for the prophylaxis and treatment of diseases associated with PI3K and particularly with PI3K-γ activity. More specifically, the azole derivatives of the present invention are useful for treatment and prophylaxis of diseases as follows: inflammatory and immunoregulatory disorders, such as asthma, atopic dermatitis, rhinitis, allergic diseases, chronic obstructive pulmonary disease (COPD), septic shock, joint diseases, autoixnmune pathologies such as rheumatoid arthritis, and Graves' disease, cancer, myocardial contractility disorders, heart failure, thromboembolism, ischemia, and atherosclerosis. The compounds of the present invention are also useful for pulmonary hypertension, renal failure, cardiac hypertrophy, as well as neurodegenerative disorders such as Parkinson's disease, Alzheimer's disease, diabetes and focal ischemia, since the diseases also relate to PI3K activity in a human or animal subject.
Owner:BAYER INTELLECTUAL PROPERTY GMBH +1
Epoxy-substituted oxopyridine derivatives and their preparation method and application in medicine
The invention relates to epoxy-substituted oxopyridine derivatives and their preparation method and application in medicine and concretely, relates to epoxy-substituted oxopyridine derivatives shown in the general formula (I), their preparation method, a pharmaceutical composition containing the derivatives and a use of the derivatives as therapeutic agents and especially as blood coagulation factor XIa (called as FXIa for short) inhibitors and in preparation of drugs for treating diseases such as thromboembolism. The definition of each substituent in the general formula (I) is the same as thedefinition in the specification.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Device for angioplasty with an embolization protection component and method therefor
InactiveUS20100262219A1Simple deployment/retrieval methodSimple deployment/retrievalStentsBalloon catheterThrombosis embolismDistal embolization
A device and method for balloon and stent angioplasty for treating atherosclerosis with a distal embolization protection component. The device includes an endoluminal dilatation balloon formed on a shaft having at least one lumen, a flexible filter mounted on the shaft, distal to the balloon and a filter deployment memory ring circling the proximal opening of the flexible filter for the purpose of controlling deployment and collapsing of the filter. When the endoluminal dilatation balloon is inflated for the purpose of dilating plaque that is blocking a blood vessel, the flexible filter deployed prior to balloon inflation, traps atherosclerotic debris and thrombi emboli that are released from the plaque, thus allowing blood flow to continue without interference during the angioplasty procedure. At the end of the procedure the flexible filter, containing the debris, is pulled and collapsed into a small profile and is pulled out of the artery as an integrated unit with the balloon. The device may or may not have a stent pre-crimped over the dilatation balloon.
