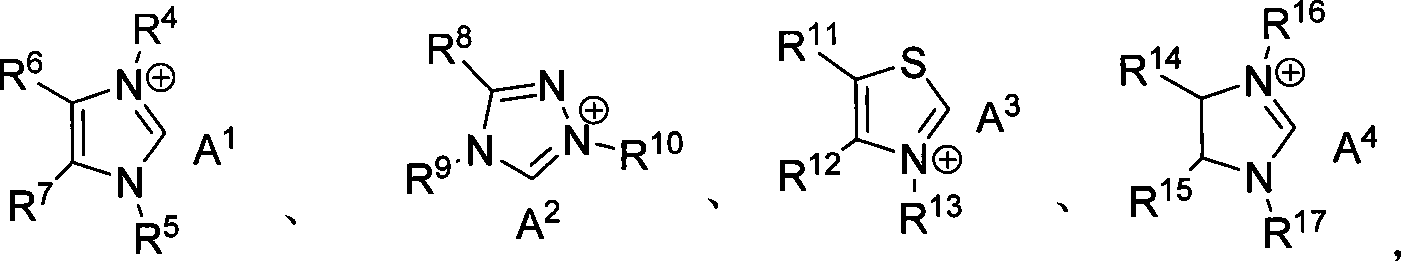
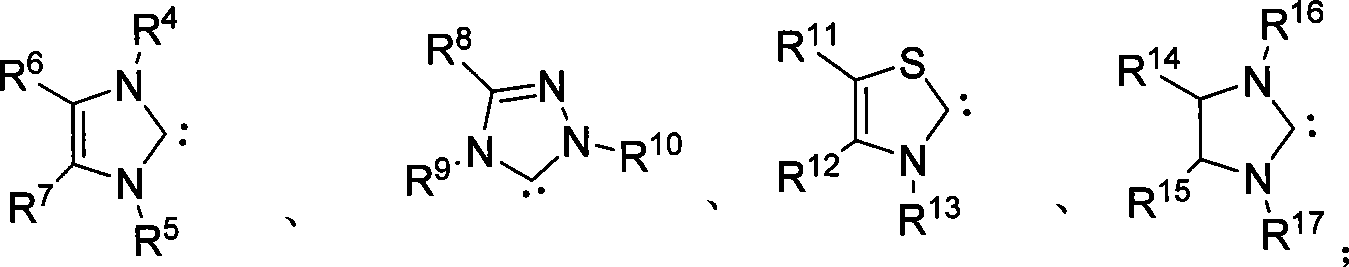
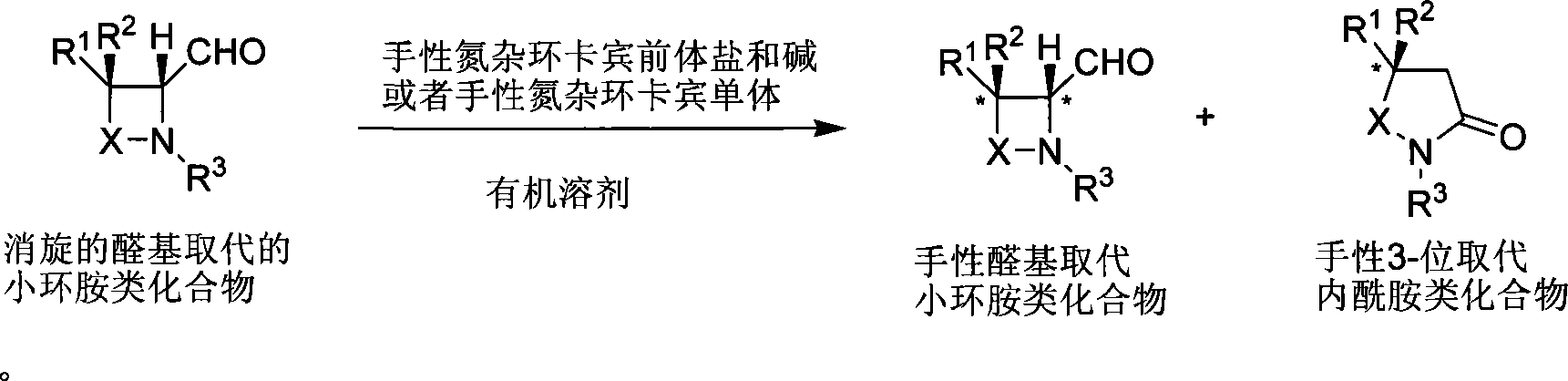
Method for synthesizing aldehyde substituted small ring amines compounds with high enantioselectivity and 3-substituted lactams compounds with optical activity
An enantioselective, lactam-based technology, applied in organic chemistry and other directions, can solve the problems of harsh reaction conditions, long reaction time, difficult operation, etc., and achieve the effects of mild reaction conditions, easy operation and good yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Kinetic resolution of aldehyde-substituted lactam compounds catalyzed by nitrogen heterocyclic carbene
[0043]
[0044] Under the protection of argon, add the precursor salt of chiral azacyclic carbene (0.005mmol), dichloromethane 2.0mL, and base (0.005mmol) successively to a dry reaction tube. After stirring for 5 minutes, add 4-aldehyde-β-lactam compound (0.5 mmol). The reaction system was stirred at 25°C, and the reaction was tracked by NMR. When the reaction conversion rate was 50%, the stirring was stopped, and the solvent was removed under reduced pressure. Column chromatography, eluent: petroleum ether / ethyl acetate=4 / 1, collection group points, the corresponding products A and B were obtained.
[0045]
[0046] Under the protection of argon, add B (0.1mmol) to a dry reaction tube, freshly distilled anhydrous methanol 1.0mL, NaBH 4 0.12mmol, stirred at room temperature, followed by TLC. After the reaction is complete, add saturated NaHCO to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com