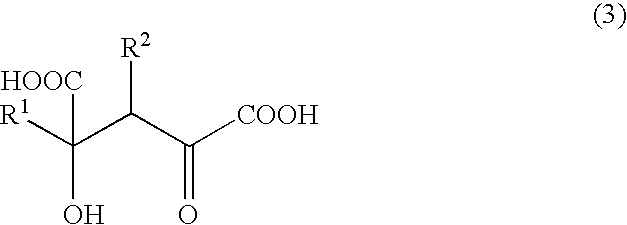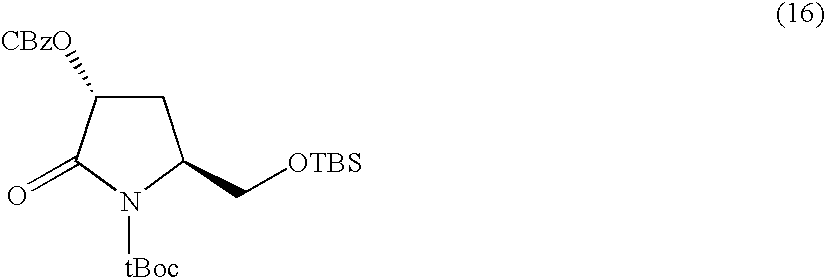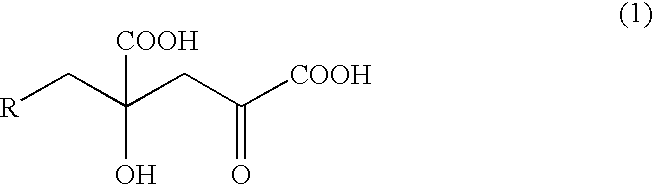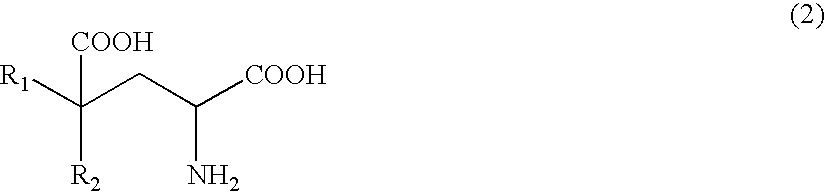Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Monatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
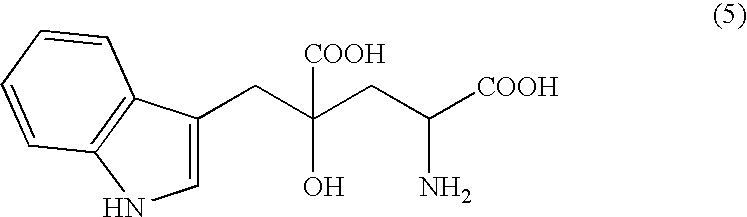
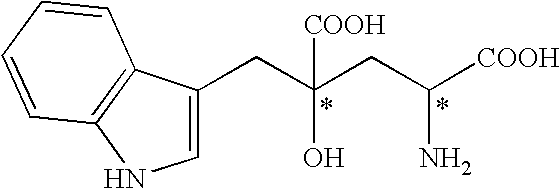
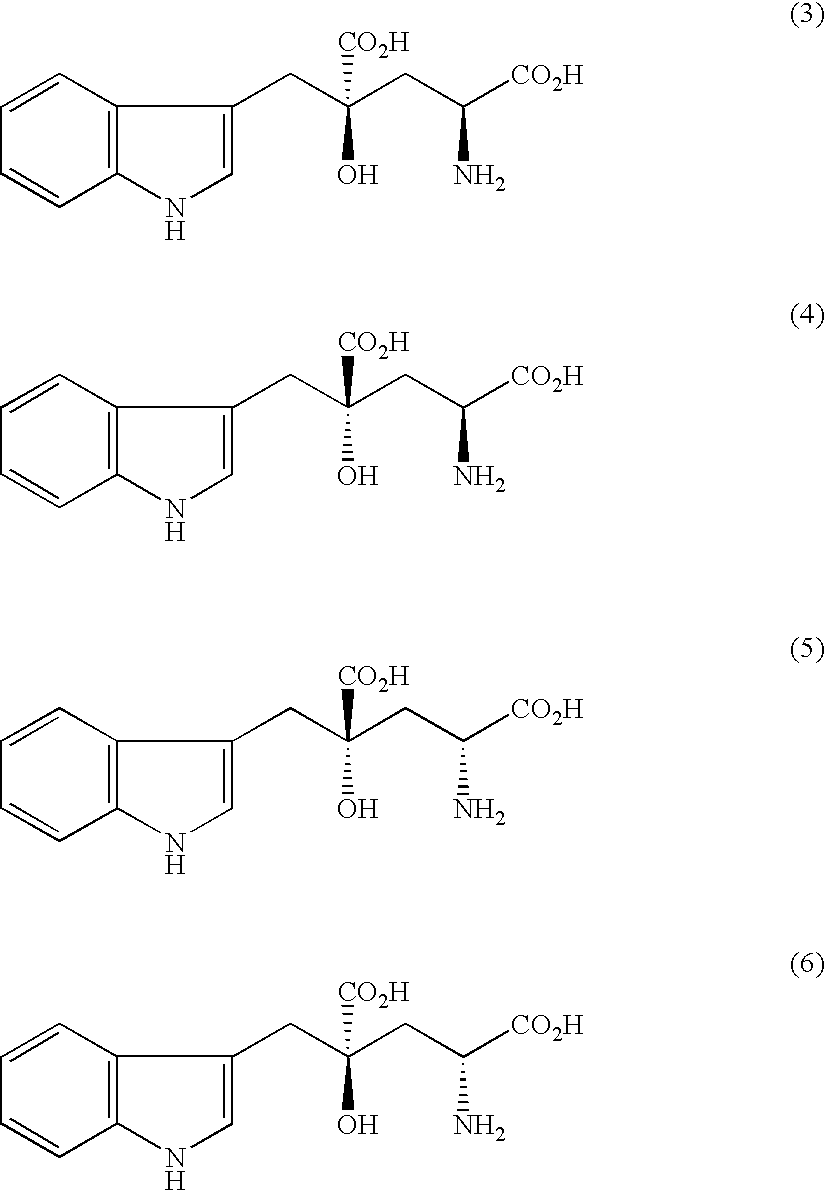
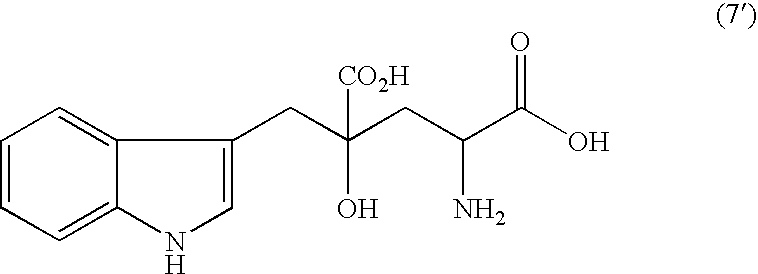
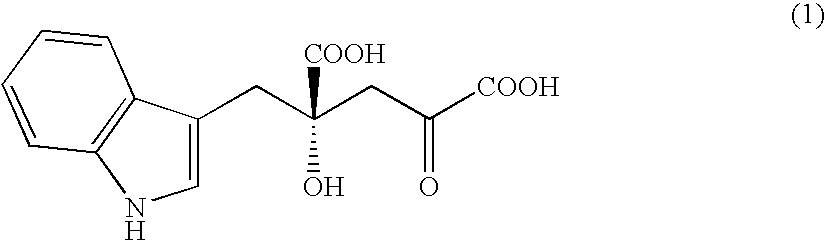
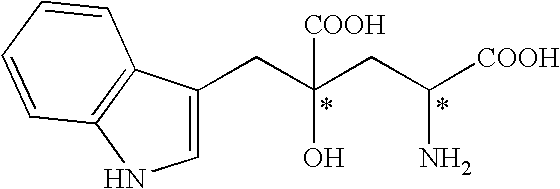
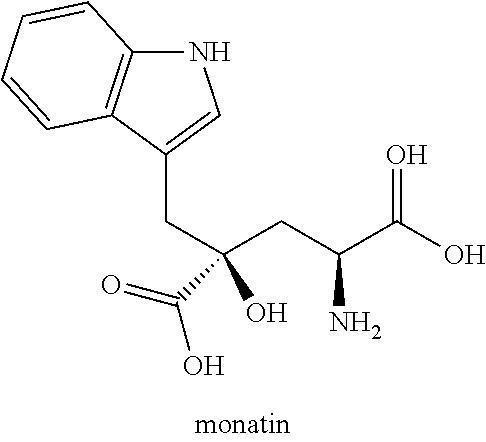
Monatin, commonly known as arruva, is a naturally occurring, high intensity sweetener isolated from the plant Sclerochiton ilicifolius, found in the Transvaal region of South Africa. Monatin contains no carbohydrate or sugar, and nearly no food energy, unlike sucrose or other nutritive sweeteners.
