Method for preparing esomeprazole impurity
A technology for esomeprazole and impurities, which is applied in the field of organic chemical synthesis, can solve the problems of few synthesis routes of benzimidazole, low ee value of the product, cumbersome operation, etc., and achieves simple operation, wide range of sources, and comprehensive synthesis routes short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
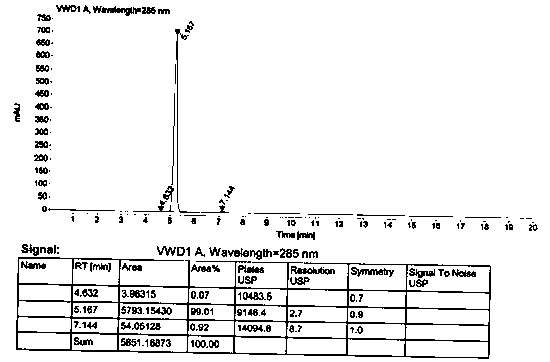
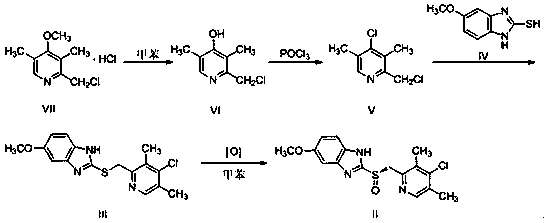
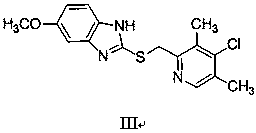
[0056] 5-methoxy-2-[(4-chloro-3,5-dimethyl-2-pyridyl)methylmercapto]-1 H -The preparation of benzimidazole (III)
[0057] Add 10g of the compound represented by formula VII and 50g of toluene to the three-necked flask, maintain 110~115°C for 12hrs, and detect the reaction solution by TLC until the reaction is complete to obtain 2-chloromethyl-4-hydroxy-3,5-dimethyl base pyridine (VI) reaction solution. Cool down to room temperature, add 13.8g of phosphorus oxychloride, maintain 100~110°C for 3 hours, detect the reaction solution with thin layer until the reaction is complete, lower the temperature, control the temperature to ≤20°C, slowly add 55g of water, add 100g of methanol, slowly add 20 %NaOH aqueous solution 70g (NaOH is 14g), after adding, slowly add 2-mercapto-5-methoxybenzimidazole (IV) 8.5g, raise the temperature to 45°C and react for 3hr, TLC detection of the reaction solution until the reaction is complete, add The reaction solution was washed with water, separat...
Embodiment 2
[0059] 5-methoxy-2-[( S )-[(4-Chloro-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1 H - preparation of benzimidazole (II)
[0060] Get the 5-methoxy-2-[(4-chloro-3,5-dimethyl-2-pyridyl) methylmercapto]-1 prepared in Example 1 H - Benzimidazole (III) 10g, add 45g toluene, 2.4g tetraisopropyl titanate, 2.8g L-diethyl tartrate, 2.5g N,N-diisopropylethylamine, heat up to 60°C and stir to dissolve, keep warm React for 30 minutes, lower the temperature, maintain the temperature at 10~15°C, slowly add 19.4g of cumene hydroperoxide (80%), keep it warm for 4hrs, detect the reaction solution by TLC until the reaction is complete, and use 960g of 10% sodium hydroxide aqueous solution ( NaOH (96g) was extracted (320g×3), the alkaline aqueous layer was combined, an appropriate amount of dichloromethane was added, and the pH was adjusted to 7~8 with dilute hydrochloric acid. Extract with dichloromethane, combine the organic layers, wash once with saturated brine, add anhydrous sodium sulfate ...
Embodiment 3
[0062] 5-methoxy-2-[(4-chloro-3,5-dimethyl-2-pyridyl)methylmercapto]-1 H -The preparation of benzimidazole (III)
[0063] Add 10 g of the compound represented by formula VII and 30 g of toluene to the three-necked flask, maintain 95~110 ° C for 15 hours, and detect the reaction solution by TLC until the reaction is complete to obtain 2-chloromethyl-4-hydroxy-3,5-dimethyl base pyridine (VI) reaction solution. Cool down to room temperature, add 10g of phosphorus oxychloride, maintain 110~115°C for 3hrs, detect the reaction solution with thin layer until the reaction is complete, cool down, slowly add 50g of water, add 100g of methanol, slowly add 80g of 15% KOH aqueous solution (KOH is 12 g), after adding, slowly add 8.1 g of 2-mercapto-5-methoxybenzimidazole (IV), raise the temperature to 55°C and react for 2.5 hrs, detect the reaction liquid by TLC until the reaction is complete, add water to wash the reaction liquid, and divide Layers and liquid separation, and the organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com