Owner:FRIMERMAN AHARON
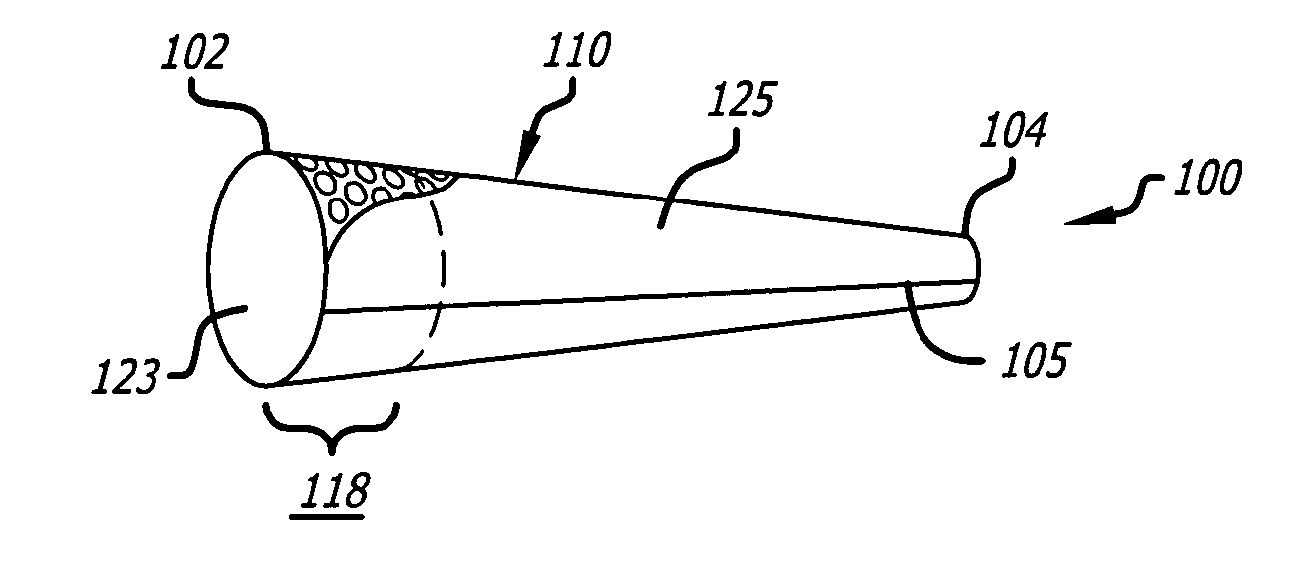
Embolectomy catheters and method for treatment
Embolectomy catheters, rapid exchange microcatheters, systems and methods for removing clots or other obstructive material (e.g., thrombus, thromboemboli, embolic fragments of atherosclerotic plaque, foreign objects, etc.) from blood vessels. This invention is particularly useable for percuatneous removal of thromboemboli or other obstructive matter from small blood vessels of the brain, during an evolving stroke or period of cerebral ischemia. In some embodiments, the embolectomy catheters of this invention are advanceable with or over a guidewire (GW) which has been pre-inserted through or around the clot. Also, in some embodiments, the embolectomy catheters include clot removal devices which are deployable from the catheter after the catheter has been advanced at least partially through the clot. The clot removal device may include a deployable wire nest that is designed to prevent a blood clot from passing therethrough. The delivery catheter may include telescoping inner and outer tubes, with the clot removal device being radially constrained by the outer tube. Retraction of the outer tube removes the constraint on the clot removal device and permits it to expand to its deployed configuration. An infusion guidewire is particularly useful in conjunction with the embolectomy catheter, and permits infusion of medicants or visualization fluids distal to the clot.
Owner:MICROVENTION INC
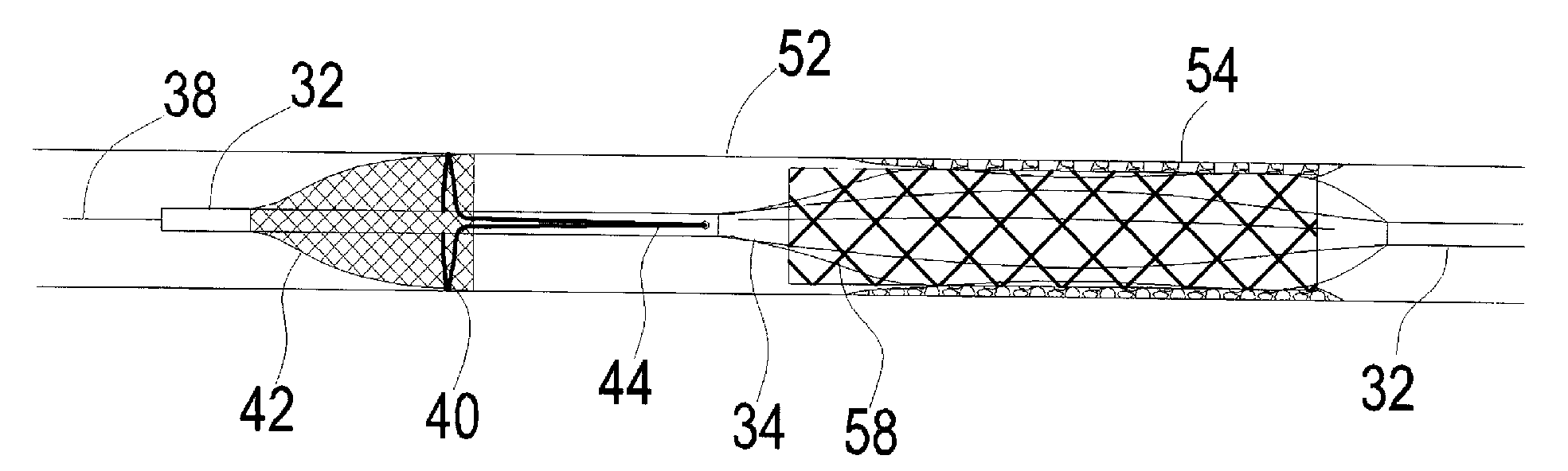
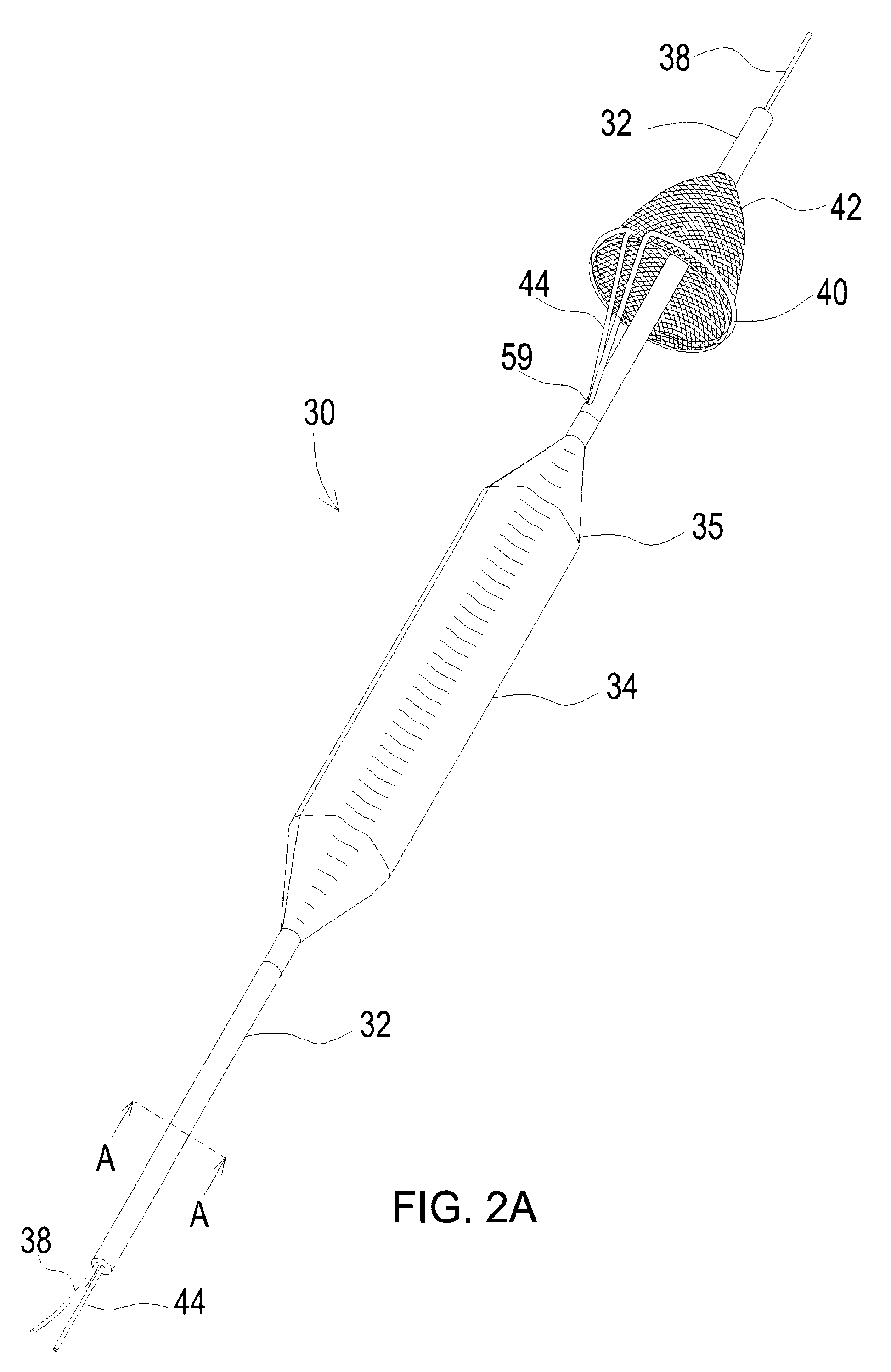
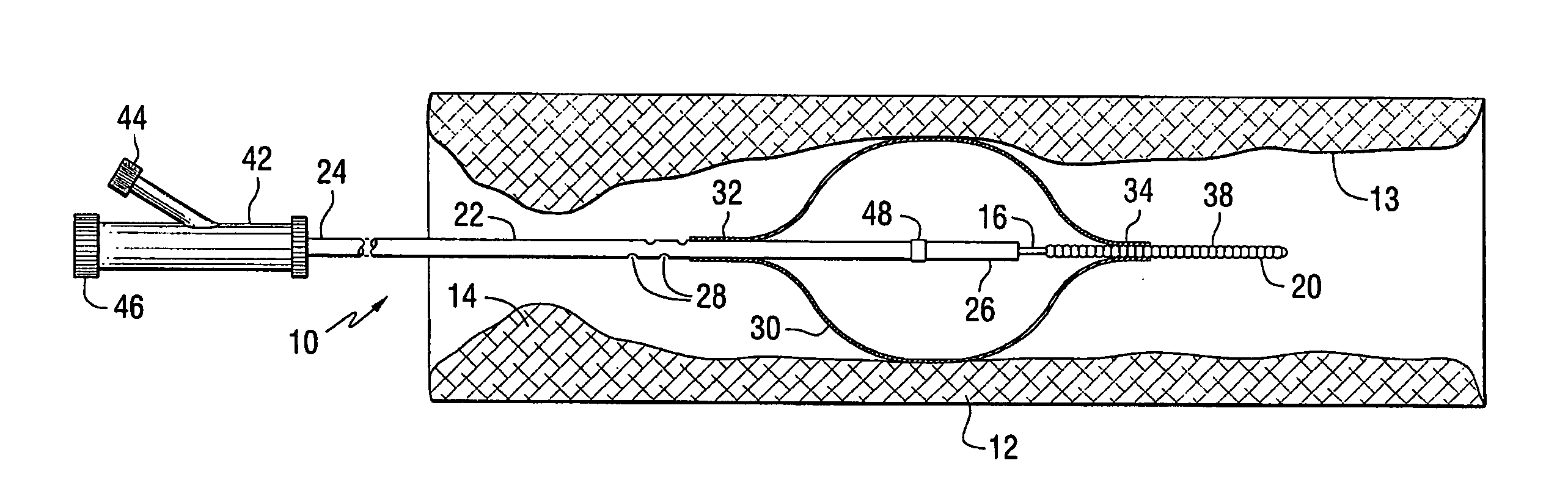
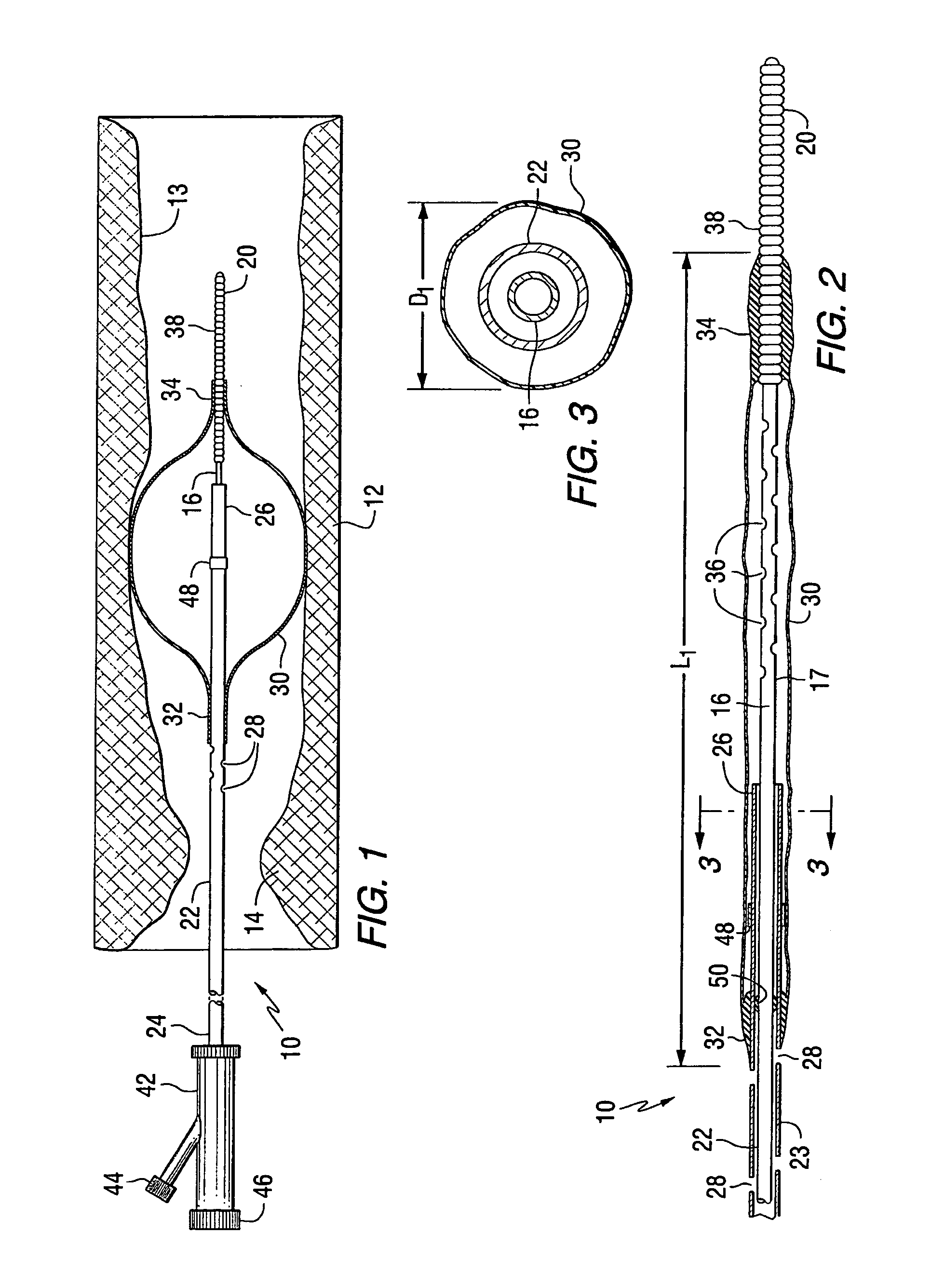
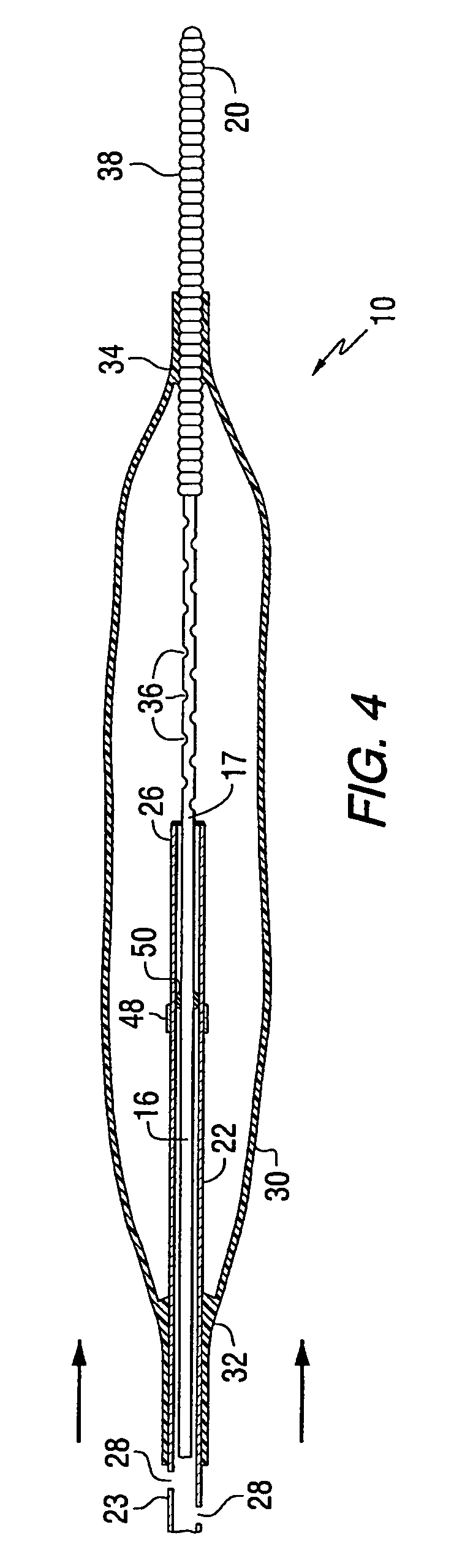
Apparatus for thromboembolic protection
A catheter apparatus captures thromboembolic material in the arterial and venous circulation. The apparatus includes an inner hollow tube and an outer hollow tube in sliding engagement with the inner hollow tube. A radially expandable segment, such as an inflatable balloon, is attached near the distal end of the catheter. One end portion of the balloon is attached to the inner tube and the other end portion is attached to the outer tube. When the catheter is advanced through a vessel to be treated, the balloon is deflated and the outer tube is slidably advanced to a storage position so that the balloon compactly surrounds the catheter. When the catheter is in place within the vessel, the outer tube is slidably advanced to a treatment position and the balloon is inflated. As the vessel is treated, the inflated balloon captures and contains thromboembolic particles that may be released, and the particles are suctioned out of the patient through apertures located in the outer hollow tube proximal to the balloon.