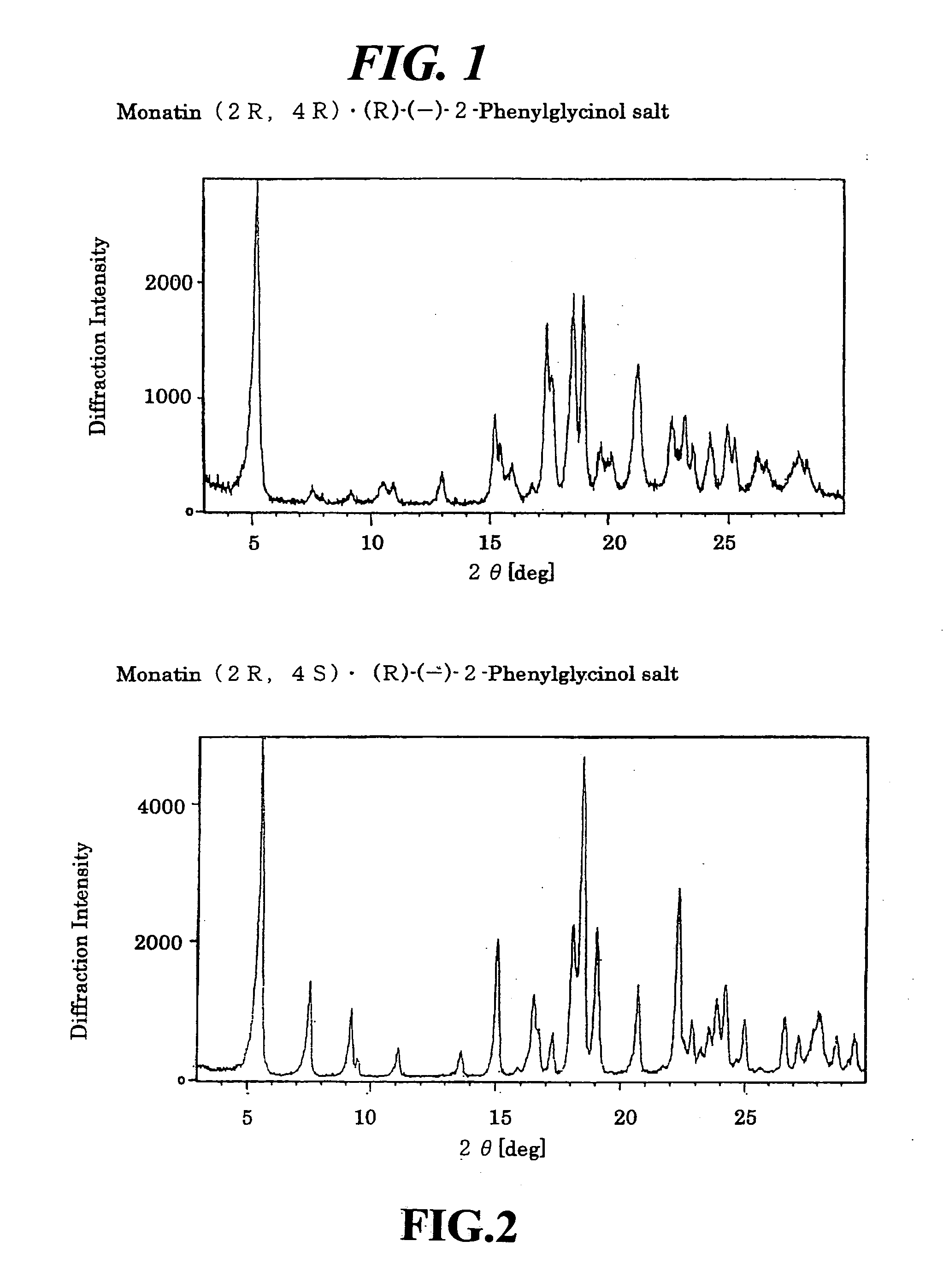
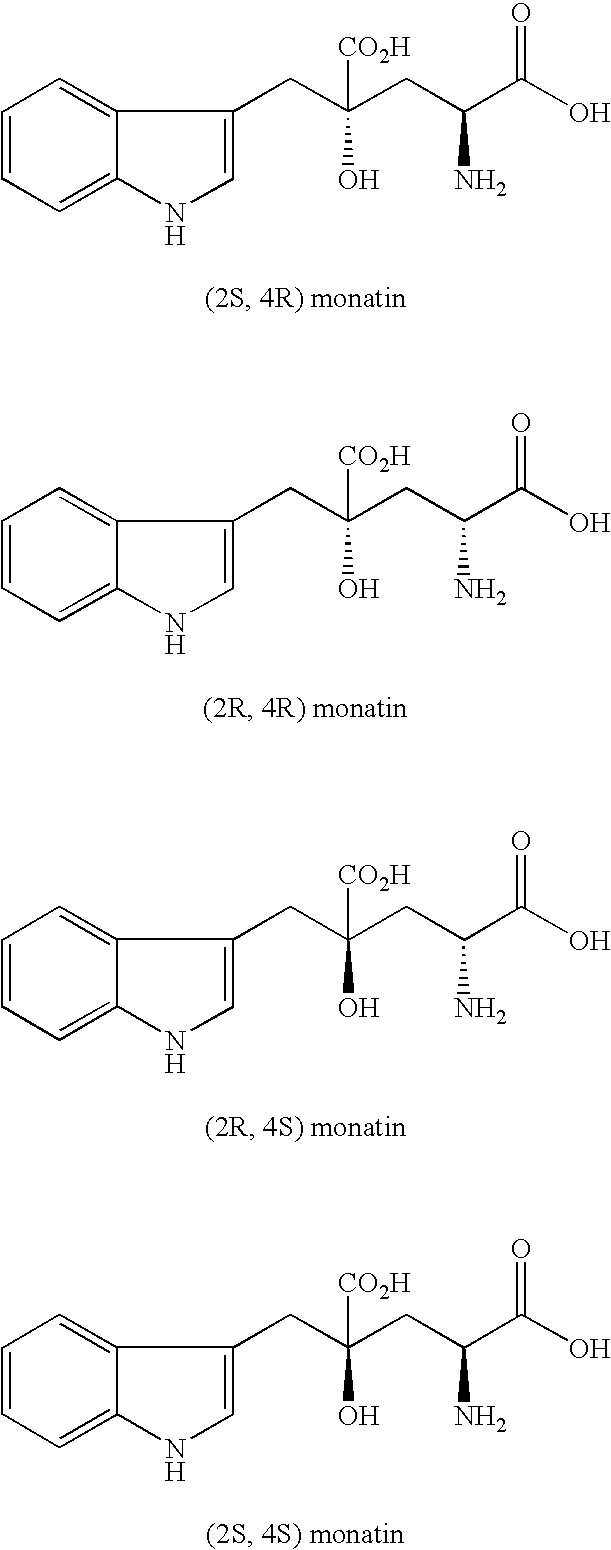
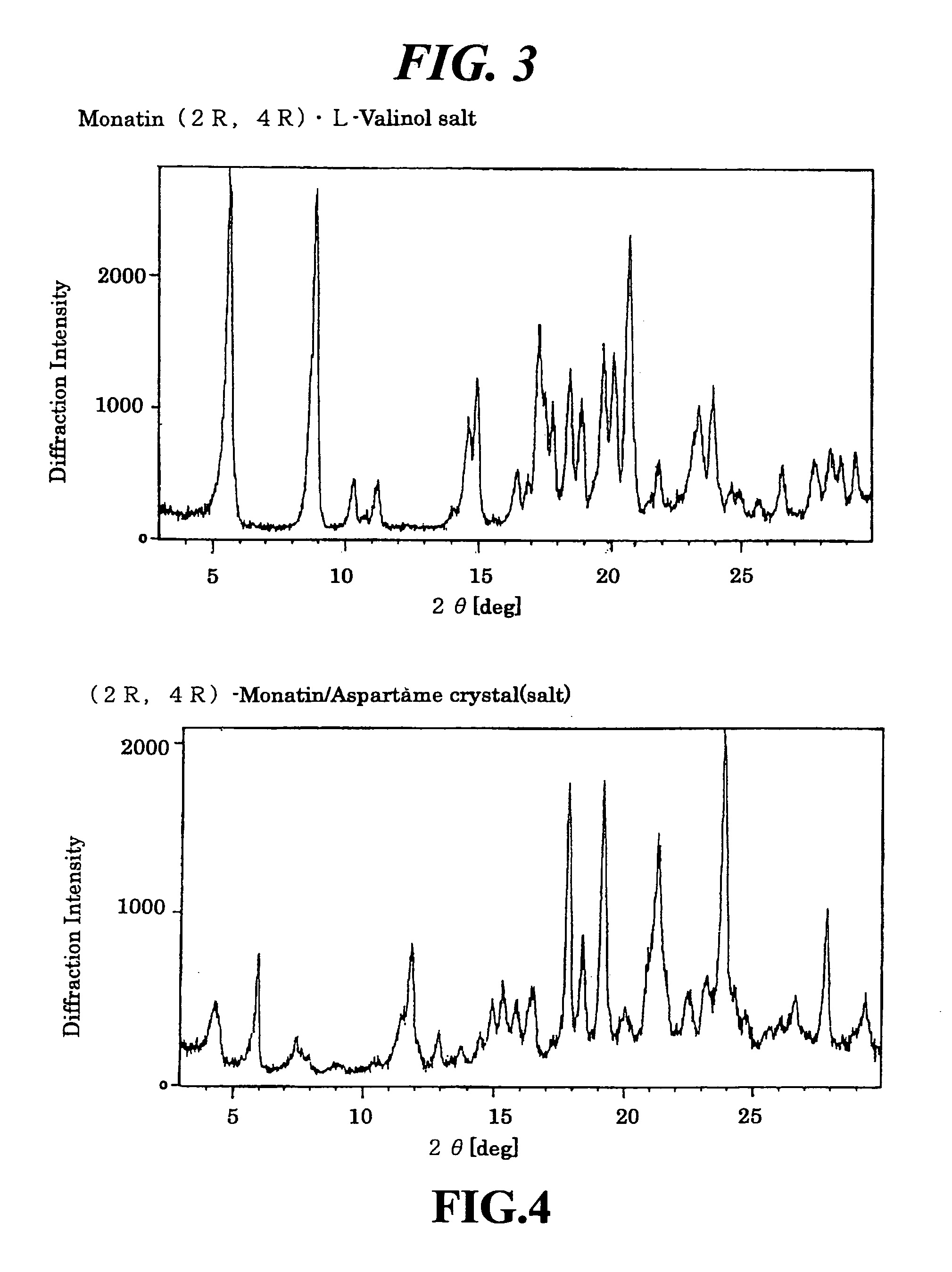
Crystals of non-natural-type stereoisomer salt of monatin and use thereof
The present invention provides salt crystals of non-natural stereoisomer forms of monatin and to the use thereof.
Owner:AJINOMOTO CO INC
Crystals of free (2R, 4R)-monatin and use thereof
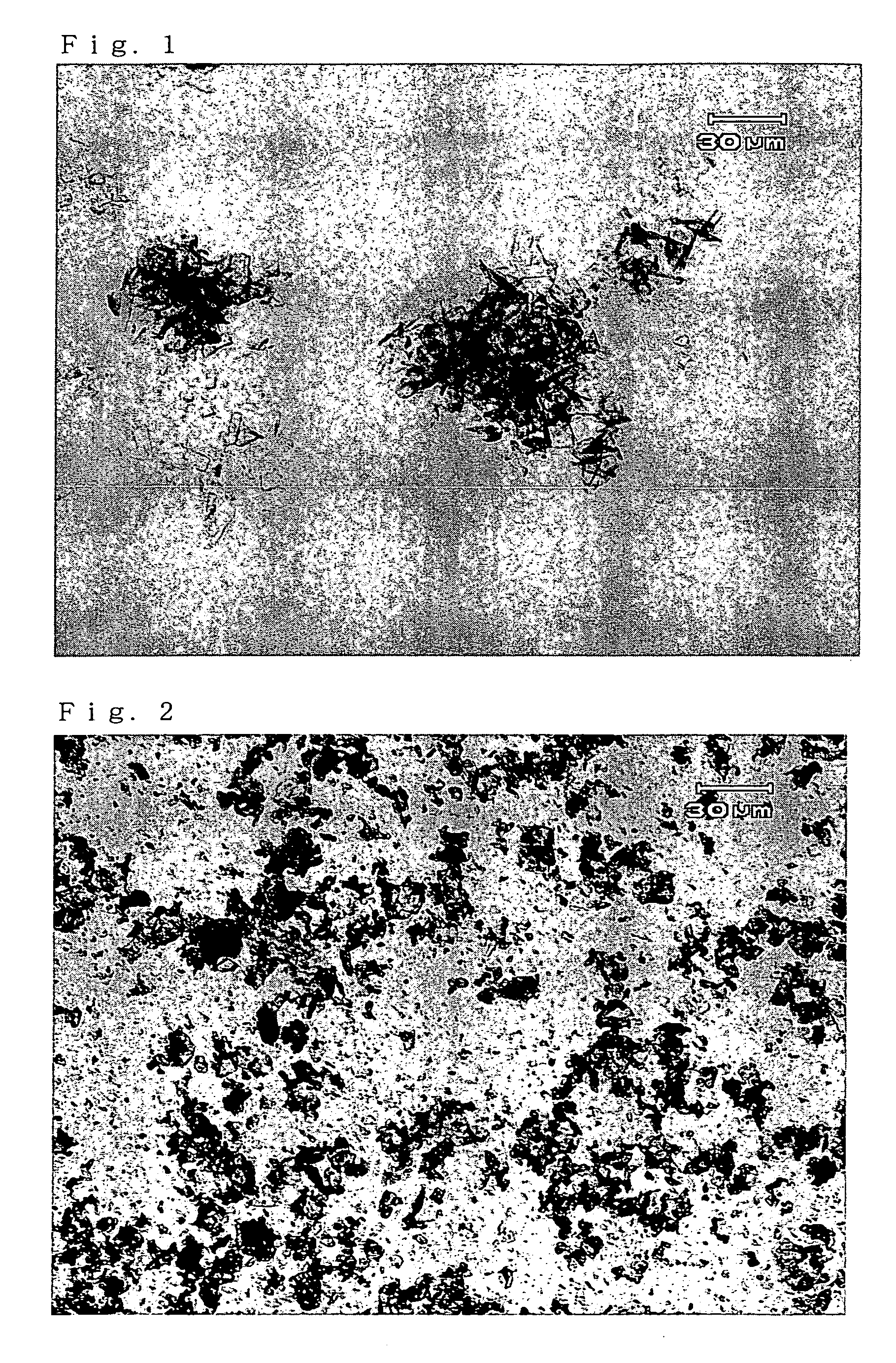
InactiveUS20050272939A1Superior in pointSuperior in sweetness intensityBiocideOrganic chemistryHigh humidityAdditive ingredient
A crystal of free (2R,4R)-monatin is useful as a sweet substance. The crystals resist absorption of water even under high humidity, are stable and exhibit a high degree of sweetness. Thus, such crystals may used as a sweetening agent or an ingredient thereof, and as an ingredient for imparting sweetness to foods and beverages.
