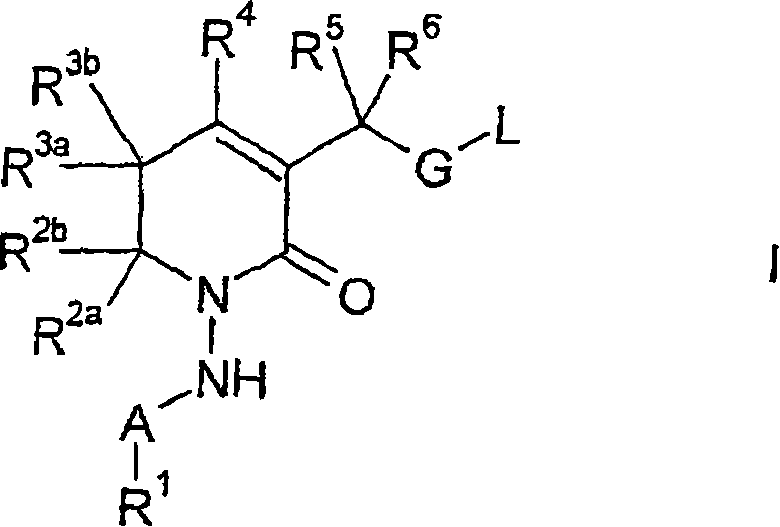
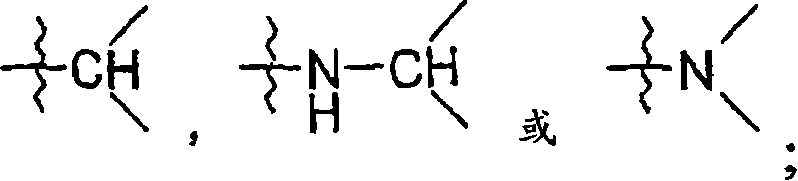
New 5,6-dihydropyrin-2-one compounds useful as inhibitors of thrombin
A compound and derivative technology, applied in the field of new pharmaceutically acceptable compounds, can solve problems such as not disclosed or implied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0905] Compounds (i)-(ix) listed below were prepared starting from the corresponding compound of Preparation 2 by hydrolysis in step (i), followed by amide coupling as described in step (ii), Method A.
[0906] Unless otherwise stated, the compounds (x)-(xxiii) listed below are starting from the corresponding compound of Preparation 2 by hydrolysis in step (i), followed by the amido-isolysis as described in step (ii), method B. made in conjunction.
[0907] Unless otherwise stated, compounds (xxiv)-(l) listed below are hydrolyzed as described in Example 1(xxvi) starting from the corresponding compound of Preparation 2, followed by hydrolysis as described in Example 1(xxiii) Prepared by amide coupling.
[0908] step (i)
[0909] To the indicated ester (0.041 mmol; see Preparation 2 above) dissolved in THF (1 mL) and water (3 drops) was added lithium hydroxide (1.5 molar equivalents). The reaction mixture was stirred at room temperature for 11 hours, then quenched with water ...
Embodiment 2
[1058] Compounds (i)-(ix) listed below were prepared starting from the corresponding compound of Example 1 according to Method A below, unless otherwise stated. Compounds (x)-(xxxiv) listed below are prepared from the corresponding compound of Example 1 according to the following method B
[1059] Method A
[1060] To a solution of the indicated benzyloxycarbonyl-protected compound (0.03 mmol; see Example 1 above) in methanol (3 mL) was added palladium-carbon (10%, 1 mass equivalent) and HCl (cone, 2-3 drops ). The suspension was hydrogenated at room temperature and pressure for 90 minutes. Pass the suspension through Celite Filter, wash with methanol (3 x 5 mL), and remove the solvent under reduced pressure. The residue was dissolved in a minimum amount of methanol and the deprotected product was precipitated with ethyl acetate. The yield is close to quantitative.
[1061] Method B
[1062] The indicated amide (0.04 mmol; see Example 1 above) was dissolved in HCl sat...
Embodiment 3
[1181] [4-chloro-2-({2-[4-methyl-1-(naphthalene-1-sulfonylamino)-2-oxo-1,2,5,6-tetrahydropyridin-3-yl] Acetylamino}methyl)benzyl]carbamate tert-butyl
[1182] [4-Methyl-1-(naphthalene-1-sulfonylamino)-2-oxo-1,2,5,6-tetrahydropyridin-3-yl] was hydrolyzed following the general procedure described in Example 1 above Ethyl acetate (0.12 mmol; see Preparation 2(iii) above), except that the solvent volume was 3 mL and the reaction time was 16 hours. The crude acid thus obtained was dissolved in DCM (2 mL) and amide coupling was carried out as described in Example 1 above (coupling with (2-aminomethyl-4-chlorobenzyl)-1-carbamic acid tert-butyl ester) , and just stirred the reaction mixture for two nights. The crude product was chromatographed (SiO 2 , 5% methanol / DCM) and preparative HPLC to afford the title compound (41%).
[1183] 1 H NMR (500MHz, CDCl 3 )δ1.50(s, 9H), 2.01(s, 3H), 2.48-2.63(m, 2H), 3.02(s, 2H), 3.72-3.79(m, 2H), 4.07(d, 2H), 4.19 -4.29(m, 2H), 5.10(br s, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com