Esomeprazole enteric pellet tablets and preparation method thereof
A technology of esomeprazole enteric and enteric-coated pellets, which is applied in the direction of pill delivery, digestive system, and drug combination, and can solve the problems of large capsule size, single dosage, and unsatisfactory stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
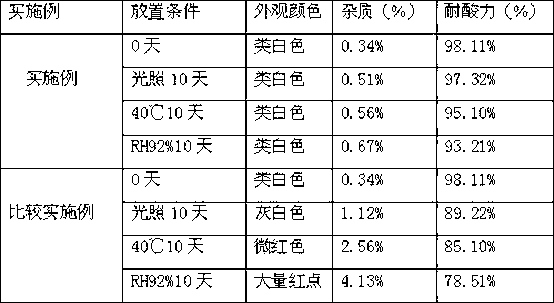
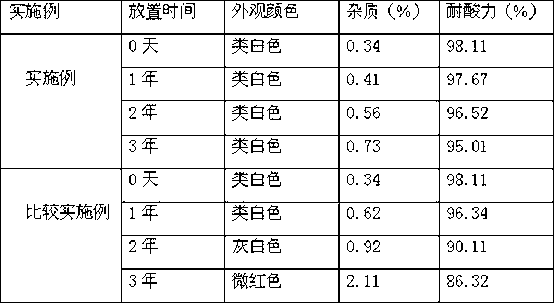
Examples
Embodiment 1
[0038] a. Drug active pellet core
[0039] Esomeprazole 20g
[0040] 30% syrup 60g
[0041] Sodium carboxymethyl starch 1g
[0042] 50g powdered sugar
[0043] Sodium bicarbonate 2g
[0044] Tween-80 1.5g
[0046] b. Isolation layer
[0047] Hydroxypropyl Methyl Cellulose 12g
[0048] Titanium dioxide 3g
[0050] Purified water 240g
[0051] c. Enteric coating layer
[0052] Udrake L30D-55 110g
[0054] Triethyl citrate 5g
[0055] Purified water 120g
[0056] d. Enteric-coated pellets
[0057] Microcrystalline Cellulose 13g
[0058] 15g pregelatinized starch
[0059] Croscarmellose Sodium 2g
[0060] Esomeprazole enteric-coated pellets 70g
[0061] Preparation:
[0062] (1) Preparation of pharmaceutically active pellet core: Prepared by centrifugal coating granulator and extrusion spheronizer, pulverized esomeprazole, disintegrant, filler, basic compound, surfactant and talcum powd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com