Owner:AJINOMOTO CO INC
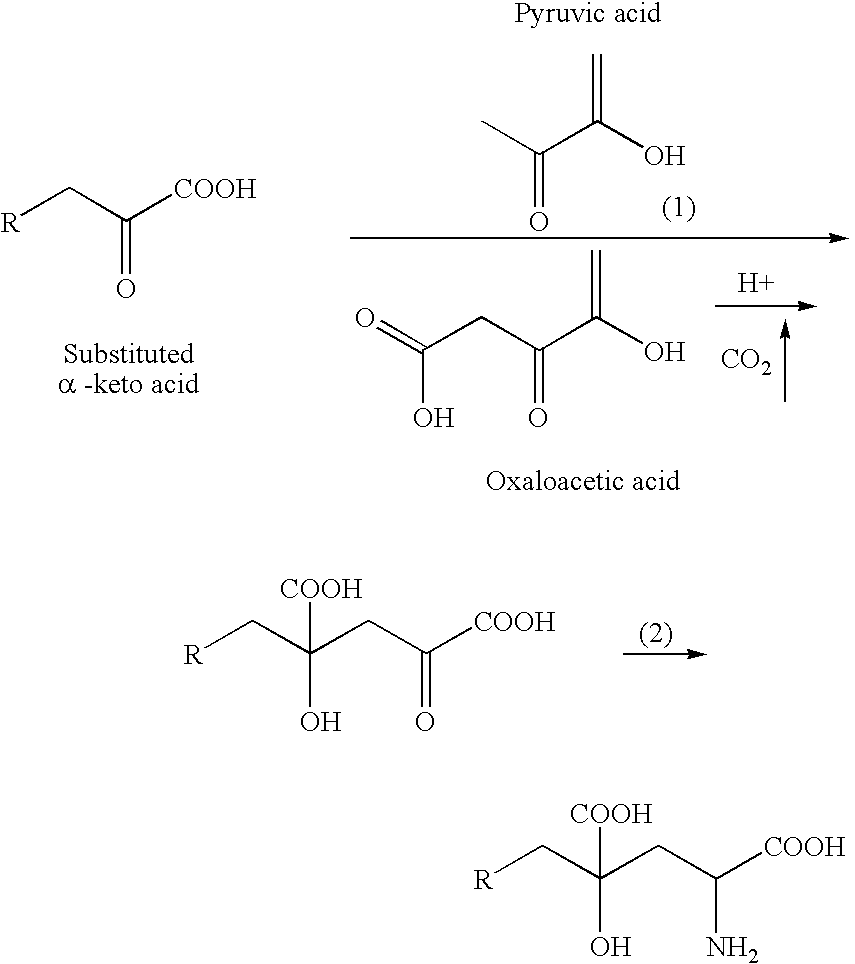
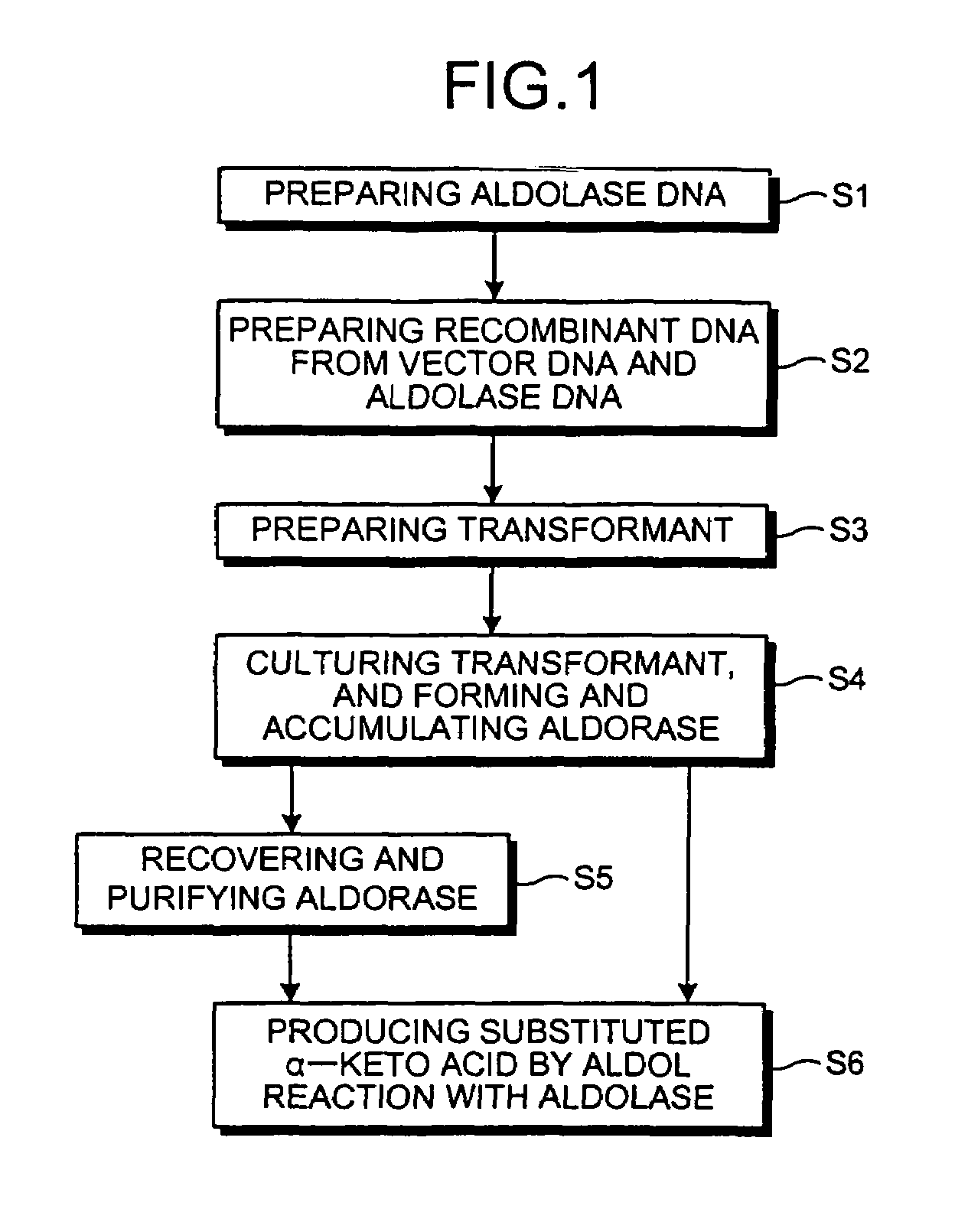
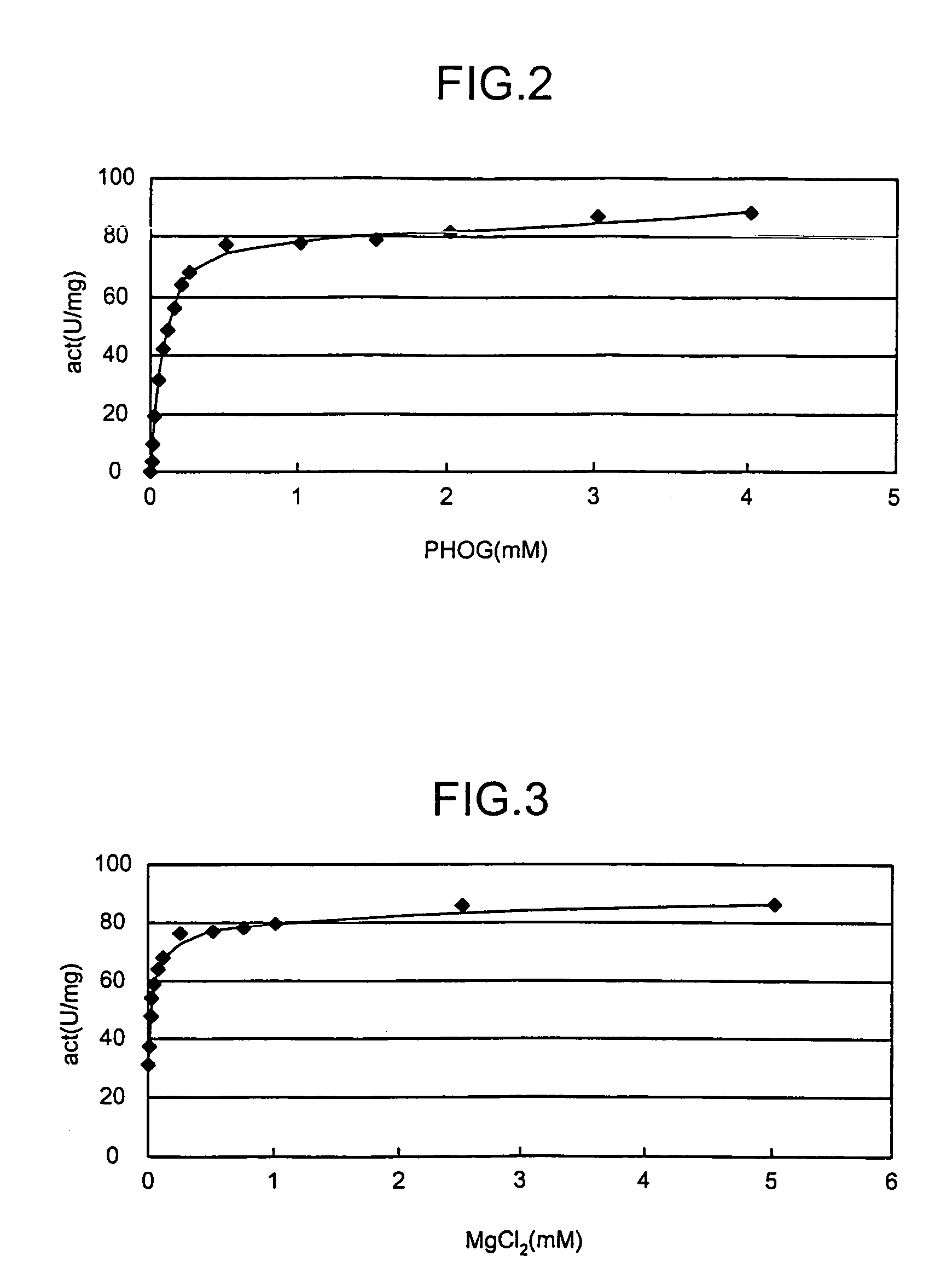
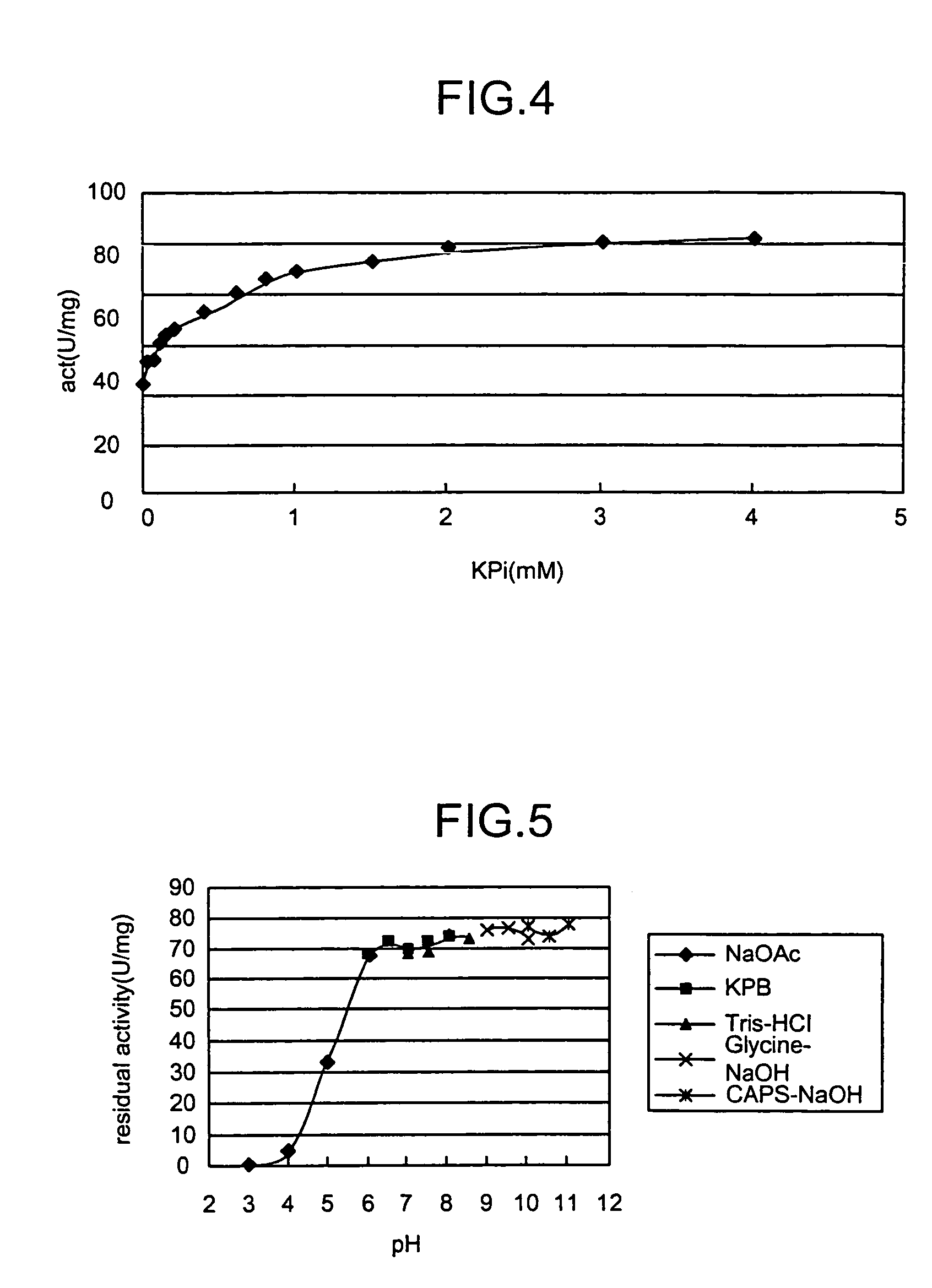
Novel aldolase and production process of substituted alpha-keto acids
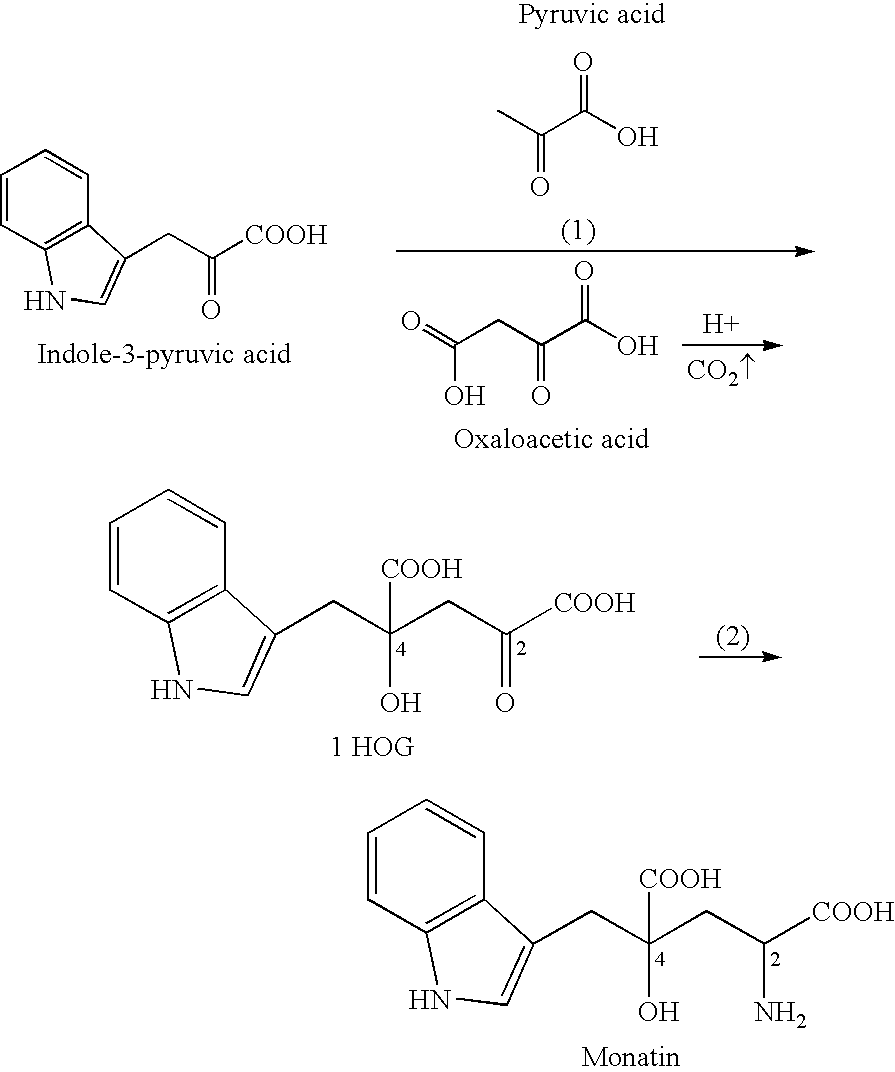
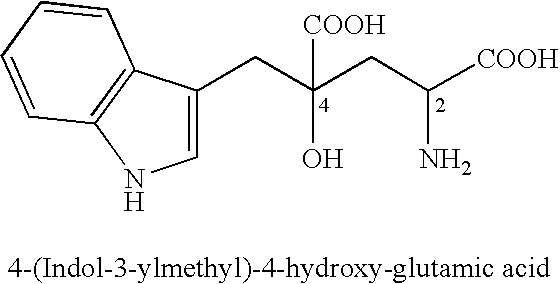
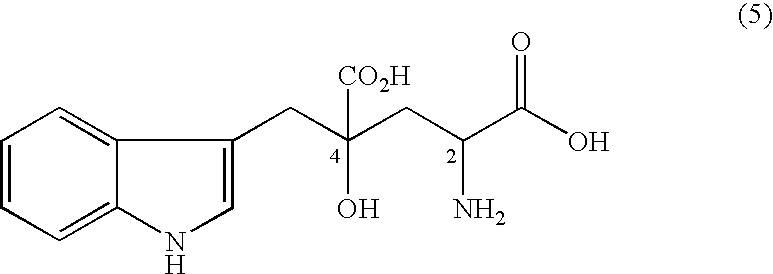
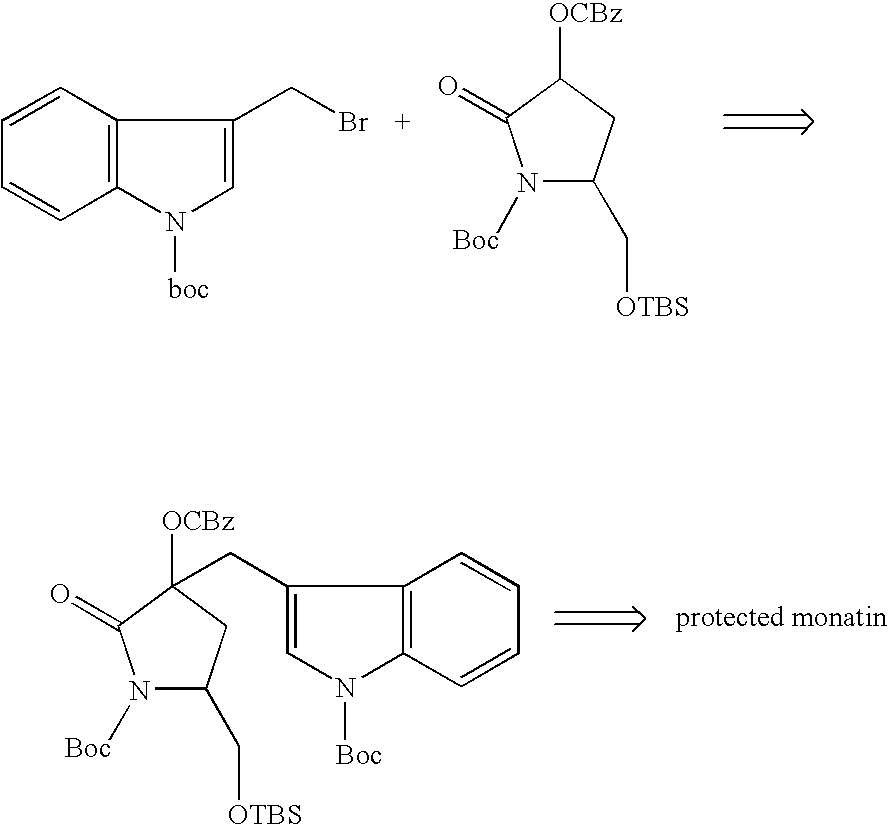
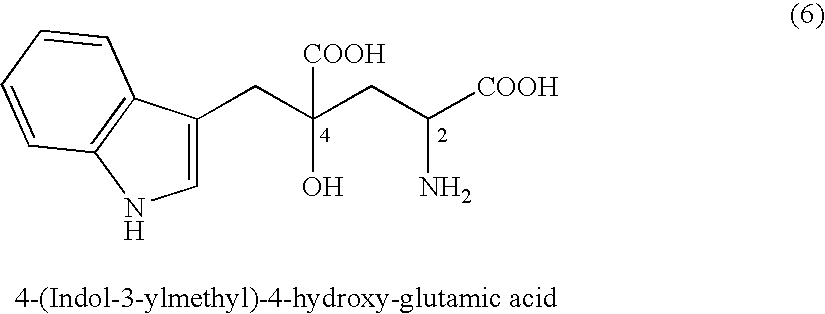
4-(Indol-3-ylmethyl)-4-hydroxy-2-oxoglutarate, which is useful as an intermediate in the synthesis of monatin, may be synthesized from indole pyruvic acid and pyruvic acid (and / or oxaloacetic acid) by using a novel aldolase derived from the genus Pseudomonas, Erwinia, Flavobacterium, or Xanthomonas.
Owner:AJINOMOTO CO INC
Products and methods for in vivo secretion of monatin
InactiveUS20070099277A1Increase amino acid transportEasy to transportBacteriaTransferasesMicroorganismIn vivo
Products and methods for the in vivo production of monatin sweetener are provided. The products include microorganisms that are genetically modified to secrete or to improve secretion of monatin; microorganisms that are genetically modified to produce monatin; and microorganisms that are genetically modified to both secrete or improve secretion of monatin and produce monatin. The methods include producing monatin in such genetically engineered microorganisms.
Owner:CARGILL INC
Novel aldolase, and method for producing optically active IHOG and monatin
The present invention relates to a method for producing optically active IHOG, which can in turn be used for the production of monatin. The present invention further relates to a method for producing optically active monatin, and aldolase used for these methods. As such, the present invention enables the synthesis of 4-(Indole-3-ylmethyl)-4-hydroxy-2-oxoglutaric acid with high optical purity, which is useful as an intermediate in the synthesis of optically active monatin, from indole pyruvic acid and pyruvic acid (or oxaloacetic acid).