Owner:WHOLEY MARK H +1
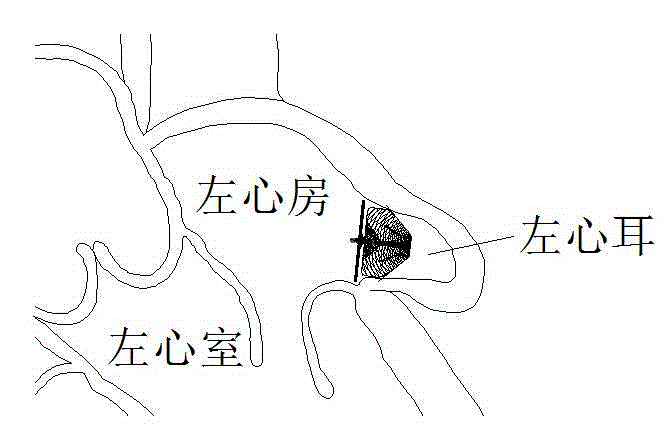
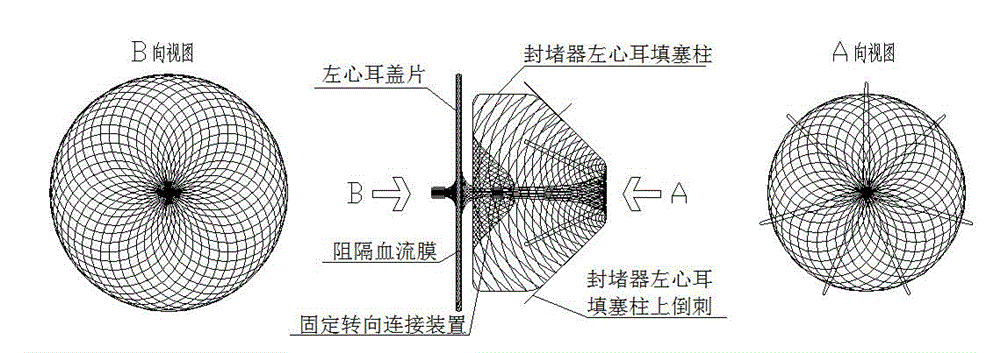
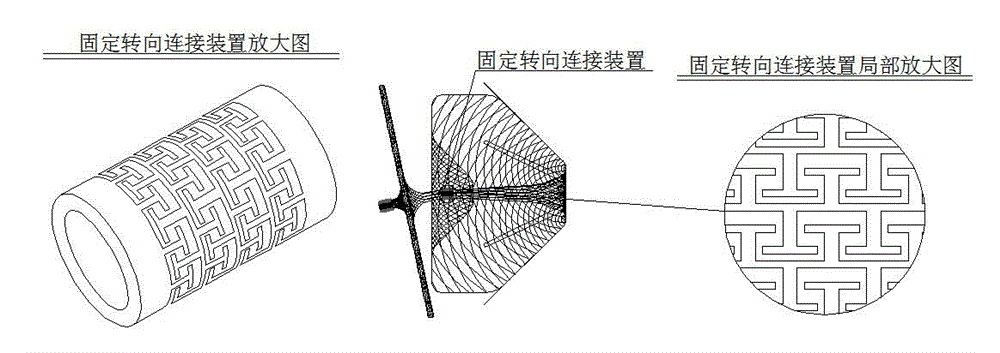
Novel left aurcle occluder and manufacturing method thereof
The invention relates to an occluder for occluding a left aurcle. The novel left aurcle occluder comprises a left aurcle packing column of the occluder, upper barbs of the left aurcle packing column of the occluder, a fixed steering connecting device, a left aurcle cover plate of the occluder, a blood flow barrier membrane, and the like. The novel left aurcle occluder is implanted into the human body by utilizing a minimally invasive therapy method and used for preventing the forming of a thrombus in the left aurcle of a patient with atrial fibrillation by occluding the left aurcle, so that the risk that long-term disability or death due to thromboembolism happens to the patient with atrial fibrillation is lowered. Meanwhile, long-term dependence of the patient with atrial fibrillation on anticoagulant drugs can be eliminated by occluding the left aurcle to provide a new treatment choice for the patient. The occluder is woven by nickel-titanium alloy wires, has a preset extensional appearance, and is used for connecting the left aurcle packing column of the occluder with the left aurcle cover plate of the occluder by virtue of the fixed steering connecting device; the upper barbs of the left aurcle packing column of the occluder are woven on the left aurcle packing column, and the blood flow barrier membrane is sewn in the left aurcle cover plate of the occluder. The novel left aurcle occluder can be used for occluding and blocking the blood flow from entering the left aurcle, the whole left aurcle occluder is smooth and flat in surface and beneficial to epithelization after being implanted.
