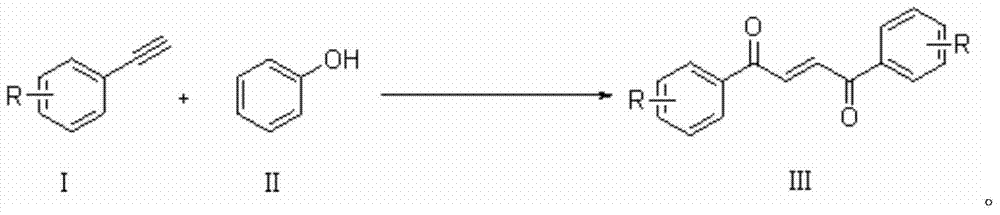
Method for synthesizing 1,4-butene diketone compound
A synthesis method and a butenedione technology, applied in 1 field, can solve the problems of metal participation, complex raw materials, harsh conditions, etc., and achieve the effects of no metal participation, simple raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017]
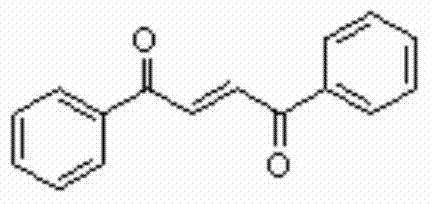
[0018] 0.5mmol phenylacetylene, 10ml CH 3 CN was added to a 50ml reaction flask, and 1mmol Selectfluor and 1mmol phenol were added to the reaction flask twice with an interval of 2 hours. After the addition was completed, it was placed at 30°C and continued to stir for 20 hours. Then, two spoons of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and the pure product was obtained by column chromatography separation (with petroleum ether / ethyl acetate volume ratio of 10 :1 as eluent). The material was a yellow solid, 53% yield.
[0019] Characterization data: R f =0.48 (petroleum ether-EtOAc, 6:1); mp108-111°C; IR (neat, cm -1 )v: 1660 (C=O), 1620 (C=C); E: Z=12:1; 1 HNMR (CDCl 3 , 500MHz) δ: 8.07-8.05 (m, 2H), 8.07 (s, 1H), 7.65-7.61 (m, 2H), 7.54-7.51 (t, J=8Hz, 2H); 13 C NMR (CDCl 3 , 125MHz) δ: 189.8, 136.9, 135.1, 133.8, 128.86, 128.85; MS (EI, 70eV): ...
Embodiment 2
[0021]
[0022] 0.5mmol phenylacetylene, 10ml CH 3 Add CN to a 50ml reaction bottle, then add 1mmol Selectfluor and 1mmol phenol, after the addition is complete, place it at 30°C and stir for 24 hours. Then, two spoonfuls of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and the pure product was obtained by column chromatography separation (with petroleum ether / ethyl acetate volume ratio of 10 :1 as eluent). The material was a yellow solid, 35% yield.
Embodiment 3
[0024]
[0025] 0.5mmol phenylacetylene, 10ml CH 3 CN was added to a 50ml reaction flask, and 1mmol Selectfluor and 1mmol phenol were added to the reaction flask twice with an interval of 2 hours. After the addition was complete, it was placed at 25°C and continued to stir for 20 hours. Then, two spoonfuls of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and the pure product was obtained by column chromatography separation (with petroleum ether / ethyl acetate volume ratio of 10 :1 as eluent). The material was a yellow solid, 48% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com