Owner:AJINOMOTO CO INC
Process for manufacturing a glutamic acid derivative and a pyroglutamic acid derivative and a novel intermediate in the manufacture thereof
InactiveUS20060009394A1Easy to manufactureEfficient and easy manufactureNervous disorderOrganic compound preparationAlkylating antineoplastic agentHydroxyproline
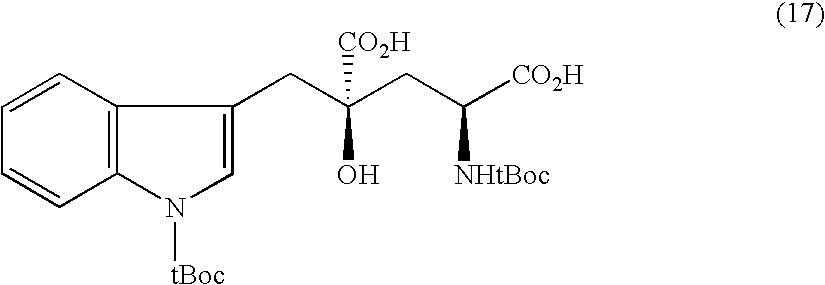
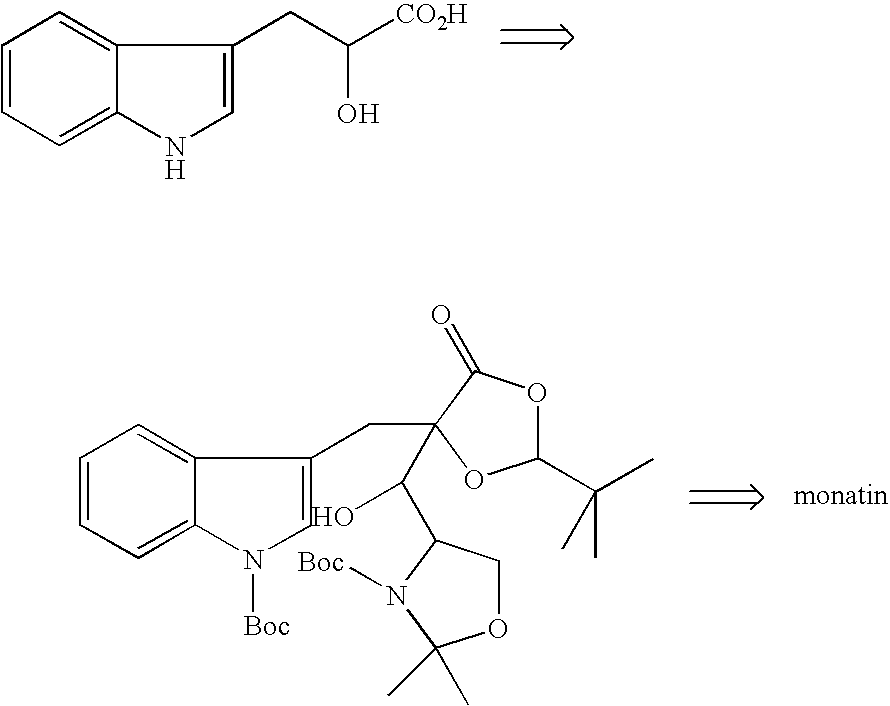
Glutamic acid derivatives, in particular monatin, may be conveniently prepared by alkylating a 4-protected hydroxyl pyroglutamic acid derivative with an alkylating agent to prepare a 4-protected hydroxyl-4-alkylglutamic acid derivative, followed by the steps of hydrolysis and deprotection. The 4-protected hydroxyl pyroglutamic acid derivative is easy to produce from hydroxyproline. The 4-protected hydroxyl pyroglutamic acid derivative is particularly suitable for use in the efficient manufacture of monatin of high optical purity, since it can be alkylated selectively at the 4-position and stereoselectively and after its alkylation, it can easily be converted to a glutamic acid derivative.
Owner:AJINOMOTO CO INC
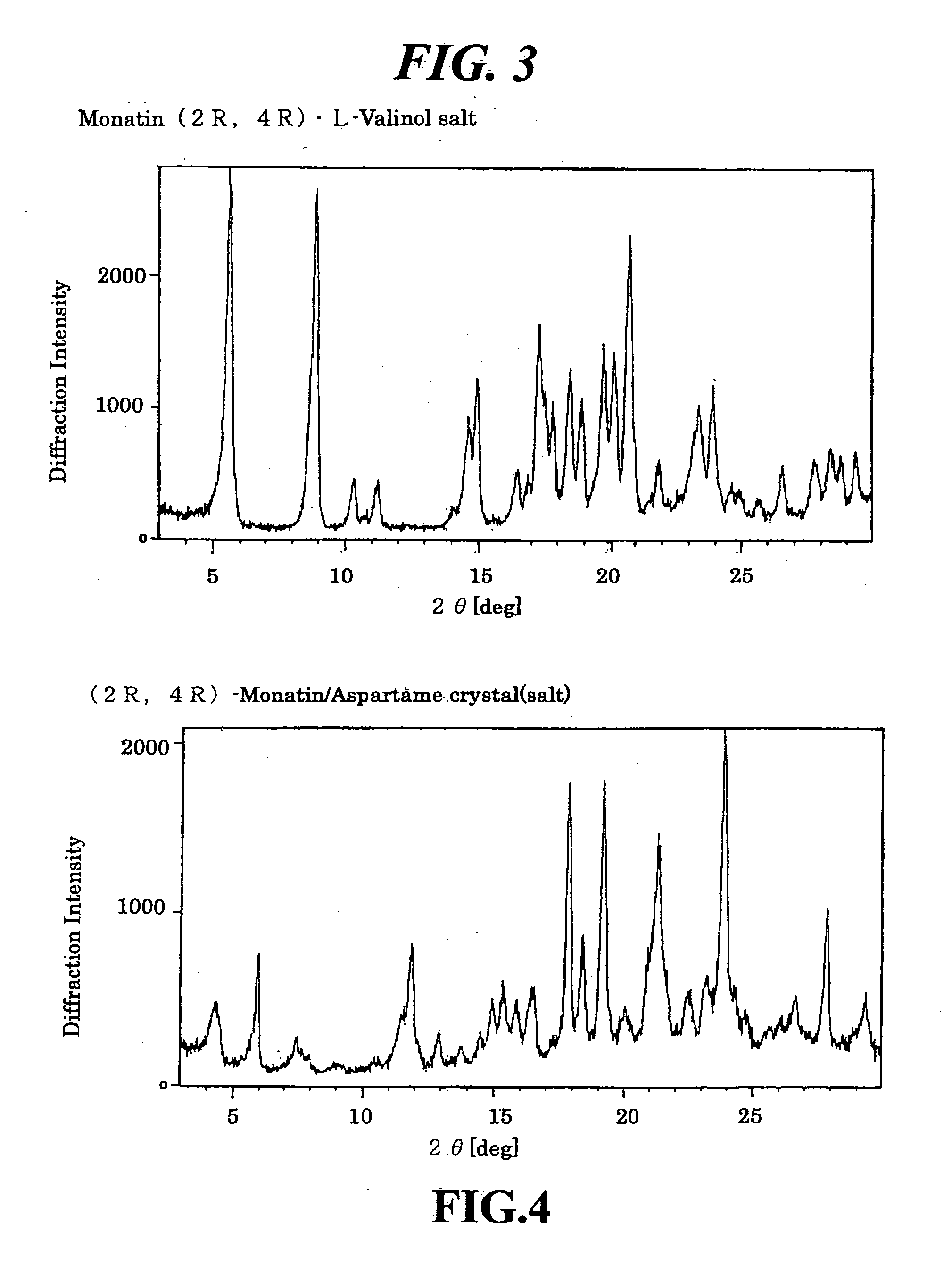
Organic amine salts of glutamic acid derivatives and their application
The present invention provides an organic amine salt of monatin, or crystal form thereof, and its application as well as a method for resolving the stereoisomers of monatin by forming its salt. The present invention further provides a salt form of monatin that is useful as a sweetening agent or as the active ingredient of a sweetener. The present invention also provides a method for preparing a salt of a particular stereoisomer of monatin with an organic amine by utilizing the difference of crystallinity or solubility of the salt of the stereoisomer of monatin with the organic amine. The present invention also relates to a use of the salt in a method for preparing a metal salt of monatin in which the organic amine is replaced by a metal such as sodium, potassium, or the like.
Owner:AJINOMOTO CO INC
Products and Methods for In Vivo Secretion of Monatin
Products and methods for the in vivo production of monatin sweetener are provided. The products include microorganisms that are genetically modified to secrete or to improve secretion of monatin; microorganisms that are genetically modified to produce monatin; and microorganisms that are genetically modified to both secrete or improve secretion of monatin and produce monatin. The methods include producing monatin in such genetically engineered microorganisms.
Owner:CARGILL INC
Method for producing monatin
InactiveUS7396941B2Efficient productionOptically-active compound separationFood preparationIsomerizationOrganic solvent
By simultaneously carrying out an isomerization reaction at position 2 of monatin in different configurations at positions 2 and 4 in the presence of an aldehyde under a condition of pH 4 to 11 in a mixture solvent of water and an organic solvent, and the crystallization of monatin in the same configurations at positions 2 and 4 or a salt thereof, monatin useful as a sweetener, particularly optically active monatin can efficiently be produced.