Owner:SHANGHAI PUSH MEDICAL DEVICE TECH
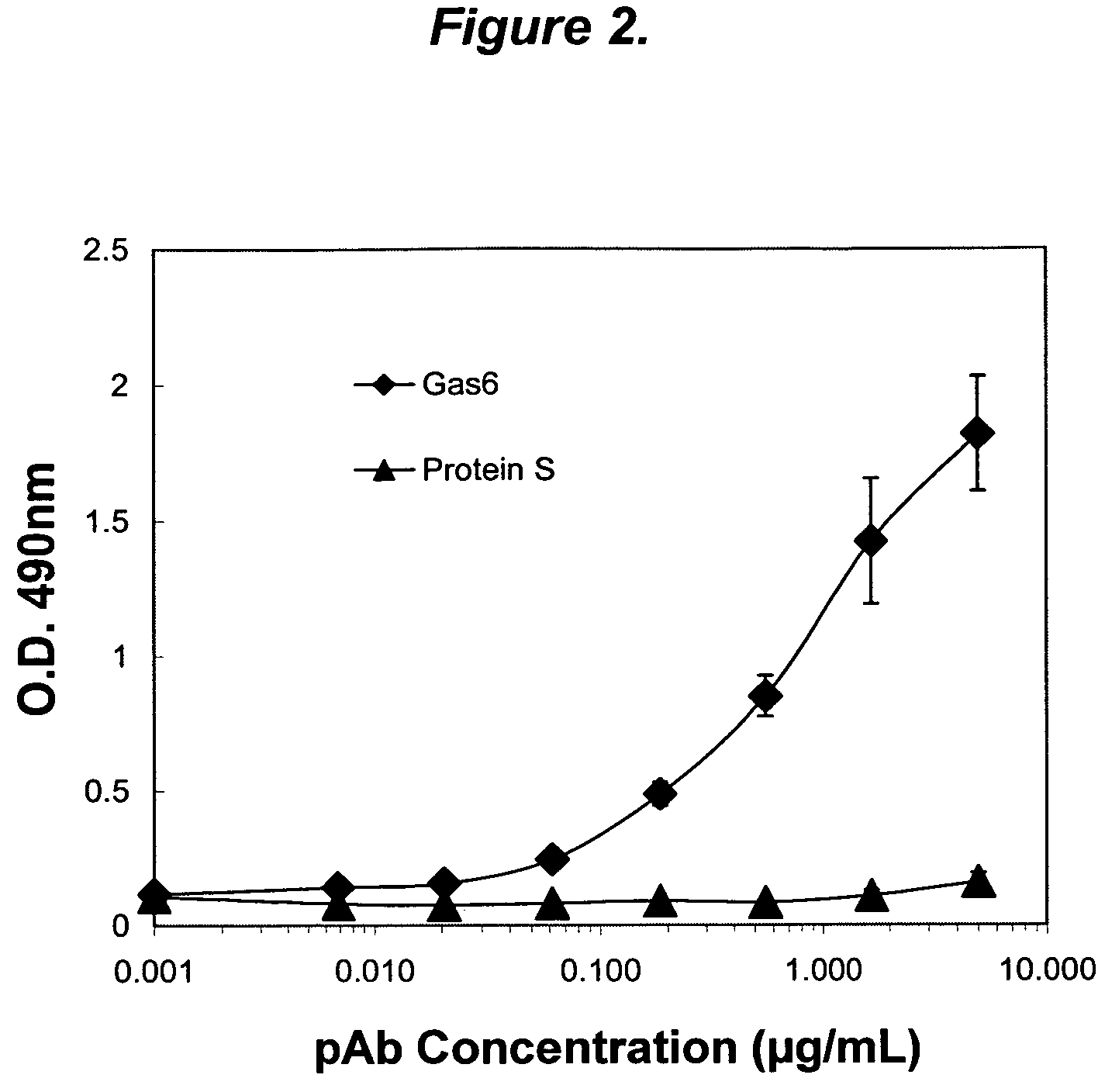
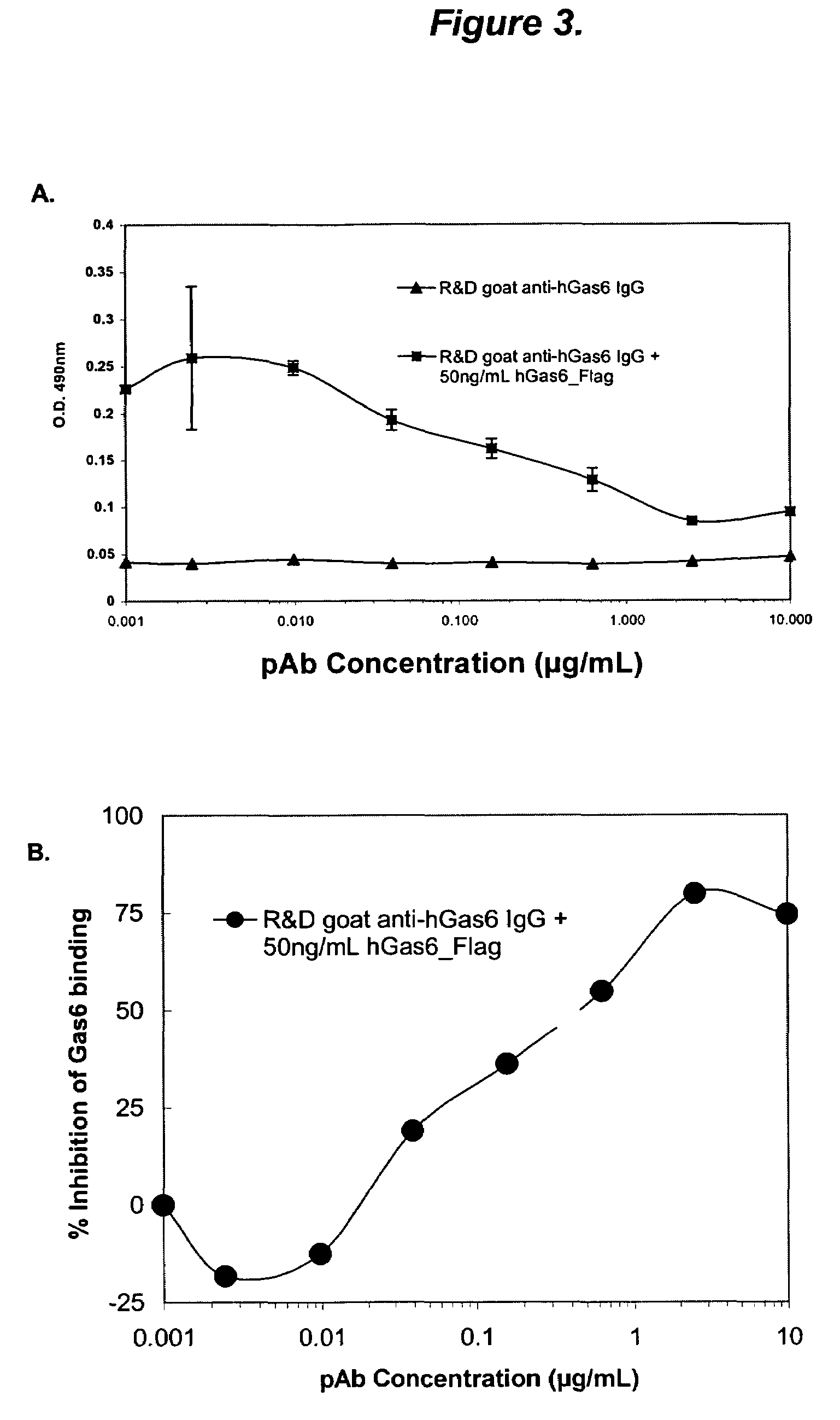
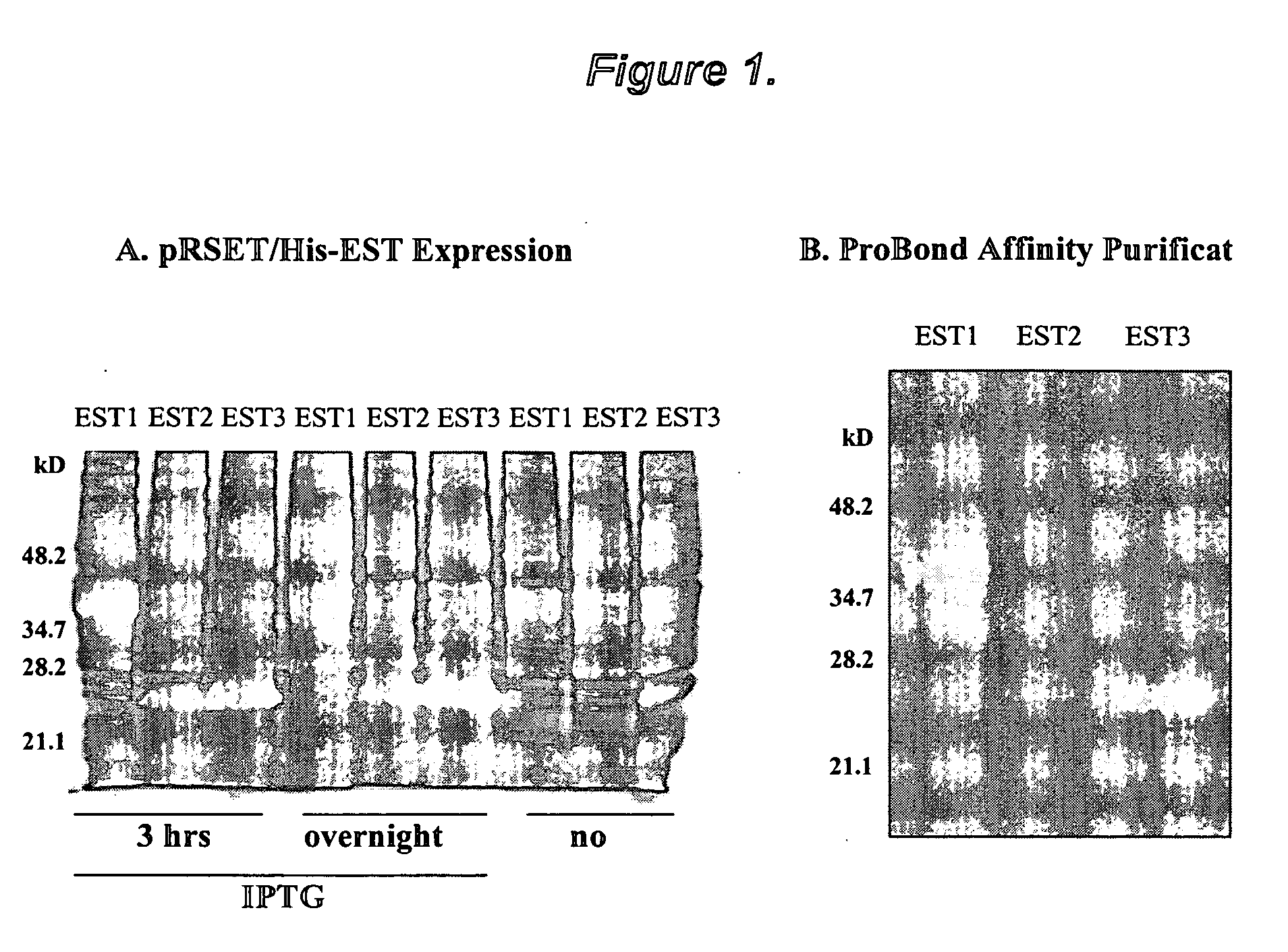
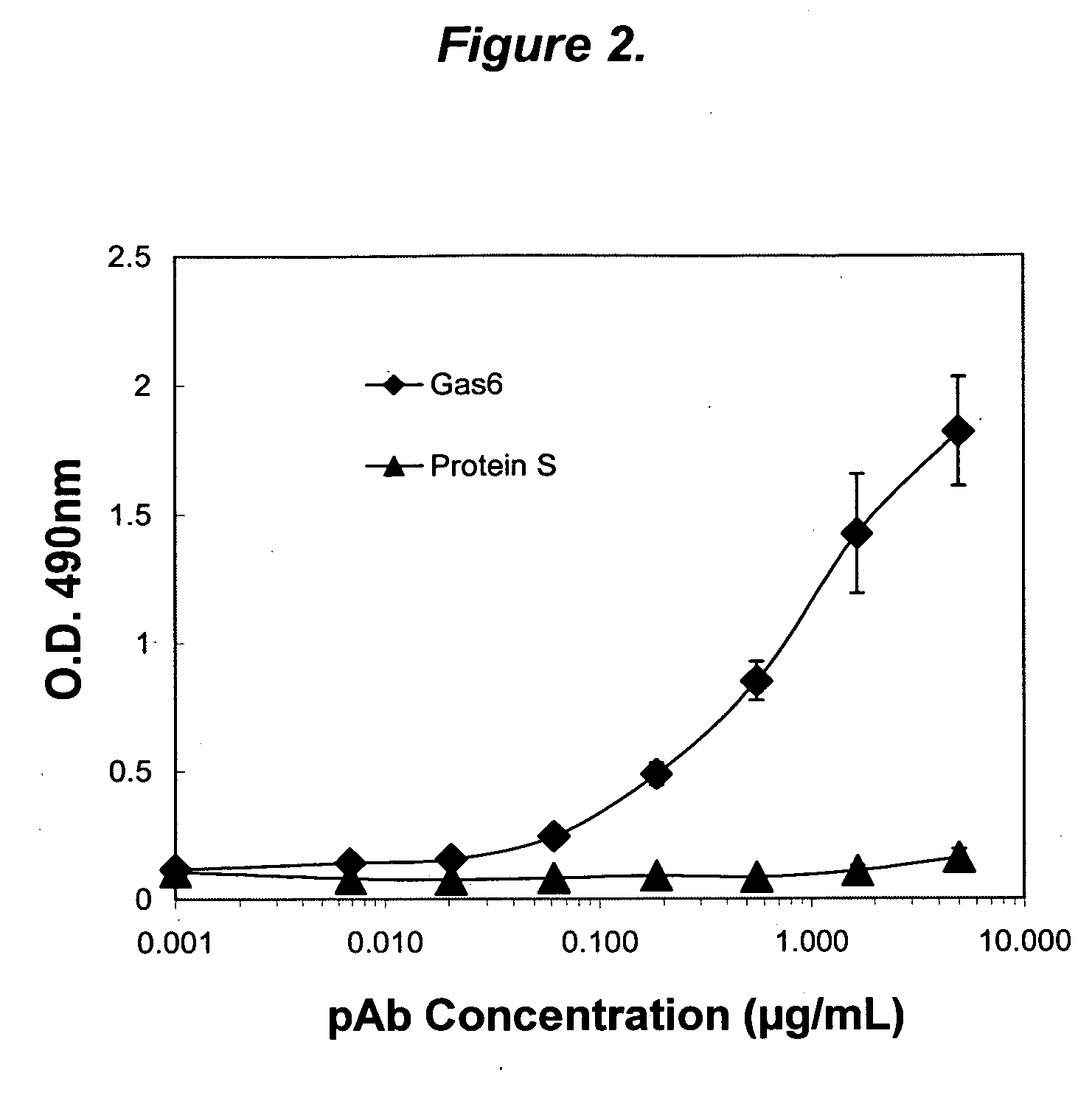
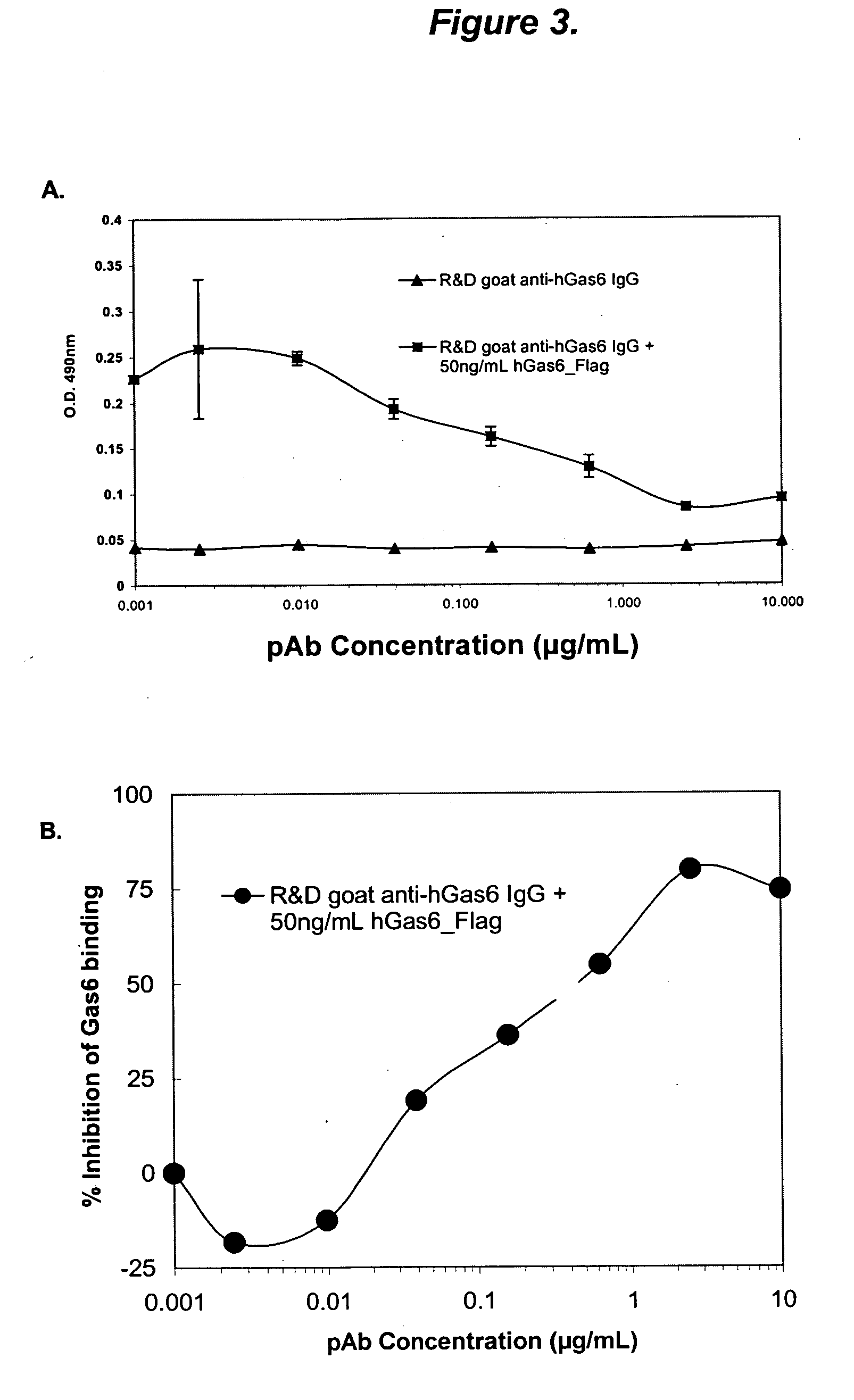
Growth arrest specific gene 6 peptides, antibodies, compositions, methods and uses
The present invention provides novel proteins and peptides from the receptor binding region of human Growth Arrest Specific Gene 6 (Gas6) and antibodies, including specified portions or variants, specific for at least one such Gas6 peptide or fragment thereof. The aforesaid peptides can be used to generate human, primate, rodent, mammalian, chimeric, humanized and / or CDR-grafted anti-Gas6 antibodies. The invention also provides for the nucleic acids encoding such peptides and anti-Gas6 antibodies, complementary nucleic acids, vectors, host cells, and methods of making and using thereof, including therapeutic formulations, administration and devices. Fifteen novel peptide sequences from the Gas6 G domain that are implicated in Gas6 interactions with its receptors are identified, isolated, and synthesized so as to allow generation of anti-Gas6 antibodies. The peptide sequences include three ESTs that encompass regions predicted to contribute to receptor binding or that can raise anti-Gas6 antibodies. This invention provides for such antibodies to be used in modulating or treating at least one Gas6-related disease in a cell, tissue, organ, animal, or patient. Such diseases may include, but are not limited to, thromboembolic disease, ischemic disease, venous thromboembolism, arterial or venous thrombosis, pulmonary embolism, restenosis, diabetic angiopathy and allograft atherosclerosis.
