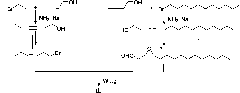Simple stereoselective synthesis method of sex pheromones of hyphantria cunea
A stereoselective, white moth technology, applied in organic chemistry and other directions, can solve the problems of complex synthesis of pheromone molecules, troublesome synthesis, low efficiency, etc., and achieve simple operation and separation, high enantioselectivity, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Step 1 Synthesis of 2-alkyne tetradecan-1-ol (2)
[0049] 2-Propargyl alcohol 1 (560mg, 10mmol) was dissolved in tetrahydrofuran (100mL) solvent, and n-butyl lithium (6.9mL, 20mmol, 2.9M in hexane) was added dropwise at -78°C under argon atmosphere. After reacting at -78°C for 60min, 1-bromoundecane (2.5mL, 11mmol) and hexamethylphosphoramide (4.5mL, 26mmol) were successively added dropwise, and then reacted at the same temperature for 5 hours. After the reaction was quenched with 20 mL of saturated sodium carbonate solution, the layers were separated, and the aqueous phase was extracted with diethyl ether (3×20 mL). The combined organic phases were dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and purified by silica gel column chromatography to obtain compound 2 (1.94 g, 92%). White solid, mp 44-45°C (n-hexane); IR(film)ν max 3342(OH), 2925, 2854, 2226(w, C≡C), 1466, 1378(w), 1137, 1015cm -1 ; 1 H-NMR (400MHz, CDCl 3 )δ0.88(t...
Embodiment 2
[0067] Step 1 Synthesis of 2-alkyne tetradecan-1-ol (2)
[0068] Under argon atmosphere, add n-butyllithium (2.9M in hexane) dropwise to the ether solution of 2-propargyl alcohol 1 at -80°C, and then add 1-bromoundecane and hexa Methylphosphoramide was reacted at the same temperature for 5 hours. After the reaction was quenched with saturated potassium carbonate aqueous solution, the layers were separated, and the aqueous phase was extracted with ether. The combined organic phases were dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by silica gel column chromatography to obtain 2 (90%) as a white solid.
[0069] Step 2 Synthesis of (Z)-2-entetradec-1-ol (3)
[0070] Under hydrogen atmosphere, at 0°C, add the hexane solution of compound 2 to the Lindella catalyst, and react at 0-5°C. After the reaction was completed, the catalyst was removed by filtration, and the filter cake was washed with ethyl acetate. The filtrate was concentrated...
Embodiment 3
[0086] Step 1 Synthesis of 2-alkyne tetradecan-1-ol (2)
[0087] Under argon atmosphere, at -78°C, add n-butyllithium (2.9M in hexane) dropwise to the tetrahydrofuran solution of 2-propargyl alcohol 1, and then add 1-bromoundecane and Hexamethylphosphoramide. After reacting at the same temperature for 2 hours, the temperature was gradually raised to 0°C, and the reaction was quenched with saturated sodium carbonate solution. The liquid was separated, the aqueous phase was extracted with ether, the combined organic phase was dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and purified by silica gel column chromatography to obtain a white solid 2 (94%).
[0088] Step 2 Synthesis of (Z)-2-entetradec-1-ol (3)
[0089]Hydrogen atmosphere, at 0°C, to Lindella catalyst (Pd / BaSO 4 / Pb 2+ ) was added the hexane solution of compound 2, and reacted at 0-5°C. After the reaction was completed, the catalyst was removed by filtration, the filter cake ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com