Owner:AJINOMOTO CO INC
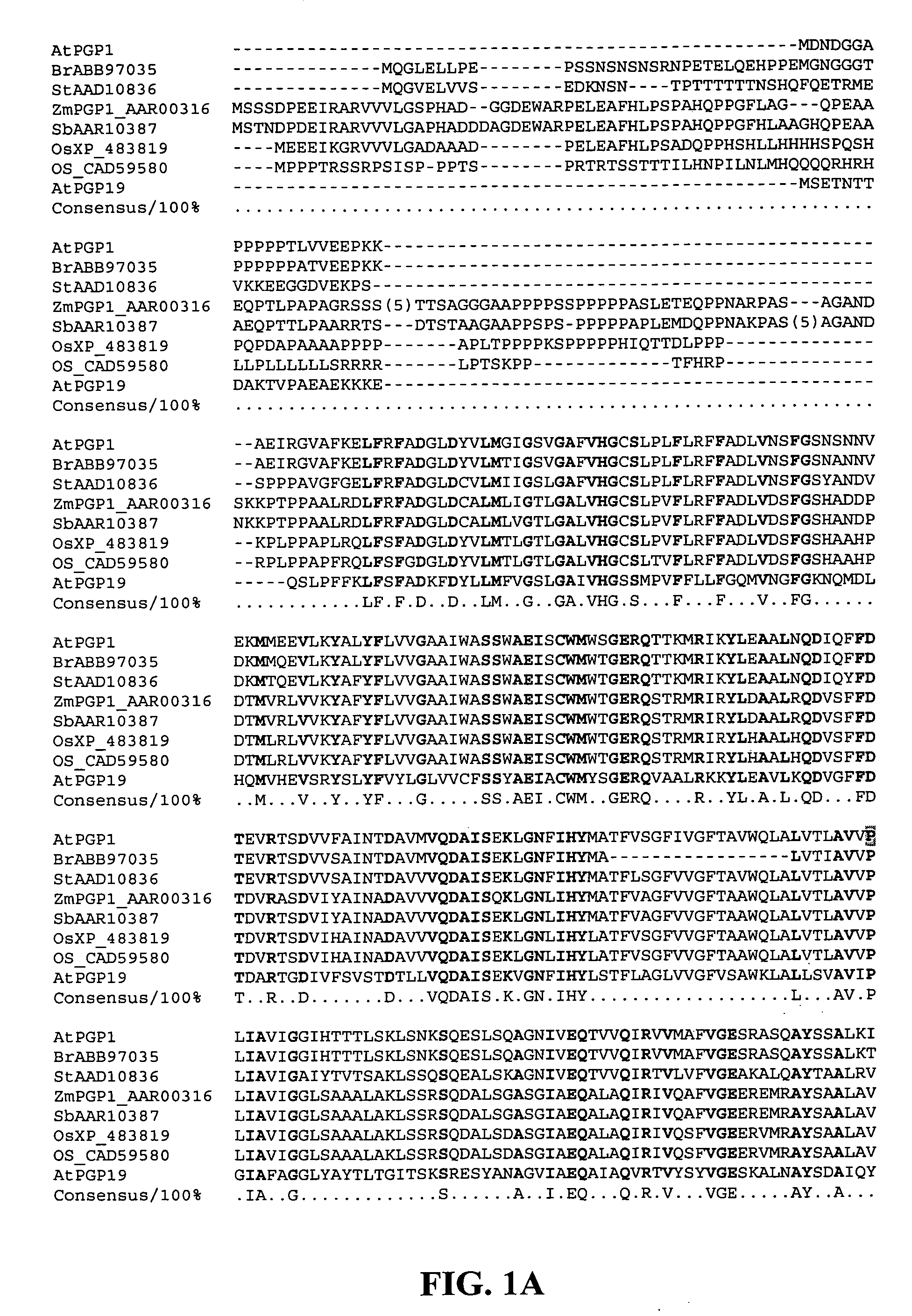
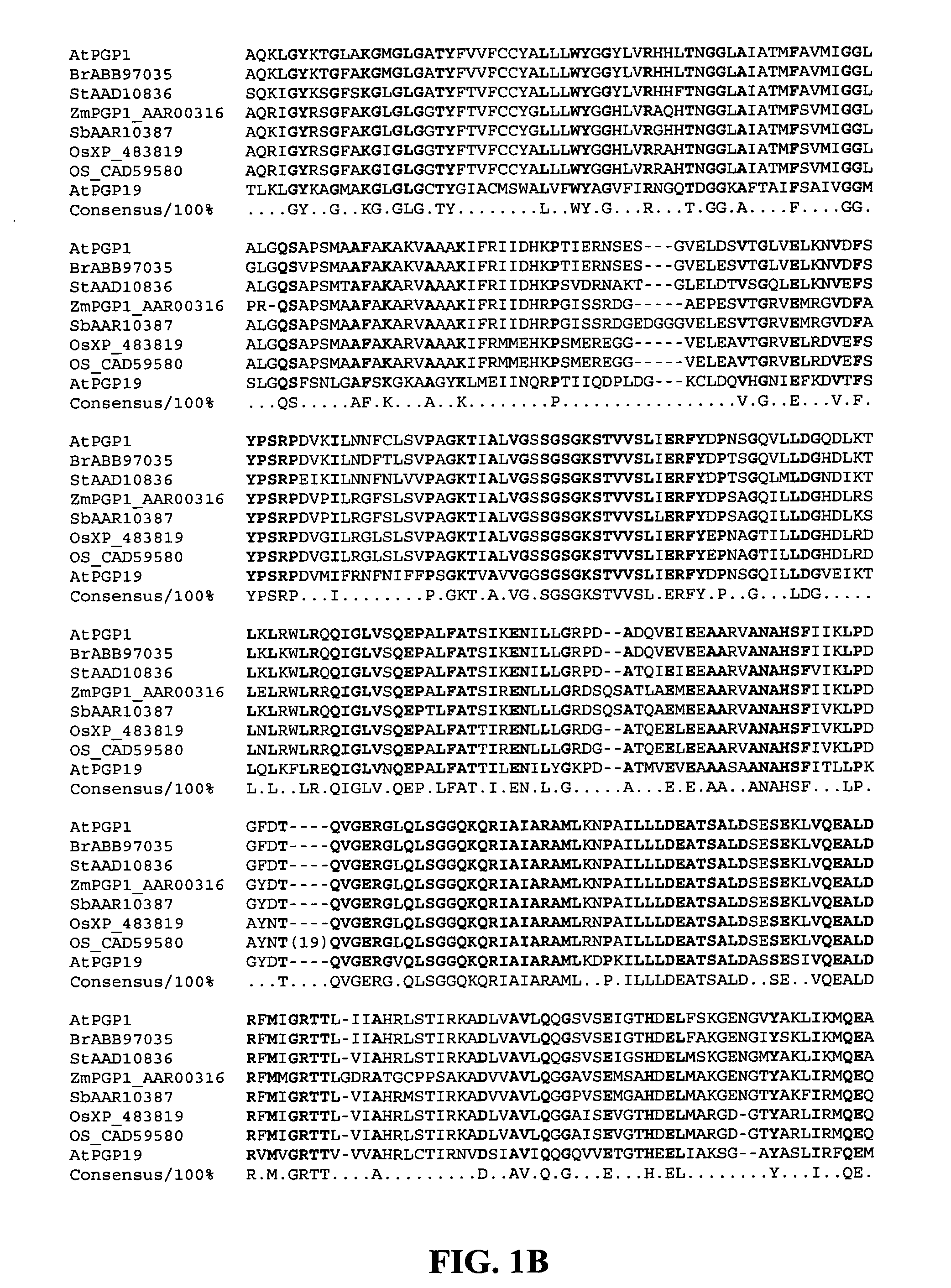
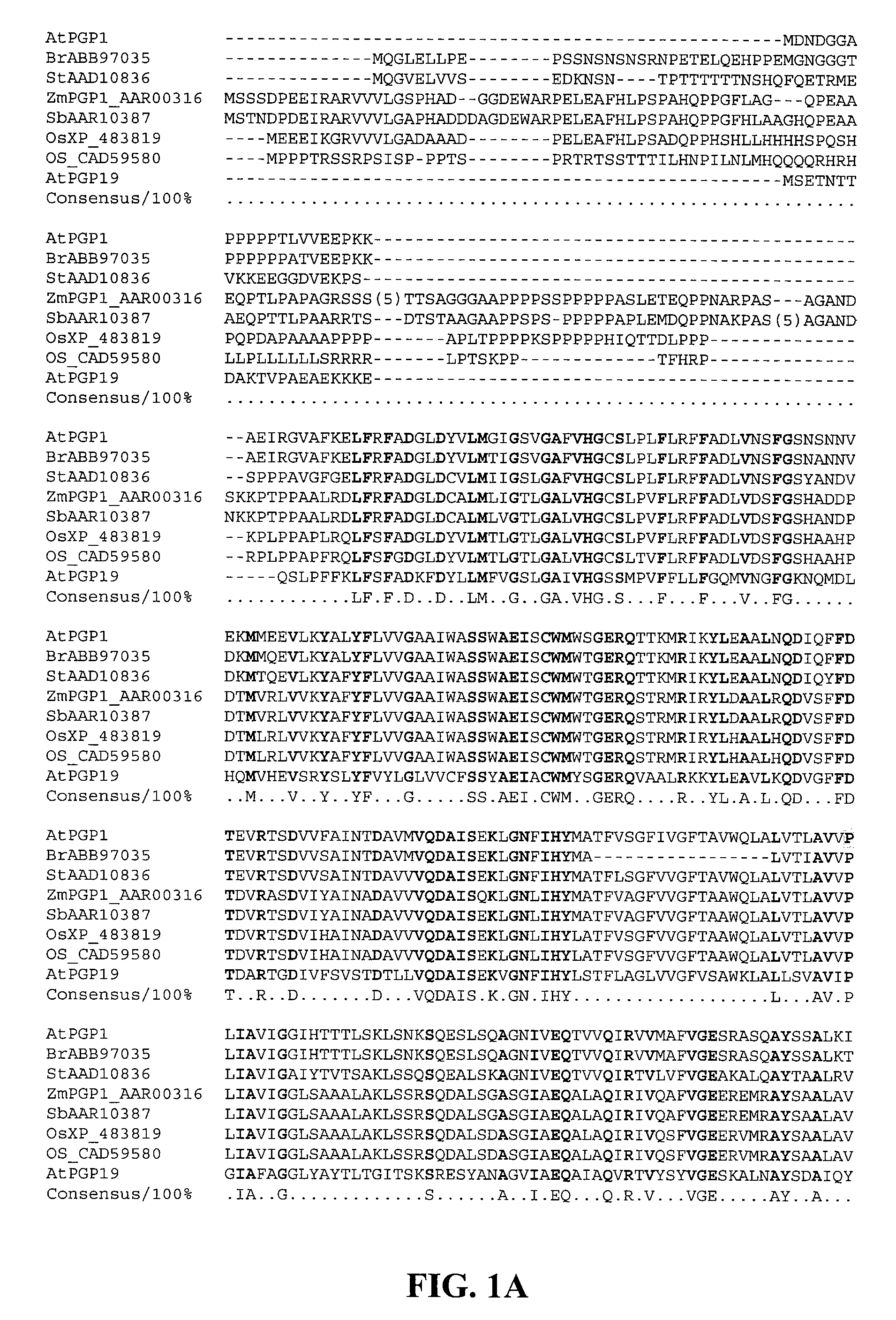
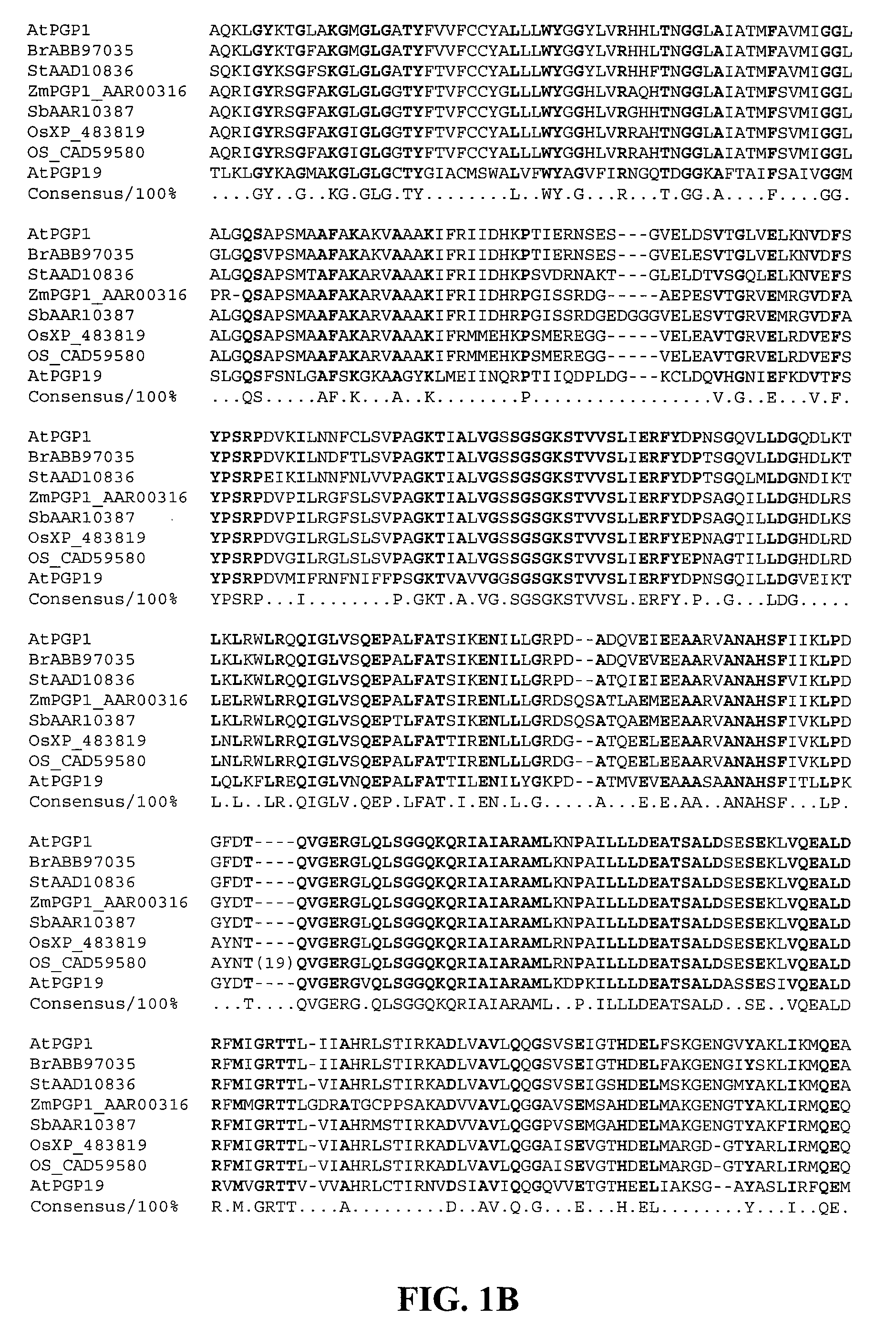
Mutated D-aminotransferase and method for producing optically active glutamic acid derivatives using the same
A D-aminotransferase can be modified so as to efficiently produce (2R, 4R)-monatin having high sweetness intensity from 4-(indol-3-ylmethyl)-4-hydroxy-2-oxoglutaric acid by substituting an amino acid at least at one of positions (positions 100, 180 to 183,243 and 244) involved in efficiently producing the (2R, 4R)-monatin in an amino acid sequence of a wild-type D-aminotransferase represented in SEQ ID NO:2.