Owner:CENTOCOR ORTHO BIOTECH
Support Stent for Transvalvular Conduit
ActiveUS20140128967A1Risk minimizationMinimal and no antigoagulationHeart valvesBone implantAxial-flow pumpThrombus
Miniature axial flow pumps are implanted inside the heart or major arteries to provide hemodynamic support. These pumps commonly utilize tubular blood conduit tubes to transport blood across the aortic valve. The valve leaflets themselves are very thin and flexible, and will seal against the conduit if it is centered within the valve orifice. If the conduit is not centered, a leaflet can be pushed to the side of the aorta, preventing the leaflets from sealing and providing a path for backflow. If the blood flow conduit remains pushed against the side of the valve for a long period of time, thrombus may form in the crevice between the conduit and the aorta, causing serious thromboembolic events such as stroke, if the thrombus breaks free. The present invention provides a conduit support device that retains the conduit centered within the annulus of the natural heart valve. A leaflet valve stent may be combined with a conduit support device comprised of a ring supported by posts attached to the valve stent ring. A blood pump may be attached to the center of a transvalvular support stent, for optimal fixation of the pump with relation to a trileaflet or bi-leaflet tissue or polymer valve.
Owner:JARVIK ROBERT
Diamine derivatives
Owner:DAIICHI SANKYO CO LTD
Growth arrest specific gene 6 peptides, antibodies, compositions, methods and uses
The present invention provides novel proteins and peptides from the receptor binding region of human Growth Arrest Specific Gene 6 (Gas6) and antibodies, including specified portions or variants, specific for at least one such Gas6 peptide or fragment thereof. The aforesaid peptides can be used to generate human, primate, rodent, mammalian, chimeric, humanized and / or CDR-grafted anti-Gas6 antibodies. The invention also provides for the nucleic acids encoding such peptides and anti-Gas6 antibodies, complementary nucleic acids, vectors, host cells, and methods of making and using thereof, including therapeutic formulations, administration and devices. Fifteen novel peptide sequences from the Gas6 G domain that are implicated in Gas6 interactions with its receptors are identified, isolated, and synthesized so as to allow generation of anti-Gas6 antibodies. The peptide sequences include three ESTs that encompass regions predicted to contribute to receptor binding or that can raise anti-Gas6 antibodies. This invention provides for such antibodies to be used in modulating or treating at least one Gas6-related disease in a cell, tissue, organ, animal, or patient. Such diseases may include, but are not limited to, thromboembolic disease, ischemic disease, venous thromboembolism, arterial or venous thrombosis, pulmonary embolism, restenosis, diabetic angiopathy and allograft atherosclerosis.
Owner:CENTOCOR ORTHO BIOTECH
Traumatism-free type method for establishing animal model of limb deep vein thrombogenesis
InactiveCN1751742AReduce mortalityEasy to manufactureIn-vivo testing preparationsVeinAnimal mortality
A method for creating the animal model of the thrombosis in deep vein of limb without wound includes such steps as reforming the intramedullary nail driving-pulling tool to obtain a quantitative beater, and simulating the surgical wounding procedure to generate different wounds on lower limb of rabbit, resulting in thrombosis in deep vein. Its advantages are simple method and low dead rate.
Owner:昆明医学院第一附属医院
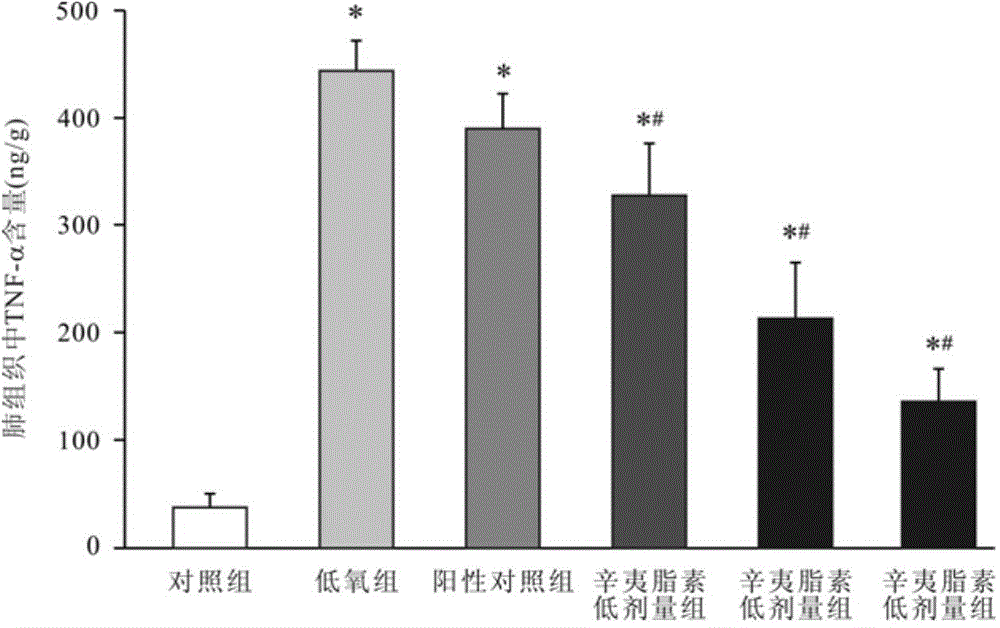
Use of fargesin and its derivative in preparation of drugs for treating or preventing pulmonary hypertension
The invention relates to a novel medical use of fargesin and its derivative and especially relates to a use of fargesin and its derivative in preparation of drugs for treating or preventing pulmonary hypertension. Fargesin, fargesin derivative, fargesin salt or fargesin solvate as a single active component or one of active components is compounded to one or more excipients, the composition is processed to form tablets, capsules, particulates, an oral liquid and an injection by conventional methods, is suitable for whole body administration and is used for preventing or treating idiopathic pulmonary hypertension, left-side cardiac disease-related pulmonary hypertension, pulmonary hypertension caused by lung disease and / or low oxygen, chronic thromboembolic pulmonary hypertension or pulmonary hypertension caused by unknown reasons.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
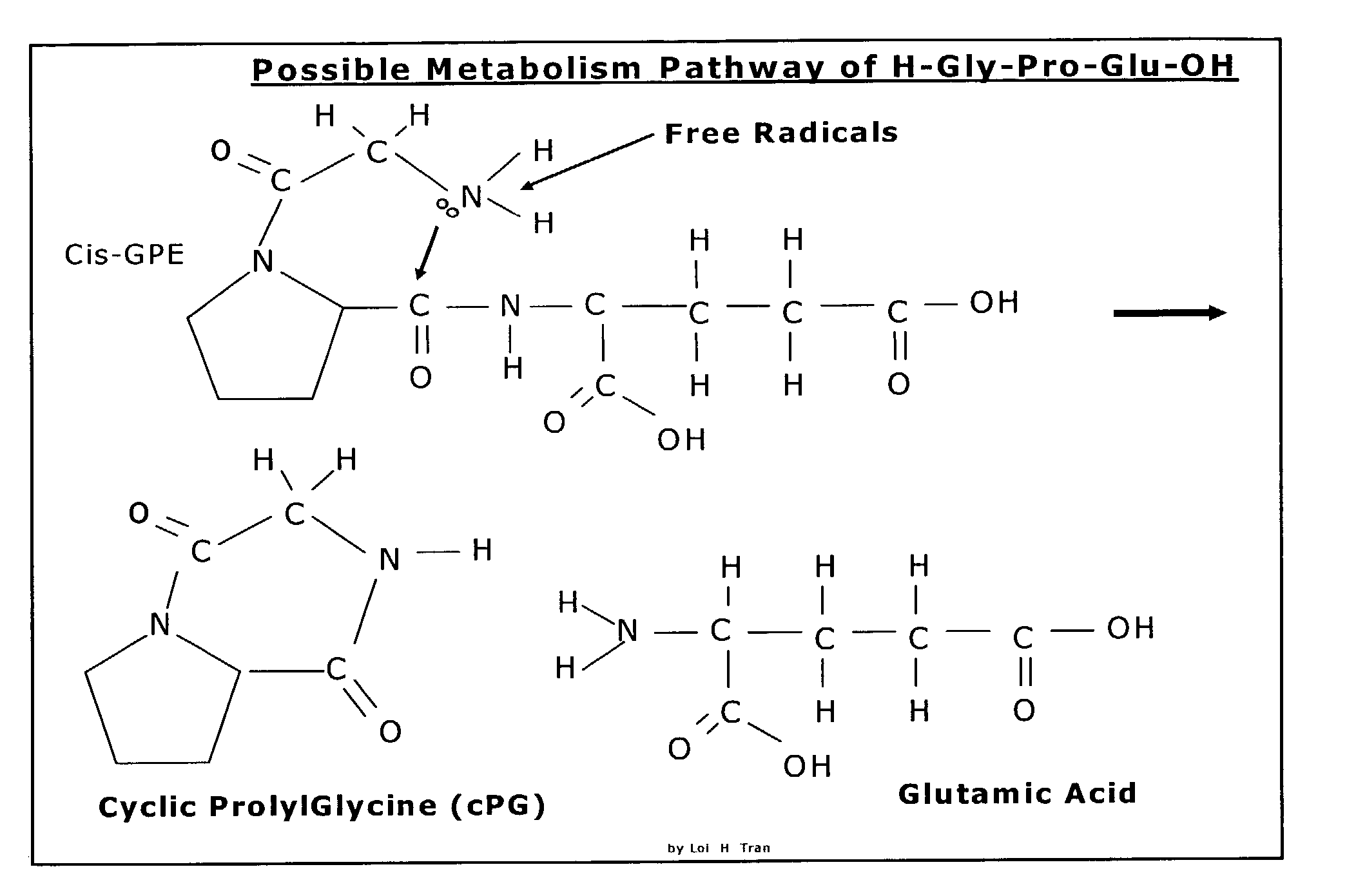
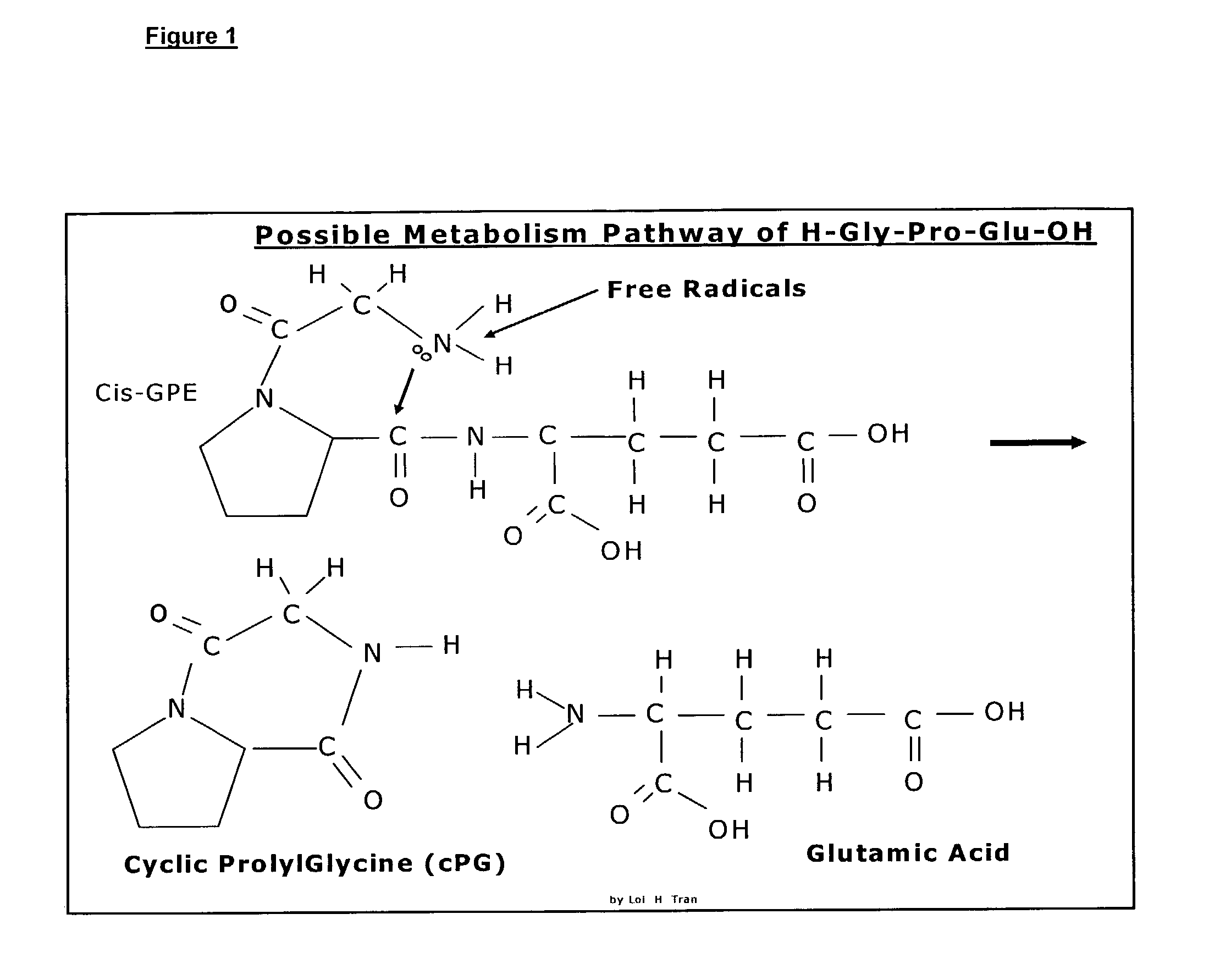
Neuroprotection and neuroegenisis by administering cyclic prolyl glycine
InactiveUS7232798B2Promote myelin productionIncrease the effective amountBiocideNervous disorderNeuroprotective factorsAnticonvulsant
The invention relates to the use of cyclic Prolyl Glycine (“cyclic PG” or “cPG”) and analogs and mimetics thereof, as neuroprotective agents for the treatment and or prevention of neurological disorders including but not limited to cerebral ischemia or cerebral infarction resulting from a range of phenomena, such as thromboembolic or hemorrhagic stroke, cerebral basospasms, hypoglycemia, cardiac arrest, status epilepticus, perinatal asphyxia, anoxia such as from drowning, pulmonary surgery, and cerebral trauma, as well as to the treatment and prevention of chronic neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and Huntington's disease, and as anticonvulsants.
Owner:NEUROBIOMED
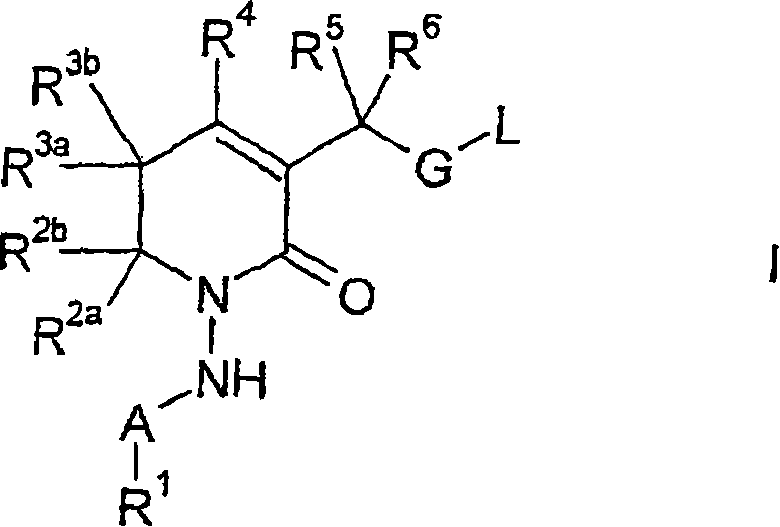
New 5,6-dihydropyrin-2-one compounds useful as inhibitors of thrombin
InactiveCN1894214AGood effectLow toxicityOrganic active ingredientsOrganic chemistryAnticoagulantThrombin activity
There is provided a compound of formula (I) wherein R<1>, R<2a>, R<2b>, R<3a>, R<3b>, R<4>, R<5>, R<6>, A, G and L have meanings given in the description, which compounds are useful as, or are useful as prodrugs of, competetive inhibitors of trypsin-like proteases, such as trombin, and thus, in particular, in the treatment of conditions where inhibition of thrombin is beneficial (e,g. conditions, such as thrombo-embolisms, where inhibition of trombin is required or desired, and / or conditions wherea anticoagulant thererapy is indicated).
Owner:ASTRAZENECA AB
Dosage forms comprising apixaban and matrix former
The invention relates to oral dosage forms for modified release of apixaban. The invention also relates to methods of preparing said dosage forms and to an agglomerated mixture of matrix former and filler for preparing an oral dosage form for use in the treatment of venous thromboembolism.
Owner:RATIOPHARM GMBH
Catheter for excising embolus and treatment method thereof
The present invention discloses embolectomy catheters (10, 10', 10'', 10''', 10''''), a quickly replaceable catheter, a system and method for removing coagulum and other block matters (for example, thrombus, thrombembolia, embolism fragment of atherosis speckle, foreign mater etc.,) the present invention especially is suitable for percutaneously removing thrombembolia or other block matters from small blood vessel of brain in apoplexy evolution process or cerebral ischemia period. In some embodiment schemes, the embolectomy catheters (10, 10', 10'', 10''', 10'''') of the invention are propelled with or on a guide wire (GW) , the guide wire is pre-inserted through or around the coagulum. And in some embodiment schemes, the embolectomy catheters comprise a coagulum removing apparatus; the apparatus expands from the catheters after the catheters is propelled to pass the coagulum partially .
Owner:MICROVENTION INC
Recyclable conformal radioactive particle cabin
PendingCN105727432ATake out the realizationInhibit sheddingX-ray/gamma-ray/particle-irradiation therapyTumour volumeHigh doses
The invention discloses a recyclable conformal radioactive particle cabin which comprises a fixing plate, a guide column and a plurality of moveable partitioning plates, wherein the guide column is longitudinally arranged at the lower end of the fixing plate; the guide column is made of a flexible material; the moveable partitioning plates are overlapped with one another up and down and are arranged on the guide column in a sliding sleeve manner. According to a preoperative plan, under the image guide, a puncture needle is penetrated into a tumor; the tip position is a tail end particle position of the preoperative plan; a needle core is pulled out, the recyclable conformal radioactive particle cabin is placed into a puncture needle tube and pushed to a particle distribution source position specified in the preoperative plan. As the guide column is made of the flexible material, the guide column can have conformal deformation along with change of the tumor, and the recyclable conformal radioactive particle cabin can be taken out after the tumor is eliminated, radioactive particles can be taken out, the consequence that the distribution sources of the radioactive particles in the tumor can be accumulated with one another to generate high-dosage areas along with reduction of the tumor size and radioactive damage of normal tissue around is caused be effectively prevented, and meanwhile particle migration and complication such as lumen obstruction and thromboembolism can be prevented.
Owner:牛洪欣
Vasculature device
A vasculature device with a wire with a shaped set portion, an electrically conductive path, an electrical connector connecting the wire to the electrically conductive path at a point distal to the shaped set portion, and a hypotube encasing the wire, electrically conductive path and electrical connector is provided. The vasculature device can be actuated from a low-profile configuration to a deployed configuration by heating of the wire, and in particular the shaped set portion of the wire, via application of an electrical current to the wire. The vasculature device is useful in removal of clots, thromboemboli and foreign bodies from the vasculature, and in particular the cerebral vasculature, and as steering wires or guidewires.