Owner:AJINOMOTO CO INC
Processes of producing glutamic acid compounds and production intermediates therefore and novel intermediate for the processes
InactiveUS20060074249A1Produced industrially efficientlyEfficient productionOrganic compound preparationAmino-carboxyl compound preparationMonatinGlutamic acid
The present invention relates to processes of producing glutamic acid compounds, for example, monatin, which are useful as, for example, production intermediates for sweetener or pharmaceutical products.
Owner:AJINOMOTO CO INC
Novel aldolase, and method for producing optically active IHOG and monatin
The present invention relates to a method for producing optically active IHOG, which can in turn be used for the production of monatin. The present invention further relates to a method for producing optically active monatin, and aldolase used for these methods. As such, the present invention enables the synthesis of 4-(Indole-3-ylmethyl)-4-hydroxy-2-oxoglutaric acid with high optical purity, which is useful as an intermediate in the synthesis of optically active monatin, from indole pyruvic acid and pyruvic acid (or oxaloacetic acid).
Owner:AJINOMOTO CO INC
Beverage composition and method of reducing degradation of monatin
A beverage comprising a first ingredient which comprises a C6-C3 phenylpropenoic carbonyl compound in an effective amount to reduce degradation of a second ingredient in the beverage, the second ingredient being monatin, and to reduce a corresponding change in a characteristic of the beverage. In addition, a method of preventing the degradation of monatin in a monatin-containing composition is provided.
Owner:PEPSICO INC
Aldolase and production process of substituted alpha-keto acids
4-(Indol-3-ylmethyl)-4-hydroxy-2-oxoglutarate, which is useful as an intermediate in the synthesis of monatin, may be synthesized from indole pyruvic acid and pyruvic acid (and / or oxaloacetic acid) by using a novel aldolase derived from the genus Pseudomonas, Erwinia, Flavobacterium, or Xanthomonas.
Owner:AJINOMOTO CO INC
Mutated D-aminotransferase and method for producing optically active glutamic acid derivatives using the same
A D-aminotransferase can be modified so as to efficiently produce (2R, 4R)-monatin having high sweetness intensity from 4-(indol-3-ylmethyl)-4-hydroxy-2-oxoglutaric acid by substituting an amino acid at least at one of positions (positions 100, 180 to 183, 243 and 244) involved in efficiently producing the (2R, 4R)-monatin in an amino acid sequence of a wild-type D-aminotransferase represented in SEQ ID NO:2.
Owner:AJINOMOTO CO INC
Beverage products with non-nutritive sweetener and bitterant
Aspects of the invention relate to beverage compositions, including, for example, concentrated and ready-to-drink formulations sweetened with at least one non-nutritive sweetener and further including a bitterant compound in an amount sufficient to reduce the lingering sweet aftertaste of the non-nutritive sweetener(s). In certain illustrative embodiments, the non-nutritive sweetener(s) may be one or more of the following: a steviol glycoside, Lo Han Guo, thaumatin, monatin, monellin, brazzein, sucralose. Another aspect of the invention relates to a method that combines a non-nutritive sweetener having a lingering sweet aftertaste with a bitterant compound to create a mixture such that when the mixture is contained in a beverage, the bitterant compound is present in an amount sufficient to reduce the lingering sweet aftertaste of the non-nutritive sweetener.
Owner:CONCENTRATE MFG OF IRELAND
Process for producing gamma-hydroxyamino acid derivatives and monatins
InactiveUS7329427B2Safe and simple procedureEasy to getOrganic chemistry methodsFood preparationAcid derivativeDicarboxylic acid
Dihydroisoxazole derivatives are conveniently converted to γ-hydroxyamino acid derivatives which are important as various synthetic intermediates by a catalytic hydrogenation reaction. High-purity monatins which may be used as sweeteners or ingredients thereof can be obtained by subjecting a 5-indolylmethyl-4,5-dihydroisoxazole-3,5-dicarboxylic acid to catalytic hydrogenation.
Owner:AJINOMOTO CO INC
Process of producing glutamate derivatives
InactiveUS7297800B2Efficient productionOrganic chemistryFermentationGlutamic Acid DerivativesMedicinal chemistry
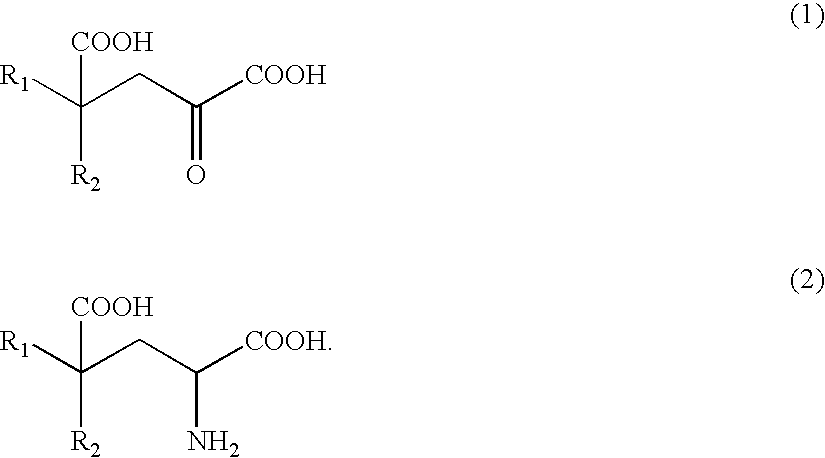
The present invention relates to a process for producing efficiently glutamic acid derivatives (including salts thereof) such as monatin by converting a substituted α-keto acid of formula (1) into a glutamic acid derivative of formula (2) in the presence of an enzyme catalyzing conversion of the same
Owner:AJINOMOTO CO INC
Organic amine salts of glutamic acid derivatives and their application
The present invention provides an organic amine salt of monatin, or crystal form thereof, and its application as well as a method for resolving the stereoisomers of monatin by forming its salt. The present invention further provides a salt form of monatin that is useful as a sweetening agent or as the active ingredient of a sweetener. The present invention also provides a method for preparing a salt of a particular stereoisomer of monatin with an organic amine by utilizing the difference of crystallinity or solubility of the salt of the stereoisomer of monatin with the organic amine. The present invention also relates to a use of the salt in a method for preparing a metal salt of monatin in which the organic amine is replaced by a metal such as sodium, potassium, or the like.
Owner:AJINOMOTO CO INC
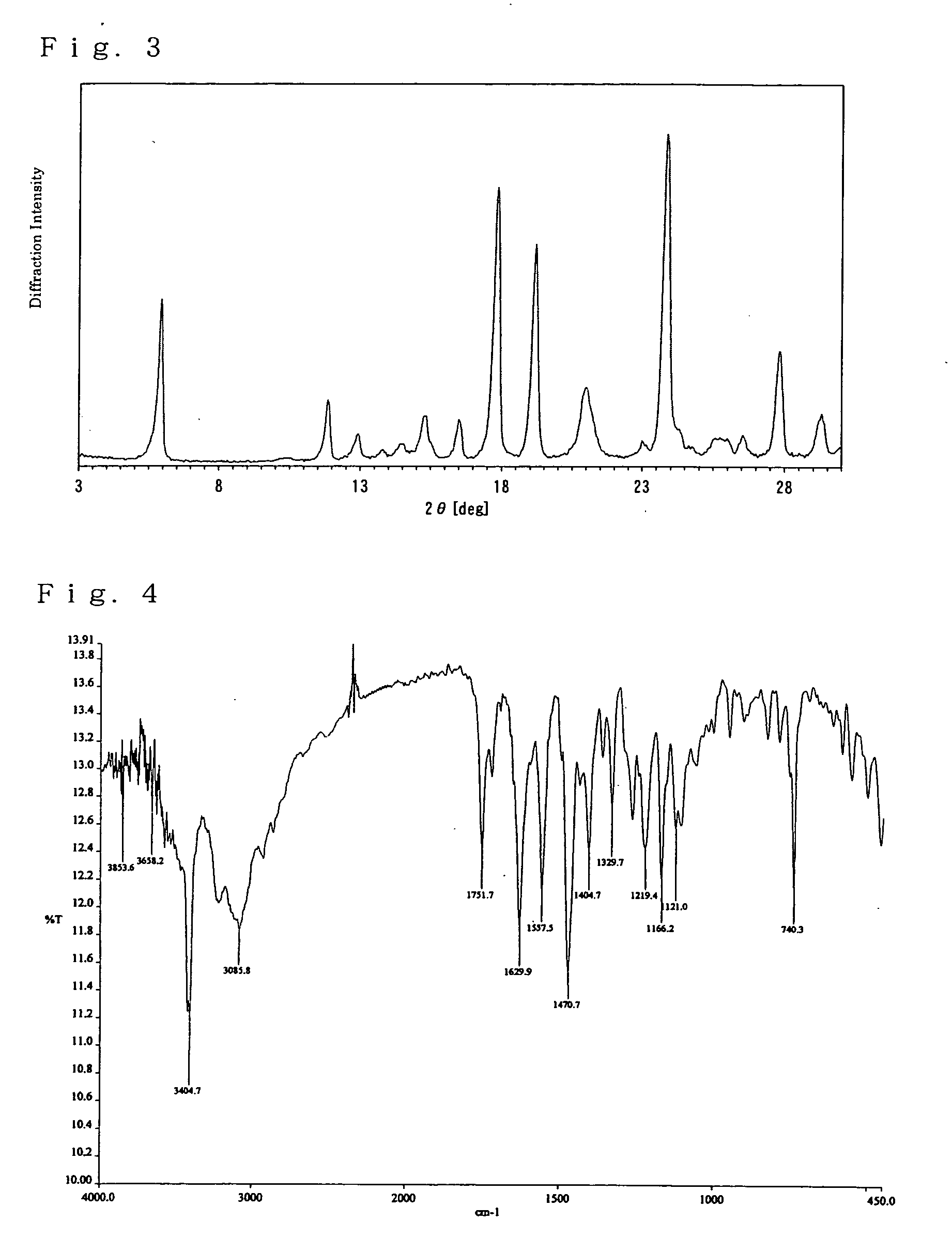
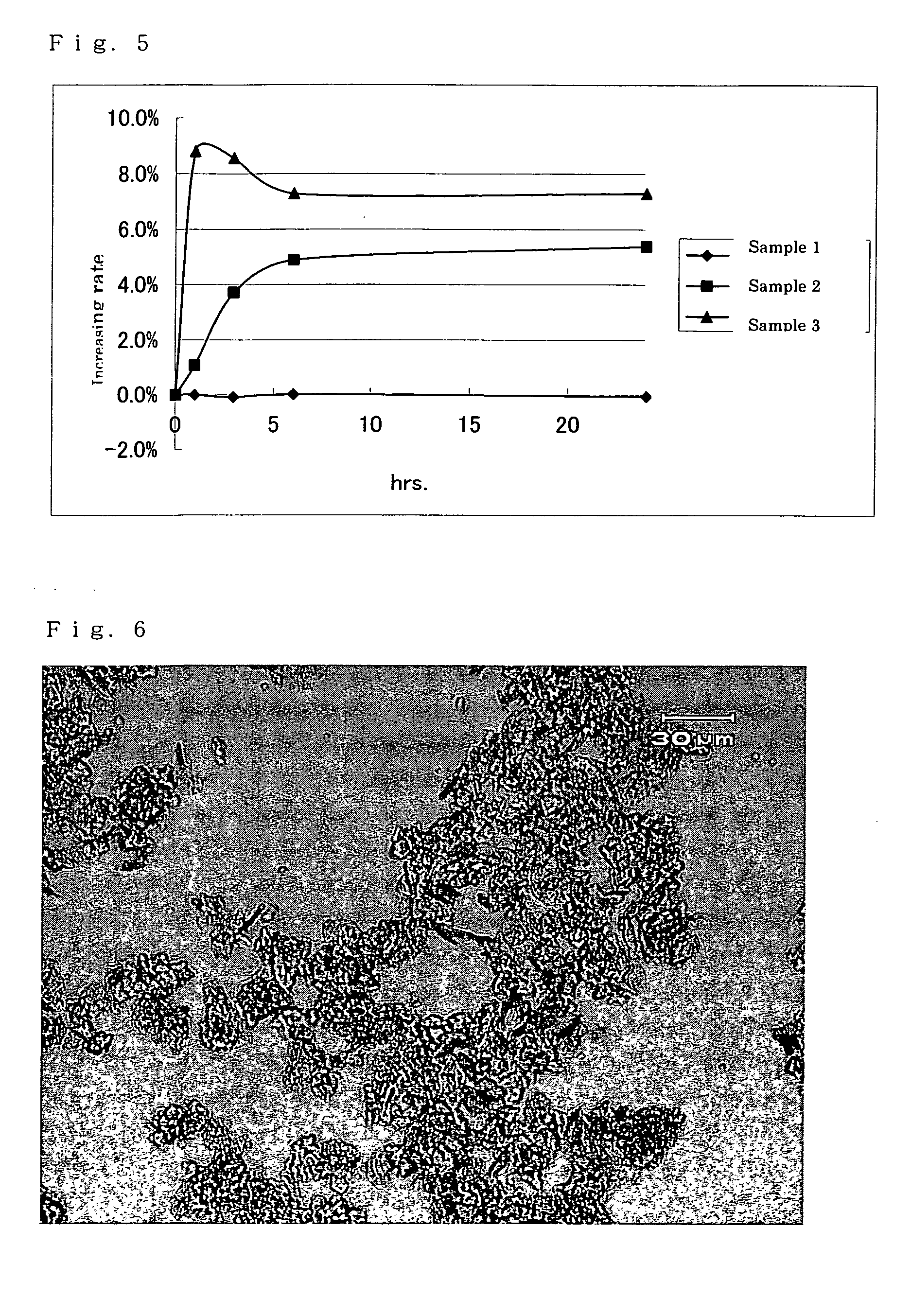
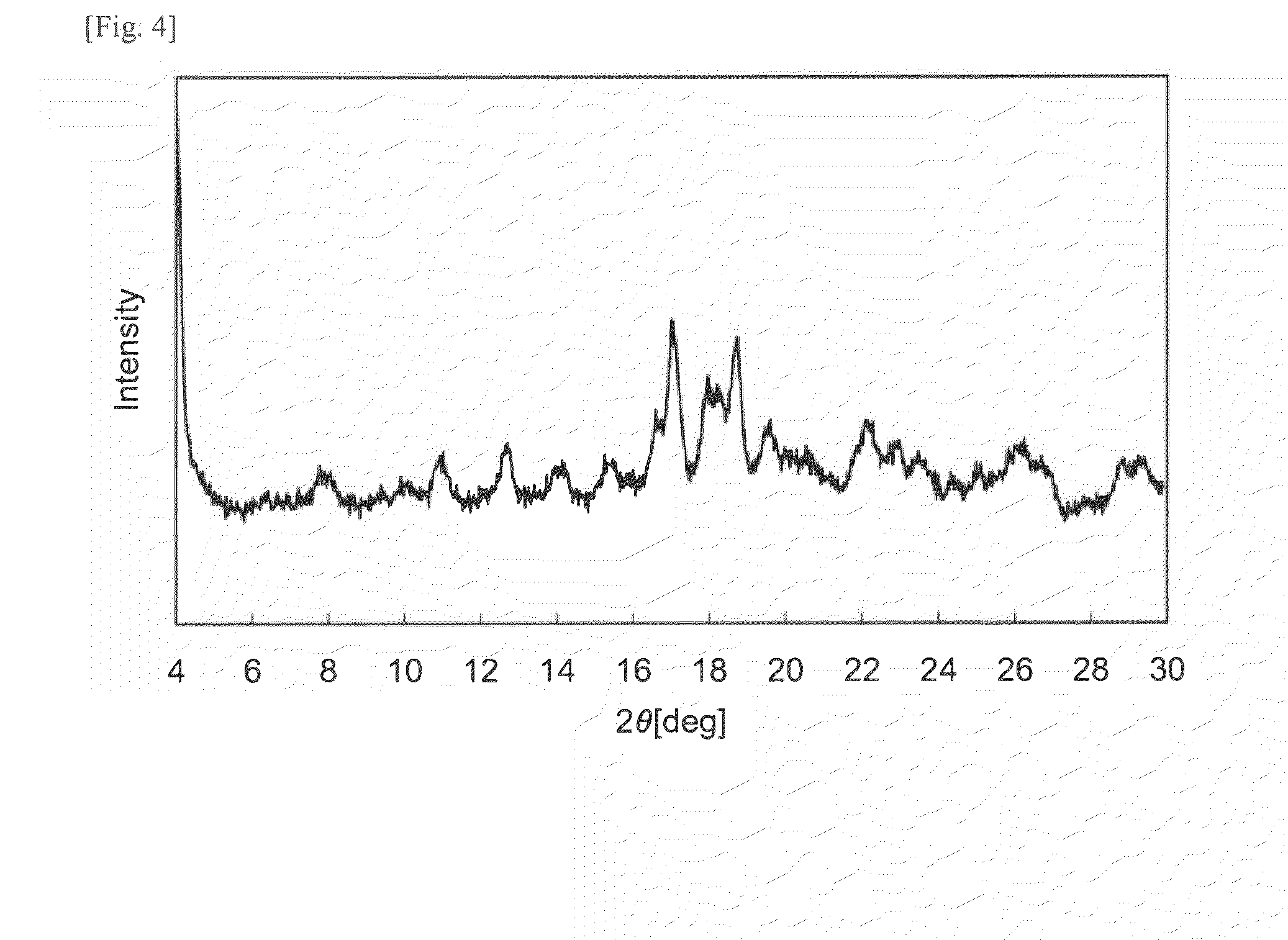
Hydrate crystals