Owner:GORE ENTERPRISE HLDG INC
Activated blood coagulation factor X (FXa) inhibitor
InactiveCN102325528AOrganic active ingredientsOrganic chemistryOral anticoagulationBlood Coagulation Factor X
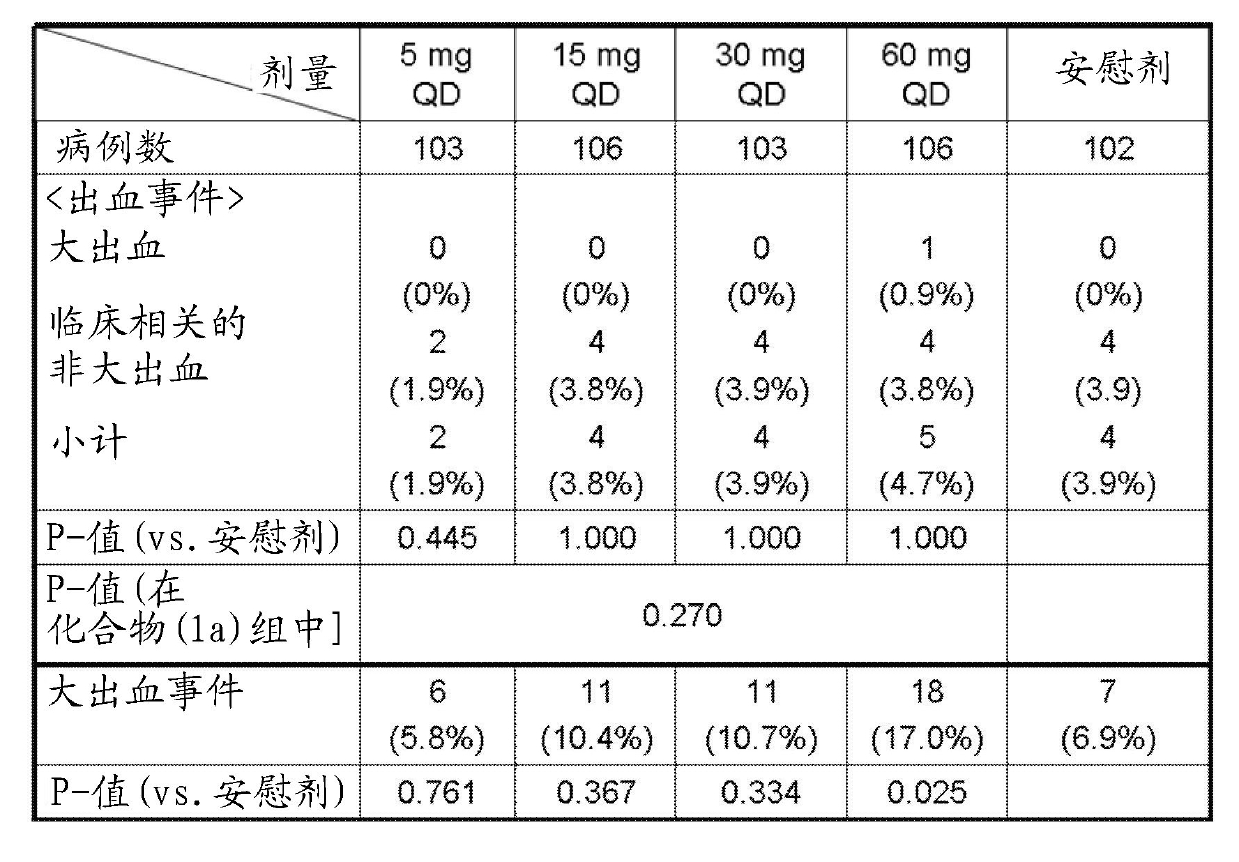
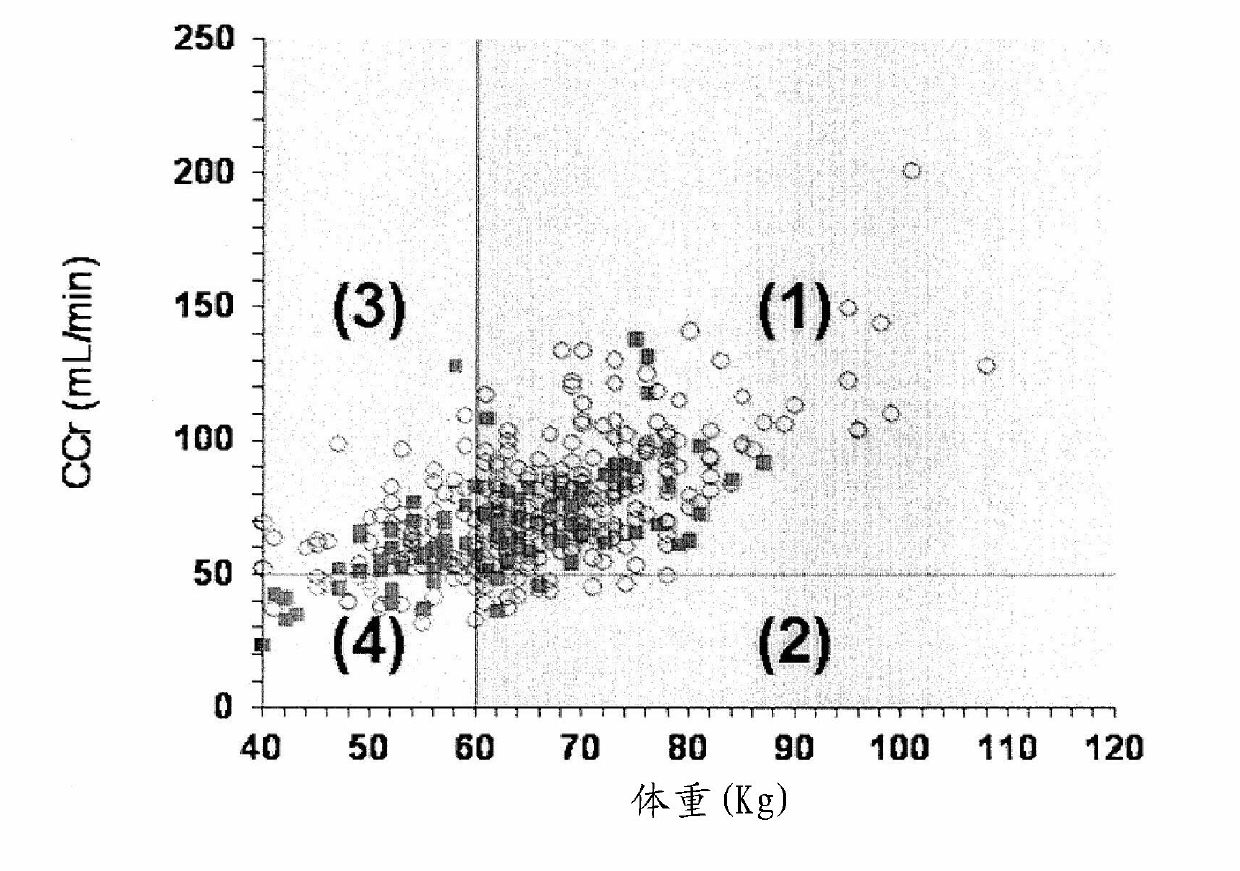
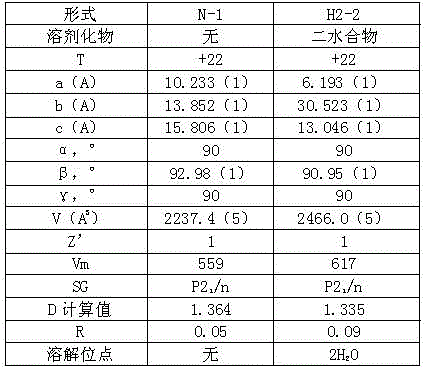
Disclosed is an activated blood coagulation factor X (FXa) inhibitor which is reduced in the risk of bleeding in the treatment of thromboembolism. Specifically disclosed is a blood coagulation inhibitor for oral administration, which comprises a compound represented by formula (1), a pharmacologically acceptable salt thereof, or a hydrate of the compound or the pharmacologically acceptable salt as an active ingredient. The blood coagulation inhibitor is characterized in that the dosage of the blood coagulation inhibitor can be determined by the following steps (A) to (D): (A) a factor associated with the risk of bleeding of the blood coagulation inhibitor is selected as a dosage determination factor; (B) a standard value for the dosage determination factor is set; (C) the level of the dosage determination factor is measured in a patient who needs the administration of the blood coagulation inhibitor; and (D) the dosage for the patient is determined by employing the standard value as a measure.
Owner:DAIICHI SANKYO CO LTD
Diamide macrocycles that are FXIA inhibitors
ActiveCN107074821APrevention of Thromboembolic DisordersOrganic chemistryUrinary disorderDual inhibitorKinin
The present invention provides compounds of Formula (I) or stereoisomers, tautomers, or pharmaceutically acceptable salts thereof, wherein all the variables are as defined herein. These compounds are selective Factor XIa inhibitors or dual inhibitors of FXIa and plasma kallikrein. The invention also relates to pharmaceutical compositions comprising these compounds and methods of treating thromboembolic and / or inflammatory disorders using the same.
Owner:BRISTOL MYERS SQUIBB CO
Apixaban composition and preparation method thereof
InactiveCN104644593AImprove solubilityPromote dissolutionOrganic active ingredientsBlood disorderMedicineThromboembolic disease
Owner:TIANJIN HANKANG PHARMA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com