New (2R,4R) monatin monosodium salt hydrate crystals characterized by having specific characteristic X-ray diffraction peaks provide general-purpose, stable, and safe monatin sodium salt crystals incorporating no organic solvent. These crystal may be prepared by a method that requires no organic solvent in the crystallization, separation, and drying steps. These crystal are useful as sweeteners and for the preparation of orally consumed products, such as foods, beverages, pharmaceutical products, topical pharmaceutical products, and feeds containing general-purpose, stable, and safe monatin sodium salt crystals.
Owner:AJINOMOTO CO INC
Beverage products with non-nutritive sweetener and bitterant
InactiveUS20080226789A1Reduce lingering sweet aftertasteFood ingredient as taste affecting agentFood preparationThaumatinBitter tastes
Aspects of the invention relate to beverage compositions, including, for example, concentrated and ready-to-drink formulations sweetened with at least one non-nutritive sweetener and further including a bitterant compound in an amount sufficient to reduce the lingering sweet aftertaste of the non-nutritive sweetener(s). In certain illustrative embodiments, the non-nutritive sweetener(s) may be one or more of the following: a steviol glycoside, Lo Han Guo, thaumatin, monatin, monellin, brazzein, sucralose. Another aspect of the invention relates to a method that combines a non-nutritive sweetener having a lingering sweet aftertaste with a bitterant compound to create a mixture such that when the mixture is contained in a beverage, the bitterant compound is present in an amount sufficient to reduce the lingering sweet aftertaste of the non-nutritive sweetener.
Owner:CONCENTRATE MFG OF IRELAND
Crystals of non-natural-type stereoisomer salt of monatin and use thereof
The present invention provides salt crystals of non-natural stereoisomer forms of monatin and to the use thereof.
Owner:AJINOMOTO CO INC
Process for manufacturing a glutamic acid derivative and a pyroglutamic acid derivative and a novel intermediate in the manufacture thereof
InactiveUS7674915B2Efficient and easy manufactureEasy to manufactureOrganic compound preparationAmino-carboxyl compound preparationAlkyl transferHydroxyproline
Glutamic acid derivatives, in particular monatin, may be conveniently prepared by alkylating a 4-protected hydroxyl pyroglutamic acid derivative with an alkylating agent to prepare a 4-protected hydroxyl-4-alkylglutamic acid derivative, followed by the steps of hydrolysis and deprotection. The 4-protected hydroxyl pyroglutamic acid derivative is easy to produce from hydroxyproline. The 4-protected hydroxyl pyroglutamic acid derivative is particularly suitable for use in the efficient manufacture of monatin of high optical purity, since it can be alkylated selectively at the 4-position and stereoselectively and after its alkylation, it can easily be converted to a glutamic acid derivative.
Owner:AJINOMOTO CO INC
Hydrate crystals
New (2R,4R) monatin monosodium salt hydrate crystals characterized by having specific characteristic X-ray diffraction peaks provide general-purpose, stable, and safe monatin sodium salt crystals incorporating no organic solvent. These crystal may be prepared by a method that requires no organic solvent in the crystallization, separation, and drying steps. These crystal are useful as sweeteners and for the preparation of orally consumed products, such as foods, beverages, pharmaceutical products, topical pharmaceutical products, and feeds containing general-purpose, stable, and safe monatin sodium salt crystals.
Owner:AJINOMOTO CO INC
Monatin hydrate crystals
New (2R,4R) monatin monosodium salt hydrate crystals characterized by having specific characteristic X-ray diffraction peaks provide general-purpose, stable, and safe monatin sodium salt crystals incorporating no organic solvent. These crystal may be prepared by a method that requires no organic solvent in the crystallization, separation, and drying steps. These crystal are useful as sweeteners and for the preparation of orally consumed products, such as foods, beverages, pharmaceutical products, topical pharmaceutical products, and feeds containing general-purpose, stable, and safe monatin sodium salt crystals.
Owner:AJINOMOTO CO INC
Process for manufacturing a glutamic acid derivative and a pyroglutamic acid derivative and a novel intermediate in the manufacture thereof
InactiveUS20100010234A1Efficient and easy manufactureEasy to manufactureSilicon organic compoundsOrganic compound preparationAlkyl transferHydroxyproline
Glutamic acid derivatives, in particular monatin, may be conveniently prepared by alkylating a 4-protected hydroxyl pyroglutamic acid derivative with an alkylating agent to prepare a 4-protected hydroxyl-4-alkylglutamic acid derivative, followed by the steps of hydrolysis and deprotection. The 4-protected hydroxyl pyroglutamic acid derivative is easy to produce from hydroxyproline. The 4-protected hydroxyl pyroglutamic acid derivative is particularly suitable for use in the efficient manufacture of monatin of high optical purity, since it can be alkylated selectively at the 4-position and stereoselectively and after its alkylation, it can easily be converted to a glutamic acid derivative.
Owner:AJINOMOTO CO INC
Chewing gum compositions comprising monatin and methods for making same
Chewing gum compositions are described that include one or more stereoisomers of monatin, a naturally occurring high-intensity sweetener.
Owner:CARGILL INC
Substance mixtures
Substance mixtures are proposed, comprising(a) terpenes,(b) propane-1,3-diol, and optionally(c) active substances selected from the group consisting of(c1) rebaudiosides or plant extracts comprising them,(c2) steviosides or plant extracts comprising them,(c3) monatin,(c4) naringin,(c5) chalcones and hydrochalcones,(c6) mogrosides or plant extracts comprising them,(c7) rubusosides or plant extracts comprising them, and(c8) glycyrrhizic acid or plant extracts comprising it.
Owner:SYMRISE GMBH & CO KG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com